User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Applications for laser-assisted drug delivery on the horizon, expert says
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Everyone wins when losers get paid
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
Bribery really is the solution to all of life’s problems
Breaking news: The United States has a bit of an obesity epidemic. Okay, maybe not so breaking news. But it’s a problem we’ve been struggling with for a very long time. Part of the issue is that there really is no secret to weight loss. Pretty much anything can work if you’re committed. The millions of diets floating around are testament to this idea.
The problem of losing weight is amplified if you don’t rake in the big bucks. Lower-income individuals often can’t afford healthy superfoods, and they’re often too busy to spend time at classes, exercising, or following programs. A group of researchers at New York University has offered up an alternate solution to encourage weight loss in low-income people: Pay them.
Specifically, pay them for losing weight. A reward, if you will. The researchers recruited several hundred lower-income people and split them into three groups. All participants received a free 1-year membership to a gym and weight-loss program, as well as food journals and fitness devices, but one group received payment (on average, about $300 overall) for attending meetings, exercising a certain amount every week, or weighing themselves twice a week. About 40% of people in this group lost 5% of their body weight after 6 months, twice as many as in the group that did not receive payment for performing these tasks.
The big winners, however, were those in the third group. They also received the free stuff, but the researchers offered them a more simple and direct bribe: Lose 5% of your weight over 6 months and we’ll pay you. The reward? About $450 on average, and it worked very well, with half this group losing the weight after 6 months. That said, after a year something like a fifth of this group put the weight back on, bringing them in line with the group that was paid to perform tasks. Still, both groups outperformed the control group, which received no money.
The takeaway from this research is pretty obvious. Pay people a fair price to do something, and they’ll do it. This is a lesson that has absolutely no relevance in the modern world. Nope, none whatsoever. We all receive completely fair wages. We all have plenty of money to pay for things. Everything is fine.
More green space, less medicine
Have you heard of the 3-30-300 rule? Proposed by urban forester Cecil Konijnendijk, it’s become the rule of thumb for urban planners and other foresters into getting more green space in populated areas. A recent study has found that people who lived within this 3-30-300 rule had better mental health and less medication use.
If you’re not an urban forester, however, you may not know what the 3-30-300 rule is. But it’s pretty simple, people should be able to see at least three trees from their home, have 30% tree canopy in their neighborhood, and have 300 Spartans to defend against the Persian army.
We may have made that last one up. It’s actually have a green space or park within 300 meters of your home.
In the new study, only 4.7% of people surveyed lived in an area that followed all three rules. About 62% of the surveyed lived with a green space at least 300 meters away, 43% had at least three trees within 15 meters from their home, and a rather pitiful 9% had adequate tree canopy coverage in their neighborhood.
Greater adherence to the 3-30-300 rule was associated with fewer visits to the psychologist, with 8.3% of the participants reporting a psychologist visit in the last year. The data come from a sample of a little over 3,000 Barcelona residents aged 15-97 who were randomly selected to participate in the Barcelona Public Health Agency Survey.
“There is an urgent need to provide citizens with more green space,” said Mark Nieuwenhuijsen, lead author of the study. “We may need to tear out asphalt and plant more trees, which would not only improve health, but also reduce heat island effects and contribute to carbon capture.”
The main goal and message is that more green space is good for everyone. So if you’re feeling a little overwhelmed, take a breather and sit somewhere green. Or call those 300 Spartans and get them to start knocking some buildings down.
Said the toilet to the engineer: Do you hear what I hear?
A mythical hero’s journey took Dorothy along the yellow brick road to find the Wizard of Oz. Huckleberry Finn used a raft to float down the Mississippi River. Luke Skywalker did most of his traveling between planets. For the rest of us, the journey may be just a bit shorter.
Also a bit less heroic. Unless, of course, you’re prepping for a colonoscopy. Yup, we’re headed to the toilet, but not just any toilet. This toilet was the subject of a presentation at the annual meeting of the Acoustical Society of America, titled “The feces thesis: Using machine learning to detect diarrhea,” and that presentation was the hero’s journey of Maia Gatlin, PhD, a research engineer at the Georgia Institute of Technology.
She and her team attached a noninvasive microphone sensor to a toilet, and now they can identify bowel diseases without collecting any identifiable information.
The audio sample of an excretion event is “transformed into a spectrogram, which essentially captures the sound in an image. Different events produce different features in the audio and the spectrogram. For example, urination creates a consistent tone, while defecation may have a singular tone. In contrast, diarrhea is more random,” they explained in the written statement.
They used a machine learning algorithm to classify each spectrogram based on its features. “The algorithm’s performance was tested against data with and without background noises to make sure it was learning the right sound features, regardless of the sensor’s environment,” Dr. Gatlin and associates wrote.
Their goal is to use the toilet sensor in areas where cholera is common to prevent the spread of disease. After that, who knows? “Perhaps someday, our algorithm can be used with existing in-home smart devices to monitor one’s own bowel movements and health!” she suggested.
That would be a heroic toilet indeed.
‘Slugging’: A TikTok skin trend that has some merit
They’ve been around for a while and show no signs of going away: videos on TikTok of people, often teens, slathering their face with petroleum jelly and claiming that it’s transformed their skin, cured their acne, or given them an amazing “glow up.”
TikTok videos mentioning petrolatum increased by 46% and Instagram videos by 93% from 2021 to 2022, reported Gabriel Santos Malave, BA, of the Icahn School of Medicine at Mount Sinai, New York, and William D. James, MD, professor of dermatology, University of Pennsylvania, Philadelphia, in a review of petroleum jelly’s uses recently published in Cutis.
The authors said that after application of a moisturizer.
In a typical demonstration, a dermatologist in the United Kingdom showed how she incorporates slugging into her routine in a TikTok video that’s had more than 1 million views.
Unlike many TikTok trends, slugging may not be entirely bad, say dermatologists.
“I think it’s a great way to keep your skin protected and moisturized, especially in those dry, cold winter months,” said dermatologist Mamina Turegano, MD, in a video posted in February 2022. That TikTok video has had more than 6 million views.
Dr. Turegano, who is in private practice in the New Orleans suburb of Metairie, La., told this news organization that she decided to post about slugging after she’d noticed that the topic was trending. Also, she had tried the technique herself when she was a resident in Washington more than a decade ago.
At the time, Dr. Turegano said that she was aware that “putting petroleum jelly on your face was not a normal thing.” But, given its history of being used in dermatology, she gave it a try and found that it worked well for her dry skin, she said.
Dr. Turegano is one among many dermatologists who have joined TikTok to dispel myths, educate, and inform. It’s important for them to be there “to engage and empower the public to become a better consumer of information out there and take ownership of their skin health,” said Jean McGee, MD, PhD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and assistant professor of dermatology at Harvard Medical School, also in Boston.
Dr. McGee and colleagues studied TikTok content on slugging and found that by far, videos that were created by health care providers were more educational. Dermatologists who posted were more likely to discuss the risks and benefits, whereas so-called “influencers” rarely posted on the risks, according to the study, published in Clinics in Dermatology.
Slugging is generally safe and effective for those who have a compromised skin barrier or “for those who have sensitive skin and can’t tolerate other products but need some form of moisturization,” said Dr. Turegano.
“Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL),” write Mr. Malave and Dr. James in Cutis. They note that petrolatum reduces TEWL by 98%, compared with only 20% to 30% for other oil-based moisturizers.
Dermatologists have often recommended a “seal and trap” regimen for dry skin or eczema. It involves a short, lukewarm shower, followed by immediately moisturizing with a petrolatum-based ointment, said Dr. McGee.
This could be safe for the face, but “other variables need to be considered,” including use of other topical medications and other skin care practices, she added.
The concept of double-layering a moisturizer and an occlusive agent can be beneficial but more typically for the hands and feet, where the skin can be severely dry and cracked, said Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington. “I would not recommend that on the face,” Dr. Friedman told this news organization.
He and other dermatologists warned about the potential for slugging – given petroleum jelly’s occlusive nature – to enhance the action of any topical steroid, retinol, or exfoliating agent.
Muneeb Shah, MD, who practices in Mooresville, N.C., is one of the most popular dermatologists on TikTok, with more than 17 million followers. He also warned in a February 2022 video about potential downsides. “Be careful after using retinol or exfoliating acids because it may actually irritate your skin more,” he says in the video.
“Slugging is awesome for some people but not for others, and not for every night,” said Whitney Bowe, MD, on a TikTok video she posted in July. She recommended it for eczema or really dry skin. Dr. Bowe, who practices with Advanced Dermatology in New York, advised those with acne-prone skin to “skip this trend.”
On a web page aimed at the general public, the American Academy of Dermatology similarly cautioned, “Avoid putting petroleum jelly on your face if you are acne-prone, as this may cause breakouts in some people.”
Acne cure or pore clogger?
And yet, plenty of TikTok users claim that it has improved their acne.
One such user posted a before and after video purporting to show that slugging had almost completely eliminated her acne and prior scarring. Not surprisingly, it has been viewed some 9 million times and got 1.5 million “likes.”
Dr. Friedman notes that it’s theoretically possible – but not likely – that acne could improve by slugging, given that acne basically is a disease of barrier disruption. “The idea here is you have disrupted skin barrier throughout the face regardless of whether you have a pimple in that spot or not, so you need to repair it,” he said. “That’s where I think slugging is somewhat on the right track, because by putting an occlusive agent on the skin, you are restoring the barrier element,” he said.
However, applying a thick, greasy ointment on the face could block pores and cause a backup of sebum and dead skin cells, and it could trap bacteria, he said. “Skin barrier protection and repair is central to acne management, but you need to do it in a safe way,” he said. He noted that that means applying an oil-free moisturizer to damp skin.
Dr. Turegano said she has seen slugging improve acne, but it’s hard to say which people with acne-prone skin would be the best candidates. Those who have used harsh products to treat acne and subsequently experienced worsening acne could potentially benefit, she said.
Even so, she said, “I’d be very cautious in anyone with acne.”
Dr. Friedman, Dr. McGee, and Dr. Turegano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
They’ve been around for a while and show no signs of going away: videos on TikTok of people, often teens, slathering their face with petroleum jelly and claiming that it’s transformed their skin, cured their acne, or given them an amazing “glow up.”
TikTok videos mentioning petrolatum increased by 46% and Instagram videos by 93% from 2021 to 2022, reported Gabriel Santos Malave, BA, of the Icahn School of Medicine at Mount Sinai, New York, and William D. James, MD, professor of dermatology, University of Pennsylvania, Philadelphia, in a review of petroleum jelly’s uses recently published in Cutis.
The authors said that after application of a moisturizer.
In a typical demonstration, a dermatologist in the United Kingdom showed how she incorporates slugging into her routine in a TikTok video that’s had more than 1 million views.
Unlike many TikTok trends, slugging may not be entirely bad, say dermatologists.
“I think it’s a great way to keep your skin protected and moisturized, especially in those dry, cold winter months,” said dermatologist Mamina Turegano, MD, in a video posted in February 2022. That TikTok video has had more than 6 million views.
Dr. Turegano, who is in private practice in the New Orleans suburb of Metairie, La., told this news organization that she decided to post about slugging after she’d noticed that the topic was trending. Also, she had tried the technique herself when she was a resident in Washington more than a decade ago.
At the time, Dr. Turegano said that she was aware that “putting petroleum jelly on your face was not a normal thing.” But, given its history of being used in dermatology, she gave it a try and found that it worked well for her dry skin, she said.
Dr. Turegano is one among many dermatologists who have joined TikTok to dispel myths, educate, and inform. It’s important for them to be there “to engage and empower the public to become a better consumer of information out there and take ownership of their skin health,” said Jean McGee, MD, PhD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and assistant professor of dermatology at Harvard Medical School, also in Boston.
Dr. McGee and colleagues studied TikTok content on slugging and found that by far, videos that were created by health care providers were more educational. Dermatologists who posted were more likely to discuss the risks and benefits, whereas so-called “influencers” rarely posted on the risks, according to the study, published in Clinics in Dermatology.
Slugging is generally safe and effective for those who have a compromised skin barrier or “for those who have sensitive skin and can’t tolerate other products but need some form of moisturization,” said Dr. Turegano.
“Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL),” write Mr. Malave and Dr. James in Cutis. They note that petrolatum reduces TEWL by 98%, compared with only 20% to 30% for other oil-based moisturizers.
Dermatologists have often recommended a “seal and trap” regimen for dry skin or eczema. It involves a short, lukewarm shower, followed by immediately moisturizing with a petrolatum-based ointment, said Dr. McGee.
This could be safe for the face, but “other variables need to be considered,” including use of other topical medications and other skin care practices, she added.
The concept of double-layering a moisturizer and an occlusive agent can be beneficial but more typically for the hands and feet, where the skin can be severely dry and cracked, said Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington. “I would not recommend that on the face,” Dr. Friedman told this news organization.
He and other dermatologists warned about the potential for slugging – given petroleum jelly’s occlusive nature – to enhance the action of any topical steroid, retinol, or exfoliating agent.
Muneeb Shah, MD, who practices in Mooresville, N.C., is one of the most popular dermatologists on TikTok, with more than 17 million followers. He also warned in a February 2022 video about potential downsides. “Be careful after using retinol or exfoliating acids because it may actually irritate your skin more,” he says in the video.
“Slugging is awesome for some people but not for others, and not for every night,” said Whitney Bowe, MD, on a TikTok video she posted in July. She recommended it for eczema or really dry skin. Dr. Bowe, who practices with Advanced Dermatology in New York, advised those with acne-prone skin to “skip this trend.”
On a web page aimed at the general public, the American Academy of Dermatology similarly cautioned, “Avoid putting petroleum jelly on your face if you are acne-prone, as this may cause breakouts in some people.”
Acne cure or pore clogger?
And yet, plenty of TikTok users claim that it has improved their acne.
One such user posted a before and after video purporting to show that slugging had almost completely eliminated her acne and prior scarring. Not surprisingly, it has been viewed some 9 million times and got 1.5 million “likes.”
Dr. Friedman notes that it’s theoretically possible – but not likely – that acne could improve by slugging, given that acne basically is a disease of barrier disruption. “The idea here is you have disrupted skin barrier throughout the face regardless of whether you have a pimple in that spot or not, so you need to repair it,” he said. “That’s where I think slugging is somewhat on the right track, because by putting an occlusive agent on the skin, you are restoring the barrier element,” he said.
However, applying a thick, greasy ointment on the face could block pores and cause a backup of sebum and dead skin cells, and it could trap bacteria, he said. “Skin barrier protection and repair is central to acne management, but you need to do it in a safe way,” he said. He noted that that means applying an oil-free moisturizer to damp skin.
Dr. Turegano said she has seen slugging improve acne, but it’s hard to say which people with acne-prone skin would be the best candidates. Those who have used harsh products to treat acne and subsequently experienced worsening acne could potentially benefit, she said.
Even so, she said, “I’d be very cautious in anyone with acne.”
Dr. Friedman, Dr. McGee, and Dr. Turegano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
They’ve been around for a while and show no signs of going away: videos on TikTok of people, often teens, slathering their face with petroleum jelly and claiming that it’s transformed their skin, cured their acne, or given them an amazing “glow up.”
TikTok videos mentioning petrolatum increased by 46% and Instagram videos by 93% from 2021 to 2022, reported Gabriel Santos Malave, BA, of the Icahn School of Medicine at Mount Sinai, New York, and William D. James, MD, professor of dermatology, University of Pennsylvania, Philadelphia, in a review of petroleum jelly’s uses recently published in Cutis.
The authors said that after application of a moisturizer.
In a typical demonstration, a dermatologist in the United Kingdom showed how she incorporates slugging into her routine in a TikTok video that’s had more than 1 million views.
Unlike many TikTok trends, slugging may not be entirely bad, say dermatologists.
“I think it’s a great way to keep your skin protected and moisturized, especially in those dry, cold winter months,” said dermatologist Mamina Turegano, MD, in a video posted in February 2022. That TikTok video has had more than 6 million views.
Dr. Turegano, who is in private practice in the New Orleans suburb of Metairie, La., told this news organization that she decided to post about slugging after she’d noticed that the topic was trending. Also, she had tried the technique herself when she was a resident in Washington more than a decade ago.
At the time, Dr. Turegano said that she was aware that “putting petroleum jelly on your face was not a normal thing.” But, given its history of being used in dermatology, she gave it a try and found that it worked well for her dry skin, she said.
Dr. Turegano is one among many dermatologists who have joined TikTok to dispel myths, educate, and inform. It’s important for them to be there “to engage and empower the public to become a better consumer of information out there and take ownership of their skin health,” said Jean McGee, MD, PhD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and assistant professor of dermatology at Harvard Medical School, also in Boston.
Dr. McGee and colleagues studied TikTok content on slugging and found that by far, videos that were created by health care providers were more educational. Dermatologists who posted were more likely to discuss the risks and benefits, whereas so-called “influencers” rarely posted on the risks, according to the study, published in Clinics in Dermatology.
Slugging is generally safe and effective for those who have a compromised skin barrier or “for those who have sensitive skin and can’t tolerate other products but need some form of moisturization,” said Dr. Turegano.
“Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL),” write Mr. Malave and Dr. James in Cutis. They note that petrolatum reduces TEWL by 98%, compared with only 20% to 30% for other oil-based moisturizers.
Dermatologists have often recommended a “seal and trap” regimen for dry skin or eczema. It involves a short, lukewarm shower, followed by immediately moisturizing with a petrolatum-based ointment, said Dr. McGee.
This could be safe for the face, but “other variables need to be considered,” including use of other topical medications and other skin care practices, she added.
