User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
U.S. reports record-breaking 1.35 million new COVID cases in a day
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
The United States reported 1.35 million new COVID-19 cases on Jan. 10, logging the highest daily total for any country in the world during the pandemic.
The United States set the previous record of 1 million cases on Jan. 3. (A large number of cases are reported on Mondays, since many states don’t provide updates over the weekend, according to Reuters.)
Still, the 7-day average for new cases has surpassed 700,000, tripling in 2 weeks as the contagious Omicron variant continues to spread across the country.
The daily record of new cases came a day after the United States crossed the grim milestone of 60 million COVID-19 cases during the pandemic, according to the latest data from Johns Hopkins University. More than 11 million new cases were reported in the past 28 days, with 5 million reported since Jan. 2.
Globally, more than 310 million cases have been reported, resulting in nearly 5.5 million COVID-19 deaths. Almost 40 million cases have been confirmed worldwide during the past month, with the United States accounting for 28% of those.
Texas became the second state to report more than 5 million cases since the pandemic began, behind California’s total of 6 million cases. Florida has reported more than 4.6 million, while New York has reported more than 4.1 million.
The United States has also hit an all-time high for hospitalizations, with nearly 146,000 COVID-19 patients in hospitals across the country, according to the latest data from the U.S. Department of Health and Human Services. The previous record was 142,000 hospitalizations in January 2021.
Jan. 11’s hospitalizations are more than twice as many as 2 weeks ago, according to CNN. About 78% of inpatient beds are in use nationwide, and 21% are being used for COVID-19 patients.
Deaths are averaging about 1,700 per day, Reuters reported, which is up from 1,400 in recent days but not much higher than earlier this winter. The peak average was 3,400 daily deaths in mid-January 2021.
The surging numbers of cases and hospitalizations across the country are straining hospitals. On Jan. 10, Virginia Gov. Ralph Northam declared a state of emergency after the number of intensive care unit hospitalizations more than doubled since Dec. 1, CNN reported. The order allows hospitals to expand bed capacity, use telehealth options, and be more flexible with staffing.
Texas is hiring at least 2,700 medical staff to help with the surge, CNN reported, and Kentucky has mobilized the National Guard to provide support.
“Omicron continues to burn through the commonwealth, growing at levels we have never seen before. Omicron is significantly more contagious than even the Delta variant,” Kentucky Gov. Andy Beshear said during a news briefing Jan. 10.
Kentucky reported its highest weekly total of cases last week and has its highest rate of positive tests, at 26%. Mr. Beshear said the state is down to 134 available adult ICU beds.
“If it spreads at the rate we are seeing, it is certainly going to fill up our hospitals,” he said.
A version of this article first appeared on WebMD.com.
Health issues in women midlife linked with health decline at 65
Having specific health issues, including depressive symptoms and cardiovascular disease, as a middle-aged woman was associated with experiencing clinically important declines in health later in life, a new study finds.
The most predictive parameters of poorer health at age 65 were cardiovascular disease, clinically significant depressive symptoms, and current smoking. Osteoarthritis, lower education level, and higher body mass index (BMI) also were associated with poorer health status 10 years on, Daniel H. Solomon, MD, MPH and colleagues wrote in their observational study, which was published in JAMA Network Open.
Determining a patient’s score on a health-related quality of life measure based on these variables might be useful in clinical practice to recognize midlife patients at increased risk for later health deterioration, Dr. Solomon, of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital, Boston, said in a statement. This measure is called the Short Form 36 (SF-36), and the researchers specifically focused on the physical component summary score (PCS) of this measure. The SF-36 is similar to the Framingham 10-year coronary heart disease risk prediction score, according to Dr. Solomon, who is a professor of medicine at Harvard Medical School, also in Boston.
Based on their risk scores, women could preemptively target modifiable risk factors before they enter old age, the investigators wrote.
“Age 55-65 may be a critical decade. A person’s health and factors during this period may set them on a path for their later adult years,” Dr. Solomon said in a statement. “The good news is that a large proportion of women at midlife are very stable and will not go on to experience declines. But being able to identify women at higher risk could help lead to interventions targeted to them.”
Study details
The study included a cohort of 1,091 women drawn from the 3,302-participant Study of Women’s Health Across the Nation (SWAN), a racially and ethnically diverse group enrolled from six U.S. sites at or immediately before transition to menopause and followed for 10 years from age 55 to 65. The study sample, consisting of 24.6% Black, 24% Japanese or Chinese, and 51.9% White, had a median baseline age of 54.8 years and median BMI of 27 kg/m2 at entry. The median baseline PCS score was 53.1 (interquartile range, 46.8-56.7).
Over 10 years, 206 (18.9%) of the women in the study experienced clinically important declines of at least 8 points in baseline characteristics at around age 55. The following were significantly associated with these declines:
- Having a higher BMI.
- Having osteoarthritis.
- Having a lower educational level.
- Being a current smoker.
- Having clinically significant depressive symptoms.
- Having cardiovascular disease.
- Having better (or higher) physical health and function score on the PCS.
The association between a higher PCS score and a greater decline might seem like an anomaly, Dr. Solomon said in an interview, but one interpretation of this finding is that women with higher or better scores at baseline have further to fall once other risk factors take effect.
With data analyzed from October 2020 to March 2021, the median 10-year change in PCS was –1.02 points, but 206 women experienced declines of 8 points or more.
Those with health declines were more likely to be Black and less likely to be Japanese. They were also more likely to have other comorbidities such as diabetes, hypertension, and osteoporosis, and to report less physical activity.
Scoring system should not replace individualized evaluation, outside expert said
Commenting on the findings, Margaret J. Nachtigall, MD, a clinical associate professor in the department of obstetrics and gynecology at New York University Langone Health, cautioned that a generalized scoring system should not replace individualized evaluation of women at midlife.
“I assess women around age 55 on a daily basis for health risk factors going forward. And while a number such as BMI can be helpful, I worry that reliance on a score could miss treating the individual,” Dr. Nachtigall said an interview. For instance, one woman might have a high BMI owing to greater muscle mass, which is heavy, while another may have a lower BMI but more fat-related weight, as well as exacerbating conditions such as hypertension that would elevate her risk. “You have to make the calculation for each person.”
Dr. Nachtigall, who was not involved in the SWAN analysis, noted, however, that a big-data scoring system might be a useful adjunct to individual patient evaluation in that “it would make physicians look at all these many risk factors to identify those prone to decline.”
Study includes racially diverse population
According to the authors, while other studies have identified similar and other risk factors such as poor sleep, most have not included such a racially diverse population and have focused on women already in their senior years when the window of opportunity may already have closed.
“As a clinician and epidemiologist, I often think about the window of opportunity at midlife, when people are vital, engaged, and resilient,” said Dr. Solomon in the statement. “If we can identify risk factors and determine who is at risk, we may be able to find interventions that can stave off health declines and help put people on a better health trajectory.”
Eric M. Ascher, DO, who practices family medicine at Lenox Hill Hospital in New York and was not involved in the SWAN research, agreed with Dr. Solomon.
“Doctors who treat chronic conditions often meet patients when they are already suffering from a medical problem,” he said in an interview. “It is key to decrease your risk factors before it is too late.”
Dr. Ascher added that many primary care providers already rely heavily on scoring systems when determining level of risk and type of intervention. “Any additional risk factor-scoring systems that are easy to implement and will prevent chronic diseases would be something providers would want to use with their patients.”
