User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
AASLD 2020: A clinical news roundup
Studies that address fundamental questions in hepatology and have the potential to change or improve clinical practice were the focus of a clinical debrief session from the virtual annual meeting of the American Association for the Study of Liver Diseases.
“We chose papers that had the highest level of evidence, such as randomized controlled trials, controlled studies, and large data sets – and some small data sets too,” said Tamar Taddei, MD, associate professor of medicine in the section of digestive disease at Yale University, New Haven, Conn.
Dr. Taddei and colleagues Silvia Vilarinho, MD, PhD; Simona Jakab, MD; and Ariel Jaffe, MD, all also from Yale, selected the papers from among 197 oral and 1,769 poster abstracts presented at AASLD 2020.
They highlighted the most important findings from presentations on autoimmune and cholestatic disease, transplantation, cirrhosis and portal hypertension, alcoholic liver disease, neoplasia, drug-induced liver injury, and COVID-19. They did not review studies focused primarily on nonalcoholic steatohepatitis or nonalcoholic fatty liver disease, viral hepatitis, or basic science, all of which were covered in separate debriefing sessions.
Cirrhosis and portal hypertension
A study from the Department of Veterans Affairs looked at the prevalence of liver disease risk factors and rates of subsequent testing for and diagnosis of cirrhosis in the Veterans Health Administration system (VHA).
The authors found that, among more than 6.65 million VHA users in 2018 with no prior diagnosis of cirrhosis, approximately half were at risk for cirrhosis, of whom about 75% were screened, and approximately 5% of those who were screened were positive for possible cirrhosis (133,636). Of the patients who screened positive, about 10% (12,566) received a diagnosis of cirrhosis, including 4,120 with liver decompensation.
“This paper underscores the importance of population-level screening in uncovering unrecognized cirrhosis, enabling intervention earlier in the course of disease,” Dr. Taddei said (Abstract #661).
A study looking at external validation of novel cirrhosis surgical risk models designed to improve prognostication for a range of common surgeries showed that the VOCAL-Penn score was superior to the Mayo Risk Score, Model for End-stage Liver Disease and MELD-sodium scores for discrimination of 30-day and 90-day postoperative mortality (Abstract #91).
“While these models are not a substitute for clinical acumen, they certainly improve our surgical risk prediction in patients with cirrhosis, a very common question in consultative hepatology,” Dr. Taddei said.
She also cited three abstracts that address the important questions regarding performing studies in patients with varices or ascites, including whether it’s safe to perform transesophageal echocardiography in patients with cirrhosis without first screening for varices, and whether nonselective beta-blockers should be continued in patients with refractory ascites.
A retrospective study of 191 patients with cirrhosis who underwent upper endoscopy within 4 years of transesophageal echocardiography had no overt gastrointestinal bleeding regardless of the presence of esophageal varices, suggesting that routine preprocedure esophagogastroduodenoscopy “is of no utility,” (Abstract #1872).
A study to determine risk of sepsis in 1,198 patients with cirrhosis found that 1-year risk of sepsis was reduced by 50% with the use of nonselective beta-blockers (Abstract #94).
The final abstract in this category touched on the use of an advance care planning video support tool to help transplant-ineligible patients with end-stage liver disease decide whether they want support measures such cardiopulmonary resuscitation or intubation. The authors found that the video decision tool was feasible and acceptable to patients, and improved their knowledge of end-of-life care. More patients randomized to the video arm opted against CPR or intubation, compared with those assigned to a verbal discussion of options (Abstract #712).
Alcohol
The reviewers highlighted two studies of alcohol use: The first was designed to determine the prevalence of early alcohol relapse (resumption within 3 months) in patients who presented with alcoholic hepatitis. The subjects included 478 patients enrolled in the STOPAH trial, and a validation set of 194 patients from the InTeam (Integrated Approaches for identifying Molecular Targets in Alcoholic Hepatitis) Consortium.
“They found that high-risk patients were younger, unemployed, and without a stable relationship. Intermediate risk were middle aged, employed, and in a stable relationship, and low-risk profiles were older, with known cirrhosis; they were mostly retired and in a stable relationship,” Dr. Taddei said.
The identification of nongenetic factors that predict early relapse may aid in personalization of treatment strategies, she said (Abstract #232).
The second study looked at fecal microbial transplant (FMT) for reducing cravings in adults with alcohol use disorder (AUD) and cirrhosis. The investigators saw a nonsignificant trend toward greater total abstinence at 6 months in patients randomized to FMT versus placebo.
“Future trials should be performed to determine the impact of FMT on altering the gut-brain axis in patients with AUD,” she said (Abstract #7).
Transplantation
The prospective controlled QUICKTRANS study by French and Belgian researchers found that patients who underwent early liver transplantation for severe alcoholic hepatitis had numerically but not significantly higher rates of relapse than patients who were transplanted after at least 6 months of abstinence, although heavy drinking was more frequent in patients who underwent early transplant.
The 2-year survival rates for both patients who underwent early transplant and those who underwent transplant after 6 months of sobriety were “identical, and excellent.” In addition, the 2-year survival rate for patients with severe alcoholic hepatitis who underwent transplant was 82.8%, compared with 28.2% for patients who were deemed ineligible for transplant according to a selection algorithm (P < .001).
“Perhaps most important is that studies in this population can be conducted in a controlled fashion across centers with reproducible transplant eligibility algorithms,” Dr. Taddei commented (Abstract #6).
The place of honor – Abstract # 1 – was reserved for a study looking at the effects on liver transplant practice of a new “safety net” policy from the Organ Procurement and Transplantation Network and United Network for Organ Sharing stating that patients awaiting liver transplantation who develop kidney failure may be given priority on the kidney transplant waiting list.
The investigators found that the new policy significantly increased the number of adult primary liver transplant alone candidates who where on dialysis at the time of listing, and did not affect either waiting list mortality or posttransplant outcomes.
The authors also saw a significant increase in kidney transplant listing after liver transplant, especially for patients who were on hemodialysis at the time of list.
In the period after implementation of the policy, there was a significantly higher probability of kidney transplant, and significant reduction in waiting list mortality.
Autoimmune & cholestatic diseases
Investigators performed an analysis of the phase 3 randomized controlled ENHANCE trial of seladelpar in patients with primary biliary cholangitis. The trial was stopped because of an adverse event ultimately deemed to be unrelated to the drug, so the analysis looked at the composite responder rate at month 3.
“The key takeaway from this study is that at the 10-mg dosage of seladelpar, 78% met a composite endpoint, 27% of patients normalized their alkaline phosphatase, and 50% normalized their ALT. There was significant improvement in pruritus,” Dr. Taddei said.
The drug was generally safe and well tolerated. A 52-week phase 3 global registration study will begin enrolling patients in early 2021 (Abstract #LO11).
In a pediatric study, investigators looked at differences in primary sclerosing cholangitis (PSC) among various population, and found that “Black and Hispanic patients have dramatically worse clinical outcomes, compared to White and Asian patients. They are more likely to be diagnosed with PSC at an advanced stage with extensive fibrosis and portal hypertensive manifestations.”
The authors suggested that the differences may be explained in part by socioeconomic disparities leading to delay in diagnosis, to a more aggressive phenotype, or both (Abstract #66).
A meta-analysis of maternal and fetal outcomes in women with autoimmune hepatitis showed that the disease is associated with increased risk of gestational diabetes, premature births, and small-for-gestational age or low-birth-weight babies.
“Pregnant women should be monitored closely before, during and after pregnancy. It’s important to know that, in the prevalence data, flares were most prevalent postpartum at 41%. These finds will help us counsel our patients with autoimmune hepatitis who become pregnant,” Dr. Taddei said (Abstract #97).
Drug-induced liver injury
A study of clinical outcomes following immune checkpoint inhibitor rechallenge in melanoma patients with resolved higher grade 3 or higher checkpoint inhibitor–induced hepatitis showed that 4 of 31 patients (13%) developed recurrence of grade 2 or greater hepatitis, and 15 of 31 (48%) developed an immune-related adverse event after rechallenge.
There was no difference in time to death between patients who were rechallenged and those who were not, and immune-related liver toxicities requiring drug discontinuation after rechallenge were uncommon.
“High-grade immune checkpoint inhibitor hepatitis should be reconsidered as an absolute contraindication for immune checkpoint inhibitor rechallenge,” Dr. Taddei said (Abstract # 116).
Neoplasia
The investigators also highlighted an abstract describing significant urban-rural and racial ethnic differences in hepatocellular carcinoma rates. A fuller description of this study can be found here (Abstract #136).
COVID-19
Finally, the reviewer highlighted a study of the clinical course of COVID-19 in patients with chronic liver disease, and to determine factors associated with adverse outcomes in patients with chronic liver disease who acquire COVID-19.
The investigators found that patients with chronic liver disease and COVID-19 have a 14% morality rate, and that alcohol-related liver disease, decompensated cirrhosis, and hepatocellular carcinoma are all risk factors for increased mortality from COVID-19.
They recommended emphasizing telemedicine, prioritizing patients with chronic liver disease for vaccination, and including these patients in prospective studies and drug trials for COVID-19 therapies.
Dr. Taddei reported having no disclosures.
Studies that address fundamental questions in hepatology and have the potential to change or improve clinical practice were the focus of a clinical debrief session from the virtual annual meeting of the American Association for the Study of Liver Diseases.
“We chose papers that had the highest level of evidence, such as randomized controlled trials, controlled studies, and large data sets – and some small data sets too,” said Tamar Taddei, MD, associate professor of medicine in the section of digestive disease at Yale University, New Haven, Conn.
Dr. Taddei and colleagues Silvia Vilarinho, MD, PhD; Simona Jakab, MD; and Ariel Jaffe, MD, all also from Yale, selected the papers from among 197 oral and 1,769 poster abstracts presented at AASLD 2020.
They highlighted the most important findings from presentations on autoimmune and cholestatic disease, transplantation, cirrhosis and portal hypertension, alcoholic liver disease, neoplasia, drug-induced liver injury, and COVID-19. They did not review studies focused primarily on nonalcoholic steatohepatitis or nonalcoholic fatty liver disease, viral hepatitis, or basic science, all of which were covered in separate debriefing sessions.
Cirrhosis and portal hypertension
A study from the Department of Veterans Affairs looked at the prevalence of liver disease risk factors and rates of subsequent testing for and diagnosis of cirrhosis in the Veterans Health Administration system (VHA).
The authors found that, among more than 6.65 million VHA users in 2018 with no prior diagnosis of cirrhosis, approximately half were at risk for cirrhosis, of whom about 75% were screened, and approximately 5% of those who were screened were positive for possible cirrhosis (133,636). Of the patients who screened positive, about 10% (12,566) received a diagnosis of cirrhosis, including 4,120 with liver decompensation.
“This paper underscores the importance of population-level screening in uncovering unrecognized cirrhosis, enabling intervention earlier in the course of disease,” Dr. Taddei said (Abstract #661).
A study looking at external validation of novel cirrhosis surgical risk models designed to improve prognostication for a range of common surgeries showed that the VOCAL-Penn score was superior to the Mayo Risk Score, Model for End-stage Liver Disease and MELD-sodium scores for discrimination of 30-day and 90-day postoperative mortality (Abstract #91).
“While these models are not a substitute for clinical acumen, they certainly improve our surgical risk prediction in patients with cirrhosis, a very common question in consultative hepatology,” Dr. Taddei said.
She also cited three abstracts that address the important questions regarding performing studies in patients with varices or ascites, including whether it’s safe to perform transesophageal echocardiography in patients with cirrhosis without first screening for varices, and whether nonselective beta-blockers should be continued in patients with refractory ascites.
A retrospective study of 191 patients with cirrhosis who underwent upper endoscopy within 4 years of transesophageal echocardiography had no overt gastrointestinal bleeding regardless of the presence of esophageal varices, suggesting that routine preprocedure esophagogastroduodenoscopy “is of no utility,” (Abstract #1872).
A study to determine risk of sepsis in 1,198 patients with cirrhosis found that 1-year risk of sepsis was reduced by 50% with the use of nonselective beta-blockers (Abstract #94).
The final abstract in this category touched on the use of an advance care planning video support tool to help transplant-ineligible patients with end-stage liver disease decide whether they want support measures such cardiopulmonary resuscitation or intubation. The authors found that the video decision tool was feasible and acceptable to patients, and improved their knowledge of end-of-life care. More patients randomized to the video arm opted against CPR or intubation, compared with those assigned to a verbal discussion of options (Abstract #712).
Alcohol
The reviewers highlighted two studies of alcohol use: The first was designed to determine the prevalence of early alcohol relapse (resumption within 3 months) in patients who presented with alcoholic hepatitis. The subjects included 478 patients enrolled in the STOPAH trial, and a validation set of 194 patients from the InTeam (Integrated Approaches for identifying Molecular Targets in Alcoholic Hepatitis) Consortium.
“They found that high-risk patients were younger, unemployed, and without a stable relationship. Intermediate risk were middle aged, employed, and in a stable relationship, and low-risk profiles were older, with known cirrhosis; they were mostly retired and in a stable relationship,” Dr. Taddei said.
