User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
The devil in the (masking) details
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
Which specialties get the biggest markups over Medicare rates?
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .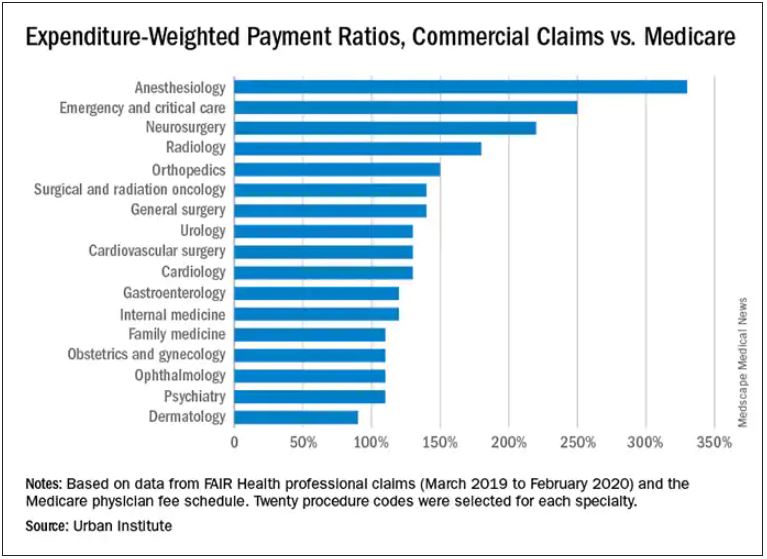
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Hot temperatures in outdoor lockboxes increase sample errors
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
SURPASS-4: ‘Twincretin’ tirzepatide surpasses insulin glargine in pivotal trial
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
FROM THE LANCET
Researchers parse which patients with T2D need SGLT2 inhibition
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
FROM DIABETES CARE
Unvaccinated people likely to catch COVID repeatedly
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
Real-world data favor invasive strategy for NSTEMI with CKD
Most patients with advanced chronic kidney disease (CKD) and non–ST-elevation myocardial infarction (NSTEMI) fare better with coronary angiography with and without revascularization than with medical therapy, a large nationwide study suggests.
“Invasive management was associated with lower mortality, major adverse cardiovascular events (MACE), and need for revascularization, with a minimal increased risk of in-hospital, postprocedural acute kidney injury (AKI) requiring dialysis and major bleeding,” said lead researcher Ankur Kalra, MD, Cleveland Clinic.
Also, similar post-discharge safety outcomes were seen at 6 months, he said in an online presentation of “key abstracts” released in advance of next month’s Transcatheter Cardiovascular Therapeutics (TCT) 2021 hybrid meeting.
Advanced CKD is an independent predictor of mortality and morbidity in patients with NSTEMI. In CKD, however, current guidelines lack evidence on the efficacy and safety of invasive versus medical management, he noted.
A rare randomized clinical trial in this high-risk population, ISCHEMIA-CKD, recently found no benefit and an increase in stroke with initial invasive management compared with optimal medical therapy.
Session co-moderator Ziad A. Ali, MD, DPhil, St. Francis Hospital & Heart Center, New York, said the current study is “incredibly clinically impactful and answers a question that’s very difficult to answer because these patients aren’t randomized in randomized controlled trials, and there’s a general avoidance, which we’ve now coined ‘renalism,’ like racism, where people don’t really want to touch these patients.”
He questioned, however, how the authors reconcile the results of ISCHEMIA-CKD, a “small but meaningful randomized controlled trial,” with their findings from a large dataset. “Perhaps this is all selection bias, even though the numbers are very large.”
Dr. Kalra replied that ISCHEMIA-CKD examined stable ischemic heart disease, whereas they looked at NSTEMI. “Even though it may fall under the same rubric, I truly believe it is a different set of patients – they are at a heightened risk for future cardiovascular events and have had an acute coronary event.”
For the study, ICD-10 coding data from 2016-2018 in the Nationwide Readmission Database was used to identify NSTEMI patients with CKD stages 3, 4, 5, and end-stage renal disease (ESRD). A total of 141,052 patients were available for in-hospital outcomes and 133,642 patients for post-discharge outcomes.
In-hospital and 6-month mortality – the study’s primary outcome – favored invasive management across all CKD stages and ESRD but did not achieve statistical significance for CKD stage 5. The number needed to treat (NNT) for CKD stages 3, 4, 5, and ESRD were 26, 56, 48, and 18, respectively.
Six-month MACE, including mortality, MI, stroke, and heart failure readmission, was significantly better in all groups with invasive management.
Kaplan-Meier curves for mortality showed similar benefits with an invasive strategy across CKD stages, again barring stage 5 disease.
With regard to in-hospital safety, stroke rates were not significantly different between the two treatment strategies across all groups.
Rates of AKI requiring dialysis, however, were lower with medical versus invasive management for CKD stage 3 (0.43% vs. 0.6%; hazard ratio, 1.39; P = .016), stage 4 (1.2% vs. 2.0%; HR 1.87; P < .001), and stage 5 (3.7% vs. 4.3%; HR 1.17; P = .527). The number needed to harm (NNH) was 588 for CKD 3 and 125 for CKD 4.
Major bleeding, defined as requiring transfusion, was lower with medical management for all CKD stages but not for ESRD. The rates are as follows:
- CKD stage 3: 2.5% vs. 2.8% (HR, 1.11; P = .078; NNH = 333)
- CKD stage 4: 2.9% vs. 4.0% (HR, 1.42; P < .001; NNH = 91)
- CKD stage 5: 2.2% vs. 4.7% (HR, 2.17; P = .008; NNH = 40)
- ESRD: 3.4% vs. 3.3% (HR, 0.97; P = .709)
“The risk of AKI requiring dialysis and bleeding, as has been shown previously in other studies, was high, but the number needed to harm was also high,” observed Dr. Kalra.
A separate analysis showed no difference in rates of AKI requiring dialysis among patients with CKD stages 3 and 4 who underwent angiography without revascularization and their peers who were medically managed.
Rates of the composite safety outcome of vascular complications, major bleeding, AKI, or stroke readmission at 6 months were not significantly different for invasive versus medical management for CKD stage 3 (both 3.3%), stage 4 (4.5% and 4.2%), stage 5 (3.9% vs. 4.3%), and ESRD (2.3% vs. 2.1%).
Besides the inherent limitations of observational studies and potential for selection bias, Dr. Kalra pointed out that the analysis relied on coding data for exact glomerular filtration rates and lacked information on contrast use, crystalloids before the procedure, and nephrotoxic medication use before or during admission. Out-of-hospital mortality was also not available in the database.
Co-moderator Allen Jeremias, MD, also with St. Francis Hospital & Heart Center, said one of the study’s strengths was that it included all comers, unlike randomized trials that typically exclude the highest risk patients.
“So, when we do these trials it’s very difficult to find the right balance, whereas this is a real-world analysis including everybody, and I think the benefits are clearly demonstrated,” he said. “So I think I’m bullish on doing complex [percutaneous coronary intervention] PCI in this patient population.”
Dr. Kalra reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most patients with advanced chronic kidney disease (CKD) and non–ST-elevation myocardial infarction (NSTEMI) fare better with coronary angiography with and without revascularization than with medical therapy, a large nationwide study suggests.
“Invasive management was associated with lower mortality, major adverse cardiovascular events (MACE), and need for revascularization, with a minimal increased risk of in-hospital, postprocedural acute kidney injury (AKI) requiring dialysis and major bleeding,” said lead researcher Ankur Kalra, MD, Cleveland Clinic.
Also, similar post-discharge safety outcomes were seen at 6 months, he said in an online presentation of “key abstracts” released in advance of next month’s Transcatheter Cardiovascular Therapeutics (TCT) 2021 hybrid meeting.
Advanced CKD is an independent predictor of mortality and morbidity in patients with NSTEMI. In CKD, however, current guidelines lack evidence on the efficacy and safety of invasive versus medical management, he noted.
A rare randomized clinical trial in this high-risk population, ISCHEMIA-CKD, recently found no benefit and an increase in stroke with initial invasive management compared with optimal medical therapy.
Session co-moderator Ziad A. Ali, MD, DPhil, St. Francis Hospital & Heart Center, New York, said the current study is “incredibly clinically impactful and answers a question that’s very difficult to answer because these patients aren’t randomized in randomized controlled trials, and there’s a general avoidance, which we’ve now coined ‘renalism,’ like racism, where people don’t really want to touch these patients.”
He questioned, however, how the authors reconcile the results of ISCHEMIA-CKD, a “small but meaningful randomized controlled trial,” with their findings from a large dataset. “Perhaps this is all selection bias, even though the numbers are very large.”
Dr. Kalra replied that ISCHEMIA-CKD examined stable ischemic heart disease, whereas they looked at NSTEMI. “Even though it may fall under the same rubric, I truly believe it is a different set of patients – they are at a heightened risk for future cardiovascular events and have had an acute coronary event.”
For the study, ICD-10 coding data from 2016-2018 in the Nationwide Readmission Database was used to identify NSTEMI patients with CKD stages 3, 4, 5, and end-stage renal disease (ESRD). A total of 141,052 patients were available for in-hospital outcomes and 133,642 patients for post-discharge outcomes.
In-hospital and 6-month mortality – the study’s primary outcome – favored invasive management across all CKD stages and ESRD but did not achieve statistical significance for CKD stage 5. The number needed to treat (NNT) for CKD stages 3, 4, 5, and ESRD were 26, 56, 48, and 18, respectively.
Six-month MACE, including mortality, MI, stroke, and heart failure readmission, was significantly better in all groups with invasive management.
Kaplan-Meier curves for mortality showed similar benefits with an invasive strategy across CKD stages, again barring stage 5 disease.
With regard to in-hospital safety, stroke rates were not significantly different between the two treatment strategies across all groups.
Rates of AKI requiring dialysis, however, were lower with medical versus invasive management for CKD stage 3 (0.43% vs. 0.6%; hazard ratio, 1.39; P = .016), stage 4 (1.2% vs. 2.0%; HR 1.87; P < .001), and stage 5 (3.7% vs. 4.3%; HR 1.17; P = .527). The number needed to harm (NNH) was 588 for CKD 3 and 125 for CKD 4.
Major bleeding, defined as requiring transfusion, was lower with medical management for all CKD stages but not for ESRD. The rates are as follows:
- CKD stage 3: 2.5% vs. 2.8% (HR, 1.11; P = .078; NNH = 333)
- CKD stage 4: 2.9% vs. 4.0% (HR, 1.42; P < .001; NNH = 91)
- CKD stage 5: 2.2% vs. 4.7% (HR, 2.17; P = .008; NNH = 40)
- ESRD: 3.4% vs. 3.3% (HR, 0.97; P = .709)
“The risk of AKI requiring dialysis and bleeding, as has been shown previously in other studies, was high, but the number needed to harm was also high,” observed Dr. Kalra.
A separate analysis showed no difference in rates of AKI requiring dialysis among patients with CKD stages 3 and 4 who underwent angiography without revascularization and their peers who were medically managed.
Rates of the composite safety outcome of vascular complications, major bleeding, AKI, or stroke readmission at 6 months were not significantly different for invasive versus medical management for CKD stage 3 (both 3.3%), stage 4 (4.5% and 4.2%), stage 5 (3.9% vs. 4.3%), and ESRD (2.3% vs. 2.1%).
Besides the inherent limitations of observational studies and potential for selection bias, Dr. Kalra pointed out that the analysis relied on coding data for exact glomerular filtration rates and lacked information on contrast use, crystalloids before the procedure, and nephrotoxic medication use before or during admission. Out-of-hospital mortality was also not available in the database.
Co-moderator Allen Jeremias, MD, also with St. Francis Hospital & Heart Center, said one of the study’s strengths was that it included all comers, unlike randomized trials that typically exclude the highest risk patients.
“So, when we do these trials it’s very difficult to find the right balance, whereas this is a real-world analysis including everybody, and I think the benefits are clearly demonstrated,” he said. “So I think I’m bullish on doing complex [percutaneous coronary intervention] PCI in this patient population.”
Dr. Kalra reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most patients with advanced chronic kidney disease (CKD) and non–ST-elevation myocardial infarction (NSTEMI) fare better with coronary angiography with and without revascularization than with medical therapy, a large nationwide study suggests.
“Invasive management was associated with lower mortality, major adverse cardiovascular events (MACE), and need for revascularization, with a minimal increased risk of in-hospital, postprocedural acute kidney injury (AKI) requiring dialysis and major bleeding,” said lead researcher Ankur Kalra, MD, Cleveland Clinic.
Also, similar post-discharge safety outcomes were seen at 6 months, he said in an online presentation of “key abstracts” released in advance of next month’s Transcatheter Cardiovascular Therapeutics (TCT) 2021 hybrid meeting.
Advanced CKD is an independent predictor of mortality and morbidity in patients with NSTEMI. In CKD, however, current guidelines lack evidence on the efficacy and safety of invasive versus medical management, he noted.
A rare randomized clinical trial in this high-risk population, ISCHEMIA-CKD, recently found no benefit and an increase in stroke with initial invasive management compared with optimal medical therapy.
Session co-moderator Ziad A. Ali, MD, DPhil, St. Francis Hospital & Heart Center, New York, said the current study is “incredibly clinically impactful and answers a question that’s very difficult to answer because these patients aren’t randomized in randomized controlled trials, and there’s a general avoidance, which we’ve now coined ‘renalism,’ like racism, where people don’t really want to touch these patients.”
He questioned, however, how the authors reconcile the results of ISCHEMIA-CKD, a “small but meaningful randomized controlled trial,” with their findings from a large dataset. “Perhaps this is all selection bias, even though the numbers are very large.”
Dr. Kalra replied that ISCHEMIA-CKD examined stable ischemic heart disease, whereas they looked at NSTEMI. “Even though it may fall under the same rubric, I truly believe it is a different set of patients – they are at a heightened risk for future cardiovascular events and have had an acute coronary event.”
For the study, ICD-10 coding data from 2016-2018 in the Nationwide Readmission Database was used to identify NSTEMI patients with CKD stages 3, 4, 5, and end-stage renal disease (ESRD). A total of 141,052 patients were available for in-hospital outcomes and 133,642 patients for post-discharge outcomes.
In-hospital and 6-month mortality – the study’s primary outcome – favored invasive management across all CKD stages and ESRD but did not achieve statistical significance for CKD stage 5. The number needed to treat (NNT) for CKD stages 3, 4, 5, and ESRD were 26, 56, 48, and 18, respectively.
Six-month MACE, including mortality, MI, stroke, and heart failure readmission, was significantly better in all groups with invasive management.
Kaplan-Meier curves for mortality showed similar benefits with an invasive strategy across CKD stages, again barring stage 5 disease.
With regard to in-hospital safety, stroke rates were not significantly different between the two treatment strategies across all groups.
Rates of AKI requiring dialysis, however, were lower with medical versus invasive management for CKD stage 3 (0.43% vs. 0.6%; hazard ratio, 1.39; P = .016), stage 4 (1.2% vs. 2.0%; HR 1.87; P < .001), and stage 5 (3.7% vs. 4.3%; HR 1.17; P = .527). The number needed to harm (NNH) was 588 for CKD 3 and 125 for CKD 4.
Major bleeding, defined as requiring transfusion, was lower with medical management for all CKD stages but not for ESRD. The rates are as follows:
- CKD stage 3: 2.5% vs. 2.8% (HR, 1.11; P = .078; NNH = 333)
- CKD stage 4: 2.9% vs. 4.0% (HR, 1.42; P < .001; NNH = 91)
- CKD stage 5: 2.2% vs. 4.7% (HR, 2.17; P = .008; NNH = 40)
- ESRD: 3.4% vs. 3.3% (HR, 0.97; P = .709)
“The risk of AKI requiring dialysis and bleeding, as has been shown previously in other studies, was high, but the number needed to harm was also high,” observed Dr. Kalra.
A separate analysis showed no difference in rates of AKI requiring dialysis among patients with CKD stages 3 and 4 who underwent angiography without revascularization and their peers who were medically managed.
Rates of the composite safety outcome of vascular complications, major bleeding, AKI, or stroke readmission at 6 months were not significantly different for invasive versus medical management for CKD stage 3 (both 3.3%), stage 4 (4.5% and 4.2%), stage 5 (3.9% vs. 4.3%), and ESRD (2.3% vs. 2.1%).
Besides the inherent limitations of observational studies and potential for selection bias, Dr. Kalra pointed out that the analysis relied on coding data for exact glomerular filtration rates and lacked information on contrast use, crystalloids before the procedure, and nephrotoxic medication use before or during admission. Out-of-hospital mortality was also not available in the database.
Co-moderator Allen Jeremias, MD, also with St. Francis Hospital & Heart Center, said one of the study’s strengths was that it included all comers, unlike randomized trials that typically exclude the highest risk patients.
“So, when we do these trials it’s very difficult to find the right balance, whereas this is a real-world analysis including everybody, and I think the benefits are clearly demonstrated,” he said. “So I think I’m bullish on doing complex [percutaneous coronary intervention] PCI in this patient population.”
Dr. Kalra reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fluoroquinolones linked to sudden death risk for those on hemodialysis
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large observational study suggests.
However, in many cases, the absolute risk is relatively small, and the antimicrobial benefits of a fluoroquinolone may outweigh the potential cardiac risks, the researchers say.
“Pathogen-directed treatment of respiratory infections is of the utmost importance. Respiratory fluoroquinolones should be prescribed whenever an amoxicillin-based antibiotic offers suboptimal antimicrobial coverage and clinicians should consider electrocardiographic monitoring,” first author Magdalene M. Assimon, PharmD, PhD, University of North Carolina, Chapel Hill, told this news organization.
The study was published online Oct. 20 in JAMA Cardiology (doi: 10.1001/jamacardio.2021.4234).
Nearly twofold increased risk
The QT interval-prolonging potential of fluoroquinolone antibiotics are well known. However, evidence linking respiratory fluoroquinolones to adverse cardiac outcomes in the hemodialysis population is limited.
These new observational findings are based on a total of 626,322 antibiotic treatment episodes among 264,968 adults (mean age, 61 years; 51% men) receiving in-center hemodialysis – with respiratory fluoroquinolone making up 40.2% of treatment episodes and amoxicillin-based antibiotic treatment episodes making up 59.8%.
The rate of SCD within 5 days of outpatient initiation of a study antibiotic was 105.7 per 100,000 people prescribed a respiratory fluoroquinolone (levofloxacin or moxifloxacin) versus with 40.0 per 100,000 prescribed amoxicillin or amoxicillin with clavulanic acid (weighted hazard ratio: 1.95; 95% confidence interval, 1.57-2.41).
The authors estimate that one additional SCD would occur during a 5-day follow-up period for every 2,273 respiratory fluoroquinolone treatment episodes. Consistent associations were seen when follow-up was extended to 7, 10, and 14 days.
“Our data suggest that curtailing respiratory fluoroquinolone prescribing may be one actionable strategy to mitigate SCD risk in the hemodialysis population. However, the associated absolute risk reduction would be relatively small,” wrote the authors.
They noted that the rate of SCD in the hemodialysis population exceeds that of the general population by more than 20-fold. Most patients undergoing hemodialysis have a least one risk factor for drug-induced QT interval prolongation.
In the current study, nearly 20% of hemodialysis patients prescribed a respiratory fluoroquinolone were taking other medications with known risk for torsades de pointes.
“Our results emphasize the importance of performing a thorough medication review and considering pharmacodynamic drug interactions before prescribing new drug therapies for any condition,” Dr. Assimon and colleagues advised.
They suggest that clinicians consider electrocardiographic monitoring before and during fluoroquinolone therapy in hemodialysis patients, especially in high-risk individuals.
Valuable study
Reached for comment, Ankur Shah, MD, of the division of kidney diseases and hypertension, Brown University, Providence, R.I., called the analysis “valuable” and said the results are “consistent with the known association of cardiac arrhythmias with respiratory fluoroquinolone use in the general population, postulated to be due to increased risk of torsades de pointes from QTc prolongation. This abnormal heart rhythm can lead to sudden cardiac death.
“Notably, the population receiving respiratory fluoroquinolones had a higher incidence of cardiac disease at baseline, but the risk persisted after adjustment for this increased burden of comorbidity,” Dr. Shah said in an interview. He was not involved in the current research.
Dr. Shah cautioned that observational data such as these should be considered more “hypothesis-generating than practice-changing, as there may be unrecognized confounders or differences in the population that received the respiratory fluoroquinolones.
“A prospective randomized trial would provide a definitive answer, but in the interim, caution should be taken in using respiratory fluoroquinolones when local bacterial resistance patterns or patient-specific data offer another option,” Dr. Shah concluded.
Dr. Assimon reported receiving grants from the Renal Research Institute (a subsidiary of Fresenius Medical Care), honoraria from the International Society of Nephrology for serving as a statistical reviewer for Kidney International Reports, and honoraria from the American Society of Nephrology for serving as an editorial fellow for the Journal of the American Society of Nephrology. Dr. Shah has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What are the cardiorenal differences between type 1 and type 2 diabetes?
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
FROM EASD 2021















