User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA fully approves Pfizer COVID-19 vaccine
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
To be, or not to be? More counseling needed for gender dysphoria
Clinicians should not blindly accept a person’s self-diagnosis as transgender and desire to medically transition without closer inspection; rather, they should make a distinction between ‘acceptance’ and conducting an in-depth, respectful, and collaborative exploration of an individual’s claims about what they believe will best promote their well-being.
These are the conclusions of two experts in ethics and clinical psychology in an extended essay published in the Journal of Medical Ethics.
“It’s not about making life harder for people who wish to transition but about improving care for all people who identify as transgender,” lead author Alessandra Lemma, DClin Psych, visiting professor in the psychoanalysis unit at University College London, said in an interview.
She stressed that the argument is neither for nor against medical transitioning per se.
“It’s an invitation to think about how the medical and mental health care communities can best support anyone considering a transition, whether they eventually pursue that course of action or not. The provision of psychotherapy, irrespective of whether the individual medically transitions or not, makes for a better outcome either way,” said Dr. Lemma, who cares for adolescents as well as adults with gender dysphoria in her private practice in London.
Reflective space has been eroded in gender identity services for the young
There has been an exponential increase in the number of adolescents who identify as transgender in Western countries in recent years. This news organization has covered the debate in detail, which has intensified worldwide in the last 12 months, regarding how best to treat youth with gender dysphoria.
This has “raised concern about how the laudable aims of gender affirmative care may be ushering children and young people too quickly into medical transitioning,” generally defined as treatment with puberty blockers in minors followed by cross-sex hormones to transition to the opposite sex, “leading subsequently to a wish to detransition with all the attendant physical and psychological complications,” wrote Dr. Lemma and her coauthor, Julian Savulescu, MD, professor of practical ethics, University of Oxford (England).
While the United Kingdom and other countries such as Finland have tightened regulations regarding the treatment of minors, “these medical interventions continue to be provided in many other countries,” they noted.
Such affirmative care has recently been interpreted by “influential sections of the transgender community” as forbidding “’questioning’ of any kind of the person’s stated gender and what will help them,” the essayists stated.
But Dr. Lemma noted that, for teenagers, this is typically a time to “try on” different identities and ways of presenting oneself to the world.
“All this requires a reflective space during the decision-making process, and this has been eroded in many gender identity services for young people especially, with a massive pressure on services.”
Family issues, trauma, and comorbid conditions can all influence people too, she noted, adding that what may be happening unconsciously may be driving the decision to modify the body.
“I cannot see that it would be harmful to anyone to have the opportunity to really think about what they are doing before making decisions about medical interventions,” she asserted.
Decision to transition must be judged to be autonomous
Dr. Lemma noted that even in those instances where medical transitioning, on balance, is the best option, it’s important to acknowledge that the process has a psychological impact.
“What matters is that in facing a major life-changing decision, an individual has the opportunity to understand the developmental and social experiences that drive their experience of gender dysphoria such that the decisions they make about medical transitioning can be said to be taken more autonomously,” she and Dr. Savulescu wrote.
And for those people who opt for full gender reassignment surgery, they are the first to say, “I don’t think I could have got through this as well as I have without psychological support,” Dr. Lemma remarked.
Ultimately, what’s important is to ensure the protection of those individuals whose needs will most likely not be met by medical transitioning, while not making it impossible for those who are suffering to get the care they need in order to transition, she concluded.
Until 2016, Dr. Lemma was professor of psychological therapies at the Tavistock and Portman NHS Foundation Trust and Essex University. During that time she worked with adult transgender individuals at the Portman clinic but not at the Gender Identity Service at the Tavistock clinic. Currently, she works in private practice with transgender individuals at the Queen Anne Street Practice, London. Dr. Savulescu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should not blindly accept a person’s self-diagnosis as transgender and desire to medically transition without closer inspection; rather, they should make a distinction between ‘acceptance’ and conducting an in-depth, respectful, and collaborative exploration of an individual’s claims about what they believe will best promote their well-being.
These are the conclusions of two experts in ethics and clinical psychology in an extended essay published in the Journal of Medical Ethics.
“It’s not about making life harder for people who wish to transition but about improving care for all people who identify as transgender,” lead author Alessandra Lemma, DClin Psych, visiting professor in the psychoanalysis unit at University College London, said in an interview.
She stressed that the argument is neither for nor against medical transitioning per se.
“It’s an invitation to think about how the medical and mental health care communities can best support anyone considering a transition, whether they eventually pursue that course of action or not. The provision of psychotherapy, irrespective of whether the individual medically transitions or not, makes for a better outcome either way,” said Dr. Lemma, who cares for adolescents as well as adults with gender dysphoria in her private practice in London.
Reflective space has been eroded in gender identity services for the young
There has been an exponential increase in the number of adolescents who identify as transgender in Western countries in recent years. This news organization has covered the debate in detail, which has intensified worldwide in the last 12 months, regarding how best to treat youth with gender dysphoria.
This has “raised concern about how the laudable aims of gender affirmative care may be ushering children and young people too quickly into medical transitioning,” generally defined as treatment with puberty blockers in minors followed by cross-sex hormones to transition to the opposite sex, “leading subsequently to a wish to detransition with all the attendant physical and psychological complications,” wrote Dr. Lemma and her coauthor, Julian Savulescu, MD, professor of practical ethics, University of Oxford (England).
While the United Kingdom and other countries such as Finland have tightened regulations regarding the treatment of minors, “these medical interventions continue to be provided in many other countries,” they noted.
Such affirmative care has recently been interpreted by “influential sections of the transgender community” as forbidding “’questioning’ of any kind of the person’s stated gender and what will help them,” the essayists stated.
But Dr. Lemma noted that, for teenagers, this is typically a time to “try on” different identities and ways of presenting oneself to the world.
“All this requires a reflective space during the decision-making process, and this has been eroded in many gender identity services for young people especially, with a massive pressure on services.”
Family issues, trauma, and comorbid conditions can all influence people too, she noted, adding that what may be happening unconsciously may be driving the decision to modify the body.
“I cannot see that it would be harmful to anyone to have the opportunity to really think about what they are doing before making decisions about medical interventions,” she asserted.
Decision to transition must be judged to be autonomous
Dr. Lemma noted that even in those instances where medical transitioning, on balance, is the best option, it’s important to acknowledge that the process has a psychological impact.
“What matters is that in facing a major life-changing decision, an individual has the opportunity to understand the developmental and social experiences that drive their experience of gender dysphoria such that the decisions they make about medical transitioning can be said to be taken more autonomously,” she and Dr. Savulescu wrote.
And for those people who opt for full gender reassignment surgery, they are the first to say, “I don’t think I could have got through this as well as I have without psychological support,” Dr. Lemma remarked.
Ultimately, what’s important is to ensure the protection of those individuals whose needs will most likely not be met by medical transitioning, while not making it impossible for those who are suffering to get the care they need in order to transition, she concluded.
Until 2016, Dr. Lemma was professor of psychological therapies at the Tavistock and Portman NHS Foundation Trust and Essex University. During that time she worked with adult transgender individuals at the Portman clinic but not at the Gender Identity Service at the Tavistock clinic. Currently, she works in private practice with transgender individuals at the Queen Anne Street Practice, London. Dr. Savulescu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should not blindly accept a person’s self-diagnosis as transgender and desire to medically transition without closer inspection; rather, they should make a distinction between ‘acceptance’ and conducting an in-depth, respectful, and collaborative exploration of an individual’s claims about what they believe will best promote their well-being.
These are the conclusions of two experts in ethics and clinical psychology in an extended essay published in the Journal of Medical Ethics.
“It’s not about making life harder for people who wish to transition but about improving care for all people who identify as transgender,” lead author Alessandra Lemma, DClin Psych, visiting professor in the psychoanalysis unit at University College London, said in an interview.
She stressed that the argument is neither for nor against medical transitioning per se.
“It’s an invitation to think about how the medical and mental health care communities can best support anyone considering a transition, whether they eventually pursue that course of action or not. The provision of psychotherapy, irrespective of whether the individual medically transitions or not, makes for a better outcome either way,” said Dr. Lemma, who cares for adolescents as well as adults with gender dysphoria in her private practice in London.
Reflective space has been eroded in gender identity services for the young
There has been an exponential increase in the number of adolescents who identify as transgender in Western countries in recent years. This news organization has covered the debate in detail, which has intensified worldwide in the last 12 months, regarding how best to treat youth with gender dysphoria.
This has “raised concern about how the laudable aims of gender affirmative care may be ushering children and young people too quickly into medical transitioning,” generally defined as treatment with puberty blockers in minors followed by cross-sex hormones to transition to the opposite sex, “leading subsequently to a wish to detransition with all the attendant physical and psychological complications,” wrote Dr. Lemma and her coauthor, Julian Savulescu, MD, professor of practical ethics, University of Oxford (England).
While the United Kingdom and other countries such as Finland have tightened regulations regarding the treatment of minors, “these medical interventions continue to be provided in many other countries,” they noted.
Such affirmative care has recently been interpreted by “influential sections of the transgender community” as forbidding “’questioning’ of any kind of the person’s stated gender and what will help them,” the essayists stated.
But Dr. Lemma noted that, for teenagers, this is typically a time to “try on” different identities and ways of presenting oneself to the world.
“All this requires a reflective space during the decision-making process, and this has been eroded in many gender identity services for young people especially, with a massive pressure on services.”
Family issues, trauma, and comorbid conditions can all influence people too, she noted, adding that what may be happening unconsciously may be driving the decision to modify the body.
“I cannot see that it would be harmful to anyone to have the opportunity to really think about what they are doing before making decisions about medical interventions,” she asserted.
Decision to transition must be judged to be autonomous
Dr. Lemma noted that even in those instances where medical transitioning, on balance, is the best option, it’s important to acknowledge that the process has a psychological impact.
“What matters is that in facing a major life-changing decision, an individual has the opportunity to understand the developmental and social experiences that drive their experience of gender dysphoria such that the decisions they make about medical transitioning can be said to be taken more autonomously,” she and Dr. Savulescu wrote.
And for those people who opt for full gender reassignment surgery, they are the first to say, “I don’t think I could have got through this as well as I have without psychological support,” Dr. Lemma remarked.
Ultimately, what’s important is to ensure the protection of those individuals whose needs will most likely not be met by medical transitioning, while not making it impossible for those who are suffering to get the care they need in order to transition, she concluded.
Until 2016, Dr. Lemma was professor of psychological therapies at the Tavistock and Portman NHS Foundation Trust and Essex University. During that time she worked with adult transgender individuals at the Portman clinic but not at the Gender Identity Service at the Tavistock clinic. Currently, she works in private practice with transgender individuals at the Queen Anne Street Practice, London. Dr. Savulescu has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shouldn’t docs who spread false COVID-19 info lose their licenses?
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
A tall, distinguished-looking physician in shirtsleeves and suspenders walked to the microphone at the Mt. Vernon, Ind., school board meeting on a Friday evening in early August. He launched into an impassioned, 7-minute attack on the public health establishment’s medical guidelines for COVID-19.
“The Center for Disease Control and the Indiana State [Department] of Health are giving you very bad scientific guidance,” said Daniel Stock, MD, a primary care physician with a concierge practice in Noblesville, Ind., He described himself as a “functional family medicine physician,” though he is not board certified in family medicine.
Dr. Stock told the school board members that COVID-19 vaccines are counterproductive because they make coronavirus infections worse. He claimed his treatment of “over 15” COVID-19 patients with vitamin D, ivermectin, and zinc has kept them out of the hospital, and that those treatments reduce mortality risk from the disease by 75%. (A study released in mid-August found that ivermectin is ineffective in treating COVID-19).
In response to Dr. Stock’s remarks, the state health department quickly issued a statement reaffirming that COVID-19 vaccines “are highly effective at preventing hospitalizations and deaths.” But by then, the YouTube video of Dr. Stock’s comments had garnered nearly 600,000 views as of Aug. 12 and had been shared over 10,000 times on Facebook. Opponents of COVID-19 vaccines and masking policies across the country have been citing his comments.
Across the country, state medical licensing boards and state and national medical associations are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians such as Dr. Stock. They fear such statements are increasing public confusion and are heightening political conflict. Physicians accused of spreading false information include public officials such as Scott Atlas, MD, who served as President Donald Trump’s COVID-19 advisor, and Kentucky Sen. Rand Paul, an ophthalmologist, whose YouTube account was temporarily suspended in August after he posted a video disputing the effectiveness of masking in stopping the spread of COVID-19.
“That’s the problem – those types of viral videos of someone somewhere who thinks they know something the rest of us don’t,” lamented Jennifer Bryan, MD, board chair of the Mississippi State Medical Association. “I don’t know any good reason why a physician should be advising against vaccination. It’s appropriate for medical boards to look into those situations.”
The Federation of State Medical Boards agrees. In July, it warned that physicians who willfully spread false information about COVID-19 risk suspension or revocation of their medical license. The federation cited a “dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians.” That’s particularly dangerous, it said, because physicians enjoy a high degree of public credibility.
Medical boards will particularly examine cases in which there is a pattern of misinformation or disinformation showing that a physician poses a continuing threat to public health, said Hank Chaudhry, DO, the federation’s CEO. In some cases, he said, boards have contacted physicians and have persuaded them to voluntarily refrain from making false public statements, without taking disciplinary action.
“Words matter,” he said. “Physicians have a really big platform, whether they realize it or not. Misinformation or disinformation in the context of COVID can not only cause harm but also death. We felt it was appropriate to remind physicians to be careful.”
Although medical leaders stress that most physicians are promoting solid science on COVID-19, the London-based Center for Countering Digital Hate, in a May report titled “The Disinformation Dozen,” named four U.S. physicians among 12 people who it said produce 65% of the misleading claims and lies about COVID-19 vaccines that abound on Facebook, Instagram, and Twitter. The leading spreader of false claims, the group said, is Joseph Mercola, MD, an Illinois-licensed osteopath living in Cape Coral, Fla. He did not respond to requests for comment.
But so far, state licensing boards and federal and state medical associations generally have been reluctant to discipline or publicly call out physicians who have spread misinformation about the causes, treatments, vaccines, and prevention strategies for COVID-19. Some of these physicians, such as Dr. Mercola, have a long history predating the COVID-19 pandemic of disseminating scientifically baseless information, often in connection with their marketing of products and services.
For instance, the Medical Licensing Board of Indiana and the state attorney general’s office, which brings medical disciplinary actions, declined to comment on Dr. Stock’s public statements at the August school board meeting. When asked about Dr. Stock, the Indiana State Medical Association, without mentioning his name, said: “We urge Hoosier physicians to share the proven facts [about public health measures recommended by the CDC and the Indiana Department of Health] with their patients and their communities.” Dr. Stock did not respond to a request for comment.
Experts say state medical boards are ill equipped and are often unwilling to address the challenge of disciplining physicians who disseminate dangerously false medical information. That enforcement gap is particularly troubling in the middle of a deadly pandemic such as this one.
“Unless you can show a harm to an individual patient, it’s pretty tough to get the boards to do much,” said Art Caplan, PhD, a professor of bioethics at New York University. “I wish they would, but they just don’t.”
That’s partly because state laws require the boards to engage in lengthy, confidential investigations and adversarial legal processes before imposing disciplinary actions. The laws generally require patients or members of the public to file a complaint before an investigation can start. Some states, however, do allow their medical boards to take rapid emergency action if a physician poses an immediate threat to patients or the public.
Another hurdle is that medical boards that seek to sanction physicians for making dangerously misleading public statements could face lawsuits alleging that such actions violate the physicians’ constitutional free speech rights or their professional autonomy.
“We have free speech, and you can get away with a lot of stuff,” said Stephen Barrett, MD, who for many years has critically documented examples of medical fraud on his website, Quackwatch. “Some doctors would sue if they were challenged by medical boards, and I’m not sure the boards would win that court fight. People have written books with advice that killed people, and I’m not aware of a single case where the author was disciplined.”
In addition, it’s not clear that U.S. physicians who are not government officials have any legal obligation – as opposed to a moral obligation – to the government or the public to promote public health, said Jonathan Moreno, PhD, a professor of medical ethics at University of Pennsylvania, Philadelphia. “Is transmitting misinformation about COVID-19 public health malpractice?” he asked. “Do we as a society see physicians having a special role as guides in an emergency? I’d like to think we do, but we don’t have a strong tradition like that in the U.S.”
But California State Sen. Richard Pan, MD, a pediatrician who represents the Sacramento area, doesn’t buy the arguments about why medical boards can’t discipline physicians for spreading misinformation. He successfully sponsored a 2019 bill that strengthens the medical board’s ability to discipline physicians who dole out medically unjustified vaccine exemptions to children.
“A medical license is a privilege. It’s an imprimatur from the state that the person is someone who upholds professional standards,” Dr. Pan said. “If someone is intentionally spreading disinformation for personal gain and that’s putting the public at risk, the medical board has a duty to act.”
There have been only a few publicly announced disciplinary actions related to COVID-19 misinformation so far.
Last December, the Oregon Medical Board, on an emergency basis, suspended the license of Steven LaTulippe, MD, of Dallas, Ore. He had publicly announced that he and his staff were not wearing masks in his clinic. In addition, he compared COVID-19 to the common cold and denied the governor’s legal authority to adopt public health protection measures. A recorded message on his office phone said he’s challenging the licensure action in court.
Last January, the Medical Board of California made Thomas Cowan, MD, of San Francisco surrender his license after Dr. Cowan posted a YouTube video, which went viral last year, that claimed that 5G Internet networks cause COVID-19. He did not respond to a request for comment.
In May, the College of Physicians and Surgeons of British Columbia reprimanded Stephen Malthouse, MD, and forbade him from speaking on issues related to COVID-19. He had written a widely circulated open letter to the province’s chief health office claiming that the pandemic was “over” and that measures to control the spread of COVID-19 were worse than the virus. He has challenged the disciplinary action in court, alleging it violates his right to free speech.
Attacking the problem from a different angle, the U.S. Federal Trade Commission has issued enforcement actions in cases in which physicians and other health care professionals engaged in deceptive business practices related to COVID-19. That approach may be applicable to a number of physicians accused of spreading COVID-19 misinformation, who allegedly have done so at least partly to sell unproven products and services to prevent or treat the disease.
In June, the FTC settled a case against Stephen Meis, MD, of Porterville, Calif. The settlement required that he stop making unsupported claims that his company’s dietary supplements effectively treat COVID-19 symptoms and that he pay $103,420 in refunds to defrauded customers.
State medical boards in the United States generally are not allowed to disclose investigations or disciplinary processes until they finalize a disciplinary action, so other investigations that have not been publicly disclosed may be pending.
A spokesperson for the Medical Board of California said the board is aware of questionable statements about COVID-19 made by several physicians and “will be looking into it.” That comment was in response to a question about statements made at a news conference last year by two Bakersfield emergency physicians, Artin Massihi, MD, and Dan Erickson, DO. They claimed that their COVID-19 testing data showed that the virus is not that dangerous. Dr. Erickson is an osteopath and is regulated by the Osteopathic Medical Board of California.
The two physicians’ news conference prompted an unusual joint statement from the American College of Emergency Medicine and the American Academy of Emergency Medicine in April 2020 declaring that they “emphatically condemn” Dr. Massihi’s and Dr. Erickson’s “reckless and untested musings.” The groups added that it appeared that the physicians issued the comments “to advance their personal financial interests without regard for the public’s health.”
Neither Dr. Massihi nor Dr. Erickson responded to a request for comment.
As for the physician dubbed by the Center for Countering Digital Hate as the world’s most influential spreader of COVID-19 misinformation on social media: No recent public complaints have been filed, and no disciplinary action has been taken against Dr. Mercola, according to a spokesman for the Illinois Department of Financial and Professional Regulation.
According to court records, Dr. Mercola faced disciplinary complaints from the Illinois board in the early 2000s for allegedly providing false and potentially harmful medical advice on his website. There is no record of any final disciplinary action taken against him.
In widely disseminated online posts, Dr. Mercola has called the COVID-19 pandemic a “scam” and said “forced vaccination” is part of a plan to re-set the global economic system. He called COVID-19 vaccines “a medical fraud,” claiming they “alter your genetic coding.” In February, the U.S. Food and Drug Administration ordered Dr. Mercola to stop saying on his website that various vitamins and dietary supplements he sells through his website are effective in preventing or treating COVID-19.
The New York Times reported in July that Dr. Mercola’s English-language Facebook page has more than 1.7 million followers, that his Spanish-language page has one million, and that he has 300,000 followers on Twitter and 400,000 on YouTube.
In August, Dr. Mercola announced that he was deleting the large archive of articles he’s written on his website but would continue to post articles every day that would be available on the site for only 48 hours. He explained his decision by saying he’s facing “blatant censorship” as part of a “McCarthyism-like attack” from “the sitting President of the United States.” He encouraged people to read his book, “The Truth about COVID-19.”
The lack of action against Dr. Mercola for his lengthy list of scientifically unfounded statements and marketing claims about COVID-19 and other medical conditions infuriates Quackwatch’s Dr. Barrett. He’s amazed that the Illinois board did not discipline Dr. Mercola despite a number of enforcement actions against him by the FTC and the FDA.
“If a doctor were to say to a patient, ‘Don’t wear a mask and don’t get vaccinated,’ the doctor would be held responsible for a bad outcome,” he said. “But if you say it to millions and as a direct result a dozen people die, shouldn’t the doctor also be held responsible for that misinformation? I think he should lose his license.”
Another of the four physicians cited in the “Disinformation Dozen” report is Sherri Tenpenny, DO, an osteopath licensed in Ohio, who has published posts on social media advocating against masking, testing, and vaccines to prevent COVID-19 infections. A spokesperson for the State Medical Board of Ohio said Dr. Tenpenny’s license expires on Oct. 1, 2021, and that any investigation would be confidential. She added that grounds for disciplinary action include “making a false, fraudulent, deceptive, or misleading statement in relation to the practice of medicine and surgery.” Dr. Tenpenny could not be reached for comment.
A third physician named in the report is Christiane Northrup, MD, an ob.gyn. formerly licensed in Maine, who has published posts advocating unproven cures for COVID-19 and claiming that vaccines increase chronic illness. Dennis Smith, executive director of the Maine Board of Licensure in Medicine, said the board received complaints about Dr. Northrup’s posts but can’t act because she withdrew her Maine license in 2015. He added that the Maine board can issue sanctions against physicians who engage in fraud, deceit, or misrepresentation or who post scientifically unfounded statements online.
The fourth physician identified in the “Disinformation Dozen” report is Rashid Buttar, DO, an osteopath practicing in Mooresville, N.C., who has claimed in social media posts that COVID-19 vaccines cause infertility and that COVID-19 tests contain living microorganisms. A spokeswoman for the North Carolina Medical Board said she could not confirm or deny the existence of any investigation of Dr. Buttar, who signed a consent order with the medical board in 2010 following charges of exorbitant fees, worthless tests and treatment, and false diagnoses. Undisclosed conditions were placed on his medical license in 2013. The spokesperson added that the board would investigate any information alleging that a physician spread false information about COVID-19.
Another physician who has caused widespread consternation over scientifically unfounded statements about COVID-19 is Simone Gold, MD, formerly an emergency department physician in Los Angeles. She founded a group called America’s Frontline Doctors, which filed a federal lawsuit in Alabama this spring to block the FDA from issuing an emergency use authorization allowing teenagers to receive COVID-19 vaccinations. She called the vaccines “an experimental biological agent whose harms are well-documented.”
Last summer, Dr. Gold and other physicians in her group held a news conference on the steps of the U.S. Supreme Court Building promoting hydroxychloroquine as a COVID-19 treatment. They declared that masks don’t work and that the virus isn’t deadly, and made other false claims. The news conference was livestreamed by conservative media outlets, was promoted on Twitter by then-President Trump and his family, and was viewed online more than 14 million times.
One of the participating physicians, Stella Immanuel, MD, of Houston, claimed in a video that went viral that she had successfully used hydroxychloroquine for more than 400 patients to cure the disease. In response, the Texas Medical Board, without naming Dr. Immanuel, warned that if it received a complaint about any physician who made a false claim about having a cure for COVID-19, it would investigate and potentially take disciplinary action.
Although no publicly known disciplinary action has been taken against Dr. Gold, she told The Washington Post last January that after participating in that July 2020 news conference, she was fired from her emergency department job at two hospitals and that she hasn’t worked as a physician since. Dr. Gold did not respond to a request for comment.
The outcome in her situation is consistent with the view of NYU’s Dr. Caplan that methods other than medical board discipline – such as action by employers, social media pressure, and reprimands from professional societies –will have to be used to hold physicians accountable for spreading COVID-19 misinformation.
“I’m disappointed to have to say it, but I don’t think medical boards are going to be effective,” he said. “We don’t know how to manage misinformation despite being in a plague. We just don’t.
A version of this article first appeared on Medscape.com.
How to pick the best face masks for children, according to the experts
One essential back-to-school item for children this fall is a face mask – the Centers for Disease Control and Prevention and the American Academy of Pediatrics both recommend them – but finding one that’s actually protective for a child is not a straightforward task, as many parents can attest.
There’s little in the way of official guidance or research to inform evidence-based recommendations on what type of face masks works best for children.
Search for children’s face masks on Amazon and you’ll run into a smorgasbord of options: masks with three, four, or five layers, different designs, and different materials. There’s one company selling a mask it calls an m95 model, a term the company devised.
It’s almost impossible to verify many of the claims being made by the manufacturers, or to know if they will fit your child’s face until you order some, which can get expensive.
But it’s worth looking for a good mask. A large study of more than 1 million people being conducted online by Facebook and Carnegie Mellon University found that students who wore face masks in school had a reduced risk for testing positive for the virus and getting sick with COVID-19 symptoms. The study was published in June in the journal Science.
Delta more contagious
The Delta variant of the new coronavirus is much more contagious than previous versions of the virus. Studies have shown that infected people carry 1,000 times more virus in their noses and throats than with the viruses that circulated last winter and spring. They shed more viral particles into the air when they talk or yell or sing, making this COVID-19–causing virus much more transmissible than in the past.
What that means, says Kimberly Prather, PhD, an aerosol scientist and distinguished professor at the Scripps Institution of Oceanography in La Jolla, California, is that if it once took about 15 minutes of proximity to an infected person to catch the infection, that window of risk is now much shorter.
“If you believe the 15-minute magical number, now if you take 1,000 times the viral load, basically in 1 second you could inhale that same amount of virus. So it’s gone from 15 minutes to 1 second,” Dr. Prather said in an online seminar on school safety she helped to organize.
A better mask
What that means is that we need to upgrade our face masks, switching away from ill-fitting fabric masks, which can offer varying degrees of protection depending on the number of layers and type of fabric that’s used, to more highly protective surgical masks or better yet, N95 respirators, which provide the highest level of filtration.
That’s harder to do for children, who have much smaller faces.
Any masks that gapes around the edges isn’t going to work well, no matter how well it filters.
“N95s are not made to fit kids. They do not come in kid sizes, so I do not recommend N95s for kids,” said Linsey Marr, an environmental engineer at Virginia Tech, who tests face masks in her lab.
Ms. Marr says parents need to consider the attributes masks in this order of priority:
Comfort: “If your kid won’t wear it, it’s not helping at all,” she said.
Fit: “Leaks around the sides are like having a hole in your mask and aerosols carrying the virus can get right through,” Ms. Marr said.
Filtration: How well the mask blocks small particles.
One option to improve fit is to layer a fabric mask over a surgical mask. The fabric mask helps to hold the edges of the surgical mask more tightly to a person’s face. The surgical mask creates better filtration.
Ms. Marr said KN94 or KN95 masks, which are being manufactured in China and Korea, are good choices. They offer nearly the same degree of filtration as an N95, and they fit closely to the face, to minimize leaks.
Check for counterfeits
The KN94 and KN95 masks for children are widely available, but Ms. Marr said parents do need to watch out for counterfeits, which don’t perform as well.
The National Institute for Occupational Safety and Health gives examples of counterfeit products here.
There’s also a type of cloth mask that has a built-in, edge-to-edge filter layer that is made for children.
“Some of these filter out more than 99% of particles and those can be very effective, if they fit well,” Ms. Marr said.
She has compiled and publicly posted a list of her recommendations for masks for children.
There’s also a new standard for face masks. It’s called ASTM F3502-21, and it’s published by an international organization that sets voluntary standards for thousands of products. To claim that a mask meets this standard, a manufacturer has to have its mask tested and demonstrate that it provides a certain level of filtration and breathability.
A version of this article first appeared on Medscape.com.
One essential back-to-school item for children this fall is a face mask – the Centers for Disease Control and Prevention and the American Academy of Pediatrics both recommend them – but finding one that’s actually protective for a child is not a straightforward task, as many parents can attest.
There’s little in the way of official guidance or research to inform evidence-based recommendations on what type of face masks works best for children.
Search for children’s face masks on Amazon and you’ll run into a smorgasbord of options: masks with three, four, or five layers, different designs, and different materials. There’s one company selling a mask it calls an m95 model, a term the company devised.
It’s almost impossible to verify many of the claims being made by the manufacturers, or to know if they will fit your child’s face until you order some, which can get expensive.
But it’s worth looking for a good mask. A large study of more than 1 million people being conducted online by Facebook and Carnegie Mellon University found that students who wore face masks in school had a reduced risk for testing positive for the virus and getting sick with COVID-19 symptoms. The study was published in June in the journal Science.
Delta more contagious
The Delta variant of the new coronavirus is much more contagious than previous versions of the virus. Studies have shown that infected people carry 1,000 times more virus in their noses and throats than with the viruses that circulated last winter and spring. They shed more viral particles into the air when they talk or yell or sing, making this COVID-19–causing virus much more transmissible than in the past.
What that means, says Kimberly Prather, PhD, an aerosol scientist and distinguished professor at the Scripps Institution of Oceanography in La Jolla, California, is that if it once took about 15 minutes of proximity to an infected person to catch the infection, that window of risk is now much shorter.
“If you believe the 15-minute magical number, now if you take 1,000 times the viral load, basically in 1 second you could inhale that same amount of virus. So it’s gone from 15 minutes to 1 second,” Dr. Prather said in an online seminar on school safety she helped to organize.
A better mask
What that means is that we need to upgrade our face masks, switching away from ill-fitting fabric masks, which can offer varying degrees of protection depending on the number of layers and type of fabric that’s used, to more highly protective surgical masks or better yet, N95 respirators, which provide the highest level of filtration.
That’s harder to do for children, who have much smaller faces.
Any masks that gapes around the edges isn’t going to work well, no matter how well it filters.
“N95s are not made to fit kids. They do not come in kid sizes, so I do not recommend N95s for kids,” said Linsey Marr, an environmental engineer at Virginia Tech, who tests face masks in her lab.
Ms. Marr says parents need to consider the attributes masks in this order of priority:
Comfort: “If your kid won’t wear it, it’s not helping at all,” she said.
Fit: “Leaks around the sides are like having a hole in your mask and aerosols carrying the virus can get right through,” Ms. Marr said.
Filtration: How well the mask blocks small particles.
One option to improve fit is to layer a fabric mask over a surgical mask. The fabric mask helps to hold the edges of the surgical mask more tightly to a person’s face. The surgical mask creates better filtration.
Ms. Marr said KN94 or KN95 masks, which are being manufactured in China and Korea, are good choices. They offer nearly the same degree of filtration as an N95, and they fit closely to the face, to minimize leaks.
Check for counterfeits
The KN94 and KN95 masks for children are widely available, but Ms. Marr said parents do need to watch out for counterfeits, which don’t perform as well.
The National Institute for Occupational Safety and Health gives examples of counterfeit products here.
There’s also a type of cloth mask that has a built-in, edge-to-edge filter layer that is made for children.
“Some of these filter out more than 99% of particles and those can be very effective, if they fit well,” Ms. Marr said.
She has compiled and publicly posted a list of her recommendations for masks for children.
There’s also a new standard for face masks. It’s called ASTM F3502-21, and it’s published by an international organization that sets voluntary standards for thousands of products. To claim that a mask meets this standard, a manufacturer has to have its mask tested and demonstrate that it provides a certain level of filtration and breathability.
A version of this article first appeared on Medscape.com.
One essential back-to-school item for children this fall is a face mask – the Centers for Disease Control and Prevention and the American Academy of Pediatrics both recommend them – but finding one that’s actually protective for a child is not a straightforward task, as many parents can attest.
There’s little in the way of official guidance or research to inform evidence-based recommendations on what type of face masks works best for children.
Search for children’s face masks on Amazon and you’ll run into a smorgasbord of options: masks with three, four, or five layers, different designs, and different materials. There’s one company selling a mask it calls an m95 model, a term the company devised.
It’s almost impossible to verify many of the claims being made by the manufacturers, or to know if they will fit your child’s face until you order some, which can get expensive.
But it’s worth looking for a good mask. A large study of more than 1 million people being conducted online by Facebook and Carnegie Mellon University found that students who wore face masks in school had a reduced risk for testing positive for the virus and getting sick with COVID-19 symptoms. The study was published in June in the journal Science.
Delta more contagious
The Delta variant of the new coronavirus is much more contagious than previous versions of the virus. Studies have shown that infected people carry 1,000 times more virus in their noses and throats than with the viruses that circulated last winter and spring. They shed more viral particles into the air when they talk or yell or sing, making this COVID-19–causing virus much more transmissible than in the past.
What that means, says Kimberly Prather, PhD, an aerosol scientist and distinguished professor at the Scripps Institution of Oceanography in La Jolla, California, is that if it once took about 15 minutes of proximity to an infected person to catch the infection, that window of risk is now much shorter.
“If you believe the 15-minute magical number, now if you take 1,000 times the viral load, basically in 1 second you could inhale that same amount of virus. So it’s gone from 15 minutes to 1 second,” Dr. Prather said in an online seminar on school safety she helped to organize.
A better mask
What that means is that we need to upgrade our face masks, switching away from ill-fitting fabric masks, which can offer varying degrees of protection depending on the number of layers and type of fabric that’s used, to more highly protective surgical masks or better yet, N95 respirators, which provide the highest level of filtration.
That’s harder to do for children, who have much smaller faces.
Any masks that gapes around the edges isn’t going to work well, no matter how well it filters.
“N95s are not made to fit kids. They do not come in kid sizes, so I do not recommend N95s for kids,” said Linsey Marr, an environmental engineer at Virginia Tech, who tests face masks in her lab.
Ms. Marr says parents need to consider the attributes masks in this order of priority:
Comfort: “If your kid won’t wear it, it’s not helping at all,” she said.
Fit: “Leaks around the sides are like having a hole in your mask and aerosols carrying the virus can get right through,” Ms. Marr said.
Filtration: How well the mask blocks small particles.
One option to improve fit is to layer a fabric mask over a surgical mask. The fabric mask helps to hold the edges of the surgical mask more tightly to a person’s face. The surgical mask creates better filtration.
Ms. Marr said KN94 or KN95 masks, which are being manufactured in China and Korea, are good choices. They offer nearly the same degree of filtration as an N95, and they fit closely to the face, to minimize leaks.
Check for counterfeits
The KN94 and KN95 masks for children are widely available, but Ms. Marr said parents do need to watch out for counterfeits, which don’t perform as well.
The National Institute for Occupational Safety and Health gives examples of counterfeit products here.
There’s also a type of cloth mask that has a built-in, edge-to-edge filter layer that is made for children.
“Some of these filter out more than 99% of particles and those can be very effective, if they fit well,” Ms. Marr said.
She has compiled and publicly posted a list of her recommendations for masks for children.
There’s also a new standard for face masks. It’s called ASTM F3502-21, and it’s published by an international organization that sets voluntary standards for thousands of products. To claim that a mask meets this standard, a manufacturer has to have its mask tested and demonstrate that it provides a certain level of filtration and breathability.
A version of this article first appeared on Medscape.com.
Mediterranean diet slows progression of atherosclerosis in CHD
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Empagliflozin gets HFrEF approval from FDA
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
Pandemic derails small success in lowering diabetes-related amputations
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.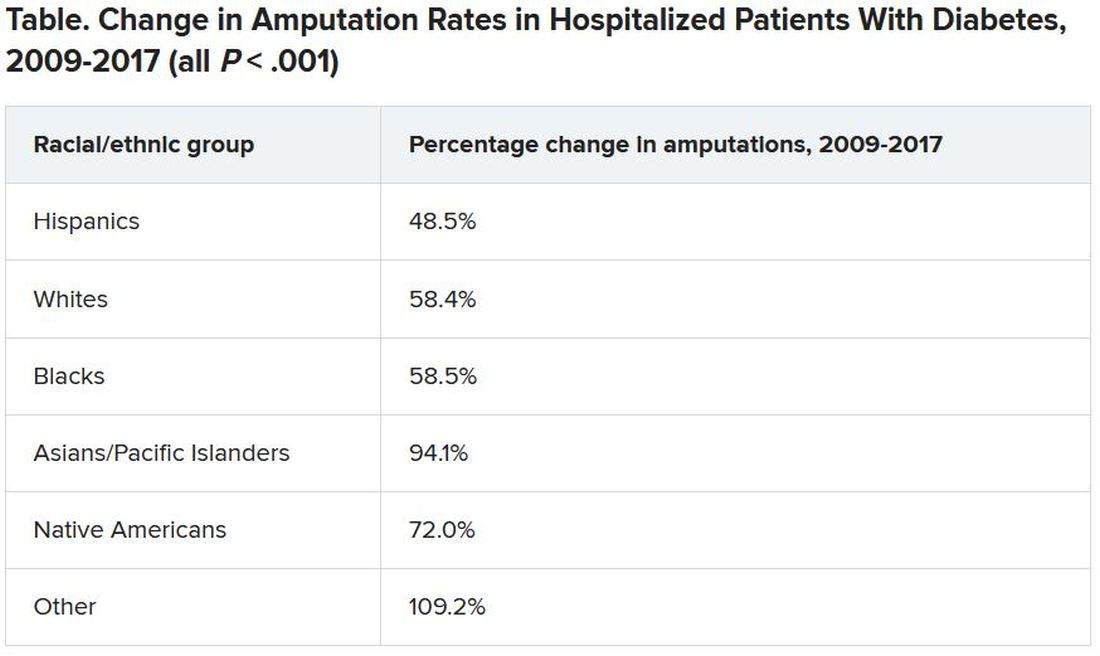
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vitamin D pills do not alter kidney function in prediabetes
However, most of these adults with prediabetes plus obesity or overweight also had sufficient serum levels of 25-hydroxyvitamin D (25[OH]D) and a low risk for adverse kidney outcomes at study entry.
“The benefits of vitamin D might be greater in people with low blood vitamin D levels and/or reduced kidney function,” lead author Sun H. Kim, MD, Stanford (Calif.) University, speculated in a statement from the American Society of Nephrology.
The study was published online August 6 in the Clinical Journal of the American Society of Nephrology.
“The D2d study is unique because we recruited individuals with high-risk prediabetes, having two out of three abnormal glucose values, and we recruited more than 2,000 participants, representing the largest vitamin D diabetes prevention trial to date,” Dr. Kim pointed out.
Although the study did not show a benefit of vitamin D supplements on kidney function outcomes, 43% of participants were already taking up to 1,000 IU of vitamin D daily when they entered the study, she noted.
A subgroup analysis of individuals who were not taking vitamin D at study entry found that vitamin D supplements were associated with lowered proteinuria, “which means that it could have a beneficial effect on kidney health,” said Dr. Kim, cautioning that “additional studies are needed to look into this further.”
Effect of vitamin D on three kidney function outcomes
Although low levels of serum 25(OH)D are associated with kidney disease, few trials have evaluated how vitamin D supplements might affect kidney function, Dr. Kim and colleagues write.
The D2d trial, they note, found that vitamin D supplements did not lower the risk of incident diabetes in people with prediabetes recruited from medical centers across the United States, as previously reported in 2019.
However, since then, meta-analyses that included the D2d trial have reported a significant 11%-12% reduction in diabetes risk in people with prediabetes who took vitamin D supplements.
The current secondary analysis of D2d aimed to investigate whether vitamin D supplements affect kidney function in people with prediabetes.
A total of 2,166 participants in D2d with complete kidney function data were included in the analysis.
The three study outcomes were change in estimated glomerular filtration rate (eGFR) from baseline, change in urine albumin-to-creatinine ratio (UACR) from baseline, and worsening Kidney Disease: Improving Global Outcomes (KDIGO) risk score (which takes eGFR and UACR into account).
At baseline, patients were a mean age of 60, had a mean body mass index (BMI) of 32 kg/m2, and 44% were women.
Most (79%) had hypertension, 52% were receiving antihypertensives, and 33% were receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Participants had a mean serum 25(OH) level of 28 ng/mL.
They had a mean eGFR of 87 mL/min/1.73 m2 and a mean UACR of 11 mg/g. Only 10% had a moderate, high, or very high KDIGO risk score.
Participants were randomized to receive a daily gel pill containing 4,000 IU vitamin D3 (cholecalciferol) or placebo.
Medication adherence was high (83%) in both groups during a median follow-up of 2.9 years.
There was no significant between-group difference in the following kidney function outcomes:
- 28 patients in the vitamin D group and 30 patients in the placebo group had a worsening KDIGO risk score.
- The mean difference in eGFR from baseline was -1.0 mL/min/1.73 m2 in the vitamin D group and -0.1 mL/min/1.73 m2 in the placebo group.
- The mean difference in UACR from baseline was 2.7 mg/g in the vitamin D group and 2.0 mg/g in the placebo group.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, most of these adults with prediabetes plus obesity or overweight also had sufficient serum levels of 25-hydroxyvitamin D (25[OH]D) and a low risk for adverse kidney outcomes at study entry.
“The benefits of vitamin D might be greater in people with low blood vitamin D levels and/or reduced kidney function,” lead author Sun H. Kim, MD, Stanford (Calif.) University, speculated in a statement from the American Society of Nephrology.
The study was published online August 6 in the Clinical Journal of the American Society of Nephrology.
“The D2d study is unique because we recruited individuals with high-risk prediabetes, having two out of three abnormal glucose values, and we recruited more than 2,000 participants, representing the largest vitamin D diabetes prevention trial to date,” Dr. Kim pointed out.
Although the study did not show a benefit of vitamin D supplements on kidney function outcomes, 43% of participants were already taking up to 1,000 IU of vitamin D daily when they entered the study, she noted.
A subgroup analysis of individuals who were not taking vitamin D at study entry found that vitamin D supplements were associated with lowered proteinuria, “which means that it could have a beneficial effect on kidney health,” said Dr. Kim, cautioning that “additional studies are needed to look into this further.”
Effect of vitamin D on three kidney function outcomes
Although low levels of serum 25(OH)D are associated with kidney disease, few trials have evaluated how vitamin D supplements might affect kidney function, Dr. Kim and colleagues write.
The D2d trial, they note, found that vitamin D supplements did not lower the risk of incident diabetes in people with prediabetes recruited from medical centers across the United States, as previously reported in 2019.
However, since then, meta-analyses that included the D2d trial have reported a significant 11%-12% reduction in diabetes risk in people with prediabetes who took vitamin D supplements.
The current secondary analysis of D2d aimed to investigate whether vitamin D supplements affect kidney function in people with prediabetes.
A total of 2,166 participants in D2d with complete kidney function data were included in the analysis.
The three study outcomes were change in estimated glomerular filtration rate (eGFR) from baseline, change in urine albumin-to-creatinine ratio (UACR) from baseline, and worsening Kidney Disease: Improving Global Outcomes (KDIGO) risk score (which takes eGFR and UACR into account).
At baseline, patients were a mean age of 60, had a mean body mass index (BMI) of 32 kg/m2, and 44% were women.
Most (79%) had hypertension, 52% were receiving antihypertensives, and 33% were receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Participants had a mean serum 25(OH) level of 28 ng/mL.
They had a mean eGFR of 87 mL/min/1.73 m2 and a mean UACR of 11 mg/g. Only 10% had a moderate, high, or very high KDIGO risk score.
Participants were randomized to receive a daily gel pill containing 4,000 IU vitamin D3 (cholecalciferol) or placebo.
Medication adherence was high (83%) in both groups during a median follow-up of 2.9 years.
There was no significant between-group difference in the following kidney function outcomes:
- 28 patients in the vitamin D group and 30 patients in the placebo group had a worsening KDIGO risk score.
- The mean difference in eGFR from baseline was -1.0 mL/min/1.73 m2 in the vitamin D group and -0.1 mL/min/1.73 m2 in the placebo group.
- The mean difference in UACR from baseline was 2.7 mg/g in the vitamin D group and 2.0 mg/g in the placebo group.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, most of these adults with prediabetes plus obesity or overweight also had sufficient serum levels of 25-hydroxyvitamin D (25[OH]D) and a low risk for adverse kidney outcomes at study entry.
“The benefits of vitamin D might be greater in people with low blood vitamin D levels and/or reduced kidney function,” lead author Sun H. Kim, MD, Stanford (Calif.) University, speculated in a statement from the American Society of Nephrology.
The study was published online August 6 in the Clinical Journal of the American Society of Nephrology.
“The D2d study is unique because we recruited individuals with high-risk prediabetes, having two out of three abnormal glucose values, and we recruited more than 2,000 participants, representing the largest vitamin D diabetes prevention trial to date,” Dr. Kim pointed out.
Although the study did not show a benefit of vitamin D supplements on kidney function outcomes, 43% of participants were already taking up to 1,000 IU of vitamin D daily when they entered the study, she noted.
A subgroup analysis of individuals who were not taking vitamin D at study entry found that vitamin D supplements were associated with lowered proteinuria, “which means that it could have a beneficial effect on kidney health,” said Dr. Kim, cautioning that “additional studies are needed to look into this further.”
Effect of vitamin D on three kidney function outcomes
Although low levels of serum 25(OH)D are associated with kidney disease, few trials have evaluated how vitamin D supplements might affect kidney function, Dr. Kim and colleagues write.
The D2d trial, they note, found that vitamin D supplements did not lower the risk of incident diabetes in people with prediabetes recruited from medical centers across the United States, as previously reported in 2019.
However, since then, meta-analyses that included the D2d trial have reported a significant 11%-12% reduction in diabetes risk in people with prediabetes who took vitamin D supplements.
The current secondary analysis of D2d aimed to investigate whether vitamin D supplements affect kidney function in people with prediabetes.
A total of 2,166 participants in D2d with complete kidney function data were included in the analysis.
The three study outcomes were change in estimated glomerular filtration rate (eGFR) from baseline, change in urine albumin-to-creatinine ratio (UACR) from baseline, and worsening Kidney Disease: Improving Global Outcomes (KDIGO) risk score (which takes eGFR and UACR into account).
At baseline, patients were a mean age of 60, had a mean body mass index (BMI) of 32 kg/m2, and 44% were women.
Most (79%) had hypertension, 52% were receiving antihypertensives, and 33% were receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Participants had a mean serum 25(OH) level of 28 ng/mL.
They had a mean eGFR of 87 mL/min/1.73 m2 and a mean UACR of 11 mg/g. Only 10% had a moderate, high, or very high KDIGO risk score.
Participants were randomized to receive a daily gel pill containing 4,000 IU vitamin D3 (cholecalciferol) or placebo.
Medication adherence was high (83%) in both groups during a median follow-up of 2.9 years.
There was no significant between-group difference in the following kidney function outcomes:
- 28 patients in the vitamin D group and 30 patients in the placebo group had a worsening KDIGO risk score.
- The mean difference in eGFR from baseline was -1.0 mL/min/1.73 m2 in the vitamin D group and -0.1 mL/min/1.73 m2 in the placebo group.
- The mean difference in UACR from baseline was 2.7 mg/g in the vitamin D group and 2.0 mg/g in the placebo group.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Why are boosters being given after 8 months? Experts weigh in
Following the White House administration’s announcement to start booster COVID-19 vaccinations for American adults in September, experts weighed in on the evidence for choosing an 8-month cutoff, how breakthrough infections figure in, and why calling one mRNA vaccine better than the other could be misleading.
Timing came up more than once at the Aug. 18 White House briefing announcing the booster plans. Reporters asked about the start time of Sept. 20 and people waiting at least 8 months after their second mRNA vaccine dose to get a booster.
Anthony Fauci, MD, chief medical adviser to the president and director of the National Institute of Allergy and Infectious Diseases, explained that late September gives the United States time to set up the logistics.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, added that 8 months is in part based on data from Israel and other countries on the waning of vaccine effectiveness over time.
“It is possible that 8 [months] is associated with the amount of time that we’ve been able to follow large groups of people, especially those who are 65 and older,” Julie Swann, PhD, said during a subsequent media briefing sponsored by Newswise on Aug. 18. “I know that Pfizer has said that they think a booster sometime between 6 and 12 months would be reasonable.”
Dr. Swann supported the administration’s booster shots plan. She said it is important “that we continue to get people the full amount of protection if it’s recommended by CDC and ACIP [Advisory Committee on Immunization Practices] that would come from a booster shot.” Dr. Swann is department head and A. Doug Allison Distinguished Professor at North Carolina State University and an adjunct professor in the joint department of biomedical engineering at the University of North Carolina at Chapel Hill.
Rising importance of breakthrough cases
Also on Aug. 18, news emerged that breakthrough cases are on the rise in seven U.S. states, likely because of the Delta variant.
These SARS-CoV-2 infections among the fully vaccinated account for 20% of cases in six of the seven states cited in a New York Times report, for example. Researchers also suggested that hospitalization and deaths associated with breakthrough cases could be higher than previously appreciated.
“It is expected that over time we will see more cases of Delta variant infections among vaccinated people. This points toward the need for booster vaccines and/or eventual modifications to the vaccine to capture new variants in the future,” Juan Wisnivesky, MD, DrPH, chief of the division of general internal medicine at Mount Sinai Health System in New York City, said during the briefing.
Vaccine comparisons unfair?
Following the release of a Mayo Clinic study reporting lower effectiveness of the Pfizer mRNA vaccine at 42% versus 76% for the Moderna product, some people started asking if one vaccine was better than the other.
“To begin with, the vaccines are not being compared side-by-side,” Dr. Wisnivesky said. “So we only know the effectiveness of each vaccine versus placebo, but we don’t know one versus the other.”
He added that different study designs, different populations, and other factors make direct comparisons difficult.
More evidence will be needed, Dr. Wisnivesky said, before public health officials can recommend that someone who received one mRNA vaccine switch to another for their booster shot.
Layering protections
Continuing to recommend masks is essential, Dr. Swann added. “With this Delta variant, it does appear that the possibility of reinfection or of a disease case breaking through vaccination can occur. So that makes it even more important to consider using nonpharmaceutical interventions while we continue to vaccinate people.”
Wearing or not wearing a mask is one of the behaviors that drive the transmission of disease, Dr. Swann said.
“What we saw across the board is that many people really wanted to go back to normal as much as they could. And we went back to normal a little bit too soon, especially given this new version of the virus that was circulating,” she said.
In poll, most favor boosters
A recent poll conducted by Medscape indicates that a majority of vaccinated physicians and nurses are ready and willing to take a COVID-19 booster vaccine. For example, 93% of 943 doctors and 87% of 1,680 nurses who responded want booster shots, either immediately or when they are authorized and recommended.
Among 510 WebMD readers responding to a similar poll, 82% indicated they wanted a booster shot.
A challenging task lies ahead
According to CDC data, as of Aug. 18, 2021, almost 169 million Americans are fully vaccinated, including the one-shot Johnson & Johnson adenovirus vaccine.
“I think it will be a challenge to get everyone who is fully vaccinated to come in for that booster,” Dr. Swann said.
Logistically speaking, Dr. Swann explained that many sites that were open for initial vaccinations, including drive-up locations and 24/7 vaccination sites, are no longer operating.
“We might see that rollout look a little bit differently. You might be able to go to your pharmacy or go to your primary care physician,” she said.
“But we may not see as many weekend events so it is going to be easier to get some people a booster than others.
“One interesting thing will also be whether a booster is effective in actually preventing you from giving a disease to someone else,” Dr. Swann said. “That could make a difference as well, because that might play into whether companies, hospitals, universities, or others require a booster.”
A version of this article first appeared on Medscape.com.
Following the White House administration’s announcement to start booster COVID-19 vaccinations for American adults in September, experts weighed in on the evidence for choosing an 8-month cutoff, how breakthrough infections figure in, and why calling one mRNA vaccine better than the other could be misleading.
Timing came up more than once at the Aug. 18 White House briefing announcing the booster plans. Reporters asked about the start time of Sept. 20 and people waiting at least 8 months after their second mRNA vaccine dose to get a booster.
Anthony Fauci, MD, chief medical adviser to the president and director of the National Institute of Allergy and Infectious Diseases, explained that late September gives the United States time to set up the logistics.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, added that 8 months is in part based on data from Israel and other countries on the waning of vaccine effectiveness over time.
“It is possible that 8 [months] is associated with the amount of time that we’ve been able to follow large groups of people, especially those who are 65 and older,” Julie Swann, PhD, said during a subsequent media briefing sponsored by Newswise on Aug. 18. “I know that Pfizer has said that they think a booster sometime between 6 and 12 months would be reasonable.”
Dr. Swann supported the administration’s booster shots plan. She said it is important “that we continue to get people the full amount of protection if it’s recommended by CDC and ACIP [Advisory Committee on Immunization Practices] that would come from a booster shot.” Dr. Swann is department head and A. Doug Allison Distinguished Professor at North Carolina State University and an adjunct professor in the joint department of biomedical engineering at the University of North Carolina at Chapel Hill.
Rising importance of breakthrough cases
Also on Aug. 18, news emerged that breakthrough cases are on the rise in seven U.S. states, likely because of the Delta variant.
These SARS-CoV-2 infections among the fully vaccinated account for 20% of cases in six of the seven states cited in a New York Times report, for example. Researchers also suggested that hospitalization and deaths associated with breakthrough cases could be higher than previously appreciated.
“It is expected that over time we will see more cases of Delta variant infections among vaccinated people. This points toward the need for booster vaccines and/or eventual modifications to the vaccine to capture new variants in the future,” Juan Wisnivesky, MD, DrPH, chief of the division of general internal medicine at Mount Sinai Health System in New York City, said during the briefing.
Vaccine comparisons unfair?
Following the release of a Mayo Clinic study reporting lower effectiveness of the Pfizer mRNA vaccine at 42% versus 76% for the Moderna product, some people started asking if one vaccine was better than the other.
“To begin with, the vaccines are not being compared side-by-side,” Dr. Wisnivesky said. “So we only know the effectiveness of each vaccine versus placebo, but we don’t know one versus the other.”
He added that different study designs, different populations, and other factors make direct comparisons difficult.
More evidence will be needed, Dr. Wisnivesky said, before public health officials can recommend that someone who received one mRNA vaccine switch to another for their booster shot.
Layering protections
Continuing to recommend masks is essential, Dr. Swann added. “With this Delta variant, it does appear that the possibility of reinfection or of a disease case breaking through vaccination can occur. So that makes it even more important to consider using nonpharmaceutical interventions while we continue to vaccinate people.”
Wearing or not wearing a mask is one of the behaviors that drive the transmission of disease, Dr. Swann said.
“What we saw across the board is that many people really wanted to go back to normal as much as they could. And we went back to normal a little bit too soon, especially given this new version of the virus that was circulating,” she said.
In poll, most favor boosters
A recent poll conducted by Medscape indicates that a majority of vaccinated physicians and nurses are ready and willing to take a COVID-19 booster vaccine. For example, 93% of 943 doctors and 87% of 1,680 nurses who responded want booster shots, either immediately or when they are authorized and recommended.
Among 510 WebMD readers responding to a similar poll, 82% indicated they wanted a booster shot.
A challenging task lies ahead
According to CDC data, as of Aug. 18, 2021, almost 169 million Americans are fully vaccinated, including the one-shot Johnson & Johnson adenovirus vaccine.
“I think it will be a challenge to get everyone who is fully vaccinated to come in for that booster,” Dr. Swann said.
Logistically speaking, Dr. Swann explained that many sites that were open for initial vaccinations, including drive-up locations and 24/7 vaccination sites, are no longer operating.
“We might see that rollout look a little bit differently. You might be able to go to your pharmacy or go to your primary care physician,” she said.
“But we may not see as many weekend events so it is going to be easier to get some people a booster than others.
“One interesting thing will also be whether a booster is effective in actually preventing you from giving a disease to someone else,” Dr. Swann said. “That could make a difference as well, because that might play into whether companies, hospitals, universities, or others require a booster.”
A version of this article first appeared on Medscape.com.
Following the White House administration’s announcement to start booster COVID-19 vaccinations for American adults in September, experts weighed in on the evidence for choosing an 8-month cutoff, how breakthrough infections figure in, and why calling one mRNA vaccine better than the other could be misleading.
Timing came up more than once at the Aug. 18 White House briefing announcing the booster plans. Reporters asked about the start time of Sept. 20 and people waiting at least 8 months after their second mRNA vaccine dose to get a booster.
Anthony Fauci, MD, chief medical adviser to the president and director of the National Institute of Allergy and Infectious Diseases, explained that late September gives the United States time to set up the logistics.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, added that 8 months is in part based on data from Israel and other countries on the waning of vaccine effectiveness over time.
“It is possible that 8 [months] is associated with the amount of time that we’ve been able to follow large groups of people, especially those who are 65 and older,” Julie Swann, PhD, said during a subsequent media briefing sponsored by Newswise on Aug. 18. “I know that Pfizer has said that they think a booster sometime between 6 and 12 months would be reasonable.”
Dr. Swann supported the administration’s booster shots plan. She said it is important “that we continue to get people the full amount of protection if it’s recommended by CDC and ACIP [Advisory Committee on Immunization Practices] that would come from a booster shot.” Dr. Swann is department head and A. Doug Allison Distinguished Professor at North Carolina State University and an adjunct professor in the joint department of biomedical engineering at the University of North Carolina at Chapel Hill.
Rising importance of breakthrough cases
Also on Aug. 18, news emerged that breakthrough cases are on the rise in seven U.S. states, likely because of the Delta variant.
These SARS-CoV-2 infections among the fully vaccinated account for 20% of cases in six of the seven states cited in a New York Times report, for example. Researchers also suggested that hospitalization and deaths associated with breakthrough cases could be higher than previously appreciated.
“It is expected that over time we will see more cases of Delta variant infections among vaccinated people. This points toward the need for booster vaccines and/or eventual modifications to the vaccine to capture new variants in the future,” Juan Wisnivesky, MD, DrPH, chief of the division of general internal medicine at Mount Sinai Health System in New York City, said during the briefing.
Vaccine comparisons unfair?
Following the release of a Mayo Clinic study reporting lower effectiveness of the Pfizer mRNA vaccine at 42% versus 76% for the Moderna product, some people started asking if one vaccine was better than the other.
“To begin with, the vaccines are not being compared side-by-side,” Dr. Wisnivesky said. “So we only know the effectiveness of each vaccine versus placebo, but we don’t know one versus the other.”
He added that different study designs, different populations, and other factors make direct comparisons difficult.
More evidence will be needed, Dr. Wisnivesky said, before public health officials can recommend that someone who received one mRNA vaccine switch to another for their booster shot.
Layering protections
Continuing to recommend masks is essential, Dr. Swann added. “With this Delta variant, it does appear that the possibility of reinfection or of a disease case breaking through vaccination can occur. So that makes it even more important to consider using nonpharmaceutical interventions while we continue to vaccinate people.”
Wearing or not wearing a mask is one of the behaviors that drive the transmission of disease, Dr. Swann said.
“What we saw across the board is that many people really wanted to go back to normal as much as they could. And we went back to normal a little bit too soon, especially given this new version of the virus that was circulating,” she said.
In poll, most favor boosters
A recent poll conducted by Medscape indicates that a majority of vaccinated physicians and nurses are ready and willing to take a COVID-19 booster vaccine. For example, 93% of 943 doctors and 87% of 1,680 nurses who responded want booster shots, either immediately or when they are authorized and recommended.
Among 510 WebMD readers responding to a similar poll, 82% indicated they wanted a booster shot.
A challenging task lies ahead
According to CDC data, as of Aug. 18, 2021, almost 169 million Americans are fully vaccinated, including the one-shot Johnson & Johnson adenovirus vaccine.
“I think it will be a challenge to get everyone who is fully vaccinated to come in for that booster,” Dr. Swann said.
Logistically speaking, Dr. Swann explained that many sites that were open for initial vaccinations, including drive-up locations and 24/7 vaccination sites, are no longer operating.
“We might see that rollout look a little bit differently. You might be able to go to your pharmacy or go to your primary care physician,” she said.
“But we may not see as many weekend events so it is going to be easier to get some people a booster than others.
“One interesting thing will also be whether a booster is effective in actually preventing you from giving a disease to someone else,” Dr. Swann said. “That could make a difference as well, because that might play into whether companies, hospitals, universities, or others require a booster.”
A version of this article first appeared on Medscape.com.
Medicinal liquor and edited mosquitoes
Drink to your health?
Whether you drink or not, most of us can agree that liquor is not the first thing that comes to mind when looking to make health improvements. But researchers have found a small exception in something traditional.
We’ve added buckwheat to pancakes, bread, and other baked goodies we made during the height of quarantine, but it’s also used to create a traditional liquor in some East Asian countries, where it is used medicinally.
Investigators have found that extracts in the Tartary buckwheat used to make the liquor induce autophagy, a process cells go through to remove proteins that are damaged or not needed anymore – sort of like a cellular spring cleaning.
To test this, the researchers treated liver and skin cells with Tartary buckwheat extract and looked to see how the cells responded with fluorescent markers. The results were clear.
“Treating cells with the extract stimulated the formation of autophagosomes, specialized cellular structures that carry out autophagy, and altered the location of proteins involved in regulating autophagy,” said senior author Takeshi Noda of Osaka (Japan) University.
Looking deeper, the researchers found that quercetin, a component of the buckwheat extract, had the same autophagic effect. And both the buckwheat and the quercetin gave the green light for liver cells to induce aggrephagy, the process of cleaning up protein aggregates.
Those protein aggregates in liver cells are closely linked to alcoholic liver disease, suggesting that quercetin could be a game changer in its treatment. In other words, liquor could help fix the problem that liquor started. Go figure.
From hospital bills to X-rated
Ralph Puglisi was an accounting manager for the University Medical Service Association (UMSA), a nonprofit that supports the massive University of South Florida health system. The association took in over $300 million in revenue in the 2019-2020 fiscal year, which is a rather large sum of money, but we’ll glide over the ethics of a “nonprofit” making a few hundred million for now.
Mr. Puglisi was in very close proximity to the money, generated from patient care, and he pled guilty to stealing it using UMSA credit cards. Now, that wouldn’t be LOTME worthy on its own, but what elevates this above garden-variety embezzlement is how the intrepid Mr. Puglisi chose to spend the millions he stole from the university health system: Adult entertainment.
And before you ask, he didn’t spend $11.5 million on something most people so inclined can find for free with judicious Google searches. What Mr. Puglisi actually did was invest in a website providing adult content through individual user profiles, one of which is believed to belong to his stepson’s fiancée, which brings a whole new level of sleaze to this enterprise. Over the course of 2 years, he visited her profile 2,800 times, an amount some might view as excessive.
While the vast majority of the embezzled money went to the adult website, Mr. Puglisi also used thousands of UMSA dollars to pay for travel, household improvements, rent, the works. Almost $44,000 was spent at a resort sometimes known as the happiest place on earth.
Then there’s Mr. Puglisi’s wife. Oh yes, this guy is married. He poured over $600,000 into a company he and his wife owned, which is a lot, but how much do you think went to the woman he married? Probably quite a bit. Go ahead, try to think of a number. It’s not like it was his money.
Did you guess $100 went into his wife’s PayPal account? No? Clearly you don’t understand the criminal mind. His stepson’s fiancée got millions, and his wife got a hundred. Now there are some priorities.
Step 1: Sit at desk. Step 2: Get in shape
Being a physician is not really a desk job, but doctors must spend a fair share of their time sitting, yes? Dealing with recalcitrant EHRs or talking on the phone to insurers or PBMs? If you are one of these physicians, or if you have patients who spend a lot of time sitting at their desks and might need to get a bit of exercise, then we’ve got a multitasking tip for you.
It came to us via one of our favorite websites, Sad and Useless. It’s the site that declares itself “the most depressive humor site on the Internet” and they’re offering up the “12 Best Exercises To Do At Your Desk.” It may not sound like much, but we think that the gang at Dunder-Mifflin would approve. And besides, who couldn’t stand to burn a few calories without having to leave the chair?
We won’t spoil your fun by going through all 12 – each one comes with step-by-step instructions and a helpful illustration or GIF – but here are just a few:
- Bending over backwards: “Agree to do something you don’t want to do. Spend twice as long as expected doing that thing. Hate yourself.”
- Fake laughter: “Hear a joke that isn’t even remotely funny. Open your mouth and make laughing sounds.”
- Bang your head: Feel the “pointlessness of your job overwhelm you” and then “bring your head forcefully down to your desk.”
Now, we here at LOTME are, of course [Bang!], highly skilled, professional wordsmithing humorists [Bang!], so when we tell you that this is a great workout [Bang!] … that this is a great workout [Bang!] … it’s great … uggh.
Wooooo. Feel the burn.
One order of mosquitoes, extra Crispr
What would it be like to have a barbecue in your backyard on a humid summer night and not get eaten alive by mosquitoes? If you’re like us, you probably thought you’d never see that day.
Mosquitoes cause itchy bites, but, more importantly, they can carry dengue, malaria, yellow fever, and Zika virus. New research shows that protection from these diseases may be possible with use of the Crispr-Cas9 gene-editing tool, which could make humans invisible to mosquitoes by taking away their light-sensing abilities and, thus, their ability to find us.
“The better we understand how they sense the human, the better we can control the mosquito in an eco-friendly manner,” Yinpeng Zhan, a postdoctoral researcher at the University of California, Santa Barbara, and the study’s lead author, told the New York Times.
After studying the mosquitoes and figuring out their hunting patterns, the researchers found that mosquitoes are attracted to dark spots more than white spots and used this to their advantage. After knocking out two of the proteins that mosquitoes need for vision – via Crispr – the little suckers could not distinguish the difference between the white and dark spots.
We’re sure mosquitoes don’t mean any harm – they’re just trying to survive and reproduce like any other species – but thanks to this new tool, gone might be the days of having to douse yourself in bug spray that smells like a mix of chemicals and melon.
Drink to your health?
Whether you drink or not, most of us can agree that liquor is not the first thing that comes to mind when looking to make health improvements. But researchers have found a small exception in something traditional.
We’ve added buckwheat to pancakes, bread, and other baked goodies we made during the height of quarantine, but it’s also used to create a traditional liquor in some East Asian countries, where it is used medicinally.
Investigators have found that extracts in the Tartary buckwheat used to make the liquor induce autophagy, a process cells go through to remove proteins that are damaged or not needed anymore – sort of like a cellular spring cleaning.
To test this, the researchers treated liver and skin cells with Tartary buckwheat extract and looked to see how the cells responded with fluorescent markers. The results were clear.
“Treating cells with the extract stimulated the formation of autophagosomes, specialized cellular structures that carry out autophagy, and altered the location of proteins involved in regulating autophagy,” said senior author Takeshi Noda of Osaka (Japan) University.
Looking deeper, the researchers found that quercetin, a component of the buckwheat extract, had the same autophagic effect. And both the buckwheat and the quercetin gave the green light for liver cells to induce aggrephagy, the process of cleaning up protein aggregates.
Those protein aggregates in liver cells are closely linked to alcoholic liver disease, suggesting that quercetin could be a game changer in its treatment. In other words, liquor could help fix the problem that liquor started. Go figure.
From hospital bills to X-rated
Ralph Puglisi was an accounting manager for the University Medical Service Association (UMSA), a nonprofit that supports the massive University of South Florida health system. The association took in over $300 million in revenue in the 2019-2020 fiscal year, which is a rather large sum of money, but we’ll glide over the ethics of a “nonprofit” making a few hundred million for now.
Mr. Puglisi was in very close proximity to the money, generated from patient care, and he pled guilty to stealing it using UMSA credit cards. Now, that wouldn’t be LOTME worthy on its own, but what elevates this above garden-variety embezzlement is how the intrepid Mr. Puglisi chose to spend the millions he stole from the university health system: Adult entertainment.
And before you ask, he didn’t spend $11.5 million on something most people so inclined can find for free with judicious Google searches. What Mr. Puglisi actually did was invest in a website providing adult content through individual user profiles, one of which is believed to belong to his stepson’s fiancée, which brings a whole new level of sleaze to this enterprise. Over the course of 2 years, he visited her profile 2,800 times, an amount some might view as excessive.
While the vast majority of the embezzled money went to the adult website, Mr. Puglisi also used thousands of UMSA dollars to pay for travel, household improvements, rent, the works. Almost $44,000 was spent at a resort sometimes known as the happiest place on earth.
Then there’s Mr. Puglisi’s wife. Oh yes, this guy is married. He poured over $600,000 into a company he and his wife owned, which is a lot, but how much do you think went to the woman he married? Probably quite a bit. Go ahead, try to think of a number. It’s not like it was his money.
Did you guess $100 went into his wife’s PayPal account? No? Clearly you don’t understand the criminal mind. His stepson’s fiancée got millions, and his wife got a hundred. Now there are some priorities.
Step 1: Sit at desk. Step 2: Get in shape
Being a physician is not really a desk job, but doctors must spend a fair share of their time sitting, yes? Dealing with recalcitrant EHRs or talking on the phone to insurers or PBMs? If you are one of these physicians, or if you have patients who spend a lot of time sitting at their desks and might need to get a bit of exercise, then we’ve got a multitasking tip for you.
It came to us via one of our favorite websites, Sad and Useless. It’s the site that declares itself “the most depressive humor site on the Internet” and they’re offering up the “12 Best Exercises To Do At Your Desk.” It may not sound like much, but we think that the gang at Dunder-Mifflin would approve. And besides, who couldn’t stand to burn a few calories without having to leave the chair?
We won’t spoil your fun by going through all 12 – each one comes with step-by-step instructions and a helpful illustration or GIF – but here are just a few:
- Bending over backwards: “Agree to do something you don’t want to do. Spend twice as long as expected doing that thing. Hate yourself.”
- Fake laughter: “Hear a joke that isn’t even remotely funny. Open your mouth and make laughing sounds.”
- Bang your head: Feel the “pointlessness of your job overwhelm you” and then “bring your head forcefully down to your desk.”
Now, we here at LOTME are, of course [Bang!], highly skilled, professional wordsmithing humorists [Bang!], so when we tell you that this is a great workout [Bang!] … that this is a great workout [Bang!] … it’s great … uggh.
Wooooo. Feel the burn.
One order of mosquitoes, extra Crispr
What would it be like to have a barbecue in your backyard on a humid summer night and not get eaten alive by mosquitoes? If you’re like us, you probably thought you’d never see that day.
Mosquitoes cause itchy bites, but, more importantly, they can carry dengue, malaria, yellow fever, and Zika virus. New research shows that protection from these diseases may be possible with use of the Crispr-Cas9 gene-editing tool, which could make humans invisible to mosquitoes by taking away their light-sensing abilities and, thus, their ability to find us.
“The better we understand how they sense the human, the better we can control the mosquito in an eco-friendly manner,” Yinpeng Zhan, a postdoctoral researcher at the University of California, Santa Barbara, and the study’s lead author, told the New York Times.
After studying the mosquitoes and figuring out their hunting patterns, the researchers found that mosquitoes are attracted to dark spots more than white spots and used this to their advantage. After knocking out two of the proteins that mosquitoes need for vision – via Crispr – the little suckers could not distinguish the difference between the white and dark spots.
We’re sure mosquitoes don’t mean any harm – they’re just trying to survive and reproduce like any other species – but thanks to this new tool, gone might be the days of having to douse yourself in bug spray that smells like a mix of chemicals and melon.
Drink to your health?
Whether you drink or not, most of us can agree that liquor is not the first thing that comes to mind when looking to make health improvements. But researchers have found a small exception in something traditional.
We’ve added buckwheat to pancakes, bread, and other baked goodies we made during the height of quarantine, but it’s also used to create a traditional liquor in some East Asian countries, where it is used medicinally.
Investigators have found that extracts in the Tartary buckwheat used to make the liquor induce autophagy, a process cells go through to remove proteins that are damaged or not needed anymore – sort of like a cellular spring cleaning.
To test this, the researchers treated liver and skin cells with Tartary buckwheat extract and looked to see how the cells responded with fluorescent markers. The results were clear.
“Treating cells with the extract stimulated the formation of autophagosomes, specialized cellular structures that carry out autophagy, and altered the location of proteins involved in regulating autophagy,” said senior author Takeshi Noda of Osaka (Japan) University.
Looking deeper, the researchers found that quercetin, a component of the buckwheat extract, had the same autophagic effect. And both the buckwheat and the quercetin gave the green light for liver cells to induce aggrephagy, the process of cleaning up protein aggregates.
Those protein aggregates in liver cells are closely linked to alcoholic liver disease, suggesting that quercetin could be a game changer in its treatment. In other words, liquor could help fix the problem that liquor started. Go figure.
From hospital bills to X-rated
Ralph Puglisi was an accounting manager for the University Medical Service Association (UMSA), a nonprofit that supports the massive University of South Florida health system. The association took in over $300 million in revenue in the 2019-2020 fiscal year, which is a rather large sum of money, but we’ll glide over the ethics of a “nonprofit” making a few hundred million for now.
Mr. Puglisi was in very close proximity to the money, generated from patient care, and he pled guilty to stealing it using UMSA credit cards. Now, that wouldn’t be LOTME worthy on its own, but what elevates this above garden-variety embezzlement is how the intrepid Mr. Puglisi chose to spend the millions he stole from the university health system: Adult entertainment.
And before you ask, he didn’t spend $11.5 million on something most people so inclined can find for free with judicious Google searches. What Mr. Puglisi actually did was invest in a website providing adult content through individual user profiles, one of which is believed to belong to his stepson’s fiancée, which brings a whole new level of sleaze to this enterprise. Over the course of 2 years, he visited her profile 2,800 times, an amount some might view as excessive.
While the vast majority of the embezzled money went to the adult website, Mr. Puglisi also used thousands of UMSA dollars to pay for travel, household improvements, rent, the works. Almost $44,000 was spent at a resort sometimes known as the happiest place on earth.
Then there’s Mr. Puglisi’s wife. Oh yes, this guy is married. He poured over $600,000 into a company he and his wife owned, which is a lot, but how much do you think went to the woman he married? Probably quite a bit. Go ahead, try to think of a number. It’s not like it was his money.
Did you guess $100 went into his wife’s PayPal account? No? Clearly you don’t understand the criminal mind. His stepson’s fiancée got millions, and his wife got a hundred. Now there are some priorities.
Step 1: Sit at desk. Step 2: Get in shape
Being a physician is not really a desk job, but doctors must spend a fair share of their time sitting, yes? Dealing with recalcitrant EHRs or talking on the phone to insurers or PBMs? If you are one of these physicians, or if you have patients who spend a lot of time sitting at their desks and might need to get a bit of exercise, then we’ve got a multitasking tip for you.
It came to us via one of our favorite websites, Sad and Useless. It’s the site that declares itself “the most depressive humor site on the Internet” and they’re offering up the “12 Best Exercises To Do At Your Desk.” It may not sound like much, but we think that the gang at Dunder-Mifflin would approve. And besides, who couldn’t stand to burn a few calories without having to leave the chair?
We won’t spoil your fun by going through all 12 – each one comes with step-by-step instructions and a helpful illustration or GIF – but here are just a few:
- Bending over backwards: “Agree to do something you don’t want to do. Spend twice as long as expected doing that thing. Hate yourself.”
- Fake laughter: “Hear a joke that isn’t even remotely funny. Open your mouth and make laughing sounds.”
- Bang your head: Feel the “pointlessness of your job overwhelm you” and then “bring your head forcefully down to your desk.”
Now, we here at LOTME are, of course [Bang!], highly skilled, professional wordsmithing humorists [Bang!], so when we tell you that this is a great workout [Bang!] … that this is a great workout [Bang!] … it’s great … uggh.
Wooooo. Feel the burn.
One order of mosquitoes, extra Crispr
What would it be like to have a barbecue in your backyard on a humid summer night and not get eaten alive by mosquitoes? If you’re like us, you probably thought you’d never see that day.
Mosquitoes cause itchy bites, but, more importantly, they can carry dengue, malaria, yellow fever, and Zika virus. New research shows that protection from these diseases may be possible with use of the Crispr-Cas9 gene-editing tool, which could make humans invisible to mosquitoes by taking away their light-sensing abilities and, thus, their ability to find us.
“The better we understand how they sense the human, the better we can control the mosquito in an eco-friendly manner,” Yinpeng Zhan, a postdoctoral researcher at the University of California, Santa Barbara, and the study’s lead author, told the New York Times.
After studying the mosquitoes and figuring out their hunting patterns, the researchers found that mosquitoes are attracted to dark spots more than white spots and used this to their advantage. After knocking out two of the proteins that mosquitoes need for vision – via Crispr – the little suckers could not distinguish the difference between the white and dark spots.
We’re sure mosquitoes don’t mean any harm – they’re just trying to survive and reproduce like any other species – but thanks to this new tool, gone might be the days of having to douse yourself in bug spray that smells like a mix of chemicals and melon.