User login
-
FDA authorizes first molecular at-home, OTC COVID-19 test
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted emergency use authorization (EUA) for the Cue COVID-19 Test for Home and Over The Counter Use (Cue OTC Test, Cue Health).
The Cue OTC Test is the first molecular diagnostic test available to consumers without a prescription.
The test detects genetic material from SARS-CoV-2 present in the nostrils and delivers results in about 20 minutes to the user’s mobile smart device via the Cue Health app.
In testing, the Cue OTC Test correctly identified 96% of positive nasal swab samples from individuals known to have symptoms and correctly identified 100% of positive samples from individuals without symptoms.
The test is intended for use in people aged 2 years and older with and without symptoms.
“With this authorization, consumers can purchase and self-administer one of the easiest, fastest, and most accurate tests without a prescription,” Clint Sever, cofounder and chief product officer of Cue Health, said in a news release.
“This FDA authorization will help us improve patient outcomes with a solution that provides the accuracy of central lab tests, with the speed and accessibility required to address emergent global health issues,” he said.
Cue Health expects to produce more than 100,000 single-use test kits per day by this summer. Dena Cook, the company’s chief communications officer, told this news organization that the company hasn’t announced pricing information yet, but the price will be “comparable” to other price points and other products on the market.
“The FDA continues to prioritize the availability of more at-home testing options in response to the pandemic,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement.
“Cue COVID-19 Test for Home and Over-the-Counter Use provides access to accurate and reliable testing at home, without a prescription. The FDA will continue to work collaboratively with test developers to advance effective testing options for doctors, clinicians, and the public,” he said.
In June, the FDA granted an EUA to Cue Health’s COVID-19 test for use in clinical and point-of-care settings.
The test is currently being used in hospitals, physicians’ offices, and dental clinics, as well as schools, essential businesses, nursing homes, and other congregate-care facilities. The test is also being distributed through a program led by the U.S. Department of Defense and the U.S. Department of Health & Human Services across several states.
A version of this article first appeared on Medscape.com.
Missed visits during pandemic cause ‘detrimental ripple effects’
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a new report from the Urban Institute.
Among the adults who postponed or missed care, 32.6% said the gap worsened one or more health conditions or limited their ability to work or perform daily activities. The findings highlight “the detrimental ripple effects of delaying or forgoing care on overall health, functioning, and well-being,” researchers write.
The survey, conducted among 4,007 U.S. adults aged 18-64 in September 2020, found that adults with one or more chronic conditions were more likely than adults without chronic conditions to have delayed or missed care (40.7% vs. 26.4%). Adults with a mental health condition were particularly likely to have delayed or gone without care, write Dulce Gonzalez, MPP, a research associate in the Health Policy Center at the Urban Institute, and colleagues.
Doctors are already seeing the consequences of the missed visits, says Jacqueline W. Fincher, MD, president of the American College of Physicians.
Two of her patients with chronic conditions missed appointments last year. By the time they resumed care in 2021, their previsit lab tests showed significant kidney deterioration.
“Lo and behold, their kidneys were in failure. … One was in the hospital for 3 days and the other one was in for 5 days,” said Dr. Fincher, who practices general internal medicine in Georgia.
Dr. Fincher’s office has been proactive about calling patients with chronic diseases who missed follow-up visits or laboratory testing or who may have run out of medication, she said.
In her experience, delays mainly have been because of patients postponing visits. “We have stayed open the whole time now,” Dr. Fincher said. Her office offers telemedicine visits and in-person visits with safety precautions.
Still, some patients have decided to postpone care during the pandemic instead of asking their primary care doctor what they should do.
“We do know that chronic problems left without appropriate follow-up can create worse problems for them in terms of stroke, heart attack, and end organ damage,” Dr. Fincher said.
Lost lives
Future studies may help researchers understand the effects of delayed and missed care during the pandemic, said Russell S. Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston.
“Although it is still early, and more data on patient outcomes will need to be collected, I anticipate that the ... delays in diagnosis, in cancer screening, and in management of chronic illness will result in lost lives and will emphasize the important role that primary care plays in saving lives,” Dr. Phillips said.
During the first several months of the pandemic, there were fewer diagnoses of hypertension, diabetes, and depression, Dr. Phillips said.
“In addition, and most importantly, the mortality rate for non-COVID conditions increased, suggesting that patients were not seeking care for symptoms of stroke or heart attack, which can be fatal if untreated,” he said. “We have also seen substantial decreases in cancer screening tests such as colonoscopy, and modeling studies suggest this will cost more lives based on delayed diagnoses of cancer.”
Vaccinating patients against COVID-19 may help primary care practices and patients get back on track, Dr. Phillips suggested.
In the meantime, some patients remain reluctant to come in. “Volumes are still lower than prepandemic, so it is challenging to overcome what is likely to be pent-up demand,” he told this news organization in an email. “Additionally, the continued burden of evaluating, testing, and monitoring patients with COVID or COVID-like symptoms makes it difficult to focus on chronic illness.”
Care most often skipped
The Urban Institute survey asked respondents about delays in prescription drugs, general doctor and specialist visits, going to a hospital, preventive health screenings or medical tests, treatment or follow-up care, dental care, mental health care or counseling, treatment or counseling for alcohol or drug use, and other types of medical care.
