User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children’s share of COVID-19 burden continues to increase
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Abemaciclib cuts early recurrence in high-risk breast cancer
First advance in 20 years
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
First advance in 20 years
First advance in 20 years
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
FROM ESMO 2020
More female specialists, but gender gap persists in pay, survey finds
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
Signs of an ‘October vaccine surprise’ alarm career scientists
who have pledged not to release any vaccine unless it’s proved safe and effective.
In podcasts, public forums, social media and medical journals, a growing number of prominent health leaders say they fear that Mr. Trump – who has repeatedly signaled his desire for the swift approval of a vaccine and his displeasure with perceived delays at the FDA – will take matters into his own hands, running roughshod over the usual regulatory process.
It would reflect another attempt by a norm-breaking administration, poised to ram through a Supreme Court nominee opposed to existing abortion rights and the Affordable Care Act, to inject politics into sensitive public health decisions. Mr. Trump has repeatedly contradicted the advice of senior scientists on COVID-19 while pushing controversial treatments for the disease.
If the executive branch were to overrule the FDA’s scientific judgment, a vaccine of limited efficacy and, worse, unknown side effects could be rushed to market.
The worries intensified over the weekend, after Alex Azar, the administration’s secretary of Health & Human Services, asserted his agency’s rule-making authority over the FDA. HHS spokesperson Caitlin Oakley said Mr. Azar’s decision had no bearing on the vaccine approval process.
Vaccines are typically approved by the FDA. Alternatively, Mr. Azar – who reports directly to Mr. Trump – can issue an emergency use authorization, even before any vaccines have been shown to be safe and effective in late-stage clinical trials.
“Yes, this scenario is certainly possible legally and politically,” said Jerry Avorn, MD, a professor of medicine at Harvard Medical School, who outlined such an event in the New England Journal of Medicine. He said it “seems frighteningly more plausible each day.”
Vaccine experts and public health officials are particularly vexed by the possibility because it could ruin the fragile public confidence in a COVID-19 vaccine. It might put scientific authorities in the position of urging people not to be vaccinated after years of coaxing hesitant parents to ignore baseless fears.
Physicians might refuse to administer a vaccine approved with inadequate data, said Preeti Malani, MD, chief health officer and professor of medicine at the University of Michigan in Ann Arbor, in a recent webinar. “You could have a safe, effective vaccine that no one wants to take.” A recent KFF poll found that 54% of Americans would not submit to a COVID-19 vaccine authorized before Election Day.
After this story was published, an HHS official said that Mr. Azar “will defer completely to the FDA” as the agency weighs whether to approve a vaccine produced through the government’s Operation Warp Speed effort.
“The idea the Secretary would approve or authorize a vaccine over the FDA’s objections is preposterous and betrays ignorance of the transparent process that we’re following for the development of the OWS vaccines,” HHS chief of staff Brian Harrison wrote in an email.
White House spokesperson Judd Deere dismissed the scientists’ concerns, saying Trump cared only about the public’s safety and health. “This false narrative that the media and Democrats have created that politics is influencing approvals is not only false but is a danger to the American public,” he said.
Usually, the FDA approves vaccines only after companies submit years of data proving that a vaccine is safe and effective. But a 2004 law allows the FDA to issue an emergency use authorization with much less evidence, as long as the vaccine “may be effective” and its “known and potential benefits” outweigh its “known and potential risks.”
Many scientists doubt a vaccine could meet those criteria before the election. But the terms might be legally vague enough to allow the administration to take such steps.
Moncef Slaoui, chief scientific adviser to Operation Warp Speed, the government program aiming to more quickly develop COVID-19 vaccines, said it’s “extremely unlikely” that vaccine trial results will be ready before the end of October.
Mr. Trump, however, has insisted repeatedly that a vaccine to fight the pandemic that has claimed 200,000 American lives will be distributed starting next month. He reiterated that claim Saturday at a campaign rally in Fayetteville, N.C.
The vaccine will be ready “in a matter of weeks,” he said. “We will end the pandemic from China.”
Although pharmaceutical companies have launched three clinical trials in the United States, no one can say with certainty when those trials will have enough data to determine whether the vaccines are safe and effective.
Officials at Moderna, whose vaccine is being tested in 30,000 volunteers, have said their studies could produce a result by the end of the year, although the final analysis could take place next spring.
Pfizer executives, who have expanded their clinical trial to 44,000 participants, boast that they could know their vaccine works by the end of October.
AstraZeneca’s U.S. vaccine trial, which was scheduled to enroll 30,000 volunteers, is on hold pending an investigation of a possible vaccine-related illness.
Scientists have warned for months that the Trump administration could try to win the election with an “October surprise,” authorizing a vaccine that hasn’t been fully tested. “I don’t think people are crazy to be thinking about all of this,” said William Schultz, a partner in a Washington, D.C., law firm who served as a former FDA commissioner for policy and as general counsel for HHS.
“You’ve got a president saying you’ll have an approval in October. Everybody’s wondering how that could happen.”
In an opinion piece published in the Wall Street Journal, conservative former FDA commissioners Scott Gottlieb and Mark McClellan argued that presidential intrusion was unlikely because the FDA’s “thorough and transparent process doesn’t lend itself to meddling. Any deviation would quickly be apparent.”
But the administration has demonstrated a willingness to bend the agency to its will. The FDA has been criticized for issuing emergency authorizations for two COVID-19 treatments that were boosted by the president but lacked strong evidence to support them: hydroxychloroquine and convalescent plasma.
Mr. Azar has sidelined the FDA in other ways, such as by blocking the agency from regulating lab-developed tests, including tests for the novel coronavirus.
Although FDA Commissioner Stephen Hahn told the Financial Times he would be willing to approve emergency use of a vaccine before large-scale studies conclude, agency officials also have pledged to ensure the safety of any COVID-19 vaccines.
A senior FDA official who oversees vaccine approvals, Peter Marks, MD, has said he will quit if his agency rubber-stamps an unproven COVID-19 vaccine.
“I think there would be an outcry from the public health community second to none, which is my worst nightmare – my worst nightmare – because we will so confuse the public,” said Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, in his weekly podcast.
Still, “even if a company did not want it to be done, even if the FDA did not want it to be done, he could still do that,” said Dr. Osterholm, in his podcast. “I hope that we’d never see that happen, but we have to entertain that’s a possibility.”
In the New England Journal editorial, Dr. Avorn and coauthor Aaron Kesselheim, MD, wondered whether Mr. Trump might invoke the 1950 Defense Production Act to force reluctant drug companies to manufacture their vaccines.
But Mr. Trump would have to sue a company to enforce the Defense Production Act, and the company would have a strong case in refusing, said Lawrence Gostin, director of Georgetown’s O’Neill Institute for National and Global Health Law.
