User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Editorial Note
The first Skin of Color column, "Diversity in Dermatology: A Society Devoted to Skin of Color," produced in collaboration with the Skin of Color Society appears on page 322. This column will be published quarterly and will serve to increase the knowledge available to dermatologists to help improve delivery of care to this underserved population.
Look for Skin of Color columns in upcoming issues of Cutis.
The first Skin of Color column, "Diversity in Dermatology: A Society Devoted to Skin of Color," produced in collaboration with the Skin of Color Society appears on page 322. This column will be published quarterly and will serve to increase the knowledge available to dermatologists to help improve delivery of care to this underserved population.
Look for Skin of Color columns in upcoming issues of Cutis.
The first Skin of Color column, "Diversity in Dermatology: A Society Devoted to Skin of Color," produced in collaboration with the Skin of Color Society appears on page 322. This column will be published quarterly and will serve to increase the knowledge available to dermatologists to help improve delivery of care to this underserved population.
Look for Skin of Color columns in upcoming issues of Cutis.
Skin Cancer Mortality in Patients With Skin of Color
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Approach to Management of Giant Basal Cell Carcinomas
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
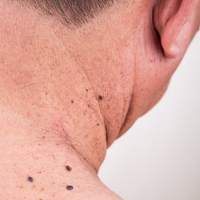
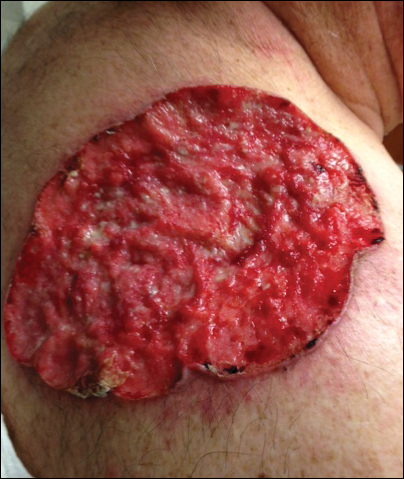
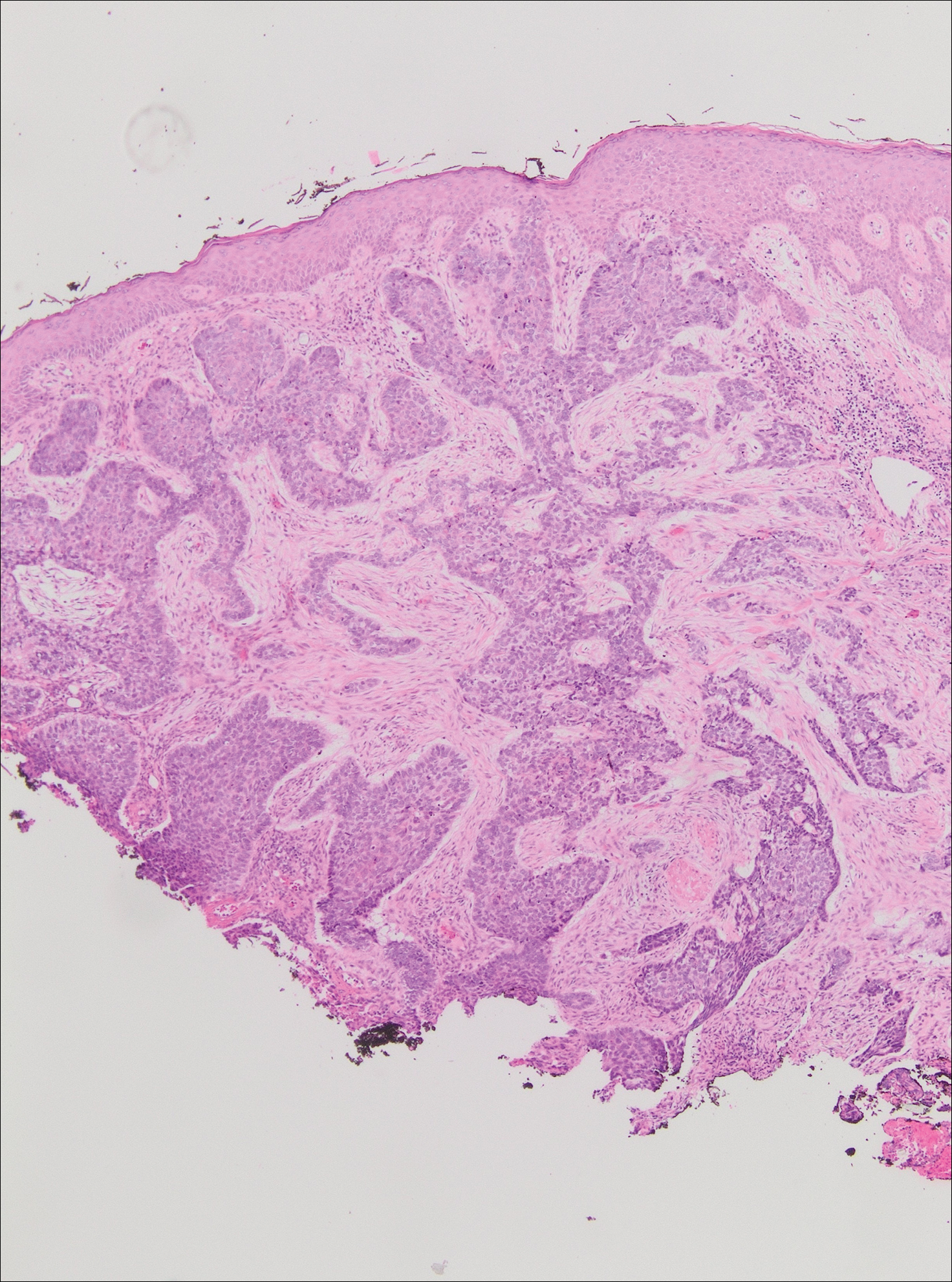
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.
The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
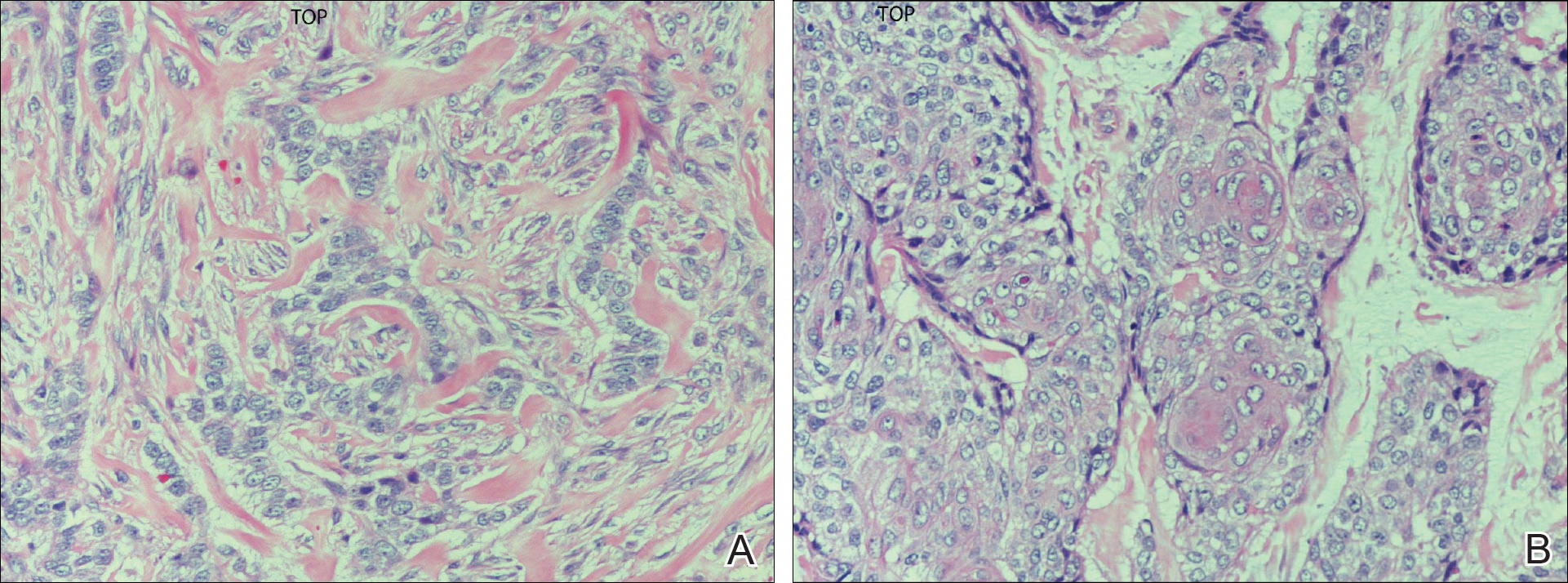
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.
The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
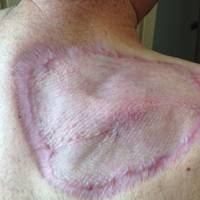
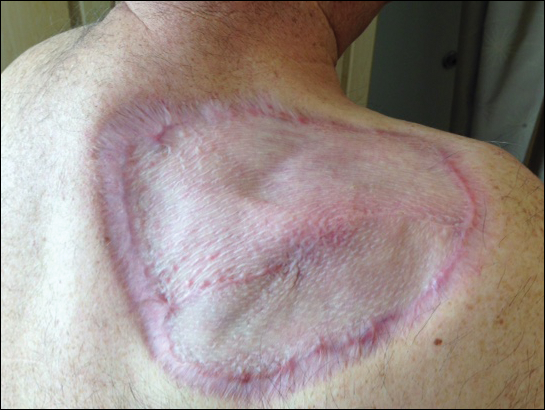
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.
Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Practice Points
- Unusually large basal cell carcinomas (BCCs) present a therapeutic challenge.
- A number of therapeutic options exist. Wide excision with margin control and complex reconstruction remains an excellent treatment option for BCC.
Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.
Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.
Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).

Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).

Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Practice Points
- Diagnosis and management of lentigo maligna (LM) and LM melanoma (LMM) is challenging due to their ill-defined margins and location mainly on the head and neck.
- Handheld reflectance confocal microscopy (RCM) has high diagnostic accuracy for LM/LMM and can be used in curved locations to assess large lesions.
- Handheld RCM can be a versatile tool in pretreatment decision-making, intraoperative surgical mapping, and posttreatment monitoring of both surgical and nonsurgical therapies for complex facial LM/LMM.
Sun Protection for Infants: Parent Behaviors and Beliefs in Miami, Florida
Sun exposure and sunburns sustained during childhood are linked to an increased risk for development of skin cancers in adulthood. In infants, the skin is particularly vulnerable and is considered to be at increased risk for UV radiation damage,1 even as early as the first 6 months of life.2 Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 To effectively teach parents proper sun-safe practices, it is essential to understand their existing perceptions and behaviors. This study sought to examine differences in infant sun-safety practices during the first 6 months of life among black, Hispanic, and non-Hispanic white (NHW) parents in Miami, Florida.
Methods
Parents presenting to the University of Miami general pediatrics clinic from February 2015 through April 2015 with a child younger than 5 years were administered a 15-item questionnaire that included items on demographics, sun-safety strategies, sunburns and tanning, beliefs and limitations regarding sunscreen, and primary information source regarding sun safety (eg, physician, Internet, media, instincts). Parents were approached by the investigators consecutively for participation in scheduled blocks, with the exception of those who were otherwise engaged in appointment-related tasks (eg, paperwork). The study was approved by the University of Miami Miller School of Medicine institutional review board. The primary objective of this study was to determine the sun protection behaviors that black and Hispanic parents in Miami, Florida, employ in infants younger than 6 months. Secondary objectives included determining if this patient population is at risk for infant sunburns and tanning, beliefs among parents regarding sunscreen's efficacy in the prevention of skin cancers, and limitations of sunscreen use.
All data were analyzed using SAS software version 9.3. Wilcoxon signed rank test, Kruskal-Wallis test, Fisher exact test, and proportional-odds cumulative logit model were used to compare nonparametric data. Parents reporting on the full first 6 months of life (ie, the child was older than 6 months at the time of study completion) were included for analysis of sun-safety strategies. All survey respondents were included for analysis of secondary objectives. Responses from parents of infants of mixed racial and ethnic backgrounds were excluded from applicable subgroup analyses.
Results
Ninety-eight parents were approached for participation in the study; 97 consented to participate and 95 completed the survey. Seventy parents had children who were at least 6 months of age and were included for analysis of the primary objectives (ie, sun-protection strategies in the first 6 months of life). The cohort included 49 Hispanic parents, 26 black parents, and 9 NHW parents; 5 parents indicated their child was of mixed racial and ethnic background. Six respondents indicated another minority group (eg, Native American, Pacific Islander). Eighty-three respondents were mothers, 72 were educated beyond high school, and 14 were Spanish-speaking only. Four reported a known family history of skin cancer.
There were notable differences in application of sunscreen, belief in the efficacy of sunscreen, and primary source of information between parents (Tables 1 and 2). Hispanic parents reported applying sunscreen more consistently than black parents (odds ratio, 4.656; 95% confidence interval, 1.154-18.782; P<.01). Hispanic parents also were more likely than black parents to believe sunscreen is effective in the prevention of skin cancers (odds ratio, 7.499; 95% confidence interval, 1.535-36.620; P<.01). Hispanic parents were more likely to report receiving information regarding sun-safety practices for infants from their pediatrician, whereas NHW parents were more likely to follow their instincts regarding how and if infants should be exposed to the sun (P<.05). No significant differences were found in the reported primary source of information in black versus Hispanic parents or in black versus NHW parents. Three percent (3/95) of respondents reported a sunburn in the infant's first 6 months of life, and 12% (11/95) reported tanning of infants' skin from sun exposure. Tanning was associated with inconsistent shade (P<.01), inconsistent clothing coverage (P<.01), and consistently allowing infants to "develop tolerance to the sun's rays by slowly increasing sun exposure each day" (P<.05).
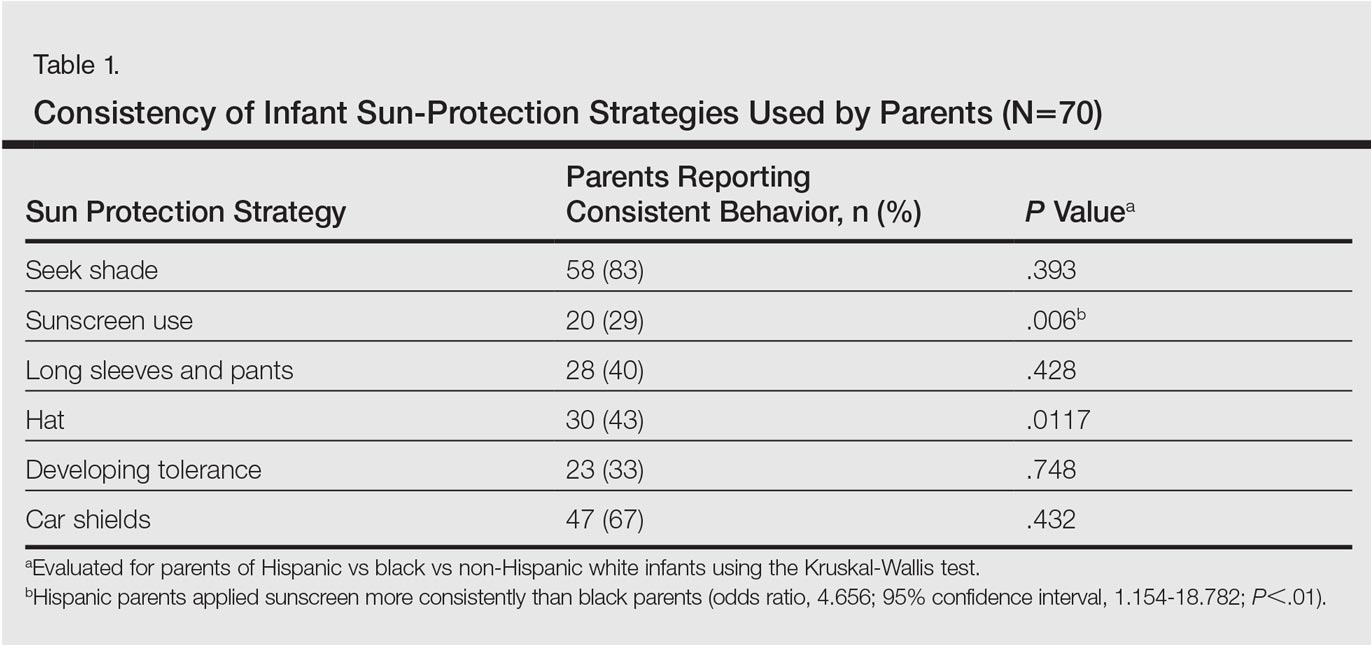
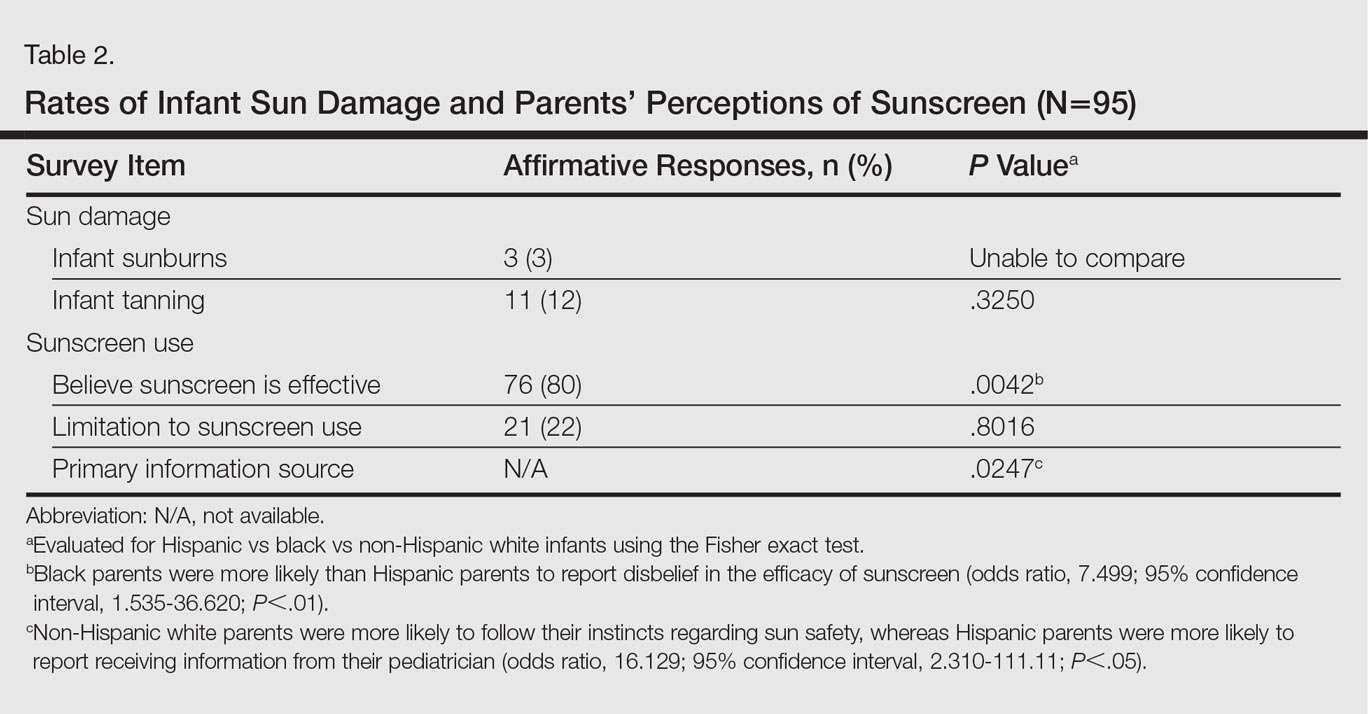
Comment
The survey results indicated suboptimal sun-protection practices among parents of black and Hispanic infants in Miami. Although the majority of respondents (83% [58/70]) reported keeping their infants in the shade, less than half of parents consistently covered their infants adequately with clothing and hats (40% [28/70] and 43% [30/70], respectively). More alarmingly, one-third of parents reported intentionally increasing their infant's level of sun exposure to develop his/her tolerance to the sun. A minority of parents reported sunburns (3%) and tanning (12%) within the first 6 months of life. Twenty-nine percent of parents (20/70) reported consistently applying sunscreen to their infants who were younger than 6 months despite limited safety data available for this age group.
Although our study included a limited sample size and represents a narrow geographic distribution, these results suggest that shortcomings in current practices in sun protection for black and Hispanic infants younger than 6 months may be a widespread problem. Black and Hispanic patients have a lower incidence of skin cancer, but the diagnosis often is delayed and the mortality is higher when skin cancer does occur.4 The common perception among laypeople as well as many health care providers that black and Hispanic individuals are not at risk for skin cancer may limit sun-safety counseling as well as the overall knowledge base of this patient demographic. As demonstrated by the results of this study, there is a need for counseling on sun-safe behaviors from a young age among this population.
Conclusion
This study highlights potential shortcomings in current sun-protection practices for black and Hispanic infants younger than 6 months. Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 Additional studies are needed to further define sun-safety behaviors in black and Hispanic children across the United States. Further, additional studies should focus on developing interventions that positively influence sun-safety behaviors in this patient population.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics. 2011;128:92-102.
- Benjes LS, Brooks DR, Zhang Z, et al. Changing patterns of sun protection between the first and second summers for very young children. Arch Dermatol. 2004;140:925-930.
- Oliveria SA, Saraiya M, Geller AC, et al. Sun exposure and risk of melanoma. Arch Dis Child. 2006;91:131-138.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
Sun exposure and sunburns sustained during childhood are linked to an increased risk for development of skin cancers in adulthood. In infants, the skin is particularly vulnerable and is considered to be at increased risk for UV radiation damage,1 even as early as the first 6 months of life.2 Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 To effectively teach parents proper sun-safe practices, it is essential to understand their existing perceptions and behaviors. This study sought to examine differences in infant sun-safety practices during the first 6 months of life among black, Hispanic, and non-Hispanic white (NHW) parents in Miami, Florida.
Methods
Parents presenting to the University of Miami general pediatrics clinic from February 2015 through April 2015 with a child younger than 5 years were administered a 15-item questionnaire that included items on demographics, sun-safety strategies, sunburns and tanning, beliefs and limitations regarding sunscreen, and primary information source regarding sun safety (eg, physician, Internet, media, instincts). Parents were approached by the investigators consecutively for participation in scheduled blocks, with the exception of those who were otherwise engaged in appointment-related tasks (eg, paperwork). The study was approved by the University of Miami Miller School of Medicine institutional review board. The primary objective of this study was to determine the sun protection behaviors that black and Hispanic parents in Miami, Florida, employ in infants younger than 6 months. Secondary objectives included determining if this patient population is at risk for infant sunburns and tanning, beliefs among parents regarding sunscreen's efficacy in the prevention of skin cancers, and limitations of sunscreen use.
All data were analyzed using SAS software version 9.3. Wilcoxon signed rank test, Kruskal-Wallis test, Fisher exact test, and proportional-odds cumulative logit model were used to compare nonparametric data. Parents reporting on the full first 6 months of life (ie, the child was older than 6 months at the time of study completion) were included for analysis of sun-safety strategies. All survey respondents were included for analysis of secondary objectives. Responses from parents of infants of mixed racial and ethnic backgrounds were excluded from applicable subgroup analyses.
Results
Ninety-eight parents were approached for participation in the study; 97 consented to participate and 95 completed the survey. Seventy parents had children who were at least 6 months of age and were included for analysis of the primary objectives (ie, sun-protection strategies in the first 6 months of life). The cohort included 49 Hispanic parents, 26 black parents, and 9 NHW parents; 5 parents indicated their child was of mixed racial and ethnic background. Six respondents indicated another minority group (eg, Native American, Pacific Islander). Eighty-three respondents were mothers, 72 were educated beyond high school, and 14 were Spanish-speaking only. Four reported a known family history of skin cancer.
There were notable differences in application of sunscreen, belief in the efficacy of sunscreen, and primary source of information between parents (Tables 1 and 2). Hispanic parents reported applying sunscreen more consistently than black parents (odds ratio, 4.656; 95% confidence interval, 1.154-18.782; P<.01). Hispanic parents also were more likely than black parents to believe sunscreen is effective in the prevention of skin cancers (odds ratio, 7.499; 95% confidence interval, 1.535-36.620; P<.01). Hispanic parents were more likely to report receiving information regarding sun-safety practices for infants from their pediatrician, whereas NHW parents were more likely to follow their instincts regarding how and if infants should be exposed to the sun (P<.05). No significant differences were found in the reported primary source of information in black versus Hispanic parents or in black versus NHW parents. Three percent (3/95) of respondents reported a sunburn in the infant's first 6 months of life, and 12% (11/95) reported tanning of infants' skin from sun exposure. Tanning was associated with inconsistent shade (P<.01), inconsistent clothing coverage (P<.01), and consistently allowing infants to "develop tolerance to the sun's rays by slowly increasing sun exposure each day" (P<.05).


Comment
The survey results indicated suboptimal sun-protection practices among parents of black and Hispanic infants in Miami. Although the majority of respondents (83% [58/70]) reported keeping their infants in the shade, less than half of parents consistently covered their infants adequately with clothing and hats (40% [28/70] and 43% [30/70], respectively). More alarmingly, one-third of parents reported intentionally increasing their infant's level of sun exposure to develop his/her tolerance to the sun. A minority of parents reported sunburns (3%) and tanning (12%) within the first 6 months of life. Twenty-nine percent of parents (20/70) reported consistently applying sunscreen to their infants who were younger than 6 months despite limited safety data available for this age group.
Although our study included a limited sample size and represents a narrow geographic distribution, these results suggest that shortcomings in current practices in sun protection for black and Hispanic infants younger than 6 months may be a widespread problem. Black and Hispanic patients have a lower incidence of skin cancer, but the diagnosis often is delayed and the mortality is higher when skin cancer does occur.4 The common perception among laypeople as well as many health care providers that black and Hispanic individuals are not at risk for skin cancer may limit sun-safety counseling as well as the overall knowledge base of this patient demographic. As demonstrated by the results of this study, there is a need for counseling on sun-safe behaviors from a young age among this population.
Conclusion
This study highlights potential shortcomings in current sun-protection practices for black and Hispanic infants younger than 6 months. Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 Additional studies are needed to further define sun-safety behaviors in black and Hispanic children across the United States. Further, additional studies should focus on developing interventions that positively influence sun-safety behaviors in this patient population.
Sun exposure and sunburns sustained during childhood are linked to an increased risk for development of skin cancers in adulthood. In infants, the skin is particularly vulnerable and is considered to be at increased risk for UV radiation damage,1 even as early as the first 6 months of life.2 Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 To effectively teach parents proper sun-safe practices, it is essential to understand their existing perceptions and behaviors. This study sought to examine differences in infant sun-safety practices during the first 6 months of life among black, Hispanic, and non-Hispanic white (NHW) parents in Miami, Florida.
Methods
Parents presenting to the University of Miami general pediatrics clinic from February 2015 through April 2015 with a child younger than 5 years were administered a 15-item questionnaire that included items on demographics, sun-safety strategies, sunburns and tanning, beliefs and limitations regarding sunscreen, and primary information source regarding sun safety (eg, physician, Internet, media, instincts). Parents were approached by the investigators consecutively for participation in scheduled blocks, with the exception of those who were otherwise engaged in appointment-related tasks (eg, paperwork). The study was approved by the University of Miami Miller School of Medicine institutional review board. The primary objective of this study was to determine the sun protection behaviors that black and Hispanic parents in Miami, Florida, employ in infants younger than 6 months. Secondary objectives included determining if this patient population is at risk for infant sunburns and tanning, beliefs among parents regarding sunscreen's efficacy in the prevention of skin cancers, and limitations of sunscreen use.
All data were analyzed using SAS software version 9.3. Wilcoxon signed rank test, Kruskal-Wallis test, Fisher exact test, and proportional-odds cumulative logit model were used to compare nonparametric data. Parents reporting on the full first 6 months of life (ie, the child was older than 6 months at the time of study completion) were included for analysis of sun-safety strategies. All survey respondents were included for analysis of secondary objectives. Responses from parents of infants of mixed racial and ethnic backgrounds were excluded from applicable subgroup analyses.
Results
Ninety-eight parents were approached for participation in the study; 97 consented to participate and 95 completed the survey. Seventy parents had children who were at least 6 months of age and were included for analysis of the primary objectives (ie, sun-protection strategies in the first 6 months of life). The cohort included 49 Hispanic parents, 26 black parents, and 9 NHW parents; 5 parents indicated their child was of mixed racial and ethnic background. Six respondents indicated another minority group (eg, Native American, Pacific Islander). Eighty-three respondents were mothers, 72 were educated beyond high school, and 14 were Spanish-speaking only. Four reported a known family history of skin cancer.
There were notable differences in application of sunscreen, belief in the efficacy of sunscreen, and primary source of information between parents (Tables 1 and 2). Hispanic parents reported applying sunscreen more consistently than black parents (odds ratio, 4.656; 95% confidence interval, 1.154-18.782; P<.01). Hispanic parents also were more likely than black parents to believe sunscreen is effective in the prevention of skin cancers (odds ratio, 7.499; 95% confidence interval, 1.535-36.620; P<.01). Hispanic parents were more likely to report receiving information regarding sun-safety practices for infants from their pediatrician, whereas NHW parents were more likely to follow their instincts regarding how and if infants should be exposed to the sun (P<.05). No significant differences were found in the reported primary source of information in black versus Hispanic parents or in black versus NHW parents. Three percent (3/95) of respondents reported a sunburn in the infant's first 6 months of life, and 12% (11/95) reported tanning of infants' skin from sun exposure. Tanning was associated with inconsistent shade (P<.01), inconsistent clothing coverage (P<.01), and consistently allowing infants to "develop tolerance to the sun's rays by slowly increasing sun exposure each day" (P<.05).


Comment
The survey results indicated suboptimal sun-protection practices among parents of black and Hispanic infants in Miami. Although the majority of respondents (83% [58/70]) reported keeping their infants in the shade, less than half of parents consistently covered their infants adequately with clothing and hats (40% [28/70] and 43% [30/70], respectively). More alarmingly, one-third of parents reported intentionally increasing their infant's level of sun exposure to develop his/her tolerance to the sun. A minority of parents reported sunburns (3%) and tanning (12%) within the first 6 months of life. Twenty-nine percent of parents (20/70) reported consistently applying sunscreen to their infants who were younger than 6 months despite limited safety data available for this age group.
Although our study included a limited sample size and represents a narrow geographic distribution, these results suggest that shortcomings in current practices in sun protection for black and Hispanic infants younger than 6 months may be a widespread problem. Black and Hispanic patients have a lower incidence of skin cancer, but the diagnosis often is delayed and the mortality is higher when skin cancer does occur.4 The common perception among laypeople as well as many health care providers that black and Hispanic individuals are not at risk for skin cancer may limit sun-safety counseling as well as the overall knowledge base of this patient demographic. As demonstrated by the results of this study, there is a need for counseling on sun-safe behaviors from a young age among this population.
Conclusion
This study highlights potential shortcomings in current sun-protection practices for black and Hispanic infants younger than 6 months. Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 Additional studies are needed to further define sun-safety behaviors in black and Hispanic children across the United States. Further, additional studies should focus on developing interventions that positively influence sun-safety behaviors in this patient population.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics. 2011;128:92-102.
- Benjes LS, Brooks DR, Zhang Z, et al. Changing patterns of sun protection between the first and second summers for very young children. Arch Dermatol. 2004;140:925-930.
- Oliveria SA, Saraiya M, Geller AC, et al. Sun exposure and risk of melanoma. Arch Dis Child. 2006;91:131-138.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics. 2011;128:92-102.
- Benjes LS, Brooks DR, Zhang Z, et al. Changing patterns of sun protection between the first and second summers for very young children. Arch Dermatol. 2004;140:925-930.
- Oliveria SA, Saraiya M, Geller AC, et al. Sun exposure and risk of melanoma. Arch Dis Child. 2006;91:131-138.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
Practice Points
- Infants of all racial and ethnic backgrounds need protection from the sun's rays. Remember to counsel parents on the importance of sun protection.
- Instruct parents to keep infants in the shade when outdoors and to dress infants in a long-sleeved shirt, pants, and a hat. Intentional sun exposure for infants is not recommended.
- The American Academy of Dermatology currently recommends that parents begin sunscreen application when their child reaches 6 months of age. Broad-spectrum barrier sunscreens containing zinc oxide or titanium dioxide are preferred and should provide a sun protection factor of 30 or greater.
Serpentine Supravenous Hyperpigmentation Following Cisplatin and Pemetrexed Chemotherapy
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
Practice Points
- A variety of dermatologic complications have been reported with cisplatin chemotherapy, including serpentine supravenous hyperpigmentation (SSH); however, pemetrexed has not been associated with SSH.
- Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.
Expanding Uses of Propranolol in Dermatology
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Cosmetic Corner: Dermatologists Weigh in on Products for Sensitive Skin
To improve patient care and outcomes, leading dermatologists offered their recommendations on products for sensitive skin. Consideration must be given to:
- Avène Cicalfate Restorative Skin Cream
Pierre Fabre Dermo-Cosmetique USA
“Sucralfate for speeding up skin repair and the soothing thermal spring waters found in this product make it perfect postprocedure for immediately cooling and calming the skin.”—Jeannette Graf, MD, New York, New York
- Cetaphil RestoraDerm Eczema Calming Body Moisturizer
Galderma Laboratories, LP
“This product is formulated for atopic skin. I personally use it on my face as a moisturizer during the cold New York City winter.”—Anthony M. Rossi, MD, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc
“I recommend Vanicream brand products to patients with sensitive skin or eczema. These products are fragrance free and have minimal ingredients.”— Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete's foot treatments, cleansing devices, redness-reducing products, and face scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on products for sensitive skin. Consideration must be given to:
- Avène Cicalfate Restorative Skin Cream
Pierre Fabre Dermo-Cosmetique USA
“Sucralfate for speeding up skin repair and the soothing thermal spring waters found in this product make it perfect postprocedure for immediately cooling and calming the skin.”—Jeannette Graf, MD, New York, New York
- Cetaphil RestoraDerm Eczema Calming Body Moisturizer
Galderma Laboratories, LP
“This product is formulated for atopic skin. I personally use it on my face as a moisturizer during the cold New York City winter.”—Anthony M. Rossi, MD, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc
“I recommend Vanicream brand products to patients with sensitive skin or eczema. These products are fragrance free and have minimal ingredients.”— Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete's foot treatments, cleansing devices, redness-reducing products, and face scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on products for sensitive skin. Consideration must be given to:
- Avène Cicalfate Restorative Skin Cream
Pierre Fabre Dermo-Cosmetique USA
“Sucralfate for speeding up skin repair and the soothing thermal spring waters found in this product make it perfect postprocedure for immediately cooling and calming the skin.”—Jeannette Graf, MD, New York, New York
- Cetaphil RestoraDerm Eczema Calming Body Moisturizer
Galderma Laboratories, LP
“This product is formulated for atopic skin. I personally use it on my face as a moisturizer during the cold New York City winter.”—Anthony M. Rossi, MD, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc
“I recommend Vanicream brand products to patients with sensitive skin or eczema. These products are fragrance free and have minimal ingredients.”— Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete's foot treatments, cleansing devices, redness-reducing products, and face scrubs will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
What’s Eating You? Cheyletiella Mites
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4
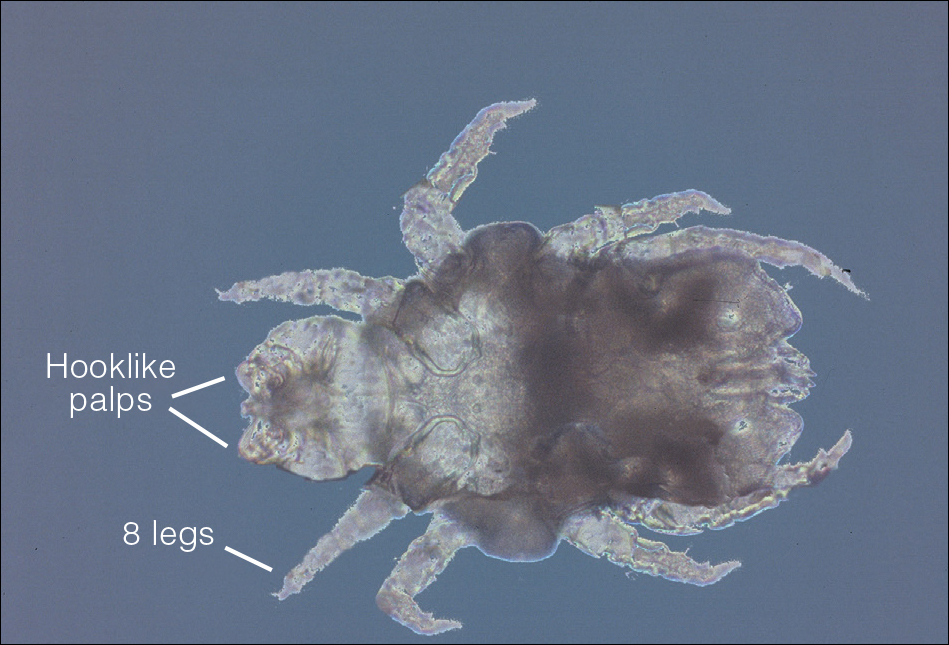
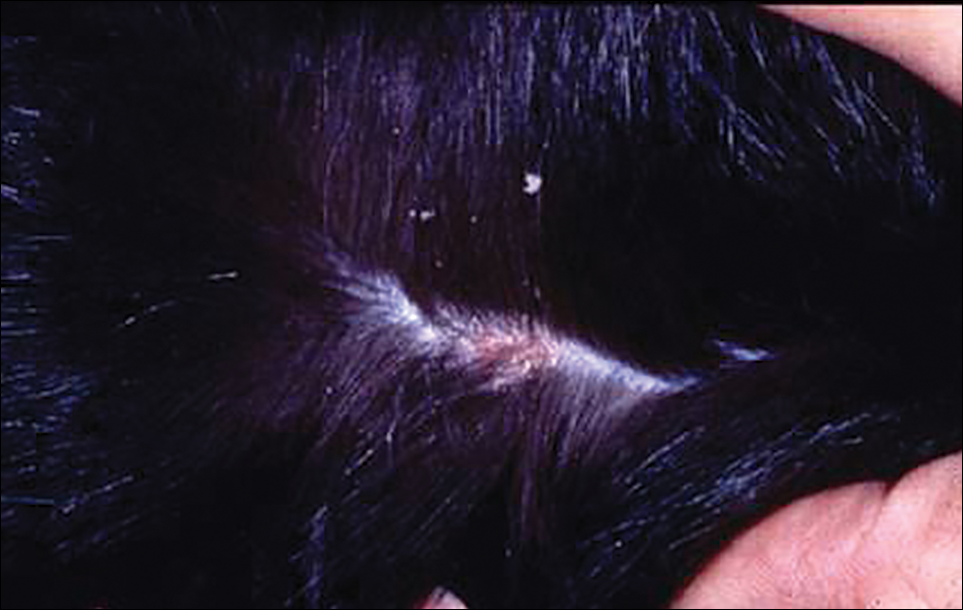
The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11
Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Practice Points
- Cheyletiella mites can cause a range of cutaneous and systemic symptoms in affected individuals.
- Diagnosis can be difficult and requires a high level of suspicion, with inquiries directed at animal exposures.
- Identification of the animal vector and treatment by a knowledgeable veterinarian is necessary to prevent recurrence in humans.
Living With Psoriasis: How the Disease Impacts the Daily Activities of Patients
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.