User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Racial morphing: A conundrum in cosmetic dermatology
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the opinion of Nazanin A. Saedi, MD, social media-induced dissatisfaction with appearance is getting out of hand in the field of cosmetic dermatology, with the emergence of apps to filter and edit images to the patient’s liking.
This, coupled with
“Overexposure of celebrity images and altered faces on social media have led to a trend of overarching brows, sculpted noses, enlarged cheeks, and sharply defined jawlines,” Dr. Saedi, cochair of the laser and aesthetics surgery center at Dermatology Associates of Plymouth Meeting, Pa., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “These trends have made people of different ethnicities morph into a similar appearance.”
At the meeting, she showed early career images of celebrities from different ethnic backgrounds, “and they all have unique features that make them look great,” said Dr. Saedi, clinical associate professor of dermatology at Thomas Jefferson University, Philadelphia. She then showed images of the same celebrities after they had undergone cosmetic procedures, “and they look so much more similar,” with overarched brows, sculpted noses, enlarged cheeks, and sharply defined jawlines. “Whereas they were all beautiful before individually, now they look very similar,” she said. “This is what we see on social media.”
Referring to the Kardashians as an example of celebrities who have had a lot of aesthetic treatments, look different than they did years ago, and are seen “more and more,” she added, “it’s this repeated overexposure to people on social media, to celebrities, that’s created this different trend of attractiveness.”
This trend also affects patients seeking cosmetic treatments, she noted. Individuals can use an app to alter their appearance, “changing the way they look to create the best version of themselves, they might say, or a filtered version of themselves,” said Dr. Saedi, one of the authors of a commentary on patient perception of beauty on social media published several years ago.
“I tell people, ‘Don’t use filters in your photos. Embrace your beauty.’ I have patients coming in who want to look like the social media photos they’ve curated, maybe larger lips or more definition in their jawline. What they don’t understand is that it takes a long time for that to happen. It’s a process.” In other cases, their desired outcome is not possible due to limits of their individual facial anatomy.
In a study published almost 20 years ago in the journal Perception, Irish researchers manipulated the familiarity of typical and distinctive faces to measure the effect on attractiveness. They found that episodic familiarity affects attractiveness ratings independently of general or structural familiarity.
“So, the more you saw a face, the more familiar that face was to you,” said Dr. Saedi, who was not involved with the study. “Over time, you felt that to be more attractive. I think that’s a lot of what’s going on in the trends that we’re seeing – both in real life and on social media. I do think we need to be more mindful of maintaining features that make an individual unique, while also maintaining their ethnic beauty.”
In an interview at the meeting, Jacqueline D. Watchmaker, MD, a board-certified cosmetic and medical dermatologist who practices in Scottsdale, Ariz., said that she identifies with the notion of racial morphing in her own clinical experience. “Patients come in and specifically ask for chiseled jawlines, high cheekbones, and bigger lips,” Dr. Watchmaker said. “It’s a tricky situation when they ask for [a treatment] you don’t think they need. I prefer a more staged approach to maintain their individuality while giving them a little bit of the aesthetic benefit that they’re looking for.”
