User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA orders stronger warnings on benzodiazepines
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
Reassuring rheumatic disease patients on value of bisphosphonates
“When we think about bisphosphonates, we have to think about whether they are good players or bad players,” Marcy B. Bolster, MD, of Harvard Medical School, Boston, said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Although bisphosphonates are a first-line treatment for many patients to reduce fracture risk, rheumatology patients have distinct concerns about these medications, said Dr. Bolster, who is director of the Rheumatology Fellowship Training Program at Massachusetts General Hospital, Boston, and medical lead for the Fracture Liaison Service there.
She shared her insights on four questions most often asked by patients in her practice:
How long do I need to take this medication?
When discussing bisphosphonates with a patient for the first time, “I typically start with the benefits, and include the risks,” she said. “Then I outline my plans for treatment, which would include treatment duration.”
Setting expectations with the patient about planned duration of therapy, reviewing risks and benefits, and preparing to be flexible if changes are needed can help relieve patients’ concerns, she said.
For example, in a hypothetical case of a 69-year-old woman with a 17% chance of a major osteoporotic fracture and 3.8% chance of hip fracture in the next 10 years based on FRAX scores, Dr. Bolster said she would treat with alendronate or zoledronic acid.
Duration must be a clinical decision individualized to the patient, she noted. Research studies support that some patients benefit from a longer duration of therapy. In the Fracture Intervention Trial (FIT) Long-Term Extension, which included 1,099 women, the risk of clinical vertebral fractures significantly declined with 10 years of alendronate treatment, compared with 5 years of treatment, she said.
In the HORIZON trial of 1,233 postmenopausal women, the risk of new morphometric vertebral fractures was significantly lower in those treated with IV zoledronic acid for 6 years versus those treated for 3 years. These studies support that patients at particularly high risk for vertebral fractures may benefit from a longer duration of bisphosphonate therapy, she said.
What should I know about infusion side effects?
Infusion side effects remain a concern, and the acute phase reaction of zoledronic acid occurs in about 30% of patients, but most of these are mild and not recurring, Dr. Bolster said. “I tell patients that 90% report mild to moderate infusion side effects, and that it usually occurs only with the first infusion,” she noted.
To potentially prevent an acute-phase reaction, Dr. Bolster has advised patients to take acetaminophen prior to infusion. “I would tend to recommend acetaminophen over NSAIDs to avoid gastric and renal toxicities,” she said.
“Determining the risks of atypical femoral fractures are challenging” but are another potential side effect that worries patients, she said. An atypical femoral fracture (AFF) is a femur fracture in the proximal third of the shaft, she said.
AFF may occur in patients with osteoporosis even in the absence of bisphosphonate use, Dr. Bolster noted. However, AFF “may occur at increased frequency in those patients with osteoporosis and prolonged bisphosphonate use,” she said. AFF is rare overall, and known risk factors include Asian race (in North America), as well as femoral bowing and glucocorticoid use, she said.
A 2019 meta-analysis favored fracture prevention benefits over potential risk associated with bisphosphonate use. Predicting the risk of AFF remains difficult given several factors, including the low incidence of AFF, the unavailability of radiographs in all studies, not accounting for potential confounding by indication in some studies, and lack of adjustment for low bone mineral density or fracture risk, she added.
Osteonecrosis of the jaw has been linked to bisphosphonate use, and some patients ask about it, Dr. Bolster said. Current data show an incidence of 1 in 10,000 to 1 in 100,000 in patients with osteoporosis, while the incidence in the general population is 1 in 100,000, she noted. The highest risk is associated with use of IV bisphosphonates, although it does occur in patients on oral bisphosphonates and denosumab (Prolia), she added. Given the relatively low risk, the American Dental Association states that there is “no need to discontinue bisphosphonates prior to procedures.” Based on current evidence, bisphosphonate treatment outweighs the low risk of medication-related osteonecrosis of the jaw in patients in need of osteoporosis treatment because of the high risk of fragility fractures in the osteoporosis population, she emphasized.
When will I need another dual x-ray absorptiometry scan?
Osteoporosis develops in fewer than 10% of older postmenopausal women using a 15-year screening interval for those with normal bone mineral density or mild osteopenia at an initial scan, with T-scores of –1.49 or higher, she noted. Therefore, the need for repeat dual x-ray absorptiometry (DXA) scans should be individualized, so some patients with normal bone density or osteopenia and few comorbidities and risk factors for osteoporosis may not need frequent DXA scans, she added.
Although little evidence exists to specifically demonstrate the value of monitoring bone mineral density during a 5-year drug treatment period, as is noted by the American College of Physicians 2017 clinical practice guideline published in Annals of Internal Medicine, Dr. Bolster said that a DXA scan showing loss of bone mineral density during treatment could indicate incorrect drug use or noncompliance, or a secondary cause for bone loss that may otherwise go unnoticed. For IV zoledronic acid in particular, a DXA scan at 3-4 years can determine whether a drug holiday is warranted, or for patients with severe osteoporosis, whether another 3 years of treatment is necessary. She suggested considering a DXA scan at 2-3 years with alendronate and at 3-4 years with IV zoledronic acid.
Will I need a new medication if I fracture while on treatment?
For patients who ask whether to change medications following a new fracture, Dr. Bolster said it is important to evaluate the patient’s compliance with the treatment regimen and also consider the presence of secondary causes of bone loss. Consideration can be given to keeping the patient on the same regimen because osteoporosis treatment regimens have demonstrated a 50%-70% fracture-risk reduction so they do not prevent all fractures, she said. “It is therefore reasonable, after confirming compliance and ruling out secondary causes of bone loss, to keep a patient on the same regimen following a fracture. For patients using denosumab, there is an increased risk of rapid bone loss and sustaining multiple vertebral fracture with missed doses or discontinuation,” she said.
It is important to evaluate patients who fracture while on therapy for secondary causes of bone loss, assess compliance, and consider strategies such as modifying the route of administration, seeking a different mechanism of action, or continuing on the same regimen, Dr. Bolster noted.
Dr. Bolster disclosed participation in clinical trials for Corbus, Cumberland, and Genentech, as well as research grants from the Rheumatology Research Foundation. She also disclosed serving on advisory boards for Gilead Sciences and Clinical Learning Designs, serving on the American College of Rheumatology’s Committee on Marketing and Communications, and holding investments in Johnson & Johnson.
Global Academy for Medical Education and this news organization are owned by the same parent company.
“When we think about bisphosphonates, we have to think about whether they are good players or bad players,” Marcy B. Bolster, MD, of Harvard Medical School, Boston, said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Although bisphosphonates are a first-line treatment for many patients to reduce fracture risk, rheumatology patients have distinct concerns about these medications, said Dr. Bolster, who is director of the Rheumatology Fellowship Training Program at Massachusetts General Hospital, Boston, and medical lead for the Fracture Liaison Service there.
She shared her insights on four questions most often asked by patients in her practice:
How long do I need to take this medication?
When discussing bisphosphonates with a patient for the first time, “I typically start with the benefits, and include the risks,” she said. “Then I outline my plans for treatment, which would include treatment duration.”
Setting expectations with the patient about planned duration of therapy, reviewing risks and benefits, and preparing to be flexible if changes are needed can help relieve patients’ concerns, she said.
For example, in a hypothetical case of a 69-year-old woman with a 17% chance of a major osteoporotic fracture and 3.8% chance of hip fracture in the next 10 years based on FRAX scores, Dr. Bolster said she would treat with alendronate or zoledronic acid.
Duration must be a clinical decision individualized to the patient, she noted. Research studies support that some patients benefit from a longer duration of therapy. In the Fracture Intervention Trial (FIT) Long-Term Extension, which included 1,099 women, the risk of clinical vertebral fractures significantly declined with 10 years of alendronate treatment, compared with 5 years of treatment, she said.
In the HORIZON trial of 1,233 postmenopausal women, the risk of new morphometric vertebral fractures was significantly lower in those treated with IV zoledronic acid for 6 years versus those treated for 3 years. These studies support that patients at particularly high risk for vertebral fractures may benefit from a longer duration of bisphosphonate therapy, she said.
What should I know about infusion side effects?
Infusion side effects remain a concern, and the acute phase reaction of zoledronic acid occurs in about 30% of patients, but most of these are mild and not recurring, Dr. Bolster said. “I tell patients that 90% report mild to moderate infusion side effects, and that it usually occurs only with the first infusion,” she noted.
To potentially prevent an acute-phase reaction, Dr. Bolster has advised patients to take acetaminophen prior to infusion. “I would tend to recommend acetaminophen over NSAIDs to avoid gastric and renal toxicities,” she said.
“Determining the risks of atypical femoral fractures are challenging” but are another potential side effect that worries patients, she said. An atypical femoral fracture (AFF) is a femur fracture in the proximal third of the shaft, she said.
AFF may occur in patients with osteoporosis even in the absence of bisphosphonate use, Dr. Bolster noted. However, AFF “may occur at increased frequency in those patients with osteoporosis and prolonged bisphosphonate use,” she said. AFF is rare overall, and known risk factors include Asian race (in North America), as well as femoral bowing and glucocorticoid use, she said.
A 2019 meta-analysis favored fracture prevention benefits over potential risk associated with bisphosphonate use. Predicting the risk of AFF remains difficult given several factors, including the low incidence of AFF, the unavailability of radiographs in all studies, not accounting for potential confounding by indication in some studies, and lack of adjustment for low bone mineral density or fracture risk, she added.
Osteonecrosis of the jaw has been linked to bisphosphonate use, and some patients ask about it, Dr. Bolster said. Current data show an incidence of 1 in 10,000 to 1 in 100,000 in patients with osteoporosis, while the incidence in the general population is 1 in 100,000, she noted. The highest risk is associated with use of IV bisphosphonates, although it does occur in patients on oral bisphosphonates and denosumab (Prolia), she added. Given the relatively low risk, the American Dental Association states that there is “no need to discontinue bisphosphonates prior to procedures.” Based on current evidence, bisphosphonate treatment outweighs the low risk of medication-related osteonecrosis of the jaw in patients in need of osteoporosis treatment because of the high risk of fragility fractures in the osteoporosis population, she emphasized.
When will I need another dual x-ray absorptiometry scan?
Osteoporosis develops in fewer than 10% of older postmenopausal women using a 15-year screening interval for those with normal bone mineral density or mild osteopenia at an initial scan, with T-scores of –1.49 or higher, she noted. Therefore, the need for repeat dual x-ray absorptiometry (DXA) scans should be individualized, so some patients with normal bone density or osteopenia and few comorbidities and risk factors for osteoporosis may not need frequent DXA scans, she added.
Although little evidence exists to specifically demonstrate the value of monitoring bone mineral density during a 5-year drug treatment period, as is noted by the American College of Physicians 2017 clinical practice guideline published in Annals of Internal Medicine, Dr. Bolster said that a DXA scan showing loss of bone mineral density during treatment could indicate incorrect drug use or noncompliance, or a secondary cause for bone loss that may otherwise go unnoticed. For IV zoledronic acid in particular, a DXA scan at 3-4 years can determine whether a drug holiday is warranted, or for patients with severe osteoporosis, whether another 3 years of treatment is necessary. She suggested considering a DXA scan at 2-3 years with alendronate and at 3-4 years with IV zoledronic acid.
Will I need a new medication if I fracture while on treatment?
For patients who ask whether to change medications following a new fracture, Dr. Bolster said it is important to evaluate the patient’s compliance with the treatment regimen and also consider the presence of secondary causes of bone loss. Consideration can be given to keeping the patient on the same regimen because osteoporosis treatment regimens have demonstrated a 50%-70% fracture-risk reduction so they do not prevent all fractures, she said. “It is therefore reasonable, after confirming compliance and ruling out secondary causes of bone loss, to keep a patient on the same regimen following a fracture. For patients using denosumab, there is an increased risk of rapid bone loss and sustaining multiple vertebral fracture with missed doses or discontinuation,” she said.
