User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
From Breakouts to Bargains: Strategies for Patient-Centered, Cost-effective Acne Care
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
Practice Points
- For mild to moderate acne, fixed-dose combination adapalene–benzoyl peroxide and clindamycin–benzoyl peroxide are highly cost-effective options for most patients.
- For moderate to severe acne, doxycycline or hormonal therapy (ie, combined oral contraceptives, spironolactone) are highly cost-effective options.
- Reduction of laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
Pruritic Papules in the Perianal and Gluteal Cleft Regions
The Diagnosis: Papular Acantholytic Dyskeratosis
The shave biopsy revealed suprabasal clefts associated with acantholytic and dyskeratotic cells as well as overlying hyperkeratosis. Direct immunofluorescence (DIF) was negative. Based on the combined clinical and histological findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD), a rare disease that clinically presents as small whitishgreyish papules with the potential to coalesce into larger plaques.1,2 The condition predominantly manifests without symptoms, though pruritus and burning have been reported in affected sites. Most cases of PAD have been reported in older adults rather than in children or adolescents; it is more prevalent in women than in men. Lesions generally are localized to the penis, vulva, scrotum, inguinal folds, and perianal region.3 More specific terms have been used to describe this presentation such as papular acantholytic dyskeratosis of the anogenital region and papular acantholytic dyskeratosis of the genital-crural region. Histologic findings of PAD include epidermal acantholysis and dyskeratosis with hyperkeratosis and parakeratosis (quiz image).
The histologic differential diagnosis of PAD is broad due to its overlapping features with other diseases such as pemphigus vulgaris, Hailey-Hailey disease (HHD), Darier disease, and Grover disease. The acantholytic pathophysiology of these conditions involves dysfunction in cell adhesion markers. The correct diagnosis can be made by considering both the clinical location of involvement and histopathologic clues.
Pemphigus is a family of disorders involving mucocutaneous blistering of an autoimmune nature (Figure 1). Pemphigus vulgaris is the most prevalent variant of the pemphigus family, with symptomatically painful involvement of mucosal and cutaneous tissue. Autoantibodies to desmoglein 3 alone or both desmoglein 1 and 3 are present. Pemphigus vulgaris displays positive DIF findings with intercellular IgG and C3.
Hailey-Hailey disease (also known as benign familial pemphigus) is an autosomal-dominant disease that shares the acantholytic feature that is common in this class of diseases and caused by a defect in cell-cell adhesion as well as a loss of function in the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Blistering lesions typically appear in the neck, axillary, inguinal, or genital regions, and they can develop into crusted, exudate-filled lesions. No autoimmunity has been associated with this disease, unlike other diseases in the pemphigus family, and mutations in the ATP2C1 gene have been linked with dysregulation of cell-cell adhesion, particularly in cadherins and calcium-dependent cell adhesion processes. Histologically, HHD will show diffuse keratinocyte acantholysis with suprabasal clefting (Figure 2).4 Dyskeratosis is mild, if present at all, and dyskeratotic keratinocytes show a well-defined nucleus with cytoplasmic preservation. In contrast to HHD, PAD typically shows more dyskeratosis.
Darier disease (also known as keratosis follicularis) is an autosomal-dominant condition that normally presents with seborrheic eruptions in intertriginous areas, usually with onset during adolescence. Darier disease is caused by a loss-of-function mutation in the ATP2A2 gene found on chromosome 12q23-24.1 that encodes for the sarco(endo)plasmic reticulum calcium ATPase2 (SERCA2) enzymes involved in calcium-dependent transport of the endoplasmic reticulum within the cell. Due to calcium dysregulation, desmosomes are unable to carry out their function in cell-cell adhesion, resulting in keratinocyte acantholysis. Histopathology of Darier disease is identical to HHD but displays more dyskeratosis than HHD (Figure 3), possibly due to the endoplasmic reticulum calcium stores that are affected in Darier disease compared to the Golgi apparatus calcium stores that are implicated in HHD.5 The lowered endoplasmic reticulum calcium stores in Darier-White disease are associated with more pronounced dyskeratosis, which is seen histologically as corps ronds. Suprabasal hyperkeratosis also is found in Darier disease. The histopathologic findings of Darier disease and PAD can be identical, but the clinical presentations are distinct, with Darier disease typically manifesting as seborrheic eruptions appearing in adolescence and PAD presenting as small white papules in the anogenital or crural regions.
Grover disease (also referred to as transient acantholytic dermatosis) has an idiopathic pathophysiology. It clinically manifests with eruptions of erythematous, pruritic, truncal papules on the chest or back. Grover disease has a predilection for White men older than 50 years, and symptoms may be exacerbated in heat and diaphoretic conditions. Histologically, Grover disease may show acantholytic features seen in pemphigus vulgaris, HHD, and Darier disease; the pattern can only follow a specific disease or consist of a combination of all disease features (Figure 4). The acantholytic pattern of Grover disease was found to be similar to pemphigus vulgaris, Darier disease, pemphigus foliaceus, and HHD 47%, 18%, 9%, and 8% of the time, respectively. In 9% of cases, Grover disease will exhibit a mixed histopathology in which its acantholytic pattern will consist of a combination of features seen in the pemphigus family of diseases.6 Biopsy results showing mixed histologic patterns or a combination of different acantholytic features are suggestive of Grover disease over PAD. Moreover, the clinical distribution helps to differentiate Grover disease from PAD.
Because the histologic characteristics of these diseases overlap, certain nuances in clinical correlations and histology allow for distinction. In our patient, the diagnosis was most consistent with PAD based on the clinical manifestation of the disease and the biopsy results. Considering solely the clinical location of the lesions, Grover disease was a less likely diagnosis because our patient’s lesions were observed in the perianal region, not the truncal region as typically seen in Grover disease. Taking into account the DIF assay results in our patient, the pemphigus family of diseases also moved lower on the differential diagnosis. Finally, because the biopsy showed more dyskeratosis than would be present in HHD and also was inconsistent with the location and onset that would be expected to be seen in Darier disease, PAD was the most probable diagnosis. Interestingly, studies have shown mosaic mutations in ATP2A2 and ATP2C1 as possible causes of PAD, suggesting that this may be an allelic variant of Darier disease and HHD.7-9 No genetic testing was performed in our patient.
- Dowd ML, Ansell LH, Husain S, et al. Papular acantholytic dyskeratosis of the genitocrural area: a rare unilateral asymptomatic intertrigo. JAAD Case Rep. 2016;2:132-134. doi:10.1016/j.jdcr.2015.11.003
- Konstantinou MP, Krasagakis K. Benign familial pemphigus (Hailey Hailey disease). StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK585136/
- Montis-Palos MC, Acebo-Mariñas E, Catón-Santarén B, et al. Papular acantholytic dermatosis in the genito-crural region: a localized form of Darier disease or Hailey-Hailey disease? Actas Dermosifiliogr (Engl Ed). 2013;104:170-172. https://doi.org/10.1016/j.adengl.2012.02.008
- Verma SB. Papular acantholytic dyskeratosis localized to the perineal and perianal area in a young male. Indian J Dermatol. 2013;58:393-395.
- Schmieder SJ, Rosario-Collazo JA. Keratosis follicularis. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm .nih.gov/books/NBK519557/
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Knopp EA, Saraceni C, Moss J, et al. Somatic ATP2A2 mutation in a case of papular acantholytic dyskeratosis: mosaic Darier disease [published online August 12, 2015]. J Cutan Pathol. 2015;42:853-857. doi:10.1111/cup.12551
- Lipoff JB, Mudgil AV, Young S, et al. Acantholytic dermatosis of the crural folds with ATP2C1 mutation is a possible variant of Hailey-Hailey Disease. J Cutan Med Surg. 2009;13:151.
- Vodo D, Malchin N, Furman M, et al. Identification of a recurrent mutation in ATP2C1 demonstrates that papular acantholytic dyskeratosis and Hailey-Hailey disease are allelic disorders. Br J Dermatol. 2018;179:1001-1002.
The Diagnosis: Papular Acantholytic Dyskeratosis
The shave biopsy revealed suprabasal clefts associated with acantholytic and dyskeratotic cells as well as overlying hyperkeratosis. Direct immunofluorescence (DIF) was negative. Based on the combined clinical and histological findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD), a rare disease that clinically presents as small whitishgreyish papules with the potential to coalesce into larger plaques.1,2 The condition predominantly manifests without symptoms, though pruritus and burning have been reported in affected sites. Most cases of PAD have been reported in older adults rather than in children or adolescents; it is more prevalent in women than in men. Lesions generally are localized to the penis, vulva, scrotum, inguinal folds, and perianal region.3 More specific terms have been used to describe this presentation such as papular acantholytic dyskeratosis of the anogenital region and papular acantholytic dyskeratosis of the genital-crural region. Histologic findings of PAD include epidermal acantholysis and dyskeratosis with hyperkeratosis and parakeratosis (quiz image).
The histologic differential diagnosis of PAD is broad due to its overlapping features with other diseases such as pemphigus vulgaris, Hailey-Hailey disease (HHD), Darier disease, and Grover disease. The acantholytic pathophysiology of these conditions involves dysfunction in cell adhesion markers. The correct diagnosis can be made by considering both the clinical location of involvement and histopathologic clues.
Pemphigus is a family of disorders involving mucocutaneous blistering of an autoimmune nature (Figure 1). Pemphigus vulgaris is the most prevalent variant of the pemphigus family, with symptomatically painful involvement of mucosal and cutaneous tissue. Autoantibodies to desmoglein 3 alone or both desmoglein 1 and 3 are present. Pemphigus vulgaris displays positive DIF findings with intercellular IgG and C3.

Hailey-Hailey disease (also known as benign familial pemphigus) is an autosomal-dominant disease that shares the acantholytic feature that is common in this class of diseases and caused by a defect in cell-cell adhesion as well as a loss of function in the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Blistering lesions typically appear in the neck, axillary, inguinal, or genital regions, and they can develop into crusted, exudate-filled lesions. No autoimmunity has been associated with this disease, unlike other diseases in the pemphigus family, and mutations in the ATP2C1 gene have been linked with dysregulation of cell-cell adhesion, particularly in cadherins and calcium-dependent cell adhesion processes. Histologically, HHD will show diffuse keratinocyte acantholysis with suprabasal clefting (Figure 2).4 Dyskeratosis is mild, if present at all, and dyskeratotic keratinocytes show a well-defined nucleus with cytoplasmic preservation. In contrast to HHD, PAD typically shows more dyskeratosis.

Darier disease (also known as keratosis follicularis) is an autosomal-dominant condition that normally presents with seborrheic eruptions in intertriginous areas, usually with onset during adolescence. Darier disease is caused by a loss-of-function mutation in the ATP2A2 gene found on chromosome 12q23-24.1 that encodes for the sarco(endo)plasmic reticulum calcium ATPase2 (SERCA2) enzymes involved in calcium-dependent transport of the endoplasmic reticulum within the cell. Due to calcium dysregulation, desmosomes are unable to carry out their function in cell-cell adhesion, resulting in keratinocyte acantholysis. Histopathology of Darier disease is identical to HHD but displays more dyskeratosis than HHD (Figure 3), possibly due to the endoplasmic reticulum calcium stores that are affected in Darier disease compared to the Golgi apparatus calcium stores that are implicated in HHD.5 The lowered endoplasmic reticulum calcium stores in Darier-White disease are associated with more pronounced dyskeratosis, which is seen histologically as corps ronds. Suprabasal hyperkeratosis also is found in Darier disease. The histopathologic findings of Darier disease and PAD can be identical, but the clinical presentations are distinct, with Darier disease typically manifesting as seborrheic eruptions appearing in adolescence and PAD presenting as small white papules in the anogenital or crural regions.

Grover disease (also referred to as transient acantholytic dermatosis) has an idiopathic pathophysiology. It clinically manifests with eruptions of erythematous, pruritic, truncal papules on the chest or back. Grover disease has a predilection for White men older than 50 years, and symptoms may be exacerbated in heat and diaphoretic conditions. Histologically, Grover disease may show acantholytic features seen in pemphigus vulgaris, HHD, and Darier disease; the pattern can only follow a specific disease or consist of a combination of all disease features (Figure 4). The acantholytic pattern of Grover disease was found to be similar to pemphigus vulgaris, Darier disease, pemphigus foliaceus, and HHD 47%, 18%, 9%, and 8% of the time, respectively. In 9% of cases, Grover disease will exhibit a mixed histopathology in which its acantholytic pattern will consist of a combination of features seen in the pemphigus family of diseases.6 Biopsy results showing mixed histologic patterns or a combination of different acantholytic features are suggestive of Grover disease over PAD. Moreover, the clinical distribution helps to differentiate Grover disease from PAD.

Because the histologic characteristics of these diseases overlap, certain nuances in clinical correlations and histology allow for distinction. In our patient, the diagnosis was most consistent with PAD based on the clinical manifestation of the disease and the biopsy results. Considering solely the clinical location of the lesions, Grover disease was a less likely diagnosis because our patient’s lesions were observed in the perianal region, not the truncal region as typically seen in Grover disease. Taking into account the DIF assay results in our patient, the pemphigus family of diseases also moved lower on the differential diagnosis. Finally, because the biopsy showed more dyskeratosis than would be present in HHD and also was inconsistent with the location and onset that would be expected to be seen in Darier disease, PAD was the most probable diagnosis. Interestingly, studies have shown mosaic mutations in ATP2A2 and ATP2C1 as possible causes of PAD, suggesting that this may be an allelic variant of Darier disease and HHD.7-9 No genetic testing was performed in our patient.
The Diagnosis: Papular Acantholytic Dyskeratosis
The shave biopsy revealed suprabasal clefts associated with acantholytic and dyskeratotic cells as well as overlying hyperkeratosis. Direct immunofluorescence (DIF) was negative. Based on the combined clinical and histological findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD), a rare disease that clinically presents as small whitishgreyish papules with the potential to coalesce into larger plaques.1,2 The condition predominantly manifests without symptoms, though pruritus and burning have been reported in affected sites. Most cases of PAD have been reported in older adults rather than in children or adolescents; it is more prevalent in women than in men. Lesions generally are localized to the penis, vulva, scrotum, inguinal folds, and perianal region.3 More specific terms have been used to describe this presentation such as papular acantholytic dyskeratosis of the anogenital region and papular acantholytic dyskeratosis of the genital-crural region. Histologic findings of PAD include epidermal acantholysis and dyskeratosis with hyperkeratosis and parakeratosis (quiz image).
The histologic differential diagnosis of PAD is broad due to its overlapping features with other diseases such as pemphigus vulgaris, Hailey-Hailey disease (HHD), Darier disease, and Grover disease. The acantholytic pathophysiology of these conditions involves dysfunction in cell adhesion markers. The correct diagnosis can be made by considering both the clinical location of involvement and histopathologic clues.
Pemphigus is a family of disorders involving mucocutaneous blistering of an autoimmune nature (Figure 1). Pemphigus vulgaris is the most prevalent variant of the pemphigus family, with symptomatically painful involvement of mucosal and cutaneous tissue. Autoantibodies to desmoglein 3 alone or both desmoglein 1 and 3 are present. Pemphigus vulgaris displays positive DIF findings with intercellular IgG and C3.

Hailey-Hailey disease (also known as benign familial pemphigus) is an autosomal-dominant disease that shares the acantholytic feature that is common in this class of diseases and caused by a defect in cell-cell adhesion as well as a loss of function in the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Blistering lesions typically appear in the neck, axillary, inguinal, or genital regions, and they can develop into crusted, exudate-filled lesions. No autoimmunity has been associated with this disease, unlike other diseases in the pemphigus family, and mutations in the ATP2C1 gene have been linked with dysregulation of cell-cell adhesion, particularly in cadherins and calcium-dependent cell adhesion processes. Histologically, HHD will show diffuse keratinocyte acantholysis with suprabasal clefting (Figure 2).4 Dyskeratosis is mild, if present at all, and dyskeratotic keratinocytes show a well-defined nucleus with cytoplasmic preservation. In contrast to HHD, PAD typically shows more dyskeratosis.

Darier disease (also known as keratosis follicularis) is an autosomal-dominant condition that normally presents with seborrheic eruptions in intertriginous areas, usually with onset during adolescence. Darier disease is caused by a loss-of-function mutation in the ATP2A2 gene found on chromosome 12q23-24.1 that encodes for the sarco(endo)plasmic reticulum calcium ATPase2 (SERCA2) enzymes involved in calcium-dependent transport of the endoplasmic reticulum within the cell. Due to calcium dysregulation, desmosomes are unable to carry out their function in cell-cell adhesion, resulting in keratinocyte acantholysis. Histopathology of Darier disease is identical to HHD but displays more dyskeratosis than HHD (Figure 3), possibly due to the endoplasmic reticulum calcium stores that are affected in Darier disease compared to the Golgi apparatus calcium stores that are implicated in HHD.5 The lowered endoplasmic reticulum calcium stores in Darier-White disease are associated with more pronounced dyskeratosis, which is seen histologically as corps ronds. Suprabasal hyperkeratosis also is found in Darier disease. The histopathologic findings of Darier disease and PAD can be identical, but the clinical presentations are distinct, with Darier disease typically manifesting as seborrheic eruptions appearing in adolescence and PAD presenting as small white papules in the anogenital or crural regions.

Grover disease (also referred to as transient acantholytic dermatosis) has an idiopathic pathophysiology. It clinically manifests with eruptions of erythematous, pruritic, truncal papules on the chest or back. Grover disease has a predilection for White men older than 50 years, and symptoms may be exacerbated in heat and diaphoretic conditions. Histologically, Grover disease may show acantholytic features seen in pemphigus vulgaris, HHD, and Darier disease; the pattern can only follow a specific disease or consist of a combination of all disease features (Figure 4). The acantholytic pattern of Grover disease was found to be similar to pemphigus vulgaris, Darier disease, pemphigus foliaceus, and HHD 47%, 18%, 9%, and 8% of the time, respectively. In 9% of cases, Grover disease will exhibit a mixed histopathology in which its acantholytic pattern will consist of a combination of features seen in the pemphigus family of diseases.6 Biopsy results showing mixed histologic patterns or a combination of different acantholytic features are suggestive of Grover disease over PAD. Moreover, the clinical distribution helps to differentiate Grover disease from PAD.

Because the histologic characteristics of these diseases overlap, certain nuances in clinical correlations and histology allow for distinction. In our patient, the diagnosis was most consistent with PAD based on the clinical manifestation of the disease and the biopsy results. Considering solely the clinical location of the lesions, Grover disease was a less likely diagnosis because our patient’s lesions were observed in the perianal region, not the truncal region as typically seen in Grover disease. Taking into account the DIF assay results in our patient, the pemphigus family of diseases also moved lower on the differential diagnosis. Finally, because the biopsy showed more dyskeratosis than would be present in HHD and also was inconsistent with the location and onset that would be expected to be seen in Darier disease, PAD was the most probable diagnosis. Interestingly, studies have shown mosaic mutations in ATP2A2 and ATP2C1 as possible causes of PAD, suggesting that this may be an allelic variant of Darier disease and HHD.7-9 No genetic testing was performed in our patient.
- Dowd ML, Ansell LH, Husain S, et al. Papular acantholytic dyskeratosis of the genitocrural area: a rare unilateral asymptomatic intertrigo. JAAD Case Rep. 2016;2:132-134. doi:10.1016/j.jdcr.2015.11.003
- Konstantinou MP, Krasagakis K. Benign familial pemphigus (Hailey Hailey disease). StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK585136/
- Montis-Palos MC, Acebo-Mariñas E, Catón-Santarén B, et al. Papular acantholytic dermatosis in the genito-crural region: a localized form of Darier disease or Hailey-Hailey disease? Actas Dermosifiliogr (Engl Ed). 2013;104:170-172. https://doi.org/10.1016/j.adengl.2012.02.008
- Verma SB. Papular acantholytic dyskeratosis localized to the perineal and perianal area in a young male. Indian J Dermatol. 2013;58:393-395.
- Schmieder SJ, Rosario-Collazo JA. Keratosis follicularis. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm .nih.gov/books/NBK519557/
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Knopp EA, Saraceni C, Moss J, et al. Somatic ATP2A2 mutation in a case of papular acantholytic dyskeratosis: mosaic Darier disease [published online August 12, 2015]. J Cutan Pathol. 2015;42:853-857. doi:10.1111/cup.12551
- Lipoff JB, Mudgil AV, Young S, et al. Acantholytic dermatosis of the crural folds with ATP2C1 mutation is a possible variant of Hailey-Hailey Disease. J Cutan Med Surg. 2009;13:151.
- Vodo D, Malchin N, Furman M, et al. Identification of a recurrent mutation in ATP2C1 demonstrates that papular acantholytic dyskeratosis and Hailey-Hailey disease are allelic disorders. Br J Dermatol. 2018;179:1001-1002.
- Dowd ML, Ansell LH, Husain S, et al. Papular acantholytic dyskeratosis of the genitocrural area: a rare unilateral asymptomatic intertrigo. JAAD Case Rep. 2016;2:132-134. doi:10.1016/j.jdcr.2015.11.003
- Konstantinou MP, Krasagakis K. Benign familial pemphigus (Hailey Hailey disease). StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK585136/
- Montis-Palos MC, Acebo-Mariñas E, Catón-Santarén B, et al. Papular acantholytic dermatosis in the genito-crural region: a localized form of Darier disease or Hailey-Hailey disease? Actas Dermosifiliogr (Engl Ed). 2013;104:170-172. https://doi.org/10.1016/j.adengl.2012.02.008
- Verma SB. Papular acantholytic dyskeratosis localized to the perineal and perianal area in a young male. Indian J Dermatol. 2013;58:393-395.
- Schmieder SJ, Rosario-Collazo JA. Keratosis follicularis. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm .nih.gov/books/NBK519557/
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Knopp EA, Saraceni C, Moss J, et al. Somatic ATP2A2 mutation in a case of papular acantholytic dyskeratosis: mosaic Darier disease [published online August 12, 2015]. J Cutan Pathol. 2015;42:853-857. doi:10.1111/cup.12551
- Lipoff JB, Mudgil AV, Young S, et al. Acantholytic dermatosis of the crural folds with ATP2C1 mutation is a possible variant of Hailey-Hailey Disease. J Cutan Med Surg. 2009;13:151.
- Vodo D, Malchin N, Furman M, et al. Identification of a recurrent mutation in ATP2C1 demonstrates that papular acantholytic dyskeratosis and Hailey-Hailey disease are allelic disorders. Br J Dermatol. 2018;179:1001-1002.
A 66-year-old man presented to the dermatology clinic with pruritus of the gluteal cleft and perianal region of several months’ duration. He had been prescribed permethrin by an outside physician, as well as oral acyclovir, triamcinolone-nystatin combination ointment, and topical zinc oxide prescribed by dermatology, without improvement. Physical examination showed several papules and erosions (<1 mm) in the perianal and gluteal cleft regions (inset). Hyperpigmented macules also were noted in the inguinal folds. A shave biopsy of a lesion from the perianal region was performed.
Results From the First Annual Association of Professors of Dermatology Program Directors Survey
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).
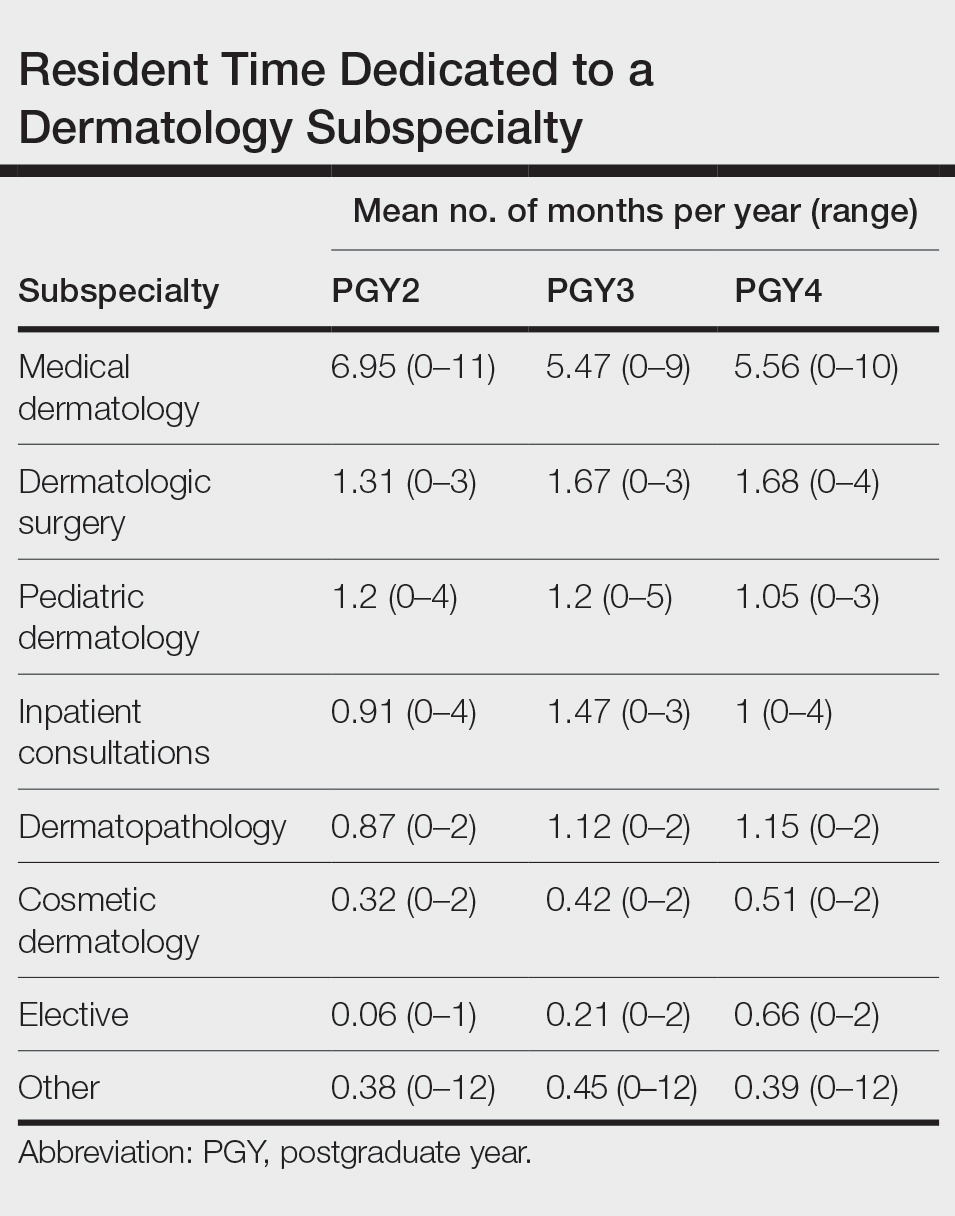
Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.
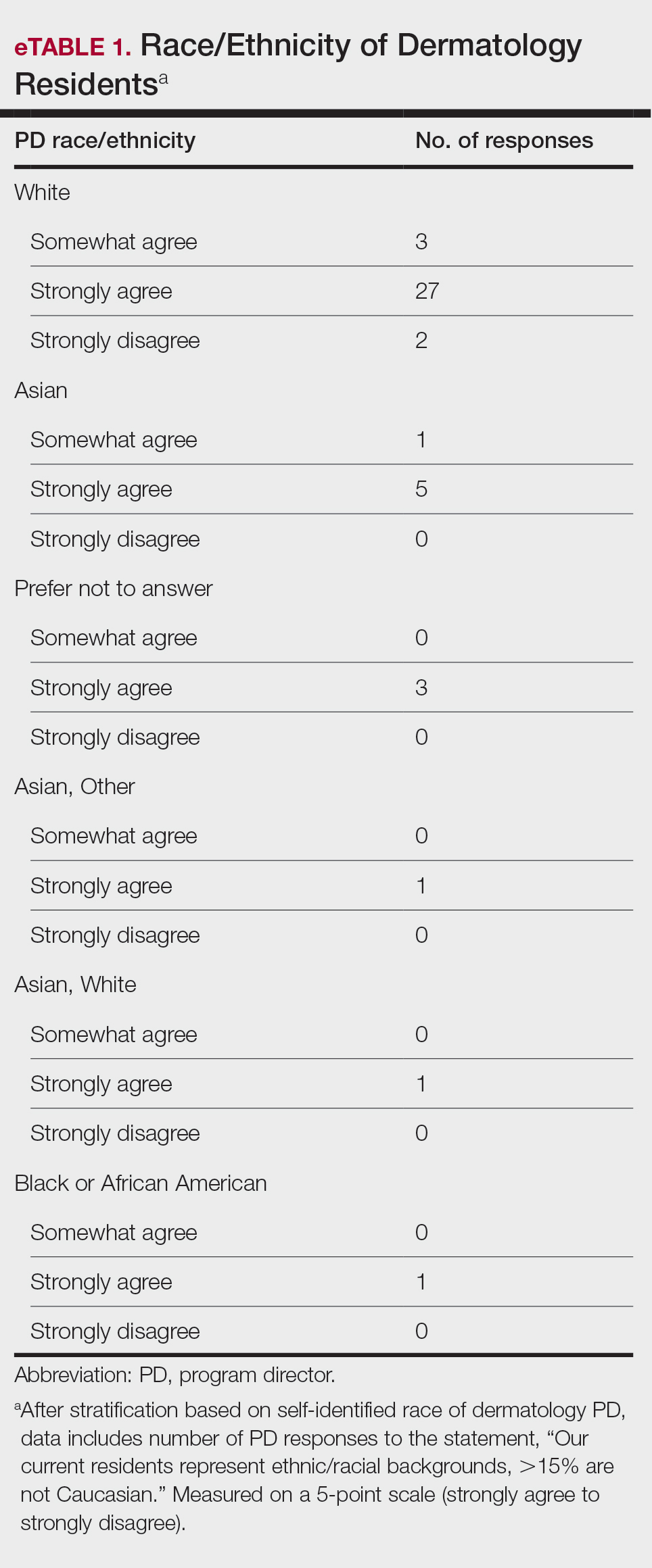
Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.
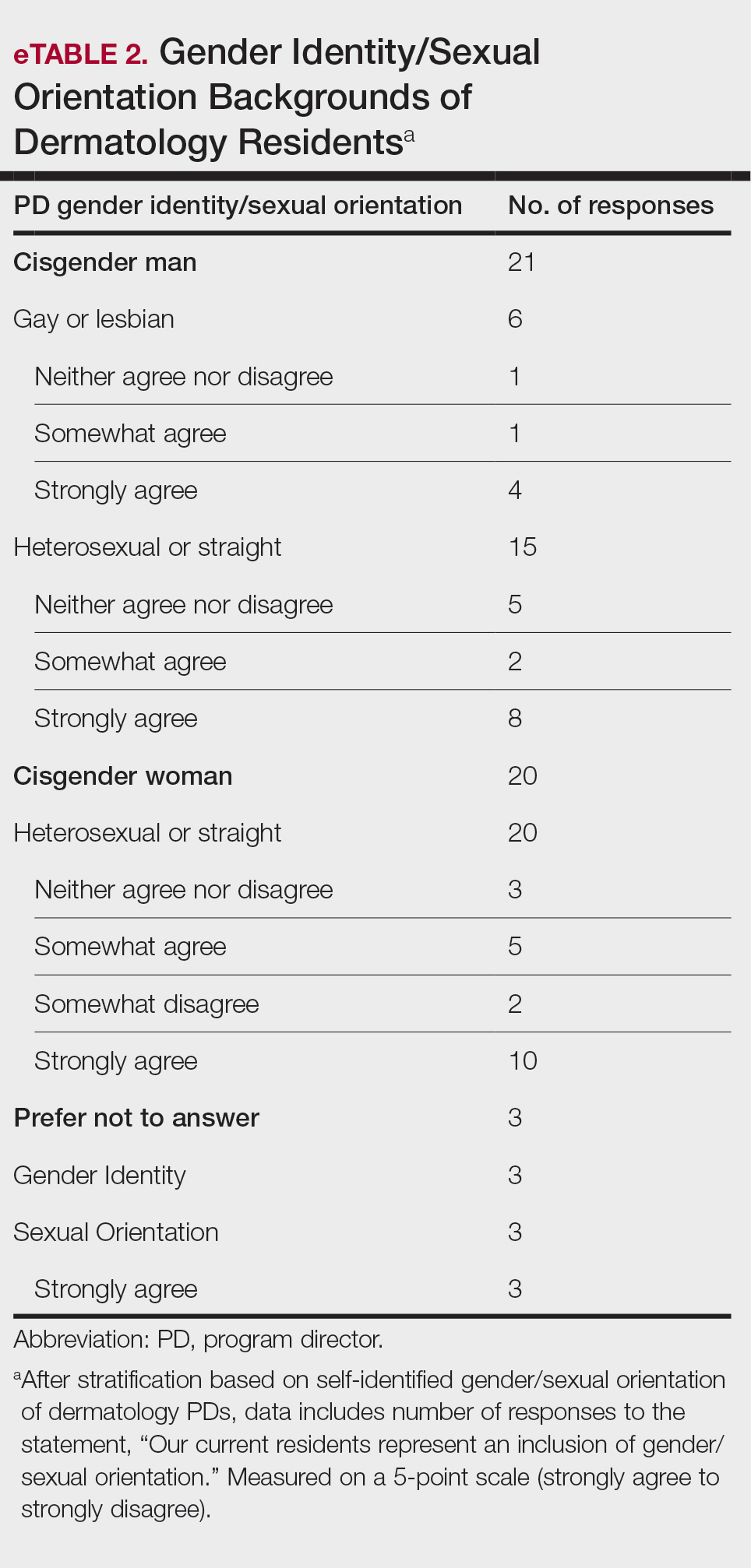
Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.
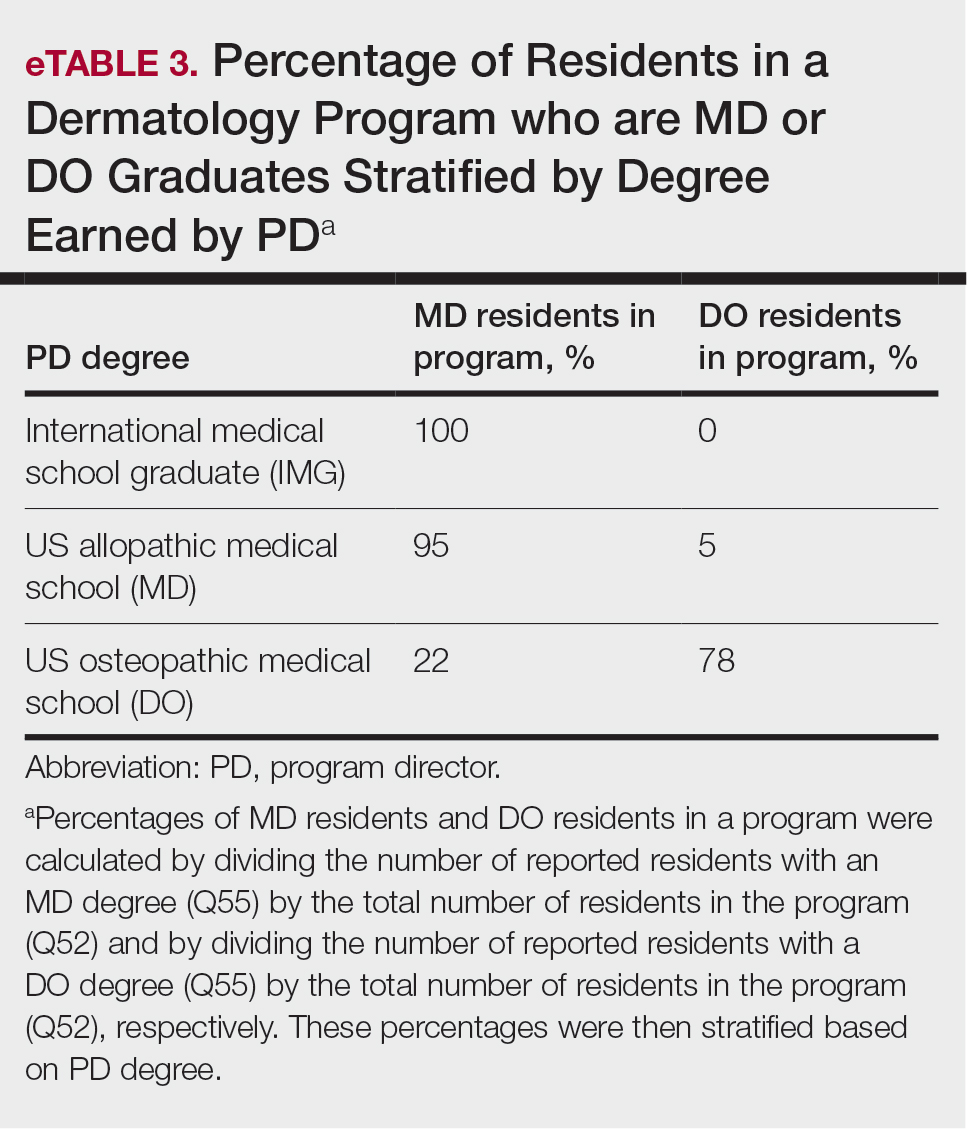
Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).

Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.

Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.

Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.

Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
Educational organizations across several specialties, including internal medicine and obstetrics and gynecology, have formal surveys1; however, the field of dermatology has been without one. This study aimed to establish a formal survey for dermatology program directors (PDs) and clinician-educators. Because the Accreditation Council for Graduate Medical Education (ACGME) and American Board of Dermatology surveys do not capture all metrics relevant to dermatology residency educators, an annual survey for our specialty may be helpful to compare dermatology-specific data among programs. Responses could provide context and perspective to faculty and residents who respond to the ACGME annual survey, as our Association of Professors of Dermatology (APD) survey asks more in-depth questions, such as how often didactics occur and who leads them. Resident commute time and faculty demographics and training also are covered. Current ad hoc surveys disseminated through listserves of various medical associations contain overlapping questions and reflect relatively low response rates; dermatology PDs may benefit from a survey with a high response rate to which they can contribute future questions and topics that reflect recent trends and current needs in graduate medical education. As future surveys are administered, the results can be captured in a centralized database accessible by dermatology PDs.
Methods
A survey of PDs from 141 ACGME-accredited dermatology residency programs was conducted by the Residency Program Director Steering Committee of the APD from November 2022 to January 2023 using a prevalidated questionnaire. Personalized survey links were created and sent individually to each PD’s email listed in the ACGME accreditation data system. All survey responses were captured anonymously, with a number assigned to keep de-identified responses separate and organized. The survey consisted of 137 survey questions addressing topics that included program characteristics, PD demographics, the impact of the COVID-19 pandemic on clinical rotation and educational conferences, available resident resources, quality improvement, clinical and didactic instruction, research content, diversity and inclusion, wellness, professionalism, evaluation systems, and graduate outcomes.
Data were collected using Qualtrics survey tools. After removing duplicate and incomplete surveys, data were analyzed using Qualtrics reports and Microsoft Excel for data plotting, averages, and range calculations.
Results
One hundred forty-one personalized survey links were created and sent individually to each program’s filed email obtained from the APD listserv. Fifty-three responses were recorded after removing duplicate or incomplete surveys (38% [53/141] response rate). As of May 2023, there were 144 ACGME-accredited dermatology residency programs due to 3 newly accredited programs in 2022-2023 academic year, which were not included in our survey population.
Program Characteristics—Forty-four respondents (83%) were from a university-based program. Fifty respondents (94%) were from programs that were ACGME accredited prior to 2020, while 3 programs (6%) were American Osteopathic Association accredited prior to singular accreditation. Seventy-one percent (38/53) of respondents had 1 or more associate PDs.
PD Demographics—Eighty-seven percent (45/52) of PDs who responded to the survey graduated from a US allopathic medical school (MD), 10% (5/52) graduated from a US osteopathic medical school (DO), and 4% (2/52) graduated from an international medical school. Seventy-four percent (35/47) of respondents were White, 17% (8/47) were Asian, and 2% (1/47) were Black or African American; this data was not provided for 4 respondents. Forty-eight percent (23/48) of PDs identified as cisgender man, 48% (23/48) identified as cisgender woman, and 4% (2/48) preferred not to answer. Eighty-one percent (38/47) of PDs identified as heterosexual or straight, 15% (7/47) identified as gay or lesbian, and 4% (2/47) preferred not to answer.
Impact of COVID-19 Pandemic on Residency Training—Due to the COVID-19 pandemic, 88% (45/51) of respondents incorporated telemedicine into the resident clinical rotation schedule. Moving forward, 75% (38/51) of respondents indicated that their programs plan to continue to incorporate telemedicine into the rotation schedule. Based on 50 responses, the average of educational conferences that became virtual at the start of the COVID-19 pandemic was 87%; based on 46 responses, the percentage of educational conferences that will remain virtual moving forward is 46%, while 90% (46/51) of respondents indicated that their programs plan to use virtual conferences in some capacity moving forward. Seventy-three percent (37/51) of respondents indicated that they plan to use virtual interviews as part of residency recruitment moving forward.
Available Resources—Twenty-four percent (11/46) of respondents indicated that residents in their program do not get protected time or time off for CORE examinations. Seventy-five percent (33/44) of PDs said their program provides funding for residents to participate in board review courses. The chief residents at 63% (31/49) of programs receive additional compensation, and 69% (34/49) provide additional administrative time to chief residents. Seventy-one percent (24/34) of PDs reported their programs have scribes for attendings, and 12% (4/34) have scribes for residents. Support staff help residents with callbacks and in-basket messages according to 76% (35/46) of respondents. The majority (98% [45/46]) of PDs indicated that residents follow-up on results and messages from patients seen in resident clinics, and 43% (20/46) of programs have residents follow-up with patients seen in faculty clinics. Only 15% (7/46) of PDs responded they have schedules with residents dedicated to handle these tasks. According to respondents, 33% (17/52) have residents who are required to travel more than 25 miles to distant clinical sites. Of them, 35% (6/17) provide accommodations.
Quality Improvement—Seventy-one percent (35/49) of respondents indicated their department has a quality improvement/patient safety team or committee, and 94% (33/35) of these teams include residents. A lecture series on quality improvement and patient safety is offered at 67% (33/49) of the respondents’ programs, while morbidity and mortality conferences are offered in 73% (36/49).
Clinical Instruction—Our survey asked PDs how many months each residency year spends on a certain rotational service. Based on 46 respondents, the average number of months dedicated to medical dermatology is 7, 5, and 6 months for postgraduate year (PGY) 2, PGY3, and PGY4, respectively. The average number of months spent in other subspecialties is provided in the Table. On average, PGY2 residents spend 8 half-days per week seeing patients in clinic, while PGY3 and PGY4 residents see patients for 7 half-days. The median and mean number of patients staffed by a single attending per hour in teaching clinics are 6 and 5.88, respectively. Respondents indicated that residents participate in the following specialty clinics: pediatric dermatology (96% [44/46]), laser/cosmetic (87% [40/44]), high-risk skin cancer (ie, immunosuppressed/transplant patient)(65% [30/44]), pigmented lesion/melanoma (52% [24/44]), connective tissue disease (52% [24/44]), teledermatology (50% [23/44]), free clinic for homeless and/or indigent populations (48% [22/44]), contact dermatitis (43% [20/44]), skin of color (43% [20/44]), oncodermatology (41% [19/44]), and bullous disease (33% [15/44]).

Additionally, in 87% (40/46) of programs, residents participate in a dedicated inpatient consultation service. Most respondents (98% [45/46]) responded that they utilize in-person consultations with a teledermatology supplement. Fifteen percent (7/46) utilize virtual teledermatology (live video-based consultations), and 57% (26/46) utilize asynchronous teledermatology (picture-based consultations). All respondents (n=46) indicated that 0% to 25% of patient encounters involving residents are teledermatology visits. Thirty-three percent (6/18) of programs have a global health special training track, 56% (10/18) have a Specialty Training and Advanced Research/Physician-Scientist Research Training track, 28% (5/18) have a diversity training track, and 50% (9/18) have a clinician educator training track.
Didactic Instruction—Five programs have a full day per week dedicated to didactics, while 36 programs have at least one half-day per week for didactics. On average, didactics in 57% (26/46) of programs are led by faculty alone, while 43% (20/46) are led at least in part by residents or fellows.

Research Content—Fifty percent (23/46) of programs have a specific research requirement for residents beyond general ACGME requirements, and 35% (16/46) require residents to participate in a longitudinal research project over the course of residency. There is a dedicated research coordinator for resident support at 63% (29/46) of programs. Dedicated biostatistics research support is available for resident projects at 42% (19/45) of programs. Additionally, at 42% (19/45) of programs, there is a dedicated faculty member for oversight of resident research.

Diversity, Equity, and Inclusion—Seventy-three percent (29/40) of programs have special diversity, equity, and inclusion programs or meetings specific to residency, 60% (24/40) have residency initiatives, and 55% (22/40) have a residency diversity committee. Eighty-six percent (42/49) of respondents strongly agreed that their current residents represent diverse ethnic and racial backgrounds (ie, >15% are not White). eTable 1 shows PD responses to this statement, which were stratified based on self-identified race. eTable 2 shows PD responses to the statement, “Our current residents represent an inclusion of gender/sexual orientation,” which were stratified based on self-identified gender identity/sexual orientation. Lastly, eTable 3 highlights the percentage of residents with an MD and DO degree, stratified based on PD degree.

Wellness—Forty-eight percent (20/42) of respondents indicated they are under stress and do not always have as much energy as before becoming a PD but do not feel burned out. Thirty-one percent (13/42) indicated they have 1 or more symptoms of burnout, such as emotional exhaustion. Eighty-six percent (36/42) are satisfied with their jobs overall (43% agree and 43% strongly agree [18/42 each]).
Evaluation System—Seventy-five percent (33/44) of programs deliver evaluations of residents by faculty online, 86% (38/44) of programs have PDs discuss evaluations in-person, and 20% (9/44) of programs have faculty evaluators discuss evaluations in-person. Seventy-seven percent (34/44) of programs have formal faculty-resident mentor-mentee programs. Clinical competency committee chair positions are filled by PDs, assistant PDs, or core faculty members 47%, 38%, and 16% of the time, respectively.
Graduation Outcomes of PGY4 Residents—About 28% (55/199) of graduating residents applied to a fellowship position, with the majority (15% [29/55]) matching into Mohs micrographic surgery and dermatologic oncology (MSDO) fellowships. Approximately 5% (9/199) and 4% (7/199) of graduates matched into dermatopathology and pediatric dermatology, respectively. The remaining 5% (10/199) of graduating residents applied to a fellowship but did not match. The majority (45% [91/199]) of residency graduates entered private practice after graduation. Approximately 21% (42/199) of graduating residents chose an academic practice with 17% (33/199), 2% (4/199), and 2% (3/199) of those positions being full-time, part-time, and adjunct, respectively.
Comment
The first annual APD survey is a novel data source and provides opportunities for areas of discussion and investigation. Evaluating the similarities and differences among dermatology residency programs across the United States can strengthen individual programs through collaboration and provide areas of cohesion among programs.
Diversity of PDs—An important area of discussion is diversity and PD demographics. Although DO students make up 1 in 4 US graduating medical students, they are not interviewed or ranked as often as MD students.2 Diversity in PD race and ethnicity may be worthy of investigation in future studies, as match rates and recruitment of diverse medical school applicants may be impacted by these demographics.
Continued Use of Telemedicine in Training—Since 2020, the benefits of virtual residency recruitment have been debated among PDs across all medical specialties. Points in favor of virtual interviews include cost savings for programs and especially for applicants, as well as time efficiency, reduced burden of travel, and reduced carbon footprint. A problem posed by virtual interviews is that candidates are unable to fully learn institutional cultures and social environments of the programs.3 Likewise, telehealth was an important means of clinical teaching for residents during the height of the COVID-19 pandemic, with benefits that included cost-effectiveness and reduction of disparities in access to dermatologic care.4 Seventy-five percent (38/51) of PDs indicated that their program plans to include telemedicine in resident clinical rotation moving forward.
Resources Available—Our survey showed that resources available for residents, delivery of lectures and program time allocated to didactics, protected academic or study time for residents, and allocation of program time for CORE examinations are highly variable across programs. This could inspire future studies to be done to determine the differences in success of the resident on CORE examinations and in digesting material.
Postgraduate Career Plans and Fellowship Matches—Residents of programs that have a home MSDO fellowship are more likely to successfully match into a MSDO fellowship.5 Based on this survey, approximately 28% of graduating residents applied to a fellowship position, with 15%, 5%, and 3% matching into desired MSDO, dermatopathology, and pediatric dermatology fellowships, respectively. Additional studies are needed to determine advantages and disadvantages that lead to residents reaching their career goals.
Limitations—Limitations of this study include a small sample size that may not adequately represent all ACGME-accredited dermatology residency programs and selection bias toward respondents who are more likely to participate in survey-based research.
Conclusion
The APD plans to continue to administer this survey on an annual basis, with updates to the content and questions based on input from PDs. This survey will continue to provide valuable information to drive collaboration among residency programs and optimize the learning experience for residents. Our hope is that the response rate will increase in coming years, allowing us to draw more generalizable conclusions. Nonetheless, the survey data allow individual dermatology residency programs to compare their specific characteristics to other programs.
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
- Maciejko L, Cope A, Mara K, et al. A national survey of obstetrics and gynecology emergency training and deficits in office emergency preparation [A53]. Obstet Gynecol. 2022;139:16S. doi:10.1097/01.AOG.0000826548.05758.26
- Lavertue SM, Terry R. A comparison of surgical subspecialty match rates in 2022 in the United States. Cureus. 2023;15:E37178. doi:10.7759/cureus.37178
- Domingo A, Rdesinski RE, Stenson A, et al. Virtual residency interviews: applicant perceptions regarding virtual interview effectiveness, advantages, and barriers. J Grad Med Educ. 2022;14:224-228. doi:10.4300/JGME-D-21-00675.1
- Rustad AM, Lio PA. Pandemic pressure: teledermatology and health care disparities. J Patient Exp. 2021;8:2374373521996982. doi:10.1177/2374373521996982
- Rickstrew J, Rajpara A, Hocker TLH. Dermatology residency program influences chance of successful surgery fellowship match. Dermatol Surg. 2021;47:1040-1042. doi:10.1097/DSS.0000000000002859
Practice Points
- The first annual Association of Professors of Dermatology program directors survey allows faculty to compare their programs to other dermatology residency programs across the United States.
- The results should inspire opportunities for growth, improvement, and collaboration among dermatology residency programs.
Effect of COVID-19 Vaccination on Disease Severity in Patients With Stable Plaque Psoriasis: A Cross-sectional Study
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.
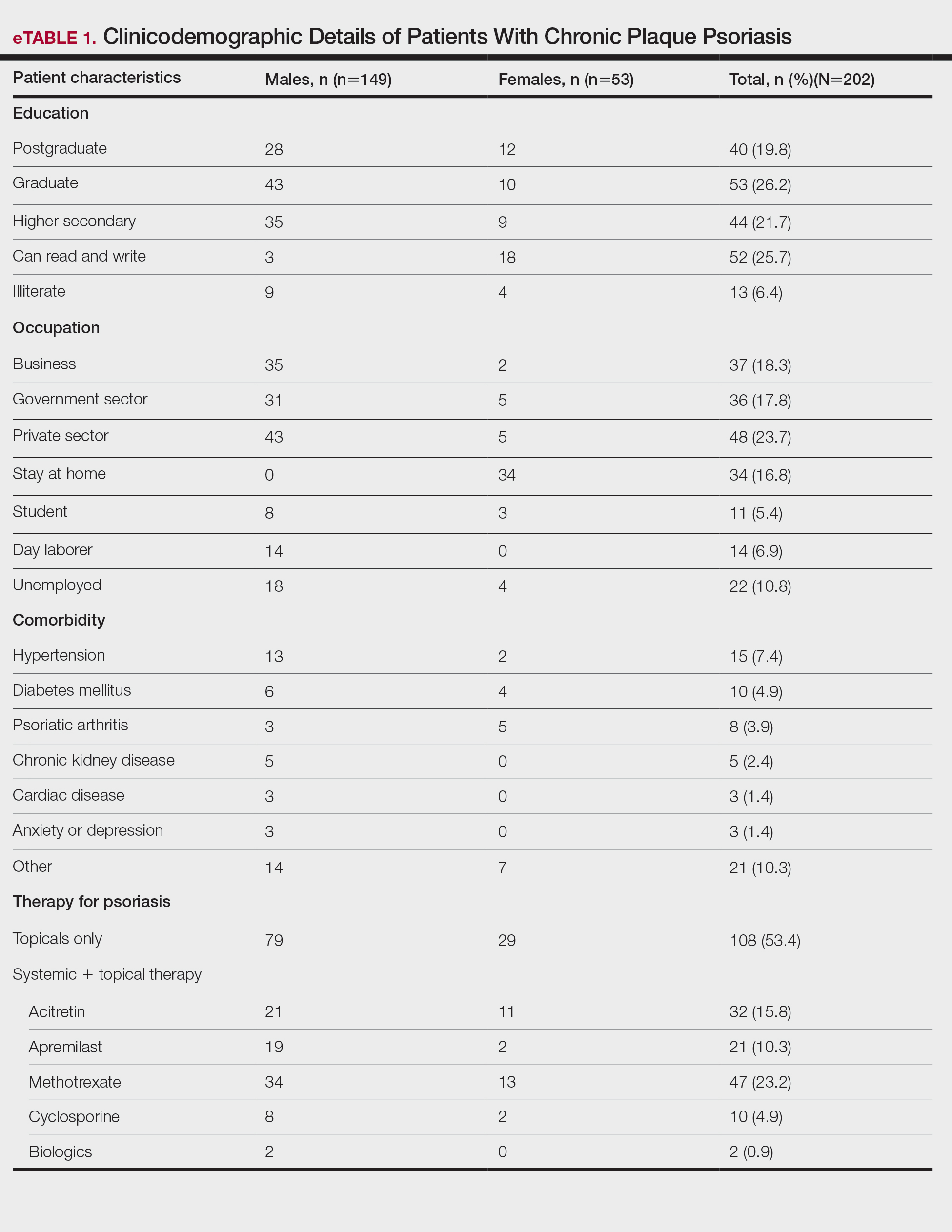
The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.

The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.

The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
Practice Points
- Vaccines are known to induce a psoriasis flare.
- Given the high risk for severe COVID infection in individuals with psoriasis who have comorbidities, vaccination with Covaxin and Covishield can be safely recommended in India for this population.
What’s Eating You? Tropical Rat Mite (Ornithonyssus bacoti)
The tropical rat mite (Ornithonyssus bacoti) belongs to the family Macronyssidae. Theses mites are commonly mistaken for red bird mites or Nordic bird mites because they belong to the same family and have similar characteristics.1 Although O bacoti is called the tropical rat mite, it also can be found in moderate climates.2,3
Characteristics
The life cycle of a tropical rat mite lasts 11 to 13 days and includes 5 stages: egg, larva, protonymph, deutonymph, and adult.1,2 The length of the mite (0.3–0.7 mm) varies with the stage of development.1 Adults can reach 0.75 to 1.40 mm, with females larger than males and possibly visible with the naked eye.1,2
Two or 3 days after a blood meal, the female mite lays approximately 100 eggs in its nest but not on the surface of a host. The eggs hatch into larvae after 1 to 4 days and go on to complete their life cyle.1 During developmental stages, mites occupy their hosts for blood meals. Mites search for their hosts at night and prefer wild or pet rodents for blood meals but are not host specific and can be found on many mammals including birds, cats, racoons, and squirrels.4
Although tropical rat mites prefer rodent hosts, they can infest humans when their preferred host is unavailable. In the United States, the first case of human dermatitis due to a tropical rate mite occurred in 1923. In Europe, rat mite dermatitis was first reported in a human in 1931, possibly due to contamination of sailing vessels.4
Infestation and Transmission
Tropical rat mites prefer wild and pet rodents as hosts because the mites are able to feed on their blood over long periods.4 During the day, the mite spends most of its time hiding in dark dry spaces; it is most active during the night, traveling to find a host for meals.3-5 If a preferred host is not present, the mite may choose to infest a human.5
Human infestation occurs most often upon close bodily contact with an infected animal or pet rodent that was sold without parasites having been eliminated.3-5 Mites are able to survive without a host for as long as 6 months; they may travel after a meal.1,2 Therefore, individuals who do not have a pet rodent can be infested if an infected wild rodent has infested their living space.1,3-5
Clinical Presentation of Infestation
Patients infested with tropical rat mites present with pruritic cutaneous lesions, most often on unclothed parts of the body that are easily exposed to mites; lesions rarely occur on the scalp.5 People of any age or gender can be infested. Rat mite bites can present as single or grouped, pruritic, erythematous papules ranging in size from 4 to 10 mm in diameter.5-7 Excoriations may be present due to excessive scratching. Although rare, vesicles or nodules have been reported.5,7
Diagnosis of the underlying cause of the cutaneous manifestations often is difficult because mites are not visible during the day, as they are less active then.2 Lesions often are misdiagnosed as an allergic response, a bacterial infection, or various forms of dermatitis.1 A parasitic cause often is not considered unless the physician or patient detects a mite or many trials of therapies fail to provide relief.1,3-5 Eliciting a thorough history may disclose that the patient has had close contact with rodents or lives in a community center, shelter, or shared space. If any of the patient’s close contacts have a similar presentation, infestation with mites should be considered.
Treatment and Prevention
Patients should be educated about treatment options and measures that need to be taken to prevent reinfection. It has been reported that tropical rat mites can survive without a blood meal for as long as 6 months; therefore, meticulous inspection and decontamination of all living spaces is required.1,4 Once identified, physicians may prescribe an antiparasitic such as permethrin or pyriproxyfen to prevent further infestation and eliminate mites on the host.5 Lindane and benzyl benzoate previously were reported to be effective but should be prescribed only in correctly diagnosed cases due to the potential adverse effects of both therapies.4,7-10 For effective treatment, physicians should thoroughly review the proper application of topical treatments with patients. Topical creams should be massaged into the skin from the head to the soles of the feet, covering all creases of the skin and between the fingers and toes. Antiparasitic creams should be left on the skin for 8 to 14 hours, and all members of the household should be examined and treated, if necessary, by a physician. A thorough bath removes tropical rat mites, but preventive measures should be taken to prevent reinfestation.4 Antihistamines or glucocorticoids also can be used as symptomatic treatment.6,8
Avoiding Reinfestation—Preventive measures should be taken to prevent reinfestation, including evaluation by an exterminator for any wild rodents to remove nests and treat the living space with an acaricide.5 Insecticides administered by exterminators, including malathion, methyl carbamate, and lindane, also have been reported to be effective for preventing reinfestation.5,7-9 A veterinarian should be consulted if the patient owns any pets to ensure proper identification of any potential tropical rat mites and treatments that may be necessary for any household pets.1
Case Report
A 68-year-old man presented to the dermatology outpatient clinic with diffuse pruritus of the skin and scalp. He reported no other symptoms and had never had a total-body skin examination. His primary care physician recently prescribed a dose pack of methylprednisolone 4-mg tablets, which relieved the symptoms except for a mild scalp itch. His wife did not experience itching, and he denied noticing mites or fleas on his pet dog. Physical examination did not reveal any contributory findings, such as erythema or rash. Ketoconazole shampoo 2% and fluocinolone solution 0.01% were prescribed for scalp pruritus; however, he could not afford those medications and therefore did not take them.
Two weeks later, the patient presented with diffuse itching that involved the scalp, trunk, and extremities. He denied groin pruritus. He reported that the itching was worse at night. His wife continued to be asymptomatic. The patient reported that his health screening was up-to-date, and he had no interval health changes. A complete blood cell count, thyroid studies, and a comprehensive metabolic panel performed recently were within reference range. He denied recent travel or taking new medications. Physical examination revealed a somewhat linear distribution of erythematous urticarial papules on the right side of the abdomen. Red dermatographic excoriations were noted on the back. No burrows were visualized. He was given intramuscular triamcinolone 60 mg and was advised to have his house evaluated for bed bugs and his pet dog evaluated by a veterinarian for mites. During the triamcinolone injection, the medical assistant observed a 1- to 2-mm red insect, which fell into his clothing and could not be further evaluated.
After 1 month, the patient had no improvement of the pruritus; instead, it became worse. During this time, his wife developed intermittent urticarial-like eruptions. He was taking oral diphenhydramine nightly and applying triamcinolone cream 0.5% that he had at home from an earlier skin problem as needed. Physical examination findings correlated with worsening symptoms. He had multiple erythematous urticarial papules—many of which were excoriated—across the chest, abdomen, buttocks, and back. The arms had multiple excoriations. The urticarial papules coalesced in the anterior axillary folds, yet no burrows were visualized. In the left anterior axillary fold adjacent to one of the urticarial papules, a 1-mm mobile mite was identified on dermoscopy. Further evaluation by microscopy showed morphologic characteristics of a tropical rat mite (Figure). The patient admitted that his house had a mouse infestation that he was struggling to eliminate. Permethrin cream 5% was prescribed. Because the patient could not afford the prescription, he was advised to use the triamcinolone cream 0.5%and oral diphenhydramine that he had at home nightly for symptomatic relief. He was advised to hire an exterminator to eradicate the mouse and mite infestation to prevent reinfestation.

Identification of Rate Mite Dermatitis
The characteristics of tropical rat mite dermatitis can be confused with many other conditions, such as infection. Even when a mite is identified, it can be difficult to classify it as a tropical rat mite. To confirm the diagnosis of tropical rat mite dermatitis, the parasite needs to be identified. Skin scrapings can be collected from pruritic lesions and examined microscopically in the hope of revealing the rat mites. The recommendation is to collect skin scrapings from the dorsal aspect of the hands or from the neck.5 Patients may report finding mites in their living space or on their bedding or clothing.
Although the tropical rat mite was reported as a vector for endemic typhus between humans, no other cases of transmission between humans have been reported since.11,12 Studies reporting non–human subject research and case reports have shown that O bacoti is a vector for Rickettsia akari, Coxiella burnetii, Francisella tularensis, Yersinia pestis, Eastern equine encephalitis virus (Alphavirus), Enterovirus (Picornaviridae), Langat virus (Flavivirus), and Hantaan orthohantavirus.5,11-17 However, no cases of these infectious diseases being transmitted naturally have been reported.5
Confirmation of O bacoti as a vector for human pathogens is difficult because it relies on identification of the mite in the clinic.5 The epidemiologic importance of the mite in transmitting infectious disease is unknown; reports of human cases of mite infestation are rare. We present this information to increase awareness and help dermatologists and other health care providers identify O bacoti.
- Beck W, Fölster-Holst R. Tropical rat mites (Ornithonyssus bacoti)—serious ectoparasites. J Dtsch Dermatol Ges. 2009;7:667-670. doi:10.1111/j.1610-0387.2009.07140.x
- Baumstark J, Beck W, Hofmann H. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology. 2007;215:66-68. doi:10.1159/000102037
- Beck W. Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings. Travel Med Infect Dis. 2008;6:245-249. doi:10.1016/j.tmaid.2008.01.002
- Beck W. Tropical rat mites as newly emerging disease pathogens in rodents and man. Trav Med Infect Dis. 2007;5:403. doi:10.1016/j.tmaid.2007.09.016
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469. doi:10.1086/515016
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678. doi:10.1001/archderm.1982.01650210056019
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Ram SM, Satija KC, Kaushik RK. Ornithonyssus bacoti infestation in laboratory personnel and veterinary students. Int J Zoonoses. 1986;13:138-140.
- Brown S, Becher J, Brady W. Treatment of ectoparasitic infections: review of the English-language literature, 1982-1992. Clin Infect Dis. 1995;20(suppl 1):S104-S109. doi:10.1093/clinids/20.supplement_1.s104
- Reeves WK, Loftis AD, Szumlas DE, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol. 2007;41:101-107. doi:10.1007/s10493-006-9040-3
- Philip CB, Hughes LE. The tropical rat mite; Liponyssus bacoti, as an experimental vector of rickettsialpox. Am J Trop Med Hyg. 1948;28:697-705. doi:10.4269/ajtmh.1948.s1-28.697
- Zemskaia AA, Pchelkina AA. Experimental infection of ticks Dermanyssus gallinae Redi Bdellonyssus bacoti Hirst with Q fever. Dokl Akad Nauk SSSR. 1955;101:391-392.
- Hopla CE. Experimental transmission of tularemia by the tropical rat mite. Am J Trop Med Hyg. 1951;31:768-783. doi:10.4269/ajtmh.1951.s1-31.768
- Clark GM, Lutz AE, Fadnessl. Observations on the ability of Haemogamasus liponyssoides Ewing and Ornithonyssus bacoti (Hirst) (Acarina, Gamasina) to retain eastern equine encephalitis virus: preliminary report. Am J Trop Med Hyg. 1966;15:107-112. doi:10.4269/ajtmh.1966.15.107
- Schwab M, Allen R, Sulkin SE. The tropical rat mite (Liponyssus bacoti) as an experimental vector of Coxsackie virus. Am J Trop Med Hyg. 1952;1:982-986. doi:10.4269/ajtmh.1952.1.982
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641. doi:10.1093/jmedent/30.3.639
The tropical rat mite (Ornithonyssus bacoti) belongs to the family Macronyssidae. Theses mites are commonly mistaken for red bird mites or Nordic bird mites because they belong to the same family and have similar characteristics.1 Although O bacoti is called the tropical rat mite, it also can be found in moderate climates.2,3
Characteristics
The life cycle of a tropical rat mite lasts 11 to 13 days and includes 5 stages: egg, larva, protonymph, deutonymph, and adult.1,2 The length of the mite (0.3–0.7 mm) varies with the stage of development.1 Adults can reach 0.75 to 1.40 mm, with females larger than males and possibly visible with the naked eye.1,2
Two or 3 days after a blood meal, the female mite lays approximately 100 eggs in its nest but not on the surface of a host. The eggs hatch into larvae after 1 to 4 days and go on to complete their life cyle.1 During developmental stages, mites occupy their hosts for blood meals. Mites search for their hosts at night and prefer wild or pet rodents for blood meals but are not host specific and can be found on many mammals including birds, cats, racoons, and squirrels.4
Although tropical rat mites prefer rodent hosts, they can infest humans when their preferred host is unavailable. In the United States, the first case of human dermatitis due to a tropical rate mite occurred in 1923. In Europe, rat mite dermatitis was first reported in a human in 1931, possibly due to contamination of sailing vessels.4
Infestation and Transmission
Tropical rat mites prefer wild and pet rodents as hosts because the mites are able to feed on their blood over long periods.4 During the day, the mite spends most of its time hiding in dark dry spaces; it is most active during the night, traveling to find a host for meals.3-5 If a preferred host is not present, the mite may choose to infest a human.5
Human infestation occurs most often upon close bodily contact with an infected animal or pet rodent that was sold without parasites having been eliminated.3-5 Mites are able to survive without a host for as long as 6 months; they may travel after a meal.1,2 Therefore, individuals who do not have a pet rodent can be infested if an infected wild rodent has infested their living space.1,3-5
Clinical Presentation of Infestation
Patients infested with tropical rat mites present with pruritic cutaneous lesions, most often on unclothed parts of the body that are easily exposed to mites; lesions rarely occur on the scalp.5 People of any age or gender can be infested. Rat mite bites can present as single or grouped, pruritic, erythematous papules ranging in size from 4 to 10 mm in diameter.5-7 Excoriations may be present due to excessive scratching. Although rare, vesicles or nodules have been reported.5,7
Diagnosis of the underlying cause of the cutaneous manifestations often is difficult because mites are not visible during the day, as they are less active then.2 Lesions often are misdiagnosed as an allergic response, a bacterial infection, or various forms of dermatitis.1 A parasitic cause often is not considered unless the physician or patient detects a mite or many trials of therapies fail to provide relief.1,3-5 Eliciting a thorough history may disclose that the patient has had close contact with rodents or lives in a community center, shelter, or shared space. If any of the patient’s close contacts have a similar presentation, infestation with mites should be considered.
Treatment and Prevention
Patients should be educated about treatment options and measures that need to be taken to prevent reinfection. It has been reported that tropical rat mites can survive without a blood meal for as long as 6 months; therefore, meticulous inspection and decontamination of all living spaces is required.1,4 Once identified, physicians may prescribe an antiparasitic such as permethrin or pyriproxyfen to prevent further infestation and eliminate mites on the host.5 Lindane and benzyl benzoate previously were reported to be effective but should be prescribed only in correctly diagnosed cases due to the potential adverse effects of both therapies.4,7-10 For effective treatment, physicians should thoroughly review the proper application of topical treatments with patients. Topical creams should be massaged into the skin from the head to the soles of the feet, covering all creases of the skin and between the fingers and toes. Antiparasitic creams should be left on the skin for 8 to 14 hours, and all members of the household should be examined and treated, if necessary, by a physician. A thorough bath removes tropical rat mites, but preventive measures should be taken to prevent reinfestation.4 Antihistamines or glucocorticoids also can be used as symptomatic treatment.6,8
Avoiding Reinfestation—Preventive measures should be taken to prevent reinfestation, including evaluation by an exterminator for any wild rodents to remove nests and treat the living space with an acaricide.5 Insecticides administered by exterminators, including malathion, methyl carbamate, and lindane, also have been reported to be effective for preventing reinfestation.5,7-9 A veterinarian should be consulted if the patient owns any pets to ensure proper identification of any potential tropical rat mites and treatments that may be necessary for any household pets.1
Case Report
A 68-year-old man presented to the dermatology outpatient clinic with diffuse pruritus of the skin and scalp. He reported no other symptoms and had never had a total-body skin examination. His primary care physician recently prescribed a dose pack of methylprednisolone 4-mg tablets, which relieved the symptoms except for a mild scalp itch. His wife did not experience itching, and he denied noticing mites or fleas on his pet dog. Physical examination did not reveal any contributory findings, such as erythema or rash. Ketoconazole shampoo 2% and fluocinolone solution 0.01% were prescribed for scalp pruritus; however, he could not afford those medications and therefore did not take them.
Two weeks later, the patient presented with diffuse itching that involved the scalp, trunk, and extremities. He denied groin pruritus. He reported that the itching was worse at night. His wife continued to be asymptomatic. The patient reported that his health screening was up-to-date, and he had no interval health changes. A complete blood cell count, thyroid studies, and a comprehensive metabolic panel performed recently were within reference range. He denied recent travel or taking new medications. Physical examination revealed a somewhat linear distribution of erythematous urticarial papules on the right side of the abdomen. Red dermatographic excoriations were noted on the back. No burrows were visualized. He was given intramuscular triamcinolone 60 mg and was advised to have his house evaluated for bed bugs and his pet dog evaluated by a veterinarian for mites. During the triamcinolone injection, the medical assistant observed a 1- to 2-mm red insect, which fell into his clothing and could not be further evaluated.
After 1 month, the patient had no improvement of the pruritus; instead, it became worse. During this time, his wife developed intermittent urticarial-like eruptions. He was taking oral diphenhydramine nightly and applying triamcinolone cream 0.5% that he had at home from an earlier skin problem as needed. Physical examination findings correlated with worsening symptoms. He had multiple erythematous urticarial papules—many of which were excoriated—across the chest, abdomen, buttocks, and back. The arms had multiple excoriations. The urticarial papules coalesced in the anterior axillary folds, yet no burrows were visualized. In the left anterior axillary fold adjacent to one of the urticarial papules, a 1-mm mobile mite was identified on dermoscopy. Further evaluation by microscopy showed morphologic characteristics of a tropical rat mite (Figure). The patient admitted that his house had a mouse infestation that he was struggling to eliminate. Permethrin cream 5% was prescribed. Because the patient could not afford the prescription, he was advised to use the triamcinolone cream 0.5%and oral diphenhydramine that he had at home nightly for symptomatic relief. He was advised to hire an exterminator to eradicate the mouse and mite infestation to prevent reinfestation.

Identification of Rate Mite Dermatitis
The characteristics of tropical rat mite dermatitis can be confused with many other conditions, such as infection. Even when a mite is identified, it can be difficult to classify it as a tropical rat mite. To confirm the diagnosis of tropical rat mite dermatitis, the parasite needs to be identified. Skin scrapings can be collected from pruritic lesions and examined microscopically in the hope of revealing the rat mites. The recommendation is to collect skin scrapings from the dorsal aspect of the hands or from the neck.5 Patients may report finding mites in their living space or on their bedding or clothing.
Although the tropical rat mite was reported as a vector for endemic typhus between humans, no other cases of transmission between humans have been reported since.11,12 Studies reporting non–human subject research and case reports have shown that O bacoti is a vector for Rickettsia akari, Coxiella burnetii, Francisella tularensis, Yersinia pestis, Eastern equine encephalitis virus (Alphavirus), Enterovirus (Picornaviridae), Langat virus (Flavivirus), and Hantaan orthohantavirus.5,11-17 However, no cases of these infectious diseases being transmitted naturally have been reported.5
Confirmation of O bacoti as a vector for human pathogens is difficult because it relies on identification of the mite in the clinic.5 The epidemiologic importance of the mite in transmitting infectious disease is unknown; reports of human cases of mite infestation are rare. We present this information to increase awareness and help dermatologists and other health care providers identify O bacoti.
The tropical rat mite (Ornithonyssus bacoti) belongs to the family Macronyssidae. Theses mites are commonly mistaken for red bird mites or Nordic bird mites because they belong to the same family and have similar characteristics.1 Although O bacoti is called the tropical rat mite, it also can be found in moderate climates.2,3
Characteristics
The life cycle of a tropical rat mite lasts 11 to 13 days and includes 5 stages: egg, larva, protonymph, deutonymph, and adult.1,2 The length of the mite (0.3–0.7 mm) varies with the stage of development.1 Adults can reach 0.75 to 1.40 mm, with females larger than males and possibly visible with the naked eye.1,2
Two or 3 days after a blood meal, the female mite lays approximately 100 eggs in its nest but not on the surface of a host. The eggs hatch into larvae after 1 to 4 days and go on to complete their life cyle.1 During developmental stages, mites occupy their hosts for blood meals. Mites search for their hosts at night and prefer wild or pet rodents for blood meals but are not host specific and can be found on many mammals including birds, cats, racoons, and squirrels.4
Although tropical rat mites prefer rodent hosts, they can infest humans when their preferred host is unavailable. In the United States, the first case of human dermatitis due to a tropical rate mite occurred in 1923. In Europe, rat mite dermatitis was first reported in a human in 1931, possibly due to contamination of sailing vessels.4
Infestation and Transmission
Tropical rat mites prefer wild and pet rodents as hosts because the mites are able to feed on their blood over long periods.4 During the day, the mite spends most of its time hiding in dark dry spaces; it is most active during the night, traveling to find a host for meals.3-5 If a preferred host is not present, the mite may choose to infest a human.5
Human infestation occurs most often upon close bodily contact with an infected animal or pet rodent that was sold without parasites having been eliminated.3-5 Mites are able to survive without a host for as long as 6 months; they may travel after a meal.1,2 Therefore, individuals who do not have a pet rodent can be infested if an infected wild rodent has infested their living space.1,3-5
Clinical Presentation of Infestation
Patients infested with tropical rat mites present with pruritic cutaneous lesions, most often on unclothed parts of the body that are easily exposed to mites; lesions rarely occur on the scalp.5 People of any age or gender can be infested. Rat mite bites can present as single or grouped, pruritic, erythematous papules ranging in size from 4 to 10 mm in diameter.5-7 Excoriations may be present due to excessive scratching. Although rare, vesicles or nodules have been reported.5,7
Diagnosis of the underlying cause of the cutaneous manifestations often is difficult because mites are not visible during the day, as they are less active then.2 Lesions often are misdiagnosed as an allergic response, a bacterial infection, or various forms of dermatitis.1 A parasitic cause often is not considered unless the physician or patient detects a mite or many trials of therapies fail to provide relief.1,3-5 Eliciting a thorough history may disclose that the patient has had close contact with rodents or lives in a community center, shelter, or shared space. If any of the patient’s close contacts have a similar presentation, infestation with mites should be considered.
Treatment and Prevention
Patients should be educated about treatment options and measures that need to be taken to prevent reinfection. It has been reported that tropical rat mites can survive without a blood meal for as long as 6 months; therefore, meticulous inspection and decontamination of all living spaces is required.1,4 Once identified, physicians may prescribe an antiparasitic such as permethrin or pyriproxyfen to prevent further infestation and eliminate mites on the host.5 Lindane and benzyl benzoate previously were reported to be effective but should be prescribed only in correctly diagnosed cases due to the potential adverse effects of both therapies.4,7-10 For effective treatment, physicians should thoroughly review the proper application of topical treatments with patients. Topical creams should be massaged into the skin from the head to the soles of the feet, covering all creases of the skin and between the fingers and toes. Antiparasitic creams should be left on the skin for 8 to 14 hours, and all members of the household should be examined and treated, if necessary, by a physician. A thorough bath removes tropical rat mites, but preventive measures should be taken to prevent reinfestation.4 Antihistamines or glucocorticoids also can be used as symptomatic treatment.6,8
Avoiding Reinfestation—Preventive measures should be taken to prevent reinfestation, including evaluation by an exterminator for any wild rodents to remove nests and treat the living space with an acaricide.5 Insecticides administered by exterminators, including malathion, methyl carbamate, and lindane, also have been reported to be effective for preventing reinfestation.5,7-9 A veterinarian should be consulted if the patient owns any pets to ensure proper identification of any potential tropical rat mites and treatments that may be necessary for any household pets.1
Case Report
A 68-year-old man presented to the dermatology outpatient clinic with diffuse pruritus of the skin and scalp. He reported no other symptoms and had never had a total-body skin examination. His primary care physician recently prescribed a dose pack of methylprednisolone 4-mg tablets, which relieved the symptoms except for a mild scalp itch. His wife did not experience itching, and he denied noticing mites or fleas on his pet dog. Physical examination did not reveal any contributory findings, such as erythema or rash. Ketoconazole shampoo 2% and fluocinolone solution 0.01% were prescribed for scalp pruritus; however, he could not afford those medications and therefore did not take them.
Two weeks later, the patient presented with diffuse itching that involved the scalp, trunk, and extremities. He denied groin pruritus. He reported that the itching was worse at night. His wife continued to be asymptomatic. The patient reported that his health screening was up-to-date, and he had no interval health changes. A complete blood cell count, thyroid studies, and a comprehensive metabolic panel performed recently were within reference range. He denied recent travel or taking new medications. Physical examination revealed a somewhat linear distribution of erythematous urticarial papules on the right side of the abdomen. Red dermatographic excoriations were noted on the back. No burrows were visualized. He was given intramuscular triamcinolone 60 mg and was advised to have his house evaluated for bed bugs and his pet dog evaluated by a veterinarian for mites. During the triamcinolone injection, the medical assistant observed a 1- to 2-mm red insect, which fell into his clothing and could not be further evaluated.
After 1 month, the patient had no improvement of the pruritus; instead, it became worse. During this time, his wife developed intermittent urticarial-like eruptions. He was taking oral diphenhydramine nightly and applying triamcinolone cream 0.5% that he had at home from an earlier skin problem as needed. Physical examination findings correlated with worsening symptoms. He had multiple erythematous urticarial papules—many of which were excoriated—across the chest, abdomen, buttocks, and back. The arms had multiple excoriations. The urticarial papules coalesced in the anterior axillary folds, yet no burrows were visualized. In the left anterior axillary fold adjacent to one of the urticarial papules, a 1-mm mobile mite was identified on dermoscopy. Further evaluation by microscopy showed morphologic characteristics of a tropical rat mite (Figure). The patient admitted that his house had a mouse infestation that he was struggling to eliminate. Permethrin cream 5% was prescribed. Because the patient could not afford the prescription, he was advised to use the triamcinolone cream 0.5%and oral diphenhydramine that he had at home nightly for symptomatic relief. He was advised to hire an exterminator to eradicate the mouse and mite infestation to prevent reinfestation.