The concept of double-layering a moisturizer and an occlusive agent can be beneficial but more typically for the hands and feet, where the skin can be severely dry and cracked, said Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington. “I would not recommend that on the face,” Dr. Friedman told this news organization.
He and other dermatologists warned about the potential for slugging – given petroleum jelly’s occlusive nature – to enhance the action of any topical steroid, retinol, or exfoliating agent.
Muneeb Shah, MD, who practices in Mooresville, N.C., is one of the most popular dermatologists on TikTok, with more than 17 million followers. He also warned in a February 2022 video about potential downsides. “Be careful after using retinol or exfoliating acids because it may actually irritate your skin more,” he says in the video.
“Slugging is awesome for some people but not for others, and not for every night,” said Whitney Bowe, MD, on a TikTok video she posted in July. She recommended it for eczema or really dry skin. Dr. Bowe, who practices with Advanced Dermatology in New York, advised those with acne-prone skin to “skip this trend.”
On a web page aimed at the general public, the American Academy of Dermatology similarly cautioned, “Avoid putting petroleum jelly on your face if you are acne-prone, as this may cause breakouts in some people.”
Acne cure or pore clogger?
And yet, plenty of TikTok users claim that it has improved their acne.
One such user posted a before and after video purporting to show that slugging had almost completely eliminated her acne and prior scarring. Not surprisingly, it has been viewed some 9 million times and got 1.5 million “likes.”
Dr. Friedman notes that it’s theoretically possible – but not likely – that acne could improve by slugging, given that acne basically is a disease of barrier disruption. “The idea here is you have disrupted skin barrier throughout the face regardless of whether you have a pimple in that spot or not, so you need to repair it,” he said. “That’s where I think slugging is somewhat on the right track, because by putting an occlusive agent on the skin, you are restoring the barrier element,” he said.
However, applying a thick, greasy ointment on the face could block pores and cause a backup of sebum and dead skin cells, and it could trap bacteria, he said. “Skin barrier protection and repair is central to acne management, but you need to do it in a safe way,” he said. He noted that that means applying an oil-free moisturizer to damp skin.
Dr. Turegano said she has seen slugging improve acne, but it’s hard to say which people with acne-prone skin would be the best candidates. Those who have used harsh products to treat acne and subsequently experienced worsening acne could potentially benefit, she said.
Even so, she said, “I’d be very cautious in anyone with acne.”
Dr. Friedman, Dr. McGee, and Dr. Turegano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
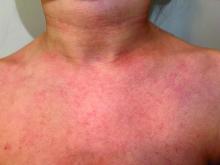
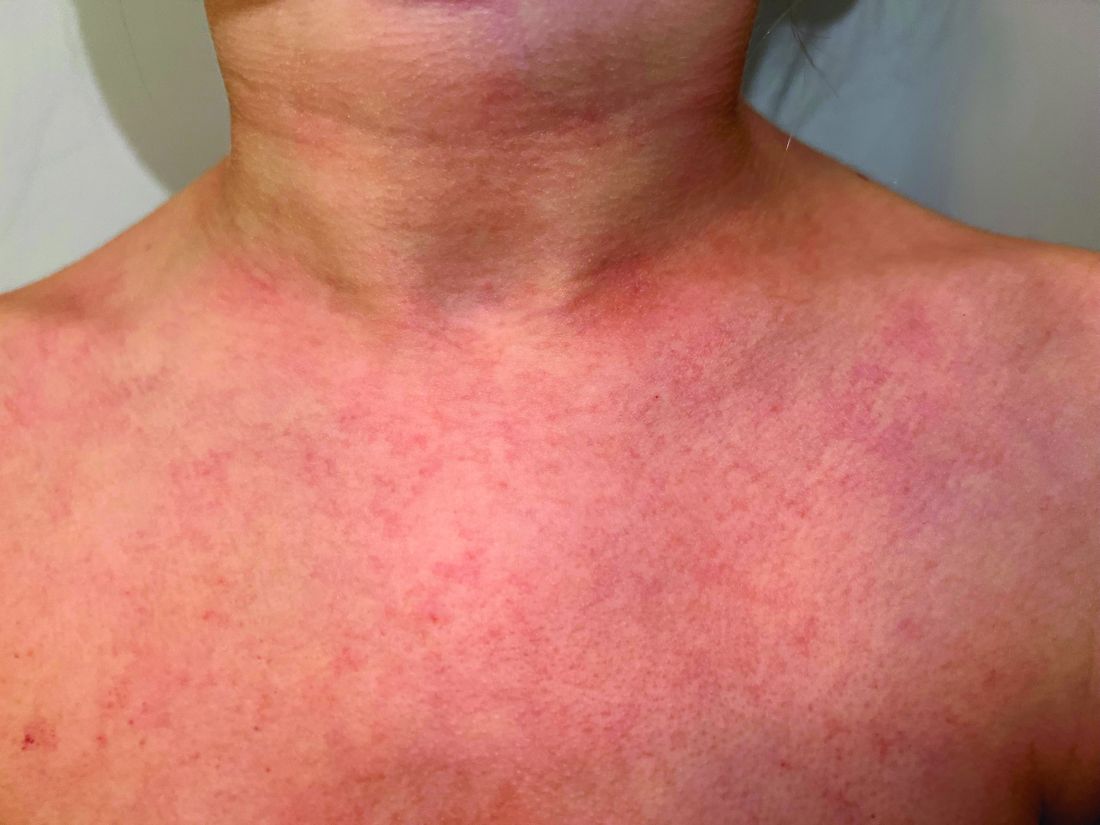
A 9-year old female presented with 1 day of fever, fatigue, and sore throat
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
Review gives weight to supplements for hair loss
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
New melting hydrogel bandage could treat burn wounds faster, with less pain
Surgically debriding burn wounds can be tedious for doctors and excruciating for patients. To change that, bioengineers have created a new hydrogel formula that dissolves rapidly from wound sites, melting off in 6 minutes or less.
“The removal of dressings, with the current standard of care, is very hard and time-consuming. It becomes very painful for the patient. People are screaming, or they’re given a lot of opioids,” said senior author O. Berk Usta, PhD, of the Center for Engineering in Medicine and Surgery at Massachusetts General Hospital, Boston. “Those are the things we wanted to minimize: the pain and the time.”
Although beneficial for all patients, a short, painless bandage change would be a particular boon for younger patients. At the pediatric burns care center at Shriners Hospitals for Children (an MGH partner), researchers “observe a lot of children who go through therapy or treatment after burns,” said Dr. Usta. The team at MGH collaborated with scientists at Tufts University, Boston, with those patients in mind, setting out to create a new hydrogel that would transform burn wound care.
A better bandage
Hydrogels provide cooling relief to burn wounds and maintain a moist environment that can speed healing. There are currently hydrogel sheets and hydrogel-infused dressings, as well as gel that is applied directly to burn wounds before being covered with protective material. These dressings must be replaced frequently to prevent infections, but that can be unbearably painful and drawn out, as dressings often stick to wounds.
Mechanical debridement can be especially difficult for second-degree burn patients, whose wounds may still retain nerve endings. Debridement tends to also remove some healthy tissue and can damage newly formed tissue, slowing down healing.
“It can take up to 2, 3 hours, and it requires multiple people working on it,” said Dr. Usta.
The new hydrogel treatment can be applied directly to a wound and it forms a protective barrier around the site in 15 seconds. The hydrogel is then covered by a protective dressing until it needs to be changed.
“After you take off the protective covering, you add another solution, which dissolves the [hydrogel] dressing, so that it can be easily removed from the burn site,” Dr. Usta said.
The solution dissolves the hydrogel in 4-6 minutes.
Hybrid gels
Many hydrogels currently used for burn wounds feature physically cross-linked molecules. This makes them strong and capable of retaining moisture, but also difficult to dissolve. The researchers used a different approach.
“This is not physical cross-linking like the traditional approaches, but rather, softer covalent bonds between the different molecules. And that’s why, when you bring in another solution, the hydrogel dissolves away,” Dr. Usta said.
The new hydrogels rely on a supramolecular assembly: a network of synthetic polymers whose connections can be reversed more easily, meaning they can be dissolved quickly. Another standout feature of the new hydrogels is their hybrid composition, displaying characteristics of both liquids and solids. The polymers are knitted together into a mesh-like network that enables water retention, with the goal of maintaining the moist environment needed for wound healing.
The supramolecular assembly is also greener, Dr. Usta explained; traditional cross-linking approaches produce a lot of toxic by-products that could harm the environment.
And whereas traditional hydrogels can require a dozen chemistry steps to produce, the new hydrogels are ready after mixing two solutions, Dr. Usta explained. This makes them easy to prepare at bedside, ideal for treating large wounds in the ER or even on battlefields.
When tested in vitro, using skin cells, and in vivo, on mice, the new hydrogels were shown to be safe to use on wounds. Additional studies on mice, as well as large animals, will focus on safety and efficacy, and may be followed by human clinical trials, said Dr. Usta.