It is important to evaluate patients who fracture while on therapy for secondary causes of bone loss, assess compliance, and consider strategies such as modifying the route of administration, seeking a different mechanism of action, or continuing on the same regimen, Dr. Bolster noted.
Dr. Bolster disclosed participation in clinical trials for Corbus, Cumberland, and Genentech, as well as research grants from the Rheumatology Research Foundation. She also disclosed serving on advisory boards for Gilead Sciences and Clinical Learning Designs, serving on the American College of Rheumatology’s Committee on Marketing and Communications, and holding investments in Johnson & Johnson.
Global Academy for Medical Education and this news organization are owned by the same parent company.
“When we think about bisphosphonates, we have to think about whether they are good players or bad players,” Marcy B. Bolster, MD, of Harvard Medical School, Boston, said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Although bisphosphonates are a first-line treatment for many patients to reduce fracture risk, rheumatology patients have distinct concerns about these medications, said Dr. Bolster, who is director of the Rheumatology Fellowship Training Program at Massachusetts General Hospital, Boston, and medical lead for the Fracture Liaison Service there.
She shared her insights on four questions most often asked by patients in her practice:
How long do I need to take this medication?
When discussing bisphosphonates with a patient for the first time, “I typically start with the benefits, and include the risks,” she said. “Then I outline my plans for treatment, which would include treatment duration.”
Setting expectations with the patient about planned duration of therapy, reviewing risks and benefits, and preparing to be flexible if changes are needed can help relieve patients’ concerns, she said.
For example, in a hypothetical case of a 69-year-old woman with a 17% chance of a major osteoporotic fracture and 3.8% chance of hip fracture in the next 10 years based on FRAX scores, Dr. Bolster said she would treat with alendronate or zoledronic acid.
Duration must be a clinical decision individualized to the patient, she noted. Research studies support that some patients benefit from a longer duration of therapy. In the Fracture Intervention Trial (FIT) Long-Term Extension, which included 1,099 women, the risk of clinical vertebral fractures significantly declined with 10 years of alendronate treatment, compared with 5 years of treatment, she said.
In the HORIZON trial of 1,233 postmenopausal women, the risk of new morphometric vertebral fractures was significantly lower in those treated with IV zoledronic acid for 6 years versus those treated for 3 years. These studies support that patients at particularly high risk for vertebral fractures may benefit from a longer duration of bisphosphonate therapy, she said.
What should I know about infusion side effects?
Infusion side effects remain a concern, and the acute phase reaction of zoledronic acid occurs in about 30% of patients, but most of these are mild and not recurring, Dr. Bolster said. “I tell patients that 90% report mild to moderate infusion side effects, and that it usually occurs only with the first infusion,” she noted.
To potentially prevent an acute-phase reaction, Dr. Bolster has advised patients to take acetaminophen prior to infusion. “I would tend to recommend acetaminophen over NSAIDs to avoid gastric and renal toxicities,” she said.
“Determining the risks of atypical femoral fractures are challenging” but are another potential side effect that worries patients, she said. An atypical femoral fracture (AFF) is a femur fracture in the proximal third of the shaft, she said.
AFF may occur in patients with osteoporosis even in the absence of bisphosphonate use, Dr. Bolster noted. However, AFF “may occur at increased frequency in those patients with osteoporosis and prolonged bisphosphonate use,” she said. AFF is rare overall, and known risk factors include Asian race (in North America), as well as femoral bowing and glucocorticoid use, she said.
A 2019 meta-analysis favored fracture prevention benefits over potential risk associated with bisphosphonate use. Predicting the risk of AFF remains difficult given several factors, including the low incidence of AFF, the unavailability of radiographs in all studies, not accounting for potential confounding by indication in some studies, and lack of adjustment for low bone mineral density or fracture risk, she added.
Osteonecrosis of the jaw has been linked to bisphosphonate use, and some patients ask about it, Dr. Bolster said. Current data show an incidence of 1 in 10,000 to 1 in 100,000 in patients with osteoporosis, while the incidence in the general population is 1 in 100,000, she noted. The highest risk is associated with use of IV bisphosphonates, although it does occur in patients on oral bisphosphonates and denosumab (Prolia), she added. Given the relatively low risk, the American Dental Association states that there is “no need to discontinue bisphosphonates prior to procedures.” Based on current evidence, bisphosphonate treatment outweighs the low risk of medication-related osteonecrosis of the jaw in patients in need of osteoporosis treatment because of the high risk of fragility fractures in the osteoporosis population, she emphasized.
When will I need another dual x-ray absorptiometry scan?
Osteoporosis develops in fewer than 10% of older postmenopausal women using a 15-year screening interval for those with normal bone mineral density or mild osteopenia at an initial scan, with T-scores of –1.49 or higher, she noted. Therefore, the need for repeat dual x-ray absorptiometry (DXA) scans should be individualized, so some patients with normal bone density or osteopenia and few comorbidities and risk factors for osteoporosis may not need frequent DXA scans, she added.
Although little evidence exists to specifically demonstrate the value of monitoring bone mineral density during a 5-year drug treatment period, as is noted by the American College of Physicians 2017 clinical practice guideline published in Annals of Internal Medicine, Dr. Bolster said that a DXA scan showing loss of bone mineral density during treatment could indicate incorrect drug use or noncompliance, or a secondary cause for bone loss that may otherwise go unnoticed. For IV zoledronic acid in particular, a DXA scan at 3-4 years can determine whether a drug holiday is warranted, or for patients with severe osteoporosis, whether another 3 years of treatment is necessary. She suggested considering a DXA scan at 2-3 years with alendronate and at 3-4 years with IV zoledronic acid.
Will I need a new medication if I fracture while on treatment?
For patients who ask whether to change medications following a new fracture, Dr. Bolster said it is important to evaluate the patient’s compliance with the treatment regimen and also consider the presence of secondary causes of bone loss. Consideration can be given to keeping the patient on the same regimen because osteoporosis treatment regimens have demonstrated a 50%-70% fracture-risk reduction so they do not prevent all fractures, she said. “It is therefore reasonable, after confirming compliance and ruling out secondary causes of bone loss, to keep a patient on the same regimen following a fracture. For patients using denosumab, there is an increased risk of rapid bone loss and sustaining multiple vertebral fracture with missed doses or discontinuation,” she said.
It is important to evaluate patients who fracture while on therapy for secondary causes of bone loss, assess compliance, and consider strategies such as modifying the route of administration, seeking a different mechanism of action, or continuing on the same regimen, Dr. Bolster noted.
Dr. Bolster disclosed participation in clinical trials for Corbus, Cumberland, and Genentech, as well as research grants from the Rheumatology Research Foundation. She also disclosed serving on advisory boards for Gilead Sciences and Clinical Learning Designs, serving on the American College of Rheumatology’s Committee on Marketing and Communications, and holding investments in Johnson & Johnson.
Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM PRD 2020
‘Paradigm shift’ in gastric junction cancers with nivolumab
Patients with gastric cancer or gastroesophageal junction cancer (GEJ) could experience significantly improved progression-free survival (PFS), and maybe overall survival (OS), with nivolumab (Opdivo) in the first-line and neoadjuvant settings, suggest data from three phase 3 trials.
However, contrasting results between the trials and question marks over the effect of the drug in all-comers leave some questions yet to be answered, despite the “practice-changing” findings, said experts discussing the new data.
The research was presented Sept. 21 at the European Society for Medical Oncology Virtual Congress 2020.
Gastric cancer and GEJ have been an area of interest for immunotherapy in recent years, as standard first-line chemotherapy is associated with poor OS at a median of less than 1 year.
Previous smaller studies have suggested that nivolumab has promising activity in the first-line setting, improving survival particularly in individuals with a combined positive score (CPS) for programmed death–ligand 1 (PD-L1) expression ≥5.
The new results come from the largest phase 3 trial of its kind to date, CheckMate 649, which involved 1,581 previously untreated patients with unresectable HER2-negative gastric cancer, GEJ, or esophageal adenocarcinoma.
Among these patients, 60% had a PD-L1 CPS ≥5.
Patients were randomly assigned to one of three treatment groups: Nivolumab plus ipilimumab (Yervoy), nivolumab plus oxaliplatin-based chemotherapy, or chemotherapy alone.
Results, after a minimum follow-up of 12 months, show that nivolumab plus chemotherapy was associated with significantly better OS than chemotherapy alone, reported Markus Moehler, MD, PhD, Johannes-Gutenberg University Clinic, Mainz, Germany.
In patients with PD-L1 CPS ≥5, median OS was 14.4 months with nivolumab-chemotherapy versus 11.1 months for chemotherapy alone (hazard ratio 0.71, P < .0001).
The figures were similar for patients with a PD-L1 CPS ≥1, at 14.0 months and 11.3 months (HR, 0.77; P = .0001), and also across the whole study population (13.8 months vs. 11.6 months; HR, 0.80, P = .0002).
PFS, however, was significantly improved with the nivolumab-chemotherapy combination only in patients with a PD-L1 CPS ≥5, at a median of 7.7 months vs 6.0 months (HR, 0.68, P < .0001).
The proportion of patients with treatment-related adverse events leading to discontinuation were 36% with nivolumab plus chemotherapy and 24% for chemotherapy alone.
At a press conference, Dr. Moehler said the benefits seen with nivolumab plus chemotherapy are “highly clinically meaningful,” and the combination “represents a new potential standard first-line treatment” for these patients.
These results are “practice changing” and are “clearly significant,” commented Salah-Eddin Al-Batran, MD, Krankenhaus Nordwest-University Cancer Center, Frankfurt, Germany, who was not involved with the study.
“However, as a physician,” he continued, “I am treating an individual patient and, for me, it’s important to know the efficacy in the patients with a CPS of 1-4, or of 0.”
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high-expressers,” he said, adding that other factors to consider will be microsatellite instability and tumor mutational burden. “I think these questions have to be addressed to give us a clear picture of how to treat the patient sitting in front of us.”
Surprisingly, the results from ATTRACTION-4, a very similar phase 3 trial conducted in Japan, Korea, and Taiwan, did not follow the same pattern.
This trial involved 724 previously untreated patients with HER2-negative gastric cancer or GEJ randomly assigned to receive nivolumab plus chemotherapy or chemotherapy alone.
Lead author Narikazu Boku, MD, PhD, National Cancer Center Hospital, Tokyo, Japan, said that, after a median follow-up of 11.6 months, the combination treatment was associated with a significant improvement in PFS, at a median 10.5 months versus 8.3 months with chemotherapy alone (HR, 0.68, P = .0007).
In contrast, there was no significant difference in OS between the nivolumab and placebo arms, at a median of 17.5 months and 17.2 months, respectively (HR, 0.90; P = .257).
Invited discussant Elizabeth Smyth, MD, from Addenbrooke’s Hospital in Cambridge, England, suggested the lack of OS benefit seen in ATTRACTION-4 could be the result of a number of factors, including that PD-L1 status was assessed on tumor cells only and there were no key endpoints based around PD-L1 status.
Moreover, the posttrial therapy could have affected the overall results, as Asian patients typically receive more subsequent therapies than those elsewhere.
Dr. Smyth also commented that both CheckMate 649 and ATTRACTION-4 represent a “paradigm shift” in the first-line treatment of gastroesophageal adenocarcinoma.
Nivolumab in adjuvant setting
The results of a third trial presented at the ESMO meeting suggest a role for nivolumab in the adjuvant setting, following neoadjuvant chemoradiation therapy in patients with resected esophageal or GEJ cancer.
This was the CheckMate 577 trial, which compared adjuvant nivolumab with placebo in 794 patients from across the globe.
Nivolumab significantly increased median disease-free survival to 22.4 months versus 11.0 months with placebo (HR, 0.69, P = .0003).
