User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Obesity, hypoxia predict severity in children with COVID-19
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
FROM THE JOURNAL OF PEDIATRICS
Diabetic retinopathy may predict greater risk of COVID-19 severity
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Medicare finalizes 2021 physician pay rule with E/M changes
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
CDC shortens COVID-19 quarantine time to 10 or 7 days, with conditions
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Noninvasive, low-cost CGM for type 2 diabetes coming in U.S. and EU
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
New AHA scientific statement on menopause and CVD risk
Changes in hormones, body composition, lipids, and vascular health during the menopause transition can increase a woman’s chance of developing cardiovascular disease (CVD) after menopause, the American Heart Association said in a scientific statement.
“This statement aims to raise awareness of both healthcare providers and women about the menopause transition as a time of increasing heart disease risk,” Samar R. El Khoudary, PhD, MPH, who chaired the writing group, said in an interview.
“As such, it emphasizes the importance of monitoring women’s health during midlife and targeting this stage as a critical window for applying early intervention strategies that aim to maintain a healthy heart and reduce the risk of heart disease,” said Dr. El Khoudary, of the University of Pittsburgh.
The statement was published online Nov. 30 in Circulation.
Evolution in knowledge
During the past 20 years, knowledge of how menopause might contribute to CVD has evolved “dramatically,” Dr. El Khoudary noted. The accumulated data consistently point to the menopause transition as a time of change in heart health.
“Importantly,” she said, the latest AHA guidelines for CVD prevention in women, published in 2011, do not include data now available on the menopause transition as a time of increased CVD risk.
“As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” said Dr. El Khoudary.
The statement provides a contemporary synthesis of the existing data on menopause and how it relates to CVD, the leading cause of death of U.S. women.
Earlier age at natural menopause has generally been found to be a marker of greater CVD risk. Iatrogenically induced menopause (bilateral oophorectomy) during the premenopausal period is also associated with higher CVD risk, the data suggest.
Vasomotor symptoms are associated with worse levels of CVD risk factors and measures of subclinical atherosclerosis. Sleep disturbance has also been linked to greater risk for subclinical CVD and worse CV health indexes in women during midlife.
Increases in central/visceral fat and decreases in lean muscle mass are more pronounced during the menopause transition. This increased central adiposity is associated with increased risk for mortality, even among those with normal body mass index, the writing group found.
Increases in lipid levels (LDL cholesterol and apolipoprotein B), metabolic syndrome risk, and vascular remodeling at midlife are driven by the menopause transition more than aging, whereas increases in blood pressure, insulin level, and glucose level are likely more influenced by chronological aging, they reported.
Lifestyle interventions
The writing group noted that, because of the increase in overall life expectancy in the United States, a significant proportion of women will spend up to 40% of their lives after menopause.
Yet data suggest that only 7.2% of women transitioning to menopause are meeting physical activity guidelines and that fewer than 20% of those women are consistently maintaining a healthy diet.
Limited data from randomized, controlled trials suggest that a multidimensional lifestyle intervention during the menopause transition can prevent weight gain and reduce blood pressure and levels of triglycerides, blood glucose, and insulin and reduce the incidence of subclinical carotid atherosclerosis, they pointed out.
“Novel data” indicate a reversal in the associations of HDL cholesterol with CVD risk over the menopause transition, suggesting that higher HDL cholesterol levels may not consistently reflect good cardiovascular health in middle-aged women, the group noted.
There are also data suggesting that starting menopause hormone therapy when younger than 60 years or within 10 years of menopause is associated with reduced CVD risk.
The group said further research is needed into the cardiometabolic effects of menopause hormone therapy, including effects associated with form, route, and duration of administration, in women traversing menopause.
They also noted that data for the primary and secondary prevention of atherosclerotic CVD and improved survival with lipid-lowering interventions “remain elusive” for women and that further study is needed to develop evidence-based recommendations tailored specifically to women.
The research had no commercial funding. Dr. El Khoudary has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Changes in hormones, body composition, lipids, and vascular health during the menopause transition can increase a woman’s chance of developing cardiovascular disease (CVD) after menopause, the American Heart Association said in a scientific statement.
“This statement aims to raise awareness of both healthcare providers and women about the menopause transition as a time of increasing heart disease risk,” Samar R. El Khoudary, PhD, MPH, who chaired the writing group, said in an interview.
“As such, it emphasizes the importance of monitoring women’s health during midlife and targeting this stage as a critical window for applying early intervention strategies that aim to maintain a healthy heart and reduce the risk of heart disease,” said Dr. El Khoudary, of the University of Pittsburgh.
The statement was published online Nov. 30 in Circulation.
Evolution in knowledge
During the past 20 years, knowledge of how menopause might contribute to CVD has evolved “dramatically,” Dr. El Khoudary noted. The accumulated data consistently point to the menopause transition as a time of change in heart health.
“Importantly,” she said, the latest AHA guidelines for CVD prevention in women, published in 2011, do not include data now available on the menopause transition as a time of increased CVD risk.
“As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” said Dr. El Khoudary.
The statement provides a contemporary synthesis of the existing data on menopause and how it relates to CVD, the leading cause of death of U.S. women.
Earlier age at natural menopause has generally been found to be a marker of greater CVD risk. Iatrogenically induced menopause (bilateral oophorectomy) during the premenopausal period is also associated with higher CVD risk, the data suggest.
Vasomotor symptoms are associated with worse levels of CVD risk factors and measures of subclinical atherosclerosis. Sleep disturbance has also been linked to greater risk for subclinical CVD and worse CV health indexes in women during midlife.
Increases in central/visceral fat and decreases in lean muscle mass are more pronounced during the menopause transition. This increased central adiposity is associated with increased risk for mortality, even among those with normal body mass index, the writing group found.
Increases in lipid levels (LDL cholesterol and apolipoprotein B), metabolic syndrome risk, and vascular remodeling at midlife are driven by the menopause transition more than aging, whereas increases in blood pressure, insulin level, and glucose level are likely more influenced by chronological aging, they reported.
Lifestyle interventions
The writing group noted that, because of the increase in overall life expectancy in the United States, a significant proportion of women will spend up to 40% of their lives after menopause.
Yet data suggest that only 7.2% of women transitioning to menopause are meeting physical activity guidelines and that fewer than 20% of those women are consistently maintaining a healthy diet.
Limited data from randomized, controlled trials suggest that a multidimensional lifestyle intervention during the menopause transition can prevent weight gain and reduce blood pressure and levels of triglycerides, blood glucose, and insulin and reduce the incidence of subclinical carotid atherosclerosis, they pointed out.
“Novel data” indicate a reversal in the associations of HDL cholesterol with CVD risk over the menopause transition, suggesting that higher HDL cholesterol levels may not consistently reflect good cardiovascular health in middle-aged women, the group noted.
There are also data suggesting that starting menopause hormone therapy when younger than 60 years or within 10 years of menopause is associated with reduced CVD risk.
The group said further research is needed into the cardiometabolic effects of menopause hormone therapy, including effects associated with form, route, and duration of administration, in women traversing menopause.
They also noted that data for the primary and secondary prevention of atherosclerotic CVD and improved survival with lipid-lowering interventions “remain elusive” for women and that further study is needed to develop evidence-based recommendations tailored specifically to women.
The research had no commercial funding. Dr. El Khoudary has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Changes in hormones, body composition, lipids, and vascular health during the menopause transition can increase a woman’s chance of developing cardiovascular disease (CVD) after menopause, the American Heart Association said in a scientific statement.
“This statement aims to raise awareness of both healthcare providers and women about the menopause transition as a time of increasing heart disease risk,” Samar R. El Khoudary, PhD, MPH, who chaired the writing group, said in an interview.
“As such, it emphasizes the importance of monitoring women’s health during midlife and targeting this stage as a critical window for applying early intervention strategies that aim to maintain a healthy heart and reduce the risk of heart disease,” said Dr. El Khoudary, of the University of Pittsburgh.
The statement was published online Nov. 30 in Circulation.
