User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
Operational changes in primary care linked with improved smoking, blood pressure outcomes
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
FROM ANNALS OF FAMILY MEDICINE
Health costs over 25 times higher for hemophilia B patients than controls
As the burden of hemophilia B in patients increases from mild to severe forms of the disease, the already high economic cost of treatment rises significantly, according to a large retrospective database study.
Researchers developed four profile categories (mild, moderate, moderate-severe, and severe) for men with hemophilia B on the basis of the frequency of hemorrhage events and factor IX replacement claims as identified from the IBM MarketScan database (June 2011–February 2019). The mean annual health care resource use (HRU) and costs were compared between 5,454 patients with hemophilia B and 1:1 demographically matched controls.
Economic burden
Total health care costs rose with increasingly severe clinical profiles, with hemophilia-related treatments being the primary cost driver, researchers led by Tyler W. Buckner, MD, of the University of Colorado at Denver, Aurora, wrote in Blood Advances.
This was particularly true among patients with more severe clinical profiles, who were more likely to be on prophylaxis with all of its associated costs.
The mean overall total costs incurred by patients with hemophilia B over the study period were $201,635 versus $7,879 for matched controls, a more than 25-fold difference (P < .001). In addition, across all four clinical profiles categories, all-cause total costs, medical costs, and pharmacy costs were significantly higher among patients with hemophilia B than matched controls (P < .001 for all), the researchers added.
Annual total health care costs also increased with increasing severity of hemophilia B clinical profiles, ranging from $80,811 and $137,455 in the mild and moderate groups to $251,619 and $632,088 in the moderate-severe and severe groups, respectively.
“Hemophilia-related treatments represented the primary cost driver. HRU was uniformly higher among patients with hemophilia B across clinical profiles, medical service types examined, and with respect to opioid use. The significant burden highlights that unmet needs remain in hemophilia B,” the researchers concluded.
This study was supported by uniQure. Dr. Buckner has received honoraria or fees for serving on advisory boards or as a consultant for uniQure. Several of the coauthors are employees of Analysis Group, which received consulting fees from uniQure to conduct this study, and two of the authors are employees of and own stock in uniQure.
As the burden of hemophilia B in patients increases from mild to severe forms of the disease, the already high economic cost of treatment rises significantly, according to a large retrospective database study.
Researchers developed four profile categories (mild, moderate, moderate-severe, and severe) for men with hemophilia B on the basis of the frequency of hemorrhage events and factor IX replacement claims as identified from the IBM MarketScan database (June 2011–February 2019). The mean annual health care resource use (HRU) and costs were compared between 5,454 patients with hemophilia B and 1:1 demographically matched controls.
Economic burden
Total health care costs rose with increasingly severe clinical profiles, with hemophilia-related treatments being the primary cost driver, researchers led by Tyler W. Buckner, MD, of the University of Colorado at Denver, Aurora, wrote in Blood Advances.
This was particularly true among patients with more severe clinical profiles, who were more likely to be on prophylaxis with all of its associated costs.
The mean overall total costs incurred by patients with hemophilia B over the study period were $201,635 versus $7,879 for matched controls, a more than 25-fold difference (P < .001). In addition, across all four clinical profiles categories, all-cause total costs, medical costs, and pharmacy costs were significantly higher among patients with hemophilia B than matched controls (P < .001 for all), the researchers added.
Annual total health care costs also increased with increasing severity of hemophilia B clinical profiles, ranging from $80,811 and $137,455 in the mild and moderate groups to $251,619 and $632,088 in the moderate-severe and severe groups, respectively.
“Hemophilia-related treatments represented the primary cost driver. HRU was uniformly higher among patients with hemophilia B across clinical profiles, medical service types examined, and with respect to opioid use. The significant burden highlights that unmet needs remain in hemophilia B,” the researchers concluded.
This study was supported by uniQure. Dr. Buckner has received honoraria or fees for serving on advisory boards or as a consultant for uniQure. Several of the coauthors are employees of Analysis Group, which received consulting fees from uniQure to conduct this study, and two of the authors are employees of and own stock in uniQure.
As the burden of hemophilia B in patients increases from mild to severe forms of the disease, the already high economic cost of treatment rises significantly, according to a large retrospective database study.
Researchers developed four profile categories (mild, moderate, moderate-severe, and severe) for men with hemophilia B on the basis of the frequency of hemorrhage events and factor IX replacement claims as identified from the IBM MarketScan database (June 2011–February 2019). The mean annual health care resource use (HRU) and costs were compared between 5,454 patients with hemophilia B and 1:1 demographically matched controls.