Identification of Rate Mite Dermatitis
The characteristics of tropical rat mite dermatitis can be confused with many other conditions, such as infection. Even when a mite is identified, it can be difficult to classify it as a tropical rat mite. To confirm the diagnosis of tropical rat mite dermatitis, the parasite needs to be identified. Skin scrapings can be collected from pruritic lesions and examined microscopically in the hope of revealing the rat mites. The recommendation is to collect skin scrapings from the dorsal aspect of the hands or from the neck.5 Patients may report finding mites in their living space or on their bedding or clothing.
Although the tropical rat mite was reported as a vector for endemic typhus between humans, no other cases of transmission between humans have been reported since.11,12 Studies reporting non–human subject research and case reports have shown that O bacoti is a vector for Rickettsia akari, Coxiella burnetii, Francisella tularensis, Yersinia pestis, Eastern equine encephalitis virus (Alphavirus), Enterovirus (Picornaviridae), Langat virus (Flavivirus), and Hantaan orthohantavirus.5,11-17 However, no cases of these infectious diseases being transmitted naturally have been reported.5
Confirmation of O bacoti as a vector for human pathogens is difficult because it relies on identification of the mite in the clinic.5 The epidemiologic importance of the mite in transmitting infectious disease is unknown; reports of human cases of mite infestation are rare. We present this information to increase awareness and help dermatologists and other health care providers identify O bacoti.
- Beck W, Fölster-Holst R. Tropical rat mites (Ornithonyssus bacoti)—serious ectoparasites. J Dtsch Dermatol Ges. 2009;7:667-670. doi:10.1111/j.1610-0387.2009.07140.x
- Baumstark J, Beck W, Hofmann H. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology. 2007;215:66-68. doi:10.1159/000102037
- Beck W. Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings. Travel Med Infect Dis. 2008;6:245-249. doi:10.1016/j.tmaid.2008.01.002
- Beck W. Tropical rat mites as newly emerging disease pathogens in rodents and man. Trav Med Infect Dis. 2007;5:403. doi:10.1016/j.tmaid.2007.09.016
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469. doi:10.1086/515016
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678. doi:10.1001/archderm.1982.01650210056019
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Ram SM, Satija KC, Kaushik RK. Ornithonyssus bacoti infestation in laboratory personnel and veterinary students. Int J Zoonoses. 1986;13:138-140.
- Brown S, Becher J, Brady W. Treatment of ectoparasitic infections: review of the English-language literature, 1982-1992. Clin Infect Dis. 1995;20(suppl 1):S104-S109. doi:10.1093/clinids/20.supplement_1.s104
- Reeves WK, Loftis AD, Szumlas DE, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol. 2007;41:101-107. doi:10.1007/s10493-006-9040-3
- Philip CB, Hughes LE. The tropical rat mite; Liponyssus bacoti, as an experimental vector of rickettsialpox. Am J Trop Med Hyg. 1948;28:697-705. doi:10.4269/ajtmh.1948.s1-28.697
- Zemskaia AA, Pchelkina AA. Experimental infection of ticks Dermanyssus gallinae Redi Bdellonyssus bacoti Hirst with Q fever. Dokl Akad Nauk SSSR. 1955;101:391-392.
- Hopla CE. Experimental transmission of tularemia by the tropical rat mite. Am J Trop Med Hyg. 1951;31:768-783. doi:10.4269/ajtmh.1951.s1-31.768
- Clark GM, Lutz AE, Fadnessl. Observations on the ability of Haemogamasus liponyssoides Ewing and Ornithonyssus bacoti (Hirst) (Acarina, Gamasina) to retain eastern equine encephalitis virus: preliminary report. Am J Trop Med Hyg. 1966;15:107-112. doi:10.4269/ajtmh.1966.15.107
- Schwab M, Allen R, Sulkin SE. The tropical rat mite (Liponyssus bacoti) as an experimental vector of Coxsackie virus. Am J Trop Med Hyg. 1952;1:982-986. doi:10.4269/ajtmh.1952.1.982
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641. doi:10.1093/jmedent/30.3.639
- Beck W, Fölster-Holst R. Tropical rat mites (Ornithonyssus bacoti)—serious ectoparasites. J Dtsch Dermatol Ges. 2009;7:667-670. doi:10.1111/j.1610-0387.2009.07140.x
- Baumstark J, Beck W, Hofmann H. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology. 2007;215:66-68. doi:10.1159/000102037
- Beck W. Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings. Travel Med Infect Dis. 2008;6:245-249. doi:10.1016/j.tmaid.2008.01.002
- Beck W. Tropical rat mites as newly emerging disease pathogens in rodents and man. Trav Med Infect Dis. 2007;5:403. doi:10.1016/j.tmaid.2007.09.016
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469. doi:10.1086/515016
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678. doi:10.1001/archderm.1982.01650210056019
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Ram SM, Satija KC, Kaushik RK. Ornithonyssus bacoti infestation in laboratory personnel and veterinary students. Int J Zoonoses. 1986;13:138-140.
- Brown S, Becher J, Brady W. Treatment of ectoparasitic infections: review of the English-language literature, 1982-1992. Clin Infect Dis. 1995;20(suppl 1):S104-S109. doi:10.1093/clinids/20.supplement_1.s104
- Reeves WK, Loftis AD, Szumlas DE, et al. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol. 2007;41:101-107. doi:10.1007/s10493-006-9040-3
- Philip CB, Hughes LE. The tropical rat mite; Liponyssus bacoti, as an experimental vector of rickettsialpox. Am J Trop Med Hyg. 1948;28:697-705. doi:10.4269/ajtmh.1948.s1-28.697
- Zemskaia AA, Pchelkina AA. Experimental infection of ticks Dermanyssus gallinae Redi Bdellonyssus bacoti Hirst with Q fever. Dokl Akad Nauk SSSR. 1955;101:391-392.
- Hopla CE. Experimental transmission of tularemia by the tropical rat mite. Am J Trop Med Hyg. 1951;31:768-783. doi:10.4269/ajtmh.1951.s1-31.768
- Clark GM, Lutz AE, Fadnessl. Observations on the ability of Haemogamasus liponyssoides Ewing and Ornithonyssus bacoti (Hirst) (Acarina, Gamasina) to retain eastern equine encephalitis virus: preliminary report. Am J Trop Med Hyg. 1966;15:107-112. doi:10.4269/ajtmh.1966.15.107
- Schwab M, Allen R, Sulkin SE. The tropical rat mite (Liponyssus bacoti) as an experimental vector of Coxsackie virus. Am J Trop Med Hyg. 1952;1:982-986. doi:10.4269/ajtmh.1952.1.982
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641. doi:10.1093/jmedent/30.3.639
Practice Points
- The tropical rat mite (Ornithonyssus bacoti) can infest humans who make bodily contact with a rodent, reside in living spaces infested with rodents, or own any pets.
- Patients infested with rat mites may present with pruritic, erythematous, cutaneous lesions with secondary excoriations that can be mistaken for an infection or dermatitis.
- The recommended treatment of rate mite infestation includes antiparasitic medications such as permethrin or pyriproxyfen. Preventive measures include proper disinfestation of living spaces.
Sarcoidosis in Post–9/11 Military Veterans
Sarcoidosis is a chronic inflammatory disease characterized by noncaseating granulomas that can affect many organ systems, most commonly the lungs and skin, with cutaneous involvement in 25% to 30% of patients in the United States.1 The etiology of sarcoidosis largely is unknown and likely is multifactorial; however, specific environmental, infectious, and pharmaceutical triggers may contribute to its pathogenesis. Sarcoidosis secondary to occupational exposures in US Military veterans historically has been discussed and investigated. Still, it was not considered a service-connected disability until the passing of the Promise to Address Comprehensive Toxics (PACT) Act2 in 2022. In this article, we review the risk factors and incidence of sarcoidosis in post–9/11 veterans as well as provide recommendations for managing presumptive service-connected sarcoidosis covered under the recently enacted PACT Act.
The PACT Act and Post–9/11 Military Veterans
Veterans of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have a history of occupational exposures to open-air burn pits, gun smoke, and recurrent high-intensity sandstorms that may cause chronic disease.3 Burn pits, which were used to dispose of solid waste on forward operating bases, released antigenic particulate matter that was detectable on air sampling.4,5 Increased respiratory disease rates in veterans that were deployed post–9/11 are well documented, but a causal relationship has not been established.6 Although burn pits cannot be directly associated with any disease at this time,5 veterans with assumed exposures can now receive a Veterans Affairs (VA) Disability Rating for presumptive conditions under the PACT Act.2 The major points of this legislation include expanding and extending eligibility for veterans with toxic exposures, providing access to toxic exposure screening for all veterans receiving VA health care, and increasing research related to toxic exposures in US servicemembers. The PACT Act expands health care benefits, making it easier for veterans exposed post–9/11 to receive coverage for 24 new presumptive diagnoses.2 Of these diagnoses, several are relevant to the practicing dermatologist. Patients with metastasis of primary cancers to the skin as well as melanoma or sarcoidosis may be eligible for coverage depending on the location and time of service. The Table lists service locations where the VA has determined servicemembers may have been exposed to burn pits or other toxins. Servicemembers with a presumptive diagnosis who served in these locations may be eligible for care under the PACT Act. Sarcoidosis is of particular concern due to its increased incidence and prevalence in military veterans compared to civilian populations. An analysis of more than 13 million veterans who received health care benefits through the Veterans Health Administration in 2019 found an annual incidence of sarcoidosis of 52 cases per 100,000 person-years and an annual prevalence of 141 cases per 100,000 individuals.7 In contrast, the United States has a reported annual incidence of sarcoidosis of 4.9 cases per 100,000 person-years and an annual prevalence of 60 cases per 100,000 individuals.8 Although the increased rates of sarcoidosis in veterans have been noted for decades, only recently have investigations provided insights into the etiology of sarcoidosis in this population.
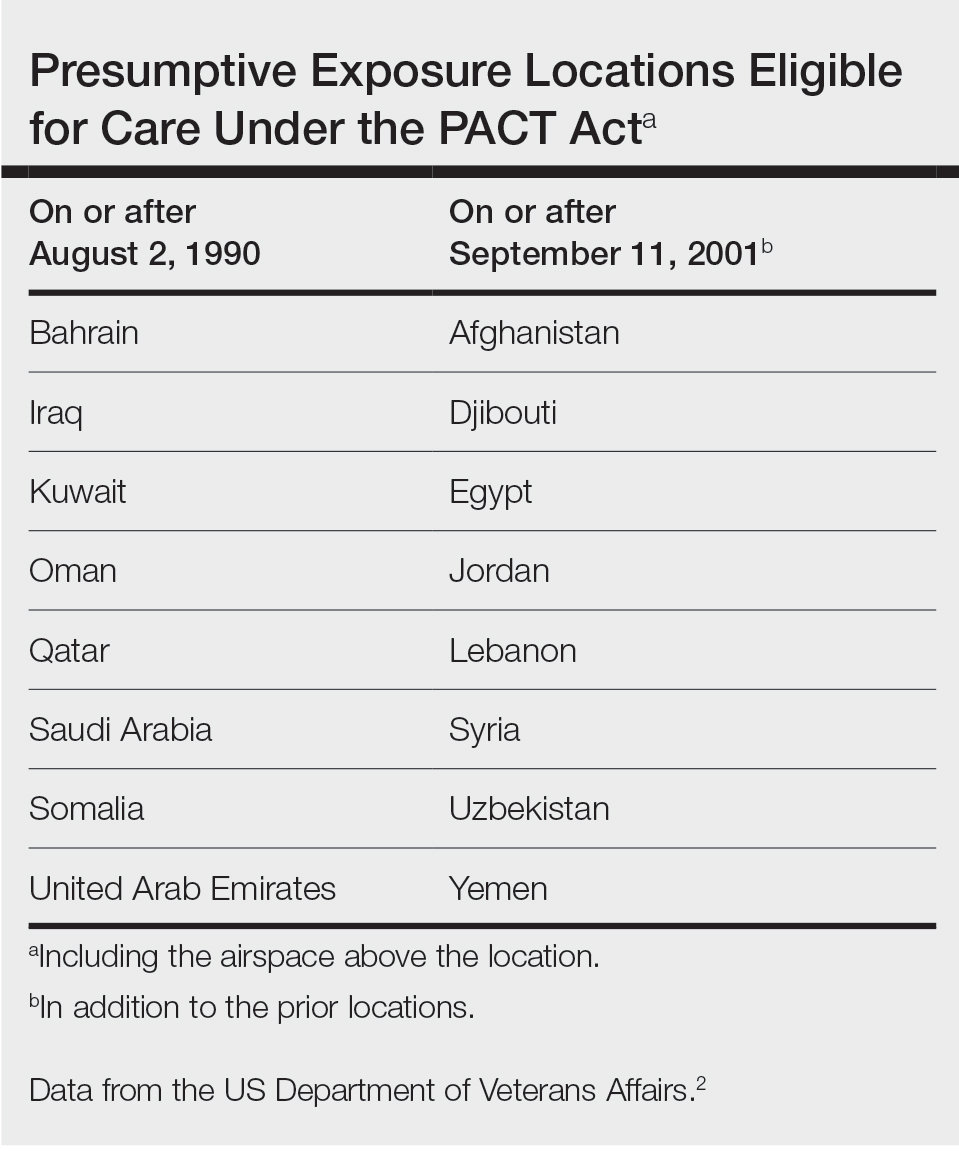
Sarcoidosis and Environmental Factors
Sarcoidosis is a multisystem granulomatous inflammatory disease that can present in any organ system9; however, it most commonly affects the lungs, skin, and eyes—all of which are subjected to direct contact with environmental toxins. The cause of sarcoidosis is unknown, but environmental exposures are theorized to play a role.9,10 It has been hypothesized that exposure to various immunologically active triggers may invoke the granulomatous inflammatory response that characterizes the disease.11 The World Trade Center disaster on 9/11 has provided insight into the potential environmental component of sarcoidosis. Firefighters who spent extensive amounts of time at the World Trade Center site experienced intense exposure to inorganic particulate matter; it was later found that there was a marked increase in the incidence of sarcoidosis or sarcoidosislike granulomatous pulmonary disease in exposed firefighters. It has been speculated that the elevated exposure to potentially antigenic particulates may have induced granulomatous inflammation, resulting in the manifestation of the disease.12 Other known occupational exposures associated with an increased risk for sarcoidosis or sarcoidosislike illness include mold, silicates, metal dust, and microbial contaminants.11 Servicemembers commonly are exposed to several of these aerosolized toxins, which theoretically could increase their risk for developing sarcoidosis.
Sarcoidosis in the Military
Servicemembers historically have faced unique environmental hazards that may increase their risk for developing sarcoidosis. Studies of naval veterans have shown relationships between occupational location and increased rates of sarcoidosis. Sailors assigned to aircraft carriers with nonskid coatings containing particulate matter such as aluminum, titanium, and silicates had a higher prevalence of sarcoidosis than those stationed on “clean” ships.13,14 Although no one trigger was identified, the increased rates of sarcoidosis in populations with extensive exposure to toxins raise concern for the possibility of occupationally induced sarcoidosis in post–9/11 veterans.
Environmental exposures during OIF and OEF may be associated with sarcoidosis. A retrospective review of lung biopsy data collected from Department of Defense military treatment facilities was conducted to identify associations between lung disease and deployment to the Middle East.15 The study included 391 military patients divided into deployed and nondeployed groups undergoing lung biopsies for various reasons from 2005 to 2012. An analysis of the reported lung histology showed an increased frequency of nonnecrotizing granulomas in those with a history of deployment to the Middle East compared to those who had never been deployed. Development of disease was not associated with confounding factors such as age, ethnicity, sex, or tobacco use, raising suspicion about similar shared toxic exposures among deployed servicemembers.15 A 2020 study of sarcoidosis in active-duty military personnel reported that the incidence of observed cases was 2-times those seen in civilian Department of Defense employees from 2005 to 2010; however, data collected in this study did not indicate an increased risk for developing sarcoidosis based on deployment to the Middle East. Still, the higher prevalence of sarcoidosis in active-duty military personnel suggests similar shared exposures in this group.16
Identification of exposures that may potentially trigger sarcoidosis is difficult due to many confounding variables; however, the Airborne Hazards and Open Burn Pit Registry questionnaire has been used to extrapolate prospective hazards of concern. Results from the questionnaire identified that only veterans exposed to convoy activity had a statistically significant (odds ratio, 1.16; 95% CI, 1.00-1.35; P=.046) increased risk for developing sarcoidosis.17 Interestingly, enlisted personnel had a higher rate of sarcoidosis than officers, comprising upwards of 78% of cases in the Military Health System from 2004 to 2013.9 This finding requires further study, but increased exposure to toxins due to occupational specialty may be the cause.
Veterans with sarcoidosis may have a unique pathophysiology, which may point to occupational exposure. Studies show that affected veterans have unique plasma metabolites and metal ions compared to civilians, with lower anti-inflammatory amino acid concentrations and downregulated GABA synthesis. The environmental exposures in OIF and OEF may have primed deployed servicemembers to develop a distinct subtype of sarcoidosis.3 Overall, there is a dearth of literature on post–9/11 veterans with sarcoidosis; therefore, further investigation is necessary to determine the actual risk for developing the disease following exposures related to military service.
Clinical Presentation and Diagnosis
Cutaneous sarcoidosis protean morphology is considered an imitator of many other skin diseases. The most common sarcoidosis-specific skin lesions include papules and papulonodules (Figure, A), lupus pernio (Figure, B), plaques (Figure, C), and subcutaneous nodules. Lesions typically present on the face, neck, trunk, and extremities and are associated with a favorable prognosis. Lupus pernio presents as centrofacial, bluish-red or violaceous nodules and can be disfiguring (Figure, B). Subcutaneous nodules occur in the subcutaneous tissue or deep dermis with minimal surface changes. Sarcoidal lesions also can occur at sites of scar tissue or trauma, within tattoos, and around foreign bodies. Other uncommon sarcoidosis-specific skin lesions include ichthyosiform, hypopigmented, atrophic, ulcerative and mucosal lesions; erythroderma; alopecia; and nail sarcoidosis.18
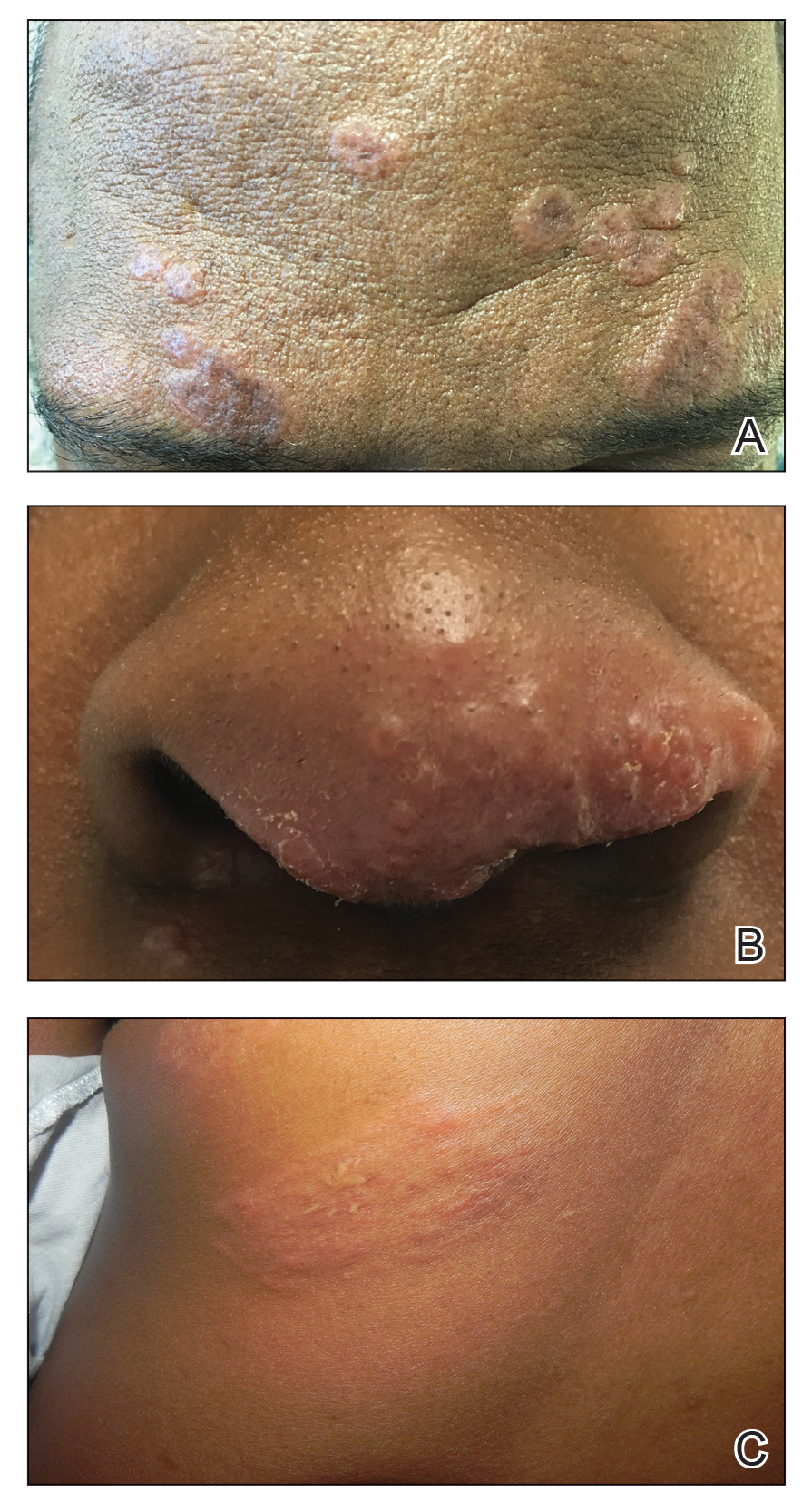
When cutaneous sarcoidosis is suspected, the skin serves as an easily accessible organ for biopsy to confirm the diagnosis.1 Sarcoidosis-specific skin lesions are histologically characterized as sarcoidal granulomas with a classic noncaseating naked appearance comprised of epithelioid histocytes with giant cells amidst a mild lymphocytic inflammatory infiltrate. Nonspecific sarcoidosis skin lesions do not contain characteristic noncaseating granulomas. Erythema nodosum is the most common nonspecific lesion and is associated with a favorable prognosis. Other nonspecific sarcoidosis skin findings include calcinosis cutis, clubbing, and vasculitis.18
Workup
Due to the systemic nature of sarcoidosis, dermatologists should initiate a comprehensive workup upon diagnosis of cutaneous sarcoidosis, which should include the following: a complete in-depth history, including occupational/environmental exposures; a complete review of systems; a military history, including time of service and location of deployments; physical examination; pulmonary function test; high-resolution chest computed tomography19; pulmonology referral for additional pulmonary function tests, including diffusion capacity for carbon monoxide and 6-minute walk test; ophthalmology referral for full ophthalmologic examination; initial cardiac screening with electrocardiogram; and a review of symptoms including assessment of heart palpitations. Any abnormalities should prompt cardiology referral for evaluation of cardiac involvement with a workup that may include transthoracic echocardiogram, Holter monitor, cardiac magnetic resonance imaging with gadolinium contrast, or cardiac positron emission tomography/computed tomography; a complete blood cell count; comprehensive metabolic panel; urinalysis, with a 24-hour urine calcium if there is a history of a kidney stone; tuberculin skin test or IFN-γ release assay to rule out tuberculosis on a case-by-case basis; thyroid testing; and 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D screening.1
Treatment
Cutaneous sarcoidosis is treated with topical or intralesional anti-inflammatory medications, immunomodulators, systemic immunosuppressants, and biologic agents. Management of cutaneous sarcoidosis should be done in an escalating approach guided by treatment response, location on the body, and patient preference. Response to therapy can take upwards of 3 months, and appropriate patient counseling is necessary to manage expectations.20 Most cutaneous sarcoidosis treatments are not approved by the US Food and Drug Administration for this purpose, and off-label use is based on available evidence and expert consensus (eTable).
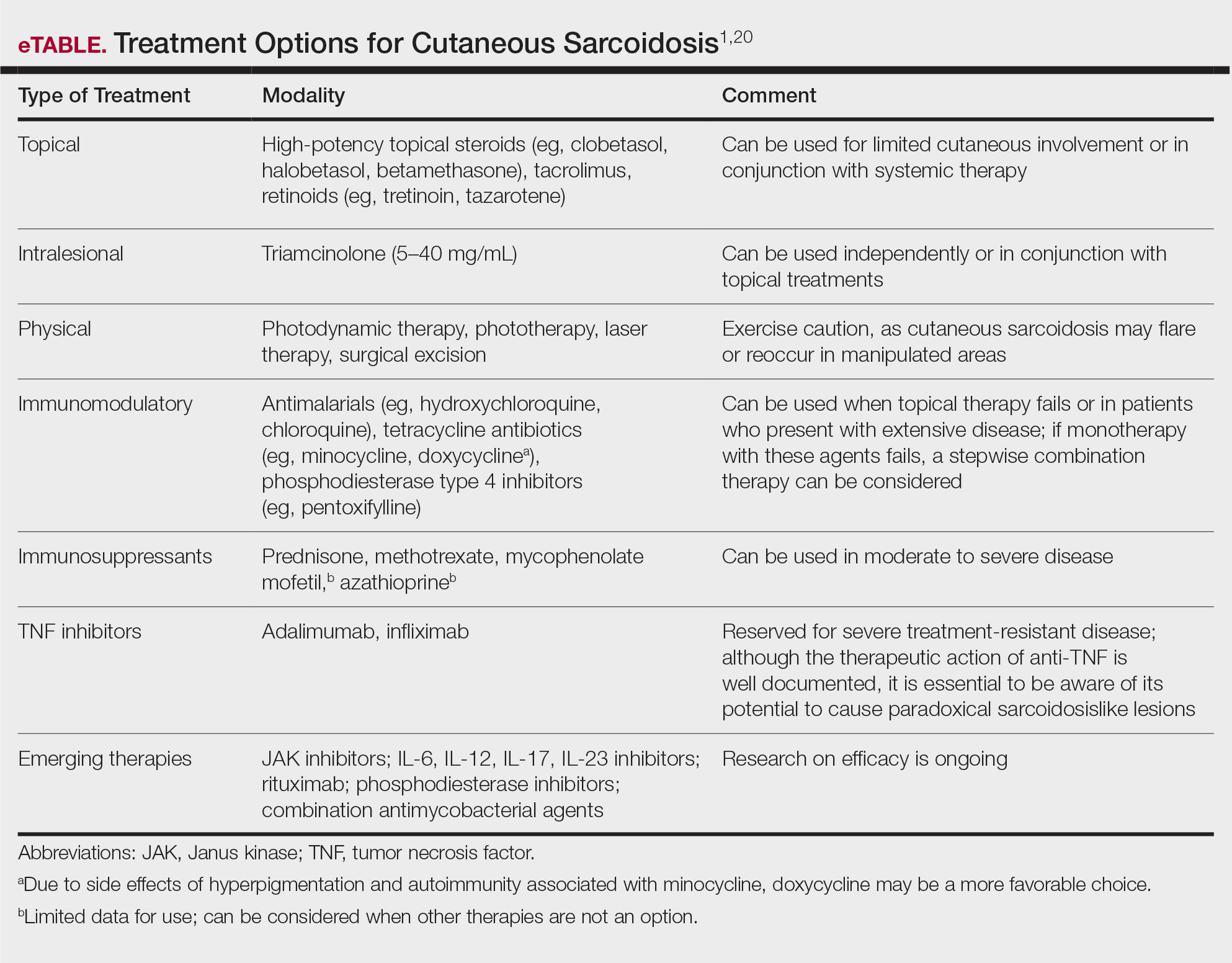
An important consideration for treating sarcoidosis in active-duty servicemembers is the use of immunosuppressants or biologics requiring refrigeration or continuous monitoring. According to Department of Defense retention standards, an active-duty servicemember may be disqualified from future service if their condition persists despite appropriate treatment and impairs their ability to perform required military duties. A medical evaluation board typically is initiated on any servicemember who starts a medication while on active duty that requires frequent monitoring by a medical provider, including immunomodulating and immunosuppressant medications.21
Final Thoughts
Military servicemembers put themselves at risk for acute bodily harm during deployment and also expose themselves to occupational hazards that may result in chronic health conditions. The VA’s coverage of new presumptive diagnoses means that veterans will receive extended care for conditions presumptively acquired during military service, including sarcoidosis. Although there are no conclusive data on whether exposure while on deployment overseas causes sarcoidosis, environmental exposures should be considered a potential cause. Patients with confirmed cutaneous sarcoidosis should undergo a complete workup for systemic sarcoidosis and be asked about their history of military service to evaluate for coverage under the PACT Act.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- US Department of Veterans Affairs. The Pact Act and your VA benefits. Updated August 15, 2023. Accessed August 18, 2023. https://www.va.gov/resources/the-pact-act-and-your-va-benefits/
- Banoei MM, Iupe I, Bazaz RD, et al. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci Rep. 2019;9:19584. doi:10.1038/s41598-019-56174-8
- Bith-Melander P, Ratliff J, Poisson C, et al. Slow burns: a qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann Psychiatry Clin Neurosci. 2021;4:1042.
- Military burn pits and cancer risk. American Cancer Society website. Revised August 25, 2022. Accessed August 18, 2023. https://www.cancer.org/healthy/cancer-causes/chemicals/burn-pits.html
- McLean J, Anderson D, Capra G, et al. The potential effects of burn pit exposure on the respiratory tract: a systematic review. Mil Med. 2021;186:672-681. doi: 10.1093/milmed/usab070
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019 [published online February 1, 2023]. Ann Am Thorac Soc. 2023. doi:10.1513/AnnalsATS.202206-515OC
- Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26:527-534. doi:10.1097/MCP.0000000000000715
- Parrish SC, Lin TK, Sicignano NM, et al. Sarcoidosis in the United States Military Health System. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35:261-267. doi:10.36141/svdld.v35i3.6949
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390/jcm9041081
- Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150. doi:10.1097/ACI.0b013e3283515173
- Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423. doi:10.1378/chest.06-2114
- Jajosky P. Sarcoidosis diagnoses among U.S. military personnel: trends and ship assignment associations. Am J Prev Med. 1998;14:176-183. doi:10.1016/s0749-3797(97)00063-9
- Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438. doi:10.1378/chest.126.5.1431
- Madar CS, Lewin-Smith MR, Franks TJ, et al. Histological diagnoses of military personnel undergoing lung biopsy after deployment to southwest Asia. Lung. 2017;195:507-515. doi:10.1007/s00408-017-0009-2
- Forbes DA, Anderson JT, Hamilton JA, et al. Relationship to deployment on sarcoidosis staging and severity in military personnel. Mil Med. 2020;185:E804-E810. doi:10.1093/milmed/usz407
- Jani N, Christie IC, Wu TD, et al. Factors associated with a diagnosis of sarcoidosis among US veterans of Iraq and Afghanistan. Sci Rep. 2022;12:22045. doi:10.1038/s41598-022-24853-8
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Motamedi M, Ferrara G, Yacyshyn E, et al. Skin disorders and interstitial lung disease: part I—screening, diagnosis, and therapeutic principles. J Am Acad Dermatol. 2023;88:751-764. doi:10.1016/j.jaad.2022.10.001
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514. doi:10.1007/s40257-022-00693-0
- US Department of Defense. DoD Instruction 6130.03, Volume 2. Medical Standards for Military Service: Retention. Published September 4, 2020. Accessed August 18, 2023. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Miscellaneous/613003v2p_MEDICAL_STANDARDS_RETENTION.PDF?ver=7gMDUq1G1dOupje6wf_-DQ%3D%3D
Sarcoidosis is a chronic inflammatory disease characterized by noncaseating granulomas that can affect many organ systems, most commonly the lungs and skin, with cutaneous involvement in 25% to 30% of patients in the United States.1 The etiology of sarcoidosis largely is unknown and likely is multifactorial; however, specific environmental, infectious, and pharmaceutical triggers may contribute to its pathogenesis. Sarcoidosis secondary to occupational exposures in US Military veterans historically has been discussed and investigated. Still, it was not considered a service-connected disability until the passing of the Promise to Address Comprehensive Toxics (PACT) Act2 in 2022. In this article, we review the risk factors and incidence of sarcoidosis in post–9/11 veterans as well as provide recommendations for managing presumptive service-connected sarcoidosis covered under the recently enacted PACT Act.
The PACT Act and Post–9/11 Military Veterans
Veterans of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have a history of occupational exposures to open-air burn pits, gun smoke, and recurrent high-intensity sandstorms that may cause chronic disease.3 Burn pits, which were used to dispose of solid waste on forward operating bases, released antigenic particulate matter that was detectable on air sampling.4,5 Increased respiratory disease rates in veterans that were deployed post–9/11 are well documented, but a causal relationship has not been established.6 Although burn pits cannot be directly associated with any disease at this time,5 veterans with assumed exposures can now receive a Veterans Affairs (VA) Disability Rating for presumptive conditions under the PACT Act.2 The major points of this legislation include expanding and extending eligibility for veterans with toxic exposures, providing access to toxic exposure screening for all veterans receiving VA health care, and increasing research related to toxic exposures in US servicemembers. The PACT Act expands health care benefits, making it easier for veterans exposed post–9/11 to receive coverage for 24 new presumptive diagnoses.2 Of these diagnoses, several are relevant to the practicing dermatologist. Patients with metastasis of primary cancers to the skin as well as melanoma or sarcoidosis may be eligible for coverage depending on the location and time of service. The Table lists service locations where the VA has determined servicemembers may have been exposed to burn pits or other toxins. Servicemembers with a presumptive diagnosis who served in these locations may be eligible for care under the PACT Act. Sarcoidosis is of particular concern due to its increased incidence and prevalence in military veterans compared to civilian populations. An analysis of more than 13 million veterans who received health care benefits through the Veterans Health Administration in 2019 found an annual incidence of sarcoidosis of 52 cases per 100,000 person-years and an annual prevalence of 141 cases per 100,000 individuals.7 In contrast, the United States has a reported annual incidence of sarcoidosis of 4.9 cases per 100,000 person-years and an annual prevalence of 60 cases per 100,000 individuals.8 Although the increased rates of sarcoidosis in veterans have been noted for decades, only recently have investigations provided insights into the etiology of sarcoidosis in this population.

Sarcoidosis and Environmental Factors
Sarcoidosis is a multisystem granulomatous inflammatory disease that can present in any organ system9; however, it most commonly affects the lungs, skin, and eyes—all of which are subjected to direct contact with environmental toxins. The cause of sarcoidosis is unknown, but environmental exposures are theorized to play a role.9,10 It has been hypothesized that exposure to various immunologically active triggers may invoke the granulomatous inflammatory response that characterizes the disease.11 The World Trade Center disaster on 9/11 has provided insight into the potential environmental component of sarcoidosis. Firefighters who spent extensive amounts of time at the World Trade Center site experienced intense exposure to inorganic particulate matter; it was later found that there was a marked increase in the incidence of sarcoidosis or sarcoidosislike granulomatous pulmonary disease in exposed firefighters. It has been speculated that the elevated exposure to potentially antigenic particulates may have induced granulomatous inflammation, resulting in the manifestation of the disease.12 Other known occupational exposures associated with an increased risk for sarcoidosis or sarcoidosislike illness include mold, silicates, metal dust, and microbial contaminants.11 Servicemembers commonly are exposed to several of these aerosolized toxins, which theoretically could increase their risk for developing sarcoidosis.
Sarcoidosis in the Military
Servicemembers historically have faced unique environmental hazards that may increase their risk for developing sarcoidosis. Studies of naval veterans have shown relationships between occupational location and increased rates of sarcoidosis. Sailors assigned to aircraft carriers with nonskid coatings containing particulate matter such as aluminum, titanium, and silicates had a higher prevalence of sarcoidosis than those stationed on “clean” ships.13,14 Although no one trigger was identified, the increased rates of sarcoidosis in populations with extensive exposure to toxins raise concern for the possibility of occupationally induced sarcoidosis in post–9/11 veterans.
Environmental exposures during OIF and OEF may be associated with sarcoidosis. A retrospective review of lung biopsy data collected from Department of Defense military treatment facilities was conducted to identify associations between lung disease and deployment to the Middle East.15 The study included 391 military patients divided into deployed and nondeployed groups undergoing lung biopsies for various reasons from 2005 to 2012. An analysis of the reported lung histology showed an increased frequency of nonnecrotizing granulomas in those with a history of deployment to the Middle East compared to those who had never been deployed. Development of disease was not associated with confounding factors such as age, ethnicity, sex, or tobacco use, raising suspicion about similar shared toxic exposures among deployed servicemembers.15 A 2020 study of sarcoidosis in active-duty military personnel reported that the incidence of observed cases was 2-times those seen in civilian Department of Defense employees from 2005 to 2010; however, data collected in this study did not indicate an increased risk for developing sarcoidosis based on deployment to the Middle East. Still, the higher prevalence of sarcoidosis in active-duty military personnel suggests similar shared exposures in this group.16
Identification of exposures that may potentially trigger sarcoidosis is difficult due to many confounding variables; however, the Airborne Hazards and Open Burn Pit Registry questionnaire has been used to extrapolate prospective hazards of concern. Results from the questionnaire identified that only veterans exposed to convoy activity had a statistically significant (odds ratio, 1.16; 95% CI, 1.00-1.35; P=.046) increased risk for developing sarcoidosis.17 Interestingly, enlisted personnel had a higher rate of sarcoidosis than officers, comprising upwards of 78% of cases in the Military Health System from 2004 to 2013.9 This finding requires further study, but increased exposure to toxins due to occupational specialty may be the cause.
Veterans with sarcoidosis may have a unique pathophysiology, which may point to occupational exposure. Studies show that affected veterans have unique plasma metabolites and metal ions compared to civilians, with lower anti-inflammatory amino acid concentrations and downregulated GABA synthesis. The environmental exposures in OIF and OEF may have primed deployed servicemembers to develop a distinct subtype of sarcoidosis.3 Overall, there is a dearth of literature on post–9/11 veterans with sarcoidosis; therefore, further investigation is necessary to determine the actual risk for developing the disease following exposures related to military service.
Clinical Presentation and Diagnosis
Cutaneous sarcoidosis protean morphology is considered an imitator of many other skin diseases. The most common sarcoidosis-specific skin lesions include papules and papulonodules (Figure, A), lupus pernio (Figure, B), plaques (Figure, C), and subcutaneous nodules. Lesions typically present on the face, neck, trunk, and extremities and are associated with a favorable prognosis. Lupus pernio presents as centrofacial, bluish-red or violaceous nodules and can be disfiguring (Figure, B). Subcutaneous nodules occur in the subcutaneous tissue or deep dermis with minimal surface changes. Sarcoidal lesions also can occur at sites of scar tissue or trauma, within tattoos, and around foreign bodies. Other uncommon sarcoidosis-specific skin lesions include ichthyosiform, hypopigmented, atrophic, ulcerative and mucosal lesions; erythroderma; alopecia; and nail sarcoidosis.18

When cutaneous sarcoidosis is suspected, the skin serves as an easily accessible organ for biopsy to confirm the diagnosis.1 Sarcoidosis-specific skin lesions are histologically characterized as sarcoidal granulomas with a classic noncaseating naked appearance comprised of epithelioid histocytes with giant cells amidst a mild lymphocytic inflammatory infiltrate. Nonspecific sarcoidosis skin lesions do not contain characteristic noncaseating granulomas. Erythema nodosum is the most common nonspecific lesion and is associated with a favorable prognosis. Other nonspecific sarcoidosis skin findings include calcinosis cutis, clubbing, and vasculitis.18
Workup
Due to the systemic nature of sarcoidosis, dermatologists should initiate a comprehensive workup upon diagnosis of cutaneous sarcoidosis, which should include the following: a complete in-depth history, including occupational/environmental exposures; a complete review of systems; a military history, including time of service and location of deployments; physical examination; pulmonary function test; high-resolution chest computed tomography19; pulmonology referral for additional pulmonary function tests, including diffusion capacity for carbon monoxide and 6-minute walk test; ophthalmology referral for full ophthalmologic examination; initial cardiac screening with electrocardiogram; and a review of symptoms including assessment of heart palpitations. Any abnormalities should prompt cardiology referral for evaluation of cardiac involvement with a workup that may include transthoracic echocardiogram, Holter monitor, cardiac magnetic resonance imaging with gadolinium contrast, or cardiac positron emission tomography/computed tomography; a complete blood cell count; comprehensive metabolic panel; urinalysis, with a 24-hour urine calcium if there is a history of a kidney stone; tuberculin skin test or IFN-γ release assay to rule out tuberculosis on a case-by-case basis; thyroid testing; and 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D screening.1
Treatment
Cutaneous sarcoidosis is treated with topical or intralesional anti-inflammatory medications, immunomodulators, systemic immunosuppressants, and biologic agents. Management of cutaneous sarcoidosis should be done in an escalating approach guided by treatment response, location on the body, and patient preference. Response to therapy can take upwards of 3 months, and appropriate patient counseling is necessary to manage expectations.20 Most cutaneous sarcoidosis treatments are not approved by the US Food and Drug Administration for this purpose, and off-label use is based on available evidence and expert consensus (eTable).

An important consideration for treating sarcoidosis in active-duty servicemembers is the use of immunosuppressants or biologics requiring refrigeration or continuous monitoring. According to Department of Defense retention standards, an active-duty servicemember may be disqualified from future service if their condition persists despite appropriate treatment and impairs their ability to perform required military duties. A medical evaluation board typically is initiated on any servicemember who starts a medication while on active duty that requires frequent monitoring by a medical provider, including immunomodulating and immunosuppressant medications.21
Final Thoughts
Military servicemembers put themselves at risk for acute bodily harm during deployment and also expose themselves to occupational hazards that may result in chronic health conditions. The VA’s coverage of new presumptive diagnoses means that veterans will receive extended care for conditions presumptively acquired during military service, including sarcoidosis. Although there are no conclusive data on whether exposure while on deployment overseas causes sarcoidosis, environmental exposures should be considered a potential cause. Patients with confirmed cutaneous sarcoidosis should undergo a complete workup for systemic sarcoidosis and be asked about their history of military service to evaluate for coverage under the PACT Act.
Sarcoidosis is a chronic inflammatory disease characterized by noncaseating granulomas that can affect many organ systems, most commonly the lungs and skin, with cutaneous involvement in 25% to 30% of patients in the United States.1 The etiology of sarcoidosis largely is unknown and likely is multifactorial; however, specific environmental, infectious, and pharmaceutical triggers may contribute to its pathogenesis. Sarcoidosis secondary to occupational exposures in US Military veterans historically has been discussed and investigated. Still, it was not considered a service-connected disability until the passing of the Promise to Address Comprehensive Toxics (PACT) Act2 in 2022. In this article, we review the risk factors and incidence of sarcoidosis in post–9/11 veterans as well as provide recommendations for managing presumptive service-connected sarcoidosis covered under the recently enacted PACT Act.
The PACT Act and Post–9/11 Military Veterans
Veterans of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) have a history of occupational exposures to open-air burn pits, gun smoke, and recurrent high-intensity sandstorms that may cause chronic disease.3 Burn pits, which were used to dispose of solid waste on forward operating bases, released antigenic particulate matter that was detectable on air sampling.4,5 Increased respiratory disease rates in veterans that were deployed post–9/11 are well documented, but a causal relationship has not been established.6 Although burn pits cannot be directly associated with any disease at this time,5 veterans with assumed exposures can now receive a Veterans Affairs (VA) Disability Rating for presumptive conditions under the PACT Act.2 The major points of this legislation include expanding and extending eligibility for veterans with toxic exposures, providing access to toxic exposure screening for all veterans receiving VA health care, and increasing research related to toxic exposures in US servicemembers. The PACT Act expands health care benefits, making it easier for veterans exposed post–9/11 to receive coverage for 24 new presumptive diagnoses.2 Of these diagnoses, several are relevant to the practicing dermatologist. Patients with metastasis of primary cancers to the skin as well as melanoma or sarcoidosis may be eligible for coverage depending on the location and time of service. The Table lists service locations where the VA has determined servicemembers may have been exposed to burn pits or other toxins. Servicemembers with a presumptive diagnosis who served in these locations may be eligible for care under the PACT Act. Sarcoidosis is of particular concern due to its increased incidence and prevalence in military veterans compared to civilian populations. An analysis of more than 13 million veterans who received health care benefits through the Veterans Health Administration in 2019 found an annual incidence of sarcoidosis of 52 cases per 100,000 person-years and an annual prevalence of 141 cases per 100,000 individuals.7 In contrast, the United States has a reported annual incidence of sarcoidosis of 4.9 cases per 100,000 person-years and an annual prevalence of 60 cases per 100,000 individuals.8 Although the increased rates of sarcoidosis in veterans have been noted for decades, only recently have investigations provided insights into the etiology of sarcoidosis in this population.

Sarcoidosis and Environmental Factors
Sarcoidosis is a multisystem granulomatous inflammatory disease that can present in any organ system9; however, it most commonly affects the lungs, skin, and eyes—all of which are subjected to direct contact with environmental toxins. The cause of sarcoidosis is unknown, but environmental exposures are theorized to play a role.9,10 It has been hypothesized that exposure to various immunologically active triggers may invoke the granulomatous inflammatory response that characterizes the disease.11 The World Trade Center disaster on 9/11 has provided insight into the potential environmental component of sarcoidosis. Firefighters who spent extensive amounts of time at the World Trade Center site experienced intense exposure to inorganic particulate matter; it was later found that there was a marked increase in the incidence of sarcoidosis or sarcoidosislike granulomatous pulmonary disease in exposed firefighters. It has been speculated that the elevated exposure to potentially antigenic particulates may have induced granulomatous inflammation, resulting in the manifestation of the disease.12 Other known occupational exposures associated with an increased risk for sarcoidosis or sarcoidosislike illness include mold, silicates, metal dust, and microbial contaminants.11 Servicemembers commonly are exposed to several of these aerosolized toxins, which theoretically could increase their risk for developing sarcoidosis.
Sarcoidosis in the Military
Servicemembers historically have faced unique environmental hazards that may increase their risk for developing sarcoidosis. Studies of naval veterans have shown relationships between occupational location and increased rates of sarcoidosis. Sailors assigned to aircraft carriers with nonskid coatings containing particulate matter such as aluminum, titanium, and silicates had a higher prevalence of sarcoidosis than those stationed on “clean” ships.13,14 Although no one trigger was identified, the increased rates of sarcoidosis in populations with extensive exposure to toxins raise concern for the possibility of occupationally induced sarcoidosis in post–9/11 veterans.
Environmental exposures during OIF and OEF may be associated with sarcoidosis. A retrospective review of lung biopsy data collected from Department of Defense military treatment facilities was conducted to identify associations between lung disease and deployment to the Middle East.15 The study included 391 military patients divided into deployed and nondeployed groups undergoing lung biopsies for various reasons from 2005 to 2012. An analysis of the reported lung histology showed an increased frequency of nonnecrotizing granulomas in those with a history of deployment to the Middle East compared to those who had never been deployed. Development of disease was not associated with confounding factors such as age, ethnicity, sex, or tobacco use, raising suspicion about similar shared toxic exposures among deployed servicemembers.15 A 2020 study of sarcoidosis in active-duty military personnel reported that the incidence of observed cases was 2-times those seen in civilian Department of Defense employees from 2005 to 2010; however, data collected in this study did not indicate an increased risk for developing sarcoidosis based on deployment to the Middle East. Still, the higher prevalence of sarcoidosis in active-duty military personnel suggests similar shared exposures in this group.16
Identification of exposures that may potentially trigger sarcoidosis is difficult due to many confounding variables; however, the Airborne Hazards and Open Burn Pit Registry questionnaire has been used to extrapolate prospective hazards of concern. Results from the questionnaire identified that only veterans exposed to convoy activity had a statistically significant (odds ratio, 1.16; 95% CI, 1.00-1.35; P=.046) increased risk for developing sarcoidosis.17 Interestingly, enlisted personnel had a higher rate of sarcoidosis than officers, comprising upwards of 78% of cases in the Military Health System from 2004 to 2013.9 This finding requires further study, but increased exposure to toxins due to occupational specialty may be the cause.
Veterans with sarcoidosis may have a unique pathophysiology, which may point to occupational exposure. Studies show that affected veterans have unique plasma metabolites and metal ions compared to civilians, with lower anti-inflammatory amino acid concentrations and downregulated GABA synthesis. The environmental exposures in OIF and OEF may have primed deployed servicemembers to develop a distinct subtype of sarcoidosis.3 Overall, there is a dearth of literature on post–9/11 veterans with sarcoidosis; therefore, further investigation is necessary to determine the actual risk for developing the disease following exposures related to military service.
Clinical Presentation and Diagnosis
Cutaneous sarcoidosis protean morphology is considered an imitator of many other skin diseases. The most common sarcoidosis-specific skin lesions include papules and papulonodules (Figure, A), lupus pernio (Figure, B), plaques (Figure, C), and subcutaneous nodules. Lesions typically present on the face, neck, trunk, and extremities and are associated with a favorable prognosis. Lupus pernio presents as centrofacial, bluish-red or violaceous nodules and can be disfiguring (Figure, B). Subcutaneous nodules occur in the subcutaneous tissue or deep dermis with minimal surface changes. Sarcoidal lesions also can occur at sites of scar tissue or trauma, within tattoos, and around foreign bodies. Other uncommon sarcoidosis-specific skin lesions include ichthyosiform, hypopigmented, atrophic, ulcerative and mucosal lesions; erythroderma; alopecia; and nail sarcoidosis.18

When cutaneous sarcoidosis is suspected, the skin serves as an easily accessible organ for biopsy to confirm the diagnosis.1 Sarcoidosis-specific skin lesions are histologically characterized as sarcoidal granulomas with a classic noncaseating naked appearance comprised of epithelioid histocytes with giant cells amidst a mild lymphocytic inflammatory infiltrate. Nonspecific sarcoidosis skin lesions do not contain characteristic noncaseating granulomas. Erythema nodosum is the most common nonspecific lesion and is associated with a favorable prognosis. Other nonspecific sarcoidosis skin findings include calcinosis cutis, clubbing, and vasculitis.18
Workup
Due to the systemic nature of sarcoidosis, dermatologists should initiate a comprehensive workup upon diagnosis of cutaneous sarcoidosis, which should include the following: a complete in-depth history, including occupational/environmental exposures; a complete review of systems; a military history, including time of service and location of deployments; physical examination; pulmonary function test; high-resolution chest computed tomography19; pulmonology referral for additional pulmonary function tests, including diffusion capacity for carbon monoxide and 6-minute walk test; ophthalmology referral for full ophthalmologic examination; initial cardiac screening with electrocardiogram; and a review of symptoms including assessment of heart palpitations. Any abnormalities should prompt cardiology referral for evaluation of cardiac involvement with a workup that may include transthoracic echocardiogram, Holter monitor, cardiac magnetic resonance imaging with gadolinium contrast, or cardiac positron emission tomography/computed tomography; a complete blood cell count; comprehensive metabolic panel; urinalysis, with a 24-hour urine calcium if there is a history of a kidney stone; tuberculin skin test or IFN-γ release assay to rule out tuberculosis on a case-by-case basis; thyroid testing; and 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D screening.1
Treatment
Cutaneous sarcoidosis is treated with topical or intralesional anti-inflammatory medications, immunomodulators, systemic immunosuppressants, and biologic agents. Management of cutaneous sarcoidosis should be done in an escalating approach guided by treatment response, location on the body, and patient preference. Response to therapy can take upwards of 3 months, and appropriate patient counseling is necessary to manage expectations.20 Most cutaneous sarcoidosis treatments are not approved by the US Food and Drug Administration for this purpose, and off-label use is based on available evidence and expert consensus (eTable).

An important consideration for treating sarcoidosis in active-duty servicemembers is the use of immunosuppressants or biologics requiring refrigeration or continuous monitoring. According to Department of Defense retention standards, an active-duty servicemember may be disqualified from future service if their condition persists despite appropriate treatment and impairs their ability to perform required military duties. A medical evaluation board typically is initiated on any servicemember who starts a medication while on active duty that requires frequent monitoring by a medical provider, including immunomodulating and immunosuppressant medications.21
Final Thoughts
Military servicemembers put themselves at risk for acute bodily harm during deployment and also expose themselves to occupational hazards that may result in chronic health conditions. The VA’s coverage of new presumptive diagnoses means that veterans will receive extended care for conditions presumptively acquired during military service, including sarcoidosis. Although there are no conclusive data on whether exposure while on deployment overseas causes sarcoidosis, environmental exposures should be considered a potential cause. Patients with confirmed cutaneous sarcoidosis should undergo a complete workup for systemic sarcoidosis and be asked about their history of military service to evaluate for coverage under the PACT Act.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- US Department of Veterans Affairs. The Pact Act and your VA benefits. Updated August 15, 2023. Accessed August 18, 2023. https://www.va.gov/resources/the-pact-act-and-your-va-benefits/
- Banoei MM, Iupe I, Bazaz RD, et al. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci Rep. 2019;9:19584. doi:10.1038/s41598-019-56174-8
- Bith-Melander P, Ratliff J, Poisson C, et al. Slow burns: a qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann Psychiatry Clin Neurosci. 2021;4:1042.
- Military burn pits and cancer risk. American Cancer Society website. Revised August 25, 2022. Accessed August 18, 2023. https://www.cancer.org/healthy/cancer-causes/chemicals/burn-pits.html
- McLean J, Anderson D, Capra G, et al. The potential effects of burn pit exposure on the respiratory tract: a systematic review. Mil Med. 2021;186:672-681. doi: 10.1093/milmed/usab070
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019 [published online February 1, 2023]. Ann Am Thorac Soc. 2023. doi:10.1513/AnnalsATS.202206-515OC
- Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26:527-534. doi:10.1097/MCP.0000000000000715
- Parrish SC, Lin TK, Sicignano NM, et al. Sarcoidosis in the United States Military Health System. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35:261-267. doi:10.36141/svdld.v35i3.6949
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390/jcm9041081
- Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150. doi:10.1097/ACI.0b013e3283515173
- Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423. doi:10.1378/chest.06-2114
- Jajosky P. Sarcoidosis diagnoses among U.S. military personnel: trends and ship assignment associations. Am J Prev Med. 1998;14:176-183. doi:10.1016/s0749-3797(97)00063-9
- Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438. doi:10.1378/chest.126.5.1431
- Madar CS, Lewin-Smith MR, Franks TJ, et al. Histological diagnoses of military personnel undergoing lung biopsy after deployment to southwest Asia. Lung. 2017;195:507-515. doi:10.1007/s00408-017-0009-2
- Forbes DA, Anderson JT, Hamilton JA, et al. Relationship to deployment on sarcoidosis staging and severity in military personnel. Mil Med. 2020;185:E804-E810. doi:10.1093/milmed/usz407
- Jani N, Christie IC, Wu TD, et al. Factors associated with a diagnosis of sarcoidosis among US veterans of Iraq and Afghanistan. Sci Rep. 2022;12:22045. doi:10.1038/s41598-022-24853-8
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Motamedi M, Ferrara G, Yacyshyn E, et al. Skin disorders and interstitial lung disease: part I—screening, diagnosis, and therapeutic principles. J Am Acad Dermatol. 2023;88:751-764. doi:10.1016/j.jaad.2022.10.001
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514. doi:10.1007/s40257-022-00693-0
- US Department of Defense. DoD Instruction 6130.03, Volume 2. Medical Standards for Military Service: Retention. Published September 4, 2020. Accessed August 18, 2023. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Miscellaneous/613003v2p_MEDICAL_STANDARDS_RETENTION.PDF?ver=7gMDUq1G1dOupje6wf_-DQ%3D%3D
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- US Department of Veterans Affairs. The Pact Act and your VA benefits. Updated August 15, 2023. Accessed August 18, 2023. https://www.va.gov/resources/the-pact-act-and-your-va-benefits/
- Banoei MM, Iupe I, Bazaz RD, et al. Metabolomic and metallomic profile differences between veterans and civilians with pulmonary sarcoidosis. Sci Rep. 2019;9:19584. doi:10.1038/s41598-019-56174-8
- Bith-Melander P, Ratliff J, Poisson C, et al. Slow burns: a qualitative study of burn pit and toxic exposures among military veterans serving in Afghanistan, Iraq and throughout the Middle East. Ann Psychiatry Clin Neurosci. 2021;4:1042.
- Military burn pits and cancer risk. American Cancer Society website. Revised August 25, 2022. Accessed August 18, 2023. https://www.cancer.org/healthy/cancer-causes/chemicals/burn-pits.html
- McLean J, Anderson D, Capra G, et al. The potential effects of burn pit exposure on the respiratory tract: a systematic review. Mil Med. 2021;186:672-681. doi: 10.1093/milmed/usab070
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019 [published online February 1, 2023]. Ann Am Thorac Soc. 2023. doi:10.1513/AnnalsATS.202206-515OC
- Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. 2020;26:527-534. doi:10.1097/MCP.0000000000000715
- Parrish SC, Lin TK, Sicignano NM, et al. Sarcoidosis in the United States Military Health System. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35:261-267. doi:10.36141/svdld.v35i3.6949
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390/jcm9041081
- Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150. doi:10.1097/ACI.0b013e3283515173
- Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423. doi:10.1378/chest.06-2114
- Jajosky P. Sarcoidosis diagnoses among U.S. military personnel: trends and ship assignment associations. Am J Prev Med. 1998;14:176-183. doi:10.1016/s0749-3797(97)00063-9
- Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438. doi:10.1378/chest.126.5.1431
- Madar CS, Lewin-Smith MR, Franks TJ, et al. Histological diagnoses of military personnel undergoing lung biopsy after deployment to southwest Asia. Lung. 2017;195:507-515. doi:10.1007/s00408-017-0009-2
- Forbes DA, Anderson JT, Hamilton JA, et al. Relationship to deployment on sarcoidosis staging and severity in military personnel. Mil Med. 2020;185:E804-E810. doi:10.1093/milmed/usz407
- Jani N, Christie IC, Wu TD, et al. Factors associated with a diagnosis of sarcoidosis among US veterans of Iraq and Afghanistan. Sci Rep. 2022;12:22045. doi:10.1038/s41598-022-24853-8
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10:766. doi:10.3390/cells10040766
- Motamedi M, Ferrara G, Yacyshyn E, et al. Skin disorders and interstitial lung disease: part I—screening, diagnosis, and therapeutic principles. J Am Acad Dermatol. 2023;88:751-764. doi:10.1016/j.jaad.2022.10.001
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514. doi:10.1007/s40257-022-00693-0
- US Department of Defense. DoD Instruction 6130.03, Volume 2. Medical Standards for Military Service: Retention. Published September 4, 2020. Accessed August 18, 2023. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Miscellaneous/613003v2p_MEDICAL_STANDARDS_RETENTION.PDF?ver=7gMDUq1G1dOupje6wf_-DQ%3D%3D
Practice Points
- Cutaneous sarcoidosis is the most common extrapulmonary manifestation of the disease.
- Cutaneous sarcoidosis can precede systemic manifestations of the disease and should prompt further workup.
- Sarcoidosis is a presumptive diagnosis under the PACT Act and may be a service-connected condition. Veterans with presumptive exposures should be referred to the US Department of Veterans Affairs.
Treatments for Hidradenitis Suppurativa Comorbidities Help With Pain Management
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
Cadaveric Split-Thickness Skin Graft With Partial Guiding Closure for Scalp Defects Extending to the Periosteum
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Complications of Body Piercings: A Systematic Review
The practice of body piercing has been present in cultures around the world for centuries. Piercings may be performed for religious or spiritual reasons or as a form of self-expression. In recent years, body piercings have become increasingly popular in all genders, with the most common sites being the ears, mouth, nose, eyebrows, nipples, navel, and genitals.1 The prevalence of body piercing in the general population is estimated to be as high as 50%.2 With the rising popularity of piercings, there also has been an increase in their associated complications, with one study noting that up to 35% of individuals with pierced ears and 30% of all pierced sites developed a complication.3 Common problems following piercing include infections, keloid formation, allergic contact dermatitis, site deformation, and tooth fractures.4 It is of utmost importance that health care professionals are aware of the potential complications associated with such a common practice. A comprehensive review of complications associated with cutaneous and mucosal piercings is lacking. We conducted a systematic review to summarize the clinical characteristics, complication types and frequency, and treatments reported for cutaneous and mucosal piercings.
METHODS
We conducted a systematic review of the literature adhering to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) reporting guidelines.5
Search Strategy, Study Eligibility Criteria, and Study Selection
A literature search of the Embase, MEDLINE, and PubMed databases was performed on June 20, 2022, using search terms related to body piercing and possible piercing-induced complications (Supplemental Information online). All studies reporting complications following body piercing were included. In vitro and animal studies were excluded. Title and abstract screening were completed by 6 independent researchers (S.C., K.K., M.M-B., K.A., T.S., I.M.M.) using Covidence online systematic review software (www.covidence.org). Six reviewers (S.C., K.K., M.M-B., K.A., T.S., I.M.M.) independently evaluated titles, abstracts, and full texts to identify relevant studies. Conflicts were resolved by the senior reviewer (I.M.M.).
Data Extraction and Synthesis
Five reviewers (S.C., K.K., M.M-B., K.A., T.S.) independently extracted data from eligible studies using a standardized extraction form that included title; authors; year of publication; sample size; and key findings, including mean age, sex, piercing location, complication type, and treatment received.
Treatment type was placed into the following categories: surgical treatments, antimicrobials, medical treatments, direct-target therapy, oral procedures, avoidance, miscellaneous therapies, and no treatment. (Data regarding treatments can be found in the Supplemental Information online.)
RESULTS
The combined search yielded 2679 studies, 617 of which underwent full-text review; 319 studies were included (Figure). Studies were published from 1950 to June 2022 and included both adult and pediatric populations.
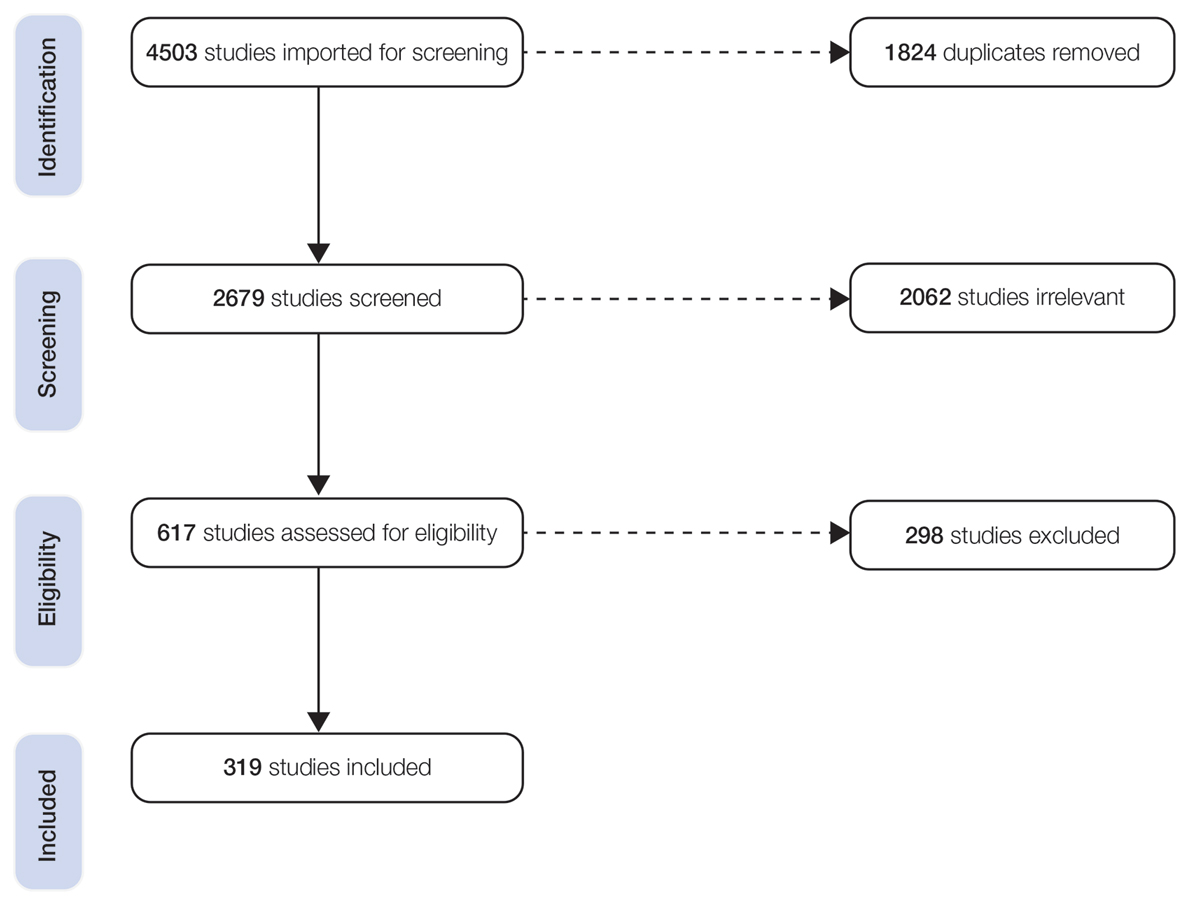
Patient Characteristics
In total, our pooled analysis included data on 30,090 complications across 36,803 pierced sites in 30,231 patients (Table 1). Demographic data are available for 55% (n=30,231) of patients. Overall, 74% (22,247/30,231) of the individuals included in our analysis were female. The mean age was 27.8 years (range, 0–76 years).
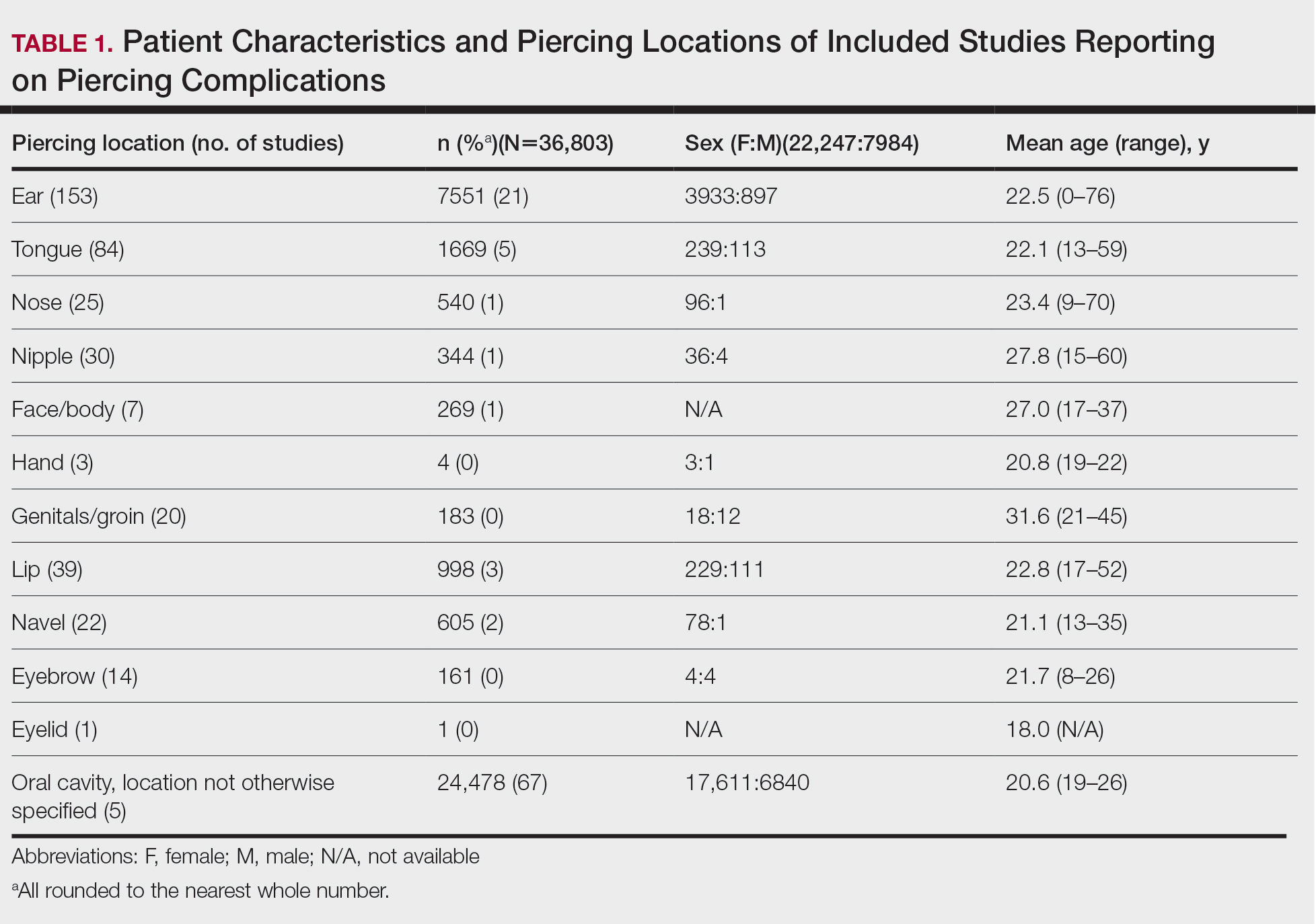
Piercing Location
Overall, 36,803 pierced sites had a reported complication. The oral cavity, location not otherwise specified, was the most common site associated with a complication, accounting for 67% (n=24,478) of complications (Table 1). Other reported sites included (in decreasing frequency) the ears (21%, n=7551), tongue (5%, n=1669), lip (3%, n=998), navel (2%, n=605), nose (1%, n=540), nipple (1%, n=344), face/body (1%, n=269), genitals/groin (0%, n=183), eyebrow (0%, n=161), hand (0%, n=4), and eyelid (0%, n=1). Piercing complications were more commonly reported among females across all piercing locations except for the eyebrow, which was equal in both sexes.
Complications
Local Infections—Local infections accounted for 36% of reported complication types (n=10,872/30,090): perichondritis (1%, n=85); abscesses (0%, n=25); bacterial colonization (1%, n=106); and local infections, not otherwise specified (98%, n=10,648)(Table 2). The majority of local infections were found to be secondary to piercings of the ear and oral cavity. The nipple was found to be a common site for abscesses (40%, n=10), whereas the tongue was found to be the most common site for bacterial colonization (69%, n=73).
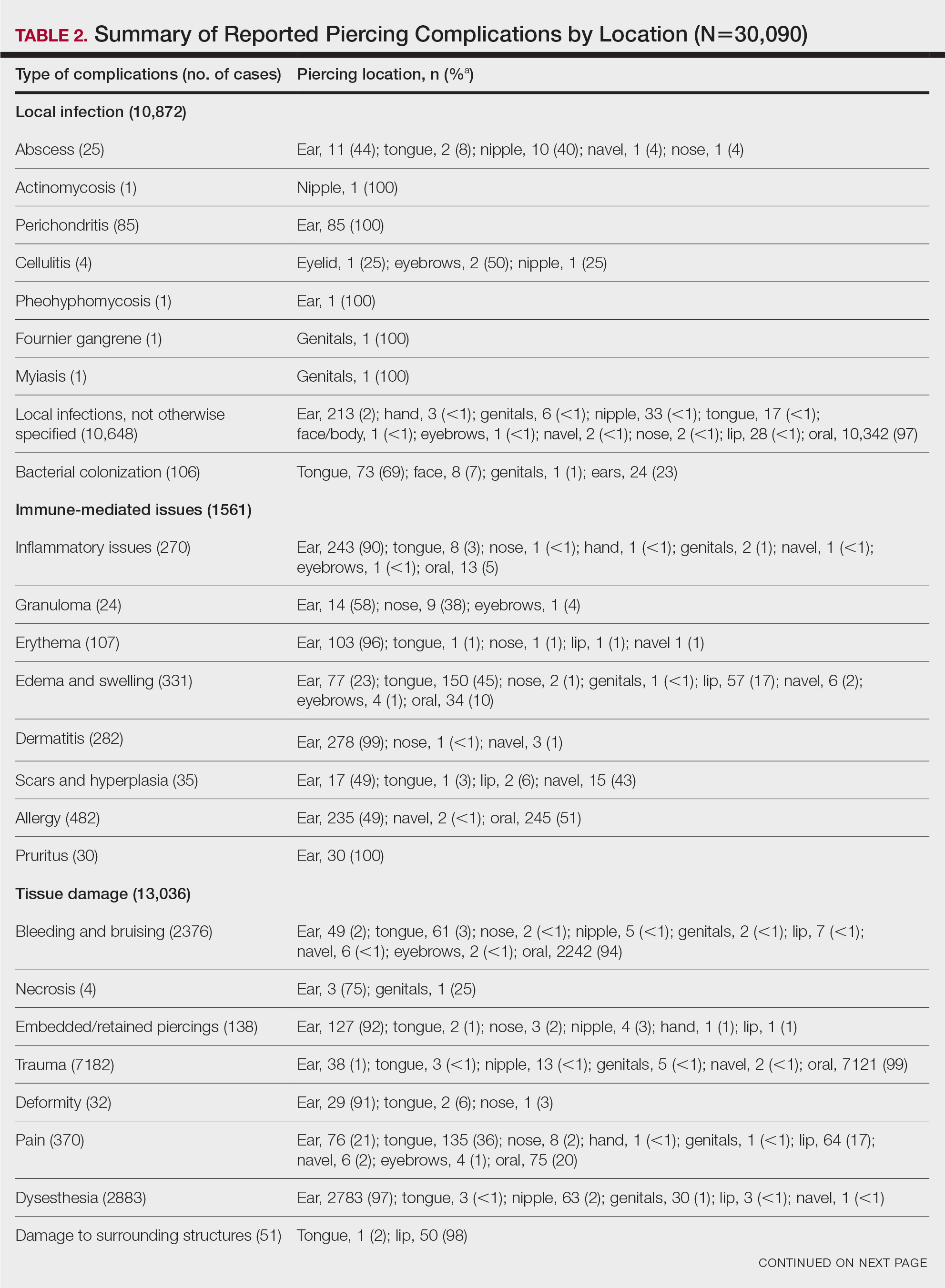
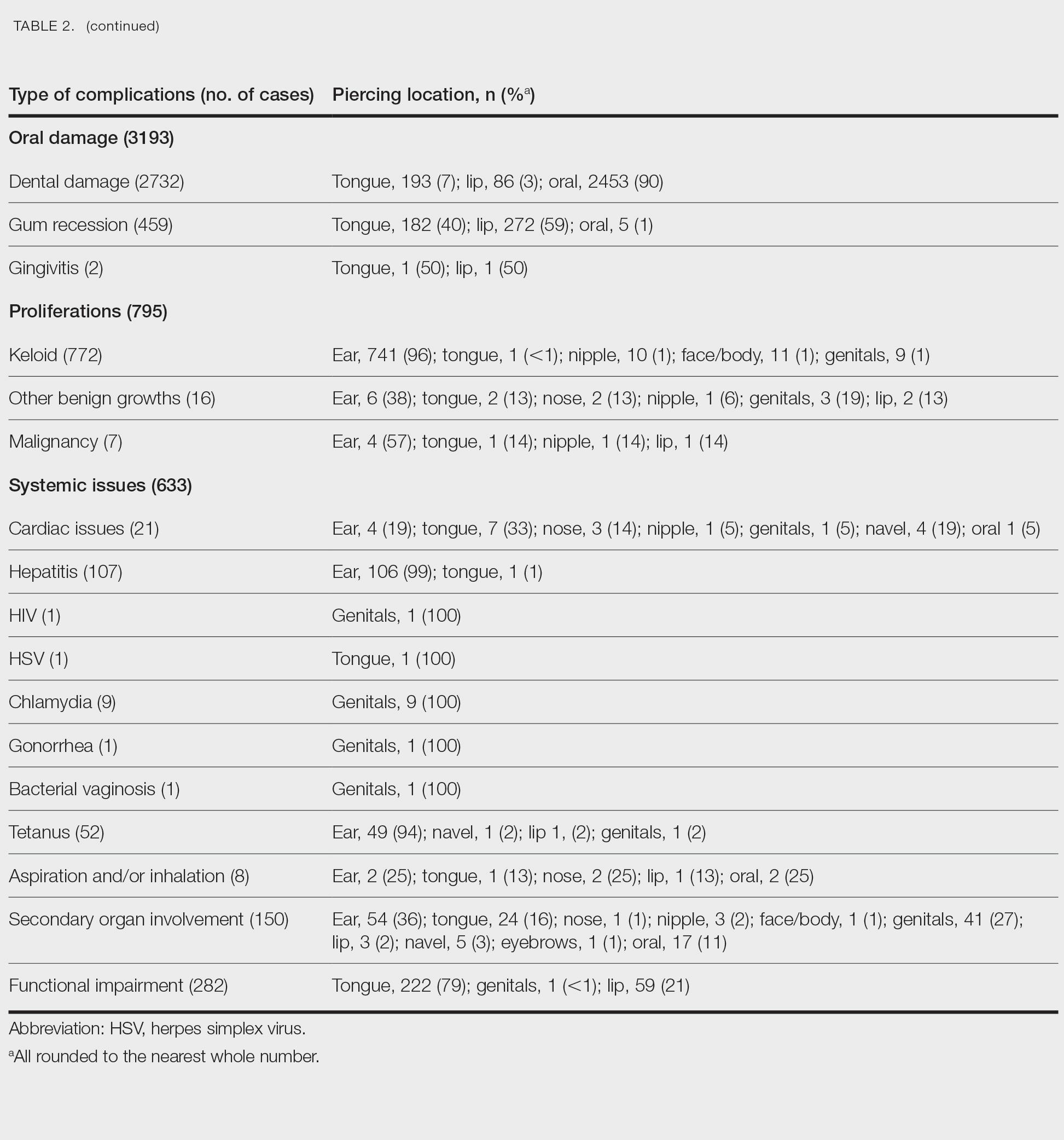
Immune-Mediated Issues—Immune-mediated issues encompassed 5% of the total reported complications (n=1561/30,090). The most commonly reported immune-mediated complications included allergies (31%, n=482), edema and swelling (21%, n=331), dermatitis (18%, n=282), and inflammatory lesions (17%, n=270). The majority were found to occur secondary to ear piercings, with the exception of edema, which mainly occurred secondary to tongue piercings (45%, n=150), and allergy, which primarily was associated with oral piercings (51%, n=245)(Table 2).
Tissue Damage—Tissue damage accounted for 43% of all complications (n=13,036/30,090). The most common forms of tissue damage were trauma (55%, n=7182), dysesthesia (22%, n=2883), bleeding and bruising (18%, n=2376), and pain (3%, n=370)(Table 2). Trauma was mainly found to be a complication in the context of oral piercings (99%, n=7121). Similarly, 94% (n=2242) of bleeding and bruising occurred secondary to oral piercings. Embedded piercings (92%, n=127), deformity (91%, n=29), and necrosis (75%, n=3) mostly occurred following ear piercings. Lip piercings were found to be the most common cause of damage to surrounding structures (98%, n=50).
Oral—Overall, 3193 intraoral complications were reported, constituting 11% of the total complications (Table 2). Oral complications included dental damage (86%, n=2732), gum recession (14%, n=459), and gingivitis (0%, n=2). Dental damage was mostly reported following oral piercings (90%, n=2453), whereas gum recession was mostly reported following lip piercings (59%, n=272).
Proliferations—Proliferations accounted for 795 (3%) of reported piercing complications. The majority (97%, n=772) were keloids, 2% (n=16) were other benign growths, and 1% (n=7) were malignancies. These complications mostly occurred secondary to ear piercings, which resulted in 741 (96%) keloids, 6 (38%) benign growths, and 4 (57%) malignancies.
Systemic—Overall, 2% (n=633) of the total complications were classified as systemic issues, including functional impairment (45%, n=282), secondary organ involvement (24%, n=150), cardiac issues (3%, n=21), and aspiration/inhalation (1%, n=8). Nonlocalized infections such as hepatitis or an increased risk thereof (17%, n=107), tetanus (8%, n=52), chlamydia (1%, n=9), HIV (0%, n=1), herpes simplex virus (0%, n=1), gonorrhea (0%, n=1), and bacterial vaginosis (0%, n=1) also were included in this category. The tongue, ear, and genitals were the locations most involved in these complications (Table 2). Secondary organ involvement mostly occurred after ear (36%, n=54) and genital piercings (27%, n=41). A total of 8 cases of piercing aspiration and/or inhalation were reported in association with piercings of the head and neck (Table 2).
COMMENT
Piercing Complications
Overall, the ear, tongue, and oral cavity were found to be the sites with the most associated complications recorded in the literature, and local infection and tissue damage were found to be the most prevalent types of complications. A plethora of treatments were used to manage piercing-induced complications, including surgical or medical treatments and avoidance (Supplemental Information). Reports by Metts6 and Escudero-Castaño et al7 provide detailed protocols and photographs of piercings.
Infections
Our review found that local infections were commonly reported complications associated with body piercings, which is consistent with other studies.1 The initial trauma inherent in the piercing process followed by the presence of an ongoing foreign body lends itself to an increased risk for developing these complications. Wound healing after piercing also varies based on the piercing location.
The rate and severity of the infection are influenced by the anatomic location of the piercing, hygiene, method of piercing, types of materials used, and aftercare.8 Piercing cartilage sites, such as the helix, concha, or nose, increases susceptibility to infections and permanent deformities. Cartilage is particularly at risk because of its avascular nature.9 Other studies have reported similar incidences of superficial localized infections; infectious complications were seen in 10% to 30% of body piercings in one study,3 while 45% of American and Australian college students reported infection at a piercing site in a second study.10
Systemic Issues
Systemic issues are potentially the most dangerous piercing-induced complications, though they were rarer in our analysis. Some serious complications included septic emboli, fatal staphylococcal toxic shock syndrome, and death. Although some systemic issues, such as staphylococcal toxic shock syndrome and septic sacroiliitis, required extensive hospital stays and complex treatment, others had lifelong repercussions, such as hepatitis and HIV. One report showed an increased incidence of endocarditis associated with body piercing, including staphylococcal endocarditis following nasal piercings, Neisseria endocarditis following tongue piercings, and Staphylococcus epidermidis endocarditis following nipple piercings.11 Moreover, Mariano et al12—who noted a case of endocarditis and meningitis associated with a nape piercing in a young female in 2015—reinforced the notion that information pertaining to the risks associated with body piercing must be better disseminated, given the potential for morbid or fatal outcomes. Finally, nonsterile piercing techniques and poor hygiene were found to contribute substantially to the increased risk for infection, so it is of utmost importance to reinforce proper practices in piercing salons.4
Immune-Mediated Issues
Because piercings are foreign bodies, they are susceptible to both acute and chronic immune responses. Our study found that allergies and dermatitis made up almost half of the immune-mediated piercing complications. It is especially important to emphasize that costume jewelry exposes our skin to a variety of contact allergens, most prominently nickel, heightening the risk for developing allergic contact dermatitis.13 Moreover, a study conducted by Brandão et al14 found that patients with pierced ears were significantly more likely to react to nickel than those without pierced ears (P=.031). Although other studies have found that allergy to metals ranges from 8.3% to 20% in the general population,15 we were not able to quantify the incidence in our study due to a lack of reporting of common benign complications, such as contact dermatitis.
Tissue Damage and Local Problems
Our review found that tissue and oral damage also were commonly reported piercing complications, with the most common pathologies being trauma, dysesthesia, bleeding/bruising, and dental damage. Laumann and Derick16 reported that bleeding, tissue trauma, and local problems were common physical health problems associated with body piercing. Severe complications, such as abscesses, toxic shock syndrome, and endocarditis, also have been reported in association with intraoral piercings.17 Moreover, other studies have shown that oral piercings are associated with several adverse oral and systemic conditions. A meta-analysis of individuals with oral piercings found a similar prevalence of dental fracture, gingival recession, and tooth wear (34%), as well as unspecified dental damage (27%) and tooth chipping (22%). Additionally, this meta-analysis reported a 3-fold increased risk for dental fracture and 7-fold increased risk for gingival recession with oral piercings.18 Another meta-analysis of oral piercing complications found a similar prevalence of dental fracture (34%), tooth wear (34%), gingival recession (33%), unspecified dental damage (27%), and tooth chipping (22%).19 Considering the extensive amount of cumulative damage, wearers of oral jewelry require periodic periodontal evaluations to monitor for dental damage and gingival recession.20 There are limited data on treatments for complications of oral piercings, and further research in this area is warranted.
Proliferations and Scars
Although proliferations and scarring were among the least common complications reported in the literature, they are some of the most cosmetically disfiguring for patients. Keloids, the most common type of growth associated with piercings, do not naturally regress and thus require some form of intervention. Given the multimodal approach used to treat keloids, as described by the evidence-based algorithm by Ogawa,21 it is not surprising that keloids also represented the complication most treated with medical therapies, such as steroids, and also with direct-target therapy, such as liquid nitrogen therapy (Supplemental Information).
Other proliferations reported in the literature include benign pyogenic granulomas22 and much less commonly malignant neoplasms such as basal cell carcinoma23 and squamous cell carcinoma.24 Although rare, treatment of piercing-associated malignancies include surgical removal, chemotherapy, and radiation therapy (Supplemental Information).
Limitations
There are several limitations to our systematic review. First, heterogeneity in study designs, patient populations, treatment interventions, and outcome measures of included studies may have affected the quality and generalizability of our results. Moreover, because the studies included in this systematic review focused on specific complications, we could not compare our results to the literature that analyzes incidence rates of piercing complications. Furthermore, not all studies included the data that we hoped to extract, and thus only available data were reported in these instances. Finally, the articles we reviewed may have included publication bias, with positive findings being more frequently published, potentially inflating certain types and sites of complications and treatment choices. Despite these limitations, our review provides essential information that must be interpreted in a clinical context.
CONCLUSION
Given that cutaneous and mucosal piercing has become more prevalent in recent years, along with an increase in the variety of piercing-induced complications, it is of utmost importance that piercing salons have proper hygiene practices in place and that patients are aware of the multitude of potential complications that can arise—whether common and benign or rare but life-threatening.
- Preslar D, Borger J. Body piercing infections. In: StatPearls. StatPearls Publishing; 2022.
- Antoszewski B, Jedrzejczak M, Kruk-Jeromin J. Complications after body piercing in patient suffering from type 1 diabetes mellitus. Int J Dermatol. 2007;46:1250-1252.
- Simplot TC, Hoffman HT. Comparison between cartilage and soft tissue ear piercing complications. Am J Otolaryngol. 1998;19:305-310.
- Meltzer DI. Complications of body piercing. Am Fam Physician. 2005;72:2029-2034.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Metts J. Common complications of body piercing. West J Med. 2002;176:85-86.
- Escudero-Castaño N, Perea-García MA, Campo-Trapero J, et al. Oral and perioral piercing complications. Open Dent J. 2008;2:133-136.
- Tweeten SS, Rickman LS. Infectious complications of body piercing. Clin Infect Dis. 1998;26:735-740.
- Gabriel OT, Anthony OO, Paul EA, et al. Trends and complications of ear piercing among selected Nigerian population. J Family Med Prim Care. 2017;6:517-521.
- Armstrong ML, Koch JR, Saunders JC, et al. The hole picture: risks, decision making, purpose, regulations, and the future of body piercing. Clin Dermatol. 2007;25:398-406.
- Millar BC, Moore JE. Antibiotic prophylaxis, body piercing and infective endocarditis. J Antimicrob Chemother. 2004;53:123-126.
- Mariano A, Pisapia R, Abdeddaim A, et al. Endocarditis and meningitis associated to nape piercing in a young female: a case report. Infez Med. 2015;23:275-279.
- Ivey LA, Limone BA, Jacob SE. Approach to the jewelry aficionado. Pediatr Dermatol. 2018;35:274-275.
- Brandão MH, Gontijo B, Girundi MA, et al. Ear piercing as a risk factor for contact allergy to nickel. J Pediatr (Rio J). 2010;86:149-154.
- Schuttelaar MLA, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy to metals in the European general population with a focus on nickel and piercings: The EDEN Fragrance Study. Contact Dermatitis. 2018;79:1-9.
- Laumann AE, Derick AJ. Tattoos and body piercings in the United States: a national data set. J Am Acad Dermatol. 2006;55:413-421.
- De Moor RJ, De Witte AM, Delmé KI, et al. Dental and oral complications of lip and tongue piercings. Br Dent J. 2005;199:506-509.
- Offen E, Allison JR. Do oral piercings cause problems in the mouth? Evid Based Dent. 2022;23:126-127.
- Passos PF, Pintor AVB, Marañón-Vásquez GA, et al. Oral manifestations arising from oral piercings: A systematic review and meta-analyses. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134:327-341.
- Covello F, Salerno C, Giovannini V, et al. Piercing and oral health: a study on the knowledge of risks and complications. Int J Environ Res Public Health. 2020;17:613.
- Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: a 2020 update of the algorithms published 10 years ago. Plast Reconstr Surg. 2022;149:E79-E94.
- Kumar Ghosh S, Bandyopadhyay D. Granuloma pyogenicum as a complication of decorative nose piercing: report of eight cases from eastern India. J Cutan Med Surg. 2012;16:197-200.
- Dreher K, Kern M, Rush L, et al. Basal cell carcinoma invasion of an ear piercing. Dermatol Online J. 2022;28.
- Stanko P, Poruban D, Mracna J, et al. Squamous cell carcinoma and piercing of the tongue—a case report. J Craniomaxillofac Surg. 2012;40:329-331.
The practice of body piercing has been present in cultures around the world for centuries. Piercings may be performed for religious or spiritual reasons or as a form of self-expression. In recent years, body piercings have become increasingly popular in all genders, with the most common sites being the ears, mouth, nose, eyebrows, nipples, navel, and genitals.1 The prevalence of body piercing in the general population is estimated to be as high as 50%.2 With the rising popularity of piercings, there also has been an increase in their associated complications, with one study noting that up to 35% of individuals with pierced ears and 30% of all pierced sites developed a complication.3 Common problems following piercing include infections, keloid formation, allergic contact dermatitis, site deformation, and tooth fractures.4 It is of utmost importance that health care professionals are aware of the potential complications associated with such a common practice. A comprehensive review of complications associated with cutaneous and mucosal piercings is lacking. We conducted a systematic review to summarize the clinical characteristics, complication types and frequency, and treatments reported for cutaneous and mucosal piercings.
METHODS
We conducted a systematic review of the literature adhering to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) reporting guidelines.5
Search Strategy, Study Eligibility Criteria, and Study Selection
A literature search of the Embase, MEDLINE, and PubMed databases was performed on June 20, 2022, using search terms related to body piercing and possible piercing-induced complications (Supplemental Information online). All studies reporting complications following body piercing were included. In vitro and animal studies were excluded. Title and abstract screening were completed by 6 independent researchers (S.C., K.K., M.M-B., K.A., T.S., I.M.M.) using Covidence online systematic review software (www.covidence.org). Six reviewers (S.C., K.K., M.M-B., K.A., T.S., I.M.M.) independently evaluated titles, abstracts, and full texts to identify relevant studies. Conflicts were resolved by the senior reviewer (I.M.M.).
Data Extraction and Synthesis
Five reviewers (S.C., K.K., M.M-B., K.A., T.S.) independently extracted data from eligible studies using a standardized extraction form that included title; authors; year of publication; sample size; and key findings, including mean age, sex, piercing location, complication type, and treatment received.
Treatment type was placed into the following categories: surgical treatments, antimicrobials, medical treatments, direct-target therapy, oral procedures, avoidance, miscellaneous therapies, and no treatment. (Data regarding treatments can be found in the Supplemental Information online.)
RESULTS
The combined search yielded 2679 studies, 617 of which underwent full-text review; 319 studies were included (Figure). Studies were published from 1950 to June 2022 and included both adult and pediatric populations.

Patient Characteristics
In total, our pooled analysis included data on 30,090 complications across 36,803 pierced sites in 30,231 patients (Table 1). Demographic data are available for 55% (n=30,231) of patients. Overall, 74% (22,247/30,231) of the individuals included in our analysis were female. The mean age was 27.8 years (range, 0–76 years).

Piercing Location
Overall, 36,803 pierced sites had a reported complication. The oral cavity, location not otherwise specified, was the most common site associated with a complication, accounting for 67% (n=24,478) of complications (Table 1). Other reported sites included (in decreasing frequency) the ears (21%, n=7551), tongue (5%, n=1669), lip (3%, n=998), navel (2%, n=605), nose (1%, n=540), nipple (1%, n=344), face/body (1%, n=269), genitals/groin (0%, n=183), eyebrow (0%, n=161), hand (0%, n=4), and eyelid (0%, n=1). Piercing complications were more commonly reported among females across all piercing locations except for the eyebrow, which was equal in both sexes.
Complications
Local Infections—Local infections accounted for 36% of reported complication types (n=10,872/30,090): perichondritis (1%, n=85); abscesses (0%, n=25); bacterial colonization (1%, n=106); and local infections, not otherwise specified (98%, n=10,648)(Table 2). The majority of local infections were found to be secondary to piercings of the ear and oral cavity. The nipple was found to be a common site for abscesses (40%, n=10), whereas the tongue was found to be the most common site for bacterial colonization (69%, n=73).


Immune-Mediated Issues—Immune-mediated issues encompassed 5% of the total reported complications (n=1561/30,090). The most commonly reported immune-mediated complications included allergies (31%, n=482), edema and swelling (21%, n=331), dermatitis (18%, n=282), and inflammatory lesions (17%, n=270). The majority were found to occur secondary to ear piercings, with the exception of edema, which mainly occurred secondary to tongue piercings (45%, n=150), and allergy, which primarily was associated with oral piercings (51%, n=245)(Table 2).
Tissue Damage—Tissue damage accounted for 43% of all complications (n=13,036/30,090). The most common forms of tissue damage were trauma (55%, n=7182), dysesthesia (22%, n=2883), bleeding and bruising (18%, n=2376), and pain (3%, n=370)(Table 2). Trauma was mainly found to be a complication in the context of oral piercings (99%, n=7121). Similarly, 94% (n=2242) of bleeding and bruising occurred secondary to oral piercings. Embedded piercings (92%, n=127), deformity (91%, n=29), and necrosis (75%, n=3) mostly occurred following ear piercings. Lip piercings were found to be the most common cause of damage to surrounding structures (98%, n=50).
Oral—Overall, 3193 intraoral complications were reported, constituting 11% of the total complications (Table 2). Oral complications included dental damage (86%, n=2732), gum recession (14%, n=459), and gingivitis (0%, n=2). Dental damage was mostly reported following oral piercings (90%, n=2453), whereas gum recession was mostly reported following lip piercings (59%, n=272).
Proliferations—Proliferations accounted for 795 (3%) of reported piercing complications. The majority (97%, n=772) were keloids, 2% (n=16) were other benign growths, and 1% (n=7) were malignancies. These complications mostly occurred secondary to ear piercings, which resulted in 741 (96%) keloids, 6 (38%) benign growths, and 4 (57%) malignancies.
Systemic—Overall, 2% (n=633) of the total complications were classified as systemic issues, including functional impairment (45%, n=282), secondary organ involvement (24%, n=150), cardiac issues (3%, n=21), and aspiration/inhalation (1%, n=8). Nonlocalized infections such as hepatitis or an increased risk thereof (17%, n=107), tetanus (8%, n=52), chlamydia (1%, n=9), HIV (0%, n=1), herpes simplex virus (0%, n=1), gonorrhea (0%, n=1), and bacterial vaginosis (0%, n=1) also were included in this category. The tongue, ear, and genitals were the locations most involved in these complications (Table 2). Secondary organ involvement mostly occurred after ear (36%, n=54) and genital piercings (27%, n=41). A total of 8 cases of piercing aspiration and/or inhalation were reported in association with piercings of the head and neck (Table 2).
COMMENT
Piercing Complications
Overall, the ear, tongue, and oral cavity were found to be the sites with the most associated complications recorded in the literature, and local infection and tissue damage were found to be the most prevalent types of complications. A plethora of treatments were used to manage piercing-induced complications, including surgical or medical treatments and avoidance (Supplemental Information). Reports by Metts6 and Escudero-Castaño et al7 provide detailed protocols and photographs of piercings.
Infections
Our review found that local infections were commonly reported complications associated with body piercings, which is consistent with other studies.1 The initial trauma inherent in the piercing process followed by the presence of an ongoing foreign body lends itself to an increased risk for developing these complications. Wound healing after piercing also varies based on the piercing location.
The rate and severity of the infection are influenced by the anatomic location of the piercing, hygiene, method of piercing, types of materials used, and aftercare.8 Piercing cartilage sites, such as the helix, concha, or nose, increases susceptibility to infections and permanent deformities. Cartilage is particularly at risk because of its avascular nature.9 Other studies have reported similar incidences of superficial localized infections; infectious complications were seen in 10% to 30% of body piercings in one study,3 while 45% of American and Australian college students reported infection at a piercing site in a second study.10
Systemic Issues
Systemic issues are potentially the most dangerous piercing-induced complications, though they were rarer in our analysis. Some serious complications included septic emboli, fatal staphylococcal toxic shock syndrome, and death. Although some systemic issues, such as staphylococcal toxic shock syndrome and septic sacroiliitis, required extensive hospital stays and complex treatment, others had lifelong repercussions, such as hepatitis and HIV. One report showed an increased incidence of endocarditis associated with body piercing, including staphylococcal endocarditis following nasal piercings, Neisseria endocarditis following tongue piercings, and Staphylococcus epidermidis endocarditis following nipple piercings.11 Moreover, Mariano et al12—who noted a case of endocarditis and meningitis associated with a nape piercing in a young female in 2015—reinforced the notion that information pertaining to the risks associated with body piercing must be better disseminated, given the potential for morbid or fatal outcomes. Finally, nonsterile piercing techniques and poor hygiene were found to contribute substantially to the increased risk for infection, so it is of utmost importance to reinforce proper practices in piercing salons.4
Immune-Mediated Issues
Because piercings are foreign bodies, they are susceptible to both acute and chronic immune responses. Our study found that allergies and dermatitis made up almost half of the immune-mediated piercing complications. It is especially important to emphasize that costume jewelry exposes our skin to a variety of contact allergens, most prominently nickel, heightening the risk for developing allergic contact dermatitis.13 Moreover, a study conducted by Brandão et al14 found that patients with pierced ears were significantly more likely to react to nickel than those without pierced ears (P=.031). Although other studies have found that allergy to metals ranges from 8.3% to 20% in the general population,15 we were not able to quantify the incidence in our study due to a lack of reporting of common benign complications, such as contact dermatitis.
Tissue Damage and Local Problems
Our review found that tissue and oral damage also were commonly reported piercing complications, with the most common pathologies being trauma, dysesthesia, bleeding/bruising, and dental damage. Laumann and Derick16 reported that bleeding, tissue trauma, and local problems were common physical health problems associated with body piercing. Severe complications, such as abscesses, toxic shock syndrome, and endocarditis, also have been reported in association with intraoral piercings.17 Moreover, other studies have shown that oral piercings are associated with several adverse oral and systemic conditions. A meta-analysis of individuals with oral piercings found a similar prevalence of dental fracture, gingival recession, and tooth wear (34%), as well as unspecified dental damage (27%) and tooth chipping (22%). Additionally, this meta-analysis reported a 3-fold increased risk for dental fracture and 7-fold increased risk for gingival recession with oral piercings.18 Another meta-analysis of oral piercing complications found a similar prevalence of dental fracture (34%), tooth wear (34%), gingival recession (33%), unspecified dental damage (27%), and tooth chipping (22%).19 Considering the extensive amount of cumulative damage, wearers of oral jewelry require periodic periodontal evaluations to monitor for dental damage and gingival recession.20 There are limited data on treatments for complications of oral piercings, and further research in this area is warranted.
Proliferations and Scars
Although proliferations and scarring were among the least common complications reported in the literature, they are some of the most cosmetically disfiguring for patients. Keloids, the most common type of growth associated with piercings, do not naturally regress and thus require some form of intervention. Given the multimodal approach used to treat keloids, as described by the evidence-based algorithm by Ogawa,21 it is not surprising that keloids also represented the complication most treated with medical therapies, such as steroids, and also with direct-target therapy, such as liquid nitrogen therapy (Supplemental Information).
Other proliferations reported in the literature include benign pyogenic granulomas22 and much less commonly malignant neoplasms such as basal cell carcinoma23 and squamous cell carcinoma.24 Although rare, treatment of piercing-associated malignancies include surgical removal, chemotherapy, and radiation therapy (Supplemental Information).
Limitations
There are several limitations to our systematic review. First, heterogeneity in study designs, patient populations, treatment interventions, and outcome measures of included studies may have affected the quality and generalizability of our results. Moreover, because the studies included in this systematic review focused on specific complications, we could not compare our results to the literature that analyzes incidence rates of piercing complications. Furthermore, not all studies included the data that we hoped to extract, and thus only available data were reported in these instances. Finally, the articles we reviewed may have included publication bias, with positive findings being more frequently published, potentially inflating certain types and sites of complications and treatment choices. Despite these limitations, our review provides essential information that must be interpreted in a clinical context.
CONCLUSION
Given that cutaneous and mucosal piercing has become more prevalent in recent years, along with an increase in the variety of piercing-induced complications, it is of utmost importance that piercing salons have proper hygiene practices in place and that patients are aware of the multitude of potential complications that can arise—whether common and benign or rare but life-threatening.
The practice of body piercing has been present in cultures around the world for centuries. Piercings may be performed for religious or spiritual reasons or as a form of self-expression. In recent years, body piercings have become increasingly popular in all genders, with the most common sites being the ears, mouth, nose, eyebrows, nipples, navel, and genitals.1 The prevalence of body piercing in the general population is estimated to be as high as 50%.2 With the rising popularity of piercings, there also has been an increase in their associated complications, with one study noting that up to 35% of individuals with pierced ears and 30% of all pierced sites developed a complication.3 Common problems following piercing include infections, keloid formation, allergic contact dermatitis, site deformation, and tooth fractures.4 It is of utmost importance that health care professionals are aware of the potential complications associated with such a common practice. A comprehensive review of complications associated with cutaneous and mucosal piercings is lacking. We conducted a systematic review to summarize the clinical characteristics, complication types and frequency, and treatments reported for cutaneous and mucosal piercings.
METHODS
We conducted a systematic review of the literature adhering to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) reporting guidelines.5
Search Strategy, Study Eligibility Criteria, and Study Selection
A literature search of the Embase, MEDLINE, and PubMed databases was performed on June 20, 2022, using search terms related to body piercing and possible piercing-induced complications (Supplemental Information online). All studies reporting complications following body piercing were included. In vitro and animal studies were excluded. Title and abstract screening were completed by 6 independent researchers (S.C., K.K., M.M-B., K.A., T.S., I.M.M.) using Covidence online systematic review software (www.covidence.org). Six reviewers (S.C., K.K., M.M-B., K.A., T.S., I.M.M.) independently evaluated titles, abstracts, and full texts to identify relevant studies. Conflicts were resolved by the senior reviewer (I.M.M.).
Data Extraction and Synthesis
Five reviewers (S.C., K.K., M.M-B., K.A., T.S.) independently extracted data from eligible studies using a standardized extraction form that included title; authors; year of publication; sample size; and key findings, including mean age, sex, piercing location, complication type, and treatment received.
Treatment type was placed into the following categories: surgical treatments, antimicrobials, medical treatments, direct-target therapy, oral procedures, avoidance, miscellaneous therapies, and no treatment. (Data regarding treatments can be found in the Supplemental Information online.)
RESULTS
The combined search yielded 2679 studies, 617 of which underwent full-text review; 319 studies were included (Figure). Studies were published from 1950 to June 2022 and included both adult and pediatric populations.

Patient Characteristics
In total, our pooled analysis included data on 30,090 complications across 36,803 pierced sites in 30,231 patients (Table 1). Demographic data are available for 55% (n=30,231) of patients. Overall, 74% (22,247/30,231) of the individuals included in our analysis were female. The mean age was 27.8 years (range, 0–76 years).

Piercing Location
Overall, 36,803 pierced sites had a reported complication. The oral cavity, location not otherwise specified, was the most common site associated with a complication, accounting for 67% (n=24,478) of complications (Table 1). Other reported sites included (in decreasing frequency) the ears (21%, n=7551), tongue (5%, n=1669), lip (3%, n=998), navel (2%, n=605), nose (1%, n=540), nipple (1%, n=344), face/body (1%, n=269), genitals/groin (0%, n=183), eyebrow (0%, n=161), hand (0%, n=4), and eyelid (0%, n=1). Piercing complications were more commonly reported among females across all piercing locations except for the eyebrow, which was equal in both sexes.
Complications
Local Infections—Local infections accounted for 36% of reported complication types (n=10,872/30,090): perichondritis (1%, n=85); abscesses (0%, n=25); bacterial colonization (1%, n=106); and local infections, not otherwise specified (98%, n=10,648)(Table 2). The majority of local infections were found to be secondary to piercings of the ear and oral cavity. The nipple was found to be a common site for abscesses (40%, n=10), whereas the tongue was found to be the most common site for bacterial colonization (69%, n=73).


Immune-Mediated Issues—Immune-mediated issues encompassed 5% of the total reported complications (n=1561/30,090). The most commonly reported immune-mediated complications included allergies (31%, n=482), edema and swelling (21%, n=331), dermatitis (18%, n=282), and inflammatory lesions (17%, n=270). The majority were found to occur secondary to ear piercings, with the exception of edema, which mainly occurred secondary to tongue piercings (45%, n=150), and allergy, which primarily was associated with oral piercings (51%, n=245)(Table 2).
Tissue Damage—Tissue damage accounted for 43% of all complications (n=13,036/30,090). The most common forms of tissue damage were trauma (55%, n=7182), dysesthesia (22%, n=2883), bleeding and bruising (18%, n=2376), and pain (3%, n=370)(Table 2). Trauma was mainly found to be a complication in the context of oral piercings (99%, n=7121). Similarly, 94% (n=2242) of bleeding and bruising occurred secondary to oral piercings. Embedded piercings (92%, n=127), deformity (91%, n=29), and necrosis (75%, n=3) mostly occurred following ear piercings. Lip piercings were found to be the most common cause of damage to surrounding structures (98%, n=50).
Oral—Overall, 3193 intraoral complications were reported, constituting 11% of the total complications (Table 2). Oral complications included dental damage (86%, n=2732), gum recession (14%, n=459), and gingivitis (0%, n=2). Dental damage was mostly reported following oral piercings (90%, n=2453), whereas gum recession was mostly reported following lip piercings (59%, n=272).
Proliferations—Proliferations accounted for 795 (3%) of reported piercing complications. The majority (97%, n=772) were keloids, 2% (n=16) were other benign growths, and 1% (n=7) were malignancies. These complications mostly occurred secondary to ear piercings, which resulted in 741 (96%) keloids, 6 (38%) benign growths, and 4 (57%) malignancies.
Systemic—Overall, 2% (n=633) of the total complications were classified as systemic issues, including functional impairment (45%, n=282), secondary organ involvement (24%, n=150), cardiac issues (3%, n=21), and aspiration/inhalation (1%, n=8). Nonlocalized infections such as hepatitis or an increased risk thereof (17%, n=107), tetanus (8%, n=52), chlamydia (1%, n=9), HIV (0%, n=1), herpes simplex virus (0%, n=1), gonorrhea (0%, n=1), and bacterial vaginosis (0%, n=1) also were included in this category. The tongue, ear, and genitals were the locations most involved in these complications (Table 2). Secondary organ involvement mostly occurred after ear (36%, n=54) and genital piercings (27%, n=41). A total of 8 cases of piercing aspiration and/or inhalation were reported in association with piercings of the head and neck (Table 2).
COMMENT
Piercing Complications
Overall, the ear, tongue, and oral cavity were found to be the sites with the most associated complications recorded in the literature, and local infection and tissue damage were found to be the most prevalent types of complications. A plethora of treatments were used to manage piercing-induced complications, including surgical or medical treatments and avoidance (Supplemental Information). Reports by Metts6 and Escudero-Castaño et al7 provide detailed protocols and photographs of piercings.
Infections
Our review found that local infections were commonly reported complications associated with body piercings, which is consistent with other studies.1 The initial trauma inherent in the piercing process followed by the presence of an ongoing foreign body lends itself to an increased risk for developing these complications. Wound healing after piercing also varies based on the piercing location.
The rate and severity of the infection are influenced by the anatomic location of the piercing, hygiene, method of piercing, types of materials used, and aftercare.8 Piercing cartilage sites, such as the helix, concha, or nose, increases susceptibility to infections and permanent deformities. Cartilage is particularly at risk because of its avascular nature.9 Other studies have reported similar incidences of superficial localized infections; infectious complications were seen in 10% to 30% of body piercings in one study,3 while 45% of American and Australian college students reported infection at a piercing site in a second study.10
Systemic Issues
Systemic issues are potentially the most dangerous piercing-induced complications, though they were rarer in our analysis. Some serious complications included septic emboli, fatal staphylococcal toxic shock syndrome, and death. Although some systemic issues, such as staphylococcal toxic shock syndrome and septic sacroiliitis, required extensive hospital stays and complex treatment, others had lifelong repercussions, such as hepatitis and HIV. One report showed an increased incidence of endocarditis associated with body piercing, including staphylococcal endocarditis following nasal piercings, Neisseria endocarditis following tongue piercings, and Staphylococcus epidermidis endocarditis following nipple piercings.11 Moreover, Mariano et al12—who noted a case of endocarditis and meningitis associated with a nape piercing in a young female in 2015—reinforced the notion that information pertaining to the risks associated with body piercing must be better disseminated, given the potential for morbid or fatal outcomes. Finally, nonsterile piercing techniques and poor hygiene were found to contribute substantially to the increased risk for infection, so it is of utmost importance to reinforce proper practices in piercing salons.4
Immune-Mediated Issues
Because piercings are foreign bodies, they are susceptible to both acute and chronic immune responses. Our study found that allergies and dermatitis made up almost half of the immune-mediated piercing complications. It is especially important to emphasize that costume jewelry exposes our skin to a variety of contact allergens, most prominently nickel, heightening the risk for developing allergic contact dermatitis.13 Moreover, a study conducted by Brandão et al14 found that patients with pierced ears were significantly more likely to react to nickel than those without pierced ears (P=.031). Although other studies have found that allergy to metals ranges from 8.3% to 20% in the general population,15 we were not able to quantify the incidence in our study due to a lack of reporting of common benign complications, such as contact dermatitis.
Tissue Damage and Local Problems
Our review found that tissue and oral damage also were commonly reported piercing complications, with the most common pathologies being trauma, dysesthesia, bleeding/bruising, and dental damage. Laumann and Derick16 reported that bleeding, tissue trauma, and local problems were common physical health problems associated with body piercing. Severe complications, such as abscesses, toxic shock syndrome, and endocarditis, also have been reported in association with intraoral piercings.17 Moreover, other studies have shown that oral piercings are associated with several adverse oral and systemic conditions. A meta-analysis of individuals with oral piercings found a similar prevalence of dental fracture, gingival recession, and tooth wear (34%), as well as unspecified dental damage (27%) and tooth chipping (22%). Additionally, this meta-analysis reported a 3-fold increased risk for dental fracture and 7-fold increased risk for gingival recession with oral piercings.18 Another meta-analysis of oral piercing complications found a similar prevalence of dental fracture (34%), tooth wear (34%), gingival recession (33%), unspecified dental damage (27%), and tooth chipping (22%).19 Considering the extensive amount of cumulative damage, wearers of oral jewelry require periodic periodontal evaluations to monitor for dental damage and gingival recession.20 There are limited data on treatments for complications of oral piercings, and further research in this area is warranted.
Proliferations and Scars
Although proliferations and scarring were among the least common complications reported in the literature, they are some of the most cosmetically disfiguring for patients. Keloids, the most common type of growth associated with piercings, do not naturally regress and thus require some form of intervention. Given the multimodal approach used to treat keloids, as described by the evidence-based algorithm by Ogawa,21 it is not surprising that keloids also represented the complication most treated with medical therapies, such as steroids, and also with direct-target therapy, such as liquid nitrogen therapy (Supplemental Information).
Other proliferations reported in the literature include benign pyogenic granulomas22 and much less commonly malignant neoplasms such as basal cell carcinoma23 and squamous cell carcinoma.24 Although rare, treatment of piercing-associated malignancies include surgical removal, chemotherapy, and radiation therapy (Supplemental Information).
Limitations
There are several limitations to our systematic review. First, heterogeneity in study designs, patient populations, treatment interventions, and outcome measures of included studies may have affected the quality and generalizability of our results. Moreover, because the studies included in this systematic review focused on specific complications, we could not compare our results to the literature that analyzes incidence rates of piercing complications. Furthermore, not all studies included the data that we hoped to extract, and thus only available data were reported in these instances. Finally, the articles we reviewed may have included publication bias, with positive findings being more frequently published, potentially inflating certain types and sites of complications and treatment choices. Despite these limitations, our review provides essential information that must be interpreted in a clinical context.
CONCLUSION
Given that cutaneous and mucosal piercing has become more prevalent in recent years, along with an increase in the variety of piercing-induced complications, it is of utmost importance that piercing salons have proper hygiene practices in place and that patients are aware of the multitude of potential complications that can arise—whether common and benign or rare but life-threatening.
- Preslar D, Borger J. Body piercing infections. In: StatPearls. StatPearls Publishing; 2022.
- Antoszewski B, Jedrzejczak M, Kruk-Jeromin J. Complications after body piercing in patient suffering from type 1 diabetes mellitus. Int J Dermatol. 2007;46:1250-1252.
- Simplot TC, Hoffman HT. Comparison between cartilage and soft tissue ear piercing complications. Am J Otolaryngol. 1998;19:305-310.
- Meltzer DI. Complications of body piercing. Am Fam Physician. 2005;72:2029-2034.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Metts J. Common complications of body piercing. West J Med. 2002;176:85-86.
- Escudero-Castaño N, Perea-García MA, Campo-Trapero J, et al. Oral and perioral piercing complications. Open Dent J. 2008;2:133-136.
- Tweeten SS, Rickman LS. Infectious complications of body piercing. Clin Infect Dis. 1998;26:735-740.
- Gabriel OT, Anthony OO, Paul EA, et al. Trends and complications of ear piercing among selected Nigerian population. J Family Med Prim Care. 2017;6:517-521.
- Armstrong ML, Koch JR, Saunders JC, et al. The hole picture: risks, decision making, purpose, regulations, and the future of body piercing. Clin Dermatol. 2007;25:398-406.
- Millar BC, Moore JE. Antibiotic prophylaxis, body piercing and infective endocarditis. J Antimicrob Chemother. 2004;53:123-126.
- Mariano A, Pisapia R, Abdeddaim A, et al. Endocarditis and meningitis associated to nape piercing in a young female: a case report. Infez Med. 2015;23:275-279.
- Ivey LA, Limone BA, Jacob SE. Approach to the jewelry aficionado. Pediatr Dermatol. 2018;35:274-275.
- Brandão MH, Gontijo B, Girundi MA, et al. Ear piercing as a risk factor for contact allergy to nickel. J Pediatr (Rio J). 2010;86:149-154.
- Schuttelaar MLA, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy to metals in the European general population with a focus on nickel and piercings: The EDEN Fragrance Study. Contact Dermatitis. 2018;79:1-9.
- Laumann AE, Derick AJ. Tattoos and body piercings in the United States: a national data set. J Am Acad Dermatol. 2006;55:413-421.
- De Moor RJ, De Witte AM, Delmé KI, et al. Dental and oral complications of lip and tongue piercings. Br Dent J. 2005;199:506-509.
- Offen E, Allison JR. Do oral piercings cause problems in the mouth? Evid Based Dent. 2022;23:126-127.
- Passos PF, Pintor AVB, Marañón-Vásquez GA, et al. Oral manifestations arising from oral piercings: A systematic review and meta-analyses. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134:327-341.
- Covello F, Salerno C, Giovannini V, et al. Piercing and oral health: a study on the knowledge of risks and complications. Int J Environ Res Public Health. 2020;17:613.
- Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: a 2020 update of the algorithms published 10 years ago. Plast Reconstr Surg. 2022;149:E79-E94.
- Kumar Ghosh S, Bandyopadhyay D. Granuloma pyogenicum as a complication of decorative nose piercing: report of eight cases from eastern India. J Cutan Med Surg. 2012;16:197-200.
- Dreher K, Kern M, Rush L, et al. Basal cell carcinoma invasion of an ear piercing. Dermatol Online J. 2022;28.
- Stanko P, Poruban D, Mracna J, et al. Squamous cell carcinoma and piercing of the tongue—a case report. J Craniomaxillofac Surg. 2012;40:329-331.
- Preslar D, Borger J. Body piercing infections. In: StatPearls. StatPearls Publishing; 2022.
- Antoszewski B, Jedrzejczak M, Kruk-Jeromin J. Complications after body piercing in patient suffering from type 1 diabetes mellitus. Int J Dermatol. 2007;46:1250-1252.
- Simplot TC, Hoffman HT. Comparison between cartilage and soft tissue ear piercing complications. Am J Otolaryngol. 1998;19:305-310.
- Meltzer DI. Complications of body piercing. Am Fam Physician. 2005;72:2029-2034.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Metts J. Common complications of body piercing. West J Med. 2002;176:85-86.
- Escudero-Castaño N, Perea-García MA, Campo-Trapero J, et al. Oral and perioral piercing complications. Open Dent J. 2008;2:133-136.
- Tweeten SS, Rickman LS. Infectious complications of body piercing. Clin Infect Dis. 1998;26:735-740.
- Gabriel OT, Anthony OO, Paul EA, et al. Trends and complications of ear piercing among selected Nigerian population. J Family Med Prim Care. 2017;6:517-521.
- Armstrong ML, Koch JR, Saunders JC, et al. The hole picture: risks, decision making, purpose, regulations, and the future of body piercing. Clin Dermatol. 2007;25:398-406.
- Millar BC, Moore JE. Antibiotic prophylaxis, body piercing and infective endocarditis. J Antimicrob Chemother. 2004;53:123-126.
- Mariano A, Pisapia R, Abdeddaim A, et al. Endocarditis and meningitis associated to nape piercing in a young female: a case report. Infez Med. 2015;23:275-279.
- Ivey LA, Limone BA, Jacob SE. Approach to the jewelry aficionado. Pediatr Dermatol. 2018;35:274-275.
- Brandão MH, Gontijo B, Girundi MA, et al. Ear piercing as a risk factor for contact allergy to nickel. J Pediatr (Rio J). 2010;86:149-154.
- Schuttelaar MLA, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy to metals in the European general population with a focus on nickel and piercings: The EDEN Fragrance Study. Contact Dermatitis. 2018;79:1-9.
- Laumann AE, Derick AJ. Tattoos and body piercings in the United States: a national data set. J Am Acad Dermatol. 2006;55:413-421.
- De Moor RJ, De Witte AM, Delmé KI, et al. Dental and oral complications of lip and tongue piercings. Br Dent J. 2005;199:506-509.
- Offen E, Allison JR. Do oral piercings cause problems in the mouth? Evid Based Dent. 2022;23:126-127.
- Passos PF, Pintor AVB, Marañón-Vásquez GA, et al. Oral manifestations arising from oral piercings: A systematic review and meta-analyses. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134:327-341.
- Covello F, Salerno C, Giovannini V, et al. Piercing and oral health: a study on the knowledge of risks and complications. Int J Environ Res Public Health. 2020;17:613.
- Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: a 2020 update of the algorithms published 10 years ago. Plast Reconstr Surg. 2022;149:E79-E94.
- Kumar Ghosh S, Bandyopadhyay D. Granuloma pyogenicum as a complication of decorative nose piercing: report of eight cases from eastern India. J Cutan Med Surg. 2012;16:197-200.
- Dreher K, Kern M, Rush L, et al. Basal cell carcinoma invasion of an ear piercing. Dermatol Online J. 2022;28.
- Stanko P, Poruban D, Mracna J, et al. Squamous cell carcinoma and piercing of the tongue—a case report. J Craniomaxillofac Surg. 2012;40:329-331.
Practice Points
- Intraoral piercings of the tongue, lip, gingiva, or mucosa are associated with the most acute and chronic complications.
- Tissue damage is a common complication associated with cutaneous and mucocutaneous piercings, including trauma, bleeding and bruising, or dysesthesia.
- Given the rapid rise in the popularity of piercings, general practitioners and dermatologists should be aware of the multitude of acute or chronic complications associated with body piercings as well as effective treatment modalities.
Disseminated Papules and Nodules on the Skin and Oral Mucosa in an Infant
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.
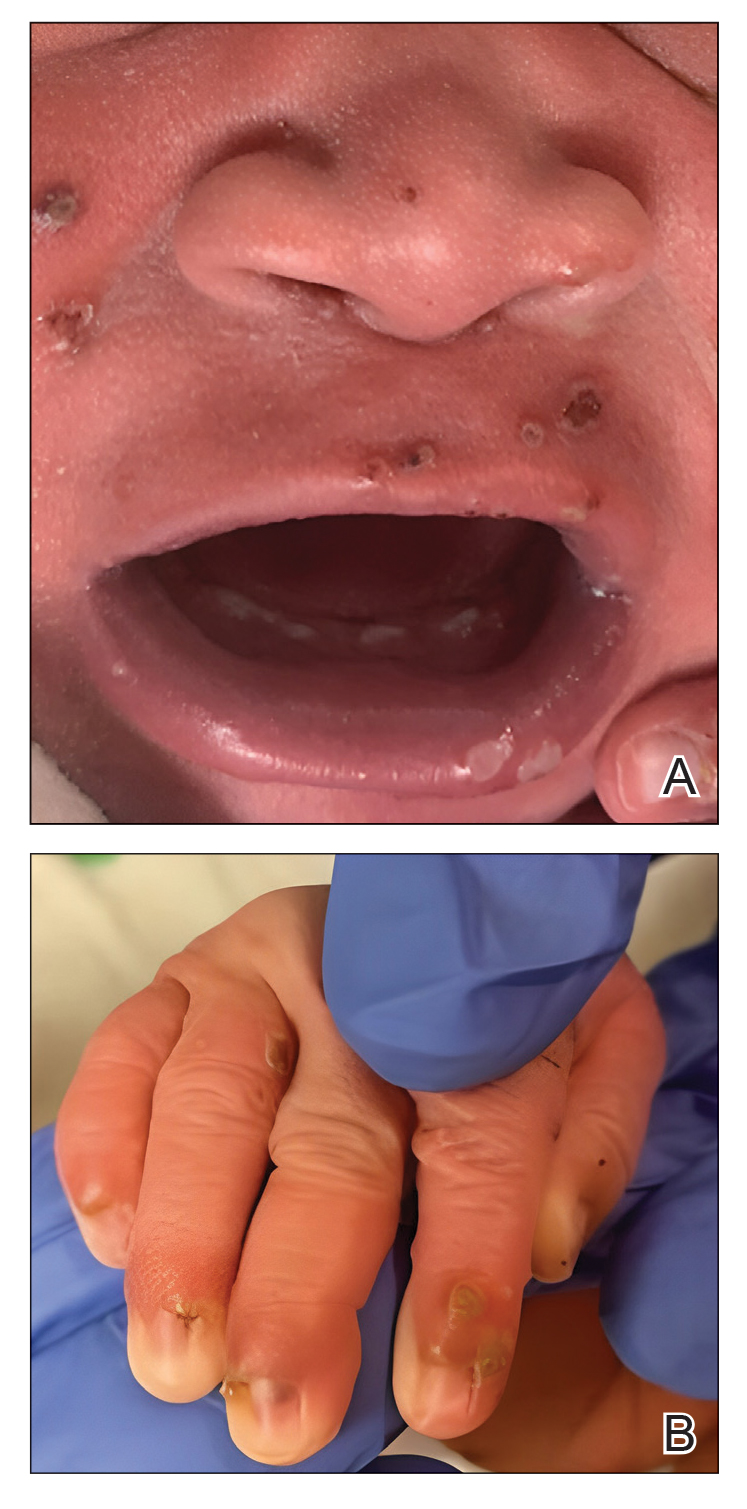
Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
A 38-week-old infant boy presented at birth with disseminated, nonblanching, purple to dark red papules and nodules on the skin and oral mucosa. He was born spontaneously after an uncomplicated pregnancy. The mother experienced an episode of oral herpes simplex virus during pregnancy. The infant was otherwise healthy. Laboratory tests including a complete blood cell count and routine serum biochemical analyses were within reference range; however, an infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies. Ophthalmologic and auditory screenings were normal.