Treatment-related adverse events leading to discontinuation were reported in 9% of nivolumab patients versus 3% on placebo, reported Ronan J. Kelly, MD, chief of oncology at Baylor Scott & White Health in Dallas.
Interestingly, patient-reported health status on the EQ-5D-3L visual analogue scale (VAS) and utility index were similar between nivolumab- and placebo-treated patients, with both groups experiencing clinically meaningful improvements.
Dr. Kelly said this is “the first adjuvant therapy to provide a statistically significant and clinically meaningful improvement” in patients with esophageal cancer or GEJ cancer following neoadjuvant chemoradiation.
Consequently, the results “represent the first advance in years for this group of patients, potentially establishing adjuvant nivolumab as a new standard of care.”
However, the invited discussant raised several issues with the trial design. Preoperative chemoradiation is not “universally accepted” as the standard of care in this setting, disease-free survival is not currently validated as a major endpoint in gastroesophageal cancers, and the median follow-up was short, commented Andrés Cervantes, MD, PhD, University of Valencia (Spain), and president-elect of ESMO.
In addition, there was no differentiation between esophageal squamous cell and adenocarcinoma histologies.
Nevertheless, CheckMate 577 is the first positive adjuvant study for checkpoint inhibitors in gastrointestinal tumors and, crucially, the results “are independent of PD-L1 status,” Dr. Cervantes said.
CheckMate 649 was funded by Bristol-Myers Squibb. ATTRACTION-4 was funded by Ono Pharmaceutical and Bristol-Myers Squibb. CheckMate 577 was funded by Bristol-Myers Squibb. Many of the presenters reported relationships with pharmaceutical companies.
This article first appeared on Medscape.com.
Patients with gastric cancer or gastroesophageal junction cancer (GEJ) could experience significantly improved progression-free survival (PFS), and maybe overall survival (OS), with nivolumab (Opdivo) in the first-line and neoadjuvant settings, suggest data from three phase 3 trials.
However, contrasting results between the trials and question marks over the effect of the drug in all-comers leave some questions yet to be answered, despite the “practice-changing” findings, said experts discussing the new data.
The research was presented Sept. 21 at the European Society for Medical Oncology Virtual Congress 2020.
Gastric cancer and GEJ have been an area of interest for immunotherapy in recent years, as standard first-line chemotherapy is associated with poor OS at a median of less than 1 year.
Previous smaller studies have suggested that nivolumab has promising activity in the first-line setting, improving survival particularly in individuals with a combined positive score (CPS) for programmed death–ligand 1 (PD-L1) expression ≥5.
The new results come from the largest phase 3 trial of its kind to date, CheckMate 649, which involved 1,581 previously untreated patients with unresectable HER2-negative gastric cancer, GEJ, or esophageal adenocarcinoma.
Among these patients, 60% had a PD-L1 CPS ≥5.
Patients were randomly assigned to one of three treatment groups: Nivolumab plus ipilimumab (Yervoy), nivolumab plus oxaliplatin-based chemotherapy, or chemotherapy alone.
Results, after a minimum follow-up of 12 months, show that nivolumab plus chemotherapy was associated with significantly better OS than chemotherapy alone, reported Markus Moehler, MD, PhD, Johannes-Gutenberg University Clinic, Mainz, Germany.
In patients with PD-L1 CPS ≥5, median OS was 14.4 months with nivolumab-chemotherapy versus 11.1 months for chemotherapy alone (hazard ratio 0.71, P < .0001).
The figures were similar for patients with a PD-L1 CPS ≥1, at 14.0 months and 11.3 months (HR, 0.77; P = .0001), and also across the whole study population (13.8 months vs. 11.6 months; HR, 0.80, P = .0002).
PFS, however, was significantly improved with the nivolumab-chemotherapy combination only in patients with a PD-L1 CPS ≥5, at a median of 7.7 months vs 6.0 months (HR, 0.68, P < .0001).
The proportion of patients with treatment-related adverse events leading to discontinuation were 36% with nivolumab plus chemotherapy and 24% for chemotherapy alone.
At a press conference, Dr. Moehler said the benefits seen with nivolumab plus chemotherapy are “highly clinically meaningful,” and the combination “represents a new potential standard first-line treatment” for these patients.
These results are “practice changing” and are “clearly significant,” commented Salah-Eddin Al-Batran, MD, Krankenhaus Nordwest-University Cancer Center, Frankfurt, Germany, who was not involved with the study.
“However, as a physician,” he continued, “I am treating an individual patient and, for me, it’s important to know the efficacy in the patients with a CPS of 1-4, or of 0.”
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high-expressers,” he said, adding that other factors to consider will be microsatellite instability and tumor mutational burden. “I think these questions have to be addressed to give us a clear picture of how to treat the patient sitting in front of us.”
Surprisingly, the results from ATTRACTION-4, a very similar phase 3 trial conducted in Japan, Korea, and Taiwan, did not follow the same pattern.
This trial involved 724 previously untreated patients with HER2-negative gastric cancer or GEJ randomly assigned to receive nivolumab plus chemotherapy or chemotherapy alone.
Lead author Narikazu Boku, MD, PhD, National Cancer Center Hospital, Tokyo, Japan, said that, after a median follow-up of 11.6 months, the combination treatment was associated with a significant improvement in PFS, at a median 10.5 months versus 8.3 months with chemotherapy alone (HR, 0.68, P = .0007).
In contrast, there was no significant difference in OS between the nivolumab and placebo arms, at a median of 17.5 months and 17.2 months, respectively (HR, 0.90; P = .257).
Invited discussant Elizabeth Smyth, MD, from Addenbrooke’s Hospital in Cambridge, England, suggested the lack of OS benefit seen in ATTRACTION-4 could be the result of a number of factors, including that PD-L1 status was assessed on tumor cells only and there were no key endpoints based around PD-L1 status.
Moreover, the posttrial therapy could have affected the overall results, as Asian patients typically receive more subsequent therapies than those elsewhere.
Dr. Smyth also commented that both CheckMate 649 and ATTRACTION-4 represent a “paradigm shift” in the first-line treatment of gastroesophageal adenocarcinoma.
Nivolumab in adjuvant setting
The results of a third trial presented at the ESMO meeting suggest a role for nivolumab in the adjuvant setting, following neoadjuvant chemoradiation therapy in patients with resected esophageal or GEJ cancer.
This was the CheckMate 577 trial, which compared adjuvant nivolumab with placebo in 794 patients from across the globe.
Nivolumab significantly increased median disease-free survival to 22.4 months versus 11.0 months with placebo (HR, 0.69, P = .0003).
Treatment-related adverse events leading to discontinuation were reported in 9% of nivolumab patients versus 3% on placebo, reported Ronan J. Kelly, MD, chief of oncology at Baylor Scott & White Health in Dallas.
Interestingly, patient-reported health status on the EQ-5D-3L visual analogue scale (VAS) and utility index were similar between nivolumab- and placebo-treated patients, with both groups experiencing clinically meaningful improvements.
Dr. Kelly said this is “the first adjuvant therapy to provide a statistically significant and clinically meaningful improvement” in patients with esophageal cancer or GEJ cancer following neoadjuvant chemoradiation.
Consequently, the results “represent the first advance in years for this group of patients, potentially establishing adjuvant nivolumab as a new standard of care.”
However, the invited discussant raised several issues with the trial design. Preoperative chemoradiation is not “universally accepted” as the standard of care in this setting, disease-free survival is not currently validated as a major endpoint in gastroesophageal cancers, and the median follow-up was short, commented Andrés Cervantes, MD, PhD, University of Valencia (Spain), and president-elect of ESMO.
In addition, there was no differentiation between esophageal squamous cell and adenocarcinoma histologies.
Nevertheless, CheckMate 577 is the first positive adjuvant study for checkpoint inhibitors in gastrointestinal tumors and, crucially, the results “are independent of PD-L1 status,” Dr. Cervantes said.
CheckMate 649 was funded by Bristol-Myers Squibb. ATTRACTION-4 was funded by Ono Pharmaceutical and Bristol-Myers Squibb. CheckMate 577 was funded by Bristol-Myers Squibb. Many of the presenters reported relationships with pharmaceutical companies.
This article first appeared on Medscape.com.
Patients with gastric cancer or gastroesophageal junction cancer (GEJ) could experience significantly improved progression-free survival (PFS), and maybe overall survival (OS), with nivolumab (Opdivo) in the first-line and neoadjuvant settings, suggest data from three phase 3 trials.
However, contrasting results between the trials and question marks over the effect of the drug in all-comers leave some questions yet to be answered, despite the “practice-changing” findings, said experts discussing the new data.
The research was presented Sept. 21 at the European Society for Medical Oncology Virtual Congress 2020.
Gastric cancer and GEJ have been an area of interest for immunotherapy in recent years, as standard first-line chemotherapy is associated with poor OS at a median of less than 1 year.
Previous smaller studies have suggested that nivolumab has promising activity in the first-line setting, improving survival particularly in individuals with a combined positive score (CPS) for programmed death–ligand 1 (PD-L1) expression ≥5.
The new results come from the largest phase 3 trial of its kind to date, CheckMate 649, which involved 1,581 previously untreated patients with unresectable HER2-negative gastric cancer, GEJ, or esophageal adenocarcinoma.
Among these patients, 60% had a PD-L1 CPS ≥5.
Patients were randomly assigned to one of three treatment groups: Nivolumab plus ipilimumab (Yervoy), nivolumab plus oxaliplatin-based chemotherapy, or chemotherapy alone.
Results, after a minimum follow-up of 12 months, show that nivolumab plus chemotherapy was associated with significantly better OS than chemotherapy alone, reported Markus Moehler, MD, PhD, Johannes-Gutenberg University Clinic, Mainz, Germany.
In patients with PD-L1 CPS ≥5, median OS was 14.4 months with nivolumab-chemotherapy versus 11.1 months for chemotherapy alone (hazard ratio 0.71, P < .0001).
The figures were similar for patients with a PD-L1 CPS ≥1, at 14.0 months and 11.3 months (HR, 0.77; P = .0001), and also across the whole study population (13.8 months vs. 11.6 months; HR, 0.80, P = .0002).
PFS, however, was significantly improved with the nivolumab-chemotherapy combination only in patients with a PD-L1 CPS ≥5, at a median of 7.7 months vs 6.0 months (HR, 0.68, P < .0001).
The proportion of patients with treatment-related adverse events leading to discontinuation were 36% with nivolumab plus chemotherapy and 24% for chemotherapy alone.
At a press conference, Dr. Moehler said the benefits seen with nivolumab plus chemotherapy are “highly clinically meaningful,” and the combination “represents a new potential standard first-line treatment” for these patients.
These results are “practice changing” and are “clearly significant,” commented Salah-Eddin Al-Batran, MD, Krankenhaus Nordwest-University Cancer Center, Frankfurt, Germany, who was not involved with the study.
“However, as a physician,” he continued, “I am treating an individual patient and, for me, it’s important to know the efficacy in the patients with a CPS of 1-4, or of 0.”
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high-expressers,” he said, adding that other factors to consider will be microsatellite instability and tumor mutational burden. “I think these questions have to be addressed to give us a clear picture of how to treat the patient sitting in front of us.”
Surprisingly, the results from ATTRACTION-4, a very similar phase 3 trial conducted in Japan, Korea, and Taiwan, did not follow the same pattern.
This trial involved 724 previously untreated patients with HER2-negative gastric cancer or GEJ randomly assigned to receive nivolumab plus chemotherapy or chemotherapy alone.
Lead author Narikazu Boku, MD, PhD, National Cancer Center Hospital, Tokyo, Japan, said that, after a median follow-up of 11.6 months, the combination treatment was associated with a significant improvement in PFS, at a median 10.5 months versus 8.3 months with chemotherapy alone (HR, 0.68, P = .0007).
In contrast, there was no significant difference in OS between the nivolumab and placebo arms, at a median of 17.5 months and 17.2 months, respectively (HR, 0.90; P = .257).