Detailed analyses of larger at-risk populations are needed to validate these risk factors and identify others, the authors said.
SWAN is supported by the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Heath’s Office of Research on Women’s Health. Dr. Solomon reported financial ties to Amgen, AbbVie and Moderna, UpToDate, and Arthritis & Rheumatology; as well as serving on the board of directors for the Childhood Arthritis and Rheumatology Research Alliance and an advisory committee for the Food and Drug Administration outside of this work. Dr. Nachtigall and Dr. Ascher disclosed no conflicts of interest with regard to their comments.
Having specific health issues, including depressive symptoms and cardiovascular disease, as a middle-aged woman was associated with experiencing clinically important declines in health later in life, a new study finds.
The most predictive parameters of poorer health at age 65 were cardiovascular disease, clinically significant depressive symptoms, and current smoking. Osteoarthritis, lower education level, and higher body mass index (BMI) also were associated with poorer health status 10 years on, Daniel H. Solomon, MD, MPH and colleagues wrote in their observational study, which was published in JAMA Network Open.
Determining a patient’s score on a health-related quality of life measure based on these variables might be useful in clinical practice to recognize midlife patients at increased risk for later health deterioration, Dr. Solomon, of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital, Boston, said in a statement. This measure is called the Short Form 36 (SF-36), and the researchers specifically focused on the physical component summary score (PCS) of this measure. The SF-36 is similar to the Framingham 10-year coronary heart disease risk prediction score, according to Dr. Solomon, who is a professor of medicine at Harvard Medical School, also in Boston.
Based on their risk scores, women could preemptively target modifiable risk factors before they enter old age, the investigators wrote.
“Age 55-65 may be a critical decade. A person’s health and factors during this period may set them on a path for their later adult years,” Dr. Solomon said in a statement. “The good news is that a large proportion of women at midlife are very stable and will not go on to experience declines. But being able to identify women at higher risk could help lead to interventions targeted to them.”
Study details
The study included a cohort of 1,091 women drawn from the 3,302-participant Study of Women’s Health Across the Nation (SWAN), a racially and ethnically diverse group enrolled from six U.S. sites at or immediately before transition to menopause and followed for 10 years from age 55 to 65. The study sample, consisting of 24.6% Black, 24% Japanese or Chinese, and 51.9% White, had a median baseline age of 54.8 years and median BMI of 27 kg/m2 at entry. The median baseline PCS score was 53.1 (interquartile range, 46.8-56.7).
Over 10 years, 206 (18.9%) of the women in the study experienced clinically important declines of at least 8 points in baseline characteristics at around age 55. The following were significantly associated with these declines:
- Having a higher BMI.
- Having osteoarthritis.
- Having a lower educational level.
- Being a current smoker.
- Having clinically significant depressive symptoms.
- Having cardiovascular disease.
- Having better (or higher) physical health and function score on the PCS.
The association between a higher PCS score and a greater decline might seem like an anomaly, Dr. Solomon said in an interview, but one interpretation of this finding is that women with higher or better scores at baseline have further to fall once other risk factors take effect.
With data analyzed from October 2020 to March 2021, the median 10-year change in PCS was –1.02 points, but 206 women experienced declines of 8 points or more.
Those with health declines were more likely to be Black and less likely to be Japanese. They were also more likely to have other comorbidities such as diabetes, hypertension, and osteoporosis, and to report less physical activity.
Scoring system should not replace individualized evaluation, outside expert said
Commenting on the findings, Margaret J. Nachtigall, MD, a clinical associate professor in the department of obstetrics and gynecology at New York University Langone Health, cautioned that a generalized scoring system should not replace individualized evaluation of women at midlife.
“I assess women around age 55 on a daily basis for health risk factors going forward. And while a number such as BMI can be helpful, I worry that reliance on a score could miss treating the individual,” Dr. Nachtigall said an interview. For instance, one woman might have a high BMI owing to greater muscle mass, which is heavy, while another may have a lower BMI but more fat-related weight, as well as exacerbating conditions such as hypertension that would elevate her risk. “You have to make the calculation for each person.”
Dr. Nachtigall, who was not involved in the SWAN analysis, noted, however, that a big-data scoring system might be a useful adjunct to individual patient evaluation in that “it would make physicians look at all these many risk factors to identify those prone to decline.”
Study includes racially diverse population
According to the authors, while other studies have identified similar and other risk factors such as poor sleep, most have not included such a racially diverse population and have focused on women already in their senior years when the window of opportunity may already have closed.
“As a clinician and epidemiologist, I often think about the window of opportunity at midlife, when people are vital, engaged, and resilient,” said Dr. Solomon in the statement. “If we can identify risk factors and determine who is at risk, we may be able to find interventions that can stave off health declines and help put people on a better health trajectory.”
Eric M. Ascher, DO, who practices family medicine at Lenox Hill Hospital in New York and was not involved in the SWAN research, agreed with Dr. Solomon.
“Doctors who treat chronic conditions often meet patients when they are already suffering from a medical problem,” he said in an interview. “It is key to decrease your risk factors before it is too late.”
Dr. Ascher added that many primary care providers already rely heavily on scoring systems when determining level of risk and type of intervention. “Any additional risk factor-scoring systems that are easy to implement and will prevent chronic diseases would be something providers would want to use with their patients.”
Detailed analyses of larger at-risk populations are needed to validate these risk factors and identify others, the authors said.
SWAN is supported by the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Heath’s Office of Research on Women’s Health. Dr. Solomon reported financial ties to Amgen, AbbVie and Moderna, UpToDate, and Arthritis & Rheumatology; as well as serving on the board of directors for the Childhood Arthritis and Rheumatology Research Alliance and an advisory committee for the Food and Drug Administration outside of this work. Dr. Nachtigall and Dr. Ascher disclosed no conflicts of interest with regard to their comments.
Having specific health issues, including depressive symptoms and cardiovascular disease, as a middle-aged woman was associated with experiencing clinically important declines in health later in life, a new study finds.
The most predictive parameters of poorer health at age 65 were cardiovascular disease, clinically significant depressive symptoms, and current smoking. Osteoarthritis, lower education level, and higher body mass index (BMI) also were associated with poorer health status 10 years on, Daniel H. Solomon, MD, MPH and colleagues wrote in their observational study, which was published in JAMA Network Open.
Determining a patient’s score on a health-related quality of life measure based on these variables might be useful in clinical practice to recognize midlife patients at increased risk for later health deterioration, Dr. Solomon, of the division of rheumatology, inflammation, and immunity at Brigham and Women’s Hospital, Boston, said in a statement. This measure is called the Short Form 36 (SF-36), and the researchers specifically focused on the physical component summary score (PCS) of this measure. The SF-36 is similar to the Framingham 10-year coronary heart disease risk prediction score, according to Dr. Solomon, who is a professor of medicine at Harvard Medical School, also in Boston.
Based on their risk scores, women could preemptively target modifiable risk factors before they enter old age, the investigators wrote.
“Age 55-65 may be a critical decade. A person’s health and factors during this period may set them on a path for their later adult years,” Dr. Solomon said in a statement. “The good news is that a large proportion of women at midlife are very stable and will not go on to experience declines. But being able to identify women at higher risk could help lead to interventions targeted to them.”