Also, he noted that Mr. Trump could not invoke the Defense Production Act unless a vaccine were “scientifically justified and approved by the FDA.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
who have pledged not to release any vaccine unless it’s proved safe and effective.
In podcasts, public forums, social media and medical journals, a growing number of prominent health leaders say they fear that Mr. Trump – who has repeatedly signaled his desire for the swift approval of a vaccine and his displeasure with perceived delays at the FDA – will take matters into his own hands, running roughshod over the usual regulatory process.
It would reflect another attempt by a norm-breaking administration, poised to ram through a Supreme Court nominee opposed to existing abortion rights and the Affordable Care Act, to inject politics into sensitive public health decisions. Mr. Trump has repeatedly contradicted the advice of senior scientists on COVID-19 while pushing controversial treatments for the disease.
If the executive branch were to overrule the FDA’s scientific judgment, a vaccine of limited efficacy and, worse, unknown side effects could be rushed to market.
The worries intensified over the weekend, after Alex Azar, the administration’s secretary of Health & Human Services, asserted his agency’s rule-making authority over the FDA. HHS spokesperson Caitlin Oakley said Mr. Azar’s decision had no bearing on the vaccine approval process.
Vaccines are typically approved by the FDA. Alternatively, Mr. Azar – who reports directly to Mr. Trump – can issue an emergency use authorization, even before any vaccines have been shown to be safe and effective in late-stage clinical trials.
“Yes, this scenario is certainly possible legally and politically,” said Jerry Avorn, MD, a professor of medicine at Harvard Medical School, who outlined such an event in the New England Journal of Medicine. He said it “seems frighteningly more plausible each day.”
Vaccine experts and public health officials are particularly vexed by the possibility because it could ruin the fragile public confidence in a COVID-19 vaccine. It might put scientific authorities in the position of urging people not to be vaccinated after years of coaxing hesitant parents to ignore baseless fears.
Physicians might refuse to administer a vaccine approved with inadequate data, said Preeti Malani, MD, chief health officer and professor of medicine at the University of Michigan in Ann Arbor, in a recent webinar. “You could have a safe, effective vaccine that no one wants to take.” A recent KFF poll found that 54% of Americans would not submit to a COVID-19 vaccine authorized before Election Day.
After this story was published, an HHS official said that Mr. Azar “will defer completely to the FDA” as the agency weighs whether to approve a vaccine produced through the government’s Operation Warp Speed effort.
“The idea the Secretary would approve or authorize a vaccine over the FDA’s objections is preposterous and betrays ignorance of the transparent process that we’re following for the development of the OWS vaccines,” HHS chief of staff Brian Harrison wrote in an email.
White House spokesperson Judd Deere dismissed the scientists’ concerns, saying Trump cared only about the public’s safety and health. “This false narrative that the media and Democrats have created that politics is influencing approvals is not only false but is a danger to the American public,” he said.
Usually, the FDA approves vaccines only after companies submit years of data proving that a vaccine is safe and effective. But a 2004 law allows the FDA to issue an emergency use authorization with much less evidence, as long as the vaccine “may be effective” and its “known and potential benefits” outweigh its “known and potential risks.”
Many scientists doubt a vaccine could meet those criteria before the election. But the terms might be legally vague enough to allow the administration to take such steps.
Moncef Slaoui, chief scientific adviser to Operation Warp Speed, the government program aiming to more quickly develop COVID-19 vaccines, said it’s “extremely unlikely” that vaccine trial results will be ready before the end of October.
Mr. Trump, however, has insisted repeatedly that a vaccine to fight the pandemic that has claimed 200,000 American lives will be distributed starting next month. He reiterated that claim Saturday at a campaign rally in Fayetteville, N.C.
The vaccine will be ready “in a matter of weeks,” he said. “We will end the pandemic from China.”
Although pharmaceutical companies have launched three clinical trials in the United States, no one can say with certainty when those trials will have enough data to determine whether the vaccines are safe and effective.
Officials at Moderna, whose vaccine is being tested in 30,000 volunteers, have said their studies could produce a result by the end of the year, although the final analysis could take place next spring.
Pfizer executives, who have expanded their clinical trial to 44,000 participants, boast that they could know their vaccine works by the end of October.
AstraZeneca’s U.S. vaccine trial, which was scheduled to enroll 30,000 volunteers, is on hold pending an investigation of a possible vaccine-related illness.
Scientists have warned for months that the Trump administration could try to win the election with an “October surprise,” authorizing a vaccine that hasn’t been fully tested. “I don’t think people are crazy to be thinking about all of this,” said William Schultz, a partner in a Washington, D.C., law firm who served as a former FDA commissioner for policy and as general counsel for HHS.
“You’ve got a president saying you’ll have an approval in October. Everybody’s wondering how that could happen.”
In an opinion piece published in the Wall Street Journal, conservative former FDA commissioners Scott Gottlieb and Mark McClellan argued that presidential intrusion was unlikely because the FDA’s “thorough and transparent process doesn’t lend itself to meddling. Any deviation would quickly be apparent.”
But the administration has demonstrated a willingness to bend the agency to its will. The FDA has been criticized for issuing emergency authorizations for two COVID-19 treatments that were boosted by the president but lacked strong evidence to support them: hydroxychloroquine and convalescent plasma.
Mr. Azar has sidelined the FDA in other ways, such as by blocking the agency from regulating lab-developed tests, including tests for the novel coronavirus.
Although FDA Commissioner Stephen Hahn told the Financial Times he would be willing to approve emergency use of a vaccine before large-scale studies conclude, agency officials also have pledged to ensure the safety of any COVID-19 vaccines.
A senior FDA official who oversees vaccine approvals, Peter Marks, MD, has said he will quit if his agency rubber-stamps an unproven COVID-19 vaccine.
“I think there would be an outcry from the public health community second to none, which is my worst nightmare – my worst nightmare – because we will so confuse the public,” said Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, in his weekly podcast.
Still, “even if a company did not want it to be done, even if the FDA did not want it to be done, he could still do that,” said Dr. Osterholm, in his podcast. “I hope that we’d never see that happen, but we have to entertain that’s a possibility.”
In the New England Journal editorial, Dr. Avorn and coauthor Aaron Kesselheim, MD, wondered whether Mr. Trump might invoke the 1950 Defense Production Act to force reluctant drug companies to manufacture their vaccines.
But Mr. Trump would have to sue a company to enforce the Defense Production Act, and the company would have a strong case in refusing, said Lawrence Gostin, director of Georgetown’s O’Neill Institute for National and Global Health Law.
Also, he noted that Mr. Trump could not invoke the Defense Production Act unless a vaccine were “scientifically justified and approved by the FDA.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
who have pledged not to release any vaccine unless it’s proved safe and effective.