Dr. Saedi disclosed ties with AbbVie, Aerolase, Allergan, Alma, Cartessa, Cynosure, Galderma Laboratories, LP, Grand Cosmetics, Revelle Aesthetics, and Revision Skincare. Dr. Watchmaker reported having no financial disclosures.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
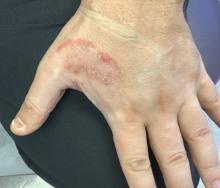
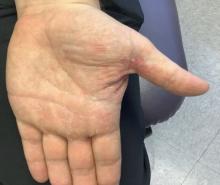
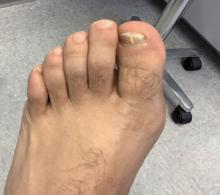
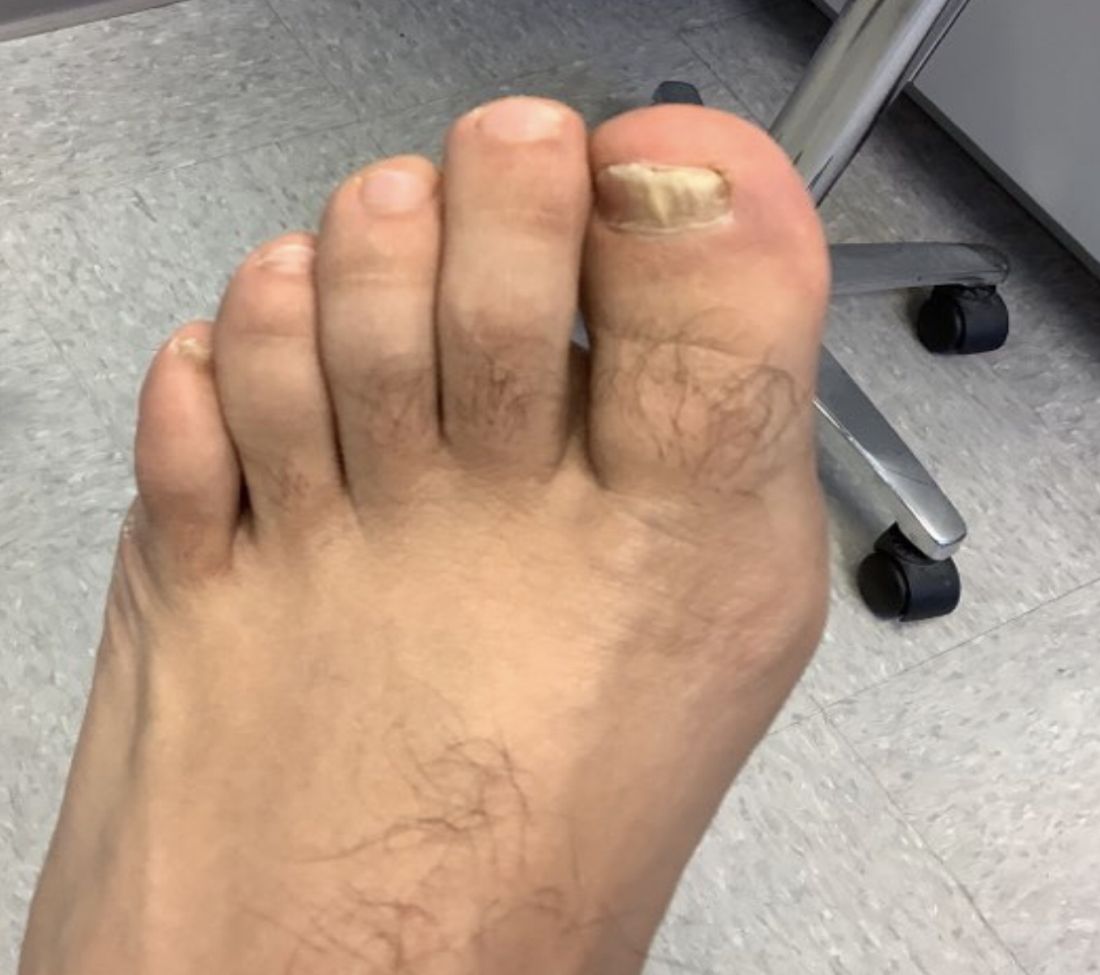
White male presents with pruritic, scaly, erythematous patches on his feet and left hand
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Biologics show signs of delaying arthritis in psoriasis patients
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
FROM LANCET RHEUMATOLOGY
We have seen the future of healthy muffins, and its name is Roselle
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
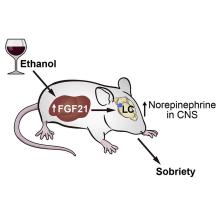
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Specialty and age may contribute to suicidal thoughts among physicians
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
Measles exposures in Kentucky have CDC on alert
The Centers for Disease Control and Prevention has issued a Health Alert Network (HAN) health advisory notifying clinicians and public health officials of a confirmed measles case in an individual who for 2 days (February 17-18) attended a large religious gathering that was attended by an estimated 20,000 people at Asbury University in Wilmore, Ky.