“The next phase of the project will be to look at whether these dressings will help wound healing by creating a moist environment,” said Dr. Usta.
The researchers are also exploring how to manufacture individual prewrapped hydrogels that could be applied in a clinical setting – or even in people’s homes. The consumer market is “another possibility,” said Dr. Usta, particularly among patients with “smaller, more superficial burns” or patients whose large burn wounds are still healing once they leave the hospital.
This research was supported by the National Institutes of Health, National Science Foundation, Massachusetts General Hospital Executive Committee on Research Interim Support Fund, and Shriners Hospitals.
A version of this article first appeared on Medscape.com.
Surgically debriding burn wounds can be tedious for doctors and excruciating for patients. To change that, bioengineers have created a new hydrogel formula that dissolves rapidly from wound sites, melting off in 6 minutes or less.
“The removal of dressings, with the current standard of care, is very hard and time-consuming. It becomes very painful for the patient. People are screaming, or they’re given a lot of opioids,” said senior author O. Berk Usta, PhD, of the Center for Engineering in Medicine and Surgery at Massachusetts General Hospital, Boston. “Those are the things we wanted to minimize: the pain and the time.”
Although beneficial for all patients, a short, painless bandage change would be a particular boon for younger patients. At the pediatric burns care center at Shriners Hospitals for Children (an MGH partner), researchers “observe a lot of children who go through therapy or treatment after burns,” said Dr. Usta. The team at MGH collaborated with scientists at Tufts University, Boston, with those patients in mind, setting out to create a new hydrogel that would transform burn wound care.
A better bandage
Hydrogels provide cooling relief to burn wounds and maintain a moist environment that can speed healing. There are currently hydrogel sheets and hydrogel-infused dressings, as well as gel that is applied directly to burn wounds before being covered with protective material. These dressings must be replaced frequently to prevent infections, but that can be unbearably painful and drawn out, as dressings often stick to wounds.
Mechanical debridement can be especially difficult for second-degree burn patients, whose wounds may still retain nerve endings. Debridement tends to also remove some healthy tissue and can damage newly formed tissue, slowing down healing.
“It can take up to 2, 3 hours, and it requires multiple people working on it,” said Dr. Usta.
The new hydrogel treatment can be applied directly to a wound and it forms a protective barrier around the site in 15 seconds. The hydrogel is then covered by a protective dressing until it needs to be changed.
“After you take off the protective covering, you add another solution, which dissolves the [hydrogel] dressing, so that it can be easily removed from the burn site,” Dr. Usta said.
The solution dissolves the hydrogel in 4-6 minutes.
Hybrid gels
Many hydrogels currently used for burn wounds feature physically cross-linked molecules. This makes them strong and capable of retaining moisture, but also difficult to dissolve. The researchers used a different approach.
“This is not physical cross-linking like the traditional approaches, but rather, softer covalent bonds between the different molecules. And that’s why, when you bring in another solution, the hydrogel dissolves away,” Dr. Usta said.
The new hydrogels rely on a supramolecular assembly: a network of synthetic polymers whose connections can be reversed more easily, meaning they can be dissolved quickly. Another standout feature of the new hydrogels is their hybrid composition, displaying characteristics of both liquids and solids. The polymers are knitted together into a mesh-like network that enables water retention, with the goal of maintaining the moist environment needed for wound healing.
The supramolecular assembly is also greener, Dr. Usta explained; traditional cross-linking approaches produce a lot of toxic by-products that could harm the environment.
And whereas traditional hydrogels can require a dozen chemistry steps to produce, the new hydrogels are ready after mixing two solutions, Dr. Usta explained. This makes them easy to prepare at bedside, ideal for treating large wounds in the ER or even on battlefields.
When tested in vitro, using skin cells, and in vivo, on mice, the new hydrogels were shown to be safe to use on wounds. Additional studies on mice, as well as large animals, will focus on safety and efficacy, and may be followed by human clinical trials, said Dr. Usta.
“The next phase of the project will be to look at whether these dressings will help wound healing by creating a moist environment,” said Dr. Usta.
The researchers are also exploring how to manufacture individual prewrapped hydrogels that could be applied in a clinical setting – or even in people’s homes. The consumer market is “another possibility,” said Dr. Usta, particularly among patients with “smaller, more superficial burns” or patients whose large burn wounds are still healing once they leave the hospital.
This research was supported by the National Institutes of Health, National Science Foundation, Massachusetts General Hospital Executive Committee on Research Interim Support Fund, and Shriners Hospitals.
A version of this article first appeared on Medscape.com.
Surgically debriding burn wounds can be tedious for doctors and excruciating for patients. To change that, bioengineers have created a new hydrogel formula that dissolves rapidly from wound sites, melting off in 6 minutes or less.
“The removal of dressings, with the current standard of care, is very hard and time-consuming. It becomes very painful for the patient. People are screaming, or they’re given a lot of opioids,” said senior author O. Berk Usta, PhD, of the Center for Engineering in Medicine and Surgery at Massachusetts General Hospital, Boston. “Those are the things we wanted to minimize: the pain and the time.”
Although beneficial for all patients, a short, painless bandage change would be a particular boon for younger patients. At the pediatric burns care center at Shriners Hospitals for Children (an MGH partner), researchers “observe a lot of children who go through therapy or treatment after burns,” said Dr. Usta. The team at MGH collaborated with scientists at Tufts University, Boston, with those patients in mind, setting out to create a new hydrogel that would transform burn wound care.
A better bandage
Hydrogels provide cooling relief to burn wounds and maintain a moist environment that can speed healing. There are currently hydrogel sheets and hydrogel-infused dressings, as well as gel that is applied directly to burn wounds before being covered with protective material. These dressings must be replaced frequently to prevent infections, but that can be unbearably painful and drawn out, as dressings often stick to wounds.
Mechanical debridement can be especially difficult for second-degree burn patients, whose wounds may still retain nerve endings. Debridement tends to also remove some healthy tissue and can damage newly formed tissue, slowing down healing.
“It can take up to 2, 3 hours, and it requires multiple people working on it,” said Dr. Usta.
The new hydrogel treatment can be applied directly to a wound and it forms a protective barrier around the site in 15 seconds. The hydrogel is then covered by a protective dressing until it needs to be changed.
“After you take off the protective covering, you add another solution, which dissolves the [hydrogel] dressing, so that it can be easily removed from the burn site,” Dr. Usta said.
The solution dissolves the hydrogel in 4-6 minutes.
Hybrid gels
Many hydrogels currently used for burn wounds feature physically cross-linked molecules. This makes them strong and capable of retaining moisture, but also difficult to dissolve. The researchers used a different approach.
“This is not physical cross-linking like the traditional approaches, but rather, softer covalent bonds between the different molecules. And that’s why, when you bring in another solution, the hydrogel dissolves away,” Dr. Usta said.
The new hydrogels rely on a supramolecular assembly: a network of synthetic polymers whose connections can be reversed more easily, meaning they can be dissolved quickly. Another standout feature of the new hydrogels is their hybrid composition, displaying characteristics of both liquids and solids. The polymers are knitted together into a mesh-like network that enables water retention, with the goal of maintaining the moist environment needed for wound healing.
The supramolecular assembly is also greener, Dr. Usta explained; traditional cross-linking approaches produce a lot of toxic by-products that could harm the environment.
And whereas traditional hydrogels can require a dozen chemistry steps to produce, the new hydrogels are ready after mixing two solutions, Dr. Usta explained. This makes them easy to prepare at bedside, ideal for treating large wounds in the ER or even on battlefields.
When tested in vitro, using skin cells, and in vivo, on mice, the new hydrogels were shown to be safe to use on wounds. Additional studies on mice, as well as large animals, will focus on safety and efficacy, and may be followed by human clinical trials, said Dr. Usta.
“The next phase of the project will be to look at whether these dressings will help wound healing by creating a moist environment,” said Dr. Usta.
The researchers are also exploring how to manufacture individual prewrapped hydrogels that could be applied in a clinical setting – or even in people’s homes. The consumer market is “another possibility,” said Dr. Usta, particularly among patients with “smaller, more superficial burns” or patients whose large burn wounds are still healing once they leave the hospital.
This research was supported by the National Institutes of Health, National Science Foundation, Massachusetts General Hospital Executive Committee on Research Interim Support Fund, and Shriners Hospitals.
A version of this article first appeared on Medscape.com.
FROM BIOACTIVE MATERIALS
Employers use patient assistance programs to offset their own costs
Anna Sutton was shocked when she received a letter from her husband’s job-based health plan stating that Humira, an expensive drug used to treat her daughter’s juvenile arthritis, was now on a long list of medications considered “nonessential benefits.”
The July 2021 letter said the family could either participate in a new effort overseen by a company called SaveOnSP and get the drug free of charge or be saddled with a monthly copayment that could top $1,000.
“It really gave us no choice,” said Mrs. Sutton, of Woodinville, Wash. She added that “every single [Food and Drug Administration]–approved medication for juvenile arthritis” was on the list of nonessential benefits.
Mrs. Sutton had unwittingly become part of a strategy that employers are using to deal with the high cost of drugs prescribed to treat conditions such as arthritis, psoriasis, cancer, and hemophilia.
Those employers are tapping into dollars provided through programs they have previously criticized: patient financial assistance initiatives set up by drugmakers, which some benefit managers have complained encourage patients to stay on expensive brand-name drugs when less expensive options might be available.
Now, though, employers, or the vendors and insurers they hire specifically to oversee such efforts, are seeking that money to offset their own costs. Drugmakers object, saying the money was intended primarily for patients. But some benefit brokers and companies like SaveOnSP say they can help trim employers’ spending on insurance – which, they say, could be the difference between an employer offering coverage to workers or not.
It’s the latest twist in a long-running dispute between the drug industry and insurers over which group is more to blame for rising costs to patients. And patients are, again, caught in the middle.
Patient advocates say the term “nonessential” stresses patients out even though it doesn’t mean the drugs – often called “specialty” drugs because of their high prices or the way they are made – are unnecessary.
Some advocates fear the new strategies could be “a way to weed out those with costly health care needs,” said Rachel Klein, deputy executive director of the AIDS Institute, a nonprofit advocacy group. Workers who rely on the drugs may feel pressured to change insurers or jobs.
Two versions of the new strategy are in play. Both are used mainly by self-insured employers that hire vendors, like SaveOnSP, which then work with the employers’ pharmacy benefit managers, such as Express Scripts/Cigna, to implement the strategy. There are also smaller vendors, like SHARx and Payer Matrix, some of which work directly with employers.
In one approach, insurers or employers continue to cover the drugs but designate them as “nonessential,” which allows the health plans to bypass annual limits set by the Affordable Care Act on how much patients can pay in out-of-pocket costs for drugs. The employer or hired vendor then raises the copay required of the worker, often sharply, but offers to substantially cut or eliminate that copay if the patient participates in the new effort. Workers who agree enroll in drugmaker financial assistance programs meant to cover the drug copays, and the vendor monitoring the effort aims to capture the maximum amount the drugmaker provides annually, according to a lawsuit filed in May by drugmaker Johnson & Johnson against SaveOnSP, which is based in Elma, N.Y.
The employer must still cover part of the cost of the drug, but the amount is reduced by the amount of copay assistance that is accessed. That assistance can vary widely and be as much as $20,000 a year for some drugs.
In the other approach, employers don’t bother naming drugs nonessential; they simply drop coverage for specific drugs or classes of drugs. Then, the outside vendor helps patients provide the financial and other information needed to apply for free medication from drugmakers through charity programs intended for uninsured patients.
“We’re seeing it in every state at this point,” said Becky Burns, chief operating officer and chief financial officer at the Bleeding and Clotting Disorders Institute in Peoria, Ill., a federally funded hemophilia treatment center.
The strategies are mostly being used in self-insured employer health plans, which are governed by federal laws that give broad flexibility to employers in designing health benefits.
Still, some patient advocates say these programs can lead to delays for patients in accessing medications while applications are processed – and sometimes unexpected bills for consumers.
“We have patients get billed after they max out their assistance,” said Kollet Koulianos, vice president of payer relations at the National Hemophilia Foundation. Once she gets involved, vendors often claim the bills were sent in error.
Even though only about 2% of the workforce needs the drugs, which can cost thousands of dollars a dose, they can lead to a hefty financial liability for self-insured employers, said Drew Mann, a benefits consultant in Knoxville, Tenn., whose clientele includes employers that use variations of these programs.
Before employer health plans took advantage of such assistance, patients often signed up for these programs on their own, receiving coupons that covered their share of the drug’s cost. In that circumstance, drugmakers often paid less than they do under the new employer schemes because a patient’s out-of-pocket costs were capped at lower amounts.
Brokers and the CEOs of firms offering the new programs say that in most cases patients continue to get their drugs, often with little or no out-of-pocket costs.
If workers do not qualify for charity because their income is too high, or for another reason, the employer might make an exception and pay the claim or look for an alternative solution, Mr. Mann said. Patient groups noted that some specialty drugs may not have any alternatives.
How this practice will play out in the long run remains uncertain. Drugmakers offer both copay assistance and charity care in part because they know many patients, even those with insurance, cannot afford their products. The programs are also good public relations and a tax write-off. But the new emphasis by some employers on maximizing the amount they or their insurers can collect from the programs could cause some drugmakers to take issue with the new strategies or even reconsider their programs.
“Even though our client, like most manufacturers, provides billions in discounts and rebates to health insurers as part of their negotiations, the insurers also want this additional pool of funds, which is meant to help people who can’t meet the copay,” said Harry Sandick, a lawyer representing J&J.
J&J’s lawsuit, filed in U.S. District Court in New Jersey, alleges that patients are “coerced” into participating in copay assistance programs after their drugs are deemed “nonessential” and therefore are “no longer subject to the ACA’s annual out-of-pocket maximum.”
Once patients enroll, the money from the drugmaker goes to the insurer or employer plan, with SaveOnSP retaining 25%, according to the lawsuit. It claims J&J has lost $100 million to these efforts.
None of that money counts toward patients’ deductibles or out-of-pocket maximums for the year.
In addition to the lawsuit over the copay assistance program efforts, there has been other reaction to the new employer strategies. In an October letter to physicians, the Johnson & Johnson Patient Assistance Foundation, a separate entity, said it will no longer offer free medications to patients with insurance starting in January, citing the rise of such “alternative funding programs.”
Still, J&J spokesperson L.D. Platt said the drugmaker has plans, also in January, to roll out other assistance to patients who may be “underinsured” so they won’t be affected by the foundation’s decision.
In a statement, SaveOnSP said that employers object to drug companies’ “using their employees’ ongoing need for these drugs as an excuse to keep hiking the drugs’ prices” and that the firm simply “advises these employers on how to fight back against rising prices while getting employees the drugs they need at no cost to the employees.”
In a court filing, SaveOnSP said drugmakers have another option if they don’t like efforts by insurers and employers to max out what they can get from the programs: reduce the amount of assistance available. J&J, the filing said, did just that when it recently cut its allotted amount of copay assistance for psoriasis drugs Stelara and Tremfya from $20,000 to $6,000 per participant annually. The filing noted that SaveOnSP participants would still have no copay for those drugs.
For Mrs. Sutton’s part, her family did participate in the program offered through her husband’s work-based insurance plan, agreeing to have SaveOnSP monitor their enrollment and payments from the drugmaker.
So far, her 15-year-old daughter has continued to get Humira, and she has not been billed a copay.
Even so, “the whole process seems kind of slimy to me,” she said. “The patients are caught in the middle between the drug industry and the insurance industry, each trying to get as much money as possible out of the other.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anna Sutton was shocked when she received a letter from her husband’s job-based health plan stating that Humira, an expensive drug used to treat her daughter’s juvenile arthritis, was now on a long list of medications considered “nonessential benefits.”
The July 2021 letter said the family could either participate in a new effort overseen by a company called SaveOnSP and get the drug free of charge or be saddled with a monthly copayment that could top $1,000.
“It really gave us no choice,” said Mrs. Sutton, of Woodinville, Wash. She added that “every single [Food and Drug Administration]–approved medication for juvenile arthritis” was on the list of nonessential benefits.
Mrs. Sutton had unwittingly become part of a strategy that employers are using to deal with the high cost of drugs prescribed to treat conditions such as arthritis, psoriasis, cancer, and hemophilia.
Those employers are tapping into dollars provided through programs they have previously criticized: patient financial assistance initiatives set up by drugmakers, which some benefit managers have complained encourage patients to stay on expensive brand-name drugs when less expensive options might be available.
Now, though, employers, or the vendors and insurers they hire specifically to oversee such efforts, are seeking that money to offset their own costs. Drugmakers object, saying the money was intended primarily for patients. But some benefit brokers and companies like SaveOnSP say they can help trim employers’ spending on insurance – which, they say, could be the difference between an employer offering coverage to workers or not.
It’s the latest twist in a long-running dispute between the drug industry and insurers over which group is more to blame for rising costs to patients. And patients are, again, caught in the middle.
Patient advocates say the term “nonessential” stresses patients out even though it doesn’t mean the drugs – often called “specialty” drugs because of their high prices or the way they are made – are unnecessary.
Some advocates fear the new strategies could be “a way to weed out those with costly health care needs,” said Rachel Klein, deputy executive director of the AIDS Institute, a nonprofit advocacy group. Workers who rely on the drugs may feel pressured to change insurers or jobs.
Two versions of the new strategy are in play. Both are used mainly by self-insured employers that hire vendors, like SaveOnSP, which then work with the employers’ pharmacy benefit managers, such as Express Scripts/Cigna, to implement the strategy. There are also smaller vendors, like SHARx and Payer Matrix, some of which work directly with employers.
In one approach, insurers or employers continue to cover the drugs but designate them as “nonessential,” which allows the health plans to bypass annual limits set by the Affordable Care Act on how much patients can pay in out-of-pocket costs for drugs. The employer or hired vendor then raises the copay required of the worker, often sharply, but offers to substantially cut or eliminate that copay if the patient participates in the new effort. Workers who agree enroll in drugmaker financial assistance programs meant to cover the drug copays, and the vendor monitoring the effort aims to capture the maximum amount the drugmaker provides annually, according to a lawsuit filed in May by drugmaker Johnson & Johnson against SaveOnSP, which is based in Elma, N.Y.
The employer must still cover part of the cost of the drug, but the amount is reduced by the amount of copay assistance that is accessed. That assistance can vary widely and be as much as $20,000 a year for some drugs.
In the other approach, employers don’t bother naming drugs nonessential; they simply drop coverage for specific drugs or classes of drugs. Then, the outside vendor helps patients provide the financial and other information needed to apply for free medication from drugmakers through charity programs intended for uninsured patients.
“We’re seeing it in every state at this point,” said Becky Burns, chief operating officer and chief financial officer at the Bleeding and Clotting Disorders Institute in Peoria, Ill., a federally funded hemophilia treatment center.
The strategies are mostly being used in self-insured employer health plans, which are governed by federal laws that give broad flexibility to employers in designing health benefits.
Still, some patient advocates say these programs can lead to delays for patients in accessing medications while applications are processed – and sometimes unexpected bills for consumers.
“We have patients get billed after they max out their assistance,” said Kollet Koulianos, vice president of payer relations at the National Hemophilia Foundation. Once she gets involved, vendors often claim the bills were sent in error.
Even though only about 2% of the workforce needs the drugs, which can cost thousands of dollars a dose, they can lead to a hefty financial liability for self-insured employers, said Drew Mann, a benefits consultant in Knoxville, Tenn., whose clientele includes employers that use variations of these programs.
Before employer health plans took advantage of such assistance, patients often signed up for these programs on their own, receiving coupons that covered their share of the drug’s cost. In that circumstance, drugmakers often paid less than they do under the new employer schemes because a patient’s out-of-pocket costs were capped at lower amounts.
Brokers and the CEOs of firms offering the new programs say that in most cases patients continue to get their drugs, often with little or no out-of-pocket costs.
If workers do not qualify for charity because their income is too high, or for another reason, the employer might make an exception and pay the claim or look for an alternative solution, Mr. Mann said. Patient groups noted that some specialty drugs may not have any alternatives.
How this practice will play out in the long run remains uncertain. Drugmakers offer both copay assistance and charity care in part because they know many patients, even those with insurance, cannot afford their products. The programs are also good public relations and a tax write-off. But the new emphasis by some employers on maximizing the amount they or their insurers can collect from the programs could cause some drugmakers to take issue with the new strategies or even reconsider their programs.
“Even though our client, like most manufacturers, provides billions in discounts and rebates to health insurers as part of their negotiations, the insurers also want this additional pool of funds, which is meant to help people who can’t meet the copay,” said Harry Sandick, a lawyer representing J&J.
J&J’s lawsuit, filed in U.S. District Court in New Jersey, alleges that patients are “coerced” into participating in copay assistance programs after their drugs are deemed “nonessential” and therefore are “no longer subject to the ACA’s annual out-of-pocket maximum.”
Once patients enroll, the money from the drugmaker goes to the insurer or employer plan, with SaveOnSP retaining 25%, according to the lawsuit. It claims J&J has lost $100 million to these efforts.
None of that money counts toward patients’ deductibles or out-of-pocket maximums for the year.
In addition to the lawsuit over the copay assistance program efforts, there has been other reaction to the new employer strategies. In an October letter to physicians, the Johnson & Johnson Patient Assistance Foundation, a separate entity, said it will no longer offer free medications to patients with insurance starting in January, citing the rise of such “alternative funding programs.”
Still, J&J spokesperson L.D. Platt said the drugmaker has plans, also in January, to roll out other assistance to patients who may be “underinsured” so they won’t be affected by the foundation’s decision.
In a statement, SaveOnSP said that employers object to drug companies’ “using their employees’ ongoing need for these drugs as an excuse to keep hiking the drugs’ prices” and that the firm simply “advises these employers on how to fight back against rising prices while getting employees the drugs they need at no cost to the employees.”
In a court filing, SaveOnSP said drugmakers have another option if they don’t like efforts by insurers and employers to max out what they can get from the programs: reduce the amount of assistance available. J&J, the filing said, did just that when it recently cut its allotted amount of copay assistance for psoriasis drugs Stelara and Tremfya from $20,000 to $6,000 per participant annually. The filing noted that SaveOnSP participants would still have no copay for those drugs.
For Mrs. Sutton’s part, her family did participate in the program offered through her husband’s work-based insurance plan, agreeing to have SaveOnSP monitor their enrollment and payments from the drugmaker.
So far, her 15-year-old daughter has continued to get Humira, and she has not been billed a copay.
Even so, “the whole process seems kind of slimy to me,” she said. “The patients are caught in the middle between the drug industry and the insurance industry, each trying to get as much money as possible out of the other.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anna Sutton was shocked when she received a letter from her husband’s job-based health plan stating that Humira, an expensive drug used to treat her daughter’s juvenile arthritis, was now on a long list of medications considered “nonessential benefits.”
The July 2021 letter said the family could either participate in a new effort overseen by a company called SaveOnSP and get the drug free of charge or be saddled with a monthly copayment that could top $1,000.
“It really gave us no choice,” said Mrs. Sutton, of Woodinville, Wash. She added that “every single [Food and Drug Administration]–approved medication for juvenile arthritis” was on the list of nonessential benefits.
Mrs. Sutton had unwittingly become part of a strategy that employers are using to deal with the high cost of drugs prescribed to treat conditions such as arthritis, psoriasis, cancer, and hemophilia.
Those employers are tapping into dollars provided through programs they have previously criticized: patient financial assistance initiatives set up by drugmakers, which some benefit managers have complained encourage patients to stay on expensive brand-name drugs when less expensive options might be available.
Now, though, employers, or the vendors and insurers they hire specifically to oversee such efforts, are seeking that money to offset their own costs. Drugmakers object, saying the money was intended primarily for patients. But some benefit brokers and companies like SaveOnSP say they can help trim employers’ spending on insurance – which, they say, could be the difference between an employer offering coverage to workers or not.
It’s the latest twist in a long-running dispute between the drug industry and insurers over which group is more to blame for rising costs to patients. And patients are, again, caught in the middle.
Patient advocates say the term “nonessential” stresses patients out even though it doesn’t mean the drugs – often called “specialty” drugs because of their high prices or the way they are made – are unnecessary.
Some advocates fear the new strategies could be “a way to weed out those with costly health care needs,” said Rachel Klein, deputy executive director of the AIDS Institute, a nonprofit advocacy group. Workers who rely on the drugs may feel pressured to change insurers or jobs.
Two versions of the new strategy are in play. Both are used mainly by self-insured employers that hire vendors, like SaveOnSP, which then work with the employers’ pharmacy benefit managers, such as Express Scripts/Cigna, to implement the strategy. There are also smaller vendors, like SHARx and Payer Matrix, some of which work directly with employers.
In one approach, insurers or employers continue to cover the drugs but designate them as “nonessential,” which allows the health plans to bypass annual limits set by the Affordable Care Act on how much patients can pay in out-of-pocket costs for drugs. The employer or hired vendor then raises the copay required of the worker, often sharply, but offers to substantially cut or eliminate that copay if the patient participates in the new effort. Workers who agree enroll in drugmaker financial assistance programs meant to cover the drug copays, and the vendor monitoring the effort aims to capture the maximum amount the drugmaker provides annually, according to a lawsuit filed in May by drugmaker Johnson & Johnson against SaveOnSP, which is based in Elma, N.Y.
The employer must still cover part of the cost of the drug, but the amount is reduced by the amount of copay assistance that is accessed. That assistance can vary widely and be as much as $20,000 a year for some drugs.
In the other approach, employers don’t bother naming drugs nonessential; they simply drop coverage for specific drugs or classes of drugs. Then, the outside vendor helps patients provide the financial and other information needed to apply for free medication from drugmakers through charity programs intended for uninsured patients.
“We’re seeing it in every state at this point,” said Becky Burns, chief operating officer and chief financial officer at the Bleeding and Clotting Disorders Institute in Peoria, Ill., a federally funded hemophilia treatment center.
The strategies are mostly being used in self-insured employer health plans, which are governed by federal laws that give broad flexibility to employers in designing health benefits.
Still, some patient advocates say these programs can lead to delays for patients in accessing medications while applications are processed – and sometimes unexpected bills for consumers.
“We have patients get billed after they max out their assistance,” said Kollet Koulianos, vice president of payer relations at the National Hemophilia Foundation. Once she gets involved, vendors often claim the bills were sent in error.
Even though only about 2% of the workforce needs the drugs, which can cost thousands of dollars a dose, they can lead to a hefty financial liability for self-insured employers, said Drew Mann, a benefits consultant in Knoxville, Tenn., whose clientele includes employers that use variations of these programs.
Before employer health plans took advantage of such assistance, patients often signed up for these programs on their own, receiving coupons that covered their share of the drug’s cost. In that circumstance, drugmakers often paid less than they do under the new employer schemes because a patient’s out-of-pocket costs were capped at lower amounts.
Brokers and the CEOs of firms offering the new programs say that in most cases patients continue to get their drugs, often with little or no out-of-pocket costs.
If workers do not qualify for charity because their income is too high, or for another reason, the employer might make an exception and pay the claim or look for an alternative solution, Mr. Mann said. Patient groups noted that some specialty drugs may not have any alternatives.
How this practice will play out in the long run remains uncertain. Drugmakers offer both copay assistance and charity care in part because they know many patients, even those with insurance, cannot afford their products. The programs are also good public relations and a tax write-off. But the new emphasis by some employers on maximizing the amount they or their insurers can collect from the programs could cause some drugmakers to take issue with the new strategies or even reconsider their programs.
“Even though our client, like most manufacturers, provides billions in discounts and rebates to health insurers as part of their negotiations, the insurers also want this additional pool of funds, which is meant to help people who can’t meet the copay,” said Harry Sandick, a lawyer representing J&J.
J&J’s lawsuit, filed in U.S. District Court in New Jersey, alleges that patients are “coerced” into participating in copay assistance programs after their drugs are deemed “nonessential” and therefore are “no longer subject to the ACA’s annual out-of-pocket maximum.”
Once patients enroll, the money from the drugmaker goes to the insurer or employer plan, with SaveOnSP retaining 25%, according to the lawsuit. It claims J&J has lost $100 million to these efforts.
None of that money counts toward patients’ deductibles or out-of-pocket maximums for the year.
In addition to the lawsuit over the copay assistance program efforts, there has been other reaction to the new employer strategies. In an October letter to physicians, the Johnson & Johnson Patient Assistance Foundation, a separate entity, said it will no longer offer free medications to patients with insurance starting in January, citing the rise of such “alternative funding programs.”
Still, J&J spokesperson L.D. Platt said the drugmaker has plans, also in January, to roll out other assistance to patients who may be “underinsured” so they won’t be affected by the foundation’s decision.
In a statement, SaveOnSP said that employers object to drug companies’ “using their employees’ ongoing need for these drugs as an excuse to keep hiking the drugs’ prices” and that the firm simply “advises these employers on how to fight back against rising prices while getting employees the drugs they need at no cost to the employees.”
In a court filing, SaveOnSP said drugmakers have another option if they don’t like efforts by insurers and employers to max out what they can get from the programs: reduce the amount of assistance available. J&J, the filing said, did just that when it recently cut its allotted amount of copay assistance for psoriasis drugs Stelara and Tremfya from $20,000 to $6,000 per participant annually. The filing noted that SaveOnSP participants would still have no copay for those drugs.
For Mrs. Sutton’s part, her family did participate in the program offered through her husband’s work-based insurance plan, agreeing to have SaveOnSP monitor their enrollment and payments from the drugmaker.
So far, her 15-year-old daughter has continued to get Humira, and she has not been billed a copay.
Even so, “the whole process seems kind of slimy to me,” she said. “The patients are caught in the middle between the drug industry and the insurance industry, each trying to get as much money as possible out of the other.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Florida doc dies by suicide after allegedly drugging and raping patients
according to a police statement.
A week later, a Collier County Sheriff’s deputy found Dr. Salata’s body near his Naples home with a gunshot wound to the head, according to police. The medical examiner later ruled it a suicide.
Dr. Salata co-owned Pura Vida Medical Spa in Naples with his wife Jill Salata, a certified family nurse practitioner. They specialized in cosmetic treatment and surgery.
Naples police said that they arrested Dr. Salata after two female patients accused the doctor of allegedly drugging and raping them while they were still unconscious.
Both victims described being given nitrous oxide, also called laughing gas, for sedation and pain from the cosmetic procedure. The first victim, age 51, said Dr. Salata prescribed alprazolam (Xanax) to take before the procedure and then also gave her nitrous oxide and tequila, causing her to black out, according to NBC2 News.
The second victim, age 72, told police that as the nitrous oxide was wearing off, she found Dr. Salata performing sexual intercourse. The victim felt shocked after the sedation subsided about what had taken place, contacted police, and submitted to a sexual assault examination, according to the police statement.
At Dr. Salata’s November 22 hearing before Judge Michael Provost, a prosecutor asked the judge whether Dr. Salata should surrender his firearms; Provost reportedly dismissed the idea.
“It is disappointing and frustrating that Dr. Salata has escaped justice,” said one victim’s attorney, Adam Horowitz, in a blog post. “Yet, we are relieved that no other women will be assaulted by Dr. Salata again. It took tremendous courage for my client to tell her truth. She was ready to hold him accountable in court.”
Horowitz says he plans to file a civil lawsuit on behalf of his client against Dr. Salata’s estate. The Naples police are continuing their investigation into the victims’ cases, which now includes a third woman, said spokesman Lt. Bryan McGinn.
Meanwhile, the Pura Vida Medical Spa has closed permanently and its website has been deleted. One reviewer named Soul F. wrote on the spa’s Yelp page: “And now may God have mercy on this rapist’s soul. Amen.”
A version of this article first appeared on Medscape.com.
according to a police statement.
A week later, a Collier County Sheriff’s deputy found Dr. Salata’s body near his Naples home with a gunshot wound to the head, according to police. The medical examiner later ruled it a suicide.
Dr. Salata co-owned Pura Vida Medical Spa in Naples with his wife Jill Salata, a certified family nurse practitioner. They specialized in cosmetic treatment and surgery.
Naples police said that they arrested Dr. Salata after two female patients accused the doctor of allegedly drugging and raping them while they were still unconscious.
Both victims described being given nitrous oxide, also called laughing gas, for sedation and pain from the cosmetic procedure. The first victim, age 51, said Dr. Salata prescribed alprazolam (Xanax) to take before the procedure and then also gave her nitrous oxide and tequila, causing her to black out, according to NBC2 News.
The second victim, age 72, told police that as the nitrous oxide was wearing off, she found Dr. Salata performing sexual intercourse. The victim felt shocked after the sedation subsided about what had taken place, contacted police, and submitted to a sexual assault examination, according to the police statement.
At Dr. Salata’s November 22 hearing before Judge Michael Provost, a prosecutor asked the judge whether Dr. Salata should surrender his firearms; Provost reportedly dismissed the idea.
“It is disappointing and frustrating that Dr. Salata has escaped justice,” said one victim’s attorney, Adam Horowitz, in a blog post. “Yet, we are relieved that no other women will be assaulted by Dr. Salata again. It took tremendous courage for my client to tell her truth. She was ready to hold him accountable in court.”
Horowitz says he plans to file a civil lawsuit on behalf of his client against Dr. Salata’s estate. The Naples police are continuing their investigation into the victims’ cases, which now includes a third woman, said spokesman Lt. Bryan McGinn.
Meanwhile, the Pura Vida Medical Spa has closed permanently and its website has been deleted. One reviewer named Soul F. wrote on the spa’s Yelp page: “And now may God have mercy on this rapist’s soul. Amen.”
A version of this article first appeared on Medscape.com.
according to a police statement.
A week later, a Collier County Sheriff’s deputy found Dr. Salata’s body near his Naples home with a gunshot wound to the head, according to police. The medical examiner later ruled it a suicide.
Dr. Salata co-owned Pura Vida Medical Spa in Naples with his wife Jill Salata, a certified family nurse practitioner. They specialized in cosmetic treatment and surgery.
Naples police said that they arrested Dr. Salata after two female patients accused the doctor of allegedly drugging and raping them while they were still unconscious.
Both victims described being given nitrous oxide, also called laughing gas, for sedation and pain from the cosmetic procedure. The first victim, age 51, said Dr. Salata prescribed alprazolam (Xanax) to take before the procedure and then also gave her nitrous oxide and tequila, causing her to black out, according to NBC2 News.
The second victim, age 72, told police that as the nitrous oxide was wearing off, she found Dr. Salata performing sexual intercourse. The victim felt shocked after the sedation subsided about what had taken place, contacted police, and submitted to a sexual assault examination, according to the police statement.
At Dr. Salata’s November 22 hearing before Judge Michael Provost, a prosecutor asked the judge whether Dr. Salata should surrender his firearms; Provost reportedly dismissed the idea.
“It is disappointing and frustrating that Dr. Salata has escaped justice,” said one victim’s attorney, Adam Horowitz, in a blog post. “Yet, we are relieved that no other women will be assaulted by Dr. Salata again. It took tremendous courage for my client to tell her truth. She was ready to hold him accountable in court.”
Horowitz says he plans to file a civil lawsuit on behalf of his client against Dr. Salata’s estate. The Naples police are continuing their investigation into the victims’ cases, which now includes a third woman, said spokesman Lt. Bryan McGinn.
Meanwhile, the Pura Vida Medical Spa has closed permanently and its website has been deleted. One reviewer named Soul F. wrote on the spa’s Yelp page: “And now may God have mercy on this rapist’s soul. Amen.”
A version of this article first appeared on Medscape.com.
What are the risk factors for Mohs surgery–related anxiety?
confirmed by a health care provider (HCP), results from a single-center survey demonstrated.
“Higher patient-reported anxiety in hospital settings is significantly linked to lower patient satisfaction with the quality of care and higher patient-reported postoperative pain,” corresponding author Ally-Khan Somani, MD, PhD, and colleagues wrote in the study, which was published online in Dermatologic Surgery. “Identifying factors associated with perioperative patient anxiety could improve outcomes and patient satisfaction.”
Dr. Somani, director of dermatologic surgery and cutaneous oncology in the department of dermatology at the University of Indiana, Indianapolis, and coauthors surveyed 145 patients who underwent Mohs micrographic surgery (MMS) at the university from February 2018 to March 2020. They collected patient self-reported demographics, medical history, and administered a 10-point visual analog scale assessment of anxiety at multiple stages. They also sought HCP-perceived assessments of anxiety and used a stepwise regression mode to explore factors that potentially contributed to anxiety outcomes. The mean age of the 145 patients was 63 years, 60% were female, and 77% had no self-reported anxiety confirmed by a prior HCP’s diagnosis.
Two-thirds of patients (66%) received a pre-MMS consultation with the surgeon, 59% had a history of skin cancer removal surgery, and 86% had 1-2 layers removed during the current MMS.
Prior to MMS, the researchers found that significant risk factors for increased anxiety included younger age, female sex, and self-reported history of anxiety confirmed by an HCP (P < .05), while intraoperatively, HCP-perceived patient anxiety increased with younger patient age and more layers removed. Following MMS, patient anxiety increased significantly with more layers removed and higher self-reported preoperative anxiety levels. “Although existing research is divided regarding the efficacy of pre-MMS consultation for anxiety reduction, these findings suggest that patient-reported and HCP-perceived anxiety were not significantly affected by in-person pre-MMS consultation with the surgeon,” Dr. Somani and colleagues wrote. “Thus, routinely recommending consultations may not be the best approach for improving anxiety outcomes.”
They acknowledged certain limitations of their analysis, including its single-center design, enrollment of demographically similar patients, and the fact that no objective measurements of anxiety such as heart rate or blood pressure were taken.
“One of the main benefits of Mohs surgery is that we are able to operate under local anesthesia, but this also means that our patients are acutely aware of everything going on around them,” said Patricia M. Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and was asked to comment on the study.
“I think it is so important that this study is primarily focusing on the patient experience,” she said. “While this study did not find that a pre-op consult impacted patient anxiety levels, I do think we can infer that it is critical to connect with your patients on some level prior to surgery, as it helps you tailor your process to make the day more tolerable for them [such as] playing music, determining the need for an oral anxiolytic, etc.”
Neither the researchers nor Dr. Richey reported having financial disclosures.
confirmed by a health care provider (HCP), results from a single-center survey demonstrated.
“Higher patient-reported anxiety in hospital settings is significantly linked to lower patient satisfaction with the quality of care and higher patient-reported postoperative pain,” corresponding author Ally-Khan Somani, MD, PhD, and colleagues wrote in the study, which was published online in Dermatologic Surgery. “Identifying factors associated with perioperative patient anxiety could improve outcomes and patient satisfaction.”
Dr. Somani, director of dermatologic surgery and cutaneous oncology in the department of dermatology at the University of Indiana, Indianapolis, and coauthors surveyed 145 patients who underwent Mohs micrographic surgery (MMS) at the university from February 2018 to March 2020. They collected patient self-reported demographics, medical history, and administered a 10-point visual analog scale assessment of anxiety at multiple stages. They also sought HCP-perceived assessments of anxiety and used a stepwise regression mode to explore factors that potentially contributed to anxiety outcomes. The mean age of the 145 patients was 63 years, 60% were female, and 77% had no self-reported anxiety confirmed by a prior HCP’s diagnosis.
Two-thirds of patients (66%) received a pre-MMS consultation with the surgeon, 59% had a history of skin cancer removal surgery, and 86% had 1-2 layers removed during the current MMS.
Prior to MMS, the researchers found that significant risk factors for increased anxiety included younger age, female sex, and self-reported history of anxiety confirmed by an HCP (P < .05), while intraoperatively, HCP-perceived patient anxiety increased with younger patient age and more layers removed. Following MMS, patient anxiety increased significantly with more layers removed and higher self-reported preoperative anxiety levels. “Although existing research is divided regarding the efficacy of pre-MMS consultation for anxiety reduction, these findings suggest that patient-reported and HCP-perceived anxiety were not significantly affected by in-person pre-MMS consultation with the surgeon,” Dr. Somani and colleagues wrote. “Thus, routinely recommending consultations may not be the best approach for improving anxiety outcomes.”
They acknowledged certain limitations of their analysis, including its single-center design, enrollment of demographically similar patients, and the fact that no objective measurements of anxiety such as heart rate or blood pressure were taken.
“One of the main benefits of Mohs surgery is that we are able to operate under local anesthesia, but this also means that our patients are acutely aware of everything going on around them,” said Patricia M. Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and was asked to comment on the study.
“I think it is so important that this study is primarily focusing on the patient experience,” she said. “While this study did not find that a pre-op consult impacted patient anxiety levels, I do think we can infer that it is critical to connect with your patients on some level prior to surgery, as it helps you tailor your process to make the day more tolerable for them [such as] playing music, determining the need for an oral anxiolytic, etc.”
Neither the researchers nor Dr. Richey reported having financial disclosures.
confirmed by a health care provider (HCP), results from a single-center survey demonstrated.
“Higher patient-reported anxiety in hospital settings is significantly linked to lower patient satisfaction with the quality of care and higher patient-reported postoperative pain,” corresponding author Ally-Khan Somani, MD, PhD, and colleagues wrote in the study, which was published online in Dermatologic Surgery. “Identifying factors associated with perioperative patient anxiety could improve outcomes and patient satisfaction.”
Dr. Somani, director of dermatologic surgery and cutaneous oncology in the department of dermatology at the University of Indiana, Indianapolis, and coauthors surveyed 145 patients who underwent Mohs micrographic surgery (MMS) at the university from February 2018 to March 2020. They collected patient self-reported demographics, medical history, and administered a 10-point visual analog scale assessment of anxiety at multiple stages. They also sought HCP-perceived assessments of anxiety and used a stepwise regression mode to explore factors that potentially contributed to anxiety outcomes. The mean age of the 145 patients was 63 years, 60% were female, and 77% had no self-reported anxiety confirmed by a prior HCP’s diagnosis.
Two-thirds of patients (66%) received a pre-MMS consultation with the surgeon, 59% had a history of skin cancer removal surgery, and 86% had 1-2 layers removed during the current MMS.
Prior to MMS, the researchers found that significant risk factors for increased anxiety included younger age, female sex, and self-reported history of anxiety confirmed by an HCP (P < .05), while intraoperatively, HCP-perceived patient anxiety increased with younger patient age and more layers removed. Following MMS, patient anxiety increased significantly with more layers removed and higher self-reported preoperative anxiety levels. “Although existing research is divided regarding the efficacy of pre-MMS consultation for anxiety reduction, these findings suggest that patient-reported and HCP-perceived anxiety were not significantly affected by in-person pre-MMS consultation with the surgeon,” Dr. Somani and colleagues wrote. “Thus, routinely recommending consultations may not be the best approach for improving anxiety outcomes.”
They acknowledged certain limitations of their analysis, including its single-center design, enrollment of demographically similar patients, and the fact that no objective measurements of anxiety such as heart rate or blood pressure were taken.
“One of the main benefits of Mohs surgery is that we are able to operate under local anesthesia, but this also means that our patients are acutely aware of everything going on around them,” said Patricia M. Richey, MD, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and was asked to comment on the study.
“I think it is so important that this study is primarily focusing on the patient experience,” she said. “While this study did not find that a pre-op consult impacted patient anxiety levels, I do think we can infer that it is critical to connect with your patients on some level prior to surgery, as it helps you tailor your process to make the day more tolerable for them [such as] playing music, determining the need for an oral anxiolytic, etc.”
Neither the researchers nor Dr. Richey reported having financial disclosures.
FROM DERMATOLOGIC SURGERY
Consider quality of life, comorbidities in hidradenitis suppurativa
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY