User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Social determinants of health may drive CVD risk in Black Americans
Investigators analyzed 20 years of data on over 50,500 U.S. adults drawn from the National Health and Nutrition Examination Surveys (NHANES) and found that, in the overall population, body mass index and hemoglobin A1c were significantly increased between 1999 and 2018, while serum total cholesterol and cigarette smoking were significantly decreased. Mean systolic blood pressure decreased between 1999 and 2010, but then increased after 2010.
The mean age- and sex-adjusted estimated 10-year risk for atherosclerotic cardiovascular disease (ASCVD) was consistently higher in Black participants vs. White participants, but the difference was attenuated after further adjusting for education, income, home ownership, employment, health insurance, and access to health care.
“These findings are helpful to guide the development of national public health policies for targeted interventions aimed at eliminating health disparities,” Jiang He, MD, PhD, Joseph S. Copes Chair and professor of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
“Interventions on social determinants of cardiovascular health should be tested in rigorous designed intervention trials,” said Dr. He, director of the Tulane University Translational Science Institute.
The study was published online Oct. 5 in JAMA.
‘Flattened’ CVD mortality?
Recent data show that the CVD mortality rate flattened, while the total number of cardiovascular deaths increased in the U.S. general population from 2010 to 2018, “but the reasons for this deceleration in the decline of CVD mortality are not entirely understood,” Dr. He said.
Moreover, “racial and ethnic differences in CVD mortality persist in the U.S. general population [but] the secular trends of cardiovascular risk factors among U.S. subpopulations with various racial and ethnic backgrounds and socioeconomic status are [also] not well understood,” he added. The effects of social determinants of health, such as education, income, home ownership, employment, health insurance, and access to health care on racial/ethnic differences in CVD risk, “are not well documented.”
To investigate these questions, the researchers drew on data from NHANES, a series of cross-sectional surveys in nationally representative samples of the U.S. population aged 20 years and older. The surveys are conducted in 2-year cycles and include data from 10 cycles conducted from 1999-2000 to 2017-2018 (n = 50,571, mean age 49.0-51.8 years; 48.2%-51.3% female).
Every 2 years, participants provided sociodemographic information, including age, race/ethnicity, sex, education, income, employment, housing, health insurance, and access to health care, as well as medical history and medication use. They underwent a physical examination that included weight and height, blood pressure, lipid levels, plasma glucose, and hemoglobin A1c.
Social determinants of health
Between 1999-2000 and 2017-2018, age- and sex-adjusted mean BMI and hemoglobin A1c increased, while mean serum total cholesterol and prevalence of smoking decreased (all P < .001).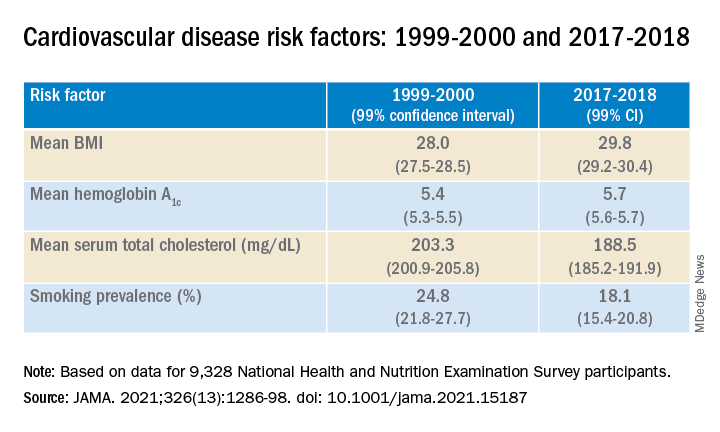
Age- and sex-adjusted 10-year atherosclerotic cardiovascular disease (ASCVD) risk decreased from 7.6% (6.9%-8.2%) in 1999-2000 to 6.5% (6.1%-6.8%) in 2011-2012, with no significant changes thereafter.
When the researchers looked at specific racial and ethnic groups, they found that age- and sex-adjusted BMI, systolic BP, and hemoglobin A1c were “consistently higher” in non-Hispanic Black participants compared with non-Hispanic White participants, but total cholesterol was lower (all P < .001).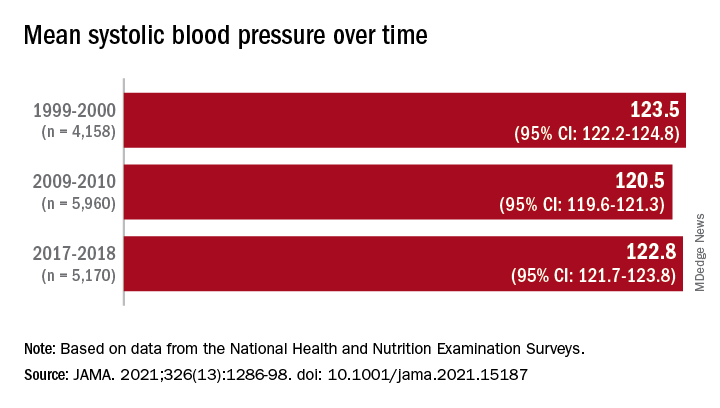
Participants with at least a college education or high family income had “consistently lower levels” of cardiovascular risk factors. And although the mean age- and sex-adjusted 10-year risk for ASCVD was significantly higher in non-Hispanic Black vs. non-Hispanic White participants (difference, 1.4% [1.0%-1.7%] in 1999-2008 and 2.0% [1.7%-2.4%] in 2009-2018), the difference was attenuated (by –0.3% in 1999-2008 and 0.7% in 2009-2018) after the researchers further adjusted for education, income, home ownership, employment, health insurance, and access to health care.
The differences in cardiovascular risk factors between Black and White participants “may have been moderated by social determinants of health,” the authors noted.
Provide appropriate education
Commenting on the study in an interview, Mary Ann McLaughlin, MD, MPH, associate professor of medicine, cardiology, Icahn School of Medicine at Mount Sinai, New York, pointed out that two important cardiovascular risk factors associated with being overweight – hypertension and diabetes – remained higher in the Black population compared with the White population in this analysis.
“Physicians and health care systems should provide appropriate education and resources regarding risk factor modification regarding diet, exercise, and blood pressure control,” advised Dr. McLaughlin, who was not involved with the study.
“Importantly, smoking rates and cholesterol levels are lower in the Black population, compared to the White population, when adjusted for many important socioeconomic factors,” she pointed out.
Dr. McLaughlin added that other “important social determinants of health, such as neighborhood and access to healthy food, were not measured and should be addressed by physicians when optimizing cardiovascular risk.”
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute and by the National Institute of General Medical Sciences. One of the researchers, Joshua D. Bundy, PhD, was supported by a grant from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. He and the other coauthors and Dr. McLaughlin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed 20 years of data on over 50,500 U.S. adults drawn from the National Health and Nutrition Examination Surveys (NHANES) and found that, in the overall population, body mass index and hemoglobin A1c were significantly increased between 1999 and 2018, while serum total cholesterol and cigarette smoking were significantly decreased. Mean systolic blood pressure decreased between 1999 and 2010, but then increased after 2010.
The mean age- and sex-adjusted estimated 10-year risk for atherosclerotic cardiovascular disease (ASCVD) was consistently higher in Black participants vs. White participants, but the difference was attenuated after further adjusting for education, income, home ownership, employment, health insurance, and access to health care.
“These findings are helpful to guide the development of national public health policies for targeted interventions aimed at eliminating health disparities,” Jiang He, MD, PhD, Joseph S. Copes Chair and professor of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
“Interventions on social determinants of cardiovascular health should be tested in rigorous designed intervention trials,” said Dr. He, director of the Tulane University Translational Science Institute.
The study was published online Oct. 5 in JAMA.
‘Flattened’ CVD mortality?
Recent data show that the CVD mortality rate flattened, while the total number of cardiovascular deaths increased in the U.S. general population from 2010 to 2018, “but the reasons for this deceleration in the decline of CVD mortality are not entirely understood,” Dr. He said.
Moreover, “racial and ethnic differences in CVD mortality persist in the U.S. general population [but] the secular trends of cardiovascular risk factors among U.S. subpopulations with various racial and ethnic backgrounds and socioeconomic status are [also] not well understood,” he added. The effects of social determinants of health, such as education, income, home ownership, employment, health insurance, and access to health care on racial/ethnic differences in CVD risk, “are not well documented.”
To investigate these questions, the researchers drew on data from NHANES, a series of cross-sectional surveys in nationally representative samples of the U.S. population aged 20 years and older. The surveys are conducted in 2-year cycles and include data from 10 cycles conducted from 1999-2000 to 2017-2018 (n = 50,571, mean age 49.0-51.8 years; 48.2%-51.3% female).
Every 2 years, participants provided sociodemographic information, including age, race/ethnicity, sex, education, income, employment, housing, health insurance, and access to health care, as well as medical history and medication use. They underwent a physical examination that included weight and height, blood pressure, lipid levels, plasma glucose, and hemoglobin A1c.
Social determinants of health
Between 1999-2000 and 2017-2018, age- and sex-adjusted mean BMI and hemoglobin A1c increased, while mean serum total cholesterol and prevalence of smoking decreased (all P < .001).
Age- and sex-adjusted 10-year atherosclerotic cardiovascular disease (ASCVD) risk decreased from 7.6% (6.9%-8.2%) in 1999-2000 to 6.5% (6.1%-6.8%) in 2011-2012, with no significant changes thereafter.
When the researchers looked at specific racial and ethnic groups, they found that age- and sex-adjusted BMI, systolic BP, and hemoglobin A1c were “consistently higher” in non-Hispanic Black participants compared with non-Hispanic White participants, but total cholesterol was lower (all P < .001).
Participants with at least a college education or high family income had “consistently lower levels” of cardiovascular risk factors. And although the mean age- and sex-adjusted 10-year risk for ASCVD was significantly higher in non-Hispanic Black vs. non-Hispanic White participants (difference, 1.4% [1.0%-1.7%] in 1999-2008 and 2.0% [1.7%-2.4%] in 2009-2018), the difference was attenuated (by –0.3% in 1999-2008 and 0.7% in 2009-2018) after the researchers further adjusted for education, income, home ownership, employment, health insurance, and access to health care.
The differences in cardiovascular risk factors between Black and White participants “may have been moderated by social determinants of health,” the authors noted.
Provide appropriate education
Commenting on the study in an interview, Mary Ann McLaughlin, MD, MPH, associate professor of medicine, cardiology, Icahn School of Medicine at Mount Sinai, New York, pointed out that two important cardiovascular risk factors associated with being overweight – hypertension and diabetes – remained higher in the Black population compared with the White population in this analysis.
“Physicians and health care systems should provide appropriate education and resources regarding risk factor modification regarding diet, exercise, and blood pressure control,” advised Dr. McLaughlin, who was not involved with the study.
“Importantly, smoking rates and cholesterol levels are lower in the Black population, compared to the White population, when adjusted for many important socioeconomic factors,” she pointed out.
Dr. McLaughlin added that other “important social determinants of health, such as neighborhood and access to healthy food, were not measured and should be addressed by physicians when optimizing cardiovascular risk.”
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute and by the National Institute of General Medical Sciences. One of the researchers, Joshua D. Bundy, PhD, was supported by a grant from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. He and the other coauthors and Dr. McLaughlin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed 20 years of data on over 50,500 U.S. adults drawn from the National Health and Nutrition Examination Surveys (NHANES) and found that, in the overall population, body mass index and hemoglobin A1c were significantly increased between 1999 and 2018, while serum total cholesterol and cigarette smoking were significantly decreased. Mean systolic blood pressure decreased between 1999 and 2010, but then increased after 2010.
The mean age- and sex-adjusted estimated 10-year risk for atherosclerotic cardiovascular disease (ASCVD) was consistently higher in Black participants vs. White participants, but the difference was attenuated after further adjusting for education, income, home ownership, employment, health insurance, and access to health care.
“These findings are helpful to guide the development of national public health policies for targeted interventions aimed at eliminating health disparities,” Jiang He, MD, PhD, Joseph S. Copes Chair and professor of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, said in an interview.
“Interventions on social determinants of cardiovascular health should be tested in rigorous designed intervention trials,” said Dr. He, director of the Tulane University Translational Science Institute.
The study was published online Oct. 5 in JAMA.
‘Flattened’ CVD mortality?
Recent data show that the CVD mortality rate flattened, while the total number of cardiovascular deaths increased in the U.S. general population from 2010 to 2018, “but the reasons for this deceleration in the decline of CVD mortality are not entirely understood,” Dr. He said.
Moreover, “racial and ethnic differences in CVD mortality persist in the U.S. general population [but] the secular trends of cardiovascular risk factors among U.S. subpopulations with various racial and ethnic backgrounds and socioeconomic status are [also] not well understood,” he added. The effects of social determinants of health, such as education, income, home ownership, employment, health insurance, and access to health care on racial/ethnic differences in CVD risk, “are not well documented.”
To investigate these questions, the researchers drew on data from NHANES, a series of cross-sectional surveys in nationally representative samples of the U.S. population aged 20 years and older. The surveys are conducted in 2-year cycles and include data from 10 cycles conducted from 1999-2000 to 2017-2018 (n = 50,571, mean age 49.0-51.8 years; 48.2%-51.3% female).
Every 2 years, participants provided sociodemographic information, including age, race/ethnicity, sex, education, income, employment, housing, health insurance, and access to health care, as well as medical history and medication use. They underwent a physical examination that included weight and height, blood pressure, lipid levels, plasma glucose, and hemoglobin A1c.
Social determinants of health
Between 1999-2000 and 2017-2018, age- and sex-adjusted mean BMI and hemoglobin A1c increased, while mean serum total cholesterol and prevalence of smoking decreased (all P < .001).
Age- and sex-adjusted 10-year atherosclerotic cardiovascular disease (ASCVD) risk decreased from 7.6% (6.9%-8.2%) in 1999-2000 to 6.5% (6.1%-6.8%) in 2011-2012, with no significant changes thereafter.
When the researchers looked at specific racial and ethnic groups, they found that age- and sex-adjusted BMI, systolic BP, and hemoglobin A1c were “consistently higher” in non-Hispanic Black participants compared with non-Hispanic White participants, but total cholesterol was lower (all P < .001).
Participants with at least a college education or high family income had “consistently lower levels” of cardiovascular risk factors. And although the mean age- and sex-adjusted 10-year risk for ASCVD was significantly higher in non-Hispanic Black vs. non-Hispanic White participants (difference, 1.4% [1.0%-1.7%] in 1999-2008 and 2.0% [1.7%-2.4%] in 2009-2018), the difference was attenuated (by –0.3% in 1999-2008 and 0.7% in 2009-2018) after the researchers further adjusted for education, income, home ownership, employment, health insurance, and access to health care.
