User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
Candida Esophagitis Associated With Adalimumab for Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
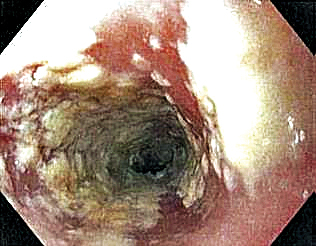
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.
Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
Practice Points
- Adalimumab is an effective treatment for patients with hidradenitis suppurativa.
- There is risk for opportunistic infections with adalimumab, and patients should be monitored closely.
We’re all vaccinated: Can we go back to the office (unmasked) now?
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
‘Major update’ of BP guidance for kidney disease; treat to 120 mm Hg
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
JAMA editor resigns over controversial podcast
JAMA editor in chief Howard Bauchner, MD, apologized to JAMA staff and stakeholders and asked for and received Dr. Livingston’s resignation, according to a statement from AMA CEO James Madara.
More than 2,000 people have signed a petition on Change.org calling for an investigation at JAMA over the podcast, called “Structural Racism for Doctors: What Is It?”
It appears they are now getting their wish. Dr. Bauchner announced that the journal’s oversight committee is investigating how the podcast and a tweet promoting the episode were developed, reviewed, and ultimately posted.
“This investigation and report of its findings will be thorough and completed rapidly,” Dr. Bauchner said.
Dr. Livingston, the host of the podcast, has been heavily criticized across social media. During the podcast, Dr. Livingston, who is White, said: “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released last week, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Dr. Katz is an editor at JAMA Internal Medicine and CEO of NYC Health + Hospitals in New York.
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
The incident was met with anger and confusion in the medical community.
Herbert C. Smitherman, MD, vice dean of diversity and community affairs at Wayne State University, Detroit, noted after hearing the podcast that it was a symptom of a much larger problem.
“At its core, this podcast had racist tendencies. Those attitudes are why you don’t have as many articles by Black and Brown people in JAMA,” he said. “People’s attitudes, whether conscious or unconscious, are what drive the policies and practices which create the structural racism.”
Dr. Katz responded to the backlash last week with the following statement: “Systemic racism exists in our country. The disparate effects of the pandemic have made this painfully clear in New York City and across the country.
“As clinicians, we must understand how these structures and policies have a direct impact on the health outcomes of the patients and communities we serve. It is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it, or that we should avoid the term ‘systematic racism’ because it makes people uncomfortable. We must and can do better.”
JAMA, an independent arm of the AMA, is taking other steps to address concerns. Its executive publisher, Thomas Easley, held an employee town hall this week, and said JAMA acknowledges that “structural racism is real, pernicious, and pervasive in health care.” The journal is also starting an “end-to-end review” of all editorial processes across all JAMA publications. Finally, the journal will also create a new associate editor’s position who will provide “insight and counsel” on racism and structural racism in health care.
A version of this article first appeared on WebMD.com .
JAMA editor in chief Howard Bauchner, MD, apologized to JAMA staff and stakeholders and asked for and received Dr. Livingston’s resignation, according to a statement from AMA CEO James Madara.
More than 2,000 people have signed a petition on Change.org calling for an investigation at JAMA over the podcast, called “Structural Racism for Doctors: What Is It?”
It appears they are now getting their wish. Dr. Bauchner announced that the journal’s oversight committee is investigating how the podcast and a tweet promoting the episode were developed, reviewed, and ultimately posted.
“This investigation and report of its findings will be thorough and completed rapidly,” Dr. Bauchner said.
Dr. Livingston, the host of the podcast, has been heavily criticized across social media. During the podcast, Dr. Livingston, who is White, said: “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released last week, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Dr. Katz is an editor at JAMA Internal Medicine and CEO of NYC Health + Hospitals in New York.
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
The incident was met with anger and confusion in the medical community.
Herbert C. Smitherman, MD, vice dean of diversity and community affairs at Wayne State University, Detroit, noted after hearing the podcast that it was a symptom of a much larger problem.
“At its core, this podcast had racist tendencies. Those attitudes are why you don’t have as many articles by Black and Brown people in JAMA,” he said. “People’s attitudes, whether conscious or unconscious, are what drive the policies and practices which create the structural racism.”
Dr. Katz responded to the backlash last week with the following statement: “Systemic racism exists in our country. The disparate effects of the pandemic have made this painfully clear in New York City and across the country.
“As clinicians, we must understand how these structures and policies have a direct impact on the health outcomes of the patients and communities we serve. It is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it, or that we should avoid the term ‘systematic racism’ because it makes people uncomfortable. We must and can do better.”
JAMA, an independent arm of the AMA, is taking other steps to address concerns. Its executive publisher, Thomas Easley, held an employee town hall this week, and said JAMA acknowledges that “structural racism is real, pernicious, and pervasive in health care.” The journal is also starting an “end-to-end review” of all editorial processes across all JAMA publications. Finally, the journal will also create a new associate editor’s position who will provide “insight and counsel” on racism and structural racism in health care.
A version of this article first appeared on WebMD.com .
JAMA editor in chief Howard Bauchner, MD, apologized to JAMA staff and stakeholders and asked for and received Dr. Livingston’s resignation, according to a statement from AMA CEO James Madara.
More than 2,000 people have signed a petition on Change.org calling for an investigation at JAMA over the podcast, called “Structural Racism for Doctors: What Is It?”
It appears they are now getting their wish. Dr. Bauchner announced that the journal’s oversight committee is investigating how the podcast and a tweet promoting the episode were developed, reviewed, and ultimately posted.
“This investigation and report of its findings will be thorough and completed rapidly,” Dr. Bauchner said.
Dr. Livingston, the host of the podcast, has been heavily criticized across social media. During the podcast, Dr. Livingston, who is White, said: “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released last week, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Dr. Katz is an editor at JAMA Internal Medicine and CEO of NYC Health + Hospitals in New York.
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
The incident was met with anger and confusion in the medical community.
Herbert C. Smitherman, MD, vice dean of diversity and community affairs at Wayne State University, Detroit, noted after hearing the podcast that it was a symptom of a much larger problem.
“At its core, this podcast had racist tendencies. Those attitudes are why you don’t have as many articles by Black and Brown people in JAMA,” he said. “People’s attitudes, whether conscious or unconscious, are what drive the policies and practices which create the structural racism.”
Dr. Katz responded to the backlash last week with the following statement: “Systemic racism exists in our country. The disparate effects of the pandemic have made this painfully clear in New York City and across the country.
“As clinicians, we must understand how these structures and policies have a direct impact on the health outcomes of the patients and communities we serve. It is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it, or that we should avoid the term ‘systematic racism’ because it makes people uncomfortable. We must and can do better.”
JAMA, an independent arm of the AMA, is taking other steps to address concerns. Its executive publisher, Thomas Easley, held an employee town hall this week, and said JAMA acknowledges that “structural racism is real, pernicious, and pervasive in health care.” The journal is also starting an “end-to-end review” of all editorial processes across all JAMA publications. Finally, the journal will also create a new associate editor’s position who will provide “insight and counsel” on racism and structural racism in health care.
A version of this article first appeared on WebMD.com .
Assessing Psychological Interventions for Hidradenitis Suppurativa as a First Step Toward Patient-Centered Practice
Hidradenitis suppurativa (HS)(also known as acne inversa) is a chronic, recurrent, and debilitating inflammatory dermatologic disease of the hair follicle. It usually presents after puberty, with painful, deep-seated, inflamed lesions in apocrine gland–bearing areas of the body, most commonly the axillae and inguinal and anogenital regions.1
Hidradenitis suppurativa patients have a high rate of psychologic and psychiatric comorbidities that often are interrelated and multidirectional. Approximately 1 in 4 adults with HS also experience depression (prevalence among all HS patients, 16.9%), and 1 in 5 experience anxiety (prevalence, 4.9%).2,3 Hidradenitis suppurativa has been associated with bipolar disorder, schizophrenia, and suicidality.2,4
These comorbidity factors have a remarkable impact on HS patients’ quality of life (QOL). Compared to other diseases, including psoriasis, stroke, and conditions that create candidacy for heart transplantation, HS was identified as the most impairing condition.5,6 It is estimated that more than 50% of HS patients experience a very or extremely large effect on their QOL, as measured by the dermatology life quality index.6
Pain, a major component of low QOL in HS patients, has an adverse impact on emotional health. Hidradenitis suppurativa causes body image dissatisfaction, leading to shame, embarrassment, lack of self-confidence, stigmatization, and social isolation.7-9 Furthermore, patients with HS have an increased risk for antidepressant drug use, completed suicide, and suicidal behavior compared to the general population.10
Focusing therapy on physical manifestations of HS only while ignoring the psychologic aspect could lead to a vicious cycle in which stress triggers flares, leading to worsening HS, leading to more stress, and so on.11 Therefore, psychological support for HS patients is critical, and we believe it should be an integral part of managing the disease.
There is no evidence to support effective therapeutic intervention for psychological aspects of HS. We conducted a PubMed search of articles indexed for MEDLINE using the term hidradenitis in combination with psychology, psychological, mindfulness, and cognitive behavioral therapy. No relevant articles were found. Most articles on HS focused on the low QOL associated with the disease and patient coping mechanisms. However, there are a number of psychological therapies to consider and evaluate for the management of HS.
Psychological Therapies to Consider in HS
Cognitive Behavioral Treatment
Cognitive behavioral treatment has been successfully used to manage skin diseases other than HS.12 Patients’ shame and stigmatization due to body dissatisfaction often cause social isolation, which might appear as social anxiety.9,13 Cognitive behavioral treatment, or compassion-focused therapy, could increase patients’ self-acceptance and reduce shameful feelings.13
Group Therapy
Alternatively, group therapy might be beneficial for HS patients. Research has shown that most HS patients know others affected by the same disease or attend an HS support group, and patients value the support of peers with the disease.13 Therefore, group therapy meetings with HS patients that are directed by a health care professional might reduce feelings of shame and stigmatization and increase feelings of social acceptance.
Mindfulness
Another approach for managing psychological aspects of skin diseases that might be useful in HS is mindfulness-based stress reduction (MBSR), developed by Kabat-Zinn and colleagues,14 which helps patients develop mindfulness through training in meditation. It is an intensive, structured, patient-centered approach that has been successfully used in a variety of settings.14,15
Current evidence supports the use of MBSR in the adjunct treatment of chronic pain, anxiety, and depression—symptoms that have a great impact on HS patients’ QOL.16 Furthermore, MBSR is offered in a group setting, which is potentially an opportunity for peer support and understanding; social support has been reported to be highly beneficial for HS patients.17
Can the Placebo Effect Aid in Managing HS?
A recent review that assessed the placebo effect in randomized clinical trials (RCTs) of treatments for cutaneous disease demonstrated that the placebo effect in HS therapy trials is higher than in RCTs of therapies for psoriasis and eczema. This finding highlights the importance of the physician-patient relationship when managing HS, which can result in greater treatment adherence and more patient education, empowerment, and encouragement toward beneficial lifestyle changes.18
Complementary psychological interventions for managing HS might maximize the placebo effect in clinical practice.18 The placebo effect in RCTs is higher for HS treatments than for psoriasis treatments, and if patients with psoriasis improved with psychological interventions,12 it would be reasonable to expect an improvement in QOL with psychological interventions for HS.
Final Thoughts
Although a number of studies have been published in the medical literature regarding psychological intervention in psoriasis management,12 we found no clinical studies assessing the psychological management of HS. We conclude that more research is necessary to develop psychological interventions targeting HS patients because a multidisciplinary and patient-centered approach is essential for the management of HS.
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Patel KR, Lee HH, Rastogi S, et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:737-744.
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Sampogna F, Fania L, Mazzanti C, et al. The broad-spectrum impact of hidradenitis suppurativa on quality of life: a comparison with psoriasis. Dermatology. 2019;235:308-314.
- von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809-813.
- Esmann S, Jemec GBE. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332.
- Schneider-Burrus S, Jost A, Peters EMJ, et al. Association of hidradenitis suppurativa with body image. JAMA Dermatol. 2018;154:447-451.
- Koumaki D, Efthymiou O, Bozi E, et al. Perspectives on perceived stigma and self-stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol. 2019;12:785-790.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 2014;6:112.
- Qureshi AA, Awosika O, Baruffi F, et al. Psychological therapies in management of psoriatic skin disease: a systematic review. Am J Clin Dermatol. 2019;20:607-624.
- Keary E, Hevey D, Tobin AM. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol. 2020;182:342-347.
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936-943.
- Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22:716-721.
- Gotink RA, Chu P, Busschbach JJV, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344.
- Golbari NM, Porter ML, Kimball AM. Online communications among hidradenitis suppurativa patients reflect community needs. J Am Acad Dermatol. 2019;80:1760-1762.
- Ali AA, Seng EK, Alavi A, et al. Exploring changes in placebo treatment arms in hidradenitis suppurativa randomized clinical trials: a systematic review. J Am Acad Dermatol. 2020;82:45-53.
Hidradenitis suppurativa (HS)(also known as acne inversa) is a chronic, recurrent, and debilitating inflammatory dermatologic disease of the hair follicle. It usually presents after puberty, with painful, deep-seated, inflamed lesions in apocrine gland–bearing areas of the body, most commonly the axillae and inguinal and anogenital regions.1
Hidradenitis suppurativa patients have a high rate of psychologic and psychiatric comorbidities that often are interrelated and multidirectional. Approximately 1 in 4 adults with HS also experience depression (prevalence among all HS patients, 16.9%), and 1 in 5 experience anxiety (prevalence, 4.9%).2,3 Hidradenitis suppurativa has been associated with bipolar disorder, schizophrenia, and suicidality.2,4
These comorbidity factors have a remarkable impact on HS patients’ quality of life (QOL). Compared to other diseases, including psoriasis, stroke, and conditions that create candidacy for heart transplantation, HS was identified as the most impairing condition.5,6 It is estimated that more than 50% of HS patients experience a very or extremely large effect on their QOL, as measured by the dermatology life quality index.6
Pain, a major component of low QOL in HS patients, has an adverse impact on emotional health. Hidradenitis suppurativa causes body image dissatisfaction, leading to shame, embarrassment, lack of self-confidence, stigmatization, and social isolation.7-9 Furthermore, patients with HS have an increased risk for antidepressant drug use, completed suicide, and suicidal behavior compared to the general population.10
Focusing therapy on physical manifestations of HS only while ignoring the psychologic aspect could lead to a vicious cycle in which stress triggers flares, leading to worsening HS, leading to more stress, and so on.11 Therefore, psychological support for HS patients is critical, and we believe it should be an integral part of managing the disease.
There is no evidence to support effective therapeutic intervention for psychological aspects of HS. We conducted a PubMed search of articles indexed for MEDLINE using the term hidradenitis in combination with psychology, psychological, mindfulness, and cognitive behavioral therapy. No relevant articles were found. Most articles on HS focused on the low QOL associated with the disease and patient coping mechanisms. However, there are a number of psychological therapies to consider and evaluate for the management of HS.
Psychological Therapies to Consider in HS
Cognitive Behavioral Treatment
Cognitive behavioral treatment has been successfully used to manage skin diseases other than HS.12 Patients’ shame and stigmatization due to body dissatisfaction often cause social isolation, which might appear as social anxiety.9,13 Cognitive behavioral treatment, or compassion-focused therapy, could increase patients’ self-acceptance and reduce shameful feelings.13
Group Therapy
Alternatively, group therapy might be beneficial for HS patients. Research has shown that most HS patients know others affected by the same disease or attend an HS support group, and patients value the support of peers with the disease.13 Therefore, group therapy meetings with HS patients that are directed by a health care professional might reduce feelings of shame and stigmatization and increase feelings of social acceptance.
Mindfulness
Another approach for managing psychological aspects of skin diseases that might be useful in HS is mindfulness-based stress reduction (MBSR), developed by Kabat-Zinn and colleagues,14 which helps patients develop mindfulness through training in meditation. It is an intensive, structured, patient-centered approach that has been successfully used in a variety of settings.14,15
Current evidence supports the use of MBSR in the adjunct treatment of chronic pain, anxiety, and depression—symptoms that have a great impact on HS patients’ QOL.16 Furthermore, MBSR is offered in a group setting, which is potentially an opportunity for peer support and understanding; social support has been reported to be highly beneficial for HS patients.17
Can the Placebo Effect Aid in Managing HS?
A recent review that assessed the placebo effect in randomized clinical trials (RCTs) of treatments for cutaneous disease demonstrated that the placebo effect in HS therapy trials is higher than in RCTs of therapies for psoriasis and eczema. This finding highlights the importance of the physician-patient relationship when managing HS, which can result in greater treatment adherence and more patient education, empowerment, and encouragement toward beneficial lifestyle changes.18
Complementary psychological interventions for managing HS might maximize the placebo effect in clinical practice.18 The placebo effect in RCTs is higher for HS treatments than for psoriasis treatments, and if patients with psoriasis improved with psychological interventions,12 it would be reasonable to expect an improvement in QOL with psychological interventions for HS.
Final Thoughts
Although a number of studies have been published in the medical literature regarding psychological intervention in psoriasis management,12 we found no clinical studies assessing the psychological management of HS. We conclude that more research is necessary to develop psychological interventions targeting HS patients because a multidisciplinary and patient-centered approach is essential for the management of HS.
Hidradenitis suppurativa (HS)(also known as acne inversa) is a chronic, recurrent, and debilitating inflammatory dermatologic disease of the hair follicle. It usually presents after puberty, with painful, deep-seated, inflamed lesions in apocrine gland–bearing areas of the body, most commonly the axillae and inguinal and anogenital regions.1
Hidradenitis suppurativa patients have a high rate of psychologic and psychiatric comorbidities that often are interrelated and multidirectional. Approximately 1 in 4 adults with HS also experience depression (prevalence among all HS patients, 16.9%), and 1 in 5 experience anxiety (prevalence, 4.9%).2,3 Hidradenitis suppurativa has been associated with bipolar disorder, schizophrenia, and suicidality.2,4
These comorbidity factors have a remarkable impact on HS patients’ quality of life (QOL). Compared to other diseases, including psoriasis, stroke, and conditions that create candidacy for heart transplantation, HS was identified as the most impairing condition.5,6 It is estimated that more than 50% of HS patients experience a very or extremely large effect on their QOL, as measured by the dermatology life quality index.6
Pain, a major component of low QOL in HS patients, has an adverse impact on emotional health. Hidradenitis suppurativa causes body image dissatisfaction, leading to shame, embarrassment, lack of self-confidence, stigmatization, and social isolation.7-9 Furthermore, patients with HS have an increased risk for antidepressant drug use, completed suicide, and suicidal behavior compared to the general population.10
Focusing therapy on physical manifestations of HS only while ignoring the psychologic aspect could lead to a vicious cycle in which stress triggers flares, leading to worsening HS, leading to more stress, and so on.11 Therefore, psychological support for HS patients is critical, and we believe it should be an integral part of managing the disease.
There is no evidence to support effective therapeutic intervention for psychological aspects of HS. We conducted a PubMed search of articles indexed for MEDLINE using the term hidradenitis in combination with psychology, psychological, mindfulness, and cognitive behavioral therapy. No relevant articles were found. Most articles on HS focused on the low QOL associated with the disease and patient coping mechanisms. However, there are a number of psychological therapies to consider and evaluate for the management of HS.
Psychological Therapies to Consider in HS
Cognitive Behavioral Treatment
Cognitive behavioral treatment has been successfully used to manage skin diseases other than HS.12 Patients’ shame and stigmatization due to body dissatisfaction often cause social isolation, which might appear as social anxiety.9,13 Cognitive behavioral treatment, or compassion-focused therapy, could increase patients’ self-acceptance and reduce shameful feelings.13
Group Therapy
Alternatively, group therapy might be beneficial for HS patients. Research has shown that most HS patients know others affected by the same disease or attend an HS support group, and patients value the support of peers with the disease.13 Therefore, group therapy meetings with HS patients that are directed by a health care professional might reduce feelings of shame and stigmatization and increase feelings of social acceptance.
Mindfulness
Another approach for managing psychological aspects of skin diseases that might be useful in HS is mindfulness-based stress reduction (MBSR), developed by Kabat-Zinn and colleagues,14 which helps patients develop mindfulness through training in meditation. It is an intensive, structured, patient-centered approach that has been successfully used in a variety of settings.14,15
Current evidence supports the use of MBSR in the adjunct treatment of chronic pain, anxiety, and depression—symptoms that have a great impact on HS patients’ QOL.16 Furthermore, MBSR is offered in a group setting, which is potentially an opportunity for peer support and understanding; social support has been reported to be highly beneficial for HS patients.17
Can the Placebo Effect Aid in Managing HS?
A recent review that assessed the placebo effect in randomized clinical trials (RCTs) of treatments for cutaneous disease demonstrated that the placebo effect in HS therapy trials is higher than in RCTs of therapies for psoriasis and eczema. This finding highlights the importance of the physician-patient relationship when managing HS, which can result in greater treatment adherence and more patient education, empowerment, and encouragement toward beneficial lifestyle changes.18
Complementary psychological interventions for managing HS might maximize the placebo effect in clinical practice.18 The placebo effect in RCTs is higher for HS treatments than for psoriasis treatments, and if patients with psoriasis improved with psychological interventions,12 it would be reasonable to expect an improvement in QOL with psychological interventions for HS.
Final Thoughts
Although a number of studies have been published in the medical literature regarding psychological intervention in psoriasis management,12 we found no clinical studies assessing the psychological management of HS. We conclude that more research is necessary to develop psychological interventions targeting HS patients because a multidisciplinary and patient-centered approach is essential for the management of HS.
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Patel KR, Lee HH, Rastogi S, et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:737-744.
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Sampogna F, Fania L, Mazzanti C, et al. The broad-spectrum impact of hidradenitis suppurativa on quality of life: a comparison with psoriasis. Dermatology. 2019;235:308-314.
- von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809-813.
- Esmann S, Jemec GBE. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332.
- Schneider-Burrus S, Jost A, Peters EMJ, et al. Association of hidradenitis suppurativa with body image. JAMA Dermatol. 2018;154:447-451.
- Koumaki D, Efthymiou O, Bozi E, et al. Perspectives on perceived stigma and self-stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol. 2019;12:785-790.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 2014;6:112.
- Qureshi AA, Awosika O, Baruffi F, et al. Psychological therapies in management of psoriatic skin disease: a systematic review. Am J Clin Dermatol. 2019;20:607-624.
- Keary E, Hevey D, Tobin AM. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol. 2020;182:342-347.
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936-943.
- Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22:716-721.
- Gotink RA, Chu P, Busschbach JJV, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344.
- Golbari NM, Porter ML, Kimball AM. Online communications among hidradenitis suppurativa patients reflect community needs. J Am Acad Dermatol. 2019;80:1760-1762.
- Ali AA, Seng EK, Alavi A, et al. Exploring changes in placebo treatment arms in hidradenitis suppurativa randomized clinical trials: a systematic review. J Am Acad Dermatol. 2020;82:45-53.
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Patel KR, Lee HH, Rastogi S, et al. Association between hidradenitis suppurativa, depression, anxiety, and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:737-744.
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945.
- Huilaja L, Tiri H, Jokelainen J, et al. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish nationwide registry study. J Invest Dermatol. 2018;138:46-51.
- Sampogna F, Fania L, Mazzanti C, et al. The broad-spectrum impact of hidradenitis suppurativa on quality of life: a comparison with psoriasis. Dermatology. 2019;235:308-314.
- von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809-813.
- Esmann S, Jemec GBE. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332.
- Schneider-Burrus S, Jost A, Peters EMJ, et al. Association of hidradenitis suppurativa with body image. JAMA Dermatol. 2018;154:447-451.
- Koumaki D, Efthymiou O, Bozi E, et al. Perspectives on perceived stigma and self-stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol. 2019;12:785-790.
- Thorlacius L, Cohen AD, Gislason GH, et al. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138:52-57.
- Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 2014;6:112.
- Qureshi AA, Awosika O, Baruffi F, et al. Psychological therapies in management of psoriatic skin disease: a systematic review. Am J Clin Dermatol. 2019;20:607-624.
- Keary E, Hevey D, Tobin AM. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol. 2020;182:342-347.
- Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936-943.
- Evans S, Ferrando S, Findler M, et al. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22:716-721.
- Gotink RA, Chu P, Busschbach JJV, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344.
- Golbari NM, Porter ML, Kimball AM. Online communications among hidradenitis suppurativa patients reflect community needs. J Am Acad Dermatol. 2019;80:1760-1762.
- Ali AA, Seng EK, Alavi A, et al. Exploring changes in placebo treatment arms in hidradenitis suppurativa randomized clinical trials: a systematic review. J Am Acad Dermatol. 2020;82:45-53.
PRACTICE POINTS
- Although hidradenitis suppurativa (HS) has high rates of psychological comorbidities, management of the psychological aspects of the disease has not been studied extensively.
- Complementary psychological interventions should be evaluated for the management of HS.
JAMA podcast on racism in medicine faces backlash
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Survey finds practice gaps in counseling women with hidradenitis suppurativa about pregnancy
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
FROM JAMA DERMATOLOGY
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
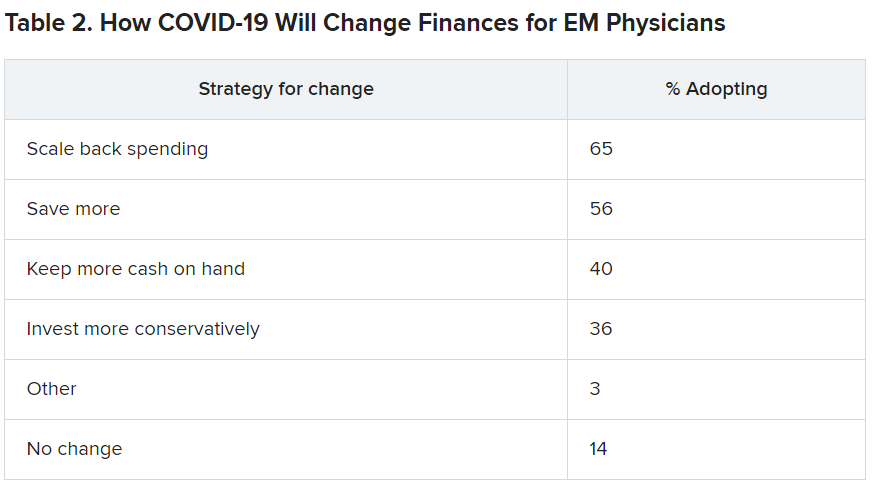
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many ED visits may be preventable for NSCLC patients
The leading cause of ED visits in the study was the cancer itself, although many visits were unrelated to non–small cell lung cancer (NSCLC) or cancer therapy.
Less than 10% of ED visits were related to cancer therapy, but visits were much more common among patients receiving chemotherapy than among those receiving immunotherapy or tyrosine kinase inhibitors (TKIs).
Manan P. Shah, MD, and Joel W. Neal, MD, PhD, both of Stanford (Calif. ) University, reported these results in JCO Oncology Practice.
The authors noted that, in the United States, care of patients with cancer, among all diseases, leads to the highest per-person cost. Unplanned hospital visits are among the largest drivers of increased spending in advanced cancer care, accounting for 48% of spending. However, that spending may not be indicative of better quality care, but rather of inefficiency, according to the authors.
One registry spanning 2009-2012 and including more than 400,000 newly diagnosed cancer patients found lung cancer to have the third-highest rate of unplanned hospitalizations (after hepatobiliary and pancreatic cancer). Those findings were published in JCO Oncology Practice in 2018.
While the reason for presentation to the ED is often the cancer or its therapy in this population, there is a paucity of research on the relative contribution of factors leading to unplanned hospital visits.
Common precipitants of medical emergencies in advanced stages of lung cancer include pulmonary embolism, obstructive pneumonia, spinal cord compression caused by metastasis, and tumor progression or pleural effusion leading to respiratory failure.
Lung cancer therapies, such as TKIs, immunotherapy, and cytotoxic chemotherapy, can also cause various medical emergencies arising out of skin reactions, gastrointestinal dysfunction, organ inflammatory processes, and bone marrow suppression.
Identifying drivers of unplanned ED visits
The primary goals of Dr. Shah and Dr. Neal’s study were to understand the drivers of unplanned ED visits and identify potential strategies to prevent them.
Drawing from the Stanford Medicine Research Data Repository, the authors identified 97 NSCLC patients with 173 ED visits at Stanford.
Patients were sorted according to which of the three therapies they had been receiving in the 3 months prior to the unplanned visit – TKIs (n = 47), immunotherapy (n = 24), or chemotherapy (n = 26). Patients receiving a combination of chemotherapy and immunotherapy were classified under the immunotherapy category.
ED visits were divided into four categories: cancer related, therapy related, unrelated to cancer or therapy, and rule-out (when an outpatient provider sent the patient to rule out medico-oncologic emergencies such as pulmonary embolism or cord compression).
If the patient’s main complaint(s) began 2 or more days before presentation, the diagnostics or therapeutics could have been provided in an outpatient setting (e.g., in clinic or urgent care), and the patient was discharged directly from the ED, the encounter was classified as potentially preventable. Among these preventable encounters, those made during business hours were also labeled unnecessary because they could have been managed through the Stanford sick call system for same-day urgent visits.
Leading cause is cancer
Overall, the leading cause of ED visits was NSCLC itself (54%). The patient’s cancer contributed to 61% of ED visits in the TKI group, 49% in the immunotherapy group, and 42% in the chemotherapy group.
Many ED visits were deemed unrelated to cancer or its therapies – 30% in the TKI group, 26% in the immunotherapy group, and 32% in the chemotherapy group.
Rule-out cases contributed to 7% of ED visits in the TKI group, 14% in the immunotherapy group, and 5% in the chemotherapy group.
Overall, 9% of ED visits were therapy related. The smallest proportion of these was observed in the TKI group (2%), which was significantly smaller than in the immunotherapy group (12%), a rate also significantly smaller than in the chemotherapy group (21%, P < .001).
Most unplanned ED visits did not lead to admissions (55%), were for complaints that began 2 or more days prior to presentation (53%), led to diagnostics or therapeutics that could have been administered in an outpatient setting (48%), and were during business hours (52%).
As a result, 24% of visits were classified as preventable, and 10% were deemed unnecessary.
Preventive strategies
“Our study suggests that TKIs lead to fewer emergency room visits than immunotherapy and chemotherapy,” Dr. Shah said in an interview.
“Overall, this may not necessarily change which therapy we prescribe,” he added, “as TKI therapy is often first line for patients with targeted mutations. However, recognizing that those on chemotherapy or immunotherapy are at higher risk for emergency room visits, we may target preventative strategies, for example, nursing phone calls, telemonitoring of symptoms, and frequent video visits toward this high-risk population.”
Dr. Shah and Dr. Neal said it’s “reassuring” that TKIs and immunotherapy are small drivers of unplanned hospital care. However, they also said efforts aimed at reducing chemotherapy-related ED visits are warranted.
The authors speculated that early intervention, extension of ambulatory care, and patient education about outpatient avenues of care could eliminate a significant proportion (at least 20%) of unplanned ED visits by NSCLC patients.
There was no specific funding for this study. Dr. Shah disclosed no conflicts of interest. Dr. Neal disclosed relationships with many companies, including this news organization.
The leading cause of ED visits in the study was the cancer itself, although many visits were unrelated to non–small cell lung cancer (NSCLC) or cancer therapy.
Less than 10% of ED visits were related to cancer therapy, but visits were much more common among patients receiving chemotherapy than among those receiving immunotherapy or tyrosine kinase inhibitors (TKIs).
Manan P. Shah, MD, and Joel W. Neal, MD, PhD, both of Stanford (Calif. ) University, reported these results in JCO Oncology Practice.
The authors noted that, in the United States, care of patients with cancer, among all diseases, leads to the highest per-person cost. Unplanned hospital visits are among the largest drivers of increased spending in advanced cancer care, accounting for 48% of spending. However, that spending may not be indicative of better quality care, but rather of inefficiency, according to the authors.
One registry spanning 2009-2012 and including more than 400,000 newly diagnosed cancer patients found lung cancer to have the third-highest rate of unplanned hospitalizations (after hepatobiliary and pancreatic cancer). Those findings were published in JCO Oncology Practice in 2018.
While the reason for presentation to the ED is often the cancer or its therapy in this population, there is a paucity of research on the relative contribution of factors leading to unplanned hospital visits.
Common precipitants of medical emergencies in advanced stages of lung cancer include pulmonary embolism, obstructive pneumonia, spinal cord compression caused by metastasis, and tumor progression or pleural effusion leading to respiratory failure.
Lung cancer therapies, such as TKIs, immunotherapy, and cytotoxic chemotherapy, can also cause various medical emergencies arising out of skin reactions, gastrointestinal dysfunction, organ inflammatory processes, and bone marrow suppression.
Identifying drivers of unplanned ED visits
The primary goals of Dr. Shah and Dr. Neal’s study were to understand the drivers of unplanned ED visits and identify potential strategies to prevent them.
Drawing from the Stanford Medicine Research Data Repository, the authors identified 97 NSCLC patients with 173 ED visits at Stanford.
Patients were sorted according to which of the three therapies they had been receiving in the 3 months prior to the unplanned visit – TKIs (n = 47), immunotherapy (n = 24), or chemotherapy (n = 26). Patients receiving a combination of chemotherapy and immunotherapy were classified under the immunotherapy category.
ED visits were divided into four categories: cancer related, therapy related, unrelated to cancer or therapy, and rule-out (when an outpatient provider sent the patient to rule out medico-oncologic emergencies such as pulmonary embolism or cord compression).
If the patient’s main complaint(s) began 2 or more days before presentation, the diagnostics or therapeutics could have been provided in an outpatient setting (e.g., in clinic or urgent care), and the patient was discharged directly from the ED, the encounter was classified as potentially preventable. Among these preventable encounters, those made during business hours were also labeled unnecessary because they could have been managed through the Stanford sick call system for same-day urgent visits.
Leading cause is cancer
Overall, the leading cause of ED visits was NSCLC itself (54%). The patient’s cancer contributed to 61% of ED visits in the TKI group, 49% in the immunotherapy group, and 42% in the chemotherapy group.
Many ED visits were deemed unrelated to cancer or its therapies – 30% in the TKI group, 26% in the immunotherapy group, and 32% in the chemotherapy group.
Rule-out cases contributed to 7% of ED visits in the TKI group, 14% in the immunotherapy group, and 5% in the chemotherapy group.
Overall, 9% of ED visits were therapy related. The smallest proportion of these was observed in the TKI group (2%), which was significantly smaller than in the immunotherapy group (12%), a rate also significantly smaller than in the chemotherapy group (21%, P < .001).
Most unplanned ED visits did not lead to admissions (55%), were for complaints that began 2 or more days prior to presentation (53%), led to diagnostics or therapeutics that could have been administered in an outpatient setting (48%), and were during business hours (52%).
As a result, 24% of visits were classified as preventable, and 10% were deemed unnecessary.
Preventive strategies
“Our study suggests that TKIs lead to fewer emergency room visits than immunotherapy and chemotherapy,” Dr. Shah said in an interview.
“Overall, this may not necessarily change which therapy we prescribe,” he added, “as TKI therapy is often first line for patients with targeted mutations. However, recognizing that those on chemotherapy or immunotherapy are at higher risk for emergency room visits, we may target preventative strategies, for example, nursing phone calls, telemonitoring of symptoms, and frequent video visits toward this high-risk population.”
Dr. Shah and Dr. Neal said it’s “reassuring” that TKIs and immunotherapy are small drivers of unplanned hospital care. However, they also said efforts aimed at reducing chemotherapy-related ED visits are warranted.
The authors speculated that early intervention, extension of ambulatory care, and patient education about outpatient avenues of care could eliminate a significant proportion (at least 20%) of unplanned ED visits by NSCLC patients.
There was no specific funding for this study. Dr. Shah disclosed no conflicts of interest. Dr. Neal disclosed relationships with many companies, including this news organization.
The leading cause of ED visits in the study was the cancer itself, although many visits were unrelated to non–small cell lung cancer (NSCLC) or cancer therapy.
Less than 10% of ED visits were related to cancer therapy, but visits were much more common among patients receiving chemotherapy than among those receiving immunotherapy or tyrosine kinase inhibitors (TKIs).
Manan P. Shah, MD, and Joel W. Neal, MD, PhD, both of Stanford (Calif. ) University, reported these results in JCO Oncology Practice.
The authors noted that, in the United States, care of patients with cancer, among all diseases, leads to the highest per-person cost. Unplanned hospital visits are among the largest drivers of increased spending in advanced cancer care, accounting for 48% of spending. However, that spending may not be indicative of better quality care, but rather of inefficiency, according to the authors.
One registry spanning 2009-2012 and including more than 400,000 newly diagnosed cancer patients found lung cancer to have the third-highest rate of unplanned hospitalizations (after hepatobiliary and pancreatic cancer). Those findings were published in JCO Oncology Practice in 2018.
While the reason for presentation to the ED is often the cancer or its therapy in this population, there is a paucity of research on the relative contribution of factors leading to unplanned hospital visits.
Common precipitants of medical emergencies in advanced stages of lung cancer include pulmonary embolism, obstructive pneumonia, spinal cord compression caused by metastasis, and tumor progression or pleural effusion leading to respiratory failure.
Lung cancer therapies, such as TKIs, immunotherapy, and cytotoxic chemotherapy, can also cause various medical emergencies arising out of skin reactions, gastrointestinal dysfunction, organ inflammatory processes, and bone marrow suppression.
Identifying drivers of unplanned ED visits
The primary goals of Dr. Shah and Dr. Neal’s study were to understand the drivers of unplanned ED visits and identify potential strategies to prevent them.
Drawing from the Stanford Medicine Research Data Repository, the authors identified 97 NSCLC patients with 173 ED visits at Stanford.
Patients were sorted according to which of the three therapies they had been receiving in the 3 months prior to the unplanned visit – TKIs (n = 47), immunotherapy (n = 24), or chemotherapy (n = 26). Patients receiving a combination of chemotherapy and immunotherapy were classified under the immunotherapy category.
ED visits were divided into four categories: cancer related, therapy related, unrelated to cancer or therapy, and rule-out (when an outpatient provider sent the patient to rule out medico-oncologic emergencies such as pulmonary embolism or cord compression).
If the patient’s main complaint(s) began 2 or more days before presentation, the diagnostics or therapeutics could have been provided in an outpatient setting (e.g., in clinic or urgent care), and the patient was discharged directly from the ED, the encounter was classified as potentially preventable. Among these preventable encounters, those made during business hours were also labeled unnecessary because they could have been managed through the Stanford sick call system for same-day urgent visits.
Leading cause is cancer
Overall, the leading cause of ED visits was NSCLC itself (54%). The patient’s cancer contributed to 61% of ED visits in the TKI group, 49% in the immunotherapy group, and 42% in the chemotherapy group.
Many ED visits were deemed unrelated to cancer or its therapies – 30% in the TKI group, 26% in the immunotherapy group, and 32% in the chemotherapy group.
Rule-out cases contributed to 7% of ED visits in the TKI group, 14% in the immunotherapy group, and 5% in the chemotherapy group.
Overall, 9% of ED visits were therapy related. The smallest proportion of these was observed in the TKI group (2%), which was significantly smaller than in the immunotherapy group (12%), a rate also significantly smaller than in the chemotherapy group (21%, P < .001).
Most unplanned ED visits did not lead to admissions (55%), were for complaints that began 2 or more days prior to presentation (53%), led to diagnostics or therapeutics that could have been administered in an outpatient setting (48%), and were during business hours (52%).
As a result, 24% of visits were classified as preventable, and 10% were deemed unnecessary.
Preventive strategies
“Our study suggests that TKIs lead to fewer emergency room visits than immunotherapy and chemotherapy,” Dr. Shah said in an interview.
“Overall, this may not necessarily change which therapy we prescribe,” he added, “as TKI therapy is often first line for patients with targeted mutations. However, recognizing that those on chemotherapy or immunotherapy are at higher risk for emergency room visits, we may target preventative strategies, for example, nursing phone calls, telemonitoring of symptoms, and frequent video visits toward this high-risk population.”
Dr. Shah and Dr. Neal said it’s “reassuring” that TKIs and immunotherapy are small drivers of unplanned hospital care. However, they also said efforts aimed at reducing chemotherapy-related ED visits are warranted.
The authors speculated that early intervention, extension of ambulatory care, and patient education about outpatient avenues of care could eliminate a significant proportion (at least 20%) of unplanned ED visits by NSCLC patients.
There was no specific funding for this study. Dr. Shah disclosed no conflicts of interest. Dr. Neal disclosed relationships with many companies, including this news organization.
FROM JCO ONCOLOGY PRACTICE
Temper enthusiasm for long-term treatment with bisphosphonates?
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.