Study details
The study included a cohort of 1,091 women drawn from the 3,302-participant Study of Women’s Health Across the Nation (SWAN), a racially and ethnically diverse group enrolled from six U.S. sites at or immediately before transition to menopause and followed for 10 years from age 55 to 65. The study sample, consisting of 24.6% Black, 24% Japanese or Chinese, and 51.9% White, had a median baseline age of 54.8 years and median BMI of 27 kg/m2 at entry. The median baseline PCS score was 53.1 (interquartile range, 46.8-56.7).
Over 10 years, 206 (18.9%) of the women in the study experienced clinically important declines of at least 8 points in baseline characteristics at around age 55. The following were significantly associated with these declines:
- Having a higher BMI.
- Having osteoarthritis.
- Having a lower educational level.
- Being a current smoker.
- Having clinically significant depressive symptoms.
- Having cardiovascular disease.
- Having better (or higher) physical health and function score on the PCS.
The association between a higher PCS score and a greater decline might seem like an anomaly, Dr. Solomon said in an interview, but one interpretation of this finding is that women with higher or better scores at baseline have further to fall once other risk factors take effect.
With data analyzed from October 2020 to March 2021, the median 10-year change in PCS was –1.02 points, but 206 women experienced declines of 8 points or more.
Those with health declines were more likely to be Black and less likely to be Japanese. They were also more likely to have other comorbidities such as diabetes, hypertension, and osteoporosis, and to report less physical activity.
Scoring system should not replace individualized evaluation, outside expert said
Commenting on the findings, Margaret J. Nachtigall, MD, a clinical associate professor in the department of obstetrics and gynecology at New York University Langone Health, cautioned that a generalized scoring system should not replace individualized evaluation of women at midlife.
“I assess women around age 55 on a daily basis for health risk factors going forward. And while a number such as BMI can be helpful, I worry that reliance on a score could miss treating the individual,” Dr. Nachtigall said an interview. For instance, one woman might have a high BMI owing to greater muscle mass, which is heavy, while another may have a lower BMI but more fat-related weight, as well as exacerbating conditions such as hypertension that would elevate her risk. “You have to make the calculation for each person.”
Dr. Nachtigall, who was not involved in the SWAN analysis, noted, however, that a big-data scoring system might be a useful adjunct to individual patient evaluation in that “it would make physicians look at all these many risk factors to identify those prone to decline.”
Study includes racially diverse population
According to the authors, while other studies have identified similar and other risk factors such as poor sleep, most have not included such a racially diverse population and have focused on women already in their senior years when the window of opportunity may already have closed.
“As a clinician and epidemiologist, I often think about the window of opportunity at midlife, when people are vital, engaged, and resilient,” said Dr. Solomon in the statement. “If we can identify risk factors and determine who is at risk, we may be able to find interventions that can stave off health declines and help put people on a better health trajectory.”
Eric M. Ascher, DO, who practices family medicine at Lenox Hill Hospital in New York and was not involved in the SWAN research, agreed with Dr. Solomon.
“Doctors who treat chronic conditions often meet patients when they are already suffering from a medical problem,” he said in an interview. “It is key to decrease your risk factors before it is too late.”
Dr. Ascher added that many primary care providers already rely heavily on scoring systems when determining level of risk and type of intervention. “Any additional risk factor-scoring systems that are easy to implement and will prevent chronic diseases would be something providers would want to use with their patients.”
Detailed analyses of larger at-risk populations are needed to validate these risk factors and identify others, the authors said.
SWAN is supported by the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Heath’s Office of Research on Women’s Health. Dr. Solomon reported financial ties to Amgen, AbbVie and Moderna, UpToDate, and Arthritis & Rheumatology; as well as serving on the board of directors for the Childhood Arthritis and Rheumatology Research Alliance and an advisory committee for the Food and Drug Administration outside of this work. Dr. Nachtigall and Dr. Ascher disclosed no conflicts of interest with regard to their comments.
FROM JAMA NETWORK OPEN
Heavy snoring in early pregnancy linked to increased insulin resistance
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
FROM SLEEP
Pig heart successfully transplanted to man
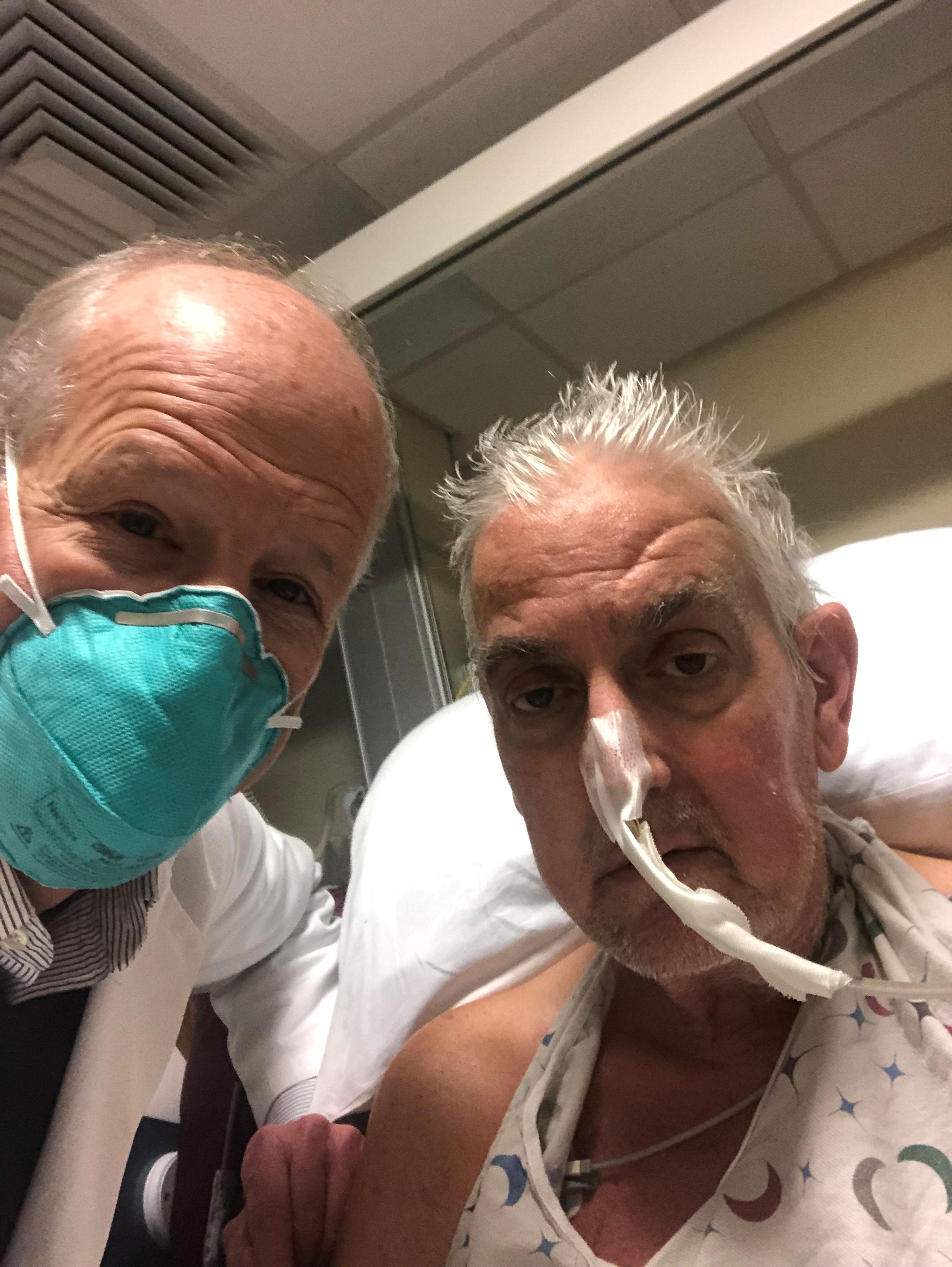
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
COVID-vaccine myocarditis: Rare, mild, and usually in young men
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
AHA advice for diabetes patients to stay heart healthy
A new document from the American Heart Association summarizes the latest research on cardiovascular risk factor management in type 2 diabetes, including medications, lifestyle, and social determinants of health.