The differences in cardiovascular risk factors between Black and White participants “may have been moderated by social determinants of health,” the authors noted.
Provide appropriate education
Commenting on the study in an interview, Mary Ann McLaughlin, MD, MPH, associate professor of medicine, cardiology, Icahn School of Medicine at Mount Sinai, New York, pointed out that two important cardiovascular risk factors associated with being overweight – hypertension and diabetes – remained higher in the Black population compared with the White population in this analysis.
“Physicians and health care systems should provide appropriate education and resources regarding risk factor modification regarding diet, exercise, and blood pressure control,” advised Dr. McLaughlin, who was not involved with the study.
“Importantly, smoking rates and cholesterol levels are lower in the Black population, compared to the White population, when adjusted for many important socioeconomic factors,” she pointed out.
Dr. McLaughlin added that other “important social determinants of health, such as neighborhood and access to healthy food, were not measured and should be addressed by physicians when optimizing cardiovascular risk.”
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute and by the National Institute of General Medical Sciences. One of the researchers, Joshua D. Bundy, PhD, was supported by a grant from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. He and the other coauthors and Dr. McLaughlin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy not warranted in COVID-19 outpatients
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
USPSTF statement on aspirin: poor messaging at best
: “The USPSTF concludes with moderate certainty that initiating aspirin use for the primary prevention of CVD events in adults age 60 years or older has no net benefit.” I take no issue with the data and appreciate the efforts of the researchers, but at a minimum the public statement is incomplete. At most, it’s dangerously poor messaging.
As physicians, we understand how best to apply this information, but most laypeople, some at significant cardiovascular risk, closed their medicine cabinets this morning and left their aspirin bottle unopened on the shelf. Some of these patients have never spent an hour in the hospital for cardiac-related issues, but they have mitigated their risk for myocardial infarction by purposely poisoning their platelets daily with 81 mg of aspirin. And they should continue to do so.
Don’t forget the calcium score
Take, for instance, my patient Jack, who is typical of many patients I’ve seen throughout the years. Jack is 68 years old and has never had a cardiac event or a gastrointestinal bleed. His daily routine includes a walk, a statin, and a baby aspirin because his CT coronary artery calcium (CAC) score was 10,000 at age 58.
He first visited me 10 years ago because his father died of a myocardial infarction in his late 50s. Jack’s left ventricular ejection fraction is normal and his stress ECG shows 1-mm ST-segment depression at 8 minutes on a Bruce protocol stress test, without angina. Because Jack is well-educated and keeps up with the latest cardiology recommendations, he is precisely the type of patient who may be harmed by this new USPSTF statement by stopping his aspirin.
In October 2020, an analysis from the DALLAS Heart Study showed that persons with a CAC score greater than 100 had a higher cumulative incidence of bleeding and of atherosclerotic cardiovascular disease (ASCVD) events compared with those with no coronary calcium. After adjustment for clinical risk factors, the association between CAC and bleeding was attenuated and no longer statistically significant, whereas the relationship between CAC and ASCVD remained.
I asked one of the investigators, Amit Khera, MD, MSc, from UT Southwestern Medical Center, about the latest recommendations. He emphasized that both the American College of Cardiology/American Heart Association prevention guidelines and the USPSTF statement say that aspirin could still be considered among patients who are at higher risk for cardiovascular events. The USPSTF delineated this as a 10-year ASCVD risk greater than 10%.
Dr. Khera, who was an author of the 2019 guidelines, explained that the guideline committee purposely did not make specific recommendations as to what demarcated higher risk because the data were not clear at that time. Since then, a couple of papers, including the Dallas Heart Study analysis published in JAMA Cardiology, showed that patients at low bleeding risk with a calcium score above 100 may get a net benefit from aspirin. “Thus, in my patients who have a high calcium score and low bleeding risk, I do discuss the option to start or continue aspirin,” he said.
One size does not fit all
I watched ABC World News Tonight on Tuesday, October 12, and was immediately troubled about the coverage of the USPSTF statement. With viewership for the “Big Three” networks in the millions, the message to discontinue aspirin may have unintended consequences for many at-risk patients. The blood-thinning effects of a single dose of aspirin last about 10 days; it will be interesting to see if the rates of myocardial infarction increase over time. This could have been avoided with a better-worded statement – I’m concerned that the lack of nuance could spell big trouble for some.
In JAMA Cardiology, Dr. Khera and colleagues wrote that, “Aspirin use is not a one-size-fits-all therapy.” All physicians likely agree with that opinion. The USPSTF statement should have included the point that if you have a high CT coronary artery calcium score and a low bleeding risk, aspirin still fits very well even if you haven’t experienced a cardiac event. At a minimum, the USPSTF statement should have included the suggestion for patients to consult their physician for advice before discontinuing aspirin therapy.
I hope patients like Jack get the right message.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology.
A version of this article first appeared on Medscape.com.
: “The USPSTF concludes with moderate certainty that initiating aspirin use for the primary prevention of CVD events in adults age 60 years or older has no net benefit.” I take no issue with the data and appreciate the efforts of the researchers, but at a minimum the public statement is incomplete. At most, it’s dangerously poor messaging.
As physicians, we understand how best to apply this information, but most laypeople, some at significant cardiovascular risk, closed their medicine cabinets this morning and left their aspirin bottle unopened on the shelf. Some of these patients have never spent an hour in the hospital for cardiac-related issues, but they have mitigated their risk for myocardial infarction by purposely poisoning their platelets daily with 81 mg of aspirin. And they should continue to do so.
Don’t forget the calcium score
Take, for instance, my patient Jack, who is typical of many patients I’ve seen throughout the years. Jack is 68 years old and has never had a cardiac event or a gastrointestinal bleed. His daily routine includes a walk, a statin, and a baby aspirin because his CT coronary artery calcium (CAC) score was 10,000 at age 58.
He first visited me 10 years ago because his father died of a myocardial infarction in his late 50s. Jack’s left ventricular ejection fraction is normal and his stress ECG shows 1-mm ST-segment depression at 8 minutes on a Bruce protocol stress test, without angina. Because Jack is well-educated and keeps up with the latest cardiology recommendations, he is precisely the type of patient who may be harmed by this new USPSTF statement by stopping his aspirin.
In October 2020, an analysis from the DALLAS Heart Study showed that persons with a CAC score greater than 100 had a higher cumulative incidence of bleeding and of atherosclerotic cardiovascular disease (ASCVD) events compared with those with no coronary calcium. After adjustment for clinical risk factors, the association between CAC and bleeding was attenuated and no longer statistically significant, whereas the relationship between CAC and ASCVD remained.
I asked one of the investigators, Amit Khera, MD, MSc, from UT Southwestern Medical Center, about the latest recommendations. He emphasized that both the American College of Cardiology/American Heart Association prevention guidelines and the USPSTF statement say that aspirin could still be considered among patients who are at higher risk for cardiovascular events. The USPSTF delineated this as a 10-year ASCVD risk greater than 10%.
Dr. Khera, who was an author of the 2019 guidelines, explained that the guideline committee purposely did not make specific recommendations as to what demarcated higher risk because the data were not clear at that time. Since then, a couple of papers, including the Dallas Heart Study analysis published in JAMA Cardiology, showed that patients at low bleeding risk with a calcium score above 100 may get a net benefit from aspirin. “Thus, in my patients who have a high calcium score and low bleeding risk, I do discuss the option to start or continue aspirin,” he said.
One size does not fit all
I watched ABC World News Tonight on Tuesday, October 12, and was immediately troubled about the coverage of the USPSTF statement. With viewership for the “Big Three” networks in the millions, the message to discontinue aspirin may have unintended consequences for many at-risk patients. The blood-thinning effects of a single dose of aspirin last about 10 days; it will be interesting to see if the rates of myocardial infarction increase over time. This could have been avoided with a better-worded statement – I’m concerned that the lack of nuance could spell big trouble for some.
In JAMA Cardiology, Dr. Khera and colleagues wrote that, “Aspirin use is not a one-size-fits-all therapy.” All physicians likely agree with that opinion. The USPSTF statement should have included the point that if you have a high CT coronary artery calcium score and a low bleeding risk, aspirin still fits very well even if you haven’t experienced a cardiac event. At a minimum, the USPSTF statement should have included the suggestion for patients to consult their physician for advice before discontinuing aspirin therapy.
I hope patients like Jack get the right message.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology.
A version of this article first appeared on Medscape.com.
: “The USPSTF concludes with moderate certainty that initiating aspirin use for the primary prevention of CVD events in adults age 60 years or older has no net benefit.” I take no issue with the data and appreciate the efforts of the researchers, but at a minimum the public statement is incomplete. At most, it’s dangerously poor messaging.
As physicians, we understand how best to apply this information, but most laypeople, some at significant cardiovascular risk, closed their medicine cabinets this morning and left their aspirin bottle unopened on the shelf. Some of these patients have never spent an hour in the hospital for cardiac-related issues, but they have mitigated their risk for myocardial infarction by purposely poisoning their platelets daily with 81 mg of aspirin. And they should continue to do so.
Don’t forget the calcium score
Take, for instance, my patient Jack, who is typical of many patients I’ve seen throughout the years. Jack is 68 years old and has never had a cardiac event or a gastrointestinal bleed. His daily routine includes a walk, a statin, and a baby aspirin because his CT coronary artery calcium (CAC) score was 10,000 at age 58.
He first visited me 10 years ago because his father died of a myocardial infarction in his late 50s. Jack’s left ventricular ejection fraction is normal and his stress ECG shows 1-mm ST-segment depression at 8 minutes on a Bruce protocol stress test, without angina. Because Jack is well-educated and keeps up with the latest cardiology recommendations, he is precisely the type of patient who may be harmed by this new USPSTF statement by stopping his aspirin.
In October 2020, an analysis from the DALLAS Heart Study showed that persons with a CAC score greater than 100 had a higher cumulative incidence of bleeding and of atherosclerotic cardiovascular disease (ASCVD) events compared with those with no coronary calcium. After adjustment for clinical risk factors, the association between CAC and bleeding was attenuated and no longer statistically significant, whereas the relationship between CAC and ASCVD remained.
I asked one of the investigators, Amit Khera, MD, MSc, from UT Southwestern Medical Center, about the latest recommendations. He emphasized that both the American College of Cardiology/American Heart Association prevention guidelines and the USPSTF statement say that aspirin could still be considered among patients who are at higher risk for cardiovascular events. The USPSTF delineated this as a 10-year ASCVD risk greater than 10%.
Dr. Khera, who was an author of the 2019 guidelines, explained that the guideline committee purposely did not make specific recommendations as to what demarcated higher risk because the data were not clear at that time. Since then, a couple of papers, including the Dallas Heart Study analysis published in JAMA Cardiology, showed that patients at low bleeding risk with a calcium score above 100 may get a net benefit from aspirin. “Thus, in my patients who have a high calcium score and low bleeding risk, I do discuss the option to start or continue aspirin,” he said.
One size does not fit all
I watched ABC World News Tonight on Tuesday, October 12, and was immediately troubled about the coverage of the USPSTF statement. With viewership for the “Big Three” networks in the millions, the message to discontinue aspirin may have unintended consequences for many at-risk patients. The blood-thinning effects of a single dose of aspirin last about 10 days; it will be interesting to see if the rates of myocardial infarction increase over time. This could have been avoided with a better-worded statement – I’m concerned that the lack of nuance could spell big trouble for some.
In JAMA Cardiology, Dr. Khera and colleagues wrote that, “Aspirin use is not a one-size-fits-all therapy.” All physicians likely agree with that opinion. The USPSTF statement should have included the point that if you have a high CT coronary artery calcium score and a low bleeding risk, aspirin still fits very well even if you haven’t experienced a cardiac event. At a minimum, the USPSTF statement should have included the suggestion for patients to consult their physician for advice before discontinuing aspirin therapy.
I hope patients like Jack get the right message.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology.
A version of this article first appeared on Medscape.com.
Broken heart syndrome: on the rise, especially in women 50-74
As a pediatric kidney doctor, Elaine S. Kamil, MD, is used to long hours helping children and teens with a variety of issues, some very serious, and also makes time to give back to her specialty.
In late 2013, she was in Washington, D.C., planning a meeting of the American Society of Nephrology. When the organizers decided at the last minute that another session was needed, she stayed late, putting it together. Then she hopped on a plane and returned home to Los Angeles on a Saturday night.
Right after midnight, Dr. Kamil knew something was wrong.
“I had really severe chest pain,” she says. “I have reflux, and I know what that feels like. This was much more intense. It really hurt.” She debated: “Should I wake up my husband?”
Soon, the pain got so bad, she had to.
At the hospital, an electrocardiogram was slightly abnormal, as was a blood test that measures damage to the heart. Next, she got an angiogram, an imaging technique to visualize the heart. Once doctors looked at the image on the screen during the angiogram, they knew the diagnosis: Broken heart syndrome, known medically as takotsubo cardiomyopathy or stress-induced cardiomyopathy. As the name suggests, it’s triggered by extreme stress or loss.
The telltale clue to the diagnosis is the appearance of the walls of the heart’s left ventricle, its main pumping chamber. When the condition is present, the left ventricle changes shape, developing a narrow neck and a round bottom, resembling an octopus pot called takotsubo used by fishermen in Japan, where the condition was first recognized in 1990.
Like most who are affected, Dr. Kamil, now 74, is fine now. She is still actively working, as a researcher and professor emerita at Cedars-Sinai Medical Center and a health sciences clinical professor of pediatrics at UCLA. But she focuses more now on stress reduction.
Study: condition on the rise
New research from Cedars-Sinai suggests that broken heart syndrome, while still not common, is not as rare as once thought. And it’s on the rise, especially among middle-age and older women.
This ‘’middle” group – women ages 50 to 74 – had the greatest rate of increase over the years studied, 2006-2017, says Susan Cheng, MD, lead author of the study, published in the Journal of the American Heart Association. She is the director of the Institute for Research on Healthy Aging at the Smidt Heart Institute at Cedars-Sinai Medical Center.
Dr. Cheng and her team used national hospital inpatient data collected from more than 135,000 men and women diagnosed with the condition during the 12 years of the study. More than 88% of all cases were women, especially in those age 50 or older. When the researchers looked more closely, they found the diagnosis has been increasing at least 6 to 10 times more rapidly for women in the 50-to-74 age group than in any other group.
For every case of the condition in younger women, or in men of all age groups, the researchers found an additional 10 cases for middle-aged women and six additional cases for older women. For example, while the syndrome occurred in 15 younger women per million per year, it occurred in 128 middle aged women per year.
The age groups found most at risk was surprising, says Dr. Cheng, who expected the risk would be highest in the oldest age group of women, those over 75.
While doctors are more aware of the condition now, “it’s not just the increased recognition,” she says. “There is something going on” driving the continual increase. It probably has something to do with environmental changes, she says.
Hormones and hormonal differences between men and women aren’t the whole story either, she says. Her team will study it further, hoping eventually to find who might be more likely to get the condition by talking to those who have had it and collecting clues. “There probably is some underlying genetic predisposition,” she says.
“The neural hormones that drive the flight-or-fight response (such as adrenaline) are definitely elevated,” she says. “The brain and the heart are talking to each other.”
Experts say these surging stress hormones essentially “stun” the heart, affecting how it functions. The question is, what makes women particularly more susceptible to being excessively triggered when exposed to stress? That is unclear, Dr. Cheng says.
While the condition is a frightening experience, ‘’the overall prognosis is much better than having a garden-variety heart attack,” she says.
But researchers are still figuring out long-term outcomes, and she can’t tell patients if they are likely to have another episode.
Research findings reflected in practice
Other cardiologists say they are not surprised by the new findings.
“I think it’s very consistent with what I am seeing clinically,” says Tracy Stevens, MD, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, MO. In the last 5 years, she has diagnosed at least 100 cases, she says. The increase is partly but not entirely due to increased awareness by doctors of the condition, she agrees.
If a postmenopausal woman comes to the hospital with chest pain, the condition is more likely now than in the past to be suspected, says Dr. Stevens, who’s also the medical director of the Muriel I. Kauffman Women’s Heart Center at Saint Luke’s. The octopus pot-like image is hard to miss.
“What we see at the base of the left ventricle is, it is squeezing like crazy, it is ballooning.”
“We probably see at least five to ten a month,” says Kevin Bybee, MD, an associate professor of medicine at the University of Missouri-Kansas City School of Medicine.
The increase in numbers found by the Los Angeles researchers may not even capture the true picture of how many people have gotten this condition, he says. He suspects some women whose deaths are blamed on sudden cardiac death might actually have had broken heart syndrome.
“I have always wondered how many don’t make it to the hospital.”
Dr. Bybee, who’s also medical director of cardiovascular services at St. Luke’s South in Overland Park, KS, became interested in the syndrome during his fellowship at Mayo Clinic when he diagnosed three patients in just 2 months. He and his team published the case histories of seven patients in 2004. Since then, many more reports have been published.
Researchers from Texas used the same national database as the Cedars researchers to look at cases from 2005 to 2014, and also found an increase. But study co-author Abhijeet Dhoble, MD, a cardiologist and associate professor of medicine at UT Health Science Center and Memorial Hermann-Texas Medical Center in Houston, believes more recognition explains most of the increase.
And the pandemic is now playing a role in driving up cases, he says.
“In the last 2 years, we have been noticing increasing numbers of cases, probably due to the pandemic,” he says.
Profiles of cases
Over the years, Dr. Bybee has collected information on what is happening before the heart begins to go haywire.
“Fifteen to twenty percent of the time, there is no obvious trigger,” he says.
Other times, a stressful emotional event, such as the death of a spouse or a severe car accident, can trigger it.
One patient with an extreme fear of public speaking had to give a talk in front of a large group when she was new to a job. Another woman lost money at a casino before it happened, Dr. Bybee says. Yet another patient took her dog out for a walk in the woods, and the dog got caught in a raccoon trap.
Fierce arguments as well as surprise parties have triggered the condition, Dr. Bybee says. Physical problems such as asthma or sepsis, a life-threatening complication of an infection, can also trigger broken heart.
“It’s challenging because this is unpredictable,” he says.
Treatments and recovery
The condition is rarely fatal, say experts from Harvard and Mayo Clinic, but some can have complications such as heart failure.
There are no standard guidelines for treatment, Dr. Dhoble, of Memorial Hermann, says. “We give medications to keep blood pressures in the optimal range.” Doctors may also prescribe lipid-lowering medicines and blood thinner medications. “Most patients recover within 3 to 7 days.”
“Usually within a month, their [heart] function returns to normal,” Dr. Stevens says.
Getting one’s full energy back can take longer, as Dr. Kamil found. “It was about 6 months before I was up to speed.”
Survivors talk
Looking back, Dr. Kamil realizes now how much stress she was under before her episode.
“I took care of chronically ill kids,” she says, and worried about them. “I’m kind of a mother hen.”
Besides patient care and her cross-county meeting planning, she was flying back and forth to Florida to tend to her mother, who had chronic health problems. She was also managing that year’s annual media prize at a San Diego university that she and her husband established after the death of their adult son several years before.
“I was busy with that, and it is a bittersweet experience,” she says.
She is trying to take her cardiologist’s advice to slow down.
“I used to be notorious for saying, ‘I need to get one more thing done,’” she says.
Joanie Simpson says she, too, has slowed down. She was diagnosed with broken heart in 2016, after a cascade of stressful events. Her son was facing back surgery, her son-in-law had lost his job, and her tiny Yorkshire terrier Meha died. And she and her husband, Benny, had issues with their rental property.
Now 66 and retired in Camp Wood, Texas, she has learned to enjoy life and worry a little less. Music is one way.
“We’re Parrotheads,” she says, referencing the nickname given to fans of singer Jimmy Buffett. “We listen to Buffett and to ’60s, ’70s, ’80s music. We dance around the house. We aren’t big tavern goers, so we dance around the living room and hope we don’t fall over the coffee table. So far, so good.”
They have plans to buy a small pontoon boat and go fishing. Benny especially loves that idea, she says, laughing, as he finds it’s the only time she stops talking.
Reducing the what-ifs
Patients have a common question and worry: What if it happens again?
“I definitely worried more about it in the beginning,” Dr. Kamil says. “Could I have permanent heart damage? Will I be a cardiac cripple?” Her worry has eased.
If you suspect the condition, ‘’get yourself to a provider who knows about it,” she says.
Cardiologists are very likely to suspect the condition, Dr. Bybee says, as are doctors working in a large-volume emergency department.
Dr. Stevens, of St. Luke’s, is straightforward, telling her patients what is known and what is not about the condition. She recommends her patients go to cardiac rehab.
“It gives them that confidence to know what they can do,” she says.
She also gives lifestyle advice, suggesting patients get a home blood pressure cuff and use it. She suggests paying attention to good nutrition and exercise and not lifting anything so heavy that grunting is necessary.
Focus on protecting heart health, Dr. Cheng tells patients. She encourages them to find the stress reduction plan that works for them. Most important, she tells patients to understand that it is not their fault.
A version of this article first appeared on WebMD.com.
As a pediatric kidney doctor, Elaine S. Kamil, MD, is used to long hours helping children and teens with a variety of issues, some very serious, and also makes time to give back to her specialty.
In late 2013, she was in Washington, D.C., planning a meeting of the American Society of Nephrology. When the organizers decided at the last minute that another session was needed, she stayed late, putting it together. Then she hopped on a plane and returned home to Los Angeles on a Saturday night.
Right after midnight, Dr. Kamil knew something was wrong.
“I had really severe chest pain,” she says. “I have reflux, and I know what that feels like. This was much more intense. It really hurt.” She debated: “Should I wake up my husband?”
Soon, the pain got so bad, she had to.
At the hospital, an electrocardiogram was slightly abnormal, as was a blood test that measures damage to the heart. Next, she got an angiogram, an imaging technique to visualize the heart. Once doctors looked at the image on the screen during the angiogram, they knew the diagnosis: Broken heart syndrome, known medically as takotsubo cardiomyopathy or stress-induced cardiomyopathy. As the name suggests, it’s triggered by extreme stress or loss.
The telltale clue to the diagnosis is the appearance of the walls of the heart’s left ventricle, its main pumping chamber. When the condition is present, the left ventricle changes shape, developing a narrow neck and a round bottom, resembling an octopus pot called takotsubo used by fishermen in Japan, where the condition was first recognized in 1990.
Like most who are affected, Dr. Kamil, now 74, is fine now. She is still actively working, as a researcher and professor emerita at Cedars-Sinai Medical Center and a health sciences clinical professor of pediatrics at UCLA. But she focuses more now on stress reduction.
Study: condition on the rise
New research from Cedars-Sinai suggests that broken heart syndrome, while still not common, is not as rare as once thought. And it’s on the rise, especially among middle-age and older women.
This ‘’middle” group – women ages 50 to 74 – had the greatest rate of increase over the years studied, 2006-2017, says Susan Cheng, MD, lead author of the study, published in the Journal of the American Heart Association. She is the director of the Institute for Research on Healthy Aging at the Smidt Heart Institute at Cedars-Sinai Medical Center.
Dr. Cheng and her team used national hospital inpatient data collected from more than 135,000 men and women diagnosed with the condition during the 12 years of the study. More than 88% of all cases were women, especially in those age 50 or older. When the researchers looked more closely, they found the diagnosis has been increasing at least 6 to 10 times more rapidly for women in the 50-to-74 age group than in any other group.
For every case of the condition in younger women, or in men of all age groups, the researchers found an additional 10 cases for middle-aged women and six additional cases for older women. For example, while the syndrome occurred in 15 younger women per million per year, it occurred in 128 middle aged women per year.
The age groups found most at risk was surprising, says Dr. Cheng, who expected the risk would be highest in the oldest age group of women, those over 75.
While doctors are more aware of the condition now, “it’s not just the increased recognition,” she says. “There is something going on” driving the continual increase. It probably has something to do with environmental changes, she says.
Hormones and hormonal differences between men and women aren’t the whole story either, she says. Her team will study it further, hoping eventually to find who might be more likely to get the condition by talking to those who have had it and collecting clues. “There probably is some underlying genetic predisposition,” she says.
“The neural hormones that drive the flight-or-fight response (such as adrenaline) are definitely elevated,” she says. “The brain and the heart are talking to each other.”
Experts say these surging stress hormones essentially “stun” the heart, affecting how it functions. The question is, what makes women particularly more susceptible to being excessively triggered when exposed to stress? That is unclear, Dr. Cheng says.
While the condition is a frightening experience, ‘’the overall prognosis is much better than having a garden-variety heart attack,” she says.
But researchers are still figuring out long-term outcomes, and she can’t tell patients if they are likely to have another episode.
Research findings reflected in practice
Other cardiologists say they are not surprised by the new findings.
“I think it’s very consistent with what I am seeing clinically,” says Tracy Stevens, MD, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, MO. In the last 5 years, she has diagnosed at least 100 cases, she says. The increase is partly but not entirely due to increased awareness by doctors of the condition, she agrees.
If a postmenopausal woman comes to the hospital with chest pain, the condition is more likely now than in the past to be suspected, says Dr. Stevens, who’s also the medical director of the Muriel I. Kauffman Women’s Heart Center at Saint Luke’s. The octopus pot-like image is hard to miss.
“What we see at the base of the left ventricle is, it is squeezing like crazy, it is ballooning.”
“We probably see at least five to ten a month,” says Kevin Bybee, MD, an associate professor of medicine at the University of Missouri-Kansas City School of Medicine.
The increase in numbers found by the Los Angeles researchers may not even capture the true picture of how many people have gotten this condition, he says. He suspects some women whose deaths are blamed on sudden cardiac death might actually have had broken heart syndrome.
“I have always wondered how many don’t make it to the hospital.”
Dr. Bybee, who’s also medical director of cardiovascular services at St. Luke’s South in Overland Park, KS, became interested in the syndrome during his fellowship at Mayo Clinic when he diagnosed three patients in just 2 months. He and his team published the case histories of seven patients in 2004. Since then, many more reports have been published.
Researchers from Texas used the same national database as the Cedars researchers to look at cases from 2005 to 2014, and also found an increase. But study co-author Abhijeet Dhoble, MD, a cardiologist and associate professor of medicine at UT Health Science Center and Memorial Hermann-Texas Medical Center in Houston, believes more recognition explains most of the increase.
And the pandemic is now playing a role in driving up cases, he says.
“In the last 2 years, we have been noticing increasing numbers of cases, probably due to the pandemic,” he says.
Profiles of cases
Over the years, Dr. Bybee has collected information on what is happening before the heart begins to go haywire.
“Fifteen to twenty percent of the time, there is no obvious trigger,” he says.
Other times, a stressful emotional event, such as the death of a spouse or a severe car accident, can trigger it.
One patient with an extreme fear of public speaking had to give a talk in front of a large group when she was new to a job. Another woman lost money at a casino before it happened, Dr. Bybee says. Yet another patient took her dog out for a walk in the woods, and the dog got caught in a raccoon trap.
Fierce arguments as well as surprise parties have triggered the condition, Dr. Bybee says. Physical problems such as asthma or sepsis, a life-threatening complication of an infection, can also trigger broken heart.
“It’s challenging because this is unpredictable,” he says.
Treatments and recovery
The condition is rarely fatal, say experts from Harvard and Mayo Clinic, but some can have complications such as heart failure.
There are no standard guidelines for treatment, Dr. Dhoble, of Memorial Hermann, says. “We give medications to keep blood pressures in the optimal range.” Doctors may also prescribe lipid-lowering medicines and blood thinner medications. “Most patients recover within 3 to 7 days.”
“Usually within a month, their [heart] function returns to normal,” Dr. Stevens says.
Getting one’s full energy back can take longer, as Dr. Kamil found. “It was about 6 months before I was up to speed.”
Survivors talk
Looking back, Dr. Kamil realizes now how much stress she was under before her episode.
“I took care of chronically ill kids,” she says, and worried about them. “I’m kind of a mother hen.”
Besides patient care and her cross-county meeting planning, she was flying back and forth to Florida to tend to her mother, who had chronic health problems. She was also managing that year’s annual media prize at a San Diego university that she and her husband established after the death of their adult son several years before.
“I was busy with that, and it is a bittersweet experience,” she says.
She is trying to take her cardiologist’s advice to slow down.
“I used to be notorious for saying, ‘I need to get one more thing done,’” she says.
Joanie Simpson says she, too, has slowed down. She was diagnosed with broken heart in 2016, after a cascade of stressful events. Her son was facing back surgery, her son-in-law had lost his job, and her tiny Yorkshire terrier Meha died. And she and her husband, Benny, had issues with their rental property.
Now 66 and retired in Camp Wood, Texas, she has learned to enjoy life and worry a little less. Music is one way.
“We’re Parrotheads,” she says, referencing the nickname given to fans of singer Jimmy Buffett. “We listen to Buffett and to ’60s, ’70s, ’80s music. We dance around the house. We aren’t big tavern goers, so we dance around the living room and hope we don’t fall over the coffee table. So far, so good.”
They have plans to buy a small pontoon boat and go fishing. Benny especially loves that idea, she says, laughing, as he finds it’s the only time she stops talking.
Reducing the what-ifs
Patients have a common question and worry: What if it happens again?
“I definitely worried more about it in the beginning,” Dr. Kamil says. “Could I have permanent heart damage? Will I be a cardiac cripple?” Her worry has eased.