In podcasts, public forums, social media and medical journals, a growing number of prominent health leaders say they fear that Mr. Trump – who has repeatedly signaled his desire for the swift approval of a vaccine and his displeasure with perceived delays at the FDA – will take matters into his own hands, running roughshod over the usual regulatory process.
It would reflect another attempt by a norm-breaking administration, poised to ram through a Supreme Court nominee opposed to existing abortion rights and the Affordable Care Act, to inject politics into sensitive public health decisions. Mr. Trump has repeatedly contradicted the advice of senior scientists on COVID-19 while pushing controversial treatments for the disease.
If the executive branch were to overrule the FDA’s scientific judgment, a vaccine of limited efficacy and, worse, unknown side effects could be rushed to market.
The worries intensified over the weekend, after Alex Azar, the administration’s secretary of Health & Human Services, asserted his agency’s rule-making authority over the FDA. HHS spokesperson Caitlin Oakley said Mr. Azar’s decision had no bearing on the vaccine approval process.
Vaccines are typically approved by the FDA. Alternatively, Mr. Azar – who reports directly to Mr. Trump – can issue an emergency use authorization, even before any vaccines have been shown to be safe and effective in late-stage clinical trials.
“Yes, this scenario is certainly possible legally and politically,” said Jerry Avorn, MD, a professor of medicine at Harvard Medical School, who outlined such an event in the New England Journal of Medicine. He said it “seems frighteningly more plausible each day.”
Vaccine experts and public health officials are particularly vexed by the possibility because it could ruin the fragile public confidence in a COVID-19 vaccine. It might put scientific authorities in the position of urging people not to be vaccinated after years of coaxing hesitant parents to ignore baseless fears.
Physicians might refuse to administer a vaccine approved with inadequate data, said Preeti Malani, MD, chief health officer and professor of medicine at the University of Michigan in Ann Arbor, in a recent webinar. “You could have a safe, effective vaccine that no one wants to take.” A recent KFF poll found that 54% of Americans would not submit to a COVID-19 vaccine authorized before Election Day.
After this story was published, an HHS official said that Mr. Azar “will defer completely to the FDA” as the agency weighs whether to approve a vaccine produced through the government’s Operation Warp Speed effort.
“The idea the Secretary would approve or authorize a vaccine over the FDA’s objections is preposterous and betrays ignorance of the transparent process that we’re following for the development of the OWS vaccines,” HHS chief of staff Brian Harrison wrote in an email.
White House spokesperson Judd Deere dismissed the scientists’ concerns, saying Trump cared only about the public’s safety and health. “This false narrative that the media and Democrats have created that politics is influencing approvals is not only false but is a danger to the American public,” he said.
Usually, the FDA approves vaccines only after companies submit years of data proving that a vaccine is safe and effective. But a 2004 law allows the FDA to issue an emergency use authorization with much less evidence, as long as the vaccine “may be effective” and its “known and potential benefits” outweigh its “known and potential risks.”
Many scientists doubt a vaccine could meet those criteria before the election. But the terms might be legally vague enough to allow the administration to take such steps.
Moncef Slaoui, chief scientific adviser to Operation Warp Speed, the government program aiming to more quickly develop COVID-19 vaccines, said it’s “extremely unlikely” that vaccine trial results will be ready before the end of October.
Mr. Trump, however, has insisted repeatedly that a vaccine to fight the pandemic that has claimed 200,000 American lives will be distributed starting next month. He reiterated that claim Saturday at a campaign rally in Fayetteville, N.C.
The vaccine will be ready “in a matter of weeks,” he said. “We will end the pandemic from China.”
Although pharmaceutical companies have launched three clinical trials in the United States, no one can say with certainty when those trials will have enough data to determine whether the vaccines are safe and effective.
Officials at Moderna, whose vaccine is being tested in 30,000 volunteers, have said their studies could produce a result by the end of the year, although the final analysis could take place next spring.
Pfizer executives, who have expanded their clinical trial to 44,000 participants, boast that they could know their vaccine works by the end of October.
AstraZeneca’s U.S. vaccine trial, which was scheduled to enroll 30,000 volunteers, is on hold pending an investigation of a possible vaccine-related illness.
Scientists have warned for months that the Trump administration could try to win the election with an “October surprise,” authorizing a vaccine that hasn’t been fully tested. “I don’t think people are crazy to be thinking about all of this,” said William Schultz, a partner in a Washington, D.C., law firm who served as a former FDA commissioner for policy and as general counsel for HHS.
“You’ve got a president saying you’ll have an approval in October. Everybody’s wondering how that could happen.”
In an opinion piece published in the Wall Street Journal, conservative former FDA commissioners Scott Gottlieb and Mark McClellan argued that presidential intrusion was unlikely because the FDA’s “thorough and transparent process doesn’t lend itself to meddling. Any deviation would quickly be apparent.”
But the administration has demonstrated a willingness to bend the agency to its will. The FDA has been criticized for issuing emergency authorizations for two COVID-19 treatments that were boosted by the president but lacked strong evidence to support them: hydroxychloroquine and convalescent plasma.
Mr. Azar has sidelined the FDA in other ways, such as by blocking the agency from regulating lab-developed tests, including tests for the novel coronavirus.
Although FDA Commissioner Stephen Hahn told the Financial Times he would be willing to approve emergency use of a vaccine before large-scale studies conclude, agency officials also have pledged to ensure the safety of any COVID-19 vaccines.
A senior FDA official who oversees vaccine approvals, Peter Marks, MD, has said he will quit if his agency rubber-stamps an unproven COVID-19 vaccine.
“I think there would be an outcry from the public health community second to none, which is my worst nightmare – my worst nightmare – because we will so confuse the public,” said Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, in his weekly podcast.
Still, “even if a company did not want it to be done, even if the FDA did not want it to be done, he could still do that,” said Dr. Osterholm, in his podcast. “I hope that we’d never see that happen, but we have to entertain that’s a possibility.”
In the New England Journal editorial, Dr. Avorn and coauthor Aaron Kesselheim, MD, wondered whether Mr. Trump might invoke the 1950 Defense Production Act to force reluctant drug companies to manufacture their vaccines.
But Mr. Trump would have to sue a company to enforce the Defense Production Act, and the company would have a strong case in refusing, said Lawrence Gostin, director of Georgetown’s O’Neill Institute for National and Global Health Law.
Also, he noted that Mr. Trump could not invoke the Defense Production Act unless a vaccine were “scientifically justified and approved by the FDA.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 Screening and Testing Among Patients With Neurologic Dysfunction: The Neuro-COVID-19 Time-out Process and Checklist
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.
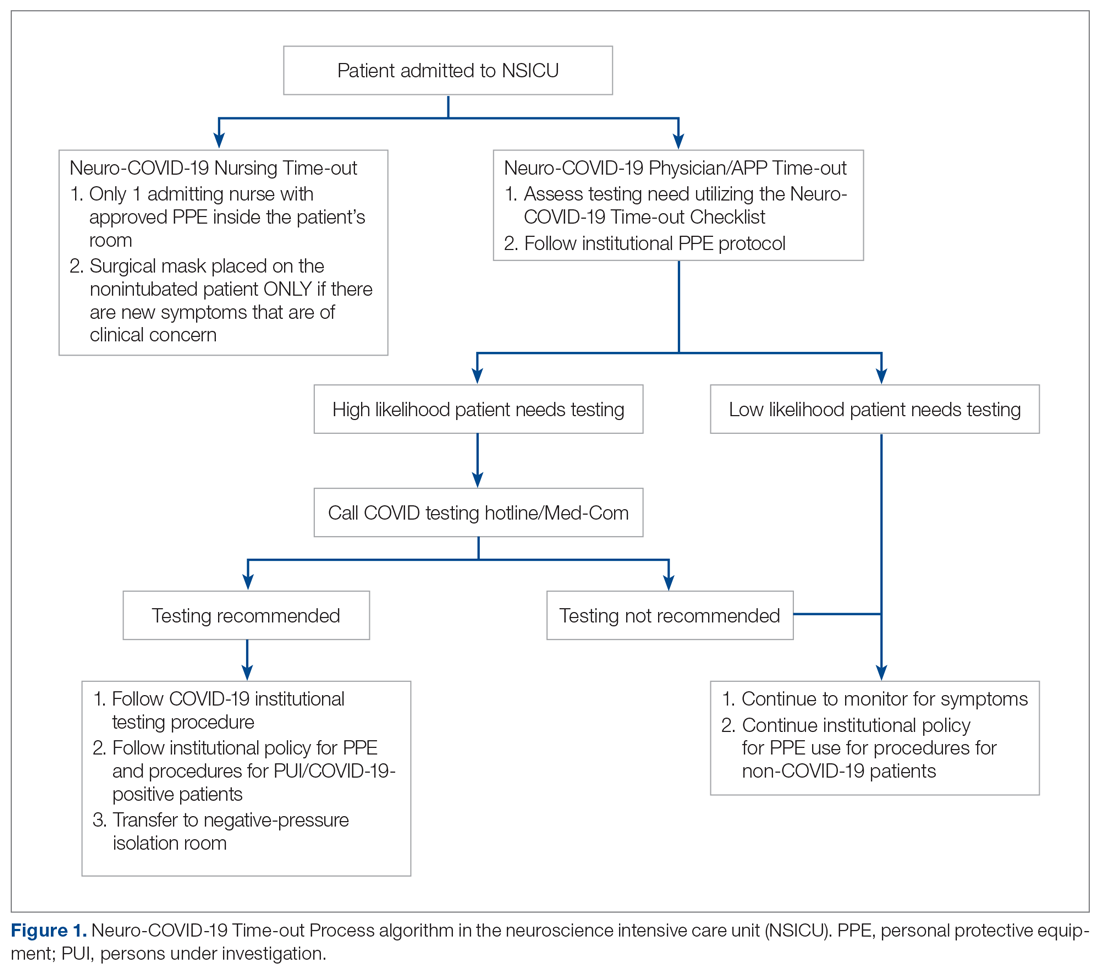
The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.
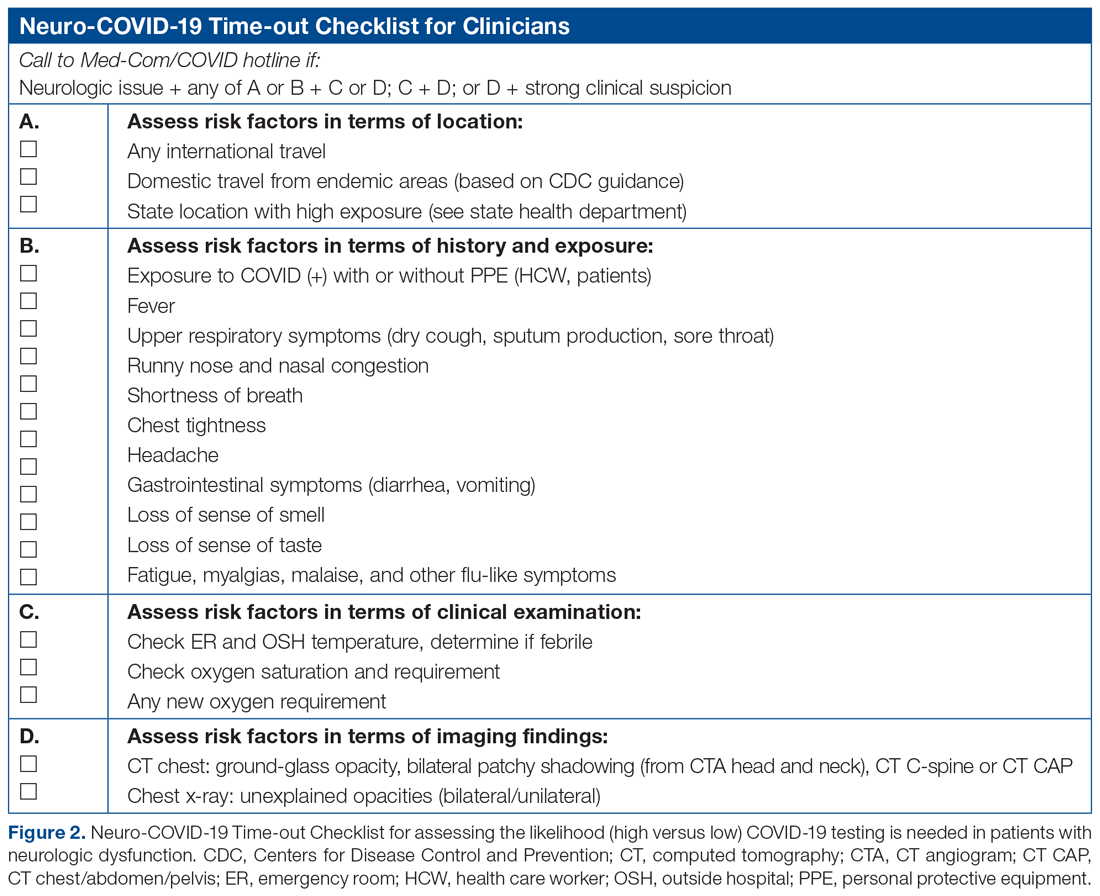
NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).
