User login
In Case You Missed It: COVID
Pandemic hit Black children harder, study shows
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Children and COVID: A look back as the fourth year begins
With 3 years of the COVID-19 experience now past, it’s safe to say that SARS-CoV-2 changed American society in ways that could not have been predicted when the first U.S. cases were reported in January of 2020.
Who would have guessed back then that not one but two vaccines would be developed, approved, and widely distributed before the end of the year? Or that those vaccines would be rejected by large segments of the population on ideological grounds? Could anyone have predicted in early 2020 that schools in 21 states would be forbidden by law to require COVID-19 vaccination in students?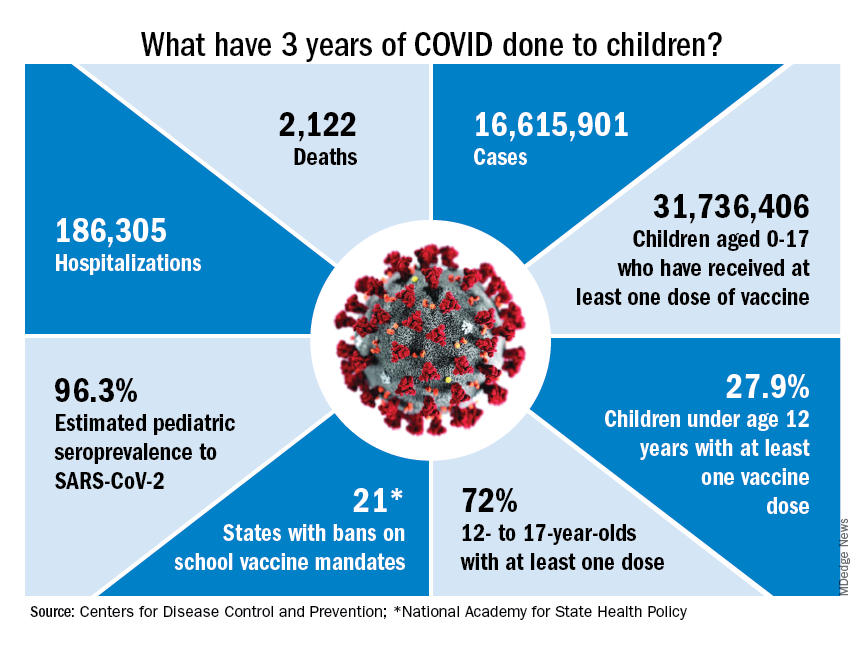
Vaccination is generally considered to be an activity of childhood, but that practice has been turned upside down with COVID-19. Among Americans aged 65 years and older, 95% have received at least one dose of vaccine, versus 27.9% of children younger than 12 years old, according to the Centers for Disease Control and Prevention.
The vaccine situation for children mirrors that of the population as a whole. The oldest children have the highest vaccination rates, and the rates decline along with age: 72.0% of those aged 12-17 years have received at least one dose, compared with 39.8% of 5- to 11-year-olds, 10.5% of 2- to 4-year-olds, and 8.0% of children under age 2, the CDC said on its COVID Data Tracker.
The youngest children were, of course, the last ones to be eligible for the vaccine, but their uptake has been much slower since emergency use was authorized in June of 2022. In the nearly 9 months since then, 9.5% of children aged 4 and under have received at least one dose, versus 66% of children aged 12-15 years in the first 9 months (May 2021 to March 2022).
Altogether, a total of 31.7 million, or 43%, of all children under age 18 had received at least one dose of COVID-19 vaccine as of March 8, 2023, according to the most recent CDC data.
Incidence: Counting COVID
Vaccination and other prevention efforts have tried to stem the tide, but what has COVID actually done to children since the Trump administration declared a nationwide emergency on March 13, 2020?
- 16.6 million cases.
- 186,035 new hospital admissions.
- 2,122 deaths.
Even the proportion of total COVID cases in children, 17.2%, is less than might be expected, given their relatively undervaccinated status.
Seroprevalence estimates seem to support the undercounting of pediatric cases. A survey of commercial laboratories working with the CDC put the seroprevalance of SARS-CoV-2 antibodies in children at 96.3% as of late 2022, based on tests of almost 27,000 specimens performed over an 8-week period from mid-October to mid-December. That would put the number of infected children at 65.7 million children.
Since Omicron
There has not been another major COVID-19 surge since the winter of 2021-2022, when the weekly rate of new cases reached 1,900 per 100,000 population in children aged 16-17 years in early January 2022 – the highest seen among children of any of the CDC’s age groups (0-4, 5-11, 12-15, 16-17) during the entire pandemic. Since the Omicron surge, the highest weekly rate was 221 per 100,000 during the week of May 15-21, again in 16- to 17-year-olds, the CDC reports.
The widely anticipated surge of COVID in the fall and winter of 2022 and 2023 – the so-called “tripledemic” involving influenza and respiratory syncytial virus – did not occur, possibly because so many Americans were vaccinated or previously infected, experts suggested. New-case rates, emergency room visits, and hospitalizations in children have continued to drop as winter comes to a close, CDC data show.
With 3 years of the COVID-19 experience now past, it’s safe to say that SARS-CoV-2 changed American society in ways that could not have been predicted when the first U.S. cases were reported in January of 2020.
Who would have guessed back then that not one but two vaccines would be developed, approved, and widely distributed before the end of the year? Or that those vaccines would be rejected by large segments of the population on ideological grounds? Could anyone have predicted in early 2020 that schools in 21 states would be forbidden by law to require COVID-19 vaccination in students?
Vaccination is generally considered to be an activity of childhood, but that practice has been turned upside down with COVID-19. Among Americans aged 65 years and older, 95% have received at least one dose of vaccine, versus 27.9% of children younger than 12 years old, according to the Centers for Disease Control and Prevention.
The vaccine situation for children mirrors that of the population as a whole. The oldest children have the highest vaccination rates, and the rates decline along with age: 72.0% of those aged 12-17 years have received at least one dose, compared with 39.8% of 5- to 11-year-olds, 10.5% of 2- to 4-year-olds, and 8.0% of children under age 2, the CDC said on its COVID Data Tracker.
The youngest children were, of course, the last ones to be eligible for the vaccine, but their uptake has been much slower since emergency use was authorized in June of 2022. In the nearly 9 months since then, 9.5% of children aged 4 and under have received at least one dose, versus 66% of children aged 12-15 years in the first 9 months (May 2021 to March 2022).
Altogether, a total of 31.7 million, or 43%, of all children under age 18 had received at least one dose of COVID-19 vaccine as of March 8, 2023, according to the most recent CDC data.
Incidence: Counting COVID
Vaccination and other prevention efforts have tried to stem the tide, but what has COVID actually done to children since the Trump administration declared a nationwide emergency on March 13, 2020?
- 16.6 million cases.
- 186,035 new hospital admissions.
- 2,122 deaths.
Even the proportion of total COVID cases in children, 17.2%, is less than might be expected, given their relatively undervaccinated status.
Seroprevalence estimates seem to support the undercounting of pediatric cases. A survey of commercial laboratories working with the CDC put the seroprevalance of SARS-CoV-2 antibodies in children at 96.3% as of late 2022, based on tests of almost 27,000 specimens performed over an 8-week period from mid-October to mid-December. That would put the number of infected children at 65.7 million children.
Since Omicron
There has not been another major COVID-19 surge since the winter of 2021-2022, when the weekly rate of new cases reached 1,900 per 100,000 population in children aged 16-17 years in early January 2022 – the highest seen among children of any of the CDC’s age groups (0-4, 5-11, 12-15, 16-17) during the entire pandemic. Since the Omicron surge, the highest weekly rate was 221 per 100,000 during the week of May 15-21, again in 16- to 17-year-olds, the CDC reports.
The widely anticipated surge of COVID in the fall and winter of 2022 and 2023 – the so-called “tripledemic” involving influenza and respiratory syncytial virus – did not occur, possibly because so many Americans were vaccinated or previously infected, experts suggested. New-case rates, emergency room visits, and hospitalizations in children have continued to drop as winter comes to a close, CDC data show.
With 3 years of the COVID-19 experience now past, it’s safe to say that SARS-CoV-2 changed American society in ways that could not have been predicted when the first U.S. cases were reported in January of 2020.
Who would have guessed back then that not one but two vaccines would be developed, approved, and widely distributed before the end of the year? Or that those vaccines would be rejected by large segments of the population on ideological grounds? Could anyone have predicted in early 2020 that schools in 21 states would be forbidden by law to require COVID-19 vaccination in students?
Vaccination is generally considered to be an activity of childhood, but that practice has been turned upside down with COVID-19. Among Americans aged 65 years and older, 95% have received at least one dose of vaccine, versus 27.9% of children younger than 12 years old, according to the Centers for Disease Control and Prevention.
The vaccine situation for children mirrors that of the population as a whole. The oldest children have the highest vaccination rates, and the rates decline along with age: 72.0% of those aged 12-17 years have received at least one dose, compared with 39.8% of 5- to 11-year-olds, 10.5% of 2- to 4-year-olds, and 8.0% of children under age 2, the CDC said on its COVID Data Tracker.
The youngest children were, of course, the last ones to be eligible for the vaccine, but their uptake has been much slower since emergency use was authorized in June of 2022. In the nearly 9 months since then, 9.5% of children aged 4 and under have received at least one dose, versus 66% of children aged 12-15 years in the first 9 months (May 2021 to March 2022).
Altogether, a total of 31.7 million, or 43%, of all children under age 18 had received at least one dose of COVID-19 vaccine as of March 8, 2023, according to the most recent CDC data.
Incidence: Counting COVID
Vaccination and other prevention efforts have tried to stem the tide, but what has COVID actually done to children since the Trump administration declared a nationwide emergency on March 13, 2020?
- 16.6 million cases.
- 186,035 new hospital admissions.
- 2,122 deaths.
Even the proportion of total COVID cases in children, 17.2%, is less than might be expected, given their relatively undervaccinated status.
Seroprevalence estimates seem to support the undercounting of pediatric cases. A survey of commercial laboratories working with the CDC put the seroprevalance of SARS-CoV-2 antibodies in children at 96.3% as of late 2022, based on tests of almost 27,000 specimens performed over an 8-week period from mid-October to mid-December. That would put the number of infected children at 65.7 million children.
Since Omicron
There has not been another major COVID-19 surge since the winter of 2021-2022, when the weekly rate of new cases reached 1,900 per 100,000 population in children aged 16-17 years in early January 2022 – the highest seen among children of any of the CDC’s age groups (0-4, 5-11, 12-15, 16-17) during the entire pandemic. Since the Omicron surge, the highest weekly rate was 221 per 100,000 during the week of May 15-21, again in 16- to 17-year-olds, the CDC reports.
The widely anticipated surge of COVID in the fall and winter of 2022 and 2023 – the so-called “tripledemic” involving influenza and respiratory syncytial virus – did not occur, possibly because so many Americans were vaccinated or previously infected, experts suggested. New-case rates, emergency room visits, and hospitalizations in children have continued to drop as winter comes to a close, CDC data show.
COVID raises risk for long-term GI complications
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Factors linked with increased VTE risk in COVID outpatients
Though VTE risk is well studied and significant in those hospitalized with COVID, little is known about the risk in the outpatient setting, said the authors of the new research published online in JAMA Network Open.
The study was conducted at two integrated health care delivery systems in northern and southern California. Data were gathered from the Kaiser Permanente Virtual Data Warehouse and electronic health records.
Nearly 400,000 patients studied
Researchers, led by Margaret Fang, MD, with the division of hospital medicine, University of California, San Francisco, identified 398,530 outpatients with COVID-19 from Jan. 1, 2020, through Jan. 31, 2021.
VTE risk was low overall for ambulatory COVID patients.
“It is a reassuring study,” Dr. Fang said in an interview.
The researchers found that the risk is highest in the first 30 days after COVID-19 diagnosis (unadjusted rate, 0.58; 95% confidence interval, 0.51-0.67 per 100 person-years vs. 0.09; 95% CI, 0.08-0.11 per 100 person-years after 30 days).
Factors linked with high VTE risk
They also found that several factors were linked with a higher risk of blood clots in the study population, including being at least 55 years old; being male; having a history of blood clots or thrombophilia; and a body mass index (BMI) of at least 30 kg/m2.
The authors write, “These findings may help identify subsets of patients with COVID-19 who could benefit from VTE preventive strategies and more intensive short-term surveillance.”
Are routine anticoagulants justified?
Previously, randomized clinical trials have found that hospitalized patients with moderate COVID-19 may benefit from therapeutically dosed heparin anticoagulants but that therapeutic anticoagulation had no net benefit – and perhaps could even harm – patients who were critically ill with COVID.
“[M]uch less is known about the optimal thromboprophylaxis strategy for people with milder presentations of COVID-19 who do not require hospitalization,” they write.
Mild COVID VTE risk similar to general population
The authors note that rates of blood clots linked with COVID-19 are not much higher than the average blood clot rate in the general population, which is about 0.1-0.2 per 100 person-years.
Therefore, the results don’t justify routine administration of anticoagulation given the costs, inconvenience, and bleeding risks, they acknowledge.
Dr. Fang told this publication that it’s hard to know what to tell patients, given the overall low VTE risk. She said their study wasn’t designed to advise when to give prophylaxis.
Physicians should inform patients of their higher risk
“We should tell our patients who fall into these risk categories that blood clot is a concern after the development of COVID, especially in those first 30 days. And some people might benefit from increased surveillance,” Dr. Fang said.
”I think this study would support ongoing studies that look at whether selected patients benefit from VTE prophylaxis, for example low-dose anticoagulants,” she said.
Dr. Fang said the subgroup factors they found increased risk of blood clots for all patients, not just COVID-19 patients. It’s not clear why factors such as being male may increase blood clot risk, though that is consistent with previous literature, but higher risk with higher BMI might be related to a combination of inflammation or decreased mobility, she said.
Unanswered questions
Robert H. Hopkins Jr., MD, says the study helps answer a couple of important questions – that the VTE risk in nonhospitalized COVID-19 patients is low and when and for which patients risk may be highest.
However, there are several unanswered questions that argue against routine initiation of anticoagulants, notes the professor of internal medicine and pediatrics chief, division of general internal medicine, at University of Arkansas for Medical Sciences, Little Rock.
One is the change in the COVID variant landscape.
“We do not know whether rates of VTE are same or lower or higher with current circulating variants,” Dr. Hopkins said.
The authors acknowledge this as a limitation. Study data predate Omicron and subvariants, which appear to lower clinical severity, so it’s unclear whether VTE risk is different in this Omicron era.
Dr. Hopkins added another unknown: “We do not know whether vaccination affects rates of VTE in ambulatory breakthrough infection.”
Dr. Hopkins and the authors also note the lack of a control group in the study, to better compare risk.
Coauthor Dr. Prasad reports consultant fees from EpiExcellence LLC outside the submitted work. Coauthor Dr. Go reports grants paid to the division of research, Kaiser Permanente Northern California, from CSL Behring, Novartis, Bristol Meyers Squibb/Pfizer Alliance, and Janssen outside the submitted work.
The research was funded through Patient-Centered Outcomes Research Institute.
Dr. Hopkins reports no relevant financial relationships.
Though VTE risk is well studied and significant in those hospitalized with COVID, little is known about the risk in the outpatient setting, said the authors of the new research published online in JAMA Network Open.
The study was conducted at two integrated health care delivery systems in northern and southern California. Data were gathered from the Kaiser Permanente Virtual Data Warehouse and electronic health records.
Nearly 400,000 patients studied
Researchers, led by Margaret Fang, MD, with the division of hospital medicine, University of California, San Francisco, identified 398,530 outpatients with COVID-19 from Jan. 1, 2020, through Jan. 31, 2021.
VTE risk was low overall for ambulatory COVID patients.
“It is a reassuring study,” Dr. Fang said in an interview.
The researchers found that the risk is highest in the first 30 days after COVID-19 diagnosis (unadjusted rate, 0.58; 95% confidence interval, 0.51-0.67 per 100 person-years vs. 0.09; 95% CI, 0.08-0.11 per 100 person-years after 30 days).
Factors linked with high VTE risk
They also found that several factors were linked with a higher risk of blood clots in the study population, including being at least 55 years old; being male; having a history of blood clots or thrombophilia; and a body mass index (BMI) of at least 30 kg/m2.