Given that large numbers of people might have been exposed to the attendee (who was not vaccinated) and that the individual had a history of recent international travel, the CDC has encouraged clinicians to be vigilant for patients presenting with symptoms that meet the measles case definition. A steady increase in measles cases from 49 in 2021 to 121 in 2022 in children who were not fully vaccinated – coupled with outbreaks in Ohio and Minnesota – underscores the potential gravity of the CDC advisory as well as the need to mitigate the risk of ongoing or secondary transmission.
Currently, little is known about the individual who contracted measles other than the fact that he is a resident of Jessamine County, Ky., according to a news release issued by the Kentucky Department of Public Health. It is the third confirmed case in Kentucky over the past 3 months. State and national health officials are concerned that the individual might have transmitted measles to attendees visiting from other states.
David Sugerman, MD, MPH, a medical officer in CDC’s division of viral diseases and lead for the measles, rubella, and cytomegalovirus team, noted that the timing of the alert coincides with the period in which persons who had had contact with the initial case patient might be expected to develop symptoms.
For clinicians, “It’s really about considering measles in any un- or undervaccinated patient that arrives at a clinic and recently traveled internationally,” Dr. Sugerman told this news organization. He explained that “when doctors are seeing patients, they’re not going to necessarily share that information off the bat when they present with fever or rash, or if their child has fever and rash, or that they traveled internationally. So, eliciting that history from the patient or their parents is really critical.”
The CDC recommends that measles be considered in anyone presenting with a febrile illness and symptoms that are clinically compatible with measles (that is, rash, cough, coryza, or conjunctivitis), as well as in patients who have recently traveled abroad, especially to countries with ongoing outbreaks, including India, Somalia, and Yemen.
“In general, if they’ve traveled internationally and they are undervaccinated, measles should be part of the differential diagnosis,” Sugerman said. He also emphasized the need to follow airborne isolation precautions in addition to general infection control measures.
Immediate triage is critical, especially since overcrowded waiting rooms might be filled with patients who are not yet eligible for vaccination or are not up to date or fully vaccinated.
“Measles is under airborne isolation criteria and precautions, and therefore, [patients] need to be placed as soon as possible into a negative pressure or airborne infection isolation room – and that should be a single room,” he explained. He noted, “In some settings, there may not be a negative pressure room, e.g., an outpatient pediatrics or family medicine office.”
Dr. Sugerman said that in these circumstances, patients should be placed in a room with masked health care providers who have received two doses of measles, mumps, and rubella (MMR) vaccine and that they should wear an N95 mask when entering the room and interviewing the patient.
Clinicians should follow CDC’s testing recommendations and collect a nasopharyngeal or throat swab or a urine specimen for PCR testing and a blood specimen for serology. In addition, they should immediately report cases to local and state public health authorities.
For all patients, it’s critical to be up to date on MMR vaccines, especially persons who are going to be traveling internationally. “We recommend that when they’ve got infants traveling with them who are 6-11 months of age, that they get a first dose (which we consider a zero dose), because they need a routine dose at 12-15 months, and then 4-6 years,” said Dr. Sugerman. He said that it’s safe for adults who are unsure of their status to receive an MMR dose as well.
Dr. Sugerman stressed that despite major strides, “we just don’t have enough coverage in all individuals in this country. Because people are traveling as often as they are, it can be imported. Until measles is eliminated globally, there’s going to be an ongoing risk of importation and potential spread amongst others in their household or community, especially amongst individuals who are not fully vaccinated and, in particular, amongst those who are unvaccinated,” he said.
Dr. Sugerman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued a Health Alert Network (HAN) health advisory notifying clinicians and public health officials of a confirmed measles case in an individual who for 2 days (February 17-18) attended a large religious gathering that was attended by an estimated 20,000 people at Asbury University in Wilmore, Ky.
