User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
A hot dog a day takes 36 minutes away
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
Eyes on ESC ‘21: Hope for EMPEROR-Preserved, guidelines remade
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
AHA targets rising prevalence of obstructive sleep apnea in children
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
U.S. kidney transplants grow in number and success
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Prevalence of youth-onset diabetes climbing, type 2 disease more so in racial/ethnic minorities
The prevalence of youth-onset diabetes in the United States rose significantly from 2001 to 2017, with rates of type 2 diabetes climbing disproportionately among racial/ethnic minorities, according to investigators.
In individuals aged 19 years or younger, prevalence rates of type 1 and type 2 diabetes increased 45.1% and 95.3%, respectively, reported lead author Jean M. Lawrence, ScD, MPH, MSSA, program director of the division of diabetes, endocrinology, and metabolic diseases at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md., and colleagues.
“Elucidating differences in diabetes prevalence trends by diabetes type and demographic characteristics is essential to describe the burden of disease and to estimate current and future resource needs,” Dr. Lawrence and colleagues wrote in JAMA.
The retrospective analysis was a part of the ongoing SEARCH study, which includes data from individuals in six areas across the United States: Colorado, California, Ohio, South Carolina, Washington state, and Arizona/New Mexico (Indian Health Services). In the present report, three prevalence years were evaluated: 2001, 2009, and 2017. For each year, approximately 3.5 million youths were included. Findings were reported in terms of diabetes type, race/ethnicity, age at diagnosis, and sex.
Absolute prevalence of type 1 diabetes per 1,000 youths increased from 1.48 in 2001, to 1.93 in 2009, and finally 2.15 in 2017. Across the 16-year period, this represents an absolute increase of 0.67 (95% confidence interval, 0.64-0.70), and a relative increase of 45.1% (95% CI, 40.0%-50.4%). In absolute terms, prevalence increased most among non-Hispanic White (0.93 per 1,000) and non-Hispanic Black (0.89 per 1,000) youths.
While type 2 diabetes was comparatively less common than type 1 diabetes, absolute prevalence per 1,000 youths increased to a greater degree, rising from 0.34 in 2001 to 0.46 in 2009 and to 0.67 in 2017. This amounts to relative increase across the period of 95.3% (95% CI, 77.0%-115.4%). Absolute increases were disproportionate among racial/ethnic minorities, particularly Black and Hispanic youths, who had absolute increases per 1,000 youths of 0.85 (95% CI, 0.74-0.97) and 0.57 (95% CI, 0.51-0.64), respectively, compared with 0.05 (95% CI, 0.03-0.07) for White youths.
“Increases [among Black and Hispanic youths] were not linear,” the investigators noted. “Hispanic youths had a significantly greater increase in the first interval compared with the second interval, while Black youths had no significant increase in the first interval and a significant increase in the second interval.”
Dr. Lawrence and colleagues offered several possible factors driving these trends in type 2 diabetes.
“Changes in anthropometric risk factors appear to play a significant role,” they wrote, noting that “Black and Mexican American teenagers experienced the greatest increase in prevalence of obesity/severe obesity from 1999 to 2018, which may contribute to race and ethnicity differences. Other contributing factors may include increases in exposure to maternal obesity and diabetes (gestational and type 2 diabetes) and exposure to environmental chemicals.”
According to Megan Kelsey, MD, associate professor of pediatric endocrinology, director of lifestyle medicine endocrinology, and medical director of the bariatric surgery center at Children’s Hospital Colorado, Aurora, the increased rates of type 2 diabetes reported by the study are alarming, yet they pale in comparison with what’s been happening since the pandemic began.
“Individual institutions have reported anywhere between a 50% – which is basically what we’re seeing at our hospital – to a 300% increase in new diagnoses [of type 2 diabetes] in a single-year time period,” Dr. Kelsey said in an interview. “So what is reported [in the present study] doesn’t even get at what’s been going on over the past year and a half.”
Dr. Kelsey offered some speculative drivers of this recent surge in cases, including stress, weight gain caused by sedentary behavior and more access to food, and the possibility that SARS-CoV-2 may infect pancreatic islet beta cells, thereby interfering with insulin production.
Type 2 diabetes is particularly concerning among young people, Dr. Kelsey noted, as it is more challenging to manage than adult-onset disease.
Young patients “also develop complications much sooner than you’d expect,” she added. “So we really need to understand why these rates are increasing, how we can identify kids at risk, and how we can better prevent it, so we aren’t stuck with a disease that’s really difficult to treat.”
To this end, the NIH recently opened applications for investigators to participate in a prospective longitudinal study of youth-onset type 2 diabetes. Young people at risk of diabetes will be followed through puberty, a period of increased risk, according to Dr. Kelsey.
“The goal will be to take kids who don’t yet have [type 2] diabetes, but are at risk, and try to better understand, as some of them progress to developing diabetes, what is going on,” Dr. Kelsey said. “What are other factors that we can use to better predict who’s going to develop diabetes? And can we use the information from this [upcoming] study to understand how to better prevent it? Because nothing that has been tried so far has worked.”
The study was supported by the Centers for Disease Control and Prevention, NIDDK, and others. The investigators and Dr. Kelsey reported no conflicts of interest.
The prevalence of youth-onset diabetes in the United States rose significantly from 2001 to 2017, with rates of type 2 diabetes climbing disproportionately among racial/ethnic minorities, according to investigators.
In individuals aged 19 years or younger, prevalence rates of type 1 and type 2 diabetes increased 45.1% and 95.3%, respectively, reported lead author Jean M. Lawrence, ScD, MPH, MSSA, program director of the division of diabetes, endocrinology, and metabolic diseases at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md., and colleagues.
“Elucidating differences in diabetes prevalence trends by diabetes type and demographic characteristics is essential to describe the burden of disease and to estimate current and future resource needs,” Dr. Lawrence and colleagues wrote in JAMA.
The retrospective analysis was a part of the ongoing SEARCH study, which includes data from individuals in six areas across the United States: Colorado, California, Ohio, South Carolina, Washington state, and Arizona/New Mexico (Indian Health Services). In the present report, three prevalence years were evaluated: 2001, 2009, and 2017. For each year, approximately 3.5 million youths were included. Findings were reported in terms of diabetes type, race/ethnicity, age at diagnosis, and sex.
Absolute prevalence of type 1 diabetes per 1,000 youths increased from 1.48 in 2001, to 1.93 in 2009, and finally 2.15 in 2017. Across the 16-year period, this represents an absolute increase of 0.67 (95% confidence interval, 0.64-0.70), and a relative increase of 45.1% (95% CI, 40.0%-50.4%). In absolute terms, prevalence increased most among non-Hispanic White (0.93 per 1,000) and non-Hispanic Black (0.89 per 1,000) youths.
While type 2 diabetes was comparatively less common than type 1 diabetes, absolute prevalence per 1,000 youths increased to a greater degree, rising from 0.34 in 2001 to 0.46 in 2009 and to 0.67 in 2017. This amounts to relative increase across the period of 95.3% (95% CI, 77.0%-115.4%). Absolute increases were disproportionate among racial/ethnic minorities, particularly Black and Hispanic youths, who had absolute increases per 1,000 youths of 0.85 (95% CI, 0.74-0.97) and 0.57 (95% CI, 0.51-0.64), respectively, compared with 0.05 (95% CI, 0.03-0.07) for White youths.
“Increases [among Black and Hispanic youths] were not linear,” the investigators noted. “Hispanic youths had a significantly greater increase in the first interval compared with the second interval, while Black youths had no significant increase in the first interval and a significant increase in the second interval.”
Dr. Lawrence and colleagues offered several possible factors driving these trends in type 2 diabetes.
“Changes in anthropometric risk factors appear to play a significant role,” they wrote, noting that “Black and Mexican American teenagers experienced the greatest increase in prevalence of obesity/severe obesity from 1999 to 2018, which may contribute to race and ethnicity differences. Other contributing factors may include increases in exposure to maternal obesity and diabetes (gestational and type 2 diabetes) and exposure to environmental chemicals.”
According to Megan Kelsey, MD, associate professor of pediatric endocrinology, director of lifestyle medicine endocrinology, and medical director of the bariatric surgery center at Children’s Hospital Colorado, Aurora, the increased rates of type 2 diabetes reported by the study are alarming, yet they pale in comparison with what’s been happening since the pandemic began.
“Individual institutions have reported anywhere between a 50% – which is basically what we’re seeing at our hospital – to a 300% increase in new diagnoses [of type 2 diabetes] in a single-year time period,” Dr. Kelsey said in an interview. “So what is reported [in the present study] doesn’t even get at what’s been going on over the past year and a half.”
Dr. Kelsey offered some speculative drivers of this recent surge in cases, including stress, weight gain caused by sedentary behavior and more access to food, and the possibility that SARS-CoV-2 may infect pancreatic islet beta cells, thereby interfering with insulin production.
Type 2 diabetes is particularly concerning among young people, Dr. Kelsey noted, as it is more challenging to manage than adult-onset disease.
Young patients “also develop complications much sooner than you’d expect,” she added. “So we really need to understand why these rates are increasing, how we can identify kids at risk, and how we can better prevent it, so we aren’t stuck with a disease that’s really difficult to treat.”
To this end, the NIH recently opened applications for investigators to participate in a prospective longitudinal study of youth-onset type 2 diabetes. Young people at risk of diabetes will be followed through puberty, a period of increased risk, according to Dr. Kelsey.
“The goal will be to take kids who don’t yet have [type 2] diabetes, but are at risk, and try to better understand, as some of them progress to developing diabetes, what is going on,” Dr. Kelsey said. “What are other factors that we can use to better predict who’s going to develop diabetes? And can we use the information from this [upcoming] study to understand how to better prevent it? Because nothing that has been tried so far has worked.”
The study was supported by the Centers for Disease Control and Prevention, NIDDK, and others. The investigators and Dr. Kelsey reported no conflicts of interest.
The prevalence of youth-onset diabetes in the United States rose significantly from 2001 to 2017, with rates of type 2 diabetes climbing disproportionately among racial/ethnic minorities, according to investigators.
In individuals aged 19 years or younger, prevalence rates of type 1 and type 2 diabetes increased 45.1% and 95.3%, respectively, reported lead author Jean M. Lawrence, ScD, MPH, MSSA, program director of the division of diabetes, endocrinology, and metabolic diseases at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md., and colleagues.
“Elucidating differences in diabetes prevalence trends by diabetes type and demographic characteristics is essential to describe the burden of disease and to estimate current and future resource needs,” Dr. Lawrence and colleagues wrote in JAMA.
The retrospective analysis was a part of the ongoing SEARCH study, which includes data from individuals in six areas across the United States: Colorado, California, Ohio, South Carolina, Washington state, and Arizona/New Mexico (Indian Health Services). In the present report, three prevalence years were evaluated: 2001, 2009, and 2017. For each year, approximately 3.5 million youths were included. Findings were reported in terms of diabetes type, race/ethnicity, age at diagnosis, and sex.
Absolute prevalence of type 1 diabetes per 1,000 youths increased from 1.48 in 2001, to 1.93 in 2009, and finally 2.15 in 2017. Across the 16-year period, this represents an absolute increase of 0.67 (95% confidence interval, 0.64-0.70), and a relative increase of 45.1% (95% CI, 40.0%-50.4%). In absolute terms, prevalence increased most among non-Hispanic White (0.93 per 1,000) and non-Hispanic Black (0.89 per 1,000) youths.
While type 2 diabetes was comparatively less common than type 1 diabetes, absolute prevalence per 1,000 youths increased to a greater degree, rising from 0.34 in 2001 to 0.46 in 2009 and to 0.67 in 2017. This amounts to relative increase across the period of 95.3% (95% CI, 77.0%-115.4%). Absolute increases were disproportionate among racial/ethnic minorities, particularly Black and Hispanic youths, who had absolute increases per 1,000 youths of 0.85 (95% CI, 0.74-0.97) and 0.57 (95% CI, 0.51-0.64), respectively, compared with 0.05 (95% CI, 0.03-0.07) for White youths.
“Increases [among Black and Hispanic youths] were not linear,” the investigators noted. “Hispanic youths had a significantly greater increase in the first interval compared with the second interval, while Black youths had no significant increase in the first interval and a significant increase in the second interval.”
Dr. Lawrence and colleagues offered several possible factors driving these trends in type 2 diabetes.
“Changes in anthropometric risk factors appear to play a significant role,” they wrote, noting that “Black and Mexican American teenagers experienced the greatest increase in prevalence of obesity/severe obesity from 1999 to 2018, which may contribute to race and ethnicity differences. Other contributing factors may include increases in exposure to maternal obesity and diabetes (gestational and type 2 diabetes) and exposure to environmental chemicals.”
According to Megan Kelsey, MD, associate professor of pediatric endocrinology, director of lifestyle medicine endocrinology, and medical director of the bariatric surgery center at Children’s Hospital Colorado, Aurora, the increased rates of type 2 diabetes reported by the study are alarming, yet they pale in comparison with what’s been happening since the pandemic began.
“Individual institutions have reported anywhere between a 50% – which is basically what we’re seeing at our hospital – to a 300% increase in new diagnoses [of type 2 diabetes] in a single-year time period,” Dr. Kelsey said in an interview. “So what is reported [in the present study] doesn’t even get at what’s been going on over the past year and a half.”
Dr. Kelsey offered some speculative drivers of this recent surge in cases, including stress, weight gain caused by sedentary behavior and more access to food, and the possibility that SARS-CoV-2 may infect pancreatic islet beta cells, thereby interfering with insulin production.
Type 2 diabetes is particularly concerning among young people, Dr. Kelsey noted, as it is more challenging to manage than adult-onset disease.
Young patients “also develop complications much sooner than you’d expect,” she added. “So we really need to understand why these rates are increasing, how we can identify kids at risk, and how we can better prevent it, so we aren’t stuck with a disease that’s really difficult to treat.”
To this end, the NIH recently opened applications for investigators to participate in a prospective longitudinal study of youth-onset type 2 diabetes. Young people at risk of diabetes will be followed through puberty, a period of increased risk, according to Dr. Kelsey.
“The goal will be to take kids who don’t yet have [type 2] diabetes, but are at risk, and try to better understand, as some of them progress to developing diabetes, what is going on,” Dr. Kelsey said. “What are other factors that we can use to better predict who’s going to develop diabetes? And can we use the information from this [upcoming] study to understand how to better prevent it? Because nothing that has been tried so far has worked.”
The study was supported by the Centers for Disease Control and Prevention, NIDDK, and others. The investigators and Dr. Kelsey reported no conflicts of interest.
FROM JAMA
Flavonoid-rich foods, aided by gut bacteria, tied to lower BP
, an association that is partially explained by bacteria in an individual’s gut microbiome, new research suggests.
In a population-based study of more than 900 individuals, those with the highest intake of flavonoid-containing foods had significantly lower systolic blood pressure and pulse pressure, as well as greater gut microbial diversity, compared with those with the lowest intakes.
Up to 15% of this observed association was explained by the gut microbiome, suggesting that these microbes play a key role in metabolizing flavonoids to enhance their cardioprotective effects, according to the researchers.
The study was published online in the journal Hypertension.
“We know what we eat plays a critical role in shaping our gut microbiome, but little is known about the relative importance of plant foods and specific constituents called flavonoids,” lead researcher Aedin Cassidy, PhD, chair and professor of nutrition and medicine at the Institute for Global Food Security, Queen’s University, Belfast, Northern Ireland, said in an interview.
“Unlike many other food constituents, flavonoids are predominantly metabolized in the gut, suggesting that the gut microbiome may be more important in enhancing their biological activity than for other things we eat,” Dr. Cassidy said.
“There is mounting evidence from population-based studies and clinical trials that a higher intake of flavonoids and flavonoid-rich foods can improve heart health, but for the first time, we provide data highlighting the key role of the gut microbiome in explaining the association between such foods and blood pressure,” she noted. “This is one of the first studies to address this.”
For this analysis, Dr. Cassidy and her group sought to assess to what extent the composition of the gut microbiome might explain the association of habitual flavonoid and flavonoid-rich food intake with systolic and diastolic blood pressure in a community-based sample of 904 individuals aged 25-82 years from Germany’s PopGen biobank.
The researchers evaluated participants’ food intake, gut microbiome, and blood pressure levels together with other clinical and molecular phenotyping at regular follow-up examinations.
Participants’ intake of flavonoid-rich foods during the previous year was calculated from a self-reported food questionnaire detailing the frequency and quantity eaten of 112 foods, and flavonoid values were assigned to foods according to United States Department of Agriculture data on flavonoid content in food.
Participants’ gut microbiome was assessed by fecal bacterial DNA extracted from stool samples.
After an overnight fast, participants’ blood pressure levels were measured three times in 3-minute intervals after an initial 5-minute rest period. Researchers also collected participants’ diet and lifestyle information.
Analysis of the data showed the following:
- Eating 1.5 servings of berries per day (about 1 cup) was associated with a 4.1–mm Hg reduction in systolic BP; 12% of this association was explained by gut microbiome factors.
- Drinking three glasses of red wine per week was associated with a 3.7–mm Hg reduction in systolic BP; 15% of this association was explained by the gut microbiome.
“These blood pressure–lowering effects are achievable with simple changes to the daily diet,” Dr. Cassidy said.
“Incorporating flavonoid-rich foods into the diet can have clinically relevant reductions in systolic blood pressure and pulse pressure, and a healthy gut microbiome is important to break down flavonoids to a more cardioprotective form,” she said.
“Our findings indicate future trials should look at participants according to metabolic profile in order to more accurately study the roles of metabolism and the gut microbiome in regulating the effects of flavonoids on blood pressure,” said Dr. Cassidy.
“A better understanding of the highly individual variability of flavonoid metabolism could very well explain why some people have greater cardiovascular protection benefits from flavonoid-rich foods than others.”
‘Interesting’ data
“The data are interesting,” David Jenkins, MD, PhD, DSc, professor of medicine and nutrition at the University of Toronto, said in an interview.
“Berries and red wine appear to be associated with lower systolic blood pressures. Lower blood pressures have been found in general in people who consume more plant-based diets, especially those high in fruits and vegetables,” noted Dr. Jenkins, who was not involved with this study.
“Berries and grapes high in polyphenols may have many health benefits as antioxidants, and in a recent study have been shown to reduce cardiovascular mortality. The change in chronic microflora is also of interest as this will change with increased fruit and vegetable consumption,” he said.
Perhaps one word of caveat, Dr. Jenkins added: “Alcohol has been found to increase blood pressure and the risk of stroke. Presumably the beneficial effects as seen here were when wine is consumed in moderation.”
Supports recommendations
The study by Cassidy and colleagues supports the dietary recommendations from the American Heart Association (AHA) for heart health, Penny M. Kris-Etherton, PhD, RDN, professor of nutritional sciences, Penn State University, University Park, Pa., and chair, AHA Council on Lifestyle and Cardiometabolic Health, said in an interview.
“The AHA recommends a healthy dietary pattern that emphasizes a variety of plant foods including fruits, vegetables, whole grains, legumes, nuts, and seeds and is low in sodium, saturated fat, and added sugars. Lean protein foods, including plant protein foods, are recommended, and red meat should be limited. If alcohol is consumed it should be done in moderation,” Dr. Kris-Etherton said.
“Based on these AHA dietary recommendations, a wide variety of plant foods will promote consumption of many flavonoids that have demonstrated CVD benefits, such as lowering systolic blood pressure as reported by the authors, as well as promoting healthy endothelial function and having antithrombotic, anti-inflammatory and antioxidant effects,” she said in email.
“This recommended dietary pattern will have other cardiovascular health benefits, such as decreasing LDL cholesterol, due to its very healthy nutrient profile. The exciting new finding reported by Cassidy et al. is that the effects of dietary flavonoids on lowering systolic blood pressure are modulated by the gut microbiome,” Dr. Kris-Etherton said.
“Further research needs to be done to confirm these findings and to identify how different foods affect specific gut bacteria that benefit cardiovascular health.”
The research was funded by grants from the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Cassidy and Dr. Jenkins have disclosed no relevant financial relationships. Dr. Kris-Etherton is a spokesperson for the AHA.
A version of this article first appeared on Medscape.com.
, an association that is partially explained by bacteria in an individual’s gut microbiome, new research suggests.
In a population-based study of more than 900 individuals, those with the highest intake of flavonoid-containing foods had significantly lower systolic blood pressure and pulse pressure, as well as greater gut microbial diversity, compared with those with the lowest intakes.
Up to 15% of this observed association was explained by the gut microbiome, suggesting that these microbes play a key role in metabolizing flavonoids to enhance their cardioprotective effects, according to the researchers.
The study was published online in the journal Hypertension.
“We know what we eat plays a critical role in shaping our gut microbiome, but little is known about the relative importance of plant foods and specific constituents called flavonoids,” lead researcher Aedin Cassidy, PhD, chair and professor of nutrition and medicine at the Institute for Global Food Security, Queen’s University, Belfast, Northern Ireland, said in an interview.
“Unlike many other food constituents, flavonoids are predominantly metabolized in the gut, suggesting that the gut microbiome may be more important in enhancing their biological activity than for other things we eat,” Dr. Cassidy said.
“There is mounting evidence from population-based studies and clinical trials that a higher intake of flavonoids and flavonoid-rich foods can improve heart health, but for the first time, we provide data highlighting the key role of the gut microbiome in explaining the association between such foods and blood pressure,” she noted. “This is one of the first studies to address this.”
For this analysis, Dr. Cassidy and her group sought to assess to what extent the composition of the gut microbiome might explain the association of habitual flavonoid and flavonoid-rich food intake with systolic and diastolic blood pressure in a community-based sample of 904 individuals aged 25-82 years from Germany’s PopGen biobank.
The researchers evaluated participants’ food intake, gut microbiome, and blood pressure levels together with other clinical and molecular phenotyping at regular follow-up examinations.
Participants’ intake of flavonoid-rich foods during the previous year was calculated from a self-reported food questionnaire detailing the frequency and quantity eaten of 112 foods, and flavonoid values were assigned to foods according to United States Department of Agriculture data on flavonoid content in food.
Participants’ gut microbiome was assessed by fecal bacterial DNA extracted from stool samples.
After an overnight fast, participants’ blood pressure levels were measured three times in 3-minute intervals after an initial 5-minute rest period. Researchers also collected participants’ diet and lifestyle information.
Analysis of the data showed the following:
- Eating 1.5 servings of berries per day (about 1 cup) was associated with a 4.1–mm Hg reduction in systolic BP; 12% of this association was explained by gut microbiome factors.
- Drinking three glasses of red wine per week was associated with a 3.7–mm Hg reduction in systolic BP; 15% of this association was explained by the gut microbiome.
“These blood pressure–lowering effects are achievable with simple changes to the daily diet,” Dr. Cassidy said.
“Incorporating flavonoid-rich foods into the diet can have clinically relevant reductions in systolic blood pressure and pulse pressure, and a healthy gut microbiome is important to break down flavonoids to a more cardioprotective form,” she said.
“Our findings indicate future trials should look at participants according to metabolic profile in order to more accurately study the roles of metabolism and the gut microbiome in regulating the effects of flavonoids on blood pressure,” said Dr. Cassidy.
“A better understanding of the highly individual variability of flavonoid metabolism could very well explain why some people have greater cardiovascular protection benefits from flavonoid-rich foods than others.”
‘Interesting’ data
“The data are interesting,” David Jenkins, MD, PhD, DSc, professor of medicine and nutrition at the University of Toronto, said in an interview.
“Berries and red wine appear to be associated with lower systolic blood pressures. Lower blood pressures have been found in general in people who consume more plant-based diets, especially those high in fruits and vegetables,” noted Dr. Jenkins, who was not involved with this study.
“Berries and grapes high in polyphenols may have many health benefits as antioxidants, and in a recent study have been shown to reduce cardiovascular mortality. The change in chronic microflora is also of interest as this will change with increased fruit and vegetable consumption,” he said.
Perhaps one word of caveat, Dr. Jenkins added: “Alcohol has been found to increase blood pressure and the risk of stroke. Presumably the beneficial effects as seen here were when wine is consumed in moderation.”
Supports recommendations
The study by Cassidy and colleagues supports the dietary recommendations from the American Heart Association (AHA) for heart health, Penny M. Kris-Etherton, PhD, RDN, professor of nutritional sciences, Penn State University, University Park, Pa., and chair, AHA Council on Lifestyle and Cardiometabolic Health, said in an interview.
“The AHA recommends a healthy dietary pattern that emphasizes a variety of plant foods including fruits, vegetables, whole grains, legumes, nuts, and seeds and is low in sodium, saturated fat, and added sugars. Lean protein foods, including plant protein foods, are recommended, and red meat should be limited. If alcohol is consumed it should be done in moderation,” Dr. Kris-Etherton said.
“Based on these AHA dietary recommendations, a wide variety of plant foods will promote consumption of many flavonoids that have demonstrated CVD benefits, such as lowering systolic blood pressure as reported by the authors, as well as promoting healthy endothelial function and having antithrombotic, anti-inflammatory and antioxidant effects,” she said in email.
“This recommended dietary pattern will have other cardiovascular health benefits, such as decreasing LDL cholesterol, due to its very healthy nutrient profile. The exciting new finding reported by Cassidy et al. is that the effects of dietary flavonoids on lowering systolic blood pressure are modulated by the gut microbiome,” Dr. Kris-Etherton said.
“Further research needs to be done to confirm these findings and to identify how different foods affect specific gut bacteria that benefit cardiovascular health.”
The research was funded by grants from the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Cassidy and Dr. Jenkins have disclosed no relevant financial relationships. Dr. Kris-Etherton is a spokesperson for the AHA.
A version of this article first appeared on Medscape.com.
, an association that is partially explained by bacteria in an individual’s gut microbiome, new research suggests.
In a population-based study of more than 900 individuals, those with the highest intake of flavonoid-containing foods had significantly lower systolic blood pressure and pulse pressure, as well as greater gut microbial diversity, compared with those with the lowest intakes.
Up to 15% of this observed association was explained by the gut microbiome, suggesting that these microbes play a key role in metabolizing flavonoids to enhance their cardioprotective effects, according to the researchers.
The study was published online in the journal Hypertension.
“We know what we eat plays a critical role in shaping our gut microbiome, but little is known about the relative importance of plant foods and specific constituents called flavonoids,” lead researcher Aedin Cassidy, PhD, chair and professor of nutrition and medicine at the Institute for Global Food Security, Queen’s University, Belfast, Northern Ireland, said in an interview.
“Unlike many other food constituents, flavonoids are predominantly metabolized in the gut, suggesting that the gut microbiome may be more important in enhancing their biological activity than for other things we eat,” Dr. Cassidy said.
“There is mounting evidence from population-based studies and clinical trials that a higher intake of flavonoids and flavonoid-rich foods can improve heart health, but for the first time, we provide data highlighting the key role of the gut microbiome in explaining the association between such foods and blood pressure,” she noted. “This is one of the first studies to address this.”
For this analysis, Dr. Cassidy and her group sought to assess to what extent the composition of the gut microbiome might explain the association of habitual flavonoid and flavonoid-rich food intake with systolic and diastolic blood pressure in a community-based sample of 904 individuals aged 25-82 years from Germany’s PopGen biobank.
The researchers evaluated participants’ food intake, gut microbiome, and blood pressure levels together with other clinical and molecular phenotyping at regular follow-up examinations.
Participants’ intake of flavonoid-rich foods during the previous year was calculated from a self-reported food questionnaire detailing the frequency and quantity eaten of 112 foods, and flavonoid values were assigned to foods according to United States Department of Agriculture data on flavonoid content in food.
Participants’ gut microbiome was assessed by fecal bacterial DNA extracted from stool samples.
After an overnight fast, participants’ blood pressure levels were measured three times in 3-minute intervals after an initial 5-minute rest period. Researchers also collected participants’ diet and lifestyle information.
Analysis of the data showed the following:
- Eating 1.5 servings of berries per day (about 1 cup) was associated with a 4.1–mm Hg reduction in systolic BP; 12% of this association was explained by gut microbiome factors.
- Drinking three glasses of red wine per week was associated with a 3.7–mm Hg reduction in systolic BP; 15% of this association was explained by the gut microbiome.
“These blood pressure–lowering effects are achievable with simple changes to the daily diet,” Dr. Cassidy said.
“Incorporating flavonoid-rich foods into the diet can have clinically relevant reductions in systolic blood pressure and pulse pressure, and a healthy gut microbiome is important to break down flavonoids to a more cardioprotective form,” she said.
“Our findings indicate future trials should look at participants according to metabolic profile in order to more accurately study the roles of metabolism and the gut microbiome in regulating the effects of flavonoids on blood pressure,” said Dr. Cassidy.
“A better understanding of the highly individual variability of flavonoid metabolism could very well explain why some people have greater cardiovascular protection benefits from flavonoid-rich foods than others.”
‘Interesting’ data
“The data are interesting,” David Jenkins, MD, PhD, DSc, professor of medicine and nutrition at the University of Toronto, said in an interview.
“Berries and red wine appear to be associated with lower systolic blood pressures. Lower blood pressures have been found in general in people who consume more plant-based diets, especially those high in fruits and vegetables,” noted Dr. Jenkins, who was not involved with this study.
“Berries and grapes high in polyphenols may have many health benefits as antioxidants, and in a recent study have been shown to reduce cardiovascular mortality. The change in chronic microflora is also of interest as this will change with increased fruit and vegetable consumption,” he said.
Perhaps one word of caveat, Dr. Jenkins added: “Alcohol has been found to increase blood pressure and the risk of stroke. Presumably the beneficial effects as seen here were when wine is consumed in moderation.”
Supports recommendations
The study by Cassidy and colleagues supports the dietary recommendations from the American Heart Association (AHA) for heart health, Penny M. Kris-Etherton, PhD, RDN, professor of nutritional sciences, Penn State University, University Park, Pa., and chair, AHA Council on Lifestyle and Cardiometabolic Health, said in an interview.
“The AHA recommends a healthy dietary pattern that emphasizes a variety of plant foods including fruits, vegetables, whole grains, legumes, nuts, and seeds and is low in sodium, saturated fat, and added sugars. Lean protein foods, including plant protein foods, are recommended, and red meat should be limited. If alcohol is consumed it should be done in moderation,” Dr. Kris-Etherton said.
“Based on these AHA dietary recommendations, a wide variety of plant foods will promote consumption of many flavonoids that have demonstrated CVD benefits, such as lowering systolic blood pressure as reported by the authors, as well as promoting healthy endothelial function and having antithrombotic, anti-inflammatory and antioxidant effects,” she said in email.
“This recommended dietary pattern will have other cardiovascular health benefits, such as decreasing LDL cholesterol, due to its very healthy nutrient profile. The exciting new finding reported by Cassidy et al. is that the effects of dietary flavonoids on lowering systolic blood pressure are modulated by the gut microbiome,” Dr. Kris-Etherton said.
“Further research needs to be done to confirm these findings and to identify how different foods affect specific gut bacteria that benefit cardiovascular health.”
The research was funded by grants from the German Research Foundation and the German Federal Ministry of Education and Research. Dr. Cassidy and Dr. Jenkins have disclosed no relevant financial relationships. Dr. Kris-Etherton is a spokesperson for the AHA.
A version of this article first appeared on Medscape.com.
US Preventive Services Task Force lowers diabetes screening age for overweight
The United States Preventive Services Task Force has updated its recommendation on the age of screening for prediabetes and type 2 diabetes in the primary care setting – lowering the age from 40 to 35 years for asymptomatic patients who are overweight or obese and encouraging greater interventions when patients do show a risk.
“The USPSTF concludes with moderate certainty that screening for prediabetes and type 2 diabetes and offering or referring patients with prediabetes to effective preventive interventions has a moderate net benefit,” the task force concludes in its recommendation, published Aug. 24 in JAMA.
“Clinicians should offer or refer patients with prediabetes to effective preventive interventions,” they write.
Experts commenting on the issue strongly emphasize that it’s not just the screening, but the subsequent intervention that is needed to make a difference.
“If young adults newly identified with abnormal glucose metabolism do not receive the needed intensive behavioral change support, screening may provide no benefit,” write Richard W. Grant, MD, MPH, and colleagues in an editorial published with the recommendation.
“Given the role of our obesogenic and physically inactive society in the shift toward earlier onset of diabetes, efforts to increase screening and recognition of abnormal glucose metabolism must be coupled with robust public health measures to address the underlying contributors.”
BMI cutoff lower for at-risk ethnic populations
The recommendation, which updates the task force’s 2015 guideline, carries a “B” classification, meaning the USPSTF has high certainty that the net benefit is moderate. It now specifies screening from age 35to 70 for persons classified as overweight (body mass index at least 25) or obese (BMI at least 30) and recommends referral to preventive interventions when patients are found to have prediabetes.
In addition to recommendations of lifestyle changes, such as diet and physical activity, the task force also endorses the diabetes drug metformin as a beneficial intervention in the prevention or delay of diabetes, while noting fewer overall health benefits from metformin than from the lifestyle changes.
A lower BMI cutoff of at least 23 is recommended for diabetes screening of Asian Americans, and, importantly, screening for prediabetes and diabetes should be considered at an even earlier age if the patient is from a population with a disproportionately high prevalence of diabetes, including American Indian/Alaska Native, Black, Hawaiian/Pacific Islander, Hispanic/Latino, the task force recommends.
Screening tests should include fasting plasma glucose, hemoglobin A1c, or an oral glucose tolerance test. Although screening every 3 years “may be a reasonable approach for adults with normal blood glucose levels,” the task force adds that “the optimal screening interval for adults with an initial normal glucose test result is uncertain.”
Data review: Few with prediabetes know they have it
The need for the update was prompted by troubling data showing increasing diabetes rates despite early signs that can and should be identified and acted upon in the primary care setting to prevent disease progression.
Data from the Centers for Disease Control and Prevention, for instance, show that while 13% of all U.S. adults 18 years or older have diabetes and 35% meet criteria for prediabetes, as many as 21% of those with diabetes were not aware of or did not report having the disease. Furthermore, only a small fraction – 15% of those with prediabetes – said they had been told by a health professional that they had this condition, the task force notes.
The task force’s final recommendation was based on a systematic review of evidence regarding the screening of asymptomatic, nonpregnant adults and the harms and benefits of interventions, such as physical activity, behavioral counseling, or pharmacotherapy.
Among key evidence supporting the lower age was a 2014 study showing that the number of people necessary to obtain one positive test for diabetes with screening sharply drops from 80 among those aged 30-34 years to just 31 among those aged 36-39.
Opportunistic universal screening of eligible people aged 35 and older would yield a ratio of 1 out of just 15 to spot a positive test, the authors of that study reported.
In addition, a large cohort study in more than 77,000 people with prediabetes strongly links the risk of developing diabetes with increases in A1c level and with increasing BMI.
ADA recommendations differ
The new recommendations differ from American Diabetes Association guidelines, which call for diabetes screening at all ages for people who are overweight or obese and who have one or more risk factors, such as physical inactivity or a first-degree relative with diabetes. If results are normal, repeat screening at least every 3 years is recommended.
The ADA further recommends universal screening for all adults 45 years and older, regardless of their risk factors.
For the screening of adults over 45, the ADA recommends using a fasting plasma glucose level, 2-hour plasma glucose level during a 75-g oral glucose tolerance test, or A1c level, regardless of risk factors.
The American Association of Clinical Endocrinology also recommends universal screening for prediabetes and diabetes for all adults 45 years or older, regardless of risk factors, and also advises screening those who have risk factors for diabetes regardless of age.
Screening of little benefit without behavior change support
In an interview, Dr. Grant added that broad efforts are essential as those at the practice level have clearly not succeeded.
“The medical model of individual counseling and referral has not really been effective, and so we really need to think in terms of large-scale public health action,” said Dr. Grant, of the division of research, Kaiser Permanente Northern California, Oakland.
His editorial details the sweeping, multifactorial efforts that are needed.
“To turn this recommendation into action – that is, to translate screening activities into improved clinical outcomes – change is needed at the patient-clinician level (recognizing and encouraging eligible individuals to be screened), health care system level (reducing screening barriers and ensuring access to robust lifestyle programs), and societal level (applying effective public health interventions to reduce obesity and increase exercise),” they write.
A top priority has to be a focus on individuals of diverse backgrounds and issues such as access to healthy programs in minority communities, Dr. Grant noted.
“Newly diagnosed adults are more likely to be African-American and Latinx,” he said.
“We really need to invest in healthier communities for low-income, non-White communities to reverse the persistent health care disparities in these communities.”
While the challenges may appear daunting, history shows they are not necessarily insurmountable – as evidenced in the campaign to discourage tobacco smoking.
“National smoking cessation efforts are one example of a mostly successful public health campaign that has made a difference in health behaviors,” Grant noted.
The recommendation is also posted on the USPSTF web site .
Dr. Grant reports receiving grants from the National Institutes of Health and the Patient-Centered Outcomes Research Institute.
The United States Preventive Services Task Force has updated its recommendation on the age of screening for prediabetes and type 2 diabetes in the primary care setting – lowering the age from 40 to 35 years for asymptomatic patients who are overweight or obese and encouraging greater interventions when patients do show a risk.
“The USPSTF concludes with moderate certainty that screening for prediabetes and type 2 diabetes and offering or referring patients with prediabetes to effective preventive interventions has a moderate net benefit,” the task force concludes in its recommendation, published Aug. 24 in JAMA.
“Clinicians should offer or refer patients with prediabetes to effective preventive interventions,” they write.
Experts commenting on the issue strongly emphasize that it’s not just the screening, but the subsequent intervention that is needed to make a difference.
“If young adults newly identified with abnormal glucose metabolism do not receive the needed intensive behavioral change support, screening may provide no benefit,” write Richard W. Grant, MD, MPH, and colleagues in an editorial published with the recommendation.
“Given the role of our obesogenic and physically inactive society in the shift toward earlier onset of diabetes, efforts to increase screening and recognition of abnormal glucose metabolism must be coupled with robust public health measures to address the underlying contributors.”
BMI cutoff lower for at-risk ethnic populations
The recommendation, which updates the task force’s 2015 guideline, carries a “B” classification, meaning the USPSTF has high certainty that the net benefit is moderate. It now specifies screening from age 35to 70 for persons classified as overweight (body mass index at least 25) or obese (BMI at least 30) and recommends referral to preventive interventions when patients are found to have prediabetes.
In addition to recommendations of lifestyle changes, such as diet and physical activity, the task force also endorses the diabetes drug metformin as a beneficial intervention in the prevention or delay of diabetes, while noting fewer overall health benefits from metformin than from the lifestyle changes.
A lower BMI cutoff of at least 23 is recommended for diabetes screening of Asian Americans, and, importantly, screening for prediabetes and diabetes should be considered at an even earlier age if the patient is from a population with a disproportionately high prevalence of diabetes, including American Indian/Alaska Native, Black, Hawaiian/Pacific Islander, Hispanic/Latino, the task force recommends.
Screening tests should include fasting plasma glucose, hemoglobin A1c, or an oral glucose tolerance test. Although screening every 3 years “may be a reasonable approach for adults with normal blood glucose levels,” the task force adds that “the optimal screening interval for adults with an initial normal glucose test result is uncertain.”
Data review: Few with prediabetes know they have it
The need for the update was prompted by troubling data showing increasing diabetes rates despite early signs that can and should be identified and acted upon in the primary care setting to prevent disease progression.
Data from the Centers for Disease Control and Prevention, for instance, show that while 13% of all U.S. adults 18 years or older have diabetes and 35% meet criteria for prediabetes, as many as 21% of those with diabetes were not aware of or did not report having the disease. Furthermore, only a small fraction – 15% of those with prediabetes – said they had been told by a health professional that they had this condition, the task force notes.
The task force’s final recommendation was based on a systematic review of evidence regarding the screening of asymptomatic, nonpregnant adults and the harms and benefits of interventions, such as physical activity, behavioral counseling, or pharmacotherapy.
Among key evidence supporting the lower age was a 2014 study showing that the number of people necessary to obtain one positive test for diabetes with screening sharply drops from 80 among those aged 30-34 years to just 31 among those aged 36-39.
Opportunistic universal screening of eligible people aged 35 and older would yield a ratio of 1 out of just 15 to spot a positive test, the authors of that study reported.
In addition, a large cohort study in more than 77,000 people with prediabetes strongly links the risk of developing diabetes with increases in A1c level and with increasing BMI.
ADA recommendations differ
The new recommendations differ from American Diabetes Association guidelines, which call for diabetes screening at all ages for people who are overweight or obese and who have one or more risk factors, such as physical inactivity or a first-degree relative with diabetes. If results are normal, repeat screening at least every 3 years is recommended.
The ADA further recommends universal screening for all adults 45 years and older, regardless of their risk factors.
For the screening of adults over 45, the ADA recommends using a fasting plasma glucose level, 2-hour plasma glucose level during a 75-g oral glucose tolerance test, or A1c level, regardless of risk factors.
The American Association of Clinical Endocrinology also recommends universal screening for prediabetes and diabetes for all adults 45 years or older, regardless of risk factors, and also advises screening those who have risk factors for diabetes regardless of age.
Screening of little benefit without behavior change support
In an interview, Dr. Grant added that broad efforts are essential as those at the practice level have clearly not succeeded.
“The medical model of individual counseling and referral has not really been effective, and so we really need to think in terms of large-scale public health action,” said Dr. Grant, of the division of research, Kaiser Permanente Northern California, Oakland.
His editorial details the sweeping, multifactorial efforts that are needed.
“To turn this recommendation into action – that is, to translate screening activities into improved clinical outcomes – change is needed at the patient-clinician level (recognizing and encouraging eligible individuals to be screened), health care system level (reducing screening barriers and ensuring access to robust lifestyle programs), and societal level (applying effective public health interventions to reduce obesity and increase exercise),” they write.
A top priority has to be a focus on individuals of diverse backgrounds and issues such as access to healthy programs in minority communities, Dr. Grant noted.
“Newly diagnosed adults are more likely to be African-American and Latinx,” he said.
“We really need to invest in healthier communities for low-income, non-White communities to reverse the persistent health care disparities in these communities.”
While the challenges may appear daunting, history shows they are not necessarily insurmountable – as evidenced in the campaign to discourage tobacco smoking.
“National smoking cessation efforts are one example of a mostly successful public health campaign that has made a difference in health behaviors,” Grant noted.
The recommendation is also posted on the USPSTF web site .
Dr. Grant reports receiving grants from the National Institutes of Health and the Patient-Centered Outcomes Research Institute.
The United States Preventive Services Task Force has updated its recommendation on the age of screening for prediabetes and type 2 diabetes in the primary care setting – lowering the age from 40 to 35 years for asymptomatic patients who are overweight or obese and encouraging greater interventions when patients do show a risk.
“The USPSTF concludes with moderate certainty that screening for prediabetes and type 2 diabetes and offering or referring patients with prediabetes to effective preventive interventions has a moderate net benefit,” the task force concludes in its recommendation, published Aug. 24 in JAMA.
“Clinicians should offer or refer patients with prediabetes to effective preventive interventions,” they write.
Experts commenting on the issue strongly emphasize that it’s not just the screening, but the subsequent intervention that is needed to make a difference.
“If young adults newly identified with abnormal glucose metabolism do not receive the needed intensive behavioral change support, screening may provide no benefit,” write Richard W. Grant, MD, MPH, and colleagues in an editorial published with the recommendation.
“Given the role of our obesogenic and physically inactive society in the shift toward earlier onset of diabetes, efforts to increase screening and recognition of abnormal glucose metabolism must be coupled with robust public health measures to address the underlying contributors.”
BMI cutoff lower for at-risk ethnic populations
The recommendation, which updates the task force’s 2015 guideline, carries a “B” classification, meaning the USPSTF has high certainty that the net benefit is moderate. It now specifies screening from age 35to 70 for persons classified as overweight (body mass index at least 25) or obese (BMI at least 30) and recommends referral to preventive interventions when patients are found to have prediabetes.
In addition to recommendations of lifestyle changes, such as diet and physical activity, the task force also endorses the diabetes drug metformin as a beneficial intervention in the prevention or delay of diabetes, while noting fewer overall health benefits from metformin than from the lifestyle changes.
A lower BMI cutoff of at least 23 is recommended for diabetes screening of Asian Americans, and, importantly, screening for prediabetes and diabetes should be considered at an even earlier age if the patient is from a population with a disproportionately high prevalence of diabetes, including American Indian/Alaska Native, Black, Hawaiian/Pacific Islander, Hispanic/Latino, the task force recommends.
Screening tests should include fasting plasma glucose, hemoglobin A1c, or an oral glucose tolerance test. Although screening every 3 years “may be a reasonable approach for adults with normal blood glucose levels,” the task force adds that “the optimal screening interval for adults with an initial normal glucose test result is uncertain.”
Data review: Few with prediabetes know they have it
The need for the update was prompted by troubling data showing increasing diabetes rates despite early signs that can and should be identified and acted upon in the primary care setting to prevent disease progression.
Data from the Centers for Disease Control and Prevention, for instance, show that while 13% of all U.S. adults 18 years or older have diabetes and 35% meet criteria for prediabetes, as many as 21% of those with diabetes were not aware of or did not report having the disease. Furthermore, only a small fraction – 15% of those with prediabetes – said they had been told by a health professional that they had this condition, the task force notes.
The task force’s final recommendation was based on a systematic review of evidence regarding the screening of asymptomatic, nonpregnant adults and the harms and benefits of interventions, such as physical activity, behavioral counseling, or pharmacotherapy.
Among key evidence supporting the lower age was a 2014 study showing that the number of people necessary to obtain one positive test for diabetes with screening sharply drops from 80 among those aged 30-34 years to just 31 among those aged 36-39.
Opportunistic universal screening of eligible people aged 35 and older would yield a ratio of 1 out of just 15 to spot a positive test, the authors of that study reported.
In addition, a large cohort study in more than 77,000 people with prediabetes strongly links the risk of developing diabetes with increases in A1c level and with increasing BMI.
ADA recommendations differ
The new recommendations differ from American Diabetes Association guidelines, which call for diabetes screening at all ages for people who are overweight or obese and who have one or more risk factors, such as physical inactivity or a first-degree relative with diabetes. If results are normal, repeat screening at least every 3 years is recommended.
The ADA further recommends universal screening for all adults 45 years and older, regardless of their risk factors.
For the screening of adults over 45, the ADA recommends using a fasting plasma glucose level, 2-hour plasma glucose level during a 75-g oral glucose tolerance test, or A1c level, regardless of risk factors.
The American Association of Clinical Endocrinology also recommends universal screening for prediabetes and diabetes for all adults 45 years or older, regardless of risk factors, and also advises screening those who have risk factors for diabetes regardless of age.
Screening of little benefit without behavior change support
In an interview, Dr. Grant added that broad efforts are essential as those at the practice level have clearly not succeeded.
“The medical model of individual counseling and referral has not really been effective, and so we really need to think in terms of large-scale public health action,” said Dr. Grant, of the division of research, Kaiser Permanente Northern California, Oakland.
His editorial details the sweeping, multifactorial efforts that are needed.
“To turn this recommendation into action – that is, to translate screening activities into improved clinical outcomes – change is needed at the patient-clinician level (recognizing and encouraging eligible individuals to be screened), health care system level (reducing screening barriers and ensuring access to robust lifestyle programs), and societal level (applying effective public health interventions to reduce obesity and increase exercise),” they write.
A top priority has to be a focus on individuals of diverse backgrounds and issues such as access to healthy programs in minority communities, Dr. Grant noted.
“Newly diagnosed adults are more likely to be African-American and Latinx,” he said.
“We really need to invest in healthier communities for low-income, non-White communities to reverse the persistent health care disparities in these communities.”
While the challenges may appear daunting, history shows they are not necessarily insurmountable – as evidenced in the campaign to discourage tobacco smoking.
“National smoking cessation efforts are one example of a mostly successful public health campaign that has made a difference in health behaviors,” Grant noted.
The recommendation is also posted on the USPSTF web site .
Dr. Grant reports receiving grants from the National Institutes of Health and the Patient-Centered Outcomes Research Institute.
FROM JAMA
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.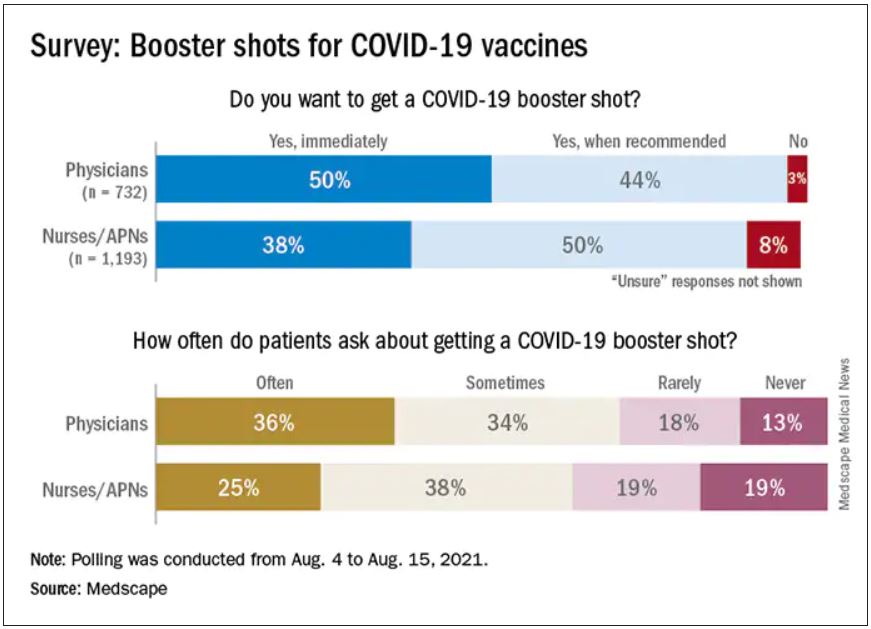
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
Plastic barriers may not stop COVID-19 spread, experts say
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
Plastic barriers that separate people in stores, restaurants, and classrooms may not be as effective at stopping the spread of COVID-19 as originally thought, according to The New York Times.
Scientists who study air flow, ventilation, and aerosol droplets say the barriers may not help, and in fact, could make the situation worse by blocking normal air flow, the newspaper reported.
Typically, as people interact and breathe in a room, currents and ventilation systems recirculate the air and disperse the exhaled particles. With plastic barriers, however, particles could get trapped in “dead zones” and build up.
“If you have a forest of barriers in a classroom, it’s going to interfere with proper ventilation of that room,” Linsey Marr, professor of civil and environmental engineering at Virginia Tech, told the newspaper.
“Everybody’s aerosols are going to be trapped and stuck there and building up, and they will end up spreading beyond your own desk,” she said.
Several variables factor into the efficacy of plastic barriers, The New York Times reported. Shields may stop big respiratory droplets from coughs and sneezes, for instance, but they may not do much to prevent small aerosol particles from viruses such as COVID-19 from spreading.
“We have shown this effect of blocking larger particles, but also that the smaller aerosols travel over the screen and become mixed in the room air within about 5 minutes,” Catherine Noakes, professor of environment engineering at the University of Leeds, told the newspaper.
“This means if people are interacting for more than a few minutes, they would likely be exposed to the virus regardless of the screen,” she said.
The effectiveness of plastic barriers likely also depends on the location and setup, the newspaper reported. A bus driver with a large barrier, for instance, may be able to avoid inhaling the particles that passengers are exhaling. A bank cashier or store clerk behind a large barrier may also be partly protected.
Even still, scientists say more research is needed. For instance, taller barriers are more likely to be effective. However, a large number of barriers in one room could likely block air flow.
Researchers have recommended that schools and offices focus on ventilation, masks, and vaccines to slow the spread of the coronavirus.
“Air flow in rooms is pretty complicated,” Richard Corsi, dean of engineering at the University of California at Davis, told the newspaper.
“Every room is different in terms of the arrangement of furniture, the height of the walls and ceilings, the vents, where the bookshelves are,” he said. “All of these things have a huge impact on the actual flow and air distribution in a room.”
A version of this article first appeared on WebMD.com.
SGLT2 inhibitor use rising in patients with DKD
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
FROM DIABETES CARE