Evolution in knowledge
During the past 20 years, knowledge of how menopause might contribute to CVD has evolved “dramatically,” Dr. El Khoudary noted. The accumulated data consistently point to the menopause transition as a time of change in heart health.
“Importantly,” she said, the latest AHA guidelines for CVD prevention in women, published in 2011, do not include data now available on the menopause transition as a time of increased CVD risk.
“As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” said Dr. El Khoudary.
The statement provides a contemporary synthesis of the existing data on menopause and how it relates to CVD, the leading cause of death of U.S. women.
Earlier age at natural menopause has generally been found to be a marker of greater CVD risk. Iatrogenically induced menopause (bilateral oophorectomy) during the premenopausal period is also associated with higher CVD risk, the data suggest.
Vasomotor symptoms are associated with worse levels of CVD risk factors and measures of subclinical atherosclerosis. Sleep disturbance has also been linked to greater risk for subclinical CVD and worse CV health indexes in women during midlife.
Increases in central/visceral fat and decreases in lean muscle mass are more pronounced during the menopause transition. This increased central adiposity is associated with increased risk for mortality, even among those with normal body mass index, the writing group found.
Increases in lipid levels (LDL cholesterol and apolipoprotein B), metabolic syndrome risk, and vascular remodeling at midlife are driven by the menopause transition more than aging, whereas increases in blood pressure, insulin level, and glucose level are likely more influenced by chronological aging, they reported.
Lifestyle interventions
The writing group noted that, because of the increase in overall life expectancy in the United States, a significant proportion of women will spend up to 40% of their lives after menopause.
Yet data suggest that only 7.2% of women transitioning to menopause are meeting physical activity guidelines and that fewer than 20% of those women are consistently maintaining a healthy diet.
Limited data from randomized, controlled trials suggest that a multidimensional lifestyle intervention during the menopause transition can prevent weight gain and reduce blood pressure and levels of triglycerides, blood glucose, and insulin and reduce the incidence of subclinical carotid atherosclerosis, they pointed out.
“Novel data” indicate a reversal in the associations of HDL cholesterol with CVD risk over the menopause transition, suggesting that higher HDL cholesterol levels may not consistently reflect good cardiovascular health in middle-aged women, the group noted.
There are also data suggesting that starting menopause hormone therapy when younger than 60 years or within 10 years of menopause is associated with reduced CVD risk.
The group said further research is needed into the cardiometabolic effects of menopause hormone therapy, including effects associated with form, route, and duration of administration, in women traversing menopause.
They also noted that data for the primary and secondary prevention of atherosclerotic CVD and improved survival with lipid-lowering interventions “remain elusive” for women and that further study is needed to develop evidence-based recommendations tailored specifically to women.
The research had no commercial funding. Dr. El Khoudary has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pandemic increases need for home-based care with remote monitoring of patients
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
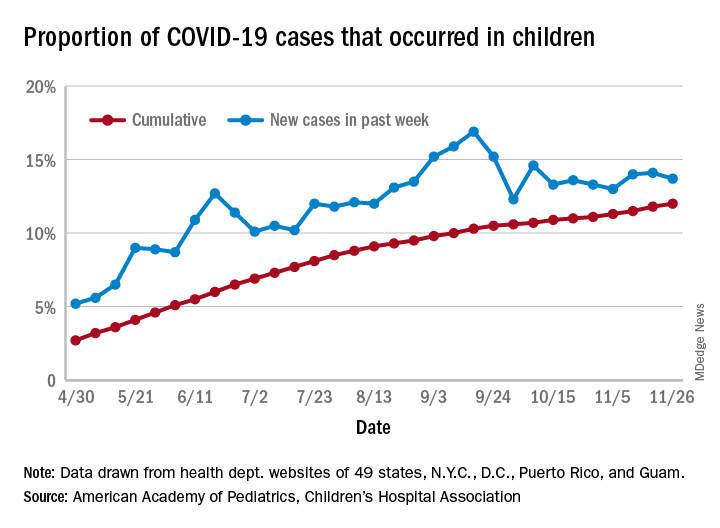
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
ACIP: Health workers, long-term care residents first tier for COVID-19 vaccine
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
What to do when anticoagulation fails cancer patients
When a patient with cancer develops venous thromboembolism despite anticoagulation, how to help them comes down to clinical judgment, according to hematologist Neil Zakai, MD, associate professor at the University of Vermont, Burlington.
“Unfortunately,” when it comes to “anticoagulation failure, we are entering an evidence free-zone,” with no large trials to guide management and only a few guiding principles, he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The first thing is to check if there was an inciting incident, such as medical noncompliance, an infection, or an interruption of anticoagulation. Dr. Zakai said he’s even had cancer patients develop heparin-induced thrombocytopenia when switched to enoxaparin from a direct oral anticoagulants (DOAC) for a procedure.
Once the underlying problem is addressed, patients may be able to continue with their original anticoagulant.
However, cancer progression is the main reason anticoagulation fails. “In general, it is very difficult to control cancer thrombosis if you can’t control cancer progression,” Dr. Zakai said.
In those cases, he steps up anticoagulation. Prophylactic dosing is increased to full treatment dosing, and patients on a DOAC are generally switched to a low molecular weight heparin (LMWH).
If patients are already on LMWH once daily, they will be bumped up to twice daily dosing; for instance, enoxaparin 1 mg/kg b.i.d. instead of 1.5 mg/kg q.d. Dr. Zakai said he’s gone as high at 2 or even 2.5 mg/kg to control thrombosis, without excessive bleeding.
In general, anticoagulation for thrombosis prophylaxis continues as long as the cancer is active, and certainly while patients are on hormonal treatments such as tamoxifen, which increases the risk.
Dr. Zakai stressed that both thrombosis and bleeding risk change for cancer patients over time, and treatment needs to keep up.
“I continuously assess the risk and benefit of anticoagulation. At certain times” such as during and for a few months after hospitalization, thrombosis risk increases; at other times, bleeding risk is higher. “You need to actively change your anticoagulation during those periods,” and tailor therapy based on transient risk factors. “People with cancer have peaks and troughs for their risk that we don’t take advantage of,” he said.
Dr. Zakai generally favors apixaban or enoxaparin for prophylaxis, carefully monitoring patients for bleeding and, for the DOAC, drug interactions with antiemetics, dexamethasone, and certain chemotherapy drugs.
He noted a recent trial that found a 59% reduction in venous thromboembolism risk in ambulatory cancer patients with apixaban 2.5 mg twice daily over 6 months, versus placebo, and a 6% absolute reduction, but at the cost of a twofold increase in bleeding risk, with an absolute 1.7% increase.
Dr. Zakai cautioned that patients in trials are selected for higher VTE and lower bleeding risks, so outcomes might “poorly reflect real world populations.” Dr. Zakai did not have any industry disclosures. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
When a patient with cancer develops venous thromboembolism despite anticoagulation, how to help them comes down to clinical judgment, according to hematologist Neil Zakai, MD, associate professor at the University of Vermont, Burlington.
“Unfortunately,” when it comes to “anticoagulation failure, we are entering an evidence free-zone,” with no large trials to guide management and only a few guiding principles, he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The first thing is to check if there was an inciting incident, such as medical noncompliance, an infection, or an interruption of anticoagulation. Dr. Zakai said he’s even had cancer patients develop heparin-induced thrombocytopenia when switched to enoxaparin from a direct oral anticoagulants (DOAC) for a procedure.
Once the underlying problem is addressed, patients may be able to continue with their original anticoagulant.
However, cancer progression is the main reason anticoagulation fails. “In general, it is very difficult to control cancer thrombosis if you can’t control cancer progression,” Dr. Zakai said.
In those cases, he steps up anticoagulation. Prophylactic dosing is increased to full treatment dosing, and patients on a DOAC are generally switched to a low molecular weight heparin (LMWH).
If patients are already on LMWH once daily, they will be bumped up to twice daily dosing; for instance, enoxaparin 1 mg/kg b.i.d. instead of 1.5 mg/kg q.d. Dr. Zakai said he’s gone as high at 2 or even 2.5 mg/kg to control thrombosis, without excessive bleeding.
In general, anticoagulation for thrombosis prophylaxis continues as long as the cancer is active, and certainly while patients are on hormonal treatments such as tamoxifen, which increases the risk.
Dr. Zakai stressed that both thrombosis and bleeding risk change for cancer patients over time, and treatment needs to keep up.
“I continuously assess the risk and benefit of anticoagulation. At certain times” such as during and for a few months after hospitalization, thrombosis risk increases; at other times, bleeding risk is higher. “You need to actively change your anticoagulation during those periods,” and tailor therapy based on transient risk factors. “People with cancer have peaks and troughs for their risk that we don’t take advantage of,” he said.
Dr. Zakai generally favors apixaban or enoxaparin for prophylaxis, carefully monitoring patients for bleeding and, for the DOAC, drug interactions with antiemetics, dexamethasone, and certain chemotherapy drugs.
He noted a recent trial that found a 59% reduction in venous thromboembolism risk in ambulatory cancer patients with apixaban 2.5 mg twice daily over 6 months, versus placebo, and a 6% absolute reduction, but at the cost of a twofold increase in bleeding risk, with an absolute 1.7% increase.
Dr. Zakai cautioned that patients in trials are selected for higher VTE and lower bleeding risks, so outcomes might “poorly reflect real world populations.” Dr. Zakai did not have any industry disclosures. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
When a patient with cancer develops venous thromboembolism despite anticoagulation, how to help them comes down to clinical judgment, according to hematologist Neil Zakai, MD, associate professor at the University of Vermont, Burlington.
“Unfortunately,” when it comes to “anticoagulation failure, we are entering an evidence free-zone,” with no large trials to guide management and only a few guiding principles, he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The first thing is to check if there was an inciting incident, such as medical noncompliance, an infection, or an interruption of anticoagulation. Dr. Zakai said he’s even had cancer patients develop heparin-induced thrombocytopenia when switched to enoxaparin from a direct oral anticoagulants (DOAC) for a procedure.
Once the underlying problem is addressed, patients may be able to continue with their original anticoagulant.
However, cancer progression is the main reason anticoagulation fails. “In general, it is very difficult to control cancer thrombosis if you can’t control cancer progression,” Dr. Zakai said.
In those cases, he steps up anticoagulation. Prophylactic dosing is increased to full treatment dosing, and patients on a DOAC are generally switched to a low molecular weight heparin (LMWH).
If patients are already on LMWH once daily, they will be bumped up to twice daily dosing; for instance, enoxaparin 1 mg/kg b.i.d. instead of 1.5 mg/kg q.d. Dr. Zakai said he’s gone as high at 2 or even 2.5 mg/kg to control thrombosis, without excessive bleeding.
In general, anticoagulation for thrombosis prophylaxis continues as long as the cancer is active, and certainly while patients are on hormonal treatments such as tamoxifen, which increases the risk.
Dr. Zakai stressed that both thrombosis and bleeding risk change for cancer patients over time, and treatment needs to keep up.
“I continuously assess the risk and benefit of anticoagulation. At certain times” such as during and for a few months after hospitalization, thrombosis risk increases; at other times, bleeding risk is higher. “You need to actively change your anticoagulation during those periods,” and tailor therapy based on transient risk factors. “People with cancer have peaks and troughs for their risk that we don’t take advantage of,” he said.
Dr. Zakai generally favors apixaban or enoxaparin for prophylaxis, carefully monitoring patients for bleeding and, for the DOAC, drug interactions with antiemetics, dexamethasone, and certain chemotherapy drugs.
He noted a recent trial that found a 59% reduction in venous thromboembolism risk in ambulatory cancer patients with apixaban 2.5 mg twice daily over 6 months, versus placebo, and a 6% absolute reduction, but at the cost of a twofold increase in bleeding risk, with an absolute 1.7% increase.
Dr. Zakai cautioned that patients in trials are selected for higher VTE and lower bleeding risks, so outcomes might “poorly reflect real world populations.” Dr. Zakai did not have any industry disclosures. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
FROM 2020 UNNH

