User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Granulomatous Cheilitis Mimicking Angioedema
To the Editor:
Granulomatous cheilitis (GC), also known as Miescher cheilitis, belongs to a larger class of diseases known as orofacial granulomatoses (OFGs), a set of diseases distinguished by their clinical and pathologic features of facial edema and granulomatous inflammation.1-3 Granulomatous cheilitis, a monosymptomatic variant of a more extensive disease known as Melkersson-Rosenthal syndrome (MRS), presents with labial swelling mimicking angioedema. Timely diagnosis of GC and MRS reduces the number of unnecessary tests, health care costs, and unnecessary patient burden. We present a case of idiopathic persistent swelling of the upper lip that was originally misdiagnosed as angioedema.
A 13-year-old white adolescent boy was referred to the allergy-immunology clinic for an alternate opinion regarding a presumed diagnosis of angioedema. He presented with prominent persistent swelling of the upper lip of 1 year’s duration associated with fissuring and discomfort while eating, which led to weight loss of more than 4.5 kg. The patient denied any history of facial asymmetry, paralysis, dental infections, or gastrointestinal tract symptoms. Additionally, he was not on any medications. His parents reported variable symptomatic worsening associated with egg ingestion, but avoiding egg did not provide any symptomatic relief. The swelling was unresponsive to multiple and prolonged courses of antihistamines and oral glucocorticoids. The patient’s medical history revealed no similar episodes of unexplained swelling, and family history was negative for angioedema. On examination, the upper lip was tender with a firm rubbery consistency. No other areas of swelling were noted. Angular cheilosis and minor labial mucosal ulcerations also were observed (Figure).
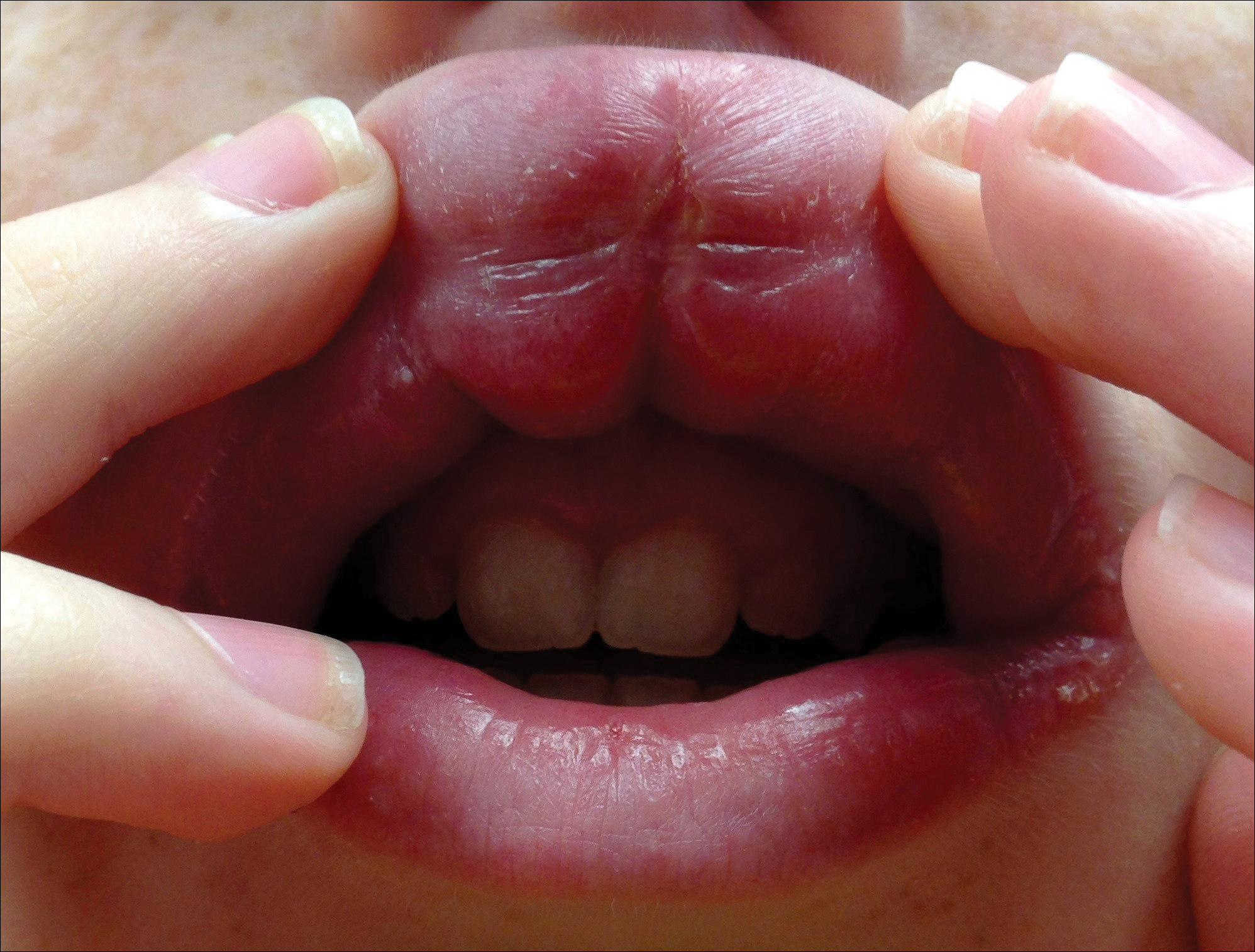
The persistent nature of the lip swelling and findings of fissures were not consistent with angioedema. Furthermore, prior laboratory studies did not reveal evidence of hereditary or acquired angioedema, and a complete blood cell count with differential was within reference range. Although the clinical suspicion for egg allergy was low, a blood test for serum-specific IgE showed a mild reactivity to egg allergen. The patient was referred to an oral surgeon for biopsy, which revealed dermal foci of noncaseating granulomas consistent with the preliminary diagnosis of GC.
Intralesional triamcinolone injections were initiated with marked improvement. Shortly after the initial improvement, however, the symptoms recurred, which necessitated several additional intralesional triamcinolone injections, again with remarkable improvement. Approximately 1.5 years later, the patient presented with recurrence of the lip swelling and admitted to having episodic diarrhea and abdominal cramps. He was referred to a pediatric gastroenterologist and a colonoscopy with biopsy confirmed Crohn disease. He was started on azathioprine followed by infliximab. A few months after this treatment was initiated, both his lip swelling and gastrointestinal tract symptoms remarkably improved. He has been maintained on this regimen and in the most recent follow-up had no recurrence of GC. He is scheduled to have another colonoscopy.
Granulomatous cheilitis is a rare chronic inflammatory condition characterized clinically by persistent lip swelling and histologically by granulomatous inflammation in the absence of systemic granulomatous disorders.4 Granulomatous cheilitis falls under the umbrella of OFGs. When it is paired with facial paralysis and fissuring of the tongue, it is specifically referred to as MRS. The prevalence of GC has historically been difficult to ascertain. In a review, an estimated incidence of 0.08% in the general population was reported with no predilection for race, sex, or age.4,5 Initially, the swelling of GC can be misdiagnosed as angioedema; therefore, it is imperative to include OFG and GC in the differential diagnosis of facial angioedema.3 Other possible diagnoses to consider include contact dermatitis, foreign-body reactions, infection, and reactions to medications such as angiotensin-converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs.5 Chronic lymphedema and other granulomatous diseases also should be considered in the differential diagnosis. Isolated lymphedema of the head and neck, though rare, typically is seen following surgical or radiological interventions for cancer. Lymphatic fibrosis also can occur in the setting of chronic inflammatory skin conditions but is not typically the first presenting symptom, as was seen in our patient.6 Although granulomatous diseases such as sarcoidosis may be difficult to clinically and histologically differentiate from GC, isolated orofacial swelling in sarcoidosis is rare. If clinical suspicion for sarcoidosis does exist, however, a negative chest radiograph as well as serum calcium and angiotensin-converting enzyme levels within reference range may help differentiate GC from sarcoidosis. In our patient, the clinical suspicion for sarcoidosis was low given his clinical history, young age, and race.
The etiology of MRS and GC currently is unknown. Genetic factors, food allergies, infectious processes, and aberrant immunologic functions all have been proposed as possible mechanisms.1-3,7,8 Genetic factors, such as HLA antigen subtypes, have been investigated but have not shown a definitive correlation.8 Numerous food allergens have been suggested as causative factors in OFG via a type of delayed hypersensitivity reaction,7 with cinnamon and benzoate reported as 2 of the most cited entities.9,10 Currently, it is believed that both of these mechanisms may play an exacerbating role to an otherwise unknown disease process.7,8 The infectious process most often associated with GC is Mycobacterium tuberculosis; however, similar to genetics and food allergens, causality has not been determined.4,7 At the present time, the best evidence points to an immunologic basis of GC with the inciting event being a random influx of inflammatory cells.7,11
There is a known association between GC and Crohn disease, especially when oral lesions are present.1,9 Granulomatous cheilitis can be considered an extraintestinal manifestation of Crohn disease.Up to 20% of OFG patients eventually go on to develop Crohn disease, with some reports being even higher when OFG presents in childhood.1,9 One study proposed that both GC and Crohn disease patients shared similar histopathologic and immunopathologic features including a helper T cell (TH1)–predominant inflammatory reaction.11
The treatment of GC is challenging, with most evidence coming from sporadic case reports. Given the relatively high rate of cinnamon and benzoate hypersensitivity seen in GC patients, it has been postulated that a diet lacking in them will improve the disease. At least one study has reported positive clinical outcomes from diets lacking in cinnamon and benzoate and in fact recommended it as a potential first-line treatment.10 The mainstay of treatment, however, is corticosteroids, but continued use is discouraged due to their large side-effect profile.12 Currently, the most agreed upon treatment for patients with isolated GC is intralesional triamcinolone injections.12 Despite the robust initial response often seen with triamcinolone injections, it is not uncommon for the benefit to be short-lived, requiring additional treatments.1,5,12 Newer medical therapies that have shown promise largely are centered on anti–tumor necrosis factor α medications such as infliximab and adalimumab.13,14 It is postulated that due to the potential overlapping pathophysiology between Crohn disease and GC, there may be utility in using the same treatments.13 In situations where medical therapy fails or in extremely disfiguring cases of GC and MRS, surgical cheiloplasty is performed to reduce lip size and improve cosmetic appearance.12 In a small study, reduction cheiloplasty gave satisfactory functional and cosmetic outcomes in all 7 patients reviewed at a median follow-up of 6.5 years.15
This case emphasizes the importance of paying close attention to history and physical examination features in developing any differential diagnosis. In this patient, persistent orofacial swelling with associated mucosal ulcerations were sufficient to exclude drug-induced, idiopathic, hereditary, and acquired angioedema. The clinical history coupled with the biopsy results yielded a confident diagnosis of GC. Furthermore, similar presentations should raise concern for a subclinical inflammatory bowel disease such as Crohn disease.
- Rose AE, Leger M, Chu J, et al. Cheilitis granulomatosa. Dermatol Online J. 2011;17:15.
- Vibhute NA, Vibhute AH, Nilima DR. Cheilitis granulomatosa: a case report with review of literature. Indian J Dermatol. 2013;58:242.
- Kakimoto C, Sparks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213.
- Withey S, Pracy P, Vaz F, et al. Sensory deprivation as a consequence of severe head and neck lymphoedema. J Laryngol Otol. 2001;115:62-64.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Gibson J, Wray D. Human leucocyte antigen typing in orofacial. Br J Dermatol. 2000;143:1119-1121.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- White A, Nunes C, Escudier M, et al. Improvement in orofacial granulomatosis on a cinnamon- and benzoate-free diet. Inflamm Bowel Dis. 2006;12:508-514.
- Freysdottir J, Zhang S, Tilakaratne WM, et al. Oral biopsies from patients with orofacial granulomatosis with histology resembling Crohn’s disease have a prominent Th1 environment. Inflamm Bowel Dis. 2007;13:439-445.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Peitsch WK, Kemmler N, Goerdt S, et al. Infliximab: a novel treatment option for refractory orofacial granulomatosis. Acta Derm Venereol. 2007;87:265-266.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
- Kruse-Lösler B, Presser D, Metze D, et al. Surgical treatment of persistent macrocheilia in patients with Melkersson-Rosenthal syndrome and cheilitis granulomatosa. Arch Dermatol. 2005;141:1085-1091.
To the Editor:
Granulomatous cheilitis (GC), also known as Miescher cheilitis, belongs to a larger class of diseases known as orofacial granulomatoses (OFGs), a set of diseases distinguished by their clinical and pathologic features of facial edema and granulomatous inflammation.1-3 Granulomatous cheilitis, a monosymptomatic variant of a more extensive disease known as Melkersson-Rosenthal syndrome (MRS), presents with labial swelling mimicking angioedema. Timely diagnosis of GC and MRS reduces the number of unnecessary tests, health care costs, and unnecessary patient burden. We present a case of idiopathic persistent swelling of the upper lip that was originally misdiagnosed as angioedema.
A 13-year-old white adolescent boy was referred to the allergy-immunology clinic for an alternate opinion regarding a presumed diagnosis of angioedema. He presented with prominent persistent swelling of the upper lip of 1 year’s duration associated with fissuring and discomfort while eating, which led to weight loss of more than 4.5 kg. The patient denied any history of facial asymmetry, paralysis, dental infections, or gastrointestinal tract symptoms. Additionally, he was not on any medications. His parents reported variable symptomatic worsening associated with egg ingestion, but avoiding egg did not provide any symptomatic relief. The swelling was unresponsive to multiple and prolonged courses of antihistamines and oral glucocorticoids. The patient’s medical history revealed no similar episodes of unexplained swelling, and family history was negative for angioedema. On examination, the upper lip was tender with a firm rubbery consistency. No other areas of swelling were noted. Angular cheilosis and minor labial mucosal ulcerations also were observed (Figure).

The persistent nature of the lip swelling and findings of fissures were not consistent with angioedema. Furthermore, prior laboratory studies did not reveal evidence of hereditary or acquired angioedema, and a complete blood cell count with differential was within reference range. Although the clinical suspicion for egg allergy was low, a blood test for serum-specific IgE showed a mild reactivity to egg allergen. The patient was referred to an oral surgeon for biopsy, which revealed dermal foci of noncaseating granulomas consistent with the preliminary diagnosis of GC.
Intralesional triamcinolone injections were initiated with marked improvement. Shortly after the initial improvement, however, the symptoms recurred, which necessitated several additional intralesional triamcinolone injections, again with remarkable improvement. Approximately 1.5 years later, the patient presented with recurrence of the lip swelling and admitted to having episodic diarrhea and abdominal cramps. He was referred to a pediatric gastroenterologist and a colonoscopy with biopsy confirmed Crohn disease. He was started on azathioprine followed by infliximab. A few months after this treatment was initiated, both his lip swelling and gastrointestinal tract symptoms remarkably improved. He has been maintained on this regimen and in the most recent follow-up had no recurrence of GC. He is scheduled to have another colonoscopy.
Granulomatous cheilitis is a rare chronic inflammatory condition characterized clinically by persistent lip swelling and histologically by granulomatous inflammation in the absence of systemic granulomatous disorders.4 Granulomatous cheilitis falls under the umbrella of OFGs. When it is paired with facial paralysis and fissuring of the tongue, it is specifically referred to as MRS. The prevalence of GC has historically been difficult to ascertain. In a review, an estimated incidence of 0.08% in the general population was reported with no predilection for race, sex, or age.4,5 Initially, the swelling of GC can be misdiagnosed as angioedema; therefore, it is imperative to include OFG and GC in the differential diagnosis of facial angioedema.3 Other possible diagnoses to consider include contact dermatitis, foreign-body reactions, infection, and reactions to medications such as angiotensin-converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs.5 Chronic lymphedema and other granulomatous diseases also should be considered in the differential diagnosis. Isolated lymphedema of the head and neck, though rare, typically is seen following surgical or radiological interventions for cancer. Lymphatic fibrosis also can occur in the setting of chronic inflammatory skin conditions but is not typically the first presenting symptom, as was seen in our patient.6 Although granulomatous diseases such as sarcoidosis may be difficult to clinically and histologically differentiate from GC, isolated orofacial swelling in sarcoidosis is rare. If clinical suspicion for sarcoidosis does exist, however, a negative chest radiograph as well as serum calcium and angiotensin-converting enzyme levels within reference range may help differentiate GC from sarcoidosis. In our patient, the clinical suspicion for sarcoidosis was low given his clinical history, young age, and race.
The etiology of MRS and GC currently is unknown. Genetic factors, food allergies, infectious processes, and aberrant immunologic functions all have been proposed as possible mechanisms.1-3,7,8 Genetic factors, such as HLA antigen subtypes, have been investigated but have not shown a definitive correlation.8 Numerous food allergens have been suggested as causative factors in OFG via a type of delayed hypersensitivity reaction,7 with cinnamon and benzoate reported as 2 of the most cited entities.9,10 Currently, it is believed that both of these mechanisms may play an exacerbating role to an otherwise unknown disease process.7,8 The infectious process most often associated with GC is Mycobacterium tuberculosis; however, similar to genetics and food allergens, causality has not been determined.4,7 At the present time, the best evidence points to an immunologic basis of GC with the inciting event being a random influx of inflammatory cells.7,11
There is a known association between GC and Crohn disease, especially when oral lesions are present.1,9 Granulomatous cheilitis can be considered an extraintestinal manifestation of Crohn disease.Up to 20% of OFG patients eventually go on to develop Crohn disease, with some reports being even higher when OFG presents in childhood.1,9 One study proposed that both GC and Crohn disease patients shared similar histopathologic and immunopathologic features including a helper T cell (TH1)–predominant inflammatory reaction.11
The treatment of GC is challenging, with most evidence coming from sporadic case reports. Given the relatively high rate of cinnamon and benzoate hypersensitivity seen in GC patients, it has been postulated that a diet lacking in them will improve the disease. At least one study has reported positive clinical outcomes from diets lacking in cinnamon and benzoate and in fact recommended it as a potential first-line treatment.10 The mainstay of treatment, however, is corticosteroids, but continued use is discouraged due to their large side-effect profile.12 Currently, the most agreed upon treatment for patients with isolated GC is intralesional triamcinolone injections.12 Despite the robust initial response often seen with triamcinolone injections, it is not uncommon for the benefit to be short-lived, requiring additional treatments.1,5,12 Newer medical therapies that have shown promise largely are centered on anti–tumor necrosis factor α medications such as infliximab and adalimumab.13,14 It is postulated that due to the potential overlapping pathophysiology between Crohn disease and GC, there may be utility in using the same treatments.13 In situations where medical therapy fails or in extremely disfiguring cases of GC and MRS, surgical cheiloplasty is performed to reduce lip size and improve cosmetic appearance.12 In a small study, reduction cheiloplasty gave satisfactory functional and cosmetic outcomes in all 7 patients reviewed at a median follow-up of 6.5 years.15
This case emphasizes the importance of paying close attention to history and physical examination features in developing any differential diagnosis. In this patient, persistent orofacial swelling with associated mucosal ulcerations were sufficient to exclude drug-induced, idiopathic, hereditary, and acquired angioedema. The clinical history coupled with the biopsy results yielded a confident diagnosis of GC. Furthermore, similar presentations should raise concern for a subclinical inflammatory bowel disease such as Crohn disease.
To the Editor:
Granulomatous cheilitis (GC), also known as Miescher cheilitis, belongs to a larger class of diseases known as orofacial granulomatoses (OFGs), a set of diseases distinguished by their clinical and pathologic features of facial edema and granulomatous inflammation.1-3 Granulomatous cheilitis, a monosymptomatic variant of a more extensive disease known as Melkersson-Rosenthal syndrome (MRS), presents with labial swelling mimicking angioedema. Timely diagnosis of GC and MRS reduces the number of unnecessary tests, health care costs, and unnecessary patient burden. We present a case of idiopathic persistent swelling of the upper lip that was originally misdiagnosed as angioedema.
A 13-year-old white adolescent boy was referred to the allergy-immunology clinic for an alternate opinion regarding a presumed diagnosis of angioedema. He presented with prominent persistent swelling of the upper lip of 1 year’s duration associated with fissuring and discomfort while eating, which led to weight loss of more than 4.5 kg. The patient denied any history of facial asymmetry, paralysis, dental infections, or gastrointestinal tract symptoms. Additionally, he was not on any medications. His parents reported variable symptomatic worsening associated with egg ingestion, but avoiding egg did not provide any symptomatic relief. The swelling was unresponsive to multiple and prolonged courses of antihistamines and oral glucocorticoids. The patient’s medical history revealed no similar episodes of unexplained swelling, and family history was negative for angioedema. On examination, the upper lip was tender with a firm rubbery consistency. No other areas of swelling were noted. Angular cheilosis and minor labial mucosal ulcerations also were observed (Figure).

The persistent nature of the lip swelling and findings of fissures were not consistent with angioedema. Furthermore, prior laboratory studies did not reveal evidence of hereditary or acquired angioedema, and a complete blood cell count with differential was within reference range. Although the clinical suspicion for egg allergy was low, a blood test for serum-specific IgE showed a mild reactivity to egg allergen. The patient was referred to an oral surgeon for biopsy, which revealed dermal foci of noncaseating granulomas consistent with the preliminary diagnosis of GC.
Intralesional triamcinolone injections were initiated with marked improvement. Shortly after the initial improvement, however, the symptoms recurred, which necessitated several additional intralesional triamcinolone injections, again with remarkable improvement. Approximately 1.5 years later, the patient presented with recurrence of the lip swelling and admitted to having episodic diarrhea and abdominal cramps. He was referred to a pediatric gastroenterologist and a colonoscopy with biopsy confirmed Crohn disease. He was started on azathioprine followed by infliximab. A few months after this treatment was initiated, both his lip swelling and gastrointestinal tract symptoms remarkably improved. He has been maintained on this regimen and in the most recent follow-up had no recurrence of GC. He is scheduled to have another colonoscopy.
Granulomatous cheilitis is a rare chronic inflammatory condition characterized clinically by persistent lip swelling and histologically by granulomatous inflammation in the absence of systemic granulomatous disorders.4 Granulomatous cheilitis falls under the umbrella of OFGs. When it is paired with facial paralysis and fissuring of the tongue, it is specifically referred to as MRS. The prevalence of GC has historically been difficult to ascertain. In a review, an estimated incidence of 0.08% in the general population was reported with no predilection for race, sex, or age.4,5 Initially, the swelling of GC can be misdiagnosed as angioedema; therefore, it is imperative to include OFG and GC in the differential diagnosis of facial angioedema.3 Other possible diagnoses to consider include contact dermatitis, foreign-body reactions, infection, and reactions to medications such as angiotensin-converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs.5 Chronic lymphedema and other granulomatous diseases also should be considered in the differential diagnosis. Isolated lymphedema of the head and neck, though rare, typically is seen following surgical or radiological interventions for cancer. Lymphatic fibrosis also can occur in the setting of chronic inflammatory skin conditions but is not typically the first presenting symptom, as was seen in our patient.6 Although granulomatous diseases such as sarcoidosis may be difficult to clinically and histologically differentiate from GC, isolated orofacial swelling in sarcoidosis is rare. If clinical suspicion for sarcoidosis does exist, however, a negative chest radiograph as well as serum calcium and angiotensin-converting enzyme levels within reference range may help differentiate GC from sarcoidosis. In our patient, the clinical suspicion for sarcoidosis was low given his clinical history, young age, and race.
The etiology of MRS and GC currently is unknown. Genetic factors, food allergies, infectious processes, and aberrant immunologic functions all have been proposed as possible mechanisms.1-3,7,8 Genetic factors, such as HLA antigen subtypes, have been investigated but have not shown a definitive correlation.8 Numerous food allergens have been suggested as causative factors in OFG via a type of delayed hypersensitivity reaction,7 with cinnamon and benzoate reported as 2 of the most cited entities.9,10 Currently, it is believed that both of these mechanisms may play an exacerbating role to an otherwise unknown disease process.7,8 The infectious process most often associated with GC is Mycobacterium tuberculosis; however, similar to genetics and food allergens, causality has not been determined.4,7 At the present time, the best evidence points to an immunologic basis of GC with the inciting event being a random influx of inflammatory cells.7,11
There is a known association between GC and Crohn disease, especially when oral lesions are present.1,9 Granulomatous cheilitis can be considered an extraintestinal manifestation of Crohn disease.Up to 20% of OFG patients eventually go on to develop Crohn disease, with some reports being even higher when OFG presents in childhood.1,9 One study proposed that both GC and Crohn disease patients shared similar histopathologic and immunopathologic features including a helper T cell (TH1)–predominant inflammatory reaction.11
The treatment of GC is challenging, with most evidence coming from sporadic case reports. Given the relatively high rate of cinnamon and benzoate hypersensitivity seen in GC patients, it has been postulated that a diet lacking in them will improve the disease. At least one study has reported positive clinical outcomes from diets lacking in cinnamon and benzoate and in fact recommended it as a potential first-line treatment.10 The mainstay of treatment, however, is corticosteroids, but continued use is discouraged due to their large side-effect profile.12 Currently, the most agreed upon treatment for patients with isolated GC is intralesional triamcinolone injections.12 Despite the robust initial response often seen with triamcinolone injections, it is not uncommon for the benefit to be short-lived, requiring additional treatments.1,5,12 Newer medical therapies that have shown promise largely are centered on anti–tumor necrosis factor α medications such as infliximab and adalimumab.13,14 It is postulated that due to the potential overlapping pathophysiology between Crohn disease and GC, there may be utility in using the same treatments.13 In situations where medical therapy fails or in extremely disfiguring cases of GC and MRS, surgical cheiloplasty is performed to reduce lip size and improve cosmetic appearance.12 In a small study, reduction cheiloplasty gave satisfactory functional and cosmetic outcomes in all 7 patients reviewed at a median follow-up of 6.5 years.15
This case emphasizes the importance of paying close attention to history and physical examination features in developing any differential diagnosis. In this patient, persistent orofacial swelling with associated mucosal ulcerations were sufficient to exclude drug-induced, idiopathic, hereditary, and acquired angioedema. The clinical history coupled with the biopsy results yielded a confident diagnosis of GC. Furthermore, similar presentations should raise concern for a subclinical inflammatory bowel disease such as Crohn disease.
- Rose AE, Leger M, Chu J, et al. Cheilitis granulomatosa. Dermatol Online J. 2011;17:15.
- Vibhute NA, Vibhute AH, Nilima DR. Cheilitis granulomatosa: a case report with review of literature. Indian J Dermatol. 2013;58:242.
- Kakimoto C, Sparks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213.
- Withey S, Pracy P, Vaz F, et al. Sensory deprivation as a consequence of severe head and neck lymphoedema. J Laryngol Otol. 2001;115:62-64.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Gibson J, Wray D. Human leucocyte antigen typing in orofacial. Br J Dermatol. 2000;143:1119-1121.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- White A, Nunes C, Escudier M, et al. Improvement in orofacial granulomatosis on a cinnamon- and benzoate-free diet. Inflamm Bowel Dis. 2006;12:508-514.
- Freysdottir J, Zhang S, Tilakaratne WM, et al. Oral biopsies from patients with orofacial granulomatosis with histology resembling Crohn’s disease have a prominent Th1 environment. Inflamm Bowel Dis. 2007;13:439-445.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Peitsch WK, Kemmler N, Goerdt S, et al. Infliximab: a novel treatment option for refractory orofacial granulomatosis. Acta Derm Venereol. 2007;87:265-266.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
- Kruse-Lösler B, Presser D, Metze D, et al. Surgical treatment of persistent macrocheilia in patients with Melkersson-Rosenthal syndrome and cheilitis granulomatosa. Arch Dermatol. 2005;141:1085-1091.
- Rose AE, Leger M, Chu J, et al. Cheilitis granulomatosa. Dermatol Online J. 2011;17:15.
- Vibhute NA, Vibhute AH, Nilima DR. Cheilitis granulomatosa: a case report with review of literature. Indian J Dermatol. 2013;58:242.
- Kakimoto C, Sparks C, White AA. Melkersson-Rosenthal syndrome: a form of pseudoangioedema. Ann Allergy Asthma Immunol. 2007;99:185-189.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review [published online September 22, 2013]. Head Neck Pathol. 2014;8:209-213.
- Withey S, Pracy P, Vaz F, et al. Sensory deprivation as a consequence of severe head and neck lymphoedema. J Laryngol Otol. 2001;115:62-64.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Gibson J, Wray D. Human leucocyte antigen typing in orofacial. Br J Dermatol. 2000;143:1119-1121.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- White A, Nunes C, Escudier M, et al. Improvement in orofacial granulomatosis on a cinnamon- and benzoate-free diet. Inflamm Bowel Dis. 2006;12:508-514.
- Freysdottir J, Zhang S, Tilakaratne WM, et al. Oral biopsies from patients with orofacial granulomatosis with histology resembling Crohn’s disease have a prominent Th1 environment. Inflamm Bowel Dis. 2007;13:439-445.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Peitsch WK, Kemmler N, Goerdt S, et al. Infliximab: a novel treatment option for refractory orofacial granulomatosis. Acta Derm Venereol. 2007;87:265-266.
- Ruiz Villaverde R, Sánchez Cano D. Successful treatment of granulomatous cheilitis with adalimumab. Int J Dermatol. 2012;51:118-120.
- Kruse-Lösler B, Presser D, Metze D, et al. Surgical treatment of persistent macrocheilia in patients with Melkersson-Rosenthal syndrome and cheilitis granulomatosa. Arch Dermatol. 2005;141:1085-1091.
Practice Points
- Granulomatous cheilitis (GC) is a rare diagnosis that can present as an isolated disease or in association with another disease, most commonly an inflammatory bowel disease (ie, Crohn disease).
- Often misdiagnosed as angioedema, GC can be differentiated primarily based on history and clinical examination.
- Intervention such as intralesional steroid injection is effective in the primary form; however, treatment of the underlying condition, such as Crohn disease, is needed when the 2 conditions are associated.
Debunking Acne Myths: Should Patients With Oily Skin Use a Moisturizer?
Myth: Moisturizers Make Acne Worse in Patients With Oily Skin
Excessive sebum production can lead to oily skin that appears greasy and shiny, which contributes to the development of acne on the face. Acne patients with oily skin may be deterred from using moisturizers out of fear that their condition will worsen, yet therapeutic moisturizers have been shown to maintain hydration and overall integrity of the stratum corneum.
In a study of patient experiences with oily skin, 68% (n=37) of participants said their skin felt unclean, dirty, or grimy. Some participants noted a feeling of having clogged pores or an additional layer of skin, and others reported that their skin felt oily or greasy to the touch. The study also reported that participants with oily skin felt self-conscious, which impacted their daily life. These domains also are affected by having acne.
In the same study, 18% (n=10) of participants reported washing their face 6 to 15 times per day, 50% (n=27) washed their face 3 to 5 times per day, and 42% (n=23) washed their face 1 to 2 times per day. Instead of applying heavy moisturizers, acne patients with oily skin may feel the need to constantly wash their face. Gentle face washing is recommended to help improve and prevent acne, but patients who wash their face excessively are at risk for skin barrier impairment and development of dry skin.
Acne patients can use noncomedogenic moisturizers to prevent and alleviate skin irritation and soothe the skin by slowing the evaporation of water. Many moisturizers on the market claim to be suitable for acne treatment and may independently contribute to improving the signs and symptoms of acne. It is important for dermatologists to direct patients with oily skin to oil-free moisturizers containing ingredients such as dimethicone, which is known to reduce transepidermal water loss without a greasy feel and contains both occlusive and emollient properties. Dimethicone is suitable for use in patients with acne and sensitive skin and is noncomedogenic and hypoallergenic. Many oil-free moisturizers also contain certain metals and botanical extracts, such as aloe vera and witch hazel, that are known to have anti-inflammatory and skin-soothing properties. Some liquid face cleansers also moisturize, which may be all that is needed in patients with oily skin.
It also is important to inform patients with oily skin that common acne treatments such as benzoyl peroxide, retinoids, salicylic acid, and oral isotretinoin commonly cause dry skin or irritation, leading to barrier disruption in the stratum corneum and subsequently causing increased transepidermal water loss and inflammation. Concomitant use of noncomedogenic moisturizers can enhance treatment efficacy, alleviate dryness, and improve skin comfort in acne patients who are taking these medications.
Expert Commentary
An often forgotten element of acne vulgaris is that it is in fact a disease of barrier dysfunction and disruption. As mentioned above, many of the medications used to treat this chronic inflammatory disease are either directly cytotoxic to keratinocytes (benzoyl peroxide) or alter the thickness and composition of the stratum corneum (retinoids), impairing its protective functions. The inflammatory cascade associated with acne itself can impair the barrier, synergizing with the array of aforementioned medications. Both etiological factors disrupt an often overlooked yet crucial component of the skin barrier, the cutaneous microbiota. The altered landscape, or petri dish if you will, unhinges the balance between the >500 species of organisms living in harmony on the skin, decreasing bacterial diversity and facilitating the overgrowth of specific organisms, here specifically certain types of Propionibacterium acnes, which contribute to the ongoing inflammatory cascade. If that's not enough, sebum, which is certainly in excess in acne, contributes very little to barrier function and skin hydration but can be used to cause a different form of disruption by P acnes, which when converted into short-chain fatty acids can impair cutaneous immune tolerance ultimately creating, you guessed it, more inflammation (thank Dr. Rich Gallo for tying this all together). All in all, the barrier is a mess, highlighting the need for barrier repair with a moisturizer to restore the "balance" on every level: Repair and replace the stratum corneum, restore the tools for the right bacteria to grow (water, carbs, lipids, etc). Moisturizers are a must in acne!
—Adam Friedman, MD (Washington, DC)
Arbuckle R, Atkinson MJ, Clark M, et al. Patient experiences with oily skin: the qualitative development of content for two new patient reported outcome questionnaires [published online October 16, 2008]. Health Qual Life Outcomes. 2008;6:80.
Bikowski J. The use of therapeutic moisturizers in various dermatologic disorders. Cutis. 2001;68(suppl 5):3-11.
Chularojanamontri L, Tuchinda P, Kulthanan K, et al. Moisturizers for acne: what are their constituents? J Clin Aesthet Dermatol. 2014;7:36-44.
Goodman G. Cleansing and moisturizing in acne patients. Am J Clin Dermatol. 2009;10(suppl 1):1-6.
Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin [published online December 6, 2014]. J Dermatol. 2015;42:181-188.
Myth: Moisturizers Make Acne Worse in Patients With Oily Skin
Excessive sebum production can lead to oily skin that appears greasy and shiny, which contributes to the development of acne on the face. Acne patients with oily skin may be deterred from using moisturizers out of fear that their condition will worsen, yet therapeutic moisturizers have been shown to maintain hydration and overall integrity of the stratum corneum.
In a study of patient experiences with oily skin, 68% (n=37) of participants said their skin felt unclean, dirty, or grimy. Some participants noted a feeling of having clogged pores or an additional layer of skin, and others reported that their skin felt oily or greasy to the touch. The study also reported that participants with oily skin felt self-conscious, which impacted their daily life. These domains also are affected by having acne.
In the same study, 18% (n=10) of participants reported washing their face 6 to 15 times per day, 50% (n=27) washed their face 3 to 5 times per day, and 42% (n=23) washed their face 1 to 2 times per day. Instead of applying heavy moisturizers, acne patients with oily skin may feel the need to constantly wash their face. Gentle face washing is recommended to help improve and prevent acne, but patients who wash their face excessively are at risk for skin barrier impairment and development of dry skin.
Acne patients can use noncomedogenic moisturizers to prevent and alleviate skin irritation and soothe the skin by slowing the evaporation of water. Many moisturizers on the market claim to be suitable for acne treatment and may independently contribute to improving the signs and symptoms of acne. It is important for dermatologists to direct patients with oily skin to oil-free moisturizers containing ingredients such as dimethicone, which is known to reduce transepidermal water loss without a greasy feel and contains both occlusive and emollient properties. Dimethicone is suitable for use in patients with acne and sensitive skin and is noncomedogenic and hypoallergenic. Many oil-free moisturizers also contain certain metals and botanical extracts, such as aloe vera and witch hazel, that are known to have anti-inflammatory and skin-soothing properties. Some liquid face cleansers also moisturize, which may be all that is needed in patients with oily skin.
It also is important to inform patients with oily skin that common acne treatments such as benzoyl peroxide, retinoids, salicylic acid, and oral isotretinoin commonly cause dry skin or irritation, leading to barrier disruption in the stratum corneum and subsequently causing increased transepidermal water loss and inflammation. Concomitant use of noncomedogenic moisturizers can enhance treatment efficacy, alleviate dryness, and improve skin comfort in acne patients who are taking these medications.
Expert Commentary
An often forgotten element of acne vulgaris is that it is in fact a disease of barrier dysfunction and disruption. As mentioned above, many of the medications used to treat this chronic inflammatory disease are either directly cytotoxic to keratinocytes (benzoyl peroxide) or alter the thickness and composition of the stratum corneum (retinoids), impairing its protective functions. The inflammatory cascade associated with acne itself can impair the barrier, synergizing with the array of aforementioned medications. Both etiological factors disrupt an often overlooked yet crucial component of the skin barrier, the cutaneous microbiota. The altered landscape, or petri dish if you will, unhinges the balance between the >500 species of organisms living in harmony on the skin, decreasing bacterial diversity and facilitating the overgrowth of specific organisms, here specifically certain types of Propionibacterium acnes, which contribute to the ongoing inflammatory cascade. If that's not enough, sebum, which is certainly in excess in acne, contributes very little to barrier function and skin hydration but can be used to cause a different form of disruption by P acnes, which when converted into short-chain fatty acids can impair cutaneous immune tolerance ultimately creating, you guessed it, more inflammation (thank Dr. Rich Gallo for tying this all together). All in all, the barrier is a mess, highlighting the need for barrier repair with a moisturizer to restore the "balance" on every level: Repair and replace the stratum corneum, restore the tools for the right bacteria to grow (water, carbs, lipids, etc). Moisturizers are a must in acne!
—Adam Friedman, MD (Washington, DC)
Myth: Moisturizers Make Acne Worse in Patients With Oily Skin
Excessive sebum production can lead to oily skin that appears greasy and shiny, which contributes to the development of acne on the face. Acne patients with oily skin may be deterred from using moisturizers out of fear that their condition will worsen, yet therapeutic moisturizers have been shown to maintain hydration and overall integrity of the stratum corneum.
In a study of patient experiences with oily skin, 68% (n=37) of participants said their skin felt unclean, dirty, or grimy. Some participants noted a feeling of having clogged pores or an additional layer of skin, and others reported that their skin felt oily or greasy to the touch. The study also reported that participants with oily skin felt self-conscious, which impacted their daily life. These domains also are affected by having acne.
In the same study, 18% (n=10) of participants reported washing their face 6 to 15 times per day, 50% (n=27) washed their face 3 to 5 times per day, and 42% (n=23) washed their face 1 to 2 times per day. Instead of applying heavy moisturizers, acne patients with oily skin may feel the need to constantly wash their face. Gentle face washing is recommended to help improve and prevent acne, but patients who wash their face excessively are at risk for skin barrier impairment and development of dry skin.
Acne patients can use noncomedogenic moisturizers to prevent and alleviate skin irritation and soothe the skin by slowing the evaporation of water. Many moisturizers on the market claim to be suitable for acne treatment and may independently contribute to improving the signs and symptoms of acne. It is important for dermatologists to direct patients with oily skin to oil-free moisturizers containing ingredients such as dimethicone, which is known to reduce transepidermal water loss without a greasy feel and contains both occlusive and emollient properties. Dimethicone is suitable for use in patients with acne and sensitive skin and is noncomedogenic and hypoallergenic. Many oil-free moisturizers also contain certain metals and botanical extracts, such as aloe vera and witch hazel, that are known to have anti-inflammatory and skin-soothing properties. Some liquid face cleansers also moisturize, which may be all that is needed in patients with oily skin.
It also is important to inform patients with oily skin that common acne treatments such as benzoyl peroxide, retinoids, salicylic acid, and oral isotretinoin commonly cause dry skin or irritation, leading to barrier disruption in the stratum corneum and subsequently causing increased transepidermal water loss and inflammation. Concomitant use of noncomedogenic moisturizers can enhance treatment efficacy, alleviate dryness, and improve skin comfort in acne patients who are taking these medications.
Expert Commentary
An often forgotten element of acne vulgaris is that it is in fact a disease of barrier dysfunction and disruption. As mentioned above, many of the medications used to treat this chronic inflammatory disease are either directly cytotoxic to keratinocytes (benzoyl peroxide) or alter the thickness and composition of the stratum corneum (retinoids), impairing its protective functions. The inflammatory cascade associated with acne itself can impair the barrier, synergizing with the array of aforementioned medications. Both etiological factors disrupt an often overlooked yet crucial component of the skin barrier, the cutaneous microbiota. The altered landscape, or petri dish if you will, unhinges the balance between the >500 species of organisms living in harmony on the skin, decreasing bacterial diversity and facilitating the overgrowth of specific organisms, here specifically certain types of Propionibacterium acnes, which contribute to the ongoing inflammatory cascade. If that's not enough, sebum, which is certainly in excess in acne, contributes very little to barrier function and skin hydration but can be used to cause a different form of disruption by P acnes, which when converted into short-chain fatty acids can impair cutaneous immune tolerance ultimately creating, you guessed it, more inflammation (thank Dr. Rich Gallo for tying this all together). All in all, the barrier is a mess, highlighting the need for barrier repair with a moisturizer to restore the "balance" on every level: Repair and replace the stratum corneum, restore the tools for the right bacteria to grow (water, carbs, lipids, etc). Moisturizers are a must in acne!
—Adam Friedman, MD (Washington, DC)
Arbuckle R, Atkinson MJ, Clark M, et al. Patient experiences with oily skin: the qualitative development of content for two new patient reported outcome questionnaires [published online October 16, 2008]. Health Qual Life Outcomes. 2008;6:80.
Bikowski J. The use of therapeutic moisturizers in various dermatologic disorders. Cutis. 2001;68(suppl 5):3-11.
Chularojanamontri L, Tuchinda P, Kulthanan K, et al. Moisturizers for acne: what are their constituents? J Clin Aesthet Dermatol. 2014;7:36-44.
Goodman G. Cleansing and moisturizing in acne patients. Am J Clin Dermatol. 2009;10(suppl 1):1-6.
Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin [published online December 6, 2014]. J Dermatol. 2015;42:181-188.
Arbuckle R, Atkinson MJ, Clark M, et al. Patient experiences with oily skin: the qualitative development of content for two new patient reported outcome questionnaires [published online October 16, 2008]. Health Qual Life Outcomes. 2008;6:80.
Bikowski J. The use of therapeutic moisturizers in various dermatologic disorders. Cutis. 2001;68(suppl 5):3-11.
Chularojanamontri L, Tuchinda P, Kulthanan K, et al. Moisturizers for acne: what are their constituents? J Clin Aesthet Dermatol. 2014;7:36-44.
Goodman G. Cleansing and moisturizing in acne patients. Am J Clin Dermatol. 2009;10(suppl 1):1-6.
Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin [published online December 6, 2014]. J Dermatol. 2015;42:181-188.
CDC Offers Free Webinar on Bleeding Disorders in Women and Girls
Public health professionals, clinicians, and researchers are invited to a free webinar being hosted by CDC Division of Blood Disorders on “Women and Girls with Bleeding Disorders: Challenges in Diagnosis and Management.” The webinar will take place on May 18, 2017, from 2 pm to 3 pm. Register online.
Public health professionals, clinicians, and researchers are invited to a free webinar being hosted by CDC Division of Blood Disorders on “Women and Girls with Bleeding Disorders: Challenges in Diagnosis and Management.” The webinar will take place on May 18, 2017, from 2 pm to 3 pm. Register online.
Public health professionals, clinicians, and researchers are invited to a free webinar being hosted by CDC Division of Blood Disorders on “Women and Girls with Bleeding Disorders: Challenges in Diagnosis and Management.” The webinar will take place on May 18, 2017, from 2 pm to 3 pm. Register online.
Rare Neuro-Immune Disorders Symposium Is Planned
The Transverse Myelitis Association has announced that registration is now open for the 2017 Rare Neuro-Immune Disorders Symposium to take place October 20-21, 2017, in Columbus, Ohio. This education and advocacy conference is for individuals, families, and caregivers affected by acute disseminated encephalomyelitis (ADEM), neuromyelitis optica spectrum disorder (NMOSD), optic neuritis (ON), and transverse myelitis including the subtype acute flaccid myelitis.
The Transverse Myelitis Association has announced that registration is now open for the 2017 Rare Neuro-Immune Disorders Symposium to take place October 20-21, 2017, in Columbus, Ohio. This education and advocacy conference is for individuals, families, and caregivers affected by acute disseminated encephalomyelitis (ADEM), neuromyelitis optica spectrum disorder (NMOSD), optic neuritis (ON), and transverse myelitis including the subtype acute flaccid myelitis.
The Transverse Myelitis Association has announced that registration is now open for the 2017 Rare Neuro-Immune Disorders Symposium to take place October 20-21, 2017, in Columbus, Ohio. This education and advocacy conference is for individuals, families, and caregivers affected by acute disseminated encephalomyelitis (ADEM), neuromyelitis optica spectrum disorder (NMOSD), optic neuritis (ON), and transverse myelitis including the subtype acute flaccid myelitis.
Registration Is Open for Spinal CSF Leak Foundation Symposium
The Spinal CSF Leak Foundation will host an Intracranial Hypotension Symposium on October 14, 2017, in Santa Monica, California, in partnership with Cedars-Sinai. Registration is now open.
The Spinal CSF Leak Foundation will host an Intracranial Hypotension Symposium on October 14, 2017, in Santa Monica, California, in partnership with Cedars-Sinai. Registration is now open.
The Spinal CSF Leak Foundation will host an Intracranial Hypotension Symposium on October 14, 2017, in Santa Monica, California, in partnership with Cedars-Sinai. Registration is now open.
Pulmonary Hypertension Association and ATS to Host PH Program
On May 20, 2017, in Washington DC, the Pulmonary Hypertension Association (PHA) and the American Thoracic Society will host a program in which participants can learn from leading pulmonary hypertension experts and meet other patients, families and caregivers. The event is part of a Meet the Experts session hosted by PHA and the American Thoracic Society’s Public Advisory Roundtable.
On May 20, 2017, in Washington DC, the Pulmonary Hypertension Association (PHA) and the American Thoracic Society will host a program in which participants can learn from leading pulmonary hypertension experts and meet other patients, families and caregivers. The event is part of a Meet the Experts session hosted by PHA and the American Thoracic Society’s Public Advisory Roundtable.
On May 20, 2017, in Washington DC, the Pulmonary Hypertension Association (PHA) and the American Thoracic Society will host a program in which participants can learn from leading pulmonary hypertension experts and meet other patients, families and caregivers. The event is part of a Meet the Experts session hosted by PHA and the American Thoracic Society’s Public Advisory Roundtable.
Platelet Disorder Support Association to Present Annual ITP Update
PDSA will present the 17th annual update on immune thrombocytopenia (ITP) for patients, caregivers, and the medical community on July 28-30, 2017, in Chandler, Arizona.
PDSA will present the 17th annual update on immune thrombocytopenia (ITP) for patients, caregivers, and the medical community on July 28-30, 2017, in Chandler, Arizona.
PDSA will present the 17th annual update on immune thrombocytopenia (ITP) for patients, caregivers, and the medical community on July 28-30, 2017, in Chandler, Arizona.
Organic Acidemia Association Launches Natural History Study With NORD
In collaboration with NORD, the Organic Acidemia Association has launched an international natural history study to support research on rare organic acid disorders, which cause multiple life-threatening conditions.
In collaboration with NORD, the Organic Acidemia Association has launched an international natural history study to support research on rare organic acid disorders, which cause multiple life-threatening conditions.
In collaboration with NORD, the Organic Acidemia Association has launched an international natural history study to support research on rare organic acid disorders, which cause multiple life-threatening conditions.
Wyck Foundation and Myotonic Dystrophy Foundation Offer Training Fellowships
September 1, 2017, is the deadline for applications for training fellowships being offered by the Wyck Foundation and Myotonic Dystrophy Foundation.
September 1, 2017, is the deadline for applications for training fellowships being offered by the Wyck Foundation and Myotonic Dystrophy Foundation.
September 1, 2017, is the deadline for applications for training fellowships being offered by the Wyck Foundation and Myotonic Dystrophy Foundation.