User login
Eruptive Melanocytic Nevi During Azathioprine Therapy for Antisynthetase Syndrome
Case Report
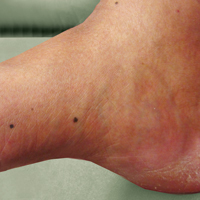
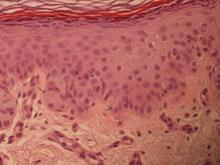
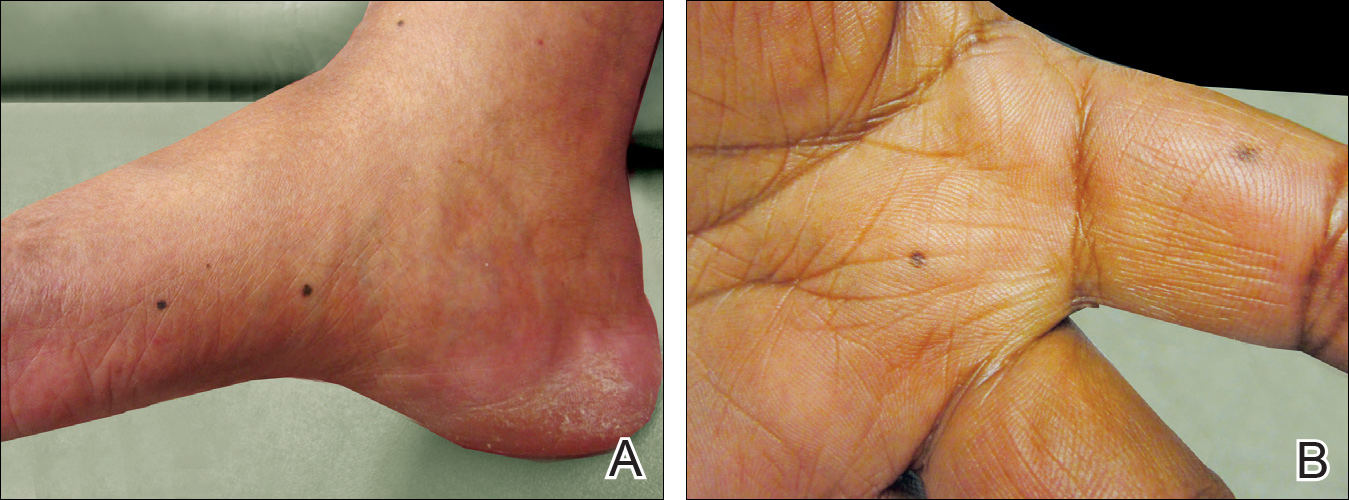
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.
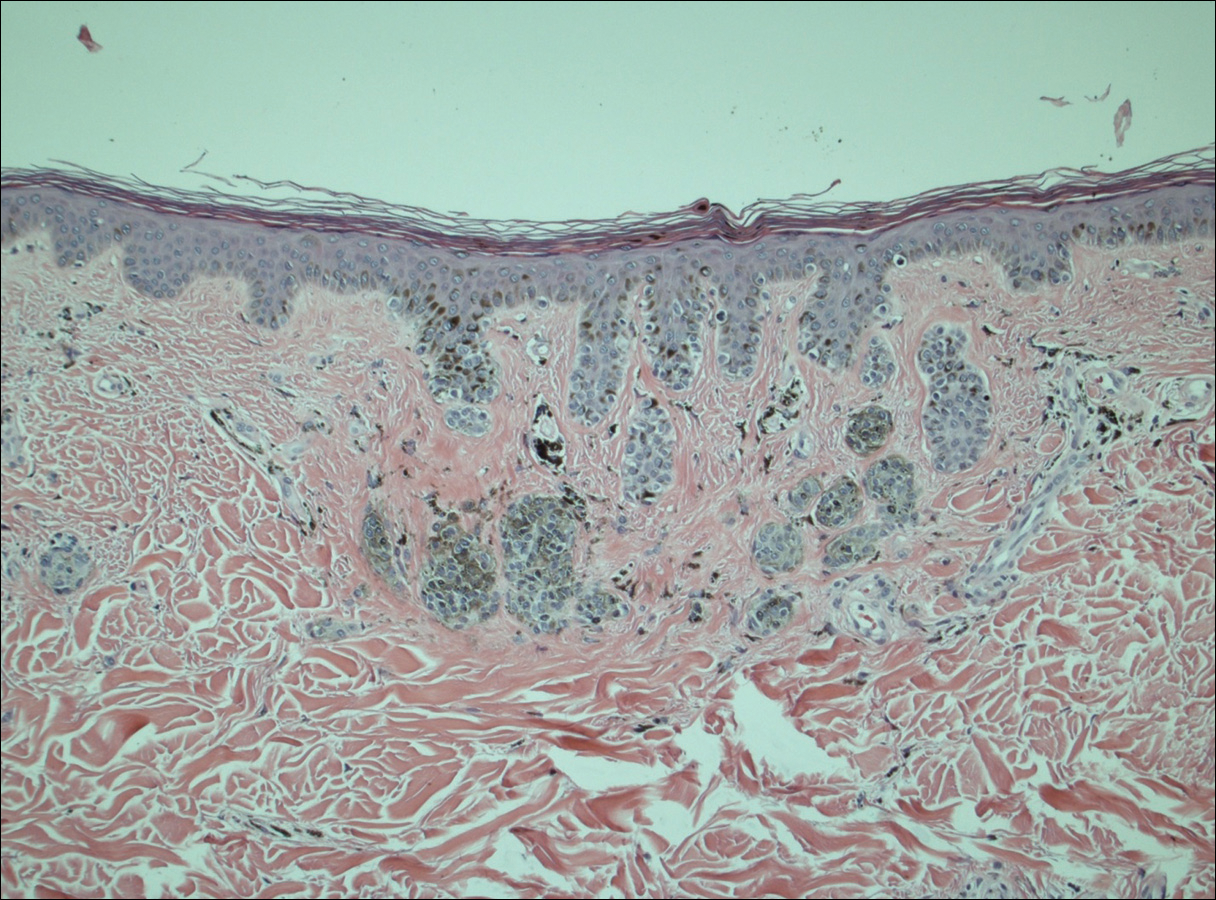
Comment
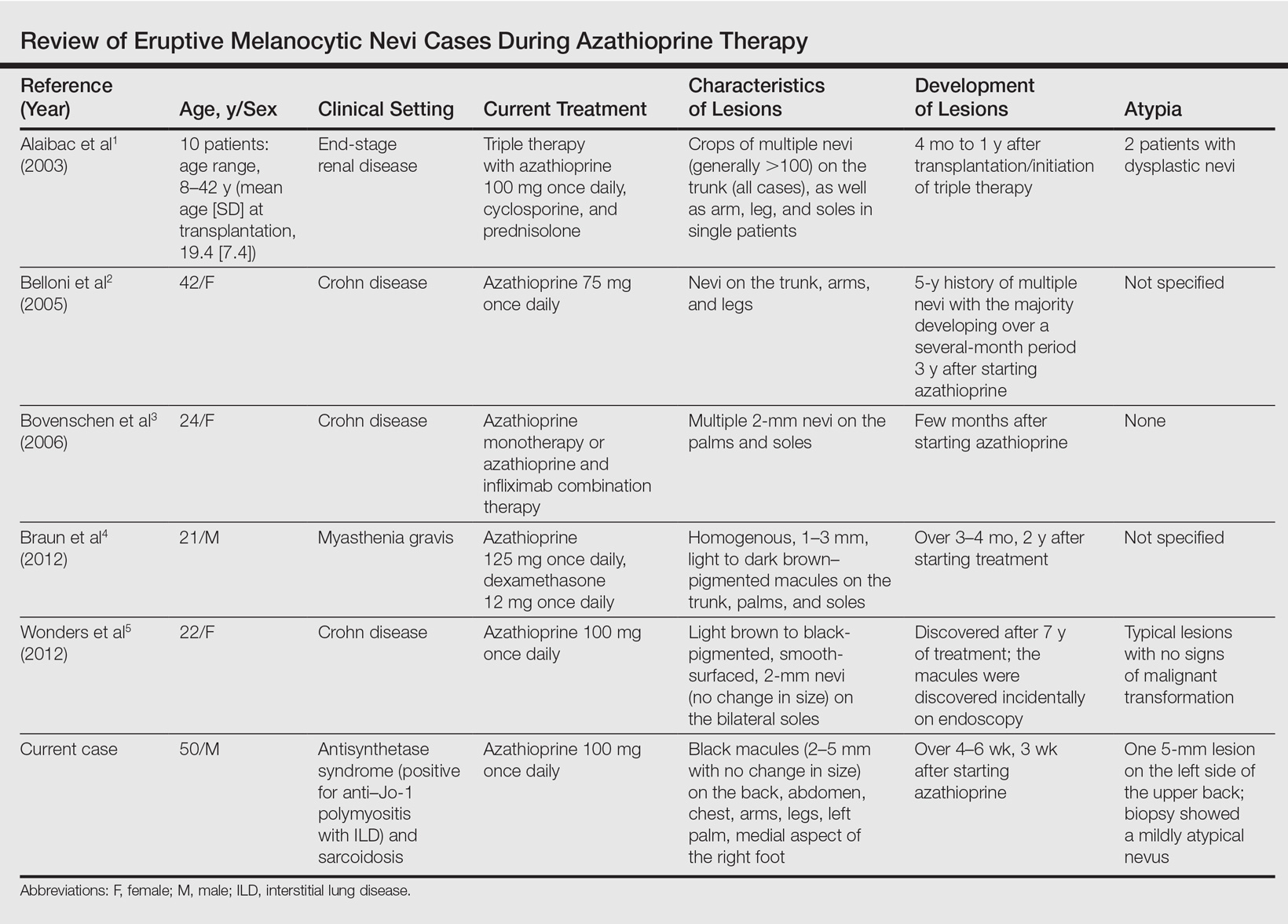
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Practice Points
- A theoretical risk exists in the setting of eruptive melanocytic nevi (EMN) given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.
- Follow patients with EMN with regular skin examinations and biopsies of atypical-appearing lesions given the increased risk for melanoma in this population.
Cutaneous Adnexal Carcinoma With Apocrine Differentiation
Differentiation between a primary adnexal carcinoma and a metastatic carcinoma to the skin is a challenging yet critical task for dermatologists and pathologists. Carcinomas that have metastasized to the skin are a sign of widespread systemic involvement and poor prognosis, while primary adnexal carcinomas tend to progress with an indolent clinical course. Although many patients with cutaneous metastases from an internal primary neoplasm can expect a median survival of no more than 12 months,1 patients with primary adnexal carcinomas are reported to have a 5-year survival rate of 95.5% for localized disease and 85% with spread to regional lymph nodes.2 We report a case of multiple cutaneous neoplasms of unknown primary origin in a 71-year-old man and describe our approach to identification of the possible primary site as well as management of the disease.
Case Report
A 71-year-old man initially presented to his primary physician for evaluation of a mass on the left side of the neck of 3 months' duration. On physical examination, a firm 2.5×3.0-cm nodule was noted at the anterior border of the trapezius muscle. Palpation of the thyroid revealed an additional right-sided nodule. The submandibular and parotid glands were unremarkable to palpation. The patient was referred to general surgery for biopsy, which revealed an infiltrating, moderately differentiated adenocarcinoma with extensive lymphatic permeation. Immunohistochemical staining for cytokeratin (CK) 7 was positive, while CK20 and thyroid transcription factor 1 were negative. A positron emission tomography/computed tomography (CT) fusion scan demonstrated 3 areas of enhanced uptake: one in the right side of the thyroid, a second corresponding to the mass on the left side of the neck at the level of the trapezius muscle, and a third in the left masseter muscle. Surgical excision with negative margins with possible chemotherapy was recommended; however, the patient declined treatment and was lost to follow-up until 2 years later when he presented to his primary physician with an additional lesion on his scalp.
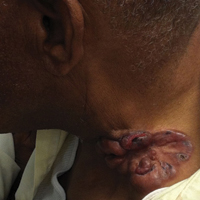
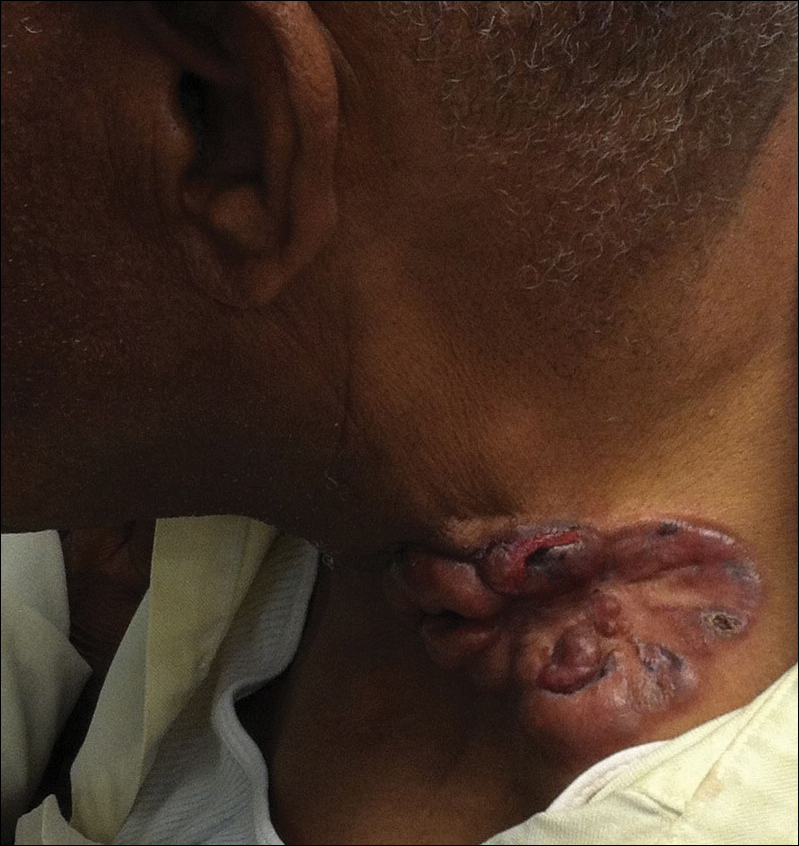
Four years after the biopsy, the patient presented to the dermatology department with additional tumor nodules including a 4-cm, annular, indurated, focally eroded plaque on the left side of the lateral neck (Figure 1); 3 separate 1-cm nodules on the right side of the lateral neck; and an ulcerated, crusted, 10×8-cm plaque on the posterior aspect of the scalp. Despite the extensive lesions, the patient remained in good health and reported no recent weight loss or signs or symptoms of systemic involvement. The posterior scalp lesion, which developed 2 years after the initial appearance of the mass on the neck and was thought to represent a possible metastasis of the tumor, was biopsied and showed diffuse infiltration of the dermis by poorly differentiated tumor cells with vacuolated cytoplasm arranged in nests and cords and sometimes in a single-file arrangement (Figure 2). A CT scan demonstrated pretracheal lymphadenopathy as well as small intraparenchymal and subpleural pulmonary nodules throughout both lung fields.
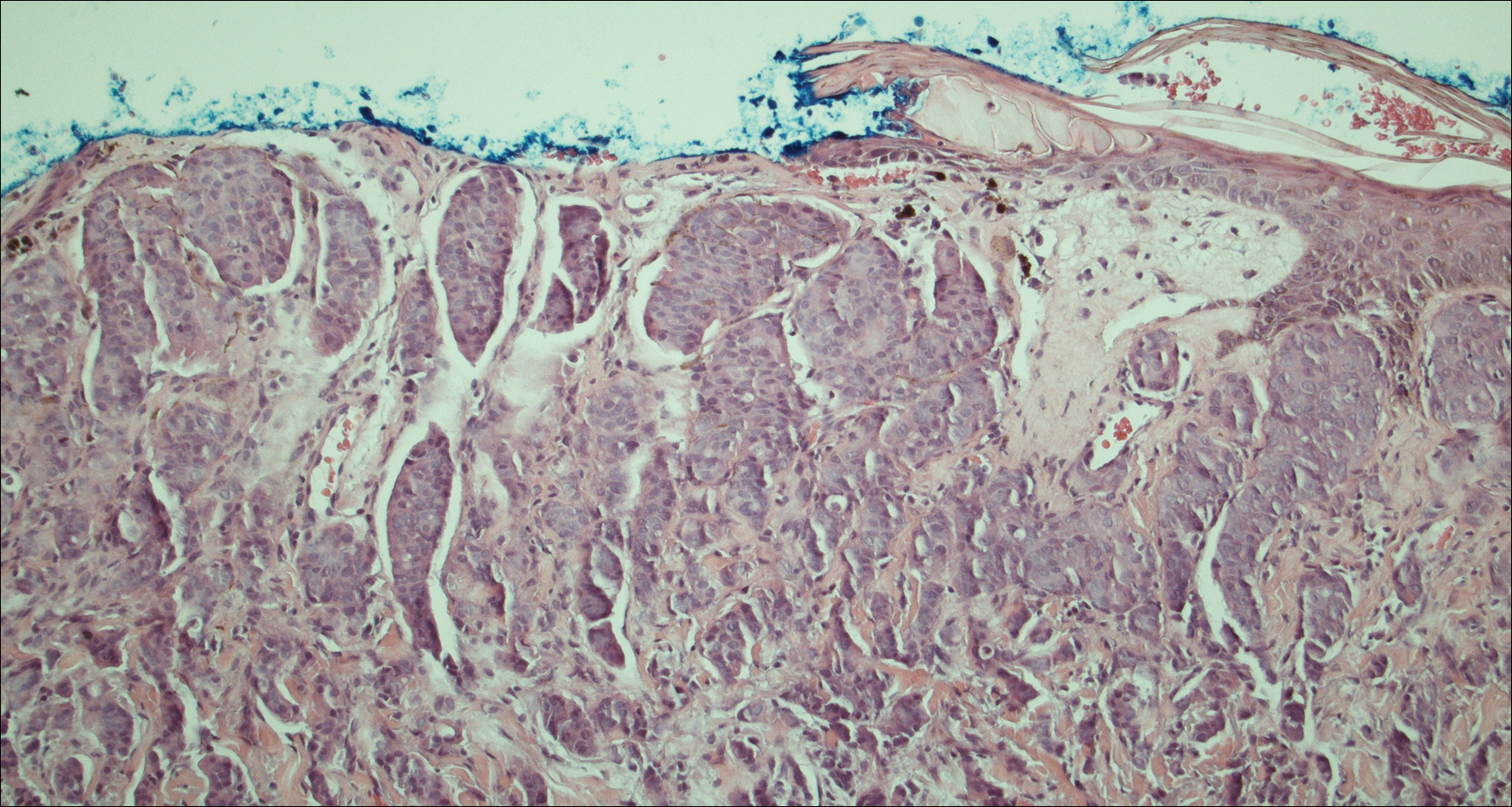
Another scalp biopsy was taken. Tumor cells were negative on mucicarmine staining. Additional immunohistochemical staining, including a periodic acid-Schiff stain with diastase digestion for epithelial mucin revealed minimal luminal positivity. Immunostaining was positive for CK7, carcinoembryonic antigen, CD15, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, and negative for CK20, podoplanin, thyroid transcription factor 1, S-100 protein, p63, and prostate specific antigen. ERBB2 (formerly HER2/neu) staining was negative according to fluorescence in situ hybridization analysis. Tumor cells showed a Ki-67 nuclear proliferation index of greater than 50%, indicating progression to aggressive carcinoma.
Based on the histological and immunochemical studies, the differential diagnosis included primary cutaneous apocrine carcinoma versus breast carcinoma; however, the prolonged clinical progression of these lesions favored a primary cutaneous adnexal tumor over a metastatic adenocarcinoma. Nevertheless, despite the initially indolent growth of the lesions over the first 5 years, the Ki-67 proliferation index and presence of widespread metastases on the posterior scalp indicated progression to an aggressive carcinoma. Chemotherapy was recommended as the treatment of choice. At his most recent follow-up visit 4 months later, the patient chose to begin treatment with tamoxifen and refused other treatment options.
Comment
The distinction between primary adnexal and metastatic adenocarcinomas of the skin is challenging both clinically and histologically. Some pathologists have argued that metastatic breast carcinomas and primary cutaneous apocrine carcinomas are essentially indistinguishable.3 Patients with cutaneous metastases, which occur in approximately 5.3% of all malignancies,4 typically can expect survival of no more than 12 months from the time of detection.1 In contrast, primary apocrine carcinomas of the skin, though much less common, carry a remarkably better prognosis, with 5-year relative survival rates of 95.5% and 85.5% reported for patients with localized disease and spread to regional lymph nodes, respectively.2
Fewer than 100 cases of primary cutaneous adnexal (apocrine) carcinomas have been reported overall, with the earliest known report dating back to 1944.5 According to the literature, primary apocrine carcinomas were diagnosed at a median age of 66 years and were slightly more common in females than males.2,6 Apocrine carcinomas were seen most frequently on the head, neck, and trunk,2 generally presenting in the form of asymptomatic nodules or plaques of 2 to 3 cm in size, with gradual progression occurring over months to years.6 Approximately 40% of patients have been reported with positive regional lymph nodes at diagnosis. Treatment of apocrine carcinoma typically has involved local excision with clear margins with or without lymph node dissection. Chemotherapy and radiation therapy have shown no proven benefit.7
Currently, there is no standardized approach to evaluating patients with possible cutaneous metastasis versus primary cutaneous adnexal carcinomas. Imaging studies such as mammography and abdominal CT typically reveal an internal primary cancer in one-third of patients. However, additional studies such as gastrointestinal radiography, chest and pelvic CT, barium enema, and intravenous pyelogram have shown to be of limited value.8 Although specificity and sensitivity of immunohistochemistry is limited, a number of immunomarkers, including CK7 and CK20, are routinely studied to narrow the differential diagnosis of a cutaneous neoplasm of unclear origin. Urothelial, gastric, colorectal, and pancreatic carcinomas generally are positive for CK20; CK7-positive adenocarcinomas include salivary, non-small cell lung, breast, ovarian, pancreatic, endometrial, and transitional cell adenocarcinomas. Carcinomas negative for both CK7 and CK20 include colorectal, hepatocellular, renal cell, prostate, and squamous cell carcinoma of the lung.
The presence of positive staining for estrogen and progesterone receptors as well as GCDFP-15 and mammaglobin raised the possibility of primary breast adenocarcinoma in our patient, but given that these markers can be positive in primary cutaneous adnexal tumors, immunohistochemistry results were not able to provide a definitive primary site. The overall staining pattern was nearly identical to 26 cases of primary cutaneous cribriform apocrine carcinoma, which was found to be positive for CK7 and carcinoembryonic antigen, and negative for CK20 and S-100. The only difference was in GCDFP-15 staining, which was positive in our case and negative in the cases of cribriform apocrine carcinoma.9 Histologic features favoring a primary apocrine origin include normal apocrine glands in the vicinity, glandular structures with decapitation secretion high in the dermis, and intracytoplasmic iron granules.10 Additionally, positive estrogen receptor staining appears to be much more common in apocrine carcinomas (5/10) than in eccrine carcinomas (1/7).11
A number of other markers have been investigated for possible diagnostic utility for distinction between primary adnexal carcinomas and metastatic adenocarcinomas. The nuclear transcription factor p63, which plays a role in keratinocyte differentiation, is preferentially expressed in a number of primary adnexal carcinomas and is purported to be the most sensitive marker overall, with a sensitivity of 78% to 91%.12-14 However, p63 has shown incomplete specificity for primary adnexal neoplasms, having been reported as positive in 11% to 22% of adenocarcinomas metastatic to skin.15-18 Nestin and CK15, which are expressed in hair follicle progenitor cells, also are potential specific markers for some primary adnexal lesions, specifically eccrine carcinoma, porocarcinoma, hidradenocarcinoma, and microcystic adnexal carcinoma; however, in one report, none of the apocrine carcinomas were positive for p63, cytokeratin 15, or D2-40.19 Thus, while markers for some primary adnexal neoplasms are emerging, specific tests at the immunohistochemical level for the apocrine carcinoma subgroup are still lacking.
Conclusion
In summary, a conclusive distinction between primary cutaneous apocrine carcinoma and metastatic adenocarcinoma to the skin remains challenging. Although new markers provide more specificity and sensitivity for neoplasms of eccrine origin, these markers do not appear to differentiate between primary apocrine carcinoma and metastatic breast carcinoma. In this case, as in other recent reports, diagnosis remained dependent on the clinical course of the patient. Although considerable progress has been made regarding immunohistochemical analysis of these cases, additional markers, especially ones more specific for primary skin cancers with apocrine differentiation, are still needed.
- Nashan D, Müller ML, Braun-Falco M, et al. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135:1-14.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:141-142.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Horn RC. Malignant papillary cystadenoma of sweat glands with metastases to the regional lymph nodes. Surgery. 1944;16:348-355.
- Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
- Vasilakaki T, Skafida E, Moustou E, et al. Primary cutaneous apocrine carcinoma of sweat glands: a rare case report [published online December 17, 2011]. Case Rep Oncol. 2011;4:597-601.
- Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993;329:257-263.
- Rutten A, Kutzner H, Mentzel T, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol. 2009;61:644-651.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever's Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2009.
- Le LP, Dias-Santagata D, Pawlak AC, et al. Apocrine-eccrine carcinomas: molecular and immunohistochemical analyses. PLoS One. 2012;7:e47290.
- Levrero M, De Laurenzi V, Costanzo A, et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113:1661-1670.
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156-3161.
- Reis-Filho JS, Torio B, Albergaria A, et al. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517-523.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Liang H, Wu H, Giorgadze TA, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Mahalingam M, Nguyen LP, Richards JE, et al. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23:713-719.
Differentiation between a primary adnexal carcinoma and a metastatic carcinoma to the skin is a challenging yet critical task for dermatologists and pathologists. Carcinomas that have metastasized to the skin are a sign of widespread systemic involvement and poor prognosis, while primary adnexal carcinomas tend to progress with an indolent clinical course. Although many patients with cutaneous metastases from an internal primary neoplasm can expect a median survival of no more than 12 months,1 patients with primary adnexal carcinomas are reported to have a 5-year survival rate of 95.5% for localized disease and 85% with spread to regional lymph nodes.2 We report a case of multiple cutaneous neoplasms of unknown primary origin in a 71-year-old man and describe our approach to identification of the possible primary site as well as management of the disease.
Case Report
A 71-year-old man initially presented to his primary physician for evaluation of a mass on the left side of the neck of 3 months' duration. On physical examination, a firm 2.5×3.0-cm nodule was noted at the anterior border of the trapezius muscle. Palpation of the thyroid revealed an additional right-sided nodule. The submandibular and parotid glands were unremarkable to palpation. The patient was referred to general surgery for biopsy, which revealed an infiltrating, moderately differentiated adenocarcinoma with extensive lymphatic permeation. Immunohistochemical staining for cytokeratin (CK) 7 was positive, while CK20 and thyroid transcription factor 1 were negative. A positron emission tomography/computed tomography (CT) fusion scan demonstrated 3 areas of enhanced uptake: one in the right side of the thyroid, a second corresponding to the mass on the left side of the neck at the level of the trapezius muscle, and a third in the left masseter muscle. Surgical excision with negative margins with possible chemotherapy was recommended; however, the patient declined treatment and was lost to follow-up until 2 years later when he presented to his primary physician with an additional lesion on his scalp.
Four years after the biopsy, the patient presented to the dermatology department with additional tumor nodules including a 4-cm, annular, indurated, focally eroded plaque on the left side of the lateral neck (Figure 1); 3 separate 1-cm nodules on the right side of the lateral neck; and an ulcerated, crusted, 10×8-cm plaque on the posterior aspect of the scalp. Despite the extensive lesions, the patient remained in good health and reported no recent weight loss or signs or symptoms of systemic involvement. The posterior scalp lesion, which developed 2 years after the initial appearance of the mass on the neck and was thought to represent a possible metastasis of the tumor, was biopsied and showed diffuse infiltration of the dermis by poorly differentiated tumor cells with vacuolated cytoplasm arranged in nests and cords and sometimes in a single-file arrangement (Figure 2). A CT scan demonstrated pretracheal lymphadenopathy as well as small intraparenchymal and subpleural pulmonary nodules throughout both lung fields.


Another scalp biopsy was taken. Tumor cells were negative on mucicarmine staining. Additional immunohistochemical staining, including a periodic acid-Schiff stain with diastase digestion for epithelial mucin revealed minimal luminal positivity. Immunostaining was positive for CK7, carcinoembryonic antigen, CD15, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, and negative for CK20, podoplanin, thyroid transcription factor 1, S-100 protein, p63, and prostate specific antigen. ERBB2 (formerly HER2/neu) staining was negative according to fluorescence in situ hybridization analysis. Tumor cells showed a Ki-67 nuclear proliferation index of greater than 50%, indicating progression to aggressive carcinoma.
Based on the histological and immunochemical studies, the differential diagnosis included primary cutaneous apocrine carcinoma versus breast carcinoma; however, the prolonged clinical progression of these lesions favored a primary cutaneous adnexal tumor over a metastatic adenocarcinoma. Nevertheless, despite the initially indolent growth of the lesions over the first 5 years, the Ki-67 proliferation index and presence of widespread metastases on the posterior scalp indicated progression to an aggressive carcinoma. Chemotherapy was recommended as the treatment of choice. At his most recent follow-up visit 4 months later, the patient chose to begin treatment with tamoxifen and refused other treatment options.
Comment
The distinction between primary adnexal and metastatic adenocarcinomas of the skin is challenging both clinically and histologically. Some pathologists have argued that metastatic breast carcinomas and primary cutaneous apocrine carcinomas are essentially indistinguishable.3 Patients with cutaneous metastases, which occur in approximately 5.3% of all malignancies,4 typically can expect survival of no more than 12 months from the time of detection.1 In contrast, primary apocrine carcinomas of the skin, though much less common, carry a remarkably better prognosis, with 5-year relative survival rates of 95.5% and 85.5% reported for patients with localized disease and spread to regional lymph nodes, respectively.2
Fewer than 100 cases of primary cutaneous adnexal (apocrine) carcinomas have been reported overall, with the earliest known report dating back to 1944.5 According to the literature, primary apocrine carcinomas were diagnosed at a median age of 66 years and were slightly more common in females than males.2,6 Apocrine carcinomas were seen most frequently on the head, neck, and trunk,2 generally presenting in the form of asymptomatic nodules or plaques of 2 to 3 cm in size, with gradual progression occurring over months to years.6 Approximately 40% of patients have been reported with positive regional lymph nodes at diagnosis. Treatment of apocrine carcinoma typically has involved local excision with clear margins with or without lymph node dissection. Chemotherapy and radiation therapy have shown no proven benefit.7
Currently, there is no standardized approach to evaluating patients with possible cutaneous metastasis versus primary cutaneous adnexal carcinomas. Imaging studies such as mammography and abdominal CT typically reveal an internal primary cancer in one-third of patients. However, additional studies such as gastrointestinal radiography, chest and pelvic CT, barium enema, and intravenous pyelogram have shown to be of limited value.8 Although specificity and sensitivity of immunohistochemistry is limited, a number of immunomarkers, including CK7 and CK20, are routinely studied to narrow the differential diagnosis of a cutaneous neoplasm of unclear origin. Urothelial, gastric, colorectal, and pancreatic carcinomas generally are positive for CK20; CK7-positive adenocarcinomas include salivary, non-small cell lung, breast, ovarian, pancreatic, endometrial, and transitional cell adenocarcinomas. Carcinomas negative for both CK7 and CK20 include colorectal, hepatocellular, renal cell, prostate, and squamous cell carcinoma of the lung.
The presence of positive staining for estrogen and progesterone receptors as well as GCDFP-15 and mammaglobin raised the possibility of primary breast adenocarcinoma in our patient, but given that these markers can be positive in primary cutaneous adnexal tumors, immunohistochemistry results were not able to provide a definitive primary site. The overall staining pattern was nearly identical to 26 cases of primary cutaneous cribriform apocrine carcinoma, which was found to be positive for CK7 and carcinoembryonic antigen, and negative for CK20 and S-100. The only difference was in GCDFP-15 staining, which was positive in our case and negative in the cases of cribriform apocrine carcinoma.9 Histologic features favoring a primary apocrine origin include normal apocrine glands in the vicinity, glandular structures with decapitation secretion high in the dermis, and intracytoplasmic iron granules.10 Additionally, positive estrogen receptor staining appears to be much more common in apocrine carcinomas (5/10) than in eccrine carcinomas (1/7).11
A number of other markers have been investigated for possible diagnostic utility for distinction between primary adnexal carcinomas and metastatic adenocarcinomas. The nuclear transcription factor p63, which plays a role in keratinocyte differentiation, is preferentially expressed in a number of primary adnexal carcinomas and is purported to be the most sensitive marker overall, with a sensitivity of 78% to 91%.12-14 However, p63 has shown incomplete specificity for primary adnexal neoplasms, having been reported as positive in 11% to 22% of adenocarcinomas metastatic to skin.15-18 Nestin and CK15, which are expressed in hair follicle progenitor cells, also are potential specific markers for some primary adnexal lesions, specifically eccrine carcinoma, porocarcinoma, hidradenocarcinoma, and microcystic adnexal carcinoma; however, in one report, none of the apocrine carcinomas were positive for p63, cytokeratin 15, or D2-40.19 Thus, while markers for some primary adnexal neoplasms are emerging, specific tests at the immunohistochemical level for the apocrine carcinoma subgroup are still lacking.
Conclusion
In summary, a conclusive distinction between primary cutaneous apocrine carcinoma and metastatic adenocarcinoma to the skin remains challenging. Although new markers provide more specificity and sensitivity for neoplasms of eccrine origin, these markers do not appear to differentiate between primary apocrine carcinoma and metastatic breast carcinoma. In this case, as in other recent reports, diagnosis remained dependent on the clinical course of the patient. Although considerable progress has been made regarding immunohistochemical analysis of these cases, additional markers, especially ones more specific for primary skin cancers with apocrine differentiation, are still needed.
Differentiation between a primary adnexal carcinoma and a metastatic carcinoma to the skin is a challenging yet critical task for dermatologists and pathologists. Carcinomas that have metastasized to the skin are a sign of widespread systemic involvement and poor prognosis, while primary adnexal carcinomas tend to progress with an indolent clinical course. Although many patients with cutaneous metastases from an internal primary neoplasm can expect a median survival of no more than 12 months,1 patients with primary adnexal carcinomas are reported to have a 5-year survival rate of 95.5% for localized disease and 85% with spread to regional lymph nodes.2 We report a case of multiple cutaneous neoplasms of unknown primary origin in a 71-year-old man and describe our approach to identification of the possible primary site as well as management of the disease.
Case Report
A 71-year-old man initially presented to his primary physician for evaluation of a mass on the left side of the neck of 3 months' duration. On physical examination, a firm 2.5×3.0-cm nodule was noted at the anterior border of the trapezius muscle. Palpation of the thyroid revealed an additional right-sided nodule. The submandibular and parotid glands were unremarkable to palpation. The patient was referred to general surgery for biopsy, which revealed an infiltrating, moderately differentiated adenocarcinoma with extensive lymphatic permeation. Immunohistochemical staining for cytokeratin (CK) 7 was positive, while CK20 and thyroid transcription factor 1 were negative. A positron emission tomography/computed tomography (CT) fusion scan demonstrated 3 areas of enhanced uptake: one in the right side of the thyroid, a second corresponding to the mass on the left side of the neck at the level of the trapezius muscle, and a third in the left masseter muscle. Surgical excision with negative margins with possible chemotherapy was recommended; however, the patient declined treatment and was lost to follow-up until 2 years later when he presented to his primary physician with an additional lesion on his scalp.
Four years after the biopsy, the patient presented to the dermatology department with additional tumor nodules including a 4-cm, annular, indurated, focally eroded plaque on the left side of the lateral neck (Figure 1); 3 separate 1-cm nodules on the right side of the lateral neck; and an ulcerated, crusted, 10×8-cm plaque on the posterior aspect of the scalp. Despite the extensive lesions, the patient remained in good health and reported no recent weight loss or signs or symptoms of systemic involvement. The posterior scalp lesion, which developed 2 years after the initial appearance of the mass on the neck and was thought to represent a possible metastasis of the tumor, was biopsied and showed diffuse infiltration of the dermis by poorly differentiated tumor cells with vacuolated cytoplasm arranged in nests and cords and sometimes in a single-file arrangement (Figure 2). A CT scan demonstrated pretracheal lymphadenopathy as well as small intraparenchymal and subpleural pulmonary nodules throughout both lung fields.


Another scalp biopsy was taken. Tumor cells were negative on mucicarmine staining. Additional immunohistochemical staining, including a periodic acid-Schiff stain with diastase digestion for epithelial mucin revealed minimal luminal positivity. Immunostaining was positive for CK7, carcinoembryonic antigen, CD15, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, and negative for CK20, podoplanin, thyroid transcription factor 1, S-100 protein, p63, and prostate specific antigen. ERBB2 (formerly HER2/neu) staining was negative according to fluorescence in situ hybridization analysis. Tumor cells showed a Ki-67 nuclear proliferation index of greater than 50%, indicating progression to aggressive carcinoma.
Based on the histological and immunochemical studies, the differential diagnosis included primary cutaneous apocrine carcinoma versus breast carcinoma; however, the prolonged clinical progression of these lesions favored a primary cutaneous adnexal tumor over a metastatic adenocarcinoma. Nevertheless, despite the initially indolent growth of the lesions over the first 5 years, the Ki-67 proliferation index and presence of widespread metastases on the posterior scalp indicated progression to an aggressive carcinoma. Chemotherapy was recommended as the treatment of choice. At his most recent follow-up visit 4 months later, the patient chose to begin treatment with tamoxifen and refused other treatment options.
Comment
The distinction between primary adnexal and metastatic adenocarcinomas of the skin is challenging both clinically and histologically. Some pathologists have argued that metastatic breast carcinomas and primary cutaneous apocrine carcinomas are essentially indistinguishable.3 Patients with cutaneous metastases, which occur in approximately 5.3% of all malignancies,4 typically can expect survival of no more than 12 months from the time of detection.1 In contrast, primary apocrine carcinomas of the skin, though much less common, carry a remarkably better prognosis, with 5-year relative survival rates of 95.5% and 85.5% reported for patients with localized disease and spread to regional lymph nodes, respectively.2
Fewer than 100 cases of primary cutaneous adnexal (apocrine) carcinomas have been reported overall, with the earliest known report dating back to 1944.5 According to the literature, primary apocrine carcinomas were diagnosed at a median age of 66 years and were slightly more common in females than males.2,6 Apocrine carcinomas were seen most frequently on the head, neck, and trunk,2 generally presenting in the form of asymptomatic nodules or plaques of 2 to 3 cm in size, with gradual progression occurring over months to years.6 Approximately 40% of patients have been reported with positive regional lymph nodes at diagnosis. Treatment of apocrine carcinoma typically has involved local excision with clear margins with or without lymph node dissection. Chemotherapy and radiation therapy have shown no proven benefit.7
Currently, there is no standardized approach to evaluating patients with possible cutaneous metastasis versus primary cutaneous adnexal carcinomas. Imaging studies such as mammography and abdominal CT typically reveal an internal primary cancer in one-third of patients. However, additional studies such as gastrointestinal radiography, chest and pelvic CT, barium enema, and intravenous pyelogram have shown to be of limited value.8 Although specificity and sensitivity of immunohistochemistry is limited, a number of immunomarkers, including CK7 and CK20, are routinely studied to narrow the differential diagnosis of a cutaneous neoplasm of unclear origin. Urothelial, gastric, colorectal, and pancreatic carcinomas generally are positive for CK20; CK7-positive adenocarcinomas include salivary, non-small cell lung, breast, ovarian, pancreatic, endometrial, and transitional cell adenocarcinomas. Carcinomas negative for both CK7 and CK20 include colorectal, hepatocellular, renal cell, prostate, and squamous cell carcinoma of the lung.
The presence of positive staining for estrogen and progesterone receptors as well as GCDFP-15 and mammaglobin raised the possibility of primary breast adenocarcinoma in our patient, but given that these markers can be positive in primary cutaneous adnexal tumors, immunohistochemistry results were not able to provide a definitive primary site. The overall staining pattern was nearly identical to 26 cases of primary cutaneous cribriform apocrine carcinoma, which was found to be positive for CK7 and carcinoembryonic antigen, and negative for CK20 and S-100. The only difference was in GCDFP-15 staining, which was positive in our case and negative in the cases of cribriform apocrine carcinoma.9 Histologic features favoring a primary apocrine origin include normal apocrine glands in the vicinity, glandular structures with decapitation secretion high in the dermis, and intracytoplasmic iron granules.10 Additionally, positive estrogen receptor staining appears to be much more common in apocrine carcinomas (5/10) than in eccrine carcinomas (1/7).11
A number of other markers have been investigated for possible diagnostic utility for distinction between primary adnexal carcinomas and metastatic adenocarcinomas. The nuclear transcription factor p63, which plays a role in keratinocyte differentiation, is preferentially expressed in a number of primary adnexal carcinomas and is purported to be the most sensitive marker overall, with a sensitivity of 78% to 91%.12-14 However, p63 has shown incomplete specificity for primary adnexal neoplasms, having been reported as positive in 11% to 22% of adenocarcinomas metastatic to skin.15-18 Nestin and CK15, which are expressed in hair follicle progenitor cells, also are potential specific markers for some primary adnexal lesions, specifically eccrine carcinoma, porocarcinoma, hidradenocarcinoma, and microcystic adnexal carcinoma; however, in one report, none of the apocrine carcinomas were positive for p63, cytokeratin 15, or D2-40.19 Thus, while markers for some primary adnexal neoplasms are emerging, specific tests at the immunohistochemical level for the apocrine carcinoma subgroup are still lacking.
Conclusion
In summary, a conclusive distinction between primary cutaneous apocrine carcinoma and metastatic adenocarcinoma to the skin remains challenging. Although new markers provide more specificity and sensitivity for neoplasms of eccrine origin, these markers do not appear to differentiate between primary apocrine carcinoma and metastatic breast carcinoma. In this case, as in other recent reports, diagnosis remained dependent on the clinical course of the patient. Although considerable progress has been made regarding immunohistochemical analysis of these cases, additional markers, especially ones more specific for primary skin cancers with apocrine differentiation, are still needed.
- Nashan D, Müller ML, Braun-Falco M, et al. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135:1-14.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:141-142.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Horn RC. Malignant papillary cystadenoma of sweat glands with metastases to the regional lymph nodes. Surgery. 1944;16:348-355.
- Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
- Vasilakaki T, Skafida E, Moustou E, et al. Primary cutaneous apocrine carcinoma of sweat glands: a rare case report [published online December 17, 2011]. Case Rep Oncol. 2011;4:597-601.
- Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993;329:257-263.
- Rutten A, Kutzner H, Mentzel T, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol. 2009;61:644-651.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever's Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2009.
- Le LP, Dias-Santagata D, Pawlak AC, et al. Apocrine-eccrine carcinomas: molecular and immunohistochemical analyses. PLoS One. 2012;7:e47290.
- Levrero M, De Laurenzi V, Costanzo A, et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113:1661-1670.
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156-3161.
- Reis-Filho JS, Torio B, Albergaria A, et al. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517-523.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Liang H, Wu H, Giorgadze TA, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Mahalingam M, Nguyen LP, Richards JE, et al. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23:713-719.
- Nashan D, Müller ML, Braun-Falco M, et al. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135:1-14.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:141-142.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Horn RC. Malignant papillary cystadenoma of sweat glands with metastases to the regional lymph nodes. Surgery. 1944;16:348-355.
- Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
- Vasilakaki T, Skafida E, Moustou E, et al. Primary cutaneous apocrine carcinoma of sweat glands: a rare case report [published online December 17, 2011]. Case Rep Oncol. 2011;4:597-601.
- Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993;329:257-263.
- Rutten A, Kutzner H, Mentzel T, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol. 2009;61:644-651.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever's Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2009.
- Le LP, Dias-Santagata D, Pawlak AC, et al. Apocrine-eccrine carcinomas: molecular and immunohistochemical analyses. PLoS One. 2012;7:e47290.
- Levrero M, De Laurenzi V, Costanzo A, et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113:1661-1670.
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156-3161.
- Reis-Filho JS, Torio B, Albergaria A, et al. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517-523.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Liang H, Wu H, Giorgadze TA, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Mahalingam M, Nguyen LP, Richards JE, et al. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23:713-719.
Practice Points
- Despite advances in immunohistochemical analysis, differentiating between primary apocrine carcinoma and metastatic breast carcinoma remains largely dependent on the clinical course of the patient.
- Treatment of apocrine carcinoma typically involves local excision with clear margins with or without lymph node dissection.
Multicentric Primary Extramammary Paget Disease: A Toker Cell Disorder?
Extramammary Paget disease (EMPD), which was first described by Crocker1 in a patient with erythematous patches on the penis and scrotum, is morphologically identical to mammary Paget disease (MPD) of the nipple. The principal difference between EMPD and MPD is anatomic location. Extramammary Paget disease predominantly affects apocrine gland–bearing areas including the vulva, scrotum, and perianal areas. Although EMPD is not a common condition, it must be considered in the differential diagnosis for patients with chronic genital or perianal dermatitis. Primary EMPD must be distinguished from secondary epithelial involvement by an underlying invasive carcinoma that originates from sites such as the gastrointestinal or genitourinary systems (secondary EMPD).
Although multicentric primary EMPD is not uncommon among Eastern Asians, as there have been several reports in the literature from Japan,2-5 multicentric EMPD in white individuals is rare.6 We report a case of primary EMPD that was established when no underlying malignancies were detected.
Case Report
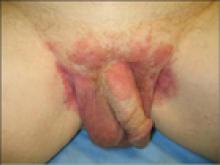
A 63-year-old white man presented to the dermatology clinic with a pruritic rash involving the groin of 8 years’ duration. Over-the-counter antifungal agents provided no improvement. Confluent erythematous and macerated plaques on the scrotum, shaft of the penis, bilateral inguinal areas, and perineum were noted on clinical examination (Figure 1). There also were well-demarcated, 4×5-cm, erythematous, velvety plaques on the right axilla and a small erythematous plaque on the left axilla. Systemic workup including colonoscopy, cystoscopy, and magnetic resonance imaging of the abdomen and pelvis were unremarkable. A positron emission tomography–computed tomography scan revealed foci of hypermetabolic activity in the bilateral inguinal nodes that were interpreted as evidence of an inflammatory process.
Fourteen biopsies were taken from the clinically involved regions in the genital area to delineate the extent of involvement. They all showed intraepidermal carcinoma characterized by large cells with clear cytoplasm and large hyperchromatic nuclei throughout the epidermis, which were more abundant in the basilar and lower portions of the epidermis (Figure 2). There was no evidence of underlying carcinoma in the dermis.
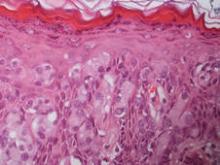
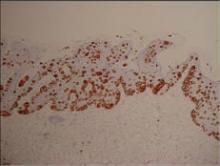
On immunohistochemical staining, the neoplastic cells were diffusely and strongly positive for cytokeratin 7 (Figure 3) and moderately positive for carcinoembryonic antigen, estrogen and progesterone receptors, and ERBB2 (formerly HER-2/neu). Additional immunostaining for cytokeratin 20 and gross cystic disease fluid protein 15 were negative. High-molecular-weight keratin staining highlighted background epithelium and spared neoplastic cells. Mucicarmine staining highlighted mucin within the cytoplasm of neoplastic cells. Ultrastructural studies showed evidence of apocrine differentiation in the neoplastic cells with deeply indented, bean-shaped nuclei and clear cytoplasm with inconspicuous organelles. Clinically unaffected skin from the periumbilical region also was biopsied. The histology demonstrated Toker cells characterized by clear cytoplasm, small vesicular nuclei, and the absence of hyperchromasia (Figure 4).
The patient was treated with 6 cycles of 5-aminolevulinic acid 20% photodynamic therapy but showed no improvement. Imiquimod cream 5% resulted in mild clinical improvement in the axillae but caused severe irritation in the groin and was discontinued after 3 months. A radiology consultation obtained 6 years prior to presentation determined that the risk associated with radiation therapy outweighed any possible benefits. Although localized disease persisted, repeated biopsies and positron emission tomography–computed tomography scans did not show any evidence of invasion or metastasis, respectively. Three years after initial presentation, groin lesions progressed to become more erythematous and vegetative. The corresponding histology demonstrated EMPD. An ancillary test (polymerase chain reaction analysis) for low- and high-risk human papillomavirus was negative.
Comment
Primary EMPD has been well documented in the literature to have a favorable prognosis if adequately treated. The few cases reported from Japan on EMPD without underlying adenocarcinoma had good outcomes.2,3 Further reports of multicentric EMPD involving 2 or 3 sites in the axillae and/or genital region did not reveal any progression to invasive carcinomas after adequate follow-up.3-9 Indeed, many of these cases, particularly those in the genital region, were aggressively treated with surgery, topical chemotherapy, immunomodulatory agents, and radiation; therefore, their natural course is not known.2 A Japanese studyconducted at 75 medical institutions (1987-1991) included 46 EMPD patients with multiple (ie, 2 or 3) sites of involvement.10 Some of the patients with a combination of genital and axillary lesions were followed without any treatment. None of the EMPD patients with axillary involvement developed invasive carcinoma after 4- to 12-year follow-up.10
Toker cells, which were first described in 1970,11 have been recognized as precursors to EMPD.12-14 Toker cells are intraepithelial cells with clear to pale-staining cytoplasm that are smaller in size than Paget cells but larger than neighboring keratinocytes. They are found in approximately 10% of normal nipples.11,14 Toker cells show vesicular chromatin, whereas Paget cells are hyperchromatic with prominent nucleoli.12 Willman et al15 examined 11 vulvectomies for the presence of Toker cells in association with mammarylike glands of the vulva. They demonstrated the presence of Toker cells in 4 (36%) of the samples.15 Additionally, Van der Putte et al16 observed Toker cells in an areolar lesion in a 47-year-old woman with MPD without underlying adenocarcinoma, suggesting that cases of MPD and EMPD confined to epithelial cells may be derived from Toker cells. Toker cells have been associated in the pathogenesis of 2 other benign entities, including clear cell papulosis17,18 and cutaneous hamartoma with pagetoid cells.19
Primary EMPD has the potential to develop in several regions of the skin that contain apocrine glands. Our patient presented with persistent genital lesions for many years without any concerns of axillary disease. Interestingly, biopsies from normal-appearing skin in the periumbilical region revealed clear cells (Figure 4). Likewise, Toker cells have been described in biopsies from clinically unaffected skin of the axillae in some cases where EMPD was identified in the genital regions.3,5 Genital lesions were the main clinical presentation and preceded axillary involvement in most instances.3,5,9 Therefore, biopsies from uninvolved apocrine sites (ie, axillary and periumbilical skin) should be considered in these patients. Based on our observation, we speculate that multicentric primary EMPD starts with Toker cell hyperplasia with a propensity to evolve into neoplasia in sites with apocrine or mammarylike glands.
Primary and secondary EMPD cannot be distinguished by histopathology and immunohistochemistry has a limited role.20,21 However, immunostaining with CDX2 (a caudal-type homeobox protein) might be helpful in differentiating primary EMPD from secondary EMPD extending from underlying anorectal adenocarcinomas.22 Primary EMPD has been treated with surgical excision.2,3,8 Despite the high recurrence rate after surgery, the prognosis for localized primary EMPD disease has been favorable.
Conclusion
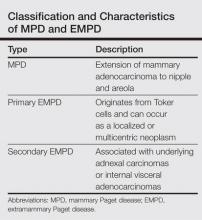
Our case suggests that Toker cell hyperplasia is a precursor to primary EMPD. In patients with established EMPD, other apocrine gland–bearing areas should be examined for multicentric disease. Lastly, the clinical course in our patient supports the hypothesis that primary multicentric EMPD has a favorable outcome. The Table lists the characteristics of MPD and EMPD and describes their development with respect to Toker cells. More studies are required to further outline the cytologic characteristics of Toker cells and to distinguish primary EMPD from secondary EMPD.
1. Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Path Soc London. 1889;40:187-191.
2. Kawatsu T, Miki Y. Triple extramammary Paget’s disease. Arch Dermatol. 1971;104:316-319.
3. Makino T, Nakamura S, Nakayama H, et al. Genital Paget’s disease with clear cells in the epidermis of the axilla. J Cutan Pathol. 1998;25:568-571.
4. Inui S, Fukuhara S, Asada H, et al. Double involvement of extramammary Paget’s disease in the genitalia and axilla. J Dermatol. 2000;27:409-412.
5. Kitajima S, Yamamoto K, Tsuji T, et al. Triple extramammary Paget’s disease. Dermatol Surg. 1997;23:1035-1038.
6. Van Hamme C, Marot L, Dachelet C, et al. Paget’s extramammary disease of the axillae and perineum [in French]. Ann Dermatol Venereol. 2002;129(5, pt 1):717-719.
7. Kao GF, Graham JH, Helwig EB. Paget’s disease of the ectopic breast with an underlying intraductal carcinoma: report of a case. J Cutan Pathol. 1986;13:59-66.
8. Murrell TW Jr, McMullan FH. Extramammary Paget’s disease. a report of two cases. Arch Dermatol. 1962;85:600-613.
9. Koseki S, Mitsuhashi Y, Yoshikawa K, et al. A case of triple extramammary Paget’s disease. J Dermatol. 1997;24:535-538.
10. Ishihara K. Statistical study of extramammary Paget’s disease in Japan [in Japanese]. Skin Cancer. 1994;9:38.
11. Toker C. Clear cells of the nipple epidermis. Cancer. 1970;25:601-610.
12. Marucci G, Betts CM, Golouh R, et al. Toker cells are probably precursors of Paget cell carcinoma: a morphological and ultrastructural description. Virchows Archiv. 2002;441:117-123.
13. Belousova IE, Kazakov DV, Michal M, et al. Vulvar toker cells: the long-awaited missing link: a proposal for an origin-based histogenetic classification of extramammary Paget disease. Am J Dermatopathol. 2006;28:84-86.
14. Val-Bernal JF. Diego C, Rodriguez-Villar D, et al. The nipple-areola complex epidermis: a prospective systemic study in adult autopsies. Am J Dermatopathology. 2010;32:787-93.
15. Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
16. Van der Putte SC, Toonstra J, Hennipman A. Mammary Paget’s disease confined to the areola and associated with multifocal Toker cell hyperplasia. Am J Dermatopathol. 1995;17:487-493.
17. Kuo TT, Chan HL, Hsueh S. Clear cell papulosis of the skin. a new entity with histogenetic implications for cutaneous Paget’s disease. Am J Surg Pathol. 1987;11:827-834.
18. Kuo TT, Huang CL, Chan HL, et al. Clear cell papulosis: report of three cases of a newly recognized disease. J Am Acad Dermatol. 1995;33(2, pt 1):230-233.
19. Piérard-Franchimont C, Dosal FL, Estrada JA, et al. Cutaneous hamartoma with pagetoid cells. Am J Dermatopathol. 1991;13:158-161.
20. Belcher RW. Extramammary Paget’s disease. enzyme histochemical and electron microscopic study. Arch Pathol. 1972;94:59-64.
21. Ordóñez NG, Awalt H, Mackay B. Mammary and extramammary Paget’s disease. an immunocytochemical and ultrastructural study. Cancer. 1987;59:1173-1183.
22. Perrotto J, Abbott JJ, Ceilley RI, et al. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am J Dermatopathol. 2010;32:137-143.
23. Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease. a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
Extramammary Paget disease (EMPD), which was first described by Crocker1 in a patient with erythematous patches on the penis and scrotum, is morphologically identical to mammary Paget disease (MPD) of the nipple. The principal difference between EMPD and MPD is anatomic location. Extramammary Paget disease predominantly affects apocrine gland–bearing areas including the vulva, scrotum, and perianal areas. Although EMPD is not a common condition, it must be considered in the differential diagnosis for patients with chronic genital or perianal dermatitis. Primary EMPD must be distinguished from secondary epithelial involvement by an underlying invasive carcinoma that originates from sites such as the gastrointestinal or genitourinary systems (secondary EMPD).
Although multicentric primary EMPD is not uncommon among Eastern Asians, as there have been several reports in the literature from Japan,2-5 multicentric EMPD in white individuals is rare.6 We report a case of primary EMPD that was established when no underlying malignancies were detected.
Case Report
A 63-year-old white man presented to the dermatology clinic with a pruritic rash involving the groin of 8 years’ duration. Over-the-counter antifungal agents provided no improvement. Confluent erythematous and macerated plaques on the scrotum, shaft of the penis, bilateral inguinal areas, and perineum were noted on clinical examination (Figure 1). There also were well-demarcated, 4×5-cm, erythematous, velvety plaques on the right axilla and a small erythematous plaque on the left axilla. Systemic workup including colonoscopy, cystoscopy, and magnetic resonance imaging of the abdomen and pelvis were unremarkable. A positron emission tomography–computed tomography scan revealed foci of hypermetabolic activity in the bilateral inguinal nodes that were interpreted as evidence of an inflammatory process.
Fourteen biopsies were taken from the clinically involved regions in the genital area to delineate the extent of involvement. They all showed intraepidermal carcinoma characterized by large cells with clear cytoplasm and large hyperchromatic nuclei throughout the epidermis, which were more abundant in the basilar and lower portions of the epidermis (Figure 2). There was no evidence of underlying carcinoma in the dermis.
On immunohistochemical staining, the neoplastic cells were diffusely and strongly positive for cytokeratin 7 (Figure 3) and moderately positive for carcinoembryonic antigen, estrogen and progesterone receptors, and ERBB2 (formerly HER-2/neu). Additional immunostaining for cytokeratin 20 and gross cystic disease fluid protein 15 were negative. High-molecular-weight keratin staining highlighted background epithelium and spared neoplastic cells. Mucicarmine staining highlighted mucin within the cytoplasm of neoplastic cells. Ultrastructural studies showed evidence of apocrine differentiation in the neoplastic cells with deeply indented, bean-shaped nuclei and clear cytoplasm with inconspicuous organelles. Clinically unaffected skin from the periumbilical region also was biopsied. The histology demonstrated Toker cells characterized by clear cytoplasm, small vesicular nuclei, and the absence of hyperchromasia (Figure 4).
The patient was treated with 6 cycles of 5-aminolevulinic acid 20% photodynamic therapy but showed no improvement. Imiquimod cream 5% resulted in mild clinical improvement in the axillae but caused severe irritation in the groin and was discontinued after 3 months. A radiology consultation obtained 6 years prior to presentation determined that the risk associated with radiation therapy outweighed any possible benefits. Although localized disease persisted, repeated biopsies and positron emission tomography–computed tomography scans did not show any evidence of invasion or metastasis, respectively. Three years after initial presentation, groin lesions progressed to become more erythematous and vegetative. The corresponding histology demonstrated EMPD. An ancillary test (polymerase chain reaction analysis) for low- and high-risk human papillomavirus was negative.
Comment
Primary EMPD has been well documented in the literature to have a favorable prognosis if adequately treated. The few cases reported from Japan on EMPD without underlying adenocarcinoma had good outcomes.2,3 Further reports of multicentric EMPD involving 2 or 3 sites in the axillae and/or genital region did not reveal any progression to invasive carcinomas after adequate follow-up.3-9 Indeed, many of these cases, particularly those in the genital region, were aggressively treated with surgery, topical chemotherapy, immunomodulatory agents, and radiation; therefore, their natural course is not known.2 A Japanese studyconducted at 75 medical institutions (1987-1991) included 46 EMPD patients with multiple (ie, 2 or 3) sites of involvement.10 Some of the patients with a combination of genital and axillary lesions were followed without any treatment. None of the EMPD patients with axillary involvement developed invasive carcinoma after 4- to 12-year follow-up.10
Toker cells, which were first described in 1970,11 have been recognized as precursors to EMPD.12-14 Toker cells are intraepithelial cells with clear to pale-staining cytoplasm that are smaller in size than Paget cells but larger than neighboring keratinocytes. They are found in approximately 10% of normal nipples.11,14 Toker cells show vesicular chromatin, whereas Paget cells are hyperchromatic with prominent nucleoli.12 Willman et al15 examined 11 vulvectomies for the presence of Toker cells in association with mammarylike glands of the vulva. They demonstrated the presence of Toker cells in 4 (36%) of the samples.15 Additionally, Van der Putte et al16 observed Toker cells in an areolar lesion in a 47-year-old woman with MPD without underlying adenocarcinoma, suggesting that cases of MPD and EMPD confined to epithelial cells may be derived from Toker cells. Toker cells have been associated in the pathogenesis of 2 other benign entities, including clear cell papulosis17,18 and cutaneous hamartoma with pagetoid cells.19
Primary EMPD has the potential to develop in several regions of the skin that contain apocrine glands. Our patient presented with persistent genital lesions for many years without any concerns of axillary disease. Interestingly, biopsies from normal-appearing skin in the periumbilical region revealed clear cells (Figure 4). Likewise, Toker cells have been described in biopsies from clinically unaffected skin of the axillae in some cases where EMPD was identified in the genital regions.3,5 Genital lesions were the main clinical presentation and preceded axillary involvement in most instances.3,5,9 Therefore, biopsies from uninvolved apocrine sites (ie, axillary and periumbilical skin) should be considered in these patients. Based on our observation, we speculate that multicentric primary EMPD starts with Toker cell hyperplasia with a propensity to evolve into neoplasia in sites with apocrine or mammarylike glands.
Primary and secondary EMPD cannot be distinguished by histopathology and immunohistochemistry has a limited role.20,21 However, immunostaining with CDX2 (a caudal-type homeobox protein) might be helpful in differentiating primary EMPD from secondary EMPD extending from underlying anorectal adenocarcinomas.22 Primary EMPD has been treated with surgical excision.2,3,8 Despite the high recurrence rate after surgery, the prognosis for localized primary EMPD disease has been favorable.
Conclusion
Our case suggests that Toker cell hyperplasia is a precursor to primary EMPD. In patients with established EMPD, other apocrine gland–bearing areas should be examined for multicentric disease. Lastly, the clinical course in our patient supports the hypothesis that primary multicentric EMPD has a favorable outcome. The Table lists the characteristics of MPD and EMPD and describes their development with respect to Toker cells. More studies are required to further outline the cytologic characteristics of Toker cells and to distinguish primary EMPD from secondary EMPD.
Extramammary Paget disease (EMPD), which was first described by Crocker1 in a patient with erythematous patches on the penis and scrotum, is morphologically identical to mammary Paget disease (MPD) of the nipple. The principal difference between EMPD and MPD is anatomic location. Extramammary Paget disease predominantly affects apocrine gland–bearing areas including the vulva, scrotum, and perianal areas. Although EMPD is not a common condition, it must be considered in the differential diagnosis for patients with chronic genital or perianal dermatitis. Primary EMPD must be distinguished from secondary epithelial involvement by an underlying invasive carcinoma that originates from sites such as the gastrointestinal or genitourinary systems (secondary EMPD).
Although multicentric primary EMPD is not uncommon among Eastern Asians, as there have been several reports in the literature from Japan,2-5 multicentric EMPD in white individuals is rare.6 We report a case of primary EMPD that was established when no underlying malignancies were detected.
Case Report
A 63-year-old white man presented to the dermatology clinic with a pruritic rash involving the groin of 8 years’ duration. Over-the-counter antifungal agents provided no improvement. Confluent erythematous and macerated plaques on the scrotum, shaft of the penis, bilateral inguinal areas, and perineum were noted on clinical examination (Figure 1). There also were well-demarcated, 4×5-cm, erythematous, velvety plaques on the right axilla and a small erythematous plaque on the left axilla. Systemic workup including colonoscopy, cystoscopy, and magnetic resonance imaging of the abdomen and pelvis were unremarkable. A positron emission tomography–computed tomography scan revealed foci of hypermetabolic activity in the bilateral inguinal nodes that were interpreted as evidence of an inflammatory process.
Fourteen biopsies were taken from the clinically involved regions in the genital area to delineate the extent of involvement. They all showed intraepidermal carcinoma characterized by large cells with clear cytoplasm and large hyperchromatic nuclei throughout the epidermis, which were more abundant in the basilar and lower portions of the epidermis (Figure 2). There was no evidence of underlying carcinoma in the dermis.
On immunohistochemical staining, the neoplastic cells were diffusely and strongly positive for cytokeratin 7 (Figure 3) and moderately positive for carcinoembryonic antigen, estrogen and progesterone receptors, and ERBB2 (formerly HER-2/neu). Additional immunostaining for cytokeratin 20 and gross cystic disease fluid protein 15 were negative. High-molecular-weight keratin staining highlighted background epithelium and spared neoplastic cells. Mucicarmine staining highlighted mucin within the cytoplasm of neoplastic cells. Ultrastructural studies showed evidence of apocrine differentiation in the neoplastic cells with deeply indented, bean-shaped nuclei and clear cytoplasm with inconspicuous organelles. Clinically unaffected skin from the periumbilical region also was biopsied. The histology demonstrated Toker cells characterized by clear cytoplasm, small vesicular nuclei, and the absence of hyperchromasia (Figure 4).
The patient was treated with 6 cycles of 5-aminolevulinic acid 20% photodynamic therapy but showed no improvement. Imiquimod cream 5% resulted in mild clinical improvement in the axillae but caused severe irritation in the groin and was discontinued after 3 months. A radiology consultation obtained 6 years prior to presentation determined that the risk associated with radiation therapy outweighed any possible benefits. Although localized disease persisted, repeated biopsies and positron emission tomography–computed tomography scans did not show any evidence of invasion or metastasis, respectively. Three years after initial presentation, groin lesions progressed to become more erythematous and vegetative. The corresponding histology demonstrated EMPD. An ancillary test (polymerase chain reaction analysis) for low- and high-risk human papillomavirus was negative.
Comment
Primary EMPD has been well documented in the literature to have a favorable prognosis if adequately treated. The few cases reported from Japan on EMPD without underlying adenocarcinoma had good outcomes.2,3 Further reports of multicentric EMPD involving 2 or 3 sites in the axillae and/or genital region did not reveal any progression to invasive carcinomas after adequate follow-up.3-9 Indeed, many of these cases, particularly those in the genital region, were aggressively treated with surgery, topical chemotherapy, immunomodulatory agents, and radiation; therefore, their natural course is not known.2 A Japanese studyconducted at 75 medical institutions (1987-1991) included 46 EMPD patients with multiple (ie, 2 or 3) sites of involvement.10 Some of the patients with a combination of genital and axillary lesions were followed without any treatment. None of the EMPD patients with axillary involvement developed invasive carcinoma after 4- to 12-year follow-up.10
Toker cells, which were first described in 1970,11 have been recognized as precursors to EMPD.12-14 Toker cells are intraepithelial cells with clear to pale-staining cytoplasm that are smaller in size than Paget cells but larger than neighboring keratinocytes. They are found in approximately 10% of normal nipples.11,14 Toker cells show vesicular chromatin, whereas Paget cells are hyperchromatic with prominent nucleoli.12 Willman et al15 examined 11 vulvectomies for the presence of Toker cells in association with mammarylike glands of the vulva. They demonstrated the presence of Toker cells in 4 (36%) of the samples.15 Additionally, Van der Putte et al16 observed Toker cells in an areolar lesion in a 47-year-old woman with MPD without underlying adenocarcinoma, suggesting that cases of MPD and EMPD confined to epithelial cells may be derived from Toker cells. Toker cells have been associated in the pathogenesis of 2 other benign entities, including clear cell papulosis17,18 and cutaneous hamartoma with pagetoid cells.19
Primary EMPD has the potential to develop in several regions of the skin that contain apocrine glands. Our patient presented with persistent genital lesions for many years without any concerns of axillary disease. Interestingly, biopsies from normal-appearing skin in the periumbilical region revealed clear cells (Figure 4). Likewise, Toker cells have been described in biopsies from clinically unaffected skin of the axillae in some cases where EMPD was identified in the genital regions.3,5 Genital lesions were the main clinical presentation and preceded axillary involvement in most instances.3,5,9 Therefore, biopsies from uninvolved apocrine sites (ie, axillary and periumbilical skin) should be considered in these patients. Based on our observation, we speculate that multicentric primary EMPD starts with Toker cell hyperplasia with a propensity to evolve into neoplasia in sites with apocrine or mammarylike glands.
Primary and secondary EMPD cannot be distinguished by histopathology and immunohistochemistry has a limited role.20,21 However, immunostaining with CDX2 (a caudal-type homeobox protein) might be helpful in differentiating primary EMPD from secondary EMPD extending from underlying anorectal adenocarcinomas.22 Primary EMPD has been treated with surgical excision.2,3,8 Despite the high recurrence rate after surgery, the prognosis for localized primary EMPD disease has been favorable.
Conclusion
Our case suggests that Toker cell hyperplasia is a precursor to primary EMPD. In patients with established EMPD, other apocrine gland–bearing areas should be examined for multicentric disease. Lastly, the clinical course in our patient supports the hypothesis that primary multicentric EMPD has a favorable outcome. The Table lists the characteristics of MPD and EMPD and describes their development with respect to Toker cells. More studies are required to further outline the cytologic characteristics of Toker cells and to distinguish primary EMPD from secondary EMPD.
1. Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Path Soc London. 1889;40:187-191.
2. Kawatsu T, Miki Y. Triple extramammary Paget’s disease. Arch Dermatol. 1971;104:316-319.
3. Makino T, Nakamura S, Nakayama H, et al. Genital Paget’s disease with clear cells in the epidermis of the axilla. J Cutan Pathol. 1998;25:568-571.
4. Inui S, Fukuhara S, Asada H, et al. Double involvement of extramammary Paget’s disease in the genitalia and axilla. J Dermatol. 2000;27:409-412.
5. Kitajima S, Yamamoto K, Tsuji T, et al. Triple extramammary Paget’s disease. Dermatol Surg. 1997;23:1035-1038.
6. Van Hamme C, Marot L, Dachelet C, et al. Paget’s extramammary disease of the axillae and perineum [in French]. Ann Dermatol Venereol. 2002;129(5, pt 1):717-719.
7. Kao GF, Graham JH, Helwig EB. Paget’s disease of the ectopic breast with an underlying intraductal carcinoma: report of a case. J Cutan Pathol. 1986;13:59-66.
8. Murrell TW Jr, McMullan FH. Extramammary Paget’s disease. a report of two cases. Arch Dermatol. 1962;85:600-613.
9. Koseki S, Mitsuhashi Y, Yoshikawa K, et al. A case of triple extramammary Paget’s disease. J Dermatol. 1997;24:535-538.
10. Ishihara K. Statistical study of extramammary Paget’s disease in Japan [in Japanese]. Skin Cancer. 1994;9:38.
11. Toker C. Clear cells of the nipple epidermis. Cancer. 1970;25:601-610.
12. Marucci G, Betts CM, Golouh R, et al. Toker cells are probably precursors of Paget cell carcinoma: a morphological and ultrastructural description. Virchows Archiv. 2002;441:117-123.
13. Belousova IE, Kazakov DV, Michal M, et al. Vulvar toker cells: the long-awaited missing link: a proposal for an origin-based histogenetic classification of extramammary Paget disease. Am J Dermatopathol. 2006;28:84-86.
14. Val-Bernal JF. Diego C, Rodriguez-Villar D, et al. The nipple-areola complex epidermis: a prospective systemic study in adult autopsies. Am J Dermatopathology. 2010;32:787-93.
15. Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
16. Van der Putte SC, Toonstra J, Hennipman A. Mammary Paget’s disease confined to the areola and associated with multifocal Toker cell hyperplasia. Am J Dermatopathol. 1995;17:487-493.
17. Kuo TT, Chan HL, Hsueh S. Clear cell papulosis of the skin. a new entity with histogenetic implications for cutaneous Paget’s disease. Am J Surg Pathol. 1987;11:827-834.
18. Kuo TT, Huang CL, Chan HL, et al. Clear cell papulosis: report of three cases of a newly recognized disease. J Am Acad Dermatol. 1995;33(2, pt 1):230-233.
19. Piérard-Franchimont C, Dosal FL, Estrada JA, et al. Cutaneous hamartoma with pagetoid cells. Am J Dermatopathol. 1991;13:158-161.
20. Belcher RW. Extramammary Paget’s disease. enzyme histochemical and electron microscopic study. Arch Pathol. 1972;94:59-64.
21. Ordóñez NG, Awalt H, Mackay B. Mammary and extramammary Paget’s disease. an immunocytochemical and ultrastructural study. Cancer. 1987;59:1173-1183.
22. Perrotto J, Abbott JJ, Ceilley RI, et al. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am J Dermatopathol. 2010;32:137-143.
23. Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease. a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
1. Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Path Soc London. 1889;40:187-191.
2. Kawatsu T, Miki Y. Triple extramammary Paget’s disease. Arch Dermatol. 1971;104:316-319.
3. Makino T, Nakamura S, Nakayama H, et al. Genital Paget’s disease with clear cells in the epidermis of the axilla. J Cutan Pathol. 1998;25:568-571.
4. Inui S, Fukuhara S, Asada H, et al. Double involvement of extramammary Paget’s disease in the genitalia and axilla. J Dermatol. 2000;27:409-412.
5. Kitajima S, Yamamoto K, Tsuji T, et al. Triple extramammary Paget’s disease. Dermatol Surg. 1997;23:1035-1038.
6. Van Hamme C, Marot L, Dachelet C, et al. Paget’s extramammary disease of the axillae and perineum [in French]. Ann Dermatol Venereol. 2002;129(5, pt 1):717-719.
7. Kao GF, Graham JH, Helwig EB. Paget’s disease of the ectopic breast with an underlying intraductal carcinoma: report of a case. J Cutan Pathol. 1986;13:59-66.
8. Murrell TW Jr, McMullan FH. Extramammary Paget’s disease. a report of two cases. Arch Dermatol. 1962;85:600-613.
9. Koseki S, Mitsuhashi Y, Yoshikawa K, et al. A case of triple extramammary Paget’s disease. J Dermatol. 1997;24:535-538.
10. Ishihara K. Statistical study of extramammary Paget’s disease in Japan [in Japanese]. Skin Cancer. 1994;9:38.
11. Toker C. Clear cells of the nipple epidermis. Cancer. 1970;25:601-610.
12. Marucci G, Betts CM, Golouh R, et al. Toker cells are probably precursors of Paget cell carcinoma: a morphological and ultrastructural description. Virchows Archiv. 2002;441:117-123.
13. Belousova IE, Kazakov DV, Michal M, et al. Vulvar toker cells: the long-awaited missing link: a proposal for an origin-based histogenetic classification of extramammary Paget disease. Am J Dermatopathol. 2006;28:84-86.
14. Val-Bernal JF. Diego C, Rodriguez-Villar D, et al. The nipple-areola complex epidermis: a prospective systemic study in adult autopsies. Am J Dermatopathology. 2010;32:787-93.
15. Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
16. Van der Putte SC, Toonstra J, Hennipman A. Mammary Paget’s disease confined to the areola and associated with multifocal Toker cell hyperplasia. Am J Dermatopathol. 1995;17:487-493.
17. Kuo TT, Chan HL, Hsueh S. Clear cell papulosis of the skin. a new entity with histogenetic implications for cutaneous Paget’s disease. Am J Surg Pathol. 1987;11:827-834.
18. Kuo TT, Huang CL, Chan HL, et al. Clear cell papulosis: report of three cases of a newly recognized disease. J Am Acad Dermatol. 1995;33(2, pt 1):230-233.
19. Piérard-Franchimont C, Dosal FL, Estrada JA, et al. Cutaneous hamartoma with pagetoid cells. Am J Dermatopathol. 1991;13:158-161.
20. Belcher RW. Extramammary Paget’s disease. enzyme histochemical and electron microscopic study. Arch Pathol. 1972;94:59-64.
21. Ordóñez NG, Awalt H, Mackay B. Mammary and extramammary Paget’s disease. an immunocytochemical and ultrastructural study. Cancer. 1987;59:1173-1183.
22. Perrotto J, Abbott JJ, Ceilley RI, et al. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am J Dermatopathol. 2010;32:137-143.
23. Jones RE Jr, Austin C, Ackerman AB. Extramammary Paget’s disease. a critical reexamination. Am J Dermatopathol. 1979;1:101-132.
Practice Points
- Toker cells are epithelial clear cells found in apocrine gland–bearing areas of the skin.
- Toker cell hyperplasia may be a precursor to primary extramammary Paget disease (EMPD).
- In patients with established EMPD, it is important to examine other parts of the skin with apocrine glands for multicentric disease.
- Primary multicentric EMPD has a favorable outcome.