User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Silent brain infarcts found in 3% of AFib patients, tied to cognitive decline
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
Patients with atrial fibrillation, even those on oral anticoagulant therapy, developed clinically silent brain infarctions at a striking rate of close to 3% per year, according to results from SWISS-AF, a prospective of study of 1,227 Swiss patients followed with serial MR brain scans over a 2 year period.
The results also showed that these brain infarctions – which occurred in 68 (5.5%) of the atrial fibrillation (AFib) patients, including 58 (85%) who did not have any strokes or transient ischemic attacks during follow-up – appeared to represent enough pathology to link with a small but statistically significant decline in three separate cognitive measures, compared with patients who did not develop brain infarctions during follow-up.
“Cognitive decline may go unrecognized for a long time in clinical practice because usually no one tests for it,” plus “the absolute declines were small and probably not appreciable” in the everyday behavior of affected patients, David Conen, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. But “we were surprised to see a significant change after just 2 years. We expect much larger effects to develop over time,” he said during a press briefing.
Another key finding was that roughly half the patients had large cortical or noncortical infarcts, which usually have a thromboembolic cause, but the other half had small noncortical infarcts that likely have a different etiology involving the microvasculature. Causes for those small infarcts might include localized atherosclerotic disease or amyloidosis, proposed Dr. Conen, a cardiologist at McMaster University, Hamilton, Ont.
This finding also suggests that, as a consequence, anticoagulation alone may not be enough to prevent this brain damage in Afib patients. “It calls for a more comprehensive approach to prevention,” with attention to atherosclerotic cardiovascular disease risk factors in AFib patients, including interventions that address hypertension, diabetes, hyperlipidemia, and smoking cessation. “Anticoagulation in AFib patients is critical, but it also is not enough,” Dr. Conen said.
These data “are very important. The two pillars for taking care of AFib patients have traditionally been to manage the patient’s stroke risk and to treat symptoms. Dr. Conen’s data suggest that simply starting anticoagulation is not sufficient, and it stresses the importance of continued management of hypertension, diabetes, and other medical and social issues,” commented Fred Kusumoto, MD, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla.
“The risk factors associated with the development of cardiovascular disease are similar to those associated with the development of AFib and heart failure. It is important to understand the importance of managing hypertension, diabetes, and obesity; encouraging exercise and a healthy diet; and stopping smoking in all AFib patients as well as in the general population. Many clinicians have not emphasized the importance of continually addressing these behaviors,” Dr. Kusumoto said in an interview.
The SWISS-AF (Swiss Atrial Fibrillation Cohort) study enrolled 2,415 AFib patients at 14 Swiss centers during 2014-2017, and obtained both a baseline brain MR scan and baseline cognitive-test results for 1,737 patients (J Am Coll Cardiol. 2019 Mar;73[9]:989-99). Patients retook the cognitive tests annually, and 1,227 had a second MR brain scan after 2 years in the study, the cohort that supplied the data Dr. Conen presented. At baseline, these patients averaged 71 years of age, just over a quarter were women, and 90% were on an oral anticoagulant, with 84% on an oral anticoagulant at 2-year follow-up. Treatment split roughly equally between direct-acting oral anticoagulants and vitamin K antagonists like warfarin.
Among the 68 patients with evidence for an incident brain infarct after 2 years, 59 (87%) were on treatment with an OAC, and 51 (75%) who were both on treatment with a direct-acting oral anticoagulant and developed their brain infarct without also having a stroke or transient ischemic attack, which Dr. Conen called a “silent event.” The cognitive tests that showed statistically significant declines after 2 years in the patients with silent brain infarcts compared with those without a new infarct were the Trail Making Test parts A and B, and the animal-naming verbal fluency test. The two other tests applied were the Montreal Cognitive Assessment and the Digital Symbol Substitution Test.
Results from several prior studies also indicated a relationship between AFib and cognitive decline, but SWISS-AF is “the largest study to rigorously examine the incidence of silent brain infarcts in AFib patients,” commented Christine M. Albert, MD, chair of cardiology at the Smidt Heart Institute of Cedars-Sinai Medical Center in Los Angeles. “Silent infarcts could be the cause, at least in part, for the cognitive decline and dementia associated with AFib,” she noted. But divining the therapeutic implications of the finding will require further investigation that looks at factors such as the impact of anticoagulant type, other treatment that addresses AFib such as ablation and rate control, the duration and type of AFib, and the prevalence of hypertension and other stroke risk factors, she said as a designated discussant for Dr. Conen’s report.
SWISS-AF received no commercial funding. Dr. Conen has been a speaker on behalf of Servier. Dr. Kusumoto had no disclosures. Dr. Albert has been a consultant to Roche Diagnostics and has received research funding from Abbott, Roche Diagnostics, and St. Jude Medical.
FROM HEART RHYTHM 2020
COVID-19 in pregnancy: Supplement oxygen if saturation dips below 94%
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
Oxygen supplementation for pregnant women with COVID-19 should begin when saturations fall below 94%, according to physicians in the divisions of maternal-fetal medicine and surgical critical care at the University of Texas Medical Branch at Galveston.
That’s a bit higher than the 92% cut point for nonpregnant women, but necessary due to the increased oxygen demand and oxygen partial pressure in pregnancy. The goal is a saturation of 94%-96%, said Luis Pacheco, MD, a maternal-fetal medicine and critical care specialist at the university, and associates.
so Dr. Pacheco and associates addressed the issue in a commentary in Obstetrics & Gynecology.
Women on respiratory support should lie prone if under 20 weeks’ gestation to help with posterior lung recruitment and oxygenation.
If conventional oxygen therapy isn’t enough, high-flow nasal cannula (HFNC) at 60 L/min and 100% oxygen should be the next step, not positive-pressure ventilation. Positive pressure, another option, kicks off aerosols that increase the risk of viral transmission to medical staff. “This makes high-flow nasal cannula the first-line option for patients not responding to conventional oxygen therapy but who are not yet candidates for endotracheal intubation,” the team said. If women do well, the fraction of inspired oxygen should be weaned before the nasal cannula flow is decreased.
However, if they continue to struggle with dyspnea, tachypnea, and oxygen saturation after 30-60 minutes on HFNC, it’s time for mechanical ventilation, and fast. “Delays in recognizing early failure of high-flow nasal cannula ... may result in life-threatening hypoxemia at the time of induction and intubation (especially in pregnant patients with difficult airway anatomy),” the authors said.
For birth, Dr. Pacheco and associates recommended controlled delivery, likely cesarean, if respiration continues to deteriorate despite intubation, especially after 28 weeks’ gestation, instead of waiting for fetal distress and an ICU delivery. A single course of steroids is reasonable to help fetal lung development beforehand, if indicated.
As for fluid strategy during respiratory support, pregnant women are at higher risk for pulmonary edema with lung inflammation, so the authors cautioned against giving maintenance fluids, and said “if daily positive fluid balances are present, combined with worsening respiratory status, the use of furosemide (10-20 mg intravenously every 12 hours) may be indicated.”
For women stable on conventional oxygen therapy or HFNC, they suggested daily nonstress tests starting at 25 weeks’ gestation instead of continuous monitoring, to minimize the COVID-19 transmission risk for staff.
The team cautioned against nebulized treatments and sputum-inducing agents when possible as this may aerosolize the virus.
There was no external funding for the report, and the authors didn’t have any relevant financial disclosures.
SOURCE: Pacheco LD et al. Obstet Gynecol. 2020 Apr 29. doi: 10.1097/AOG.0000000000003929.
OBSTETRICS & GYNECOLOGY
With life in the balance, a pediatric palliative care program expands its work to adults
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
Evidence builds linking anticoagulation to COVID-19 survival
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
Operation Quack Hack: FDA moves to stop fraudulent COVID-19 products
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.
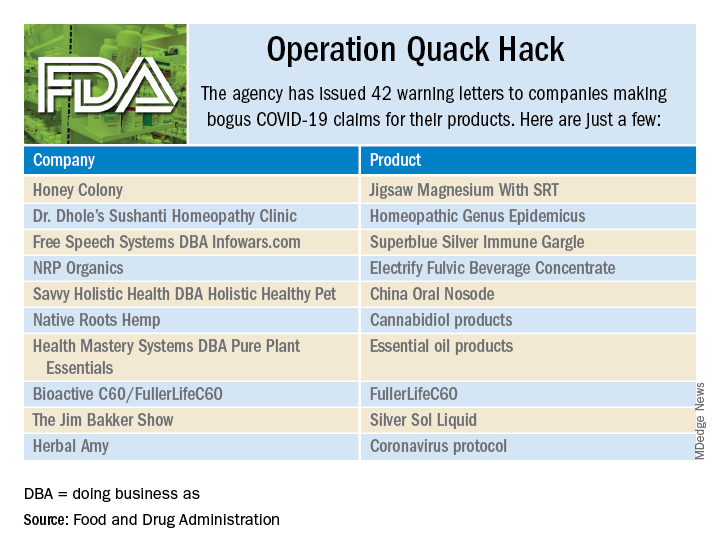
As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
Coronary CT angiography gives superior MI risk prediction
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
FROM ACC 2020
U.S. ‘deaths of despair’ from COVID-19 could top 75,000, experts warn
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
UNTOUCHED: Inappropriate shocks cut by subcutaneous ICD improvements
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
FROM HEART RHYTHM 2020
Novel inflammatory syndrome in children possibly linked to COVID-19
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
Volunteering during the pandemic: What doctors need to know
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.






