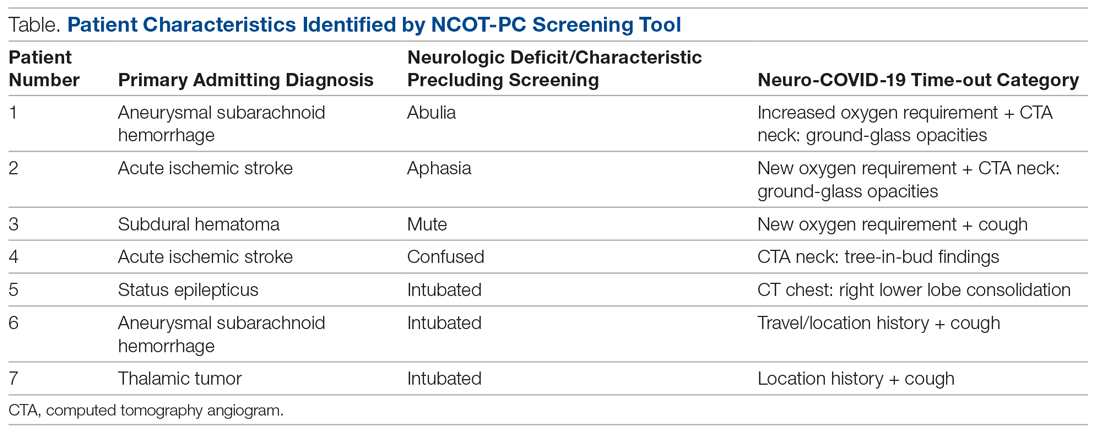
Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
Clinical Utility of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swab Testing in Lower Respiratory Tract Infections
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).
In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.
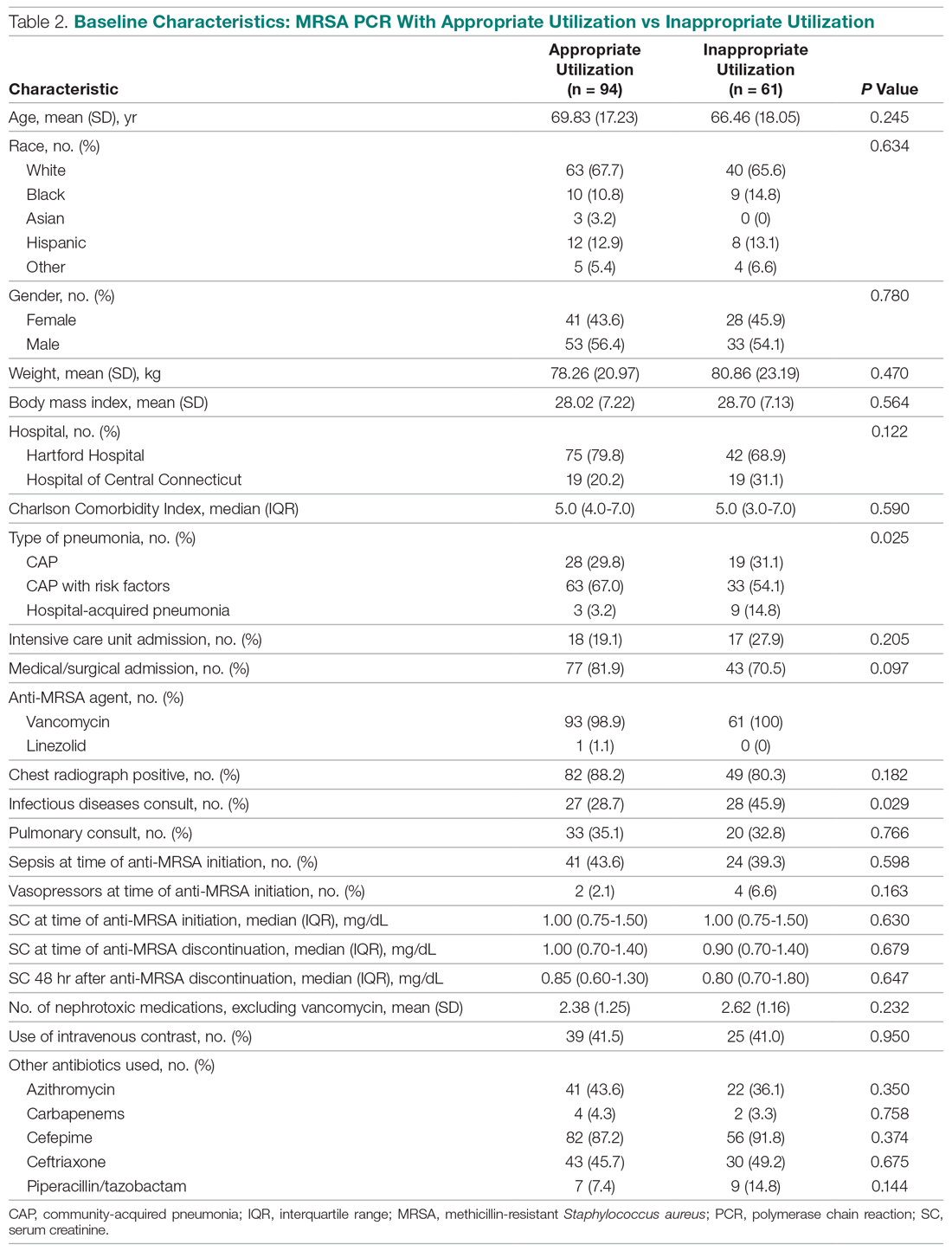
Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.
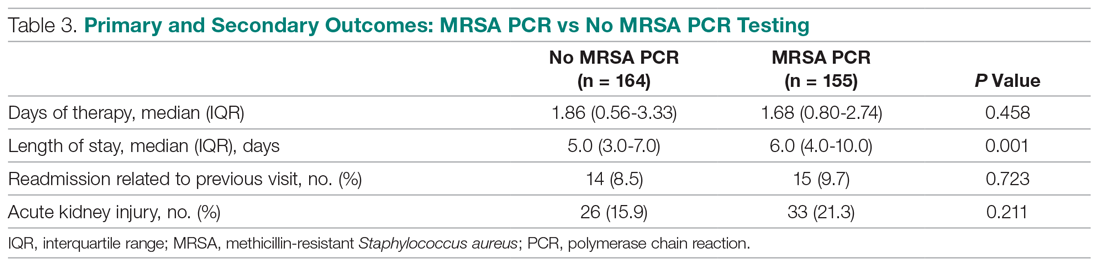
In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.
