User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Here’s what ICUs are putting up against COVID-19
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.
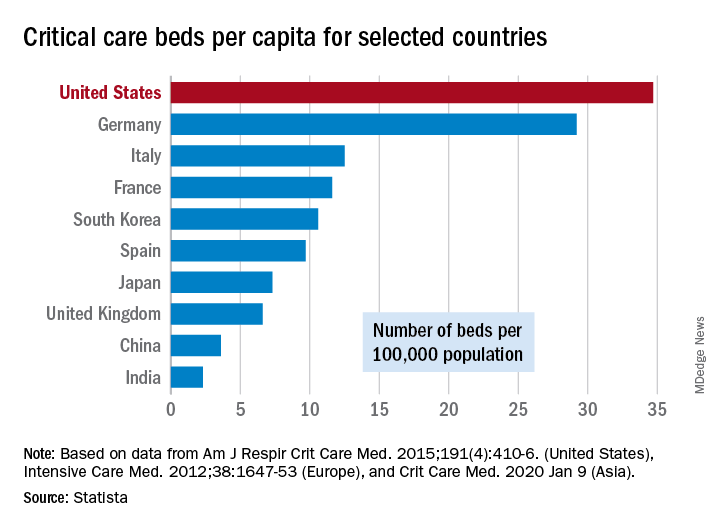
That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
Study supports genetic testing for all breast cancer patients age 65 and younger
Current National Comprehensive Cancer Network (NCCN) criteria may prevent genetic testing in “a substantial proportion” of women who carry germline pathogenic variants in breast cancer predisposition genes, according to investigators.
They found that, by expanding NCCN criteria to include germline genetic testing for all women diagnosed with breast cancer at age 65 or younger, the sensitivity of testing for nine well-established breast cancer predisposition genes would improve from 70% to more than 90%. The sensitivity for detection of BRCA1 and BRCA2 only would improve from 87% to greater than 98%.
Siddhartha Yadav, MD, of the Mayo Clinic, Rochester, Minn., and colleagues reported these findings in the Journal of Clinical Oncology.
“In a large unselected series of women with breast cancer, we demonstrate that expanding the NCCN testing criteria to include all women diagnosed with breast cancer at or before the age of 65 years has the potential to improve the sensitivity of germline genetic testing without the need for evaluation of all women with breast cancer,” Dr. Yadav and colleagues wrote.
Robert Pilarski, who was vice-chair of the panel that drew up the NCCN guidelines, said in an interview that the guideline authors tried to achieve a balance.
“We’ve known that NCCN misses cases and indications, but it comes down to whether the goal is to test all women with mutations or to have criteria that are a cost-effective and reasonable compromise to capture as many patients as possible,” said Mr. Pilarski, a licensed genetic counselor at the Ohio State University Wexner Medical Center in Columbus.
Current NCCN criteria for genetic/familial high-risk assessment for breast, ovarian, and pancreatic cancer recommend testing for individuals with blood relatives who have known or likely pathogenic variants, as well as patients with breast cancer diagnosed at age 45 or younger, patients aged 46-50 years with unknown or limited family history, patients with a second breast cancer diagnosed at any age, patients with triple-negative breast cancer diagnosed at age 60 or younger, and patients with breast cancer diagnosed at any age if they are of Ashkenazi Jewish ancestry.
But as Dr. Yadav and colleagues note, two recent studies (J Clin Oncol. 2019 Feb 20;37[6]:453-60; Ann Surg Oncol. 2018 Oct;25[10]:2925-31) suggested that up to 50% of germline pathogenic variants could be missed if testing were based solely on NCCN criteria.
Based on these findings, the American Society of Breast Surgeons issued a consensus guideline on genetic testing for hereditary breast cancer (Ann Surg Oncol. 2019 Oct;26[10]:3025-31), which states that, “genetic testing should be made available to all patients with a personal history of breast cancer.”
“Without question, if your goal is to identify everyone with a mutation, you’d have to test every cancer patient,” Mr. Pilarski said. “At this point, the ASBrS [American Society of Breast Surgeons] are the only group that have proposed that, and a lot of us feel that’s going too far at this point in time, and so the issue becomes what’s reasonable before that, and I think this paper is a great step forward.”
Cutting through the confusion
To see whether tweaking the existing guidelines could help clarify the issues surrounding genetic testing for breast cancer, Dr. Yadav and colleagues looked at a cohort of patients from the Mayo Clinic Breast Cancer Study. This prospective registry was open to all women evaluated at the Mayo Clinic Rochester for a first diagnosis of invasive breast cancer or ductal carcinoma in situ from May 2000 through May 2016.
The women were evaluated for germline pathogenic variants in nine breast cancer predisposition genes: ATM, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, and TP53.
The researchers found that, of the 3,907 women in the sample, 1,872 (47.9%) would have been recommended for testing under the NCCN criteria, but the remaining 2,035 would not.
Women who met NCCN criteria were significantly more likely to carry a pathogenic variant (9% vs. 3.5%, P less than .001). However, 29.9% of women with pathogenic variants in the nine-gene panel and 13.1% of those with pathogenic variants in BRCA1 or BRCA2 did not qualify for testing by NCCN criteria.
The sensitivity of NCCN criteria was 70% for the nine-gene panel and 87% for BRCA 1 and BRCA 2, with a 53% specificity.
But if the criteria were expanded to include all women age 65 years and younger with a breast cancer diagnosis, the sensitivity for the nine-gene panel would increase to 92.1%, and the sensitivity for BRCA1 and BRCA2 only would climb to greater than 98.1%, with a specificity of approximately 22% for each test combination.
The authors acknowledged that they did not assess the cost-effectiveness of the testing criteria.
This study was supported by grants from the National Institutes of Health and the Breast Cancer Research Foundation. Authors disclosed relationships with Grail, bioTheranostics, Myriad Genetics, and other companies. Mr. Pilarski reported no conflicts of interest.
SOURCE: Yadav S et al. J Clin Oncol. 2020 Mar 3. doi: 10.1200/JCO.19.02190
Current National Comprehensive Cancer Network (NCCN) criteria may prevent genetic testing in “a substantial proportion” of women who carry germline pathogenic variants in breast cancer predisposition genes, according to investigators.
They found that, by expanding NCCN criteria to include germline genetic testing for all women diagnosed with breast cancer at age 65 or younger, the sensitivity of testing for nine well-established breast cancer predisposition genes would improve from 70% to more than 90%. The sensitivity for detection of BRCA1 and BRCA2 only would improve from 87% to greater than 98%.
Siddhartha Yadav, MD, of the Mayo Clinic, Rochester, Minn., and colleagues reported these findings in the Journal of Clinical Oncology.
“In a large unselected series of women with breast cancer, we demonstrate that expanding the NCCN testing criteria to include all women diagnosed with breast cancer at or before the age of 65 years has the potential to improve the sensitivity of germline genetic testing without the need for evaluation of all women with breast cancer,” Dr. Yadav and colleagues wrote.
Robert Pilarski, who was vice-chair of the panel that drew up the NCCN guidelines, said in an interview that the guideline authors tried to achieve a balance.
“We’ve known that NCCN misses cases and indications, but it comes down to whether the goal is to test all women with mutations or to have criteria that are a cost-effective and reasonable compromise to capture as many patients as possible,” said Mr. Pilarski, a licensed genetic counselor at the Ohio State University Wexner Medical Center in Columbus.
Current NCCN criteria for genetic/familial high-risk assessment for breast, ovarian, and pancreatic cancer recommend testing for individuals with blood relatives who have known or likely pathogenic variants, as well as patients with breast cancer diagnosed at age 45 or younger, patients aged 46-50 years with unknown or limited family history, patients with a second breast cancer diagnosed at any age, patients with triple-negative breast cancer diagnosed at age 60 or younger, and patients with breast cancer diagnosed at any age if they are of Ashkenazi Jewish ancestry.
But as Dr. Yadav and colleagues note, two recent studies (J Clin Oncol. 2019 Feb 20;37[6]:453-60; Ann Surg Oncol. 2018 Oct;25[10]:2925-31) suggested that up to 50% of germline pathogenic variants could be missed if testing were based solely on NCCN criteria.
Based on these findings, the American Society of Breast Surgeons issued a consensus guideline on genetic testing for hereditary breast cancer (Ann Surg Oncol. 2019 Oct;26[10]:3025-31), which states that, “genetic testing should be made available to all patients with a personal history of breast cancer.”
“Without question, if your goal is to identify everyone with a mutation, you’d have to test every cancer patient,” Mr. Pilarski said. “At this point, the ASBrS [American Society of Breast Surgeons] are the only group that have proposed that, and a lot of us feel that’s going too far at this point in time, and so the issue becomes what’s reasonable before that, and I think this paper is a great step forward.”
Cutting through the confusion
To see whether tweaking the existing guidelines could help clarify the issues surrounding genetic testing for breast cancer, Dr. Yadav and colleagues looked at a cohort of patients from the Mayo Clinic Breast Cancer Study. This prospective registry was open to all women evaluated at the Mayo Clinic Rochester for a first diagnosis of invasive breast cancer or ductal carcinoma in situ from May 2000 through May 2016.
The women were evaluated for germline pathogenic variants in nine breast cancer predisposition genes: ATM, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, and TP53.
The researchers found that, of the 3,907 women in the sample, 1,872 (47.9%) would have been recommended for testing under the NCCN criteria, but the remaining 2,035 would not.
Women who met NCCN criteria were significantly more likely to carry a pathogenic variant (9% vs. 3.5%, P less than .001). However, 29.9% of women with pathogenic variants in the nine-gene panel and 13.1% of those with pathogenic variants in BRCA1 or BRCA2 did not qualify for testing by NCCN criteria.
The sensitivity of NCCN criteria was 70% for the nine-gene panel and 87% for BRCA 1 and BRCA 2, with a 53% specificity.
But if the criteria were expanded to include all women age 65 years and younger with a breast cancer diagnosis, the sensitivity for the nine-gene panel would increase to 92.1%, and the sensitivity for BRCA1 and BRCA2 only would climb to greater than 98.1%, with a specificity of approximately 22% for each test combination.
The authors acknowledged that they did not assess the cost-effectiveness of the testing criteria.
This study was supported by grants from the National Institutes of Health and the Breast Cancer Research Foundation. Authors disclosed relationships with Grail, bioTheranostics, Myriad Genetics, and other companies. Mr. Pilarski reported no conflicts of interest.
SOURCE: Yadav S et al. J Clin Oncol. 2020 Mar 3. doi: 10.1200/JCO.19.02190
Current National Comprehensive Cancer Network (NCCN) criteria may prevent genetic testing in “a substantial proportion” of women who carry germline pathogenic variants in breast cancer predisposition genes, according to investigators.
They found that, by expanding NCCN criteria to include germline genetic testing for all women diagnosed with breast cancer at age 65 or younger, the sensitivity of testing for nine well-established breast cancer predisposition genes would improve from 70% to more than 90%. The sensitivity for detection of BRCA1 and BRCA2 only would improve from 87% to greater than 98%.
Siddhartha Yadav, MD, of the Mayo Clinic, Rochester, Minn., and colleagues reported these findings in the Journal of Clinical Oncology.
“In a large unselected series of women with breast cancer, we demonstrate that expanding the NCCN testing criteria to include all women diagnosed with breast cancer at or before the age of 65 years has the potential to improve the sensitivity of germline genetic testing without the need for evaluation of all women with breast cancer,” Dr. Yadav and colleagues wrote.
Robert Pilarski, who was vice-chair of the panel that drew up the NCCN guidelines, said in an interview that the guideline authors tried to achieve a balance.
“We’ve known that NCCN misses cases and indications, but it comes down to whether the goal is to test all women with mutations or to have criteria that are a cost-effective and reasonable compromise to capture as many patients as possible,” said Mr. Pilarski, a licensed genetic counselor at the Ohio State University Wexner Medical Center in Columbus.
Current NCCN criteria for genetic/familial high-risk assessment for breast, ovarian, and pancreatic cancer recommend testing for individuals with blood relatives who have known or likely pathogenic variants, as well as patients with breast cancer diagnosed at age 45 or younger, patients aged 46-50 years with unknown or limited family history, patients with a second breast cancer diagnosed at any age, patients with triple-negative breast cancer diagnosed at age 60 or younger, and patients with breast cancer diagnosed at any age if they are of Ashkenazi Jewish ancestry.
But as Dr. Yadav and colleagues note, two recent studies (J Clin Oncol. 2019 Feb 20;37[6]:453-60; Ann Surg Oncol. 2018 Oct;25[10]:2925-31) suggested that up to 50% of germline pathogenic variants could be missed if testing were based solely on NCCN criteria.
Based on these findings, the American Society of Breast Surgeons issued a consensus guideline on genetic testing for hereditary breast cancer (Ann Surg Oncol. 2019 Oct;26[10]:3025-31), which states that, “genetic testing should be made available to all patients with a personal history of breast cancer.”
“Without question, if your goal is to identify everyone with a mutation, you’d have to test every cancer patient,” Mr. Pilarski said. “At this point, the ASBrS [American Society of Breast Surgeons] are the only group that have proposed that, and a lot of us feel that’s going too far at this point in time, and so the issue becomes what’s reasonable before that, and I think this paper is a great step forward.”
Cutting through the confusion
To see whether tweaking the existing guidelines could help clarify the issues surrounding genetic testing for breast cancer, Dr. Yadav and colleagues looked at a cohort of patients from the Mayo Clinic Breast Cancer Study. This prospective registry was open to all women evaluated at the Mayo Clinic Rochester for a first diagnosis of invasive breast cancer or ductal carcinoma in situ from May 2000 through May 2016.
The women were evaluated for germline pathogenic variants in nine breast cancer predisposition genes: ATM, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, and TP53.
The researchers found that, of the 3,907 women in the sample, 1,872 (47.9%) would have been recommended for testing under the NCCN criteria, but the remaining 2,035 would not.
Women who met NCCN criteria were significantly more likely to carry a pathogenic variant (9% vs. 3.5%, P less than .001). However, 29.9% of women with pathogenic variants in the nine-gene panel and 13.1% of those with pathogenic variants in BRCA1 or BRCA2 did not qualify for testing by NCCN criteria.
The sensitivity of NCCN criteria was 70% for the nine-gene panel and 87% for BRCA 1 and BRCA 2, with a 53% specificity.
But if the criteria were expanded to include all women age 65 years and younger with a breast cancer diagnosis, the sensitivity for the nine-gene panel would increase to 92.1%, and the sensitivity for BRCA1 and BRCA2 only would climb to greater than 98.1%, with a specificity of approximately 22% for each test combination.
The authors acknowledged that they did not assess the cost-effectiveness of the testing criteria.
This study was supported by grants from the National Institutes of Health and the Breast Cancer Research Foundation. Authors disclosed relationships with Grail, bioTheranostics, Myriad Genetics, and other companies. Mr. Pilarski reported no conflicts of interest.
SOURCE: Yadav S et al. J Clin Oncol. 2020 Mar 3. doi: 10.1200/JCO.19.02190
FROM THE JOURNAL OF CLINICAL ONCOLOGY
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
Flattening the curve: Viral graphic shows COVID-19 containment needs
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.
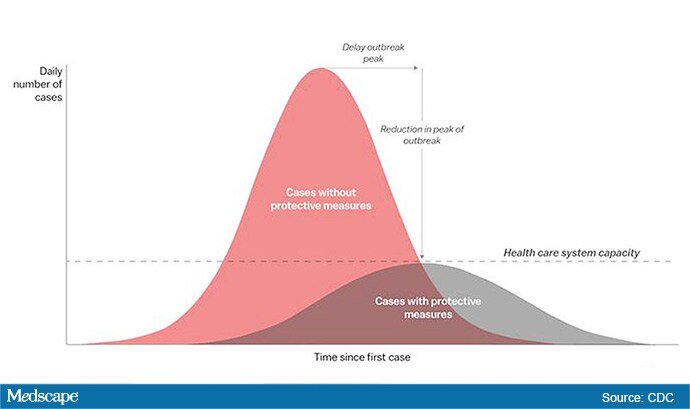
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
So you have a COVID-19 patient: How do you treat them?
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Review highlights shortage of data on elderly cancer patients
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
FROM JOURNAL OF GERIATRIC ONCOLOGY
President declares national emergency for COVID-19, ramps up testing capability
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
Liver cancer risk reduced by aspirin in chronic viral hepatitis
The risk of liver cancer and liver-related death in patients with chronic viral hepatitis was substantially reduced with the use of low-dose aspirin, results from a nationwide study from Sweden suggest.
The risk of hepatocellular carcinoma (HCC) was reduced by 31% compared with no aspirin use, and liver-related mortality dropped by 27%, as long as aspirin use continued.
“We were excited to find for the first time in a nationwide Western population that low-dose aspirin use was associated with substantial reduction in risk of developing incident HCC,” lead author Tracey G. Simon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School in Boston, told Medscape Medical News.
The study was published in the March 12 issue of the New England Journal of Medicine.
HCC is the fourth-leading cause of cancer mortality worldwide, and is driven mostly by viral hepatitis B (HBV) and viral hepatitis C (HCV) infection, noted Jennifer A. Flemming, MD, of Queen’s University, Kingston, Canada, an expert not involved with the study. HCC is also one of the only cancers to show a rising incidence over the past several decades, she added .
However, the results of this do not change clinical practice. “It is premature to prescribe low dose ASA [acetylsalicylic acid] in patients with viral hepatitis for the sole indication of HCC prevention in routine clinical practice without support from prospective randomized data,” she said.
“The results of this study make it clear that a prospective randomized study comparing ASA to placebo in patients with viral hepatitis without an indication for low-dose ASA is justified to evaluate the risk of incident HCC,” she told Medscape Medical News.
The study authors agree, and they also emphasize that the findings from this observational study “should not yet change clinical practice.”
More research is needed in populations with compensated and decompensated cirrhosis to determine the optimal timing of aspirin initiation — or cessation of therapy — that will maximize benefit and prevent adverse events, said Simon.
Study Details
Although several earlier studies have suggested a duration-dependent benefit of aspirin use in preventing HCC in smaller populations, this study is the first to confirm a duration-response relationship with low-dose aspirin use in an unselected European population with confirmed viral hepatitis, Simon pointed out.
For their study, Simon and colleagues used the Swedish Register for Surveillance of Communicable Diseases database to identify 50,275 adults diagnosed between 2005 and 2015 with acute and chronic HBV and HCV infection. Some 13,276 adults had HBV and 36,999 had HCV, and this included 14,205 low-dose (75 mg or 160 mg) aspirin users and 36,070 nonusers.
The analysis showed that in aspirin users, the 10-year cumulative incidence of HCC was 4% compared with 8.3% in nonusers. After multivariable adjustment, aspirin users had a risk of HCC that was 31% lower compared with nonusers (adjusted subhazard ratio, 0.69; 95% confidence interval [CI], 0.62 - 0.76).
Patients taking low-dose aspirin had a 10-year liver-related mortality of 11% compared with 17.9% among nonusers. The adjusted risk of liver-related mortality was 27% lower in aspirin users than in nonusers.
There was no significant difference in the 10-year risk of gastrointestinal bleeding between users and nonusers of aspirin (7.8% and 6.9%, respectively). In addition, the analysis showed that the risks of any gastrointestinal bleeding were similar among aspirin-users with compensated cirrhosis and those without cirrhosis (8.3% and 7.5%, respectively).
Notably, the risk of HCC was significantly lower after 3 to 5 years of aspirin use and after 5 or more years of use (adjusted hazard ratio [HR], 0.66, 0.57, respectively) compared with short-term use (3 months to <1 year; adjusted HR, 0.90) or with intermittent, discontinued, or no aspirin use. But when those with chronic viral hepatitis stopped taking aspirin, their risk of HCC rose to become 22% higher compared with peers who continued to use aspirin.
The risk of liver-related death also rose by 31% in aspirin users who stopped taking aspirin compared with those who did not stop (subhazard ratio, 1.31). Again, this relationship appeared to be duration-dependent, with the risk of incident HCC rising sharply among those who discontinued aspirin and increasing in magnitude over time.
The consistency of aspirin use also influenced risk. In individuals who had an on-again, off-again pattern of aspirin use, the incidence of HCC was 5.9% compared with 1.1% in those who used it consistently.
“Our results were consistent regardless of sex, cause of hepatitis, or underlying compensated cirrhosis,” the authors write. “The consistent duration-response associations lend further credence to a potential causal relationship.”
Limitations of the Study
The current study findings are not new, but this is the best-designed study to date, commented Flemming. Still, there were a number of limitations, she noted. Although cirrhosis is the strongest risk factor for HCC in patients with viral hepatitis, for instance, it was assessed only at cohort entry, and not during the median 8 years of follow-up. There was also a lack of information about sustained virologic response (SVR) rates.
Since less than 25% of patients with HCV received HCV therapy, this indicates they were likely treated with interferon-based therapy, Flemming suggested. Interferon-based therapy is associated with much lower SVR rates than direct acting antiviral (DAA) therapy, which can produce SVR in approximately 95% of patients, she pointed out.
“Therefore, a large proportion of the study patients were likely viremic and at a higher baseline risk of HCC than contemporary HCV populations.”
Evidence from a number of studies indicates that achieving SVR with DAA therapy is associated with a 70% risk reduction for incident HCC and liver-related events, Flemming said. “Whether the use of ASA in patients who have achieved SVR provides the same HCC risk reduction and decrease in hepatic outcomes is unknown.”
Also, the study did not provide information on the specific type of HBV therapy used in patients with HBV, Flemming noted. When considering the prevention of HCC in patients with chronic HBV infection, recent data support a differential protective effect of tenofovir disoproxil fumarate (multiple brands) compared with entecavir (Baraclude, Bristol-Myers Squibb), she pointed out. As previously reported by Medscape Medical News, these data also indicate that tenofovir may be more effective than entecavir in reducing the risk of liver failure and all-cause mortality.
This study was funded by the US National Institutes of Health, Nyckelfonden, Region Stockholm County, the American Association for the Study of Liver Diseases, Boston Nutrition Obesity Research Council, Region Örebro County, and Karolinska Institutet. Simon has disclosed no relevant financial relationships. A number of study coauthors disclosed having relationships with industry; the full list can be found with the original article. Flemming reported relationships with Gilead Sciences Canada, AbbVie, and Lupin Pharmaceuticals.
This article first appeared on Medscape.com.
N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMoa1912035.
The risk of liver cancer and liver-related death in patients with chronic viral hepatitis was substantially reduced with the use of low-dose aspirin, results from a nationwide study from Sweden suggest.
The risk of hepatocellular carcinoma (HCC) was reduced by 31% compared with no aspirin use, and liver-related mortality dropped by 27%, as long as aspirin use continued.
“We were excited to find for the first time in a nationwide Western population that low-dose aspirin use was associated with substantial reduction in risk of developing incident HCC,” lead author Tracey G. Simon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School in Boston, told Medscape Medical News.
The study was published in the March 12 issue of the New England Journal of Medicine.
HCC is the fourth-leading cause of cancer mortality worldwide, and is driven mostly by viral hepatitis B (HBV) and viral hepatitis C (HCV) infection, noted Jennifer A. Flemming, MD, of Queen’s University, Kingston, Canada, an expert not involved with the study. HCC is also one of the only cancers to show a rising incidence over the past several decades, she added .
However, the results of this do not change clinical practice. “It is premature to prescribe low dose ASA [acetylsalicylic acid] in patients with viral hepatitis for the sole indication of HCC prevention in routine clinical practice without support from prospective randomized data,” she said.
“The results of this study make it clear that a prospective randomized study comparing ASA to placebo in patients with viral hepatitis without an indication for low-dose ASA is justified to evaluate the risk of incident HCC,” she told Medscape Medical News.
The study authors agree, and they also emphasize that the findings from this observational study “should not yet change clinical practice.”
More research is needed in populations with compensated and decompensated cirrhosis to determine the optimal timing of aspirin initiation — or cessation of therapy — that will maximize benefit and prevent adverse events, said Simon.
Study Details
Although several earlier studies have suggested a duration-dependent benefit of aspirin use in preventing HCC in smaller populations, this study is the first to confirm a duration-response relationship with low-dose aspirin use in an unselected European population with confirmed viral hepatitis, Simon pointed out.
For their study, Simon and colleagues used the Swedish Register for Surveillance of Communicable Diseases database to identify 50,275 adults diagnosed between 2005 and 2015 with acute and chronic HBV and HCV infection. Some 13,276 adults had HBV and 36,999 had HCV, and this included 14,205 low-dose (75 mg or 160 mg) aspirin users and 36,070 nonusers.
The analysis showed that in aspirin users, the 10-year cumulative incidence of HCC was 4% compared with 8.3% in nonusers. After multivariable adjustment, aspirin users had a risk of HCC that was 31% lower compared with nonusers (adjusted subhazard ratio, 0.69; 95% confidence interval [CI], 0.62 - 0.76).
Patients taking low-dose aspirin had a 10-year liver-related mortality of 11% compared with 17.9% among nonusers. The adjusted risk of liver-related mortality was 27% lower in aspirin users than in nonusers.
There was no significant difference in the 10-year risk of gastrointestinal bleeding between users and nonusers of aspirin (7.8% and 6.9%, respectively). In addition, the analysis showed that the risks of any gastrointestinal bleeding were similar among aspirin-users with compensated cirrhosis and those without cirrhosis (8.3% and 7.5%, respectively).
Notably, the risk of HCC was significantly lower after 3 to 5 years of aspirin use and after 5 or more years of use (adjusted hazard ratio [HR], 0.66, 0.57, respectively) compared with short-term use (3 months to <1 year; adjusted HR, 0.90) or with intermittent, discontinued, or no aspirin use. But when those with chronic viral hepatitis stopped taking aspirin, their risk of HCC rose to become 22% higher compared with peers who continued to use aspirin.
The risk of liver-related death also rose by 31% in aspirin users who stopped taking aspirin compared with those who did not stop (subhazard ratio, 1.31). Again, this relationship appeared to be duration-dependent, with the risk of incident HCC rising sharply among those who discontinued aspirin and increasing in magnitude over time.
The consistency of aspirin use also influenced risk. In individuals who had an on-again, off-again pattern of aspirin use, the incidence of HCC was 5.9% compared with 1.1% in those who used it consistently.
“Our results were consistent regardless of sex, cause of hepatitis, or underlying compensated cirrhosis,” the authors write. “The consistent duration-response associations lend further credence to a potential causal relationship.”
Limitations of the Study
The current study findings are not new, but this is the best-designed study to date, commented Flemming. Still, there were a number of limitations, she noted. Although cirrhosis is the strongest risk factor for HCC in patients with viral hepatitis, for instance, it was assessed only at cohort entry, and not during the median 8 years of follow-up. There was also a lack of information about sustained virologic response (SVR) rates.
Since less than 25% of patients with HCV received HCV therapy, this indicates they were likely treated with interferon-based therapy, Flemming suggested. Interferon-based therapy is associated with much lower SVR rates than direct acting antiviral (DAA) therapy, which can produce SVR in approximately 95% of patients, she pointed out.
“Therefore, a large proportion of the study patients were likely viremic and at a higher baseline risk of HCC than contemporary HCV populations.”
Evidence from a number of studies indicates that achieving SVR with DAA therapy is associated with a 70% risk reduction for incident HCC and liver-related events, Flemming said. “Whether the use of ASA in patients who have achieved SVR provides the same HCC risk reduction and decrease in hepatic outcomes is unknown.”
Also, the study did not provide information on the specific type of HBV therapy used in patients with HBV, Flemming noted. When considering the prevention of HCC in patients with chronic HBV infection, recent data support a differential protective effect of tenofovir disoproxil fumarate (multiple brands) compared with entecavir (Baraclude, Bristol-Myers Squibb), she pointed out. As previously reported by Medscape Medical News, these data also indicate that tenofovir may be more effective than entecavir in reducing the risk of liver failure and all-cause mortality.
This study was funded by the US National Institutes of Health, Nyckelfonden, Region Stockholm County, the American Association for the Study of Liver Diseases, Boston Nutrition Obesity Research Council, Region Örebro County, and Karolinska Institutet. Simon has disclosed no relevant financial relationships. A number of study coauthors disclosed having relationships with industry; the full list can be found with the original article. Flemming reported relationships with Gilead Sciences Canada, AbbVie, and Lupin Pharmaceuticals.
This article first appeared on Medscape.com.
N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMoa1912035.
The risk of liver cancer and liver-related death in patients with chronic viral hepatitis was substantially reduced with the use of low-dose aspirin, results from a nationwide study from Sweden suggest.
The risk of hepatocellular carcinoma (HCC) was reduced by 31% compared with no aspirin use, and liver-related mortality dropped by 27%, as long as aspirin use continued.
“We were excited to find for the first time in a nationwide Western population that low-dose aspirin use was associated with substantial reduction in risk of developing incident HCC,” lead author Tracey G. Simon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School in Boston, told Medscape Medical News.
The study was published in the March 12 issue of the New England Journal of Medicine.
HCC is the fourth-leading cause of cancer mortality worldwide, and is driven mostly by viral hepatitis B (HBV) and viral hepatitis C (HCV) infection, noted Jennifer A. Flemming, MD, of Queen’s University, Kingston, Canada, an expert not involved with the study. HCC is also one of the only cancers to show a rising incidence over the past several decades, she added .
However, the results of this do not change clinical practice. “It is premature to prescribe low dose ASA [acetylsalicylic acid] in patients with viral hepatitis for the sole indication of HCC prevention in routine clinical practice without support from prospective randomized data,” she said.
“The results of this study make it clear that a prospective randomized study comparing ASA to placebo in patients with viral hepatitis without an indication for low-dose ASA is justified to evaluate the risk of incident HCC,” she told Medscape Medical News.
The study authors agree, and they also emphasize that the findings from this observational study “should not yet change clinical practice.”
More research is needed in populations with compensated and decompensated cirrhosis to determine the optimal timing of aspirin initiation — or cessation of therapy — that will maximize benefit and prevent adverse events, said Simon.
Study Details
Although several earlier studies have suggested a duration-dependent benefit of aspirin use in preventing HCC in smaller populations, this study is the first to confirm a duration-response relationship with low-dose aspirin use in an unselected European population with confirmed viral hepatitis, Simon pointed out.
For their study, Simon and colleagues used the Swedish Register for Surveillance of Communicable Diseases database to identify 50,275 adults diagnosed between 2005 and 2015 with acute and chronic HBV and HCV infection. Some 13,276 adults had HBV and 36,999 had HCV, and this included 14,205 low-dose (75 mg or 160 mg) aspirin users and 36,070 nonusers.
The analysis showed that in aspirin users, the 10-year cumulative incidence of HCC was 4% compared with 8.3% in nonusers. After multivariable adjustment, aspirin users had a risk of HCC that was 31% lower compared with nonusers (adjusted subhazard ratio, 0.69; 95% confidence interval [CI], 0.62 - 0.76).
Patients taking low-dose aspirin had a 10-year liver-related mortality of 11% compared with 17.9% among nonusers. The adjusted risk of liver-related mortality was 27% lower in aspirin users than in nonusers.
There was no significant difference in the 10-year risk of gastrointestinal bleeding between users and nonusers of aspirin (7.8% and 6.9%, respectively). In addition, the analysis showed that the risks of any gastrointestinal bleeding were similar among aspirin-users with compensated cirrhosis and those without cirrhosis (8.3% and 7.5%, respectively).
Notably, the risk of HCC was significantly lower after 3 to 5 years of aspirin use and after 5 or more years of use (adjusted hazard ratio [HR], 0.66, 0.57, respectively) compared with short-term use (3 months to <1 year; adjusted HR, 0.90) or with intermittent, discontinued, or no aspirin use. But when those with chronic viral hepatitis stopped taking aspirin, their risk of HCC rose to become 22% higher compared with peers who continued to use aspirin.
The risk of liver-related death also rose by 31% in aspirin users who stopped taking aspirin compared with those who did not stop (subhazard ratio, 1.31). Again, this relationship appeared to be duration-dependent, with the risk of incident HCC rising sharply among those who discontinued aspirin and increasing in magnitude over time.
The consistency of aspirin use also influenced risk. In individuals who had an on-again, off-again pattern of aspirin use, the incidence of HCC was 5.9% compared with 1.1% in those who used it consistently.
“Our results were consistent regardless of sex, cause of hepatitis, or underlying compensated cirrhosis,” the authors write. “The consistent duration-response associations lend further credence to a potential causal relationship.”
Limitations of the Study
The current study findings are not new, but this is the best-designed study to date, commented Flemming. Still, there were a number of limitations, she noted. Although cirrhosis is the strongest risk factor for HCC in patients with viral hepatitis, for instance, it was assessed only at cohort entry, and not during the median 8 years of follow-up. There was also a lack of information about sustained virologic response (SVR) rates.
Since less than 25% of patients with HCV received HCV therapy, this indicates they were likely treated with interferon-based therapy, Flemming suggested. Interferon-based therapy is associated with much lower SVR rates than direct acting antiviral (DAA) therapy, which can produce SVR in approximately 95% of patients, she pointed out.
“Therefore, a large proportion of the study patients were likely viremic and at a higher baseline risk of HCC than contemporary HCV populations.”
Evidence from a number of studies indicates that achieving SVR with DAA therapy is associated with a 70% risk reduction for incident HCC and liver-related events, Flemming said. “Whether the use of ASA in patients who have achieved SVR provides the same HCC risk reduction and decrease in hepatic outcomes is unknown.”
Also, the study did not provide information on the specific type of HBV therapy used in patients with HBV, Flemming noted. When considering the prevention of HCC in patients with chronic HBV infection, recent data support a differential protective effect of tenofovir disoproxil fumarate (multiple brands) compared with entecavir (Baraclude, Bristol-Myers Squibb), she pointed out. As previously reported by Medscape Medical News, these data also indicate that tenofovir may be more effective than entecavir in reducing the risk of liver failure and all-cause mortality.
This study was funded by the US National Institutes of Health, Nyckelfonden, Region Stockholm County, the American Association for the Study of Liver Diseases, Boston Nutrition Obesity Research Council, Region Örebro County, and Karolinska Institutet. Simon has disclosed no relevant financial relationships. A number of study coauthors disclosed having relationships with industry; the full list can be found with the original article. Flemming reported relationships with Gilead Sciences Canada, AbbVie, and Lupin Pharmaceuticals.
This article first appeared on Medscape.com.
N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMoa1912035.
Lombardy ICU capacity stressed to breaking point by COVID-19 outbreak
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.
Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
REPORTING FROM JAMA LIVE STREAM
Internist reports from COVID-19 front lines near Seattle
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.