The identification of nongenetic factors that predict early relapse may aid in personalization of treatment strategies, she said (Abstract #232).
The second study looked at fecal microbial transplant (FMT) for reducing cravings in adults with alcohol use disorder (AUD) and cirrhosis. The investigators saw a nonsignificant trend toward greater total abstinence at 6 months in patients randomized to FMT versus placebo.
“Future trials should be performed to determine the impact of FMT on altering the gut-brain axis in patients with AUD,” she said (Abstract #7).
Transplantation
The prospective controlled QUICKTRANS study by French and Belgian researchers found that patients who underwent early liver transplantation for severe alcoholic hepatitis had numerically but not significantly higher rates of relapse than patients who were transplanted after at least 6 months of abstinence, although heavy drinking was more frequent in patients who underwent early transplant.
The 2-year survival rates for both patients who underwent early transplant and those who underwent transplant after 6 months of sobriety were “identical, and excellent.” In addition, the 2-year survival rate for patients with severe alcoholic hepatitis who underwent transplant was 82.8%, compared with 28.2% for patients who were deemed ineligible for transplant according to a selection algorithm (P < .001).
“Perhaps most important is that studies in this population can be conducted in a controlled fashion across centers with reproducible transplant eligibility algorithms,” Dr. Taddei commented (Abstract #6).
The place of honor – Abstract # 1 – was reserved for a study looking at the effects on liver transplant practice of a new “safety net” policy from the Organ Procurement and Transplantation Network and United Network for Organ Sharing stating that patients awaiting liver transplantation who develop kidney failure may be given priority on the kidney transplant waiting list.
The investigators found that the new policy significantly increased the number of adult primary liver transplant alone candidates who where on dialysis at the time of listing, and did not affect either waiting list mortality or posttransplant outcomes.
The authors also saw a significant increase in kidney transplant listing after liver transplant, especially for patients who were on hemodialysis at the time of list.
In the period after implementation of the policy, there was a significantly higher probability of kidney transplant, and significant reduction in waiting list mortality.
Autoimmune & cholestatic diseases
Investigators performed an analysis of the phase 3 randomized controlled ENHANCE trial of seladelpar in patients with primary biliary cholangitis. The trial was stopped because of an adverse event ultimately deemed to be unrelated to the drug, so the analysis looked at the composite responder rate at month 3.
“The key takeaway from this study is that at the 10-mg dosage of seladelpar, 78% met a composite endpoint, 27% of patients normalized their alkaline phosphatase, and 50% normalized their ALT. There was significant improvement in pruritus,” Dr. Taddei said.
The drug was generally safe and well tolerated. A 52-week phase 3 global registration study will begin enrolling patients in early 2021 (Abstract #LO11).
In a pediatric study, investigators looked at differences in primary sclerosing cholangitis (PSC) among various population, and found that “Black and Hispanic patients have dramatically worse clinical outcomes, compared to White and Asian patients. They are more likely to be diagnosed with PSC at an advanced stage with extensive fibrosis and portal hypertensive manifestations.”
The authors suggested that the differences may be explained in part by socioeconomic disparities leading to delay in diagnosis, to a more aggressive phenotype, or both (Abstract #66).
A meta-analysis of maternal and fetal outcomes in women with autoimmune hepatitis showed that the disease is associated with increased risk of gestational diabetes, premature births, and small-for-gestational age or low-birth-weight babies.
“Pregnant women should be monitored closely before, during and after pregnancy. It’s important to know that, in the prevalence data, flares were most prevalent postpartum at 41%. These finds will help us counsel our patients with autoimmune hepatitis who become pregnant,” Dr. Taddei said (Abstract #97).
Drug-induced liver injury
A study of clinical outcomes following immune checkpoint inhibitor rechallenge in melanoma patients with resolved higher grade 3 or higher checkpoint inhibitor–induced hepatitis showed that 4 of 31 patients (13%) developed recurrence of grade 2 or greater hepatitis, and 15 of 31 (48%) developed an immune-related adverse event after rechallenge.
There was no difference in time to death between patients who were rechallenged and those who were not, and immune-related liver toxicities requiring drug discontinuation after rechallenge were uncommon.
“High-grade immune checkpoint inhibitor hepatitis should be reconsidered as an absolute contraindication for immune checkpoint inhibitor rechallenge,” Dr. Taddei said (Abstract # 116).
Neoplasia
The investigators also highlighted an abstract describing significant urban-rural and racial ethnic differences in hepatocellular carcinoma rates. A fuller description of this study can be found here (Abstract #136).
COVID-19
Finally, the reviewer highlighted a study of the clinical course of COVID-19 in patients with chronic liver disease, and to determine factors associated with adverse outcomes in patients with chronic liver disease who acquire COVID-19.
The investigators found that patients with chronic liver disease and COVID-19 have a 14% morality rate, and that alcohol-related liver disease, decompensated cirrhosis, and hepatocellular carcinoma are all risk factors for increased mortality from COVID-19.
They recommended emphasizing telemedicine, prioritizing patients with chronic liver disease for vaccination, and including these patients in prospective studies and drug trials for COVID-19 therapies.
Dr. Taddei reported having no disclosures.
Studies that address fundamental questions in hepatology and have the potential to change or improve clinical practice were the focus of a clinical debrief session from the virtual annual meeting of the American Association for the Study of Liver Diseases.
“We chose papers that had the highest level of evidence, such as randomized controlled trials, controlled studies, and large data sets – and some small data sets too,” said Tamar Taddei, MD, associate professor of medicine in the section of digestive disease at Yale University, New Haven, Conn.
Dr. Taddei and colleagues Silvia Vilarinho, MD, PhD; Simona Jakab, MD; and Ariel Jaffe, MD, all also from Yale, selected the papers from among 197 oral and 1,769 poster abstracts presented at AASLD 2020.
They highlighted the most important findings from presentations on autoimmune and cholestatic disease, transplantation, cirrhosis and portal hypertension, alcoholic liver disease, neoplasia, drug-induced liver injury, and COVID-19. They did not review studies focused primarily on nonalcoholic steatohepatitis or nonalcoholic fatty liver disease, viral hepatitis, or basic science, all of which were covered in separate debriefing sessions.
Cirrhosis and portal hypertension
A study from the Department of Veterans Affairs looked at the prevalence of liver disease risk factors and rates of subsequent testing for and diagnosis of cirrhosis in the Veterans Health Administration system (VHA).
The authors found that, among more than 6.65 million VHA users in 2018 with no prior diagnosis of cirrhosis, approximately half were at risk for cirrhosis, of whom about 75% were screened, and approximately 5% of those who were screened were positive for possible cirrhosis (133,636). Of the patients who screened positive, about 10% (12,566) received a diagnosis of cirrhosis, including 4,120 with liver decompensation.
“This paper underscores the importance of population-level screening in uncovering unrecognized cirrhosis, enabling intervention earlier in the course of disease,” Dr. Taddei said (Abstract #661).
A study looking at external validation of novel cirrhosis surgical risk models designed to improve prognostication for a range of common surgeries showed that the VOCAL-Penn score was superior to the Mayo Risk Score, Model for End-stage Liver Disease and MELD-sodium scores for discrimination of 30-day and 90-day postoperative mortality (Abstract #91).
“While these models are not a substitute for clinical acumen, they certainly improve our surgical risk prediction in patients with cirrhosis, a very common question in consultative hepatology,” Dr. Taddei said.
She also cited three abstracts that address the important questions regarding performing studies in patients with varices or ascites, including whether it’s safe to perform transesophageal echocardiography in patients with cirrhosis without first screening for varices, and whether nonselective beta-blockers should be continued in patients with refractory ascites.
A retrospective study of 191 patients with cirrhosis who underwent upper endoscopy within 4 years of transesophageal echocardiography had no overt gastrointestinal bleeding regardless of the presence of esophageal varices, suggesting that routine preprocedure esophagogastroduodenoscopy “is of no utility,” (Abstract #1872).
A study to determine risk of sepsis in 1,198 patients with cirrhosis found that 1-year risk of sepsis was reduced by 50% with the use of nonselective beta-blockers (Abstract #94).
The final abstract in this category touched on the use of an advance care planning video support tool to help transplant-ineligible patients with end-stage liver disease decide whether they want support measures such cardiopulmonary resuscitation or intubation. The authors found that the video decision tool was feasible and acceptable to patients, and improved their knowledge of end-of-life care. More patients randomized to the video arm opted against CPR or intubation, compared with those assigned to a verbal discussion of options (Abstract #712).
Alcohol
The reviewers highlighted two studies of alcohol use: The first was designed to determine the prevalence of early alcohol relapse (resumption within 3 months) in patients who presented with alcoholic hepatitis. The subjects included 478 patients enrolled in the STOPAH trial, and a validation set of 194 patients from the InTeam (Integrated Approaches for identifying Molecular Targets in Alcoholic Hepatitis) Consortium.
“They found that high-risk patients were younger, unemployed, and without a stable relationship. Intermediate risk were middle aged, employed, and in a stable relationship, and low-risk profiles were older, with known cirrhosis; they were mostly retired and in a stable relationship,” Dr. Taddei said.
The identification of nongenetic factors that predict early relapse may aid in personalization of treatment strategies, she said (Abstract #232).
The second study looked at fecal microbial transplant (FMT) for reducing cravings in adults with alcohol use disorder (AUD) and cirrhosis. The investigators saw a nonsignificant trend toward greater total abstinence at 6 months in patients randomized to FMT versus placebo.
“Future trials should be performed to determine the impact of FMT on altering the gut-brain axis in patients with AUD,” she said (Abstract #7).
Transplantation
The prospective controlled QUICKTRANS study by French and Belgian researchers found that patients who underwent early liver transplantation for severe alcoholic hepatitis had numerically but not significantly higher rates of relapse than patients who were transplanted after at least 6 months of abstinence, although heavy drinking was more frequent in patients who underwent early transplant.
The 2-year survival rates for both patients who underwent early transplant and those who underwent transplant after 6 months of sobriety were “identical, and excellent.” In addition, the 2-year survival rate for patients with severe alcoholic hepatitis who underwent transplant was 82.8%, compared with 28.2% for patients who were deemed ineligible for transplant according to a selection algorithm (P < .001).
“Perhaps most important is that studies in this population can be conducted in a controlled fashion across centers with reproducible transplant eligibility algorithms,” Dr. Taddei commented (Abstract #6).
The place of honor – Abstract # 1 – was reserved for a study looking at the effects on liver transplant practice of a new “safety net” policy from the Organ Procurement and Transplantation Network and United Network for Organ Sharing stating that patients awaiting liver transplantation who develop kidney failure may be given priority on the kidney transplant waiting list.
The investigators found that the new policy significantly increased the number of adult primary liver transplant alone candidates who where on dialysis at the time of listing, and did not affect either waiting list mortality or posttransplant outcomes.
The authors also saw a significant increase in kidney transplant listing after liver transplant, especially for patients who were on hemodialysis at the time of list.
In the period after implementation of the policy, there was a significantly higher probability of kidney transplant, and significant reduction in waiting list mortality.
Autoimmune & cholestatic diseases
Investigators performed an analysis of the phase 3 randomized controlled ENHANCE trial of seladelpar in patients with primary biliary cholangitis. The trial was stopped because of an adverse event ultimately deemed to be unrelated to the drug, so the analysis looked at the composite responder rate at month 3.
“The key takeaway from this study is that at the 10-mg dosage of seladelpar, 78% met a composite endpoint, 27% of patients normalized their alkaline phosphatase, and 50% normalized their ALT. There was significant improvement in pruritus,” Dr. Taddei said.
The drug was generally safe and well tolerated. A 52-week phase 3 global registration study will begin enrolling patients in early 2021 (Abstract #LO11).
In a pediatric study, investigators looked at differences in primary sclerosing cholangitis (PSC) among various population, and found that “Black and Hispanic patients have dramatically worse clinical outcomes, compared to White and Asian patients. They are more likely to be diagnosed with PSC at an advanced stage with extensive fibrosis and portal hypertensive manifestations.”
The authors suggested that the differences may be explained in part by socioeconomic disparities leading to delay in diagnosis, to a more aggressive phenotype, or both (Abstract #66).
A meta-analysis of maternal and fetal outcomes in women with autoimmune hepatitis showed that the disease is associated with increased risk of gestational diabetes, premature births, and small-for-gestational age or low-birth-weight babies.
“Pregnant women should be monitored closely before, during and after pregnancy. It’s important to know that, in the prevalence data, flares were most prevalent postpartum at 41%. These finds will help us counsel our patients with autoimmune hepatitis who become pregnant,” Dr. Taddei said (Abstract #97).
Drug-induced liver injury
A study of clinical outcomes following immune checkpoint inhibitor rechallenge in melanoma patients with resolved higher grade 3 or higher checkpoint inhibitor–induced hepatitis showed that 4 of 31 patients (13%) developed recurrence of grade 2 or greater hepatitis, and 15 of 31 (48%) developed an immune-related adverse event after rechallenge.