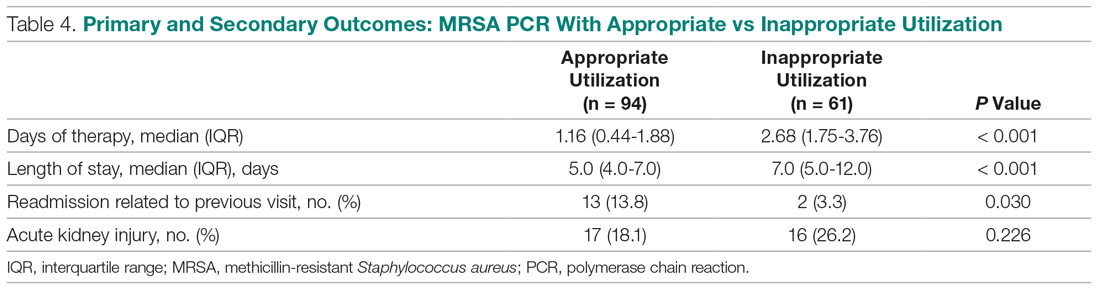
A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.
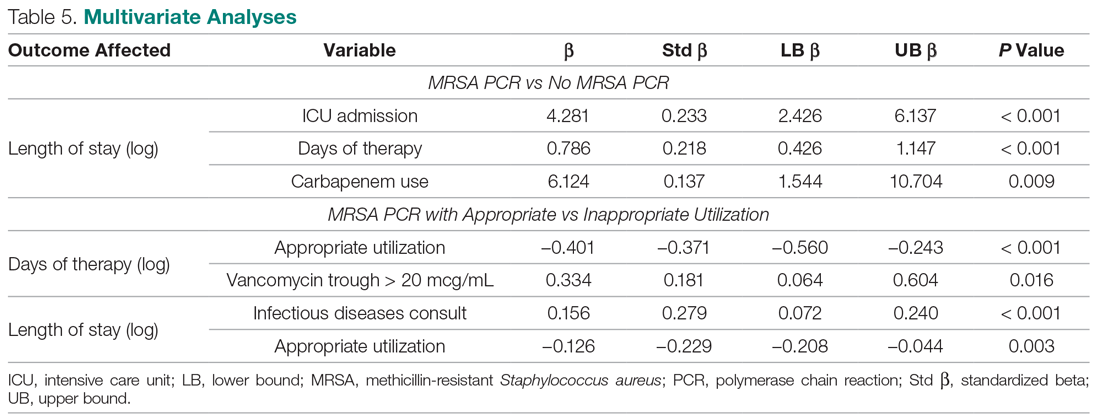
Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
“I Really Didn’t Want To Come In”: The Unseen Effects of COVID-19 on Children
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
The Children’s Hospital of Philadelphia, Philadelphia, PA.
The effects of COVID-19 on children’s health are multifaceted. In comparison to adults, children typically experience far milder physical consequences when infected with the virus. A notable exception is the newly described multisystem inflammatory syndrome associated with COVID-19 (MIS-C), which has proven to be a source of significant morbidity among the children it affects.1 Nevertheless, even those children not infected with COVID-19 have suffered due to the disease. School closures have deprived children of opportunities for social and academic growth and, in some cases, the provision of food, social services, medication administration, and many different therapies. Social distancing rules have limited play among children, which is crucial to their development and mental health. The impact on children who have lost family members, including parents, is monumental. Amidst all of this observable suffering, however, the pandemic poses a less visible threat to the health of children.
It is well documented that concern about exposure to COVID-19 has led many adults to avoid emergency departments (EDs) around the world. We believe parents may be avoiding ED visits for their children for the same reason. In the United States, ED volumes dropped approximately 50% during spring 2020.2 While EDs saw increasing, and at times overwhelming, numbers of patients with COVID-19, the number of patients presenting with other life-threatening medical issues, including heart attacks and strokes, declined.3,4 Data from the National Center for Health Statistics this past spring revealed nationwide increases in deaths due to nonrespiratory causes such as diabetes, heart disease, and stroke.5 ED avoidance and unprecedented lack of access to outpatient care, though with the intent to reduce overall risk, are likely significant contributors to these deaths.
Pediatric patients, especially the most vulnerable, are similarly at risk for deleterious health-related consequences from ED avoidance and from limited access to primary and outpatient specialty care. Data from Europe indicate dramatic drops in pediatric ED (PED) volumes, as well as an increase in the proportion of ED visits leading to hospitalization.6,7 These studies suggest that when patients do ultimately present to the PED, they may be more seriously ill.
At our institution, we have seen many COVID-19-negative patients whose medical care has been negatively influenced by the pandemic. A few months ago, a 1-month-old infant with an underlying health condition presented to the PED in extremis after weeks of progressively worsening feeding issues. The infant had been closely followed by the primary care provider (PCP) and subspecialty team via phone calls, televisits, and some office visits. Both physicians and parents had tried to resolve the feeding issues within the outpatient context, explicitly hoping to avoid potential exposure of this fragile patient to COVID-19 in the hospital. On eventual presentation to the PED, the infant was profoundly dehydrated, with significant electrolyte derangement and an acute abdomen, requiring admission to the intensive care unit. Ultimately, a new diagnosis of Hirschsprung disease was made, and the infant was hospitalized for several weeks for weight gain.
Later this summer, a school-aged child with a history of poorly controlled type 1 diabetes presented to an affiliated community hospital comatose and with Kussmaul respirations. Prior to the pandemic, a school nurse administered the child’s morning insulin. Since school closed, the patient had been responsible for administering this dose of insulin while the parents worked outside the home. Despite close and frequent communication between the patient’s endocrinology team and the family, the patient’s glucose and ketone levels began to rise. The parent administered repeated boluses of insulin at home in an attempt to avoid the perceived exposure risk associated with an ED visit. On presentation to the PED, the patient was profoundly altered, with a pH of 7.0. When transfer to a tertiary care center was recommended, the patient’s parent expressed persistent concerns about COVID-19 exposure in the larger hospital, although ultimately consent to transfer was given.
A third case from this summer provides an example of a different type of patient affected by COVID-19: the neonate whose birth circumstances were altered due to the virus. A 3-day-old, full-term infant presented to the ED with hypothermia after PCP referral. The parents had considered both home birth and hospital delivery earlier in the pregnancy, ultimately opting for home birth due to concerns about COVID-19 exposure in the hospital. The pregnancy and delivery were uncomplicated. The neonate did not receive the first hepatitis B vaccine, erythromycin eye ointment, or vitamin K after delivery. In the first 3 days of life, the patient had voided once and stooled once per day. The patient’s mother, inexperienced with breastfeeding and without access to a lactation consultant, was unsure about latch or emptying of her breasts. At the first pediatrician visit, the infant was noted to be hypothermic to 35°C, intermittently bradycardic to the 80s, and with diminished arousal. In the PED, a full sepsis work-up was initiated. Though multiple attempts were made by different providers, only a minimal amount of blood could be drawn, presumably due to dehydration. Of note, the neonate received vitamin K subcutaneously prior to lumbar puncture.
Pediatricians across the country have gone to great lengths to protect their patients and to provide high-quality care both inside and outside the office during this unprecedented time. Nevertheless, these 3 cases illustrate the detrimental effects of COVID-19 on the delivery of pediatric health care. The first 2 cases in particular demonstrate the limitations of even close and consistent phone and televisit follow-up. Telehealth has provided a lifeline for patients and families during the pandemic, and, in most cases, has provided an excellent temporary substitution for office visits. There are, however, limitations to care without physical evaluation. Had the children in the first 2 cases been evaluated in person sooner, they may have been referred to a higher level of care more expediently. Likewise, in all 3 cases, parental reservations about exposing their children to COVID-19 through a trip to the hospital, however well-intentioned, likely played a role in the eventual severity of illness with which each child presented to the hospital.
If we are encountering children in the PED with severe illness due to delayed presentation to care, what about the children we aren’t seeing? As COVID-19 cases rise daily in the United States, we must be aware of the possibility of ED avoidance. We propose a multimodal approach to combat this dangerous phenomenon. Inpatient and ED-based pediatricians must maintain clear and open lines of communication with outpatient colleagues so that we can partner in considering which cases warrant prompt ED evaluation, even in the midst of a pandemic. All pediatricians must remind families that our hospitals remain open and ready to treat children safely. We must promote community awareness of the numerous safety precautions we take every day so that patients and families can feel comfortable seeking care at the hospital; the message of ED and hospital safety must be even more robust for caregivers of our particularly vulnerable children. As always, how we communicate with patients and their families matters. Validating and addressing concerns about COVID-19 exposure, while providing reassurance about the safety of our hospitals, could save children’s lives.
Acknowledgment: Thank you to Dr. Cynthia Mollen and Dr. Kathy Shaw for their reviews of the manuscript.
Corresponding author: Regina L. Toto, MD, Department of Pediatrics, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd., Philadelphia, PA 19104; [email protected].
Financial disclosures: None.
Keywords: coronavirus; pediatric; children; access to care; emergency department.
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
1. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608.
2. Wong LE, Hawkins JE, Langness S, et al. Where are all the patients? addressing COVID-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0193
3. Moroni F, Gramegna M, Ajello S, et al. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. JACC. 2020. doi:10.1016/j.jaccas.2020.04.010
4. Deerberg-Wittram J, Knothe C. Do not stay home: we are ready for you. NEJM Catalyst. 2020. doi:10.1056/CAT.20.0146
5. Woolf SH, Chapman DA, Sabo RT, et al. Excess deaths From COVID-19 and other causes, March-April 2020. JAMA. 2020. doi:10.1001.jama.2020.11787
6. Lazzerini M, Barbi E, Apicella A, et al. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4:E10-1.
7. Happle C, Dopfer C, Wetzke M, et al. Covid-19 related reduction in paediatric emergency healthcare utilization--a concerning trend. BMC Pediatrics. [under review]. 2020. doi:10.21203/rs.3.rs-2
Systemic Corticosteroids in Critically Ill Patients With COVID-19
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
Effect of a Smartphone App Plus an Accelerometer on Physical Activity and Functional Recovery During Hospitalization After Orthopedic Surgery
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
Study Overview
Objective. To investigate the potential of Hospital Fit (a smartphone application with an accelerometer) to enhance physical activity levels and functional recovery following orthopedic surgery.
Design. Nonrandomized, quasi-experimental pilot study.
Settings and participants. Patients scheduled for an elective total knee arthroplasty (TKA) or total hip arthroplasty (THA) at the orthopedic ward of Maastricht University Medical Center in Maastricht, the Netherlands, were invited to participate. Patients scheduled for surgery between January 2017 and December 2018 were recruited for the control group at a rate of 1 patient per week (due to a limited number of accelerometers available). After development of Hospital Fit was completed in December 2018 (and sufficient accelerators had become available), patients scheduled for surgery between February 2019 and May 2019 were recruited for the intervention group. The ratio of patients included in the control and intervention group was set at 2:1, respectively.
At preoperative physiotherapy screenings (scheduled 6 weeks before surgery), patients received verbal and written information about the study. Patients were eligible if they met the following inclusion criteria: receiving physiotherapy after elective TKA or THA; able to walk independently 2 weeks prior to surgery, as scored on the Functional Ambulation Categories (FAC > 3); were expected to be discharged to their own home; were aged 18 years and older; and had a sufficient understanding of the Dutch language. Exclusion criteria were: the presence of contraindications to walking or wearing an accelerometer on the upper leg; admission to the intensive care unit; impaired cognition (delirium/dementia), as reported by the attending doctor; a life expectancy of less than 3 months; and previous participation in this study. Patients were contacted on the day of their surgery, and written informed consent was obtained prior to the initiation of any study activities.
Intervention. Once enrolled, all patients followed a standardized clinical care pathway for TKA or THA (see original article for additional details). Postoperative physiotherapy was administered to all participating patients, starting within 4 hours after surgery. The physiotherapy treatment was aimed at increasing physical activity levels and enhancing functional recovery. Control group patients only received physiotherapy (twice daily, 30 minutes per session) and had their physical activity levels monitored with an accelerometer, without receiving feedback, until functional recovery was achieved, as measured with the modified Iowa Level of Assistance Scale (mILAS). Intervention group patients used Hospital Fit in addition to physiotherapy. Hospital Fit consists of a smartphone-based app, connected to a MOX activity monitor via Bluetooth (device contains a tri-axial accelerometer sensor in a small waterproof housing attached to the upper leg). Hospital Fit enables objective activity monitoring, provides patients and their physiotherapists insights and real-time feedback on the number of minutes spent standing and walking per day, and offers a tailored exercise program supported by videos aimed at stimulating self-management.
Measures. The primary outcome measure was the time spent physically active (total number of minutes standing and walking) per day until discharge. Physical activity was monitored 24 hours a day; days with ≥ 20 hours of wear time were considered valid measurement days and were included in the analysis. After the last treatment session, the accelerometer was removed, and the raw tri-axial accelerometer data were uploaded and processed to classify minutes as “active” (standing and walking) or “sedentary” (lying and sitting). The secondary outcome measures were the achievement of functional recovery on postoperative day 1 (POD1). Functional recovery was assessed by the physiotherapist during each treatment session using the mILAS and was reported in the electronic health record. In the intervention group, it was also reported in the app. The achievement of functional recovery on POD1 was defined as having reached a total mILAS-score of 0 on or before POD1, using a dichotomized outcome (0 = mILAS = 0 > POD1; 1 = mILAS = 0 ≤ POD1).
The independent variables measured were: Hospital Fit use (control versus the intervention group), age, sex, body mass index (BMI), type of surgery (TKA or THA), and comorbidities assessed by the American Society of Anesthesiologists (ASA) classification (ASA class ≤ 2 versus ASA class = 3; a higher score indicates being less fit for surgery). The medical and demographic data measured were the type of walking aid used and length of stay, with the day of surgery being defined as day 1.
Analysis. Data analysis was performed according to the intention-to-treat principle. Missing values were not substituted; drop-outs were not replaced. Descriptive statistics were presented as means (SD) or as 95% confidence intervals (CI) for continuous variables. The median and interquartile ranges (IQR) were used to present non-normally distributed data. The frequencies and percentages were used to present categorical variables. A multiple linear regression analysis was performed to determine the association between the time spent physically active per day and Hospital Fit use, corrected for potential confounding factors (age, sex, BMI, ASA class, and type of surgery). A multiple logistic regression analysis was performed additionally to determine the association between the achievement of functional recovery on POD1 and Hospital Fit use, corrected for potential confounding factors. For all statistical analyses, the level of significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 23.0.0.2; IBM Corporation, Armonk, NY).
Main results. Ninety-seven patients were recruited; after excluding 9 patients because of missing data, 88 were included for analysis, with 61 (69%) in the control group and 27 (31%) in the intervention group. A median (IQR) number of 1.00 (0) valid measurement days (≥ 20 hr wear time) was collected. Physical activity data for 84 patients (95%) was available on POD1 (n = 61 control group, n = 23 intervention group). On postoperative day 2 (POD2), the majority of patients were discharged (n = 61, 69%), and data for only 23 patients (26%) were available (n = 17 control, n = 6 intervention). From postoperative day 3 to day 7, data of valid measurement days were available for just 1 patient (intervention group). Due to the large reduction in valid measurement days from POD2 onward, data from these days were not included in the analysis.
Results of the multiple linear regression analysis showed that, corrected for age, patients who used Hospital Fit stood and walked an average of 28.43 minutes (95% CI, 5.55-51.32) more on POD1 than patients who did not use Hospital Fit. Also, the model showed that an increase in age led to a decrease in the number of minutes standing and walking on POD1. The results of the multiple logistic regression analysis also showed that, corrected for ASA class, the odds of achieving functional recovery on POD1 were 3.08 times higher (95% CI, 1.14-8.31) for patients who used Hospital Fit compared to patients who did not use Hospital Fit. Including ASA class in the model shows that a lower ASA class increased the odds ratio for a functional recovery on POD1.
Conclusion. A smartphone app combined with an accelerometer demonstrates the potential to enhance patients’ physical activity levels and functional recovery during hospitalization following joint replacement surgery.
Commentary
Although the beneficial effects of physical activity during hospitalization after surgery are well documented, patients continue to spend between 92% and 96% of their time lying or sitting.1-3 Therefore, strategies aimed at increasing the amount of time spent standing and walking are needed. Postoperative physiotherapy aims to enhance physical activity levels and functional recovery of activities of daily living, which are essential to function independently at home.4-7 Physiotherapists may be able to advise patients more effectively on their physical activity behavior if continuous physical activity monitoring with real-time feedback is implemented in standard care. Although mobile health (mHealth) tools are being used to monitor physical activity in support of outpatient physiotherapy within the orthopedic rehabilitation pathway,8-10 there is currently no mHealth tool available that offers hospitalized patients and their physiotherapists essential strategies to enhance their physical activity levels and support their recovery process. In addition, because hospitalized patients frequently use walking aids and often have impaired gait, the algorithm of most available activity monitors is not validated for use in this population.
This study, therefore, is an important contribution to the literature, as it describes a preliminary evaluation of a novel mHealth tool—Hospital Fit—consisting of a smartphone application connected to an accelerometer whose algorithm has been validated to differentiate between lying/sitting and standing/walking among hospitalized patients. Briefly, results from this study showed an increase in the time spent standing and walking, as well as higher odds of functional recovery on POD1 from the introduction of Hospital Fit. While guidelines on the recommended amount of physical activity during hospitalization do not yet exist, an average improvement of 28 minutes (39%) standing and walking on POD1 can be considered a clinically relevant contribution to prevent the negative effects of inactivity.
This study has limitations, particularly related to the study design, which is acknowledged by the authors. The current study was a nonrandomized, quasi-experimental pilot study implemented at a single medical center, and therefore, the results have limited generalizability and more importantly, may not only be attributable to the introduction of Hospital Fit. In addition, as there was lag in patient recruitment where patients were initially selected for the control group over the course of 1 year, followed by selection of patients for the intervention group over 4 months (once Hospital Fit was developed), it is possible that awareness on the importance of physical activity during hospitalization increased among patients and health care professionals, which may have resulted in a bias in favor of the intervention group (and thus a potentially slight overestimation of results). Also, as individual functionalities of Hospital Fit were not investigated, relationships between each functionality and physical activity could not be established. As the authors indicated, future research is needed to determine the effectiveness of Hospital Fit (ie, a larger, cluster randomized controlled trial in a population of hospitalized patients with a longer length of stay). This study design would also enable investigation of the effect of individual functionalities of Hospital Fit on physical activity.
Applications for Clinical Practice
mHealth tools have the potential to increase patient awareness, support personalized care, and stimulate self-management. This study highlights the potential for a novel mHealth tool—Hospital Fit—to improve the amount of physical activity and shorten the time to functional recovery in hospitalized patients following orthopedic surgery. Further, mHealth tools like Hospital Fit may have a greater impact when the hospital stay of a patient permits the use of the tool for a longer period of time. More broadly, continuous objective monitoring through mHealth tools may provide patients and their physiotherapists enhanced and more detailed data to support and create more personalized recovery goals and related strategies.
Katrina F. Mateo, PhD, MPH
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.
1. Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. J Rehabil Res Dev. 2008;45:551-558.
2. Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol Ser A Biol Sci Med Sci. 2013;68:331–337.
3. Evensen S, Sletvold O, Lydersen S, Taraldsen K. Physical activity among hospitalized older adults – an observational study. BMC Geriatr. 2017;17:110.
4. Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473-478.
5. Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27:161-166.
6. Hoogeboom TJ, van Meeteren NL, Schank K, et al. Risk factors for delayed inpatient functional recovery after total knee arthroplasty. Biomed Res Int. 2015:2015:167643.
7. Lenssen AF, Crijns YH, Waltje EM, et al. Efficiency of immediate postoperative inpatient physical therapy following total knee arthroplasty: an RCT. BMC Musculoskelet Disord. 2006;7:71.
8. Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34:2253-2259.
9. Ramkumar PN, Haeberle HS, Bloomfield MR, et al. Artificial Intelligence and arthroplasty at a single institution: Real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplast. 2019;34:2204-2209.
10. Correia FD, Nogueira A, Magalhães I, et al, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6:e13111.