Economic burden
Total health care costs rose with increasingly severe clinical profiles, with hemophilia-related treatments being the primary cost driver, researchers led by Tyler W. Buckner, MD, of the University of Colorado at Denver, Aurora, wrote in Blood Advances.
This was particularly true among patients with more severe clinical profiles, who were more likely to be on prophylaxis with all of its associated costs.
The mean overall total costs incurred by patients with hemophilia B over the study period were $201,635 versus $7,879 for matched controls, a more than 25-fold difference (P < .001). In addition, across all four clinical profiles categories, all-cause total costs, medical costs, and pharmacy costs were significantly higher among patients with hemophilia B than matched controls (P < .001 for all), the researchers added.
Annual total health care costs also increased with increasing severity of hemophilia B clinical profiles, ranging from $80,811 and $137,455 in the mild and moderate groups to $251,619 and $632,088 in the moderate-severe and severe groups, respectively.
“Hemophilia-related treatments represented the primary cost driver. HRU was uniformly higher among patients with hemophilia B across clinical profiles, medical service types examined, and with respect to opioid use. The significant burden highlights that unmet needs remain in hemophilia B,” the researchers concluded.
This study was supported by uniQure. Dr. Buckner has received honoraria or fees for serving on advisory boards or as a consultant for uniQure. Several of the coauthors are employees of Analysis Group, which received consulting fees from uniQure to conduct this study, and two of the authors are employees of and own stock in uniQure.
FROM BLOOD ADVANCES
A ‘mess’ of a diagnosis: Is it type 2 MI or a nonischemic imposter?
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Finerenone scores second pivotal-trial success in patients with diabetic kidney disease
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Two treatments show early promise for hypothalamic obesity
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
FROM ENDO 2021
‘Malicious peer review’ destroyed doc’s career, he says
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Possible obesity effect detected in cancer death rates
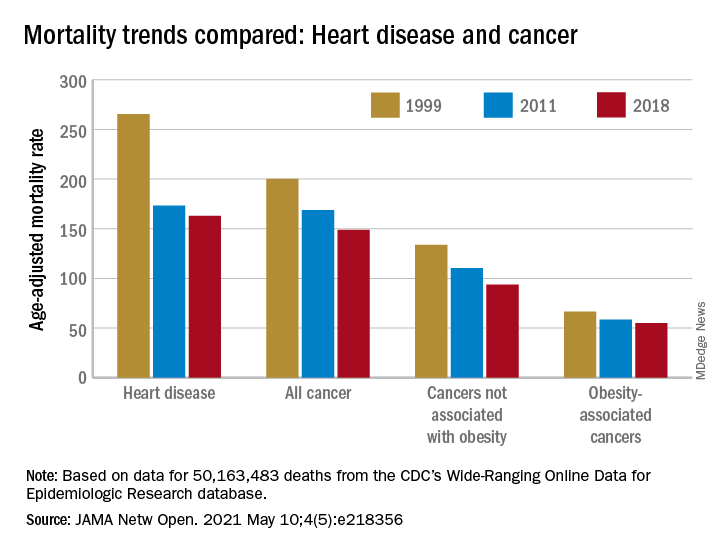
“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.

“By integrating 20 years of cancer mortality data, we demonstrated that trends in obesity-associated cancer mortality showed signs of recent deceleration, consistent with recent findings for heart disease mortality,” Christy L. Avery, PhD, and associates wrote in JAMA Network Open.
Improvements in mortality related to heart disease slowed after 2011, a phenomenon that has been associated with rising obesity rates. The age-adjusted mortality rate (AAMR) declined at an average of 3.8 deaths per 100,000 persons from 1999 to 2011 but only 0.7 deaths per 100,000 from 2011 to 2018, based on data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER).
To understand trends in cancer mortality and their possible connection with obesity, data for 1999-2018 from the WONDER database were divided into obesity-associated and non–obesity-associated categories and compared with heart disease mortality, they explained. The database included more than 50 million deaths that matched inclusion criteria.
The analysis showed there was difference between obesity-associated and non–obesity-associated cancers that was obscured when all cancer deaths were considered together. The average annual change in AAMR for obesity-associated cancers slowed from –1.19 deaths per 100,000 in 1999-2011 to –0.83 in 2011-2018, Dr. Avery and associates reported.
For non–obesity-associated cancers, the annual change in AAMR increased from –1.62 per 100,000 for 1999-2011 to –2.29 for 2011-2018, following the trend for all cancers: –1.48 per 100,000 during 1999-2011 and –1.77 in 2011-2018, they said.
“The largest mortality decreases were observed for melanoma of the skin and lung cancer, two cancers not associated with obesity. For obesity-associated cancers, stable or increasing mortality rates have been observed for liver and pancreatic cancer among both men and women as well as for uterine cancer among women,” the investigators wrote.
