User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
‘Worrisome’ rates of suicidal thoughts and behaviors in children with epilepsy
new research suggests. In a study of more than 100 youth with the disorder, more than 40% had depression, 30% had anxiety, and about 1 in 10 exhibited signs of suicidal thoughts and behaviors.
These rates “are really worrisome” and highlight the need to screen all children and young adults with epilepsy for psychiatric disorders, said study author Tatiana Falcone, MD, assistant professor of neurology and child and adolescent psychiatry at the Cleveland Clinic.
“It’s very important to screen for suicidality and for depression and anxiety, even when patients aren’t reporting symptoms,” said Dr. Falcone.
Previous research shows children with epilepsy will attend the emergency room with symptoms such as headache or stomachache “when the main reason for the visit was the kid was suicidal,” Dr. Falcone said. “Unless you ask the specific question: ‘Are you having thoughts about hurting yourself?’ this will go unreported,” she added.
The findings were presented at the American Epilepsy Society’s 74th Annual Meeting, which was held online this year because of the COVID-19 pandemic.
Red flag
Not much is known about suicidality in children and youth with epilepsy except that depression and anxiety – the most common psychiatric comorbidities in this population – appear to contribute to suicidal thoughts.
Dr. Falcone said that she and her colleagues often see children and adolescents with epilepsy in their clinic who have attempted suicide. In recent years, the clinicians have increased efforts to try to identify them before they carry out a successful suicide attempt, said lead investigator Anjali Dagar, MD, clinical research psychiatry fellow at Cleveland Clinic.
The study included 119 patients aged 10-24 years (mean age, 15.8 years; 54.6% female). All attended an epilepsy clinic or underwent testing in the pediatric epilepsy monitoring unit at the Cleveland Clinic and did not have a psychiatric diagnosis.
Epilepsy severity ranged among study participants. About half were drug resistant and were at the center for surgical evaluation and the others were newly diagnosed.
Participants filled out questionnaires to self-report psychiatric conditions. The validated screening tools included the Center for Epidemiological Studies Depression Scale for Children (CES-DC), the Screen for Child Anxiety Related Emotional Disorders (SCARED), and the Ask Suicide–Screening Questions (ASQ).
A score of 15 or higher on the CES-DC indicates a risk for depression. On the SCARED test, a score higher than 32 indicates anxiety. Recent research has shown that anxiety is a main risk factor “in moving people from contemplating suicide to actually carrying it out,” Dr. Falcone said.
The ASQ includes four questions about suicidal thoughts and whether respondents have tried to hurt themselves. Dr. Dagar noted that a positive response to any of these questions should raise a red flag.
Very high rates
Results showed that almost one-third (30.2%) of the participants scored positive for anxiety on SCARED and 41.2% scored positive for depression on the CSE-DC. These are “very high” rates, Dr. Falcone said. For comparison, the rate of reported anxiety is less than 10% in school surveys.
In addition, the Centers for Disease Control and Prevention reports about 3% of 2- to 17-year-olds in the general population have depression. Even compared with other chronic illnesses (including diabetes, heart disease, and cancer), children with epilepsy have a higher rate of depression, said Dr. Falcone.
More than 1 in 10 (10.9%) participants in the study exhibited signs of suicidality, as shown by having at least one positive response on the ASQ. “That’s a lot,” and much higher than the estimated rate in the general teen population, Dr. Falcone noted.
She noted that “these are just general kids with epilepsy” who had not been previously diagnosed with a psychiatric disorder.
“Depression, anxiety, and suicidality are very frequent comorbidities in patients with epilepsy; and even if a patient is not reporting any symptoms, we should be asking these questions to help them,” she said.
Study participants who had at least one positive response on the ASQ had a mean score of 32.1 on the SCARED, compared with a mean score of 18.3 for those who did not have a positive response on the ASQ (P = .003).
“We wanted to see if there was a direct association in our sample between anxiety and suicidal thoughts, and we found [that] yes there was,” Dr. Falcone said. There was also an association with depression. More than 26% of participants who scored 16 or higher on the CES-DC indicated at least one positive response on the ASQ. This is significantly higher than those who scored 15 or below on the CES-DC (P < .0001).
Bidirectional relationship
The findings suggest that either depression or anxiety may contribute to suicidal thoughts or behaviors, Dr. Dagar said. “It’s like two hands. It could be anxiety leading to suicidality, or it could be depression, or it could be both.”
Dr. Falcone noted that children with epilepsy who aren’t sure when they’ll get their next seizure, or who are bullied at school for being different, may be especially prone to anxiety or depression.
There’s a bit of a “chicken-and-egg” relationship between depression and epilepsy, a disorder affecting electrical signals in the brain, she said. Previous research has shown that a “bidirectional relationship” is involved.
“Even in patients with depression who are not diagnosed with epilepsy, the incidence of epilepsy is 3% higher just because you have depression,” Dr. Falcone said.
Suicidal youth tend to attempt suicide more than once. Dr. Falcone and colleagues are trying to intervene “at different levels,” be that in the hospital or as an outpatient, to prevent this from happening. “We want to find out what different things we can do to engage them and improve the probability they don’t reattempt,” she said.
All children and youth with epilepsy should be screened for anxiety, depression, and suicidal thoughts and behaviors. From age 10 years, children with epilepsy should be screened at least once a year, but those with a psychiatric disorder should be screened more often, Dr. Falcone added. The investigators note their findings need to be confirmed in larger, more diverse studies.
Importance of screening
Michael Privitera, MD, director of the Epilepsy Center and professor of neurology at the University of Cincinnati Gardner Neuroscience Institute, said the findings reinforce that, as with adults, depression and anxiety are common in children with epilepsy.
“Neurologists should take advantage of the many psychiatric screening tools available to identify these problems in their pediatric and adult patients,” Dr. Privitera said. Even more importantly, screening may help identify those who may be at highest risk of suicide.
The study was funded by the Health Resources Services Administration. The investigators and Dr. Privitera have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. In a study of more than 100 youth with the disorder, more than 40% had depression, 30% had anxiety, and about 1 in 10 exhibited signs of suicidal thoughts and behaviors.
These rates “are really worrisome” and highlight the need to screen all children and young adults with epilepsy for psychiatric disorders, said study author Tatiana Falcone, MD, assistant professor of neurology and child and adolescent psychiatry at the Cleveland Clinic.
“It’s very important to screen for suicidality and for depression and anxiety, even when patients aren’t reporting symptoms,” said Dr. Falcone.
Previous research shows children with epilepsy will attend the emergency room with symptoms such as headache or stomachache “when the main reason for the visit was the kid was suicidal,” Dr. Falcone said. “Unless you ask the specific question: ‘Are you having thoughts about hurting yourself?’ this will go unreported,” she added.
The findings were presented at the American Epilepsy Society’s 74th Annual Meeting, which was held online this year because of the COVID-19 pandemic.
Red flag
Not much is known about suicidality in children and youth with epilepsy except that depression and anxiety – the most common psychiatric comorbidities in this population – appear to contribute to suicidal thoughts.
Dr. Falcone said that she and her colleagues often see children and adolescents with epilepsy in their clinic who have attempted suicide. In recent years, the clinicians have increased efforts to try to identify them before they carry out a successful suicide attempt, said lead investigator Anjali Dagar, MD, clinical research psychiatry fellow at Cleveland Clinic.
The study included 119 patients aged 10-24 years (mean age, 15.8 years; 54.6% female). All attended an epilepsy clinic or underwent testing in the pediatric epilepsy monitoring unit at the Cleveland Clinic and did not have a psychiatric diagnosis.
Epilepsy severity ranged among study participants. About half were drug resistant and were at the center for surgical evaluation and the others were newly diagnosed.
Participants filled out questionnaires to self-report psychiatric conditions. The validated screening tools included the Center for Epidemiological Studies Depression Scale for Children (CES-DC), the Screen for Child Anxiety Related Emotional Disorders (SCARED), and the Ask Suicide–Screening Questions (ASQ).
A score of 15 or higher on the CES-DC indicates a risk for depression. On the SCARED test, a score higher than 32 indicates anxiety. Recent research has shown that anxiety is a main risk factor “in moving people from contemplating suicide to actually carrying it out,” Dr. Falcone said.
The ASQ includes four questions about suicidal thoughts and whether respondents have tried to hurt themselves. Dr. Dagar noted that a positive response to any of these questions should raise a red flag.
Very high rates
Results showed that almost one-third (30.2%) of the participants scored positive for anxiety on SCARED and 41.2% scored positive for depression on the CSE-DC. These are “very high” rates, Dr. Falcone said. For comparison, the rate of reported anxiety is less than 10% in school surveys.
In addition, the Centers for Disease Control and Prevention reports about 3% of 2- to 17-year-olds in the general population have depression. Even compared with other chronic illnesses (including diabetes, heart disease, and cancer), children with epilepsy have a higher rate of depression, said Dr. Falcone.
More than 1 in 10 (10.9%) participants in the study exhibited signs of suicidality, as shown by having at least one positive response on the ASQ. “That’s a lot,” and much higher than the estimated rate in the general teen population, Dr. Falcone noted.
She noted that “these are just general kids with epilepsy” who had not been previously diagnosed with a psychiatric disorder.
“Depression, anxiety, and suicidality are very frequent comorbidities in patients with epilepsy; and even if a patient is not reporting any symptoms, we should be asking these questions to help them,” she said.
Study participants who had at least one positive response on the ASQ had a mean score of 32.1 on the SCARED, compared with a mean score of 18.3 for those who did not have a positive response on the ASQ (P = .003).
“We wanted to see if there was a direct association in our sample between anxiety and suicidal thoughts, and we found [that] yes there was,” Dr. Falcone said. There was also an association with depression. More than 26% of participants who scored 16 or higher on the CES-DC indicated at least one positive response on the ASQ. This is significantly higher than those who scored 15 or below on the CES-DC (P < .0001).
Bidirectional relationship
The findings suggest that either depression or anxiety may contribute to suicidal thoughts or behaviors, Dr. Dagar said. “It’s like two hands. It could be anxiety leading to suicidality, or it could be depression, or it could be both.”
Dr. Falcone noted that children with epilepsy who aren’t sure when they’ll get their next seizure, or who are bullied at school for being different, may be especially prone to anxiety or depression.
There’s a bit of a “chicken-and-egg” relationship between depression and epilepsy, a disorder affecting electrical signals in the brain, she said. Previous research has shown that a “bidirectional relationship” is involved.
“Even in patients with depression who are not diagnosed with epilepsy, the incidence of epilepsy is 3% higher just because you have depression,” Dr. Falcone said.
Suicidal youth tend to attempt suicide more than once. Dr. Falcone and colleagues are trying to intervene “at different levels,” be that in the hospital or as an outpatient, to prevent this from happening. “We want to find out what different things we can do to engage them and improve the probability they don’t reattempt,” she said.
All children and youth with epilepsy should be screened for anxiety, depression, and suicidal thoughts and behaviors. From age 10 years, children with epilepsy should be screened at least once a year, but those with a psychiatric disorder should be screened more often, Dr. Falcone added. The investigators note their findings need to be confirmed in larger, more diverse studies.
Importance of screening
Michael Privitera, MD, director of the Epilepsy Center and professor of neurology at the University of Cincinnati Gardner Neuroscience Institute, said the findings reinforce that, as with adults, depression and anxiety are common in children with epilepsy.
“Neurologists should take advantage of the many psychiatric screening tools available to identify these problems in their pediatric and adult patients,” Dr. Privitera said. Even more importantly, screening may help identify those who may be at highest risk of suicide.
The study was funded by the Health Resources Services Administration. The investigators and Dr. Privitera have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. In a study of more than 100 youth with the disorder, more than 40% had depression, 30% had anxiety, and about 1 in 10 exhibited signs of suicidal thoughts and behaviors.
These rates “are really worrisome” and highlight the need to screen all children and young adults with epilepsy for psychiatric disorders, said study author Tatiana Falcone, MD, assistant professor of neurology and child and adolescent psychiatry at the Cleveland Clinic.
“It’s very important to screen for suicidality and for depression and anxiety, even when patients aren’t reporting symptoms,” said Dr. Falcone.
Previous research shows children with epilepsy will attend the emergency room with symptoms such as headache or stomachache “when the main reason for the visit was the kid was suicidal,” Dr. Falcone said. “Unless you ask the specific question: ‘Are you having thoughts about hurting yourself?’ this will go unreported,” she added.
The findings were presented at the American Epilepsy Society’s 74th Annual Meeting, which was held online this year because of the COVID-19 pandemic.
Red flag
Not much is known about suicidality in children and youth with epilepsy except that depression and anxiety – the most common psychiatric comorbidities in this population – appear to contribute to suicidal thoughts.
Dr. Falcone said that she and her colleagues often see children and adolescents with epilepsy in their clinic who have attempted suicide. In recent years, the clinicians have increased efforts to try to identify them before they carry out a successful suicide attempt, said lead investigator Anjali Dagar, MD, clinical research psychiatry fellow at Cleveland Clinic.
The study included 119 patients aged 10-24 years (mean age, 15.8 years; 54.6% female). All attended an epilepsy clinic or underwent testing in the pediatric epilepsy monitoring unit at the Cleveland Clinic and did not have a psychiatric diagnosis.
Epilepsy severity ranged among study participants. About half were drug resistant and were at the center for surgical evaluation and the others were newly diagnosed.
Participants filled out questionnaires to self-report psychiatric conditions. The validated screening tools included the Center for Epidemiological Studies Depression Scale for Children (CES-DC), the Screen for Child Anxiety Related Emotional Disorders (SCARED), and the Ask Suicide–Screening Questions (ASQ).
A score of 15 or higher on the CES-DC indicates a risk for depression. On the SCARED test, a score higher than 32 indicates anxiety. Recent research has shown that anxiety is a main risk factor “in moving people from contemplating suicide to actually carrying it out,” Dr. Falcone said.
The ASQ includes four questions about suicidal thoughts and whether respondents have tried to hurt themselves. Dr. Dagar noted that a positive response to any of these questions should raise a red flag.
Very high rates
Results showed that almost one-third (30.2%) of the participants scored positive for anxiety on SCARED and 41.2% scored positive for depression on the CSE-DC. These are “very high” rates, Dr. Falcone said. For comparison, the rate of reported anxiety is less than 10% in school surveys.
In addition, the Centers for Disease Control and Prevention reports about 3% of 2- to 17-year-olds in the general population have depression. Even compared with other chronic illnesses (including diabetes, heart disease, and cancer), children with epilepsy have a higher rate of depression, said Dr. Falcone.
More than 1 in 10 (10.9%) participants in the study exhibited signs of suicidality, as shown by having at least one positive response on the ASQ. “That’s a lot,” and much higher than the estimated rate in the general teen population, Dr. Falcone noted.
She noted that “these are just general kids with epilepsy” who had not been previously diagnosed with a psychiatric disorder.
“Depression, anxiety, and suicidality are very frequent comorbidities in patients with epilepsy; and even if a patient is not reporting any symptoms, we should be asking these questions to help them,” she said.
Study participants who had at least one positive response on the ASQ had a mean score of 32.1 on the SCARED, compared with a mean score of 18.3 for those who did not have a positive response on the ASQ (P = .003).
“We wanted to see if there was a direct association in our sample between anxiety and suicidal thoughts, and we found [that] yes there was,” Dr. Falcone said. There was also an association with depression. More than 26% of participants who scored 16 or higher on the CES-DC indicated at least one positive response on the ASQ. This is significantly higher than those who scored 15 or below on the CES-DC (P < .0001).
Bidirectional relationship
The findings suggest that either depression or anxiety may contribute to suicidal thoughts or behaviors, Dr. Dagar said. “It’s like two hands. It could be anxiety leading to suicidality, or it could be depression, or it could be both.”
Dr. Falcone noted that children with epilepsy who aren’t sure when they’ll get their next seizure, or who are bullied at school for being different, may be especially prone to anxiety or depression.
There’s a bit of a “chicken-and-egg” relationship between depression and epilepsy, a disorder affecting electrical signals in the brain, she said. Previous research has shown that a “bidirectional relationship” is involved.
“Even in patients with depression who are not diagnosed with epilepsy, the incidence of epilepsy is 3% higher just because you have depression,” Dr. Falcone said.
Suicidal youth tend to attempt suicide more than once. Dr. Falcone and colleagues are trying to intervene “at different levels,” be that in the hospital or as an outpatient, to prevent this from happening. “We want to find out what different things we can do to engage them and improve the probability they don’t reattempt,” she said.
All children and youth with epilepsy should be screened for anxiety, depression, and suicidal thoughts and behaviors. From age 10 years, children with epilepsy should be screened at least once a year, but those with a psychiatric disorder should be screened more often, Dr. Falcone added. The investigators note their findings need to be confirmed in larger, more diverse studies.
Importance of screening
Michael Privitera, MD, director of the Epilepsy Center and professor of neurology at the University of Cincinnati Gardner Neuroscience Institute, said the findings reinforce that, as with adults, depression and anxiety are common in children with epilepsy.
“Neurologists should take advantage of the many psychiatric screening tools available to identify these problems in their pediatric and adult patients,” Dr. Privitera said. Even more importantly, screening may help identify those who may be at highest risk of suicide.
The study was funded by the Health Resources Services Administration. The investigators and Dr. Privitera have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AES 2020
To D or not to D? Vitamin D doesn’t reduce falls in older adults
Higher doses of vitamin D supplementation not only show no benefit in the prevention of falls in older adults at increased risk of falling, compared with the lowest doses, but they appear to increase the risk, new research shows.
Based on the findings, supplemental vitamin D above the minimum dose of 200 IU/day likely has little benefit, lead author Lawrence J. Appel, MD, MPH, told this news organization.
“In the absence of any benefit of 1,000 IU/day versus 2,000 IU/day [of vitamin D supplementation] on falls, along with the potential for harm from doses above 1,000 IU/day, it is hard to recommend a dose above 200 IU/day in older-aged persons, unless there is a compelling reason,” asserted Dr. Appel, director of the Welch Center for Prevention, Epidemiology, and Clinical Research at Johns Hopkins Bloomberg School of Public Health in Baltimore.
“More is not always better – and it may even be worse,” when it comes to vitamin D’s role in the prevention of falls, he said.
The research, published in Annals of Internal Medicine, adds important evidence in the ongoing struggle to prevent falls, says Bruce R. Troen, MD, in an accompanying editorial.
“Falls and their deleterious consequences remain a substantial risk for older adults and a huge challenge for health care teams,” writes Dr. Troen, a physician-investigator with the Veterans Affairs Western New York Healthcare System.
However, commenting in an interview, Dr. Troen cautions: “There are many epidemiological studies that are correlative, not causative, that do show a likelihood for benefit [with vitamin D supplementation]. … Therefore, there’s no reason for clinicians to discontinue vitamin D in individuals because of this study.”
“If you’re monitoring an older adult who is frail and has multiple comorbidities, you want to know what their vitamin D level is [and] provide them an appropriate supplement if needed,” he emphasized.
Some guidelines already reflect the lack of evidence of any role of vitamin D supplementation in the prevention of falls, including those of the 2018 U.S. Preventive Services Task Force, which, in a reversal of its 2012 recommendation, now does not recommend vitamin D supplementation for fall prevention in older persons without osteoporosis or vitamin D deficiency, Dr. Appel and colleagues note.
No prevention of falls regardless of baseline vitamin D
As part of STURDY (Study to understand fall reduction and vitamin D in you), Dr. Appel and colleagues enrolled 688 community-dwelling participants who had an elevated risk of falling, defined as a serum 25-hydroxyvitamin D [25(OH)D] level of 25 to 72.5 nmol/L (10-29 ng/dL).
Participants were a mean age of 77.2 years and had a mean total 25(OH)D level of 55.3 nmol/L at enrollment.
They were randomized to one of four doses of vitamin D3, including 200 IU/day (the control group), or 1,000, 2,000, or 4,000 IU/day.
The highest doses were found to be associated with worse – not better – outcomes including a shorter time to hospitalization or death, compared with the 1,000-IU/day group. The higher-dose groups were therefore switched to a dose of 1,000 IU/day or lower, and all participants were followed for up to 2 years.
Overall, 63% experienced falls over the course of the study, which, though high, was consistent with the study’s criteria of participants having an elevated fall risk.
Of the 667 participants who completed the trial, no benefit in prevention of falling was seen across any of the doses, compared with the control group dose of 200 IU/day, regardless of participants’ baseline vitamin D levels.
Safety analyses showed that even in the 1,000-IU/day group, a higher risk of first serious fall and first fall with hospitalization was seen compared with the 200-IU/day group.
A limitation is that the study did not have a placebo group, however, “200 IU/day is a very small dose, probably homeopathic,” Dr. Appel said. “It was likely close to a placebo,” he said.
Caveats: comorbidities, subgroups
In his editorial, Dr. Troen notes other studies, including VITAL (Vitamin D and Omega-3 Trial) also found no reduction in falls with higher vitamin D doses; however, that study did not show any significant risks with the higher doses.
He adds that the current study lacks information on subsets of participants.
“We don’t have enough information about the existing comorbidities and medications that these people are on to be able to pull back the layers. Maybe there is a subgroup that should not be getting 4,000 IU, whereas another subgroup may not be harmed and you may decide that patient can benefit,” he said.
Furthermore, the trial doesn’t address groups such as nursing home residents.
“I have, for instance, 85-year-olds with vitamin D levels of maybe 20 nmol/L with multiple medical issues, but levels that low were not included in the study, so this is a tricky business, but the bottom line is first, do no harm,” he said.
“We really need trials that factor in the multiple different aspects so we can come up, hopefully, with a holistic and interdisciplinary approach, which is usually the best way to optimize care for frail older adults,” he concluded.
The study received funding from the National Institute of Aging.
A version of this article originally appeared on Medscape.com.
Higher doses of vitamin D supplementation not only show no benefit in the prevention of falls in older adults at increased risk of falling, compared with the lowest doses, but they appear to increase the risk, new research shows.
Based on the findings, supplemental vitamin D above the minimum dose of 200 IU/day likely has little benefit, lead author Lawrence J. Appel, MD, MPH, told this news organization.
“In the absence of any benefit of 1,000 IU/day versus 2,000 IU/day [of vitamin D supplementation] on falls, along with the potential for harm from doses above 1,000 IU/day, it is hard to recommend a dose above 200 IU/day in older-aged persons, unless there is a compelling reason,” asserted Dr. Appel, director of the Welch Center for Prevention, Epidemiology, and Clinical Research at Johns Hopkins Bloomberg School of Public Health in Baltimore.
“More is not always better – and it may even be worse,” when it comes to vitamin D’s role in the prevention of falls, he said.
The research, published in Annals of Internal Medicine, adds important evidence in the ongoing struggle to prevent falls, says Bruce R. Troen, MD, in an accompanying editorial.
“Falls and their deleterious consequences remain a substantial risk for older adults and a huge challenge for health care teams,” writes Dr. Troen, a physician-investigator with the Veterans Affairs Western New York Healthcare System.
However, commenting in an interview, Dr. Troen cautions: “There are many epidemiological studies that are correlative, not causative, that do show a likelihood for benefit [with vitamin D supplementation]. … Therefore, there’s no reason for clinicians to discontinue vitamin D in individuals because of this study.”
“If you’re monitoring an older adult who is frail and has multiple comorbidities, you want to know what their vitamin D level is [and] provide them an appropriate supplement if needed,” he emphasized.
Some guidelines already reflect the lack of evidence of any role of vitamin D supplementation in the prevention of falls, including those of the 2018 U.S. Preventive Services Task Force, which, in a reversal of its 2012 recommendation, now does not recommend vitamin D supplementation for fall prevention in older persons without osteoporosis or vitamin D deficiency, Dr. Appel and colleagues note.
No prevention of falls regardless of baseline vitamin D
As part of STURDY (Study to understand fall reduction and vitamin D in you), Dr. Appel and colleagues enrolled 688 community-dwelling participants who had an elevated risk of falling, defined as a serum 25-hydroxyvitamin D [25(OH)D] level of 25 to 72.5 nmol/L (10-29 ng/dL).
Participants were a mean age of 77.2 years and had a mean total 25(OH)D level of 55.3 nmol/L at enrollment.
They were randomized to one of four doses of vitamin D3, including 200 IU/day (the control group), or 1,000, 2,000, or 4,000 IU/day.
The highest doses were found to be associated with worse – not better – outcomes including a shorter time to hospitalization or death, compared with the 1,000-IU/day group. The higher-dose groups were therefore switched to a dose of 1,000 IU/day or lower, and all participants were followed for up to 2 years.
Overall, 63% experienced falls over the course of the study, which, though high, was consistent with the study’s criteria of participants having an elevated fall risk.
Of the 667 participants who completed the trial, no benefit in prevention of falling was seen across any of the doses, compared with the control group dose of 200 IU/day, regardless of participants’ baseline vitamin D levels.
Safety analyses showed that even in the 1,000-IU/day group, a higher risk of first serious fall and first fall with hospitalization was seen compared with the 200-IU/day group.
A limitation is that the study did not have a placebo group, however, “200 IU/day is a very small dose, probably homeopathic,” Dr. Appel said. “It was likely close to a placebo,” he said.
Caveats: comorbidities, subgroups
In his editorial, Dr. Troen notes other studies, including VITAL (Vitamin D and Omega-3 Trial) also found no reduction in falls with higher vitamin D doses; however, that study did not show any significant risks with the higher doses.
He adds that the current study lacks information on subsets of participants.
“We don’t have enough information about the existing comorbidities and medications that these people are on to be able to pull back the layers. Maybe there is a subgroup that should not be getting 4,000 IU, whereas another subgroup may not be harmed and you may decide that patient can benefit,” he said.
Furthermore, the trial doesn’t address groups such as nursing home residents.
“I have, for instance, 85-year-olds with vitamin D levels of maybe 20 nmol/L with multiple medical issues, but levels that low were not included in the study, so this is a tricky business, but the bottom line is first, do no harm,” he said.
“We really need trials that factor in the multiple different aspects so we can come up, hopefully, with a holistic and interdisciplinary approach, which is usually the best way to optimize care for frail older adults,” he concluded.
The study received funding from the National Institute of Aging.
A version of this article originally appeared on Medscape.com.
Higher doses of vitamin D supplementation not only show no benefit in the prevention of falls in older adults at increased risk of falling, compared with the lowest doses, but they appear to increase the risk, new research shows.
Based on the findings, supplemental vitamin D above the minimum dose of 200 IU/day likely has little benefit, lead author Lawrence J. Appel, MD, MPH, told this news organization.
“In the absence of any benefit of 1,000 IU/day versus 2,000 IU/day [of vitamin D supplementation] on falls, along with the potential for harm from doses above 1,000 IU/day, it is hard to recommend a dose above 200 IU/day in older-aged persons, unless there is a compelling reason,” asserted Dr. Appel, director of the Welch Center for Prevention, Epidemiology, and Clinical Research at Johns Hopkins Bloomberg School of Public Health in Baltimore.
“More is not always better – and it may even be worse,” when it comes to vitamin D’s role in the prevention of falls, he said.
The research, published in Annals of Internal Medicine, adds important evidence in the ongoing struggle to prevent falls, says Bruce R. Troen, MD, in an accompanying editorial.
“Falls and their deleterious consequences remain a substantial risk for older adults and a huge challenge for health care teams,” writes Dr. Troen, a physician-investigator with the Veterans Affairs Western New York Healthcare System.
However, commenting in an interview, Dr. Troen cautions: “There are many epidemiological studies that are correlative, not causative, that do show a likelihood for benefit [with vitamin D supplementation]. … Therefore, there’s no reason for clinicians to discontinue vitamin D in individuals because of this study.”
“If you’re monitoring an older adult who is frail and has multiple comorbidities, you want to know what their vitamin D level is [and] provide them an appropriate supplement if needed,” he emphasized.
Some guidelines already reflect the lack of evidence of any role of vitamin D supplementation in the prevention of falls, including those of the 2018 U.S. Preventive Services Task Force, which, in a reversal of its 2012 recommendation, now does not recommend vitamin D supplementation for fall prevention in older persons without osteoporosis or vitamin D deficiency, Dr. Appel and colleagues note.
No prevention of falls regardless of baseline vitamin D
As part of STURDY (Study to understand fall reduction and vitamin D in you), Dr. Appel and colleagues enrolled 688 community-dwelling participants who had an elevated risk of falling, defined as a serum 25-hydroxyvitamin D [25(OH)D] level of 25 to 72.5 nmol/L (10-29 ng/dL).
Participants were a mean age of 77.2 years and had a mean total 25(OH)D level of 55.3 nmol/L at enrollment.
They were randomized to one of four doses of vitamin D3, including 200 IU/day (the control group), or 1,000, 2,000, or 4,000 IU/day.
The highest doses were found to be associated with worse – not better – outcomes including a shorter time to hospitalization or death, compared with the 1,000-IU/day group. The higher-dose groups were therefore switched to a dose of 1,000 IU/day or lower, and all participants were followed for up to 2 years.
Overall, 63% experienced falls over the course of the study, which, though high, was consistent with the study’s criteria of participants having an elevated fall risk.
Of the 667 participants who completed the trial, no benefit in prevention of falling was seen across any of the doses, compared with the control group dose of 200 IU/day, regardless of participants’ baseline vitamin D levels.
Safety analyses showed that even in the 1,000-IU/day group, a higher risk of first serious fall and first fall with hospitalization was seen compared with the 200-IU/day group.
A limitation is that the study did not have a placebo group, however, “200 IU/day is a very small dose, probably homeopathic,” Dr. Appel said. “It was likely close to a placebo,” he said.
Caveats: comorbidities, subgroups
In his editorial, Dr. Troen notes other studies, including VITAL (Vitamin D and Omega-3 Trial) also found no reduction in falls with higher vitamin D doses; however, that study did not show any significant risks with the higher doses.
He adds that the current study lacks information on subsets of participants.
“We don’t have enough information about the existing comorbidities and medications that these people are on to be able to pull back the layers. Maybe there is a subgroup that should not be getting 4,000 IU, whereas another subgroup may not be harmed and you may decide that patient can benefit,” he said.
Furthermore, the trial doesn’t address groups such as nursing home residents.
“I have, for instance, 85-year-olds with vitamin D levels of maybe 20 nmol/L with multiple medical issues, but levels that low were not included in the study, so this is a tricky business, but the bottom line is first, do no harm,” he said.
“We really need trials that factor in the multiple different aspects so we can come up, hopefully, with a holistic and interdisciplinary approach, which is usually the best way to optimize care for frail older adults,” he concluded.
The study received funding from the National Institute of Aging.
A version of this article originally appeared on Medscape.com.
Demand for COVID vaccines expected to get heated – and fast
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
New child COVID-19 cases down in last weekly count
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 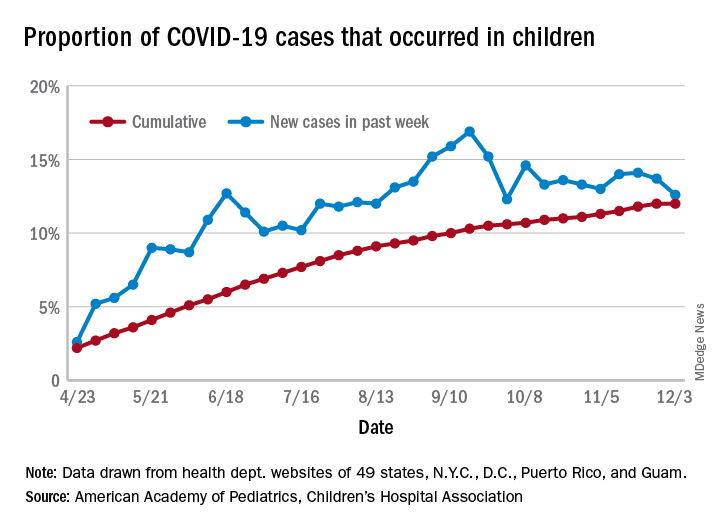
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
New residency matching sets record, says NRMP
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
COVID-19 fuels surge in overdose-related cardiac arrests
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Biden chooses California Attorney General Xavier Becerra to head HHS
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
PPE shortage crisis continues at most hospitals, survey shows
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
Infant’s COVID-19–related myocardial injury reversed
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
FROM JACC CASE REPORTS
Key clinical point: Children presenting with COVID-19 should be tested for heart failure.
Major finding: A 2-month-old infant with COVID-19 had acute but reversible myocardial injury.
Study details: Single case report.
Disclosures: Dr. Sharma, MD, has no relevant financial relationships to disclose.
Source: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.