Invited discussant Elizabeth Smyth, MD, from Addenbrooke’s Hospital in Cambridge, England, suggested the lack of OS benefit seen in ATTRACTION-4 could be the result of a number of factors, including that PD-L1 status was assessed on tumor cells only and there were no key endpoints based around PD-L1 status.
Moreover, the posttrial therapy could have affected the overall results, as Asian patients typically receive more subsequent therapies than those elsewhere.
Dr. Smyth also commented that both CheckMate 649 and ATTRACTION-4 represent a “paradigm shift” in the first-line treatment of gastroesophageal adenocarcinoma.
Nivolumab in adjuvant setting
The results of a third trial presented at the ESMO meeting suggest a role for nivolumab in the adjuvant setting, following neoadjuvant chemoradiation therapy in patients with resected esophageal or GEJ cancer.
This was the CheckMate 577 trial, which compared adjuvant nivolumab with placebo in 794 patients from across the globe.
Nivolumab significantly increased median disease-free survival to 22.4 months versus 11.0 months with placebo (HR, 0.69, P = .0003).
Treatment-related adverse events leading to discontinuation were reported in 9% of nivolumab patients versus 3% on placebo, reported Ronan J. Kelly, MD, chief of oncology at Baylor Scott & White Health in Dallas.
Interestingly, patient-reported health status on the EQ-5D-3L visual analogue scale (VAS) and utility index were similar between nivolumab- and placebo-treated patients, with both groups experiencing clinically meaningful improvements.
Dr. Kelly said this is “the first adjuvant therapy to provide a statistically significant and clinically meaningful improvement” in patients with esophageal cancer or GEJ cancer following neoadjuvant chemoradiation.
Consequently, the results “represent the first advance in years for this group of patients, potentially establishing adjuvant nivolumab as a new standard of care.”
However, the invited discussant raised several issues with the trial design. Preoperative chemoradiation is not “universally accepted” as the standard of care in this setting, disease-free survival is not currently validated as a major endpoint in gastroesophageal cancers, and the median follow-up was short, commented Andrés Cervantes, MD, PhD, University of Valencia (Spain), and president-elect of ESMO.
In addition, there was no differentiation between esophageal squamous cell and adenocarcinoma histologies.
Nevertheless, CheckMate 577 is the first positive adjuvant study for checkpoint inhibitors in gastrointestinal tumors and, crucially, the results “are independent of PD-L1 status,” Dr. Cervantes said.
CheckMate 649 was funded by Bristol-Myers Squibb. ATTRACTION-4 was funded by Ono Pharmaceutical and Bristol-Myers Squibb. CheckMate 577 was funded by Bristol-Myers Squibb. Many of the presenters reported relationships with pharmaceutical companies.
This article first appeared on Medscape.com.
FROM ESMO 2020
J&J’s one-shot COVID-19 vaccine advances to phase 3 testing
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
New first-line standard of care for esophageal cancer?
Pembrolizumab (Keytruda) is already approved for use in the treatment of esophageal cancer, but in the second-line setting.
New results show that it also improves outcomes when used in the first-line setting, and the investigators suggest that pembrolizumab in combination with chemotherapy should be the new standard of care.
The results come from an interim analysis of the phase 3 KEYNOTE-590 trial, conducted with 740 patients who had advanced cancers of the esophagus or esophagogastric junction (EGJ). The patients were followed for a median of 10.8 months.
The findings show that the combination offered patients a modest but distinct survival benefit over chemotherapy alone.
Median overall survival (OS), one of two primary endpoints for the trial, was 12.4 months for pembrolizumab plus chemotherapy, compared with 9.8 months for patients who received chemotherapy plus placebo.
This difference translated into a hazard ratio (HR) for death with pembrolizumab of 0.73 (P < .0001), reported Peter Enzinger, MD, from the Dana-Farber Cancer Institute in Boston. Progression-free survival (PFS), the trial’s other primary endpoint, was superior with the combination, at a median of 6.3 months compared with 5.8 months for chemotherapy alone, translating into an HR for progression with pembrolizumab of 0.65 (P < .0001).
“Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy in patients with locally advanced unresectable or metastastic esophageal cancer, including the EGJ, regardless of the histology and biomarker status,” Enzinger concluded.
He was speaking at a press briefing prior to a presentation of the data in a presidential symposium at the European Society of Medical Oncology (ESMO) Virtual Congress 2020. (The data were presented in oral session by Ken Kato, MD, from the National Cancer Center Hospital in Tokyo, Japan.)
Little change in treatment
“Unfortunately, for esophageal cancer, the standard of care has remained relatively unchanged for a long period of time,” Enzinger said.
Standard first-line therapy for patients with advanced esophageal, EGJ, or gastric adenocarcinoma is primarily a chemotherapy doublet consisting of a fluoropyrimidine plus a platinum agent.
Pembrolizumab was previously shown to have some activity as monotherapy against advanced or metastatic esophageal cancer in the third line in the KEYNOTE-180 trial. It yielded an overall response rate (ORR) of 14%. A median duration of response was not reached among patients with esophageal squamous cell carcinoma (ESCC) and tumors with programmed cell death–ligand-1 (PD-L1) that had combined positive scores (CPSs) of 10 or higher.
Another previous trial, KEYNOTE-181, showed that pembrolizumab monotherapy in the second line was associated with an ORR of 22% vs 7% for chemotherapy and respective median OS of 10.3 vs 6.7 months. This trial led to US Food and Drug Administration approval of pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic ESCC with PD-L1 CPS ≥10 who have experienced disease progression after at least one prior line of therapy.
The trial now being reported, KEYNOTE-590, assessed use of the drug in the first-line setting. It was designed to see whether combining standard-of-care chemotherapy with pembrolizumab would improve outcomes.
Study details
Patients were randomly assigned to receive chemotherapy with 5-fluorauracil (5-FU) 800 mg/m2 intravenously on days 1–5 every 3 weeks for up to 35 cycles and cisplatin 80 mg/m2 IV every 3 weeks for up to 6 cycles, plus either pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles or saline placebo IV.
Rates of treatment-related adverse events of grade 3 or higher were similar between study arms, occurring in 71.9% with the combination and in 67.6% with chemotherapy alone. Adverse events leading to drug discontinuation occurred in 19.5% and 11.6%, respectively. Fatal adverse events occurred in 2.4% of patients who received the combination and in 1.4% of patients who received chemotherapy and placebo.
Immune-mediated adverse events of grade 3 or higher and infusion reactions occurred in 7% and 2.2% of patients, respectively.
Patient-reported quality of life was similar between the groups.
Mixed histologies muddy results
The improvement in OS was observed across all patient populations, including patients with ESCC, esophageal adenocarcinoma (EAC), and EGJ tumors, the researchers noted.
However, invited discussant Andres Cervantes, MD, PhD, from the University of Valencia, Spain, commented that ESCC and EAC have very different histologies and that including patients with both in a single trial can make the results very confusing.
He pointed out that, in the KEYNOTE-590 population, 73% of patients had ESCCs and 27% had EACs.
The OS improvement was seen regardless of PD-L1 expression, although the best results occurred in patients with high expression.
However, Cervantes noted that PD-L1 expression was not used as a stratification factor prior to randomization, even though it was included in the complex, step-wise statistical plan for the study.
Another expert, Salah-Eddin Al-Batran, MD, from the Krankenhaus Nordwest in Frankfurt, Germany, questioned the researchers’ recommendation that all patients with esophageal cancer receive this regimen, regardless of PD-L1 expression.
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high [PD-L1] expressers,” he said at the press briefing.
He said the trial report also leaves open the question of efficacy in tumors with microsatellite instability and/or high tumor mutational burden.
“I think these questions have to be addressed to give us a clear picture of how to treat a patient sitting in front of us as doctors,” he said.
Also adding to the concerns about this trial is the fact that the “selected chemotherapy schedule is not much currently used as the standard of care,” said Cervantes, although he added that it “is acceptable for this protocol.”
Despite these caveats, the trial was clearly positive, Cervantes said. The greatest benefit appeared to accrue for patients with ESCC.
“The addition of pembrolizumab to platinum-based chemotherapy significantly increases response rate, PFS, and OS in patients with advanced esophageal carcinomas over chemotherapy alone,” he said, and therefore he agreed with the investigators that “this is a new standard of care.”
The study was funded by Merck Sharp & Dohme. Enzinger disclosed honoraria and advisory or consulting roles for Merck and others. Cervantes disclosed consulting or advising and research funding from Merck Serono and others. He is president-elect of ESMO. Al-Batran has disclosed no relevant financial relationships.
This story first appeared on Medscape.com.
Pembrolizumab (Keytruda) is already approved for use in the treatment of esophageal cancer, but in the second-line setting.
New results show that it also improves outcomes when used in the first-line setting, and the investigators suggest that pembrolizumab in combination with chemotherapy should be the new standard of care.
The results come from an interim analysis of the phase 3 KEYNOTE-590 trial, conducted with 740 patients who had advanced cancers of the esophagus or esophagogastric junction (EGJ). The patients were followed for a median of 10.8 months.
The findings show that the combination offered patients a modest but distinct survival benefit over chemotherapy alone.
Median overall survival (OS), one of two primary endpoints for the trial, was 12.4 months for pembrolizumab plus chemotherapy, compared with 9.8 months for patients who received chemotherapy plus placebo.
This difference translated into a hazard ratio (HR) for death with pembrolizumab of 0.73 (P < .0001), reported Peter Enzinger, MD, from the Dana-Farber Cancer Institute in Boston. Progression-free survival (PFS), the trial’s other primary endpoint, was superior with the combination, at a median of 6.3 months compared with 5.8 months for chemotherapy alone, translating into an HR for progression with pembrolizumab of 0.65 (P < .0001).
“Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy in patients with locally advanced unresectable or metastastic esophageal cancer, including the EGJ, regardless of the histology and biomarker status,” Enzinger concluded.
He was speaking at a press briefing prior to a presentation of the data in a presidential symposium at the European Society of Medical Oncology (ESMO) Virtual Congress 2020. (The data were presented in oral session by Ken Kato, MD, from the National Cancer Center Hospital in Tokyo, Japan.)
Little change in treatment
“Unfortunately, for esophageal cancer, the standard of care has remained relatively unchanged for a long period of time,” Enzinger said.
Standard first-line therapy for patients with advanced esophageal, EGJ, or gastric adenocarcinoma is primarily a chemotherapy doublet consisting of a fluoropyrimidine plus a platinum agent.
Pembrolizumab was previously shown to have some activity as monotherapy against advanced or metastatic esophageal cancer in the third line in the KEYNOTE-180 trial. It yielded an overall response rate (ORR) of 14%. A median duration of response was not reached among patients with esophageal squamous cell carcinoma (ESCC) and tumors with programmed cell death–ligand-1 (PD-L1) that had combined positive scores (CPSs) of 10 or higher.
Another previous trial, KEYNOTE-181, showed that pembrolizumab monotherapy in the second line was associated with an ORR of 22% vs 7% for chemotherapy and respective median OS of 10.3 vs 6.7 months. This trial led to US Food and Drug Administration approval of pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic ESCC with PD-L1 CPS ≥10 who have experienced disease progression after at least one prior line of therapy.
The trial now being reported, KEYNOTE-590, assessed use of the drug in the first-line setting. It was designed to see whether combining standard-of-care chemotherapy with pembrolizumab would improve outcomes.
Study details
Patients were randomly assigned to receive chemotherapy with 5-fluorauracil (5-FU) 800 mg/m2 intravenously on days 1–5 every 3 weeks for up to 35 cycles and cisplatin 80 mg/m2 IV every 3 weeks for up to 6 cycles, plus either pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles or saline placebo IV.
Rates of treatment-related adverse events of grade 3 or higher were similar between study arms, occurring in 71.9% with the combination and in 67.6% with chemotherapy alone. Adverse events leading to drug discontinuation occurred in 19.5% and 11.6%, respectively. Fatal adverse events occurred in 2.4% of patients who received the combination and in 1.4% of patients who received chemotherapy and placebo.
Immune-mediated adverse events of grade 3 or higher and infusion reactions occurred in 7% and 2.2% of patients, respectively.
Patient-reported quality of life was similar between the groups.
Mixed histologies muddy results
The improvement in OS was observed across all patient populations, including patients with ESCC, esophageal adenocarcinoma (EAC), and EGJ tumors, the researchers noted.
However, invited discussant Andres Cervantes, MD, PhD, from the University of Valencia, Spain, commented that ESCC and EAC have very different histologies and that including patients with both in a single trial can make the results very confusing.
He pointed out that, in the KEYNOTE-590 population, 73% of patients had ESCCs and 27% had EACs.
The OS improvement was seen regardless of PD-L1 expression, although the best results occurred in patients with high expression.
However, Cervantes noted that PD-L1 expression was not used as a stratification factor prior to randomization, even though it was included in the complex, step-wise statistical plan for the study.
Another expert, Salah-Eddin Al-Batran, MD, from the Krankenhaus Nordwest in Frankfurt, Germany, questioned the researchers’ recommendation that all patients with esophageal cancer receive this regimen, regardless of PD-L1 expression.
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high [PD-L1] expressers,” he said at the press briefing.
He said the trial report also leaves open the question of efficacy in tumors with microsatellite instability and/or high tumor mutational burden.
“I think these questions have to be addressed to give us a clear picture of how to treat a patient sitting in front of us as doctors,” he said.
Also adding to the concerns about this trial is the fact that the “selected chemotherapy schedule is not much currently used as the standard of care,” said Cervantes, although he added that it “is acceptable for this protocol.”
Despite these caveats, the trial was clearly positive, Cervantes said. The greatest benefit appeared to accrue for patients with ESCC.
“The addition of pembrolizumab to platinum-based chemotherapy significantly increases response rate, PFS, and OS in patients with advanced esophageal carcinomas over chemotherapy alone,” he said, and therefore he agreed with the investigators that “this is a new standard of care.”
The study was funded by Merck Sharp & Dohme. Enzinger disclosed honoraria and advisory or consulting roles for Merck and others. Cervantes disclosed consulting or advising and research funding from Merck Serono and others. He is president-elect of ESMO. Al-Batran has disclosed no relevant financial relationships.
This story first appeared on Medscape.com.
Pembrolizumab (Keytruda) is already approved for use in the treatment of esophageal cancer, but in the second-line setting.
New results show that it also improves outcomes when used in the first-line setting, and the investigators suggest that pembrolizumab in combination with chemotherapy should be the new standard of care.
The results come from an interim analysis of the phase 3 KEYNOTE-590 trial, conducted with 740 patients who had advanced cancers of the esophagus or esophagogastric junction (EGJ). The patients were followed for a median of 10.8 months.
The findings show that the combination offered patients a modest but distinct survival benefit over chemotherapy alone.
Median overall survival (OS), one of two primary endpoints for the trial, was 12.4 months for pembrolizumab plus chemotherapy, compared with 9.8 months for patients who received chemotherapy plus placebo.
This difference translated into a hazard ratio (HR) for death with pembrolizumab of 0.73 (P < .0001), reported Peter Enzinger, MD, from the Dana-Farber Cancer Institute in Boston. Progression-free survival (PFS), the trial’s other primary endpoint, was superior with the combination, at a median of 6.3 months compared with 5.8 months for chemotherapy alone, translating into an HR for progression with pembrolizumab of 0.65 (P < .0001).
“Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy in patients with locally advanced unresectable or metastastic esophageal cancer, including the EGJ, regardless of the histology and biomarker status,” Enzinger concluded.
He was speaking at a press briefing prior to a presentation of the data in a presidential symposium at the European Society of Medical Oncology (ESMO) Virtual Congress 2020. (The data were presented in oral session by Ken Kato, MD, from the National Cancer Center Hospital in Tokyo, Japan.)
Little change in treatment
“Unfortunately, for esophageal cancer, the standard of care has remained relatively unchanged for a long period of time,” Enzinger said.
Standard first-line therapy for patients with advanced esophageal, EGJ, or gastric adenocarcinoma is primarily a chemotherapy doublet consisting of a fluoropyrimidine plus a platinum agent.
Pembrolizumab was previously shown to have some activity as monotherapy against advanced or metastatic esophageal cancer in the third line in the KEYNOTE-180 trial. It yielded an overall response rate (ORR) of 14%. A median duration of response was not reached among patients with esophageal squamous cell carcinoma (ESCC) and tumors with programmed cell death–ligand-1 (PD-L1) that had combined positive scores (CPSs) of 10 or higher.
Another previous trial, KEYNOTE-181, showed that pembrolizumab monotherapy in the second line was associated with an ORR of 22% vs 7% for chemotherapy and respective median OS of 10.3 vs 6.7 months. This trial led to US Food and Drug Administration approval of pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic ESCC with PD-L1 CPS ≥10 who have experienced disease progression after at least one prior line of therapy.
The trial now being reported, KEYNOTE-590, assessed use of the drug in the first-line setting. It was designed to see whether combining standard-of-care chemotherapy with pembrolizumab would improve outcomes.
Study details
Patients were randomly assigned to receive chemotherapy with 5-fluorauracil (5-FU) 800 mg/m2 intravenously on days 1–5 every 3 weeks for up to 35 cycles and cisplatin 80 mg/m2 IV every 3 weeks for up to 6 cycles, plus either pembrolizumab 200 mg IV every 3 weeks for up to 35 cycles or saline placebo IV.
Rates of treatment-related adverse events of grade 3 or higher were similar between study arms, occurring in 71.9% with the combination and in 67.6% with chemotherapy alone. Adverse events leading to drug discontinuation occurred in 19.5% and 11.6%, respectively. Fatal adverse events occurred in 2.4% of patients who received the combination and in 1.4% of patients who received chemotherapy and placebo.
Immune-mediated adverse events of grade 3 or higher and infusion reactions occurred in 7% and 2.2% of patients, respectively.
Patient-reported quality of life was similar between the groups.
Mixed histologies muddy results
The improvement in OS was observed across all patient populations, including patients with ESCC, esophageal adenocarcinoma (EAC), and EGJ tumors, the researchers noted.
However, invited discussant Andres Cervantes, MD, PhD, from the University of Valencia, Spain, commented that ESCC and EAC have very different histologies and that including patients with both in a single trial can make the results very confusing.
He pointed out that, in the KEYNOTE-590 population, 73% of patients had ESCCs and 27% had EACs.
The OS improvement was seen regardless of PD-L1 expression, although the best results occurred in patients with high expression.
However, Cervantes noted that PD-L1 expression was not used as a stratification factor prior to randomization, even though it was included in the complex, step-wise statistical plan for the study.
Another expert, Salah-Eddin Al-Batran, MD, from the Krankenhaus Nordwest in Frankfurt, Germany, questioned the researchers’ recommendation that all patients with esophageal cancer receive this regimen, regardless of PD-L1 expression.
“We have to be sure that we do not inflate the results for the all-comers by the very responsive group of high [PD-L1] expressers,” he said at the press briefing.
He said the trial report also leaves open the question of efficacy in tumors with microsatellite instability and/or high tumor mutational burden.
“I think these questions have to be addressed to give us a clear picture of how to treat a patient sitting in front of us as doctors,” he said.
Also adding to the concerns about this trial is the fact that the “selected chemotherapy schedule is not much currently used as the standard of care,” said Cervantes, although he added that it “is acceptable for this protocol.”
Despite these caveats, the trial was clearly positive, Cervantes said. The greatest benefit appeared to accrue for patients with ESCC.
“The addition of pembrolizumab to platinum-based chemotherapy significantly increases response rate, PFS, and OS in patients with advanced esophageal carcinomas over chemotherapy alone,” he said, and therefore he agreed with the investigators that “this is a new standard of care.”
The study was funded by Merck Sharp & Dohme. Enzinger disclosed honoraria and advisory or consulting roles for Merck and others. Cervantes disclosed consulting or advising and research funding from Merck Serono and others. He is president-elect of ESMO. Al-Batran has disclosed no relevant financial relationships.
This story first appeared on Medscape.com.
FROM ESMO 2020
CDC playbook prepares states for rollout of COVID-19 vaccine if one is approved
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
Health Care Disparities Among Adolescents and Adults With Sickle Cell Disease: A Community-Based Needs Assessment to Inform Intervention Strategies
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23
The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.
Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.
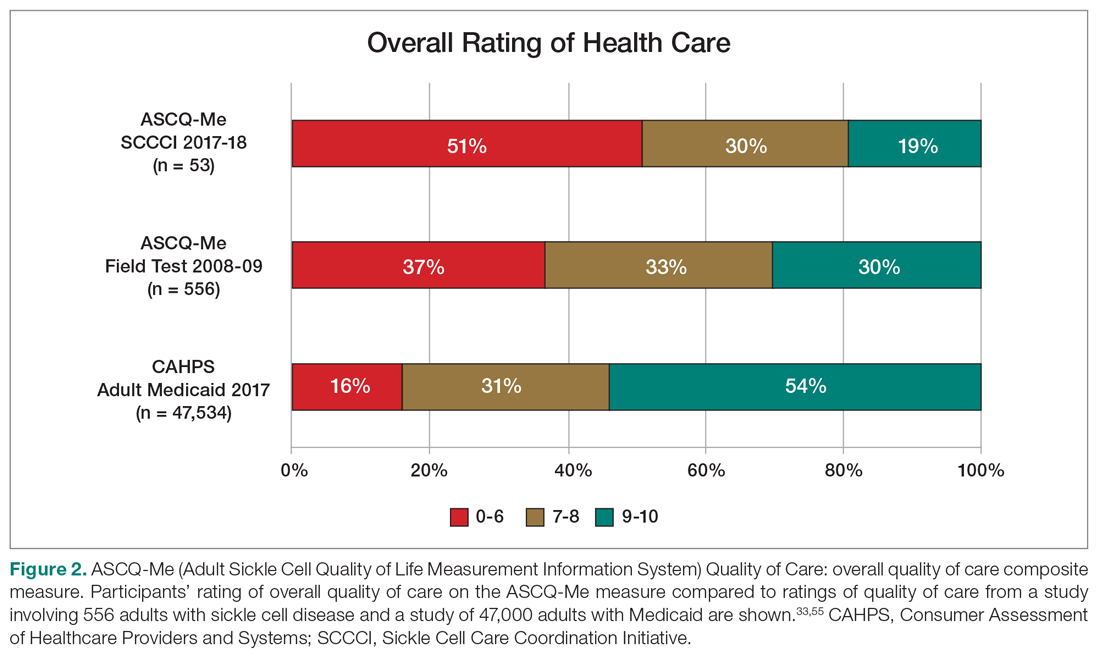
Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”
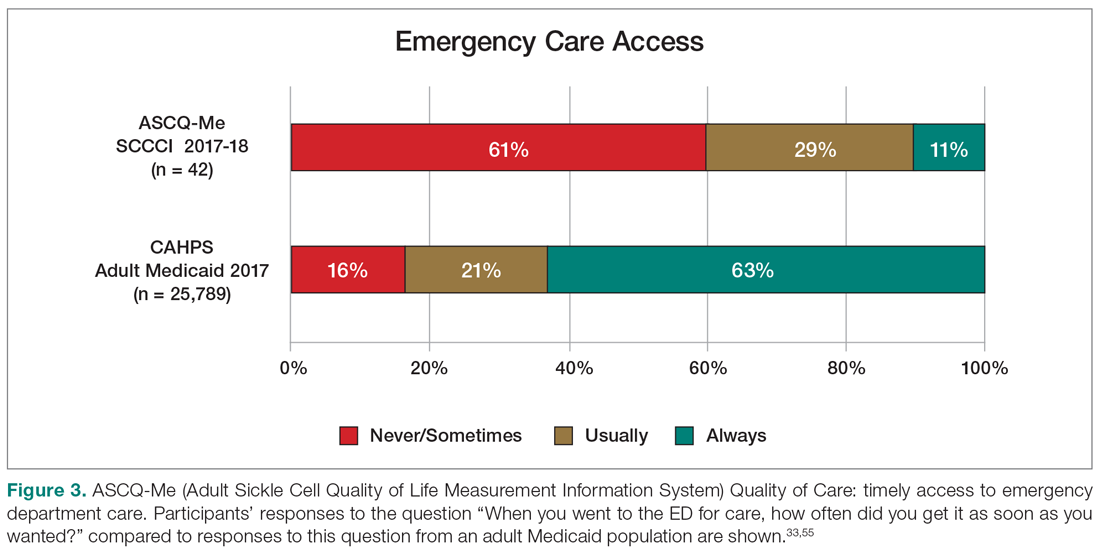
On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.
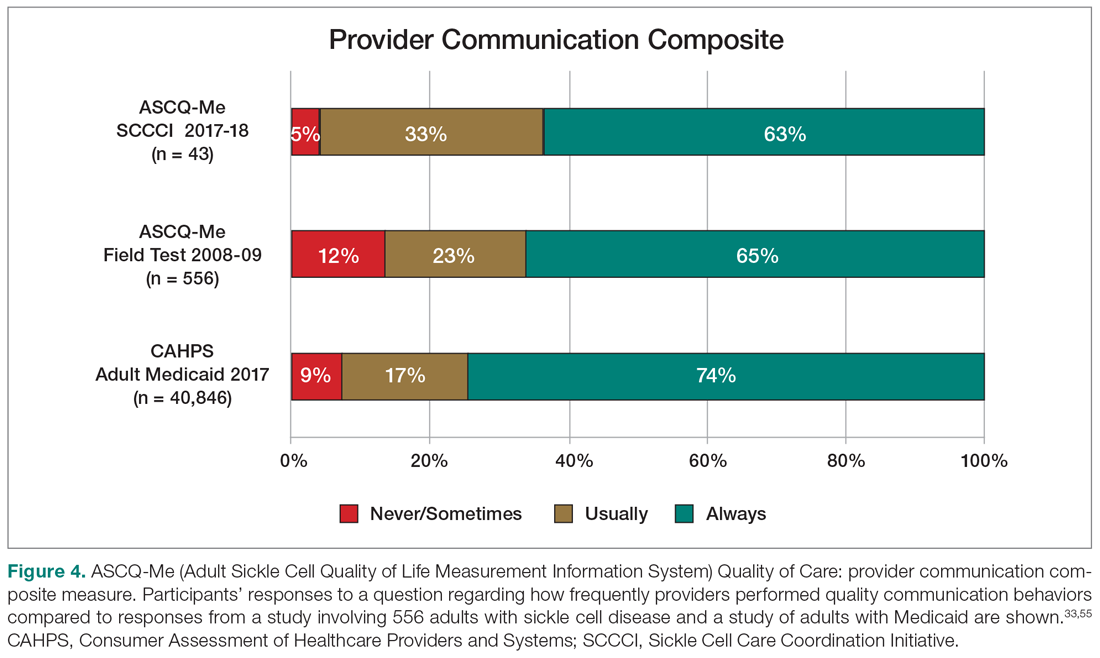
Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).
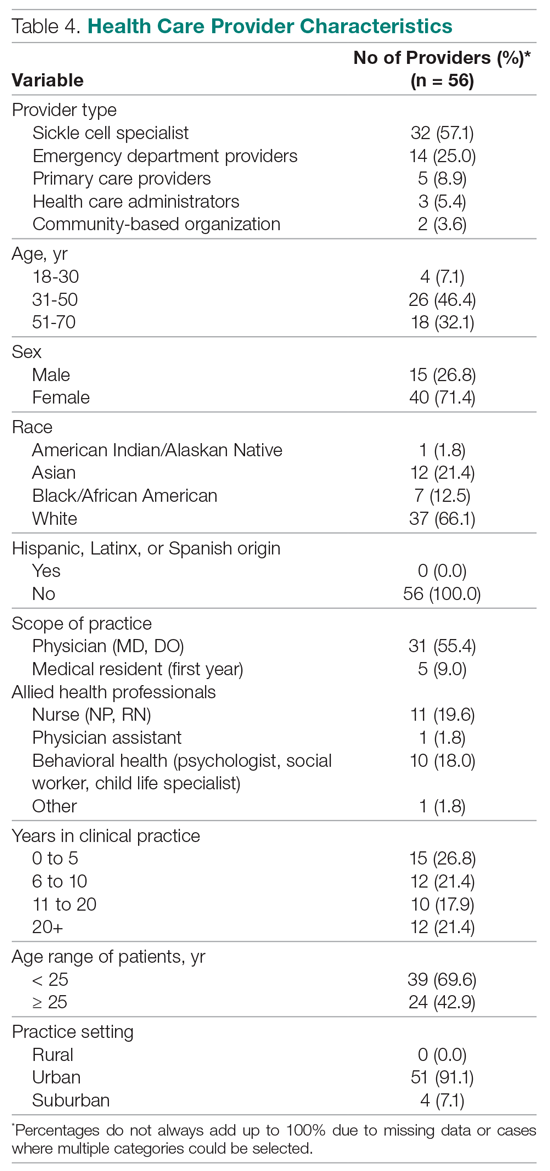
Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23

The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.

Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.

Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”

On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.

Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).

Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23

The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.

Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.

Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”

On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.

Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).

Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
Lenvatinib combo may offer hope after immunotherapy in melanoma
Patients with advanced melanoma who have progressed on anti–programmed death 1/PD-ligand 1 (PD-L1) immunotherapy could substantially extend their overall survival (OS) with a combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and pembrolizumab (Keytruda), suggests an open-label, single arm study.
The research was presented Sept. 19 at the European Society for Medical Oncology Virtual Congress 2020.
In LEAP-004 trial, over 100 patients with stage 3 or 4 melanoma who had progressed after immunotherapy were given lenvatinib plus pembrolizumab, which yielded a median progression-free survival (PFS) of more than 4 months and a median OS of more than a year. Median follow-up was 12 months.
Presenting the findings, Ana Maria Arance Fernandez, MD, PhD, Hospital Clínic de Barcelona, Spain, said lenvatinib plus pembrolizumab has “promising” antitumor activity in patients with advanced melanoma with confirmed progression on a PD-1 inhibitor given alone or in combination. “These results are encouraging given the stringent definition of progression on prior anti-PD-1 therapy and the enrollment of poor-risk patients.”
Dr. Arance Fernandez added that “these data support lenvatinib plus pembrolizumab as a potential treatment regimen for this population of high unmet medical need.”
Bartosz Chmielowski, MD, PhD, Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, who was not involved in the study, discussed the findings.
He highlighted that the patients were not randomly assigned in LEAP-004, with all of them receiving the same therapy.
Nevertheless, the response rate was “quite impressive for this patient population.”
He also drew comparison with previous data with nivolumab (Opdivo) alone or in combination with ipilimumab (Yervoy) in a similar population, noting that the overall survival was less than half that seen in the current trial, “which makes these results even more important.”
“It tells us that this combination might be an option with disease progression on anti-PD-1,” Dr. Chmielowski noted.
Dr. Arance Fernandez pointed out that patients with advanced melanoma who progress on standard-of-care treatment with anti-PD-1 therapy or a cytotoxic T-lymphocyte–associated protein 4 (CTLA4) inhibitor plus anti-PD-1 “have very limited therapeutic options available and there is no approved regimen in this indication.”
Response rate, PFS, and OS
Previous studies have indicated that adding an anti-PD-1 drug to lenvatinib achieves superior antitumor activity than either treatment alone, with promising results in phase 1/2b data in pretreated metastatic melanoma.
LEAP-004 therefore enrolled patients with unresectable stage 3 or 4 melanoma, who had disease progression within 12 weeks of their last dose of anti-PD-(L)1 therapy either alone or with a CTLA4 inhibitor. There was no limit on the number of prior treatments.
The patients received pembrolizumab 200 mg IV for up to 35 cycles plus lenvatinib 20 mg daily until progression, unacceptable toxicity, or patient or physician decision.
They were imaged at baseline and every 9 weeks through to week 54, then every 12 weeks until week 102, and then every 24 weeks.
From February to September 2019, 103 patients were enrolled, all of whom received at least one dose of lenvatinib plus pembrolizumab. The median age of the patients was 63 years, and 53.4% were male.
Dr. Arance Fernandez pointed out that this was a high-risk population, with 20.4% having a lactate dehydrogenase level twice the upper limit of normal and 14.6% having brain metastasis, while the median sum of target lesions was 100 mm.
A BRAFv600 mutation was identified in 36.9% of patients, and 64.1% were PD-L1 positive.
Nearly one third (28.2%) had received a prior anti-CTLA4 plus anti-PD-(L)1 combination, and 19.5% had undergone four or more prior lines of therapy.
The overall response rate to lenvatinib plus pembrolizumab was 21.4%, with 1.9% having a complete response and 19.4% a partial response. This was seen across subgroups, including by age and disease stage.
Dr. Arance Fernandez said the overall response rate was even higher in patients who had previously been treated with an anti-CTLA4 plus anti-PD-(L)1 combination, at 31%.
However, Dr. Chmielowski warned that “we must interpret this result with caution since only 29 patients were in this subpopulation.”
The median duration of response (per blinded independent committee review) across the study population was 6.3 months, with 72.6% still responding at 6 months.
The median PFS was 4.2 months with the combination therapy, with 41.7% of patients progression free at 6 months, and 26.2% at 9 months.
Median overall survival was 13.9 months, with 77.3% of patients still alive at 6 months and 65.4% alive at 9 months.
Although 96.1% of patients experienced at least one treatment-related adverse event of any grade, only 44.7% had grade 3 or higher events, and only in 7.8% of cases did that lead to treatment discontinuation.
The most common adverse events were hypertension (56.3%), diarrhea (35.9%), nausea (34%), and hypothyroidism (33%), although, in the vast majority of cases, these events were grade 1 or 2.
LEAP presents challenges
Dr. Chmielowski would like to see treatment in this setting individualized somehow.
“It will be also important to come up with personalized immunotherapy so that, based on the mechanism of resistance in patient populations, we would be able to choose the subsequent treatments,” he commented.
Dr. Arance Fernandez explained that lenvatinib inhibits multiple tyrosine kinases involved in angiogenesis, cell proliferation, and immune modulation, and has demonstrated immunomodulatory activity in the tumor microenvironment.
However, Dr. Arance Fernandez noted that, as resistance to immunotherapy is “multifactorial,” it may be that a combination treatment will be more effective in these patients.
The study was funded by Merck. Dr. Arance Fernandez has financial ties to Merck and multiple other drug companies. Dr. Chmielowski has financial ties to Merck Serono and multiple other companies.
This article first appeared on Medscape.com.
Patients with advanced melanoma who have progressed on anti–programmed death 1/PD-ligand 1 (PD-L1) immunotherapy could substantially extend their overall survival (OS) with a combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and pembrolizumab (Keytruda), suggests an open-label, single arm study.
The research was presented Sept. 19 at the European Society for Medical Oncology Virtual Congress 2020.
In LEAP-004 trial, over 100 patients with stage 3 or 4 melanoma who had progressed after immunotherapy were given lenvatinib plus pembrolizumab, which yielded a median progression-free survival (PFS) of more than 4 months and a median OS of more than a year. Median follow-up was 12 months.
