User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Equitable Post-COVID-19 Care: A Practical Framework to Integrate Health Equity in Diabetes Management
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
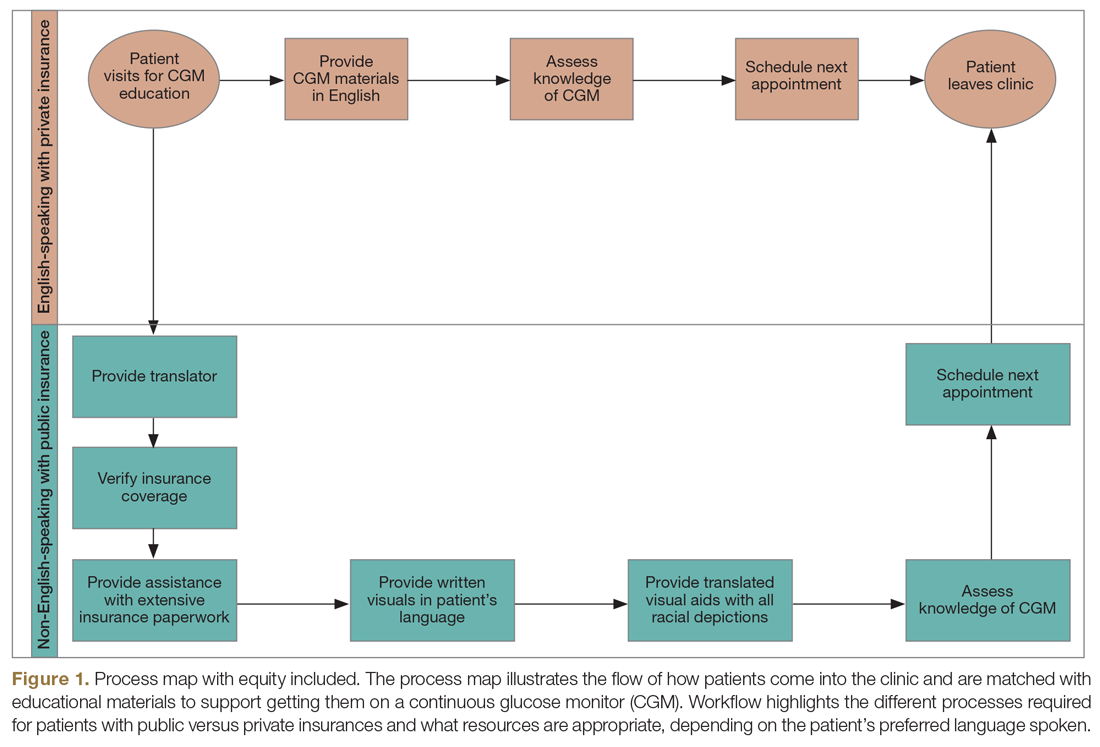
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.
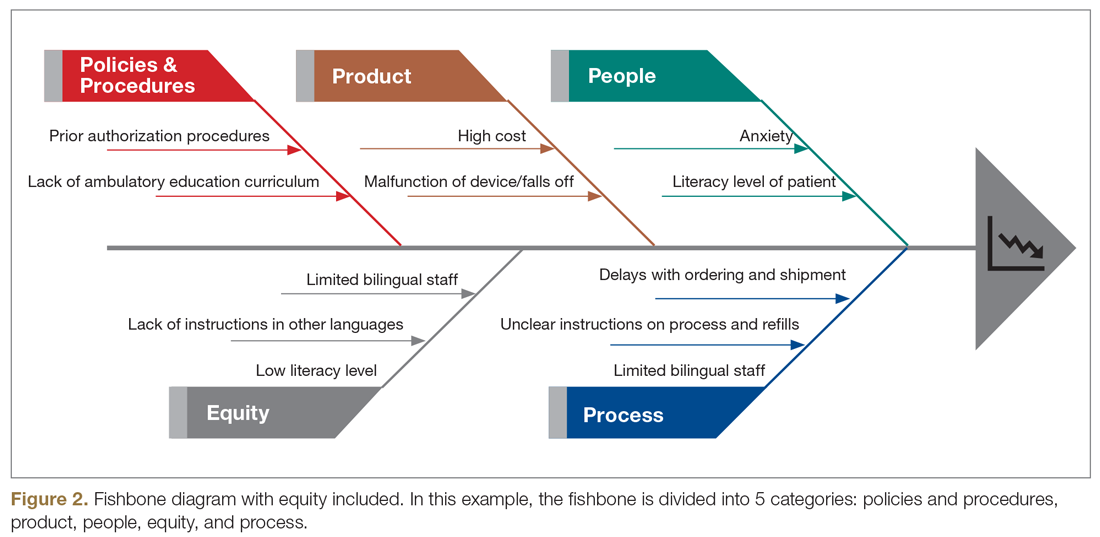
Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.
Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
AMA takes on vaccine misinformation, physician vaccines, racism
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
Combo DAA treatments may benefit patients with resistant HCV genotype 3
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
Patients with hepatitis C virus (HCV) genotype 3 infection have shown resistance to direct-acting antiviral (DAA) treatments. However, a meta-analysis of 34 research reports found that DAA combo treatment can be effective in achieving sustained virologic response (SVR) in patients with HCV genotype 3, according to a study published online in Annals of Hepatology.
This study aimed to analyze the effectiveness of four regimens: sofosbuvir (SOF)/daclatasvir (DCV) with or without ribavirin (RBV); SOF/velpatasvir (VEL) with or without RBV; SOF/VEL/voxilaprevir (VOX);and glecaprevir (GLE)/pibrentasvir (PIB) in the treatment of HCV genotype 3–infected patients in real-world situations, according to Liwei Zhuang, of Beijing Ditan Hospital, Capital Medical University, and colleagues.
A total of 34 studies, comprising 7,328 patients from 22 countries, met the inclusion criteria and formed the basis of the analysis.
Promising results
The pooled SVR rate after 12 or 24 weeks of treatment for the four regimens was 92.1%.
For each regimen, the SVR rate was 91.2% in patients treated with SOF/DCV with or without RBV; 95.1% in patients treated with SOF/VEL with or without RBV; 85.0% in patients treated with SOF/VEL/VOX; and 98.5% in patients treated with GLE/PIB.
In addition, the pooled SVR rate of the four regimens was 95.2% in patients without cirrhosis and 89.4% in patients with cirrhosis, and the pooled SVR rate was 94.4% in treatment-naive patients and 88.0% in treatment-experienced patients. All results were within 95% confidence intervals.
The researchers pointed out that their meta-analysis had limitations. “We think that no strong conclusions can be drawn due to high heterogeneity in four DAA regimens administration in real-world setting from 22 countries, as well as small numbers of patients treated with SOF + VEL + VOX and GLE + PIB. More studies are needed in the future in order to better analyze the antiviral effectiveness of DAAs in GT3 HCV patients in real-world studies,” they authors stated.
However, they also concluded that “the antiviral effectiveness of treatment regimens for HCV-GT3 [genotype 3] infection, including SOF + DCV ± RBV, SOF + VEL ± RBV, GLE + PIB, and SOF + VEL + VOX, was good. The SVR rate of GLE + PIB was higher, and the treatment duration was shorter than other regimens.”
The study was funded by the Chinese government and public institutions. The authors reported that they had no conflicts of interest.
SOURCE: Zhuang L et al. Ann Hepatol. 2020 Oct 12. doi: 10.1016/j.aohep.2020.09.012.