Given that large numbers of people might have been exposed to the attendee (who was not vaccinated) and that the individual had a history of recent international travel, the CDC has encouraged clinicians to be vigilant for patients presenting with symptoms that meet the measles case definition. A steady increase in measles cases from 49 in 2021 to 121 in 2022 in children who were not fully vaccinated – coupled with outbreaks in Ohio and Minnesota – underscores the potential gravity of the CDC advisory as well as the need to mitigate the risk of ongoing or secondary transmission.
Currently, little is known about the individual who contracted measles other than the fact that he is a resident of Jessamine County, Ky., according to a news release issued by the Kentucky Department of Public Health. It is the third confirmed case in Kentucky over the past 3 months. State and national health officials are concerned that the individual might have transmitted measles to attendees visiting from other states.
David Sugerman, MD, MPH, a medical officer in CDC’s division of viral diseases and lead for the measles, rubella, and cytomegalovirus team, noted that the timing of the alert coincides with the period in which persons who had had contact with the initial case patient might be expected to develop symptoms.
For clinicians, “It’s really about considering measles in any un- or undervaccinated patient that arrives at a clinic and recently traveled internationally,” Dr. Sugerman told this news organization. He explained that “when doctors are seeing patients, they’re not going to necessarily share that information off the bat when they present with fever or rash, or if their child has fever and rash, or that they traveled internationally. So, eliciting that history from the patient or their parents is really critical.”
The CDC recommends that measles be considered in anyone presenting with a febrile illness and symptoms that are clinically compatible with measles (that is, rash, cough, coryza, or conjunctivitis), as well as in patients who have recently traveled abroad, especially to countries with ongoing outbreaks, including India, Somalia, and Yemen.
“In general, if they’ve traveled internationally and they are undervaccinated, measles should be part of the differential diagnosis,” Sugerman said. He also emphasized the need to follow airborne isolation precautions in addition to general infection control measures.
Immediate triage is critical, especially since overcrowded waiting rooms might be filled with patients who are not yet eligible for vaccination or are not up to date or fully vaccinated.
“Measles is under airborne isolation criteria and precautions, and therefore, [patients] need to be placed as soon as possible into a negative pressure or airborne infection isolation room – and that should be a single room,” he explained. He noted, “In some settings, there may not be a negative pressure room, e.g., an outpatient pediatrics or family medicine office.”
Dr. Sugerman said that in these circumstances, patients should be placed in a room with masked health care providers who have received two doses of measles, mumps, and rubella (MMR) vaccine and that they should wear an N95 mask when entering the room and interviewing the patient.
Clinicians should follow CDC’s testing recommendations and collect a nasopharyngeal or throat swab or a urine specimen for PCR testing and a blood specimen for serology. In addition, they should immediately report cases to local and state public health authorities.
For all patients, it’s critical to be up to date on MMR vaccines, especially persons who are going to be traveling internationally. “We recommend that when they’ve got infants traveling with them who are 6-11 months of age, that they get a first dose (which we consider a zero dose), because they need a routine dose at 12-15 months, and then 4-6 years,” said Dr. Sugerman. He said that it’s safe for adults who are unsure of their status to receive an MMR dose as well.
Dr. Sugerman stressed that despite major strides, “we just don’t have enough coverage in all individuals in this country. Because people are traveling as often as they are, it can be imported. Until measles is eliminated globally, there’s going to be an ongoing risk of importation and potential spread amongst others in their household or community, especially amongst individuals who are not fully vaccinated and, in particular, amongst those who are unvaccinated,” he said.
Dr. Sugerman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued a Health Alert Network (HAN) health advisory notifying clinicians and public health officials of a confirmed measles case in an individual who for 2 days (February 17-18) attended a large religious gathering that was attended by an estimated 20,000 people at Asbury University in Wilmore, Ky.
Given that large numbers of people might have been exposed to the attendee (who was not vaccinated) and that the individual had a history of recent international travel, the CDC has encouraged clinicians to be vigilant for patients presenting with symptoms that meet the measles case definition. A steady increase in measles cases from 49 in 2021 to 121 in 2022 in children who were not fully vaccinated – coupled with outbreaks in Ohio and Minnesota – underscores the potential gravity of the CDC advisory as well as the need to mitigate the risk of ongoing or secondary transmission.