If you suspect the condition, ‘’get yourself to a provider who knows about it,” she says.
Cardiologists are very likely to suspect the condition, Dr. Bybee says, as are doctors working in a large-volume emergency department.
Dr. Stevens, of St. Luke’s, is straightforward, telling her patients what is known and what is not about the condition. She recommends her patients go to cardiac rehab.
“It gives them that confidence to know what they can do,” she says.
She also gives lifestyle advice, suggesting patients get a home blood pressure cuff and use it. She suggests paying attention to good nutrition and exercise and not lifting anything so heavy that grunting is necessary.
Focus on protecting heart health, Dr. Cheng tells patients. She encourages them to find the stress reduction plan that works for them. Most important, she tells patients to understand that it is not their fault.
A version of this article first appeared on WebMD.com.
As a pediatric kidney doctor, Elaine S. Kamil, MD, is used to long hours helping children and teens with a variety of issues, some very serious, and also makes time to give back to her specialty.
In late 2013, she was in Washington, D.C., planning a meeting of the American Society of Nephrology. When the organizers decided at the last minute that another session was needed, she stayed late, putting it together. Then she hopped on a plane and returned home to Los Angeles on a Saturday night.
Right after midnight, Dr. Kamil knew something was wrong.
“I had really severe chest pain,” she says. “I have reflux, and I know what that feels like. This was much more intense. It really hurt.” She debated: “Should I wake up my husband?”
Soon, the pain got so bad, she had to.
At the hospital, an electrocardiogram was slightly abnormal, as was a blood test that measures damage to the heart. Next, she got an angiogram, an imaging technique to visualize the heart. Once doctors looked at the image on the screen during the angiogram, they knew the diagnosis: Broken heart syndrome, known medically as takotsubo cardiomyopathy or stress-induced cardiomyopathy. As the name suggests, it’s triggered by extreme stress or loss.
The telltale clue to the diagnosis is the appearance of the walls of the heart’s left ventricle, its main pumping chamber. When the condition is present, the left ventricle changes shape, developing a narrow neck and a round bottom, resembling an octopus pot called takotsubo used by fishermen in Japan, where the condition was first recognized in 1990.
Like most who are affected, Dr. Kamil, now 74, is fine now. She is still actively working, as a researcher and professor emerita at Cedars-Sinai Medical Center and a health sciences clinical professor of pediatrics at UCLA. But she focuses more now on stress reduction.
Study: condition on the rise
New research from Cedars-Sinai suggests that broken heart syndrome, while still not common, is not as rare as once thought. And it’s on the rise, especially among middle-age and older women.
This ‘’middle” group – women ages 50 to 74 – had the greatest rate of increase over the years studied, 2006-2017, says Susan Cheng, MD, lead author of the study, published in the Journal of the American Heart Association. She is the director of the Institute for Research on Healthy Aging at the Smidt Heart Institute at Cedars-Sinai Medical Center.
Dr. Cheng and her team used national hospital inpatient data collected from more than 135,000 men and women diagnosed with the condition during the 12 years of the study. More than 88% of all cases were women, especially in those age 50 or older. When the researchers looked more closely, they found the diagnosis has been increasing at least 6 to 10 times more rapidly for women in the 50-to-74 age group than in any other group.
For every case of the condition in younger women, or in men of all age groups, the researchers found an additional 10 cases for middle-aged women and six additional cases for older women. For example, while the syndrome occurred in 15 younger women per million per year, it occurred in 128 middle aged women per year.
The age groups found most at risk was surprising, says Dr. Cheng, who expected the risk would be highest in the oldest age group of women, those over 75.
While doctors are more aware of the condition now, “it’s not just the increased recognition,” she says. “There is something going on” driving the continual increase. It probably has something to do with environmental changes, she says.
Hormones and hormonal differences between men and women aren’t the whole story either, she says. Her team will study it further, hoping eventually to find who might be more likely to get the condition by talking to those who have had it and collecting clues. “There probably is some underlying genetic predisposition,” she says.
“The neural hormones that drive the flight-or-fight response (such as adrenaline) are definitely elevated,” she says. “The brain and the heart are talking to each other.”
Experts say these surging stress hormones essentially “stun” the heart, affecting how it functions. The question is, what makes women particularly more susceptible to being excessively triggered when exposed to stress? That is unclear, Dr. Cheng says.
While the condition is a frightening experience, ‘’the overall prognosis is much better than having a garden-variety heart attack,” she says.
But researchers are still figuring out long-term outcomes, and she can’t tell patients if they are likely to have another episode.
Research findings reflected in practice
Other cardiologists say they are not surprised by the new findings.
“I think it’s very consistent with what I am seeing clinically,” says Tracy Stevens, MD, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, MO. In the last 5 years, she has diagnosed at least 100 cases, she says. The increase is partly but not entirely due to increased awareness by doctors of the condition, she agrees.
If a postmenopausal woman comes to the hospital with chest pain, the condition is more likely now than in the past to be suspected, says Dr. Stevens, who’s also the medical director of the Muriel I. Kauffman Women’s Heart Center at Saint Luke’s. The octopus pot-like image is hard to miss.
“What we see at the base of the left ventricle is, it is squeezing like crazy, it is ballooning.”
“We probably see at least five to ten a month,” says Kevin Bybee, MD, an associate professor of medicine at the University of Missouri-Kansas City School of Medicine.
The increase in numbers found by the Los Angeles researchers may not even capture the true picture of how many people have gotten this condition, he says. He suspects some women whose deaths are blamed on sudden cardiac death might actually have had broken heart syndrome.
“I have always wondered how many don’t make it to the hospital.”
Dr. Bybee, who’s also medical director of cardiovascular services at St. Luke’s South in Overland Park, KS, became interested in the syndrome during his fellowship at Mayo Clinic when he diagnosed three patients in just 2 months. He and his team published the case histories of seven patients in 2004. Since then, many more reports have been published.
Researchers from Texas used the same national database as the Cedars researchers to look at cases from 2005 to 2014, and also found an increase. But study co-author Abhijeet Dhoble, MD, a cardiologist and associate professor of medicine at UT Health Science Center and Memorial Hermann-Texas Medical Center in Houston, believes more recognition explains most of the increase.
And the pandemic is now playing a role in driving up cases, he says.
“In the last 2 years, we have been noticing increasing numbers of cases, probably due to the pandemic,” he says.
Profiles of cases
Over the years, Dr. Bybee has collected information on what is happening before the heart begins to go haywire.
“Fifteen to twenty percent of the time, there is no obvious trigger,” he says.
Other times, a stressful emotional event, such as the death of a spouse or a severe car accident, can trigger it.
One patient with an extreme fear of public speaking had to give a talk in front of a large group when she was new to a job. Another woman lost money at a casino before it happened, Dr. Bybee says. Yet another patient took her dog out for a walk in the woods, and the dog got caught in a raccoon trap.
Fierce arguments as well as surprise parties have triggered the condition, Dr. Bybee says. Physical problems such as asthma or sepsis, a life-threatening complication of an infection, can also trigger broken heart.
“It’s challenging because this is unpredictable,” he says.
Treatments and recovery
The condition is rarely fatal, say experts from Harvard and Mayo Clinic, but some can have complications such as heart failure.
There are no standard guidelines for treatment, Dr. Dhoble, of Memorial Hermann, says. “We give medications to keep blood pressures in the optimal range.” Doctors may also prescribe lipid-lowering medicines and blood thinner medications. “Most patients recover within 3 to 7 days.”
“Usually within a month, their [heart] function returns to normal,” Dr. Stevens says.
Getting one’s full energy back can take longer, as Dr. Kamil found. “It was about 6 months before I was up to speed.”
Survivors talk
Looking back, Dr. Kamil realizes now how much stress she was under before her episode.
“I took care of chronically ill kids,” she says, and worried about them. “I’m kind of a mother hen.”
Besides patient care and her cross-county meeting planning, she was flying back and forth to Florida to tend to her mother, who had chronic health problems. She was also managing that year’s annual media prize at a San Diego university that she and her husband established after the death of their adult son several years before.
“I was busy with that, and it is a bittersweet experience,” she says.
She is trying to take her cardiologist’s advice to slow down.
“I used to be notorious for saying, ‘I need to get one more thing done,’” she says.
Joanie Simpson says she, too, has slowed down. She was diagnosed with broken heart in 2016, after a cascade of stressful events. Her son was facing back surgery, her son-in-law had lost his job, and her tiny Yorkshire terrier Meha died. And she and her husband, Benny, had issues with their rental property.
Now 66 and retired in Camp Wood, Texas, she has learned to enjoy life and worry a little less. Music is one way.
“We’re Parrotheads,” she says, referencing the nickname given to fans of singer Jimmy Buffett. “We listen to Buffett and to ’60s, ’70s, ’80s music. We dance around the house. We aren’t big tavern goers, so we dance around the living room and hope we don’t fall over the coffee table. So far, so good.”
They have plans to buy a small pontoon boat and go fishing. Benny especially loves that idea, she says, laughing, as he finds it’s the only time she stops talking.
Reducing the what-ifs
Patients have a common question and worry: What if it happens again?
“I definitely worried more about it in the beginning,” Dr. Kamil says. “Could I have permanent heart damage? Will I be a cardiac cripple?” Her worry has eased.
If you suspect the condition, ‘’get yourself to a provider who knows about it,” she says.
Cardiologists are very likely to suspect the condition, Dr. Bybee says, as are doctors working in a large-volume emergency department.
Dr. Stevens, of St. Luke’s, is straightforward, telling her patients what is known and what is not about the condition. She recommends her patients go to cardiac rehab.
“It gives them that confidence to know what they can do,” she says.
She also gives lifestyle advice, suggesting patients get a home blood pressure cuff and use it. She suggests paying attention to good nutrition and exercise and not lifting anything so heavy that grunting is necessary.
Focus on protecting heart health, Dr. Cheng tells patients. She encourages them to find the stress reduction plan that works for them. Most important, she tells patients to understand that it is not their fault.
A version of this article first appeared on WebMD.com.
Estimating insulin resistance may help predict stroke, death in T2D
Calculating the estimated glucose disposal rate (eGDR) as a proxy for the level of insulin resistance may be useful way to determine if someone with type 2 diabetes (T2D) is at risk for having a first stroke, Swedish researchers have found.
In a large population-based study, the lower the eGDR score went, the higher the risk for having a first stroke became.
The eGDR score was also predictive of the chance of dying from any or a cardiovascular cause, Alexander Zabala, MD, reported at the annual meeting of the European Association for the Study of Diabetes (Abstract OP 01-4).
The link between insulin resistance and an increased risk for stroke has been known for some time, and not just in people with T2D. However, the current way of determining insulin resistance is not suitable for widespread practice.
“The goal standard technique for measuring insulin resistance is the euglycemic clamp method,” said Dr. Zabala, an internal medical resident at Södersjukhuset hospital and researcher at the Karolinska Institutet in Stockholm.
“For that reason, [the eGDR], a method based on readily available clinical factors – waist circumference, hypertension, and glycosylated hemoglobin was developed,” he explained. Body mass index can also be used in place of waist circumference, he qualified.
The eGDR has already been proven to be very precise in people with type 1 diabetes, said Dr. Zabala, and could be an “excellent tool to measure insulin resistance in a large patient population.”
Investigating the link between eGDR and first stroke risk
The aim of the study he presented was to see if changes in the eGDR were associated with changes in the risk of someone with T2D experiencing a first stroke, or dying from a cardiovascular or other cause.
An observational cohort was formed by first considering data on all adult patients with T2D who were logged in the Swedish National Diabetes Registry (NDR) during 2004-2016. Then anyone with a history of stroke, or with any missing data on the clinical variables needed to calculate the eGDR, were excluded.
This resulted in an overall population of 104,697 individuals, aged a mean of 63 years, who had developed T2D at around the age of 59 years. About 44% of the study population were women. The mean eGDR for the whole population was 5.6 mg/kg per min.
The study subjects were grouped according to four eGDR levels: 24,706 were in the lowest quartile of eGDR (less than 4 mg/kg per min), signifying the highest level of insulin resistance, and 18,762 were in the upper quartile of eGDR (greater than 8 mg/kg per min), signifying the lowest level of insulin resistance. The middle two groups had an eGDR between 4 and 6 mg/kg per min (40,187), and 6 and 8 mg/kg/min (21,042).
Data from the NDR were then combined with the Swedish Cause of Death register, the Swedish In-patient Care Diagnoses registry, and the Longitudinal Database for Health Insurance and Labour Market Studies (LISA) to determine the rates of stroke, ischemic stroke, hemorrhagic stroke, all-cause mortality, and cardiovascular mortality.
Increasing insulin resistance ups risk for stroke, death
After a median follow-up of 5.6 years, 4% (4,201) of the study population had had a stroke.
“We clearly see an increased occurrence of first-time stroke in the group with the lowest eGDR, indicating worst insulin resistance, in comparison with the group with the highest eGDR, indicating less insulin resistance,” Dr. Zabala reported.
After adjustment for potential confounding factors, including age at baseline, gender, diabetes duration, among other variables, the risk for stroke was lowest in those with a high eGDR value and highest for those with a low eGDR value.
Using individuals with the lowest eGDR (less than 4 mg/kg per min) and thus greatest risk of stroke as the reference, adjusted hazard ratios (aHR) for first-time stroke were: 0.60, 0.68, and 0.77 for those with an eGDR of greater than 8, 6-8, and 4-6 mg/kg per min, respectively.
The corresponding values for risk of ischemic stroke were 0.55, 0.68, and 0.75. Regarding hemorrhagic stroke, there was no statistically significant correlation between eGDR levels and stroke occurrence. This was due to the small number of cases recorded.
As for all-cause and cardiovascular mortality, a similar pattern was seen, with higher rates of death linked to increasing insulin resistance. Adjusted hazard ratios according to increasing insulin resistance (decreasing eGDR scores) for all-cause death were 0.68, 0.75, and 0.82 and for cardiovascular mortality were 0.65, 0.75, and 0.82.
A sensitivity analysis, using BMI instead of waist circumference to calculate the eGDR, showed a similar pattern, and “interestingly, a correlation between eGDR levels and risk of hemorrhagic stroke.” Dr. Zabala said.
Limitations and take-homes
Of course, this is an observational cohort study, so no conclusions on causality can be made and there are no data on the use of anti-diabetic treatments specifically. But there are strengths such as covering almost all adults with T2D in Sweden and a relatively long-follow-up time.
The findings suggest that “eGDR, which may reflect insulin resistance may be a useful risk marker for stroke and death in people with type 2 diabetes,” said Dr. Zabala.
“You had a very large cohort, and that certainly makes your results very valid,” observed Peter Novodvorsky, MUDr. (Hons), PhD, MRCP, a consultant diabetologist in Trenčín, Slovakia.