FROM ANNALS OF HEPATOLOGY
Dapagliflozin Reduces Adverse Renal and Cardiovascular Events in Patients With Chronic Kidney Disease
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
Study Overview
Objective. To assess whether dapagliflozin added to guideline-recommended therapies is effective and safe over the long-term to reduce the rate of renal and cardiovascular events in patients across multiple chronic kidney disease (CKD) stages, with and without type 2 diabetes.
Design. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) was a randomized, double-blind, parallel-group, placebo-controlled, multicenter event-driven, clinical trial sponsored by Astra-Zeneca. It was conducted at 386 sites in 21 countries from February 2, 2017, to June 12, 2020. A recruitment period of 24 months and a total study duration of 45 months were initially planned. The primary efficacy analysis was based on the intention-to-treat population. This was the first randomized controlled trial designed to assess the effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on renal and cardiovascular outcomes in patients with CKD.
Setting and participants. This trial randomly assigned 4304 adult participants with CKD stages 2 to 4 (an estimated glomerular filtration rate [GFR] of 25 to 75 mL/min/1.73 m2 of body-surface area) and elevated urinary albumin excretion (urinary albumin-to-creatinine ratio of 200 to 5000, measured in mg of albumin per g of creatinine) to receive dapagliflozin (10 mg once daily) or placebo. Exclusion criteria included type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, recent immunosuppressive therapy for primary or secondary kidney disease, New York Heart Association class IV congestive heart failure, myocardial infarction, unstable angina, stroke or transient ischemic attacks, or recent coronary revascularization or valvular repair/replacement. All participants received a stable dose of renin–angiotensin system inhibitor for 4 weeks prior to screening, and the vast majority received a maximum tolerated dose at enrollment. Randomization was monitored to ensure that at least 30% of participants recruited did not have diabetes and that no more than 10% had stage 2 CKD. Participants were randomly assigned to receive dapagliflozin (n = 2152) or matching placebo (n = 2152) to ensure a 1:1 ratio of the 2 regimens. Dapagliflozin and placebo had identical appearance and administration schedules. All participants and trial personnel (except members of the independent data monitoring committee) were unaware of the trial-group assignments. After randomization, in-person study visits were conducted at 2 weeks, at 2, 4, and 8 months, and at 4-month intervals thereafter.
Main outcome measures. The primary outcome was a composite of the first occurrence of either a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes. Secondary outcomes, in hierarchical order, were: (1) the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes; (2) a composite cardiovascular outcome defined as hospitalization for heart failure or death from cardiovascular causes; and (3) death from any cause. All outcomes were assessed by time-to-event analyses.
Given the extensive prior experience with dapagliflozin, only selected adverse events were recorded. These included serious adverse events, adverse events resulting in the discontinuation of dapagliflozin or placebo, and adverse events of interest to dapagliflozin (eg, volume depletion symptoms, renal events, major hypoglycemia, fractures, diabetic ketoacidosis, events leading to higher risk of lower limb amputation, and lower limb amputations).
Main results. On March 26, 2020, the independent data monitoring committee recommended stopping the trial because of clear efficacy on the basis of 408 primary outcome events. The participants were 61.8 ± 12.1 years of age, and 1425 participants (33.1%) were female. The baseline mean estimated GFR was 43.1 ± 12.4 mL/min/1.73 m2, the median urinary albumin-to-creatinine ratio was 949, and 2906 participants (67.5%) had type 2 diabetes. Over a median of 2.4 years, a primary outcome event occurred in 197 participants (9.2%) in the dapagliflozin group and 312 (14.5%) in the placebo group (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.51-0.72; P < 0.001). The number of participants who needed to be treated during the trial period to prevent 1 primary outcome event was 19 (95% CI, 15-27). The beneficial effect of dapagliflozin compared with placebo was consistent across all 8 prespecified subgroups (ie, age, sex, race, geographic region, type 2 diabetes, estimated GFR, urinary albumin-to-creatinine ratio, and systolic blood pressure) for the primary outcome. The effects of dapagliflozin were similar in participants with type 2 diabetes and in those without type 2 diabetes.
The incidence of each secondary outcome was similarly lower in the dapagliflozin-treated group than in the placebo group. The HR for the composite kidney outcome of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56 (95% CI, 0.45-0.68; P < 0.001), and the HR for the composite cardiovascular outcome of hospitalization for heart failure or death from cardiovascular causes was 0.71 (95% CI, 0.55-0.92; P = 0.009). Death occurred in 101 participants (4.7%) in the dapagliflozin group and 146 participants (6.8%) in the placebo group (HR, 0.69; 95% CI, 0.53-0.88; P = 0.004). The known safety profile of dapagliflozin was confirmed by the similar overall incidences of adverse events and serious adverse events in the dapagliflozin and placebo groups.
Conclusion. In patients with CKD, with or without type 2 diabetes, the risk of a composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes was significantly lowered by dapagliflozin treatment.
Commentary
Although SGLT2 inhibitors were designed to reduce plasma glucose and hemoglobin A1c (HbA1c) by increasing urinary glucose excretion in a non-insulin-dependent fashion, an increasing number of clinical trials have demonstrated their possible cardiovascular and renal benefits that extend beyond glycemic control. In 2008, the US Food and Drug Administration (FDA) issued a guidance recommending the evaluation of long-term cardiovascular outcomes prior to approval and commercialization of new antidiabetic therapies to ensure minimum cardiovascular risks following the discovery of cardiovascular safety issues associated with antidiabetic compounds, including rosiglitazone, after drug approval. No one foresaw that this recommendation would lead to the discovery of new classes of antidiabetic drugs (glucagon-like peptide 1 [GLP1] and SGLT2 inhibitors) that improve cardiovascular outcomes. A series of clinical trials of SGLT2 inhibitors, including empagliflozin,1 canagliflozin,2 and dapagliflozin,3 showed a reduction in cardiovascular death and hospitalization due to heart failure among patients with type 2 diabetes. Furthermore, a meta-analysis from 2019 found that SGLT2 inhibitors reduced the risk of a composite of cardiovascular death or hospitalization for heart failure by 23% and the risk of progression of kidney failure by 45% in patients with diabetes.4 Thus, the strong and consistent evidence from these large and well-designed outcome trials led the American Diabetes Association in its most recent guidelines to recommend adding SGLT2 inhibitors to metformin for the treatment of patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease, heart failure, or CKD, regardless of baseline HbA1c levels or HbA1c target.5 As a result of the compelling effects of SGLT2 inhibitors on cardiovascular outcomes in diabetic patients, as well as increasing evidence that these clinical effects were independent of glycemic control, several subsequent trials were conducted to evaluate whether this new class of drugs may improve clinical outcomes in nondiabetic patients.
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) was the first clinical trial to investigate the effect of SGLT2 inhibitors on cardiovascular disease in nondiabetic patients. Findings from DAPA-HF showed that dapagliflozin reduced the risk of worsening heart failure or death from cardiovascular causes, independent of the presence of underlying diabetes. This initial finding resonates with a growing body of evidence6,7 that supports the use of SGLT2 inhibitors as an adjunctive therapy for heart failure in the absence of diabetes.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed that long-term administration of canagliflozin conferred cardiovascular, as well as renal, protection in patients with type 2 diabetes with CKD.8 Similar to the protective effects on heart failure, the renal benefits of SGLT2 inhibitors appeared to be independent of their blood glucose-lowering effects. Thus, these recent discoveries led to the design of the DAPA-CKD trial to further assess the long-term efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with CKD precipitated by causes other than type 2 diabetes. Although diabetes is the most common cause for CKD, it nonetheless only accounts for 40% of all CKD etiologies. To date, the only classes of medication that have been shown to slow a decline in kidney function in patients with diabetes are angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Given that CKD is an important contributor to illness, is associated with diminished quality of life and reduced life expectancy, and increases health care costs, the findings of the DAPA-CKD trial are particularly significant as they show a renal benefit of dapagliflozin treatment across CKD stages that is independent of underlying diabetes. Therefore, SGLT2 inhibitors may offer a new and unique treatment option for millions of patients with CKD worldwide for whom ACE inhibitors and ARBs were otherwise the only treatments to prevent kidney failure. Moreover, with a number-needed-to-treat of 19 to prevent 1 composite renal vascular event over a period of 2.4 years, dapagliflozin requires a much lower number needed to treat compared to ACE inhibitors and ARBs in similar patients.