Dental care was the most common type of care that adults delayed or did not receive because of the pandemic (25.3%), followed by general doctor or specialist visits (20.6%) and preventive health screenings or medical tests (15.5%).
Black adults were more likely than White or Hispanic/Latinx adults to have delayed or forgone care (39.7% vs. 34.3% and 35.5%), the researchers found. Compared with adults with higher incomes, adults with lower incomes were more likely to have missed multiple types of care (26.6% vs. 20.3%).
The report by the Urban Institute researchers was supported by the Robert Wood Johnson Foundation. Dr. Phillips is an adviser to two telemedicine companies, Bicycle Health and Grow Health. Dr. Fincher has disclosed no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Call to action on obesity amid COVID-19 pandemic
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Hundreds of thousands of deaths worldwide from COVID-19 could have been avoided if obesity rates were lower, a new report says.
An analysis by the World Obesity Federation found that of the 2.5 million COVID-19 deaths reported by the end of February 2021, almost 90% (2.2 million) were in countries where more than half the population is classified as overweight.
The report, released to coincide with World Obesity Day, calls for obesity to be recognized as a disease in its own right around the world, and for people with obesity to be included in priority lists for COVID-19 testing and vaccination.
“Overweight is a highly significant predictor of developing complications from COVID-19, including the need for hospitalization, for intensive care and for mechanical ventilation,” the WOF notes in the report.
It adds that in countries where less than half the adult population is classified as overweight (body mass index > 25 mg/kg2), for example, Vietnam, the likelihood of death from COVID-19 is a small fraction – around one-tenth – of the level seen in countries where more than half the population is classified as overweight.
And while it acknowledges that figures for COVID-19 deaths are affected by the age structure of national populations and a country’s relative wealth and reporting capacity, “our findings appear to be independent of these contributory factors. Furthermore, other studies have found that overweight remains a highly significant predictor of the need for COVID-19 health care after accounting for these other influences.”
As an example, based on the U.K. experience, where an estimated 36% of COVID-19 hospitalizations have been attributed to lack of physical activity and excess body weight, it can be suggested that up to a third of the costs – between $6 trillion and $7 trillion over the longer period – might be attributable to these predisposing risks.
The report said the prevalence of obesity in the United Kingdom is expected to rise from 27.8% in 2016 to more than 35% by 2025.
Rachel Batterham, lead adviser on obesity at the Royal College of Physicians, commented: “The link between high levels of obesity and deaths from COVID-19 in the U.K. is indisputable, as is the urgent need to address the factors that lead so many people to be living with obesity.
“With 30% of COVID-19 hospitalizations in the U.K. directly attributed to overweight and obesity, and three-quarters of all critically ill patients having overweight or obesity, the human and financial costs are high.”
Window of opportunity to prioritize obesity as a disease
WOF says that evolving evidence on the close association between COVID-19 and underlying obesity “provides a new urgency … for political and collective action.”
“Obesity is a disease that does not receive prioritization commensurate with its prevalence and impact, which is rising fastest in emerging economies. It is a gateway to many other noncommunicable diseases and mental-health illness and is now a major factor in COVID-19 complications and mortality.”
The WOF also shows that COVID-19 is not a special case, noting that several other respiratory viruses lead to more severe consequences in people living with excess bodyweight, giving good reasons to expect the next pandemic to have similar effects. “For these reasons we need to recognize overweight as a major risk factor for infectious diseases including respiratory viruses.”
“To prevent pandemic health crises in future requires action now: we call on all readers to support the World Obesity Federation’s call for stronger, more resilient economies that prioritize investment in people’s health.”
There is, it stresses, “a window of opportunity to advocate for, fund and implement these actions in all countries to ensure better, more resilient and sustainable health for all, “now and in our postCOVID-19 future.”
It proposes a ROOTS approach:
- Recognize that obesity is a disease in its own right.
- Obesity monitoring and surveillance must be enhanced.
- Obesity prevention strategies must be developed.
- Treatment of obesity.
- Systems-based approaches should be applied.
A version of this article first appeared on Medscape.com.
Potential COVID-19 variant surge looms over U.S.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Another coronavirus surge may be on the way in the United States as daily COVID-19 cases continue to plateau around 60,000, states begin to lift restrictions, and people embark on spring break trips this week, according to CNN.
Outbreaks will likely stem from the B.1.1.7 variant, which was first identified in the United Kingdom, and gain momentum during the next 6-14 weeks.
“Four weeks ago, the B.1.1.7 variant made up about 1%-4% of the virus that we were seeing in communities across the country. Today it’s up to 30%-40%,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told NBC’s Meet the Press on March 7.
Dr. Osterholm compared the current situation with the “eye of the hurricane,” where the skies appear clear but more storms are on the way. Across Europe, 27 countries are seeing significant B.1.1.7 case increases, and 10 are getting hit hard, he said.
“What we’ve seen in Europe, when we hit that 50% mark, you see cases surge,” he said. “So right now, we do have to keep America as safe as we can from this virus by not letting up on any of the public health measures we’ve taken.”