The authors write, “These findings may help identify subsets of patients with COVID-19 who could benefit from VTE preventive strategies and more intensive short-term surveillance.”
Are routine anticoagulants justified?
Previously, randomized clinical trials have found that hospitalized patients with moderate COVID-19 may benefit from therapeutically dosed heparin anticoagulants but that therapeutic anticoagulation had no net benefit – and perhaps could even harm – patients who were critically ill with COVID.
“[M]uch less is known about the optimal thromboprophylaxis strategy for people with milder presentations of COVID-19 who do not require hospitalization,” they write.
Mild COVID VTE risk similar to general population
The authors note that rates of blood clots linked with COVID-19 are not much higher than the average blood clot rate in the general population, which is about 0.1-0.2 per 100 person-years.
Therefore, the results don’t justify routine administration of anticoagulation given the costs, inconvenience, and bleeding risks, they acknowledge.
Dr. Fang told this publication that it’s hard to know what to tell patients, given the overall low VTE risk. She said their study wasn’t designed to advise when to give prophylaxis.
Physicians should inform patients of their higher risk
“We should tell our patients who fall into these risk categories that blood clot is a concern after the development of COVID, especially in those first 30 days. And some people might benefit from increased surveillance,” Dr. Fang said.
”I think this study would support ongoing studies that look at whether selected patients benefit from VTE prophylaxis, for example low-dose anticoagulants,” she said.
Dr. Fang said the subgroup factors they found increased risk of blood clots for all patients, not just COVID-19 patients. It’s not clear why factors such as being male may increase blood clot risk, though that is consistent with previous literature, but higher risk with higher BMI might be related to a combination of inflammation or decreased mobility, she said.
Unanswered questions
Robert H. Hopkins Jr., MD, says the study helps answer a couple of important questions – that the VTE risk in nonhospitalized COVID-19 patients is low and when and for which patients risk may be highest.
However, there are several unanswered questions that argue against routine initiation of anticoagulants, notes the professor of internal medicine and pediatrics chief, division of general internal medicine, at University of Arkansas for Medical Sciences, Little Rock.
One is the change in the COVID variant landscape.
“We do not know whether rates of VTE are same or lower or higher with current circulating variants,” Dr. Hopkins said.
The authors acknowledge this as a limitation. Study data predate Omicron and subvariants, which appear to lower clinical severity, so it’s unclear whether VTE risk is different in this Omicron era.
Dr. Hopkins added another unknown: “We do not know whether vaccination affects rates of VTE in ambulatory breakthrough infection.”
Dr. Hopkins and the authors also note the lack of a control group in the study, to better compare risk.
Coauthor Dr. Prasad reports consultant fees from EpiExcellence LLC outside the submitted work. Coauthor Dr. Go reports grants paid to the division of research, Kaiser Permanente Northern California, from CSL Behring, Novartis, Bristol Meyers Squibb/Pfizer Alliance, and Janssen outside the submitted work.
The research was funded through Patient-Centered Outcomes Research Institute.
Dr. Hopkins reports no relevant financial relationships.
Though VTE risk is well studied and significant in those hospitalized with COVID, little is known about the risk in the outpatient setting, said the authors of the new research published online in JAMA Network Open.
The study was conducted at two integrated health care delivery systems in northern and southern California. Data were gathered from the Kaiser Permanente Virtual Data Warehouse and electronic health records.
Nearly 400,000 patients studied
Researchers, led by Margaret Fang, MD, with the division of hospital medicine, University of California, San Francisco, identified 398,530 outpatients with COVID-19 from Jan. 1, 2020, through Jan. 31, 2021.
VTE risk was low overall for ambulatory COVID patients.
“It is a reassuring study,” Dr. Fang said in an interview.
The researchers found that the risk is highest in the first 30 days after COVID-19 diagnosis (unadjusted rate, 0.58; 95% confidence interval, 0.51-0.67 per 100 person-years vs. 0.09; 95% CI, 0.08-0.11 per 100 person-years after 30 days).
Factors linked with high VTE risk
They also found that several factors were linked with a higher risk of blood clots in the study population, including being at least 55 years old; being male; having a history of blood clots or thrombophilia; and a body mass index (BMI) of at least 30 kg/m2.
The authors write, “These findings may help identify subsets of patients with COVID-19 who could benefit from VTE preventive strategies and more intensive short-term surveillance.”
Are routine anticoagulants justified?
Previously, randomized clinical trials have found that hospitalized patients with moderate COVID-19 may benefit from therapeutically dosed heparin anticoagulants but that therapeutic anticoagulation had no net benefit – and perhaps could even harm – patients who were critically ill with COVID.
“[M]uch less is known about the optimal thromboprophylaxis strategy for people with milder presentations of COVID-19 who do not require hospitalization,” they write.
Mild COVID VTE risk similar to general population
The authors note that rates of blood clots linked with COVID-19 are not much higher than the average blood clot rate in the general population, which is about 0.1-0.2 per 100 person-years.
Therefore, the results don’t justify routine administration of anticoagulation given the costs, inconvenience, and bleeding risks, they acknowledge.
Dr. Fang told this publication that it’s hard to know what to tell patients, given the overall low VTE risk. She said their study wasn’t designed to advise when to give prophylaxis.
Physicians should inform patients of their higher risk
“We should tell our patients who fall into these risk categories that blood clot is a concern after the development of COVID, especially in those first 30 days. And some people might benefit from increased surveillance,” Dr. Fang said.
”I think this study would support ongoing studies that look at whether selected patients benefit from VTE prophylaxis, for example low-dose anticoagulants,” she said.
Dr. Fang said the subgroup factors they found increased risk of blood clots for all patients, not just COVID-19 patients. It’s not clear why factors such as being male may increase blood clot risk, though that is consistent with previous literature, but higher risk with higher BMI might be related to a combination of inflammation or decreased mobility, she said.
Unanswered questions
Robert H. Hopkins Jr., MD, says the study helps answer a couple of important questions – that the VTE risk in nonhospitalized COVID-19 patients is low and when and for which patients risk may be highest.
However, there are several unanswered questions that argue against routine initiation of anticoagulants, notes the professor of internal medicine and pediatrics chief, division of general internal medicine, at University of Arkansas for Medical Sciences, Little Rock.
One is the change in the COVID variant landscape.
“We do not know whether rates of VTE are same or lower or higher with current circulating variants,” Dr. Hopkins said.
The authors acknowledge this as a limitation. Study data predate Omicron and subvariants, which appear to lower clinical severity, so it’s unclear whether VTE risk is different in this Omicron era.
Dr. Hopkins added another unknown: “We do not know whether vaccination affects rates of VTE in ambulatory breakthrough infection.”
Dr. Hopkins and the authors also note the lack of a control group in the study, to better compare risk.
Coauthor Dr. Prasad reports consultant fees from EpiExcellence LLC outside the submitted work. Coauthor Dr. Go reports grants paid to the division of research, Kaiser Permanente Northern California, from CSL Behring, Novartis, Bristol Meyers Squibb/Pfizer Alliance, and Janssen outside the submitted work.
The research was funded through Patient-Centered Outcomes Research Institute.
Dr. Hopkins reports no relevant financial relationships.
FROM JAMA NETWORK OPEN
FREEDOM COVID: Full-dose anticoagulation cut mortality but missed primary endpoint
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
Study conducted in noncritically ill
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
AT ACC 2023
‘Breakthrough’ study: Diabetes drug helps prevent long COVID
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
Effect of the COVID-19 Pandemic on Resources, Other Diseases, and Healthcare Workers’ Experience
Introduction
The COVID-19 pandemic has changed the healthcare system in a multitude of ways, affecting healthcare capacity, treatment of other illnesses, and wellness as well as professional retention of healthcare workers.1-3 During the peak of the COVID-19 pandemic, healthcare capacity was tested and resources were used up quickly.1 As the pandemic has progressed, healthcare systems have had to decide how to proceed with lessons learned, reassessing the environment of care delivery, healthcare supply chains, workforce structures, communication systems, and scientific collaboration as well as policy frameworks in healthcare.4
There have been both immediate effects and long-term consequences of the delay in care for other conditions.2,5 One stark example of this is in cancer care, where screening and procedures were postponed or canceled due to the pandemic with a resulting predicted 2% increase in cancer mortality in the next 10 years.2 The care of heart disease, chronic illnesses, and other viruses has also been similarly negatively impacted by the COVID-19 pandemic due to similar delays in diagnosis and treatment.5-7
The impact on healthcare workers has also been profound.3 Occupational stress from the pandemic has correlated with increased depression and posttraumatic stress disorder (PTSD) among other mental health diseases in healthcare workers.3 In a survey of neurosurgery residents, 26.1% of physicians reported feeling burnt out, and 65.8% were worried that they would not be able to reach surgical milestones.8,9 Among respiratory therapists, a hard hit group during this time, 79% reported burnout.10 Additionally, more healthcare workers left the field during the pandemic, with 15 million lost jobs. Future recovery of jobs looks bleak in some settings, like long-term care and among assistants and aides.11 Overall, the long-term outcomes of these resource, disease, and mental health disruptions need to be assessed and solutions created to maintain a quality and effective healthcare system, with ample resources and measures to account for disease increases and address the impact on providers.
Healthcare Capacity and Resources
With COVID-19 affecting over 100 million in the United States as of March 1, 2023, the impact on healthcare resources since the start of the pandemic has been immense.12 With 5% to 38% of hospitalized patients being admitted to the intensive care unit (ICU) and 75% to 88% of those patients requiring mechanical ventilation, a huge strain was placed on resources during and after the pandemic.1
The question of balancing resources for other hospital needs while tending to patients with COVID-19 has been an ongoing discussion at many levels.1 One core resource concern is the lack of staff. In a survey of 77 different countries, including physicians (41%), nurses (40%), respiratory therapists (11%), and advanced practice providers (8%), 15% reported insufficient intensivists and 32% reported insufficient ICU nursing staff during March and April of 2020.1 A lack of hospital and care space that led to reallocation of limited-care acute care space was a concern. Thirteen percent reported a shortage of hospital ICU beds, while others reported the conversion of postoperative recovery rooms (20%) and operating rooms (12%) for patients with COVID-19.1
Along with staff and care space concerns, hospital survey respondents reported that healthcare equipment was also challenged. Access to COVID-19 testing was one concern, with only 35% of respondents reporting availability for all patients at the beginning of the pandemic, and 56% reporting availability for only select patients based on symptom severity.1 Access to personal protective equipment (PPE) was also affected, with PPE always available according to 83% to 95% of respondents but just 35% having access to N95 masks.1 Additionally, 26% reported that there were no respirators in their hospital, and 11% reported limited ventilators.1
Although resource depletion is a problem, studies have looked at public health measures that helped to mitigate this issue. With proper public health planning and implementation, such as physical distancing, aggressive testing, contact tracing, and increased hospital capacity, by freeing up existing resources or adding additional support, public health modeling showed that resources may be able to withstand the increase.13 Development of reallocation models at local, state, national, and international levels is an important step to be able to deal with future public health crises.14
The long-term impact from the pandemic includes disruption in the physical environment of healthcare, production, supply chain, staff structure, and workforce alterations.4 For example, the physical shape of healthcare facilities is changing to accommodate increasing volumes and decrease the risk of spreading disease.4 To accommodate the burden on staffing structure and workforce alteration, telehealth gained a prominent role.4 All in all, the pandemic has changed the healthcare system; however, institutions, organizations, and policy makers need to evaluate which measures were impactful and should be considered for long-term inclusion in healthcare practice.
Impact on Other Diseases: Cancer, Heart Disease, Chronic Illnesses, and Other Viruses
The treatment of other new and existing conditions has also been affected by the pandemic. Cancer, especially, is a disease of concern. Elective surgeries and screening were halted or altered during the pandemic, which is modeled to lead to higher cancer mortality in years to come.2 The most affected cancers were breast, lung, and colorectal cancer.2 A study of colorectal cancer screening showed that colonoscopies were delayed due to COVID-19 and that gastroenterology visits declined by 49% to 61%.15 This will likely lead to delayed cancer diagnoses and possible increases in mortality.15 Breast cancer screening was also delayed and many patients continued to avoid it for various reasons such as fears of contracting COVID-19 infection in healthcare facilities, and the economic effects of the pandemic such as job loss and healthcare coverage loss.16 These delays will result in an estimated potential 0.52% overall increase in breast cancer deaths by 2030.17
A study of 368 patients from Spain showed a 56.5% decrease in hospital admissions, usually related to heart attacks, in March and April of 2020, compared to January and February 2020.18,19 For other chronic illnesses, the pandemic resulted in decreased preventative care and management.20 The care of other infections similarly suffered. The World Health Organization announced that the number of patients receiving treatment for tuberculosis (TB) dropped by 1 million, setting the disease mitigation back considerably.20 An estimated 500,000 more people died in 2020 from TB.21 The drastic shift in focus to COVID-19 care during this period will continue to have a profound impact on other diseases like these for many years post-pandemic.
Provider Experience and Mental Health Outcomes
The impact on provider experiences and mental health has been immense. One study of 510 healthcare providers (HCPs) and first responders found that occupational stress from the pandemic correlated with psychiatric symptoms, including depression, PTSD, insomnia, and generalized anxiety.3 Occupational stress also correlated with one’s likelihood to leave the medical field and trouble doing work they had once loved.3 Half of the healthcare workers surveyed indicated a decreased likelihood of staying in their current profession after the pandemic.3
Other studies have also looked at specific subspecialties and impact on trainees during the pandemic. In neurosurgery, for example, resident burnout is high, at 26.1%.9 Additionally, the lack of surgeries in the pandemic made 65.8% of neurosurgery residents anxious about meeting career milestones.9 Respiratory therapists, a highly impacted group, also experienced burnout, reporting higher levels in those who worked more in the ICU. Another study identified several themes in the concerns reported by healthcare workers during the pandemic era including “changes in personal life and enhanced negative affect,” “gaining experience, normalization, and adaptation to the pandemic,” and “mental health considerations.”22
Some studies have investigated ways to mitigate this dissatisfaction with the healthcare field post-pandemic. Intrapreneurship, reverse mentoring, and democratized learning all had a reported positive impact on employee experience and retention during this time.23 Intrapreneurship describes entrepreneurship within an existing organization, while reverse mentoring and democratized learning refer to newer employees teaching older employees and communicative learning on a breadth of topics. Other studies have examined the necessity of having mental health resources available, and that these resources need to be multi-stage and individualistic as well as specific to certain stressors HCPs faced during the pandemic.22
Conclusion and Future Directions
The COVID-19 pandemic had stark effects on the healthcare system, impacting resources and capacity, care of other diseases, and provider mental health and experiences.1-3 After the chaos of the pandemic, many questions remain. What needs to be done now by health systems and HCPs? How can we learn from the challenges and the effects on capacity to change the healthcare workflow in times of crisis and in the present? How do we mitigate the impact of the pandemic on diagnosis and management of diseases? And how do we continue to provide healthcare workers with proper mental health and professional resources now, not just in times of stress, and encourage the future generation to pursue careers in healthcare?
These are all the questions the pandemic has left us with, and more studies and initiatives are needed to investigate solutions to these issues. The COVID-19 pandemic left behind valuable lessons and changed the healthcare system, disease management, and staffing for many. Now is the time to pick up the pieces and strategize on how to make our existing system more effective for workers and patients post pandemic.
Wahlster S, Sharma M, Lewis AK, et al. The coronavirus disease 2019 pandemic's effect on critical care resources and health-care providers: a global survey. Chest. 2021;159(2):619-633. doi:10.1016/j.chest.2020.09.070
Malagón T, Yong JHE, Tope P, Miller WH Jr, Franco EL; McGill task force on the impact of COVID-19 on cancer control and care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer. 2022;150(8):1244-1254. doi:10.1002/ijc.33884
Hendrickson RC, Slevin RA, Hoerster KD, et al. The impact of the COVID-19 pandemic on mental health, occupational functioning, and professional retention among health care workers and first responders. J Gen Intern Med. 2022;37(2):397-408. doi:10.1007/s11606-021-07252-z
Davis B, Bankhead-Kendall BK, Dumas RP. A review of COVID-19's impact on modern medical systems from a health organization management perspective. Health Technol (Berl). 2022;12(4):815-824. doi:10.1007/s12553-022-00660-z
Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368-2371. doi:10.1056/NEJMms2009984
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: the impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Jalili M, Niroomand M, Hadavand F, Zeinali K, Fotouhi A. Burnout among healthcare professionals during COVID-19 pandemic: a cross-sectional study. Int Arch Occup Environ Health. 2021;94(6):1345-1352. doi:10.1007/s00420-021-01695-x
Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142. doi:10.1016/j.jocn.2020.08.012
Miller AG, Roberts KJ, Smith BJ, et al. Prevalence of burnout among respiratory therapists amidst the COVID-19 pandemic. Respir Care. 2021;respcare.09283. doi:10.4187/respcare.09283
Frogner BK, Dill JS. Tracking turnover among health care workers during the COVID-19 pandemic: a cross-sectional study. JAMA Health Forum. 2022;3(4):e220371. doi:10.1001/jamahealthforum.2022.0371
CDC COVID data tracker. Centers for Disease Control and Prevention. Accessed December 22, 2022. http://covid-data-tracker/#datatracker-home.
Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ. 2020;192(24):E640-E646. doi:10.1503/cmaj.200715
Kaul V, Chahal J, Schrarstzhaupt IN, et al. Lessons learned from a global perspective of COVID-19. Clin Chest Med. 2022 Nov. 24. [online ahead of print]. doi:10.1016/j.ccm.2022.11.020
Issaka RB, Somsouk M. Colorectal cancer screening and prevention in the COVID-19 Era. JAMA Health Forum. 2020;1(5):e200588. doi:10.1001/jamahealthforum.2020.0588
Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59(1):1-11. doi:10.1016/j.rcl.2020.09.008
Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113(11):1484-1494. doi:10.1093/jnci/djab097
Jiménez-Blanco Bravo M, Cordero Pereda D, Sánchez Vega D, et al. Heart failure in the time of COVID-19. Cardiology. 2020;145(8):481-484. doi:10.1159/000509181
Frankfurter C, Buchan TA, Kobulnik J, et al. Reduced rate of hospital presentations for heart failure during the COVID-19 pandemic in Toronto, Canada. Can J Cardiol. 2020;36(10):1680-1684. doi:10.1016/j.cjca.2020.07.006
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: The impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Eftekhar Ardebili M, Naserbakht M, Bernstein C, Alazmani-Noodeh F, Hakimi H, Ranjbar H. Healthcare providers experience of working during the COVID-19 pandemic: a qualitative study. Am J Infect Control. 2021;49(5):547-554. doi:10.1016/j.ajic.2020.10.001
Jayathilake HD, Daud D, Eaw HC, Annuar N. Employee development and retention of generation-Z employees in the post-covid-19 workplace: a conceptual framework. Benchmarking: An International Journal. 2021;28(7):2343-2364. doi:10.1108/bij-06-2020-0311
Introduction
The COVID-19 pandemic has changed the healthcare system in a multitude of ways, affecting healthcare capacity, treatment of other illnesses, and wellness as well as professional retention of healthcare workers.1-3 During the peak of the COVID-19 pandemic, healthcare capacity was tested and resources were used up quickly.1 As the pandemic has progressed, healthcare systems have had to decide how to proceed with lessons learned, reassessing the environment of care delivery, healthcare supply chains, workforce structures, communication systems, and scientific collaboration as well as policy frameworks in healthcare.4
There have been both immediate effects and long-term consequences of the delay in care for other conditions.2,5 One stark example of this is in cancer care, where screening and procedures were postponed or canceled due to the pandemic with a resulting predicted 2% increase in cancer mortality in the next 10 years.2 The care of heart disease, chronic illnesses, and other viruses has also been similarly negatively impacted by the COVID-19 pandemic due to similar delays in diagnosis and treatment.5-7
The impact on healthcare workers has also been profound.3 Occupational stress from the pandemic has correlated with increased depression and posttraumatic stress disorder (PTSD) among other mental health diseases in healthcare workers.3 In a survey of neurosurgery residents, 26.1% of physicians reported feeling burnt out, and 65.8% were worried that they would not be able to reach surgical milestones.8,9 Among respiratory therapists, a hard hit group during this time, 79% reported burnout.10 Additionally, more healthcare workers left the field during the pandemic, with 15 million lost jobs. Future recovery of jobs looks bleak in some settings, like long-term care and among assistants and aides.11 Overall, the long-term outcomes of these resource, disease, and mental health disruptions need to be assessed and solutions created to maintain a quality and effective healthcare system, with ample resources and measures to account for disease increases and address the impact on providers.
Healthcare Capacity and Resources
With COVID-19 affecting over 100 million in the United States as of March 1, 2023, the impact on healthcare resources since the start of the pandemic has been immense.12 With 5% to 38% of hospitalized patients being admitted to the intensive care unit (ICU) and 75% to 88% of those patients requiring mechanical ventilation, a huge strain was placed on resources during and after the pandemic.1
The question of balancing resources for other hospital needs while tending to patients with COVID-19 has been an ongoing discussion at many levels.1 One core resource concern is the lack of staff. In a survey of 77 different countries, including physicians (41%), nurses (40%), respiratory therapists (11%), and advanced practice providers (8%), 15% reported insufficient intensivists and 32% reported insufficient ICU nursing staff during March and April of 2020.1 A lack of hospital and care space that led to reallocation of limited-care acute care space was a concern. Thirteen percent reported a shortage of hospital ICU beds, while others reported the conversion of postoperative recovery rooms (20%) and operating rooms (12%) for patients with COVID-19.1
Along with staff and care space concerns, hospital survey respondents reported that healthcare equipment was also challenged. Access to COVID-19 testing was one concern, with only 35% of respondents reporting availability for all patients at the beginning of the pandemic, and 56% reporting availability for only select patients based on symptom severity.1 Access to personal protective equipment (PPE) was also affected, with PPE always available according to 83% to 95% of respondents but just 35% having access to N95 masks.1 Additionally, 26% reported that there were no respirators in their hospital, and 11% reported limited ventilators.1
Although resource depletion is a problem, studies have looked at public health measures that helped to mitigate this issue. With proper public health planning and implementation, such as physical distancing, aggressive testing, contact tracing, and increased hospital capacity, by freeing up existing resources or adding additional support, public health modeling showed that resources may be able to withstand the increase.13 Development of reallocation models at local, state, national, and international levels is an important step to be able to deal with future public health crises.14
The long-term impact from the pandemic includes disruption in the physical environment of healthcare, production, supply chain, staff structure, and workforce alterations.4 For example, the physical shape of healthcare facilities is changing to accommodate increasing volumes and decrease the risk of spreading disease.4 To accommodate the burden on staffing structure and workforce alteration, telehealth gained a prominent role.4 All in all, the pandemic has changed the healthcare system; however, institutions, organizations, and policy makers need to evaluate which measures were impactful and should be considered for long-term inclusion in healthcare practice.
Impact on Other Diseases: Cancer, Heart Disease, Chronic Illnesses, and Other Viruses
The treatment of other new and existing conditions has also been affected by the pandemic. Cancer, especially, is a disease of concern. Elective surgeries and screening were halted or altered during the pandemic, which is modeled to lead to higher cancer mortality in years to come.2 The most affected cancers were breast, lung, and colorectal cancer.2 A study of colorectal cancer screening showed that colonoscopies were delayed due to COVID-19 and that gastroenterology visits declined by 49% to 61%.15 This will likely lead to delayed cancer diagnoses and possible increases in mortality.15 Breast cancer screening was also delayed and many patients continued to avoid it for various reasons such as fears of contracting COVID-19 infection in healthcare facilities, and the economic effects of the pandemic such as job loss and healthcare coverage loss.16 These delays will result in an estimated potential 0.52% overall increase in breast cancer deaths by 2030.17
A study of 368 patients from Spain showed a 56.5% decrease in hospital admissions, usually related to heart attacks, in March and April of 2020, compared to January and February 2020.18,19 For other chronic illnesses, the pandemic resulted in decreased preventative care and management.20 The care of other infections similarly suffered. The World Health Organization announced that the number of patients receiving treatment for tuberculosis (TB) dropped by 1 million, setting the disease mitigation back considerably.20 An estimated 500,000 more people died in 2020 from TB.21 The drastic shift in focus to COVID-19 care during this period will continue to have a profound impact on other diseases like these for many years post-pandemic.
Provider Experience and Mental Health Outcomes
The impact on provider experiences and mental health has been immense. One study of 510 healthcare providers (HCPs) and first responders found that occupational stress from the pandemic correlated with psychiatric symptoms, including depression, PTSD, insomnia, and generalized anxiety.3 Occupational stress also correlated with one’s likelihood to leave the medical field and trouble doing work they had once loved.3 Half of the healthcare workers surveyed indicated a decreased likelihood of staying in their current profession after the pandemic.3
Other studies have also looked at specific subspecialties and impact on trainees during the pandemic. In neurosurgery, for example, resident burnout is high, at 26.1%.9 Additionally, the lack of surgeries in the pandemic made 65.8% of neurosurgery residents anxious about meeting career milestones.9 Respiratory therapists, a highly impacted group, also experienced burnout, reporting higher levels in those who worked more in the ICU. Another study identified several themes in the concerns reported by healthcare workers during the pandemic era including “changes in personal life and enhanced negative affect,” “gaining experience, normalization, and adaptation to the pandemic,” and “mental health considerations.”22
Some studies have investigated ways to mitigate this dissatisfaction with the healthcare field post-pandemic. Intrapreneurship, reverse mentoring, and democratized learning all had a reported positive impact on employee experience and retention during this time.23 Intrapreneurship describes entrepreneurship within an existing organization, while reverse mentoring and democratized learning refer to newer employees teaching older employees and communicative learning on a breadth of topics. Other studies have examined the necessity of having mental health resources available, and that these resources need to be multi-stage and individualistic as well as specific to certain stressors HCPs faced during the pandemic.22
Conclusion and Future Directions
The COVID-19 pandemic had stark effects on the healthcare system, impacting resources and capacity, care of other diseases, and provider mental health and experiences.1-3 After the chaos of the pandemic, many questions remain. What needs to be done now by health systems and HCPs? How can we learn from the challenges and the effects on capacity to change the healthcare workflow in times of crisis and in the present? How do we mitigate the impact of the pandemic on diagnosis and management of diseases? And how do we continue to provide healthcare workers with proper mental health and professional resources now, not just in times of stress, and encourage the future generation to pursue careers in healthcare?
These are all the questions the pandemic has left us with, and more studies and initiatives are needed to investigate solutions to these issues. The COVID-19 pandemic left behind valuable lessons and changed the healthcare system, disease management, and staffing for many. Now is the time to pick up the pieces and strategize on how to make our existing system more effective for workers and patients post pandemic.
Introduction
The COVID-19 pandemic has changed the healthcare system in a multitude of ways, affecting healthcare capacity, treatment of other illnesses, and wellness as well as professional retention of healthcare workers.1-3 During the peak of the COVID-19 pandemic, healthcare capacity was tested and resources were used up quickly.1 As the pandemic has progressed, healthcare systems have had to decide how to proceed with lessons learned, reassessing the environment of care delivery, healthcare supply chains, workforce structures, communication systems, and scientific collaboration as well as policy frameworks in healthcare.4
There have been both immediate effects and long-term consequences of the delay in care for other conditions.2,5 One stark example of this is in cancer care, where screening and procedures were postponed or canceled due to the pandemic with a resulting predicted 2% increase in cancer mortality in the next 10 years.2 The care of heart disease, chronic illnesses, and other viruses has also been similarly negatively impacted by the COVID-19 pandemic due to similar delays in diagnosis and treatment.5-7
The impact on healthcare workers has also been profound.3 Occupational stress from the pandemic has correlated with increased depression and posttraumatic stress disorder (PTSD) among other mental health diseases in healthcare workers.3 In a survey of neurosurgery residents, 26.1% of physicians reported feeling burnt out, and 65.8% were worried that they would not be able to reach surgical milestones.8,9 Among respiratory therapists, a hard hit group during this time, 79% reported burnout.10 Additionally, more healthcare workers left the field during the pandemic, with 15 million lost jobs. Future recovery of jobs looks bleak in some settings, like long-term care and among assistants and aides.11 Overall, the long-term outcomes of these resource, disease, and mental health disruptions need to be assessed and solutions created to maintain a quality and effective healthcare system, with ample resources and measures to account for disease increases and address the impact on providers.
Healthcare Capacity and Resources
With COVID-19 affecting over 100 million in the United States as of March 1, 2023, the impact on healthcare resources since the start of the pandemic has been immense.12 With 5% to 38% of hospitalized patients being admitted to the intensive care unit (ICU) and 75% to 88% of those patients requiring mechanical ventilation, a huge strain was placed on resources during and after the pandemic.1
The question of balancing resources for other hospital needs while tending to patients with COVID-19 has been an ongoing discussion at many levels.1 One core resource concern is the lack of staff. In a survey of 77 different countries, including physicians (41%), nurses (40%), respiratory therapists (11%), and advanced practice providers (8%), 15% reported insufficient intensivists and 32% reported insufficient ICU nursing staff during March and April of 2020.1 A lack of hospital and care space that led to reallocation of limited-care acute care space was a concern. Thirteen percent reported a shortage of hospital ICU beds, while others reported the conversion of postoperative recovery rooms (20%) and operating rooms (12%) for patients with COVID-19.1
Along with staff and care space concerns, hospital survey respondents reported that healthcare equipment was also challenged. Access to COVID-19 testing was one concern, with only 35% of respondents reporting availability for all patients at the beginning of the pandemic, and 56% reporting availability for only select patients based on symptom severity.1 Access to personal protective equipment (PPE) was also affected, with PPE always available according to 83% to 95% of respondents but just 35% having access to N95 masks.1 Additionally, 26% reported that there were no respirators in their hospital, and 11% reported limited ventilators.1
Although resource depletion is a problem, studies have looked at public health measures that helped to mitigate this issue. With proper public health planning and implementation, such as physical distancing, aggressive testing, contact tracing, and increased hospital capacity, by freeing up existing resources or adding additional support, public health modeling showed that resources may be able to withstand the increase.13 Development of reallocation models at local, state, national, and international levels is an important step to be able to deal with future public health crises.14
The long-term impact from the pandemic includes disruption in the physical environment of healthcare, production, supply chain, staff structure, and workforce alterations.4 For example, the physical shape of healthcare facilities is changing to accommodate increasing volumes and decrease the risk of spreading disease.4 To accommodate the burden on staffing structure and workforce alteration, telehealth gained a prominent role.4 All in all, the pandemic has changed the healthcare system; however, institutions, organizations, and policy makers need to evaluate which measures were impactful and should be considered for long-term inclusion in healthcare practice.
Impact on Other Diseases: Cancer, Heart Disease, Chronic Illnesses, and Other Viruses
The treatment of other new and existing conditions has also been affected by the pandemic. Cancer, especially, is a disease of concern. Elective surgeries and screening were halted or altered during the pandemic, which is modeled to lead to higher cancer mortality in years to come.2 The most affected cancers were breast, lung, and colorectal cancer.2 A study of colorectal cancer screening showed that colonoscopies were delayed due to COVID-19 and that gastroenterology visits declined by 49% to 61%.15 This will likely lead to delayed cancer diagnoses and possible increases in mortality.15 Breast cancer screening was also delayed and many patients continued to avoid it for various reasons such as fears of contracting COVID-19 infection in healthcare facilities, and the economic effects of the pandemic such as job loss and healthcare coverage loss.16 These delays will result in an estimated potential 0.52% overall increase in breast cancer deaths by 2030.17
A study of 368 patients from Spain showed a 56.5% decrease in hospital admissions, usually related to heart attacks, in March and April of 2020, compared to January and February 2020.18,19 For other chronic illnesses, the pandemic resulted in decreased preventative care and management.20 The care of other infections similarly suffered. The World Health Organization announced that the number of patients receiving treatment for tuberculosis (TB) dropped by 1 million, setting the disease mitigation back considerably.20 An estimated 500,000 more people died in 2020 from TB.21 The drastic shift in focus to COVID-19 care during this period will continue to have a profound impact on other diseases like these for many years post-pandemic.
Provider Experience and Mental Health Outcomes
The impact on provider experiences and mental health has been immense. One study of 510 healthcare providers (HCPs) and first responders found that occupational stress from the pandemic correlated with psychiatric symptoms, including depression, PTSD, insomnia, and generalized anxiety.3 Occupational stress also correlated with one’s likelihood to leave the medical field and trouble doing work they had once loved.3 Half of the healthcare workers surveyed indicated a decreased likelihood of staying in their current profession after the pandemic.3
Other studies have also looked at specific subspecialties and impact on trainees during the pandemic. In neurosurgery, for example, resident burnout is high, at 26.1%.9 Additionally, the lack of surgeries in the pandemic made 65.8% of neurosurgery residents anxious about meeting career milestones.9 Respiratory therapists, a highly impacted group, also experienced burnout, reporting higher levels in those who worked more in the ICU. Another study identified several themes in the concerns reported by healthcare workers during the pandemic era including “changes in personal life and enhanced negative affect,” “gaining experience, normalization, and adaptation to the pandemic,” and “mental health considerations.”22
Some studies have investigated ways to mitigate this dissatisfaction with the healthcare field post-pandemic. Intrapreneurship, reverse mentoring, and democratized learning all had a reported positive impact on employee experience and retention during this time.23 Intrapreneurship describes entrepreneurship within an existing organization, while reverse mentoring and democratized learning refer to newer employees teaching older employees and communicative learning on a breadth of topics. Other studies have examined the necessity of having mental health resources available, and that these resources need to be multi-stage and individualistic as well as specific to certain stressors HCPs faced during the pandemic.22
Conclusion and Future Directions
The COVID-19 pandemic had stark effects on the healthcare system, impacting resources and capacity, care of other diseases, and provider mental health and experiences.1-3 After the chaos of the pandemic, many questions remain. What needs to be done now by health systems and HCPs? How can we learn from the challenges and the effects on capacity to change the healthcare workflow in times of crisis and in the present? How do we mitigate the impact of the pandemic on diagnosis and management of diseases? And how do we continue to provide healthcare workers with proper mental health and professional resources now, not just in times of stress, and encourage the future generation to pursue careers in healthcare?
These are all the questions the pandemic has left us with, and more studies and initiatives are needed to investigate solutions to these issues. The COVID-19 pandemic left behind valuable lessons and changed the healthcare system, disease management, and staffing for many. Now is the time to pick up the pieces and strategize on how to make our existing system more effective for workers and patients post pandemic.
Wahlster S, Sharma M, Lewis AK, et al. The coronavirus disease 2019 pandemic's effect on critical care resources and health-care providers: a global survey. Chest. 2021;159(2):619-633. doi:10.1016/j.chest.2020.09.070
Malagón T, Yong JHE, Tope P, Miller WH Jr, Franco EL; McGill task force on the impact of COVID-19 on cancer control and care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer. 2022;150(8):1244-1254. doi:10.1002/ijc.33884
Hendrickson RC, Slevin RA, Hoerster KD, et al. The impact of the COVID-19 pandemic on mental health, occupational functioning, and professional retention among health care workers and first responders. J Gen Intern Med. 2022;37(2):397-408. doi:10.1007/s11606-021-07252-z
Davis B, Bankhead-Kendall BK, Dumas RP. A review of COVID-19's impact on modern medical systems from a health organization management perspective. Health Technol (Berl). 2022;12(4):815-824. doi:10.1007/s12553-022-00660-z
Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368-2371. doi:10.1056/NEJMms2009984
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: the impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Jalili M, Niroomand M, Hadavand F, Zeinali K, Fotouhi A. Burnout among healthcare professionals during COVID-19 pandemic: a cross-sectional study. Int Arch Occup Environ Health. 2021;94(6):1345-1352. doi:10.1007/s00420-021-01695-x
Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142. doi:10.1016/j.jocn.2020.08.012
Miller AG, Roberts KJ, Smith BJ, et al. Prevalence of burnout among respiratory therapists amidst the COVID-19 pandemic. Respir Care. 2021;respcare.09283. doi:10.4187/respcare.09283
Frogner BK, Dill JS. Tracking turnover among health care workers during the COVID-19 pandemic: a cross-sectional study. JAMA Health Forum. 2022;3(4):e220371. doi:10.1001/jamahealthforum.2022.0371
CDC COVID data tracker. Centers for Disease Control and Prevention. Accessed December 22, 2022. http://covid-data-tracker/#datatracker-home.
Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ. 2020;192(24):E640-E646. doi:10.1503/cmaj.200715
Kaul V, Chahal J, Schrarstzhaupt IN, et al. Lessons learned from a global perspective of COVID-19. Clin Chest Med. 2022 Nov. 24. [online ahead of print]. doi:10.1016/j.ccm.2022.11.020
Issaka RB, Somsouk M. Colorectal cancer screening and prevention in the COVID-19 Era. JAMA Health Forum. 2020;1(5):e200588. doi:10.1001/jamahealthforum.2020.0588
Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59(1):1-11. doi:10.1016/j.rcl.2020.09.008
Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113(11):1484-1494. doi:10.1093/jnci/djab097
Jiménez-Blanco Bravo M, Cordero Pereda D, Sánchez Vega D, et al. Heart failure in the time of COVID-19. Cardiology. 2020;145(8):481-484. doi:10.1159/000509181
Frankfurter C, Buchan TA, Kobulnik J, et al. Reduced rate of hospital presentations for heart failure during the COVID-19 pandemic in Toronto, Canada. Can J Cardiol. 2020;36(10):1680-1684. doi:10.1016/j.cjca.2020.07.006
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: The impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Eftekhar Ardebili M, Naserbakht M, Bernstein C, Alazmani-Noodeh F, Hakimi H, Ranjbar H. Healthcare providers experience of working during the COVID-19 pandemic: a qualitative study. Am J Infect Control. 2021;49(5):547-554. doi:10.1016/j.ajic.2020.10.001
Jayathilake HD, Daud D, Eaw HC, Annuar N. Employee development and retention of generation-Z employees in the post-covid-19 workplace: a conceptual framework. Benchmarking: An International Journal. 2021;28(7):2343-2364. doi:10.1108/bij-06-2020-0311
Wahlster S, Sharma M, Lewis AK, et al. The coronavirus disease 2019 pandemic's effect on critical care resources and health-care providers: a global survey. Chest. 2021;159(2):619-633. doi:10.1016/j.chest.2020.09.070
Malagón T, Yong JHE, Tope P, Miller WH Jr, Franco EL; McGill task force on the impact of COVID-19 on cancer control and care. Predicted long-term impact of COVID-19 pandemic-related care delays on cancer mortality in Canada. Int J Cancer. 2022;150(8):1244-1254. doi:10.1002/ijc.33884
Hendrickson RC, Slevin RA, Hoerster KD, et al. The impact of the COVID-19 pandemic on mental health, occupational functioning, and professional retention among health care workers and first responders. J Gen Intern Med. 2022;37(2):397-408. doi:10.1007/s11606-021-07252-z
Davis B, Bankhead-Kendall BK, Dumas RP. A review of COVID-19's impact on modern medical systems from a health organization management perspective. Health Technol (Berl). 2022;12(4):815-824. doi:10.1007/s12553-022-00660-z
Rosenbaum L. The untold toll - the pandemic's effects on patients without COVID-19. N Engl J Med. 2020;382(24):2368-2371. doi:10.1056/NEJMms2009984
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: the impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Jalili M, Niroomand M, Hadavand F, Zeinali K, Fotouhi A. Burnout among healthcare professionals during COVID-19 pandemic: a cross-sectional study. Int Arch Occup Environ Health. 2021;94(6):1345-1352. doi:10.1007/s00420-021-01695-x
Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142. doi:10.1016/j.jocn.2020.08.012
Miller AG, Roberts KJ, Smith BJ, et al. Prevalence of burnout among respiratory therapists amidst the COVID-19 pandemic. Respir Care. 2021;respcare.09283. doi:10.4187/respcare.09283
Frogner BK, Dill JS. Tracking turnover among health care workers during the COVID-19 pandemic: a cross-sectional study. JAMA Health Forum. 2022;3(4):e220371. doi:10.1001/jamahealthforum.2022.0371
CDC COVID data tracker. Centers for Disease Control and Prevention. Accessed December 22, 2022. http://covid-data-tracker/#datatracker-home.
Barrett K, Khan YA, Mac S, Ximenes R, Naimark DMJ, Sander B. Estimation of COVID-19-induced depletion of hospital resources in Ontario, Canada. CMAJ. 2020;192(24):E640-E646. doi:10.1503/cmaj.200715
Kaul V, Chahal J, Schrarstzhaupt IN, et al. Lessons learned from a global perspective of COVID-19. Clin Chest Med. 2022 Nov. 24. [online ahead of print]. doi:10.1016/j.ccm.2022.11.020
Issaka RB, Somsouk M. Colorectal cancer screening and prevention in the COVID-19 Era. JAMA Health Forum. 2020;1(5):e200588. doi:10.1001/jamahealthforum.2020.0588
Freer PE. The impact of the COVID-19 pandemic on breast imaging. Radiol Clin North Am. 2021;59(1):1-11. doi:10.1016/j.rcl.2020.09.008
Alagoz O, Lowry KP, Kurian AW, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst. 2021;113(11):1484-1494. doi:10.1093/jnci/djab097
Jiménez-Blanco Bravo M, Cordero Pereda D, Sánchez Vega D, et al. Heart failure in the time of COVID-19. Cardiology. 2020;145(8):481-484. doi:10.1159/000509181
Frankfurter C, Buchan TA, Kobulnik J, et al. Reduced rate of hospital presentations for heart failure during the COVID-19 pandemic in Toronto, Canada. Can J Cardiol. 2020;36(10):1680-1684. doi:10.1016/j.cjca.2020.07.006
Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and chronic disease: The impact now and in the future. Prev Chronic Dis. 2021;18:E62. doi:10.5888/pcd18.210086
Roberts L. How COVID hurt the fight against other dangerous diseases. Nature. 2021;592(7855):502-504. doi:10.1038/d41586-021-01022-x
Eftekhar Ardebili M, Naserbakht M, Bernstein C, Alazmani-Noodeh F, Hakimi H, Ranjbar H. Healthcare providers experience of working during the COVID-19 pandemic: a qualitative study. Am J Infect Control. 2021;49(5):547-554. doi:10.1016/j.ajic.2020.10.001
Jayathilake HD, Daud D, Eaw HC, Annuar N. Employee development and retention of generation-Z employees in the post-covid-19 workplace: a conceptual framework. Benchmarking: An International Journal. 2021;28(7):2343-2364. doi:10.1108/bij-06-2020-0311
One in four parents lied about kids’ COVID status: Survey
More than 1 in 4 parents lied to school officials about their children’s COVID-19 status or refused to comply with public health rules during the height of the pandemic, a new study found. Researchers said they suspected the 26% of parents who misrepresented their children’s health status may have undercounted the actual figure.
“If anything, 26% is probably the minimum” of parents who misled school officials, said Angela Fagerlin, PhD, a researcher at the University of Utah Medical School, Salt Lake City.
In the survey, many parents said they considered it their right as parents to make their own decision about their children’s health status, said Dr. Fagerlin, who is also the chair of the department of population health sciences at the University of Utah School of Medicine.
“It appears that many parents were concerned about their children missing school,” she said. “At the same time, they’re potentially exposing other kids to a serious illness.”
In the survey, parents were asked whether they lied or misrepresented information about their children on seven different COVID-19 topics, including illness and vaccination status and if they followed quarantine protocols. Researchers tallied survey responses collected in December 2021 from 580 parents, whose average age was 36 and of whom 70% were women. Results were published in the journal JAMA Network Open.
Overall, 24% of parents said they lied to people that their children were with while knowing or suspecting the children had COVID. About half of parents cited at least one of the following reasons for doing so: parental freedom, child did not feel very sick, or wanted the child’s life to feel “normal.”
About 20% of parents said they avoided testing when they thought their child had COVID, and parents also reported allowing children to break quarantine rules at a similar rate. More than half of parents who avoided testing said they were worried testing would hurt or feel uncomfortable.
About 4 in 10 parents who lied about their child’s illness status or who lied about whether their child should be in quarantine said they did so because of guidance from a public figure such as a celebrity or politician. At least 3 in 10 said they lied because they could not miss work to stay home with their child.
“We need to do a better job of providing support mechanisms like paid sick leave for family illness so that parents don’t feel like their only option is to engage in misrepresentation or non-adherence to public health guidelines during a future infectious disease outbreak that matches or exceeds the magnitude of COVID-19,” says researcher Andrea Gurmankin Levy, PhD, of Middlesex (Conn.) Community College.
A version of this article first appeared on WebMD.com.
More than 1 in 4 parents lied to school officials about their children’s COVID-19 status or refused to comply with public health rules during the height of the pandemic, a new study found. Researchers said they suspected the 26% of parents who misrepresented their children’s health status may have undercounted the actual figure.
“If anything, 26% is probably the minimum” of parents who misled school officials, said Angela Fagerlin, PhD, a researcher at the University of Utah Medical School, Salt Lake City.
In the survey, many parents said they considered it their right as parents to make their own decision about their children’s health status, said Dr. Fagerlin, who is also the chair of the department of population health sciences at the University of Utah School of Medicine.
“It appears that many parents were concerned about their children missing school,” she said. “At the same time, they’re potentially exposing other kids to a serious illness.”
In the survey, parents were asked whether they lied or misrepresented information about their children on seven different COVID-19 topics, including illness and vaccination status and if they followed quarantine protocols. Researchers tallied survey responses collected in December 2021 from 580 parents, whose average age was 36 and of whom 70% were women. Results were published in the journal JAMA Network Open.
Overall, 24% of parents said they lied to people that their children were with while knowing or suspecting the children had COVID. About half of parents cited at least one of the following reasons for doing so: parental freedom, child did not feel very sick, or wanted the child’s life to feel “normal.”
About 20% of parents said they avoided testing when they thought their child had COVID, and parents also reported allowing children to break quarantine rules at a similar rate. More than half of parents who avoided testing said they were worried testing would hurt or feel uncomfortable.
About 4 in 10 parents who lied about their child’s illness status or who lied about whether their child should be in quarantine said they did so because of guidance from a public figure such as a celebrity or politician. At least 3 in 10 said they lied because they could not miss work to stay home with their child.
“We need to do a better job of providing support mechanisms like paid sick leave for family illness so that parents don’t feel like their only option is to engage in misrepresentation or non-adherence to public health guidelines during a future infectious disease outbreak that matches or exceeds the magnitude of COVID-19,” says researcher Andrea Gurmankin Levy, PhD, of Middlesex (Conn.) Community College.
A version of this article first appeared on WebMD.com.
More than 1 in 4 parents lied to school officials about their children’s COVID-19 status or refused to comply with public health rules during the height of the pandemic, a new study found. Researchers said they suspected the 26% of parents who misrepresented their children’s health status may have undercounted the actual figure.
“If anything, 26% is probably the minimum” of parents who misled school officials, said Angela Fagerlin, PhD, a researcher at the University of Utah Medical School, Salt Lake City.
In the survey, many parents said they considered it their right as parents to make their own decision about their children’s health status, said Dr. Fagerlin, who is also the chair of the department of population health sciences at the University of Utah School of Medicine.
“It appears that many parents were concerned about their children missing school,” she said. “At the same time, they’re potentially exposing other kids to a serious illness.”
In the survey, parents were asked whether they lied or misrepresented information about their children on seven different COVID-19 topics, including illness and vaccination status and if they followed quarantine protocols. Researchers tallied survey responses collected in December 2021 from 580 parents, whose average age was 36 and of whom 70% were women. Results were published in the journal JAMA Network Open.
Overall, 24% of parents said they lied to people that their children were with while knowing or suspecting the children had COVID. About half of parents cited at least one of the following reasons for doing so: parental freedom, child did not feel very sick, or wanted the child’s life to feel “normal.”
About 20% of parents said they avoided testing when they thought their child had COVID, and parents also reported allowing children to break quarantine rules at a similar rate. More than half of parents who avoided testing said they were worried testing would hurt or feel uncomfortable.
About 4 in 10 parents who lied about their child’s illness status or who lied about whether their child should be in quarantine said they did so because of guidance from a public figure such as a celebrity or politician. At least 3 in 10 said they lied because they could not miss work to stay home with their child.
“We need to do a better job of providing support mechanisms like paid sick leave for family illness so that parents don’t feel like their only option is to engage in misrepresentation or non-adherence to public health guidelines during a future infectious disease outbreak that matches or exceeds the magnitude of COVID-19,” says researcher Andrea Gurmankin Levy, PhD, of Middlesex (Conn.) Community College.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Even mild COVID is hard on the brain
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
Celiac disease appears to double COVID-19 hospitalization risk
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY


