User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Boys may carry the weight, or overweight, of adults’ infertility
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
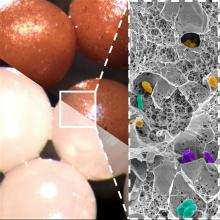
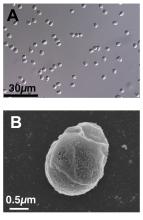
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Clinical trials: Top priority for long COVID
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
H. pylori eradication therapy curbs risk for stomach cancer
People with H. pylori who were treated had about a 63% lower risk of developing noncardia gastric adenocarcinoma (NCGA) after 8 years of follow-up, compared with peers with H. pylori who were not treated.
The U.S. data align with previous studies, conducted mostly in Asia, that found that treating the infection can reduce stomach cancer incidence.
The KPNC study shows the “potential for stomach cancer prevention in U.S. populations through H. pylori screening and treatment,” study investigator Dan Li, MD, gastroenterologist with the Kaiser Permanente Medical Group and Kaiser Permanente Division of Research in Oakland, Calif., said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health in New York, who wasn’t involved in the research, said that the study is significant because “it is the first to show this effect in a large, diverse population in the U.S., where gastric cancer incidence is lower.”
The study was published online in Gastroenterology.
Top risk factor
About 30% of people in the United States are infected with H. pylori, which is the No. 1 known risk factor for stomach cancer, Dr. Li said.
The study cohort included 716,567 KPNC members who underwent H. pylori testing and/or treatment between 1997 and 2015.
Among H. pylori–infected individuals (based on positive nonserology test results), the subdistribution hazard ratio was 6.07 for untreated individuals and 2.68 for treated individuals, compared with H. pylori–negative individuals.
It’s not surprising that people who were treated for the infection still had a higher risk of NCGA than people who had never had the infection, Dr. Li said.
“This is likely because many people with chronic H. pylori infection had already developed some precancerous changes in their stomach before they were treated. This finding suggests that H. pylori ideally should be treated before precancerous changes develop,” he said.
When compared directly with H. pylori–positive/untreated individuals, the risk for NCGA in H. pylori–positive/treated individuals was somewhat lower at less than 8 years follow-up (sHR, 0.95) and significantly lower at 8+ years of follow-up (sHR, 0.37).
“After 7-10 years of follow-up, people with H. pylori who received treatment had nearly half the risk of developing stomach cancer as the general population,” Dr. Li said. “This is likely because most people infected with H. pylori in the general population are not screened nor treated. This highlights the impact screening and treatment can have.”
The data also show that cumulative incidence curves for H. pylori–positive/untreated and H. pylori–positive/treated largely overlapped during the first 7 years of follow-up and started to separate after 8 years.
At 10 years, cumulative NCGA incidence rates for H. pylori–positive/untreated, H. pylori–positive/treated, and H. pylori negative were 31.0, 19.7, and 3.5 per 10,000 persons, respectively (P < .0001).
This study shows that treating H. pylori reduces stomach cancer incidence in the United States, thus “filling an important research and knowledge gap,” Dr. Li said.
In the United States, Asian, Black, and Hispanic adults are much more likely to be infected with H. pylori, and they have a two- to threefold higher risk of developing stomach cancer, he noted.
“This suggests it may be reasonable to consider targeted screening and treatment in these high-risk groups. However, the optimal strategy for population-based H. pylori screening has not been established, and more research is needed to determine who should be screened for H. pylori and at what age screening should begin,” Dr. Li said.
Strong data, jury out on universal screening
For additional comment, this news organization reached out to Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y.
The study shows that the treatment of H. pylori “absolutely will decrease your risk of certain types of gastric carcinoma down the line. It does take a while to show that, 7 years, but this study shows that very clearly,” Dr. Glatt said.
“People who have definitely been shown to have H. pylori should be treated,” Dr. Glatt said.
“I don’t think this study yet supports that everybody should be screened, but it does make sense that people who have upper GI symptoms consistent with H. pylori should be checked for H. pylori and then appropriately treated, he noted.
Routine screening for H. pylori is recommended in countries with high incidence of gastric cancer, but not in the United States, Dr. Kim noted.
“Given the risk reduction of cancer with H. pylori treatment, consideration should be made in the U.S. for asymptomatic individuals with a family history of gastric cancer or immigrants from high-incidence countries,” she added.
The study was funded by the Kaiser Permanente Northern California Community Health Research Grants Program, the Permanente Medical Group Delivery Science & Applied Research Program, and the Permanente Medical Group. Dr. Li, Dr. Glatt, and Dr. Kim have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with H. pylori who were treated had about a 63% lower risk of developing noncardia gastric adenocarcinoma (NCGA) after 8 years of follow-up, compared with peers with H. pylori who were not treated.
The U.S. data align with previous studies, conducted mostly in Asia, that found that treating the infection can reduce stomach cancer incidence.
The KPNC study shows the “potential for stomach cancer prevention in U.S. populations through H. pylori screening and treatment,” study investigator Dan Li, MD, gastroenterologist with the Kaiser Permanente Medical Group and Kaiser Permanente Division of Research in Oakland, Calif., said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health in New York, who wasn’t involved in the research, said that the study is significant because “it is the first to show this effect in a large, diverse population in the U.S., where gastric cancer incidence is lower.”
The study was published online in Gastroenterology.
Top risk factor
About 30% of people in the United States are infected with H. pylori, which is the No. 1 known risk factor for stomach cancer, Dr. Li said.
The study cohort included 716,567 KPNC members who underwent H. pylori testing and/or treatment between 1997 and 2015.
Among H. pylori–infected individuals (based on positive nonserology test results), the subdistribution hazard ratio was 6.07 for untreated individuals and 2.68 for treated individuals, compared with H. pylori–negative individuals.
It’s not surprising that people who were treated for the infection still had a higher risk of NCGA than people who had never had the infection, Dr. Li said.
“This is likely because many people with chronic H. pylori infection had already developed some precancerous changes in their stomach before they were treated. This finding suggests that H. pylori ideally should be treated before precancerous changes develop,” he said.
When compared directly with H. pylori–positive/untreated individuals, the risk for NCGA in H. pylori–positive/treated individuals was somewhat lower at less than 8 years follow-up (sHR, 0.95) and significantly lower at 8+ years of follow-up (sHR, 0.37).
“After 7-10 years of follow-up, people with H. pylori who received treatment had nearly half the risk of developing stomach cancer as the general population,” Dr. Li said. “This is likely because most people infected with H. pylori in the general population are not screened nor treated. This highlights the impact screening and treatment can have.”
The data also show that cumulative incidence curves for H. pylori–positive/untreated and H. pylori–positive/treated largely overlapped during the first 7 years of follow-up and started to separate after 8 years.
At 10 years, cumulative NCGA incidence rates for H. pylori–positive/untreated, H. pylori–positive/treated, and H. pylori negative were 31.0, 19.7, and 3.5 per 10,000 persons, respectively (P < .0001).
This study shows that treating H. pylori reduces stomach cancer incidence in the United States, thus “filling an important research and knowledge gap,” Dr. Li said.
In the United States, Asian, Black, and Hispanic adults are much more likely to be infected with H. pylori, and they have a two- to threefold higher risk of developing stomach cancer, he noted.
“This suggests it may be reasonable to consider targeted screening and treatment in these high-risk groups. However, the optimal strategy for population-based H. pylori screening has not been established, and more research is needed to determine who should be screened for H. pylori and at what age screening should begin,” Dr. Li said.
Strong data, jury out on universal screening
For additional comment, this news organization reached out to Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y.
The study shows that the treatment of H. pylori “absolutely will decrease your risk of certain types of gastric carcinoma down the line. It does take a while to show that, 7 years, but this study shows that very clearly,” Dr. Glatt said.
“People who have definitely been shown to have H. pylori should be treated,” Dr. Glatt said.
“I don’t think this study yet supports that everybody should be screened, but it does make sense that people who have upper GI symptoms consistent with H. pylori should be checked for H. pylori and then appropriately treated, he noted.
Routine screening for H. pylori is recommended in countries with high incidence of gastric cancer, but not in the United States, Dr. Kim noted.
“Given the risk reduction of cancer with H. pylori treatment, consideration should be made in the U.S. for asymptomatic individuals with a family history of gastric cancer or immigrants from high-incidence countries,” she added.
The study was funded by the Kaiser Permanente Northern California Community Health Research Grants Program, the Permanente Medical Group Delivery Science & Applied Research Program, and the Permanente Medical Group. Dr. Li, Dr. Glatt, and Dr. Kim have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with H. pylori who were treated had about a 63% lower risk of developing noncardia gastric adenocarcinoma (NCGA) after 8 years of follow-up, compared with peers with H. pylori who were not treated.
The U.S. data align with previous studies, conducted mostly in Asia, that found that treating the infection can reduce stomach cancer incidence.
The KPNC study shows the “potential for stomach cancer prevention in U.S. populations through H. pylori screening and treatment,” study investigator Dan Li, MD, gastroenterologist with the Kaiser Permanente Medical Group and Kaiser Permanente Division of Research in Oakland, Calif., said in an interview.
Judith Kim, MD, a gastroenterologist at NYU Langone Health in New York, who wasn’t involved in the research, said that the study is significant because “it is the first to show this effect in a large, diverse population in the U.S., where gastric cancer incidence is lower.”
The study was published online in Gastroenterology.
Top risk factor
About 30% of people in the United States are infected with H. pylori, which is the No. 1 known risk factor for stomach cancer, Dr. Li said.
The study cohort included 716,567 KPNC members who underwent H. pylori testing and/or treatment between 1997 and 2015.
Among H. pylori–infected individuals (based on positive nonserology test results), the subdistribution hazard ratio was 6.07 for untreated individuals and 2.68 for treated individuals, compared with H. pylori–negative individuals.
It’s not surprising that people who were treated for the infection still had a higher risk of NCGA than people who had never had the infection, Dr. Li said.
“This is likely because many people with chronic H. pylori infection had already developed some precancerous changes in their stomach before they were treated. This finding suggests that H. pylori ideally should be treated before precancerous changes develop,” he said.
When compared directly with H. pylori–positive/untreated individuals, the risk for NCGA in H. pylori–positive/treated individuals was somewhat lower at less than 8 years follow-up (sHR, 0.95) and significantly lower at 8+ years of follow-up (sHR, 0.37).
“After 7-10 years of follow-up, people with H. pylori who received treatment had nearly half the risk of developing stomach cancer as the general population,” Dr. Li said. “This is likely because most people infected with H. pylori in the general population are not screened nor treated. This highlights the impact screening and treatment can have.”
The data also show that cumulative incidence curves for H. pylori–positive/untreated and H. pylori–positive/treated largely overlapped during the first 7 years of follow-up and started to separate after 8 years.
At 10 years, cumulative NCGA incidence rates for H. pylori–positive/untreated, H. pylori–positive/treated, and H. pylori negative were 31.0, 19.7, and 3.5 per 10,000 persons, respectively (P < .0001).
This study shows that treating H. pylori reduces stomach cancer incidence in the United States, thus “filling an important research and knowledge gap,” Dr. Li said.
In the United States, Asian, Black, and Hispanic adults are much more likely to be infected with H. pylori, and they have a two- to threefold higher risk of developing stomach cancer, he noted.
“This suggests it may be reasonable to consider targeted screening and treatment in these high-risk groups. However, the optimal strategy for population-based H. pylori screening has not been established, and more research is needed to determine who should be screened for H. pylori and at what age screening should begin,” Dr. Li said.
Strong data, jury out on universal screening
For additional comment, this news organization reached out to Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America and chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau in Oceanside, N.Y.
The study shows that the treatment of H. pylori “absolutely will decrease your risk of certain types of gastric carcinoma down the line. It does take a while to show that, 7 years, but this study shows that very clearly,” Dr. Glatt said.
“People who have definitely been shown to have H. pylori should be treated,” Dr. Glatt said.
“I don’t think this study yet supports that everybody should be screened, but it does make sense that people who have upper GI symptoms consistent with H. pylori should be checked for H. pylori and then appropriately treated, he noted.
Routine screening for H. pylori is recommended in countries with high incidence of gastric cancer, but not in the United States, Dr. Kim noted.
“Given the risk reduction of cancer with H. pylori treatment, consideration should be made in the U.S. for asymptomatic individuals with a family history of gastric cancer or immigrants from high-incidence countries,” she added.
The study was funded by the Kaiser Permanente Northern California Community Health Research Grants Program, the Permanente Medical Group Delivery Science & Applied Research Program, and the Permanente Medical Group. Dr. Li, Dr. Glatt, and Dr. Kim have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM GASTROENTEROLOGY
New drugs in primary care: Lessons learned from COVID-19
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
AT INTERNAL MEDICINE 2023
Medical-level empathy? Yup, ChatGPT can fake that
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
FDA approves first RSV vaccine for older adults
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older, the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older, the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older, the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
New outbreaks of Marburg virus disease: What clinicians need to know
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
White House to end COVID vaccine mandate for federal workers
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
The move means vaccines will no longer be required for workers who are federal employees, federal contractors, Head Start early education employees, workers at Medicare-certified health care facilities, and those who work at U.S. borders. International air travelers will no longer be required to prove their vaccination status. The requirement will be lifted at the end of the day on May 11, which is also when the federal public health emergency declaration ends.
“While vaccination remains one of the most important tools in advancing the health and safety of employees and promoting the efficiency of workplaces, we are now in a different phase of our response when these measures are no longer necessary,” an announcement from the White House stated.
White House officials credited vaccine requirements with saving millions of lives, noting that the rules ensured “the safety of workers in critical workforces including those in the healthcare and education sectors, protecting themselves and the populations they serve, and strengthening their ability to provide services without disruptions to operations.”
More than 100 million people were subject to the vaccine requirement, The Associated Press reported. All but 2% of those covered by the mandate had received at least one dose or had a pending or approved exception on file by January 2022, the Biden administration said, noting that COVID deaths have dropped 95% since January 2021 and hospitalizations are down nearly 91%.
In January, vaccine requirements were lifted for U.S. military members.
On the government-run website Safer Federal Workforce, which helped affected organizations put federal COVID rules into place, agencies were told to “take no action to implement or enforce the COVID-19 vaccination requirement” at this time.
A version of this article first appeared on WebMD.com.
Long-COVID rate may be higher with rheumatic diseases
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
MANCHESTER, England – Data from the COVAD-2 e-survey suggest that people with a rheumatic disease are twice as likely as are those without to experience long-term effects after contracting COVID-19.
The prevalence of post–COVID-19 condition (PCC), the term the World Health Organization advocates for describing the widely popularized term long COVID, was 10.8% among people with autoimmune rheumatic diseases (AIRDs) vs. 5.3% among those with no autoimmune condition (designated as “healthy controls”). The odds ratio was 2.1, with a 95% confidence interval of 1.4-3.2 and a P-value of .002.
The prevalence in people with nonrheumatic autoimmune diseases was also higher than it was in the control participants but still lower, at 7.3%, than in those with AIRDs.
“Our findings highlight the importance of close monitoring for PCC,” Arvind Nune, MBBCh, MSc, said in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
They also show the need for “appropriate referral for optimized multidisciplinary care for patients with autoimmune rheumatic diseases during the recovery period following COVID-19,” added Dr. Nune, who works for Southport (England) and Ormskirk Hospital NHS Trust.
In an interview, he noted that it was patients who had a severe COVID-19 course or had other coexisting conditions that appeared to experience more long-term effects than did their less-affected counterparts.
Commenting on the study, Jeffrey A. Sparks, MD, MMSc, told this news organization: “This is one of the first studies to find that the prevalence of long COVID is higher among people with systemic rheumatic diseases than those without.”
Dr. Sparks, who is based at Brigham and Women’s Hospital and Harvard Medical School in Boston, added: “Since the symptoms of long COVID and rheumatic diseases can overlap substantially, more work will need to be done to determine whether COVID may have induced flares, new symptoms, or whether the finding is due to the presence of the chronic rheumatic disease.”
The COVAD study
Using an electronic survey platform, the COVAD study has been set up to look at the long-term efficacy and safety of COVID-19 vaccinations in patients with AIRDs. It’s now a large international effort involving more than 150 collaborating clinics in 106 countries.
A huge amount of data has been collected. “We collected demographics, details of autoimmune disease, including treatment, comorbidity, COVID infection, vaccination history and outcomes, date on flares, and validated patient-reported outcomes, including pain, fatigue, physical function, and quality of life,” Dr. Nune said in his presentation.
A total of 12,358 people who were invited to participate responded to the e-survey. Of them, 2,640 were confirmed to have COVID-19. Because the analysis aimed to look at PCC, anyone who had completed the survey less than 3 months after infection was excluded. This left 1,677 eligible respondents, of whom, an overall 8.7% (n = 136) were identified as having PCC.
“The [WHO] definition for PCC was employed, which is persistent signs or symptoms beyond 3 months of COVID-19 infection lasting at least 2 months,” Dr. Nune told this news organization.
“Symptoms could be anything from fatigue to breathlessness to arthralgias,” he added. However, the focus of the present analysis was to look at how many people were experiencing the condition rather than specific symptoms.
A higher risk for PCC was seen in women than in men (OR, 2.9; 95% CI, 1.1-7.7; P = .037) in the entire cohort.
In addition, those with comorbidities were found to have a greater chance of long-term sequelae from COVID-19 than were those without comorbid disease (OR, 2.8; 95% CI, 1.4-5.7; P = .005).
Patients who experienced more severe acute COVID-19, such as those who needed intensive care treatment, oxygen therapy, or advanced treatment for COVID-19 with monoclonal antibodies, were significantly more likely to later have PCC than were those who did not (OR, 3.8; 95% CI, 1.1-13.6; P = .039).
Having PCC was also associated with poorer patient-reported outcomes for physical function, compared with not having PCC. “However, no association with disease flares of underlying rheumatic diseases or immunosuppressive drugs used were noted,” Dr. Nune said.
These new findings from the COVAD study should be published soon. Dr. Nune suggested that the findings might be used to help identify patients early so that they can be referred to the appropriate services in good time.
The COVAD study was independently supported. Dr. Nune reports no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Sparks has received research support from Bristol-Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
AT BSR 2023