In January, the CDC warned that B.1.1.7 variant cases would increase in 2021 and become the dominant variant in the country by this month. The United States has now reported more than 3,000 cases across 46 states, according to the latest CDC tally updated on March 7. More than 600 cases have been found in Florida, followed by more than 400 in Michigan.
The CDC has said the tally doesn’t represent the total number of B.1.1.7 cases in the United States, only the ones that have been identified by analyzing samples through genomic sequencing.
“Where it has hit in the U.K. and now elsewhere in Europe, it has been catastrophic,” Celine Gounder, MD, an infectious disease specialist with New York University Langone Health, told CNN on March 7.
The variant is more transmissible than the original novel coronavirus, and the cases in the United States are “increasing exponentially,” she said.
“It has driven up rates of hospitalizations and deaths and it’s very difficult to control,” Dr. Gounder said.
Vaccination numbers aren’t yet high enough to stop the predicted surge, she added. The United States has shipped more than 116 million vaccine doses, according to the latest CDC update on March 7. Nearly 59 million people have received at least one dose, and 30.6 million people have received two vaccine doses. About 9% of the U.S. population has been fully vaccinated.
States shouldn’t ease restrictions until the vaccination numbers are much higher and daily COVID-19 cases fall below 10,000 – and maybe “considerably less than that,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNN on March 4.
Several states have already begun to lift COVID-19 safety protocols, with Texas and Mississippi removing mask mandates last week. Businesses in Texas will be able to reopen at full capacity on March 10. For now, public health officials are urging Americans to continue to wear masks, avoid crowds, and follow social distancing guidelines as vaccines roll out across the country.
“This is sort of like we’ve been running this really long marathon, and we’re 100 yards from the finish line and we sit down and we give up,” Dr. Gounder told CNN on Sunday. ‘We’re almost there, we just need to give ourselves a bit more time to get a larger proportion of the population covered with vaccines.”
A version of this article first appeared on WebMD.com.
Five-day course of oral antiviral appears to stop SARS-CoV-2 in its tracks
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
A single pill of the investigational drug molnupiravir taken twice a day for 5 days eliminated SARS-CoV-2 from the nasopharynx of 49 participants.
That led Carlos del Rio, MD, distinguished professor of medicine at Emory University, Atlanta, to suggest a future in which a drug like molnupiravir could be taken in the first few days of symptoms to prevent severe disease, similar to Tamiflu for influenza.
“I think it’s critically important,” he said of the data. Emory University was involved in the trial of molnupiravir but Dr. del Rio was not part of that team. “This drug offers the first antiviral oral drug that then could be used in an outpatient setting.”
Still, Dr. del Rio said it’s too soon to call this particular drug the breakthrough clinicians need to keep people out of the ICU. “It has the potential to be practice changing; it’s not practice changing at the moment.”
Wendy Painter, MD, of Ridgeback Biotherapeutics, who presented the data at the Conference on Retroviruses and Opportunistic Infections, agreed. While the data are promising, “We will need to see if people get better from actual illness” to assess the real value of the drug in clinical care.
“That’s a phase 3 objective we’ll need to prove,” she said in an interview.
Phase 2/3 efficacy and safety studies of the drug are now underway in hospitalized and nonhospitalized patients.
In a brief prerecorded presentation of the data, Dr. Painter laid out what researchers know so far: Preclinical studies suggest that molnupiravir is effective against a number of viruses, including coronaviruses and specifically SARS-CoV-2. It prevents a virus from replicating by inducing viral error catastrophe (Proc Natl Acad Sci U S A. 2002 Oct 15;99[21]:13374-6) – essentially overloading the virus with replication and mutation until the virus burns itself out and can’t produce replicable copies.
In this phase 2a, randomized, double-blind, controlled trial, researchers recruited 202 adults who were treated at an outpatient clinic with fever or other symptoms of a respiratory virus and confirmed SARS-CoV-2 infection by day 4. Participants were randomly assigned to three different groups: 200 mg of molnupiravir, 400 mg, or 800 mg. The 200-mg arm was matched 1:1 with a placebo-controlled group, and the other two groups had three participants in the active group for every one control.
Participants took the pills twice daily for 5 days, and then were followed for a total of 28 days to monitor for complications or adverse events. At days 3, 5, 7, 14, and 28, researchers also took nasopharyngeal swabs for polymerase chain reaction tests, to sequence the virus, and to grow cultures of SARS-CoV-2 to see if the virus that’s present is actually capable of infecting others.
Notably, the pills do not have to be refrigerated at any point in the process, alleviating the cold-chain challenges that have plagued vaccines.
“There’s an urgent need for an easily produced, transported, stored, and administered antiviral drug against SARS-CoV-2,” Dr. Painter said.
Of the 202 people recruited, 182 had swabs that could be evaluated, of which 78 showed infection at baseline. The results are based on labs of those 78 participants.
By day 3, 28% of patients in the placebo arm had SARS-CoV-2 in their nasopharynx, compared with 20.4% of patients receiving any dose of molnupiravir. But by day 5, none of the participants receiving the active drug had evidence of SARS-CoV-2 in their nasopharynx. In comparison, 24% of people in the placebo arm still had detectable virus.