There was no difference in time to death between patients who were rechallenged and those who were not, and immune-related liver toxicities requiring drug discontinuation after rechallenge were uncommon.
“High-grade immune checkpoint inhibitor hepatitis should be reconsidered as an absolute contraindication for immune checkpoint inhibitor rechallenge,” Dr. Taddei said (Abstract # 116).
Neoplasia
The investigators also highlighted an abstract describing significant urban-rural and racial ethnic differences in hepatocellular carcinoma rates. A fuller description of this study can be found here (Abstract #136).
COVID-19
Finally, the reviewer highlighted a study of the clinical course of COVID-19 in patients with chronic liver disease, and to determine factors associated with adverse outcomes in patients with chronic liver disease who acquire COVID-19.
The investigators found that patients with chronic liver disease and COVID-19 have a 14% morality rate, and that alcohol-related liver disease, decompensated cirrhosis, and hepatocellular carcinoma are all risk factors for increased mortality from COVID-19.
They recommended emphasizing telemedicine, prioritizing patients with chronic liver disease for vaccination, and including these patients in prospective studies and drug trials for COVID-19 therapies.
Dr. Taddei reported having no disclosures.
FROM THE LIVER MEETING DIGITAL EXPERIENCE
A closer look at migraine aura
Migraine aura sometimes accompanies or precedes migraine pain, but the phenomenon is difficult to treat and poorly understood. However, some evidence points to potential neurological mechanisms, and migraine aura is associated with cardiovascular disease risk.
Andrea Harriott, MD, PhD, said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England, which was conducted virtually. Dr. Harriott is assistant professor of neurology at Massachusetts General Hospital in Boston.
Somewhere between 20% and 40% of patients with migraine experience aura. It is most often visual, though it can also include sensory, aphasic, and motor symptoms. Visual aura usually begins as a flickering zigzag pattern in the central visual field that moves slowly toward the periphery and often leaves a scotoma. Typical duration is 15-30 minutes. Aura symptoms are more common in females.
Research in the 1940s conducted by the Brazilian researcher Aristides de Azevedo Pacheco Leão, PhD, then at Harvard Medical School, Boston, showed evidence of CSD in rabbits after electrical or mechanical stimulation. He observed a wave of vasodilation and increased blood flow over the cortex that spread over nearly the entire dorsolateral cortex within 3-6 minutes.
In the 1940s and 1950s, researchers sketched on paper the visual disturbance over 10 minutes, tracking the expanding spectrum across the visual field, from the center toward the periphery. The resulting scotoma advanced across the visual cortex at a rate very similar to that of the cortical spreading observed by Dr. Leão, “potentially linking this electrical event that was described with the aura event of migraine,” said Dr. Harriott. Those researchers hypothesized that the aura was produced by a strong excitation phase, followed by a wave of total inhibition.
More recent functional magnetic resonance imaging studies have also shown that CSD-like disturbances occur when patients experience migraine aura. In one study, researchers observed an initial increase and then a decrease in the blood oxygenation level dependent (BOLD) signal, which spread slowly across the visual cortex and correlated with the aura event. “This study was really important in confirming that a CSD-like phenomenon was likely the underlying perturbation that produced the visual aura of migraine,” said Dr. Harriott.
Despite the evidence that CSD causes migraine aura, its connection to migraine pain hasn’t been firmly established. But Dr. Harriott presented some evidence linking the two. Migraine aura is usually followed by pain, and aura precedes migraine attacks 78%-93% of the time. Cephalic allodynia occurs in migraine about 70% to 80% of the time, and migraine with aura is more often associated with severe cutaneous allodynia than is migraine without aura. Finally, migraine patients with comorbidities have more severe disability, and more frequent cutaneous allodynia and aura than does the general migraine population (40% vs. 29%).
All of that suggests that activation of trigeminal nociceptors is involved with migraine aura, according to Dr. Harriott. Preclinical studies have also suggested links between CSD and activation of trigeminal nociceptors, with both immunohistochemical and electrophysiological lines of evidence. “These data suggest that spreading depression actually activates trigeminal nociceptors that we know are involved in signal pain in the head and neck, and that we know are involved in cephalic allodynia as well,” Dr. Harriott said.
The evidence impressed Allan Purdy, MD, professor of medicine at Dalhousie University, Halifax, N.S., who was the discussant for the presentation. “It’s an excellent case that CSD is a remarkably good correlate for aura,” he said during the session.
Along with potential impacts on migraine pain, aura is also associated with cardiovascular risk. “This is really important to know about in our clinical population,” said Dr. Harriott.
Meta-analyses of case control and cohort studies have shown associations between migraine aura and vascular disorders such as ischemic stroke. One meta-analysis showed about a twofold increased risk associated with migraine compared with the nonmigraine population. This difference was driven by migraine with aura (relative risk [RR], 2.25; 95% confidence interval [CI], 1.53-3.33) rather than migraine without aura (RR, 1.24; 95% CI, 0.86-1.79). Migraine generally is associated with greater risk of myocardial infarction (adjusted hazard ratio, 1.33; 95% CI, 1.08-1.64), and that association may be stronger in the aura phenotype.
There doesn’t appear to be evidence that traditional risk factors for heart disease – such as hypertension, diabetes, or high cholesterol – play a role in the association between aura and heart disease. One possibility is that variables like platelet activation, hypercoagulable state, or genetic susceptibility could be responsible.
The risks associated with migraine aura should be noted, but with a caveat, according to Dr. Purdy. “Even though the relative risk is high, the absolute risk is still relatively low, and patients with migraine with aura, who smoke or are female and over 45, those are the cases where the worry comes in.”
Dr. Harriott and Dr. Purdy have nothing to disclose.
Migraine aura sometimes accompanies or precedes migraine pain, but the phenomenon is difficult to treat and poorly understood. However, some evidence points to potential neurological mechanisms, and migraine aura is associated with cardiovascular disease risk.
Andrea Harriott, MD, PhD, said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England, which was conducted virtually. Dr. Harriott is assistant professor of neurology at Massachusetts General Hospital in Boston.
Somewhere between 20% and 40% of patients with migraine experience aura. It is most often visual, though it can also include sensory, aphasic, and motor symptoms. Visual aura usually begins as a flickering zigzag pattern in the central visual field that moves slowly toward the periphery and often leaves a scotoma. Typical duration is 15-30 minutes. Aura symptoms are more common in females.
Research in the 1940s conducted by the Brazilian researcher Aristides de Azevedo Pacheco Leão, PhD, then at Harvard Medical School, Boston, showed evidence of CSD in rabbits after electrical or mechanical stimulation. He observed a wave of vasodilation and increased blood flow over the cortex that spread over nearly the entire dorsolateral cortex within 3-6 minutes.
In the 1940s and 1950s, researchers sketched on paper the visual disturbance over 10 minutes, tracking the expanding spectrum across the visual field, from the center toward the periphery. The resulting scotoma advanced across the visual cortex at a rate very similar to that of the cortical spreading observed by Dr. Leão, “potentially linking this electrical event that was described with the aura event of migraine,” said Dr. Harriott. Those researchers hypothesized that the aura was produced by a strong excitation phase, followed by a wave of total inhibition.
More recent functional magnetic resonance imaging studies have also shown that CSD-like disturbances occur when patients experience migraine aura. In one study, researchers observed an initial increase and then a decrease in the blood oxygenation level dependent (BOLD) signal, which spread slowly across the visual cortex and correlated with the aura event. “This study was really important in confirming that a CSD-like phenomenon was likely the underlying perturbation that produced the visual aura of migraine,” said Dr. Harriott.
Despite the evidence that CSD causes migraine aura, its connection to migraine pain hasn’t been firmly established. But Dr. Harriott presented some evidence linking the two. Migraine aura is usually followed by pain, and aura precedes migraine attacks 78%-93% of the time. Cephalic allodynia occurs in migraine about 70% to 80% of the time, and migraine with aura is more often associated with severe cutaneous allodynia than is migraine without aura. Finally, migraine patients with comorbidities have more severe disability, and more frequent cutaneous allodynia and aura than does the general migraine population (40% vs. 29%).
All of that suggests that activation of trigeminal nociceptors is involved with migraine aura, according to Dr. Harriott. Preclinical studies have also suggested links between CSD and activation of trigeminal nociceptors, with both immunohistochemical and electrophysiological lines of evidence. “These data suggest that spreading depression actually activates trigeminal nociceptors that we know are involved in signal pain in the head and neck, and that we know are involved in cephalic allodynia as well,” Dr. Harriott said.
The evidence impressed Allan Purdy, MD, professor of medicine at Dalhousie University, Halifax, N.S., who was the discussant for the presentation. “It’s an excellent case that CSD is a remarkably good correlate for aura,” he said during the session.
Along with potential impacts on migraine pain, aura is also associated with cardiovascular risk. “This is really important to know about in our clinical population,” said Dr. Harriott.
Meta-analyses of case control and cohort studies have shown associations between migraine aura and vascular disorders such as ischemic stroke. One meta-analysis showed about a twofold increased risk associated with migraine compared with the nonmigraine population. This difference was driven by migraine with aura (relative risk [RR], 2.25; 95% confidence interval [CI], 1.53-3.33) rather than migraine without aura (RR, 1.24; 95% CI, 0.86-1.79). Migraine generally is associated with greater risk of myocardial infarction (adjusted hazard ratio, 1.33; 95% CI, 1.08-1.64), and that association may be stronger in the aura phenotype.
There doesn’t appear to be evidence that traditional risk factors for heart disease – such as hypertension, diabetes, or high cholesterol – play a role in the association between aura and heart disease. One possibility is that variables like platelet activation, hypercoagulable state, or genetic susceptibility could be responsible.
The risks associated with migraine aura should be noted, but with a caveat, according to Dr. Purdy. “Even though the relative risk is high, the absolute risk is still relatively low, and patients with migraine with aura, who smoke or are female and over 45, those are the cases where the worry comes in.”
Dr. Harriott and Dr. Purdy have nothing to disclose.
Migraine aura sometimes accompanies or precedes migraine pain, but the phenomenon is difficult to treat and poorly understood. However, some evidence points to potential neurological mechanisms, and migraine aura is associated with cardiovascular disease risk.
Andrea Harriott, MD, PhD, said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England, which was conducted virtually. Dr. Harriott is assistant professor of neurology at Massachusetts General Hospital in Boston.
Somewhere between 20% and 40% of patients with migraine experience aura. It is most often visual, though it can also include sensory, aphasic, and motor symptoms. Visual aura usually begins as a flickering zigzag pattern in the central visual field that moves slowly toward the periphery and often leaves a scotoma. Typical duration is 15-30 minutes. Aura symptoms are more common in females.
Research in the 1940s conducted by the Brazilian researcher Aristides de Azevedo Pacheco Leão, PhD, then at Harvard Medical School, Boston, showed evidence of CSD in rabbits after electrical or mechanical stimulation. He observed a wave of vasodilation and increased blood flow over the cortex that spread over nearly the entire dorsolateral cortex within 3-6 minutes.
In the 1940s and 1950s, researchers sketched on paper the visual disturbance over 10 minutes, tracking the expanding spectrum across the visual field, from the center toward the periphery. The resulting scotoma advanced across the visual cortex at a rate very similar to that of the cortical spreading observed by Dr. Leão, “potentially linking this electrical event that was described with the aura event of migraine,” said Dr. Harriott. Those researchers hypothesized that the aura was produced by a strong excitation phase, followed by a wave of total inhibition.
More recent functional magnetic resonance imaging studies have also shown that CSD-like disturbances occur when patients experience migraine aura. In one study, researchers observed an initial increase and then a decrease in the blood oxygenation level dependent (BOLD) signal, which spread slowly across the visual cortex and correlated with the aura event. “This study was really important in confirming that a CSD-like phenomenon was likely the underlying perturbation that produced the visual aura of migraine,” said Dr. Harriott.
Despite the evidence that CSD causes migraine aura, its connection to migraine pain hasn’t been firmly established. But Dr. Harriott presented some evidence linking the two. Migraine aura is usually followed by pain, and aura precedes migraine attacks 78%-93% of the time. Cephalic allodynia occurs in migraine about 70% to 80% of the time, and migraine with aura is more often associated with severe cutaneous allodynia than is migraine without aura. Finally, migraine patients with comorbidities have more severe disability, and more frequent cutaneous allodynia and aura than does the general migraine population (40% vs. 29%).
All of that suggests that activation of trigeminal nociceptors is involved with migraine aura, according to Dr. Harriott. Preclinical studies have also suggested links between CSD and activation of trigeminal nociceptors, with both immunohistochemical and electrophysiological lines of evidence. “These data suggest that spreading depression actually activates trigeminal nociceptors that we know are involved in signal pain in the head and neck, and that we know are involved in cephalic allodynia as well,” Dr. Harriott said.
The evidence impressed Allan Purdy, MD, professor of medicine at Dalhousie University, Halifax, N.S., who was the discussant for the presentation. “It’s an excellent case that CSD is a remarkably good correlate for aura,” he said during the session.
