User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Cutaneous Myoepithelial Carcinoma With Disseminated Metastases
Cutaneous myoepithelial tumors are rare neoplasms but are being increasingly recognized and reported in the literature.1-7 Myoepithelial tumors are related to benign mixed tumors of the skin but lack the epithelial ductules that are present in mixed tumors. Cutaneous myoepithelial tumors may show a variety of architectural, cytological, and stromal features. Their immunophenotype usually is characterized by coexpression of an epithelial marker (eg, keratin, epithelial membrane antigen [EMA]) and S-100 protein; they also may express a variety of other myoepithelial markers, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin.7 EWS RNA binding protein 1 (EWSR1) and pleomorphic adenoma gene 1 (PLAG1) gene rearrangement has been detected in subsets of these tumors on in situ hybridization.8-10
Malignant myoepithelial tumors of the skin, also referred to as cutaneous myoepithelial carcinomas, are exceedingly rare. Including the current case, a search of PubMed articles indexed for MEDLINE and Google Scholar using the terms myoepithelial carcinoma and cutaneous revealed 12 cases that have been reported in the literature (Table).1-7,11-13 These tumors often occur in the head and neck areas and the lower extremities and display a bimodal age distribution, generally occurring in patients younger than 21 years and older than 50 years of age; they also show a slight female predominance. Available follow-up data from the literature have shown local recurrence or metastasis in 3 cases3,4,6; however, in one case the metastatic focus was not histologically identified.4 Cutaneous myoepithelial carcinoma presenting with metastatic disease further limits treatment options. Here, we describe a case of metastatic cutaneous myoepithelial carcinoma in a 47-year-old man, a rare example of cutaneous myoepithelial carcinoma with histologically documented metastatic disease at the initial presentation.
Case Report
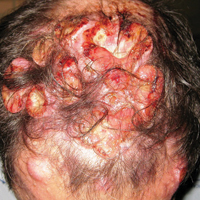
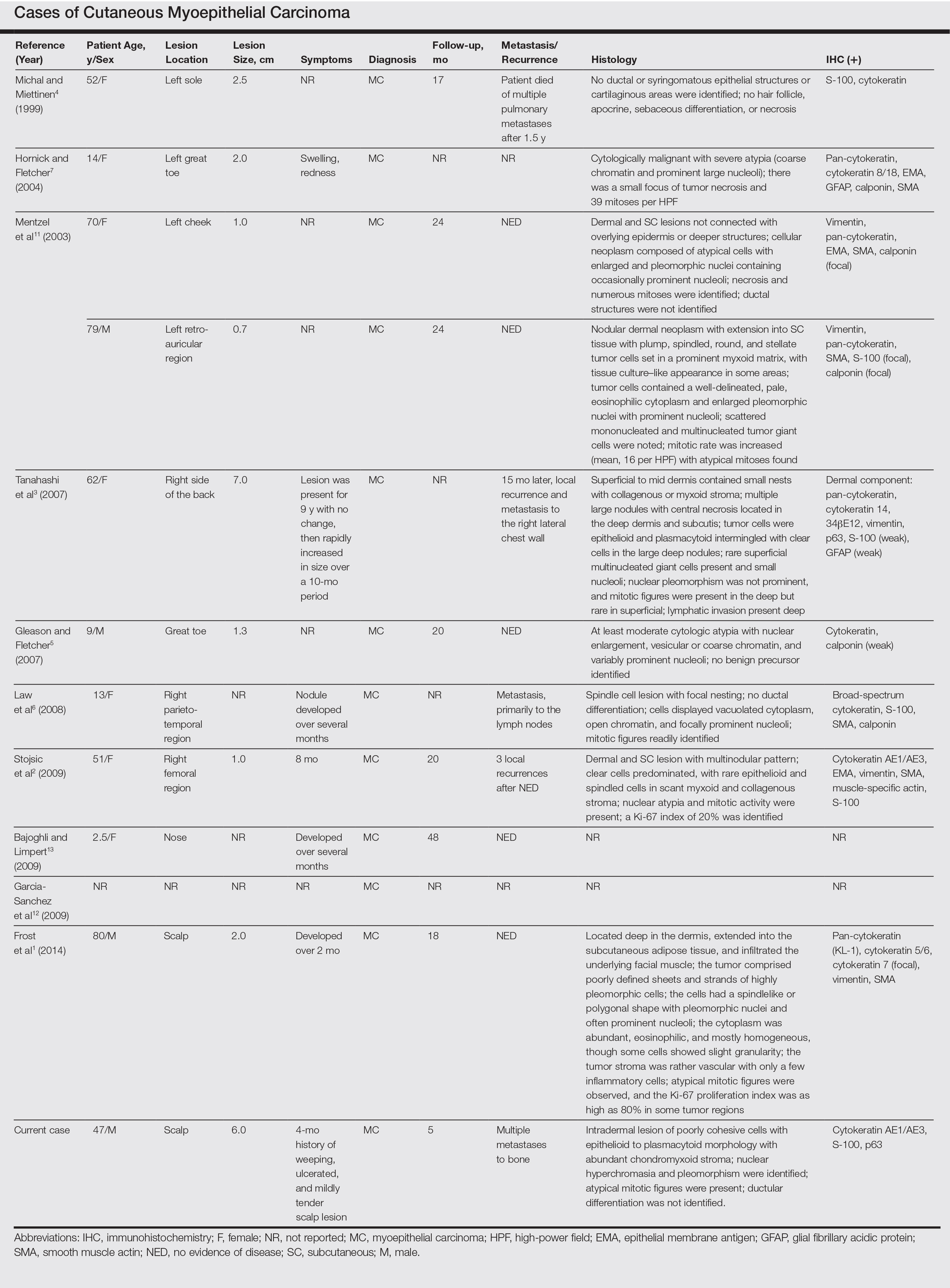
A 47-year-old man who underwent a renal transplant 19 years prior presented with a weeping, ulcerated, mildly tender lesion on the scalp of 4 months’ duration with neck and back pain of 3 months’ duration. Physical examination demonstrated a 6-cm area of ulceration on the anterior crown of the scalp with adjacent enlarged keratoacanthomalike craters and satellite nodules (Figure 1). He was previously diagnosed with basal cell carcinoma (BCC) of the scalp at an outside institution 4 years prior and was treated with radiation therapy. The prior scalp biopsy for BCC diagnosis was unavailable for review. The patient had a history of chronic eczematous dermatitis in the waistband area that had been present for 19 years and another BCC with nodular and infiltrative patterns on the left helix. Of note, he also had been taking long-term immunosuppressant medications (ie, cyclosporine, azathioprine) for maintenance following the renal transplant.
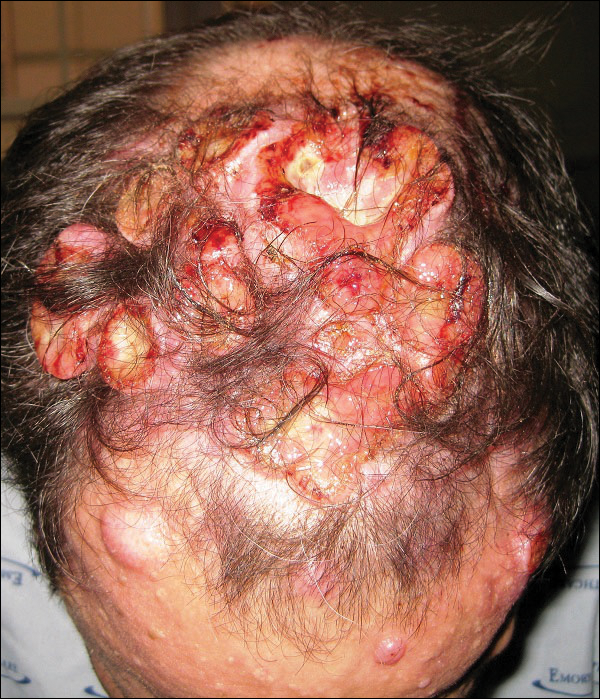
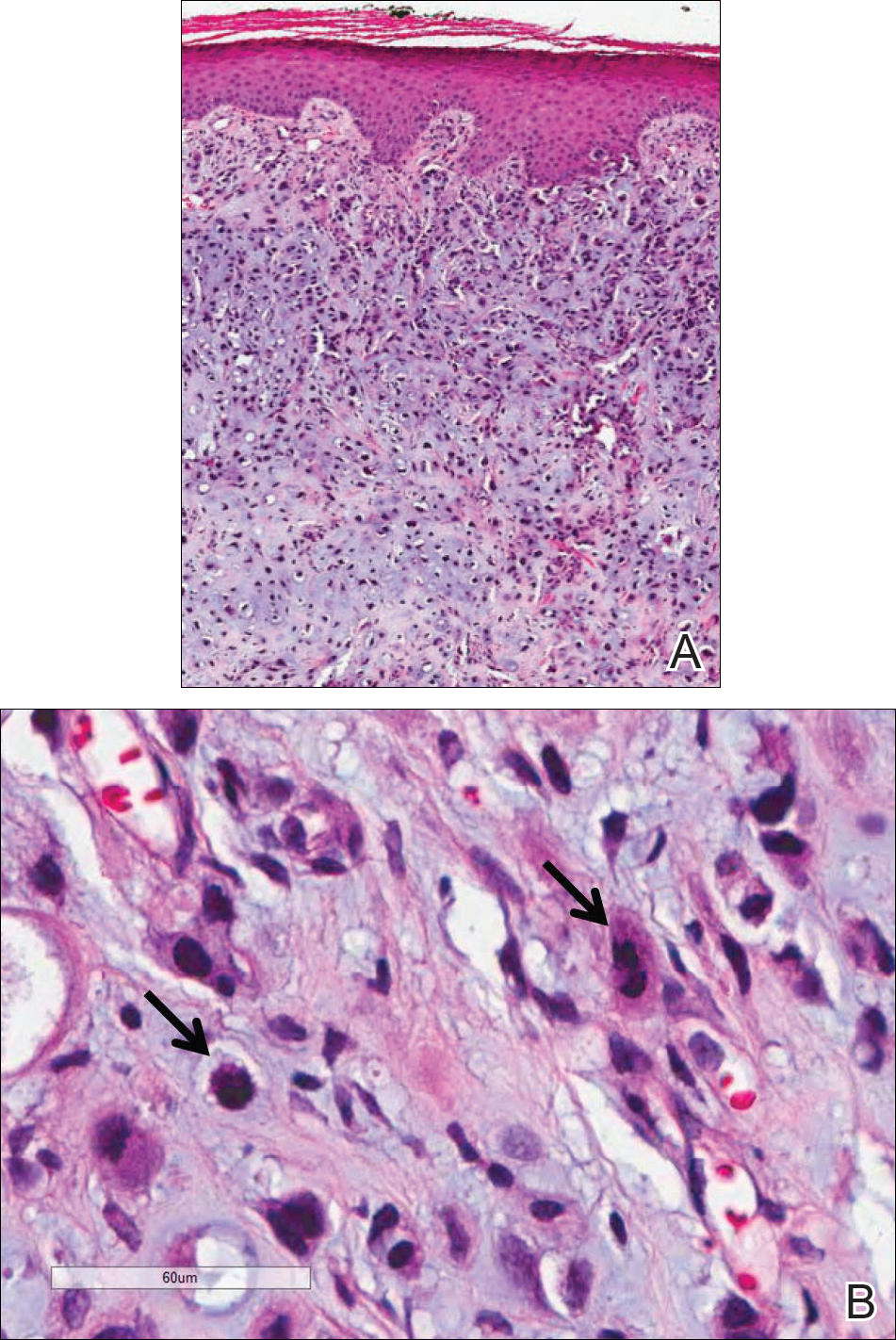
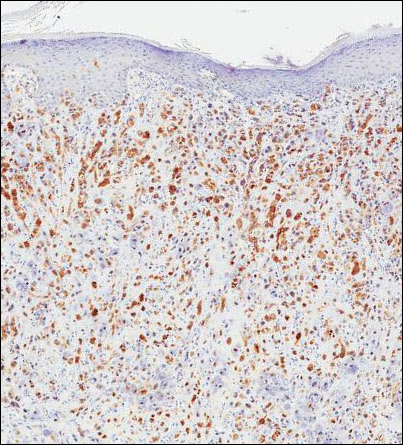
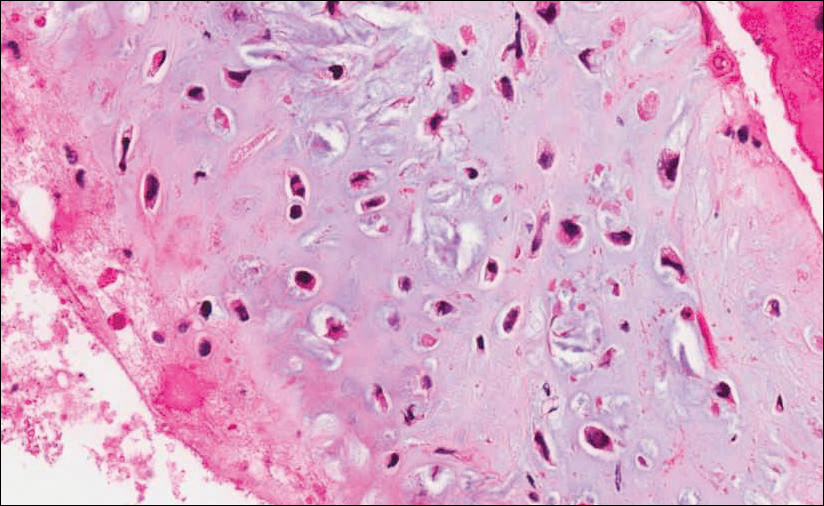
Because of the extensive ulceration of the primary lesion, a shave biopsy of the scalp was performed on an adjacent satellite nodule. Histopathologic findings showed an intradermal neoplasm characterized by poorly cohesive cells exhibiting epithelioid to plasmacytoid morphologic features surrounded by abundant chondromyxoid stroma. Ductular differentiation was not identified (Figure 2A). The neoplastic cells displayed hyperchromatic nuclei with marked nuclear pleomorphism and atypical mitotic figures (Figure 2B). On immunohistochemistry the tumor cells stained positive for cytokeratin AE1/AE3 (Figure 3), S-100 protein (Figure 4), and p63, and were negative for calponin, desmin, melan-A, cytokeratin 7, and brachyury (Figure 5).
Radiographic imaging was performed due to the patient’s history of neck and back pain. Magnetic resonance imaging showed innumerable slightly expansile, T1-hypointense, T2-hyperintense, and robustly enhancing lesions involving the cervical, thoracic, lumbar, and sacral spine, as well as the thoracic ribs and bilateral iliac bones. There was no evidence of soft tissue tumor around the bone lesions. Ventral cervical spinal cord compression was detected at the C4 vertebra, causing a symptomatic radiculopathy; however, due to widely metastatic disease, the patient was not considered appropriate for neurosurgical intervention of the compression. Computerized tomography of the chest, abdomen, and pelvis did not identify any visceral source of malignancy, though multiple bilaterally enlarged cervical lymph nodes were identified on magnetic resonance imaging.

Fine needle aspiration of a left iliac bone lesion demonstrated neoplastic cells and chondromyxoid stroma essentially identical to the features shown in the skin biopsy (Figure 6). Given the morphologic features of the tumor and coexpression of cytokeratin and S-100 protein, the findings were interpreted as primary cutaneous myoepithelial carcinoma with disseminated metastatic lesions. The patient began treatment with carboplatin and paclitaxel chemotherapy. To combat the symptomatic bone pain and upper extremity radiculopathy, palliative radiation was administered to the cervical spine, lumbar spine, and right sacrum (30 Gy to each site in 10 fractions at 3 Gy per fraction). Despite the attempted chemotherapy and radiation, the patient continued to decline, and after 2 months, he elected to pursue palliative care. The patient died after 3 months in palliative care (5 months after the initial presentation).
Comment
Myoepithelial cells normally surround ducts in secretory organs, such as the breasts, salivary glands, and cutaneous sweat glands. Myoepithelial neoplasms are well recognized in the salivary glands14,15; however, myoepithelial neoplasms also can arise in other sites, including the soft tissue4,5,16-18 and skin.1-3,7,11,19,20 Myoepithelioma of soft tissue was first described by Burke et al21 in 1995 and later described in the skin by Fernandez-Figueras et al22 in 1998. Since then, diagnostic criteria for cutaneous myoepithelial neoplasms have evolved, suggesting a spectrum of disease rather than a single distinct entity.11 Most often, cutaneous myoepithelial carcinomas arise as soft nodular lesions in the head and neck areas or extremities of adults. The nodules typically are nontender and range in size from 0.5 to 18.0 cm. Our review of the literature revealed 11 additional cases of cutaneous myoepithelial carcinomas have been reported, ranging in size from 0.7 to 7.0 cm (Table). In our case, the main lesion was 6 cm, mildly tender, ulcerated, and accompanied by satellite nodules.
Histologically, cutaneous myoepithelial tumors typically are well-defined, dermal-based nodules with no connection to the overlying epidermis. Similar to myoepithelial tumors of other sites, they can be diagnostically challenging due to the heterogeneity of both their architectural and cytological features. The presence of a chondromyxoid or hyalinized stroma is common but not always present. Neoplastic myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show growth patterns in clusters, cords, glands, or sheets. Focal epithelial cells can be present. Although benign myoepithelial neoplasms with overt ductal differentiation are consistent with cutaneous mixed tumors (chondroid syringomas), those without ducts are characterized as myoepitheliomas. It is uncertain if cases with only focal ductal differentiation should be classified as mixed tumors or as myoepitheliomas. Malignant myoepithelial tumors show infiltrative borders, nuclear pleomorphism, coarse nuclear chromatin, prominent nucleoli, and increased mitotic activity. A 2003 study by Hornick and Fletcher16 found that cytologic atypia was the primary predictor of malignant behavior for myoepithelial neoplasms of the soft tissue.
Despite a wide variety of expression patterns, immunohistochemistry is critical in demonstrating myoepithelial differentiation and establishing a diagnosis of a myoepithelial neoplasm. Most cases display coexpression of epithelial markers, including keratins and/or EMA as well as S-100 protein. Myogenic markers also may be variably expressed; however, the absence of myogenic markers does not exclude the diagnosis of a myoepithelial tumor. Commonly expressed epithelial markers are cytokeratin AE1/AE3, cytokeratin 8/18, and EMA, while commonly expressed myogenic markers include muscle specific actin and smooth muscle actin.5,7,11,19 Myoepithelial tumors also may express calponin, p63, and glial fibrillary acidic protein.16
Molecular studies also can aid in the diagnosis of myoepithelial tumors. A study by Antonescu et al8 demonstrated EWSR1 gene rearrangement in 45% (30/66) of extrasalivary myoepithelial tumors and the absence of EWSR1 gene rearrangement in salivary gland myoepithelial tumors. The authors also showed that EWSR1-negative tumors were more likely to be superficially located, display ductal differentiation, and possess a benign clinical course.8 In another study, Bahrami et al23 suggested that a subset of mixed tumors, specifically those with tubuloductal differentiation, are genetically linked to salivary gland pleomorphic adenomas, which was achieved by the coexpression of the PLAG1 protein and PLAG1 gene rearrangement on immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Of the 19 cases evaluated, 11 (58%) expressed nuclear staining for PLAG1 immunohistochemistry; 8 of those 11 showed positive gene rearrangement for PLAG1 using FISH. These findings raise the possibility that cutaneous mixed tumors may be more closely related to those of the salivary glands, while deep myoepithelial tumors that lack ductal differentiation may represent a distinct group. Similar to the study by Antonescu et al,8 Flucke et al10 investigated EWSR1 gene rearrangement but limited their sample to cutaneous tumors, including myoepitheliomas, mixed tumors, and myoepithelial carcinoma. The authors found that 44% of cases (7/16) expressed EWSR1; this expression suggests that cutaneous myoepithelial tumors may have a genetic relationship to their soft tissue, bone, and visceral counterparts.10
Myoepithelial tumors display a broad spectrum of morphologic features; however, one of the most common growth patterns is that of oval to round cells forming cords and chains in a chondromyxoid stroma. As such, the histopathologic differential diagnosis for myoepithelial tumors includes other epithelioid or round-cell neoplasms with similar growth patterns including extraskeletal myxoid chondrosarcoma (EMC), ossifying fibromyxoid tumor of soft parts, and extra-axial soft tissue chordoma. Extraskeletal myxoid chondrosarcoma bears the closest similarity to myoepithelial tumors both histologically and by ancillary studies. It typically possesses cords or chains of small round tumor cells set in a chondromyxoid or myxoid background. In contrast to myoepithelial tumors, which typically have more abundant cytoplasm and can show at least focal areas of spindle cell growth, the cells of EMC are more uniform, small, round cells with relatively scant cytoplasm. Extraskeletal myxoid chondrosarcomas lack the typical myoepithelial coexpression of cytokeratin and S-100 protein, with a minority of EMCs expressing S-100 protein but rarely cytokeratin. Most cases of EMC possess a balanced t(9;22) translocation involving the EWSR1 gene,24 a finding that could lead to confusion with soft tissue myoepithelial tumors, which also may show EWSR1 rearrangement on FISH. Ossifying fibromyxoid tumor of soft parts is also composed of round cells arranged in cords in a myxoid or fibrous stroma; the majority of cases also display a peripheral rim of mature bone, a feature that is not typically seen in myoepithelial tumors. Similar to myoepithelial tumors, ossifying fibromyxoid tumor of soft parts often is positive for S-100 protein; however, it rarely is positive for cytokeratins. Ossifying fibromyxoid tumor of soft parts has been shown to have a rearrangement of the PHD finger protein 1 (PHF1) gene in approximately half of cases, a molecular finding that has not been reported for myoepithelial tumors.25 Finally, extra-axial soft tissue chordomas, though quite rare, may possess striking similarities to myoepithelial tumors both histopathologically and immunohistochemically. Chordomas are composed of epithelioid cells arranged in nests, nodules, and chains with a variably myxoid background. A variable amount of cells with bubbly cytoplasm (known as physaliphorous cells) can be seen. High mitotic activity is not a characteristic feature in chordomas. They classically coexpress cytokeratins and S-100 protein, similar to myoepithelial tumors.
Because cutaneous myoepithelial tumors are relatively rare, a well-defined standard of care for treatment is lacking. Surgical excision is the primary treatment method in most reported cases in the literature.17,19 Miller et al29 reported the successful treatment of recurrent cutaneous myoepitheliomas with Mohs micrographic surgery. Chemotherapy may be useful in the setting of metastatic myoepithelial carcinomas in adults, but reported results are inconsistent.30,31 Radiation treatment of recurrent or metastatic disease has not been shown to be effective. A study of children treated with surgical resection and chemotherapy using ifosfamide, cisplatin, and etoposide followed by radiation therapy showed positive results.32
Our case highlights several challenges that may arise in establishing a diagnosis of cutaneous myoepithelial carcinoma with disseminated metastases. The diagnostic difficulty in our case was compounded by the advanced nature of the lesion at the time of presentation. Given the rarity of metastatic cutaneous myoepithelial carcinomas and the lack of a prior primary diagnosis of a malignant myoepithelioma, the index of suspicion for this entity was not high. A report of myoepithelial carcinoma of the parotid gland metastatic to the skin has been reported,33 but in the absence of salivary gland involvement or other visceral lesions, metastasis from any source other than our patient’s cutaneous scalp lesion is unlikely. The histopathologic features in combination with the characteristic immunophenotype, unique clinical setting, and radiographic findings were essential to arriving at the correct diagnosis. Unlike previously reported metastatic lesions, our case is unique in that metastatic lesions were identified at the time of initial clinical presentation.
Conclusion
Cutaneous myoepithelial carcinomas are exceedingly rare tumors with a wide range of histopathologic and immunohistochemical findings. In challenging cases, studies for EWSR1 or PLAG1 gene rearrangement can be helpful. Furthermore, this case illustrates the potential for widespread dissemination of myoepithelial carcinomas requiring clinical evaluation and imaging studies to exclude metastatic lesions.
- Frost MW, Steiniche T, Damsgaard TE, et al. Primary cutaneous myoepithelial carcinoma: a case report and review of the literature. APMIS. 2014;122:369-379.
- Stojsic Z, Brasanac D, Boricic I, et al. Clear cell myoepithelial carcinoma of the skin. a case report. J Cutan Pathol. 2009;36:680-683.
- Tanahashi J, Kashima K, Daa T, et al. A case of cutaneous myoepithelial carcinoma. J Cutan Pathol. 2007;34:648-653.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. report of 12 cases. Virchows Arch. 1999;434:393-400.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Law RM, Viglione MP, Barrett TL. Metastatic myoepithelial carcinoma in a child. J Cutan Pathol. 2008;35:779-781.
- Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14-24.
- Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-1124.
- Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52:675-682.
- Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444-1450.
- Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294-302.
- Garcia-Sanchez S, Elices M, Nieto S. Cutaneous myoepithelial carcinoma (malignant myoepithelial tumor of skin). Virchows Archiv. 2009;455(suppl 1):1-482.
- Bajoghli A, Limpert J. Treatment of cutaneous malignant myoepithelioma on the nasal ala using Mohs micrographic surgery in a two and a half year old child. J Invest Dermatol. 2009;129:S44.
- Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-806.
- Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands. a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196.
- Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22.
- Neto AG, Pineda-Daboin K, Luna MA. Myoepithelioma of the soft tissue of the head and neck: a case report and review of the literature. Head Neck. 2004;26:470-473.
- Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348-355.
- Dix BT, Hentges MJ, Saltrick KR, et al. Cutaneous myoepithelioma in the foot: case report. Foot Ankle Spec. 2013;6:239-241.
- Burke T, Sahin A, Johnson DE, et al. Myoepithelioma of the retroperitoneum. Ultrastruct Pathol. 1995;19:269-274.
- Fernandez-Figueras MT, Puig L, Trias I, et al. Benign myoepithelioma of the skin. Am J Dermatopathol. 1998;20:208-212.
- Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer. 2012;51:140-148.
- Panagopoulos I, Mertens F, Isaksson M, et al. Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2002;35:340-352.
- Graham RP, Weiss SW, Sukov WR, et al. PHF1 rearrangements in ossifying fibromyxoid tumors of soft parts: a fluorescence in situ hybridization study of 41 cases with emphasis on the malignant variant. Am J Surg Pathol. 2013;37:1751-1755.
- Dabska M. Parachordoma: a new clinicopathologic entity. Cancer. 1977;40:1586-1592.
- Fletcher CDM, Mertens F, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Lauer SR, Edgar MA, Gardner JM, et al. Soft tissue chordomas: a clinicopathologic analysis of 11 cases. Am J Surg Pathol. 2013;37:719-726.
- Miller TD, McCalmont T, Tope WD. Recurrent cutaneous myoepithelioma treated using Mohs micrographic surgery: case report and review of the literature. Dermatol Surg. 2009;35:139-143.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Noronha V, Cooper DL, Higgins SA, et al. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7:270-271.
- Bisogno G, Tagarelli A, Schiavetti A, et al. Myoepithelial carcinoma treatment in children: a report from the TREP project. Pediatr Blood Cancer. 2014;61:643-646.
- He DQ, Hua CG, Tang XF, et al. Cutaneous metastasis from a parotid myoepithelial carcinoma: a case report and review of the literature. J Cutan Pathol. 2008;35:1138-1143.
Cutaneous myoepithelial tumors are rare neoplasms but are being increasingly recognized and reported in the literature.1-7 Myoepithelial tumors are related to benign mixed tumors of the skin but lack the epithelial ductules that are present in mixed tumors. Cutaneous myoepithelial tumors may show a variety of architectural, cytological, and stromal features. Their immunophenotype usually is characterized by coexpression of an epithelial marker (eg, keratin, epithelial membrane antigen [EMA]) and S-100 protein; they also may express a variety of other myoepithelial markers, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin.7 EWS RNA binding protein 1 (EWSR1) and pleomorphic adenoma gene 1 (PLAG1) gene rearrangement has been detected in subsets of these tumors on in situ hybridization.8-10
Malignant myoepithelial tumors of the skin, also referred to as cutaneous myoepithelial carcinomas, are exceedingly rare. Including the current case, a search of PubMed articles indexed for MEDLINE and Google Scholar using the terms myoepithelial carcinoma and cutaneous revealed 12 cases that have been reported in the literature (Table).1-7,11-13 These tumors often occur in the head and neck areas and the lower extremities and display a bimodal age distribution, generally occurring in patients younger than 21 years and older than 50 years of age; they also show a slight female predominance. Available follow-up data from the literature have shown local recurrence or metastasis in 3 cases3,4,6; however, in one case the metastatic focus was not histologically identified.4 Cutaneous myoepithelial carcinoma presenting with metastatic disease further limits treatment options. Here, we describe a case of metastatic cutaneous myoepithelial carcinoma in a 47-year-old man, a rare example of cutaneous myoepithelial carcinoma with histologically documented metastatic disease at the initial presentation.

Case Report
A 47-year-old man who underwent a renal transplant 19 years prior presented with a weeping, ulcerated, mildly tender lesion on the scalp of 4 months’ duration with neck and back pain of 3 months’ duration. Physical examination demonstrated a 6-cm area of ulceration on the anterior crown of the scalp with adjacent enlarged keratoacanthomalike craters and satellite nodules (Figure 1). He was previously diagnosed with basal cell carcinoma (BCC) of the scalp at an outside institution 4 years prior and was treated with radiation therapy. The prior scalp biopsy for BCC diagnosis was unavailable for review. The patient had a history of chronic eczematous dermatitis in the waistband area that had been present for 19 years and another BCC with nodular and infiltrative patterns on the left helix. Of note, he also had been taking long-term immunosuppressant medications (ie, cyclosporine, azathioprine) for maintenance following the renal transplant.

Because of the extensive ulceration of the primary lesion, a shave biopsy of the scalp was performed on an adjacent satellite nodule. Histopathologic findings showed an intradermal neoplasm characterized by poorly cohesive cells exhibiting epithelioid to plasmacytoid morphologic features surrounded by abundant chondromyxoid stroma. Ductular differentiation was not identified (Figure 2A). The neoplastic cells displayed hyperchromatic nuclei with marked nuclear pleomorphism and atypical mitotic figures (Figure 2B). On immunohistochemistry the tumor cells stained positive for cytokeratin AE1/AE3 (Figure 3), S-100 protein (Figure 4), and p63, and were negative for calponin, desmin, melan-A, cytokeratin 7, and brachyury (Figure 5).
Radiographic imaging was performed due to the patient’s history of neck and back pain. Magnetic resonance imaging showed innumerable slightly expansile, T1-hypointense, T2-hyperintense, and robustly enhancing lesions involving the cervical, thoracic, lumbar, and sacral spine, as well as the thoracic ribs and bilateral iliac bones. There was no evidence of soft tissue tumor around the bone lesions. Ventral cervical spinal cord compression was detected at the C4 vertebra, causing a symptomatic radiculopathy; however, due to widely metastatic disease, the patient was not considered appropriate for neurosurgical intervention of the compression. Computerized tomography of the chest, abdomen, and pelvis did not identify any visceral source of malignancy, though multiple bilaterally enlarged cervical lymph nodes were identified on magnetic resonance imaging.




Fine needle aspiration of a left iliac bone lesion demonstrated neoplastic cells and chondromyxoid stroma essentially identical to the features shown in the skin biopsy (Figure 6). Given the morphologic features of the tumor and coexpression of cytokeratin and S-100 protein, the findings were interpreted as primary cutaneous myoepithelial carcinoma with disseminated metastatic lesions. The patient began treatment with carboplatin and paclitaxel chemotherapy. To combat the symptomatic bone pain and upper extremity radiculopathy, palliative radiation was administered to the cervical spine, lumbar spine, and right sacrum (30 Gy to each site in 10 fractions at 3 Gy per fraction). Despite the attempted chemotherapy and radiation, the patient continued to decline, and after 2 months, he elected to pursue palliative care. The patient died after 3 months in palliative care (5 months after the initial presentation).

Comment
Myoepithelial cells normally surround ducts in secretory organs, such as the breasts, salivary glands, and cutaneous sweat glands. Myoepithelial neoplasms are well recognized in the salivary glands14,15; however, myoepithelial neoplasms also can arise in other sites, including the soft tissue4,5,16-18 and skin.1-3,7,11,19,20 Myoepithelioma of soft tissue was first described by Burke et al21 in 1995 and later described in the skin by Fernandez-Figueras et al22 in 1998. Since then, diagnostic criteria for cutaneous myoepithelial neoplasms have evolved, suggesting a spectrum of disease rather than a single distinct entity.11 Most often, cutaneous myoepithelial carcinomas arise as soft nodular lesions in the head and neck areas or extremities of adults. The nodules typically are nontender and range in size from 0.5 to 18.0 cm. Our review of the literature revealed 11 additional cases of cutaneous myoepithelial carcinomas have been reported, ranging in size from 0.7 to 7.0 cm (Table). In our case, the main lesion was 6 cm, mildly tender, ulcerated, and accompanied by satellite nodules.
Histologically, cutaneous myoepithelial tumors typically are well-defined, dermal-based nodules with no connection to the overlying epidermis. Similar to myoepithelial tumors of other sites, they can be diagnostically challenging due to the heterogeneity of both their architectural and cytological features. The presence of a chondromyxoid or hyalinized stroma is common but not always present. Neoplastic myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show growth patterns in clusters, cords, glands, or sheets. Focal epithelial cells can be present. Although benign myoepithelial neoplasms with overt ductal differentiation are consistent with cutaneous mixed tumors (chondroid syringomas), those without ducts are characterized as myoepitheliomas. It is uncertain if cases with only focal ductal differentiation should be classified as mixed tumors or as myoepitheliomas. Malignant myoepithelial tumors show infiltrative borders, nuclear pleomorphism, coarse nuclear chromatin, prominent nucleoli, and increased mitotic activity. A 2003 study by Hornick and Fletcher16 found that cytologic atypia was the primary predictor of malignant behavior for myoepithelial neoplasms of the soft tissue.
Despite a wide variety of expression patterns, immunohistochemistry is critical in demonstrating myoepithelial differentiation and establishing a diagnosis of a myoepithelial neoplasm. Most cases display coexpression of epithelial markers, including keratins and/or EMA as well as S-100 protein. Myogenic markers also may be variably expressed; however, the absence of myogenic markers does not exclude the diagnosis of a myoepithelial tumor. Commonly expressed epithelial markers are cytokeratin AE1/AE3, cytokeratin 8/18, and EMA, while commonly expressed myogenic markers include muscle specific actin and smooth muscle actin.5,7,11,19 Myoepithelial tumors also may express calponin, p63, and glial fibrillary acidic protein.16
Molecular studies also can aid in the diagnosis of myoepithelial tumors. A study by Antonescu et al8 demonstrated EWSR1 gene rearrangement in 45% (30/66) of extrasalivary myoepithelial tumors and the absence of EWSR1 gene rearrangement in salivary gland myoepithelial tumors. The authors also showed that EWSR1-negative tumors were more likely to be superficially located, display ductal differentiation, and possess a benign clinical course.8 In another study, Bahrami et al23 suggested that a subset of mixed tumors, specifically those with tubuloductal differentiation, are genetically linked to salivary gland pleomorphic adenomas, which was achieved by the coexpression of the PLAG1 protein and PLAG1 gene rearrangement on immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Of the 19 cases evaluated, 11 (58%) expressed nuclear staining for PLAG1 immunohistochemistry; 8 of those 11 showed positive gene rearrangement for PLAG1 using FISH. These findings raise the possibility that cutaneous mixed tumors may be more closely related to those of the salivary glands, while deep myoepithelial tumors that lack ductal differentiation may represent a distinct group. Similar to the study by Antonescu et al,8 Flucke et al10 investigated EWSR1 gene rearrangement but limited their sample to cutaneous tumors, including myoepitheliomas, mixed tumors, and myoepithelial carcinoma. The authors found that 44% of cases (7/16) expressed EWSR1; this expression suggests that cutaneous myoepithelial tumors may have a genetic relationship to their soft tissue, bone, and visceral counterparts.10
Myoepithelial tumors display a broad spectrum of morphologic features; however, one of the most common growth patterns is that of oval to round cells forming cords and chains in a chondromyxoid stroma. As such, the histopathologic differential diagnosis for myoepithelial tumors includes other epithelioid or round-cell neoplasms with similar growth patterns including extraskeletal myxoid chondrosarcoma (EMC), ossifying fibromyxoid tumor of soft parts, and extra-axial soft tissue chordoma. Extraskeletal myxoid chondrosarcoma bears the closest similarity to myoepithelial tumors both histologically and by ancillary studies. It typically possesses cords or chains of small round tumor cells set in a chondromyxoid or myxoid background. In contrast to myoepithelial tumors, which typically have more abundant cytoplasm and can show at least focal areas of spindle cell growth, the cells of EMC are more uniform, small, round cells with relatively scant cytoplasm. Extraskeletal myxoid chondrosarcomas lack the typical myoepithelial coexpression of cytokeratin and S-100 protein, with a minority of EMCs expressing S-100 protein but rarely cytokeratin. Most cases of EMC possess a balanced t(9;22) translocation involving the EWSR1 gene,24 a finding that could lead to confusion with soft tissue myoepithelial tumors, which also may show EWSR1 rearrangement on FISH. Ossifying fibromyxoid tumor of soft parts is also composed of round cells arranged in cords in a myxoid or fibrous stroma; the majority of cases also display a peripheral rim of mature bone, a feature that is not typically seen in myoepithelial tumors. Similar to myoepithelial tumors, ossifying fibromyxoid tumor of soft parts often is positive for S-100 protein; however, it rarely is positive for cytokeratins. Ossifying fibromyxoid tumor of soft parts has been shown to have a rearrangement of the PHD finger protein 1 (PHF1) gene in approximately half of cases, a molecular finding that has not been reported for myoepithelial tumors.25 Finally, extra-axial soft tissue chordomas, though quite rare, may possess striking similarities to myoepithelial tumors both histopathologically and immunohistochemically. Chordomas are composed of epithelioid cells arranged in nests, nodules, and chains with a variably myxoid background. A variable amount of cells with bubbly cytoplasm (known as physaliphorous cells) can be seen. High mitotic activity is not a characteristic feature in chordomas. They classically coexpress cytokeratins and S-100 protein, similar to myoepithelial tumors.
Because cutaneous myoepithelial tumors are relatively rare, a well-defined standard of care for treatment is lacking. Surgical excision is the primary treatment method in most reported cases in the literature.17,19 Miller et al29 reported the successful treatment of recurrent cutaneous myoepitheliomas with Mohs micrographic surgery. Chemotherapy may be useful in the setting of metastatic myoepithelial carcinomas in adults, but reported results are inconsistent.30,31 Radiation treatment of recurrent or metastatic disease has not been shown to be effective. A study of children treated with surgical resection and chemotherapy using ifosfamide, cisplatin, and etoposide followed by radiation therapy showed positive results.32
Our case highlights several challenges that may arise in establishing a diagnosis of cutaneous myoepithelial carcinoma with disseminated metastases. The diagnostic difficulty in our case was compounded by the advanced nature of the lesion at the time of presentation. Given the rarity of metastatic cutaneous myoepithelial carcinomas and the lack of a prior primary diagnosis of a malignant myoepithelioma, the index of suspicion for this entity was not high. A report of myoepithelial carcinoma of the parotid gland metastatic to the skin has been reported,33 but in the absence of salivary gland involvement or other visceral lesions, metastasis from any source other than our patient’s cutaneous scalp lesion is unlikely. The histopathologic features in combination with the characteristic immunophenotype, unique clinical setting, and radiographic findings were essential to arriving at the correct diagnosis. Unlike previously reported metastatic lesions, our case is unique in that metastatic lesions were identified at the time of initial clinical presentation.
Conclusion
Cutaneous myoepithelial carcinomas are exceedingly rare tumors with a wide range of histopathologic and immunohistochemical findings. In challenging cases, studies for EWSR1 or PLAG1 gene rearrangement can be helpful. Furthermore, this case illustrates the potential for widespread dissemination of myoepithelial carcinomas requiring clinical evaluation and imaging studies to exclude metastatic lesions.
Cutaneous myoepithelial tumors are rare neoplasms but are being increasingly recognized and reported in the literature.1-7 Myoepithelial tumors are related to benign mixed tumors of the skin but lack the epithelial ductules that are present in mixed tumors. Cutaneous myoepithelial tumors may show a variety of architectural, cytological, and stromal features. Their immunophenotype usually is characterized by coexpression of an epithelial marker (eg, keratin, epithelial membrane antigen [EMA]) and S-100 protein; they also may express a variety of other myoepithelial markers, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin.7 EWS RNA binding protein 1 (EWSR1) and pleomorphic adenoma gene 1 (PLAG1) gene rearrangement has been detected in subsets of these tumors on in situ hybridization.8-10
Malignant myoepithelial tumors of the skin, also referred to as cutaneous myoepithelial carcinomas, are exceedingly rare. Including the current case, a search of PubMed articles indexed for MEDLINE and Google Scholar using the terms myoepithelial carcinoma and cutaneous revealed 12 cases that have been reported in the literature (Table).1-7,11-13 These tumors often occur in the head and neck areas and the lower extremities and display a bimodal age distribution, generally occurring in patients younger than 21 years and older than 50 years of age; they also show a slight female predominance. Available follow-up data from the literature have shown local recurrence or metastasis in 3 cases3,4,6; however, in one case the metastatic focus was not histologically identified.4 Cutaneous myoepithelial carcinoma presenting with metastatic disease further limits treatment options. Here, we describe a case of metastatic cutaneous myoepithelial carcinoma in a 47-year-old man, a rare example of cutaneous myoepithelial carcinoma with histologically documented metastatic disease at the initial presentation.

Case Report
A 47-year-old man who underwent a renal transplant 19 years prior presented with a weeping, ulcerated, mildly tender lesion on the scalp of 4 months’ duration with neck and back pain of 3 months’ duration. Physical examination demonstrated a 6-cm area of ulceration on the anterior crown of the scalp with adjacent enlarged keratoacanthomalike craters and satellite nodules (Figure 1). He was previously diagnosed with basal cell carcinoma (BCC) of the scalp at an outside institution 4 years prior and was treated with radiation therapy. The prior scalp biopsy for BCC diagnosis was unavailable for review. The patient had a history of chronic eczematous dermatitis in the waistband area that had been present for 19 years and another BCC with nodular and infiltrative patterns on the left helix. Of note, he also had been taking long-term immunosuppressant medications (ie, cyclosporine, azathioprine) for maintenance following the renal transplant.

Because of the extensive ulceration of the primary lesion, a shave biopsy of the scalp was performed on an adjacent satellite nodule. Histopathologic findings showed an intradermal neoplasm characterized by poorly cohesive cells exhibiting epithelioid to plasmacytoid morphologic features surrounded by abundant chondromyxoid stroma. Ductular differentiation was not identified (Figure 2A). The neoplastic cells displayed hyperchromatic nuclei with marked nuclear pleomorphism and atypical mitotic figures (Figure 2B). On immunohistochemistry the tumor cells stained positive for cytokeratin AE1/AE3 (Figure 3), S-100 protein (Figure 4), and p63, and were negative for calponin, desmin, melan-A, cytokeratin 7, and brachyury (Figure 5).
Radiographic imaging was performed due to the patient’s history of neck and back pain. Magnetic resonance imaging showed innumerable slightly expansile, T1-hypointense, T2-hyperintense, and robustly enhancing lesions involving the cervical, thoracic, lumbar, and sacral spine, as well as the thoracic ribs and bilateral iliac bones. There was no evidence of soft tissue tumor around the bone lesions. Ventral cervical spinal cord compression was detected at the C4 vertebra, causing a symptomatic radiculopathy; however, due to widely metastatic disease, the patient was not considered appropriate for neurosurgical intervention of the compression. Computerized tomography of the chest, abdomen, and pelvis did not identify any visceral source of malignancy, though multiple bilaterally enlarged cervical lymph nodes were identified on magnetic resonance imaging.




Fine needle aspiration of a left iliac bone lesion demonstrated neoplastic cells and chondromyxoid stroma essentially identical to the features shown in the skin biopsy (Figure 6). Given the morphologic features of the tumor and coexpression of cytokeratin and S-100 protein, the findings were interpreted as primary cutaneous myoepithelial carcinoma with disseminated metastatic lesions. The patient began treatment with carboplatin and paclitaxel chemotherapy. To combat the symptomatic bone pain and upper extremity radiculopathy, palliative radiation was administered to the cervical spine, lumbar spine, and right sacrum (30 Gy to each site in 10 fractions at 3 Gy per fraction). Despite the attempted chemotherapy and radiation, the patient continued to decline, and after 2 months, he elected to pursue palliative care. The patient died after 3 months in palliative care (5 months after the initial presentation).

Comment
Myoepithelial cells normally surround ducts in secretory organs, such as the breasts, salivary glands, and cutaneous sweat glands. Myoepithelial neoplasms are well recognized in the salivary glands14,15; however, myoepithelial neoplasms also can arise in other sites, including the soft tissue4,5,16-18 and skin.1-3,7,11,19,20 Myoepithelioma of soft tissue was first described by Burke et al21 in 1995 and later described in the skin by Fernandez-Figueras et al22 in 1998. Since then, diagnostic criteria for cutaneous myoepithelial neoplasms have evolved, suggesting a spectrum of disease rather than a single distinct entity.11 Most often, cutaneous myoepithelial carcinomas arise as soft nodular lesions in the head and neck areas or extremities of adults. The nodules typically are nontender and range in size from 0.5 to 18.0 cm. Our review of the literature revealed 11 additional cases of cutaneous myoepithelial carcinomas have been reported, ranging in size from 0.7 to 7.0 cm (Table). In our case, the main lesion was 6 cm, mildly tender, ulcerated, and accompanied by satellite nodules.
Histologically, cutaneous myoepithelial tumors typically are well-defined, dermal-based nodules with no connection to the overlying epidermis. Similar to myoepithelial tumors of other sites, they can be diagnostically challenging due to the heterogeneity of both their architectural and cytological features. The presence of a chondromyxoid or hyalinized stroma is common but not always present. Neoplastic myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show growth patterns in clusters, cords, glands, or sheets. Focal epithelial cells can be present. Although benign myoepithelial neoplasms with overt ductal differentiation are consistent with cutaneous mixed tumors (chondroid syringomas), those without ducts are characterized as myoepitheliomas. It is uncertain if cases with only focal ductal differentiation should be classified as mixed tumors or as myoepitheliomas. Malignant myoepithelial tumors show infiltrative borders, nuclear pleomorphism, coarse nuclear chromatin, prominent nucleoli, and increased mitotic activity. A 2003 study by Hornick and Fletcher16 found that cytologic atypia was the primary predictor of malignant behavior for myoepithelial neoplasms of the soft tissue.
Despite a wide variety of expression patterns, immunohistochemistry is critical in demonstrating myoepithelial differentiation and establishing a diagnosis of a myoepithelial neoplasm. Most cases display coexpression of epithelial markers, including keratins and/or EMA as well as S-100 protein. Myogenic markers also may be variably expressed; however, the absence of myogenic markers does not exclude the diagnosis of a myoepithelial tumor. Commonly expressed epithelial markers are cytokeratin AE1/AE3, cytokeratin 8/18, and EMA, while commonly expressed myogenic markers include muscle specific actin and smooth muscle actin.5,7,11,19 Myoepithelial tumors also may express calponin, p63, and glial fibrillary acidic protein.16
Molecular studies also can aid in the diagnosis of myoepithelial tumors. A study by Antonescu et al8 demonstrated EWSR1 gene rearrangement in 45% (30/66) of extrasalivary myoepithelial tumors and the absence of EWSR1 gene rearrangement in salivary gland myoepithelial tumors. The authors also showed that EWSR1-negative tumors were more likely to be superficially located, display ductal differentiation, and possess a benign clinical course.8 In another study, Bahrami et al23 suggested that a subset of mixed tumors, specifically those with tubuloductal differentiation, are genetically linked to salivary gland pleomorphic adenomas, which was achieved by the coexpression of the PLAG1 protein and PLAG1 gene rearrangement on immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Of the 19 cases evaluated, 11 (58%) expressed nuclear staining for PLAG1 immunohistochemistry; 8 of those 11 showed positive gene rearrangement for PLAG1 using FISH. These findings raise the possibility that cutaneous mixed tumors may be more closely related to those of the salivary glands, while deep myoepithelial tumors that lack ductal differentiation may represent a distinct group. Similar to the study by Antonescu et al,8 Flucke et al10 investigated EWSR1 gene rearrangement but limited their sample to cutaneous tumors, including myoepitheliomas, mixed tumors, and myoepithelial carcinoma. The authors found that 44% of cases (7/16) expressed EWSR1; this expression suggests that cutaneous myoepithelial tumors may have a genetic relationship to their soft tissue, bone, and visceral counterparts.10
Myoepithelial tumors display a broad spectrum of morphologic features; however, one of the most common growth patterns is that of oval to round cells forming cords and chains in a chondromyxoid stroma. As such, the histopathologic differential diagnosis for myoepithelial tumors includes other epithelioid or round-cell neoplasms with similar growth patterns including extraskeletal myxoid chondrosarcoma (EMC), ossifying fibromyxoid tumor of soft parts, and extra-axial soft tissue chordoma. Extraskeletal myxoid chondrosarcoma bears the closest similarity to myoepithelial tumors both histologically and by ancillary studies. It typically possesses cords or chains of small round tumor cells set in a chondromyxoid or myxoid background. In contrast to myoepithelial tumors, which typically have more abundant cytoplasm and can show at least focal areas of spindle cell growth, the cells of EMC are more uniform, small, round cells with relatively scant cytoplasm. Extraskeletal myxoid chondrosarcomas lack the typical myoepithelial coexpression of cytokeratin and S-100 protein, with a minority of EMCs expressing S-100 protein but rarely cytokeratin. Most cases of EMC possess a balanced t(9;22) translocation involving the EWSR1 gene,24 a finding that could lead to confusion with soft tissue myoepithelial tumors, which also may show EWSR1 rearrangement on FISH. Ossifying fibromyxoid tumor of soft parts is also composed of round cells arranged in cords in a myxoid or fibrous stroma; the majority of cases also display a peripheral rim of mature bone, a feature that is not typically seen in myoepithelial tumors. Similar to myoepithelial tumors, ossifying fibromyxoid tumor of soft parts often is positive for S-100 protein; however, it rarely is positive for cytokeratins. Ossifying fibromyxoid tumor of soft parts has been shown to have a rearrangement of the PHD finger protein 1 (PHF1) gene in approximately half of cases, a molecular finding that has not been reported for myoepithelial tumors.25 Finally, extra-axial soft tissue chordomas, though quite rare, may possess striking similarities to myoepithelial tumors both histopathologically and immunohistochemically. Chordomas are composed of epithelioid cells arranged in nests, nodules, and chains with a variably myxoid background. A variable amount of cells with bubbly cytoplasm (known as physaliphorous cells) can be seen. High mitotic activity is not a characteristic feature in chordomas. They classically coexpress cytokeratins and S-100 protein, similar to myoepithelial tumors.
Because cutaneous myoepithelial tumors are relatively rare, a well-defined standard of care for treatment is lacking. Surgical excision is the primary treatment method in most reported cases in the literature.17,19 Miller et al29 reported the successful treatment of recurrent cutaneous myoepitheliomas with Mohs micrographic surgery. Chemotherapy may be useful in the setting of metastatic myoepithelial carcinomas in adults, but reported results are inconsistent.30,31 Radiation treatment of recurrent or metastatic disease has not been shown to be effective. A study of children treated with surgical resection and chemotherapy using ifosfamide, cisplatin, and etoposide followed by radiation therapy showed positive results.32
Our case highlights several challenges that may arise in establishing a diagnosis of cutaneous myoepithelial carcinoma with disseminated metastases. The diagnostic difficulty in our case was compounded by the advanced nature of the lesion at the time of presentation. Given the rarity of metastatic cutaneous myoepithelial carcinomas and the lack of a prior primary diagnosis of a malignant myoepithelioma, the index of suspicion for this entity was not high. A report of myoepithelial carcinoma of the parotid gland metastatic to the skin has been reported,33 but in the absence of salivary gland involvement or other visceral lesions, metastasis from any source other than our patient’s cutaneous scalp lesion is unlikely. The histopathologic features in combination with the characteristic immunophenotype, unique clinical setting, and radiographic findings were essential to arriving at the correct diagnosis. Unlike previously reported metastatic lesions, our case is unique in that metastatic lesions were identified at the time of initial clinical presentation.
Conclusion
Cutaneous myoepithelial carcinomas are exceedingly rare tumors with a wide range of histopathologic and immunohistochemical findings. In challenging cases, studies for EWSR1 or PLAG1 gene rearrangement can be helpful. Furthermore, this case illustrates the potential for widespread dissemination of myoepithelial carcinomas requiring clinical evaluation and imaging studies to exclude metastatic lesions.
- Frost MW, Steiniche T, Damsgaard TE, et al. Primary cutaneous myoepithelial carcinoma: a case report and review of the literature. APMIS. 2014;122:369-379.
- Stojsic Z, Brasanac D, Boricic I, et al. Clear cell myoepithelial carcinoma of the skin. a case report. J Cutan Pathol. 2009;36:680-683.
- Tanahashi J, Kashima K, Daa T, et al. A case of cutaneous myoepithelial carcinoma. J Cutan Pathol. 2007;34:648-653.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. report of 12 cases. Virchows Arch. 1999;434:393-400.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Law RM, Viglione MP, Barrett TL. Metastatic myoepithelial carcinoma in a child. J Cutan Pathol. 2008;35:779-781.
- Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14-24.
- Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-1124.
- Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52:675-682.
- Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444-1450.
- Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294-302.
- Garcia-Sanchez S, Elices M, Nieto S. Cutaneous myoepithelial carcinoma (malignant myoepithelial tumor of skin). Virchows Archiv. 2009;455(suppl 1):1-482.
- Bajoghli A, Limpert J. Treatment of cutaneous malignant myoepithelioma on the nasal ala using Mohs micrographic surgery in a two and a half year old child. J Invest Dermatol. 2009;129:S44.
- Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-806.
- Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands. a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196.
- Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22.
- Neto AG, Pineda-Daboin K, Luna MA. Myoepithelioma of the soft tissue of the head and neck: a case report and review of the literature. Head Neck. 2004;26:470-473.
- Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348-355.
- Dix BT, Hentges MJ, Saltrick KR, et al. Cutaneous myoepithelioma in the foot: case report. Foot Ankle Spec. 2013;6:239-241.
- Burke T, Sahin A, Johnson DE, et al. Myoepithelioma of the retroperitoneum. Ultrastruct Pathol. 1995;19:269-274.
- Fernandez-Figueras MT, Puig L, Trias I, et al. Benign myoepithelioma of the skin. Am J Dermatopathol. 1998;20:208-212.
- Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer. 2012;51:140-148.
- Panagopoulos I, Mertens F, Isaksson M, et al. Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2002;35:340-352.
- Graham RP, Weiss SW, Sukov WR, et al. PHF1 rearrangements in ossifying fibromyxoid tumors of soft parts: a fluorescence in situ hybridization study of 41 cases with emphasis on the malignant variant. Am J Surg Pathol. 2013;37:1751-1755.
- Dabska M. Parachordoma: a new clinicopathologic entity. Cancer. 1977;40:1586-1592.
- Fletcher CDM, Mertens F, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Lauer SR, Edgar MA, Gardner JM, et al. Soft tissue chordomas: a clinicopathologic analysis of 11 cases. Am J Surg Pathol. 2013;37:719-726.
- Miller TD, McCalmont T, Tope WD. Recurrent cutaneous myoepithelioma treated using Mohs micrographic surgery: case report and review of the literature. Dermatol Surg. 2009;35:139-143.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Noronha V, Cooper DL, Higgins SA, et al. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7:270-271.
- Bisogno G, Tagarelli A, Schiavetti A, et al. Myoepithelial carcinoma treatment in children: a report from the TREP project. Pediatr Blood Cancer. 2014;61:643-646.
- He DQ, Hua CG, Tang XF, et al. Cutaneous metastasis from a parotid myoepithelial carcinoma: a case report and review of the literature. J Cutan Pathol. 2008;35:1138-1143.
- Frost MW, Steiniche T, Damsgaard TE, et al. Primary cutaneous myoepithelial carcinoma: a case report and review of the literature. APMIS. 2014;122:369-379.
- Stojsic Z, Brasanac D, Boricic I, et al. Clear cell myoepithelial carcinoma of the skin. a case report. J Cutan Pathol. 2009;36:680-683.
- Tanahashi J, Kashima K, Daa T, et al. A case of cutaneous myoepithelial carcinoma. J Cutan Pathol. 2007;34:648-653.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. report of 12 cases. Virchows Arch. 1999;434:393-400.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Law RM, Viglione MP, Barrett TL. Metastatic myoepithelial carcinoma in a child. J Cutan Pathol. 2008;35:779-781.
- Hornick JL, Fletcher CD. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004;35:14-24.
- Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. a molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114-1124.
- Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes Chromosomes Cancer. 2013;52:675-682.
- Flucke U, Palmedo G, Blankenhorn N, et al. EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: a study of 18 cases. Mod Pathol. 2011;24:1444-1450.
- Mentzel T, Requena L, Kaddu S, et al. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol. 2003;30:294-302.
- Garcia-Sanchez S, Elices M, Nieto S. Cutaneous myoepithelial carcinoma (malignant myoepithelial tumor of skin). Virchows Archiv. 2009;455(suppl 1):1-482.
- Bajoghli A, Limpert J. Treatment of cutaneous malignant myoepithelioma on the nasal ala using Mohs micrographic surgery in a two and a half year old child. J Invest Dermatol. 2009;129:S44.
- Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801-806.
- Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands. a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196.
- Kilpatrick SE, Hitchcock MG, Kraus MD, et al. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22.
- Neto AG, Pineda-Daboin K, Luna MA. Myoepithelioma of the soft tissue of the head and neck: a case report and review of the literature. Head Neck. 2004;26:470-473.
- Kutzner H, Mentzel T, Kaddu S, et al. Cutaneous myoepithelioma: an under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol. 2001;25:348-355.
- Dix BT, Hentges MJ, Saltrick KR, et al. Cutaneous myoepithelioma in the foot: case report. Foot Ankle Spec. 2013;6:239-241.
- Burke T, Sahin A, Johnson DE, et al. Myoepithelioma of the retroperitoneum. Ultrastruct Pathol. 1995;19:269-274.
- Fernandez-Figueras MT, Puig L, Trias I, et al. Benign myoepithelioma of the skin. Am J Dermatopathol. 1998;20:208-212.
- Bahrami A, Dalton JD, Krane JF, et al. A subset of cutaneous and soft tissue mixed tumors are genetically linked to their salivary gland counterpart. Genes Chromosomes Cancer. 2012;51:140-148.
- Panagopoulos I, Mertens F, Isaksson M, et al. Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2002;35:340-352.
- Graham RP, Weiss SW, Sukov WR, et al. PHF1 rearrangements in ossifying fibromyxoid tumors of soft parts: a fluorescence in situ hybridization study of 41 cases with emphasis on the malignant variant. Am J Surg Pathol. 2013;37:1751-1755.
- Dabska M. Parachordoma: a new clinicopathologic entity. Cancer. 1977;40:1586-1592.
- Fletcher CDM, Mertens F, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002.
- Lauer SR, Edgar MA, Gardner JM, et al. Soft tissue chordomas: a clinicopathologic analysis of 11 cases. Am J Surg Pathol. 2013;37:719-726.
- Miller TD, McCalmont T, Tope WD. Recurrent cutaneous myoepithelioma treated using Mohs micrographic surgery: case report and review of the literature. Dermatol Surg. 2009;35:139-143.
- Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31:1813-1824.
- Noronha V, Cooper DL, Higgins SA, et al. Metastatic myoepithelial carcinoma of the vulva treated with carboplatin and paclitaxel. Lancet Oncol. 2006;7:270-271.
- Bisogno G, Tagarelli A, Schiavetti A, et al. Myoepithelial carcinoma treatment in children: a report from the TREP project. Pediatr Blood Cancer. 2014;61:643-646.
- He DQ, Hua CG, Tang XF, et al. Cutaneous metastasis from a parotid myoepithelial carcinoma: a case report and review of the literature. J Cutan Pathol. 2008;35:1138-1143.
Practice Points
- Cutaneous myoepithelial carcinoma is a rare malignant adnexal neoplasm with metastatic potential that can present in the skin.
- Cutaneous myoepithelial carcinoma is a tumor that can occasionally show EWSR1 gene rearrangement.
- Excision with negative margins and close follow-up is recommended for cutaneous myoepithelial carcinoma.
Completeness of Facial Self-application of Sunscreen in Cosmetic Surgery Patients
UV radiation from sun exposure is a risk factor for most types of skin cancer.1 Despite comprising only 1% of the body's surface area, the periocular region is the location of approximately 5% to 10% of skin cancers described in one US study.2 The efficacy of sunscreen in preventing skin cancer is widely accepted, and the American Academy of Dermatology recommends application of broad-spectrum UVA/UVB sunscreen with a sun protection factor of 30 or higher to help prevent skin cancer.3-5
RELATED ARTICLE: Sun Protection for Infants: Parent Behaviors and Beliefs
Reducing the risk of skin cancer from sun exposure relies on many factors, including completeness of application. A number of studies have demonstrated incomplete sunscreen application on the hairline, ears, neck, and dorsal feet.6-8 The purpose of this study was to assess the completeness of facial sunscreen self-application in oculofacial surgery patients using UV photography.
Methods
This single-site, cross-sectional, qualitative study assessed the completeness of facial sunscreen self-application among patients from a single surgeon's (J.A.W.) cosmetic and tertiary-care oculofacial surgery practice at the Duke Eye Center (Durham, North Carolina) between March 2016 and May 2016. Approval from the Duke University institutional review board was obtained, and the research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act. Informed consent was obtained from all patients, and patients could elect to provide specific written consent for publication of photographs in scientific presentations and publications. Patients younger than 18 years of age; those with known sensitivity to sunscreen or its ingredients; and those with an active lesion, rash, or open wound were excluded from the study.
After obtaining informed consent, patients were photographed using a camera with a UV lens in natural outdoor lighting, first without sunscreen and again after self-application of a sunscreen of their choosing using their routine application technique. Completeness of sunscreen application was graded independently by 3 oculofacial surgeons (N.A.L., J.L., J.A.W.) as complete, partial, none, or cannot determine for 15 facial regions. The majority response was used for analysis.
Results
Forty-four patients were enrolled in the study. Six patients were disqualified due to use of mineral-based formulations (zinc oxide and/or titanium dioxide), as these sunscreens could not be visualized using UV photography. The age range of the remaining 38 patients was 28 to 74 years; 26% (10/38) were men and 74% (28/38) were women.
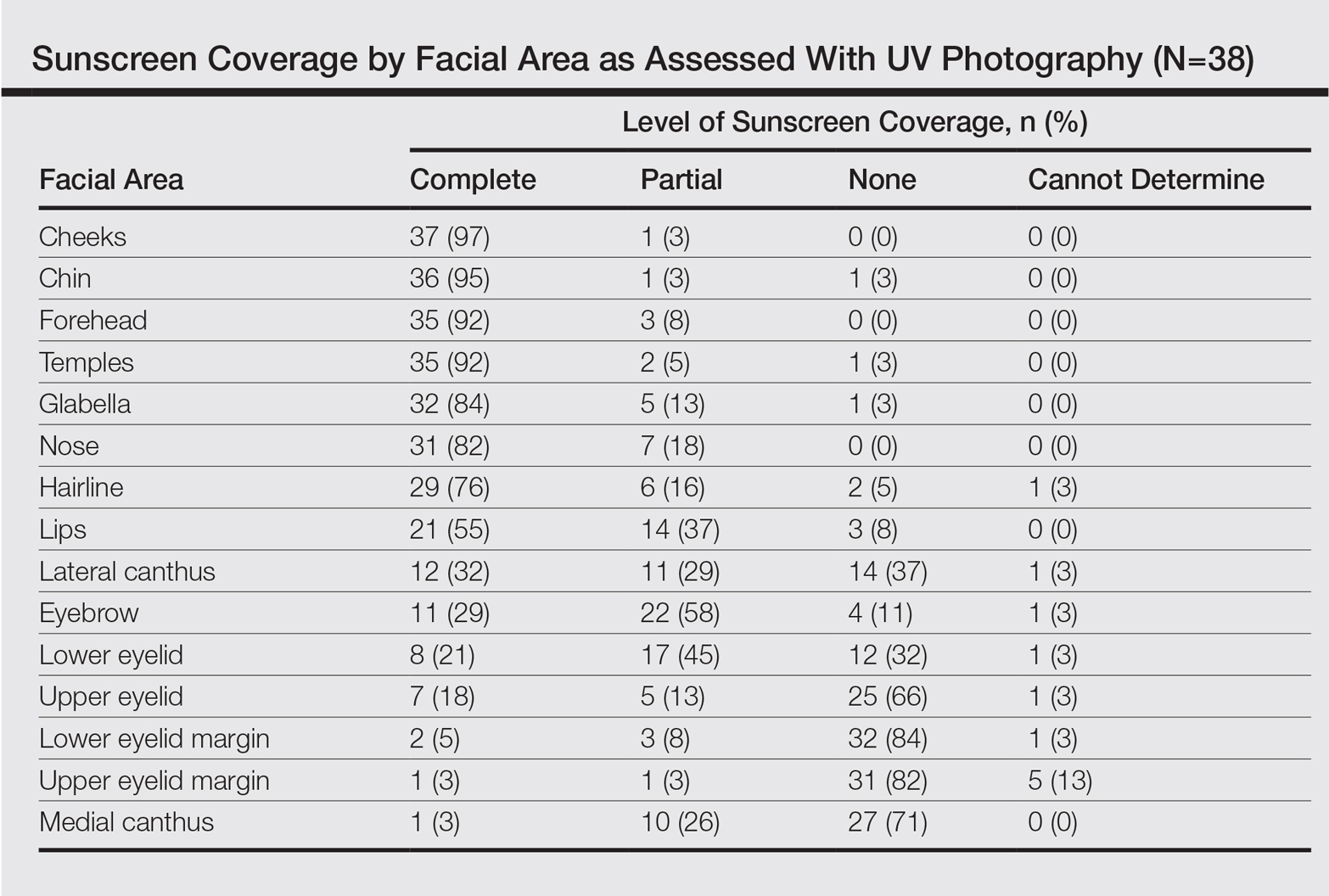
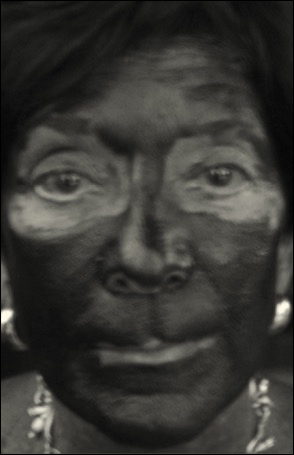
Complete sunscreen application was most frequently performed on the cheeks (97% [37/38]), chin (95% [36/38]), forehead (92% [35/38]), and temples (92% [35/38]). Complete absence of sunscreen coverage was most common on the lower eyelid margin (84% [32/38]), upper eyelid margin (82% [31/38]), medial canthus (71% 27/38]), and upper eyelid (66% [25/38])(Table)(Figure).
Comment
UV radiation-related skin cancers frequently occur in the periocular area, presumably because it is a frequent site of UV exposure. Clothing, sunglasses, and hats can be used to aid in protection from UV radiation, but these products are only regulated by the US Food and Drug Administration if the product claims to prevent skin cancer. Sunscreen is a proven method of protection from UV radiation and the prevention of skin cancer but must be properly applied for it to be effective.1,2,5,6 Incomplete sunscreen application has been demonstrated in numerous studies. Lademann et al7 studied sunscreen application among 60 beachgoers in Germany and found they typically missed the hairline, ears, and dorsal feet. In a study of 10 women with photosensitivity in England who were asked to apply sunscreen in their routine manner, Azurdia et al6 found the posterior neck, lateral neck, temples, and ears, respectively, were the most frequently missed sites. Yang et al8 assessed sunscreen application in 39 dermatologists and 41 photosensitive patients in China and found the neck, ears, dorsal hands, hairline, temples, and perioral region, respectively, were most commonly left unprotected.
Our study investigated detailed facial self-application of sunscreen and found excellent coverage of the larger facial units such as the forehead, cheeks, chin, and temples. The brow, medial canthus, lateral canthus, and upper and lower eyelids and eyelid margins were infrequently protected with sunscreen during routine application. Our opinion is that patients are unaware that eyelid sunscreen application is important. They may be afraid that the products will sting or cause damage if they get in the eyes. Although some products do sting if they get into the eyes, there is no evidence that sunscreens cause injury to the eyes. The US Food and Drug Administration does not have clear guidelines about applying sunscreens in the periocular area, but in general, mineral blocks are recommended because they have less chance of irritation. Several companies make such products that are designed to be applied to the eyelids.
Limitations of our study included a small sample size and a majority female demographic, which may have affected the results, as women generally are more familiar with the application of lotions to the face. Additionally, the patients were recruited from a tertiary-care clinic and may have had periocular malignancy or may have previously received counseling on the importance of sunscreen use.
Conclusion
Cancer reconstruction of the periocular area is challenging, and even in the best of hands, a patient's quality of life may be negatively affected by postreconstructive appearance or suboptimal function, resulting in ocular exposure. The authors recommend counseling patients on the importance of good sun protection habits, including daily application of sunscreen to the face and periocular region to prevent malignancy in these delicate areas.
- Olsen CM, Wilson LF, Green AC, et al. Cancers inAustralia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust N Z J Public Health. 2015;39:471-476.
- Cook BE Jr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106:746-750.
- van de Pols JC, Williams GM, Pandeye N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Preven. 2006;15:2546-2548.
- Skin Cancer Foundation. Basal cell carcinoma prevention guidelines. http://www.skincancer.org/skin-cancer-information/basal-cell-carcinoma/bcc-prevention-guidelines. Accessed May 24, 2017.
- American Academy of Dermatology. Basal cell carcinoma: tips for managing. https://www.aad.org/public/diseases/skin-cancer/basal-cell-carcinoma#tips. Accessed May 24, 2017.
- Azurdia RM, Pagliaro JA, Diffey BL, et al. Sunscreen application by photosensitive patients is inadequate for protection. Br J Dermatol. 1999;140:255-258.
- Lademann J, Schanzer S, Richter H, et al. Sunscreen application at the beach. J Cosmet Dermatol. 2004;3:62-68.
- Yang HP, Chen K, Chang BZ, et al. A study of the way in which dermatologists and photosensitive patients apply sunscreen in China. Photodermatol Photoimmunol Photomed. 2009;25:245-249.
UV radiation from sun exposure is a risk factor for most types of skin cancer.1 Despite comprising only 1% of the body's surface area, the periocular region is the location of approximately 5% to 10% of skin cancers described in one US study.2 The efficacy of sunscreen in preventing skin cancer is widely accepted, and the American Academy of Dermatology recommends application of broad-spectrum UVA/UVB sunscreen with a sun protection factor of 30 or higher to help prevent skin cancer.3-5
RELATED ARTICLE: Sun Protection for Infants: Parent Behaviors and Beliefs
Reducing the risk of skin cancer from sun exposure relies on many factors, including completeness of application. A number of studies have demonstrated incomplete sunscreen application on the hairline, ears, neck, and dorsal feet.6-8 The purpose of this study was to assess the completeness of facial sunscreen self-application in oculofacial surgery patients using UV photography.
Methods
This single-site, cross-sectional, qualitative study assessed the completeness of facial sunscreen self-application among patients from a single surgeon's (J.A.W.) cosmetic and tertiary-care oculofacial surgery practice at the Duke Eye Center (Durham, North Carolina) between March 2016 and May 2016. Approval from the Duke University institutional review board was obtained, and the research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act. Informed consent was obtained from all patients, and patients could elect to provide specific written consent for publication of photographs in scientific presentations and publications. Patients younger than 18 years of age; those with known sensitivity to sunscreen or its ingredients; and those with an active lesion, rash, or open wound were excluded from the study.
After obtaining informed consent, patients were photographed using a camera with a UV lens in natural outdoor lighting, first without sunscreen and again after self-application of a sunscreen of their choosing using their routine application technique. Completeness of sunscreen application was graded independently by 3 oculofacial surgeons (N.A.L., J.L., J.A.W.) as complete, partial, none, or cannot determine for 15 facial regions. The majority response was used for analysis.
Results
Forty-four patients were enrolled in the study. Six patients were disqualified due to use of mineral-based formulations (zinc oxide and/or titanium dioxide), as these sunscreens could not be visualized using UV photography. The age range of the remaining 38 patients was 28 to 74 years; 26% (10/38) were men and 74% (28/38) were women.
Complete sunscreen application was most frequently performed on the cheeks (97% [37/38]), chin (95% [36/38]), forehead (92% [35/38]), and temples (92% [35/38]). Complete absence of sunscreen coverage was most common on the lower eyelid margin (84% [32/38]), upper eyelid margin (82% [31/38]), medial canthus (71% 27/38]), and upper eyelid (66% [25/38])(Table)(Figure).


Comment
UV radiation-related skin cancers frequently occur in the periocular area, presumably because it is a frequent site of UV exposure. Clothing, sunglasses, and hats can be used to aid in protection from UV radiation, but these products are only regulated by the US Food and Drug Administration if the product claims to prevent skin cancer. Sunscreen is a proven method of protection from UV radiation and the prevention of skin cancer but must be properly applied for it to be effective.1,2,5,6 Incomplete sunscreen application has been demonstrated in numerous studies. Lademann et al7 studied sunscreen application among 60 beachgoers in Germany and found they typically missed the hairline, ears, and dorsal feet. In a study of 10 women with photosensitivity in England who were asked to apply sunscreen in their routine manner, Azurdia et al6 found the posterior neck, lateral neck, temples, and ears, respectively, were the most frequently missed sites. Yang et al8 assessed sunscreen application in 39 dermatologists and 41 photosensitive patients in China and found the neck, ears, dorsal hands, hairline, temples, and perioral region, respectively, were most commonly left unprotected.
Our study investigated detailed facial self-application of sunscreen and found excellent coverage of the larger facial units such as the forehead, cheeks, chin, and temples. The brow, medial canthus, lateral canthus, and upper and lower eyelids and eyelid margins were infrequently protected with sunscreen during routine application. Our opinion is that patients are unaware that eyelid sunscreen application is important. They may be afraid that the products will sting or cause damage if they get in the eyes. Although some products do sting if they get into the eyes, there is no evidence that sunscreens cause injury to the eyes. The US Food and Drug Administration does not have clear guidelines about applying sunscreens in the periocular area, but in general, mineral blocks are recommended because they have less chance of irritation. Several companies make such products that are designed to be applied to the eyelids.
Limitations of our study included a small sample size and a majority female demographic, which may have affected the results, as women generally are more familiar with the application of lotions to the face. Additionally, the patients were recruited from a tertiary-care clinic and may have had periocular malignancy or may have previously received counseling on the importance of sunscreen use.
Conclusion
Cancer reconstruction of the periocular area is challenging, and even in the best of hands, a patient's quality of life may be negatively affected by postreconstructive appearance or suboptimal function, resulting in ocular exposure. The authors recommend counseling patients on the importance of good sun protection habits, including daily application of sunscreen to the face and periocular region to prevent malignancy in these delicate areas.
UV radiation from sun exposure is a risk factor for most types of skin cancer.1 Despite comprising only 1% of the body's surface area, the periocular region is the location of approximately 5% to 10% of skin cancers described in one US study.2 The efficacy of sunscreen in preventing skin cancer is widely accepted, and the American Academy of Dermatology recommends application of broad-spectrum UVA/UVB sunscreen with a sun protection factor of 30 or higher to help prevent skin cancer.3-5
RELATED ARTICLE: Sun Protection for Infants: Parent Behaviors and Beliefs
Reducing the risk of skin cancer from sun exposure relies on many factors, including completeness of application. A number of studies have demonstrated incomplete sunscreen application on the hairline, ears, neck, and dorsal feet.6-8 The purpose of this study was to assess the completeness of facial sunscreen self-application in oculofacial surgery patients using UV photography.
Methods
This single-site, cross-sectional, qualitative study assessed the completeness of facial sunscreen self-application among patients from a single surgeon's (J.A.W.) cosmetic and tertiary-care oculofacial surgery practice at the Duke Eye Center (Durham, North Carolina) between March 2016 and May 2016. Approval from the Duke University institutional review board was obtained, and the research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act. Informed consent was obtained from all patients, and patients could elect to provide specific written consent for publication of photographs in scientific presentations and publications. Patients younger than 18 years of age; those with known sensitivity to sunscreen or its ingredients; and those with an active lesion, rash, or open wound were excluded from the study.
After obtaining informed consent, patients were photographed using a camera with a UV lens in natural outdoor lighting, first without sunscreen and again after self-application of a sunscreen of their choosing using their routine application technique. Completeness of sunscreen application was graded independently by 3 oculofacial surgeons (N.A.L., J.L., J.A.W.) as complete, partial, none, or cannot determine for 15 facial regions. The majority response was used for analysis.
Results
Forty-four patients were enrolled in the study. Six patients were disqualified due to use of mineral-based formulations (zinc oxide and/or titanium dioxide), as these sunscreens could not be visualized using UV photography. The age range of the remaining 38 patients was 28 to 74 years; 26% (10/38) were men and 74% (28/38) were women.
Complete sunscreen application was most frequently performed on the cheeks (97% [37/38]), chin (95% [36/38]), forehead (92% [35/38]), and temples (92% [35/38]). Complete absence of sunscreen coverage was most common on the lower eyelid margin (84% [32/38]), upper eyelid margin (82% [31/38]), medial canthus (71% 27/38]), and upper eyelid (66% [25/38])(Table)(Figure).


Comment
UV radiation-related skin cancers frequently occur in the periocular area, presumably because it is a frequent site of UV exposure. Clothing, sunglasses, and hats can be used to aid in protection from UV radiation, but these products are only regulated by the US Food and Drug Administration if the product claims to prevent skin cancer. Sunscreen is a proven method of protection from UV radiation and the prevention of skin cancer but must be properly applied for it to be effective.1,2,5,6 Incomplete sunscreen application has been demonstrated in numerous studies. Lademann et al7 studied sunscreen application among 60 beachgoers in Germany and found they typically missed the hairline, ears, and dorsal feet. In a study of 10 women with photosensitivity in England who were asked to apply sunscreen in their routine manner, Azurdia et al6 found the posterior neck, lateral neck, temples, and ears, respectively, were the most frequently missed sites. Yang et al8 assessed sunscreen application in 39 dermatologists and 41 photosensitive patients in China and found the neck, ears, dorsal hands, hairline, temples, and perioral region, respectively, were most commonly left unprotected.
Our study investigated detailed facial self-application of sunscreen and found excellent coverage of the larger facial units such as the forehead, cheeks, chin, and temples. The brow, medial canthus, lateral canthus, and upper and lower eyelids and eyelid margins were infrequently protected with sunscreen during routine application. Our opinion is that patients are unaware that eyelid sunscreen application is important. They may be afraid that the products will sting or cause damage if they get in the eyes. Although some products do sting if they get into the eyes, there is no evidence that sunscreens cause injury to the eyes. The US Food and Drug Administration does not have clear guidelines about applying sunscreens in the periocular area, but in general, mineral blocks are recommended because they have less chance of irritation. Several companies make such products that are designed to be applied to the eyelids.
Limitations of our study included a small sample size and a majority female demographic, which may have affected the results, as women generally are more familiar with the application of lotions to the face. Additionally, the patients were recruited from a tertiary-care clinic and may have had periocular malignancy or may have previously received counseling on the importance of sunscreen use.
Conclusion
Cancer reconstruction of the periocular area is challenging, and even in the best of hands, a patient's quality of life may be negatively affected by postreconstructive appearance or suboptimal function, resulting in ocular exposure. The authors recommend counseling patients on the importance of good sun protection habits, including daily application of sunscreen to the face and periocular region to prevent malignancy in these delicate areas.
- Olsen CM, Wilson LF, Green AC, et al. Cancers inAustralia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust N Z J Public Health. 2015;39:471-476.
- Cook BE Jr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106:746-750.
- van de Pols JC, Williams GM, Pandeye N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Preven. 2006;15:2546-2548.
- Skin Cancer Foundation. Basal cell carcinoma prevention guidelines. http://www.skincancer.org/skin-cancer-information/basal-cell-carcinoma/bcc-prevention-guidelines. Accessed May 24, 2017.
- American Academy of Dermatology. Basal cell carcinoma: tips for managing. https://www.aad.org/public/diseases/skin-cancer/basal-cell-carcinoma#tips. Accessed May 24, 2017.
- Azurdia RM, Pagliaro JA, Diffey BL, et al. Sunscreen application by photosensitive patients is inadequate for protection. Br J Dermatol. 1999;140:255-258.
- Lademann J, Schanzer S, Richter H, et al. Sunscreen application at the beach. J Cosmet Dermatol. 2004;3:62-68.
- Yang HP, Chen K, Chang BZ, et al. A study of the way in which dermatologists and photosensitive patients apply sunscreen in China. Photodermatol Photoimmunol Photomed. 2009;25:245-249.
- Olsen CM, Wilson LF, Green AC, et al. Cancers inAustralia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust N Z J Public Health. 2015;39:471-476.
- Cook BE Jr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106:746-750.
- van de Pols JC, Williams GM, Pandeye N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Preven. 2006;15:2546-2548.
- Skin Cancer Foundation. Basal cell carcinoma prevention guidelines. http://www.skincancer.org/skin-cancer-information/basal-cell-carcinoma/bcc-prevention-guidelines. Accessed May 24, 2017.
- American Academy of Dermatology. Basal cell carcinoma: tips for managing. https://www.aad.org/public/diseases/skin-cancer/basal-cell-carcinoma#tips. Accessed May 24, 2017.
- Azurdia RM, Pagliaro JA, Diffey BL, et al. Sunscreen application by photosensitive patients is inadequate for protection. Br J Dermatol. 1999;140:255-258.
- Lademann J, Schanzer S, Richter H, et al. Sunscreen application at the beach. J Cosmet Dermatol. 2004;3:62-68.
- Yang HP, Chen K, Chang BZ, et al. A study of the way in which dermatologists and photosensitive patients apply sunscreen in China. Photodermatol Photoimmunol Photomed. 2009;25:245-249.
Resident Pearl
- Patients may benefit from their physician taking a moment to describe the importance of applying sunscreen to the eyelids while applying it to the rest of the face.
Painful Necrotic Ulcer on the Vulva
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.
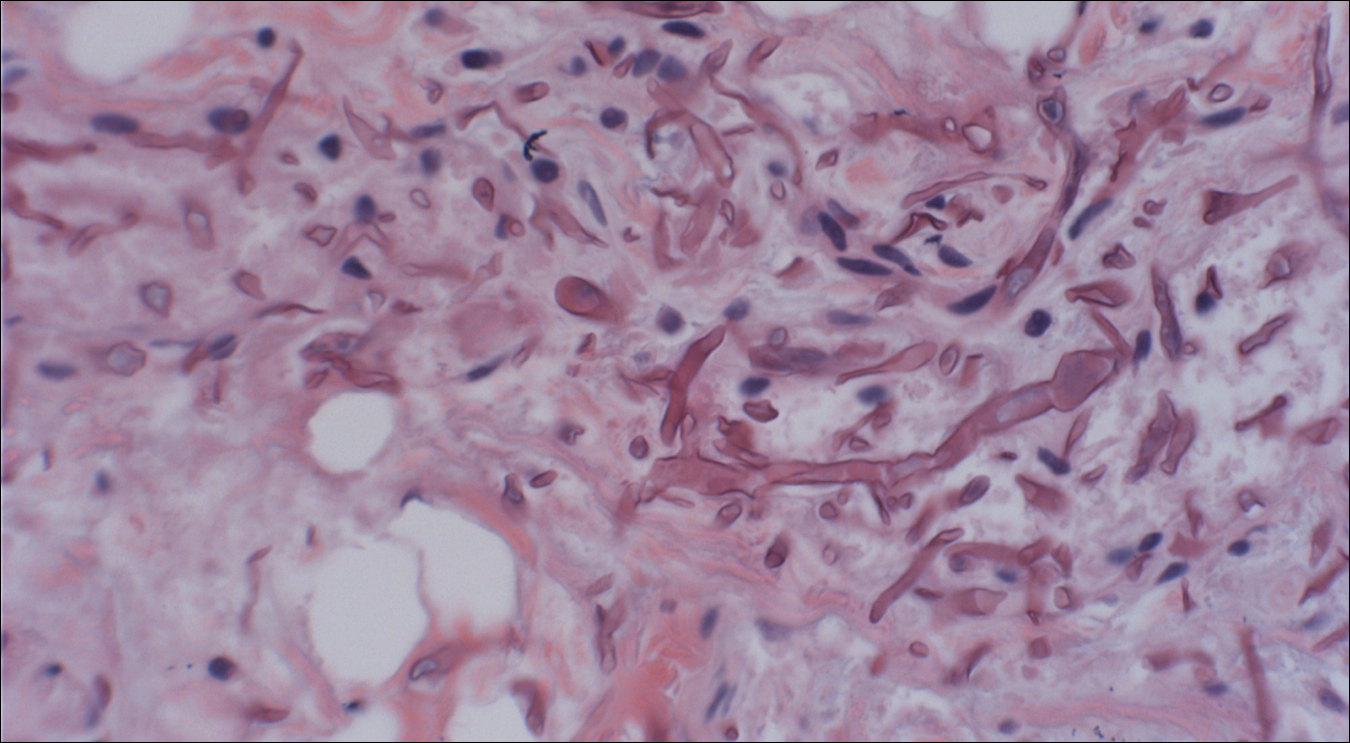
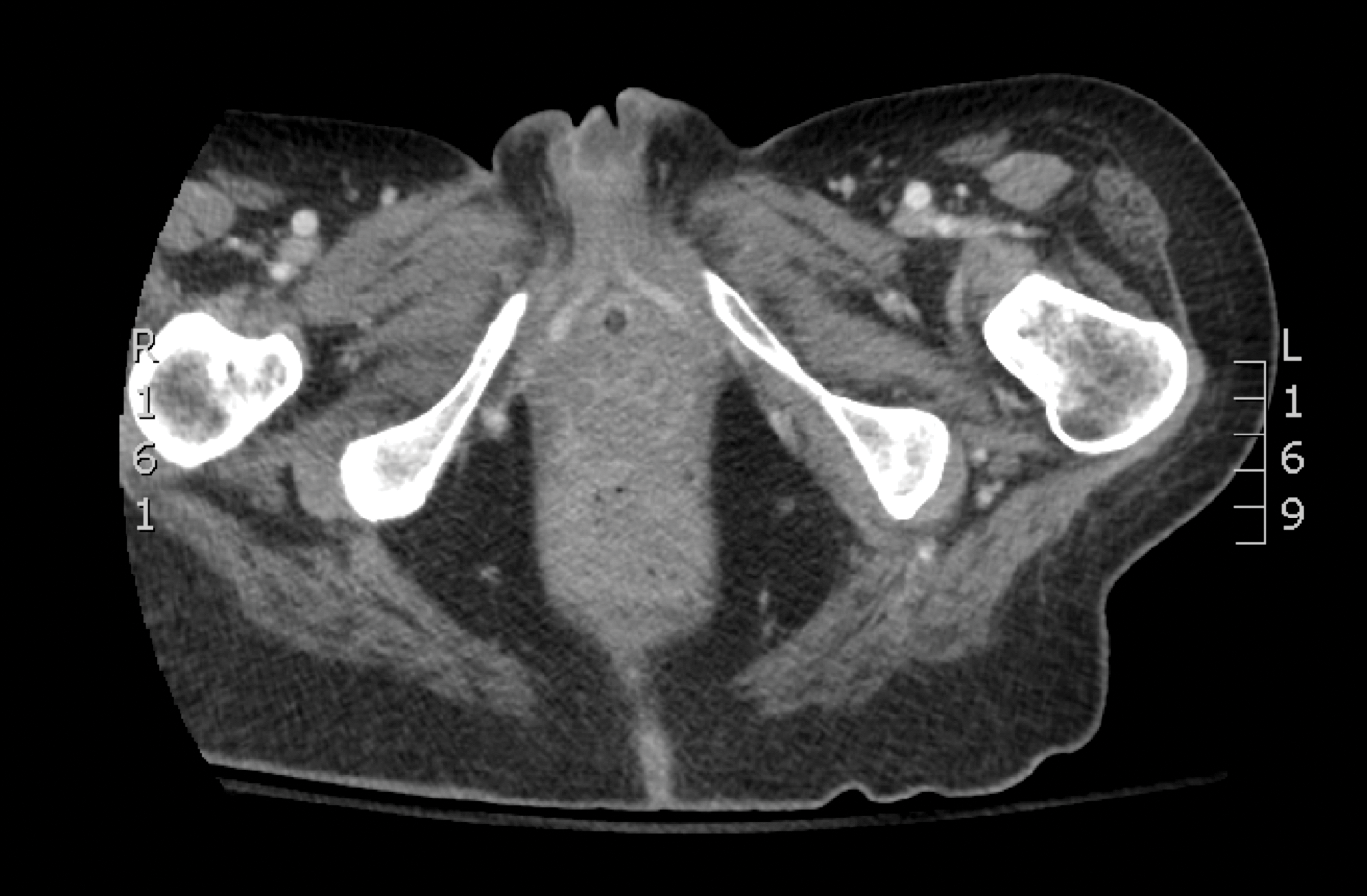
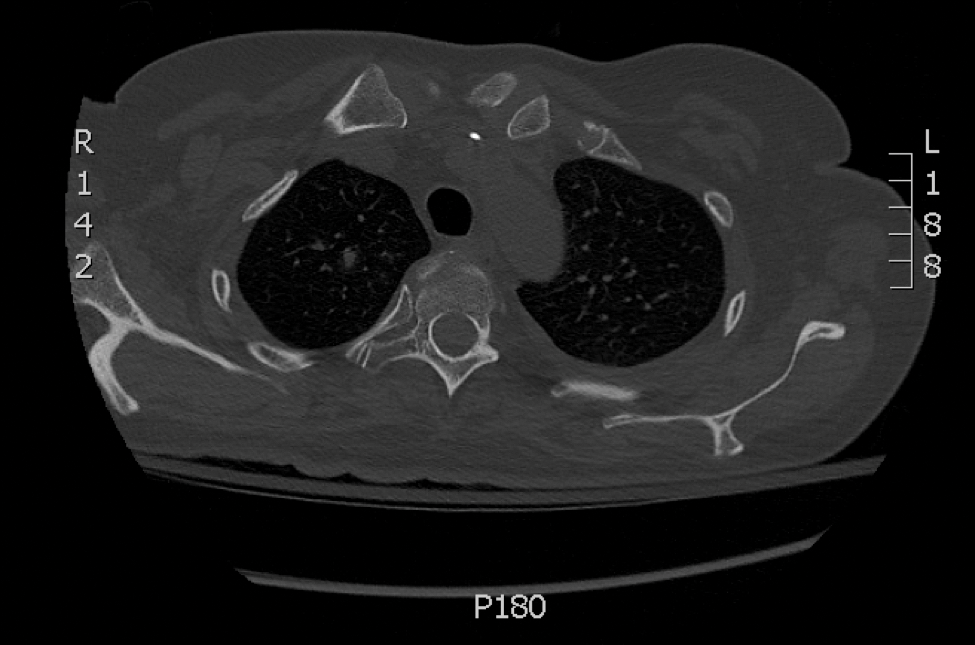
Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.



Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
The Diagnosis: Mucormycosis
Skin biopsy and histology revealed broad, wide-angle, branched, nonseptate hyphae suggestive of mucormycosis infection (Figure 1). Computed tomography of the abdomen and pelvis revealed marked stranding in the vulvar region and urothelial thickening and enhancement suggestive of infection (Figure 2). Computed tomography of the chest demonstrated multiple irregular nodules in the bilateral upper lobes consistent with disseminated mucormycosis (Figure 3). The patient was started on intravenous amphotericin B and posaconazole. Surgery was not pursued given the poor prognosis of her refractory acute lymphoblastic leukemia, pancytopenia, and disseminated fungal infection. The patient was discharged home with hospice care.



Mucormycosis is an infection caused by fungi that belong to the order Mucorales. The most common genera responsible for human disease are Rhizopus, Mucor, and Rhizomucor, which are organisms ubiquitous in nature and found in soil.1 Mucorales hyphae are widely branched and primarily nonseptate, which distinguishes them from hyphae of ascomycetous molds such as Aspergillus, which are narrowly branched and septate.
Mucormycosis primarily affects immunocompromised individuals. The overall incidence of mucormycosis is difficult to estimate, and the risk for infection varies based on the patient population. For example, the incidence of mucormycosis in hematologic malignancy ranges from 1% to 8% and from 0.4% to 16.0% in solid organ transplant recipients.2 One large series of 929 cases noted that the most common risk factors were associated with impaired immune function including diabetes mellitus and diabetic ketoacidosis (36% of cases), hematologic malignancy (17%), and solid organ (7%) or bone marrow transplantation (5%). Other risk factors include neutropenia, steroid therapy, and other immunocompromising conditions.3 Healthy individuals have a strong natural immunity to mucormycosis and rarely are affected by the disease.2
The host response to Mucorales is primarily driven by phagocyte-mediated killing via oxidative metabolites and cationic peptides called defensins.1 Thus, severely neutropenic patients are at high risk for developing mucormycosis.1 In contrast, it appears as though AIDS patients are not at increased risk for mucormycosis, supporting the theory that T lymphocytes are not involved in the host response.1 The conditions of diabetic ketoacidosis leave patients susceptible to mucormycosis for several reasons. First, hyperglycemia and low pH induce phagocyte dysfunction and thus inhibit the host response to Mucorales.4 Second, these organisms have an active ketone reductase system that may allow them to grow more readily in high glucose, acidic conditions.1 Third, diabetic ketoacidosis conditions increase serum free iron, and Mucorales utilizes host iron for cell growth and development.1 Individuals such as hemodialysis patients receiving the iron chelator deferoxamine also are at risk for mucormycosis, as Rhizopus can bind to this molecule and transport the bound iron intracellularly for growth utilization.1
Mucormycosis infection is characterized by infarction and rapid necrosis of host tissues resulting from vascular infiltration by fungal hyphae. The most common site of infection is rhino-orbital-cerebral (39%), followed by lungs (24%) and skin (19%).3 Dissemination occurs in 23% of cases.3 Inoculation most commonly occurs via inhalation of airborne fungal spores by an immunocompromised host with resultant fungal proliferation in the paranasal sinuses, bronchioles, or alveoli. Gastrointestinal tract infection is presumed to occur via ingestion of spores.5
Cutaneous infection, as in our patient, occurs via the inoculation of spores into the dermis through breaks in the skin such as from intravenous lines, urinary catheters, injection sites, surgical sites, and traumatic wounds. Cutaneous infections typically present as a single erythematous, painful, indurated papule that rapidly progresses to a necrotic ulcer with overlying black eschar. In some cases, the progression may be more indolent over the course of several weeks.2 There are few reported cases of primary vulvar mucormycosis, as in our patient.6,7 The previously reported cases involved severely immunocompromised patients who developed large necrotic lesions over the vulva that demonstrated widely branching, nonseptate hyphae on histologic examination. Each patient required extensive surgical debridement with systemic antifungal treatment.6,7
A timely diagnosis of mucormycosis often hinges on a high index of suspicion on behalf of the clinician. A fungal etiology always should be considered for an infection in an immunocompromised patient. Furthermore, nonresponse to antibiotic treatment should be an important diagnostic clue that the infection could be fungal in origin. The definitive diagnosis of mucormycosis is confirmed by tissue biopsy and the presence of broad, widely branching, nonseptate hyphae seen on histopathologic examination.
Treatment involves aggressive surgical debridement of all necrotic tissues and elimination of predisposing factors for infection such as hyperglycemia, metabolic acidosis, deferoxamine administration, and immunosuppressive medications. Early initiation of antifungal therapy with the lipid formulation of amphotericin B is recommended. Oral posaconazole or isavuconazole typically are used as step-down therapy after a favorable clinical response with initial amphotericin B treatment. Deferasirox, in contrast to deferoxamine, is an iron chelator that may reduce the pathogenicity of Mucorales and may help as an adjunctive therapy.8 In addition, hyperbaric oxygen therapy may have limited benefit in some cases.9 In spite of these treatments, the overall mortality of mucormycosis is 50% or higher and approaches nearly 100% in cases of disseminated disease, such as in our patient.1,3
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
- Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-1129.
- Cheng VC, Chan JF, Ngan AH, et al. Outbreak of intestinal infection due to Rhizopus microsporus [published online July 29, 2009]. J Clin Microbiol. 2009;47:2834-2843.
- Colon M, Romaguera J, Mendez K, et al. Mucormycosis of the vulva in an immunocompromised pediatric patient. Bol Asoc Med P R. 2013;105:65-67.
- Nomura J, Ruskin J, Sahebi F, et al. Mucormycosis of the vulva following bone marrow transplantation. Bone Marrow Transplant. 1997;19:859-860.
- Spellberg B, Andes D, Perez M, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53:3122-3125.
- Ferguson BJ, Mitchell TG, Moon R, et al. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10:551-559.
A 48-year-old woman with relapsed T-cell acute lymphoblastic leukemia was admitted to the oncology service for salvage chemotherapy and allogeneic stem cell transplant. Her admission was complicated by extended-spectrum β-lactamase-producing Escherichia coli sepsis and persistent pancytopenia, which required transfer to the intensive care unit. After 2 weeks and while still in the intensive care unit, she developed a painful necrotic vulvar ulcer over the right labia and clitoris that progressed and formed an overlying black eschar.
Recurring Yellowish Papules and Plaques on the Back
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.
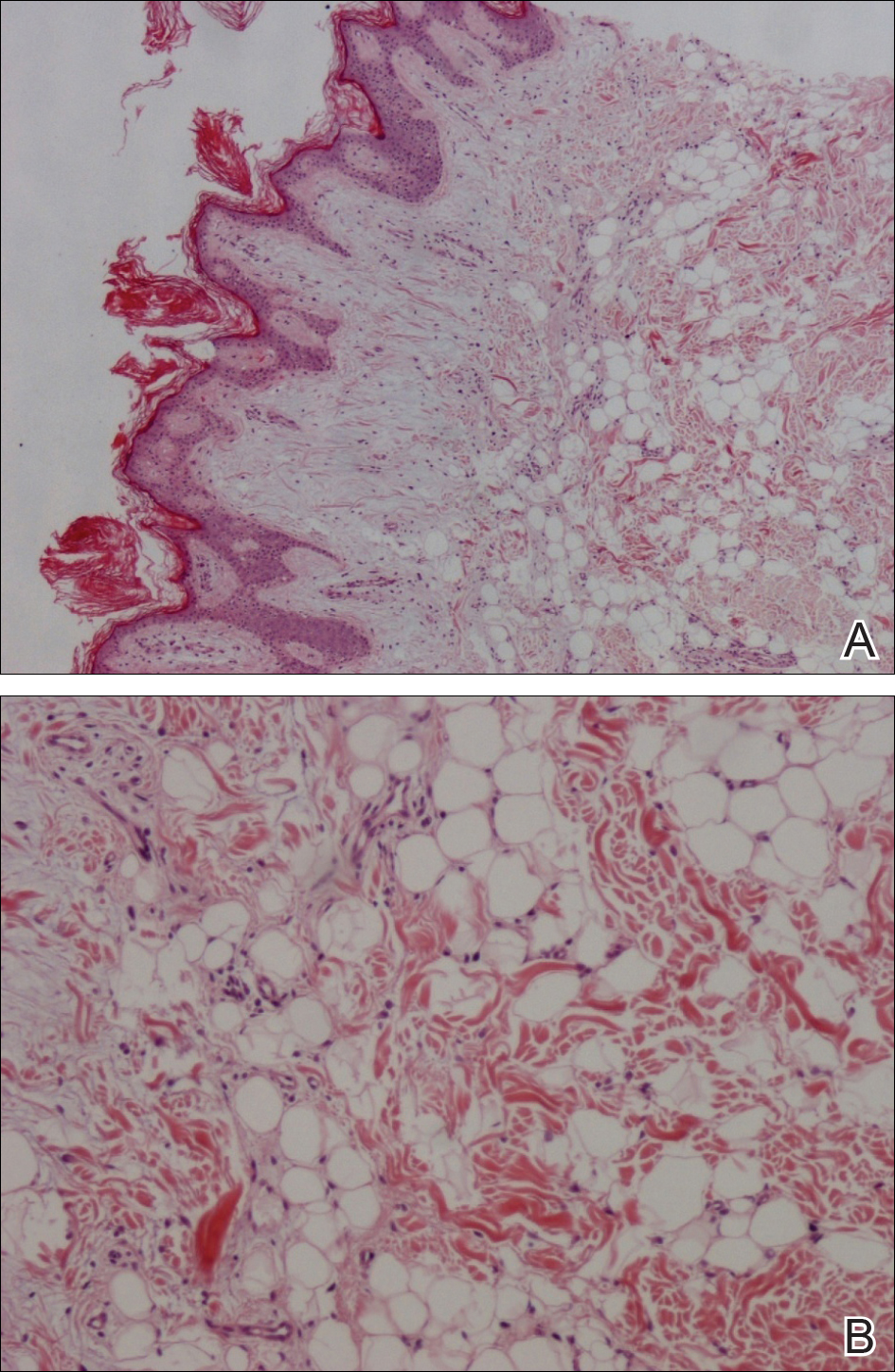
Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.

Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
The Diagnosis: Nevus Lipomatosus Cutaneous Superficialis
A punch biopsy was obtained from a skin lesion, which showed orthokeratosis, irregular acanthosis, papillomatosis, intense edema in the upper dermis, and mature fat lobules that dissected collagen fibers in the reticular dermis (Figure). Classical-type nevus lipomatosus cutaneous superficialis (NLCS) was diagnosed based on these clinical and histopathological findings. The patient was referred to the plastic surgery clinic for total excision of all lesions.

Nevus lipomatosus cutaneous superficialis is a rare hamartoma characterized by ectopic deposition of mature adipose tissue in the dermis.1 It was first described by Hoffmann and Zurhelle2 in 1921. Clinically, NLCS is classified into 2 subtypes: classical (multiple) and solitary. Classical-type NLCS is characterized by multiple pedunculated or sessile, soft, cerebriform, yellowish papules and nodules, especially in the pelvic area. Solitary-type NLCS presents as a sessile papule or nodule with no predilection for localization. Although the classical form of NLCS generally occurs in the first 2 decades of life, the solitary form usually appears in adulthood.3 Nevus lipomatosus cutaneous superficialis has no gender predilection and there is no genetic or congenital defect association.1,4
The pathogenesis of NLCS still is unknown, but some theories have been proposed, such as the development of adipose metaplasia secondary to degeneration of connective tissue, the formation of a true nevus resulting from heterotopic development of adipose tissue, and the development of mature adipocytes from pericytes in dermal vessels.1,5
Histopathology of NLCS shows clusters of ectopic mature adipose tissue in varying rates (10%-50%) between collagen bundles in the dermis. Characteristically, there is no connection between the ectopic mature adipose tissue and the subcutaneous adipose tissue.3 The differential diagnosis of NLCS includes neurofibroma, lymphangioma, sebaceous nevus, fibroepithelial polyps, leiomyoma, and lipomas.1,6
Treatment of NLCS generally involves basic surgical excision; however, patients treated with CO2 laser also have been reported in the literature.5 Because of the growth tendency and the large size of the classical form of NLCS, recurrence may occur, as in our case. In such cases, gradual surgical excision is recommended.5 We present this case to indicate that undesirable surgical results or relapse may occur in untreated patients because of lesion growth and delayed diagnosis.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
- Goucha S, Khaled A, Zéglaoui F, et al. Nevus lipomatosus cutaneous superficialis: report of eight cases. Dermatol Ther (Heidelb). 2011;1:25-30.
- Hoffmann E, Zurhelle E. Ubereinen nevus lipomatodes cutaneous superficialis der linkenglutaalgegend. Arch Dermatol Syph. 1921;130:327-333.
- Patil SB, Narchal S, Paricharak M, et al. Nevus lipomatosus cutaneous superficialis: a rare case report. Iran J Med Sci. 2014;39:304-307.
- Bancalari E, Martínez-Sánchez D, Tardío JC. Nevus lipomatosus superficialis with a folliculosebaceous component: report of 2 cases. Patholog Res Int. 2011;2011:105973.
- Kim YJ, Choi JH, Kim H, et al. Recurrence of nevus lipomatosus cutaneous superficialis after CO(2) laser treatment [published online November 14, 2012]. Arch Plast Surg. 2012;39:671-673.
- Wollina U. Photoletter to the editor - nevus lipomatosus superficialis (Hoffmann-Zurhelle). three new cases including one with ulceration and one with ipsilateral gluteal hypertrophy. J Dermatol Case Rep. 2013;7:71-73.
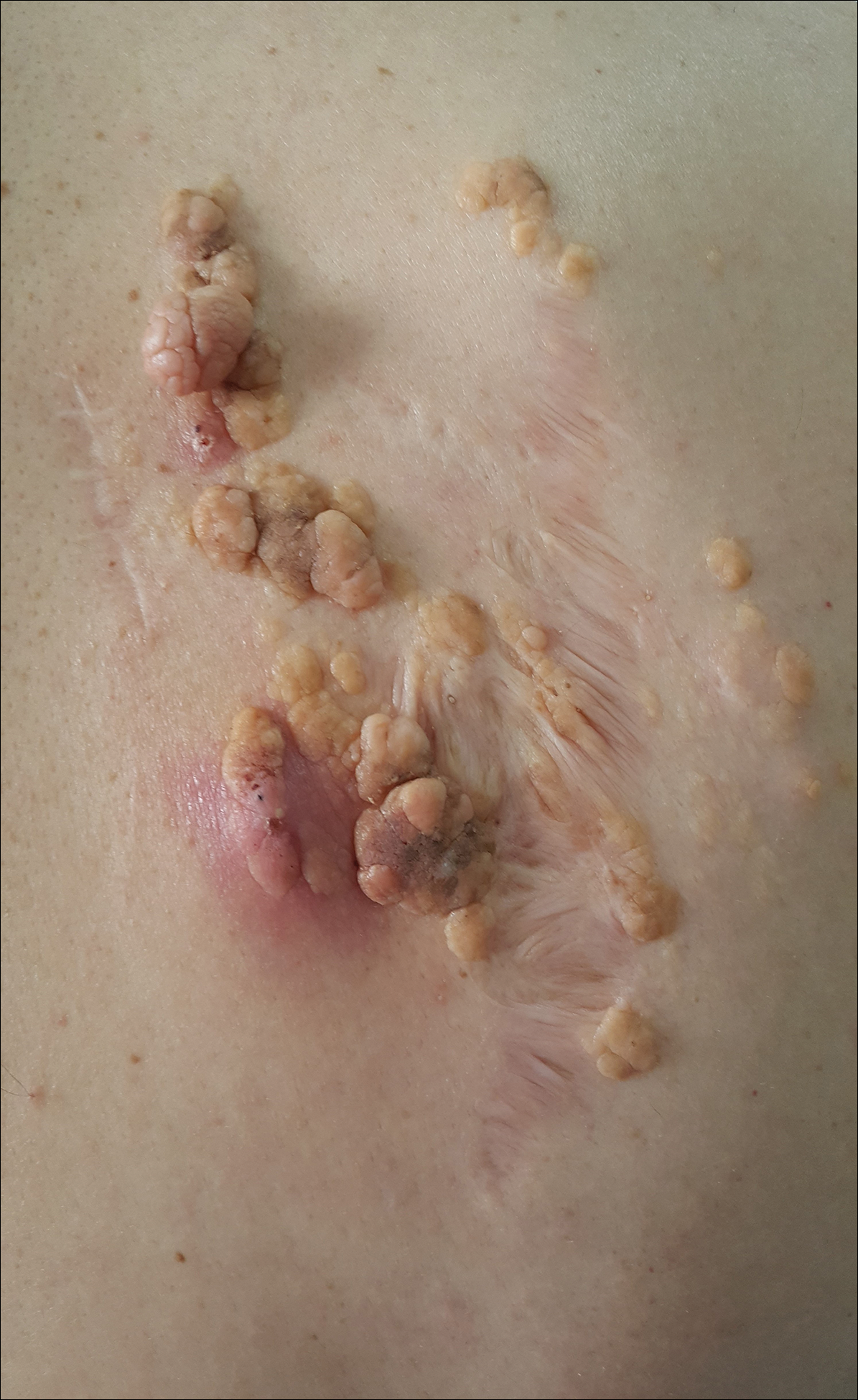
A 36-year-old man presented with a group of partially erythematous, yellowish papules and plaques ranging from 5 to 20 mm in diameter on the right side of the upper back of 20 years' duration. They were surgically excised 8 years prior but recurred and spread. The lesions occasionally were painful and tender with redness and discharge.
Five Steps for Delivering an Effective and Educational Lecture
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
As lifelong learners, physicians are encouraged and expected to share their knowledge base with budding residents and students. Effective communication is essential to the utmost delivery of clinical knowledge and pearls. Lecture delivery is important for all stages of learning, and adapting efficient techniques early in one's career is critical for the transmission of ideas and teaching points. These tips were created to help formulate guidelines for physician presentations and are open for interpretation. These well-meaning suggestions can be integrated into one's toolbox to foster an enthusiastic educational arena.
Step 1: Know Your Key Message
First and foremost, one should ruminate over the overall message of the lecture. Consider at least 3 main points you want the learner to gain and remember on completion of the lecture. Additionally, it is crucial to think about the audience who will be present for your message and how to deliver your ideas clearly and effectively. Be cognizant of the knowledge base of your listeners and gauge how much initial background information is needed; conversely, if the audience is familiar with the material, excessive introductory material may be unnecessary and cause inattentiveness. Simplicity, both within the inherent message itself and the content and layout, can ameliorate the transmission of data regardless of the audience. A mentor once told me that no slide should contain more than 13 lines of text. Furthermore, if you are counting the number of lines, then you likely need to reduce the text and simplify the slide. Each slide should contain a maximum of 3 or 4 bullet points.1 Convoluted figures should be avoided and key points should be highlighted. Overall, know your take-home message and provide the listener with simplistic text and images to convey the key ideas at their educational level.
Step 2: Prepare
Preparation is of utmost importance. Reading over the slides several times prior to the presentation is vital. You are the assumed expert on the topic and meticulously knowing the subject matter helps with the confidence of your delivery. Ease of subject matter also helps you, as the presenter, to rely less on verbatim reading of the slides and allows you to interact more with your audience. It is important to be familiar with the order of your presentation as well as the phrases and figures provided.2 Flipping back and forth through slides can be distracting to the audience and can make the order of your presentation seem incongruous, presenting as a hastily constructed lecture. If you are prepared, you can engage your audience and provide additional information that is not on the slides to maintain interest. Remember that reading the slides can reduce your voice to a monotone, subtracting enthusiasm and energy from the delivery of your talk.2 Rehearsal helps give you the freedom to confidently and proudly present your subject material.
Step 3: Be Animated
You are the main attraction and the performer of this lecture. Radiate the confidence you gained from being prepared with the ability to engage in eye contact and gestures as needed to convey your point. Regularly shift your focus around the room to attempt to involve as many people as possible in your talk.2 Your main focus should be your audience and not your slides; the slides should simply help guide your talk.3 During your presentation, you also can ask rhetorical questions that you can then answer to keep the group engaged (eg, "So, what does this tell us?" or "What would you do next?"). These questions demonstrate to your audience that you are interested in their attention and can help reciprocate the enthusiasm. Use language that involves your audience as a group participant. For example, when looking at visual aids, introduce them by saying "If we look at this table, we can see that . . ." or "This figure shows us that . . ."2,3 Additionally, be cognizant of the volume and pace of your voice. During key points, you may want to slightly raise your voice and slow your pace for emphasis. Anxiety can make all presenters speed through their material; however, try to be mindful of the rhythm of your speech. With preparation you should be able to accurately gauge the length of your presentation but also adapt to the necessary time constraints if too much time is spent on one point early on. Most would believe that all good lectures end at least a few minutes early to allow for questions and comprehension of the material as well as to provide your audience with time to move on to their next engagement or clinical duty.
Step 4: Encourage Active Participation
Active audience participation is shown by a multitude of studies to provide the highest level of comprehension.4,5 In a crossover study conducted by Bleske et al,4 30 students were divided into 2 groups and were taught 6 therapeutic topics, with 3 topics provided by conventional lecture and 3 topics taught by team-based learning. At the end of the educational series, the students were surveyed to evaluate their confidence and attitudes. Students demonstrated not only higher examination scores with team-based learning but higher confidence in their ability to transmit the information garnered through therapeutic recommendations.4 Although small, this study highlights the intuitive notion that active learning with subject material, either by sharing ideas with colleagues or having small brainstorming discussions throughout lectures, helps consolidate the information for long-term memory and comprehension.
Additionally, teaching in a medical environment can present unique challenges, as participants may feel anxiety over having right or wrong answers due to fear of inadequacy among their scholarly peers. Neher et al6 proposed a 5-step "microskills" model for teaching young physicians, and although it is intended for a clinical setting, it also can be applied to engaging and answering questions from a medical audience in general. Their model focuses on the teacher, or in our case the lecturer, asking a question and then applying the following model: (1) get a commitment, (2) probe for supporting evidence, (3) teach general rules, (4) reinforce what was done right, and (5) correct mistakes.6 After asking your question, the student commits to an answer and must then provide supporting details for their choice, thus feeling more responsible for their collaborative role in problem-solving. Based on their answer, you can then teach your general rule, provide positive feedback on what the student said accurately, and ultimately correct any erroneous information. This prototype of learning is best utilized in the clinical setting but also can enhance participant engagement in lectures while maintaining an inviting educational environment.
Step 5: Summarize
Lastly, conclude your presentation with at least 3 memorable points. What was the point of the presentation? What message do you want your audience to take with them and apply to clinical care? Reiterating the key points through repetition is crucial for long-term memory. Leave the audience with additional thoughts for exploration and subsequent discussion. How can your work or topic be further translated into additional projects for investigation? If the lecture material contains abundant clinical information beyond 3 points, a handout can be helpful to avoid having learners struggling to keep up with notes. This piece of take-home material can serve as a tool for subsequent study and to stimulate enhanced memory of the subject material provided. A strong concluding message can consolidate and remind learners of the scope of the topic and highlight the vital information that should be retained.
Final Thoughts
In summary, the clinical lecturer provides a unique teaching experience, and all physicians should feel proficient in formulating and delivering an educational lecture. These simple tips that call for the teacher to know and prepare his/her key message to deliver an animated and engaged presentation and then to summarize key findings are suggestions for the utmost transmission of data and ideas for all learners.
Acknowledgment
A special thank you to Joan E. St. Onge, MD (Miami, Florida), for her help providing resources for this topic.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
- Yeager M. 4 Steps to Giving Effective Presentations. U.S. News & World Report. http://money.usnews.com/money/blogs/outside-voices-careers/2015/04/02/4-steps-to-giving-effective-presentations. Published April 2, 2015. Accessed May 30, 2017.
- Delivering an effective presentation. University of Leicester website. http://www2.le.ac.uk/offices/ld/resources/presentations/delivering-presentation. Accessed May 30, 2017.
- James G. Fix your presentations: 21 quick tips. Inc. http://www.inc.com/geoffrey-james/how-to-fix-your-presentations-21-tips.html. Published February 29, 2012. Accessed May 30, 2017.
- Bleske BE, Remington TL, Wells TD, et al. A randomized crossover comparison of team-based learning and lecture format on learning outcomes. Am J Pharm Educ. 2016;80:120.
- Tsang A, Harris DM. Faculty and second-year medical student perceptions of active learning in an integrated curriculum. Adv Physiol Educ. 2016;40:446-453.
- Neher JO, Gordon KC, Meyer B, et al. A five-step "microskills" model of clinical teaching. J Am Board Fam Pract. 1992;5:419-424.
Phototherapy and Nondrug Therapies for Psoriasis Considered Beneficial by Patients
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Hand Rejuvenation With Calcium Hydroxylapatite
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Netherton Syndrome in Association With Vitamin D Deficiency
To the Editor:
Netherton syndrome (NS) is a rare genodermatosis that presents with erythroderma accompanied with failure to thrive in the neonatal period. Ichthyosis linearis circumflexa, or double-edged scale, is a typical skin finding. Chronic severe atopic dermatitis with diffuse generalized xerosis usually develops and often is associated with elevated IgE levels; however, a feature most associated with and crucial for the diagnosis of NS is trichorrhexis invaginata, or bamboo hair, that causes patchy hair thinning. The triad of ichthyosis linearis circumflexa, atopic dermatitis, and trichorrhexis invaginata is diagnostic of NS. Several other clinical features, including delayed growth, skeletal age delay, and short stature also can develop during its clinical course.1
Netherton syndrome is an autosomal-recessive disorder resulting from a mutation in the SPINK5 gene, which encodes a serine protease inhibitor important in skin barrier formation and immunity.2 Thus, frequent infections are common in these patients. Current treatment options include emollients and topical anti-inflammatory agents to minimize and control the classic manifestations of NS.
A 10-year-old girl with a history of allergic rhinitis and multiple food allergies presented to the dermatology clinic with a long history of diffuse generalized xerosis and erythema with areas of lichenification and scaly patches on the face, trunk, and extremities. She was born prematurely at 34 weeks and developed scaling and erythema involving most of the body shortly after birth. She exhibited severe failure to thrive that necessitated placement of a gastrostomy feeding tube at 8 months of age, resulting in satisfactory weight gain and the tube was later removed. A liver biopsy obtained at that time revealed early intrahepatic duct obstruction and early cirrhosis. She continued to have severe atopic dermatitis, poor growth, milk intolerance, and frequent infections. She had a history of dysfunctional voiding, necessitating the use of oxybutynin. The patient also was taking desmopressin to help with insensible water losses. She had no family history of dermatologic disorders.
At presentation she had diffuse scaling and erythema around the nasal vestibule and bilateral oral commissures. She also was noted to have coarse, brittle, and sparse scalp hair and eyebrows. Her current medications included hydrocortisone cream 2.5%, loratadine 10 mg daily, desmopressin 0.1 mg twice daily, and oxybutynin. Laboratory DNA analysis revealed 2 deletion mutations involving the SPINK5 gene that combined with physical findings led to the diagnosis of NS. Due to her severe growth retardation (approximately 6 SDs below the mean), she was referred to the pediatric endocrinology department. Our patient’s skeletal age was markedly delayed (6.5 years), and she was vitamin D deficient with a total vitamin D level of 16 ng/mL (reference range, 30–80 ng/mL). She is now under the care of a dietitian and taking a vitamin D supplement of 2000 IU of vitamin D3 daily. Growth hormone therapy trials have not been helpful.
An important feature of NS is growth retardation, which is multifactorial, resulting from increased caloric requirements, percutaneous fluid loss, and food allergies. Komatsu et al3 proposed that the SPINK5 inhibitory domain in addition to its role in skin barrier function is involved in regulating proteolytic processing of growth hormone in the pituitary gland. Its dysfunction may lead to a decrease in human growth hormone levels, resulting in short stature.3 This association suggested that our patient would be a good candidate for growth hormone therapy.
Furthermore, our patient was found to be vitamin D deficient, which was not surprising, as cholecalciferol (vitamin D3) is synthesized in the epidermis with UV exposure. This finding suggests that vitamin D deficiency should be suspected in patients with an impaired skin barrier. In addition to calcium regulation and bone mineralization, vitamin D plays a preventative role in cardiovascular disease, autoimmune diseases such as Crohn disease and multiple sclerosis, type 2 diabetes mellitus, infectious diseases such as tuberculosis and influenza, and many cancers.4
Vitamin D has 2 primary derivatives: (1) vitamin D3 from the skin and dietary animal sources, and (2) ergocalciferol (vitamin D2), which is obtained primarily from dietary plant sources and fortified foods. The most common test for vitamin D sufficiency is an assay for serum 25-hydroxyvitamin D (25[OH]D) concentration; 25(OH)D is derived primarily from vitamin D3, which is 3 times more potent than vitamin D2 in the production of 25(OH)D.5 The American Academy of Pediatrics recommends vitamin D replacement therapy for children with 25(OH)D levels less than 20 ng/mL (50 nmol/L) or in children who are clinically symptomatic.6 The Endocrine Society Clinical Practice Guidelines suggest screening for vitamin D deficiency only in individuals at risk.7 We suggest that serum vitamin D testing should be routine in children with NS and other atopic dermatitis conditions in which UV absorption may be impaired.
- Sun J, Linden K. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693-697.
- Bitoun E, Chavanas S, Irvine AD, et al. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352-361.
- Komatsu N, Saijoh K, Otsuki N, et al. Proteolytic processing of human growth hormone by multiple tissue kallikreins and regulation by the serine protease inhibitor Kazal-Type5 (SPINK5) protein. Clin Chim Acta. 2007;377:228-236.
- Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148.
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391.
- Madhusmita M, Pacaud D, Collett-Solberg PF, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
- Holick MF, Binkley NC, Bisckoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
To the Editor:
Netherton syndrome (NS) is a rare genodermatosis that presents with erythroderma accompanied with failure to thrive in the neonatal period. Ichthyosis linearis circumflexa, or double-edged scale, is a typical skin finding. Chronic severe atopic dermatitis with diffuse generalized xerosis usually develops and often is associated with elevated IgE levels; however, a feature most associated with and crucial for the diagnosis of NS is trichorrhexis invaginata, or bamboo hair, that causes patchy hair thinning. The triad of ichthyosis linearis circumflexa, atopic dermatitis, and trichorrhexis invaginata is diagnostic of NS. Several other clinical features, including delayed growth, skeletal age delay, and short stature also can develop during its clinical course.1
Netherton syndrome is an autosomal-recessive disorder resulting from a mutation in the SPINK5 gene, which encodes a serine protease inhibitor important in skin barrier formation and immunity.2 Thus, frequent infections are common in these patients. Current treatment options include emollients and topical anti-inflammatory agents to minimize and control the classic manifestations of NS.
A 10-year-old girl with a history of allergic rhinitis and multiple food allergies presented to the dermatology clinic with a long history of diffuse generalized xerosis and erythema with areas of lichenification and scaly patches on the face, trunk, and extremities. She was born prematurely at 34 weeks and developed scaling and erythema involving most of the body shortly after birth. She exhibited severe failure to thrive that necessitated placement of a gastrostomy feeding tube at 8 months of age, resulting in satisfactory weight gain and the tube was later removed. A liver biopsy obtained at that time revealed early intrahepatic duct obstruction and early cirrhosis. She continued to have severe atopic dermatitis, poor growth, milk intolerance, and frequent infections. She had a history of dysfunctional voiding, necessitating the use of oxybutynin. The patient also was taking desmopressin to help with insensible water losses. She had no family history of dermatologic disorders.
At presentation she had diffuse scaling and erythema around the nasal vestibule and bilateral oral commissures. She also was noted to have coarse, brittle, and sparse scalp hair and eyebrows. Her current medications included hydrocortisone cream 2.5%, loratadine 10 mg daily, desmopressin 0.1 mg twice daily, and oxybutynin. Laboratory DNA analysis revealed 2 deletion mutations involving the SPINK5 gene that combined with physical findings led to the diagnosis of NS. Due to her severe growth retardation (approximately 6 SDs below the mean), she was referred to the pediatric endocrinology department. Our patient’s skeletal age was markedly delayed (6.5 years), and she was vitamin D deficient with a total vitamin D level of 16 ng/mL (reference range, 30–80 ng/mL). She is now under the care of a dietitian and taking a vitamin D supplement of 2000 IU of vitamin D3 daily. Growth hormone therapy trials have not been helpful.
An important feature of NS is growth retardation, which is multifactorial, resulting from increased caloric requirements, percutaneous fluid loss, and food allergies. Komatsu et al3 proposed that the SPINK5 inhibitory domain in addition to its role in skin barrier function is involved in regulating proteolytic processing of growth hormone in the pituitary gland. Its dysfunction may lead to a decrease in human growth hormone levels, resulting in short stature.3 This association suggested that our patient would be a good candidate for growth hormone therapy.
Furthermore, our patient was found to be vitamin D deficient, which was not surprising, as cholecalciferol (vitamin D3) is synthesized in the epidermis with UV exposure. This finding suggests that vitamin D deficiency should be suspected in patients with an impaired skin barrier. In addition to calcium regulation and bone mineralization, vitamin D plays a preventative role in cardiovascular disease, autoimmune diseases such as Crohn disease and multiple sclerosis, type 2 diabetes mellitus, infectious diseases such as tuberculosis and influenza, and many cancers.4
Vitamin D has 2 primary derivatives: (1) vitamin D3 from the skin and dietary animal sources, and (2) ergocalciferol (vitamin D2), which is obtained primarily from dietary plant sources and fortified foods. The most common test for vitamin D sufficiency is an assay for serum 25-hydroxyvitamin D (25[OH]D) concentration; 25(OH)D is derived primarily from vitamin D3, which is 3 times more potent than vitamin D2 in the production of 25(OH)D.5 The American Academy of Pediatrics recommends vitamin D replacement therapy for children with 25(OH)D levels less than 20 ng/mL (50 nmol/L) or in children who are clinically symptomatic.6 The Endocrine Society Clinical Practice Guidelines suggest screening for vitamin D deficiency only in individuals at risk.7 We suggest that serum vitamin D testing should be routine in children with NS and other atopic dermatitis conditions in which UV absorption may be impaired.
To the Editor:
Netherton syndrome (NS) is a rare genodermatosis that presents with erythroderma accompanied with failure to thrive in the neonatal period. Ichthyosis linearis circumflexa, or double-edged scale, is a typical skin finding. Chronic severe atopic dermatitis with diffuse generalized xerosis usually develops and often is associated with elevated IgE levels; however, a feature most associated with and crucial for the diagnosis of NS is trichorrhexis invaginata, or bamboo hair, that causes patchy hair thinning. The triad of ichthyosis linearis circumflexa, atopic dermatitis, and trichorrhexis invaginata is diagnostic of NS. Several other clinical features, including delayed growth, skeletal age delay, and short stature also can develop during its clinical course.1
Netherton syndrome is an autosomal-recessive disorder resulting from a mutation in the SPINK5 gene, which encodes a serine protease inhibitor important in skin barrier formation and immunity.2 Thus, frequent infections are common in these patients. Current treatment options include emollients and topical anti-inflammatory agents to minimize and control the classic manifestations of NS.
A 10-year-old girl with a history of allergic rhinitis and multiple food allergies presented to the dermatology clinic with a long history of diffuse generalized xerosis and erythema with areas of lichenification and scaly patches on the face, trunk, and extremities. She was born prematurely at 34 weeks and developed scaling and erythema involving most of the body shortly after birth. She exhibited severe failure to thrive that necessitated placement of a gastrostomy feeding tube at 8 months of age, resulting in satisfactory weight gain and the tube was later removed. A liver biopsy obtained at that time revealed early intrahepatic duct obstruction and early cirrhosis. She continued to have severe atopic dermatitis, poor growth, milk intolerance, and frequent infections. She had a history of dysfunctional voiding, necessitating the use of oxybutynin. The patient also was taking desmopressin to help with insensible water losses. She had no family history of dermatologic disorders.
At presentation she had diffuse scaling and erythema around the nasal vestibule and bilateral oral commissures. She also was noted to have coarse, brittle, and sparse scalp hair and eyebrows. Her current medications included hydrocortisone cream 2.5%, loratadine 10 mg daily, desmopressin 0.1 mg twice daily, and oxybutynin. Laboratory DNA analysis revealed 2 deletion mutations involving the SPINK5 gene that combined with physical findings led to the diagnosis of NS. Due to her severe growth retardation (approximately 6 SDs below the mean), she was referred to the pediatric endocrinology department. Our patient’s skeletal age was markedly delayed (6.5 years), and she was vitamin D deficient with a total vitamin D level of 16 ng/mL (reference range, 30–80 ng/mL). She is now under the care of a dietitian and taking a vitamin D supplement of 2000 IU of vitamin D3 daily. Growth hormone therapy trials have not been helpful.
An important feature of NS is growth retardation, which is multifactorial, resulting from increased caloric requirements, percutaneous fluid loss, and food allergies. Komatsu et al3 proposed that the SPINK5 inhibitory domain in addition to its role in skin barrier function is involved in regulating proteolytic processing of growth hormone in the pituitary gland. Its dysfunction may lead to a decrease in human growth hormone levels, resulting in short stature.3 This association suggested that our patient would be a good candidate for growth hormone therapy.
Furthermore, our patient was found to be vitamin D deficient, which was not surprising, as cholecalciferol (vitamin D3) is synthesized in the epidermis with UV exposure. This finding suggests that vitamin D deficiency should be suspected in patients with an impaired skin barrier. In addition to calcium regulation and bone mineralization, vitamin D plays a preventative role in cardiovascular disease, autoimmune diseases such as Crohn disease and multiple sclerosis, type 2 diabetes mellitus, infectious diseases such as tuberculosis and influenza, and many cancers.4
Vitamin D has 2 primary derivatives: (1) vitamin D3 from the skin and dietary animal sources, and (2) ergocalciferol (vitamin D2), which is obtained primarily from dietary plant sources and fortified foods. The most common test for vitamin D sufficiency is an assay for serum 25-hydroxyvitamin D (25[OH]D) concentration; 25(OH)D is derived primarily from vitamin D3, which is 3 times more potent than vitamin D2 in the production of 25(OH)D.5 The American Academy of Pediatrics recommends vitamin D replacement therapy for children with 25(OH)D levels less than 20 ng/mL (50 nmol/L) or in children who are clinically symptomatic.6 The Endocrine Society Clinical Practice Guidelines suggest screening for vitamin D deficiency only in individuals at risk.7 We suggest that serum vitamin D testing should be routine in children with NS and other atopic dermatitis conditions in which UV absorption may be impaired.
- Sun J, Linden K. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693-697.
- Bitoun E, Chavanas S, Irvine AD, et al. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352-361.
- Komatsu N, Saijoh K, Otsuki N, et al. Proteolytic processing of human growth hormone by multiple tissue kallikreins and regulation by the serine protease inhibitor Kazal-Type5 (SPINK5) protein. Clin Chim Acta. 2007;377:228-236.
- Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148.
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391.
- Madhusmita M, Pacaud D, Collett-Solberg PF, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
- Holick MF, Binkley NC, Bisckoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- Sun J, Linden K. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693-697.
- Bitoun E, Chavanas S, Irvine AD, et al. Netherton syndrome: disease expression and spectrum of SPINK5 mutations in 21 families. J Invest Dermatol. 2002;118:352-361.
- Komatsu N, Saijoh K, Otsuki N, et al. Proteolytic processing of human growth hormone by multiple tissue kallikreins and regulation by the serine protease inhibitor Kazal-Type5 (SPINK5) protein. Clin Chim Acta. 2007;377:228-236.
- Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148.
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391.
- Madhusmita M, Pacaud D, Collett-Solberg PF, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
- Holick MF, Binkley NC, Bisckoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
Practice Points
- Netherton syndrome (NS) is characterized by severe atopic dermatitis, ichthyosis linearis circumflexa, and trichorrhexis invaginata.
- Children with NS are at increased risk for vitamin D deficiency.
- Consider screening patients with chronic severe dermatitis for vitamin D deficiency.