Halfway through the treatment course, differences in the presence of infectious virus were already evident. By day 3 of the 5-day course, 36.4% of participants in the 200-mg group had detectable virus in the nasopharynx, compared with 21% in the 400-mg group and just 12.5% in the 800-mg group. And although the reduction in SARS-CoV-2 was noticeable in the 200-mg and the 400-mg arms, it was only statistically significant in the 800-mg arm.
In contrast, by the end of the 5 days in the placebo groups, infectious virus varied from 18.2% in the 200-mg placebo group to 30% in the 800-mg group. This points out the variability of the disease course of SARS-CoV-2.
“You just don’t know” which infections will lead to serious disease, Dr. Painter said in an interview. “And don’t you wish we did?”
Seven participants discontinued treatment, though only four experienced adverse events. Three of those discontinued the trial because of adverse events. The study is still blinded, so it’s unclear what those events were, but Dr. Painter said that they were not thought to be related to the study drug.
The bottom line, said Dr. Painter, was that people treated with molnupiravir had starkly different outcomes in lab measures during the study.
“An average of 10 days after symptom onset, 24% of placebo patients remained culture positive” for SARS-CoV-2 – meaning there wasn’t just virus in the nasopharynx, but it was capable of replicating, Dr. Painter said. “In contrast, no infectious virus could be recovered at study day 5 in any molnupiravir-treated patients.”
A version of this article first appeared on Medscape.com.
Bleeding disorder diagnoses delayed by years in girls and women
Diagnosis of bleeding disorders in girls and women can lag behind diagnosis in boys and men by more than a decade, meaning needless delays in treatment and poor quality of life for many with hemophilia or related conditions.
“There is increasing awareness about issues faced by women and girls with inherited bleeding disorders, but disparities still exist both in both access to diagnosis and treatment,” said Roseline D’Oiron, MD, from Hôpital Bicêtre in Paris.
“Diagnosis, when it is made, is often made late, particularly in women. Indeed, a recent study from the European Hemophilia Consortium including more than 700 women with bleeding disorders showed that the median age at diagnosis was 16 years old,” she said during the annual congress of the European Association for Haemophilia and Allied Disorders.
She said that delayed diagnosis of bleeding disorders in women and girls may be caused by a lack of knowledge by patients, families, and general practitioners about family history of bleeding disorders, abnormal bleeding events, and heavy menstrual bleeding. In addition, despite the frequency and severity of heavy bleeding events, patients, their families, and caregivers may underestimate the effect on the patient’s quality of life.
Disparities documented
Dr. D’Oiron pointed to several studies showing clear sex-based disparities in time to diagnosis. For example, a study published in Haemophilia showed that in 22 girls with hemophilia A or hemophilia B, the diagnosis of severe hemophilia was delayed by a median of 6.5 months compared with the diagnosis in boys, and a diagnosis of moderate hemophilia in girls was delayed by a median of 39 months.
In a second, single-center study comparing 44 women and girls with mild hemophilia (factor VIII or factor XI levels from 5 to 50 IU/dL) with 77 men and boys with mild hemophilia, the mean age at diagnosis was 31.63 years versus 19.18 years, respectively – a delay of 12.45 years.
A third study comparing 442 girls/women and 442 boys/men with mild hemophilia in France showed a difference of 6.07 years in diagnosis: the median age for girls/women at diagnosis was 16.91 years versus10.84 years for boys/men.
Why it matters
Dr. D’Oiron described the case of a patient named Clare, who first experienced, at age 8, 12 hours of bleeding following a dental procedure. At age 12.5, she began having heavy menstrual bleeding, causing her to miss school for a few days each month, to be feel tired, and have poor-quality sleep.
Despite repeated bleeding episodes, severe anemia, and iron deficiency, her hemophilia was not suspected until after her 16th birthday, and a definitive diagnosis of hemophilia in both Clare and her mother was finally made when Clare was past 17, when a nonsense variant factor in F8, the gene encoding for factor VIII, was detected.
“For Clare, it took more the 8 years after the first bleeding symptoms, and nearly 4 years after presenting with heavy menstrual bleeding to recognize that she had a bleeding problem,” she said.
In total, Clare had about 450 days of heavy menstrual bleeding, causing her to miss an estimated 140 days of school because of the delayed diagnosis and treatment.
“In my view, this is the main argument why it is urgent for these patients to achieve diagnosis early: this is to reduce the duration [of] a very poor quality of life,” Dr. D’Oiron said.
Barriers to diagnosis
Patients and families have reported difficulty distinguishing normal bleeding from abnormal symptoms, and girls may be reluctant to discuss their symptoms with their family or peers. In addition, primary care practitioners may not recognize the severity of the symptoms and therefore may not refer patients to hematologists for further workup.
These findings emphasize the need for improved tools to help patients differentiate between normal and abnormal bleeding, using symptom recognition–based language tools that can lead to early testing and application of accurate diagnostic tools, she said.
Standardization of definitions can help to improve screening and diagnosis, Dr. D’Oiron said, pointing to a recent study in Blood Advances proposing definitions for future research in von Willebrand disease.
For example, the authors of that study proposed a definition of heavy menstrual bleeding to include any of the following:
- Bleeding lasting 8 or more days
- Bleeding that consistently soaks through one or more sanitary protections every 2 hours on multiple days
- Requires use of more than one sanitary protection item at a time
- Requires changing sanitary protection during the night
- Is associated with repeat passing of blood clots
- Has a Pictorial Blood Assessment Chart score greater than 100.
Problem and solutions
Answering the question posed in the title of her talk, Dr. D’Oiron said: “Yes, we do have a problem with the diagnosis of bleeding disorders in women and girls, but we also have solutions.”
The solutions include family and patient outreach efforts; communication to improve awareness; inclusion of general practitioners in the circle of care; and early screening, diagnosis, and treatment.
A bleeding disorders specialist who was not involved in the study said that Dr. D’Oiron’s report closely reflects what she sees in the clinic.
“I do pediatrics, and usually what happens is that I see a teenager with heavy menstrual bleeding and we take her history, and we find out that Mom and multiple female family members have had horrible menstrual bleeding, possibly many of whom have had hysterectomies for it, and then diagnosing the parents and other family members after diagnosing the girl that we’re seeing” said Veronica H. Flood, MD, from the Medical College of Wisconsin, Milwaukee.
“It is unfortunately a very real thing,” she added.
Reasons for the delay likely include lack of awareness of bleeding disorders.
“If you present to a hematologist, we think about bleeding disorders, but if you present to a primary care physician, they don’t always have that on their radar,” she said.
Additionally, a girl from a family with a history of heavy menstrual bleeding may just assume that what she is experiencing is “normal,” despite the serious affect it has on her quality of life, Dr. Flood said.
Dr. D’Oiron’s research is supported by her institution, the French Hemophilia Association, FranceCoag and Mhemon, the European Hemophilia Consortium, and the World Federation of Hemophilia. She reported advisory board or invited speaker activities for multiple companies. Dr. Flood reported having no conflicts of interest to disclose.
Diagnosis of bleeding disorders in girls and women can lag behind diagnosis in boys and men by more than a decade, meaning needless delays in treatment and poor quality of life for many with hemophilia or related conditions.
“There is increasing awareness about issues faced by women and girls with inherited bleeding disorders, but disparities still exist both in both access to diagnosis and treatment,” said Roseline D’Oiron, MD, from Hôpital Bicêtre in Paris.
“Diagnosis, when it is made, is often made late, particularly in women. Indeed, a recent study from the European Hemophilia Consortium including more than 700 women with bleeding disorders showed that the median age at diagnosis was 16 years old,” she said during the annual congress of the European Association for Haemophilia and Allied Disorders.
She said that delayed diagnosis of bleeding disorders in women and girls may be caused by a lack of knowledge by patients, families, and general practitioners about family history of bleeding disorders, abnormal bleeding events, and heavy menstrual bleeding. In addition, despite the frequency and severity of heavy bleeding events, patients, their families, and caregivers may underestimate the effect on the patient’s quality of life.
Disparities documented
Dr. D’Oiron pointed to several studies showing clear sex-based disparities in time to diagnosis. For example, a study published in Haemophilia showed that in 22 girls with hemophilia A or hemophilia B, the diagnosis of severe hemophilia was delayed by a median of 6.5 months compared with the diagnosis in boys, and a diagnosis of moderate hemophilia in girls was delayed by a median of 39 months.
In a second, single-center study comparing 44 women and girls with mild hemophilia (factor VIII or factor XI levels from 5 to 50 IU/dL) with 77 men and boys with mild hemophilia, the mean age at diagnosis was 31.63 years versus 19.18 years, respectively – a delay of 12.45 years.
A third study comparing 442 girls/women and 442 boys/men with mild hemophilia in France showed a difference of 6.07 years in diagnosis: the median age for girls/women at diagnosis was 16.91 years versus10.84 years for boys/men.
Why it matters
Dr. D’Oiron described the case of a patient named Clare, who first experienced, at age 8, 12 hours of bleeding following a dental procedure. At age 12.5, she began having heavy menstrual bleeding, causing her to miss school for a few days each month, to be feel tired, and have poor-quality sleep.
Despite repeated bleeding episodes, severe anemia, and iron deficiency, her hemophilia was not suspected until after her 16th birthday, and a definitive diagnosis of hemophilia in both Clare and her mother was finally made when Clare was past 17, when a nonsense variant factor in F8, the gene encoding for factor VIII, was detected.
“For Clare, it took more the 8 years after the first bleeding symptoms, and nearly 4 years after presenting with heavy menstrual bleeding to recognize that she had a bleeding problem,” she said.
In total, Clare had about 450 days of heavy menstrual bleeding, causing her to miss an estimated 140 days of school because of the delayed diagnosis and treatment.
“In my view, this is the main argument why it is urgent for these patients to achieve diagnosis early: this is to reduce the duration [of] a very poor quality of life,” Dr. D’Oiron said.
Barriers to diagnosis
Patients and families have reported difficulty distinguishing normal bleeding from abnormal symptoms, and girls may be reluctant to discuss their symptoms with their family or peers. In addition, primary care practitioners may not recognize the severity of the symptoms and therefore may not refer patients to hematologists for further workup.
These findings emphasize the need for improved tools to help patients differentiate between normal and abnormal bleeding, using symptom recognition–based language tools that can lead to early testing and application of accurate diagnostic tools, she said.
Standardization of definitions can help to improve screening and diagnosis, Dr. D’Oiron said, pointing to a recent study in Blood Advances proposing definitions for future research in von Willebrand disease.
For example, the authors of that study proposed a definition of heavy menstrual bleeding to include any of the following:
- Bleeding lasting 8 or more days
- Bleeding that consistently soaks through one or more sanitary protections every 2 hours on multiple days
- Requires use of more than one sanitary protection item at a time
- Requires changing sanitary protection during the night
- Is associated with repeat passing of blood clots
- Has a Pictorial Blood Assessment Chart score greater than 100.
Problem and solutions
Answering the question posed in the title of her talk, Dr. D’Oiron said: “Yes, we do have a problem with the diagnosis of bleeding disorders in women and girls, but we also have solutions.”
The solutions include family and patient outreach efforts; communication to improve awareness; inclusion of general practitioners in the circle of care; and early screening, diagnosis, and treatment.
A bleeding disorders specialist who was not involved in the study said that Dr. D’Oiron’s report closely reflects what she sees in the clinic.
“I do pediatrics, and usually what happens is that I see a teenager with heavy menstrual bleeding and we take her history, and we find out that Mom and multiple female family members have had horrible menstrual bleeding, possibly many of whom have had hysterectomies for it, and then diagnosing the parents and other family members after diagnosing the girl that we’re seeing” said Veronica H. Flood, MD, from the Medical College of Wisconsin, Milwaukee.
“It is unfortunately a very real thing,” she added.
Reasons for the delay likely include lack of awareness of bleeding disorders.
“If you present to a hematologist, we think about bleeding disorders, but if you present to a primary care physician, they don’t always have that on their radar,” she said.
Additionally, a girl from a family with a history of heavy menstrual bleeding may just assume that what she is experiencing is “normal,” despite the serious affect it has on her quality of life, Dr. Flood said.
Dr. D’Oiron’s research is supported by her institution, the French Hemophilia Association, FranceCoag and Mhemon, the European Hemophilia Consortium, and the World Federation of Hemophilia. She reported advisory board or invited speaker activities for multiple companies. Dr. Flood reported having no conflicts of interest to disclose.
Diagnosis of bleeding disorders in girls and women can lag behind diagnosis in boys and men by more than a decade, meaning needless delays in treatment and poor quality of life for many with hemophilia or related conditions.
“There is increasing awareness about issues faced by women and girls with inherited bleeding disorders, but disparities still exist both in both access to diagnosis and treatment,” said Roseline D’Oiron, MD, from Hôpital Bicêtre in Paris.
“Diagnosis, when it is made, is often made late, particularly in women. Indeed, a recent study from the European Hemophilia Consortium including more than 700 women with bleeding disorders showed that the median age at diagnosis was 16 years old,” she said during the annual congress of the European Association for Haemophilia and Allied Disorders.
She said that delayed diagnosis of bleeding disorders in women and girls may be caused by a lack of knowledge by patients, families, and general practitioners about family history of bleeding disorders, abnormal bleeding events, and heavy menstrual bleeding. In addition, despite the frequency and severity of heavy bleeding events, patients, their families, and caregivers may underestimate the effect on the patient’s quality of life.
Disparities documented
Dr. D’Oiron pointed to several studies showing clear sex-based disparities in time to diagnosis. For example, a study published in Haemophilia showed that in 22 girls with hemophilia A or hemophilia B, the diagnosis of severe hemophilia was delayed by a median of 6.5 months compared with the diagnosis in boys, and a diagnosis of moderate hemophilia in girls was delayed by a median of 39 months.
In a second, single-center study comparing 44 women and girls with mild hemophilia (factor VIII or factor XI levels from 5 to 50 IU/dL) with 77 men and boys with mild hemophilia, the mean age at diagnosis was 31.63 years versus 19.18 years, respectively – a delay of 12.45 years.
A third study comparing 442 girls/women and 442 boys/men with mild hemophilia in France showed a difference of 6.07 years in diagnosis: the median age for girls/women at diagnosis was 16.91 years versus10.84 years for boys/men.
Why it matters
Dr. D’Oiron described the case of a patient named Clare, who first experienced, at age 8, 12 hours of bleeding following a dental procedure. At age 12.5, she began having heavy menstrual bleeding, causing her to miss school for a few days each month, to be feel tired, and have poor-quality sleep.
Despite repeated bleeding episodes, severe anemia, and iron deficiency, her hemophilia was not suspected until after her 16th birthday, and a definitive diagnosis of hemophilia in both Clare and her mother was finally made when Clare was past 17, when a nonsense variant factor in F8, the gene encoding for factor VIII, was detected.
“For Clare, it took more the 8 years after the first bleeding symptoms, and nearly 4 years after presenting with heavy menstrual bleeding to recognize that she had a bleeding problem,” she said.
In total, Clare had about 450 days of heavy menstrual bleeding, causing her to miss an estimated 140 days of school because of the delayed diagnosis and treatment.
“In my view, this is the main argument why it is urgent for these patients to achieve diagnosis early: this is to reduce the duration [of] a very poor quality of life,” Dr. D’Oiron said.
Barriers to diagnosis
Patients and families have reported difficulty distinguishing normal bleeding from abnormal symptoms, and girls may be reluctant to discuss their symptoms with their family or peers. In addition, primary care practitioners may not recognize the severity of the symptoms and therefore may not refer patients to hematologists for further workup.
These findings emphasize the need for improved tools to help patients differentiate between normal and abnormal bleeding, using symptom recognition–based language tools that can lead to early testing and application of accurate diagnostic tools, she said.
Standardization of definitions can help to improve screening and diagnosis, Dr. D’Oiron said, pointing to a recent study in Blood Advances proposing definitions for future research in von Willebrand disease.
For example, the authors of that study proposed a definition of heavy menstrual bleeding to include any of the following:
- Bleeding lasting 8 or more days
- Bleeding that consistently soaks through one or more sanitary protections every 2 hours on multiple days
- Requires use of more than one sanitary protection item at a time
- Requires changing sanitary protection during the night
- Is associated with repeat passing of blood clots
- Has a Pictorial Blood Assessment Chart score greater than 100.
Problem and solutions
Answering the question posed in the title of her talk, Dr. D’Oiron said: “Yes, we do have a problem with the diagnosis of bleeding disorders in women and girls, but we also have solutions.”
The solutions include family and patient outreach efforts; communication to improve awareness; inclusion of general practitioners in the circle of care; and early screening, diagnosis, and treatment.
A bleeding disorders specialist who was not involved in the study said that Dr. D’Oiron’s report closely reflects what she sees in the clinic.
“I do pediatrics, and usually what happens is that I see a teenager with heavy menstrual bleeding and we take her history, and we find out that Mom and multiple female family members have had horrible menstrual bleeding, possibly many of whom have had hysterectomies for it, and then diagnosing the parents and other family members after diagnosing the girl that we’re seeing” said Veronica H. Flood, MD, from the Medical College of Wisconsin, Milwaukee.
“It is unfortunately a very real thing,” she added.
Reasons for the delay likely include lack of awareness of bleeding disorders.
“If you present to a hematologist, we think about bleeding disorders, but if you present to a primary care physician, they don’t always have that on their radar,” she said.
Additionally, a girl from a family with a history of heavy menstrual bleeding may just assume that what she is experiencing is “normal,” despite the serious affect it has on her quality of life, Dr. Flood said.
Dr. D’Oiron’s research is supported by her institution, the French Hemophilia Association, FranceCoag and Mhemon, the European Hemophilia Consortium, and the World Federation of Hemophilia. She reported advisory board or invited speaker activities for multiple companies. Dr. Flood reported having no conflicts of interest to disclose.
FROM EAHAD 2021
CDC: Vaccinated people can gather indoors without masks
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
People who are fully vaccinated against COVID-19 can safely gather unmasked and inside with nonvulnerable people who are not yet immunized, according to long-awaited guidance released by the CDC.
“Today’s action represents an important first step. It is not our final destination,” CDC Director Rochelle Walensky, MD, said March 8 at a White House briefing. “As more people get vaccinated, levels of COVID-19 infection decline in communities, and as our understanding of COVID immunity improves, we look forward to updating these recommendations to the public.”
According to the new guidance, people who are at least 2 weeks out from their last dose can:
- Visit with other fully vaccinated people indoors without wearing masks or physical distancing.
- Visit with unvaccinated people from a single household who are at low risk for severe COVID-19 disease indoors without wearing masks or physical distancing
- Avoid quarantine and testing following exposure to someone if they remain asymptomatic.
However, there are still restrictions that will remain until further data are collected. Those who are fully vaccinated must still:
- Wear masks and physically distance in public settings and around people at high risk for severe disease.
- Wear masks and physically distance when visiting unvaccinated people from more than one household.
- Avoid medium- and large-sized gatherings.
- Avoid travel.
People considered at high risk for severe disease include older adults and those with cancer, chronic kidney disease, COPD, Down syndrome, heart disease, heart failure, a weakened immune system, obesity, sickle cell disease, and type 2 diabetes. The category also includes pregnant women and smokers.
“In public spaces, fully vaccinated people should continue to follow guidance to protect themselves and others, including wearing a well-fitted mask, physical distancing (at least 6 feet), avoiding crowds, avoiding poorly ventilated spaces, covering coughs and sneezes, washing hands often, and following any applicable workplace or school guidance,” the guidance says. “Fully vaccinated people should still watch for symptoms of COVID-19, especially following an exposure to someone with suspected or confirmed COVID-19.”
Respecting travel restrictions is still crucial, Dr. Walensky said, given past surges and variants that have emerged after periods of increased travel.
"We would like to give the opportunity for vaccinated grandparents to visit children and grandchildren who are healthy and local,” Dr. Walensky said.
But, she said, “It’s important to realize as we’re working through this that over 90% of the population is not yet vaccinated.”
For now, there are not enough data on transmission rates from those who are vaccinated to the rest of the public. However, Anthony Fauci, MD, said at a briefing last month that preliminary data are “pointing in a very favorable direction.”
Studies from Spain and Israel published last month showed the amount of viral load – or the amount of the COVID-19 virus in someone’s body – is significantly lower if someone gets infected after they’ve been vaccinated, compared with people who get infected and didn’t have the vaccine. Lower viral load means much lower chances of passing the virus to someone else, Dr. Fauci said.
“The science of COVID-19 is complex,” Dr. Walensky said, “and our understanding of it continues to evolve.”
A version of this article first appeared on WebMD.com.
Dining restrictions, mask mandates tied to less illness, death, CDC reaffirms
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The numbers are in to back up two policies designed to restrict the spread of the COVID-19 pandemic.
Researchers at the Centers for Disease Control and Prevention) found that when states lifted restrictions on dining on premises at restaurants, rates of daily COVID-19 cases jumped 41-100 days later. COVID-19-related deaths also increased significantly after 60 days.
On the other hand, the same report demonstrates that state mask mandates slowed the spread of SARS-CoV-2 within a few weeks.
The study was published online March 5 in the CDC Morbidity and Mortality Weekly Report.
The investigators did not distinguish between outdoor and indoor restaurant dining. But they did compare COVID-19 case and death rates before and after most states banned restaurants from serving patrons on-premises in March and April 2020.
They found, for example, that COVID-19 daily cases increased by 0.9% at 41-60 days after on-premise dining was permitted. Similarly, rates jumped by 1.2% at 61-80 days, and 1.1% at 81-100 days after the restaurant restrictions were lifted.
The differences were statistically significant, with P values of .02, <.01, and .04, respectively.
COVID-19–related death rates did not increase significantly at first – but did jump 2.2% between 61 and 80 days after the return of on-premises dining, for example. Deaths also increased by 3% at 81-100 days.
Both these differences were statistically significant (P < .01).
This is not the first report where the CDC announced reservations about in-person dining. In September 2020, CDC investigators implicated the inability to wear a mask while eating and drinking as likely contributing to the heightened risk.
Masks make a difference
The CDC report also provided more evidence to back mask-wearing policies for public spaces. Between March 1 and Dec. 31, 2020, 74% of U.S. counties issued mask mandates.
Investigators found that these policies had a more immediate effect, reducing daily COVID-19 cases by 0.5% in the first 20 days. Mask mandates likewise were linked to daily cases dropping 1.1% between 21 and 40 days, 1.5% between 41 and 60 days, 1.7% between 61 and 80 days, and 1.8% between 81 and 100 days.
These decreases in daily COVID-19 cases were statistically significant (P < .01) compared with a reference period before March 1, 2020.
The CDC also linked mask mandates to lower mortality. For example, these state policies were associated with 0.7% fewer deaths at 1-20 days post implementation. The effect increased thereafter – 1.0% drop at 21-40 days, 1.4% decrease at 41-60 days, 1.6% drop between 61 and 80 days, and 1.9% fewer deaths between 81 and 100 days.
The decrease in deaths was statistically significant at 1-20 days after the mask mandate (P = .03), as well as during the other periods (each P < .01) compared with the reference period.
CDC Director Rochelle Walensky, MD, reacted to the new findings at a White House press briefing. She cited how increases in COVID-19 cases and death rates “slowed significantly within 20 days of putting mask mandates into place. This is why I’m asking you to double down on prevention measures.
“We have seen this movie before,” Dr. Walensky added. “When prevention measures like mask-wearing mandates are lifted, cases go up.”
Recently, multiple states have announced plans to roll back restrictions related to the pandemic, including mask mandates, which prompted warnings from some public health officials.
These are not the first CDC data to show that mask mandates make a difference.
In February 2021, for example, the agency pointed out that state-wide mask mandates reduced COVID-19 hospitalizations by 5.5% among adults 18-64 years old within 3 weeks of implementation.
Restrictions regarding on-premises restaurant dining and implementation of state-wide mask mandates are two tactics within a more comprehensive CDC strategy to reduce the spread of SARS-CoV-2. The researchers note that “such efforts are increasingly important given the emergence of highly transmissible SARS-CoV-2 variants in the United States.”
The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Routine vaccinations missed by older adults during pandemic
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.
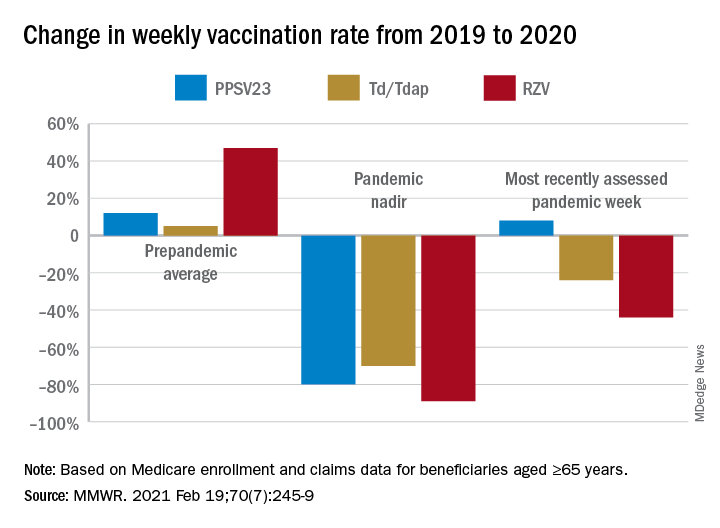
In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
FROM MMWR
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.