User login
Approach to Management of Giant Basal Cell Carcinomas
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
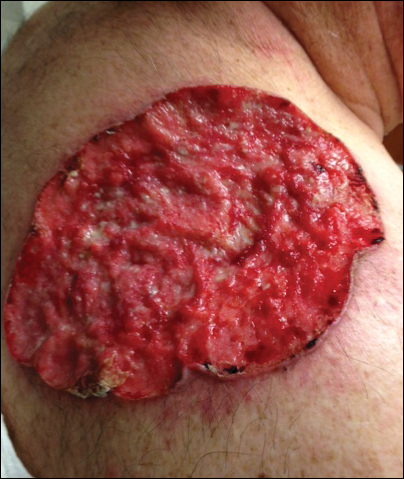
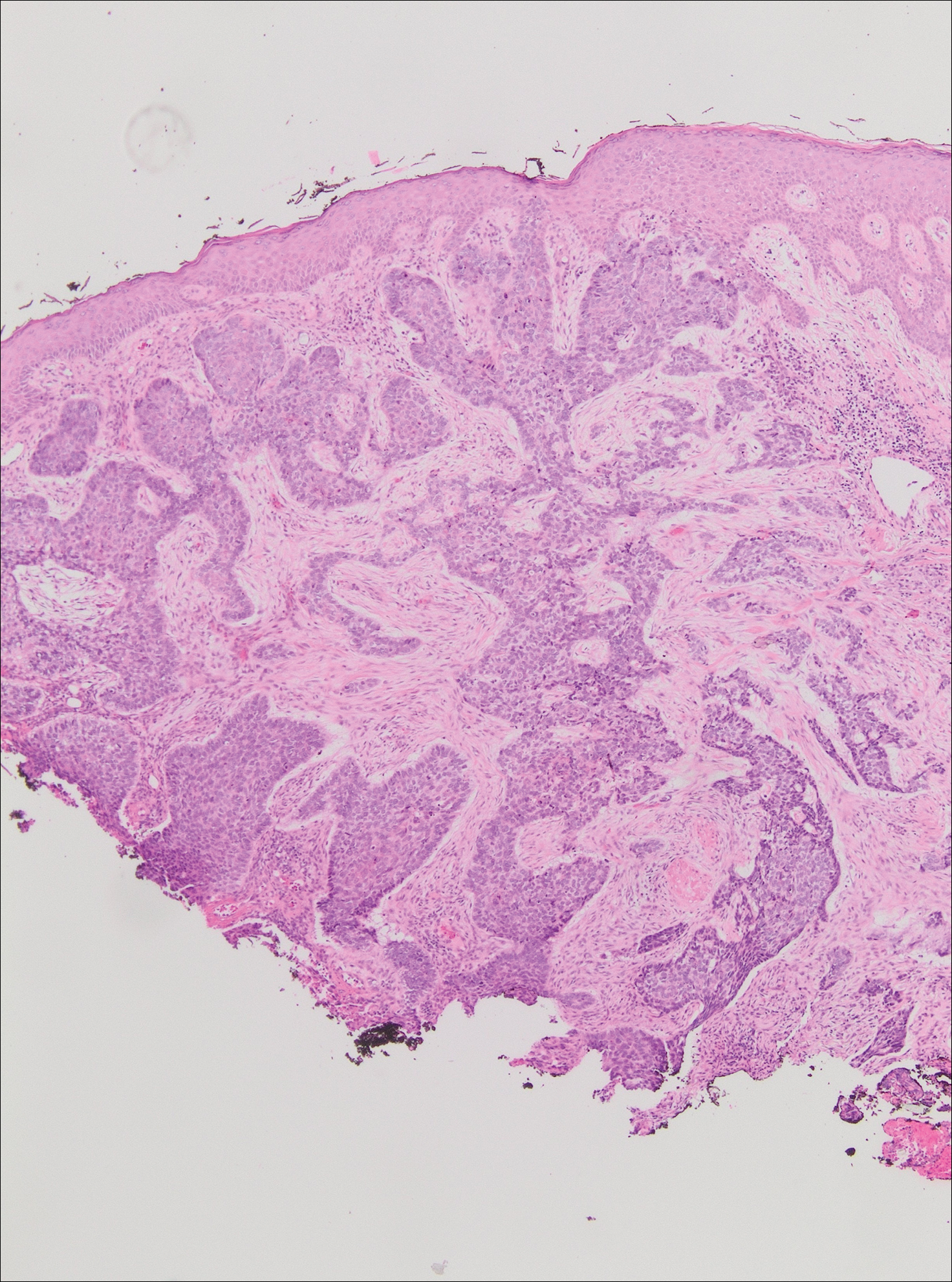
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.
The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
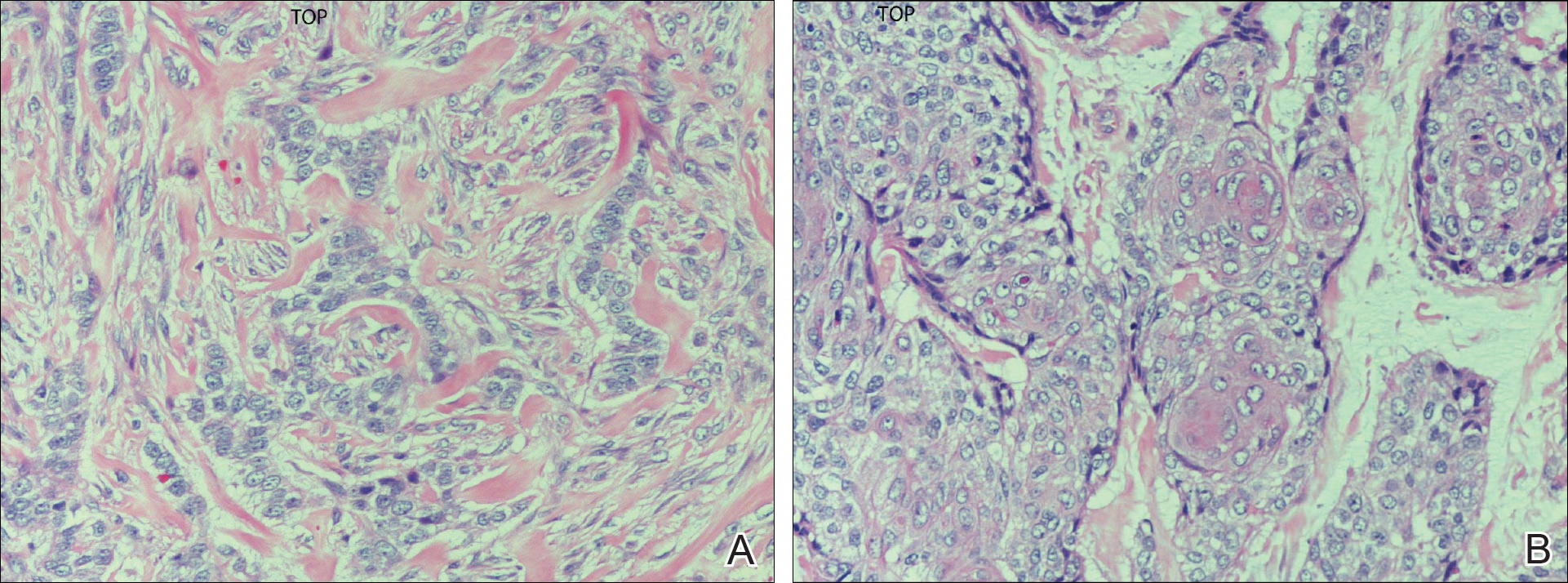
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.
The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
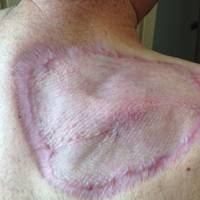
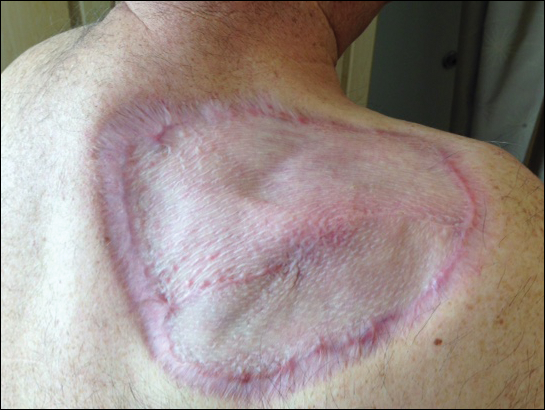
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.
Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Practice Points
- Unusually large basal cell carcinomas (BCCs) present a therapeutic challenge.
- A number of therapeutic options exist. Wide excision with margin control and complex reconstruction remains an excellent treatment option for BCC.
A Case of Human Orf Contracted From a Deer
Orf, also known as contagious ecthyma, scabby mouth, soremouth, contagious pustular dermatitis, and ovine pustular dermatitis, is caused by a member of the genus Parapoxvirus in the family Poxviridae.1,2 The virus typically infects sheep or goats and produces papulovesicular lesions on non–wool-bearing areas (eg, gums, lips, nose, groin).3,4 The infection can be transmitted to humans either by direct contact with an infected animal or by indirect contact with contaminated fomites.4,5 After an incubation period of 3 to 7 days, an erythematous maculopapular lesion develops.1,4 Orf lesions are benign and progress to complete resolution in 6 weeks.4
Case Report
A 51-year-old woman presented to her primary care physician for evaluation of a lesion on the metacarpal-phalangeal joint of the left second digit. A raised annular lesion was identified, and the patient was started on dicloxacillin therapy. On reexamination 4 days later, an enlarging 2-cm nodule with signs of central necrosis was found. The lesion was incised, and a bacterial culture of purulent exudate was obtained. Gram stain and initial culture results were negative. The patient also complained of diarrhea, which was attributed to use of dicloxacillin; this antibiotic was replaced with clarithromycin. The patient was reevaluated the next day for signs of cellulitis and ascending lymphangitis. She denied constitutional symptoms or fever and chills. The lymphangitis was present on the dorsum of the left hand to the level of the wrist. Ceftriaxone was injected intramuscularly. Three days later, an enlarging lesion with serous discharge was identified. The patient was referred for further dermatologic evaluation.
On examining the patient, we found a 2.5-cm boggy fluctuant nodule over the metacarpal-phalangeal joint of the left second digit (Figure). The patient complained of pain, and the hand showed signs of lymphangitic spread. When her history was taken, the patient indicated that she worked at a deer station. Her job involved handling deer carcasses, and her bare hands had been exposed to blood from slaughtered deer. She denied having any contact with sheep, goats, cattle, and farm structures containing those animals. Results of bacterial cultures were negative, and a differential diagnosis of deep fungal infection, atypical mycobacterial infection, or contagious ecthyma was entertained. Two 3-mm punch biopsies were performed. Stains for acid-fast bacilli and fungi were negative. Results of microscopic examination showed vacuolation of cells in the upper third of the malpighian stratum, eosinophilic inclusion bodies, and massive dermal infiltrate.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Because of the patient's clinical presentation, microscopic findings, and negative culture results, orf was diagnosed. Oral antibiotic therapy was discontinued, and daily cleansing and use of bacitracin ointment were started. The lesion resolved completely over the ensuing 3 to 4 weeks.
Comment
The diagnosis of orf is suggested by a characteristic skin lesion and a history of exposure. The typical lesion begins as a solitary papule on a finger, a hand,3 or the face.6 Orf lesions classically progress through a series of 6 clinical and histopathologic stages, each lasting approximately 1 week. In the initial maculopapular stage, an erythematous macule or papule erupts. In the target stage, the lesion becomes a papule with a red center, a white middle ring, and a red halo. In the acute stage, the lesion becomes a weeping nodule. In the regenerative stage, the lesion dries, a thin yellow crust develops, and small black dots form on the surface. In the papillomatous stage, tiny papillomas form on the surface. In the final, regressive stage, a dry crust forms.1,3,4,6 Uncomplicated lesions rarely leave a residual scar.4
The histopathology of an orf lesion evolves with the clinical stages and helps to secure the diagnosis. The maculopapular and target stages are characterized by vacuolated epidermal cells. In the maculopapular stage, cells have intracytoplasmic inclusions; in the target stage, they have both intracytoplasmic and intranuclear inclusions. The acute stage is marked by patchy areas of lost epidermis, reticular degeneration of the epidermis, and a dermal infiltrate composed primarily of lymphocytes. The regenerative stage involves epidermal regeneration and extrusion of pyknotic hair-follicle cells that form the small black dots on the surface of the lesion. In the papillomatous and regressive stages, fingerlike downward projections of epidermis are evident.1,4,6
The diagnosis of orf is further supported by a history of exposure to infected animals. The most compelling history involves exposure to sheep or goats, but the orf virus has infected other animals, including musk oxen7 and camels.8 Experimental inoculation has produced contagious ecthyma lesions in mule deer, white-tailed deer, pronghorn, and wapiti.9 That wild deer could contract orf and that the infection could be transmitted to humans through direct contact seem reasonable speculations. Our patient's clinical picture suggests that this mechanism may have been involved in her contracting the disease.
Electron microscopic views of characteristic viral particles in the cytoplasm of keratinocytes provide the definitive diagnosis of orf.1,10 Other diagnostic studies are tests of viral culture, complement fixation, and immunofluorescence.1,4,6 These tests are used mainly for epidemiology rather than clinical diagnosis.6
Complications of orf are generally rare but may include fever, chills, rigor, drenching sweat, malaise, lymphadenopathy, and lymphangitis.1,6,10 Secondary bacterial infection is the most common complication.1 Cases of erythema multiforme also have developed in the presence of orf.11
As a benign self-limited disease, orf requires no specific treatment. Antibiotics should be administered in cases of secondary bacterial infection but are otherwise unnecessary.1,2,6 Regression of the lesion may be accelerated by application of idoxuridine.10 Surgical excision also can bring about rapid resolution but is generally contraindicated because the lesion spontaneously regresses without leaving a scar.2 Corticosteroids and other immunosuppressive drugs should be avoided because they can exacerbate the lesion in its papillomatous stage.6,12 Use of topical cidofovir has been beneficial in treating patients who are immunocompromised.13
Although orf is a benign disease with a striking presentation that is easy to spot, early diagnosis can prevent unnecessary diagnostic workup and treatment.1 Orf should be considered in patients presenting with the characteristic skin lesion and a history of exposure to sheep or goats. As with our case, however, when a patient presents with a characteristic lesion and a history of exposure to other animals, orf should be kept in the differential diagnosis.
- Huerter CJ, Alvarez L, Stinson R. Orf: case report and literature review. Cleve Clin J Med. 1991;58:531-534.
- Chahidi N, de Fontaine S, Lacotte B. Human orf. Br J Plast Surg. 1993;46:532-534.
- Leavell UW, McNamara MJ, Muelling R, et al. Orf: report of 19 human cases with clinical and pathological observations. JAMA. 1968;204:109-116.
- Mendez B, Burnett JW. Orf. Cutis. 1989;44:286-287.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Bodnar MG, Miller OF, Tyler WB. Facial orf. J Am Acad Dermatol. 1999;40:815-817.
- Zarnke RL, Dieterich RA, Neiland KA, et al. Serologic and experimental investigations of contagious ecthyma in Alaska. J Wildl Dis. 1983;19:170-174.
- Azwai SM, Carter SD, Woldehiwet Z. Immune responses of the camel (Camelus dromedarius) to contagious ecthyma (orf) virus infection. Vet Microbiol. 1995;47:119-131.
- Lance WR, Hibler CP, DeMartini J. Experimental contagious ecthyma in mule deer, white-tailed deer, pronghorn and wapiti. J Wildl Dis. 1983;19:165-169.
- Lo C, Mathisen G. Human orf in Los Angeles County. West J Med. 1996;164:77-78.
- Agger WA, Webster SB. Human orf infection complicated by erythema multiforme. Cutis. 1983;31:334-338.
- Mohr BW, Katz D. Orf: a case report. Henry Ford Hosp Med J. 1989;37:79-80.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
Orf, also known as contagious ecthyma, scabby mouth, soremouth, contagious pustular dermatitis, and ovine pustular dermatitis, is caused by a member of the genus Parapoxvirus in the family Poxviridae.1,2 The virus typically infects sheep or goats and produces papulovesicular lesions on non–wool-bearing areas (eg, gums, lips, nose, groin).3,4 The infection can be transmitted to humans either by direct contact with an infected animal or by indirect contact with contaminated fomites.4,5 After an incubation period of 3 to 7 days, an erythematous maculopapular lesion develops.1,4 Orf lesions are benign and progress to complete resolution in 6 weeks.4
Case Report
A 51-year-old woman presented to her primary care physician for evaluation of a lesion on the metacarpal-phalangeal joint of the left second digit. A raised annular lesion was identified, and the patient was started on dicloxacillin therapy. On reexamination 4 days later, an enlarging 2-cm nodule with signs of central necrosis was found. The lesion was incised, and a bacterial culture of purulent exudate was obtained. Gram stain and initial culture results were negative. The patient also complained of diarrhea, which was attributed to use of dicloxacillin; this antibiotic was replaced with clarithromycin. The patient was reevaluated the next day for signs of cellulitis and ascending lymphangitis. She denied constitutional symptoms or fever and chills. The lymphangitis was present on the dorsum of the left hand to the level of the wrist. Ceftriaxone was injected intramuscularly. Three days later, an enlarging lesion with serous discharge was identified. The patient was referred for further dermatologic evaluation.
On examining the patient, we found a 2.5-cm boggy fluctuant nodule over the metacarpal-phalangeal joint of the left second digit (Figure). The patient complained of pain, and the hand showed signs of lymphangitic spread. When her history was taken, the patient indicated that she worked at a deer station. Her job involved handling deer carcasses, and her bare hands had been exposed to blood from slaughtered deer. She denied having any contact with sheep, goats, cattle, and farm structures containing those animals. Results of bacterial cultures were negative, and a differential diagnosis of deep fungal infection, atypical mycobacterial infection, or contagious ecthyma was entertained. Two 3-mm punch biopsies were performed. Stains for acid-fast bacilli and fungi were negative. Results of microscopic examination showed vacuolation of cells in the upper third of the malpighian stratum, eosinophilic inclusion bodies, and massive dermal infiltrate.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Because of the patient's clinical presentation, microscopic findings, and negative culture results, orf was diagnosed. Oral antibiotic therapy was discontinued, and daily cleansing and use of bacitracin ointment were started. The lesion resolved completely over the ensuing 3 to 4 weeks.
Comment
The diagnosis of orf is suggested by a characteristic skin lesion and a history of exposure. The typical lesion begins as a solitary papule on a finger, a hand,3 or the face.6 Orf lesions classically progress through a series of 6 clinical and histopathologic stages, each lasting approximately 1 week. In the initial maculopapular stage, an erythematous macule or papule erupts. In the target stage, the lesion becomes a papule with a red center, a white middle ring, and a red halo. In the acute stage, the lesion becomes a weeping nodule. In the regenerative stage, the lesion dries, a thin yellow crust develops, and small black dots form on the surface. In the papillomatous stage, tiny papillomas form on the surface. In the final, regressive stage, a dry crust forms.1,3,4,6 Uncomplicated lesions rarely leave a residual scar.4
The histopathology of an orf lesion evolves with the clinical stages and helps to secure the diagnosis. The maculopapular and target stages are characterized by vacuolated epidermal cells. In the maculopapular stage, cells have intracytoplasmic inclusions; in the target stage, they have both intracytoplasmic and intranuclear inclusions. The acute stage is marked by patchy areas of lost epidermis, reticular degeneration of the epidermis, and a dermal infiltrate composed primarily of lymphocytes. The regenerative stage involves epidermal regeneration and extrusion of pyknotic hair-follicle cells that form the small black dots on the surface of the lesion. In the papillomatous and regressive stages, fingerlike downward projections of epidermis are evident.1,4,6
The diagnosis of orf is further supported by a history of exposure to infected animals. The most compelling history involves exposure to sheep or goats, but the orf virus has infected other animals, including musk oxen7 and camels.8 Experimental inoculation has produced contagious ecthyma lesions in mule deer, white-tailed deer, pronghorn, and wapiti.9 That wild deer could contract orf and that the infection could be transmitted to humans through direct contact seem reasonable speculations. Our patient's clinical picture suggests that this mechanism may have been involved in her contracting the disease.
Electron microscopic views of characteristic viral particles in the cytoplasm of keratinocytes provide the definitive diagnosis of orf.1,10 Other diagnostic studies are tests of viral culture, complement fixation, and immunofluorescence.1,4,6 These tests are used mainly for epidemiology rather than clinical diagnosis.6
Complications of orf are generally rare but may include fever, chills, rigor, drenching sweat, malaise, lymphadenopathy, and lymphangitis.1,6,10 Secondary bacterial infection is the most common complication.1 Cases of erythema multiforme also have developed in the presence of orf.11
As a benign self-limited disease, orf requires no specific treatment. Antibiotics should be administered in cases of secondary bacterial infection but are otherwise unnecessary.1,2,6 Regression of the lesion may be accelerated by application of idoxuridine.10 Surgical excision also can bring about rapid resolution but is generally contraindicated because the lesion spontaneously regresses without leaving a scar.2 Corticosteroids and other immunosuppressive drugs should be avoided because they can exacerbate the lesion in its papillomatous stage.6,12 Use of topical cidofovir has been beneficial in treating patients who are immunocompromised.13
Although orf is a benign disease with a striking presentation that is easy to spot, early diagnosis can prevent unnecessary diagnostic workup and treatment.1 Orf should be considered in patients presenting with the characteristic skin lesion and a history of exposure to sheep or goats. As with our case, however, when a patient presents with a characteristic lesion and a history of exposure to other animals, orf should be kept in the differential diagnosis.
Orf, also known as contagious ecthyma, scabby mouth, soremouth, contagious pustular dermatitis, and ovine pustular dermatitis, is caused by a member of the genus Parapoxvirus in the family Poxviridae.1,2 The virus typically infects sheep or goats and produces papulovesicular lesions on non–wool-bearing areas (eg, gums, lips, nose, groin).3,4 The infection can be transmitted to humans either by direct contact with an infected animal or by indirect contact with contaminated fomites.4,5 After an incubation period of 3 to 7 days, an erythematous maculopapular lesion develops.1,4 Orf lesions are benign and progress to complete resolution in 6 weeks.4
Case Report
A 51-year-old woman presented to her primary care physician for evaluation of a lesion on the metacarpal-phalangeal joint of the left second digit. A raised annular lesion was identified, and the patient was started on dicloxacillin therapy. On reexamination 4 days later, an enlarging 2-cm nodule with signs of central necrosis was found. The lesion was incised, and a bacterial culture of purulent exudate was obtained. Gram stain and initial culture results were negative. The patient also complained of diarrhea, which was attributed to use of dicloxacillin; this antibiotic was replaced with clarithromycin. The patient was reevaluated the next day for signs of cellulitis and ascending lymphangitis. She denied constitutional symptoms or fever and chills. The lymphangitis was present on the dorsum of the left hand to the level of the wrist. Ceftriaxone was injected intramuscularly. Three days later, an enlarging lesion with serous discharge was identified. The patient was referred for further dermatologic evaluation.
On examining the patient, we found a 2.5-cm boggy fluctuant nodule over the metacarpal-phalangeal joint of the left second digit (Figure). The patient complained of pain, and the hand showed signs of lymphangitic spread. When her history was taken, the patient indicated that she worked at a deer station. Her job involved handling deer carcasses, and her bare hands had been exposed to blood from slaughtered deer. She denied having any contact with sheep, goats, cattle, and farm structures containing those animals. Results of bacterial cultures were negative, and a differential diagnosis of deep fungal infection, atypical mycobacterial infection, or contagious ecthyma was entertained. Two 3-mm punch biopsies were performed. Stains for acid-fast bacilli and fungi were negative. Results of microscopic examination showed vacuolation of cells in the upper third of the malpighian stratum, eosinophilic inclusion bodies, and massive dermal infiltrate.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Because of the patient's clinical presentation, microscopic findings, and negative culture results, orf was diagnosed. Oral antibiotic therapy was discontinued, and daily cleansing and use of bacitracin ointment were started. The lesion resolved completely over the ensuing 3 to 4 weeks.
Comment
The diagnosis of orf is suggested by a characteristic skin lesion and a history of exposure. The typical lesion begins as a solitary papule on a finger, a hand,3 or the face.6 Orf lesions classically progress through a series of 6 clinical and histopathologic stages, each lasting approximately 1 week. In the initial maculopapular stage, an erythematous macule or papule erupts. In the target stage, the lesion becomes a papule with a red center, a white middle ring, and a red halo. In the acute stage, the lesion becomes a weeping nodule. In the regenerative stage, the lesion dries, a thin yellow crust develops, and small black dots form on the surface. In the papillomatous stage, tiny papillomas form on the surface. In the final, regressive stage, a dry crust forms.1,3,4,6 Uncomplicated lesions rarely leave a residual scar.4
The histopathology of an orf lesion evolves with the clinical stages and helps to secure the diagnosis. The maculopapular and target stages are characterized by vacuolated epidermal cells. In the maculopapular stage, cells have intracytoplasmic inclusions; in the target stage, they have both intracytoplasmic and intranuclear inclusions. The acute stage is marked by patchy areas of lost epidermis, reticular degeneration of the epidermis, and a dermal infiltrate composed primarily of lymphocytes. The regenerative stage involves epidermal regeneration and extrusion of pyknotic hair-follicle cells that form the small black dots on the surface of the lesion. In the papillomatous and regressive stages, fingerlike downward projections of epidermis are evident.1,4,6
The diagnosis of orf is further supported by a history of exposure to infected animals. The most compelling history involves exposure to sheep or goats, but the orf virus has infected other animals, including musk oxen7 and camels.8 Experimental inoculation has produced contagious ecthyma lesions in mule deer, white-tailed deer, pronghorn, and wapiti.9 That wild deer could contract orf and that the infection could be transmitted to humans through direct contact seem reasonable speculations. Our patient's clinical picture suggests that this mechanism may have been involved in her contracting the disease.
Electron microscopic views of characteristic viral particles in the cytoplasm of keratinocytes provide the definitive diagnosis of orf.1,10 Other diagnostic studies are tests of viral culture, complement fixation, and immunofluorescence.1,4,6 These tests are used mainly for epidemiology rather than clinical diagnosis.6
Complications of orf are generally rare but may include fever, chills, rigor, drenching sweat, malaise, lymphadenopathy, and lymphangitis.1,6,10 Secondary bacterial infection is the most common complication.1 Cases of erythema multiforme also have developed in the presence of orf.11
As a benign self-limited disease, orf requires no specific treatment. Antibiotics should be administered in cases of secondary bacterial infection but are otherwise unnecessary.1,2,6 Regression of the lesion may be accelerated by application of idoxuridine.10 Surgical excision also can bring about rapid resolution but is generally contraindicated because the lesion spontaneously regresses without leaving a scar.2 Corticosteroids and other immunosuppressive drugs should be avoided because they can exacerbate the lesion in its papillomatous stage.6,12 Use of topical cidofovir has been beneficial in treating patients who are immunocompromised.13
Although orf is a benign disease with a striking presentation that is easy to spot, early diagnosis can prevent unnecessary diagnostic workup and treatment.1 Orf should be considered in patients presenting with the characteristic skin lesion and a history of exposure to sheep or goats. As with our case, however, when a patient presents with a characteristic lesion and a history of exposure to other animals, orf should be kept in the differential diagnosis.
- Huerter CJ, Alvarez L, Stinson R. Orf: case report and literature review. Cleve Clin J Med. 1991;58:531-534.
- Chahidi N, de Fontaine S, Lacotte B. Human orf. Br J Plast Surg. 1993;46:532-534.
- Leavell UW, McNamara MJ, Muelling R, et al. Orf: report of 19 human cases with clinical and pathological observations. JAMA. 1968;204:109-116.
- Mendez B, Burnett JW. Orf. Cutis. 1989;44:286-287.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Bodnar MG, Miller OF, Tyler WB. Facial orf. J Am Acad Dermatol. 1999;40:815-817.
- Zarnke RL, Dieterich RA, Neiland KA, et al. Serologic and experimental investigations of contagious ecthyma in Alaska. J Wildl Dis. 1983;19:170-174.
- Azwai SM, Carter SD, Woldehiwet Z. Immune responses of the camel (Camelus dromedarius) to contagious ecthyma (orf) virus infection. Vet Microbiol. 1995;47:119-131.
- Lance WR, Hibler CP, DeMartini J. Experimental contagious ecthyma in mule deer, white-tailed deer, pronghorn and wapiti. J Wildl Dis. 1983;19:165-169.
- Lo C, Mathisen G. Human orf in Los Angeles County. West J Med. 1996;164:77-78.
- Agger WA, Webster SB. Human orf infection complicated by erythema multiforme. Cutis. 1983;31:334-338.
- Mohr BW, Katz D. Orf: a case report. Henry Ford Hosp Med J. 1989;37:79-80.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Huerter CJ, Alvarez L, Stinson R. Orf: case report and literature review. Cleve Clin J Med. 1991;58:531-534.
- Chahidi N, de Fontaine S, Lacotte B. Human orf. Br J Plast Surg. 1993;46:532-534.
- Leavell UW, McNamara MJ, Muelling R, et al. Orf: report of 19 human cases with clinical and pathological observations. JAMA. 1968;204:109-116.
- Mendez B, Burnett JW. Orf. Cutis. 1989;44:286-287.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Bodnar MG, Miller OF, Tyler WB. Facial orf. J Am Acad Dermatol. 1999;40:815-817.
- Zarnke RL, Dieterich RA, Neiland KA, et al. Serologic and experimental investigations of contagious ecthyma in Alaska. J Wildl Dis. 1983;19:170-174.
- Azwai SM, Carter SD, Woldehiwet Z. Immune responses of the camel (Camelus dromedarius) to contagious ecthyma (orf) virus infection. Vet Microbiol. 1995;47:119-131.
- Lance WR, Hibler CP, DeMartini J. Experimental contagious ecthyma in mule deer, white-tailed deer, pronghorn and wapiti. J Wildl Dis. 1983;19:165-169.
- Lo C, Mathisen G. Human orf in Los Angeles County. West J Med. 1996;164:77-78.
- Agger WA, Webster SB. Human orf infection complicated by erythema multiforme. Cutis. 1983;31:334-338.
- Mohr BW, Katz D. Orf: a case report. Henry Ford Hosp Med J. 1989;37:79-80.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.