Along with potential impacts on migraine pain, aura is also associated with cardiovascular risk. “This is really important to know about in our clinical population,” said Dr. Harriott.
Meta-analyses of case control and cohort studies have shown associations between migraine aura and vascular disorders such as ischemic stroke. One meta-analysis showed about a twofold increased risk associated with migraine compared with the nonmigraine population. This difference was driven by migraine with aura (relative risk [RR], 2.25; 95% confidence interval [CI], 1.53-3.33) rather than migraine without aura (RR, 1.24; 95% CI, 0.86-1.79). Migraine generally is associated with greater risk of myocardial infarction (adjusted hazard ratio, 1.33; 95% CI, 1.08-1.64), and that association may be stronger in the aura phenotype.
There doesn’t appear to be evidence that traditional risk factors for heart disease – such as hypertension, diabetes, or high cholesterol – play a role in the association between aura and heart disease. One possibility is that variables like platelet activation, hypercoagulable state, or genetic susceptibility could be responsible.
The risks associated with migraine aura should be noted, but with a caveat, according to Dr. Purdy. “Even though the relative risk is high, the absolute risk is still relatively low, and patients with migraine with aura, who smoke or are female and over 45, those are the cases where the worry comes in.”
Dr. Harriott and Dr. Purdy have nothing to disclose.
FROM HCNE STOWE 2020
FDA expands Xofluza indication to include postexposure flu prophylaxis
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the antiviral baloxavir marboxil (Xofluza) to include postexposure prophylaxis of uncomplicated influenza in people aged 12 years and older.
“This expanded indication for Xofluza will provide an important option to help prevent influenza just in time for a flu season that is anticipated to be unlike any other because it will coincide with the coronavirus pandemic,” Debra Birnkrant, MD, director, Division of Antiviral Products, FDA Center for Drug Evaluation and Research, said in a press release.
In addition, Xofluza, which was previously available only in tablet form, is also now available as granules for mixing in water, the FDA said.
The agency first approved baloxavir marboxil in 2018 for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for no more than 48 hours.
A year later, the FDA expanded the indication to include people at high risk of developing influenza-related complications, such as those with asthma, chronic lung disease, diabetes, heart disease, or morbid obesity, as well as adults aged 65 years or older.
The safety and efficacy of Xofluza for influenza postexposure prophylaxis is supported by a randomized, double-blind, controlled trial involving 607 people aged 12 years and older. After exposure to a person with influenza in their household, they received a single dose of Xofluza or placebo.
The primary endpoint was the proportion of individuals who became infected with influenza and presented with fever and at least one respiratory symptom from day 1 to day 10.
Of the 303 people who received Xofluza, 1% of individuals met these criteria, compared with 13% of those who received placebo.
The most common adverse effects of Xofluza include diarrhea, bronchitis, nausea, sinusitis, and headache.
Hypersensitivity, including anaphylaxis, can occur in patients taking Xofluza. The antiviral is contraindicated in people with a known hypersensitivity reaction to Xofluza.
Xofluza should not be coadministered with dairy products, calcium-fortified beverages, laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminium, or zinc.
Full prescribing information is available online.
This article first appeared on Medscape.com.
50.6 million tobacco users are not a homogeneous group
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
FROM MMWR
Metformin improves most outcomes for T2D during pregnancy
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Improving Primary Care Fall Risk Management: Adoption of Practice Changes After a Geriatric Mini-Fellowship
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.
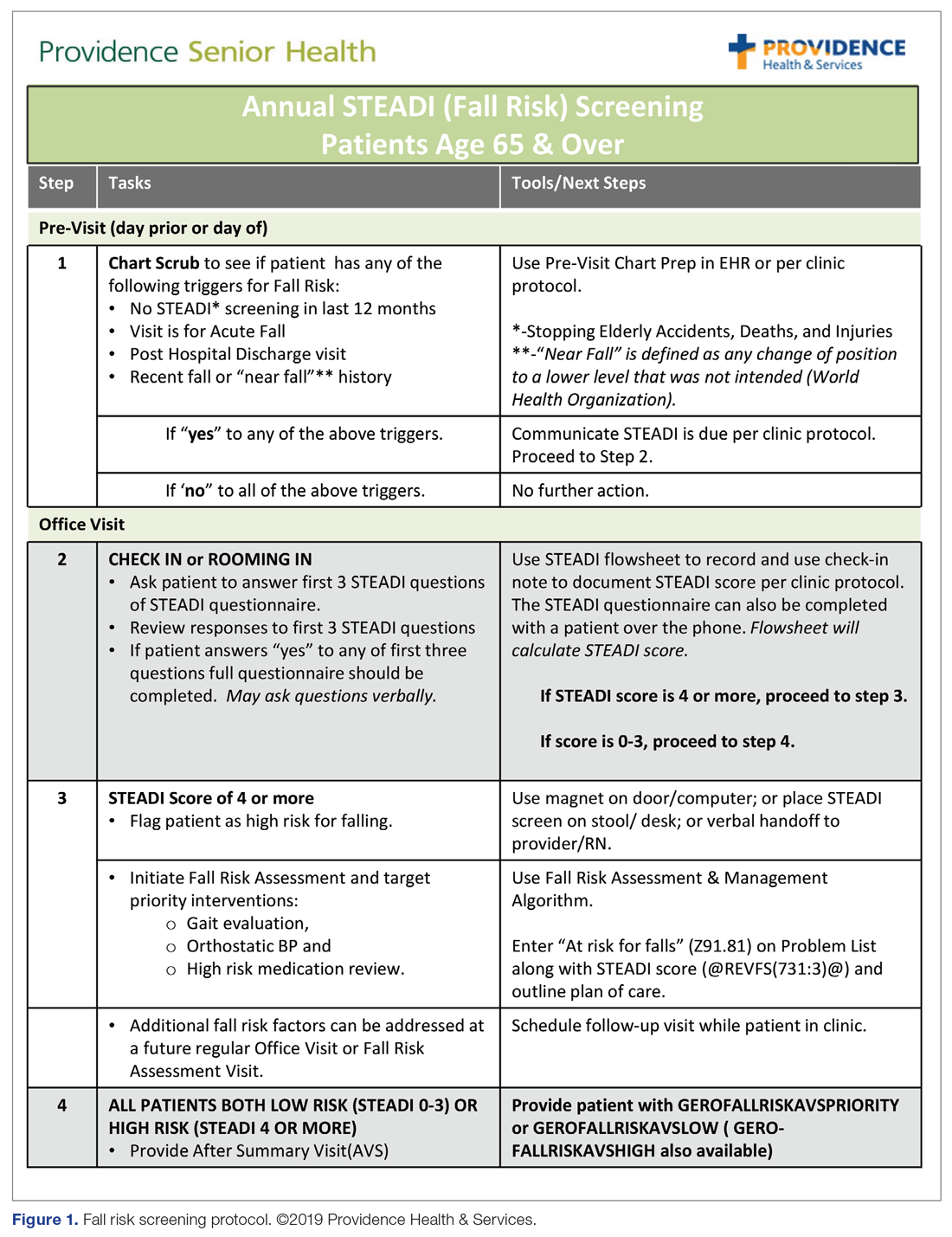
Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)
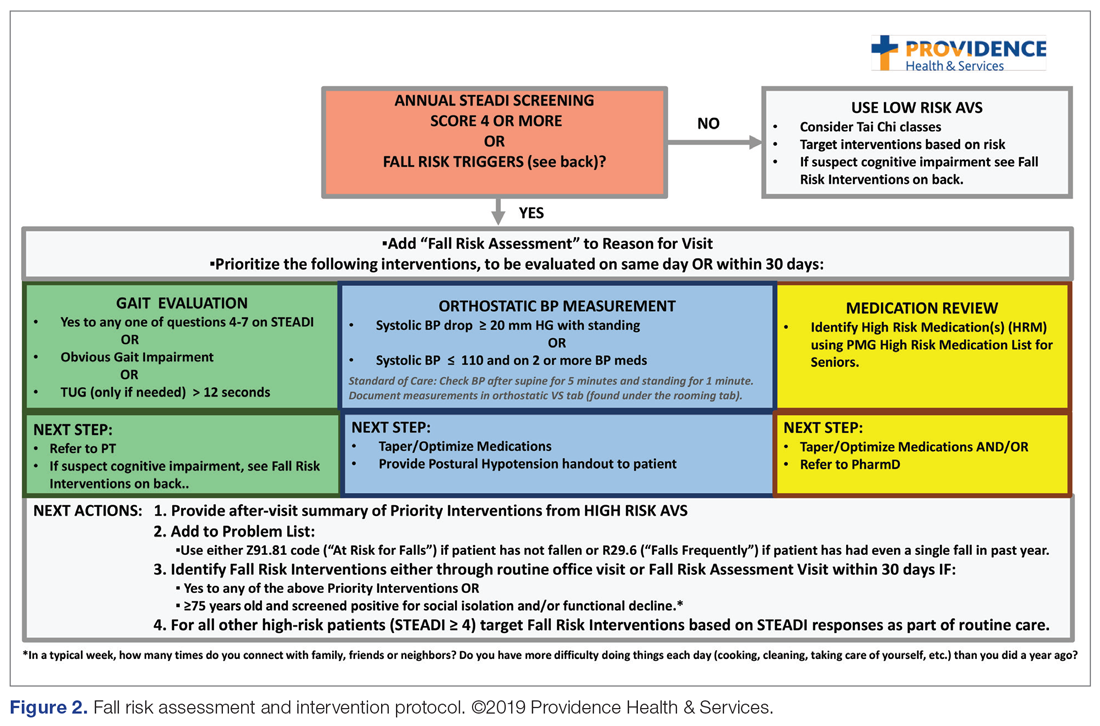
Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 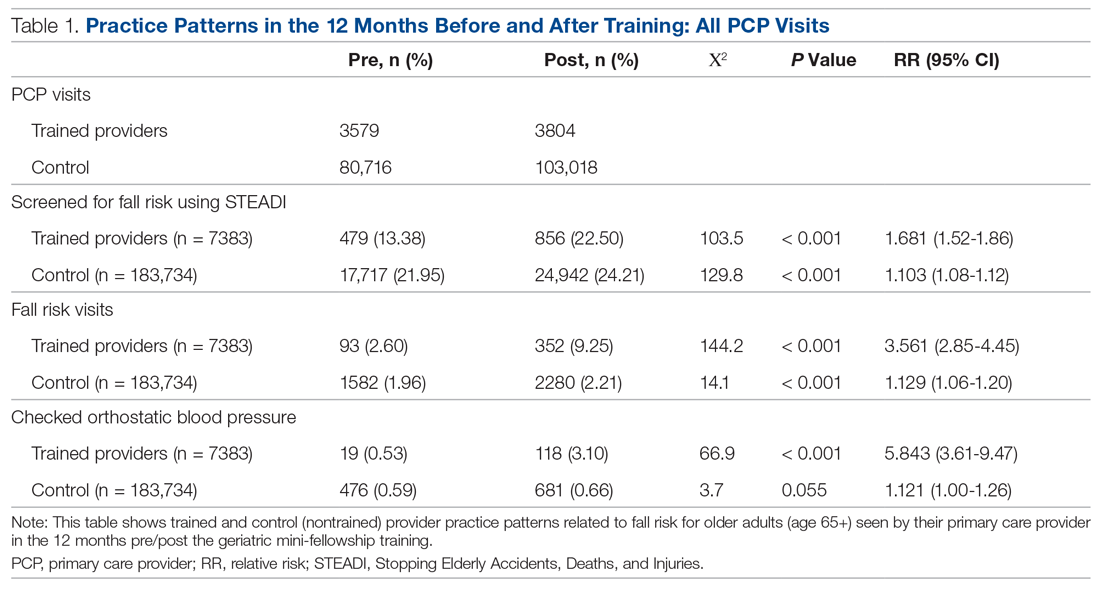
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.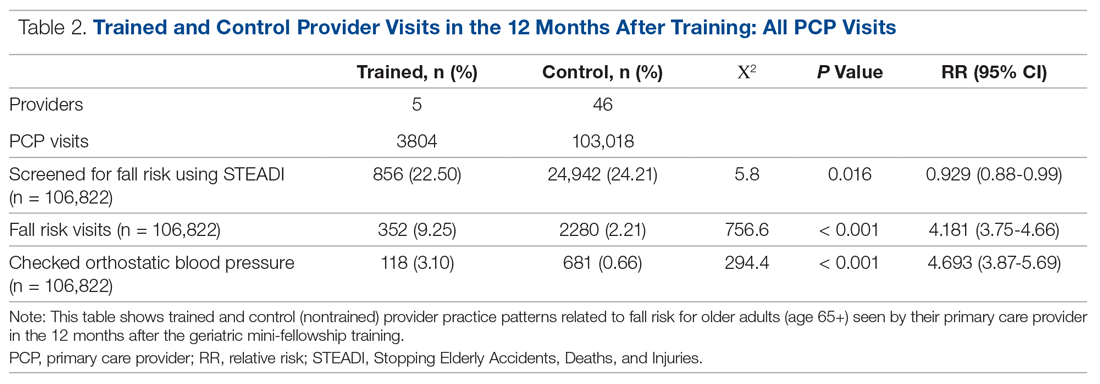
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 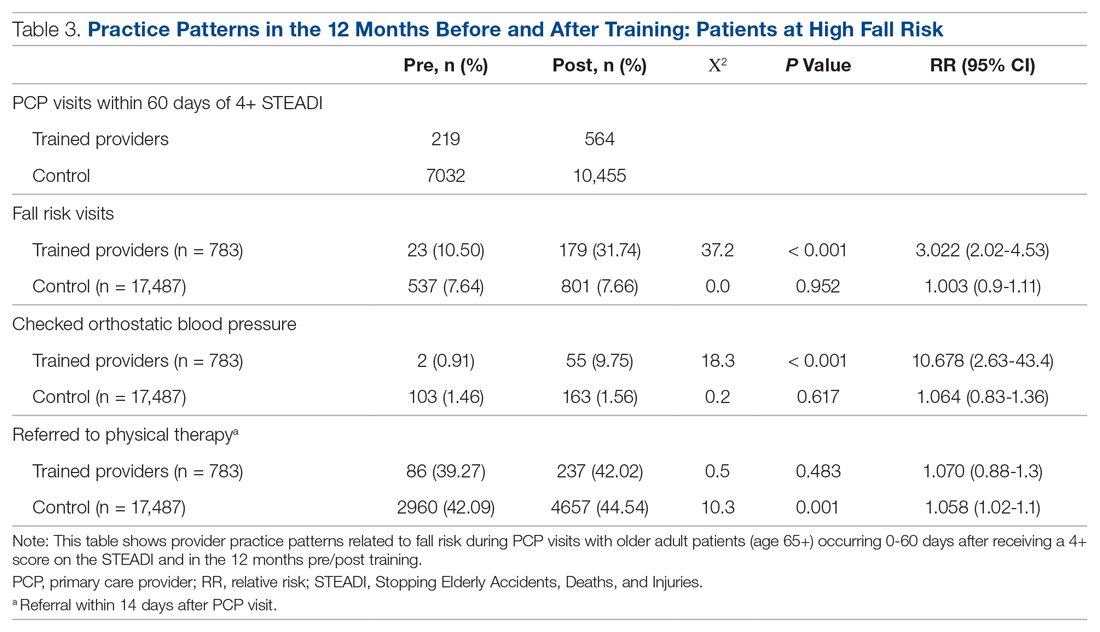
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.
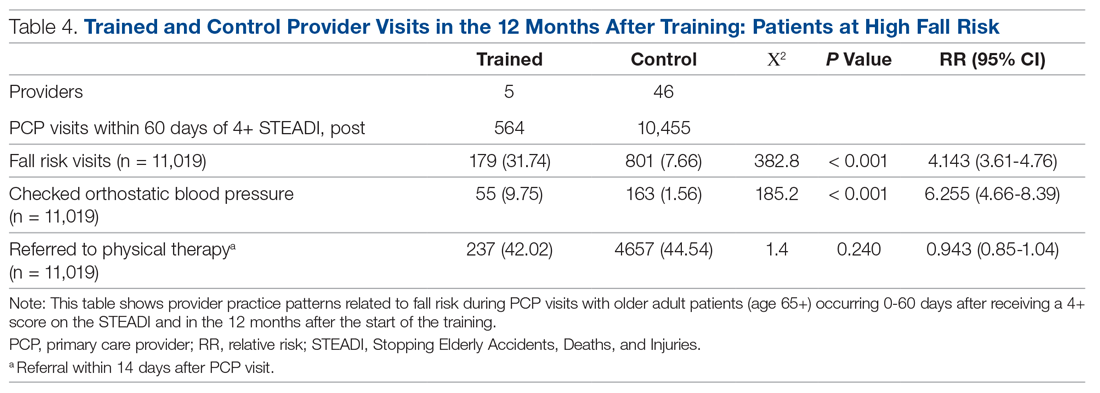
Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.

Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)

Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.

Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
From the Senior Health Program, Providence Health & Services, Oregon, Portland, OR.
Abstract
Background: Approximately 51 million adults in the United States are 65 years of age or older, yet few geriatric-trained primary care providers (PCP) serve this population. The Age-Friendly Health System framework, consisting of evidence-based 4M care (Mobility, Medication, Mentation, and what Matters), encourages all PCPs to assess mobility in older adults.
Objective: To improve PCP knowledge, confidence, and clinical practice in assessing and managing fall risk.
Methods: A 1-week educational session focusing on mobility (part of a 4-week Geriatric Mini-Fellowship) for 6 selected PCPs from a large health care system was conducted to increase knowledge and ability to address fall risk in older adults. The week included learning and practicing a Fall Risk Management Plan (FRMP) algorithm, including planning for their own practice changes. Pre- and post-test surveys assessed changes in knowledge and confidence. Patient data were compared 12 months before and after training to evaluate PCP adoption of FRMP components.
Results: The training increased provider knowledge and confidence. The trained PCPs were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to assess orthostatic blood pressure in their 65+ patients after the mini-fellowship. In high-risk patients, they were 4.1 times more likely to discuss fall risk and 6.3 times more likely to assess orthostatic blood pressure than their nontrained peers. Changes in physical therapy referral rates were not observed.
Conclusions: In-depth, skills-based geriatric educational sessions improved PCPs’ knowledge and confidence and also improved their fall risk management practices for their older patients.
Keywords: geriatrics; guidelines; Age-Friendly Health System; 4M; workforce training; practice change; fellowship.
The US population is aging rapidly. People aged 85 years and older are the largest-growing segment of the US population, and this segment is expected to increase by 123% by 2040.1 Caregiving needs increase with age as older adults develop more chronic conditions, such as hypertension, heart disease, arthritis, and dementia. However, even with increasing morbidity and dependence, a majority of older adults still live in the community rather than in institutional settings.2 These older adults seek medical care more frequently than younger people, with about 22% of patients 75 years and older having 10 or more health care visits in the previous 12 months. By 2040, nearly a quarter of the US population is expected to be 65 or older, with many of these older adults seeking regular primary care from providers who do not have formal training in the care of a population with multiple complex, chronic health conditions and increased caregiving needs.1
Despite this growing demand for health care professionals trained in the care of older adults, access to these types of clinicians is limited. In 2018, there were roughly 7000 certified geriatricians, with only 3600 of them practicing full-time.3,4 Similarly, of 290,000 certified nurse practitioners (NPs), about 9% of them have geriatric certification.5 Geriatricians, medical doctors trained in the care of older adults, and geriatric-trained NPs are part of a cadre of a geriatric-trained workforce that provides unique expertise in caring for older adults with chronic and advanced illness. They know how to manage multiple, complex geriatric syndromes like falls, dementia, and polypharmacy; understand and maximize team-based care; and focus on caring for an older person with a goal-centered versus a disease-centered approach.6
Broadly, geriatric care includes a spectrum of adults, from those who are aging healthfully to those who are the frailest. Research has suggested that approximately 30% of older adults need care by a geriatric-trained clinician, with the oldest and frailest patients needing more clinician time for assessment and treatment, care coordination, and coaching of caregivers.7 With this assumption in mind, it is projected that by 2025, there will be a national shortage of 26,980 geriatricians, with the western United States disproportionately affected by this shortage.4Rather than lamenting this shortage, Tinetti recommends a new path forward: “Our mission should not be to train enough geriatricians to provide direct care, but rather to ensure that every clinician caring for older adults is competent in geriatric principles and practices.”8 Sometimes called ”geriatricizing,” the idea is to use existing geriatric providers as a small elite training force to infuse geriatric principles and skills across their colleagues in primary care and other disciplines.8,9 Efforts of the American Geriatrics Society (AGS), with support from the John A. Hartford Foundation (JAHF), have been successful in developing geriatric training across multiple specialties, including surgery, orthopedics, and emergency medicine (www.americangeriatrics.org/programs/geriatrics-specialists-initiative).
The Age-Friendly Health System and 4M Model
To help augment this idea of equipping health care systems and their clinicians with more readily available geriatric knowledge, skills, and tools, the JAHF, along with the Institute for Healthcare Improvement (IHI), created the Age-Friendly Health System (AFHS) paradigm in 2015.10 Using the 4M model, the AFHS initiative established a set of evidence-based geriatric priorities and interventions meant to improve the care of older adults, reduce harm and duplication, and provide a framework for engaging leadership, clinical teams, and operational systems across inpatient and ambulatory settings.11 Mobility, including fall risk screening and intervention, is 1 of the 4M foundational elements of the Age-Friendly model. In addition to Mobility, the 4M model also includes 3 other key geriatric domains: Mentation (dementia, depression, and delirium), Medication (high-risk medications, polypharmacy, and deprescribing), and What Matters (goals of care conversations and understanding quality of life for older patients).11 The 4M initiative encourages adoption of a geriatric lens that looks across chronic conditions and accounts for the interplay among geriatric syndromes, such as falls, cognitive impairment, and frailty, in order to provide care better tailored to what the patient needs and desires.12 IHI and JAHF have targeted the adoption of the 4M model by 20% of US health care systems by 2020.11
Mini-Fellowship and Mobility Week
To bolster geriatric skills among community-based primary care providers (PCPs), we initiated a Geriatric Mini-Fellowship, a 4-week condensed curriculum taught over 6 months. Each week focuses on 1 of the age-friendly 4Ms, with the goal of increasing the knowledge, self-efficacy, skills, and competencies of the participating PCPs (called “fellow” hereafter) and at the same time, equipping each to become a champion of geriatric practice. This article focuses on the Mobility week, the second week of the mini-fellowship, and the effect of the week on the fellows’ practice changes.
To construct the Mobility week’s curriculum with a focus on the ambulatory setting, we relied upon national evidence-based work in fall risk management. The Centers for Disease Control and Prevention (CDC) has made fall risk screening and management in primary care a high priority. Using the clinical practice guidelines for managing fall risk developed by the American and British Geriatrics Societies (AGS/BGS), the CDC developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) toolkit.13 Foundational to the toolkit is the validated 12-item Stay Independent falls screening questionnaire (STEADI questionnaire).14 Patients who score 4 or higher (out of a total score of 14) on the questionnaire are considered at increased risk of falling. The CDC has developed a clinical algorithm that guides clinical teams through screening and assessment to help identify appropriate interventions to target specific risk factors. Research has clearly established that a multifactorial approach to fall risk intervention can be successful in reducing fall risk by as much as 25%.15-17
The significant morbidity and mortality caused by falls make training nongeriatrician clinicians on how to better address fall risk imperative. More than 25% of older adults fall each year.18 These falls contribute to rising rates of fall-related deaths,19 emergency department (ED) visits,20 and hospital readmissions.21 Initiatives like the AFHS focus on mobility and the CDC’s development of supporting clinical materials22 aim to improve primary care adoption of fall risk screening and intervention practices.23,24 The epidemic of falls must compel all PCPs, not just those practicing geriatrics, to make discussing and addressing fall risk and falls a priority.
Methods
Setting
This project took place as part of a regional primary care effort in Oregon. Providence Health & Services-Oregon is part of a multi-state integrated health care system in the western United States whose PCPs serve more than 80,000 patients aged 65 years and older per year; these patients comprise 38% of the system’s office visits each year. Regionally, there are 47 family and internal medicine clinics employing roughly 290 providers (physicians, NPs, and physician assistants). The organization has only 4 PCPs trained in geriatrics and does not offer any geriatric clinical consultation services. Six PCPs from different clinics, representing both rural and urban settings, are chosen to participate in the geriatric mini-fellowship each year.
This project was conducted as a quality improvement initiative within the organization and did not constitute human subjects research. It was not conducted under the oversight of the Institutional Review Board.
Intervention
The mini-fellowship was taught in 4 1-week blocks between April and October 2018, with a curriculum designed to be interactive and practical. The faculty was intentionally interdisciplinary to teach and model team-based practice. Each week participants were excused from their clinical practice. Approximately 160 hours of continuing medical education credits were awarded for the full mini-fellowship. As part of each weekly session, a performance improvement project (PIP) focused on that week’s topic (1 of the 4Ms) was developed by the fellow and their team members to incorporate the mini-fellowship learnings into their clinic workflows. Fellows also had 2 hours per week of dedicated administration time for a year, outside the fellowship, to work on their PIP and 4M practice changes within their clinic.
Provider Education
The week for mobility training comprised 4 daylong sessions. The first 2 days were spent learning about the epidemiology of falls; risk factors for falling; how to conduct a thorough history and assessment of fall risk; and how to create a prioritized Fall Risk Management Plan (FRMP) to decrease a patient’s individual fall risk through tailored interventions. The FRMP was adapted from the CDC STEADI toolkit.13 Core faculty were 2 geriatric-trained providers (NP and physician) and a physical therapist (PT) specializing in fall prevention.
On the third day, fellows took part in a simulated fall risk clinic, in which older adults volunteered to be patient partners, providing an opportunity to apply learnings from days 1 and 2. The clinic included the fellow observing a PT complete a mobility assessment and a pharmacist conduct a high-risk medication review. The fellow synthesized the findings of the mobility assessment and medication review, as well as their own history and assessment, to create a summary of fall risk recommendations to discuss with their volunteer patient partner. The fellows were observed and evaluated in their skills by their patient partner, course faculty, and another fellow. The patient partners, and their assigned fellow, also participated in a 45-minute fall risk presentation, led by a nurse.
On the fourth day, the fellows were joined by select clinic partners, including nurses, pharmacists, and/or medical assistants. The session included discussions among each fellow’s clinical team regarding the current state of fall risk efforts at their clinic, an analysis of barriers, and identification of opportunities to improve workflows and screening rates. Each fellow took with them an action plan tailored to their clinic to improve fall risk management practices, starting with the fellow’s own practice.

Fall Risk Management Plan
The educational sessions introduced the fellows to the FRMP. The FRMP, adapted from the STEADI toolkit, includes a process for fall risk screening (Figure 1) and stratifying a patient’s risk based on their STEADI score in order to promote 3 priority assessments (gait evaluation with PT referral if appropriate; orthostatic blood pressure; and high-risk medication review; Figure 2). Initial actions based on these priority assessments were followed over time, with additional fall risk interventions added as clinically indicated.25 The FRMP is intended to be used during routine office visits, Medicare annual wellness visits, or office visits focused on fall risk or related medical disorders (ie, fall risk visits.)

Providers and their teams were encouraged to spread out fall-related conversations with their patients over multiple visits, since many patients have multiple fall risk factors at play, in addition to other chronic medical issues, and since many interventions often require behavior changes on the part of the patient. Providers also had access to fall-related electronic health record (EHR) templates as well as a comprehensive, internal fall risk management website that included assessment tools, evidence-based resources, and patient handouts.
Assessment and Measurements
We assessed provider knowledge and comfort in their fall risk evaluation and management skills before and after the educational intervention using an 11-item multiple-choice questionnaire and a 4-item confidence questionnaire. The confidence questions used a 7-point Likert scale, with 0 indicating “no confidence” and 7 indicating ”lots of confidence.” The questions were administered via a paper survey. Qualitative comments were derived from evaluations completed at the end of the week.
The fellows’ practice of fall risk screening and management was studied from May 2018, at the completion of Mobility week, to May 2019 for the post-intervention period. A 1-year timeframe before May 2018 was used as the pre-intervention period. Eligible visit types, during which we assumed fall risk was discussed, were any office visits for patients 65+ completed by the patients’ PCPs that used fall risk as a reason for the visit or had a fall-related diagnosis code. Fall risk visits performed by other clinic providers were not counted.
Of those patients who had fall risk screenings completed and were determined to be high risk (STEADI score ≥ 4), data were analyzed to determine whether these patients had any fall-related follow-up visits to their PCP within 60 days of the STEADI screening. For these high-risk patients, data were studied to understand whether orthostatic blood pressure measurements were performed (as documented in a flowsheet) and whether a PT referral was placed. These data were compared with those from providers who practiced in clinics within the same system but who did not participate in the mini-fellowship. Data were obtained from the organization’s EHR. Additional data were measured to evaluate patterns of deprescribing of select high-risk medications, but these data are not included in this analysis.
Analysis
A paired-samples t test was used to measure changes in provider confidence levels. Data were aggregated across fellows, resulting in a mean. A chi-square test of independence was performed to examine the relationship between rates of FRMP adoption by select provider groups. Analysis included a pre- and post-intervention assessment of the fellows’ adoption of FRMP practices, as well as a comparison between the fellows’ practice patterns and those of a control group of PCPs in the organization’s other clinics who did not participate in the mini-fellowship (nontrained control group). Excluded from the control group were providers from the same clinic as the fellows; providers in clinics with a geriatric-trained provider on staff; and clinics outside of the Portland metro and Medford service areas. We used an alpha level of 0.05 for all statistical tests.
Data from 5 providers were included in the analysis of the FRMP adoption. The sixth provider changed practice settings from the clinic to the ED after completing the fellowship; her patient data were not included in the FRMP part of the analysis. EHR data included data on all visits of patients 65+, as well as data for just those 65+ patients who had been identified as being at high risk to fall based on a STEADI score of 4 or higher.
Results
Provider Questionnaire
All 6 providers responded to the pre-intervention and post-intervention tests. For the knowledge questions, fellows, as a composite, correctly answered 57% of the questions before the intervention and 79% after the intervention. Provider confidence level in delivering fall risk care was measured prior to the training (mean, 4.12 [SD, 0.62]) and at the end of the training (mean, 6.47 [SD, 0.45]), demonstrating a significant increase in confidence (t (5) = –10.46, P < 0.001).
Qualitative Comments
Providers also had the opportunity to provide comments on their experience during the Mobility week and at the end of 1 year. In general, the simulated interdisciplinary fall risk clinic was highly rated (“the highlight of the week”) as a practical strategy to embed learning principles. One fellow commented, “Putting the learning into practice helps solidify it in my brain.” Fellows also appreciated the opportunity to learn and meet with their clinic colleagues to begin work on a fall-risk focused PIP and to “have a framework for what to do for people who screen positive [for fall risk].”
FRMP Adoption
A comparison of the care the fellows provided to their patients 65+ in the 12 months pre- and post-training shows the fellows demonstrated significant changes in practice patterns. The fellows were 1.7 times more likely to screen for fall risk; 3.6 times more likely to discuss fall risk; and 5.8 times more likely to check orthostatic blood pressure than prior to the mini-fellowship (Table 1). 
The control providers also demonstrated significant increases in fall risk screening and discussion of fall risk between the pre- and post-intervention periods; however, the relative risk (RR) was between 1.10 and 1.13 for this group. For the control group, checking orthostatic blood pressure did not significantly change. In the 12 months after training (Table 2), the fellows were 4.2 times more likely to discuss fall risk and almost 5 times more likely to check orthostatic blood pressure than their nontrained peers for all of their patients 65+, regardless of their risk to fall.
As shown in Table 3, for those patients determined to be at high risk of falling (STEADI score ≥ 4), fellows showed statistically significant increases in fall risk visits (RR, 3.02) and assessment of orthostatic blood pressure (RR, 10.68) before and after the mini-fellowship. The control providers did not show any changes in practice patterns between the pre- and post-period among patients at high risk to fall. 
Neither the fellows nor the control group showed changes in patterns of referral to PT. In comparing the 2 groups in the 12 months after training (Table 4), for their patients at risk of falling, the fellows were 4 times more likely to complete fall risk visits and over 6 times more likely to assess orthostatic blood pressure than their nontrained peers. Subgroup analysis of the 75+ population revealed similar trends and significance, but these results are not included here.

Discussion
This study aimed to improve not only providers’ knowledge and confidence in caring for older adults at increased risk to fall, but also their clinical practice in assessing and managing fall risk. In addition to improved knowledge and confidence, we found that the fellows increased their discussion of fall risk (through fall risk visits) and their assessment of orthostatic blood pressure for all of their patients, not just for those identified at increased risk to fall. This improvement held true for the fellows themselves before and after the intervention, but also as compared to their nontrained peers. These practice improvements for all of their 65+ patients, not just those identified as being at high risk to fall, are especially important, since studies indicate that early screening and intervention can help identify people at risk and prevent future falls.15
We were surprised that there were no significant differences in PT referrals made by the trained fellows, but this finding may have been confounded by the fact that the data included all PT referrals, regardless of diagnosis, not just those referrals that were fall-related. Furthermore, our baseline PT referral rates, at 39% for the intervention group and 42% for the control group, are higher than national data when looking at rehabilitation use by older adults.26
In comparison to a study evaluating the occurrence of fall risk–related clinical practice in primary care before any fall-related educational intervention, orthostatics were checked less frequently in our study (10% versus 30%) and there were fewer PT referrals (42%–44% versus 53%).27 However, the Phelan study took place in patients who had actually had a fall, rather than just having a higher risk for a fall, and was based on detailed chart review. Other studies23,24 found higher rates of fall risk interventions, but did not break out PT referrals specifically.
In terms of the educational intervention itself, most studies of geriatric education interventions have measured changes in knowledge, confidence, or self-efficacy as they relate to geriatric competence,28-30 and do not measure practice change as an outcome outside of intent to change or self-reported practice change.31,32 In general, practice change or longer-term health care–related outcomes have not been studied. Additionally, a range of dosages of educational interventions has been studied, from 1-hour lunchtime presentations23,32 to half-day29 or several half-day workshops,28 up to 160 hours over 10 months30 or 5 weekends over 6 months.31 The duration of our entire intervention at 160 hours over 6 months would be considered on the upper end of dosing relative to these studies, with our Mobility week intervention comprising 32 hours during 1 week. In the Warshaw study, despite 107 1-hour sessions being taught to over 60 physicians in 16 practices over 4 years, only 2 practices ultimately initiated any practice change projects.32 We believe that only curricula that embed practice change skills and opportunities, at a significant enough dose, can actually impact practice change in a sustainable manner.
Knowledge and skill acquisition among individual providers does not take place to a sufficient degree in the current health care arena, which is focused on productivity and short visit times. Consistent with other studies, we included interdisciplinary members of the primary care team for part of the mini-fellowship, although other studies used models that train across disciplines for the entirety of the learning experience.28-30,33 Our educational model was strengthened by including other professionals to provide some of the education and model the ideal geriatric team, including PT, occupational therapy, and pharmacy, for the week on mobility.
Most studies exploring interventions through geriatric educational initiatives are conducted within academic institutions, with a primary focus on physician faculty and, by extension, their teaching of residents and others.34,35 We believe our integrated model, which is steeped in community-based primary care practices like Lam’s,31 offers the greatest outreach to large community-based care systems and their patients. Training providers to work with their teams to change their own practices first gives skills and expertise that help further establish them as geriatric champions within their practices, laying the groundwork for more widespread practice change at their clinics.
Limitations
In addition to the limitations described above relating to the capture of PT referrals, other limitations included the relatively short time period for follow-up data as well as the small size of the intervention group. However, we found value in the instructional depth that the small group size allowed.
While the nontrained providers did show some improvement during the same period, we believe the relative risk was not clinically significant. We suspect that the larger health system efforts to standardize screening of patients 65+ across all clinics as a core quality metric confounded these results. The data analysis also included only fall-related patient visits that occurred with a provider who was that patient’s PCP, which could have missed visits done by other PCP colleagues, RNs, or pharmacists in the same clinic, thus undercounting the true number of fall-related visits. Furthermore, counting of fall-related interventions relied upon providers documenting consistently in the EHR, which could also lead to under-represention of fall risk clinical efforts.
The data presented, while encouraging, do not reflect clinic-wide practice change patterns and are considered only proximate outcomes rather than more long-term or cost-related outcomes, as would be captured by fall-related utilization measures like emergency room visits and hospitalizations. We expect to evaluate the broader impact and these value-based outcomes in the future. All providers and teams were from the same health care system, which may not allow our results to transfer to other organizations or regions of clinical practice.
Summary
This study demonstrates that an intensive mini-fellowship model of geriatrics training improved both knowledge and confidence in the realm of fall risk assessment and intervention among PCPs who had not been formally trained in geriatrics. More importantly, the training improved the fall-related care of their patients at increased risk to fall, but also of all of their older patients, with improvements in care measured up to a year after the mini-fellowship. Although this article only describes the work done as part of the Mobility aim of the 4M AFHS model, we believe the entire mini-fellowship curriculum offers the opportunity to “geriatricize” clinicians and their teams in learning geriatric principles and skills that they can translate into their practice in a sustainable way, as Tinetti encourages.8 Future study to evaluate other process outcomes more precisely, such as PT, as well as cost- and value-based outcomes, and the influence of trained providers on their clinic partners, will further establish the value proposition of targeted, disseminated, intensive geriatrics training of primary care clinicians as a strategy of age-friendly health systems as they work to improve the care of their older adults.
Acknowledgment: We are grateful for the dedication and hard work of the 2018 Geriatric Mini-Fellowship fellows at Providence Health & Services-Oregon who made this article possible. Thanks to Drs. Stephanie Cha, Emily Puukka-Clark, Laurie Dutkiewicz, Cara Ellis, Deb Frost, Jordan Roth, and Subhechchha Shah for promoting the AFHS work within their Providence Medical Group clinics and to PMG leadership and the fellows’ clinical teams for supporting the fellows, the AFHS work, and their older patients.
Corresponding author: Colleen M. Casey, PhD, ANP-BC, Providence Health & Services, Senior Health Program, 4400 NE Halsey, 5th Floor, Portland, OR 97213; [email protected].
Financial disclosures: None.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
1. US Department of Health and Human Services. 2018 Profile of Older Americans. Administration on Aging. April 2018.
2. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. Washington, DC: US Census Bureau; 2018.
3. American Board of Medicine Specialties. 2017-2018 ABMS Board Certification Report. https://www.abms.org/board-certification/abms-board-certification-report/. Accessed November 3, 2020.
4. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and regional projections of supply and demand for geriatricians: 2013-2025. Rockville, MD: US Department of Health and Human Services; 2007.
5. American Association of Nurse Practitioners, NP Facts: The Voice of the Nurse Practitioner. 2020. https://storage.aanp.org/www/documents/NPFacts__080420.pdf.
6. Tinetti ME, Naik AD, Dodson JA, Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9-10.
7. Fried LP, Hall WJ. Editorial: leading on behalf of an aging society. J Am Geriatr Soc. 2008;56:1791-1795.
8. Tinetti M. Mainstream or extinction: can defining who we are save geriatrics? J Am Geriatr Soc. 2016;64:1400-1404.
9. Jafari P, Kostas T, Levine S, et al. ECHO-Chicago Geriatrics: using telementoring to “geriatricize” the primary care workforce. Gerontol Geriatr Educ. 2020;41:333-341.
10. Fulmer T, Mate KS, Berman A. The Age-Friendly Health System imperative. J Am Geriatr Soc. 2018;66:22-24.
11. Mate KS, Berman A, Laderman M, et al. Creating Age-Friendly Health Systems - A vision for better care of older adults. Healthc (Amst). 2018;6:4-6.
12. Tinetti ME, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261-275.
13. Stevens JA, Phelan EA. Development of STEADI: a fall prevention resource for health care providers. Health Promot Pract. 2013;14:706-714.
14. Rubenstein LZ, et al. Validating an evidence-based, self-rated fall risk questionnaire (FRQ) for older adults. J Safety Res. 2011;42:493-499.
15. Grossman DC, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319: 1696-1704.
16. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687-1699.
17. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012(9):CD007146.
18. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
19. Burns E, Kakara R. Deaths from falls among persons aged >=65 Years - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2018;67:509-514.
20. Shankar KN, Liu SW, Ganz DA. Trends and characteristics of emergency department visits for fall-related injuries in older adults, 2003-2010. West J Emerg Med. 2017;18:785-793.
21. Hoffman GJ, et al. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276.
22. Eckstrom E, Parker EM, Shakya I, Lee R. Coordinated care plan to prevent older adult falls. 2018. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2018.
23. Eckstrom E, Parker EM, Lambert GH, et al. Implementing STEADI in academic primary care to address older adult fall risk. Innov Aging. 2017;1:igx028.
24. Johnston YA, Bergen G, Bauer M, et al. Implementation of the stopping elderly accidents, deaths, and injuries initiative in primary care: an outcome evaluation. Gerontologist. 2019;59:1182-1191.
25. Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99:281-293.
26. Gell NM, Mroz TM, Patel KV. Rehabilitation services use and patient-reported outcomes among older adults in the United States. Arch Phys Med Rehabil. 2017;98:2221-2227.e3.
27. Phelan EA, Aerts S, Dowler D, et al. Adoption of evidence-based fall prevention practices in primary care for older adults with a history of falls. Front Public Health. 2016;4:190.
28. Solberg LB, Carter CS, Solberg LM. Geriatric care boot camp series: interprofessional education for a new training paradigm. Geriatr Nurs. 2019;40:579-583.
29. Solberg LB, Solberg LM, Carter CS. Geriatric care boot cAMP: an interprofessional education program for healthcare professionals. J Am Geriatr Soc. 2015;63:997-1001.
30. Coogle CL, Hackett L, Owens MG, et al. Perceived self-efficacy gains following an interprofessional faculty development programme in geriatrics education. J Interprof Care. 2016;30:483-492.
31. Lam R, Lee L, Tazkarji B, et al. Five-weekend care of the elderly certificate course: continuing professional development activity for family physicians. Can Fam Physician. 2015;61:e135-141.
32. Warshaw GA, Modawal A, Kues J, et al. Community physician education in geriatrics: applying the assessing care of vulnerable elders model with a multisite primary care group. J Am Geriatr Soc. 2010;58:1780-1785.
33. Solai LK, Kumar K, Mulvaney E, et al. Geriatric mental healthcare training: a mini-fellowship approach to interprofessional assessment and management of geriatric mental health issues. Am J Geriatr Psychiatry. 2019;27:706-711.
34. Christmas C, Park E, Schmaltz H, et al. A model intensive course in geriatric teaching for non-geriatrician educators. J Gen Intern Med. 2008;23:1048-1052.
35. Heflin MT, Bragg EJ, Fernandez H, et al. The Donald W. Reynolds Consortium for Faculty Development to Advance Geriatrics Education (FD~AGE): a model for dissemination of subspecialty educational expertise. Acad Med. 2012;87:618-626.
Blood pressure treatment reduces bleeding in ICH
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a systematic review and meta-analysis shows, although it does reduce hematoma growth in these patients.
Despite the negative finding, the investigators observed broad variation in treatment effect among the studies they reviewed. They also found that target-based blood pressure treatment tended to improve function more than fixed-dose treatment.
“These data provide a strong message that early blood pressure–lowering treatment can control bleeding. This was not clear beforehand,” Craig Anderson, PhD, professor of neurology and epidemiology at the University of New South Wales, Sydney, said in an interview.
“But these data also indicate that the management of blood pressure in ICH is complex,” he added. Timing, type of drug, and type of patient must be considered, he said. “We need more data to allow better individualizing of such therapy.”
The results were presented at the European Stroke Organisation–World Stroke Organisation (ESO-WSO) Conference 2020.
Controversy about the efficacy of blood pressure reduction for patients with ICH continues, despite studies that have examined this question. In this analysis, Dr. Anderson and colleagues sought to examine the evidence from randomized controlled trials in this area and identify potentially overlooked heterogeneity among trials.
The investigators conducted a systematic review and meta-analysis of studies in the Cochrane Central Register of Controlled Trials, EMBASE, and MEDLINE databases. They searched for randomized controlled trials of blood pressure management for adults with acute ICH, focusing on studies in which patients were enrolled within 7 days of ICH onset. These studies compared intensive blood pressure management with guideline-based management.
Investigators chose function, defined as Modified Rankin Scale (mRS) score at 90 days, as their primary outcome. Radiologic outcomes included absolute (>6 mL) and proportional (>33%) hematoma growth at 24 hours. They used the intention to treat dataset from each trial in their statistical analyses and created generalized linear mixed models with prespecified covariables using a one-stage approach.
Variation by drug
A total of 7,094 studies were identified, of which 50 were eligible for inclusion. Their analysis encompassed 16 studies for which the respective investigators were willing to share patient-level data. The analysis included data on 6,221 patients. The mean age of the patients was 64.2 years, 36.4% were women, and the median time from symptom onset to randomization was 3.8 hours.
Mean National Institutes of Health Stroke Scale score was approximately 11. Mean systolic blood pressure at baseline was 177 mm Hg, and mean hematoma volume was approximately 10.6 mL.
The difference in blood pressure between the intensive and guideline groups was approximately 8 mm Hg at 1 hour and 12 mm Hg at 24 hours.
Intensive blood pressure management did not affect function at 90 days. The adjusted odds ratio for unfavorable shift in mRS scores was 0.97 (95% CI, 0.88-1.06; P = .503). Intensive blood pressure management did, however, reduce hematoma growth (absolute aOR, 0.75; 95% CI, 0.60-0.92; P = .007; relative aOR, 0.82; 95% CI, 0.68-0.99; P = .034).
In prespecified subgroup analyses, they found a trend toward adverse outcomes among patients who received renin-angiotensin blockers and a trend toward benefit for patients who received alpha- or beta-receptor antagonists or calcium channel blockers. They did not observe a clear association between time of treatment and outcome.
In addition to hematoma growth, other factors influence prognosis after ICH, such as the patient’s status before ICH (for example, cardiovascular risk factors, age, and hypertensive effects on the brain, kidneys, and heart), the location of ICH and its effects on surrounding structures, and complications of care in hospitals, such as infection and bleeding, said Dr. Anderson.
They are conducting two ongoing clinical trials in patients with ICH. One, INTERACT3, is evaluating a “care bundle” quality control package that includes early intensive blood pressure lowering for patients with large ICH who undergo surgery.
The other, INTERACT4, is evaluating early blood pressure control in the ambulance for patients with suspected acute stroke. At least one-fifth of those patients will have ICH, said Dr. Anderson.
Prevention is essential
Among patients with ICH, much of the bleeding occurs before presentation at the hospital, Louis R. Caplan, MD, a neurologist at Beth Israel Deaconess Medical Center, Boston, said in an interview. Furthermore, the bleeding mainly occurs in the deep part of the brain where most of the important motor tracts are. “If those tracts are already hit, a little extra blood isn’t going to change things,” said Dr. Caplan, who was not involved in the research.
In addition, blood is pushed from inside the brain to the periphery until the pressure outside the brain is equal to the pressure inside it. “You can decrease the amount of bleeding significantly, but it probably doesn’t affect the outcome,” said Dr. Caplan.
One factor in patients’ apparent lack of functional improvement is that the mRS is not sensitive to minor changes in disability, he said. “You have to show a pretty important change for it to make a difference,” said Dr. Caplan.
In addition, recovery from a hemorrhage takes much longer than recovery from an infarct. Examining the population at 6 months would have been preferable to examining them at 90 days, but the investigators might not have 6-month data, said Dr. Caplan.
“The main thing is really prevention,” he concluded.
The study was conducted with funding from Takeda. Dr. Anderson reported receiving funding from the National Health and Medical Research Council of Australia and speaker fees from Takeda. Dr. Caplan has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ESO-WSO CONFERENCE 2020
Statins beneficial in elderly, guidelines should be strengthened
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
Contrary to historical evidence, two new studies show.
“By contrast with previous historical studies, our data show that LDL cholesterol is an important risk factor for myocardial infarction and atherosclerotic cardiovascular disease in a contemporary primary prevention cohort of individuals aged 70 to 100 years,” Borge Nordestgaard, MD, of the University of Copenhagen, and colleagues noted in the first of the two studies, published this week in the Lancet.
“By lowering LDL cholesterol in healthy individuals aged 70-100 years, the potential for preventing myocardial infarctions and atherosclerotic cardiovascular disease is huge, and at a substantially lower number needed to treat when compared with those aged 20-69 years,” they added.
“These findings support the concept of the cumulative burden of LDL cholesterol over one’s lifetime and the progressive increase in risk for atherosclerotic cardiovascular disease, including myocardial infarction, with age,” added Frederick J. Raal, PhD, and Farzahna Mohamed, MB BCh, of the University of the Witwatersrand, Johannesburg, South Africa, in an editorial published with both new studies in the Lancet (2020 Nov 10. doi: 10.1016/S0140-6736[20]32333-3).
The studies underscore the need for clinicians to consider continued risks associated with elevated LDL cholesterol in older age, they stressed, adding that statins are also beneficial for younger persons at risk to prevent conditions from worsening.
“The average age of patients in all the trials analyzed was older than 60 years, an age when atherosclerotic cardiovascular disease is already well established,” the editorialists wrote.
“Lipid-lowering therapy should be initiated at a younger age, preferably before age 40 years, in those at risk to delay the onset of atherosclerosis, rather than try to manage the condition once fully established or advanced,” they stressed.
No RCTs have included patients older than 70
For persons aged 40-75 years, elevated LDL cholesterol levels are a known risk factor for MI and atherosclerotic cardiovascular disease, and there is consensus in guidelines regarding treatment with statins.
However, the risk for people older than 70 is controversial. Some studies show little or no association between elevated LDL cholesterol levels and an increased risk for MI.
Contributing to the uncertainty is that few of the randomized, controlled trials that have investigated the question have included patients aged older than 70 years.
As a consequence, many practice guidelines have noted that the level of evidence in older patients is low, and some organizations have lowered the strength of recommendations regarding the treatment for older patients in comparison with younger patients.
Primary prevention: CV events increase with elevated LDL cholesterol in older age
Dr. Nordestgaard and colleagues studied data on 91,131 people living in Copenhagen who did not have atherosclerotic cardiovascular disease or diabetes at baseline and were not taking statins.
Of the participants, 10,592 were aged 70-79 years, and 3,188 participants were aged 80-100 years.
Over an average follow-up period of 7.7 years, 1,515 participants had a first MI, and 3,389 developed atherosclerotic cardiovascular disease.
In the primary-prevention cohort, after multivariate adjustment, the risk of having a heart attack per 1.0 mmol/L increase in LDL cholesterol was increased in the group overall (hazard ratio, 1.34). The increased risk was observed for all age groups, including those aged 80-100 years (HR, 1.28), 70-79 (HR, 1.25), 60-69 (HR, 1.29), 50-59 (HR, 1.28), and 20-49 (HR, 1.68).
Risk for atherosclerotic cardiovascular disease was also raised per 1.0 mmol/L increase in LDL cholesterol overall (HR, 1.16) and in all age groups, particularly those aged 70-100 years.
Greater elevations in LDL cholesterol (5.0 mmol/L or higher, indicative of possible familial hypercholesterolemia) were associated with a notably higher risk for heart attack after multivariate adjustment in people aged 80-100 (HR, 2.99). Risk was also higher among those aged 70-79 (HR, 1.82).
The highest incidence was in those older than 70. The rate was 8.5 heart attacks per 1,000 people per year among those aged 80-100 and 5.2 heart attacks per 1,000 in those aged 70-79. The rates were 2.5 per 1,000 among those 60-69, 1.8 for those aged 50-59, and 0.8 for those aged 20-49.
“The absolute risk [of cardiovascular events] is of course much higher in the elderly than those under the age of 75, but what was a surprise was how clear our results were on a relative risk scale, that the risk associated with elevated LDL [cholesterol] was as high in people aged 80-100 as the younger patients,” Dr. Nordestgaard said in an interview.
With regard to the benefits of cholesterol-lowering drugs, the study showed that the number needed to prevent one heart attack over 5 years was 80 among those aged 80-100; the number was 439 for people aged 50-59.
With regard to stronger statins, when moderate-intensity statins were used, the number needed to treat to prevent one cardiovascular disease event of any type dropped to 42 for patients aged 80-100. It was 88 for those aged 70-79, 164 for those aged 60-69, 345 for those aged 50-59, and 769 for those aged 20-49.
“The clinical significance of this is that it appears those in older age groups indeed benefit from cholesterol-lowering therapy,” Dr. Nordestgaard said. “I think many people have this idea that LDL [cholesterol] is not important over the age of about 70-75, but that’s not the case.”
“These robust findings are novel,” he and his colleagues stressed.
Despite these observational findings, the South African editorialists noted that “whether lipid-lowering therapy should be initiated for primary prevention in people aged 75 years or older is unclear,” owing to the host of risks and benefits that need to be balanced.
The findings of an ongoing randomized, placebo-controlled trial (STAREE) may answer this question, they wrote. It is investigating primary prevention in 18,000 older patients (≥70 years) who are being randomly assigned to receive atorvastatin 40 mg/d or placebo. The study is seeking to determine whether statin treatment extends the length of a disability-free life, which will be assessed on the basis of survival outside permanent residential care. Results are expected in 2022-2023.
Unequivocal reductions in events in elderly, comparable with younger patients
In the second study (Lancet. 2020 Nov 10. doi: 10.1016/S0140-6736[20]32332-1), Baris Gencer, MD, of Brigham and Women’s Hospital, Boston, =and colleagues evaluated the effects of statins and other cholesterol-lowering drugs, including ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors, in older versus younger patients.
The systematic review and meta-analysis of 29 randomized controlled trials, also published in the Lancet, were presented virtually as a poster as part of the 2020 American Heart Association scientific session. It included data on 244,090 patients, including 21,492 aged 75 years and older.
The meta-analysis included studies of cardiovascular outcomes of a guideline-recommended LDL cholesterol–lowering drug, with a median follow-up of at least 2 years and inclusion of data on patients aged 75 years and older.
The results showed that over a median follow-up of 2.2 to 6 years, statin use by older patients was associated with a relative risk reduction of major vascular events of 26% per 1 mmol/L reduction in LDL cholesterol (P = .0019), which was comparable with a risk reduction of 15% per 1 mmol/L reduction in LDL cholesterol for patients younger than 75 years (P = .37, compared with older patients).
Treatment of older patients with LDL cholesterol–lowering drugs was also associated with significantly improved outcomes in cardiovascular death (risk ratio, 0.85), MI (RR, 0.80), stroke (RR, 0.73), and coronary revascularization (RR, 0.80).
“We found an unequivocal reduction in the risk of major vascular events with both statin and nonstatin LDL cholesterol-lowering treatments, which was similar to that seen in younger patients,” the authors wrote.
“Cholesterol-lowering medications are affordable drugs that have reduced risk of heart disease for millions of people worldwide, but until now, their benefits for older people have remained less certain,” said lead author Marc Sabatine, MD, also of Brigham and Women’s Hospital, in a Lancet press release.
“Our analysis indicates that these therapies are as effective in reducing cardiovascular events and deaths in people aged 75 years and over as they are in younger people. We found no offsetting safety concerns, and together, these results should strengthen guideline recommendations for the use of cholesterol-lowering medications, including statin and nonstatin therapy, in elderly people.”
The editorialists agreed: “More than 80% of fatal cardiovascular events occur in individuals older than 65 years, and the incidence of cardiovascular events is increasing in those older than 80 years; therefore, the findings of Gencer and colleagues’ study should encourage the use of lipid-lowering therapy in older patients.”
The authors of the two studies have disclosed no relevant financial relationships. Dr. Raal has received research grants, honoraria, or consulting fees for advisory board membership, professional input, and lectures on lipid-lowering drug therapy from Amgen, Regeneron, Sanofi, Novartis, and the Medicines Company.
A version of this article originally appeared on Medscape.com.
IDSA updates COVID guidelines for antibodies, antivirals, other drugs
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
Finerenone’s heart benefits hold up in T2D patients without CVD
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
Finerenone, the first nonsteroidal mineralocorticoid receptor antagonist to complete a phase 3 trial, showed cardiovascular benefits in patients with type 2 diabetes and chronic kidney disease, regardless of whether they entered the study with a history of cardiovascular disease, in follow-up analyses of the FIDELIO-DKD trial, which included 5,674 patients.
“Finerenone demonstrated benefits for primary and secondary cardiovascular disease protection,” said Gerasimos Filippatos, MD, at the American Heart Association scientific sessions. Finerenone treatment cut the rate of cardiovascular death, nonfatal MI or stroke, or heart failure hospitalization, when compared with placebo, by a relative 15% among patients with a history of cardiovascular disease (CVD), and by a relative 14% in patients without this history, differences that met a statistical test for consistency. But the absolute, drug-associated increments in benefit over placebo differed between the two CVD subgroups because of a sharp underlying difference in event rates.
In contrast, the analyses reported by Dr. Filippatos and associates from the FIDELIO-DKD study showed significant heterogeneity based on the presence or absence of CVD for the study’s primary endpoint, a composite renal metric that tallied the combined rate of death from renal causes, renal failure, or a sustained drop in estimated glomerular filtration rate of at least 40%. Researchers enrolled patients into FIDELIO-DKD based on having type 2 diabetes (T2D) and chronic kidney disease (CKD). The prevalence of a history of CVD was 46%.
Among patients with a history of CVD, the composite adverse CVD outcome occurred at a rate of 8.5/100 patient-years in patients on placebo and in 7.18/100 patients years among those on finerenone during a median of 2.6 years of follow-up, a 1.32/100–patient-year absolute between-group difference. Among patients in a primary prevention setting, incident CVD event rates during follow-up were roughly half that in the secondary prevention patients. The upshot was that, in the placebo group, the rate was 3.92/100 patient- years, and in those on finerenone was 3.43/100 patient-years, a 0.49/100–patient-year absolute difference.
CVD history produced heterogeneity for the primary endpoint
In the analysis that focused on the study’s primary, renal endpoint, among patients identified as having CVD at study entry, the outcome occurred at a rate of 9.06/100 patient-years in the placebo subgroup and at a rate of 6.6/100 patient years in those who received finerenone, a significant 30% relative risk reduction and an absolute between-group difference of 2.46/100 patient-years.
In contrast, among patients without a CVD history, the composite renal endpoint occurred at a rate of 9.1/100 patient-years in the placebo patients and 8.42/100 patient-years in those on finerenone, a 6% relative risk reduction that was not significant, and a 0.68/100–patient-year absolute difference. This disparity in the primary event rate between the two treatment arms reached statistical significance (P = .016), the investigators reported in the published version of the report in Circulation that simultaneously appeared online.
“The totality of evidence suggests that finerenone could be used in patients with T2D with or without a history of CVD,” explained Dr. Filippatos in an interview. “The P-interaction for the composite kidney outcome is significant, but it is not corrected for multiple testing; therefore, it might be a false-chance finding and must be interpreted cautiously.
Furthermore, in another prespecified kidney composite outcome the results were consistent in patients with and without a history of CVD. In sum, all the FIDELIO-DKD analyses so far are “suggestive of a beneficial effect in patients without a history of CVD.”
Despite these patients receiving guideline directed therapies, “there remains a high unmet medical need in patients with T2D and CKD,” added Dr. Filippatos, professor of cardiology at the University of Athens. “We use multiple treatments for patients with heart failure, and we should use the same mindset for treating patients with T2D and CKD. The costs of dialysis and kidney transplant are very high, so it is important to consider options that slow progression of CKD in these patients.”
In FIDELIO-DKD, virtually all patients were on background therapy with a renin-angiotensin-system (RAS) inhibitor, so the trial’s results suggest that treatment should at least involve dual therapy with finerenone and a RAS inhibitor. Fewer than 5% were on background therapy with a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a drug class recently established as another key agent for treating CKD in patients with T2D, setting up the prospect for triple therapy, although this approach has not yet undergone prospective testing.
Combining RAS inhibition, finerenone, and an SGLT2 inhibitor is “potentially a marriage made in diabetes heaven,” commented Deepak L. Bhatt, MD, a professor of medicine at Harvard Medical School, Boston, who has not participated in finerenone studies.
Finerenone looks better for safety
Regardless of subgroup analyses based on history of CVD, the findings from all patients enrolled in FIDELIO-DKD were positive for the both the primary renal outcome and key secondary outcome of composite CVD events. In the total randomized cohort, treatment with finerenone on top of optimized treatment with an ACE inhibitor or angiotensin receptor blocker (RAS inhibition) led to a significant 18% relative risk reduction, compared with placebo, for the primary renal endpoint, and a significant 14% relative drop in the key secondary CVD outcome. Those results were published in October in the New England Journal of Medicine.
For treating patients with T2D and CKD ,finerenone overall “looks like a major advance,” Dr. Bhatt said in an interview.
In addition to the positive efficacy results, several experts also focused on what they saw as superior safety of finerenone in the trial, compared with the historical safety of the steroidal mineralocorticoid receptor antagonists (MRAs) now in use: spironolactone and eplerenone.
“I’m a big believer in spironolactone, but it has issues with side effects, and eplerenone never seemed to catch on,” said Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Hospital in Boston.
“A lot of physicians like these MRAs, but acknowledge that side effects have kept these drugs from being used to the extent they should.” The existing MRAs, especially spironolactone, have become a key drug class for treating heart failure with reduced ejection fraction (and, some claim, for also treating heart failure with preserved ejection fraction), as well as treatment-resistant hypertension and primary aldosteronism. By design, FIDELIO-DKD did not enroll patients with heart failure because treatment with an MRA is indicated for those with heart failure with reduced ejection fraction.
The spironolactone adverse effect that generates the greatest concern is hyperkalemia. During his discussion of FIDELIO-DKD as designated discussant, Christoph Wanner, MD, noted a recent study in which the incidence of hyperkalemia severe enough to cause study discontinuation was 23% among patients treated with spironolactone for heart failure, which contrasts with the 2.3% rate in FIDELIO-DKD among finerenone recipients. This hyperkalemia incidence from finerenone also improved on the historical performance of other drugs, like aliskiren (Tekturna), said Dr. Wanner, professor and head of nephrology at the University of Würzburg (Germany).
The FIDELIO-DKD results place finerenone alongside the RAS- and SGLT2-inhibitor drug classes as appropriate treatments for most patients with T2D and CKD. “We have entered a new era of effective treatment for diabetic kidney disease,” Dr. Wanner declared.
“The overall safety profile of finerenone looked better, including hyperkalemia,” said Dr. Bhatt. “Hyperkalemia with spironolactone is not necessarily as bad as the perception. With careful monitoring of spironolactone, the hyperkalemia is manageable. But the perception is that it’s bad, and along with gynecomastia it’s a real killer.”
While some dismiss gynecomastia as a major concern (for men) with spironolactone treatment, “if medical students learn one thing about spironolactone, it’s that it can cause gynecomastia,” adding to the negative image that the approved MRAs carry, Dr. Bhatt said.
“The hyperkalemia was manageable. This is very important because of past problems with potassium when using spironolactone,” Dr. Filippatos said. Finerenone also looks “more cardiorenal protective” than the steroidal MRAs, exerting renal benefits in FIDELIO-DKD never previously described for a steroidal MRA.
Some of the uncertainty about the efficacy of finerenone in patients with a history of cardiovascular disease will lift when results are available in about another year from the FIGARO-DKD pivotal trial of finerenone, which enrolled more than 7,000 patients with T2D and CKD (entry criteria very similar to FIDELIO-CKD). A big difference is that FIGARO-DKD has a composite CVD event metric as its primary endpoint, and includes hospitalization for heart failure as one facet of the composite.
FIDELIO-DKD was sponsored by Bayer. Dr. Filippatos has been a lecturer on behalf of, served as a researcher for, or both for Bayer and also for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Bhatt has received research funding from Bayer and also from several other companies, and he also is an adviser to several companies. Dr. Wanner has received honoraria from Bayer, and also from AstraZeneca, Boehringer Ingelheim, FMC, Gilead, GlaxoSmithKline, Lilly, and Merck.
FROM AHA 2020