User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
SSaSS: Salt substitute shows clear reduction in stroke, CV events, death
Switching from regular salt to a low-sodium salt substitute has major public health benefits, including a reduction in stroke, cardiovascular events, and death, a new landmark study shows.
The Salt Substitute and Stroke Study (SSaSS) was conducted in 21,000 people with a history of stroke or high blood pressure in rural China, with half of them using a lower-sodium salt substitute instead of regular salt.
Results showed that after 5 years, those using the salt substitute had a 14% reduction in stroke, a 13% reduction in major cardiovascular events, and a 12% reduction in death. These benefits were achieved without any apparent adverse effects.
The trial was presented by Bruce Neal, MB, George Institute for Global Health, Sydney, Australia, on Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021. They were simultaneously published online in the New England Journal of Medicine.
“This is one of the largest dietary intervention trials ever conducted and has shown very clear evidence of protection against stroke, cardiovascular events, and premature death, with no adverse effects with a very simple and low-cost intervention,” Dr. Neal concluded. “This is a very easy thing to work into the diet. You just replace regular salt with a substitute that looks and tastes almost identical,” he added.
Addressing the issue of whether these results are generalizable to other populations, Dr. Neal said, “We believe the results are relevant to everyone who eats salt.
“The way the body manages sodium and potassium and their association with blood pressure is highly consistent across different populations,” he said. “Almost everyone, with the exception of a few people with serious kidney disease, should be avoiding salt or switching to a salt substitute and expect to see some benefit of this.”
Commentators at the ESC presentation lauded the study as “magnificent,” with “extraordinary” results and “very powerful implications.”
Designated discussant, hypertension expert Bryan Williams, MD, University College London, said the SSaSS was “probably the most important study with regards to public health that we will see.” He described the reductions in stroke, cardiovascular events, and death as “extraordinary for such a simple intervention.”
Dr. Williams added: “Those who have doubted the benefits of salt restriction must now admit they were wrong. The debate stops here. The data are in. Global health interventions to implement these findings must now begin.”
He also highlighted the large number of events in the trial. “This was a large, pragmatic, long-duration study in a high-risk population, and with 5,000 cardiovascular events it gives enormous power to show benefits.”
Chair of the ESC session, Barbara Casadei, MD, DPhil, John Radcliffe Hospital, Oxford (England), said the SSaSS “will change the way we think about salt and be remembered for years to come.”
Noting that the benefits were seen in all subgroups across the study, Bertram Pitt, MD, University of Michigan, Ann Arbor, was particularly excited about the stroke reduction seen in patients with diabetes, noting that several recent trials of new diabetes drugs have not managed to show a reduction in stroke.
“For patients with diabetes, this is a really important intervention,” he stated.
However, an editorial accompanying the NEJM publication gave a somewhat less enthusiastic response to the study than the ESC commentators.
Julie R. Ingelfinger, MD, deputy editor of the journal, points out that serial monitoring of potassium levels was not performed in the trial, so it is possible that hyperkalemic episodes were not detected, and persons with a history of medical conditions that may be associated with hyperkalemia were not studied.
She also noted that because the salt substitute was distributed to families, it would have been instructive to have data on the household members without risk factors, but no such data were obtained.
“Overall, the SSaSS provides some intriguing hints, but wider effectiveness is hard to predict, given limited generalizability,” she concluded.
Cluster-randomized trial
The SSaSS was an open-label, cluster-randomized trial involving 20,995 people from 600 villages in rural China who had a history of stroke or were 60 years of age or older and had uncontrolled hypertension. Patients with a history of severe kidney disease and those taking potassium supplements or potassium-sparing diuretics were excluded.
They were randomly assigned in a 1:1 ratio to the intervention group, in which the participants used a salt substitute (roughly 75% sodium chloride and 25% potassium chloride), or to the control group, in which the participants continued to use regular salt (100% sodium chloride).
Results showed that after a mean follow-up of 4.74 years, systolic blood pressure was reduced by 3.3 mm Hg in the salt substitute group.
The rate of stroke, the primary endpoint, was 29.14 events per 1,000 person-years in the salt substitute group vs. 33.65 events per 1,000 person-years with regular salt (rate ratio, 0.86; 95% confidence interval, 0.77-0.96; P = .006).
The rates of major cardiovascular events were 49.09 events per 1,000 person-years in the salt substitute group vs. 56.29 events per 1,000 person-years in those using regular salt (rate ratio, 0.87; 95% CI, 0.80-0.94; P < .001).
And the rate of death was 39.28 events per 1,000 person-years with the salt substitute vs. 44.61 events per 1,000 person-years with regular salt (rate ratio, 0.88; 95% CI, 0.82-0.95; P < .001).
The rate of serious adverse events attributed to hyperkalemia was not significantly higher with the salt substitute than with regular salt (3.35 events vs. 3.30 events per 1,000 person-years; rate ratio, 1.04; 95% CI, 0.80-1.37; P = .76).
Dr. Neal reported that 7%-8% of the control group started using salt substitute over the study period, so these results have likely underestimated the true effect of switching to a salt substitute product.
Noting that about 10 million cardiovascular events occur each year in China, he said the study results suggested that using salt substitute instead of regular salt could prevent about 10% of these events.
Food manufacturers must make changes
Dr. Neal acknowledged that a limitation of the study was the fact it was conducted in a single country, which would raise issues of generalizability. But he said he believes the results are generalizable to other populations.
Those who would get the most benefit from switching to a salt substitute are those who consume large amounts of discretionary salt – salt added at home at the time of cooking for preservation of food or seasoning. “This is salt that is easy to replace with salt substitute,” Dr. Neal noted.
“There are more than 5 billion people in the world that consume more than 50% of their salt intake as discretionary salt – mainly in the developing world. These people would expect to get significant health benefits from a switch to salt substitute.”
He pointed out that salt substitute is low cost and is easy to manufacture. “Salt substitutes cost around 50% more than regular salt, but this translates into just a dollar or two per person per year to make the switch.”
Dr. Neal said the results also apply to higher-income countries but must be implemented by governments and food manufactures, as most salt in these countries comes from processed foods.
“This study provides strong evidence to take to the food industry,” he concluded. “We would like to see food manufacturers switch to using salt substitute and for salt substitute products to be widely available on supermarket shelves. We also urge governments to take action to promote use of salt substitutes over regular salt. This could take the form of taxing regular salt or subsidies for use of salt substitutes.”
The SSaSS was supported by grants from the National Health and Medical Research Council of Australia. Dr. Neal reports no disclosures. Dr. Ingelfinger is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
Switching from regular salt to a low-sodium salt substitute has major public health benefits, including a reduction in stroke, cardiovascular events, and death, a new landmark study shows.
The Salt Substitute and Stroke Study (SSaSS) was conducted in 21,000 people with a history of stroke or high blood pressure in rural China, with half of them using a lower-sodium salt substitute instead of regular salt.
Results showed that after 5 years, those using the salt substitute had a 14% reduction in stroke, a 13% reduction in major cardiovascular events, and a 12% reduction in death. These benefits were achieved without any apparent adverse effects.
The trial was presented by Bruce Neal, MB, George Institute for Global Health, Sydney, Australia, on Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021. They were simultaneously published online in the New England Journal of Medicine.
“This is one of the largest dietary intervention trials ever conducted and has shown very clear evidence of protection against stroke, cardiovascular events, and premature death, with no adverse effects with a very simple and low-cost intervention,” Dr. Neal concluded. “This is a very easy thing to work into the diet. You just replace regular salt with a substitute that looks and tastes almost identical,” he added.
Addressing the issue of whether these results are generalizable to other populations, Dr. Neal said, “We believe the results are relevant to everyone who eats salt.
“The way the body manages sodium and potassium and their association with blood pressure is highly consistent across different populations,” he said. “Almost everyone, with the exception of a few people with serious kidney disease, should be avoiding salt or switching to a salt substitute and expect to see some benefit of this.”
Commentators at the ESC presentation lauded the study as “magnificent,” with “extraordinary” results and “very powerful implications.”
Designated discussant, hypertension expert Bryan Williams, MD, University College London, said the SSaSS was “probably the most important study with regards to public health that we will see.” He described the reductions in stroke, cardiovascular events, and death as “extraordinary for such a simple intervention.”
Dr. Williams added: “Those who have doubted the benefits of salt restriction must now admit they were wrong. The debate stops here. The data are in. Global health interventions to implement these findings must now begin.”
He also highlighted the large number of events in the trial. “This was a large, pragmatic, long-duration study in a high-risk population, and with 5,000 cardiovascular events it gives enormous power to show benefits.”
Chair of the ESC session, Barbara Casadei, MD, DPhil, John Radcliffe Hospital, Oxford (England), said the SSaSS “will change the way we think about salt and be remembered for years to come.”
Noting that the benefits were seen in all subgroups across the study, Bertram Pitt, MD, University of Michigan, Ann Arbor, was particularly excited about the stroke reduction seen in patients with diabetes, noting that several recent trials of new diabetes drugs have not managed to show a reduction in stroke.
“For patients with diabetes, this is a really important intervention,” he stated.
However, an editorial accompanying the NEJM publication gave a somewhat less enthusiastic response to the study than the ESC commentators.
Julie R. Ingelfinger, MD, deputy editor of the journal, points out that serial monitoring of potassium levels was not performed in the trial, so it is possible that hyperkalemic episodes were not detected, and persons with a history of medical conditions that may be associated with hyperkalemia were not studied.
She also noted that because the salt substitute was distributed to families, it would have been instructive to have data on the household members without risk factors, but no such data were obtained.
“Overall, the SSaSS provides some intriguing hints, but wider effectiveness is hard to predict, given limited generalizability,” she concluded.
Cluster-randomized trial
The SSaSS was an open-label, cluster-randomized trial involving 20,995 people from 600 villages in rural China who had a history of stroke or were 60 years of age or older and had uncontrolled hypertension. Patients with a history of severe kidney disease and those taking potassium supplements or potassium-sparing diuretics were excluded.
They were randomly assigned in a 1:1 ratio to the intervention group, in which the participants used a salt substitute (roughly 75% sodium chloride and 25% potassium chloride), or to the control group, in which the participants continued to use regular salt (100% sodium chloride).
Results showed that after a mean follow-up of 4.74 years, systolic blood pressure was reduced by 3.3 mm Hg in the salt substitute group.
The rate of stroke, the primary endpoint, was 29.14 events per 1,000 person-years in the salt substitute group vs. 33.65 events per 1,000 person-years with regular salt (rate ratio, 0.86; 95% confidence interval, 0.77-0.96; P = .006).
The rates of major cardiovascular events were 49.09 events per 1,000 person-years in the salt substitute group vs. 56.29 events per 1,000 person-years in those using regular salt (rate ratio, 0.87; 95% CI, 0.80-0.94; P < .001).
And the rate of death was 39.28 events per 1,000 person-years with the salt substitute vs. 44.61 events per 1,000 person-years with regular salt (rate ratio, 0.88; 95% CI, 0.82-0.95; P < .001).
The rate of serious adverse events attributed to hyperkalemia was not significantly higher with the salt substitute than with regular salt (3.35 events vs. 3.30 events per 1,000 person-years; rate ratio, 1.04; 95% CI, 0.80-1.37; P = .76).
Dr. Neal reported that 7%-8% of the control group started using salt substitute over the study period, so these results have likely underestimated the true effect of switching to a salt substitute product.
Noting that about 10 million cardiovascular events occur each year in China, he said the study results suggested that using salt substitute instead of regular salt could prevent about 10% of these events.
Food manufacturers must make changes
Dr. Neal acknowledged that a limitation of the study was the fact it was conducted in a single country, which would raise issues of generalizability. But he said he believes the results are generalizable to other populations.
Those who would get the most benefit from switching to a salt substitute are those who consume large amounts of discretionary salt – salt added at home at the time of cooking for preservation of food or seasoning. “This is salt that is easy to replace with salt substitute,” Dr. Neal noted.
“There are more than 5 billion people in the world that consume more than 50% of their salt intake as discretionary salt – mainly in the developing world. These people would expect to get significant health benefits from a switch to salt substitute.”
He pointed out that salt substitute is low cost and is easy to manufacture. “Salt substitutes cost around 50% more than regular salt, but this translates into just a dollar or two per person per year to make the switch.”
Dr. Neal said the results also apply to higher-income countries but must be implemented by governments and food manufactures, as most salt in these countries comes from processed foods.
“This study provides strong evidence to take to the food industry,” he concluded. “We would like to see food manufacturers switch to using salt substitute and for salt substitute products to be widely available on supermarket shelves. We also urge governments to take action to promote use of salt substitutes over regular salt. This could take the form of taxing regular salt or subsidies for use of salt substitutes.”
The SSaSS was supported by grants from the National Health and Medical Research Council of Australia. Dr. Neal reports no disclosures. Dr. Ingelfinger is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
Switching from regular salt to a low-sodium salt substitute has major public health benefits, including a reduction in stroke, cardiovascular events, and death, a new landmark study shows.
The Salt Substitute and Stroke Study (SSaSS) was conducted in 21,000 people with a history of stroke or high blood pressure in rural China, with half of them using a lower-sodium salt substitute instead of regular salt.
Results showed that after 5 years, those using the salt substitute had a 14% reduction in stroke, a 13% reduction in major cardiovascular events, and a 12% reduction in death. These benefits were achieved without any apparent adverse effects.
The trial was presented by Bruce Neal, MB, George Institute for Global Health, Sydney, Australia, on Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021. They were simultaneously published online in the New England Journal of Medicine.
“This is one of the largest dietary intervention trials ever conducted and has shown very clear evidence of protection against stroke, cardiovascular events, and premature death, with no adverse effects with a very simple and low-cost intervention,” Dr. Neal concluded. “This is a very easy thing to work into the diet. You just replace regular salt with a substitute that looks and tastes almost identical,” he added.
Addressing the issue of whether these results are generalizable to other populations, Dr. Neal said, “We believe the results are relevant to everyone who eats salt.
“The way the body manages sodium and potassium and their association with blood pressure is highly consistent across different populations,” he said. “Almost everyone, with the exception of a few people with serious kidney disease, should be avoiding salt or switching to a salt substitute and expect to see some benefit of this.”
Commentators at the ESC presentation lauded the study as “magnificent,” with “extraordinary” results and “very powerful implications.”
Designated discussant, hypertension expert Bryan Williams, MD, University College London, said the SSaSS was “probably the most important study with regards to public health that we will see.” He described the reductions in stroke, cardiovascular events, and death as “extraordinary for such a simple intervention.”
Dr. Williams added: “Those who have doubted the benefits of salt restriction must now admit they were wrong. The debate stops here. The data are in. Global health interventions to implement these findings must now begin.”
He also highlighted the large number of events in the trial. “This was a large, pragmatic, long-duration study in a high-risk population, and with 5,000 cardiovascular events it gives enormous power to show benefits.”
Chair of the ESC session, Barbara Casadei, MD, DPhil, John Radcliffe Hospital, Oxford (England), said the SSaSS “will change the way we think about salt and be remembered for years to come.”
Noting that the benefits were seen in all subgroups across the study, Bertram Pitt, MD, University of Michigan, Ann Arbor, was particularly excited about the stroke reduction seen in patients with diabetes, noting that several recent trials of new diabetes drugs have not managed to show a reduction in stroke.
“For patients with diabetes, this is a really important intervention,” he stated.
However, an editorial accompanying the NEJM publication gave a somewhat less enthusiastic response to the study than the ESC commentators.
Julie R. Ingelfinger, MD, deputy editor of the journal, points out that serial monitoring of potassium levels was not performed in the trial, so it is possible that hyperkalemic episodes were not detected, and persons with a history of medical conditions that may be associated with hyperkalemia were not studied.
She also noted that because the salt substitute was distributed to families, it would have been instructive to have data on the household members without risk factors, but no such data were obtained.
“Overall, the SSaSS provides some intriguing hints, but wider effectiveness is hard to predict, given limited generalizability,” she concluded.
Cluster-randomized trial
The SSaSS was an open-label, cluster-randomized trial involving 20,995 people from 600 villages in rural China who had a history of stroke or were 60 years of age or older and had uncontrolled hypertension. Patients with a history of severe kidney disease and those taking potassium supplements or potassium-sparing diuretics were excluded.
They were randomly assigned in a 1:1 ratio to the intervention group, in which the participants used a salt substitute (roughly 75% sodium chloride and 25% potassium chloride), or to the control group, in which the participants continued to use regular salt (100% sodium chloride).
Results showed that after a mean follow-up of 4.74 years, systolic blood pressure was reduced by 3.3 mm Hg in the salt substitute group.
The rate of stroke, the primary endpoint, was 29.14 events per 1,000 person-years in the salt substitute group vs. 33.65 events per 1,000 person-years with regular salt (rate ratio, 0.86; 95% confidence interval, 0.77-0.96; P = .006).
The rates of major cardiovascular events were 49.09 events per 1,000 person-years in the salt substitute group vs. 56.29 events per 1,000 person-years in those using regular salt (rate ratio, 0.87; 95% CI, 0.80-0.94; P < .001).
And the rate of death was 39.28 events per 1,000 person-years with the salt substitute vs. 44.61 events per 1,000 person-years with regular salt (rate ratio, 0.88; 95% CI, 0.82-0.95; P < .001).
The rate of serious adverse events attributed to hyperkalemia was not significantly higher with the salt substitute than with regular salt (3.35 events vs. 3.30 events per 1,000 person-years; rate ratio, 1.04; 95% CI, 0.80-1.37; P = .76).
Dr. Neal reported that 7%-8% of the control group started using salt substitute over the study period, so these results have likely underestimated the true effect of switching to a salt substitute product.
Noting that about 10 million cardiovascular events occur each year in China, he said the study results suggested that using salt substitute instead of regular salt could prevent about 10% of these events.
Food manufacturers must make changes
Dr. Neal acknowledged that a limitation of the study was the fact it was conducted in a single country, which would raise issues of generalizability. But he said he believes the results are generalizable to other populations.
Those who would get the most benefit from switching to a salt substitute are those who consume large amounts of discretionary salt – salt added at home at the time of cooking for preservation of food or seasoning. “This is salt that is easy to replace with salt substitute,” Dr. Neal noted.
“There are more than 5 billion people in the world that consume more than 50% of their salt intake as discretionary salt – mainly in the developing world. These people would expect to get significant health benefits from a switch to salt substitute.”
He pointed out that salt substitute is low cost and is easy to manufacture. “Salt substitutes cost around 50% more than regular salt, but this translates into just a dollar or two per person per year to make the switch.”
Dr. Neal said the results also apply to higher-income countries but must be implemented by governments and food manufactures, as most salt in these countries comes from processed foods.
“This study provides strong evidence to take to the food industry,” he concluded. “We would like to see food manufacturers switch to using salt substitute and for salt substitute products to be widely available on supermarket shelves. We also urge governments to take action to promote use of salt substitutes over regular salt. This could take the form of taxing regular salt or subsidies for use of salt substitutes.”
The SSaSS was supported by grants from the National Health and Medical Research Council of Australia. Dr. Neal reports no disclosures. Dr. Ingelfinger is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
‘High normal’ sodium, poor hydration linked to heart failure
– a heart failure (HF) precursor – and for HF itself, in older age, a new study suggests.
Compared with middle-aged adults in the Atherosclerosis Risk in Communities (ARIC) study with normal serum sodium, those with levels of 142-146 mmol/L were more likely to have left ventricular hypertrophy or HF when they were in their 70s and 80s, independent of other risk factors.
Natalia Dmitrieva, PhD, a research scientist at the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md., presented the study findings in an e-poster on Aug. 27 at the European Society of Cardiology (ESC) Congress 2021.
“Our study suggests that maintaining good hydration can prevent or at least slow down the changes within the heart that lead to heart failure,” she said in a statement from the ESC.
It “suggests that all adults should aim for eight to ten glasses of liquid [daily] and keep salt intake low,” Dr. Dmitrieva said in an interview.
However, people should not rely completely on thirst, she cautioned, especially in middle age, when thirst sensation starts to deteriorate. And too much fluid intake can be harmful and even dangerous.
Normal serum sodium is usually defined as 135-146 mmol/L, Dr. Dmitrieva explained, and this study involved only patients in ARIC with sodium levels in this range, to try to exclude patients with genetic or water-salt balance diseases.
The findings suggest that a serum sodium level of 142-146 mmol/L, which would not be flagged as abnormal by a test lab, “can be used by clinicians as a warning sign” for a patient’s increased risk for HF, she noted.
Clinicians should explain this risk to patients and advise them to drink at least 2 L per day. However, people should not try to reduce their sodium levels by drinking more than 2 to 3 L per day, she cautioned, which can be harmful and even deadly, and they should consult their doctors.
Watch hydration
“An important finding of this study is that sodium values considered ‘normal’ may also be deleterious,” Jacob Joseph, MD, director, heart failure program, VA Boston Healthcare System, who was not involved with this study, said in an interview.
“These results are similar to studies we have conducted in heart failure with preserved ejection fraction,” noted Dr. Joseph, associate professor of medicine at Harvard Medical School, Boston.
Their studies showed a U-shaped relationship between serum sodium values and adverse outcomes, “indicating an ‘optimal’ range of serum sodium value that was narrower than the accepted normal laboratory value range,” he noted.
The study by Dmitrieva et al. was observational and the findings would need to be verified in a randomized controlled trial, Dr. Joseph pointed out; however, the research “supports the idea that even a high normal sodium level may indicate risk of future heart failure.
“Hence, patients should pay attention to hydration,” he continued, and “clinicians should not assume that a sodium level of 142 mmol/L is appropriate and should ensure that patients are paying attention to hydration.
“In today’s busy and stress-filled lifestyle, it is easy to forget about adequate fluid intake,” Dr. Joseph added.
More than 15,000 adults followed for 25 Years
To investigate the relationship between serum sodium, hydration, and future heart failure, Dr. Dmitrieva and colleagues analyzed data from 15,792 adults in ARIC who were 44-66 years of age at study entry, with serum sodium levels from 135 to 146 mmol/L.
The participants were evaluated over five visits until they reached 70-90 years.
They were divided into four groups based on their average serum sodium concentrations at study visits one and two (conducted in the first 3 years): 135 -139.5 mmol/L, 140-141.5 mmol/L, 142-143.5 mmol/L, and 144-146 mmol/L.
The researchers determined the percentage of people in each group who developed HF and left ventricular hypertrophy at visit five (25 years after study enrollment).
Patients with higher serum sodium levels had a significantly higher risk for HF and left ventricular hypertrophy, after adjustment for other risk factors, including age, blood pressure, kidney function, blood cholesterol, blood glucose, body mass index, sex, and smoking status.
Every 1 mmol/L increase in serum sodium concentration in midlife was associated with 1.20 and 1.11 increased odds of developing left ventricular hypertrophy and HF, respectively, 25 years later.
“More studies are needed to find out what proportion of people with serum sodium 142 mmol/L and higher have this [serum sodium] level because they do not drink enough and will be able to reduce it by making sure they consistently drink 2 to 2.5 L per day,” said Dr. Dmitrieva.
“It is likely that for some people, other factors that are related to genetics or diseases affecting water-salt balance could be causing their increased serum sodium levels,” she speculated.
The study was funded by the Intramural Program of the National Heart, Lung, and Blood Institute. The authors and Dr. Joseph have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
– a heart failure (HF) precursor – and for HF itself, in older age, a new study suggests.
Compared with middle-aged adults in the Atherosclerosis Risk in Communities (ARIC) study with normal serum sodium, those with levels of 142-146 mmol/L were more likely to have left ventricular hypertrophy or HF when they were in their 70s and 80s, independent of other risk factors.
Natalia Dmitrieva, PhD, a research scientist at the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md., presented the study findings in an e-poster on Aug. 27 at the European Society of Cardiology (ESC) Congress 2021.
“Our study suggests that maintaining good hydration can prevent or at least slow down the changes within the heart that lead to heart failure,” she said in a statement from the ESC.
It “suggests that all adults should aim for eight to ten glasses of liquid [daily] and keep salt intake low,” Dr. Dmitrieva said in an interview.
However, people should not rely completely on thirst, she cautioned, especially in middle age, when thirst sensation starts to deteriorate. And too much fluid intake can be harmful and even dangerous.
Normal serum sodium is usually defined as 135-146 mmol/L, Dr. Dmitrieva explained, and this study involved only patients in ARIC with sodium levels in this range, to try to exclude patients with genetic or water-salt balance diseases.
The findings suggest that a serum sodium level of 142-146 mmol/L, which would not be flagged as abnormal by a test lab, “can be used by clinicians as a warning sign” for a patient’s increased risk for HF, she noted.
Clinicians should explain this risk to patients and advise them to drink at least 2 L per day. However, people should not try to reduce their sodium levels by drinking more than 2 to 3 L per day, she cautioned, which can be harmful and even deadly, and they should consult their doctors.
Watch hydration
“An important finding of this study is that sodium values considered ‘normal’ may also be deleterious,” Jacob Joseph, MD, director, heart failure program, VA Boston Healthcare System, who was not involved with this study, said in an interview.
“These results are similar to studies we have conducted in heart failure with preserved ejection fraction,” noted Dr. Joseph, associate professor of medicine at Harvard Medical School, Boston.
Their studies showed a U-shaped relationship between serum sodium values and adverse outcomes, “indicating an ‘optimal’ range of serum sodium value that was narrower than the accepted normal laboratory value range,” he noted.
The study by Dmitrieva et al. was observational and the findings would need to be verified in a randomized controlled trial, Dr. Joseph pointed out; however, the research “supports the idea that even a high normal sodium level may indicate risk of future heart failure.
“Hence, patients should pay attention to hydration,” he continued, and “clinicians should not assume that a sodium level of 142 mmol/L is appropriate and should ensure that patients are paying attention to hydration.
“In today’s busy and stress-filled lifestyle, it is easy to forget about adequate fluid intake,” Dr. Joseph added.
More than 15,000 adults followed for 25 Years
To investigate the relationship between serum sodium, hydration, and future heart failure, Dr. Dmitrieva and colleagues analyzed data from 15,792 adults in ARIC who were 44-66 years of age at study entry, with serum sodium levels from 135 to 146 mmol/L.
The participants were evaluated over five visits until they reached 70-90 years.
They were divided into four groups based on their average serum sodium concentrations at study visits one and two (conducted in the first 3 years): 135 -139.5 mmol/L, 140-141.5 mmol/L, 142-143.5 mmol/L, and 144-146 mmol/L.
The researchers determined the percentage of people in each group who developed HF and left ventricular hypertrophy at visit five (25 years after study enrollment).
Patients with higher serum sodium levels had a significantly higher risk for HF and left ventricular hypertrophy, after adjustment for other risk factors, including age, blood pressure, kidney function, blood cholesterol, blood glucose, body mass index, sex, and smoking status.
Every 1 mmol/L increase in serum sodium concentration in midlife was associated with 1.20 and 1.11 increased odds of developing left ventricular hypertrophy and HF, respectively, 25 years later.
“More studies are needed to find out what proportion of people with serum sodium 142 mmol/L and higher have this [serum sodium] level because they do not drink enough and will be able to reduce it by making sure they consistently drink 2 to 2.5 L per day,” said Dr. Dmitrieva.
“It is likely that for some people, other factors that are related to genetics or diseases affecting water-salt balance could be causing their increased serum sodium levels,” she speculated.
The study was funded by the Intramural Program of the National Heart, Lung, and Blood Institute. The authors and Dr. Joseph have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
– a heart failure (HF) precursor – and for HF itself, in older age, a new study suggests.
Compared with middle-aged adults in the Atherosclerosis Risk in Communities (ARIC) study with normal serum sodium, those with levels of 142-146 mmol/L were more likely to have left ventricular hypertrophy or HF when they were in their 70s and 80s, independent of other risk factors.
Natalia Dmitrieva, PhD, a research scientist at the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md., presented the study findings in an e-poster on Aug. 27 at the European Society of Cardiology (ESC) Congress 2021.
“Our study suggests that maintaining good hydration can prevent or at least slow down the changes within the heart that lead to heart failure,” she said in a statement from the ESC.
It “suggests that all adults should aim for eight to ten glasses of liquid [daily] and keep salt intake low,” Dr. Dmitrieva said in an interview.
However, people should not rely completely on thirst, she cautioned, especially in middle age, when thirst sensation starts to deteriorate. And too much fluid intake can be harmful and even dangerous.
Normal serum sodium is usually defined as 135-146 mmol/L, Dr. Dmitrieva explained, and this study involved only patients in ARIC with sodium levels in this range, to try to exclude patients with genetic or water-salt balance diseases.
The findings suggest that a serum sodium level of 142-146 mmol/L, which would not be flagged as abnormal by a test lab, “can be used by clinicians as a warning sign” for a patient’s increased risk for HF, she noted.
Clinicians should explain this risk to patients and advise them to drink at least 2 L per day. However, people should not try to reduce their sodium levels by drinking more than 2 to 3 L per day, she cautioned, which can be harmful and even deadly, and they should consult their doctors.
Watch hydration
“An important finding of this study is that sodium values considered ‘normal’ may also be deleterious,” Jacob Joseph, MD, director, heart failure program, VA Boston Healthcare System, who was not involved with this study, said in an interview.
“These results are similar to studies we have conducted in heart failure with preserved ejection fraction,” noted Dr. Joseph, associate professor of medicine at Harvard Medical School, Boston.
Their studies showed a U-shaped relationship between serum sodium values and adverse outcomes, “indicating an ‘optimal’ range of serum sodium value that was narrower than the accepted normal laboratory value range,” he noted.
The study by Dmitrieva et al. was observational and the findings would need to be verified in a randomized controlled trial, Dr. Joseph pointed out; however, the research “supports the idea that even a high normal sodium level may indicate risk of future heart failure.
“Hence, patients should pay attention to hydration,” he continued, and “clinicians should not assume that a sodium level of 142 mmol/L is appropriate and should ensure that patients are paying attention to hydration.
“In today’s busy and stress-filled lifestyle, it is easy to forget about adequate fluid intake,” Dr. Joseph added.
More than 15,000 adults followed for 25 Years
To investigate the relationship between serum sodium, hydration, and future heart failure, Dr. Dmitrieva and colleagues analyzed data from 15,792 adults in ARIC who were 44-66 years of age at study entry, with serum sodium levels from 135 to 146 mmol/L.
The participants were evaluated over five visits until they reached 70-90 years.
They were divided into four groups based on their average serum sodium concentrations at study visits one and two (conducted in the first 3 years): 135 -139.5 mmol/L, 140-141.5 mmol/L, 142-143.5 mmol/L, and 144-146 mmol/L.
The researchers determined the percentage of people in each group who developed HF and left ventricular hypertrophy at visit five (25 years after study enrollment).
Patients with higher serum sodium levels had a significantly higher risk for HF and left ventricular hypertrophy, after adjustment for other risk factors, including age, blood pressure, kidney function, blood cholesterol, blood glucose, body mass index, sex, and smoking status.
Every 1 mmol/L increase in serum sodium concentration in midlife was associated with 1.20 and 1.11 increased odds of developing left ventricular hypertrophy and HF, respectively, 25 years later.
“More studies are needed to find out what proportion of people with serum sodium 142 mmol/L and higher have this [serum sodium] level because they do not drink enough and will be able to reduce it by making sure they consistently drink 2 to 2.5 L per day,” said Dr. Dmitrieva.
“It is likely that for some people, other factors that are related to genetics or diseases affecting water-salt balance could be causing their increased serum sodium levels,” she speculated.
The study was funded by the Intramural Program of the National Heart, Lung, and Blood Institute. The authors and Dr. Joseph have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Coffee drinking in midlife tied to heart benefits
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Among middle-aged people without heart disease, drinking up to three cups of coffee per day was linked with a lower risk for stroke or death over the next decade, along with better heart structure and function, in a large, observational study.
Specifically, light-to-moderate coffee drinking, defined as 0.5 to 3 cups per day, was associated with a 21% lower risk for stroke, a 17% lower risk for death from cardiovascular disease (CVD), and a 12% lower risk for death from all causes, as well as more favorable cardiac MRI findings, compared with nondrinkers (< 0.5 cup per day) during a median 11-year follow-up.
Heavy coffee drinkers, defined as those consuming more than three cups per day, on the other hand, likewise had more favorable cardiac MRI findings, but with similar (not lower) rates of stroke and CVD or all-cause mortality compared with nondrinkers.
Judit Simon, MD, presented these findings, from close to 500,000 participants in the UK Biobank study, at a press conference before an e-poster session at the virtual annual congress of the European Society of Cardiology.
“To our knowledge, this is the largest study to systematically assess the cardiovascular effects of regular coffee consumption in a population without diagnosed heart disease,” Dr. Simon, a PhD student at the Heart and Vascular Centre, Semmelweis University, Budapest, Hungary, said in an ESC press release.
The results “suggest that regular coffee consumption is safe, as even high daily intake was not associated with adverse cardiovascular outcomes and all-cause mortality after a follow-up of 10 to 15 years,” she said.
The imaging analysis showed that “compared with participants who did not drink coffee regularly, daily consumers had healthier sized and better functioning hearts,” Dr. Simon continued, “consistent with reversing the detrimental effects of aging on the heart.”
“The observed benefits might be partly explained by positive alterations in cardiac structure and function,” she speculated, adding that further studies are needed to explain the underlying mechanisms.
Instant coffee most popular
In this population, the coffee drinkers mostly drank instant coffee (55%), followed by filtered/ground (23%), decaffeinated (20%), or other types of coffee (2%), Dr. Simon said in an interview.
Risk for myocardial infarction (MI) or heart failure did not significantly differ for different categories of coffee intake, she added. The researchers did not study the effect of coffee consumption on atrial fibrillation (AF), she noted.
Study limitations, Dr. Simon acknowledged, include that it was observational, so it cannot show causation, and that coffee consumption was self-reported in a questionnaire.
Invited to comment, Alice H. Lichtenstein, DSc, who was not involved with the research, said, “Consistent with prior data, this new study indicates there is no adverse effect of coffee consumption on cardiovascular health and there may be a benefit.”
However, “because of the nature of the data, it would not be recommended that an individual starting drinking coffee to improve cardiovascular health,” added Dr. Lichtenstein, director and senior scientist at the Cardiovascular Nutrition Laboratory at Tufts University, Boston.
But if people already drink coffee, “it is fine to continue, assuming that the coffee drinks are not high in added sugar and cream,” she said in an interview.
Coffee intake, CVD outcomes, and heart structure
To study the relationship between coffee intake and incident MI, stroke, and death, as well as heart structure, the researchers examined data from the UK Biobank, which recruited 500,000 people aged 40-69 years in 2006-2010 from across the United Kingdom.
They identified 468,629 participants with no signs of heart disease at recruitment and an average age of 56 years, of whom 56% were women.
The participants were divided into three groups based on usual coffee intake: none (22% of participants), light-to-moderate (58%), and high (20%).
Median tea intake was three cups per day overall, four cups per day in noncoffee drinkers, three cups per day in light-to-moderate coffee drinkers, and one cup per day in high coffee drinkers.
Compared to not drinking coffee, light-to-moderate coffee consumption was associated with lower risks for all-cause death (hazard ratio [HR], 0.88; P < .001), CVD death (HR, 0.83; P = .006), and stroke (HR, 0.79; P = .037), over a median follow-up of 11 years, after adjustment for sex; weight; height; smoking status; physical activity; high blood pressure; diabetes; cholesterol level; socioeconomic status; and usual intake of alcohol, meat, tea, fruit, and vegetables.
In the 30,650 participants who had cardiac MRI data, the study found that compared with not drinking coffee, both light-to-moderate and high coffee consumption were associated with significantly increased left and right ventricular end-systolic and end-diastolic volumes, and with greater left ventricular mass (all P < .001).
These differences were small but significant, Dr. Simon stressed, because this was a cohort of healthy patients who did not have CVD (heart failure, MI, stroke, AF) at baseline, although some had hypertension or diabetes.
Press conference chairperson, Steen Dalby Kristensen, MD, professor and cardiologist, Aarhus University Hospital, Denmark, a coffee lover himself, wanted to know if an amount such as two, three, or four cups of coffee was optimal to see these heart benefits, and whether there were differences in benefits seen with drinking different types of coffee.
The analysis did not identify an optimal coffee intake, Dr. Simon said. Compared with not drinking coffee, she continued, drinking instant coffee was associated with a lower risk for all-cause mortality, but not CVD mortality or stroke.
Drinking filtered coffee was associated with lower risks for all three outcomes, but there was no significant difference in risk for MI. Drinking decaffeinated coffee was associated with a lower risk for all-cause and CVD mortality.
“Decaffeinated coffee contains a small amount of caffeine,” Dr. Simon pointed out. “Something other than caffeine might have this protective impact,” she suggested.
The researchers and Dr. Lichtenstein declared having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FIDELITY: Finerenone benefits patients with T2D across CKD spectrum
New data on using the nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone to treat patients with type 2 diabetes and chronic kidney disease did more than further confirm this new drug’s efficacy in these patients for slowing progression to end-stage renal disease and reducing hospitalizations for heart failure.
It also strengthened the case for clinicians to be much more proactive in collecting urine specimens from patients with type 2 diabetes (T2D) to find those with albuminuria whose kidney function has not yet dropped below 60 mL/min per 1.73 m2, a population that the data show finerenone can help.
The FIDELITY prespecified meta-analysis combined data from two related pivotal trials of finerenone (Kerendia) in a total of more than 13,000 patients with T2D and chronic kidney disease (CKD). Each of these two trials, FIDELIO-DKD and FIGARO-DKD, identified patients with CKD by either of two methods, or a total of four different criteria.
In sum, the two trials enrolled patients with an estimated glomerular filtration rate (eGFR) of 25-90 mL/min per 1.73 m2 and a urinary albumin-to-creatinine ratio (UACR) of 30-299, or an eGFR of 25-75 mL/min per 1.73 m2 and a UACR of 300-5,000. The result was that 40% of enrolled patients had an eGFR of at least 60, levels that are considered normal, but they also had some level of albuminuria that defined them as having CKD.
The results showed that during a median follow-up of 36 months, patients with a normal eGFR and albuminuria had their combined incidence of cardiovascular disease events (cardiovascular death, MI, stroke, or hospitalization for heart failure) reduced by roughly the same amount as seen in patients with lower levels of eGFR and renal function, a finding that reimagines how clinicians need to routinely screen patients with T2D for CKD, Gerasimos Filippatos, MD, reported at the virtual annual congress of the European Society of Cardiology.
“Measuring UACR in patients with type 2 diabetes is important to identify patients who will benefit from finerenone treatment independent of their eGFR,” said Dr. Filippatos, professor of medicine at the University of Athens and director of the heart failure unit at Attikon University Hospital in Athens.
The combined FIDELITY analysis showed a significant overall cut in the combined cardiovascular disease endpoint of 14% relative to placebo, which reflected a 1.7% absolute reduction in events between the two arms during 3 years of treatment. The primary driver of this benefit was the significant drop in hospitalizations for heart failure on finerenone compared with placebo, which fell by a relative 22% and by an absolute 1.1%, Dr. Filippatos reported.
Routinely screening for albuminuria is ‘practice changing’
“This is really practice changing information for cardiologists,” said Rajiv L. Agarwal, MD, a copresenter of the FIDELITY analysis and a lead investigator of the two finerenone trials.
When cardiologists and possibly other specialists see patients with T2D, they traditionally have focused on measuring left ventricular ejection fraction and checking for other indications of heart failure. The new results from FIDELIO-DKD and FIGARO-DKD showed that finerenone treatment can prevent heart failure onset or worsening in patients with T2D with finerenone, which clinicians can accomplish by “simply measuring UACR,” as well as eGFR, and then treating patients with abnormal levels of either, explained Dr. Agarwal, a nephrologist and professor of medicine at Indiana University in Indianapolis.
“Diabetologists know that when they see patients with diabetes they need to collect a urine sample to check for albuminuria. But when some other clinicians see a patient with type 2 diabetes and a normal eGFR, they often think that the patient is okay and don’t get a urine specimen,” noted Bertram Pitt, MD, another collaborator of the finerenone trials and a heart failure specialist affiliated with the University of Michigan in Ann Arbor.
“We need to pay more attention to UACR and albuminuria; traditionally clinicians have mostly looked at eGFR,” agreed Dipti Itchhaporia, MD, a cardiologist at the Carlton Heart and Vascular Institute of Hoag Hospital in Newport Beach, Calif. UACR “is a marker that should be shared” between endocrinologists, nephrologists, and cardiologists as they together care for patients with T2D, suggested Dr. Itchhaporia, president of the American College of Cardiology.
Two pivotal trials with consistent findings
The FIDELITY analysis combined data from the FIDELIO-DKD trial, reported in 2020, and from the FIGARO-DKD trial that was first reported during the current congress as well as in a simultaneous report published online.
Results from the two trials were very consistent, although the primary endpoint in FIDELIO-DKD was a composite measure of renal disease with the combined cardiovascular disease metric a secondary endpoint, while this got flipped in FIGARO-DKD which had the cardiovascular disease composite as its primary endpoint as the combined renal outcomes as a secondary endpoint.
In addition to showing a consistent, significant reduction in both combined cardiovascular disease events and in the specific endpoint of hospitalization for heart failure, the two trials also showed a consistent benefit for slowing renal disease progression, including significantly fewer patients developing end-stage kidney disease. In the combined FIDELITY analysis, treatment with finerenone cut the incidence of end-stage kidney disease by a significant 20% compared with placebo, and by an absolute reduction of 0.6%.
Another common finding was a relatively low incidence of hyperkalemia compared with what’s usually seen using a steroidal MRA, spironolactone or eplerenone. In the combined analysis treatment with finerenone produced a 14% incidence of any hyperkalemia compared with 7% among placebo-treated patients, and the rate of patients stopping their treatment because of hyperkalemia was 1.7% on finerenone and 0.6% on placebo.
“Finerenone is much better tolerated” than the steroidal MRAs in causing clinically significant hyperkalemia, noted Dr. Pitt. “There are a lot of misconceptions” about the potassium-raising potential of MRAs, and “people get frightened” by the potential. Spreading the message of finerenone’s relative safety “will take a lot of education,” he acknowledged. Routine monitoring of potassium levels is a key step to minimizing the risk for hyperkalemia when using finerenone, he added.
Suggested benefit from combination treatment
Another intriguing observation from FIDELITY derived from the fact that roughly 7% of enrolled patients were also on treatment with a sodium-glucose cotransporter 2 (SGLT2) inhibitor at entry, and about 7% were on treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist, and in both subgroups the incidence of the composite cardiovascular disease endpoint appeared to suggest additive effects of agents from either of these classes when combined with finerenone. Although the numbers of patients on combined treatment were too low to show a definitive result, “our expectation is that we will see an additive effect,” said Dr. Pitt. Ideally, patients with T2D and CKD “should be on both” an SGLT2 inhibitor and finerenone, he predicted.
SGLT2 inhibitors have now been embraced as a key treatment for patients with T2D or with heart failure with reduced ejection fraction, and the preliminary data suggest that combining these agents with finerenone can provide additional benefit, agreed Dr. Itchhaporia. Aside from the need for more evidence to prove this, there are also practical considerations of “How do we pay for all these fantastic therapies?” She expressed optimism that cost-benefit analyses will eventually show that the additive benefits justify the added cost.
Based largely on results from FIDELIO-DKD, finerenone received marketing approval from the Food and Drug Administration in July 2021 for the indication of treating patients with T2D and chronic kidney disease.
FIGARO-DKD, FIDELIO-DKD, and FIDELITY were sponsored by Bayer, the company that markets finerenone. Dr. Filippatos has received lecture fees from Bayer, and has had financial relationships with Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Agarwal received travel support from and has been a consultant to Bayer and to numerous other companies. Dr. Pitt has been a consultant to Bayer and to numerous other companies. Dr. Itchhaporia had no disclosures.
New data on using the nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone to treat patients with type 2 diabetes and chronic kidney disease did more than further confirm this new drug’s efficacy in these patients for slowing progression to end-stage renal disease and reducing hospitalizations for heart failure.
It also strengthened the case for clinicians to be much more proactive in collecting urine specimens from patients with type 2 diabetes (T2D) to find those with albuminuria whose kidney function has not yet dropped below 60 mL/min per 1.73 m2, a population that the data show finerenone can help.
The FIDELITY prespecified meta-analysis combined data from two related pivotal trials of finerenone (Kerendia) in a total of more than 13,000 patients with T2D and chronic kidney disease (CKD). Each of these two trials, FIDELIO-DKD and FIGARO-DKD, identified patients with CKD by either of two methods, or a total of four different criteria.
In sum, the two trials enrolled patients with an estimated glomerular filtration rate (eGFR) of 25-90 mL/min per 1.73 m2 and a urinary albumin-to-creatinine ratio (UACR) of 30-299, or an eGFR of 25-75 mL/min per 1.73 m2 and a UACR of 300-5,000. The result was that 40% of enrolled patients had an eGFR of at least 60, levels that are considered normal, but they also had some level of albuminuria that defined them as having CKD.
The results showed that during a median follow-up of 36 months, patients with a normal eGFR and albuminuria had their combined incidence of cardiovascular disease events (cardiovascular death, MI, stroke, or hospitalization for heart failure) reduced by roughly the same amount as seen in patients with lower levels of eGFR and renal function, a finding that reimagines how clinicians need to routinely screen patients with T2D for CKD, Gerasimos Filippatos, MD, reported at the virtual annual congress of the European Society of Cardiology.
“Measuring UACR in patients with type 2 diabetes is important to identify patients who will benefit from finerenone treatment independent of their eGFR,” said Dr. Filippatos, professor of medicine at the University of Athens and director of the heart failure unit at Attikon University Hospital in Athens.
The combined FIDELITY analysis showed a significant overall cut in the combined cardiovascular disease endpoint of 14% relative to placebo, which reflected a 1.7% absolute reduction in events between the two arms during 3 years of treatment. The primary driver of this benefit was the significant drop in hospitalizations for heart failure on finerenone compared with placebo, which fell by a relative 22% and by an absolute 1.1%, Dr. Filippatos reported.
Routinely screening for albuminuria is ‘practice changing’
“This is really practice changing information for cardiologists,” said Rajiv L. Agarwal, MD, a copresenter of the FIDELITY analysis and a lead investigator of the two finerenone trials.
When cardiologists and possibly other specialists see patients with T2D, they traditionally have focused on measuring left ventricular ejection fraction and checking for other indications of heart failure. The new results from FIDELIO-DKD and FIGARO-DKD showed that finerenone treatment can prevent heart failure onset or worsening in patients with T2D with finerenone, which clinicians can accomplish by “simply measuring UACR,” as well as eGFR, and then treating patients with abnormal levels of either, explained Dr. Agarwal, a nephrologist and professor of medicine at Indiana University in Indianapolis.
“Diabetologists know that when they see patients with diabetes they need to collect a urine sample to check for albuminuria. But when some other clinicians see a patient with type 2 diabetes and a normal eGFR, they often think that the patient is okay and don’t get a urine specimen,” noted Bertram Pitt, MD, another collaborator of the finerenone trials and a heart failure specialist affiliated with the University of Michigan in Ann Arbor.
“We need to pay more attention to UACR and albuminuria; traditionally clinicians have mostly looked at eGFR,” agreed Dipti Itchhaporia, MD, a cardiologist at the Carlton Heart and Vascular Institute of Hoag Hospital in Newport Beach, Calif. UACR “is a marker that should be shared” between endocrinologists, nephrologists, and cardiologists as they together care for patients with T2D, suggested Dr. Itchhaporia, president of the American College of Cardiology.
Two pivotal trials with consistent findings
The FIDELITY analysis combined data from the FIDELIO-DKD trial, reported in 2020, and from the FIGARO-DKD trial that was first reported during the current congress as well as in a simultaneous report published online.
Results from the two trials were very consistent, although the primary endpoint in FIDELIO-DKD was a composite measure of renal disease with the combined cardiovascular disease metric a secondary endpoint, while this got flipped in FIGARO-DKD which had the cardiovascular disease composite as its primary endpoint as the combined renal outcomes as a secondary endpoint.
In addition to showing a consistent, significant reduction in both combined cardiovascular disease events and in the specific endpoint of hospitalization for heart failure, the two trials also showed a consistent benefit for slowing renal disease progression, including significantly fewer patients developing end-stage kidney disease. In the combined FIDELITY analysis, treatment with finerenone cut the incidence of end-stage kidney disease by a significant 20% compared with placebo, and by an absolute reduction of 0.6%.
Another common finding was a relatively low incidence of hyperkalemia compared with what’s usually seen using a steroidal MRA, spironolactone or eplerenone. In the combined analysis treatment with finerenone produced a 14% incidence of any hyperkalemia compared with 7% among placebo-treated patients, and the rate of patients stopping their treatment because of hyperkalemia was 1.7% on finerenone and 0.6% on placebo.
“Finerenone is much better tolerated” than the steroidal MRAs in causing clinically significant hyperkalemia, noted Dr. Pitt. “There are a lot of misconceptions” about the potassium-raising potential of MRAs, and “people get frightened” by the potential. Spreading the message of finerenone’s relative safety “will take a lot of education,” he acknowledged. Routine monitoring of potassium levels is a key step to minimizing the risk for hyperkalemia when using finerenone, he added.
Suggested benefit from combination treatment
Another intriguing observation from FIDELITY derived from the fact that roughly 7% of enrolled patients were also on treatment with a sodium-glucose cotransporter 2 (SGLT2) inhibitor at entry, and about 7% were on treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist, and in both subgroups the incidence of the composite cardiovascular disease endpoint appeared to suggest additive effects of agents from either of these classes when combined with finerenone. Although the numbers of patients on combined treatment were too low to show a definitive result, “our expectation is that we will see an additive effect,” said Dr. Pitt. Ideally, patients with T2D and CKD “should be on both” an SGLT2 inhibitor and finerenone, he predicted.
SGLT2 inhibitors have now been embraced as a key treatment for patients with T2D or with heart failure with reduced ejection fraction, and the preliminary data suggest that combining these agents with finerenone can provide additional benefit, agreed Dr. Itchhaporia. Aside from the need for more evidence to prove this, there are also practical considerations of “How do we pay for all these fantastic therapies?” She expressed optimism that cost-benefit analyses will eventually show that the additive benefits justify the added cost.
Based largely on results from FIDELIO-DKD, finerenone received marketing approval from the Food and Drug Administration in July 2021 for the indication of treating patients with T2D and chronic kidney disease.
FIGARO-DKD, FIDELIO-DKD, and FIDELITY were sponsored by Bayer, the company that markets finerenone. Dr. Filippatos has received lecture fees from Bayer, and has had financial relationships with Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Agarwal received travel support from and has been a consultant to Bayer and to numerous other companies. Dr. Pitt has been a consultant to Bayer and to numerous other companies. Dr. Itchhaporia had no disclosures.
New data on using the nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone to treat patients with type 2 diabetes and chronic kidney disease did more than further confirm this new drug’s efficacy in these patients for slowing progression to end-stage renal disease and reducing hospitalizations for heart failure.
It also strengthened the case for clinicians to be much more proactive in collecting urine specimens from patients with type 2 diabetes (T2D) to find those with albuminuria whose kidney function has not yet dropped below 60 mL/min per 1.73 m2, a population that the data show finerenone can help.
The FIDELITY prespecified meta-analysis combined data from two related pivotal trials of finerenone (Kerendia) in a total of more than 13,000 patients with T2D and chronic kidney disease (CKD). Each of these two trials, FIDELIO-DKD and FIGARO-DKD, identified patients with CKD by either of two methods, or a total of four different criteria.
In sum, the two trials enrolled patients with an estimated glomerular filtration rate (eGFR) of 25-90 mL/min per 1.73 m2 and a urinary albumin-to-creatinine ratio (UACR) of 30-299, or an eGFR of 25-75 mL/min per 1.73 m2 and a UACR of 300-5,000. The result was that 40% of enrolled patients had an eGFR of at least 60, levels that are considered normal, but they also had some level of albuminuria that defined them as having CKD.
The results showed that during a median follow-up of 36 months, patients with a normal eGFR and albuminuria had their combined incidence of cardiovascular disease events (cardiovascular death, MI, stroke, or hospitalization for heart failure) reduced by roughly the same amount as seen in patients with lower levels of eGFR and renal function, a finding that reimagines how clinicians need to routinely screen patients with T2D for CKD, Gerasimos Filippatos, MD, reported at the virtual annual congress of the European Society of Cardiology.
“Measuring UACR in patients with type 2 diabetes is important to identify patients who will benefit from finerenone treatment independent of their eGFR,” said Dr. Filippatos, professor of medicine at the University of Athens and director of the heart failure unit at Attikon University Hospital in Athens.
The combined FIDELITY analysis showed a significant overall cut in the combined cardiovascular disease endpoint of 14% relative to placebo, which reflected a 1.7% absolute reduction in events between the two arms during 3 years of treatment. The primary driver of this benefit was the significant drop in hospitalizations for heart failure on finerenone compared with placebo, which fell by a relative 22% and by an absolute 1.1%, Dr. Filippatos reported.
Routinely screening for albuminuria is ‘practice changing’
“This is really practice changing information for cardiologists,” said Rajiv L. Agarwal, MD, a copresenter of the FIDELITY analysis and a lead investigator of the two finerenone trials.
When cardiologists and possibly other specialists see patients with T2D, they traditionally have focused on measuring left ventricular ejection fraction and checking for other indications of heart failure. The new results from FIDELIO-DKD and FIGARO-DKD showed that finerenone treatment can prevent heart failure onset or worsening in patients with T2D with finerenone, which clinicians can accomplish by “simply measuring UACR,” as well as eGFR, and then treating patients with abnormal levels of either, explained Dr. Agarwal, a nephrologist and professor of medicine at Indiana University in Indianapolis.
“Diabetologists know that when they see patients with diabetes they need to collect a urine sample to check for albuminuria. But when some other clinicians see a patient with type 2 diabetes and a normal eGFR, they often think that the patient is okay and don’t get a urine specimen,” noted Bertram Pitt, MD, another collaborator of the finerenone trials and a heart failure specialist affiliated with the University of Michigan in Ann Arbor.
“We need to pay more attention to UACR and albuminuria; traditionally clinicians have mostly looked at eGFR,” agreed Dipti Itchhaporia, MD, a cardiologist at the Carlton Heart and Vascular Institute of Hoag Hospital in Newport Beach, Calif. UACR “is a marker that should be shared” between endocrinologists, nephrologists, and cardiologists as they together care for patients with T2D, suggested Dr. Itchhaporia, president of the American College of Cardiology.
Two pivotal trials with consistent findings
The FIDELITY analysis combined data from the FIDELIO-DKD trial, reported in 2020, and from the FIGARO-DKD trial that was first reported during the current congress as well as in a simultaneous report published online.
Results from the two trials were very consistent, although the primary endpoint in FIDELIO-DKD was a composite measure of renal disease with the combined cardiovascular disease metric a secondary endpoint, while this got flipped in FIGARO-DKD which had the cardiovascular disease composite as its primary endpoint as the combined renal outcomes as a secondary endpoint.
In addition to showing a consistent, significant reduction in both combined cardiovascular disease events and in the specific endpoint of hospitalization for heart failure, the two trials also showed a consistent benefit for slowing renal disease progression, including significantly fewer patients developing end-stage kidney disease. In the combined FIDELITY analysis, treatment with finerenone cut the incidence of end-stage kidney disease by a significant 20% compared with placebo, and by an absolute reduction of 0.6%.
Another common finding was a relatively low incidence of hyperkalemia compared with what’s usually seen using a steroidal MRA, spironolactone or eplerenone. In the combined analysis treatment with finerenone produced a 14% incidence of any hyperkalemia compared with 7% among placebo-treated patients, and the rate of patients stopping their treatment because of hyperkalemia was 1.7% on finerenone and 0.6% on placebo.
“Finerenone is much better tolerated” than the steroidal MRAs in causing clinically significant hyperkalemia, noted Dr. Pitt. “There are a lot of misconceptions” about the potassium-raising potential of MRAs, and “people get frightened” by the potential. Spreading the message of finerenone’s relative safety “will take a lot of education,” he acknowledged. Routine monitoring of potassium levels is a key step to minimizing the risk for hyperkalemia when using finerenone, he added.
Suggested benefit from combination treatment
Another intriguing observation from FIDELITY derived from the fact that roughly 7% of enrolled patients were also on treatment with a sodium-glucose cotransporter 2 (SGLT2) inhibitor at entry, and about 7% were on treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist, and in both subgroups the incidence of the composite cardiovascular disease endpoint appeared to suggest additive effects of agents from either of these classes when combined with finerenone. Although the numbers of patients on combined treatment were too low to show a definitive result, “our expectation is that we will see an additive effect,” said Dr. Pitt. Ideally, patients with T2D and CKD “should be on both” an SGLT2 inhibitor and finerenone, he predicted.
SGLT2 inhibitors have now been embraced as a key treatment for patients with T2D or with heart failure with reduced ejection fraction, and the preliminary data suggest that combining these agents with finerenone can provide additional benefit, agreed Dr. Itchhaporia. Aside from the need for more evidence to prove this, there are also practical considerations of “How do we pay for all these fantastic therapies?” She expressed optimism that cost-benefit analyses will eventually show that the additive benefits justify the added cost.
Based largely on results from FIDELIO-DKD, finerenone received marketing approval from the Food and Drug Administration in July 2021 for the indication of treating patients with T2D and chronic kidney disease.
FIGARO-DKD, FIDELIO-DKD, and FIDELITY were sponsored by Bayer, the company that markets finerenone. Dr. Filippatos has received lecture fees from Bayer, and has had financial relationships with Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Agarwal received travel support from and has been a consultant to Bayer and to numerous other companies. Dr. Pitt has been a consultant to Bayer and to numerous other companies. Dr. Itchhaporia had no disclosures.
FROM ESC CONGRESS 2021
Dapagliflozin in HFrEF may cut arrhythmias, sudden death: DAPA-HF
Dapagliflozin might reduce the risk for ventricular arrhythmias and sudden death in patients with heart failure and reduced ejection fraction (HFrEF), a post hoc analysis of the DAPA-HF trial suggests.
The addition of dapagliflozin to standard therapy reduced the relative risk for the primary composite endpoint of any serious ventricular arrhythmia, resuscitated cardiac arrest, or sudden death by 21%, compared with placebo (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99). The absolute risk reduction was 1.5% (5.9% vs. 7.4%).
The benefit was consistent in a competing-risks analysis that included all-cause mortality (HR, 0.80; P = .043) and across the individual components of the composite outcome, James Curtain, MD, Cardiovascular Research Centre of Glasgow, said at the annual congress of the European Society of Cardiology.
As previously reported from the main trial, treatment with the sodium-glucose cotransporter 2 (SGLT2) inhibitor cut the primary endpoint of cardiovascular death or worsening heart failure by 26% among 4,744 patients with HFrEF and in New York Heart Association functional class 2-4.
Cochair of the late-breaking science session, Lars Lund, MD, Karolinska Institute, Stockholm, pointed out that dapagliflozin reduced sudden cardiac deaths and related events to an extent similar to that observed for cardiovascular deaths, total mortality, and the main trial’s primary endpoint.
“So does that mean it has any particular effect on arrhythmic events or does it mean, such as a beta-blocker, for example, [it] reduces calcium transience and improves handling of calcium, or does it have an effect simply by improving heart failure?” he asked.
Dr. Curtain replied they are still trying to understand the effects of this new class of drug but that studies have shown dapagliflozin and other SGLT2 inhibitors have favorable effects on adverse cardiac remodeling, which contributes to sudden death and ventricular arrhythmia. They’ve also been shown to reduce cardiac chamber size, left ventricular hypertrophy, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels over time, consistent with a reduction in myocardial wall stress. “So it could indeed be one of several mechanisms by which they may exert a beneficial cardiac effect.”
Speaking with this news organization, Dr. Curtain pointed out that the Kaplan-Meier curves for the composite outcome began to separate early on, but that the clearest separation was after 9 months, suggestive of a positive action on adverse cardiac remodeling over time.
“This would improve the patients’ heart failure situation, but also thick ventricles are a key risk factor for the occurrence of sudden death and ventricular arrhythmias,” he said. “The effects on adverse cardiac remodeling, given its plausibility in terms of our Kaplan-Meier curves, are one [mechanism] that I’d look to in the first instance, but I’m sure there are more than one actions at play.”
According to the new analysis, the primary outcome occurred in 315 (6.6%) patients; there were 203 adjudicated sudden deaths (64%), 104 investigator-reported ventricular arrhythmias (33%), and 8 resuscitated cardiac arrests (3%). Independent predictors of the primary outcome were higher NT-proBNP levels (odds ratio, 1.54), previous ventricular arrhythmia (OR, 1.93), previous myocardial infarction (OR, 1.42), male sex (OR, 1.53), and higher body mass index (OR, 1.03).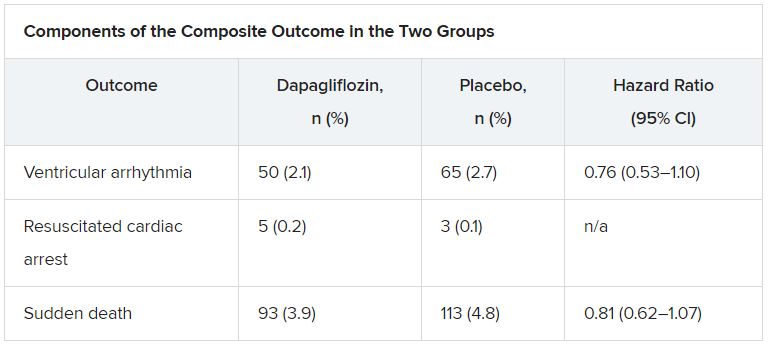
The effect of dapagliflozin on the primary outcome was consistent in several sensitivity analyses and “generally consistent” across key subgroups, Dr. Curtain said.
During a discussion of the results, session cochair Mitja Lainscak, MD, General Hospital Murska Sobota, Slovenia, called out two exceptions. “With regard to patients with implanted ICDs, the effect was neutral, and in the patients without diabetes, the benefit was less than in diabetic patients. Any explanations for that?”
Dr. Curtain responded that “it’s important to note that in the subgroup analyses the point estimates were all on the side favoring dapagliflozin and the interaction test was not significant in that subgroup. The numbers of patients who were in the defibrillator group were modest, and there was a relatively smaller number of events, so it may be harder to show benefit in that group.”
In the dapagliflozin and placebo groups, the event rates per 100 person-years were 3.9 and 5.8, respectively, in patients with diabetes, and 4.1 and 4.7, respectively, in those without diabetes (P for interaction = .273).
Event rates per 100 person-years were 5.8 and 5.9, respectively, in patients with a defibrillator at baseline, and 3.5 and 4.9, respectively, in those without a defibrillator (P for interaction = .174).
Asked to comment on the study, which was simultaneously published in the European Heart Journal, Milton Packer, MD, Baylor University Medical Center, Dallas, said he had “very little confidence” in the findings.
“This was entirely post hoc and the investigators combined events – with markedly different levels of clinical importance – in order to achieve a P value less than 0.05,” he told this news organization. “If one takes asymptomatic ventricular arrhythmias out of the analysis, the effect is no longer statistically significant. Furthermore, half of sudden deaths in patients with heart failure are not related to a ventricular arrhythmia.”
The authors note in their report that the analysis was not prespecified and the findings should be regarded as “hypothesis generating and require confirmation,” but also point out that a recent meta-analysis showed that SGLT2 inhibitor use was associated with a lower risk for ventricular tachycardia. Other limitations to the post hoc analysis are that adverse-event reporting likely underestimated the true prevalence of ventricular arrhythmias, and that these events were not adjudicated.
DAPA-HF was funded by AstraZeneca. Dr. Curtain reports no relevant financial relationships. Disclosures for the coauthors are listed in the paper.
A version of this article first appeared on Medscape.com.
Dapagliflozin might reduce the risk for ventricular arrhythmias and sudden death in patients with heart failure and reduced ejection fraction (HFrEF), a post hoc analysis of the DAPA-HF trial suggests.
The addition of dapagliflozin to standard therapy reduced the relative risk for the primary composite endpoint of any serious ventricular arrhythmia, resuscitated cardiac arrest, or sudden death by 21%, compared with placebo (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99). The absolute risk reduction was 1.5% (5.9% vs. 7.4%).
The benefit was consistent in a competing-risks analysis that included all-cause mortality (HR, 0.80; P = .043) and across the individual components of the composite outcome, James Curtain, MD, Cardiovascular Research Centre of Glasgow, said at the annual congress of the European Society of Cardiology.
As previously reported from the main trial, treatment with the sodium-glucose cotransporter 2 (SGLT2) inhibitor cut the primary endpoint of cardiovascular death or worsening heart failure by 26% among 4,744 patients with HFrEF and in New York Heart Association functional class 2-4.
Cochair of the late-breaking science session, Lars Lund, MD, Karolinska Institute, Stockholm, pointed out that dapagliflozin reduced sudden cardiac deaths and related events to an extent similar to that observed for cardiovascular deaths, total mortality, and the main trial’s primary endpoint.
“So does that mean it has any particular effect on arrhythmic events or does it mean, such as a beta-blocker, for example, [it] reduces calcium transience and improves handling of calcium, or does it have an effect simply by improving heart failure?” he asked.
Dr. Curtain replied they are still trying to understand the effects of this new class of drug but that studies have shown dapagliflozin and other SGLT2 inhibitors have favorable effects on adverse cardiac remodeling, which contributes to sudden death and ventricular arrhythmia. They’ve also been shown to reduce cardiac chamber size, left ventricular hypertrophy, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels over time, consistent with a reduction in myocardial wall stress. “So it could indeed be one of several mechanisms by which they may exert a beneficial cardiac effect.”
Speaking with this news organization, Dr. Curtain pointed out that the Kaplan-Meier curves for the composite outcome began to separate early on, but that the clearest separation was after 9 months, suggestive of a positive action on adverse cardiac remodeling over time.
“This would improve the patients’ heart failure situation, but also thick ventricles are a key risk factor for the occurrence of sudden death and ventricular arrhythmias,” he said. “The effects on adverse cardiac remodeling, given its plausibility in terms of our Kaplan-Meier curves, are one [mechanism] that I’d look to in the first instance, but I’m sure there are more than one actions at play.”
According to the new analysis, the primary outcome occurred in 315 (6.6%) patients; there were 203 adjudicated sudden deaths (64%), 104 investigator-reported ventricular arrhythmias (33%), and 8 resuscitated cardiac arrests (3%). Independent predictors of the primary outcome were higher NT-proBNP levels (odds ratio, 1.54), previous ventricular arrhythmia (OR, 1.93), previous myocardial infarction (OR, 1.42), male sex (OR, 1.53), and higher body mass index (OR, 1.03).
The effect of dapagliflozin on the primary outcome was consistent in several sensitivity analyses and “generally consistent” across key subgroups, Dr. Curtain said.
During a discussion of the results, session cochair Mitja Lainscak, MD, General Hospital Murska Sobota, Slovenia, called out two exceptions. “With regard to patients with implanted ICDs, the effect was neutral, and in the patients without diabetes, the benefit was less than in diabetic patients. Any explanations for that?”
Dr. Curtain responded that “it’s important to note that in the subgroup analyses the point estimates were all on the side favoring dapagliflozin and the interaction test was not significant in that subgroup. The numbers of patients who were in the defibrillator group were modest, and there was a relatively smaller number of events, so it may be harder to show benefit in that group.”
In the dapagliflozin and placebo groups, the event rates per 100 person-years were 3.9 and 5.8, respectively, in patients with diabetes, and 4.1 and 4.7, respectively, in those without diabetes (P for interaction = .273).
Event rates per 100 person-years were 5.8 and 5.9, respectively, in patients with a defibrillator at baseline, and 3.5 and 4.9, respectively, in those without a defibrillator (P for interaction = .174).
Asked to comment on the study, which was simultaneously published in the European Heart Journal, Milton Packer, MD, Baylor University Medical Center, Dallas, said he had “very little confidence” in the findings.
“This was entirely post hoc and the investigators combined events – with markedly different levels of clinical importance – in order to achieve a P value less than 0.05,” he told this news organization. “If one takes asymptomatic ventricular arrhythmias out of the analysis, the effect is no longer statistically significant. Furthermore, half of sudden deaths in patients with heart failure are not related to a ventricular arrhythmia.”
The authors note in their report that the analysis was not prespecified and the findings should be regarded as “hypothesis generating and require confirmation,” but also point out that a recent meta-analysis showed that SGLT2 inhibitor use was associated with a lower risk for ventricular tachycardia. Other limitations to the post hoc analysis are that adverse-event reporting likely underestimated the true prevalence of ventricular arrhythmias, and that these events were not adjudicated.
DAPA-HF was funded by AstraZeneca. Dr. Curtain reports no relevant financial relationships. Disclosures for the coauthors are listed in the paper.
A version of this article first appeared on Medscape.com.
Dapagliflozin might reduce the risk for ventricular arrhythmias and sudden death in patients with heart failure and reduced ejection fraction (HFrEF), a post hoc analysis of the DAPA-HF trial suggests.
The addition of dapagliflozin to standard therapy reduced the relative risk for the primary composite endpoint of any serious ventricular arrhythmia, resuscitated cardiac arrest, or sudden death by 21%, compared with placebo (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99). The absolute risk reduction was 1.5% (5.9% vs. 7.4%).
The benefit was consistent in a competing-risks analysis that included all-cause mortality (HR, 0.80; P = .043) and across the individual components of the composite outcome, James Curtain, MD, Cardiovascular Research Centre of Glasgow, said at the annual congress of the European Society of Cardiology.
As previously reported from the main trial, treatment with the sodium-glucose cotransporter 2 (SGLT2) inhibitor cut the primary endpoint of cardiovascular death or worsening heart failure by 26% among 4,744 patients with HFrEF and in New York Heart Association functional class 2-4.
Cochair of the late-breaking science session, Lars Lund, MD, Karolinska Institute, Stockholm, pointed out that dapagliflozin reduced sudden cardiac deaths and related events to an extent similar to that observed for cardiovascular deaths, total mortality, and the main trial’s primary endpoint.
“So does that mean it has any particular effect on arrhythmic events or does it mean, such as a beta-blocker, for example, [it] reduces calcium transience and improves handling of calcium, or does it have an effect simply by improving heart failure?” he asked.
Dr. Curtain replied they are still trying to understand the effects of this new class of drug but that studies have shown dapagliflozin and other SGLT2 inhibitors have favorable effects on adverse cardiac remodeling, which contributes to sudden death and ventricular arrhythmia. They’ve also been shown to reduce cardiac chamber size, left ventricular hypertrophy, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels over time, consistent with a reduction in myocardial wall stress. “So it could indeed be one of several mechanisms by which they may exert a beneficial cardiac effect.”
Speaking with this news organization, Dr. Curtain pointed out that the Kaplan-Meier curves for the composite outcome began to separate early on, but that the clearest separation was after 9 months, suggestive of a positive action on adverse cardiac remodeling over time.
“This would improve the patients’ heart failure situation, but also thick ventricles are a key risk factor for the occurrence of sudden death and ventricular arrhythmias,” he said. “The effects on adverse cardiac remodeling, given its plausibility in terms of our Kaplan-Meier curves, are one [mechanism] that I’d look to in the first instance, but I’m sure there are more than one actions at play.”
According to the new analysis, the primary outcome occurred in 315 (6.6%) patients; there were 203 adjudicated sudden deaths (64%), 104 investigator-reported ventricular arrhythmias (33%), and 8 resuscitated cardiac arrests (3%). Independent predictors of the primary outcome were higher NT-proBNP levels (odds ratio, 1.54), previous ventricular arrhythmia (OR, 1.93), previous myocardial infarction (OR, 1.42), male sex (OR, 1.53), and higher body mass index (OR, 1.03).
The effect of dapagliflozin on the primary outcome was consistent in several sensitivity analyses and “generally consistent” across key subgroups, Dr. Curtain said.
During a discussion of the results, session cochair Mitja Lainscak, MD, General Hospital Murska Sobota, Slovenia, called out two exceptions. “With regard to patients with implanted ICDs, the effect was neutral, and in the patients without diabetes, the benefit was less than in diabetic patients. Any explanations for that?”
Dr. Curtain responded that “it’s important to note that in the subgroup analyses the point estimates were all on the side favoring dapagliflozin and the interaction test was not significant in that subgroup. The numbers of patients who were in the defibrillator group were modest, and there was a relatively smaller number of events, so it may be harder to show benefit in that group.”
In the dapagliflozin and placebo groups, the event rates per 100 person-years were 3.9 and 5.8, respectively, in patients with diabetes, and 4.1 and 4.7, respectively, in those without diabetes (P for interaction = .273).
Event rates per 100 person-years were 5.8 and 5.9, respectively, in patients with a defibrillator at baseline, and 3.5 and 4.9, respectively, in those without a defibrillator (P for interaction = .174).
Asked to comment on the study, which was simultaneously published in the European Heart Journal, Milton Packer, MD, Baylor University Medical Center, Dallas, said he had “very little confidence” in the findings.
“This was entirely post hoc and the investigators combined events – with markedly different levels of clinical importance – in order to achieve a P value less than 0.05,” he told this news organization. “If one takes asymptomatic ventricular arrhythmias out of the analysis, the effect is no longer statistically significant. Furthermore, half of sudden deaths in patients with heart failure are not related to a ventricular arrhythmia.”
The authors note in their report that the analysis was not prespecified and the findings should be regarded as “hypothesis generating and require confirmation,” but also point out that a recent meta-analysis showed that SGLT2 inhibitor use was associated with a lower risk for ventricular tachycardia. Other limitations to the post hoc analysis are that adverse-event reporting likely underestimated the true prevalence of ventricular arrhythmias, and that these events were not adjudicated.
DAPA-HF was funded by AstraZeneca. Dr. Curtain reports no relevant financial relationships. Disclosures for the coauthors are listed in the paper.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2021
Stop blaming the unvaccinated
As politicians battle over masks and mandates, heated rhetoric has been used to describe the fourth heartbreaking surge in COVID as a “pandemic of the unvaccinated.”
While it may serve to further divide red and blue states, I disagree with the assertion that the current surge in cases is driven simply by the unvaccinated. Why? First, the premise would assume complete efficacy with our vaccinated population, which is statistically incorrect (at least 15 million of the U.S. population never completed a second round of injections), which means they were not considered “fully vaccinated.”
Alternately, we need to examine what has occurred in nations with significantly higher vaccination rates than ours (the United Kingdom and Israel) to realize that variants have overrun the dramatic success achieved in those countries as well. Israel, once considered to be the most vaccinated country in the world, is facing a brutal fourth wave of COVID that has sent the country spiraling into another heartbreaking lockdown.
The unvaccinated could hardly be blamed for what is happening in either of these highly vaccinated countries.
The concept of blame
So why use blame? It defeats the purpose of encouraging those who are hesitant or possibly misinformed or disenfranchised to move forward. It lacks compassion. It does not encompass the art and science of nursing (for example, the University of Southern Indiana), such as those that hospitals have used to frame optimal nursing care. I abhor the idea of labeling because it denies the prospect of future comprehension.
Labeling reminds me of one of the saddest cases in my career.
An unfortunate case
I was the nurse caring for a man from a motor vehicular accident where an entire family was brutally killed. My patient was alleged to be the cause, with a blood alcohol level of 0.40%+ post hydration, intubated and ventilated, with a flailed chest and multiple orthopedic injuries as well as blunt head trauma. He was secured to the bed with handcuffs, although that was unnecessary. Multiple times I was asked how I could possibly care for such an individual, by the police and even a few colleagues. But it was not my place to judge the man.
He was in pain, and he was dying. I comforted him for the 2 weeks it took his battered body to pass into the next realm. No one visited him except the police, eagerly waiting for the man to wake up to explain the tragic events that occurred. It was my job to ease what pain I could and protect him from labels. Did he deserve the labels? Who knew? I did not care. I cared about his writhing and his physical anguish.
The comparison
Blame did not help the situation then, nor does it help us move forward now. As nurses, we seek to work within a framework of understanding. As we tire of caring for thousands of COVID patients, we do not stop to ask if they “deserve” care or if they have taken precautions and lived reasonably prior to seeking assistance for disease. We would not be nurses if we did this.
Think about Gov. Greg Abbott, who has asked that Texans not be allowed to mandate masks for children returning to school. He has recently been diagnosed with COVID, despite assuring the public he is fully vaccinated. Politically, his diagnosis could be visualized as a fiasco for a purple state where he has been adamant in denying the efficacy of masks for children.
Yet, his diagnosis should not be fodder for the press. The first concern should be his health and well-being, similar for any man of his age and potential comorbidity.
Conclusion
We should be people first, human beings that remain interconnected by our need for care and survival, not conservatives, independents, or liberals, not “vaccinated or unvaccinated,” not seen as “breakthrough” infections, or the immunosuppressed possibly unable to mount a robust response to COVID.
Labels do not define the ability to effectively defeat coronavirus or variants, as highly vaccinated countries have demonstrated in recent months. We are in the midst of a global pandemic, and the battle is raging onward.
In fact, the longer this pandemic continues, the more likely it is we will need to live with this as an endemic disease, so we should stop blaming those who become ill and need support.
It could be any of us.
A version of this article first appeared on Medscape.com.
As politicians battle over masks and mandates, heated rhetoric has been used to describe the fourth heartbreaking surge in COVID as a “pandemic of the unvaccinated.”
While it may serve to further divide red and blue states, I disagree with the assertion that the current surge in cases is driven simply by the unvaccinated. Why? First, the premise would assume complete efficacy with our vaccinated population, which is statistically incorrect (at least 15 million of the U.S. population never completed a second round of injections), which means they were not considered “fully vaccinated.”
Alternately, we need to examine what has occurred in nations with significantly higher vaccination rates than ours (the United Kingdom and Israel) to realize that variants have overrun the dramatic success achieved in those countries as well. Israel, once considered to be the most vaccinated country in the world, is facing a brutal fourth wave of COVID that has sent the country spiraling into another heartbreaking lockdown.
The unvaccinated could hardly be blamed for what is happening in either of these highly vaccinated countries.
The concept of blame
So why use blame? It defeats the purpose of encouraging those who are hesitant or possibly misinformed or disenfranchised to move forward. It lacks compassion. It does not encompass the art and science of nursing (for example, the University of Southern Indiana), such as those that hospitals have used to frame optimal nursing care. I abhor the idea of labeling because it denies the prospect of future comprehension.
Labeling reminds me of one of the saddest cases in my career.
An unfortunate case
I was the nurse caring for a man from a motor vehicular accident where an entire family was brutally killed. My patient was alleged to be the cause, with a blood alcohol level of 0.40%+ post hydration, intubated and ventilated, with a flailed chest and multiple orthopedic injuries as well as blunt head trauma. He was secured to the bed with handcuffs, although that was unnecessary. Multiple times I was asked how I could possibly care for such an individual, by the police and even a few colleagues. But it was not my place to judge the man.
He was in pain, and he was dying. I comforted him for the 2 weeks it took his battered body to pass into the next realm. No one visited him except the police, eagerly waiting for the man to wake up to explain the tragic events that occurred. It was my job to ease what pain I could and protect him from labels. Did he deserve the labels? Who knew? I did not care. I cared about his writhing and his physical anguish.
The comparison
Blame did not help the situation then, nor does it help us move forward now. As nurses, we seek to work within a framework of understanding. As we tire of caring for thousands of COVID patients, we do not stop to ask if they “deserve” care or if they have taken precautions and lived reasonably prior to seeking assistance for disease. We would not be nurses if we did this.
Think about Gov. Greg Abbott, who has asked that Texans not be allowed to mandate masks for children returning to school. He has recently been diagnosed with COVID, despite assuring the public he is fully vaccinated. Politically, his diagnosis could be visualized as a fiasco for a purple state where he has been adamant in denying the efficacy of masks for children.
Yet, his diagnosis should not be fodder for the press. The first concern should be his health and well-being, similar for any man of his age and potential comorbidity.
Conclusion
We should be people first, human beings that remain interconnected by our need for care and survival, not conservatives, independents, or liberals, not “vaccinated or unvaccinated,” not seen as “breakthrough” infections, or the immunosuppressed possibly unable to mount a robust response to COVID.
Labels do not define the ability to effectively defeat coronavirus or variants, as highly vaccinated countries have demonstrated in recent months. We are in the midst of a global pandemic, and the battle is raging onward.
In fact, the longer this pandemic continues, the more likely it is we will need to live with this as an endemic disease, so we should stop blaming those who become ill and need support.
It could be any of us.
A version of this article first appeared on Medscape.com.
As politicians battle over masks and mandates, heated rhetoric has been used to describe the fourth heartbreaking surge in COVID as a “pandemic of the unvaccinated.”
While it may serve to further divide red and blue states, I disagree with the assertion that the current surge in cases is driven simply by the unvaccinated. Why? First, the premise would assume complete efficacy with our vaccinated population, which is statistically incorrect (at least 15 million of the U.S. population never completed a second round of injections), which means they were not considered “fully vaccinated.”
Alternately, we need to examine what has occurred in nations with significantly higher vaccination rates than ours (the United Kingdom and Israel) to realize that variants have overrun the dramatic success achieved in those countries as well. Israel, once considered to be the most vaccinated country in the world, is facing a brutal fourth wave of COVID that has sent the country spiraling into another heartbreaking lockdown.
The unvaccinated could hardly be blamed for what is happening in either of these highly vaccinated countries.
The concept of blame
So why use blame? It defeats the purpose of encouraging those who are hesitant or possibly misinformed or disenfranchised to move forward. It lacks compassion. It does not encompass the art and science of nursing (for example, the University of Southern Indiana), such as those that hospitals have used to frame optimal nursing care. I abhor the idea of labeling because it denies the prospect of future comprehension.
Labeling reminds me of one of the saddest cases in my career.
An unfortunate case
I was the nurse caring for a man from a motor vehicular accident where an entire family was brutally killed. My patient was alleged to be the cause, with a blood alcohol level of 0.40%+ post hydration, intubated and ventilated, with a flailed chest and multiple orthopedic injuries as well as blunt head trauma. He was secured to the bed with handcuffs, although that was unnecessary. Multiple times I was asked how I could possibly care for such an individual, by the police and even a few colleagues. But it was not my place to judge the man.
He was in pain, and he was dying. I comforted him for the 2 weeks it took his battered body to pass into the next realm. No one visited him except the police, eagerly waiting for the man to wake up to explain the tragic events that occurred. It was my job to ease what pain I could and protect him from labels. Did he deserve the labels? Who knew? I did not care. I cared about his writhing and his physical anguish.
The comparison
Blame did not help the situation then, nor does it help us move forward now. As nurses, we seek to work within a framework of understanding. As we tire of caring for thousands of COVID patients, we do not stop to ask if they “deserve” care or if they have taken precautions and lived reasonably prior to seeking assistance for disease. We would not be nurses if we did this.
Think about Gov. Greg Abbott, who has asked that Texans not be allowed to mandate masks for children returning to school. He has recently been diagnosed with COVID, despite assuring the public he is fully vaccinated. Politically, his diagnosis could be visualized as a fiasco for a purple state where he has been adamant in denying the efficacy of masks for children.
Yet, his diagnosis should not be fodder for the press. The first concern should be his health and well-being, similar for any man of his age and potential comorbidity.
Conclusion
We should be people first, human beings that remain interconnected by our need for care and survival, not conservatives, independents, or liberals, not “vaccinated or unvaccinated,” not seen as “breakthrough” infections, or the immunosuppressed possibly unable to mount a robust response to COVID.
Labels do not define the ability to effectively defeat coronavirus or variants, as highly vaccinated countries have demonstrated in recent months. We are in the midst of a global pandemic, and the battle is raging onward.
In fact, the longer this pandemic continues, the more likely it is we will need to live with this as an endemic disease, so we should stop blaming those who become ill and need support.
It could be any of us.
A version of this article first appeared on Medscape.com.
EMPEROR-Preserved: Empagliflozin scores HFpEF breakthrough
Updated August 30, 2021
The SGLT2 inhibitor empagliflozin achieved in EMPEROR-Preserved what no other agent could previously do: unequivocally cut the incidence of cardiovascular death or hospitalization in patients with heart failure and preserved ejection fraction (HFpEF).
Treatment with empagliflozin (Jardiance) led to a significant 21% relative reduction in the rate of cardiovascular death or hospitalization for heart failure (HHF), compared with placebo, among 5,988 randomized patients with HFpEF during a median 26 months of follow-up, proving that patients with HFpEF finally have a treatment that gives them clinically meaningful benefit, and paving the way to an abrupt change in management of these patients, experts said.
“This is the first trial to show unequivocal benefits of any drug on major heart failure outcomes in patients with HFpEF,” Stefan D. Anker, MD, PhD, declared at the virtual annual congress of the European Society of Cardiology.
The 21% relative reduction, which reflected a cut in the absolute rate of the trial’s primary composite endpoint of 3.3% compared with placebo, was driven mainly by a significant 27% relative reduction in the incidence of HHF (P < .001). Empagliflozin treatment, on top of standard therapy for patients with HFpEF, also resulted in a nonsignificant 9% relative risk reduction in the incidence of cardiovascular death, but it had no discernible impact on the rate of death from any cause, said Dr. Anker, professor of cardiology at Charité Medical University in Berlin.
Concurrently with his talk at the meeting, the results were published online in the New England Journal of Medicine.
Practice will change ‘quickly’
“This will definitely change our practice, and quite quickly,” said Carlos Aguiar, MD, chair of the Advanced Heart Failure and Heart Transplantation Unit at Hospital Santa Cruz in Carnaxide, Portugal, who was not involved in the study.
Transition to routine use of empagliflozin in patients with HFpEF should be swift because it has already become a mainstay of treatment for patients with heart failure with reduced ejection fraction (HFrEF) based on evidence for empagliflozin in EMPEROR-Reduced. A second sodium-glucose cotransporter 2 (SGLT2 ) inhibitor, dapagliflozin (Farxiga), is also an option for treating HFrEF based on results in the DAPA-HF trial, and the DELIVER trial, still in progress, is testing dapagliflozin as a HFpEF treatment in about 6,000 patients, with results expected in 2022.
About half of the patients in EMPEROR-Preserved had diabetes, and the treatment effects on HFpEF were similar regardless of patients’ diabetes status. Empagliflozin, like other members of the SGLT2 inhibitor class, boosts urinary excretion of glucose and received initial regulatory approval as an agent for glycemic control in patients with type 2 diabetes. Empagliflozin also has U.S.-approved marketing indications for treating patients with HFrEF whether or not they also have diabetes, and for reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease.
“We already use this drug class in cardiovascular medicine and to treat patients with type 2 diabetes, and we have been eager to find a treatment for patients with HFpEF. This is something that will be really significant,” said Dr. Aguiar.
Heart failure clinicians have “become familiar prescribing” SGLT2 inhibitors following approval of HFrEF indications for some of these agents, noted Mary Norine Walsh, MD, a heart failure specialist with Ascension Medical Group in Indianapolis. The new results “are good news because there have been so few options” for patients with HFpEF, she said in an interview.
EMPEROR-Preserved “is the first phase 3 clinical trial that exclusively enrolled patients with heart failure and an ejection fraction of more than 40% to meet its primary outcome,” and the results “represent a major win against a medical condition that had previously proven formidable,” Mark H. Drazner, MD, said in an editorial that accompanied the published results.
The trial’s findings “should contribute to a change in clinical practice given the paucity of therapeutic options available for patients with HFpEF,” wrote Dr. Drazner, a heart failure specialist who is professor and clinical chief of cardiology at UT Southwestern Medical Center in Dallas.
Theresa A, McDonagh, MD, MBChB, who chaired the panel that just released revised guidelines from the European Society of Cardiology for managing patients with heart failure, predicted that empagliflozin treatment for patients with HFpEF will soon show up in guidelines. It will likely receive a “should be considered” ranking despite being a single study because of the impressive size of the treatment effect and lack of well-supported alternative treatments, she commented as a discussant of the trial during its presentation at the congress. If the DELIVER trial with dapagliflozin shows a similar effect, the recommendation would likely become even stronger, added Dr. McDonagh, a heart failure specialist and professor of cardiology at King’s College, London.
More women enrolled than ever before
EMPEROR-Preserved enrolled adults with chronic HFpEF in New York Heart Association functional class II-IV and a left ventricular ejection fraction greater than 40% starting in 2017 at more than 600 sites in more than 20 countries worldwide including the United States. As background therapy, more than 80% of patients received treatment with either an ACE inhibitor or angiotensin receptor blocker (in some instances in the form of sacubitril/valsartan), more than 80% were on a beta-blocker, and about a third were taking a mineralocorticoid receptor antagonist, making them “very well treated HFpEF patients,” Dr. Anker said.
One of the most notable features of enrollment was that 45% of participants were women, giving this trial the highest inclusion of women compared with all prior studies in patients with HFpEF or with HFrEF, said Dr. Walsh. “HFpEF is very prevalent in woman,” she noted, and having this high participation rate of women in the study increases its relevance to these patients. “It’s important to be able to tell women that patients like you were in the study so we can more easily apply the lessons from the trial to you. That can’t be stressed enough,” she said.
The primary outcome occurred in 415 (13.8%) of the 2,997 patients in the empagliflozin group and in 511 (17.1%) of 2,991 patients who received placebo (hazard ratio, 0.79; 95% confidence interval, 0.69-0.90; P < .001).
The study showed a safety profile consistent with prior experience with empagliflozin, Dr. Anker added.
Pooling EMPEROR-Preserved with EMPEROR-Reduced
The investigators who ran EMPEROR-Preserved designed the trial to closely parallel the EMPEROR-Reduced trial in patients with HFrEF, and they included a prespecified analysis (EMPEROR-Pooled) that combined the more than 9,700 patients in the two studies. This showed a consistent and robust benefit from empagliflozin for reducing HHF across a wide spectrum of patients with heart failure, ranging from patients with left ventricular ejection fractions of less than 25% to patients with ejection fractions as high as 64%. However, the analysis also showed that patients with ejection fractions of 65% or greater received no discernible benefit from empagliflozin, Milton Packer, MD, reported in a separate talk at the congress.
“The findings demonstrate the benefits of empagliflozin across a broad range of patients with heart failure who have ejection fractions of less than 60%-65%,” said Dr. Packer, a researcher at Baylor University Medical Center in Dallas.
This apparent attenuation of an effect at higher ejection fractions “has been observed in other HFpEF trials, most recently in the PARAGON-HF trial” of sacubitril/valsartan (Entresto), he noted. Additional analyses led by Dr. Packer showed that in patients with ejection fractions below 65% the HHF benefit from empagliflozin consistently surpassed the benefit seen with sacubitril/valsartan in PARAGON-HF. But he recommended using both drugs in patients with HFpEF and an ejection fraction up to about 60%.
“If I had a patient with HFpEF I would use both drugs as well as beta-blockers and mineralocorticoid receptor antagonists,” he said during a press briefing.
Another finding from analysis of the EMPEROR-Reduced and EMPEROR-Preserved trials together was that patients with reduced ejection fractions showed a significant 49% relative reduction in the incidence of serious renal outcomes, but this effect was completely blunted in EMPEROR-Preserved.
“Ejection fraction influences the effects of empagliflozin on major renal outcomes,” concluded Dr. Packer in a report on this analysis published simultaneously with the main EMPEROR-Preserved findings (N Engl J Med. 2021 Aug 27. doi: 10.1056/NEJMc2112411). “These data from the EMPEROR trials are unique. We have no comparable data” from any of the other reported studies of SGLT2 inhibitors,” he said.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and by Eli Lilly, the two companies that jointly market empagliflozin (Jardiance). Dr. Anker has received personal fees from Boehringer Ingelheim and from several other companies, and he has received grants and personal fees from Abbott Vascular and Vifor. Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. McDonagh has has recent financial relationships with AstraZeneca, Cprpus, Novartis, Pfizer, and Vifor. Dr. Aguiar and Dr. Walsh had no disclosures.
Updated August 30, 2021
The SGLT2 inhibitor empagliflozin achieved in EMPEROR-Preserved what no other agent could previously do: unequivocally cut the incidence of cardiovascular death or hospitalization in patients with heart failure and preserved ejection fraction (HFpEF).
Treatment with empagliflozin (Jardiance) led to a significant 21% relative reduction in the rate of cardiovascular death or hospitalization for heart failure (HHF), compared with placebo, among 5,988 randomized patients with HFpEF during a median 26 months of follow-up, proving that patients with HFpEF finally have a treatment that gives them clinically meaningful benefit, and paving the way to an abrupt change in management of these patients, experts said.
“This is the first trial to show unequivocal benefits of any drug on major heart failure outcomes in patients with HFpEF,” Stefan D. Anker, MD, PhD, declared at the virtual annual congress of the European Society of Cardiology.
The 21% relative reduction, which reflected a cut in the absolute rate of the trial’s primary composite endpoint of 3.3% compared with placebo, was driven mainly by a significant 27% relative reduction in the incidence of HHF (P < .001). Empagliflozin treatment, on top of standard therapy for patients with HFpEF, also resulted in a nonsignificant 9% relative risk reduction in the incidence of cardiovascular death, but it had no discernible impact on the rate of death from any cause, said Dr. Anker, professor of cardiology at Charité Medical University in Berlin.
Concurrently with his talk at the meeting, the results were published online in the New England Journal of Medicine.
Practice will change ‘quickly’
“This will definitely change our practice, and quite quickly,” said Carlos Aguiar, MD, chair of the Advanced Heart Failure and Heart Transplantation Unit at Hospital Santa Cruz in Carnaxide, Portugal, who was not involved in the study.
Transition to routine use of empagliflozin in patients with HFpEF should be swift because it has already become a mainstay of treatment for patients with heart failure with reduced ejection fraction (HFrEF) based on evidence for empagliflozin in EMPEROR-Reduced. A second sodium-glucose cotransporter 2 (SGLT2 ) inhibitor, dapagliflozin (Farxiga), is also an option for treating HFrEF based on results in the DAPA-HF trial, and the DELIVER trial, still in progress, is testing dapagliflozin as a HFpEF treatment in about 6,000 patients, with results expected in 2022.
About half of the patients in EMPEROR-Preserved had diabetes, and the treatment effects on HFpEF were similar regardless of patients’ diabetes status. Empagliflozin, like other members of the SGLT2 inhibitor class, boosts urinary excretion of glucose and received initial regulatory approval as an agent for glycemic control in patients with type 2 diabetes. Empagliflozin also has U.S.-approved marketing indications for treating patients with HFrEF whether or not they also have diabetes, and for reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease.
“We already use this drug class in cardiovascular medicine and to treat patients with type 2 diabetes, and we have been eager to find a treatment for patients with HFpEF. This is something that will be really significant,” said Dr. Aguiar.
Heart failure clinicians have “become familiar prescribing” SGLT2 inhibitors following approval of HFrEF indications for some of these agents, noted Mary Norine Walsh, MD, a heart failure specialist with Ascension Medical Group in Indianapolis. The new results “are good news because there have been so few options” for patients with HFpEF, she said in an interview.
EMPEROR-Preserved “is the first phase 3 clinical trial that exclusively enrolled patients with heart failure and an ejection fraction of more than 40% to meet its primary outcome,” and the results “represent a major win against a medical condition that had previously proven formidable,” Mark H. Drazner, MD, said in an editorial that accompanied the published results.
The trial’s findings “should contribute to a change in clinical practice given the paucity of therapeutic options available for patients with HFpEF,” wrote Dr. Drazner, a heart failure specialist who is professor and clinical chief of cardiology at UT Southwestern Medical Center in Dallas.
Theresa A, McDonagh, MD, MBChB, who chaired the panel that just released revised guidelines from the European Society of Cardiology for managing patients with heart failure, predicted that empagliflozin treatment for patients with HFpEF will soon show up in guidelines. It will likely receive a “should be considered” ranking despite being a single study because of the impressive size of the treatment effect and lack of well-supported alternative treatments, she commented as a discussant of the trial during its presentation at the congress. If the DELIVER trial with dapagliflozin shows a similar effect, the recommendation would likely become even stronger, added Dr. McDonagh, a heart failure specialist and professor of cardiology at King’s College, London.
More women enrolled than ever before
EMPEROR-Preserved enrolled adults with chronic HFpEF in New York Heart Association functional class II-IV and a left ventricular ejection fraction greater than 40% starting in 2017 at more than 600 sites in more than 20 countries worldwide including the United States. As background therapy, more than 80% of patients received treatment with either an ACE inhibitor or angiotensin receptor blocker (in some instances in the form of sacubitril/valsartan), more than 80% were on a beta-blocker, and about a third were taking a mineralocorticoid receptor antagonist, making them “very well treated HFpEF patients,” Dr. Anker said.
One of the most notable features of enrollment was that 45% of participants were women, giving this trial the highest inclusion of women compared with all prior studies in patients with HFpEF or with HFrEF, said Dr. Walsh. “HFpEF is very prevalent in woman,” she noted, and having this high participation rate of women in the study increases its relevance to these patients. “It’s important to be able to tell women that patients like you were in the study so we can more easily apply the lessons from the trial to you. That can’t be stressed enough,” she said.
The primary outcome occurred in 415 (13.8%) of the 2,997 patients in the empagliflozin group and in 511 (17.1%) of 2,991 patients who received placebo (hazard ratio, 0.79; 95% confidence interval, 0.69-0.90; P < .001).
The study showed a safety profile consistent with prior experience with empagliflozin, Dr. Anker added.
Pooling EMPEROR-Preserved with EMPEROR-Reduced
The investigators who ran EMPEROR-Preserved designed the trial to closely parallel the EMPEROR-Reduced trial in patients with HFrEF, and they included a prespecified analysis (EMPEROR-Pooled) that combined the more than 9,700 patients in the two studies. This showed a consistent and robust benefit from empagliflozin for reducing HHF across a wide spectrum of patients with heart failure, ranging from patients with left ventricular ejection fractions of less than 25% to patients with ejection fractions as high as 64%. However, the analysis also showed that patients with ejection fractions of 65% or greater received no discernible benefit from empagliflozin, Milton Packer, MD, reported in a separate talk at the congress.
“The findings demonstrate the benefits of empagliflozin across a broad range of patients with heart failure who have ejection fractions of less than 60%-65%,” said Dr. Packer, a researcher at Baylor University Medical Center in Dallas.
This apparent attenuation of an effect at higher ejection fractions “has been observed in other HFpEF trials, most recently in the PARAGON-HF trial” of sacubitril/valsartan (Entresto), he noted. Additional analyses led by Dr. Packer showed that in patients with ejection fractions below 65% the HHF benefit from empagliflozin consistently surpassed the benefit seen with sacubitril/valsartan in PARAGON-HF. But he recommended using both drugs in patients with HFpEF and an ejection fraction up to about 60%.
“If I had a patient with HFpEF I would use both drugs as well as beta-blockers and mineralocorticoid receptor antagonists,” he said during a press briefing.
Another finding from analysis of the EMPEROR-Reduced and EMPEROR-Preserved trials together was that patients with reduced ejection fractions showed a significant 49% relative reduction in the incidence of serious renal outcomes, but this effect was completely blunted in EMPEROR-Preserved.
“Ejection fraction influences the effects of empagliflozin on major renal outcomes,” concluded Dr. Packer in a report on this analysis published simultaneously with the main EMPEROR-Preserved findings (N Engl J Med. 2021 Aug 27. doi: 10.1056/NEJMc2112411). “These data from the EMPEROR trials are unique. We have no comparable data” from any of the other reported studies of SGLT2 inhibitors,” he said.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and by Eli Lilly, the two companies that jointly market empagliflozin (Jardiance). Dr. Anker has received personal fees from Boehringer Ingelheim and from several other companies, and he has received grants and personal fees from Abbott Vascular and Vifor. Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. McDonagh has has recent financial relationships with AstraZeneca, Cprpus, Novartis, Pfizer, and Vifor. Dr. Aguiar and Dr. Walsh had no disclosures.
Updated August 30, 2021
The SGLT2 inhibitor empagliflozin achieved in EMPEROR-Preserved what no other agent could previously do: unequivocally cut the incidence of cardiovascular death or hospitalization in patients with heart failure and preserved ejection fraction (HFpEF).
Treatment with empagliflozin (Jardiance) led to a significant 21% relative reduction in the rate of cardiovascular death or hospitalization for heart failure (HHF), compared with placebo, among 5,988 randomized patients with HFpEF during a median 26 months of follow-up, proving that patients with HFpEF finally have a treatment that gives them clinically meaningful benefit, and paving the way to an abrupt change in management of these patients, experts said.
“This is the first trial to show unequivocal benefits of any drug on major heart failure outcomes in patients with HFpEF,” Stefan D. Anker, MD, PhD, declared at the virtual annual congress of the European Society of Cardiology.
The 21% relative reduction, which reflected a cut in the absolute rate of the trial’s primary composite endpoint of 3.3% compared with placebo, was driven mainly by a significant 27% relative reduction in the incidence of HHF (P < .001). Empagliflozin treatment, on top of standard therapy for patients with HFpEF, also resulted in a nonsignificant 9% relative risk reduction in the incidence of cardiovascular death, but it had no discernible impact on the rate of death from any cause, said Dr. Anker, professor of cardiology at Charité Medical University in Berlin.
Concurrently with his talk at the meeting, the results were published online in the New England Journal of Medicine.
Practice will change ‘quickly’
“This will definitely change our practice, and quite quickly,” said Carlos Aguiar, MD, chair of the Advanced Heart Failure and Heart Transplantation Unit at Hospital Santa Cruz in Carnaxide, Portugal, who was not involved in the study.
Transition to routine use of empagliflozin in patients with HFpEF should be swift because it has already become a mainstay of treatment for patients with heart failure with reduced ejection fraction (HFrEF) based on evidence for empagliflozin in EMPEROR-Reduced. A second sodium-glucose cotransporter 2 (SGLT2 ) inhibitor, dapagliflozin (Farxiga), is also an option for treating HFrEF based on results in the DAPA-HF trial, and the DELIVER trial, still in progress, is testing dapagliflozin as a HFpEF treatment in about 6,000 patients, with results expected in 2022.
About half of the patients in EMPEROR-Preserved had diabetes, and the treatment effects on HFpEF were similar regardless of patients’ diabetes status. Empagliflozin, like other members of the SGLT2 inhibitor class, boosts urinary excretion of glucose and received initial regulatory approval as an agent for glycemic control in patients with type 2 diabetes. Empagliflozin also has U.S.-approved marketing indications for treating patients with HFrEF whether or not they also have diabetes, and for reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease.
“We already use this drug class in cardiovascular medicine and to treat patients with type 2 diabetes, and we have been eager to find a treatment for patients with HFpEF. This is something that will be really significant,” said Dr. Aguiar.
Heart failure clinicians have “become familiar prescribing” SGLT2 inhibitors following approval of HFrEF indications for some of these agents, noted Mary Norine Walsh, MD, a heart failure specialist with Ascension Medical Group in Indianapolis. The new results “are good news because there have been so few options” for patients with HFpEF, she said in an interview.
EMPEROR-Preserved “is the first phase 3 clinical trial that exclusively enrolled patients with heart failure and an ejection fraction of more than 40% to meet its primary outcome,” and the results “represent a major win against a medical condition that had previously proven formidable,” Mark H. Drazner, MD, said in an editorial that accompanied the published results.
The trial’s findings “should contribute to a change in clinical practice given the paucity of therapeutic options available for patients with HFpEF,” wrote Dr. Drazner, a heart failure specialist who is professor and clinical chief of cardiology at UT Southwestern Medical Center in Dallas.
Theresa A, McDonagh, MD, MBChB, who chaired the panel that just released revised guidelines from the European Society of Cardiology for managing patients with heart failure, predicted that empagliflozin treatment for patients with HFpEF will soon show up in guidelines. It will likely receive a “should be considered” ranking despite being a single study because of the impressive size of the treatment effect and lack of well-supported alternative treatments, she commented as a discussant of the trial during its presentation at the congress. If the DELIVER trial with dapagliflozin shows a similar effect, the recommendation would likely become even stronger, added Dr. McDonagh, a heart failure specialist and professor of cardiology at King’s College, London.
More women enrolled than ever before
EMPEROR-Preserved enrolled adults with chronic HFpEF in New York Heart Association functional class II-IV and a left ventricular ejection fraction greater than 40% starting in 2017 at more than 600 sites in more than 20 countries worldwide including the United States. As background therapy, more than 80% of patients received treatment with either an ACE inhibitor or angiotensin receptor blocker (in some instances in the form of sacubitril/valsartan), more than 80% were on a beta-blocker, and about a third were taking a mineralocorticoid receptor antagonist, making them “very well treated HFpEF patients,” Dr. Anker said.
One of the most notable features of enrollment was that 45% of participants were women, giving this trial the highest inclusion of women compared with all prior studies in patients with HFpEF or with HFrEF, said Dr. Walsh. “HFpEF is very prevalent in woman,” she noted, and having this high participation rate of women in the study increases its relevance to these patients. “It’s important to be able to tell women that patients like you were in the study so we can more easily apply the lessons from the trial to you. That can’t be stressed enough,” she said.
The primary outcome occurred in 415 (13.8%) of the 2,997 patients in the empagliflozin group and in 511 (17.1%) of 2,991 patients who received placebo (hazard ratio, 0.79; 95% confidence interval, 0.69-0.90; P < .001).
The study showed a safety profile consistent with prior experience with empagliflozin, Dr. Anker added.
Pooling EMPEROR-Preserved with EMPEROR-Reduced
The investigators who ran EMPEROR-Preserved designed the trial to closely parallel the EMPEROR-Reduced trial in patients with HFrEF, and they included a prespecified analysis (EMPEROR-Pooled) that combined the more than 9,700 patients in the two studies. This showed a consistent and robust benefit from empagliflozin for reducing HHF across a wide spectrum of patients with heart failure, ranging from patients with left ventricular ejection fractions of less than 25% to patients with ejection fractions as high as 64%. However, the analysis also showed that patients with ejection fractions of 65% or greater received no discernible benefit from empagliflozin, Milton Packer, MD, reported in a separate talk at the congress.
“The findings demonstrate the benefits of empagliflozin across a broad range of patients with heart failure who have ejection fractions of less than 60%-65%,” said Dr. Packer, a researcher at Baylor University Medical Center in Dallas.
This apparent attenuation of an effect at higher ejection fractions “has been observed in other HFpEF trials, most recently in the PARAGON-HF trial” of sacubitril/valsartan (Entresto), he noted. Additional analyses led by Dr. Packer showed that in patients with ejection fractions below 65% the HHF benefit from empagliflozin consistently surpassed the benefit seen with sacubitril/valsartan in PARAGON-HF. But he recommended using both drugs in patients with HFpEF and an ejection fraction up to about 60%.
“If I had a patient with HFpEF I would use both drugs as well as beta-blockers and mineralocorticoid receptor antagonists,” he said during a press briefing.
Another finding from analysis of the EMPEROR-Reduced and EMPEROR-Preserved trials together was that patients with reduced ejection fractions showed a significant 49% relative reduction in the incidence of serious renal outcomes, but this effect was completely blunted in EMPEROR-Preserved.
“Ejection fraction influences the effects of empagliflozin on major renal outcomes,” concluded Dr. Packer in a report on this analysis published simultaneously with the main EMPEROR-Preserved findings (N Engl J Med. 2021 Aug 27. doi: 10.1056/NEJMc2112411). “These data from the EMPEROR trials are unique. We have no comparable data” from any of the other reported studies of SGLT2 inhibitors,” he said.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and by Eli Lilly, the two companies that jointly market empagliflozin (Jardiance). Dr. Anker has received personal fees from Boehringer Ingelheim and from several other companies, and he has received grants and personal fees from Abbott Vascular and Vifor. Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. McDonagh has has recent financial relationships with AstraZeneca, Cprpus, Novartis, Pfizer, and Vifor. Dr. Aguiar and Dr. Walsh had no disclosures.
FROM ESC CONGRESS 2021
After five fatal overdoses, doctor sentenced for unlawful prescriptions; more
Doctor sentenced for unlawful prescriptions leading to five patient deaths
Darrel R. Rinehart, MD, was sentenced to 3 years in prison in June 2021 for unlawfully distributing controlled substances, primarily opioids, out of his clinic in Columbia, Tenn. Five of his patients who received prescriptions died of fatal overdoses within a year, according to the Indianapolis Star. Dr. Rinehart agreed to leave Tennessee to avoid punishment in that state before setting up his Indiana clinic.
Dr. Rinehart, 66, admitted to distributing Schedule II controlled substances to four different patients without legitimate medical purpose on 18 occasions between December 2014 and December 2015. He also admitted to knowingly distributing hydrocodone, also a Schedule II controlled substance, in January 2016 to a patient who did not have any health issues justifying the prescription. His medical license has been revoked.
Judge approves $15 million settlement in patient’s sexual assault
An incapacitated woman at Hacienda Healthcare, a long-term care center in Phoenix, Ariz., gave birth in late 2018 after being raped by one of the nursing staff, according to Insurance Journal. In June 2021, a judge approved a $15 million settlement in a lawsuit by the woman’s parents against Phillip E. Gear Jr., MD, the woman’s caregiver for 26 years at the center. The woman had been in a vegetative state at Hacienda Healthcare since childhood, and the judge ruled that she had been the victim of numerous sexual assaults prior to the birth.
The pregnancy was discovered when an employee was changing the garments of the then 29-year-old victim and saw that she was delivering a child. Employees told police they had no idea the woman was pregnant. Police have said that DNA from Nathan Sutherland, a licensed practical nurse who worked at Hacienda and has since given up his nursing license, matched a genetic sample taken from the woman’s son.
The woman’s parents, who care for her son, also sued the state of Arizona and another doctor, Thanh Nguyen, MD, who cared for their daughter. Arizona, which contracts with companies like Hacienda to provide services to people with developmental disabilities, settled last year for $7.5 million. Both Hacienda and Dr. Nguyen, who cared for the woman in the months before the birth, settled for undisclosed amounts.
The insurer for Dr. Gear, who died in late 2020, said it has no obligation to pay the amount, arguing that the doctor’s policy didn’t cover claims arising from a sexual act. The insurer also argued that Dr. Gear wasn’t the woman’s primary care physician when she gave birth and couldn’t be held responsible for sexual assault.
The judge declared the $15 million settlement reasonable, concluding that Dr. Gear’s treatment of the woman had fallen below the standard of care, which included failing to examine her regularly and to diagnose her pregnancy. Requests by the woman’s mother to have exclusively female employees tend to her were not followed, as shown by medical records.
Doctor fired for contributing to suffering and death of prisoners
Washington’s prison system will pay $3.25 million and has fired the medical director of one of its facilities, stemming from the death of an inmate.
John Kleutsch, a 57-year-old prisoner, died in late 2018 of septic shock, acute pancreatitis, and a perforated intestine caused by an improperly treated abdominal wound, according to the Seattle Times. A lawsuit filed by his wife, Julia Kleutsch, said that the staff offered him only Tylenol for his pain and that Julia Barnett, MD, the former prison medical director, refused to take him to a hospital.
Dr. Barnett, whose medical license has been indefinitely suspended, was fired in 2019 after an internal investigation found that her medical care and supervision contributed to the suffering and deaths of several men in the prison, including Mr. Kleutsch.
Mr. Kleutsch, imprisoned for child molestation, was recovering from outpatient cancer surgery and sent back to the prison infirmary to recover. The lawsuit says that Mr. Kleutsch asked staff for help when his abdominal wound became excruciatingly painful, puffy, and oozing, and that at least one nurse asked Dr. Barnett to transfer him to a hospital, but she refused. Dr. Kleutsch’s causes of death were conditions never diagnosed at the prison.
Plaintiff attorney Marta O’Brien called the case “one of the worst medical malpractice cases I have encountered” and said it showed “a systemic failure” by the Department of Corrections.
SNF pays $11 million to resolve Medicare fraud allegations
SavaSeniorCare (Sava) and related entities agreed to pay $11.2 million in May 2021 to resolve allegations that they violated the False Claims Act by making their skilled nursing facilities (SNFs) bill Medicare for rehabilitation therapy services that were not reasonable, necessary, or skilled. The payment was also to resolve allegations that Sava billed the Medicare and Medicaid programs for substandard skilled nursing services, according to the U.S. Department of Justice. Sava is based in Georgia but owns and operates SNFs across the country.
The government filed a complaint against Sava in 2015, alleging that between October 2008 and September 2012, Sava intentionally submitted false claims for rehabilitation therapy services as a result of a systematic effort to increase its Medicare and Medicaid billings. The claim alleged that Sava exerted significant pressure on its SNFs to meet unrealistic financial goals, resulting in the provision of medically unreasonable, unnecessary, or unskilled services to Medicare patients. Sava also allegedly sought to increase its Medicare payments by delaying the discharge of patients from its facilities, even though the patients were medically ready to be discharged.
Additionally, the government alleged that some of Sava’s nursing services failed to meet federal standards of care, including failing to have sufficient staffing at certain facilities, failing to follow appropriate pressure ulcer and falls protocols, and failing to appropriately administer medications.
A version of this article first appeared on Medscape.com.
Doctor sentenced for unlawful prescriptions leading to five patient deaths
Darrel R. Rinehart, MD, was sentenced to 3 years in prison in June 2021 for unlawfully distributing controlled substances, primarily opioids, out of his clinic in Columbia, Tenn. Five of his patients who received prescriptions died of fatal overdoses within a year, according to the Indianapolis Star. Dr. Rinehart agreed to leave Tennessee to avoid punishment in that state before setting up his Indiana clinic.
Dr. Rinehart, 66, admitted to distributing Schedule II controlled substances to four different patients without legitimate medical purpose on 18 occasions between December 2014 and December 2015. He also admitted to knowingly distributing hydrocodone, also a Schedule II controlled substance, in January 2016 to a patient who did not have any health issues justifying the prescription. His medical license has been revoked.
Judge approves $15 million settlement in patient’s sexual assault
An incapacitated woman at Hacienda Healthcare, a long-term care center in Phoenix, Ariz., gave birth in late 2018 after being raped by one of the nursing staff, according to Insurance Journal. In June 2021, a judge approved a $15 million settlement in a lawsuit by the woman’s parents against Phillip E. Gear Jr., MD, the woman’s caregiver for 26 years at the center. The woman had been in a vegetative state at Hacienda Healthcare since childhood, and the judge ruled that she had been the victim of numerous sexual assaults prior to the birth.
The pregnancy was discovered when an employee was changing the garments of the then 29-year-old victim and saw that she was delivering a child. Employees told police they had no idea the woman was pregnant. Police have said that DNA from Nathan Sutherland, a licensed practical nurse who worked at Hacienda and has since given up his nursing license, matched a genetic sample taken from the woman’s son.
The woman’s parents, who care for her son, also sued the state of Arizona and another doctor, Thanh Nguyen, MD, who cared for their daughter. Arizona, which contracts with companies like Hacienda to provide services to people with developmental disabilities, settled last year for $7.5 million. Both Hacienda and Dr. Nguyen, who cared for the woman in the months before the birth, settled for undisclosed amounts.
The insurer for Dr. Gear, who died in late 2020, said it has no obligation to pay the amount, arguing that the doctor’s policy didn’t cover claims arising from a sexual act. The insurer also argued that Dr. Gear wasn’t the woman’s primary care physician when she gave birth and couldn’t be held responsible for sexual assault.
The judge declared the $15 million settlement reasonable, concluding that Dr. Gear’s treatment of the woman had fallen below the standard of care, which included failing to examine her regularly and to diagnose her pregnancy. Requests by the woman’s mother to have exclusively female employees tend to her were not followed, as shown by medical records.
Doctor fired for contributing to suffering and death of prisoners
Washington’s prison system will pay $3.25 million and has fired the medical director of one of its facilities, stemming from the death of an inmate.
John Kleutsch, a 57-year-old prisoner, died in late 2018 of septic shock, acute pancreatitis, and a perforated intestine caused by an improperly treated abdominal wound, according to the Seattle Times. A lawsuit filed by his wife, Julia Kleutsch, said that the staff offered him only Tylenol for his pain and that Julia Barnett, MD, the former prison medical director, refused to take him to a hospital.
Dr. Barnett, whose medical license has been indefinitely suspended, was fired in 2019 after an internal investigation found that her medical care and supervision contributed to the suffering and deaths of several men in the prison, including Mr. Kleutsch.
Mr. Kleutsch, imprisoned for child molestation, was recovering from outpatient cancer surgery and sent back to the prison infirmary to recover. The lawsuit says that Mr. Kleutsch asked staff for help when his abdominal wound became excruciatingly painful, puffy, and oozing, and that at least one nurse asked Dr. Barnett to transfer him to a hospital, but she refused. Dr. Kleutsch’s causes of death were conditions never diagnosed at the prison.
Plaintiff attorney Marta O’Brien called the case “one of the worst medical malpractice cases I have encountered” and said it showed “a systemic failure” by the Department of Corrections.
SNF pays $11 million to resolve Medicare fraud allegations
SavaSeniorCare (Sava) and related entities agreed to pay $11.2 million in May 2021 to resolve allegations that they violated the False Claims Act by making their skilled nursing facilities (SNFs) bill Medicare for rehabilitation therapy services that were not reasonable, necessary, or skilled. The payment was also to resolve allegations that Sava billed the Medicare and Medicaid programs for substandard skilled nursing services, according to the U.S. Department of Justice. Sava is based in Georgia but owns and operates SNFs across the country.
The government filed a complaint against Sava in 2015, alleging that between October 2008 and September 2012, Sava intentionally submitted false claims for rehabilitation therapy services as a result of a systematic effort to increase its Medicare and Medicaid billings. The claim alleged that Sava exerted significant pressure on its SNFs to meet unrealistic financial goals, resulting in the provision of medically unreasonable, unnecessary, or unskilled services to Medicare patients. Sava also allegedly sought to increase its Medicare payments by delaying the discharge of patients from its facilities, even though the patients were medically ready to be discharged.
Additionally, the government alleged that some of Sava’s nursing services failed to meet federal standards of care, including failing to have sufficient staffing at certain facilities, failing to follow appropriate pressure ulcer and falls protocols, and failing to appropriately administer medications.
A version of this article first appeared on Medscape.com.
Doctor sentenced for unlawful prescriptions leading to five patient deaths
Darrel R. Rinehart, MD, was sentenced to 3 years in prison in June 2021 for unlawfully distributing controlled substances, primarily opioids, out of his clinic in Columbia, Tenn. Five of his patients who received prescriptions died of fatal overdoses within a year, according to the Indianapolis Star. Dr. Rinehart agreed to leave Tennessee to avoid punishment in that state before setting up his Indiana clinic.
Dr. Rinehart, 66, admitted to distributing Schedule II controlled substances to four different patients without legitimate medical purpose on 18 occasions between December 2014 and December 2015. He also admitted to knowingly distributing hydrocodone, also a Schedule II controlled substance, in January 2016 to a patient who did not have any health issues justifying the prescription. His medical license has been revoked.
Judge approves $15 million settlement in patient’s sexual assault
An incapacitated woman at Hacienda Healthcare, a long-term care center in Phoenix, Ariz., gave birth in late 2018 after being raped by one of the nursing staff, according to Insurance Journal. In June 2021, a judge approved a $15 million settlement in a lawsuit by the woman’s parents against Phillip E. Gear Jr., MD, the woman’s caregiver for 26 years at the center. The woman had been in a vegetative state at Hacienda Healthcare since childhood, and the judge ruled that she had been the victim of numerous sexual assaults prior to the birth.
The pregnancy was discovered when an employee was changing the garments of the then 29-year-old victim and saw that she was delivering a child. Employees told police they had no idea the woman was pregnant. Police have said that DNA from Nathan Sutherland, a licensed practical nurse who worked at Hacienda and has since given up his nursing license, matched a genetic sample taken from the woman’s son.
The woman’s parents, who care for her son, also sued the state of Arizona and another doctor, Thanh Nguyen, MD, who cared for their daughter. Arizona, which contracts with companies like Hacienda to provide services to people with developmental disabilities, settled last year for $7.5 million. Both Hacienda and Dr. Nguyen, who cared for the woman in the months before the birth, settled for undisclosed amounts.
The insurer for Dr. Gear, who died in late 2020, said it has no obligation to pay the amount, arguing that the doctor’s policy didn’t cover claims arising from a sexual act. The insurer also argued that Dr. Gear wasn’t the woman’s primary care physician when she gave birth and couldn’t be held responsible for sexual assault.
The judge declared the $15 million settlement reasonable, concluding that Dr. Gear’s treatment of the woman had fallen below the standard of care, which included failing to examine her regularly and to diagnose her pregnancy. Requests by the woman’s mother to have exclusively female employees tend to her were not followed, as shown by medical records.
Doctor fired for contributing to suffering and death of prisoners
Washington’s prison system will pay $3.25 million and has fired the medical director of one of its facilities, stemming from the death of an inmate.
John Kleutsch, a 57-year-old prisoner, died in late 2018 of septic shock, acute pancreatitis, and a perforated intestine caused by an improperly treated abdominal wound, according to the Seattle Times. A lawsuit filed by his wife, Julia Kleutsch, said that the staff offered him only Tylenol for his pain and that Julia Barnett, MD, the former prison medical director, refused to take him to a hospital.
Dr. Barnett, whose medical license has been indefinitely suspended, was fired in 2019 after an internal investigation found that her medical care and supervision contributed to the suffering and deaths of several men in the prison, including Mr. Kleutsch.
Mr. Kleutsch, imprisoned for child molestation, was recovering from outpatient cancer surgery and sent back to the prison infirmary to recover. The lawsuit says that Mr. Kleutsch asked staff for help when his abdominal wound became excruciatingly painful, puffy, and oozing, and that at least one nurse asked Dr. Barnett to transfer him to a hospital, but she refused. Dr. Kleutsch’s causes of death were conditions never diagnosed at the prison.
Plaintiff attorney Marta O’Brien called the case “one of the worst medical malpractice cases I have encountered” and said it showed “a systemic failure” by the Department of Corrections.
SNF pays $11 million to resolve Medicare fraud allegations
SavaSeniorCare (Sava) and related entities agreed to pay $11.2 million in May 2021 to resolve allegations that they violated the False Claims Act by making their skilled nursing facilities (SNFs) bill Medicare for rehabilitation therapy services that were not reasonable, necessary, or skilled. The payment was also to resolve allegations that Sava billed the Medicare and Medicaid programs for substandard skilled nursing services, according to the U.S. Department of Justice. Sava is based in Georgia but owns and operates SNFs across the country.
The government filed a complaint against Sava in 2015, alleging that between October 2008 and September 2012, Sava intentionally submitted false claims for rehabilitation therapy services as a result of a systematic effort to increase its Medicare and Medicaid billings. The claim alleged that Sava exerted significant pressure on its SNFs to meet unrealistic financial goals, resulting in the provision of medically unreasonable, unnecessary, or unskilled services to Medicare patients. Sava also allegedly sought to increase its Medicare payments by delaying the discharge of patients from its facilities, even though the patients were medically ready to be discharged.
Additionally, the government alleged that some of Sava’s nursing services failed to meet federal standards of care, including failing to have sufficient staffing at certain facilities, failing to follow appropriate pressure ulcer and falls protocols, and failing to appropriately administer medications.
A version of this article first appeared on Medscape.com.
FDA okays difelikefalin for dialysis-associated pruritus in patients with CKD
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
Some nephrologists welcomed the Aug. 23 approval of this new option for treating pruritus, a relatively common and often hard-to-resolve complication of dialysis in patients with chronic kidney disease (CKD) that can substantially impinge on quality of life for some patients, but also voiced uncertainty about the role of a new agent with a modest trial track record that may be expensive and face insurance-coverage hurdles.
“Uptake of difelikefalin will depend on awareness of itch among patients dependent on hemodialysis, and on payment policies,” predicted Daniel E. Weiner, MD, a nephrologist at Tufts Medical Center in Boston. “Pruritus is underdiagnosed among people with kidney failure, and in some patients ongoing pruritus can be highly impactful on sleep and quality of life. The clinical trial results were very encouraging that difelikefalin is effective and safe,” which makes recognition of pruritus as a significant issue for patients a key factor in uptake of the new drug, Dr. Weiner, an investigator in a difelikefalin clinical study, said in an interview.
Other nephrologists acknowledged the substantial problem that itch can pose for many patients with CKD on dialysis but questioned the weight of evidence behind difelikefalin’s approval.
Two pivotal trials with fewer than 900 total randomized patients
The data considered by the FDA primarily featured results from two pivotal trials, KALM-1 and KALM-2. KALM-1 randomized 378 patients with CKD and on hemodialysis and with moderate to severe pruritus to intravenous treatment with difelikefalin or placebo three times a week for 12 weeks with a primary endpoint of an improvement (decrease) of at least 3 points from baseline in their Worst Itching Intensity Numerical Rating Scale (WI-NRS) score, which averaged just over 7 points at baseline. After 12 weeks on treatment, 52% of patients who received difelikefalin had at least a 3-point drop, compared with 31% of patients who received placebo, a significant difference. The results appeared in a 2020 report in the New England Journal of Medicine.
Confirmatory results came in the second pivotal trial, KALM-2, a similarly designed, 12-week study that randomized 473 patients, with 54% of those in the active arm achieving at least a 3-point cut in their baseline WI-NRS score, compared with 42% of patients who received placebo, a significant difference. A report at the Kidney Week meeting sponsored by the National Kidney Foundation in October 2020 presented the KALM-2 results, but the findings have not yet appeared in a published article.
In sum, the data suggest that treatment with difelikefalin will, on average, produce a clinically meaningful effect on itch compared with placebo in about 20% of patients, with nearly half the patients who receive the active drug having a less robust response and many patients who receive no active treatment also show a meaningful cut in their pruritus severity in a trial setting, noted Paul Palevsky, MD, professor of medicine at the University of Pittsburgh and chief of the renal section at the Veterans Affairs Pittsburgh Healthcare System.
The upshot is that questions linger over which patients are the best candidates for this drug and how it might perform in real-world practice given difelikefalin’s limited track record, Dr. Palevsky said in an interview.
In addition, the labeling specifies the indication is for patients with moderate to severe pruritus, but itching severity is not routinely quantified in these patients in current practice, added Dr. Palevsky, who is also president of the National Kidney Foundation.
Dr. Weiner noted that another unknown is the appropriate duration of treatment in real-world use.
What will it cost, and will it be covered?
The drug’s price and insurance coverage will likely be a major factor in uptake of the new drug, agreed both Dr. Weiner and Dr. Palevsky, especially the coverage decision for Medicare patients by the Centers for Medicare & Medicaid Services. A corollary is whether or not coverage for difelikefalin, which patients receive as an intravenous infusion during each of their usual three-times-a-week dialysis sessions, will lie outside of the bundled dialysis reimbursement payment. If is no mechanism exists to pay for difelikefalin separately beyond the current bundled dialysis rate, “I suspect it will not get used very much unless it is very inexpensive,” predicted Dr. Weiner.
Another issue is where difelikefalin fits within the lineup of standard treatment options. “A lot of people receiving hemodialysis suffer from pruritus and have not been successfully treated. For these individuals difelikefalin could be a game changer,” Dr. Weiner said.
Other nephrologists have a more positive take on the existing treatment options.
“Start systemic therapy for patients with itch that is significantly affecting quality of life; stepping up from topical therapy just delays effective treatment,” advised Hugh C. Rayner, MD, a nephrologist affiliated with Birmingham (England) Heartland’s Hospital who was lead author on a review of pruritus treatments for patients with CKD on hemodialysis.
“Standard systemic therapy is gabapentin or pregabalin,” an approach “supported by robust evidence confirmed in a Cochrane review,” he said in an interview. The impact of difelikefalin “will be limited as its effectiveness in reducing itch is modest at best and far inferior to gabapentin and pregabalin,” Dr. Rayner added. Difelikefalin’s “main downsides will be its cost, compared with gabapentin, and its gastrointestinal side effects.”
Adverse-event profiles
In KALM-1, the most frequent adverse effects from difelikefalin treatment was diarrhea, in 10% of patients, compared with a 4% rate among patients who received placebo. Vomiting occurred at a 5% incidence on difelikefalin and in 3% of patients on placebo. All serious adverse events occurred in 26% of patients on difelikefalin and in 22% of those who received placebo. Discontinuations because of an adverse event occurred in 8% of patients on difelikefalin and in 5% of the placebo patients.
An editorial that accompanied the published KALM-1 report in 2020 said “the findings are compelling, although diarrhea, dizziness, and vomiting were frequent side effects.”
Both Dr. Weiner and Dr. Palevsky were more reserved than Dr. Rayner in their appraisal of gabapentin and pregabalin, although Dr. Palevsky admitted that he has prescribed one or the other of these two drugs to “lots of patients,” especially gabapentin. “But they are not completely benign drugs,” he cautioned, a concern echoed by Dr. Weiner.
“Antihistamines, gabapentin, and pregabalin have a high side-effect burden in patients on hemodialysis and limited efficacy, and are poor options for chronic pruritus management,” explained Dr. Weiner. “I would favor difelikefalin to chronic prescription of these other agents” because difelikefalin “appears effective and has a very low side effect burden. Very few effective treatments for pruritus do not have side effects.”
Difelikefalin is a peripherally restricted, selective kappa opioid receptor agonist that exerts antipruritic effects by activating kappa opioid receptors on peripheral neurons and immune cells. The drug’s hydrophilic, small-peptide structure restricts passive diffusion across membranes, which limits the drug’s access to kappa opioid receptors in the central nervous system and hence reduces potential adverse effects.
The FDA made this approval decision without consulting an advisory committee. The companies that will market difelikefalin (Korsuva), Cara Therapeutics and Vifor Pharma, announced that their U.S. promotional launch of the drug starts early in 2022.
The KALM-1 and KALM-2 studies were sponsored by Cara Therapeutics and Vifor Pharma, the two companies that have been jointly developing difelikefalin. Dr. Pavelsky and Dr. Rayner had no relevant disclosures. Dr. Weiner was previously an adviser to Cara and Vifor and participated as an investigator in a difelikefalin clinical study, but more recently has had no relationships with the companies.
‘Countdown to zero’: Endocrine disruptors and worldwide sperm counts
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
In medical school, I remember thinking that telling a patient “you have cancer” would be the most professionally challenging phrase I would ever utter. And don’t get me wrong – it certainly isn’t easy; but, compared with telling someone “you are infertile,” it’s a cakewalk.
Maybe it’s because people “have” cancer and cancer is something you “fight.” Or maybe because, unlike infertility, cancer has become a part of public life (think lapel pins and support groups) and is now easier to accept. On the other hand, someone “is” infertile. The condition is a source of embarrassment for the couple and is often hidden from society.
Here’s another concerning point of contrast: While the overall rate of cancer death has declined since the early 1990s, infertility is increasing. Reports now show that one in six couples have problems conceiving and the use of assisted reproductive technologies is increasing by 5%-10% per year. Many theories exist to explain these trends, chief among them the rise in average maternal age and the increasing incidence of obesity, as well as various other male- and female-specific factors.
But interestingly, recent data suggest that the most male of all male-specific factors – total sperm count – may be specifically to blame.
According to a recent meta-analysis, the average total sperm count in men declined by 59.3% between 1973 and 2011. While these data certainly have limitations – including the exclusion of non-English publications, the reliance on total sperm count and not sperm motility, and the potential bias of those patients willing to give a semen sample – the overall trend nevertheless seems to be clearly downward. What’s more concerning, if you believe the data presented, is that there does not appear to be a leveling off of the downward curve in total sperm count.
Think about that last statement. At the current rate of decline, the average sperm count will be zero in 2045. One of the lead authors on the meta-analysis, Hagai Levine, MD, MPH, goes so far as to state, “We should hope for the best and prepare for the worst.”
As a matter of personal philosophy, I’m not a huge fan of end-of-the-world predictions because they tend not to come true (think Montanism back in the 2nd century; the 2012 Mayan calendar scare; or my personal favorite, the Prophet Hen of Leeds). On the other hand, the overall trend of decreased total sperm count in the English-speaking world seems to be true and it raises the interesting question of why.
According to the Mayo Clinic, causes of decreased sperm count include everything from anatomical factors (like varicoceles and ejaculatory issues) and lifestyle issues (such as recreational drugs, weight gain, and emotional stress) to environmental exposures (heavy metal or radiation). The senior author of the aforementioned meta-analysis, Shanna Swan, PhD, has championed another theory: the widespread exposure to endocrine-disrupting chemicals in everyday plastics.
It turns out that at least two chemicals used in the plastics industry, bisphenol A and phthalates, can mimic the effect of estrogen when ingested into the body. Even low levels of these chemicals in our bodies can lead to health problems.
Consider for a moment the presence of plastics in your life: the plastic wrappings on your food, plastic containers for shampoos and beauty products, and even the coatings of our oral supplements. A study by the Centers for Disease Control and Prevention looked at the urine of people participating in the National Health and Nutrition Examination Survey and found detectable concentrations of both of these chemicals in nearly all participants.
In 2045, I intend to be retired. But in the meantime, I think we all need to be aware of the potential impact that various endocrine-disrupting chemicals could be having on humanity. We need more research. If indeed the connection between endocrine disruptors and decreased sperm count is borne out, changes in our environmental exposure to these chemicals need to be made.
Henry Rosevear, MD, is a private-practice urologist based in Colorado Springs. He comes from a long line of doctors, but before entering medicine he served in the U.S. Navy as an officer aboard the USS Pittsburgh, a fast-attack submarine based out of New London, Conn. During his time in the Navy, he served in two deployments to the Persian Gulf, including combat experience as part of Operation Iraqi Freedom. Dr. Rosevear disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.