Currently, little is known about the individual who contracted measles other than the fact that he is a resident of Jessamine County, Ky., according to a news release issued by the Kentucky Department of Public Health. It is the third confirmed case in Kentucky over the past 3 months. State and national health officials are concerned that the individual might have transmitted measles to attendees visiting from other states.
David Sugerman, MD, MPH, a medical officer in CDC’s division of viral diseases and lead for the measles, rubella, and cytomegalovirus team, noted that the timing of the alert coincides with the period in which persons who had had contact with the initial case patient might be expected to develop symptoms.
For clinicians, “It’s really about considering measles in any un- or undervaccinated patient that arrives at a clinic and recently traveled internationally,” Dr. Sugerman told this news organization. He explained that “when doctors are seeing patients, they’re not going to necessarily share that information off the bat when they present with fever or rash, or if their child has fever and rash, or that they traveled internationally. So, eliciting that history from the patient or their parents is really critical.”
The CDC recommends that measles be considered in anyone presenting with a febrile illness and symptoms that are clinically compatible with measles (that is, rash, cough, coryza, or conjunctivitis), as well as in patients who have recently traveled abroad, especially to countries with ongoing outbreaks, including India, Somalia, and Yemen.
“In general, if they’ve traveled internationally and they are undervaccinated, measles should be part of the differential diagnosis,” Sugerman said. He also emphasized the need to follow airborne isolation precautions in addition to general infection control measures.
Immediate triage is critical, especially since overcrowded waiting rooms might be filled with patients who are not yet eligible for vaccination or are not up to date or fully vaccinated.
“Measles is under airborne isolation criteria and precautions, and therefore, [patients] need to be placed as soon as possible into a negative pressure or airborne infection isolation room – and that should be a single room,” he explained. He noted, “In some settings, there may not be a negative pressure room, e.g., an outpatient pediatrics or family medicine office.”
Dr. Sugerman said that in these circumstances, patients should be placed in a room with masked health care providers who have received two doses of measles, mumps, and rubella (MMR) vaccine and that they should wear an N95 mask when entering the room and interviewing the patient.
Clinicians should follow CDC’s testing recommendations and collect a nasopharyngeal or throat swab or a urine specimen for PCR testing and a blood specimen for serology. In addition, they should immediately report cases to local and state public health authorities.
For all patients, it’s critical to be up to date on MMR vaccines, especially persons who are going to be traveling internationally. “We recommend that when they’ve got infants traveling with them who are 6-11 months of age, that they get a first dose (which we consider a zero dose), because they need a routine dose at 12-15 months, and then 4-6 years,” said Dr. Sugerman. He said that it’s safe for adults who are unsure of their status to receive an MMR dose as well.
Dr. Sugerman stressed that despite major strides, “we just don’t have enough coverage in all individuals in this country. Because people are traveling as often as they are, it can be imported. Until measles is eliminated globally, there’s going to be an ongoing risk of importation and potential spread amongst others in their household or community, especially amongst individuals who are not fully vaccinated and, in particular, amongst those who are unvaccinated,” he said.
Dr. Sugerman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA to review dupilumab for treating chronic spontaneous urticaria
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
Experts share real-world experience prescribing voclosporin, belimumab for lupus nephritis
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
FDA accepts application for topical molluscum treatment
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
Pembrolizumab before and after melanoma surgery boosts outcomes
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
, show results from the phase 2 SWOG S1801 trial.
The trial involved 319 patients with operable stage IIIB to stage IV melanoma. The investigators found that patients who received pembrolizumab both before and after surgery (i.e., neoadjuvant and adjuvant therapy) fared better than those who received the drug only after surgery: The 2-year event-free survival rates were 72% vs. 49%, respectively.
The research was published in the New England Journal of Medicine, but similar results had already been presented at the European Society for Medical Oncology 2022 annual Meeting.
“It’s not just what you give; it’s when you give it,” said lead author Sapna Patel, MD, in a press release issued by the University of Texas MD Anderson Cancer Center, echoing comments she gave at ESMO 2022.
The study, she continued, “demonstrates the same treatment for resectable melanoma given before surgery can generate better outcomes.”
On the basis of their findings, Dr. Patel, who is associate professor of melanoma medical oncology at the University of Texas MD Anderson Cancer Center, Houston, said that patients with high-risk melanoma “should start immunotherapy prior to surgery to generate an immune response while the bulk of the melanoma and the anti-tumor T cells are intact.”
The mechanism of action of PD-1 blockade “relies on the presence of preexisting anti-tumor T cells attempting to attack cancer cells,” with the immunotherapy allowing the anti-tumor cells to proliferate and mediate clinical responses.
Resection of the bulk of the tumor is therefore “likely to take away some or even most of the potential anti-tumor T cells that would proliferate after PD-1 blockade,” they write.
Likely to apply also to nivolumab
Approached for comment, Jeffrey S. Weber, MD, PhD, professor of medicine, NYU Langone Medical Center, New York, said that outside of trials, both pembrolizumab and ipilimumab (Yervoy)/nivolumab (Opdivo) are already being used neoadjuvantly.
He thinks that the findings for neoadjuvant and adjuvant pembrolizumab could also apply to nivolumab because “the drugs are quite similar in efficacy.”
Dr. Weber told this news organization that, “even though the S1801 trial was not accepted as a registration trial by the FDA, I think that its results could very well change practice and confirm it for others who already use neoadjuvant therapy for palpable stage III melanoma.”
One question that is being addressed to an extent in the NADINA trial is whether adjuvant immunotherapy can be avoided all together and patients receive only neoadjuvant therapy, although Dr. Weber said, “I doubt that will be the case.”
Study details
In this study, patients were randomly assigned to either surgery followed by 18 doses of adjuvant pembrolizumab, or to receive 3 doses of neoadjuvant pembrolizumab followed by surgery and then 15 additional doses of adjuvant pembrolizumab.
After a median duration of follow-up of 14.7 months, there were 38 events in the neoadjuvant-adjuvant group and 67 in the adjuvant-only group.
“Events” were defined as disease progression, toxic effects, or complications that precluded surgery or the initiation of adjuvant therapy within 84 days of surgery, as well as the inability to fully resect the gross disease, melanoma recurrence, and death.
The team calculated that event-free survival was significantly longer in the neoadjuvant-adjuvant group (P = .004), with 2-year event-free survival at 72% vs. 49% in the adjuvant-only group.
“The benefit of neoadjuvant pembrolizumab was seen across all subgroups of patients,” the investigators note.
At the data cut-off, there were 14 deaths in the neoadjuvant-adjuvant group vs. 22 in the adjuvant-only group, which the researchers say is too few to allow “definitive comparison” in terms of overall survival.
Definitive surgery had been performed in 88% of neoadjuvant-adjuvant patients and in 95% of those assigned to adjuvant-only pembrolizumab. The most common reason for not undergoing surgery was disease progression.
Among the patients for whom safety data were available, 7% in the neoadjuvant-adjuvant group had at least one grade 3 or 4 adverse event related to pembrolizumab, whereas 7% had at least one grade 3 or 4 adverse event related to surgery.
In the adjuvant-only arm, 4% of patients had at least one grade 3 adverse event related to surgery, with no grade 4 adverse events reported.
The rates of grade 3 or 4 adverse events during adjuvant therapy were similar in the two groups, at 12% in patients assigned to neoadjuvant-adjuvant therapy and 14% in those given adjuvant-only pembrolizumab.
“Future studies can explore deescalation strategies for both surgery and adjuvant therapy, as well as approaches for patients whose melanoma does not respond to neoadjuvant therapy,” the researchers commented.
The study was funded by the National Cancer Institute and Merck Sharp and Dohme.
Dr. Patel reports numerous relationships with industry, including with Merck, manufacturer of pembrolizumab; other coauthors also have numerous relationships with industry. Dr. Weber is a regular columnist for this news organization and lists his disclosures in his Weber on Oncology column.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE