User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Cardiologists say rights to maternity leave violated
A survey of 323 women cardiologists who were working while they were pregnant showed that nearly 75% experienced discriminatory maternity-leave practices, some of which were likely violations of the Family and Medical Leave Act (FMLA).
More than 40% saw their salaries decreased during their year of pregnancy, 38% were required to perform extra service or call before taking maternity leave, exposing them to occupational hazards such as radiation, and 40% experienced a pregnancy complication, significantly higher than the general population and other medical specialties.
Additionally, of those who performed extra service or call, 18% were placed on bedrest before delivery, compared with 7.4% who did not perform extra service or call.
More than half of respondents reported that pregnancy negatively impacted their careers, and 42.4% said they experienced pressure to return to work and a delay in promotions, both illegal practices under the FMLA.
The survey is published in the Journal of the American College of Cardiology.
“Childbearing is difficult for women in cardiology with more than double the rate of gestational complications of the U.S. population, frequent income loss out of proportion to reduced productivity, and for nearly half, has an adverse impact on their career,” lead author Martha Gulati, MD, University of Arizona, Phoenix, said in a statement.
“While many professions struggle to create environments supportive of pregnancy and child-rearing, the prevalence of illegal behavior in cardiology is quite high and presents substantial legal risk for employers,” Dr. Gulati added.
C. Noel Bairey Merz, MD, professor of cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles, and a coauthor of the survey, told this news organization that it’s not surprising that such a situation exists, even “in this day and age.”
“I’m not surprised as a woman in cardiology myself. I was told by my training director that if I took off more than my allowed sick leave when I had my first and second children, I would have to repeat the year of training, so not surprised at all. I hear this from colleagues all the time,” Dr. Bairey Merz said.
The exchange left her feeling fearful for her career.
“Who wants to repeat a year? It pushes you back from a career standpoint, financially, everything. It also made me angry. I had a colleague who busted his leg in a motorcycle accident. He was unable to do any procedures for 16 weeks, and he didn’t have to repeat the year,” she pointed out.
The challenge that pregnancy represents is frequently cited by women as a deterrent for applying for a cardiology fellowship, Laxmi S. Mehta, MD, Ohio State University, Columbus, and colleagues wrote in an accompanying editorial.
The findings from the survey “reveal restrictive maternity leave data in a profession that has historically and currently continues to have a diversity problem,” they wrote.
“Maternity and pregnancy issues are a thing in cardiology,” Dr. Mehta said in an interview. “It’s one of the reasons why women get deterred from going into the field. It makes it challenging to choose cardiology if you perceive that the culture is negative, that it’s hard to be pregnant, or to bear children, or to take care of them post partum. It is problematic and it should not be occurring now.”
Leadership that condones such restrictive policies or even promotes them through ignorance and inaction needs to be held accountable, she added.
“We need to move forward from this negativity and make it more warm and welcoming to have families, whether you are a trainee or a practicing cardiologist, male or female. We need transparent and consistent parental leave policies and things like lactation support when a woman returns to work. That is a big issue,” Dr. Mehta said.
Having cardiovascular leaders champion the cause of adequate maternity and paternity leave are crucial to creating a newer, inclusive environment in cardiology.
As an example, Dr. Mehta recounted her own experience when she was in training 17 years ago.
“When I interviewed for a cardiology fellowship, one of the female program directors asked me if I was planning to have children, because if I did, the other fellows wouldn’t like it if they had to cover for me,” she said. “I ended up doing my fellowship where the chief of cardiology encouraged me to have children. He said: ‘Have your children during training, we will support you.’ And he did. I still had to do all of the call make-up and that stuff, but I worked in a supportive environment, and it made all the difference.”
“It’s about allyship,” she added. “You will have some people who are supportive and some who are not, but when you have the chief supporting you, you have a strong ally.”
The researchers suggest that one strategy is to temporarily replace cardiologists on maternity leave with locums, or “deepen the bench of coverage for clinical work, as is done for other absences. Given the expanding coverage of parental and family medical leaves, and awareness of these issues nationally, the need for this is likely to become less of an exception and more the rule.”
For example, nine states and Washington, D.C. now provide paid parental leave, they wrote, “and there is pending legislation in others.”
Dr. Bairey Merz and Dr. Mehta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A survey of 323 women cardiologists who were working while they were pregnant showed that nearly 75% experienced discriminatory maternity-leave practices, some of which were likely violations of the Family and Medical Leave Act (FMLA).
More than 40% saw their salaries decreased during their year of pregnancy, 38% were required to perform extra service or call before taking maternity leave, exposing them to occupational hazards such as radiation, and 40% experienced a pregnancy complication, significantly higher than the general population and other medical specialties.
Additionally, of those who performed extra service or call, 18% were placed on bedrest before delivery, compared with 7.4% who did not perform extra service or call.
More than half of respondents reported that pregnancy negatively impacted their careers, and 42.4% said they experienced pressure to return to work and a delay in promotions, both illegal practices under the FMLA.
The survey is published in the Journal of the American College of Cardiology.
“Childbearing is difficult for women in cardiology with more than double the rate of gestational complications of the U.S. population, frequent income loss out of proportion to reduced productivity, and for nearly half, has an adverse impact on their career,” lead author Martha Gulati, MD, University of Arizona, Phoenix, said in a statement.
“While many professions struggle to create environments supportive of pregnancy and child-rearing, the prevalence of illegal behavior in cardiology is quite high and presents substantial legal risk for employers,” Dr. Gulati added.
C. Noel Bairey Merz, MD, professor of cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles, and a coauthor of the survey, told this news organization that it’s not surprising that such a situation exists, even “in this day and age.”
“I’m not surprised as a woman in cardiology myself. I was told by my training director that if I took off more than my allowed sick leave when I had my first and second children, I would have to repeat the year of training, so not surprised at all. I hear this from colleagues all the time,” Dr. Bairey Merz said.
The exchange left her feeling fearful for her career.
“Who wants to repeat a year? It pushes you back from a career standpoint, financially, everything. It also made me angry. I had a colleague who busted his leg in a motorcycle accident. He was unable to do any procedures for 16 weeks, and he didn’t have to repeat the year,” she pointed out.
The challenge that pregnancy represents is frequently cited by women as a deterrent for applying for a cardiology fellowship, Laxmi S. Mehta, MD, Ohio State University, Columbus, and colleagues wrote in an accompanying editorial.
The findings from the survey “reveal restrictive maternity leave data in a profession that has historically and currently continues to have a diversity problem,” they wrote.
“Maternity and pregnancy issues are a thing in cardiology,” Dr. Mehta said in an interview. “It’s one of the reasons why women get deterred from going into the field. It makes it challenging to choose cardiology if you perceive that the culture is negative, that it’s hard to be pregnant, or to bear children, or to take care of them post partum. It is problematic and it should not be occurring now.”
Leadership that condones such restrictive policies or even promotes them through ignorance and inaction needs to be held accountable, she added.
“We need to move forward from this negativity and make it more warm and welcoming to have families, whether you are a trainee or a practicing cardiologist, male or female. We need transparent and consistent parental leave policies and things like lactation support when a woman returns to work. That is a big issue,” Dr. Mehta said.
Having cardiovascular leaders champion the cause of adequate maternity and paternity leave are crucial to creating a newer, inclusive environment in cardiology.
As an example, Dr. Mehta recounted her own experience when she was in training 17 years ago.
“When I interviewed for a cardiology fellowship, one of the female program directors asked me if I was planning to have children, because if I did, the other fellows wouldn’t like it if they had to cover for me,” she said. “I ended up doing my fellowship where the chief of cardiology encouraged me to have children. He said: ‘Have your children during training, we will support you.’ And he did. I still had to do all of the call make-up and that stuff, but I worked in a supportive environment, and it made all the difference.”
“It’s about allyship,” she added. “You will have some people who are supportive and some who are not, but when you have the chief supporting you, you have a strong ally.”
The researchers suggest that one strategy is to temporarily replace cardiologists on maternity leave with locums, or “deepen the bench of coverage for clinical work, as is done for other absences. Given the expanding coverage of parental and family medical leaves, and awareness of these issues nationally, the need for this is likely to become less of an exception and more the rule.”
For example, nine states and Washington, D.C. now provide paid parental leave, they wrote, “and there is pending legislation in others.”
Dr. Bairey Merz and Dr. Mehta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A survey of 323 women cardiologists who were working while they were pregnant showed that nearly 75% experienced discriminatory maternity-leave practices, some of which were likely violations of the Family and Medical Leave Act (FMLA).
More than 40% saw their salaries decreased during their year of pregnancy, 38% were required to perform extra service or call before taking maternity leave, exposing them to occupational hazards such as radiation, and 40% experienced a pregnancy complication, significantly higher than the general population and other medical specialties.
Additionally, of those who performed extra service or call, 18% were placed on bedrest before delivery, compared with 7.4% who did not perform extra service or call.
More than half of respondents reported that pregnancy negatively impacted their careers, and 42.4% said they experienced pressure to return to work and a delay in promotions, both illegal practices under the FMLA.
The survey is published in the Journal of the American College of Cardiology.
“Childbearing is difficult for women in cardiology with more than double the rate of gestational complications of the U.S. population, frequent income loss out of proportion to reduced productivity, and for nearly half, has an adverse impact on their career,” lead author Martha Gulati, MD, University of Arizona, Phoenix, said in a statement.
“While many professions struggle to create environments supportive of pregnancy and child-rearing, the prevalence of illegal behavior in cardiology is quite high and presents substantial legal risk for employers,” Dr. Gulati added.
C. Noel Bairey Merz, MD, professor of cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles, and a coauthor of the survey, told this news organization that it’s not surprising that such a situation exists, even “in this day and age.”
“I’m not surprised as a woman in cardiology myself. I was told by my training director that if I took off more than my allowed sick leave when I had my first and second children, I would have to repeat the year of training, so not surprised at all. I hear this from colleagues all the time,” Dr. Bairey Merz said.
The exchange left her feeling fearful for her career.
“Who wants to repeat a year? It pushes you back from a career standpoint, financially, everything. It also made me angry. I had a colleague who busted his leg in a motorcycle accident. He was unable to do any procedures for 16 weeks, and he didn’t have to repeat the year,” she pointed out.
The challenge that pregnancy represents is frequently cited by women as a deterrent for applying for a cardiology fellowship, Laxmi S. Mehta, MD, Ohio State University, Columbus, and colleagues wrote in an accompanying editorial.
The findings from the survey “reveal restrictive maternity leave data in a profession that has historically and currently continues to have a diversity problem,” they wrote.
“Maternity and pregnancy issues are a thing in cardiology,” Dr. Mehta said in an interview. “It’s one of the reasons why women get deterred from going into the field. It makes it challenging to choose cardiology if you perceive that the culture is negative, that it’s hard to be pregnant, or to bear children, or to take care of them post partum. It is problematic and it should not be occurring now.”
Leadership that condones such restrictive policies or even promotes them through ignorance and inaction needs to be held accountable, she added.
“We need to move forward from this negativity and make it more warm and welcoming to have families, whether you are a trainee or a practicing cardiologist, male or female. We need transparent and consistent parental leave policies and things like lactation support when a woman returns to work. That is a big issue,” Dr. Mehta said.
Having cardiovascular leaders champion the cause of adequate maternity and paternity leave are crucial to creating a newer, inclusive environment in cardiology.
As an example, Dr. Mehta recounted her own experience when she was in training 17 years ago.
“When I interviewed for a cardiology fellowship, one of the female program directors asked me if I was planning to have children, because if I did, the other fellows wouldn’t like it if they had to cover for me,” she said. “I ended up doing my fellowship where the chief of cardiology encouraged me to have children. He said: ‘Have your children during training, we will support you.’ And he did. I still had to do all of the call make-up and that stuff, but I worked in a supportive environment, and it made all the difference.”
“It’s about allyship,” she added. “You will have some people who are supportive and some who are not, but when you have the chief supporting you, you have a strong ally.”
The researchers suggest that one strategy is to temporarily replace cardiologists on maternity leave with locums, or “deepen the bench of coverage for clinical work, as is done for other absences. Given the expanding coverage of parental and family medical leaves, and awareness of these issues nationally, the need for this is likely to become less of an exception and more the rule.”
For example, nine states and Washington, D.C. now provide paid parental leave, they wrote, “and there is pending legislation in others.”
Dr. Bairey Merz and Dr. Mehta reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Relax. The dog-tor will see you now
Patients in the emergency room who spent just 10 minutes with a trained therapy dog reported less pain, anxiety, and depression and improved well-being, researchers from the University of Saskatchewan in Canada found.
“The ER is an important community resource but also a scary place for most people,” James Stempien, MD, provincial department head of emergency medicine with the Saskatchewan Health Authority, who worked on the study, said in an interview.
“People tend to visit the ER on the worst day of their life, either for them or a loved one. Interacting with a therapy dog can make the ER visit a little calmer. We have also seen benefit for the staff that get to interact with the dogs as well,” he says.
“Thanks to our wonderful therapy dog volunteer teams, the cost is minimal and the result is priceless,” Dr. Stempien says.
The study, published in the journal PLOS One, builds on earlier “uncontrolled” studies by the Saskatchewan team.
Those studies showed that most ER patients wanted to visit with the therapy dog, if given a chance. After the encounter, patients reported feeling more comfortable, happier, and less distressed while waiting in the ER.
“A controlled trial was the natural next step,” says study investigator Colleen Dell, PhD, of One Health and Wellness at the University of Saskatchewan.
The study was done at the Royal University Hospital (RUH) in Saskatoon, Saskatchewan -- the first emergency department in Canada to introduce therapy dogs to improve the experience of waiting patients.
Nearly 200 adults visiting the ER received either a 10-minute visit with a therapy dog and its handler in addition to usual care or just usual care.
“This did not occur in patients in the ER who did not visit with a therapy dog.
“This gives us confidence in the intervention,” Dr. Dell says.
Pain is a major reason that patients come to the ER, and interactions with a therapy dog may distract from that pain, the researchers believe.
The study results lend more evidence to research that shows animals can help in medical settings, says Kara Rauscher, a licensed social worker and interim director of behavioral health for Nashville CARES in Tennessee, who wasn’t involved in the study.
“There are clearly opportunities to replicate this study in other emergency departments to strengthen our understanding of the potential benefits of these programs,” she says.
Part of her work at Nashville CARES, an AIDS service organization, has been supporting care that moves away from questions like, “What’s wrong with you?” to patient-focused questions like, “What happened to you?” It is a practice known as trauma-informed care.
“This includes bringing in therapy dogs for staff to spend time with during the workday; anecdotally, our staff reported a reduction in stress and improvements in mood,” Ms. Rauscher says.
A version of this article first appeared on WebMD.com.
Patients in the emergency room who spent just 10 minutes with a trained therapy dog reported less pain, anxiety, and depression and improved well-being, researchers from the University of Saskatchewan in Canada found.
“The ER is an important community resource but also a scary place for most people,” James Stempien, MD, provincial department head of emergency medicine with the Saskatchewan Health Authority, who worked on the study, said in an interview.
“People tend to visit the ER on the worst day of their life, either for them or a loved one. Interacting with a therapy dog can make the ER visit a little calmer. We have also seen benefit for the staff that get to interact with the dogs as well,” he says.
“Thanks to our wonderful therapy dog volunteer teams, the cost is minimal and the result is priceless,” Dr. Stempien says.
The study, published in the journal PLOS One, builds on earlier “uncontrolled” studies by the Saskatchewan team.
Those studies showed that most ER patients wanted to visit with the therapy dog, if given a chance. After the encounter, patients reported feeling more comfortable, happier, and less distressed while waiting in the ER.
“A controlled trial was the natural next step,” says study investigator Colleen Dell, PhD, of One Health and Wellness at the University of Saskatchewan.
The study was done at the Royal University Hospital (RUH) in Saskatoon, Saskatchewan -- the first emergency department in Canada to introduce therapy dogs to improve the experience of waiting patients.
Nearly 200 adults visiting the ER received either a 10-minute visit with a therapy dog and its handler in addition to usual care or just usual care.
“This did not occur in patients in the ER who did not visit with a therapy dog.
“This gives us confidence in the intervention,” Dr. Dell says.
Pain is a major reason that patients come to the ER, and interactions with a therapy dog may distract from that pain, the researchers believe.
The study results lend more evidence to research that shows animals can help in medical settings, says Kara Rauscher, a licensed social worker and interim director of behavioral health for Nashville CARES in Tennessee, who wasn’t involved in the study.
“There are clearly opportunities to replicate this study in other emergency departments to strengthen our understanding of the potential benefits of these programs,” she says.
Part of her work at Nashville CARES, an AIDS service organization, has been supporting care that moves away from questions like, “What’s wrong with you?” to patient-focused questions like, “What happened to you?” It is a practice known as trauma-informed care.
“This includes bringing in therapy dogs for staff to spend time with during the workday; anecdotally, our staff reported a reduction in stress and improvements in mood,” Ms. Rauscher says.
A version of this article first appeared on WebMD.com.
Patients in the emergency room who spent just 10 minutes with a trained therapy dog reported less pain, anxiety, and depression and improved well-being, researchers from the University of Saskatchewan in Canada found.
“The ER is an important community resource but also a scary place for most people,” James Stempien, MD, provincial department head of emergency medicine with the Saskatchewan Health Authority, who worked on the study, said in an interview.
“People tend to visit the ER on the worst day of their life, either for them or a loved one. Interacting with a therapy dog can make the ER visit a little calmer. We have also seen benefit for the staff that get to interact with the dogs as well,” he says.
“Thanks to our wonderful therapy dog volunteer teams, the cost is minimal and the result is priceless,” Dr. Stempien says.
The study, published in the journal PLOS One, builds on earlier “uncontrolled” studies by the Saskatchewan team.
Those studies showed that most ER patients wanted to visit with the therapy dog, if given a chance. After the encounter, patients reported feeling more comfortable, happier, and less distressed while waiting in the ER.
“A controlled trial was the natural next step,” says study investigator Colleen Dell, PhD, of One Health and Wellness at the University of Saskatchewan.
The study was done at the Royal University Hospital (RUH) in Saskatoon, Saskatchewan -- the first emergency department in Canada to introduce therapy dogs to improve the experience of waiting patients.
Nearly 200 adults visiting the ER received either a 10-minute visit with a therapy dog and its handler in addition to usual care or just usual care.
“This did not occur in patients in the ER who did not visit with a therapy dog.
“This gives us confidence in the intervention,” Dr. Dell says.
Pain is a major reason that patients come to the ER, and interactions with a therapy dog may distract from that pain, the researchers believe.
The study results lend more evidence to research that shows animals can help in medical settings, says Kara Rauscher, a licensed social worker and interim director of behavioral health for Nashville CARES in Tennessee, who wasn’t involved in the study.
“There are clearly opportunities to replicate this study in other emergency departments to strengthen our understanding of the potential benefits of these programs,” she says.
Part of her work at Nashville CARES, an AIDS service organization, has been supporting care that moves away from questions like, “What’s wrong with you?” to patient-focused questions like, “What happened to you?” It is a practice known as trauma-informed care.
“This includes bringing in therapy dogs for staff to spend time with during the workday; anecdotally, our staff reported a reduction in stress and improvements in mood,” Ms. Rauscher says.
A version of this article first appeared on WebMD.com.
FROM PLOS ONE
Opting out of dialysis not instant death sentence for kidney disease
, a new systematic review of cohort studies suggests.
“Our findings challenge the common misconception that the only alternative to dialysis for many patients with advanced chronic kidney disease is no care or death,” say Susan Wong, MD, of the Renal Dialysis Unit, Seattle, and colleagues in their review, published online March 14 in JAMA Network Open.
In an accompanying commentary, Christine Liu, MD, and Kurella Tamura, MD, MPH, note: “The decision to initiate dialysis or focus on active alleviation of symptoms, known as conservative care … is likely one of the consequential decisions [patients] will face.”
“[But] in reality, dialysis is viewed as the default treatment for kidney failure, and the option to forgo dialysis treatment is often not explicitly discussed,” they add.
“We believe it is time to broaden the scope of kidney replacement therapy registries to include persons who receive conservative treatment of kidney failure … and we need to address the conservative care information gap so that lack of awareness is no longer a barrier to informed decision-making,” Dr. Liu and Dr. Tamura, both from Stanford (Calif.) University, note.
The work by Dr. Wong and colleagues “dispels the notion that conservative care for kidney failure means a grim and near-immediate death. The study advances the idea that a conservative care approach can provide time and sustain quality of life to support patients’ life goals,” they emphasize.
Conservative care assessed in 41 studies
The review included 41 studies involving 5,102 patients with a mean age ranging from 60 to 87 years conducted in the United Kingdom, Europe, and Asia.
Median survival of cohorts ranged from 1 to 41 months as measured from a baseline mean estimated glomerular filtration rate (eGFR) ranging from 7 to 19 mL/min/1.73m2.
Younger patients between 70 and 79 years of age had a median survival of 7 to 41 months, the authors note, while cohorts consisting of patients 80 years of age and older had a median survival of 1 to 37 months despite overlapping ranges of baseline mean eGFRs.
During an observation period of 8-24 months, mental well-being improved, and physical well-being and overall quality of life were largely stable until late in the course of illness.
“Ten studies … provided information on the use of health care resources during follow-up,” the researchers say. Patients generally experienced one to two hospital admissions, 6-16 in-hospital days, seven to eight clinic visits, and two emergency department visits per person-year. Use of acute care services was “therefore common,” they note.
Not all studies provided information about end-of-life care, but those that did reported rates of hospice enrollment that ranged from 20% to 76%; hospitalization rates during the final month of life from 57% to 76%; in-hospital death rates of 27%-68%, and in-home death rates ranging from 12% to 71%.
This indicates substantial disparity in access to supportive care near the end of life across cohorts, the authors observe.
Nevertheless, “Most patients survived several years after the decision to forgo dialysis was made,” they stress.
“These findings not only suggest that conservative kidney management may be a viable and positive therapeutic alternative to dialysis, they also highlight the strengths of its multidisciplinary approach to care and aggressive symptom management.”
“Collectively, our findings demonstrate the need to implement systematic and unified research methods for conservative kidney management and to develop models of care and the care infrastructure to advance practice and outcomes of conservative kidney management,” they conclude.
Dr. Wong has no financial ties to industry. Dr. Tamura has reported receiving personal fees from the American Federation for Aging Research.
A version of this article first appeared on Medscape.com.
, a new systematic review of cohort studies suggests.
“Our findings challenge the common misconception that the only alternative to dialysis for many patients with advanced chronic kidney disease is no care or death,” say Susan Wong, MD, of the Renal Dialysis Unit, Seattle, and colleagues in their review, published online March 14 in JAMA Network Open.
In an accompanying commentary, Christine Liu, MD, and Kurella Tamura, MD, MPH, note: “The decision to initiate dialysis or focus on active alleviation of symptoms, known as conservative care … is likely one of the consequential decisions [patients] will face.”
“[But] in reality, dialysis is viewed as the default treatment for kidney failure, and the option to forgo dialysis treatment is often not explicitly discussed,” they add.
“We believe it is time to broaden the scope of kidney replacement therapy registries to include persons who receive conservative treatment of kidney failure … and we need to address the conservative care information gap so that lack of awareness is no longer a barrier to informed decision-making,” Dr. Liu and Dr. Tamura, both from Stanford (Calif.) University, note.
The work by Dr. Wong and colleagues “dispels the notion that conservative care for kidney failure means a grim and near-immediate death. The study advances the idea that a conservative care approach can provide time and sustain quality of life to support patients’ life goals,” they emphasize.
Conservative care assessed in 41 studies
The review included 41 studies involving 5,102 patients with a mean age ranging from 60 to 87 years conducted in the United Kingdom, Europe, and Asia.
Median survival of cohorts ranged from 1 to 41 months as measured from a baseline mean estimated glomerular filtration rate (eGFR) ranging from 7 to 19 mL/min/1.73m2.
Younger patients between 70 and 79 years of age had a median survival of 7 to 41 months, the authors note, while cohorts consisting of patients 80 years of age and older had a median survival of 1 to 37 months despite overlapping ranges of baseline mean eGFRs.
During an observation period of 8-24 months, mental well-being improved, and physical well-being and overall quality of life were largely stable until late in the course of illness.
“Ten studies … provided information on the use of health care resources during follow-up,” the researchers say. Patients generally experienced one to two hospital admissions, 6-16 in-hospital days, seven to eight clinic visits, and two emergency department visits per person-year. Use of acute care services was “therefore common,” they note.
Not all studies provided information about end-of-life care, but those that did reported rates of hospice enrollment that ranged from 20% to 76%; hospitalization rates during the final month of life from 57% to 76%; in-hospital death rates of 27%-68%, and in-home death rates ranging from 12% to 71%.
This indicates substantial disparity in access to supportive care near the end of life across cohorts, the authors observe.
Nevertheless, “Most patients survived several years after the decision to forgo dialysis was made,” they stress.
“These findings not only suggest that conservative kidney management may be a viable and positive therapeutic alternative to dialysis, they also highlight the strengths of its multidisciplinary approach to care and aggressive symptom management.”
“Collectively, our findings demonstrate the need to implement systematic and unified research methods for conservative kidney management and to develop models of care and the care infrastructure to advance practice and outcomes of conservative kidney management,” they conclude.
Dr. Wong has no financial ties to industry. Dr. Tamura has reported receiving personal fees from the American Federation for Aging Research.
A version of this article first appeared on Medscape.com.
, a new systematic review of cohort studies suggests.
“Our findings challenge the common misconception that the only alternative to dialysis for many patients with advanced chronic kidney disease is no care or death,” say Susan Wong, MD, of the Renal Dialysis Unit, Seattle, and colleagues in their review, published online March 14 in JAMA Network Open.
In an accompanying commentary, Christine Liu, MD, and Kurella Tamura, MD, MPH, note: “The decision to initiate dialysis or focus on active alleviation of symptoms, known as conservative care … is likely one of the consequential decisions [patients] will face.”
“[But] in reality, dialysis is viewed as the default treatment for kidney failure, and the option to forgo dialysis treatment is often not explicitly discussed,” they add.
“We believe it is time to broaden the scope of kidney replacement therapy registries to include persons who receive conservative treatment of kidney failure … and we need to address the conservative care information gap so that lack of awareness is no longer a barrier to informed decision-making,” Dr. Liu and Dr. Tamura, both from Stanford (Calif.) University, note.
The work by Dr. Wong and colleagues “dispels the notion that conservative care for kidney failure means a grim and near-immediate death. The study advances the idea that a conservative care approach can provide time and sustain quality of life to support patients’ life goals,” they emphasize.
Conservative care assessed in 41 studies
The review included 41 studies involving 5,102 patients with a mean age ranging from 60 to 87 years conducted in the United Kingdom, Europe, and Asia.
Median survival of cohorts ranged from 1 to 41 months as measured from a baseline mean estimated glomerular filtration rate (eGFR) ranging from 7 to 19 mL/min/1.73m2.
Younger patients between 70 and 79 years of age had a median survival of 7 to 41 months, the authors note, while cohorts consisting of patients 80 years of age and older had a median survival of 1 to 37 months despite overlapping ranges of baseline mean eGFRs.
During an observation period of 8-24 months, mental well-being improved, and physical well-being and overall quality of life were largely stable until late in the course of illness.
“Ten studies … provided information on the use of health care resources during follow-up,” the researchers say. Patients generally experienced one to two hospital admissions, 6-16 in-hospital days, seven to eight clinic visits, and two emergency department visits per person-year. Use of acute care services was “therefore common,” they note.
Not all studies provided information about end-of-life care, but those that did reported rates of hospice enrollment that ranged from 20% to 76%; hospitalization rates during the final month of life from 57% to 76%; in-hospital death rates of 27%-68%, and in-home death rates ranging from 12% to 71%.
This indicates substantial disparity in access to supportive care near the end of life across cohorts, the authors observe.
Nevertheless, “Most patients survived several years after the decision to forgo dialysis was made,” they stress.
“These findings not only suggest that conservative kidney management may be a viable and positive therapeutic alternative to dialysis, they also highlight the strengths of its multidisciplinary approach to care and aggressive symptom management.”
“Collectively, our findings demonstrate the need to implement systematic and unified research methods for conservative kidney management and to develop models of care and the care infrastructure to advance practice and outcomes of conservative kidney management,” they conclude.
Dr. Wong has no financial ties to industry. Dr. Tamura has reported receiving personal fees from the American Federation for Aging Research.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Just one extra drink a day may change the brain
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
FDA approves generic Symbicort for asthma, COPD
The U.S. Food and Drug Administration approved the first generic of Symbicort (budesonide and formoterol fumarate dihydrate) inhalation aerosol for the treatment of asthma in patients 6 years of age and older and for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
The approval was given for a complex generic drug-device combination product – a metered-dose inhaler that contains both budesonide (a corticosteroid that reduces inflammation) and formoterol (a long-acting bronchodilator that relaxes muscles in the airways to improve breathing). It is intended to be used as two inhalations, two times a day (usually morning and night, about 12 hours apart), to treat both diseases by preventing symptoms, such as wheezing for those with asthma and for improved breathing for patients with COPD.
The inhaler is approved at two strengths (160/4.5 mcg/actuation and 80/4.5 mcg/actuation), according to the March 15 FDA announcement. The device is not intended for the treatment of acute asthma.
“Today’s approval of the first generic for one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD is another step forward in our commitment to bring generic copies of complex drugs to the market, which can improve quality of life and help reduce the cost of treatment,” said Sally Choe, PhD, director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research.
The most common side effects associated with budesonide and formoterol fumarate dihydrate oral inhalation aerosol for those with asthma are nasopharyngitis pain, sinusitis, influenza, back pain, nasal congestion, stomach discomfort, vomiting, and oral candidiasis (thrush). For those with COPD, the most common side effects are nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection, the FDA reported.
The approval of this generic drug-device combination was granted to Mylan Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved the first generic of Symbicort (budesonide and formoterol fumarate dihydrate) inhalation aerosol for the treatment of asthma in patients 6 years of age and older and for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
The approval was given for a complex generic drug-device combination product – a metered-dose inhaler that contains both budesonide (a corticosteroid that reduces inflammation) and formoterol (a long-acting bronchodilator that relaxes muscles in the airways to improve breathing). It is intended to be used as two inhalations, two times a day (usually morning and night, about 12 hours apart), to treat both diseases by preventing symptoms, such as wheezing for those with asthma and for improved breathing for patients with COPD.
The inhaler is approved at two strengths (160/4.5 mcg/actuation and 80/4.5 mcg/actuation), according to the March 15 FDA announcement. The device is not intended for the treatment of acute asthma.
“Today’s approval of the first generic for one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD is another step forward in our commitment to bring generic copies of complex drugs to the market, which can improve quality of life and help reduce the cost of treatment,” said Sally Choe, PhD, director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research.
The most common side effects associated with budesonide and formoterol fumarate dihydrate oral inhalation aerosol for those with asthma are nasopharyngitis pain, sinusitis, influenza, back pain, nasal congestion, stomach discomfort, vomiting, and oral candidiasis (thrush). For those with COPD, the most common side effects are nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection, the FDA reported.
The approval of this generic drug-device combination was granted to Mylan Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved the first generic of Symbicort (budesonide and formoterol fumarate dihydrate) inhalation aerosol for the treatment of asthma in patients 6 years of age and older and for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
The approval was given for a complex generic drug-device combination product – a metered-dose inhaler that contains both budesonide (a corticosteroid that reduces inflammation) and formoterol (a long-acting bronchodilator that relaxes muscles in the airways to improve breathing). It is intended to be used as two inhalations, two times a day (usually morning and night, about 12 hours apart), to treat both diseases by preventing symptoms, such as wheezing for those with asthma and for improved breathing for patients with COPD.
The inhaler is approved at two strengths (160/4.5 mcg/actuation and 80/4.5 mcg/actuation), according to the March 15 FDA announcement. The device is not intended for the treatment of acute asthma.
“Today’s approval of the first generic for one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD is another step forward in our commitment to bring generic copies of complex drugs to the market, which can improve quality of life and help reduce the cost of treatment,” said Sally Choe, PhD, director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research.
The most common side effects associated with budesonide and formoterol fumarate dihydrate oral inhalation aerosol for those with asthma are nasopharyngitis pain, sinusitis, influenza, back pain, nasal congestion, stomach discomfort, vomiting, and oral candidiasis (thrush). For those with COPD, the most common side effects are nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection, the FDA reported.
The approval of this generic drug-device combination was granted to Mylan Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Children and COVID: Decline in new cases reaches 7th week
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.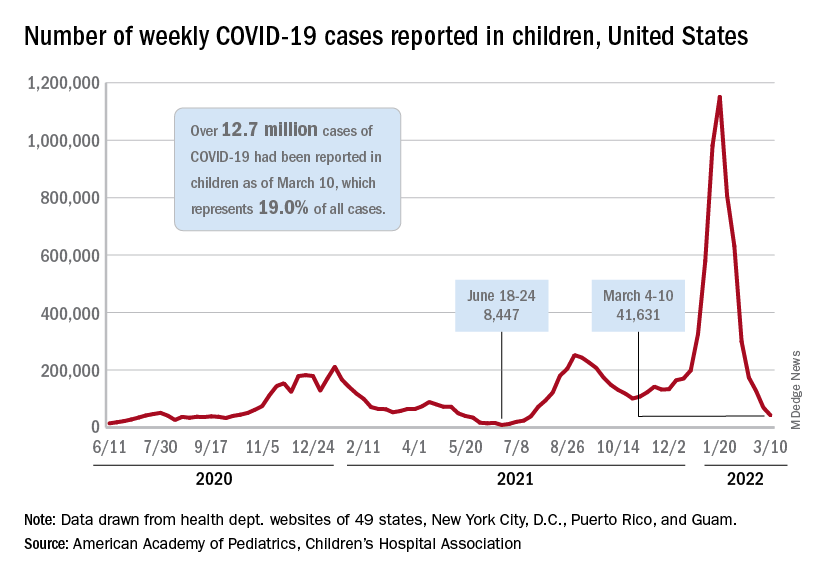
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
Death of pig heart transplant patient is more a beginning than an end
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
Air trapping common in patients with long COVID
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM RADIOLOGY
Big missed opportunities for BP control in premenopausal women
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new report shows considerable gaps in the awareness, treatment, and control of hypertension in premenopausal women in the United States, with a key driver being regular access to health care.
In a nationally representative sample of women ages 35-54 with no prior cardiovascular disease, the prevalence of hypertension increased 8% from an estimated 15.2 million women between 2011 and 2014 to 16.4 million women between 2015 and 2018.
What’s more, the percentage of women with controlled hypertension dropped over the two time periods from 55% to 50%, which is well below the government’s Million Hearts target of 70%.
Missed opportunities for hypertension control in these premenopausal women were a lack of awareness of their hypertension in 23%, ineffective treatment in 34%, and a lack of health care access in 43%; increasing to 51% in non-Hispanic Black patients and 56% in Hispanic patients.
Notably, lack of health care access affected an estimated 3.1 million women (45%) in 2011-2014 and 3.5 million women (43%) in 2015-2018.
Equally stubborn over the two time periods was the lack of effective treatment, affecting 2.1 million (31%) versus 2.8 million (34%) women, and lack of awareness, affecting 1.6 million (24%) versus 1.9 million (23%) women.
“There’s been no improvement over the past decade, and there is evidence of race/ethnic disparities,” study author Susan Hennessy, PhD, said at the recent Epidemiology, Prevention/Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
The prevalence of uncontrolled hypertension among non-Hispanic Whites was less than that of the U.S. population, at 44%, and most of the missed opportunities were due to uncontrolled blood pressure (BP), noted Dr. Hennessy, a researcher with the University of California, San Francisco School of Medicine.
However, the uncontrolled prevalence was 54% in non-Hispanic Black women and 66% in Hispanic women. “In both of these subgroups, over half of the missed opportunities occur because these women have no regular access to health care,” she said.
In women who identified as “other,” which includes non-Hispanic Asian and mixed-race populations, the uncontrolled prevalence reached 70%, and the biggest missed opportunity was in those who were untreated.
Raising awareness, empowering women, and delivery of guideline-concordant care will help premenopausal women gain control of their blood pressure, Dr. Hennessy said. “But underpinning all of this is ensuring equitable health care access, because if we fail to get women into the system, then we have no opportunity to help them lower their blood pressure.”
She reminded the audience that cardiovascular disease (CVD) is the number one killer of women in the United States and that CVD risk, mediated through hypertension, increases after menopause. Thus, managing hypertension prior to this life event is an important element of primary prevention of CVD and should be a priority.
Session moderator Sadiya S. Khan, MD, Northwestern University Feinberg School of Medicine, Chicago, told this news organization that the findings should raise “alarm and concern that hypertension is not just a disease of the old but very prevalent in younger women, particularly around the time of pregnancy. And this is a clear driver of maternal morbidity and mortality as well.”
“This idea that patients should ‘Know Your Numbers’ is really important, and we talk a lot about that for hypertension, but if you don’t have a doctor, if you don’t have someone to go to, it’s very hard to know or understand what your numbers mean,” she said. “I think that’s really the main message.”
Speaking to this news organization, Dr. Hennessy said there’s no simple solution to the problem, given that some women are not even in the system, whereas others are not being treated effectively, but that increasing opportunities to screen BP would be a start. That could be through community programs, similar to the Barbershop Hypertension trial, or by making BP devices available for home monitoring.
“Again, this is about empowering ourselves to take some level of control, but, as a system, we have to be able to make it equitable for everyone and make sure they have the right equipment, the right cuff size,” she said. “The disparities arise because of the social determinants of health, so if these women are struggling to put food on the table, they aren’t going to be able to afford a blood pressure cuff.”
During a discussion of the findings, audience members noted that the National Health and Nutrition Examination Survey (NHANES) data used for the analysis were somewhat dated. Dr. Hennessy also pointed out that NHANES blood pressure is measured up to three times during a single visit, which differs from clinical practice, and that responses were based on self-report and thus subject to recall bias.
The sample included 3,343 women aged 35-54 years with no prior cardiovascular disease, representing an estimated 31.6 million American women. Hypertension was defined by a systolic BP of at least 140 mm Hg or a diastolic BP of at least 90 mm Hg or current BP medication use.
The authors and Dr. Khan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiologist pleads guilty to abusive sexual contact
John Giacomini, MD, has pleaded guilty to one count of abusive sexual contact of a female physician he was supervising, the Department of Justice (DOJ) has announced.
Dr. Giacomini, 73, of Atherton, California, had practiced medicine and cardiology for more than 30 years and served as chief of the cardiology section at the VA Hospital in Palo Alto from 1985 to 2018.
According to the statement from DOJ, starting in the fall of 2017, Dr. Giacomini repeatedly subjected a subordinate doctor to unwanted and unwelcome sexual contact, which included hugging, kissing, and intimate touching while on VA premises.
The victim explicitly told Dr. Giacomini she was not interested in a romantic or sexual relationship with him and forcibly resisted his repeated attempts to kiss her, the statement notes.
The abuse continued, culminating in December 2017 with the incident of abusive sexual contact, the DOJ says.
Afterward, the victim resigned from her position at the VA, citing Dr. Giacomini’s behavior as her principal reason for leaving.
“As a federal employee for well over 30 years, [Dr.] Giacomini was trained throughout his career on the prevention of workplace sexual assault and sexual harassment,” the DOJ says.
“As a supervisor and manager, [Dr.] Giacomini had an obligation to the VA and to his subordinates to prevent workplace sexual harassment and disclose any harassing behavior of which he became aware. He failed to do this,” the DOJ says.
A federal grand jury indicted Dr. Giacomini in March 2020, charging him with one count of abusive sexual contact. Dr. Giacomini has now pleaded guilty to the charge, a felony.
Sentencing is scheduled for July 12. Dr. Giacomini faces a maximum sentence of 2 years in prison, a fine of $250,000, restitution, and supervised release.
A version of this article first appeared on Medscape.com.
John Giacomini, MD, has pleaded guilty to one count of abusive sexual contact of a female physician he was supervising, the Department of Justice (DOJ) has announced.
Dr. Giacomini, 73, of Atherton, California, had practiced medicine and cardiology for more than 30 years and served as chief of the cardiology section at the VA Hospital in Palo Alto from 1985 to 2018.
According to the statement from DOJ, starting in the fall of 2017, Dr. Giacomini repeatedly subjected a subordinate doctor to unwanted and unwelcome sexual contact, which included hugging, kissing, and intimate touching while on VA premises.
The victim explicitly told Dr. Giacomini she was not interested in a romantic or sexual relationship with him and forcibly resisted his repeated attempts to kiss her, the statement notes.
The abuse continued, culminating in December 2017 with the incident of abusive sexual contact, the DOJ says.
Afterward, the victim resigned from her position at the VA, citing Dr. Giacomini’s behavior as her principal reason for leaving.
“As a federal employee for well over 30 years, [Dr.] Giacomini was trained throughout his career on the prevention of workplace sexual assault and sexual harassment,” the DOJ says.
“As a supervisor and manager, [Dr.] Giacomini had an obligation to the VA and to his subordinates to prevent workplace sexual harassment and disclose any harassing behavior of which he became aware. He failed to do this,” the DOJ says.
A federal grand jury indicted Dr. Giacomini in March 2020, charging him with one count of abusive sexual contact. Dr. Giacomini has now pleaded guilty to the charge, a felony.
Sentencing is scheduled for July 12. Dr. Giacomini faces a maximum sentence of 2 years in prison, a fine of $250,000, restitution, and supervised release.
A version of this article first appeared on Medscape.com.
John Giacomini, MD, has pleaded guilty to one count of abusive sexual contact of a female physician he was supervising, the Department of Justice (DOJ) has announced.
Dr. Giacomini, 73, of Atherton, California, had practiced medicine and cardiology for more than 30 years and served as chief of the cardiology section at the VA Hospital in Palo Alto from 1985 to 2018.
According to the statement from DOJ, starting in the fall of 2017, Dr. Giacomini repeatedly subjected a subordinate doctor to unwanted and unwelcome sexual contact, which included hugging, kissing, and intimate touching while on VA premises.
The victim explicitly told Dr. Giacomini she was not interested in a romantic or sexual relationship with him and forcibly resisted his repeated attempts to kiss her, the statement notes.
The abuse continued, culminating in December 2017 with the incident of abusive sexual contact, the DOJ says.
Afterward, the victim resigned from her position at the VA, citing Dr. Giacomini’s behavior as her principal reason for leaving.
“As a federal employee for well over 30 years, [Dr.] Giacomini was trained throughout his career on the prevention of workplace sexual assault and sexual harassment,” the DOJ says.
“As a supervisor and manager, [Dr.] Giacomini had an obligation to the VA and to his subordinates to prevent workplace sexual harassment and disclose any harassing behavior of which he became aware. He failed to do this,” the DOJ says.
A federal grand jury indicted Dr. Giacomini in March 2020, charging him with one count of abusive sexual contact. Dr. Giacomini has now pleaded guilty to the charge, a felony.
Sentencing is scheduled for July 12. Dr. Giacomini faces a maximum sentence of 2 years in prison, a fine of $250,000, restitution, and supervised release.
A version of this article first appeared on Medscape.com.