The trial has several limitations in study design. For example, the management of diabetes and hypertension were left to the discretion of each trial site, in keeping with local clinical practice and guidelines. It is unknown whether this variability in the management of comorbidities that impact kidney function had an effect on the study’s results. In addition, the trial was stopped early as a result of recommendations from an independent committee due to the demonstrated efficacy of dapagliflozin. This may have reduced the statistical power to assess some of the secondary outcomes. Finally, the authors discussed an initial dip in the estimated GFR after initiation of dapagliflozin treatment, similar to that observed in other SGLT2 inhibitor clinical trials. However, they were unable to ascertain the reversibility of this effect after the discontinuation of dapagliflozin because assessment of GFR was not completed after trial closure. Nonetheless, the authors specified that the reversibility of this initial estimated GFR dip had been assessed and observed in other clinical trials involving dapagliflozin.
The nonglycemic benefits of SGLT2 inhibitors, including improvement in renal outcomes, have strong implications for the future management of patients with CKD. If this indication is approved by the FDA and recommended by clinical guidelines, the ease of SGLT2 inhibitor prescription (eg, minimal drug-drug interaction, no titration), treatment administration (orally once daily), and safety profile may lead to wide use of SGLT2 inhibitors by generalists, nephrologists, and endocrinologists in preserving or improving renal outcomes in patients at risk for end-stage kidney disease. Given that SGLT2 inhibitors are a new class of pharmacologic therapeutics, patient education should include a discussion of the possible side effects, such as euglycemic ketoacidosis, genital and urinary tract infection, and foot and leg amputation. Finally, as Strandberg and colleagues reported in a recent commentary,9 the safety of SGLT2 inhibitors in older adults with multimorbidity, frailty, and polypharmacy remains unclear. Thus, future studies of SGLT2 inhibitors are needed to better evaluate their clinical effects in older adults.
Applications for Clinical Practice
This trial enrolled a dedicated patient population with CKD and demonstrated a benefit of dapagliflozin in reducing renal and cardiovascular outcomes, regardless of baseline diabetes status. These drugs (dapagliflozin as well as other SGLT2 inhibitors) will likely have a prominent role in future CKD management guidelines. Until then, several barriers remain before SGLT2 inhibitors can be widely used in clinical practice. Among these barriers are FDA approval for their use in patients with and without diabetes with an estimated GFR < 30 mL/min/1.73 m2 and lowering the costs of this class of drugs.
—Rachel Litke, MD, PhD
Icahn School of Medicine at Mount Sinai
Fred Ko, MD, MS
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
4. Zelniker TA, Wiviott SD, Raz I, Sabatine MS. SGLT-2 inhibitors for people with type 2 diabetes - Authors’ reply. Lancet. 2019;394:560-561.
5. American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111-S34.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413-1424.
7. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829.
8. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
9. Strandberg TE, Petrovic M, Benetos A. SGLT-2 inhibitors for people with type 2 diabetes. Lancet. 2019;394:560.
Marijuana use tied to repeat MI, stroke after percutaneous coronary intervention
in separate studies.
Rhushik Bhuva, MD, presented the recurrent-MI results from a national U.S. study, and Sang Gune K. Yoo, MD, presented the PCI study, which used data from a Michigan cohort. The studies were presented at the American Heart Association scientific sessions.
Both studies “add to our accumulating knowledge of the cardiovascular risks of marijuana,” Ersilia M. DeFilippis, MD, a cardiology fellow at Columbia University Irvine Medical Center, New York, who was not involved with this research, said in an interview.
Dr. DeFilippis and the two study authors say clinicians and patients need to be more aware of cardiovascular risks from smoking marijuana, and they call for more patient screening, counseling, and research.
Need for screening and counseling
Marijuana is a Schedule 1 controlled substance in the United States, which makes it illegal to conduct rigorous controlled trials of marijuana products. Existing knowledge is therefore based on observational studies, Dr. DeFilippis noted.
She was lead author of a review of marijuana use by patients with cardiovascular disease. The review was published in the Journal of the American College of Cardiology. An AHA scientific statement about marijuana and cardiovascular health was published in Circulation.
Both documents drew attention to risks from marijuana use in patients with cardiovascular disease.
Until more data are available, “I think it is absolutely critical” that cardiologists and general providers screen patients for marijuana use, “either at the time of their MI or ideally prior to that, when they are making a cardiovascular risk assessment,” said Dr. DeFilippis.
That is also the time to “counsel patients, especially those who have had an MI, about risks associated with continuing to use marijuana.”
Importantly, providers and patients need to be aware that “cannabinoids, through the cytochrome P450 system, can interact with well-known cardiovascular medications, which we know provide benefit in the post-MI period,” she added. “For example, marijuana can interfere with beta-blockers, statins, antiarrhythmics, and certain anticoagulants.”
Dr. Bhuva, a cardiology fellow with the Wright Center for Community Health, Scranton, Pa., said that it is “concerning” that “recurrent heart attacks and cardiac interventions [were] higher among cannabis users, even though they were younger and had fewer risk factors for heart disease.
“Spreading awareness regarding the potential risk of recurrent heart attacks in middle-aged, African American, and male cannabis users and screening them at an earlier age for potential risk factors of future heart attacks should be encouraged among clinicians,” he urged in a statement from the AHA.
Dr. Yoo, an internal medicine resident at the University of Michigan, Ann Arbor, pointed out that, in their study of patients who underwent PCI after MI or because they had coronary artery disease, those who smoked or vaped marijuana were younger and were more likely to be male. They were less likely to have traditional cardiovascular risk factors except for smoking tobacco, which was highly prevalent.
After propensity matching, patients who used marijuana had a 1.5-fold increased risk of in-hospital bleeding and an 11-fold higher risk for in-hospital stroke following PCI.
However, the absolute number of strokes in PCI was small, and the confidence interval was wide (indicating a large uncertainty), Dr. Yoo said in an interview.
These risks “should not deter patients from undergoing these [lifesaving] procedures,” he said; however, clinicians should be aware of these risks with marijuana use and should screen and counsel patients about this.
Hospitalized patients with prior MI
Dr. Bhuva and colleagues identified patients from the National Inpatient Sample who were hospitalized in the United States from 2007 to 2014 and who had experienced a prior MI and had undergone revascularization with PCI or coronary artery bypass grafting (CABG).
There were about 8 million hospital stays per year. The database did not specify the type of marijuana that patients used.
During the 8-year study period, many states legalized or decriminalized medical and/or recreational marijuana, and marijuana use increased steadily, from 0.2% to 0.7%.
Compared with nonusers, those who used marijuana were younger (median age, 53 vs. 72 years), and there were more men (77% vs. 62%) or Black persons (34% vs. 10%) (all P < .001). Fewer marijuana users had hypertension (72% vs. 75%), diabetes (24% vs. 33%), or dyslipidemia (51% vs. 58%) (all P < .001). More marijuana users underwent a repeat MI (67% vs. 41%).
On the other hand, marijuana users, who were younger and healthier than the other patients, were less likely to die during hospitalization for a recurrent MI (0.8% vs. 2.5%), and their hospital costs were lower.
The researchers acknowledged that study limitations include lack of information about marijuana type (smoked, edible, medicinal, or recreational) or dose, as well as the time from marijuana use to cardiac event.
In-Hospital outcomes after PCI
Dr. Yoo and colleagues analyzed data from patients who underwent PCI from Jan. 1, 2013, to Oct. 1, 2016, at Michigan’s 48 nonfederal hospitals, which are part of the Blue Cross Blue Shield Michigan Cardiovascular Consortium PCI registry.
In this cohort, 3,970 patients (3.5%) had smoked or vaped marijuana in the month prior to PCI, and 109,507 patients had not done so. The marijuana users were younger (mean age, 54 vs. 66 years) and were more likely to be male (79% vs. 67%) and to smoke cigarettes (73% vs. 27%).
They were less likely to have hypertension, type 2 diabetes, dyslipidemia, cerebrovascular disease, or prior CABG and were equally likely to have had a prior MI (36%).
Compared with nonusers, marijuana users were more likely to present with non–ST-elevation MI (30% vs. 23%) or ST-elevation MI (27% vs. 16%) and were less likely to present with angina.
Using propensity score matching, the researchers matched 3,803 marijuana users with the same number of nonusers.
In the matched cohort, patients who used marijuana had a greater risk of in-hospital bleeding (adjusted odds ratio, 1.54; 95% confidence interval, 1.20-1.97; P < .001) or stroke (aOR, 11.01; 95% CI, 1.32-91.67; P = .026) following PCI.
Marijuana users had a lower risk for acute kidney injury (2.2% vs. 2.9%; P = .007). Transfusion and mortality rates were similar in both groups.
The researchers acknowledged study limitations, including the fact that it did not include marijuana edibles, that the results may not be generalizable, and that marijuana use is now likely more common in Michigan following legalization of recreational marijuana in 2018.
Dr. Bhuva, Dr. Yoo, and Dr. DeFilippis have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
in separate studies.
Rhushik Bhuva, MD, presented the recurrent-MI results from a national U.S. study, and Sang Gune K. Yoo, MD, presented the PCI study, which used data from a Michigan cohort. The studies were presented at the American Heart Association scientific sessions.
Both studies “add to our accumulating knowledge of the cardiovascular risks of marijuana,” Ersilia M. DeFilippis, MD, a cardiology fellow at Columbia University Irvine Medical Center, New York, who was not involved with this research, said in an interview.
Dr. DeFilippis and the two study authors say clinicians and patients need to be more aware of cardiovascular risks from smoking marijuana, and they call for more patient screening, counseling, and research.
Need for screening and counseling
Marijuana is a Schedule 1 controlled substance in the United States, which makes it illegal to conduct rigorous controlled trials of marijuana products. Existing knowledge is therefore based on observational studies, Dr. DeFilippis noted.
She was lead author of a review of marijuana use by patients with cardiovascular disease. The review was published in the Journal of the American College of Cardiology. An AHA scientific statement about marijuana and cardiovascular health was published in Circulation.
Both documents drew attention to risks from marijuana use in patients with cardiovascular disease.
Until more data are available, “I think it is absolutely critical” that cardiologists and general providers screen patients for marijuana use, “either at the time of their MI or ideally prior to that, when they are making a cardiovascular risk assessment,” said Dr. DeFilippis.
That is also the time to “counsel patients, especially those who have had an MI, about risks associated with continuing to use marijuana.”
Importantly, providers and patients need to be aware that “cannabinoids, through the cytochrome P450 system, can interact with well-known cardiovascular medications, which we know provide benefit in the post-MI period,” she added. “For example, marijuana can interfere with beta-blockers, statins, antiarrhythmics, and certain anticoagulants.”
Dr. Bhuva, a cardiology fellow with the Wright Center for Community Health, Scranton, Pa., said that it is “concerning” that “recurrent heart attacks and cardiac interventions [were] higher among cannabis users, even though they were younger and had fewer risk factors for heart disease.
“Spreading awareness regarding the potential risk of recurrent heart attacks in middle-aged, African American, and male cannabis users and screening them at an earlier age for potential risk factors of future heart attacks should be encouraged among clinicians,” he urged in a statement from the AHA.
Dr. Yoo, an internal medicine resident at the University of Michigan, Ann Arbor, pointed out that, in their study of patients who underwent PCI after MI or because they had coronary artery disease, those who smoked or vaped marijuana were younger and were more likely to be male. They were less likely to have traditional cardiovascular risk factors except for smoking tobacco, which was highly prevalent.
After propensity matching, patients who used marijuana had a 1.5-fold increased risk of in-hospital bleeding and an 11-fold higher risk for in-hospital stroke following PCI.
However, the absolute number of strokes in PCI was small, and the confidence interval was wide (indicating a large uncertainty), Dr. Yoo said in an interview.
These risks “should not deter patients from undergoing these [lifesaving] procedures,” he said; however, clinicians should be aware of these risks with marijuana use and should screen and counsel patients about this.
Hospitalized patients with prior MI
Dr. Bhuva and colleagues identified patients from the National Inpatient Sample who were hospitalized in the United States from 2007 to 2014 and who had experienced a prior MI and had undergone revascularization with PCI or coronary artery bypass grafting (CABG).
There were about 8 million hospital stays per year. The database did not specify the type of marijuana that patients used.
During the 8-year study period, many states legalized or decriminalized medical and/or recreational marijuana, and marijuana use increased steadily, from 0.2% to 0.7%.
Compared with nonusers, those who used marijuana were younger (median age, 53 vs. 72 years), and there were more men (77% vs. 62%) or Black persons (34% vs. 10%) (all P < .001). Fewer marijuana users had hypertension (72% vs. 75%), diabetes (24% vs. 33%), or dyslipidemia (51% vs. 58%) (all P < .001). More marijuana users underwent a repeat MI (67% vs. 41%).
On the other hand, marijuana users, who were younger and healthier than the other patients, were less likely to die during hospitalization for a recurrent MI (0.8% vs. 2.5%), and their hospital costs were lower.
The researchers acknowledged that study limitations include lack of information about marijuana type (smoked, edible, medicinal, or recreational) or dose, as well as the time from marijuana use to cardiac event.
In-Hospital outcomes after PCI
Dr. Yoo and colleagues analyzed data from patients who underwent PCI from Jan. 1, 2013, to Oct. 1, 2016, at Michigan’s 48 nonfederal hospitals, which are part of the Blue Cross Blue Shield Michigan Cardiovascular Consortium PCI registry.
In this cohort, 3,970 patients (3.5%) had smoked or vaped marijuana in the month prior to PCI, and 109,507 patients had not done so. The marijuana users were younger (mean age, 54 vs. 66 years) and were more likely to be male (79% vs. 67%) and to smoke cigarettes (73% vs. 27%).
They were less likely to have hypertension, type 2 diabetes, dyslipidemia, cerebrovascular disease, or prior CABG and were equally likely to have had a prior MI (36%).
Compared with nonusers, marijuana users were more likely to present with non–ST-elevation MI (30% vs. 23%) or ST-elevation MI (27% vs. 16%) and were less likely to present with angina.
Using propensity score matching, the researchers matched 3,803 marijuana users with the same number of nonusers.
In the matched cohort, patients who used marijuana had a greater risk of in-hospital bleeding (adjusted odds ratio, 1.54; 95% confidence interval, 1.20-1.97; P < .001) or stroke (aOR, 11.01; 95% CI, 1.32-91.67; P = .026) following PCI.
Marijuana users had a lower risk for acute kidney injury (2.2% vs. 2.9%; P = .007). Transfusion and mortality rates were similar in both groups.
The researchers acknowledged study limitations, including the fact that it did not include marijuana edibles, that the results may not be generalizable, and that marijuana use is now likely more common in Michigan following legalization of recreational marijuana in 2018.
Dr. Bhuva, Dr. Yoo, and Dr. DeFilippis have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
in separate studies.
Rhushik Bhuva, MD, presented the recurrent-MI results from a national U.S. study, and Sang Gune K. Yoo, MD, presented the PCI study, which used data from a Michigan cohort. The studies were presented at the American Heart Association scientific sessions.
Both studies “add to our accumulating knowledge of the cardiovascular risks of marijuana,” Ersilia M. DeFilippis, MD, a cardiology fellow at Columbia University Irvine Medical Center, New York, who was not involved with this research, said in an interview.
Dr. DeFilippis and the two study authors say clinicians and patients need to be more aware of cardiovascular risks from smoking marijuana, and they call for more patient screening, counseling, and research.
Need for screening and counseling
Marijuana is a Schedule 1 controlled substance in the United States, which makes it illegal to conduct rigorous controlled trials of marijuana products. Existing knowledge is therefore based on observational studies, Dr. DeFilippis noted.
She was lead author of a review of marijuana use by patients with cardiovascular disease. The review was published in the Journal of the American College of Cardiology. An AHA scientific statement about marijuana and cardiovascular health was published in Circulation.
Both documents drew attention to risks from marijuana use in patients with cardiovascular disease.
Until more data are available, “I think it is absolutely critical” that cardiologists and general providers screen patients for marijuana use, “either at the time of their MI or ideally prior to that, when they are making a cardiovascular risk assessment,” said Dr. DeFilippis.
That is also the time to “counsel patients, especially those who have had an MI, about risks associated with continuing to use marijuana.”
Importantly, providers and patients need to be aware that “cannabinoids, through the cytochrome P450 system, can interact with well-known cardiovascular medications, which we know provide benefit in the post-MI period,” she added. “For example, marijuana can interfere with beta-blockers, statins, antiarrhythmics, and certain anticoagulants.”
Dr. Bhuva, a cardiology fellow with the Wright Center for Community Health, Scranton, Pa., said that it is “concerning” that “recurrent heart attacks and cardiac interventions [were] higher among cannabis users, even though they were younger and had fewer risk factors for heart disease.
“Spreading awareness regarding the potential risk of recurrent heart attacks in middle-aged, African American, and male cannabis users and screening them at an earlier age for potential risk factors of future heart attacks should be encouraged among clinicians,” he urged in a statement from the AHA.
Dr. Yoo, an internal medicine resident at the University of Michigan, Ann Arbor, pointed out that, in their study of patients who underwent PCI after MI or because they had coronary artery disease, those who smoked or vaped marijuana were younger and were more likely to be male. They were less likely to have traditional cardiovascular risk factors except for smoking tobacco, which was highly prevalent.
After propensity matching, patients who used marijuana had a 1.5-fold increased risk of in-hospital bleeding and an 11-fold higher risk for in-hospital stroke following PCI.
However, the absolute number of strokes in PCI was small, and the confidence interval was wide (indicating a large uncertainty), Dr. Yoo said in an interview.
These risks “should not deter patients from undergoing these [lifesaving] procedures,” he said; however, clinicians should be aware of these risks with marijuana use and should screen and counsel patients about this.
Hospitalized patients with prior MI
Dr. Bhuva and colleagues identified patients from the National Inpatient Sample who were hospitalized in the United States from 2007 to 2014 and who had experienced a prior MI and had undergone revascularization with PCI or coronary artery bypass grafting (CABG).
There were about 8 million hospital stays per year. The database did not specify the type of marijuana that patients used.
During the 8-year study period, many states legalized or decriminalized medical and/or recreational marijuana, and marijuana use increased steadily, from 0.2% to 0.7%.
Compared with nonusers, those who used marijuana were younger (median age, 53 vs. 72 years), and there were more men (77% vs. 62%) or Black persons (34% vs. 10%) (all P < .001). Fewer marijuana users had hypertension (72% vs. 75%), diabetes (24% vs. 33%), or dyslipidemia (51% vs. 58%) (all P < .001). More marijuana users underwent a repeat MI (67% vs. 41%).
On the other hand, marijuana users, who were younger and healthier than the other patients, were less likely to die during hospitalization for a recurrent MI (0.8% vs. 2.5%), and their hospital costs were lower.
The researchers acknowledged that study limitations include lack of information about marijuana type (smoked, edible, medicinal, or recreational) or dose, as well as the time from marijuana use to cardiac event.
In-Hospital outcomes after PCI
Dr. Yoo and colleagues analyzed data from patients who underwent PCI from Jan. 1, 2013, to Oct. 1, 2016, at Michigan’s 48 nonfederal hospitals, which are part of the Blue Cross Blue Shield Michigan Cardiovascular Consortium PCI registry.
In this cohort, 3,970 patients (3.5%) had smoked or vaped marijuana in the month prior to PCI, and 109,507 patients had not done so. The marijuana users were younger (mean age, 54 vs. 66 years) and were more likely to be male (79% vs. 67%) and to smoke cigarettes (73% vs. 27%).
They were less likely to have hypertension, type 2 diabetes, dyslipidemia, cerebrovascular disease, or prior CABG and were equally likely to have had a prior MI (36%).
Compared with nonusers, marijuana users were more likely to present with non–ST-elevation MI (30% vs. 23%) or ST-elevation MI (27% vs. 16%) and were less likely to present with angina.
Using propensity score matching, the researchers matched 3,803 marijuana users with the same number of nonusers.
In the matched cohort, patients who used marijuana had a greater risk of in-hospital bleeding (adjusted odds ratio, 1.54; 95% confidence interval, 1.20-1.97; P < .001) or stroke (aOR, 11.01; 95% CI, 1.32-91.67; P = .026) following PCI.
Marijuana users had a lower risk for acute kidney injury (2.2% vs. 2.9%; P = .007). Transfusion and mortality rates were similar in both groups.
The researchers acknowledged study limitations, including the fact that it did not include marijuana edibles, that the results may not be generalizable, and that marijuana use is now likely more common in Michigan following legalization of recreational marijuana in 2018.
Dr. Bhuva, Dr. Yoo, and Dr. DeFilippis have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From AHA 2020
Pfizer files for FDA emergency use authorization of COVID vaccine
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Aspirin and statins in chronic hepatitis B: It’s complicated
For patients with chronic hepatitis B, the protective effects of aspirin against hepatocellular carcinoma (HCC) can vary with cirrhosis status and statin treatment, a pair of new studies finds.
One study showed that, although aspirin is linked to a reduction in risk for HCC in these patients, comedication with statins could explain some of that effect. The other showed that cirrhosis dampens the risk-reduction benefit of aspirin.
Currently, there is a link between a reduction in HCC risk and aspirin or statins in patients with chronic hepatitis B, said investigator Won-Mook Choi, MD, PhD, from the University of Ulsan College of Medicine, in Seoul, Republic of Korea.
In one of their analyses, Choi and his colleagues teased out the contribution of each drug and found that the decrease in HCC risk conferred by statins is similar whether or not patients also take aspirin.
“Only statins showed consistent and significant dose-dependent reductions in the risk of HCC, regardless of study design,” said Choi, who presented the findings at The Liver Meeting 2020.
The second study, which looked at the association between aspirin and the risk for HCC in patients with and without cirrhosis, was presented by Heejoon Jang, MD, from the Seoul National University College of Medicine.
Aspirin was shown to be associated with a reduced risk for HCC, but cirrhosis “had a substantial effect on this association,” erasing the benefit of aspirin, Jang reported.
Statins and aspirin
Statins and aspirin are more likely to be prescribed together for patients with chronic hepatitis B but no cirrhosis, said Choi. For that reason, he and his colleagues analyzed data from the Korean National Health Insurance Service database from 2005 to 2015.
In their nested case-control analysis, 17,150 patients with HCC were matched for sex, age, and other factors to 817,675 patients without HCC. All participants had chronic hepatitis B without cirrhosis and had never received antiviral treatment.
The team also analyzed the incidence of HCC in two historic cohorts of patients with chronic hepatitis B but no cirrhosis, one consisting of 673,107 people who took aspirin and the other with 588,045 who took statins.
The nested case-control analysis showed an 11% risk reduction with aspirin use (adjusted odds ratio [OR], 0.89; 95% CI, 0.85 - 0.94) and a 61% risk reduction with statin use (adjusted OR, 0.39; 95% CI, 0.36 - 0.40). There was a dose-response effect with statins, but not with aspirin.
The historic cohort analysis showed a 33% reduction in the risk for HCC with aspirin (adjusted hazard ratio [HR], 0.67; 95% CI, 0.63 - 0.72) and a 67% reduction with statins (adjusted HR, 0.33; 95% CI, 0.30 - 0.37). However, stratified analyses by drug showed a statin benefit with or without aspirin (P < .001 for both), but no aspirin benefit without statins.
Cirrhosis and aspirin
To assess the interaction between cirrhosis and aspirin, Jang and his colleagues identified 329,635 patients with chronic hepatitis B in the Korean National Health Insurance Service database.
A total of 20,200 had taken aspirin for at least 90 consecutive days, and the rest had never received antiplatelet therapy. Treated and untreated patients were matched for several factors, and HCC incidence was assessed after a median follow-up of 6.7 years.
Among the 2,697 patients who developed HCC during follow-up, the cumulative incidence of HCC was significantly lower for those who took aspirin than for those who did not (P < .001). There was a 15% reduction in the risk for HCC in the aspirin group (adjusted HR, 0.85; 95% CI, 0.78 - 0.92).
However, in patients with cirrhosis, the benefit of aspirin disappeared. Patients without cirrhosis still had a 13% reduction in risk for HCC (adjusted HR, 0.87: 95% CI, 0.79 - 0.95). This group also had a slightly elevated risk for major bleeding (adjusted HR, 1.1; 95% CI, 1.03 - 1.28).
The findings from these two studies add to a growing body of literature that shows the promise of statins and aspirin, which are both readily available and relatively safe, said Amit Singal, MD, from the UT Southwestern Medical Center in Dallas, who was not involved with either study.
“The studies are relatively simple but really do tackle an area of immense need in the field,” he said. Short of having higher-quality data, however, statins and aspirin aren’t quite ready to become bespoke chemotherapies in the clinic, he added, although the results show promise for future randomized trials.
The subgroup analyses that looked at cirrhosis and the interplay of aspirin and statins can help with the planning of such trials, which “is really important for trial design,” Singal noted.
He also pointed to studies that, unlike these results, have found a benefit of aspirin in patients with cirrhosis, underscoring the need for randomized trials. However, “each study does provide a data point that can help to inform those trials,” he said.
Choi and Jang have disclosed no relevant financial relationships. Singal is a consultant for Genentech, Bayer, Eisai, Exelixis, Bristol-Myers Squibb, Roche, Glycotest, FujiFilm, GRAIL, and Exact Sciences, primarily in relation to HCC treatment and screening, not chemoprevention.
This article first appeared on Medscape.com.
For patients with chronic hepatitis B, the protective effects of aspirin against hepatocellular carcinoma (HCC) can vary with cirrhosis status and statin treatment, a pair of new studies finds.
One study showed that, although aspirin is linked to a reduction in risk for HCC in these patients, comedication with statins could explain some of that effect. The other showed that cirrhosis dampens the risk-reduction benefit of aspirin.
Currently, there is a link between a reduction in HCC risk and aspirin or statins in patients with chronic hepatitis B, said investigator Won-Mook Choi, MD, PhD, from the University of Ulsan College of Medicine, in Seoul, Republic of Korea.
In one of their analyses, Choi and his colleagues teased out the contribution of each drug and found that the decrease in HCC risk conferred by statins is similar whether or not patients also take aspirin.
“Only statins showed consistent and significant dose-dependent reductions in the risk of HCC, regardless of study design,” said Choi, who presented the findings at The Liver Meeting 2020.
The second study, which looked at the association between aspirin and the risk for HCC in patients with and without cirrhosis, was presented by Heejoon Jang, MD, from the Seoul National University College of Medicine.
Aspirin was shown to be associated with a reduced risk for HCC, but cirrhosis “had a substantial effect on this association,” erasing the benefit of aspirin, Jang reported.
Statins and aspirin
Statins and aspirin are more likely to be prescribed together for patients with chronic hepatitis B but no cirrhosis, said Choi. For that reason, he and his colleagues analyzed data from the Korean National Health Insurance Service database from 2005 to 2015.
In their nested case-control analysis, 17,150 patients with HCC were matched for sex, age, and other factors to 817,675 patients without HCC. All participants had chronic hepatitis B without cirrhosis and had never received antiviral treatment.
The team also analyzed the incidence of HCC in two historic cohorts of patients with chronic hepatitis B but no cirrhosis, one consisting of 673,107 people who took aspirin and the other with 588,045 who took statins.
The nested case-control analysis showed an 11% risk reduction with aspirin use (adjusted odds ratio [OR], 0.89; 95% CI, 0.85 - 0.94) and a 61% risk reduction with statin use (adjusted OR, 0.39; 95% CI, 0.36 - 0.40). There was a dose-response effect with statins, but not with aspirin.
The historic cohort analysis showed a 33% reduction in the risk for HCC with aspirin (adjusted hazard ratio [HR], 0.67; 95% CI, 0.63 - 0.72) and a 67% reduction with statins (adjusted HR, 0.33; 95% CI, 0.30 - 0.37). However, stratified analyses by drug showed a statin benefit with or without aspirin (P < .001 for both), but no aspirin benefit without statins.
Cirrhosis and aspirin
To assess the interaction between cirrhosis and aspirin, Jang and his colleagues identified 329,635 patients with chronic hepatitis B in the Korean National Health Insurance Service database.
A total of 20,200 had taken aspirin for at least 90 consecutive days, and the rest had never received antiplatelet therapy. Treated and untreated patients were matched for several factors, and HCC incidence was assessed after a median follow-up of 6.7 years.
Among the 2,697 patients who developed HCC during follow-up, the cumulative incidence of HCC was significantly lower for those who took aspirin than for those who did not (P < .001). There was a 15% reduction in the risk for HCC in the aspirin group (adjusted HR, 0.85; 95% CI, 0.78 - 0.92).
However, in patients with cirrhosis, the benefit of aspirin disappeared. Patients without cirrhosis still had a 13% reduction in risk for HCC (adjusted HR, 0.87: 95% CI, 0.79 - 0.95). This group also had a slightly elevated risk for major bleeding (adjusted HR, 1.1; 95% CI, 1.03 - 1.28).
The findings from these two studies add to a growing body of literature that shows the promise of statins and aspirin, which are both readily available and relatively safe, said Amit Singal, MD, from the UT Southwestern Medical Center in Dallas, who was not involved with either study.
“The studies are relatively simple but really do tackle an area of immense need in the field,” he said. Short of having higher-quality data, however, statins and aspirin aren’t quite ready to become bespoke chemotherapies in the clinic, he added, although the results show promise for future randomized trials.
The subgroup analyses that looked at cirrhosis and the interplay of aspirin and statins can help with the planning of such trials, which “is really important for trial design,” Singal noted.
He also pointed to studies that, unlike these results, have found a benefit of aspirin in patients with cirrhosis, underscoring the need for randomized trials. However, “each study does provide a data point that can help to inform those trials,” he said.
Choi and Jang have disclosed no relevant financial relationships. Singal is a consultant for Genentech, Bayer, Eisai, Exelixis, Bristol-Myers Squibb, Roche, Glycotest, FujiFilm, GRAIL, and Exact Sciences, primarily in relation to HCC treatment and screening, not chemoprevention.
This article first appeared on Medscape.com.
For patients with chronic hepatitis B, the protective effects of aspirin against hepatocellular carcinoma (HCC) can vary with cirrhosis status and statin treatment, a pair of new studies finds.
One study showed that, although aspirin is linked to a reduction in risk for HCC in these patients, comedication with statins could explain some of that effect. The other showed that cirrhosis dampens the risk-reduction benefit of aspirin.
Currently, there is a link between a reduction in HCC risk and aspirin or statins in patients with chronic hepatitis B, said investigator Won-Mook Choi, MD, PhD, from the University of Ulsan College of Medicine, in Seoul, Republic of Korea.
In one of their analyses, Choi and his colleagues teased out the contribution of each drug and found that the decrease in HCC risk conferred by statins is similar whether or not patients also take aspirin.
“Only statins showed consistent and significant dose-dependent reductions in the risk of HCC, regardless of study design,” said Choi, who presented the findings at The Liver Meeting 2020.
The second study, which looked at the association between aspirin and the risk for HCC in patients with and without cirrhosis, was presented by Heejoon Jang, MD, from the Seoul National University College of Medicine.
Aspirin was shown to be associated with a reduced risk for HCC, but cirrhosis “had a substantial effect on this association,” erasing the benefit of aspirin, Jang reported.
Statins and aspirin
Statins and aspirin are more likely to be prescribed together for patients with chronic hepatitis B but no cirrhosis, said Choi. For that reason, he and his colleagues analyzed data from the Korean National Health Insurance Service database from 2005 to 2015.
In their nested case-control analysis, 17,150 patients with HCC were matched for sex, age, and other factors to 817,675 patients without HCC. All participants had chronic hepatitis B without cirrhosis and had never received antiviral treatment.
The team also analyzed the incidence of HCC in two historic cohorts of patients with chronic hepatitis B but no cirrhosis, one consisting of 673,107 people who took aspirin and the other with 588,045 who took statins.
The nested case-control analysis showed an 11% risk reduction with aspirin use (adjusted odds ratio [OR], 0.89; 95% CI, 0.85 - 0.94) and a 61% risk reduction with statin use (adjusted OR, 0.39; 95% CI, 0.36 - 0.40). There was a dose-response effect with statins, but not with aspirin.
The historic cohort analysis showed a 33% reduction in the risk for HCC with aspirin (adjusted hazard ratio [HR], 0.67; 95% CI, 0.63 - 0.72) and a 67% reduction with statins (adjusted HR, 0.33; 95% CI, 0.30 - 0.37). However, stratified analyses by drug showed a statin benefit with or without aspirin (P < .001 for both), but no aspirin benefit without statins.
Cirrhosis and aspirin
To assess the interaction between cirrhosis and aspirin, Jang and his colleagues identified 329,635 patients with chronic hepatitis B in the Korean National Health Insurance Service database.
A total of 20,200 had taken aspirin for at least 90 consecutive days, and the rest had never received antiplatelet therapy. Treated and untreated patients were matched for several factors, and HCC incidence was assessed after a median follow-up of 6.7 years.
Among the 2,697 patients who developed HCC during follow-up, the cumulative incidence of HCC was significantly lower for those who took aspirin than for those who did not (P < .001). There was a 15% reduction in the risk for HCC in the aspirin group (adjusted HR, 0.85; 95% CI, 0.78 - 0.92).
However, in patients with cirrhosis, the benefit of aspirin disappeared. Patients without cirrhosis still had a 13% reduction in risk for HCC (adjusted HR, 0.87: 95% CI, 0.79 - 0.95). This group also had a slightly elevated risk for major bleeding (adjusted HR, 1.1; 95% CI, 1.03 - 1.28).
The findings from these two studies add to a growing body of literature that shows the promise of statins and aspirin, which are both readily available and relatively safe, said Amit Singal, MD, from the UT Southwestern Medical Center in Dallas, who was not involved with either study.
“The studies are relatively simple but really do tackle an area of immense need in the field,” he said. Short of having higher-quality data, however, statins and aspirin aren’t quite ready to become bespoke chemotherapies in the clinic, he added, although the results show promise for future randomized trials.
The subgroup analyses that looked at cirrhosis and the interplay of aspirin and statins can help with the planning of such trials, which “is really important for trial design,” Singal noted.
He also pointed to studies that, unlike these results, have found a benefit of aspirin in patients with cirrhosis, underscoring the need for randomized trials. However, “each study does provide a data point that can help to inform those trials,” he said.
Choi and Jang have disclosed no relevant financial relationships. Singal is a consultant for Genentech, Bayer, Eisai, Exelixis, Bristol-Myers Squibb, Roche, Glycotest, FujiFilm, GRAIL, and Exact Sciences, primarily in relation to HCC treatment and screening, not chemoprevention.
This article first appeared on Medscape.com.
FDA authorizes baricitinib combo for COVID-19
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
Pronounced racial differences in HBsAg loss after stopping nucleos(t)ide
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
Loss of the hepatitis B surface antigen (HBsAg), a marker for functional cure of hepatitis B infection, is nearly six times more common among White patients than Asian patients following cessation of therapy with a nucleotide or nucleoside analogue, investigators in the RETRACT-B study group report.
Among 1,541 patients in a global retrospective cohort, the cumulative rate of HBsAg loss 4 years after cessation of therapy with entecavir (ETV), tenofovir disoproxil fumarate (TDF), or other nucleoside/nucleotide analogue (“nuc” or NA) was 11% in Asian patients, compared with 41% in Whites, which translated in multivariate analysis into a hazard ratio (HR) of 5.8 (P < .001), said Grishma Hirode, a clinical research associate and PhD candidate at the Toronto Centre for Liver Disease.
“On univariate Cox regression, the rate of S [antigen] loss was significantly higher among older patients, among [Whites], and among tenofovir-treated patients prior to stopping,” she said during the virtual annual meeting of the American Association for the Study of Liver Diseases.
Although NAs are effective at suppressing hepatitis B viral activity, functional cure as indicated by HBsAg loss is uncommon, Ms. Hirode noted.
“Finite use of antiviral therapy has been proposed as an alternative to long-term therapy, and the rationale for stopping nuc therapy is to induce a durable virologic remission in the form of an inactive carrier state, and ideally a functional cure,” she said.
The RETRACT-B (Response after End of Treatment with Antivirals in Chronic Hepatitis B) study group, comprising liver treatment centers in Canada, Europe, Hong Kong, and Taiwan, studies outcomes following cessation of nucleos(t)ide analogue therapy.
The investigators looked at data on 1,541 patients, including those with both hepatitis B e-antigen (HBeAg) positive and HBeAg-negative disease at the start of therapy, all of whom were HBeAg negative at the time of antiviral cessation and had undetectable serum HBV DNA. Patients with hepatitis C, hepatitis D and/or HIV co-infection were excluded, as were patients who had received interferon treatment less than 12 months before stopping.
The mean age at baseline was 53 years. Men comprised 73% of the sample. In all, 88% of patients were Asian, 10% White, and 2% other.
In patients for whom genotype data was known, 0.5% had type A, 43% type B, 11% type C, and 2% type D.
Nearly two-thirds of patients (60%) were on ETV at the time of drug cessation, 29% were on TDF, and 11% were on other agents.
In all, 5% of patients had cirrhosis at the time of nucleos(t)ide cessation, the mean HBsAg was 2.6 log10 IU/mL, and the mean alanine aminotransferase (ALT) level was 0.6 times the upper limit of normal.
The median duration of NA therapy was 3 years.
The cumulative rates of HBsAg loss over time among all patients was 3% at 1 year, 8% at 2 years. 12% at 3 years, and 14% at 4 years. Cumulative rates of antigen loss at year 4 were significantly greater for patients 50 and older vs. those younger than 50 (18% vs. 9%, respectively, P = .01), Whites vs. Asians (41% vs. 11%, P < .001), and in those who had been on TDF vs. ETV (17% vs. 12%, P = .001). There was no significant difference in cumulative HBsAg loss between patients who were HBeAg positive or negative at the start of NA therapy.
Cumulative rates of retreatment were 30% at 1 year, 43% at 2 years, 50% at 3 years, and 56% at 4 years. The only significant predictor for retreatment was age, with patients 50 and older being significantly more likely to be retreated by year 4 (63% vs. 45%, respectively, P < .001).
In a univariate model for HBsAg loss, the HR for age 50 and older was 1.7 (P = .01), the HR for White vs. Asian patients was 5.5 (P < .001), and the HR for TDF vs. ETV was 2.0 (P = .001).
A univariate model for retreatment showed an HR of 1.6 for patients 50 and older; all other parameters (sex, race, NA type, and HBeAg status at start of therapy) were not significantly different.
In multivariate models, only race/ethnicity remained significant as a predictor for HBsAg loss, with a HR of 5.8 for Whites vs. Asians (P < .001), and only age 50 and older remained significant as a predictor for retreatment, with a HR of 1.6 (P < .001).
The 4-year cumulative rate of virologic relapse, defined as an HBV DNA of 2000 IU/mL or higher) was 74%, the rate of combined DNA plus ALT relapse (ALT 2 or more times the upper limit of normal) was 56%, and the rate of ALT flares (5 or more times the upper limit of normal) was 33%.
In all, 15 patients (1%) experienced hepatic decompensation, and 12 (0.96%) died, with 9 of the deaths reported as liver-related.
Race/ethnicity differences previously seen
Liver specialist Anna Suk-Fong Lok, MD, professor of medicine at the University of Michigan in Ann Arbor, who was not involved in the study, said that the findings are not especially surprising.
“When the studies came out from Asian countries showing that patients who were taken off treatment had a higher rate of S antigen loss than patients who stayed on treatment, the rate of S antigen loss was not all that impressive, but when you look at the European studies the rate of S antigen loss was very high,” she said in an interview.
“The question of course is ‘Why?’ I don’t think we understand completely why. We can speculate, but none of these type studies give us a definitive answer,” she said.
Possible reasons for the racial differences in HBsAg loss include differences in hepatitis B genotype, she said.
“Another possibility is that Asian patients may have been infected either at the time of birth or as a young kid, so they may have been infected for a much longer period of time than [Whites], who usually acquire infections as adults,” Dr. Lok said.
There may also be differences between patient populations in immune responses following cessation of antiviral therapy, she added.
The study was supported by the RETRACT-B group. Ms. Hirode and Dr. Lok reported no relevant disclosures.
SOURCE: Hirode G et al. AASLD 2020. Abstract 23.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
Harnessing the HIV care continuum model to improve HCV treatment success
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Better linkage to care with providers who are familiar with both the HCV and HIV treatment cascade may not only improve access to HCV treatment, but it may also support patient retention, treatment adherence, and achievement of sustained virologic response (SVR) and viral suppression, said Stephanie LaMoy, CAN Community Health, North Point, Florida. She presented the results of a pilot study at the virtual Association of Nurses in AIDS Care 2020 Annual Meeting.
In an effort to identify strategies most important for improving care access among their patients with HCV, LaMoy and her colleagues assessed 12-month patient data collected from three of their clinics. These data were evaluated for HCV treatment access, engagement, and outcomes.
The pilot study included 126 patients who were reactive and another 24 HCV-positive patients who were referred from other sources. Active HCV infections requiring treatment were reported in 144 patients.
A total of 59 patients were linked to care but did not initiate treatment for their active infection. LaMoy said there were multiple causes, including homelessness, substance abuse, and inability to maintain contact.
In contrast, 85 patients with HCV infection started treatment, but 35 of these patients did not complete their regimen. Out of the 50 patients who reported completing treatment, 30 did not return to the clinic to confirm sustained viral suppression.
According to LaMoy, this raised a red flag, causing the investigators to consider a different approach to care.
HIV care continuum model and its role in HCV
To improve the rate at which patients with HCV infection complete treatment within their clinics, the researchers formed a panel to determine necessary interventions that could reduce barriers to care.
The HIV care continuum came into play. They chose this model based on knowledge that HCV and HIV share the same care continuum with similar goals in diagnosis, linkage to care, retention, and suppression.
Based on the consensus of the panel and consideration of the HIV care continuum model, they identified a number of interventions needed to mitigate HCV treatment barriers. These included the incorporation of peer navigators or linkage-to-care (LCC) coordinators, use of the mobile medical unit, greater implementation of onsite lab visits, and medication-assisted treatment.
The LCC coordinators proved to be particularly important, as these team members helped assist patients with social and financial support to address challenges with access to treatment. These coordinators can also help patients gain access to specialized providers, ultimately improving the chance of successful HCV management.
Additionally, LCC coordinators may help identify and reduce barriers associated with housing, transportation, and nutrition. Frequent patient contact by the LCC coordinators can encourage adherence and promote risk reduction education, such as providing referrals to needle exchange services.
“Linking individuals to care with providers who are familiar with the treatment cascade could help improve retention and should be a top priority for those involved in HCV screening and treatment,” said LaMoy. “An environment with knowledge, lack of judgment, and a tenacious need to heal the community that welcomes those with barriers to care is exactly what is needed for the patients in our program.”
National, community challenges fuel barriers to HCV treatment access
Substance use, trauma histories, and mental health problems can negatively affect care engagement and must be addressed before the benefits of HCV therapy can be realized.
Addressing these issues isn’t always easy, said Kathleen Bernock, FNP-BC, AACRN, AAHIVS, of the Bedford-Stuyvesant Family Health Center in New York City, in an email to Medscape Medical News. She pointed out that several states have harsh restrictions on who is able to access HCV treatment, and some states will not approve certain medications for people who actively use drugs.
“Even for states without these restrictions, many health systems are difficult to navigate and may not be welcoming to persons actively using,” said Bernock. Trauma-informed care can also be difficult to translate into clinics, she added.
“Decentralizing care to the communities most affected would greatly help mitigate these barriers,” suggested Bernock. Decentralization, she explained, might include co-locating services such as syringe exchanges, utilizing community health workers and patient navigators, and expanding capacity-to-treat to community-based providers.
“[And] with the expansion of telehealth services in the US,” said Bernock, “we now have even more avenues to reach people that we never had before.”
LaMoy and Bernock have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.