Dr. Novodvorsky, who chaired the session, picked up on the lack of information about how many people were taking newer diabetes drugs, such as the glucagon-like peptide 1 receptor antagonists and sodium glucose-lowering transport 2 inhibitors.
“As we all know, these might have protective effects which are not necessarily related to the glucose lowering or insulin resistance-lowering” effects, so could have influenced the results. In terms of how practical the eGDR is for clinical practice, Dr. Zabala observed in a press release: “eGDR could be used to help T2D patients better understand and manage their risk of stroke and death.
“It could also be of importance in research. In this era of personalized medicine, better stratification of type 2 diabetes patients will help optimize clinical trials and further vital research into treatment, diagnosis, care and prevention.”
The research was a collaboration between the Karolinska Institutet, Gothenburg University and the Swedish National Diabetes Registry. Dr. Zabala and coauthors reported having no conflicts of interest.
Calculating the estimated glucose disposal rate (eGDR) as a proxy for the level of insulin resistance may be useful way to determine if someone with type 2 diabetes (T2D) is at risk for having a first stroke, Swedish researchers have found.
In a large population-based study, the lower the eGDR score went, the higher the risk for having a first stroke became.
The eGDR score was also predictive of the chance of dying from any or a cardiovascular cause, Alexander Zabala, MD, reported at the annual meeting of the European Association for the Study of Diabetes (Abstract OP 01-4).
The link between insulin resistance and an increased risk for stroke has been known for some time, and not just in people with T2D. However, the current way of determining insulin resistance is not suitable for widespread practice.
“The goal standard technique for measuring insulin resistance is the euglycemic clamp method,” said Dr. Zabala, an internal medical resident at Södersjukhuset hospital and researcher at the Karolinska Institutet in Stockholm.
“For that reason, [the eGDR], a method based on readily available clinical factors – waist circumference, hypertension, and glycosylated hemoglobin was developed,” he explained. Body mass index can also be used in place of waist circumference, he qualified.
The eGDR has already been proven to be very precise in people with type 1 diabetes, said Dr. Zabala, and could be an “excellent tool to measure insulin resistance in a large patient population.”
Investigating the link between eGDR and first stroke risk
The aim of the study he presented was to see if changes in the eGDR were associated with changes in the risk of someone with T2D experiencing a first stroke, or dying from a cardiovascular or other cause.
An observational cohort was formed by first considering data on all adult patients with T2D who were logged in the Swedish National Diabetes Registry (NDR) during 2004-2016. Then anyone with a history of stroke, or with any missing data on the clinical variables needed to calculate the eGDR, were excluded.
This resulted in an overall population of 104,697 individuals, aged a mean of 63 years, who had developed T2D at around the age of 59 years. About 44% of the study population were women. The mean eGDR for the whole population was 5.6 mg/kg per min.
The study subjects were grouped according to four eGDR levels: 24,706 were in the lowest quartile of eGDR (less than 4 mg/kg per min), signifying the highest level of insulin resistance, and 18,762 were in the upper quartile of eGDR (greater than 8 mg/kg per min), signifying the lowest level of insulin resistance. The middle two groups had an eGDR between 4 and 6 mg/kg per min (40,187), and 6 and 8 mg/kg/min (21,042).
Data from the NDR were then combined with the Swedish Cause of Death register, the Swedish In-patient Care Diagnoses registry, and the Longitudinal Database for Health Insurance and Labour Market Studies (LISA) to determine the rates of stroke, ischemic stroke, hemorrhagic stroke, all-cause mortality, and cardiovascular mortality.
Increasing insulin resistance ups risk for stroke, death
After a median follow-up of 5.6 years, 4% (4,201) of the study population had had a stroke.
“We clearly see an increased occurrence of first-time stroke in the group with the lowest eGDR, indicating worst insulin resistance, in comparison with the group with the highest eGDR, indicating less insulin resistance,” Dr. Zabala reported.
After adjustment for potential confounding factors, including age at baseline, gender, diabetes duration, among other variables, the risk for stroke was lowest in those with a high eGDR value and highest for those with a low eGDR value.
Using individuals with the lowest eGDR (less than 4 mg/kg per min) and thus greatest risk of stroke as the reference, adjusted hazard ratios (aHR) for first-time stroke were: 0.60, 0.68, and 0.77 for those with an eGDR of greater than 8, 6-8, and 4-6 mg/kg per min, respectively.
The corresponding values for risk of ischemic stroke were 0.55, 0.68, and 0.75. Regarding hemorrhagic stroke, there was no statistically significant correlation between eGDR levels and stroke occurrence. This was due to the small number of cases recorded.
As for all-cause and cardiovascular mortality, a similar pattern was seen, with higher rates of death linked to increasing insulin resistance. Adjusted hazard ratios according to increasing insulin resistance (decreasing eGDR scores) for all-cause death were 0.68, 0.75, and 0.82 and for cardiovascular mortality were 0.65, 0.75, and 0.82.
A sensitivity analysis, using BMI instead of waist circumference to calculate the eGDR, showed a similar pattern, and “interestingly, a correlation between eGDR levels and risk of hemorrhagic stroke.” Dr. Zabala said.
Limitations and take-homes
Of course, this is an observational cohort study, so no conclusions on causality can be made and there are no data on the use of anti-diabetic treatments specifically. But there are strengths such as covering almost all adults with T2D in Sweden and a relatively long-follow-up time.
The findings suggest that “eGDR, which may reflect insulin resistance may be a useful risk marker for stroke and death in people with type 2 diabetes,” said Dr. Zabala.
“You had a very large cohort, and that certainly makes your results very valid,” observed Peter Novodvorsky, MUDr. (Hons), PhD, MRCP, a consultant diabetologist in Trenčín, Slovakia.
Dr. Novodvorsky, who chaired the session, picked up on the lack of information about how many people were taking newer diabetes drugs, such as the glucagon-like peptide 1 receptor antagonists and sodium glucose-lowering transport 2 inhibitors.
“As we all know, these might have protective effects which are not necessarily related to the glucose lowering or insulin resistance-lowering” effects, so could have influenced the results. In terms of how practical the eGDR is for clinical practice, Dr. Zabala observed in a press release: “eGDR could be used to help T2D patients better understand and manage their risk of stroke and death.
“It could also be of importance in research. In this era of personalized medicine, better stratification of type 2 diabetes patients will help optimize clinical trials and further vital research into treatment, diagnosis, care and prevention.”
The research was a collaboration between the Karolinska Institutet, Gothenburg University and the Swedish National Diabetes Registry. Dr. Zabala and coauthors reported having no conflicts of interest.
Calculating the estimated glucose disposal rate (eGDR) as a proxy for the level of insulin resistance may be useful way to determine if someone with type 2 diabetes (T2D) is at risk for having a first stroke, Swedish researchers have found.
In a large population-based study, the lower the eGDR score went, the higher the risk for having a first stroke became.
The eGDR score was also predictive of the chance of dying from any or a cardiovascular cause, Alexander Zabala, MD, reported at the annual meeting of the European Association for the Study of Diabetes (Abstract OP 01-4).
The link between insulin resistance and an increased risk for stroke has been known for some time, and not just in people with T2D. However, the current way of determining insulin resistance is not suitable for widespread practice.
“The goal standard technique for measuring insulin resistance is the euglycemic clamp method,” said Dr. Zabala, an internal medical resident at Södersjukhuset hospital and researcher at the Karolinska Institutet in Stockholm.
“For that reason, [the eGDR], a method based on readily available clinical factors – waist circumference, hypertension, and glycosylated hemoglobin was developed,” he explained. Body mass index can also be used in place of waist circumference, he qualified.
The eGDR has already been proven to be very precise in people with type 1 diabetes, said Dr. Zabala, and could be an “excellent tool to measure insulin resistance in a large patient population.”
Investigating the link between eGDR and first stroke risk
The aim of the study he presented was to see if changes in the eGDR were associated with changes in the risk of someone with T2D experiencing a first stroke, or dying from a cardiovascular or other cause.
An observational cohort was formed by first considering data on all adult patients with T2D who were logged in the Swedish National Diabetes Registry (NDR) during 2004-2016. Then anyone with a history of stroke, or with any missing data on the clinical variables needed to calculate the eGDR, were excluded.
This resulted in an overall population of 104,697 individuals, aged a mean of 63 years, who had developed T2D at around the age of 59 years. About 44% of the study population were women. The mean eGDR for the whole population was 5.6 mg/kg per min.
The study subjects were grouped according to four eGDR levels: 24,706 were in the lowest quartile of eGDR (less than 4 mg/kg per min), signifying the highest level of insulin resistance, and 18,762 were in the upper quartile of eGDR (greater than 8 mg/kg per min), signifying the lowest level of insulin resistance. The middle two groups had an eGDR between 4 and 6 mg/kg per min (40,187), and 6 and 8 mg/kg/min (21,042).
Data from the NDR were then combined with the Swedish Cause of Death register, the Swedish In-patient Care Diagnoses registry, and the Longitudinal Database for Health Insurance and Labour Market Studies (LISA) to determine the rates of stroke, ischemic stroke, hemorrhagic stroke, all-cause mortality, and cardiovascular mortality.
Increasing insulin resistance ups risk for stroke, death
After a median follow-up of 5.6 years, 4% (4,201) of the study population had had a stroke.
“We clearly see an increased occurrence of first-time stroke in the group with the lowest eGDR, indicating worst insulin resistance, in comparison with the group with the highest eGDR, indicating less insulin resistance,” Dr. Zabala reported.
After adjustment for potential confounding factors, including age at baseline, gender, diabetes duration, among other variables, the risk for stroke was lowest in those with a high eGDR value and highest for those with a low eGDR value.
Using individuals with the lowest eGDR (less than 4 mg/kg per min) and thus greatest risk of stroke as the reference, adjusted hazard ratios (aHR) for first-time stroke were: 0.60, 0.68, and 0.77 for those with an eGDR of greater than 8, 6-8, and 4-6 mg/kg per min, respectively.
The corresponding values for risk of ischemic stroke were 0.55, 0.68, and 0.75. Regarding hemorrhagic stroke, there was no statistically significant correlation between eGDR levels and stroke occurrence. This was due to the small number of cases recorded.
As for all-cause and cardiovascular mortality, a similar pattern was seen, with higher rates of death linked to increasing insulin resistance. Adjusted hazard ratios according to increasing insulin resistance (decreasing eGDR scores) for all-cause death were 0.68, 0.75, and 0.82 and for cardiovascular mortality were 0.65, 0.75, and 0.82.
A sensitivity analysis, using BMI instead of waist circumference to calculate the eGDR, showed a similar pattern, and “interestingly, a correlation between eGDR levels and risk of hemorrhagic stroke.” Dr. Zabala said.
Limitations and take-homes
Of course, this is an observational cohort study, so no conclusions on causality can be made and there are no data on the use of anti-diabetic treatments specifically. But there are strengths such as covering almost all adults with T2D in Sweden and a relatively long-follow-up time.
The findings suggest that “eGDR, which may reflect insulin resistance may be a useful risk marker for stroke and death in people with type 2 diabetes,” said Dr. Zabala.
“You had a very large cohort, and that certainly makes your results very valid,” observed Peter Novodvorsky, MUDr. (Hons), PhD, MRCP, a consultant diabetologist in Trenčín, Slovakia.
Dr. Novodvorsky, who chaired the session, picked up on the lack of information about how many people were taking newer diabetes drugs, such as the glucagon-like peptide 1 receptor antagonists and sodium glucose-lowering transport 2 inhibitors.
“As we all know, these might have protective effects which are not necessarily related to the glucose lowering or insulin resistance-lowering” effects, so could have influenced the results. In terms of how practical the eGDR is for clinical practice, Dr. Zabala observed in a press release: “eGDR could be used to help T2D patients better understand and manage their risk of stroke and death.
“It could also be of importance in research. In this era of personalized medicine, better stratification of type 2 diabetes patients will help optimize clinical trials and further vital research into treatment, diagnosis, care and prevention.”
The research was a collaboration between the Karolinska Institutet, Gothenburg University and the Swedish National Diabetes Registry. Dr. Zabala and coauthors reported having no conflicts of interest.
FROM EASD 2021
The compass that points toward food
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
The new breakfast of champions
We love a good ranking system here at LOTME world headquarters, especially the food-based ones. Luckily for us (and our readers), a new study published in Nature Food offers a food-based ranking system.
Sadly, unlike the last food-related ranking we covered, the Food Compass doesn’t tell you how much life you gain or lose from each food you eat down to the precise minute. Instead, it favors a more simple rating system from 1 to 100, with healthier foods scoring higher, and even incorporates mixed foods, not just single ingredients. This makes it better at assessing and comparing food combinations, rather than trying to mix and match the many ingredients that go into even relatively simple recipes.
The top and bottom of the rankings contain the usual suspects. Legumes and nuts, at 78.6, had the highest average score among the broad food groups, followed by fruits and then vegetables. Rounding out the bottom were sweets and savory snacks at 16.4. Among the individual foods, there were perfect scores in both directions: 100 for raw raspberries, while instant noodle soup and nonchocolate, ready-to-eat, nonfat pudding (very specific there) each earned a 1.
There are a few surprises in between. Nonfat cappuccino received a green light from the investigators, great news for the coffee drinkers out there. A serving of sweet potato chips scored better than a simple grilled chicken breast, and a slice of pizza, loaded up with extra meat and a thick crust, is still more nutritious than a bowl of corn flakes.
Neither is good for you, of course, but we’re still going to take this as a sign that pizza is the ideal breakfast food. Add that to your morning coffee, and you’re ready to start the day. Move over Wheaties, there’s a new breakfast of champions.
COVID-19 resisters, please step forward
Some people have all the luck with good genes, both inside and out.
Genetically speaking, humans are 99.9% the same, but that 0.1% is where things get interesting. Because of that 0.1% difference, some people are more likely to contract diseases such as HIV, while others might be more resistant. These small differences in genetic code could be the key to finding treatments for COVID-19.
“The introduction of SARS-CoV-2 to a naive population, on a global scale, has provided yet another demonstration of the remarkable clinical variability between individuals in the course of infection, ranging from asymptomatic infections to life-threatening disease,” the researchers said in Nature Immunology.
The investigators have been scouring the world to find people who might be resistant to SARS-CoV-2 and have enrolled over 400 individuals in a “dedicated resistance study cohort,” according to ScienceAlert.
The investigators are looking at households in which families were infected but one member did not show severe symptoms, or for individuals who have been around the virus multiple times and haven’t contracted it. They are also looking at blood types.
Enrollment is ongoing, so if you’ve been in contact with COVID-19 multiple times and have not gotten sick, scientists would like to hear from you.
Better living through parasitization
How would you like to triple your life span, while maintaining a youthful appearance and gaining special social standing and privileges?
Sounds pretty good, right, so what’s the catch? Well, you have to be infected with a tapeworm ... and you have to be an ant.
If you are an ant, here’s the deal: Workers of the species Temnothorax nylanderi that have tapeworms live much longer than uninfected workers, and while living out those longer lives they do less work and receive gifts of food.
In a study conducted at Johannes Gutenberg University in Mainz, Germany, infected ants’ metabolic rates and lipid levels were similar to those of younger ants, and they appeared to remain in a permanent juvenile stage as a result of the infection, the investigators reported.
They tracked Temnothorax colonies for 3 years, at which point 95% of the uninfected workers had died but over half of the infected ants were still alive. Pretty great, right? Wrong. There was no joy in antville, for the uninfected workers had struck out. “Strained by the additional burden of their wormed-up nestmates, they seemed to be shunting care away from their queen. They were dying sooner than they might have if the colonies had remained parasite-free,” according to an article in the Atlantic.
Does this situation seem just a wee bit familiar? A small group lives longer, healthier lives and enjoys special privileges while the majority of that society works harder to support them? We’ll put it into the form of a chicken-and-egg argument: Which came first, the tapeworms or the one-percenters?
Laughing the pandemic stress away
Doomscrolling on social media has become one of the world’s favorite pastimes during the pandemic, but research shows that those memes about COVID-19 might combat the doom and gloom of the outside world.
A study recently published in Psychology of Popular Media showed that viewing memes, specifically those that were COVID-19 related, actually lessened the stress of the pandemic.
The researchers conducted a survey of 748 people aged 18-88 years. Each participant viewed three memes with text or three memes with text but no images. All three memes had similar cuteness levels (baby or adult), subject (animal or human), and caption (COVID-19–related or not). The participants were then asked to report on their stress levels and feelings before and after the memes.
The people who looked at memes felt less stressed and a higher humor level, especially the participants who received the COVID-19 memes. Study Finds said that they had more “pandemic-coping confidence” than those who got regular memes.
“While the World Health Organization recommended that people avoid too much COVID-related media for the benefit of their mental health, our research reveals that memes about COVID-19 could help people feel more confident in their ability to deal with the pandemic,” lead author Jessica Gall Myrick, PhD, said in a written statement. “The positive emotions associated with this type of content may make people feel psychologically safer and therefore better able to pay attention to the underlying messages related to health threats.”
So if you think you’ve been wasting time looking at memes during this pandemic, think again. It actually might keep you sane. Keep on scrolling!
Giving the gift of stress reduction
It’s a big week here at LOTME. You’ve just read our 100th edition, and to help celebrate that milestone – along with Count Your Buttons Day, Celebration of the Mind Day, and the International Day of the Nacho – we’re presenting an extra-special bonus feature, courtesy of Sad and Useless: The most depressive humor site on the Internet.
We hope you’ll stop your doomscrolling long enough to enjoy this stress-reducing meme. Thanks for reading!
FDA authorizes boosters for Moderna, J&J, allows mix-and-match
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
Survey spotlights double-edged sword for minority cardiologists
Survey results paint a stark picture of discrimination among racial minorities in the cardiology workforce but also a strong sense of belonging.
Among respondents to the 2015 American College of Cardiology (ACC) Professional Life Survey, which is the most recent survey, over half (52.3%) of underrepresented racial and ethnic minorities (URMs) and 45.5% of Asian or Pacific Islanders reported experiencing discrimination compared with 36.4% of Whites (both P < .01).
Nevertheless, 91.2% of URMs reported being satisfied with their career, as did 90% of Asians or Pacific Islanders and 89.1% of Whites.
Satisfaction with financial compensation also did not differ between groups, and most cardiologists believed their opportunities for advancement were similar to those of their peers.
One possible explanation is that the respondents may simply be people who’ve had better experiences, lead author Kevin L. Thomas, MD, Duke Clinical Research Institute, Durham, N.C., and colleagues told this news organization. A second hypothesis looks more to sheer determination, or grit.
“Perhaps along the sometimes circuitous pathway to being a cardiologist – which is a lot of training, a lot of standardized testing, a lot of applications – that maybe you sub-select a group of individuals who are simply more resilient based on their life experiences and things that they’ve overcome to get where they are,” he said.
Interestingly, rates of burnout were lower among URMs (22.4%) and Asians/Pacific Islanders (20.1%) than Whites (30.3%; P = .02 and P < .01, respectively). The finding is unexpected but in line with a recent report of more than 4,400 U.S. physicians finding lower odds of burnout among Asian, Hispanic/Latinx, and Black physicians.
The new study, published October 18 in the Journal of the American College of Cardiology, however, affirms that women of all racial and ethnic groups face significant headwinds in the White, male-dominated cardiology workforce.
Just 13.9% of White men reported experiencing discrimination, compared with 44.6% of URM men and 36.2% of Asians/Pacific Islander men. In comparison, 69.2% of White women reported discrimination, as did 62.7% of URM women and 57% of Asian/Pacific Islander women (both P <.01).
“When you look specifically at White men versus White women, there is a large discrepancy there, and it just shows us, I think, for a lot of different groups, we still have a long way to go in terms of trying to achieve equity and to try to be inclusive in the workplace,” Dr. Thomas said.
Men were more likely to experience race- and religion-based discrimination in the workplace, whereas nearly all women reported sex discrimination, with parenting an important second. Approximately 85% of cardiologists reported being satisfied with their family lives, although unpublished data suggest URMs were less likely to be married and to have fewer children, Dr. Thomas said.
During job negotiations, URM cardiologists were less likely to prioritize salary, benefits, and work hours for their first job (13.6%, 10.9% 19.3%) than White cardiologists (20.6%, 23.3%, 31.3%; P < .02 for all).
In subsequent negotiations, URMs placed more emphasis on salary, benefits, and work hours than Whites, whereas both URMs and Asians/Pacific Islanders placed a greater importance on travel benefits, diversity, mentoring, workspace, time to promotion, academic rank, and roles with community, institutional, or national recognition, which the authors say, “might indicate a greater need to overcome systemic barriers.”
Three-fourths of all cardiologist respondents had a mentor during training, which can take many shapes, Dr. Thomas noted. “Within my own section as an electrophysiologist, which is a very subspecialized category, we have four Black electrophysiologists, and I think it was because many of us mentored each other as we came along, and it inspired us.”
URMs are more likely to experience the so-called “minority tax” of being tapped for added responsibilities in the name of inclusivity efforts, he said, and called on individuals from the dominant culture to mentor or sponsor cardiologists from other racial groups and to carve out leadership pathways for women and minorities so they “can use their gifts to benefit the profession at large,” leading clinical trials or steering committees and serving in high-profile roles.
Although the events of 2020 sharpened attention on the issue of diversity in America, Dr. Thomas and colleagues say that more work needs to be done defining the problem and that professional organizations and health systems also should systematically collect sex, racial, and ethnic identifies of members using classifications similar to the 2020 U.S. Census.
The study was based on 2,245 respondents to the 2015 Professional Life Survey, which was not specifically designed to assess racial/ethnic diversity topics and had a response rate of 21%, which limited representatives of each group.
In all, 197 were from URMs (80 Blacks, 113 Hispanics, 4 Native Americans), 564 were Asians/Pacific Islanders, 1,447 were Whites, and 37 listed multiracial/other. More than half (58%) were men, and most were adult cardiologists (83% to 85%), followed by pediatric cardiology (6% to 10%) and cardiovascular surgery (1% to 2%).
“Further research is needed to understand these findings and their significance, because ongoing efforts within ACC and other organizations to increase diversity will fail unless this is successfully addressed,” the authors conclude.
To that end, Dr. Thomas said they are looking to develop a new survey that taps other groups like the Association of Black Cardiologists and members of the LGBTQ community.
“I’m really excited about the opportunity to develop a survey that specifically has the objective of trying to understand the experiences of systematically disadvantaged, historically marginalized groups to see if we can see the same information, but maybe through a clear lens, and then be able to develop strategies to mitigate some of the challenges that we see” he said. “So we can increase the numbers and also have a workforce that is reflective of the populations that we take care of and the nation as a whole.”
The study was funded by the American College of Cardiology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survey results paint a stark picture of discrimination among racial minorities in the cardiology workforce but also a strong sense of belonging.
Among respondents to the 2015 American College of Cardiology (ACC) Professional Life Survey, which is the most recent survey, over half (52.3%) of underrepresented racial and ethnic minorities (URMs) and 45.5% of Asian or Pacific Islanders reported experiencing discrimination compared with 36.4% of Whites (both P < .01).
Nevertheless, 91.2% of URMs reported being satisfied with their career, as did 90% of Asians or Pacific Islanders and 89.1% of Whites.
Satisfaction with financial compensation also did not differ between groups, and most cardiologists believed their opportunities for advancement were similar to those of their peers.
One possible explanation is that the respondents may simply be people who’ve had better experiences, lead author Kevin L. Thomas, MD, Duke Clinical Research Institute, Durham, N.C., and colleagues told this news organization. A second hypothesis looks more to sheer determination, or grit.
“Perhaps along the sometimes circuitous pathway to being a cardiologist – which is a lot of training, a lot of standardized testing, a lot of applications – that maybe you sub-select a group of individuals who are simply more resilient based on their life experiences and things that they’ve overcome to get where they are,” he said.
Interestingly, rates of burnout were lower among URMs (22.4%) and Asians/Pacific Islanders (20.1%) than Whites (30.3%; P = .02 and P < .01, respectively). The finding is unexpected but in line with a recent report of more than 4,400 U.S. physicians finding lower odds of burnout among Asian, Hispanic/Latinx, and Black physicians.
The new study, published October 18 in the Journal of the American College of Cardiology, however, affirms that women of all racial and ethnic groups face significant headwinds in the White, male-dominated cardiology workforce.
Just 13.9% of White men reported experiencing discrimination, compared with 44.6% of URM men and 36.2% of Asians/Pacific Islander men. In comparison, 69.2% of White women reported discrimination, as did 62.7% of URM women and 57% of Asian/Pacific Islander women (both P <.01).
“When you look specifically at White men versus White women, there is a large discrepancy there, and it just shows us, I think, for a lot of different groups, we still have a long way to go in terms of trying to achieve equity and to try to be inclusive in the workplace,” Dr. Thomas said.
Men were more likely to experience race- and religion-based discrimination in the workplace, whereas nearly all women reported sex discrimination, with parenting an important second. Approximately 85% of cardiologists reported being satisfied with their family lives, although unpublished data suggest URMs were less likely to be married and to have fewer children, Dr. Thomas said.
During job negotiations, URM cardiologists were less likely to prioritize salary, benefits, and work hours for their first job (13.6%, 10.9% 19.3%) than White cardiologists (20.6%, 23.3%, 31.3%; P < .02 for all).
In subsequent negotiations, URMs placed more emphasis on salary, benefits, and work hours than Whites, whereas both URMs and Asians/Pacific Islanders placed a greater importance on travel benefits, diversity, mentoring, workspace, time to promotion, academic rank, and roles with community, institutional, or national recognition, which the authors say, “might indicate a greater need to overcome systemic barriers.”
Three-fourths of all cardiologist respondents had a mentor during training, which can take many shapes, Dr. Thomas noted. “Within my own section as an electrophysiologist, which is a very subspecialized category, we have four Black electrophysiologists, and I think it was because many of us mentored each other as we came along, and it inspired us.”
URMs are more likely to experience the so-called “minority tax” of being tapped for added responsibilities in the name of inclusivity efforts, he said, and called on individuals from the dominant culture to mentor or sponsor cardiologists from other racial groups and to carve out leadership pathways for women and minorities so they “can use their gifts to benefit the profession at large,” leading clinical trials or steering committees and serving in high-profile roles.
Although the events of 2020 sharpened attention on the issue of diversity in America, Dr. Thomas and colleagues say that more work needs to be done defining the problem and that professional organizations and health systems also should systematically collect sex, racial, and ethnic identifies of members using classifications similar to the 2020 U.S. Census.
The study was based on 2,245 respondents to the 2015 Professional Life Survey, which was not specifically designed to assess racial/ethnic diversity topics and had a response rate of 21%, which limited representatives of each group.
In all, 197 were from URMs (80 Blacks, 113 Hispanics, 4 Native Americans), 564 were Asians/Pacific Islanders, 1,447 were Whites, and 37 listed multiracial/other. More than half (58%) were men, and most were adult cardiologists (83% to 85%), followed by pediatric cardiology (6% to 10%) and cardiovascular surgery (1% to 2%).
“Further research is needed to understand these findings and their significance, because ongoing efforts within ACC and other organizations to increase diversity will fail unless this is successfully addressed,” the authors conclude.
To that end, Dr. Thomas said they are looking to develop a new survey that taps other groups like the Association of Black Cardiologists and members of the LGBTQ community.
“I’m really excited about the opportunity to develop a survey that specifically has the objective of trying to understand the experiences of systematically disadvantaged, historically marginalized groups to see if we can see the same information, but maybe through a clear lens, and then be able to develop strategies to mitigate some of the challenges that we see” he said. “So we can increase the numbers and also have a workforce that is reflective of the populations that we take care of and the nation as a whole.”
The study was funded by the American College of Cardiology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survey results paint a stark picture of discrimination among racial minorities in the cardiology workforce but also a strong sense of belonging.
Among respondents to the 2015 American College of Cardiology (ACC) Professional Life Survey, which is the most recent survey, over half (52.3%) of underrepresented racial and ethnic minorities (URMs) and 45.5% of Asian or Pacific Islanders reported experiencing discrimination compared with 36.4% of Whites (both P < .01).
Nevertheless, 91.2% of URMs reported being satisfied with their career, as did 90% of Asians or Pacific Islanders and 89.1% of Whites.
Satisfaction with financial compensation also did not differ between groups, and most cardiologists believed their opportunities for advancement were similar to those of their peers.
One possible explanation is that the respondents may simply be people who’ve had better experiences, lead author Kevin L. Thomas, MD, Duke Clinical Research Institute, Durham, N.C., and colleagues told this news organization. A second hypothesis looks more to sheer determination, or grit.
“Perhaps along the sometimes circuitous pathway to being a cardiologist – which is a lot of training, a lot of standardized testing, a lot of applications – that maybe you sub-select a group of individuals who are simply more resilient based on their life experiences and things that they’ve overcome to get where they are,” he said.
Interestingly, rates of burnout were lower among URMs (22.4%) and Asians/Pacific Islanders (20.1%) than Whites (30.3%; P = .02 and P < .01, respectively). The finding is unexpected but in line with a recent report of more than 4,400 U.S. physicians finding lower odds of burnout among Asian, Hispanic/Latinx, and Black physicians.
The new study, published October 18 in the Journal of the American College of Cardiology, however, affirms that women of all racial and ethnic groups face significant headwinds in the White, male-dominated cardiology workforce.
Just 13.9% of White men reported experiencing discrimination, compared with 44.6% of URM men and 36.2% of Asians/Pacific Islander men. In comparison, 69.2% of White women reported discrimination, as did 62.7% of URM women and 57% of Asian/Pacific Islander women (both P <.01).
“When you look specifically at White men versus White women, there is a large discrepancy there, and it just shows us, I think, for a lot of different groups, we still have a long way to go in terms of trying to achieve equity and to try to be inclusive in the workplace,” Dr. Thomas said.
Men were more likely to experience race- and religion-based discrimination in the workplace, whereas nearly all women reported sex discrimination, with parenting an important second. Approximately 85% of cardiologists reported being satisfied with their family lives, although unpublished data suggest URMs were less likely to be married and to have fewer children, Dr. Thomas said.
During job negotiations, URM cardiologists were less likely to prioritize salary, benefits, and work hours for their first job (13.6%, 10.9% 19.3%) than White cardiologists (20.6%, 23.3%, 31.3%; P < .02 for all).
In subsequent negotiations, URMs placed more emphasis on salary, benefits, and work hours than Whites, whereas both URMs and Asians/Pacific Islanders placed a greater importance on travel benefits, diversity, mentoring, workspace, time to promotion, academic rank, and roles with community, institutional, or national recognition, which the authors say, “might indicate a greater need to overcome systemic barriers.”
Three-fourths of all cardiologist respondents had a mentor during training, which can take many shapes, Dr. Thomas noted. “Within my own section as an electrophysiologist, which is a very subspecialized category, we have four Black electrophysiologists, and I think it was because many of us mentored each other as we came along, and it inspired us.”
URMs are more likely to experience the so-called “minority tax” of being tapped for added responsibilities in the name of inclusivity efforts, he said, and called on individuals from the dominant culture to mentor or sponsor cardiologists from other racial groups and to carve out leadership pathways for women and minorities so they “can use their gifts to benefit the profession at large,” leading clinical trials or steering committees and serving in high-profile roles.
Although the events of 2020 sharpened attention on the issue of diversity in America, Dr. Thomas and colleagues say that more work needs to be done defining the problem and that professional organizations and health systems also should systematically collect sex, racial, and ethnic identifies of members using classifications similar to the 2020 U.S. Census.
The study was based on 2,245 respondents to the 2015 Professional Life Survey, which was not specifically designed to assess racial/ethnic diversity topics and had a response rate of 21%, which limited representatives of each group.
In all, 197 were from URMs (80 Blacks, 113 Hispanics, 4 Native Americans), 564 were Asians/Pacific Islanders, 1,447 were Whites, and 37 listed multiracial/other. More than half (58%) were men, and most were adult cardiologists (83% to 85%), followed by pediatric cardiology (6% to 10%) and cardiovascular surgery (1% to 2%).
“Further research is needed to understand these findings and their significance, because ongoing efforts within ACC and other organizations to increase diversity will fail unless this is successfully addressed,” the authors conclude.
To that end, Dr. Thomas said they are looking to develop a new survey that taps other groups like the Association of Black Cardiologists and members of the LGBTQ community.
“I’m really excited about the opportunity to develop a survey that specifically has the objective of trying to understand the experiences of systematically disadvantaged, historically marginalized groups to see if we can see the same information, but maybe through a clear lens, and then be able to develop strategies to mitigate some of the challenges that we see” he said. “So we can increase the numbers and also have a workforce that is reflective of the populations that we take care of and the nation as a whole.”
The study was funded by the American College of Cardiology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
No benefit from lower temps for out-of-hospital cardiac arrest
The results “do not support the use of moderate therapeutic hypothermia to improve neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest,” write the investigators led by Michel Le May, MD, from the University of Ottawa Heart Institute, Ontario, Canada.
The CAPITAL CHILL results were first presented at the American College of Cardiology (ACC) 2021 Scientific Sessions in May.
They have now been published online, October 19, in JAMA.
High rates of brain injury and death
Comatose survivors of OHCA have high rates of severe brain injury and death. Current guidelines recommend targeted temperature management at 32°C to 36°C for 24 hours. However, small studies have suggested a potential benefit of targeting lower body temperatures.
In the CAPITAL CHILL study of 367 OHCA patients who were comatose on admission, there were no statistically significant differences in the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days with mild-versus-moderate hypothermia.
The primary composite outcome occurred in 89 of 184 (48.4%) patients in the moderate hypothermia group and 83 of 183 (45.4%) patients in the mild hypothermia group — a risk difference of 3.0% (95% confidence interval [CI], 7.2% - 13.2%) and relative risk of 1.07 (95% CI, 0.86 - 1.33; P = .56).
There was also no significant difference when looking at the individual components of mortality (43.5% vs 41.0%) and poor neurologic outcome (Disability Rating Scale score >5: 4.9% vs 4.4%).
The baseline characteristics of patients were similar in the moderate and mild hypothermia groups. The lack of a significant difference in the primary outcome was consistent after adjusting for baseline covariates as well as across all subgroups.
The rates of secondary outcomes were also similar between the two groups, except for a longer length of stay in the intensive care unit in the moderate hypothermia group compared with the mild hypothermia group, which would likely add to overall costs.
The researchers note that the Targeted Hypothermia vs Targeted Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial recently reported that targeted hypothermia at 33°C did not improve survival at 180 days compared with targeted normothermia at 37.5°C or less.
The CAPITAL CHILL study “adds to the spectrum of target temperature management, as it did not find any benefit of even further lowering temperatures to 31°C,” the study team says.
They caution that most patients in the trial had cardiac arrest secondary to a primary cardiac etiology and therefore the findings may not be applicable to cardiac arrest of all etiologies.
It’s also possible that the trial was underpowered to detect clinically important differences between moderate and mild hypothermia. Also, the number of patients presenting with a nonshockable rhythm was relatively small, and further study may be worthwhile in this subgroup, they say.
For now, however, the CAPITAL CHILL results provide no support for a lower target temperature of 31°C to improve outcomes in OHCA patients, Dr. Le May and colleagues conclude.
CAPITAL CHILL was an investigator-initiated study and funding was provided by the University of Ottawa Heart Institute Cardiac Arrest Program. Dr. Le May has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results “do not support the use of moderate therapeutic hypothermia to improve neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest,” write the investigators led by Michel Le May, MD, from the University of Ottawa Heart Institute, Ontario, Canada.
The CAPITAL CHILL results were first presented at the American College of Cardiology (ACC) 2021 Scientific Sessions in May.
They have now been published online, October 19, in JAMA.
High rates of brain injury and death
Comatose survivors of OHCA have high rates of severe brain injury and death. Current guidelines recommend targeted temperature management at 32°C to 36°C for 24 hours. However, small studies have suggested a potential benefit of targeting lower body temperatures.
In the CAPITAL CHILL study of 367 OHCA patients who were comatose on admission, there were no statistically significant differences in the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days with mild-versus-moderate hypothermia.
The primary composite outcome occurred in 89 of 184 (48.4%) patients in the moderate hypothermia group and 83 of 183 (45.4%) patients in the mild hypothermia group — a risk difference of 3.0% (95% confidence interval [CI], 7.2% - 13.2%) and relative risk of 1.07 (95% CI, 0.86 - 1.33; P = .56).
There was also no significant difference when looking at the individual components of mortality (43.5% vs 41.0%) and poor neurologic outcome (Disability Rating Scale score >5: 4.9% vs 4.4%).
The baseline characteristics of patients were similar in the moderate and mild hypothermia groups. The lack of a significant difference in the primary outcome was consistent after adjusting for baseline covariates as well as across all subgroups.
The rates of secondary outcomes were also similar between the two groups, except for a longer length of stay in the intensive care unit in the moderate hypothermia group compared with the mild hypothermia group, which would likely add to overall costs.
The researchers note that the Targeted Hypothermia vs Targeted Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial recently reported that targeted hypothermia at 33°C did not improve survival at 180 days compared with targeted normothermia at 37.5°C or less.
The CAPITAL CHILL study “adds to the spectrum of target temperature management, as it did not find any benefit of even further lowering temperatures to 31°C,” the study team says.
They caution that most patients in the trial had cardiac arrest secondary to a primary cardiac etiology and therefore the findings may not be applicable to cardiac arrest of all etiologies.
It’s also possible that the trial was underpowered to detect clinically important differences between moderate and mild hypothermia. Also, the number of patients presenting with a nonshockable rhythm was relatively small, and further study may be worthwhile in this subgroup, they say.
For now, however, the CAPITAL CHILL results provide no support for a lower target temperature of 31°C to improve outcomes in OHCA patients, Dr. Le May and colleagues conclude.
CAPITAL CHILL was an investigator-initiated study and funding was provided by the University of Ottawa Heart Institute Cardiac Arrest Program. Dr. Le May has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The results “do not support the use of moderate therapeutic hypothermia to improve neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest,” write the investigators led by Michel Le May, MD, from the University of Ottawa Heart Institute, Ontario, Canada.
The CAPITAL CHILL results were first presented at the American College of Cardiology (ACC) 2021 Scientific Sessions in May.
They have now been published online, October 19, in JAMA.
High rates of brain injury and death
Comatose survivors of OHCA have high rates of severe brain injury and death. Current guidelines recommend targeted temperature management at 32°C to 36°C for 24 hours. However, small studies have suggested a potential benefit of targeting lower body temperatures.
In the CAPITAL CHILL study of 367 OHCA patients who were comatose on admission, there were no statistically significant differences in the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days with mild-versus-moderate hypothermia.
The primary composite outcome occurred in 89 of 184 (48.4%) patients in the moderate hypothermia group and 83 of 183 (45.4%) patients in the mild hypothermia group — a risk difference of 3.0% (95% confidence interval [CI], 7.2% - 13.2%) and relative risk of 1.07 (95% CI, 0.86 - 1.33; P = .56).
There was also no significant difference when looking at the individual components of mortality (43.5% vs 41.0%) and poor neurologic outcome (Disability Rating Scale score >5: 4.9% vs 4.4%).
The baseline characteristics of patients were similar in the moderate and mild hypothermia groups. The lack of a significant difference in the primary outcome was consistent after adjusting for baseline covariates as well as across all subgroups.
The rates of secondary outcomes were also similar between the two groups, except for a longer length of stay in the intensive care unit in the moderate hypothermia group compared with the mild hypothermia group, which would likely add to overall costs.
The researchers note that the Targeted Hypothermia vs Targeted Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial recently reported that targeted hypothermia at 33°C did not improve survival at 180 days compared with targeted normothermia at 37.5°C or less.
The CAPITAL CHILL study “adds to the spectrum of target temperature management, as it did not find any benefit of even further lowering temperatures to 31°C,” the study team says.
They caution that most patients in the trial had cardiac arrest secondary to a primary cardiac etiology and therefore the findings may not be applicable to cardiac arrest of all etiologies.
It’s also possible that the trial was underpowered to detect clinically important differences between moderate and mild hypothermia. Also, the number of patients presenting with a nonshockable rhythm was relatively small, and further study may be worthwhile in this subgroup, they say.
For now, however, the CAPITAL CHILL results provide no support for a lower target temperature of 31°C to improve outcomes in OHCA patients, Dr. Le May and colleagues conclude.
CAPITAL CHILL was an investigator-initiated study and funding was provided by the University of Ottawa Heart Institute Cardiac Arrest Program. Dr. Le May has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Preterm delivery raises lifetime hypertension risk
Women who had a preterm delivery were at least 1.6 times as likely to develop hypertension over the next decade as those who had full-term deliveries, based on data from a national cohort study of more than 2 million women.
Pregnancy complications such as preeclampsia and other hypertensive disorders of pregnancy have been associated with chronic hypertension as well as with preterm delivery, but the independent role of preterm delivery in chronic hypertension risk remains unclear, Casey Crump, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote. “A better understanding of the long-term hypertension risks associated with preterm delivery is needed to improve risk stratification, clinical monitoring, and CVD [cardiovascular disease] prevention in women.”
In a study published in JAMA Cardiology, the researchers reviewed data from 2,195,989 women with 4,308,286 singleton deliveries in Sweden from Jan. 1, 1973, to Dec. 31, 2015. Women with preexisting hypertension before their first pregnancy were excluded. Pregnancy duration was based on maternal reports of the last menstrual period for patients in the 1970s, and based on ultrasound estimates in the 1980s and beyond. Pregnancy duration was divided into six groups in terms of completed weeks of gestation: extremely preterm (22-27 weeks), moderately preterm (28-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), full term (39-41 weeks), and post term (≥42 weeks). Full-term delivery was used as the reference, and the three preterm groups were combined for summaries of preterm delivery (less than 37 weeks).
Overall, women who delivered at less than 37 weeks’ gestation had a 1.6-fold increased risk of hypertension (adjusted hazard ratio, 1.67) within the next 10 years, compared with women who delivered full term after controlling for preeclampsia, other hypertensive disorders of pregnancy, and maternal factors.
When further stratified by pregnancy duration, the aHRs for extremely preterm, moderately preterm, late preterm, and early term, compared with full-term deliveries were 2.23, 1.85, 1.55, and 1.26, respectively, in the first decade after delivery. Each additional week of pregnancy was associated with a mean 7% reduction in hypertension risk (a HR, 0.93).
The increased hypertension risk following preterm delivery (less than 37 weeks) persisted at 10-19 years, 20-29 years, and 30-43 years, with aHRs of 1.40, 1.20, and 1.12, respectively. Early-term delivery at 37-38 weeks also carried an increased risk of long-term hypertension compared with full-term delivery, with aHRs of 1.12 and 1.06 at 20-29 years and 30-43 years, respectively.
“Cosibling analyses suggested that these findings were only partially explained by familial (genetic and/or early-life environmental) factors that are shared determinants of both preterm delivery and hypertension,” the researchers noted. The findings suggest that preterm delivery itself may contribute to or affect the pathophysiology that leads to cardiovascular disease, they added, hypothesizing that endothelial dysfunction caused by preterm delivery may cause functional impairments in the microvasculature.
The study findings were limited by several factors including the lack of detailed records to verify hypertension and the use of data from a single country, the researchers noted. However, the results were strengthened by the large study population, the use of highly complete prenatal and birth records to minimize selection bias, and the long-term follow-up.
The results are consistent with those from previous studies, and support the recognition of preterm delivery as a lifetime risk factor for hypertension, but future studies should focus on racial and ethnic subgroups already at increased risk for both preterm delivery and hypertension, they added.
“Additional follow-up will be needed to examine these associations in older adulthood when hypertension increasingly and disproportionately affects women,” they concluded.
Data highlight the need for patient and provider education
“This study furthers our knowledge regarding long-term complications associated with the frequent pregnancy complication of preterm delivery,” Stephen S. Crane, MD, an ob.gyn. and maternal-fetal medicine specialist in private practice in Orlando, said in an interview. “Cardiovascular disease is the leading cause of death and often goes unrecognized in women. There are shared risk factors among women and men for developing CVD, the most common being hypertension. However, women have the unique risk factor of pregnancy and its attendant complications including preeclampsia, glucose intolerance, and preterm delivery. Hypertensive disorders in pregnancy often lead to indicated premature delivery, and are associated with development of chronic hypertension and subsequent CVD. However, prior data suggest that preterm delivery itself is a risk factor for developing chronic hypertension later in life.
“The current study, which evaluates one of the most complete population data sets with up to 43 years of follow-up, is the first to assess for familial determinants by cosibling analysis, and supports preterm delivery as an independent risk factor for the development of hypertension,” he said. The study results illustrate that this risk is longstanding, and that recurrent preterm birth further increases the risk of developing hypertension.
Dr. Crane said he was not surprised by the study findings, given that inflammatory processes have been linked to the development of hypertension and CVD. “Similarly, inflammatory processes have been implicated in the pathophysiology of preterm labor and inflammatory cytokines may also play a role in normal term labor. Therefore, it is not surprising that preterm delivery would be a marker for the risk of development of hypertension, as both may be responses to underlying inflammatory processes. Identification of these underlying inflammatory processes and methods for prevention will be critical if we are to decrease both the incidence of preterm birth and CVD.
“As prenatal care may be the only medical care women obtain, it is important to take this opportunity to educate patients regarding their long-term risks of developing hypertension and the need for long-term follow up. Interventions that may help reduce the risk for recurrent preterm birth and long-term risks for developing hypertension and CVD include weight loss, increased activity, and smoking cessation; the resources to achieve these goals need to be shared with patients,” he said.
“Knowledge deficits both on the part of the provider and patient may be a significant barrier to intervention that may be overcome with improved education,” said Dr. Crane. “Care providers need education regarding the long-term risks associated with a history of preterm delivery in order to better educate their patients regarding both prevention of recurrent preterm birth and the development of hypertension and CVD.” However, socioeconomic status, education level, and the inability to obtain further health care remain common barriers to intervention for many women.
“Additional research is needed to identify the causes of inflammatory processes leading to preterm delivery and risks for hypertension and CVD,” said Dr. Crane. “Only after the causes are identified can treatments be sought to successfully treat these conditions.”
The study was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health; the Swedish Research Council; the Swedish Heart-Lung Foundation; and an Avtal om Läkarutbildning och Forskning (Agreement on Medical Training and Research) (ALF) project grant from Region Skåne/Lund University. Neither the researchers nor Dr. Crane had any financial conflicts to disclose.
Women who had a preterm delivery were at least 1.6 times as likely to develop hypertension over the next decade as those who had full-term deliveries, based on data from a national cohort study of more than 2 million women.
Pregnancy complications such as preeclampsia and other hypertensive disorders of pregnancy have been associated with chronic hypertension as well as with preterm delivery, but the independent role of preterm delivery in chronic hypertension risk remains unclear, Casey Crump, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote. “A better understanding of the long-term hypertension risks associated with preterm delivery is needed to improve risk stratification, clinical monitoring, and CVD [cardiovascular disease] prevention in women.”
In a study published in JAMA Cardiology, the researchers reviewed data from 2,195,989 women with 4,308,286 singleton deliveries in Sweden from Jan. 1, 1973, to Dec. 31, 2015. Women with preexisting hypertension before their first pregnancy were excluded. Pregnancy duration was based on maternal reports of the last menstrual period for patients in the 1970s, and based on ultrasound estimates in the 1980s and beyond. Pregnancy duration was divided into six groups in terms of completed weeks of gestation: extremely preterm (22-27 weeks), moderately preterm (28-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), full term (39-41 weeks), and post term (≥42 weeks). Full-term delivery was used as the reference, and the three preterm groups were combined for summaries of preterm delivery (less than 37 weeks).
Overall, women who delivered at less than 37 weeks’ gestation had a 1.6-fold increased risk of hypertension (adjusted hazard ratio, 1.67) within the next 10 years, compared with women who delivered full term after controlling for preeclampsia, other hypertensive disorders of pregnancy, and maternal factors.
When further stratified by pregnancy duration, the aHRs for extremely preterm, moderately preterm, late preterm, and early term, compared with full-term deliveries were 2.23, 1.85, 1.55, and 1.26, respectively, in the first decade after delivery. Each additional week of pregnancy was associated with a mean 7% reduction in hypertension risk (a HR, 0.93).
The increased hypertension risk following preterm delivery (less than 37 weeks) persisted at 10-19 years, 20-29 years, and 30-43 years, with aHRs of 1.40, 1.20, and 1.12, respectively. Early-term delivery at 37-38 weeks also carried an increased risk of long-term hypertension compared with full-term delivery, with aHRs of 1.12 and 1.06 at 20-29 years and 30-43 years, respectively.
“Cosibling analyses suggested that these findings were only partially explained by familial (genetic and/or early-life environmental) factors that are shared determinants of both preterm delivery and hypertension,” the researchers noted. The findings suggest that preterm delivery itself may contribute to or affect the pathophysiology that leads to cardiovascular disease, they added, hypothesizing that endothelial dysfunction caused by preterm delivery may cause functional impairments in the microvasculature.
The study findings were limited by several factors including the lack of detailed records to verify hypertension and the use of data from a single country, the researchers noted. However, the results were strengthened by the large study population, the use of highly complete prenatal and birth records to minimize selection bias, and the long-term follow-up.
The results are consistent with those from previous studies, and support the recognition of preterm delivery as a lifetime risk factor for hypertension, but future studies should focus on racial and ethnic subgroups already at increased risk for both preterm delivery and hypertension, they added.
“Additional follow-up will be needed to examine these associations in older adulthood when hypertension increasingly and disproportionately affects women,” they concluded.
Data highlight the need for patient and provider education
“This study furthers our knowledge regarding long-term complications associated with the frequent pregnancy complication of preterm delivery,” Stephen S. Crane, MD, an ob.gyn. and maternal-fetal medicine specialist in private practice in Orlando, said in an interview. “Cardiovascular disease is the leading cause of death and often goes unrecognized in women. There are shared risk factors among women and men for developing CVD, the most common being hypertension. However, women have the unique risk factor of pregnancy and its attendant complications including preeclampsia, glucose intolerance, and preterm delivery. Hypertensive disorders in pregnancy often lead to indicated premature delivery, and are associated with development of chronic hypertension and subsequent CVD. However, prior data suggest that preterm delivery itself is a risk factor for developing chronic hypertension later in life.
“The current study, which evaluates one of the most complete population data sets with up to 43 years of follow-up, is the first to assess for familial determinants by cosibling analysis, and supports preterm delivery as an independent risk factor for the development of hypertension,” he said. The study results illustrate that this risk is longstanding, and that recurrent preterm birth further increases the risk of developing hypertension.
Dr. Crane said he was not surprised by the study findings, given that inflammatory processes have been linked to the development of hypertension and CVD. “Similarly, inflammatory processes have been implicated in the pathophysiology of preterm labor and inflammatory cytokines may also play a role in normal term labor. Therefore, it is not surprising that preterm delivery would be a marker for the risk of development of hypertension, as both may be responses to underlying inflammatory processes. Identification of these underlying inflammatory processes and methods for prevention will be critical if we are to decrease both the incidence of preterm birth and CVD.
“As prenatal care may be the only medical care women obtain, it is important to take this opportunity to educate patients regarding their long-term risks of developing hypertension and the need for long-term follow up. Interventions that may help reduce the risk for recurrent preterm birth and long-term risks for developing hypertension and CVD include weight loss, increased activity, and smoking cessation; the resources to achieve these goals need to be shared with patients,” he said.
“Knowledge deficits both on the part of the provider and patient may be a significant barrier to intervention that may be overcome with improved education,” said Dr. Crane. “Care providers need education regarding the long-term risks associated with a history of preterm delivery in order to better educate their patients regarding both prevention of recurrent preterm birth and the development of hypertension and CVD.” However, socioeconomic status, education level, and the inability to obtain further health care remain common barriers to intervention for many women.
“Additional research is needed to identify the causes of inflammatory processes leading to preterm delivery and risks for hypertension and CVD,” said Dr. Crane. “Only after the causes are identified can treatments be sought to successfully treat these conditions.”
The study was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health; the Swedish Research Council; the Swedish Heart-Lung Foundation; and an Avtal om Läkarutbildning och Forskning (Agreement on Medical Training and Research) (ALF) project grant from Region Skåne/Lund University. Neither the researchers nor Dr. Crane had any financial conflicts to disclose.
Women who had a preterm delivery were at least 1.6 times as likely to develop hypertension over the next decade as those who had full-term deliveries, based on data from a national cohort study of more than 2 million women.
Pregnancy complications such as preeclampsia and other hypertensive disorders of pregnancy have been associated with chronic hypertension as well as with preterm delivery, but the independent role of preterm delivery in chronic hypertension risk remains unclear, Casey Crump, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues wrote. “A better understanding of the long-term hypertension risks associated with preterm delivery is needed to improve risk stratification, clinical monitoring, and CVD [cardiovascular disease] prevention in women.”
In a study published in JAMA Cardiology, the researchers reviewed data from 2,195,989 women with 4,308,286 singleton deliveries in Sweden from Jan. 1, 1973, to Dec. 31, 2015. Women with preexisting hypertension before their first pregnancy were excluded. Pregnancy duration was based on maternal reports of the last menstrual period for patients in the 1970s, and based on ultrasound estimates in the 1980s and beyond. Pregnancy duration was divided into six groups in terms of completed weeks of gestation: extremely preterm (22-27 weeks), moderately preterm (28-33 weeks), late preterm (34-36 weeks), early term (37-38 weeks), full term (39-41 weeks), and post term (≥42 weeks). Full-term delivery was used as the reference, and the three preterm groups were combined for summaries of preterm delivery (less than 37 weeks).
Overall, women who delivered at less than 37 weeks’ gestation had a 1.6-fold increased risk of hypertension (adjusted hazard ratio, 1.67) within the next 10 years, compared with women who delivered full term after controlling for preeclampsia, other hypertensive disorders of pregnancy, and maternal factors.
When further stratified by pregnancy duration, the aHRs for extremely preterm, moderately preterm, late preterm, and early term, compared with full-term deliveries were 2.23, 1.85, 1.55, and 1.26, respectively, in the first decade after delivery. Each additional week of pregnancy was associated with a mean 7% reduction in hypertension risk (a HR, 0.93).
The increased hypertension risk following preterm delivery (less than 37 weeks) persisted at 10-19 years, 20-29 years, and 30-43 years, with aHRs of 1.40, 1.20, and 1.12, respectively. Early-term delivery at 37-38 weeks also carried an increased risk of long-term hypertension compared with full-term delivery, with aHRs of 1.12 and 1.06 at 20-29 years and 30-43 years, respectively.
“Cosibling analyses suggested that these findings were only partially explained by familial (genetic and/or early-life environmental) factors that are shared determinants of both preterm delivery and hypertension,” the researchers noted. The findings suggest that preterm delivery itself may contribute to or affect the pathophysiology that leads to cardiovascular disease, they added, hypothesizing that endothelial dysfunction caused by preterm delivery may cause functional impairments in the microvasculature.
The study findings were limited by several factors including the lack of detailed records to verify hypertension and the use of data from a single country, the researchers noted. However, the results were strengthened by the large study population, the use of highly complete prenatal and birth records to minimize selection bias, and the long-term follow-up.
The results are consistent with those from previous studies, and support the recognition of preterm delivery as a lifetime risk factor for hypertension, but future studies should focus on racial and ethnic subgroups already at increased risk for both preterm delivery and hypertension, they added.
“Additional follow-up will be needed to examine these associations in older adulthood when hypertension increasingly and disproportionately affects women,” they concluded.
Data highlight the need for patient and provider education
“This study furthers our knowledge regarding long-term complications associated with the frequent pregnancy complication of preterm delivery,” Stephen S. Crane, MD, an ob.gyn. and maternal-fetal medicine specialist in private practice in Orlando, said in an interview. “Cardiovascular disease is the leading cause of death and often goes unrecognized in women. There are shared risk factors among women and men for developing CVD, the most common being hypertension. However, women have the unique risk factor of pregnancy and its attendant complications including preeclampsia, glucose intolerance, and preterm delivery. Hypertensive disorders in pregnancy often lead to indicated premature delivery, and are associated with development of chronic hypertension and subsequent CVD. However, prior data suggest that preterm delivery itself is a risk factor for developing chronic hypertension later in life.
“The current study, which evaluates one of the most complete population data sets with up to 43 years of follow-up, is the first to assess for familial determinants by cosibling analysis, and supports preterm delivery as an independent risk factor for the development of hypertension,” he said. The study results illustrate that this risk is longstanding, and that recurrent preterm birth further increases the risk of developing hypertension.
Dr. Crane said he was not surprised by the study findings, given that inflammatory processes have been linked to the development of hypertension and CVD. “Similarly, inflammatory processes have been implicated in the pathophysiology of preterm labor and inflammatory cytokines may also play a role in normal term labor. Therefore, it is not surprising that preterm delivery would be a marker for the risk of development of hypertension, as both may be responses to underlying inflammatory processes. Identification of these underlying inflammatory processes and methods for prevention will be critical if we are to decrease both the incidence of preterm birth and CVD.
“As prenatal care may be the only medical care women obtain, it is important to take this opportunity to educate patients regarding their long-term risks of developing hypertension and the need for long-term follow up. Interventions that may help reduce the risk for recurrent preterm birth and long-term risks for developing hypertension and CVD include weight loss, increased activity, and smoking cessation; the resources to achieve these goals need to be shared with patients,” he said.
“Knowledge deficits both on the part of the provider and patient may be a significant barrier to intervention that may be overcome with improved education,” said Dr. Crane. “Care providers need education regarding the long-term risks associated with a history of preterm delivery in order to better educate their patients regarding both prevention of recurrent preterm birth and the development of hypertension and CVD.” However, socioeconomic status, education level, and the inability to obtain further health care remain common barriers to intervention for many women.
“Additional research is needed to identify the causes of inflammatory processes leading to preterm delivery and risks for hypertension and CVD,” said Dr. Crane. “Only after the causes are identified can treatments be sought to successfully treat these conditions.”
The study was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health; the Swedish Research Council; the Swedish Heart-Lung Foundation; and an Avtal om Läkarutbildning och Forskning (Agreement on Medical Training and Research) (ALF) project grant from Region Skåne/Lund University. Neither the researchers nor Dr. Crane had any financial conflicts to disclose.
FROM JAMA CARDIOLOGY