Presenting the findings, Ana Maria Arance Fernandez, MD, PhD, Hospital Clínic de Barcelona, Spain, said lenvatinib plus pembrolizumab has “promising” antitumor activity in patients with advanced melanoma with confirmed progression on a PD-1 inhibitor given alone or in combination. “These results are encouraging given the stringent definition of progression on prior anti-PD-1 therapy and the enrollment of poor-risk patients.”
Dr. Arance Fernandez added that “these data support lenvatinib plus pembrolizumab as a potential treatment regimen for this population of high unmet medical need.”
Bartosz Chmielowski, MD, PhD, Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, who was not involved in the study, discussed the findings.
He highlighted that the patients were not randomly assigned in LEAP-004, with all of them receiving the same therapy.
Nevertheless, the response rate was “quite impressive for this patient population.”
He also drew comparison with previous data with nivolumab (Opdivo) alone or in combination with ipilimumab (Yervoy) in a similar population, noting that the overall survival was less than half that seen in the current trial, “which makes these results even more important.”
“It tells us that this combination might be an option with disease progression on anti-PD-1,” Dr. Chmielowski noted.
Dr. Arance Fernandez pointed out that patients with advanced melanoma who progress on standard-of-care treatment with anti-PD-1 therapy or a cytotoxic T-lymphocyte–associated protein 4 (CTLA4) inhibitor plus anti-PD-1 “have very limited therapeutic options available and there is no approved regimen in this indication.”
Response rate, PFS, and OS
Previous studies have indicated that adding an anti-PD-1 drug to lenvatinib achieves superior antitumor activity than either treatment alone, with promising results in phase 1/2b data in pretreated metastatic melanoma.
LEAP-004 therefore enrolled patients with unresectable stage 3 or 4 melanoma, who had disease progression within 12 weeks of their last dose of anti-PD-(L)1 therapy either alone or with a CTLA4 inhibitor. There was no limit on the number of prior treatments.
The patients received pembrolizumab 200 mg IV for up to 35 cycles plus lenvatinib 20 mg daily until progression, unacceptable toxicity, or patient or physician decision.
They were imaged at baseline and every 9 weeks through to week 54, then every 12 weeks until week 102, and then every 24 weeks.
From February to September 2019, 103 patients were enrolled, all of whom received at least one dose of lenvatinib plus pembrolizumab. The median age of the patients was 63 years, and 53.4% were male.
Dr. Arance Fernandez pointed out that this was a high-risk population, with 20.4% having a lactate dehydrogenase level twice the upper limit of normal and 14.6% having brain metastasis, while the median sum of target lesions was 100 mm.
A BRAFv600 mutation was identified in 36.9% of patients, and 64.1% were PD-L1 positive.
Nearly one third (28.2%) had received a prior anti-CTLA4 plus anti-PD-(L)1 combination, and 19.5% had undergone four or more prior lines of therapy.
The overall response rate to lenvatinib plus pembrolizumab was 21.4%, with 1.9% having a complete response and 19.4% a partial response. This was seen across subgroups, including by age and disease stage.
Dr. Arance Fernandez said the overall response rate was even higher in patients who had previously been treated with an anti-CTLA4 plus anti-PD-(L)1 combination, at 31%.
However, Dr. Chmielowski warned that “we must interpret this result with caution since only 29 patients were in this subpopulation.”
The median duration of response (per blinded independent committee review) across the study population was 6.3 months, with 72.6% still responding at 6 months.
The median PFS was 4.2 months with the combination therapy, with 41.7% of patients progression free at 6 months, and 26.2% at 9 months.
Median overall survival was 13.9 months, with 77.3% of patients still alive at 6 months and 65.4% alive at 9 months.
Although 96.1% of patients experienced at least one treatment-related adverse event of any grade, only 44.7% had grade 3 or higher events, and only in 7.8% of cases did that lead to treatment discontinuation.
The most common adverse events were hypertension (56.3%), diarrhea (35.9%), nausea (34%), and hypothyroidism (33%), although, in the vast majority of cases, these events were grade 1 or 2.
LEAP presents challenges
Dr. Chmielowski would like to see treatment in this setting individualized somehow.
“It will be also important to come up with personalized immunotherapy so that, based on the mechanism of resistance in patient populations, we would be able to choose the subsequent treatments,” he commented.
Dr. Arance Fernandez explained that lenvatinib inhibits multiple tyrosine kinases involved in angiogenesis, cell proliferation, and immune modulation, and has demonstrated immunomodulatory activity in the tumor microenvironment.
However, Dr. Arance Fernandez noted that, as resistance to immunotherapy is “multifactorial,” it may be that a combination treatment will be more effective in these patients.
The study was funded by Merck. Dr. Arance Fernandez has financial ties to Merck and multiple other drug companies. Dr. Chmielowski has financial ties to Merck Serono and multiple other companies.
This article first appeared on Medscape.com.
Patients with advanced melanoma who have progressed on anti–programmed death 1/PD-ligand 1 (PD-L1) immunotherapy could substantially extend their overall survival (OS) with a combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and pembrolizumab (Keytruda), suggests an open-label, single arm study.
The research was presented Sept. 19 at the European Society for Medical Oncology Virtual Congress 2020.
In LEAP-004 trial, over 100 patients with stage 3 or 4 melanoma who had progressed after immunotherapy were given lenvatinib plus pembrolizumab, which yielded a median progression-free survival (PFS) of more than 4 months and a median OS of more than a year. Median follow-up was 12 months.
Presenting the findings, Ana Maria Arance Fernandez, MD, PhD, Hospital Clínic de Barcelona, Spain, said lenvatinib plus pembrolizumab has “promising” antitumor activity in patients with advanced melanoma with confirmed progression on a PD-1 inhibitor given alone or in combination. “These results are encouraging given the stringent definition of progression on prior anti-PD-1 therapy and the enrollment of poor-risk patients.”
Dr. Arance Fernandez added that “these data support lenvatinib plus pembrolizumab as a potential treatment regimen for this population of high unmet medical need.”
Bartosz Chmielowski, MD, PhD, Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, who was not involved in the study, discussed the findings.
He highlighted that the patients were not randomly assigned in LEAP-004, with all of them receiving the same therapy.
Nevertheless, the response rate was “quite impressive for this patient population.”
He also drew comparison with previous data with nivolumab (Opdivo) alone or in combination with ipilimumab (Yervoy) in a similar population, noting that the overall survival was less than half that seen in the current trial, “which makes these results even more important.”
“It tells us that this combination might be an option with disease progression on anti-PD-1,” Dr. Chmielowski noted.
Dr. Arance Fernandez pointed out that patients with advanced melanoma who progress on standard-of-care treatment with anti-PD-1 therapy or a cytotoxic T-lymphocyte–associated protein 4 (CTLA4) inhibitor plus anti-PD-1 “have very limited therapeutic options available and there is no approved regimen in this indication.”
Response rate, PFS, and OS
Previous studies have indicated that adding an anti-PD-1 drug to lenvatinib achieves superior antitumor activity than either treatment alone, with promising results in phase 1/2b data in pretreated metastatic melanoma.
LEAP-004 therefore enrolled patients with unresectable stage 3 or 4 melanoma, who had disease progression within 12 weeks of their last dose of anti-PD-(L)1 therapy either alone or with a CTLA4 inhibitor. There was no limit on the number of prior treatments.
The patients received pembrolizumab 200 mg IV for up to 35 cycles plus lenvatinib 20 mg daily until progression, unacceptable toxicity, or patient or physician decision.
They were imaged at baseline and every 9 weeks through to week 54, then every 12 weeks until week 102, and then every 24 weeks.
From February to September 2019, 103 patients were enrolled, all of whom received at least one dose of lenvatinib plus pembrolizumab. The median age of the patients was 63 years, and 53.4% were male.
Dr. Arance Fernandez pointed out that this was a high-risk population, with 20.4% having a lactate dehydrogenase level twice the upper limit of normal and 14.6% having brain metastasis, while the median sum of target lesions was 100 mm.
A BRAFv600 mutation was identified in 36.9% of patients, and 64.1% were PD-L1 positive.
Nearly one third (28.2%) had received a prior anti-CTLA4 plus anti-PD-(L)1 combination, and 19.5% had undergone four or more prior lines of therapy.
The overall response rate to lenvatinib plus pembrolizumab was 21.4%, with 1.9% having a complete response and 19.4% a partial response. This was seen across subgroups, including by age and disease stage.
Dr. Arance Fernandez said the overall response rate was even higher in patients who had previously been treated with an anti-CTLA4 plus anti-PD-(L)1 combination, at 31%.
However, Dr. Chmielowski warned that “we must interpret this result with caution since only 29 patients were in this subpopulation.”
The median duration of response (per blinded independent committee review) across the study population was 6.3 months, with 72.6% still responding at 6 months.
The median PFS was 4.2 months with the combination therapy, with 41.7% of patients progression free at 6 months, and 26.2% at 9 months.
Median overall survival was 13.9 months, with 77.3% of patients still alive at 6 months and 65.4% alive at 9 months.
Although 96.1% of patients experienced at least one treatment-related adverse event of any grade, only 44.7% had grade 3 or higher events, and only in 7.8% of cases did that lead to treatment discontinuation.
The most common adverse events were hypertension (56.3%), diarrhea (35.9%), nausea (34%), and hypothyroidism (33%), although, in the vast majority of cases, these events were grade 1 or 2.
LEAP presents challenges
Dr. Chmielowski would like to see treatment in this setting individualized somehow.
“It will be also important to come up with personalized immunotherapy so that, based on the mechanism of resistance in patient populations, we would be able to choose the subsequent treatments,” he commented.
Dr. Arance Fernandez explained that lenvatinib inhibits multiple tyrosine kinases involved in angiogenesis, cell proliferation, and immune modulation, and has demonstrated immunomodulatory activity in the tumor microenvironment.
However, Dr. Arance Fernandez noted that, as resistance to immunotherapy is “multifactorial,” it may be that a combination treatment will be more effective in these patients.
The study was funded by Merck. Dr. Arance Fernandez has financial ties to Merck and multiple other drug companies. Dr. Chmielowski has financial ties to Merck Serono and multiple other companies.
This article first appeared on Medscape.com.
FROM ESMO 2020
Three major COVID vaccine developers release detailed trial protocols
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Palbociclib plus letrozole improves PFS in advanced endometrial cancer
Adding palbociclib to letrozole significantly prolonged progression-free survival (PFS) in a phase 2 trial of patients with advanced or recurrent estrogen receptor (ER)–positive endometrial cancer.
This was the first randomized trial to evaluate the efficacy of a CDK4/6 inhibitor in combination with an aromatase inhibitor in patients with advanced or recurrent ER-positive endometrial cancer, noted study investigator Mansoor Raza Mirza, MD, PhD, of Rigshospitalet Copenhagen University Hospital.
Dr. Mirza presented results from this study, ENGOT-EN3-NSGO/PALEO, at the European Society for Medical Oncology Virtual Congress 2020.
Palbociclib is a selective inhibitor of CDK4/, both of which are involved in cell-cycle transitions, Dr. Mirza explained. He observed that endometrial endometrioid adenocarcinomas are hormone dependent, and endocrine treatment with an aromatase inhibitor is well established. Palbociclib has been shown to be superior, when combined with letrozole, to letrozole alone in ER-positive breast cancer.
For the ENGOT-EN3-NSGO/PALEO study, investigators enrolled 77 patients with ER-positive advanced/recurrent endometrial cancer. Patients had received at least one prior systemic therapy, no prior endocrine therapy (except medroxyprogesterone acetate and megestrol acetate), and no prior CDK inhibitor.
Patients were randomized 1:1 to receive oral letrozole (2.5 mg on days 1-28) and either palbociclib (125 mg on days 1-21) or placebo (125 mg on days 1-21) in a 28-day cycle until progression. Baseline characteristics were similar between the treatment arms.
Efficacy
Of the 77 patients enrolled, 73 were evaluable. The primary endpoint was PFS.
The median PFS in the intention-to-treat population was 3.0 months in the placebo arm and 8.3 months in the palbociclib arm (hazard ratio, 0.56; 95% confidence interval, 0.32-0.98; P = .0376).
Looking at stratification factors, PFS was higher in the palbociclib arm among the large majority (about 85%) of patients who had received no prior medroxyprogesterone acetate or megestrol acetate (HR, 0.55; 95% CI, 0.29-01.04; P = .0615) and among patients with relapsed disease (HR, 0.61; 95% CI, 0.34-1.09; P = .0916).
The disease control rate at 24 weeks, a secondary endpoint, was 63.6% in the palbociclib arm and 37.8% in the placebo arm.
Safety
Adverse events were more frequent in the palbociclib arm, with neutropenia being the most common.
Rates of adverse event–related dose reduction (to 100 mg or 75 mg) were 36% in the palbociclib arm and 3% in the placebo arm.
Adverse event–related discontinuation rates were 25% and 19% for palbociclib and letrozole, respectively, in the palbociclib arm and 14% and 11% for placebo and letrozole, respectively, in the placebo arm.
“The toxicity of palbociclib plus letrozole combination therapy was manageable, with most patients remaining on treatment until disease progression,” Dr. Mirza said.
He noted that an analysis of patient-reported outcomes revealed no detrimental effect on quality of life with the combination therapy.
Next steps
“Compared with placebo plus letrozole, the combination of palbociclib plus letrozole demonstrated clinically meaningful improvement in PFS,” Dr. Mirza said. “These results merit a phase 3 validation trial.”
“There is a huge rationale for using this drug in endometrial cancer,” commented study discussant Domenica Lorusso, MD, PhD, of Fondazione Policlinico Universitario Agostino Gemelli IRCCS and Catholic University of Sacred Hearth in Rome.
Dr. Lorusso said hormone receptor expression, which has been identified as a strong predictor of CDK4/6 inhibitor activity, is present in up to 90% of patients with type 1 and in about 65% of patients with type 2 endometrial cancer.
Response rates, in experience with aromatase inhibitors, have been “quite disappointing, in the 10%-20% range,” Dr. Lorusso said, with “dismal prognosis” and guidelines stating that “no standard second-line treatment has been identified.”
This research was sponsored by investigators, but Pfizer provided a study grant. Dr. Mirza disclosed relationships with Pfizer, AstraZeneca, Biocad, Clovis Oncology, Genmab, Karyopharm Therapeutics, Merck, Oncology Adventure, Roche, Seattle Genetics, Sera Prognostics, Sotio, GlaxoSmithKline, Zai Lab, and Boehringer Ingelheim. Dr. Lorusso disclosed relationships with AstraZeneca, Biocad, Clovis Oncology, Genmab, Merck, Roche, Tesaro, Amgen, Immunogen, and Pharma Mar.
SOURCE: Mirza MR et al. ESMO 2020, Abstract LBA28.
Adding palbociclib to letrozole significantly prolonged progression-free survival (PFS) in a phase 2 trial of patients with advanced or recurrent estrogen receptor (ER)–positive endometrial cancer.
This was the first randomized trial to evaluate the efficacy of a CDK4/6 inhibitor in combination with an aromatase inhibitor in patients with advanced or recurrent ER-positive endometrial cancer, noted study investigator Mansoor Raza Mirza, MD, PhD, of Rigshospitalet Copenhagen University Hospital.
Dr. Mirza presented results from this study, ENGOT-EN3-NSGO/PALEO, at the European Society for Medical Oncology Virtual Congress 2020.
Palbociclib is a selective inhibitor of CDK4/, both of which are involved in cell-cycle transitions, Dr. Mirza explained. He observed that endometrial endometrioid adenocarcinomas are hormone dependent, and endocrine treatment with an aromatase inhibitor is well established. Palbociclib has been shown to be superior, when combined with letrozole, to letrozole alone in ER-positive breast cancer.
For the ENGOT-EN3-NSGO/PALEO study, investigators enrolled 77 patients with ER-positive advanced/recurrent endometrial cancer. Patients had received at least one prior systemic therapy, no prior endocrine therapy (except medroxyprogesterone acetate and megestrol acetate), and no prior CDK inhibitor.
Patients were randomized 1:1 to receive oral letrozole (2.5 mg on days 1-28) and either palbociclib (125 mg on days 1-21) or placebo (125 mg on days 1-21) in a 28-day cycle until progression. Baseline characteristics were similar between the treatment arms.
Efficacy
Of the 77 patients enrolled, 73 were evaluable. The primary endpoint was PFS.
The median PFS in the intention-to-treat population was 3.0 months in the placebo arm and 8.3 months in the palbociclib arm (hazard ratio, 0.56; 95% confidence interval, 0.32-0.98; P = .0376).
Looking at stratification factors, PFS was higher in the palbociclib arm among the large majority (about 85%) of patients who had received no prior medroxyprogesterone acetate or megestrol acetate (HR, 0.55; 95% CI, 0.29-01.04; P = .0615) and among patients with relapsed disease (HR, 0.61; 95% CI, 0.34-1.09; P = .0916).
The disease control rate at 24 weeks, a secondary endpoint, was 63.6% in the palbociclib arm and 37.8% in the placebo arm.
Safety
Adverse events were more frequent in the palbociclib arm, with neutropenia being the most common.
Rates of adverse event–related dose reduction (to 100 mg or 75 mg) were 36% in the palbociclib arm and 3% in the placebo arm.
Adverse event–related discontinuation rates were 25% and 19% for palbociclib and letrozole, respectively, in the palbociclib arm and 14% and 11% for placebo and letrozole, respectively, in the placebo arm.
“The toxicity of palbociclib plus letrozole combination therapy was manageable, with most patients remaining on treatment until disease progression,” Dr. Mirza said.
He noted that an analysis of patient-reported outcomes revealed no detrimental effect on quality of life with the combination therapy.
Next steps
“Compared with placebo plus letrozole, the combination of palbociclib plus letrozole demonstrated clinically meaningful improvement in PFS,” Dr. Mirza said. “These results merit a phase 3 validation trial.”
“There is a huge rationale for using this drug in endometrial cancer,” commented study discussant Domenica Lorusso, MD, PhD, of Fondazione Policlinico Universitario Agostino Gemelli IRCCS and Catholic University of Sacred Hearth in Rome.
Dr. Lorusso said hormone receptor expression, which has been identified as a strong predictor of CDK4/6 inhibitor activity, is present in up to 90% of patients with type 1 and in about 65% of patients with type 2 endometrial cancer.
Response rates, in experience with aromatase inhibitors, have been “quite disappointing, in the 10%-20% range,” Dr. Lorusso said, with “dismal prognosis” and guidelines stating that “no standard second-line treatment has been identified.”
This research was sponsored by investigators, but Pfizer provided a study grant. Dr. Mirza disclosed relationships with Pfizer, AstraZeneca, Biocad, Clovis Oncology, Genmab, Karyopharm Therapeutics, Merck, Oncology Adventure, Roche, Seattle Genetics, Sera Prognostics, Sotio, GlaxoSmithKline, Zai Lab, and Boehringer Ingelheim. Dr. Lorusso disclosed relationships with AstraZeneca, Biocad, Clovis Oncology, Genmab, Merck, Roche, Tesaro, Amgen, Immunogen, and Pharma Mar.
SOURCE: Mirza MR et al. ESMO 2020, Abstract LBA28.
Adding palbociclib to letrozole significantly prolonged progression-free survival (PFS) in a phase 2 trial of patients with advanced or recurrent estrogen receptor (ER)–positive endometrial cancer.
This was the first randomized trial to evaluate the efficacy of a CDK4/6 inhibitor in combination with an aromatase inhibitor in patients with advanced or recurrent ER-positive endometrial cancer, noted study investigator Mansoor Raza Mirza, MD, PhD, of Rigshospitalet Copenhagen University Hospital.
Dr. Mirza presented results from this study, ENGOT-EN3-NSGO/PALEO, at the European Society for Medical Oncology Virtual Congress 2020.
Palbociclib is a selective inhibitor of CDK4/, both of which are involved in cell-cycle transitions, Dr. Mirza explained. He observed that endometrial endometrioid adenocarcinomas are hormone dependent, and endocrine treatment with an aromatase inhibitor is well established. Palbociclib has been shown to be superior, when combined with letrozole, to letrozole alone in ER-positive breast cancer.
For the ENGOT-EN3-NSGO/PALEO study, investigators enrolled 77 patients with ER-positive advanced/recurrent endometrial cancer. Patients had received at least one prior systemic therapy, no prior endocrine therapy (except medroxyprogesterone acetate and megestrol acetate), and no prior CDK inhibitor.
Patients were randomized 1:1 to receive oral letrozole (2.5 mg on days 1-28) and either palbociclib (125 mg on days 1-21) or placebo (125 mg on days 1-21) in a 28-day cycle until progression. Baseline characteristics were similar between the treatment arms.
Efficacy
Of the 77 patients enrolled, 73 were evaluable. The primary endpoint was PFS.
The median PFS in the intention-to-treat population was 3.0 months in the placebo arm and 8.3 months in the palbociclib arm (hazard ratio, 0.56; 95% confidence interval, 0.32-0.98; P = .0376).
Looking at stratification factors, PFS was higher in the palbociclib arm among the large majority (about 85%) of patients who had received no prior medroxyprogesterone acetate or megestrol acetate (HR, 0.55; 95% CI, 0.29-01.04; P = .0615) and among patients with relapsed disease (HR, 0.61; 95% CI, 0.34-1.09; P = .0916).
The disease control rate at 24 weeks, a secondary endpoint, was 63.6% in the palbociclib arm and 37.8% in the placebo arm.
Safety
Adverse events were more frequent in the palbociclib arm, with neutropenia being the most common.
Rates of adverse event–related dose reduction (to 100 mg or 75 mg) were 36% in the palbociclib arm and 3% in the placebo arm.
Adverse event–related discontinuation rates were 25% and 19% for palbociclib and letrozole, respectively, in the palbociclib arm and 14% and 11% for placebo and letrozole, respectively, in the placebo arm.
“The toxicity of palbociclib plus letrozole combination therapy was manageable, with most patients remaining on treatment until disease progression,” Dr. Mirza said.
He noted that an analysis of patient-reported outcomes revealed no detrimental effect on quality of life with the combination therapy.
Next steps
“Compared with placebo plus letrozole, the combination of palbociclib plus letrozole demonstrated clinically meaningful improvement in PFS,” Dr. Mirza said. “These results merit a phase 3 validation trial.”
“There is a huge rationale for using this drug in endometrial cancer,” commented study discussant Domenica Lorusso, MD, PhD, of Fondazione Policlinico Universitario Agostino Gemelli IRCCS and Catholic University of Sacred Hearth in Rome.
Dr. Lorusso said hormone receptor expression, which has been identified as a strong predictor of CDK4/6 inhibitor activity, is present in up to 90% of patients with type 1 and in about 65% of patients with type 2 endometrial cancer.
Response rates, in experience with aromatase inhibitors, have been “quite disappointing, in the 10%-20% range,” Dr. Lorusso said, with “dismal prognosis” and guidelines stating that “no standard second-line treatment has been identified.”
This research was sponsored by investigators, but Pfizer provided a study grant. Dr. Mirza disclosed relationships with Pfizer, AstraZeneca, Biocad, Clovis Oncology, Genmab, Karyopharm Therapeutics, Merck, Oncology Adventure, Roche, Seattle Genetics, Sera Prognostics, Sotio, GlaxoSmithKline, Zai Lab, and Boehringer Ingelheim. Dr. Lorusso disclosed relationships with AstraZeneca, Biocad, Clovis Oncology, Genmab, Merck, Roche, Tesaro, Amgen, Immunogen, and Pharma Mar.
SOURCE: Mirza MR et al. ESMO 2020, Abstract LBA28.
FROM ESMO 2020