Demographically, however, the slowing improvement in mortality for obesity-associated cancers did not follow the trend for heart disease. The deceleration for cancer was more pronounced for women and for non-Hispanic Whites and not seen at all in non-Hispanic Asian/Pacific Islander individuals. “For heart disease, evidence of a deceleration was consistent across sex, race, and ethnicity,” they said.
There are “longstanding disparities in obesity” among various populations in the United States, and the recent trend of obesity occurring earlier in life may be having an effect. “Whether the findings of decelerating mortality rates potentially signal a changing profile of cancer and heart disease mortality as the consequences of the obesity epidemic are realized remains to be seen,” they concluded.
The investigators reported receiving grants from the National Institutes of Health during the conduct of the study, but no other disclosures were reported.
FROM JAMA NETWORK OPEN
A simple new definition for ‘metabolically healthy obesity’?
Scientists have proposed a simple new definition for “metabolically healthy obesity” to identify individuals who do not have an increased risk of cardiovascular disease (CVD) death and total mortality.
The team – led by Anika Zembic, MPH, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany – performed an assessment of anthropometric and metabolic risk factors as well as mortality data from two cohorts that “yielded a simple definition to categorize participants with obesity as metabolically healthy or unhealthy.”
They defined “metabolically healthy” as systolic blood pressure <130 mm Hg and no use of blood pressure-lowering medication; waist-to-hip ratio <0.95 (in women) and <1.03 (in men); and no prevalent type 2 diabetes.
Based on this new definition, 42% of participants in the third U.S. National Health and Nutrition Examination Survey (NHANES-III) and 19% of participants in the UK Biobank study had metabolically healthy obesity and did not have an increased risk for CVD mortality and total mortality compared with individuals with metabolically healthy normal weight.
“People with a phenotype defined as metabolically unhealthy using this definition had significantly higher hazard ratios for [CVD] mortality and total mortality irrespective of body mass index category, and people with phenotypes defined as having metabolically healthy obesity displayed no increased risk,” the researchers noted in their article, published May 7 in JAMA Network Open.
“Our new definition may be important not only to stratify risk of mortality in people with obesity, but also in people with overweight and normal weight,” they concluded.
Thirty different definitions of ‘metabolically healthy obesity’
“To date, there is no universally accepted standard for defining [metabolically healthy obesity] and more than 30 different definitions have been used to operationalize the phenotypes in studies,” which may explain the “continued unresolved debate” about outcomes in patients with metabolically unhealthy obesity, Ayana K. April-Sanders, PhD, and Carlos J. Rodriguez, MD, MPH, from Albert Einstein College of Medicine, New York, wrote in an accompanying commentary.
The current study, they noted, suggests that waist-to-hip ratio is a better measure of central adiposity than waist circumference, and that the effect of dyslipidemia on CVD mortality may be weaker among individuals with obesity.
However, the findings may not be generalizable to other CVD outcomes, they cautioned.
And importantly, some individuals with metabolically healthy obesity will likely transition to unhealthy obesity over time due to weight gain, aging, and lack of physical activity.
Therefore, “the present study provides a prototype of how that definition can be derived, but more rigorous tests and evidence using similar techniques are needed, particularly in prospective studies,” according to Dr. April-Sanders and Dr. Rodriguez.
They call for more research to establish a standardized definition of metabolically healthy obesity and then, using that definition, to determine the prevalence of healthy and unhealthy obesity and identify factors that preserve healthy obesity.
Definition developed from NHANES cohort, validated in UK biobank
Ms. Zembic and colleagues explained that previous definitions for metabolically healthy obesity were mainly based on the absence of either metabolic syndrome or insulin resistance, but some individuals with obesity but without metabolic disease still have increased risks of CVD mortality and total mortality.
To develop a more precise definition of metabolically healthy obesity, the researchers analyzed data from 12,341 individuals in the United States who participated in NHANES-III, conducted between 1988 and 1994. The individuals were a mean age of 42 and 51% were women, and they were followed for an average of 14.5 years.
The researchers validated this definition using data from 374,079 individuals in the population-based UK Biobank cohort who were assessed in 2006 to 2010. Those individuals were a mean age of 56 and 55% were women, and they were followed for a mean of 7.8 years.
The combination of systolic blood pressure and waist-to-hip ratio had the strongest association with CVD mortality and total mortality, and the prevalence of type 2 diabetes was also associated with greater risk.
Regardless of BMI, all groups of metabolically unhealthy individuals had increased risks of CVD mortality and total mortality.
The study and some of the researchers were supported by grants from the German Federal Ministry of Education and Research.
A version of this article first appeared on Medscape.com.
Scientists have proposed a simple new definition for “metabolically healthy obesity” to identify individuals who do not have an increased risk of cardiovascular disease (CVD) death and total mortality.
The team – led by Anika Zembic, MPH, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany – performed an assessment of anthropometric and metabolic risk factors as well as mortality data from two cohorts that “yielded a simple definition to categorize participants with obesity as metabolically healthy or unhealthy.”
They defined “metabolically healthy” as systolic blood pressure <130 mm Hg and no use of blood pressure-lowering medication; waist-to-hip ratio <0.95 (in women) and <1.03 (in men); and no prevalent type 2 diabetes.
Based on this new definition, 42% of participants in the third U.S. National Health and Nutrition Examination Survey (NHANES-III) and 19% of participants in the UK Biobank study had metabolically healthy obesity and did not have an increased risk for CVD mortality and total mortality compared with individuals with metabolically healthy normal weight.
“People with a phenotype defined as metabolically unhealthy using this definition had significantly higher hazard ratios for [CVD] mortality and total mortality irrespective of body mass index category, and people with phenotypes defined as having metabolically healthy obesity displayed no increased risk,” the researchers noted in their article, published May 7 in JAMA Network Open.
“Our new definition may be important not only to stratify risk of mortality in people with obesity, but also in people with overweight and normal weight,” they concluded.
Thirty different definitions of ‘metabolically healthy obesity’
“To date, there is no universally accepted standard for defining [metabolically healthy obesity] and more than 30 different definitions have been used to operationalize the phenotypes in studies,” which may explain the “continued unresolved debate” about outcomes in patients with metabolically unhealthy obesity, Ayana K. April-Sanders, PhD, and Carlos J. Rodriguez, MD, MPH, from Albert Einstein College of Medicine, New York, wrote in an accompanying commentary.
The current study, they noted, suggests that waist-to-hip ratio is a better measure of central adiposity than waist circumference, and that the effect of dyslipidemia on CVD mortality may be weaker among individuals with obesity.
However, the findings may not be generalizable to other CVD outcomes, they cautioned.
And importantly, some individuals with metabolically healthy obesity will likely transition to unhealthy obesity over time due to weight gain, aging, and lack of physical activity.
Therefore, “the present study provides a prototype of how that definition can be derived, but more rigorous tests and evidence using similar techniques are needed, particularly in prospective studies,” according to Dr. April-Sanders and Dr. Rodriguez.
They call for more research to establish a standardized definition of metabolically healthy obesity and then, using that definition, to determine the prevalence of healthy and unhealthy obesity and identify factors that preserve healthy obesity.
Definition developed from NHANES cohort, validated in UK biobank
Ms. Zembic and colleagues explained that previous definitions for metabolically healthy obesity were mainly based on the absence of either metabolic syndrome or insulin resistance, but some individuals with obesity but without metabolic disease still have increased risks of CVD mortality and total mortality.
To develop a more precise definition of metabolically healthy obesity, the researchers analyzed data from 12,341 individuals in the United States who participated in NHANES-III, conducted between 1988 and 1994. The individuals were a mean age of 42 and 51% were women, and they were followed for an average of 14.5 years.
The researchers validated this definition using data from 374,079 individuals in the population-based UK Biobank cohort who were assessed in 2006 to 2010. Those individuals were a mean age of 56 and 55% were women, and they were followed for a mean of 7.8 years.
The combination of systolic blood pressure and waist-to-hip ratio had the strongest association with CVD mortality and total mortality, and the prevalence of type 2 diabetes was also associated with greater risk.
Regardless of BMI, all groups of metabolically unhealthy individuals had increased risks of CVD mortality and total mortality.
The study and some of the researchers were supported by grants from the German Federal Ministry of Education and Research.
A version of this article first appeared on Medscape.com.
Scientists have proposed a simple new definition for “metabolically healthy obesity” to identify individuals who do not have an increased risk of cardiovascular disease (CVD) death and total mortality.
The team – led by Anika Zembic, MPH, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany – performed an assessment of anthropometric and metabolic risk factors as well as mortality data from two cohorts that “yielded a simple definition to categorize participants with obesity as metabolically healthy or unhealthy.”
They defined “metabolically healthy” as systolic blood pressure <130 mm Hg and no use of blood pressure-lowering medication; waist-to-hip ratio <0.95 (in women) and <1.03 (in men); and no prevalent type 2 diabetes.
Based on this new definition, 42% of participants in the third U.S. National Health and Nutrition Examination Survey (NHANES-III) and 19% of participants in the UK Biobank study had metabolically healthy obesity and did not have an increased risk for CVD mortality and total mortality compared with individuals with metabolically healthy normal weight.
“People with a phenotype defined as metabolically unhealthy using this definition had significantly higher hazard ratios for [CVD] mortality and total mortality irrespective of body mass index category, and people with phenotypes defined as having metabolically healthy obesity displayed no increased risk,” the researchers noted in their article, published May 7 in JAMA Network Open.
“Our new definition may be important not only to stratify risk of mortality in people with obesity, but also in people with overweight and normal weight,” they concluded.
Thirty different definitions of ‘metabolically healthy obesity’
“To date, there is no universally accepted standard for defining [metabolically healthy obesity] and more than 30 different definitions have been used to operationalize the phenotypes in studies,” which may explain the “continued unresolved debate” about outcomes in patients with metabolically unhealthy obesity, Ayana K. April-Sanders, PhD, and Carlos J. Rodriguez, MD, MPH, from Albert Einstein College of Medicine, New York, wrote in an accompanying commentary.
The current study, they noted, suggests that waist-to-hip ratio is a better measure of central adiposity than waist circumference, and that the effect of dyslipidemia on CVD mortality may be weaker among individuals with obesity.
However, the findings may not be generalizable to other CVD outcomes, they cautioned.
And importantly, some individuals with metabolically healthy obesity will likely transition to unhealthy obesity over time due to weight gain, aging, and lack of physical activity.
Therefore, “the present study provides a prototype of how that definition can be derived, but more rigorous tests and evidence using similar techniques are needed, particularly in prospective studies,” according to Dr. April-Sanders and Dr. Rodriguez.
They call for more research to establish a standardized definition of metabolically healthy obesity and then, using that definition, to determine the prevalence of healthy and unhealthy obesity and identify factors that preserve healthy obesity.
Definition developed from NHANES cohort, validated in UK biobank
Ms. Zembic and colleagues explained that previous definitions for metabolically healthy obesity were mainly based on the absence of either metabolic syndrome or insulin resistance, but some individuals with obesity but without metabolic disease still have increased risks of CVD mortality and total mortality.
To develop a more precise definition of metabolically healthy obesity, the researchers analyzed data from 12,341 individuals in the United States who participated in NHANES-III, conducted between 1988 and 1994. The individuals were a mean age of 42 and 51% were women, and they were followed for an average of 14.5 years.
The researchers validated this definition using data from 374,079 individuals in the population-based UK Biobank cohort who were assessed in 2006 to 2010. Those individuals were a mean age of 56 and 55% were women, and they were followed for a mean of 7.8 years.
The combination of systolic blood pressure and waist-to-hip ratio had the strongest association with CVD mortality and total mortality, and the prevalence of type 2 diabetes was also associated with greater risk.
Regardless of BMI, all groups of metabolically unhealthy individuals had increased risks of CVD mortality and total mortality.
The study and some of the researchers were supported by grants from the German Federal Ministry of Education and Research.
A version of this article first appeared on Medscape.com.
Structural racism tied to psychosis risk in Black people
Social and economic disparities are linked to an increased risk for psychosis in Black and Latino communities, new research shows.
Results of a literature review of social and economic disparities in mental illness suggest that “structural racism” contributes to social and environmental conditions that affect psychosis risk.
“Black and Latino people suffer disproportionately from psychosis risk factors, at the neighborhood level and at the individual level, in large part as a result of structural racism,” study investigator Deidre M. Anglin, PhD, associate professor, department of psychology, City College of New York (N.Y.), told reporters attending a press briefing.
The social environment, which, for minorities, involves disadvantage and discrimination, may account for this increased psychosis risk, perhaps even more so than genetics, she said. Structural racism “is a critical public health threat,” Dr. Anglin added.
The findings were presented at the virtual American Psychiatric Association annual meeting and were simultaneously published online May 3 in The American Journal of Psychiatry.
Perpetual disadvantage
Dr. Anglin and colleagues examined U.S.-based evidence connecting characteristics of social environments with outcomes across the psychosis continuum – from psychotic experiences to schizophrenia.
Citing numerous studies, the researchers highlighted three key areas that reflect social and environmental conditions that may affect psychosis risk, and that disproportionately affect minorities. These were neighborhood factors, trauma in a U.S. context, and racial disparities during the prenatal and perinatal periods.
The data that were related to neighborhoods revealed “just how much racism has historically structured U.S. neighborhoods in ways that generationally perpetuate disadvantage for racially minoritized communities,” said Dr. Anglin.
“This happens through inequitable access to resources, such as health care, clean air, education, [and] employment, but also in terms of disproportionate exposure to environmental toxins and stressors,” she said.
These neighborhood factors are associated with cumulative stress that may be linked to heightened risk for psychosis, the investigators noted.
U.S. studies show that rates of adverse childhood experiences, such as abuse and emotional and physical neglect, are higher among racial and ethnic minorities.
Police victimization and gun violence disproportionately affect racial minorities and create what the investigators call “a unique type of collective trauma” in the United States. They note that Black men have a 1 in 1,000 chance of being victims of lethal force by police over their lifetimes. By comparison, White men have a 39 in 100,000 chance.
One study of a diverse sample from four large U.S. urban centers showed that those who self-reported different types of police victimization were more likely to report psychotic experiences. Another study showed that greater exposure to gun violence fatalities, regardless of police involvement, was positively associated with psychotic experiences.
Obstetric complications
A variety of obstetric complications, including infection, maternal inflammation, and stress, have been associated with increased risk for psychotic disorders in U.S. samples.
“What we saw emerge from the literature is that Black women in the U.S. are at substantially increased risk for many of these obstetrical complications compared to White women, and this is not necessarily explained by socioeconomic status,” said Dr. Anglin.
Neighborhood- and individual-level factors appear to affect the disparity in these outcomes. A recent study revealed that exposure to environmental contaminants such as air pollution is associated with higher rates of preterm birth and low birth weight differentially in Black mothers compared with other mothers, “possibly as a result of an interaction between prenatal stress and contaminants,” the investigators noted.
Research also indicates that Black women are more likely to have lower levels of cortisol during the second trimester of pregnancy compared with women of other racial and ethnic groups. Cortisol is essential for fetal growth. Evidence links lower cortisol levels in later stages of pregnancy with decreased fetal growth in individuals who develop schizophrenia.
, compared with White women of the same socioeconomic status.
Such findings “highlight a complex picture” involving maternal cortisol levels and other stress biomarkers, “potentially leading to poor birth outcomes and subsequent risk for psychotic disorders in adulthood,” the investigators noted.
The researchers call for the dismantling of structural racism and the social policies and norms it shapes. They also recommend changes in health care policy and in the approach to early intervention for psychosis among Black and other racially-minoritized groups.
“Altogether, the current evidence suggests the need to identify, address, and tackle the social determinants deeply ingrained in U.S. society, in tandem with empowering the most marginalized communities,” the researchers wrote.
“We recommend that the field of psychiatry devote considerably more effort to addressing structural racism and social determinants of psychosis in funding priorities, training, and intervention development,” they added.
Dr. Anglin suggests that mental health providers use what she called a “cultural formulation interview” that takes a person’s environmental and social context into consideration. Studies show that incorporating this into clinical practice helps reduce misdiagnosis of mental illness in Black populations, she said.
Call to action
Commenting on the findings in an interview, Ned H. Kalin, MD, editor of The American Journal of Psychiatry and professor and chair of the department of psychiatry, University of Wisconsin, Madison, said the study was well done and serves as a “call to action” to address the impact of structural racism on mental health issues and psychiatric diseases.
The article highlights the need for “collecting better data” on structural racism, said Dr. Kalin. “We know it’s a big issue, but we can’t even quantitate it, so we need some fundamental measures to use as a benchmark as we move forward, as we try to make change.”
He noted that racism “is so embedded in one’s experience and in our society that we sort of don’t even think about it as a trauma.”
In psychiatry, for example, trauma is often thought of as a loss or a traumatic event. “We don’t typically think of trauma as an experience that pervades one’s entire life,” but that needs to change, he said. “At the individual level and in the doctor’s office, being sensitive to and aware of these issues is absolutely critical.”
Dr. Anglin and Dr. Kalin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Social and economic disparities are linked to an increased risk for psychosis in Black and Latino communities, new research shows.
Results of a literature review of social and economic disparities in mental illness suggest that “structural racism” contributes to social and environmental conditions that affect psychosis risk.
“Black and Latino people suffer disproportionately from psychosis risk factors, at the neighborhood level and at the individual level, in large part as a result of structural racism,” study investigator Deidre M. Anglin, PhD, associate professor, department of psychology, City College of New York (N.Y.), told reporters attending a press briefing.
The social environment, which, for minorities, involves disadvantage and discrimination, may account for this increased psychosis risk, perhaps even more so than genetics, she said. Structural racism “is a critical public health threat,” Dr. Anglin added.
The findings were presented at the virtual American Psychiatric Association annual meeting and were simultaneously published online May 3 in The American Journal of Psychiatry.
Perpetual disadvantage
Dr. Anglin and colleagues examined U.S.-based evidence connecting characteristics of social environments with outcomes across the psychosis continuum – from psychotic experiences to schizophrenia.
Citing numerous studies, the researchers highlighted three key areas that reflect social and environmental conditions that may affect psychosis risk, and that disproportionately affect minorities. These were neighborhood factors, trauma in a U.S. context, and racial disparities during the prenatal and perinatal periods.
The data that were related to neighborhoods revealed “just how much racism has historically structured U.S. neighborhoods in ways that generationally perpetuate disadvantage for racially minoritized communities,” said Dr. Anglin.
“This happens through inequitable access to resources, such as health care, clean air, education, [and] employment, but also in terms of disproportionate exposure to environmental toxins and stressors,” she said.
These neighborhood factors are associated with cumulative stress that may be linked to heightened risk for psychosis, the investigators noted.
U.S. studies show that rates of adverse childhood experiences, such as abuse and emotional and physical neglect, are higher among racial and ethnic minorities.
Police victimization and gun violence disproportionately affect racial minorities and create what the investigators call “a unique type of collective trauma” in the United States. They note that Black men have a 1 in 1,000 chance of being victims of lethal force by police over their lifetimes. By comparison, White men have a 39 in 100,000 chance.
One study of a diverse sample from four large U.S. urban centers showed that those who self-reported different types of police victimization were more likely to report psychotic experiences. Another study showed that greater exposure to gun violence fatalities, regardless of police involvement, was positively associated with psychotic experiences.
Obstetric complications
A variety of obstetric complications, including infection, maternal inflammation, and stress, have been associated with increased risk for psychotic disorders in U.S. samples.
“What we saw emerge from the literature is that Black women in the U.S. are at substantially increased risk for many of these obstetrical complications compared to White women, and this is not necessarily explained by socioeconomic status,” said Dr. Anglin.
Neighborhood- and individual-level factors appear to affect the disparity in these outcomes. A recent study revealed that exposure to environmental contaminants such as air pollution is associated with higher rates of preterm birth and low birth weight differentially in Black mothers compared with other mothers, “possibly as a result of an interaction between prenatal stress and contaminants,” the investigators noted.
Research also indicates that Black women are more likely to have lower levels of cortisol during the second trimester of pregnancy compared with women of other racial and ethnic groups. Cortisol is essential for fetal growth. Evidence links lower cortisol levels in later stages of pregnancy with decreased fetal growth in individuals who develop schizophrenia.
, compared with White women of the same socioeconomic status.
Such findings “highlight a complex picture” involving maternal cortisol levels and other stress biomarkers, “potentially leading to poor birth outcomes and subsequent risk for psychotic disorders in adulthood,” the investigators noted.
The researchers call for the dismantling of structural racism and the social policies and norms it shapes. They also recommend changes in health care policy and in the approach to early intervention for psychosis among Black and other racially-minoritized groups.
“Altogether, the current evidence suggests the need to identify, address, and tackle the social determinants deeply ingrained in U.S. society, in tandem with empowering the most marginalized communities,” the researchers wrote.
“We recommend that the field of psychiatry devote considerably more effort to addressing structural racism and social determinants of psychosis in funding priorities, training, and intervention development,” they added.
Dr. Anglin suggests that mental health providers use what she called a “cultural formulation interview” that takes a person’s environmental and social context into consideration. Studies show that incorporating this into clinical practice helps reduce misdiagnosis of mental illness in Black populations, she said.
Call to action
Commenting on the findings in an interview, Ned H. Kalin, MD, editor of The American Journal of Psychiatry and professor and chair of the department of psychiatry, University of Wisconsin, Madison, said the study was well done and serves as a “call to action” to address the impact of structural racism on mental health issues and psychiatric diseases.
The article highlights the need for “collecting better data” on structural racism, said Dr. Kalin. “We know it’s a big issue, but we can’t even quantitate it, so we need some fundamental measures to use as a benchmark as we move forward, as we try to make change.”
He noted that racism “is so embedded in one’s experience and in our society that we sort of don’t even think about it as a trauma.”
In psychiatry, for example, trauma is often thought of as a loss or a traumatic event. “We don’t typically think of trauma as an experience that pervades one’s entire life,” but that needs to change, he said. “At the individual level and in the doctor’s office, being sensitive to and aware of these issues is absolutely critical.”
Dr. Anglin and Dr. Kalin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Social and economic disparities are linked to an increased risk for psychosis in Black and Latino communities, new research shows.
Results of a literature review of social and economic disparities in mental illness suggest that “structural racism” contributes to social and environmental conditions that affect psychosis risk.
“Black and Latino people suffer disproportionately from psychosis risk factors, at the neighborhood level and at the individual level, in large part as a result of structural racism,” study investigator Deidre M. Anglin, PhD, associate professor, department of psychology, City College of New York (N.Y.), told reporters attending a press briefing.
The social environment, which, for minorities, involves disadvantage and discrimination, may account for this increased psychosis risk, perhaps even more so than genetics, she said. Structural racism “is a critical public health threat,” Dr. Anglin added.
The findings were presented at the virtual American Psychiatric Association annual meeting and were simultaneously published online May 3 in The American Journal of Psychiatry.
Perpetual disadvantage
Dr. Anglin and colleagues examined U.S.-based evidence connecting characteristics of social environments with outcomes across the psychosis continuum – from psychotic experiences to schizophrenia.
Citing numerous studies, the researchers highlighted three key areas that reflect social and environmental conditions that may affect psychosis risk, and that disproportionately affect minorities. These were neighborhood factors, trauma in a U.S. context, and racial disparities during the prenatal and perinatal periods.
The data that were related to neighborhoods revealed “just how much racism has historically structured U.S. neighborhoods in ways that generationally perpetuate disadvantage for racially minoritized communities,” said Dr. Anglin.
“This happens through inequitable access to resources, such as health care, clean air, education, [and] employment, but also in terms of disproportionate exposure to environmental toxins and stressors,” she said.
These neighborhood factors are associated with cumulative stress that may be linked to heightened risk for psychosis, the investigators noted.
U.S. studies show that rates of adverse childhood experiences, such as abuse and emotional and physical neglect, are higher among racial and ethnic minorities.
Police victimization and gun violence disproportionately affect racial minorities and create what the investigators call “a unique type of collective trauma” in the United States. They note that Black men have a 1 in 1,000 chance of being victims of lethal force by police over their lifetimes. By comparison, White men have a 39 in 100,000 chance.
One study of a diverse sample from four large U.S. urban centers showed that those who self-reported different types of police victimization were more likely to report psychotic experiences. Another study showed that greater exposure to gun violence fatalities, regardless of police involvement, was positively associated with psychotic experiences.
Obstetric complications
A variety of obstetric complications, including infection, maternal inflammation, and stress, have been associated with increased risk for psychotic disorders in U.S. samples.
“What we saw emerge from the literature is that Black women in the U.S. are at substantially increased risk for many of these obstetrical complications compared to White women, and this is not necessarily explained by socioeconomic status,” said Dr. Anglin.
Neighborhood- and individual-level factors appear to affect the disparity in these outcomes. A recent study revealed that exposure to environmental contaminants such as air pollution is associated with higher rates of preterm birth and low birth weight differentially in Black mothers compared with other mothers, “possibly as a result of an interaction between prenatal stress and contaminants,” the investigators noted.
Research also indicates that Black women are more likely to have lower levels of cortisol during the second trimester of pregnancy compared with women of other racial and ethnic groups. Cortisol is essential for fetal growth. Evidence links lower cortisol levels in later stages of pregnancy with decreased fetal growth in individuals who develop schizophrenia.
, compared with White women of the same socioeconomic status.
Such findings “highlight a complex picture” involving maternal cortisol levels and other stress biomarkers, “potentially leading to poor birth outcomes and subsequent risk for psychotic disorders in adulthood,” the investigators noted.
The researchers call for the dismantling of structural racism and the social policies and norms it shapes. They also recommend changes in health care policy and in the approach to early intervention for psychosis among Black and other racially-minoritized groups.
“Altogether, the current evidence suggests the need to identify, address, and tackle the social determinants deeply ingrained in U.S. society, in tandem with empowering the most marginalized communities,” the researchers wrote.
“We recommend that the field of psychiatry devote considerably more effort to addressing structural racism and social determinants of psychosis in funding priorities, training, and intervention development,” they added.
Dr. Anglin suggests that mental health providers use what she called a “cultural formulation interview” that takes a person’s environmental and social context into consideration. Studies show that incorporating this into clinical practice helps reduce misdiagnosis of mental illness in Black populations, she said.
Call to action
Commenting on the findings in an interview, Ned H. Kalin, MD, editor of The American Journal of Psychiatry and professor and chair of the department of psychiatry, University of Wisconsin, Madison, said the study was well done and serves as a “call to action” to address the impact of structural racism on mental health issues and psychiatric diseases.
The article highlights the need for “collecting better data” on structural racism, said Dr. Kalin. “We know it’s a big issue, but we can’t even quantitate it, so we need some fundamental measures to use as a benchmark as we move forward, as we try to make change.”
He noted that racism “is so embedded in one’s experience and in our society that we sort of don’t even think about it as a trauma.”
In psychiatry, for example, trauma is often thought of as a loss or a traumatic event. “We don’t typically think of trauma as an experience that pervades one’s entire life,” but that needs to change, he said. “At the individual level and in the doctor’s office, being sensitive to and aware of these issues is absolutely critical.”
Dr. Anglin and Dr. Kalin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.