Despite the availability of effective therapies for improving cardiovascular risk, in the United States fewer than one in five people with type 2 diabetes and without known cardiovascular disease meet control targets for a combination of A1c, blood pressure, LDL cholesterol, and nonsmoking status.
That proportion drops to less than 1 in 10 if body mass index less than 30 kg/m2 is included among the targets, and even less than that among individuals with established atherosclerotic cardiovascular disease, Joshua J. Joseph, MD, and colleagues point out in their paper, published online Jan. 10 in Circulation.
“This new scientific statement is an urgent call to action to follow the latest evidence-based approaches and to develop new best practices to advance type 2 diabetes treatment and care and reduce cardiovascular disease risk,” wrote Dr. Joseph, assistant professor of medicine in the division of endocrinology, diabetes, and metabolism at The Ohio State University, Columbus, Ohio, and coauthors.
The statement is not a guideline but an expert analysis that may inform future clinical practice guidelines, according to a press release from the AHA.
The new statement reviews evidence through June 2020 for lifestyle management of diabetes and weight, glycemic targets and control, blood pressure management, lipid management, antithrombotic therapy, and screening for cardiovascular and renal complications, including imaging. It also discusses the clinical implications of recent cardiovascular outcomes trials of newer glucose-lowering medications.
However, Dr. Joseph and colleagues point out, clinical care and treatment account for just 10%-20% of modifiable contributors to health outcomes. The other 80%-90% relate to social determinants of health, including health-related behaviors, socioeconomic factors, environmental factors, and racism.
“If we are to continue to advance the management of cardiovascular risk factors, we must also address the [social determinants of health] in the delivery of health care,” they noted.
Overall, they advise a patient-centered approach, meaning “reframing our clinical encounters to think about patients as people who live in families, communities, and societies that must be considered in their cardiovascular risk management.”
“People with [type 2 diabetes] face numerous barriers to health including access to care and equitable care, which must be considered when developing individualized care plans with our patients,” Dr. Joseph said in the AHA press release.
Lifestyle, medications for lowering A1c, BP, lipids
For lifestyle management, the authors say, “culturally appropriate recommendations through diabetes self-management education and support and medical nutrition therapy are key to meeting individualized goals for behavioral change and diabetes self-management.”
The document summarizes recommendations from other professional societies regarding glycemic targets and glucose lowering medications, i.e., target A1c levels of either < 7% or < 6.5% for the majority, with adjustments based on individual factors, such as life expectancy. It advises on use of metformin as first-line therapy followed by a sodium-glucose cotransporter-2 inhibitor or a glucagon-like peptide-1 agonist for those with established cardiovascular disease or risk factors.
“Cost may be a barrier to taking some [type 2 diabetes] medications as prescribed; however, many of these medications are now more commonly covered by more health insurance plans,” Dr. Joseph said.
“Another barrier is recognition by patients that these newer [type 2 diabetes] medications are also effective in reducing the risk of heart disease, stroke, heart failure, and kidney disease.”
Blood pressure treatment guidelines differ between those of the AHA/American College of Cardiology (ACC) and the American Diabetes Association (ADA), most notably that the AHA/ACC guidelines advise a general target of < 130/80 mm Hg, whereas ADA advises < 140/90 mm Hg or < 130/80 mm Hg for those with high risk if it can be safely achieved.
The decision should be “patient-centered with shared decision-making,” Dr. Joseph and colleagues advised.
For lipid-lowering, the document cites the 2018 ACC/AHA cholesterol guidelines, which include advising statins as first-line therapy for both primary and secondary prevention in diabetes, with highest intensity statins used in those at highest risk. But again, treatment should be individualized, and other agents should be used for patients in whom statins don’t work or aren’t tolerated.
And while use of antiplatelets – that is, aspirin – is well established as secondary prevention in type 2 diabetes, given new data suggesting that the risk for major bleeding could outweigh the benefits for primary prevention, “the relative benefits of antithrombotic approaches need to be weighed carefully against risks using a patient-centered approach,” the authors advised.
Among the many imaging tests available to facilitate cardiovascular risk stratification in type 2 diabetes, coronary artery calcification (CAC) CT screening is one of the few with sufficient data to support routine use in selected patients. The National Lipid Association, for example, recommends escalation to high-intensity statin for CAC > 100.
“One avenue to continue to address and advance diabetes management is through breaking down the four walls of the clinic or hospital through community engagement, clinic-to-community connections, and academic-community-government partnerships that may help address and support modifiable lifestyle behaviors such as physical activity, nutrition, smoking cessation and stress management,” Dr. Joseph concluded.
The AHA receives funding primarily from individuals. Foundations and corporations, including pharmaceutical, device manufacturers, and other companies, also make donations and fund AHA programs and events. The AHA’s strict policies prevent these relationships from influencing the science content. Revenues from pharmaceutical and biotech companies, device manufacturers, and health insurance providers and the AHA’s financial information are available on the association’s website. Dr. Joseph has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new document from the American Heart Association summarizes the latest research on cardiovascular risk factor management in type 2 diabetes, including medications, lifestyle, and social determinants of health.
Despite the availability of effective therapies for improving cardiovascular risk, in the United States fewer than one in five people with type 2 diabetes and without known cardiovascular disease meet control targets for a combination of A1c, blood pressure, LDL cholesterol, and nonsmoking status.
That proportion drops to less than 1 in 10 if body mass index less than 30 kg/m2 is included among the targets, and even less than that among individuals with established atherosclerotic cardiovascular disease, Joshua J. Joseph, MD, and colleagues point out in their paper, published online Jan. 10 in Circulation.
“This new scientific statement is an urgent call to action to follow the latest evidence-based approaches and to develop new best practices to advance type 2 diabetes treatment and care and reduce cardiovascular disease risk,” wrote Dr. Joseph, assistant professor of medicine in the division of endocrinology, diabetes, and metabolism at The Ohio State University, Columbus, Ohio, and coauthors.
The statement is not a guideline but an expert analysis that may inform future clinical practice guidelines, according to a press release from the AHA.
The new statement reviews evidence through June 2020 for lifestyle management of diabetes and weight, glycemic targets and control, blood pressure management, lipid management, antithrombotic therapy, and screening for cardiovascular and renal complications, including imaging. It also discusses the clinical implications of recent cardiovascular outcomes trials of newer glucose-lowering medications.
However, Dr. Joseph and colleagues point out, clinical care and treatment account for just 10%-20% of modifiable contributors to health outcomes. The other 80%-90% relate to social determinants of health, including health-related behaviors, socioeconomic factors, environmental factors, and racism.
“If we are to continue to advance the management of cardiovascular risk factors, we must also address the [social determinants of health] in the delivery of health care,” they noted.
Overall, they advise a patient-centered approach, meaning “reframing our clinical encounters to think about patients as people who live in families, communities, and societies that must be considered in their cardiovascular risk management.”
“People with [type 2 diabetes] face numerous barriers to health including access to care and equitable care, which must be considered when developing individualized care plans with our patients,” Dr. Joseph said in the AHA press release.
Lifestyle, medications for lowering A1c, BP, lipids
For lifestyle management, the authors say, “culturally appropriate recommendations through diabetes self-management education and support and medical nutrition therapy are key to meeting individualized goals for behavioral change and diabetes self-management.”
The document summarizes recommendations from other professional societies regarding glycemic targets and glucose lowering medications, i.e., target A1c levels of either < 7% or < 6.5% for the majority, with adjustments based on individual factors, such as life expectancy. It advises on use of metformin as first-line therapy followed by a sodium-glucose cotransporter-2 inhibitor or a glucagon-like peptide-1 agonist for those with established cardiovascular disease or risk factors.
“Cost may be a barrier to taking some [type 2 diabetes] medications as prescribed; however, many of these medications are now more commonly covered by more health insurance plans,” Dr. Joseph said.
“Another barrier is recognition by patients that these newer [type 2 diabetes] medications are also effective in reducing the risk of heart disease, stroke, heart failure, and kidney disease.”
Blood pressure treatment guidelines differ between those of the AHA/American College of Cardiology (ACC) and the American Diabetes Association (ADA), most notably that the AHA/ACC guidelines advise a general target of < 130/80 mm Hg, whereas ADA advises < 140/90 mm Hg or < 130/80 mm Hg for those with high risk if it can be safely achieved.
The decision should be “patient-centered with shared decision-making,” Dr. Joseph and colleagues advised.
For lipid-lowering, the document cites the 2018 ACC/AHA cholesterol guidelines, which include advising statins as first-line therapy for both primary and secondary prevention in diabetes, with highest intensity statins used in those at highest risk. But again, treatment should be individualized, and other agents should be used for patients in whom statins don’t work or aren’t tolerated.
And while use of antiplatelets – that is, aspirin – is well established as secondary prevention in type 2 diabetes, given new data suggesting that the risk for major bleeding could outweigh the benefits for primary prevention, “the relative benefits of antithrombotic approaches need to be weighed carefully against risks using a patient-centered approach,” the authors advised.
Among the many imaging tests available to facilitate cardiovascular risk stratification in type 2 diabetes, coronary artery calcification (CAC) CT screening is one of the few with sufficient data to support routine use in selected patients. The National Lipid Association, for example, recommends escalation to high-intensity statin for CAC > 100.
“One avenue to continue to address and advance diabetes management is through breaking down the four walls of the clinic or hospital through community engagement, clinic-to-community connections, and academic-community-government partnerships that may help address and support modifiable lifestyle behaviors such as physical activity, nutrition, smoking cessation and stress management,” Dr. Joseph concluded.
The AHA receives funding primarily from individuals. Foundations and corporations, including pharmaceutical, device manufacturers, and other companies, also make donations and fund AHA programs and events. The AHA’s strict policies prevent these relationships from influencing the science content. Revenues from pharmaceutical and biotech companies, device manufacturers, and health insurance providers and the AHA’s financial information are available on the association’s website. Dr. Joseph has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new document from the American Heart Association summarizes the latest research on cardiovascular risk factor management in type 2 diabetes, including medications, lifestyle, and social determinants of health.
Despite the availability of effective therapies for improving cardiovascular risk, in the United States fewer than one in five people with type 2 diabetes and without known cardiovascular disease meet control targets for a combination of A1c, blood pressure, LDL cholesterol, and nonsmoking status.
That proportion drops to less than 1 in 10 if body mass index less than 30 kg/m2 is included among the targets, and even less than that among individuals with established atherosclerotic cardiovascular disease, Joshua J. Joseph, MD, and colleagues point out in their paper, published online Jan. 10 in Circulation.
“This new scientific statement is an urgent call to action to follow the latest evidence-based approaches and to develop new best practices to advance type 2 diabetes treatment and care and reduce cardiovascular disease risk,” wrote Dr. Joseph, assistant professor of medicine in the division of endocrinology, diabetes, and metabolism at The Ohio State University, Columbus, Ohio, and coauthors.
The statement is not a guideline but an expert analysis that may inform future clinical practice guidelines, according to a press release from the AHA.
The new statement reviews evidence through June 2020 for lifestyle management of diabetes and weight, glycemic targets and control, blood pressure management, lipid management, antithrombotic therapy, and screening for cardiovascular and renal complications, including imaging. It also discusses the clinical implications of recent cardiovascular outcomes trials of newer glucose-lowering medications.
However, Dr. Joseph and colleagues point out, clinical care and treatment account for just 10%-20% of modifiable contributors to health outcomes. The other 80%-90% relate to social determinants of health, including health-related behaviors, socioeconomic factors, environmental factors, and racism.
“If we are to continue to advance the management of cardiovascular risk factors, we must also address the [social determinants of health] in the delivery of health care,” they noted.
Overall, they advise a patient-centered approach, meaning “reframing our clinical encounters to think about patients as people who live in families, communities, and societies that must be considered in their cardiovascular risk management.”
“People with [type 2 diabetes] face numerous barriers to health including access to care and equitable care, which must be considered when developing individualized care plans with our patients,” Dr. Joseph said in the AHA press release.
Lifestyle, medications for lowering A1c, BP, lipids
For lifestyle management, the authors say, “culturally appropriate recommendations through diabetes self-management education and support and medical nutrition therapy are key to meeting individualized goals for behavioral change and diabetes self-management.”
The document summarizes recommendations from other professional societies regarding glycemic targets and glucose lowering medications, i.e., target A1c levels of either < 7% or < 6.5% for the majority, with adjustments based on individual factors, such as life expectancy. It advises on use of metformin as first-line therapy followed by a sodium-glucose cotransporter-2 inhibitor or a glucagon-like peptide-1 agonist for those with established cardiovascular disease or risk factors.
“Cost may be a barrier to taking some [type 2 diabetes] medications as prescribed; however, many of these medications are now more commonly covered by more health insurance plans,” Dr. Joseph said.
“Another barrier is recognition by patients that these newer [type 2 diabetes] medications are also effective in reducing the risk of heart disease, stroke, heart failure, and kidney disease.”
Blood pressure treatment guidelines differ between those of the AHA/American College of Cardiology (ACC) and the American Diabetes Association (ADA), most notably that the AHA/ACC guidelines advise a general target of < 130/80 mm Hg, whereas ADA advises < 140/90 mm Hg or < 130/80 mm Hg for those with high risk if it can be safely achieved.
The decision should be “patient-centered with shared decision-making,” Dr. Joseph and colleagues advised.
For lipid-lowering, the document cites the 2018 ACC/AHA cholesterol guidelines, which include advising statins as first-line therapy for both primary and secondary prevention in diabetes, with highest intensity statins used in those at highest risk. But again, treatment should be individualized, and other agents should be used for patients in whom statins don’t work or aren’t tolerated.
And while use of antiplatelets – that is, aspirin – is well established as secondary prevention in type 2 diabetes, given new data suggesting that the risk for major bleeding could outweigh the benefits for primary prevention, “the relative benefits of antithrombotic approaches need to be weighed carefully against risks using a patient-centered approach,” the authors advised.
Among the many imaging tests available to facilitate cardiovascular risk stratification in type 2 diabetes, coronary artery calcification (CAC) CT screening is one of the few with sufficient data to support routine use in selected patients. The National Lipid Association, for example, recommends escalation to high-intensity statin for CAC > 100.
“One avenue to continue to address and advance diabetes management is through breaking down the four walls of the clinic or hospital through community engagement, clinic-to-community connections, and academic-community-government partnerships that may help address and support modifiable lifestyle behaviors such as physical activity, nutrition, smoking cessation and stress management,” Dr. Joseph concluded.
The AHA receives funding primarily from individuals. Foundations and corporations, including pharmaceutical, device manufacturers, and other companies, also make donations and fund AHA programs and events. The AHA’s strict policies prevent these relationships from influencing the science content. Revenues from pharmaceutical and biotech companies, device manufacturers, and health insurance providers and the AHA’s financial information are available on the association’s website. Dr. Joseph has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At-risk Americans become eligible for fourth COVID shot this week
The Centers for Disease Control and Prevention endorsed a third dose of the Pfizer or Moderna vaccines for moderately and severely immunocompromised people on Aug. 13, which is considered part of their first immunization series rather than a booster shot.
In October, the CDC said moderately and severely immunocompromised people could receive a booster shot, or a fourth dose of the vaccine , 6 months after their third dose.
But the CDC last week shortened the timeline to 5 months for a booster shot of the Pfizer or Moderna vaccines. That means immunocompromised people could begin signing up for a fourth shot later this week, the New York Times reported.
About 2.7% of U.S. adults, or about 7 million adults, are considered immunocompromised, according to the CDC. They’re more likely to contract severe COVID-19, have a higher risk for long COVID, have lower antibody levels after vaccination, and develop serious breakthrough infections. About 40% of hospitalized breakthrough cases are among immunocompromised people.
According to CDC guidance, people are considered to be “moderately or severely immunocompromised” if they have:
- Active cancer treatment for tumors or cancers of the blood
- Had an organ transplant and are taking medicine to suppress the immune system
- Had a stem cell transplant in the last 2 years and are taking medicine to suppress the immune system
- Advanced or untreated HIV infection
- Moderate or severe primary immunodeficiency, such as DiGeorge syndrome or Wiskott-Aldrich syndrome
- Active treatment with high-dose corticosteroids or other drugs that suppress the immune response
So far, only moderately and severely immunocompromised Americans are eligible for a fourth shot. Israel has begun offering fourth doses to high-risk groups, including older adults, but the Biden administration hasn’t yet said whether the United States will follow, the Times reported.
Overall, the focus remains on getting third shots to Americans who are eligible for boosters, Rochelle Walensky, MD, the CDC director, told reporters Jan. 7. U.S. officials will remain in touch with Israel to follow their data on fourth shots.
“We will be following our own data carefully as well, to see how these boosters are working in terms of waning effectiveness, not just for infection but, importantly, for severe disease,” she said.
A version of this article first appeared on WebMD.com .
The Centers for Disease Control and Prevention endorsed a third dose of the Pfizer or Moderna vaccines for moderately and severely immunocompromised people on Aug. 13, which is considered part of their first immunization series rather than a booster shot.
In October, the CDC said moderately and severely immunocompromised people could receive a booster shot, or a fourth dose of the vaccine , 6 months after their third dose.
But the CDC last week shortened the timeline to 5 months for a booster shot of the Pfizer or Moderna vaccines. That means immunocompromised people could begin signing up for a fourth shot later this week, the New York Times reported.
About 2.7% of U.S. adults, or about 7 million adults, are considered immunocompromised, according to the CDC. They’re more likely to contract severe COVID-19, have a higher risk for long COVID, have lower antibody levels after vaccination, and develop serious breakthrough infections. About 40% of hospitalized breakthrough cases are among immunocompromised people.
According to CDC guidance, people are considered to be “moderately or severely immunocompromised” if they have:
- Active cancer treatment for tumors or cancers of the blood
- Had an organ transplant and are taking medicine to suppress the immune system
- Had a stem cell transplant in the last 2 years and are taking medicine to suppress the immune system
- Advanced or untreated HIV infection
- Moderate or severe primary immunodeficiency, such as DiGeorge syndrome or Wiskott-Aldrich syndrome
- Active treatment with high-dose corticosteroids or other drugs that suppress the immune response
So far, only moderately and severely immunocompromised Americans are eligible for a fourth shot. Israel has begun offering fourth doses to high-risk groups, including older adults, but the Biden administration hasn’t yet said whether the United States will follow, the Times reported.
Overall, the focus remains on getting third shots to Americans who are eligible for boosters, Rochelle Walensky, MD, the CDC director, told reporters Jan. 7. U.S. officials will remain in touch with Israel to follow their data on fourth shots.
“We will be following our own data carefully as well, to see how these boosters are working in terms of waning effectiveness, not just for infection but, importantly, for severe disease,” she said.
A version of this article first appeared on WebMD.com .
The Centers for Disease Control and Prevention endorsed a third dose of the Pfizer or Moderna vaccines for moderately and severely immunocompromised people on Aug. 13, which is considered part of their first immunization series rather than a booster shot.
In October, the CDC said moderately and severely immunocompromised people could receive a booster shot, or a fourth dose of the vaccine , 6 months after their third dose.
But the CDC last week shortened the timeline to 5 months for a booster shot of the Pfizer or Moderna vaccines. That means immunocompromised people could begin signing up for a fourth shot later this week, the New York Times reported.
About 2.7% of U.S. adults, or about 7 million adults, are considered immunocompromised, according to the CDC. They’re more likely to contract severe COVID-19, have a higher risk for long COVID, have lower antibody levels after vaccination, and develop serious breakthrough infections. About 40% of hospitalized breakthrough cases are among immunocompromised people.
According to CDC guidance, people are considered to be “moderately or severely immunocompromised” if they have:
- Active cancer treatment for tumors or cancers of the blood
- Had an organ transplant and are taking medicine to suppress the immune system
- Had a stem cell transplant in the last 2 years and are taking medicine to suppress the immune system
- Advanced or untreated HIV infection
- Moderate or severe primary immunodeficiency, such as DiGeorge syndrome or Wiskott-Aldrich syndrome
- Active treatment with high-dose corticosteroids or other drugs that suppress the immune response
So far, only moderately and severely immunocompromised Americans are eligible for a fourth shot. Israel has begun offering fourth doses to high-risk groups, including older adults, but the Biden administration hasn’t yet said whether the United States will follow, the Times reported.
Overall, the focus remains on getting third shots to Americans who are eligible for boosters, Rochelle Walensky, MD, the CDC director, told reporters Jan. 7. U.S. officials will remain in touch with Israel to follow their data on fourth shots.
“We will be following our own data carefully as well, to see how these boosters are working in terms of waning effectiveness, not just for infection but, importantly, for severe disease,” she said.
A version of this article first appeared on WebMD.com .
COVID-19 linked to increased diabetes risk in youth
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
FROM MMWR
As pandemic regs expire, states get tougher on telehealth: report
Among the most important restrictions that have been reinstated in some states are those barring requirements for insurers to cover telehealth and regulations that prohibit telehealth visits across state lines, unless the physician is licensed in both states.
“Only three states – Arizona, Florida, and Indiana – allow all health care providers to easily practice telehealth across state lines,” says a news release on the think tanks’ report. “Forty-seven others have arbitrary barriers in place that limit patients’ access to specialists and available appointments based purely on residency.”
“Once the [state-based] public health emergency declarations started to end or executive orders were withdrawn, many of the new flexibilities for providers, insurers, and patients were lost overnight,” Vittorio Nastasi, a policy analyst at Reason Foundation and a co-author of the report, says in the news release. “States need to adopt a number of telehealth reforms to provide their residents better access to this safe and effective virtual care.”
On a positive note, the report says, most states have removed the requirement that a patient must first see a provider in person before they can use telehealth services. The exceptions are Tennessee, Alaska, and West Virginia, which require an in-person visit before certain telehealth services can be provided.
In addition, 20 states allow nurse practitioners to conduct telehealth visits without being under the supervision of a physician. Prior to the pandemic, some states allowed only doctors to use telehealth, the report says, but, during the COVID crisis, “the acute shortage of providers in many counties adds to the need for more kinds of providers to be able to use it.”
A number of states place restrictions on the telehealth modalities that can be utilized. Under the definition by the American Telemedicine Association, telehealth includes audio-video visits, remote patient monitoring, and “store and forward” telemedicine, which entails collecting clinical information and sending it to another site for evaluation. The latter method is particularly useful for consultations with specialists, the report notes.
Coverage mandates and payment parity
The report also examines other parameters of telehealth regulations in each state, including whether they have telehealth coverage mandates and whether they require physicians to be paid the same amount for similar types of in-person and telehealth visits.
The report views insurance mandates as beneficial, but not if they require coverage of all virtual services. While telehealth can be a game changer for post-stroke care and for other “treatment-intensive conditions,” the report says, the evidence of better outcomes for other conditions treated through telehealth is far less certain. Therefore, it advises states to “protect flexibility so that new innovative models can emerge.”
Ateev Mehrotra, MD, a professor at Harvard Medical School who studies telehealth, agrees that it offers more value in some clinical situations than in others. “High value is improving quality or outcomes at a reasonable cost,” he told this news organization. “If a telemedicine visit for stroke can save a person’s life and prevent disability, let’s pay for it. A telemedicine visit for a cold may not be necessary. Mom’s chicken soup is fine.”
A little over half of the states still require payment parity, according to the report. While these regulations are intended to promote the use of telehealth, the authors note, they can increase the growth of health care costs. Moreover, they argue, it’s hard to defend equal payments for virtual visits when the overhead required to deliver them – such as office rental, utility, and labor costs – is much lower than that for in-person visits. Also, it makes no sense for health systems to charge facility fees for telehealth visits when these visits can be initiated from anywhere, they say.
Dr. Mehrotra concurs with this view. “If you see someone in your office, your fee includes all the overhead for your office, and it’s a substantial cost,” he says. “For many procedures, it’s more than half of the cost. If you have a telemedicine visit and you’re at home, why would you pay the same amount? The visit may take the same amount of time, but all the money that goes for overhead is not accounted for.”
Telemedicine across state lines
The report’s contention about the difficulty of conducting telehealth encounters across most state lines seems to be at odds with the growth in the Interstate Medical Licensure Compact, which makes it easier for physicians in one compact member state to get licensed in others. Currently, 35 states belong to the compact, Joe Knickrehm, vice president of communications for the Federation of State Medical Boards, told this news organization.
In addition, he says, “12 state boards issue a special purpose license, telemedicine license or certificate, or license to practice medicine across state lines to allow for the practice of telemedicine.”
The catch, Dr. Mehrotra says, is that, despite the streamlining of license applications in compact member states, the fees charged by the state boards are still very high – a point that the report also makes. “If I want to have broad scope of practice, I’d have to pay thousands of dollars to many states. The license fees start to add up. Also, I have to keep track of each state’s CME requirements, which are all different. Keeping up with all of that is an administration burden, and it’s a pain.”
Mr. Knickrehm contends that obtaining multiple licenses via the compact “is generally less expensive for physicians than the cost of requesting transcripts, fingerprints, and other necessary paperwork each time they apply for licensure in a new state. Physicians are seeing the benefits of an expedited process that allows them to begin practicing more quickly [in other states].”
Dr. Mehrotra says he has seen the same retrenchment in state telehealth regulations that the report references. However, he says, “CMS [the Centers for Medicare & Medicaid Services] has signaled that at least through 2022 and maybe into 2023, they’ll continue their extensions of telemedicine [pandemic regulations].” After that, Congress would have to decide whether to make the changes permanent.
“Right now, it’s hard for me to see how a payer is going to pull back on telehealth, unless there’s ample evidence of overuse of telehealth,” he argues. “With the public and providers liking telehealth, it’s hard to say on theoretical grounds that we should stop using it. That’s why Medicare and others have extended it and why Congress will too.”
A version of this article first appeared on Medscape.com.
Among the most important restrictions that have been reinstated in some states are those barring requirements for insurers to cover telehealth and regulations that prohibit telehealth visits across state lines, unless the physician is licensed in both states.
“Only three states – Arizona, Florida, and Indiana – allow all health care providers to easily practice telehealth across state lines,” says a news release on the think tanks’ report. “Forty-seven others have arbitrary barriers in place that limit patients’ access to specialists and available appointments based purely on residency.”
“Once the [state-based] public health emergency declarations started to end or executive orders were withdrawn, many of the new flexibilities for providers, insurers, and patients were lost overnight,” Vittorio Nastasi, a policy analyst at Reason Foundation and a co-author of the report, says in the news release. “States need to adopt a number of telehealth reforms to provide their residents better access to this safe and effective virtual care.”
On a positive note, the report says, most states have removed the requirement that a patient must first see a provider in person before they can use telehealth services. The exceptions are Tennessee, Alaska, and West Virginia, which require an in-person visit before certain telehealth services can be provided.
In addition, 20 states allow nurse practitioners to conduct telehealth visits without being under the supervision of a physician. Prior to the pandemic, some states allowed only doctors to use telehealth, the report says, but, during the COVID crisis, “the acute shortage of providers in many counties adds to the need for more kinds of providers to be able to use it.”
A number of states place restrictions on the telehealth modalities that can be utilized. Under the definition by the American Telemedicine Association, telehealth includes audio-video visits, remote patient monitoring, and “store and forward” telemedicine, which entails collecting clinical information and sending it to another site for evaluation. The latter method is particularly useful for consultations with specialists, the report notes.
Coverage mandates and payment parity
The report also examines other parameters of telehealth regulations in each state, including whether they have telehealth coverage mandates and whether they require physicians to be paid the same amount for similar types of in-person and telehealth visits.
The report views insurance mandates as beneficial, but not if they require coverage of all virtual services. While telehealth can be a game changer for post-stroke care and for other “treatment-intensive conditions,” the report says, the evidence of better outcomes for other conditions treated through telehealth is far less certain. Therefore, it advises states to “protect flexibility so that new innovative models can emerge.”
Ateev Mehrotra, MD, a professor at Harvard Medical School who studies telehealth, agrees that it offers more value in some clinical situations than in others. “High value is improving quality or outcomes at a reasonable cost,” he told this news organization. “If a telemedicine visit for stroke can save a person’s life and prevent disability, let’s pay for it. A telemedicine visit for a cold may not be necessary. Mom’s chicken soup is fine.”
A little over half of the states still require payment parity, according to the report. While these regulations are intended to promote the use of telehealth, the authors note, they can increase the growth of health care costs. Moreover, they argue, it’s hard to defend equal payments for virtual visits when the overhead required to deliver them – such as office rental, utility, and labor costs – is much lower than that for in-person visits. Also, it makes no sense for health systems to charge facility fees for telehealth visits when these visits can be initiated from anywhere, they say.
Dr. Mehrotra concurs with this view. “If you see someone in your office, your fee includes all the overhead for your office, and it’s a substantial cost,” he says. “For many procedures, it’s more than half of the cost. If you have a telemedicine visit and you’re at home, why would you pay the same amount? The visit may take the same amount of time, but all the money that goes for overhead is not accounted for.”
Telemedicine across state lines
The report’s contention about the difficulty of conducting telehealth encounters across most state lines seems to be at odds with the growth in the Interstate Medical Licensure Compact, which makes it easier for physicians in one compact member state to get licensed in others. Currently, 35 states belong to the compact, Joe Knickrehm, vice president of communications for the Federation of State Medical Boards, told this news organization.
In addition, he says, “12 state boards issue a special purpose license, telemedicine license or certificate, or license to practice medicine across state lines to allow for the practice of telemedicine.”
The catch, Dr. Mehrotra says, is that, despite the streamlining of license applications in compact member states, the fees charged by the state boards are still very high – a point that the report also makes. “If I want to have broad scope of practice, I’d have to pay thousands of dollars to many states. The license fees start to add up. Also, I have to keep track of each state’s CME requirements, which are all different. Keeping up with all of that is an administration burden, and it’s a pain.”
Mr. Knickrehm contends that obtaining multiple licenses via the compact “is generally less expensive for physicians than the cost of requesting transcripts, fingerprints, and other necessary paperwork each time they apply for licensure in a new state. Physicians are seeing the benefits of an expedited process that allows them to begin practicing more quickly [in other states].”
Dr. Mehrotra says he has seen the same retrenchment in state telehealth regulations that the report references. However, he says, “CMS [the Centers for Medicare & Medicaid Services] has signaled that at least through 2022 and maybe into 2023, they’ll continue their extensions of telemedicine [pandemic regulations].” After that, Congress would have to decide whether to make the changes permanent.
“Right now, it’s hard for me to see how a payer is going to pull back on telehealth, unless there’s ample evidence of overuse of telehealth,” he argues. “With the public and providers liking telehealth, it’s hard to say on theoretical grounds that we should stop using it. That’s why Medicare and others have extended it and why Congress will too.”
A version of this article first appeared on Medscape.com.
Among the most important restrictions that have been reinstated in some states are those barring requirements for insurers to cover telehealth and regulations that prohibit telehealth visits across state lines, unless the physician is licensed in both states.
“Only three states – Arizona, Florida, and Indiana – allow all health care providers to easily practice telehealth across state lines,” says a news release on the think tanks’ report. “Forty-seven others have arbitrary barriers in place that limit patients’ access to specialists and available appointments based purely on residency.”
“Once the [state-based] public health emergency declarations started to end or executive orders were withdrawn, many of the new flexibilities for providers, insurers, and patients were lost overnight,” Vittorio Nastasi, a policy analyst at Reason Foundation and a co-author of the report, says in the news release. “States need to adopt a number of telehealth reforms to provide their residents better access to this safe and effective virtual care.”
On a positive note, the report says, most states have removed the requirement that a patient must first see a provider in person before they can use telehealth services. The exceptions are Tennessee, Alaska, and West Virginia, which require an in-person visit before certain telehealth services can be provided.
In addition, 20 states allow nurse practitioners to conduct telehealth visits without being under the supervision of a physician. Prior to the pandemic, some states allowed only doctors to use telehealth, the report says, but, during the COVID crisis, “the acute shortage of providers in many counties adds to the need for more kinds of providers to be able to use it.”
A number of states place restrictions on the telehealth modalities that can be utilized. Under the definition by the American Telemedicine Association, telehealth includes audio-video visits, remote patient monitoring, and “store and forward” telemedicine, which entails collecting clinical information and sending it to another site for evaluation. The latter method is particularly useful for consultations with specialists, the report notes.
Coverage mandates and payment parity
The report also examines other parameters of telehealth regulations in each state, including whether they have telehealth coverage mandates and whether they require physicians to be paid the same amount for similar types of in-person and telehealth visits.
The report views insurance mandates as beneficial, but not if they require coverage of all virtual services. While telehealth can be a game changer for post-stroke care and for other “treatment-intensive conditions,” the report says, the evidence of better outcomes for other conditions treated through telehealth is far less certain. Therefore, it advises states to “protect flexibility so that new innovative models can emerge.”
Ateev Mehrotra, MD, a professor at Harvard Medical School who studies telehealth, agrees that it offers more value in some clinical situations than in others. “High value is improving quality or outcomes at a reasonable cost,” he told this news organization. “If a telemedicine visit for stroke can save a person’s life and prevent disability, let’s pay for it. A telemedicine visit for a cold may not be necessary. Mom’s chicken soup is fine.”
A little over half of the states still require payment parity, according to the report. While these regulations are intended to promote the use of telehealth, the authors note, they can increase the growth of health care costs. Moreover, they argue, it’s hard to defend equal payments for virtual visits when the overhead required to deliver them – such as office rental, utility, and labor costs – is much lower than that for in-person visits. Also, it makes no sense for health systems to charge facility fees for telehealth visits when these visits can be initiated from anywhere, they say.
Dr. Mehrotra concurs with this view. “If you see someone in your office, your fee includes all the overhead for your office, and it’s a substantial cost,” he says. “For many procedures, it’s more than half of the cost. If you have a telemedicine visit and you’re at home, why would you pay the same amount? The visit may take the same amount of time, but all the money that goes for overhead is not accounted for.”
Telemedicine across state lines
The report’s contention about the difficulty of conducting telehealth encounters across most state lines seems to be at odds with the growth in the Interstate Medical Licensure Compact, which makes it easier for physicians in one compact member state to get licensed in others. Currently, 35 states belong to the compact, Joe Knickrehm, vice president of communications for the Federation of State Medical Boards, told this news organization.
In addition, he says, “12 state boards issue a special purpose license, telemedicine license or certificate, or license to practice medicine across state lines to allow for the practice of telemedicine.”
The catch, Dr. Mehrotra says, is that, despite the streamlining of license applications in compact member states, the fees charged by the state boards are still very high – a point that the report also makes. “If I want to have broad scope of practice, I’d have to pay thousands of dollars to many states. The license fees start to add up. Also, I have to keep track of each state’s CME requirements, which are all different. Keeping up with all of that is an administration burden, and it’s a pain.”
Mr. Knickrehm contends that obtaining multiple licenses via the compact “is generally less expensive for physicians than the cost of requesting transcripts, fingerprints, and other necessary paperwork each time they apply for licensure in a new state. Physicians are seeing the benefits of an expedited process that allows them to begin practicing more quickly [in other states].”
Dr. Mehrotra says he has seen the same retrenchment in state telehealth regulations that the report references. However, he says, “CMS [the Centers for Medicare & Medicaid Services] has signaled that at least through 2022 and maybe into 2023, they’ll continue their extensions of telemedicine [pandemic regulations].” After that, Congress would have to decide whether to make the changes permanent.
“Right now, it’s hard for me to see how a payer is going to pull back on telehealth, unless there’s ample evidence of overuse of telehealth,” he argues. “With the public and providers liking telehealth, it’s hard to say on theoretical grounds that we should stop using it. That’s why Medicare and others have extended it and why Congress will too.”
A version of this article first appeared on Medscape.com.
Similar 10-year survival after CABG, PCI in heavy calcification
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS