User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Erratum
The article "Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna" (Cutis. 2017;99:346-352) contained an error in the author affiliations. The affiliations should have read:
All from the Dermatology Service, Memorial Sloan Kettering Cancer Center, New York, New York. Dr. Yélamos also is from the Dermatology Department, Hospital Clínic, Universitat de Barcelona, Spain. Dr. Rossi also is from the Department of Dermatology, Weill Cornell Medical College, New York.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
The article "Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna" (Cutis. 2017;99:346-352) contained an error in the author affiliations. The affiliations should have read:
All from the Dermatology Service, Memorial Sloan Kettering Cancer Center, New York, New York. Dr. Yélamos also is from the Dermatology Department, Hospital Clínic, Universitat de Barcelona, Spain. Dr. Rossi also is from the Department of Dermatology, Weill Cornell Medical College, New York.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
The article "Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna" (Cutis. 2017;99:346-352) contained an error in the author affiliations. The affiliations should have read:
All from the Dermatology Service, Memorial Sloan Kettering Cancer Center, New York, New York. Dr. Yélamos also is from the Dermatology Department, Hospital Clínic, Universitat de Barcelona, Spain. Dr. Rossi also is from the Department of Dermatology, Weill Cornell Medical College, New York.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
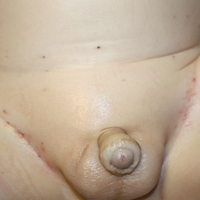
Purpuric Lesions of the Scalp, Axillae, and Groin of an Infant
The Diagnosis: Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a clonal proliferative disorder of Langerhans cells that can affect any organ, most commonly the skin and bones. It typically develops in children aged 1 to 3 years, with a male to female ratio of 2 to 1.1 Skin manifestations include purpuric papules, pustules, vesicles, erosions, and fissuring distributed predominantly on the scalp and flexural sites. Mucosal sites, particularly the oral mucosa, may be involved and usually present as erosions associated with underlying bone lesions.1 Langerhans cell histiocytosis should be considered in the differential diagnosis of recalcitrant diaper dermatitis in an infant, especially when there is purpura and erosions, as seen in our patient. Common conditions in infants such as cutaneous candidiasis (intense erythema with superficial erosions, peripheral scale and satellite pustules on flexural areas, potassium hydroxide microscopy revealing yeast forms and pseudohyphae) and seborrheic dermatitis (well-defined pink to red, moist, and often scaly patches favoring the folds) may be distinguished clinically from Hailey-Hailey disease (malodorous plaques with fissures and erosions favoring the folds), which is rare in infancy, and acrodermatitis enteropathica (erythema and erosions with scale-crust and desquamation on periorificial, acral, and intertriginous skin).
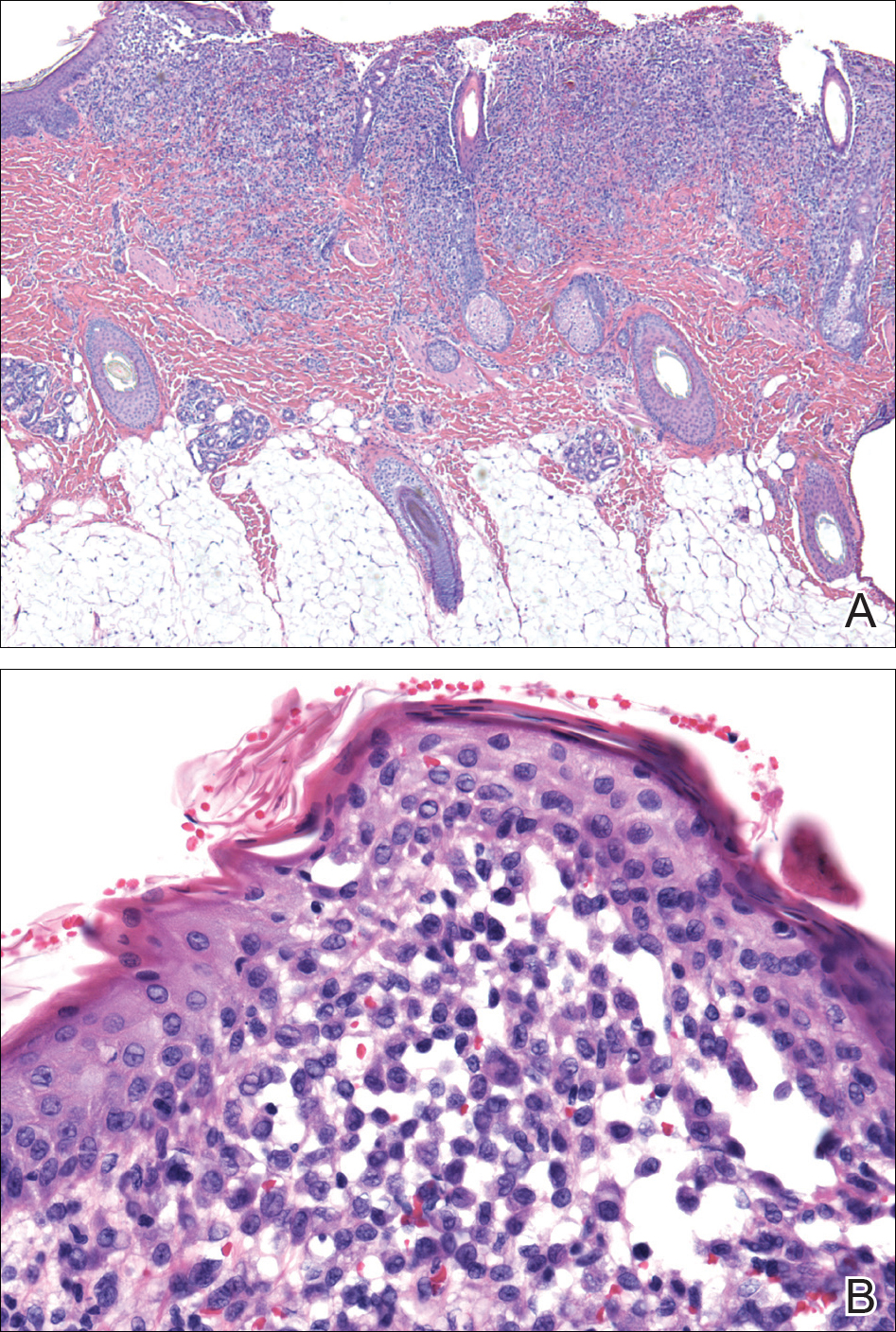
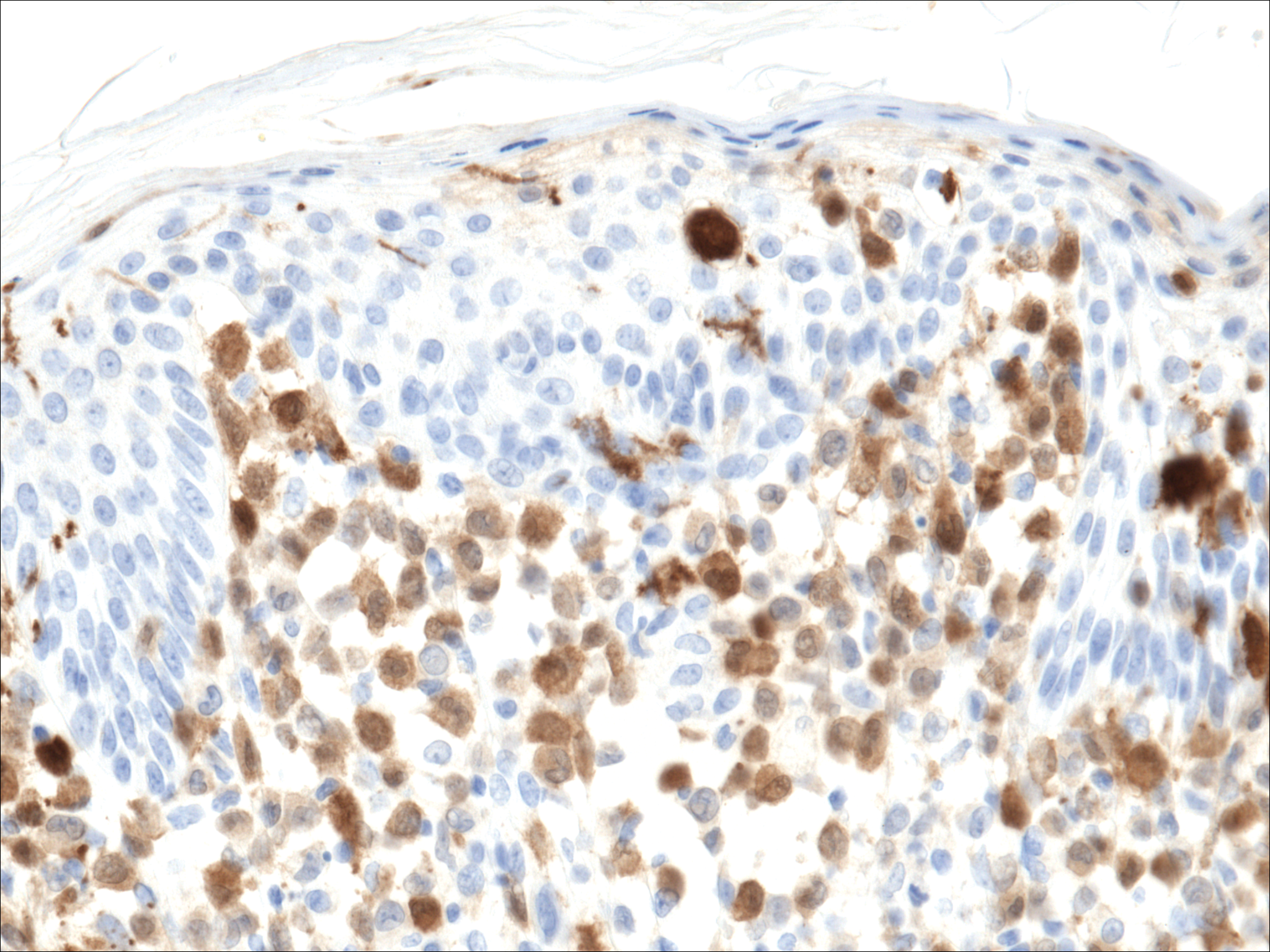
Histopathologic evaluation is instrumental in diagnosing the skin lesions of LCH. Further evaluation for systemic involvement is necessary once the diagnosis is made. Skin biopsy of the scalp and right inguinal fold revealed a wedge-shaped infiltrate of histiocytes with slightly folded nuclear contours in our patient (Figure 1). CD1a (Figure 2) and S-100 stains were markedly positive, which is characteristic of LCH. Complete blood cell count, renal function, liver function, urinalysis, and flow cytometry results were within reference range. A skeletal survey and echocardiogram were unremarkable; however, mild hepatosplenomegaly was noted on abdominal ultrasonography.
Treatment of LCH varies based on the extent of organ involvement. For isolated cutaneous disease, topical steroids, topical nitrogen mustard, phototherapy, and thalidomide may be employed.2 Multisystem disease requires chemotherapeutic agents including vinblastine and prednisone.2,3 Because more than half of patients with LCH have oncogenic BRAF V600E mutations,4 vemurafenib may have a therapeutic role in treatment. Rare case reports have documented disease response in patients with LCH and Erdheim-Chester disease.5,6
Prognosis varies based on age and extent of systemic involvement. Children younger than 2 years with multiorgan involvement have a poor prognosis (35%-55% mortality rate) compared to older children without hematopoietic, hepatosplenic, or lung involvement (100% survival rate). Additionally, response to treatment affects prognosis, as there is a 66% mortality rate in those who do not respond to treatment after 6 weeks.3 Long-term sequelae of LCH include endocrine dysfunction (ie, diabetes insipidus, growth hormone deficiencies), hearing impairment, orthopedic impairment, and neuropsychological disease; thus, multidisciplinary care often is neccessary.7
Given the multisystem involvement in our patient, he was treated with vinblastine, 6-mercaptopurine, and prednisolone with only partial and transient disease response. He was then treated with clofarabine with dramatic resolution of the mediastinal mass on follow-up positron emission tomography. The cutaneous lesions persisted and were managed with topical corticosteroids.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years [published online October 25, 2012]. Pediatr Blood Cancer. 2013;60:175-184.
- Gadner H, Grois N, Arico M, et al; Histiocyte Society. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728-734.
- Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-1500.
- Charles J, Beani JC, Fiandrino G, et al. Major response to vemurafenib in patient with severe cutaneous Langerhans cell histiocytosis harboring BRAF V600E mutation. J Am Acad Dermatol. 2014;71:E97-E99.
- Martin A, Macmillan S, Murphy D, et al. Langerhans cell histiocytosis: 23 years' paediatric experience highlights severe long-term sequelae. Scott Med J. 2014;59:149-157.
The Diagnosis: Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a clonal proliferative disorder of Langerhans cells that can affect any organ, most commonly the skin and bones. It typically develops in children aged 1 to 3 years, with a male to female ratio of 2 to 1.1 Skin manifestations include purpuric papules, pustules, vesicles, erosions, and fissuring distributed predominantly on the scalp and flexural sites. Mucosal sites, particularly the oral mucosa, may be involved and usually present as erosions associated with underlying bone lesions.1 Langerhans cell histiocytosis should be considered in the differential diagnosis of recalcitrant diaper dermatitis in an infant, especially when there is purpura and erosions, as seen in our patient. Common conditions in infants such as cutaneous candidiasis (intense erythema with superficial erosions, peripheral scale and satellite pustules on flexural areas, potassium hydroxide microscopy revealing yeast forms and pseudohyphae) and seborrheic dermatitis (well-defined pink to red, moist, and often scaly patches favoring the folds) may be distinguished clinically from Hailey-Hailey disease (malodorous plaques with fissures and erosions favoring the folds), which is rare in infancy, and acrodermatitis enteropathica (erythema and erosions with scale-crust and desquamation on periorificial, acral, and intertriginous skin).
Histopathologic evaluation is instrumental in diagnosing the skin lesions of LCH. Further evaluation for systemic involvement is necessary once the diagnosis is made. Skin biopsy of the scalp and right inguinal fold revealed a wedge-shaped infiltrate of histiocytes with slightly folded nuclear contours in our patient (Figure 1). CD1a (Figure 2) and S-100 stains were markedly positive, which is characteristic of LCH. Complete blood cell count, renal function, liver function, urinalysis, and flow cytometry results were within reference range. A skeletal survey and echocardiogram were unremarkable; however, mild hepatosplenomegaly was noted on abdominal ultrasonography.


Treatment of LCH varies based on the extent of organ involvement. For isolated cutaneous disease, topical steroids, topical nitrogen mustard, phototherapy, and thalidomide may be employed.2 Multisystem disease requires chemotherapeutic agents including vinblastine and prednisone.2,3 Because more than half of patients with LCH have oncogenic BRAF V600E mutations,4 vemurafenib may have a therapeutic role in treatment. Rare case reports have documented disease response in patients with LCH and Erdheim-Chester disease.5,6
Prognosis varies based on age and extent of systemic involvement. Children younger than 2 years with multiorgan involvement have a poor prognosis (35%-55% mortality rate) compared to older children without hematopoietic, hepatosplenic, or lung involvement (100% survival rate). Additionally, response to treatment affects prognosis, as there is a 66% mortality rate in those who do not respond to treatment after 6 weeks.3 Long-term sequelae of LCH include endocrine dysfunction (ie, diabetes insipidus, growth hormone deficiencies), hearing impairment, orthopedic impairment, and neuropsychological disease; thus, multidisciplinary care often is neccessary.7
Given the multisystem involvement in our patient, he was treated with vinblastine, 6-mercaptopurine, and prednisolone with only partial and transient disease response. He was then treated with clofarabine with dramatic resolution of the mediastinal mass on follow-up positron emission tomography. The cutaneous lesions persisted and were managed with topical corticosteroids.
The Diagnosis: Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a clonal proliferative disorder of Langerhans cells that can affect any organ, most commonly the skin and bones. It typically develops in children aged 1 to 3 years, with a male to female ratio of 2 to 1.1 Skin manifestations include purpuric papules, pustules, vesicles, erosions, and fissuring distributed predominantly on the scalp and flexural sites. Mucosal sites, particularly the oral mucosa, may be involved and usually present as erosions associated with underlying bone lesions.1 Langerhans cell histiocytosis should be considered in the differential diagnosis of recalcitrant diaper dermatitis in an infant, especially when there is purpura and erosions, as seen in our patient. Common conditions in infants such as cutaneous candidiasis (intense erythema with superficial erosions, peripheral scale and satellite pustules on flexural areas, potassium hydroxide microscopy revealing yeast forms and pseudohyphae) and seborrheic dermatitis (well-defined pink to red, moist, and often scaly patches favoring the folds) may be distinguished clinically from Hailey-Hailey disease (malodorous plaques with fissures and erosions favoring the folds), which is rare in infancy, and acrodermatitis enteropathica (erythema and erosions with scale-crust and desquamation on periorificial, acral, and intertriginous skin).
Histopathologic evaluation is instrumental in diagnosing the skin lesions of LCH. Further evaluation for systemic involvement is necessary once the diagnosis is made. Skin biopsy of the scalp and right inguinal fold revealed a wedge-shaped infiltrate of histiocytes with slightly folded nuclear contours in our patient (Figure 1). CD1a (Figure 2) and S-100 stains were markedly positive, which is characteristic of LCH. Complete blood cell count, renal function, liver function, urinalysis, and flow cytometry results were within reference range. A skeletal survey and echocardiogram were unremarkable; however, mild hepatosplenomegaly was noted on abdominal ultrasonography.


Treatment of LCH varies based on the extent of organ involvement. For isolated cutaneous disease, topical steroids, topical nitrogen mustard, phototherapy, and thalidomide may be employed.2 Multisystem disease requires chemotherapeutic agents including vinblastine and prednisone.2,3 Because more than half of patients with LCH have oncogenic BRAF V600E mutations,4 vemurafenib may have a therapeutic role in treatment. Rare case reports have documented disease response in patients with LCH and Erdheim-Chester disease.5,6
Prognosis varies based on age and extent of systemic involvement. Children younger than 2 years with multiorgan involvement have a poor prognosis (35%-55% mortality rate) compared to older children without hematopoietic, hepatosplenic, or lung involvement (100% survival rate). Additionally, response to treatment affects prognosis, as there is a 66% mortality rate in those who do not respond to treatment after 6 weeks.3 Long-term sequelae of LCH include endocrine dysfunction (ie, diabetes insipidus, growth hormone deficiencies), hearing impairment, orthopedic impairment, and neuropsychological disease; thus, multidisciplinary care often is neccessary.7
Given the multisystem involvement in our patient, he was treated with vinblastine, 6-mercaptopurine, and prednisolone with only partial and transient disease response. He was then treated with clofarabine with dramatic resolution of the mediastinal mass on follow-up positron emission tomography. The cutaneous lesions persisted and were managed with topical corticosteroids.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years [published online October 25, 2012]. Pediatr Blood Cancer. 2013;60:175-184.
- Gadner H, Grois N, Arico M, et al; Histiocyte Society. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728-734.
- Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-1500.
- Charles J, Beani JC, Fiandrino G, et al. Major response to vemurafenib in patient with severe cutaneous Langerhans cell histiocytosis harboring BRAF V600E mutation. J Am Acad Dermatol. 2014;71:E97-E99.
- Martin A, Macmillan S, Murphy D, et al. Langerhans cell histiocytosis: 23 years' paediatric experience highlights severe long-term sequelae. Scott Med J. 2014;59:149-157.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years [published online October 25, 2012]. Pediatr Blood Cancer. 2013;60:175-184.
- Gadner H, Grois N, Arico M, et al; Histiocyte Society. A randomized trial of treatment for multisystem Langerhans' cell histiocytosis. J Pediatr. 2001;138:728-734.
- Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923.
- Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495-1500.
- Charles J, Beani JC, Fiandrino G, et al. Major response to vemurafenib in patient with severe cutaneous Langerhans cell histiocytosis harboring BRAF V600E mutation. J Am Acad Dermatol. 2014;71:E97-E99.
- Martin A, Macmillan S, Murphy D, et al. Langerhans cell histiocytosis: 23 years' paediatric experience highlights severe long-term sequelae. Scott Med J. 2014;59:149-157.
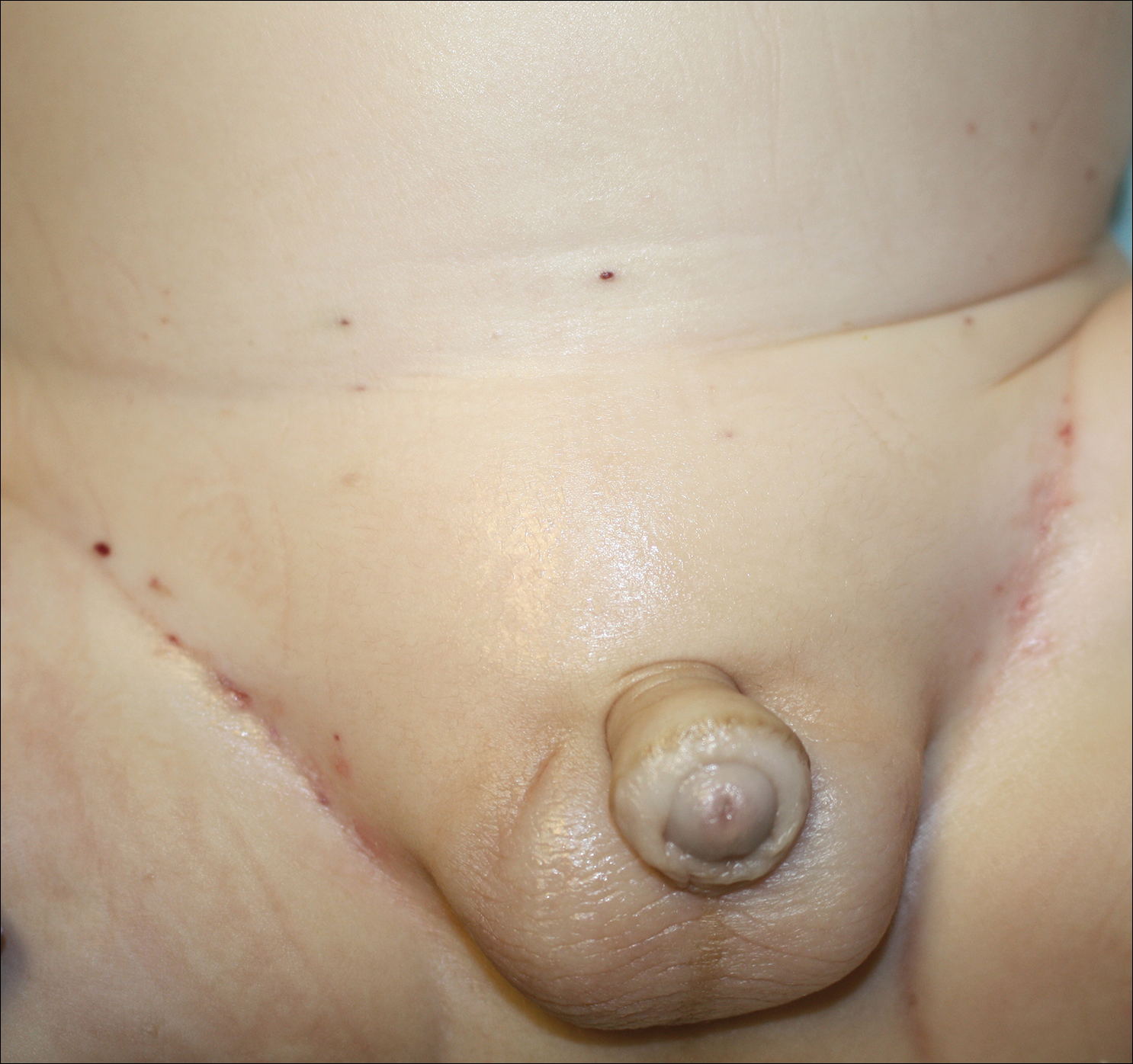
A 7-month-old boy admitted to the hospital with new-onset respiratory stridor was found to have a rash of the scalp, axillae, and groin of 1 month's duration that was unresponsive to treatment with mineral oil. Bronchoscopy revealed tracheal compression, and urgent magnetic resonance imaging of the chest demonstrated an anterior mediastinal mass. Prior to presentation, the patient was otherwise healthy with normal growth and development. On physical examination, scattered red-brown and purpuric papules with hemorrhagic crust were noted on the scalp. There were well-defined pink erosive patches and purpuric papules in the inguinal folds bilaterally and similar erosive patches in the axillae. Numerous punched out ulcerations were noted on the lower gingiva. There was no palpable lymphadenopathy. The hands, feet, penis, scrotum, and perianal area were spared. Biopsies of the skin and mediastinal mass were performed.
Local Anesthetics in Cosmetic Dermatology
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
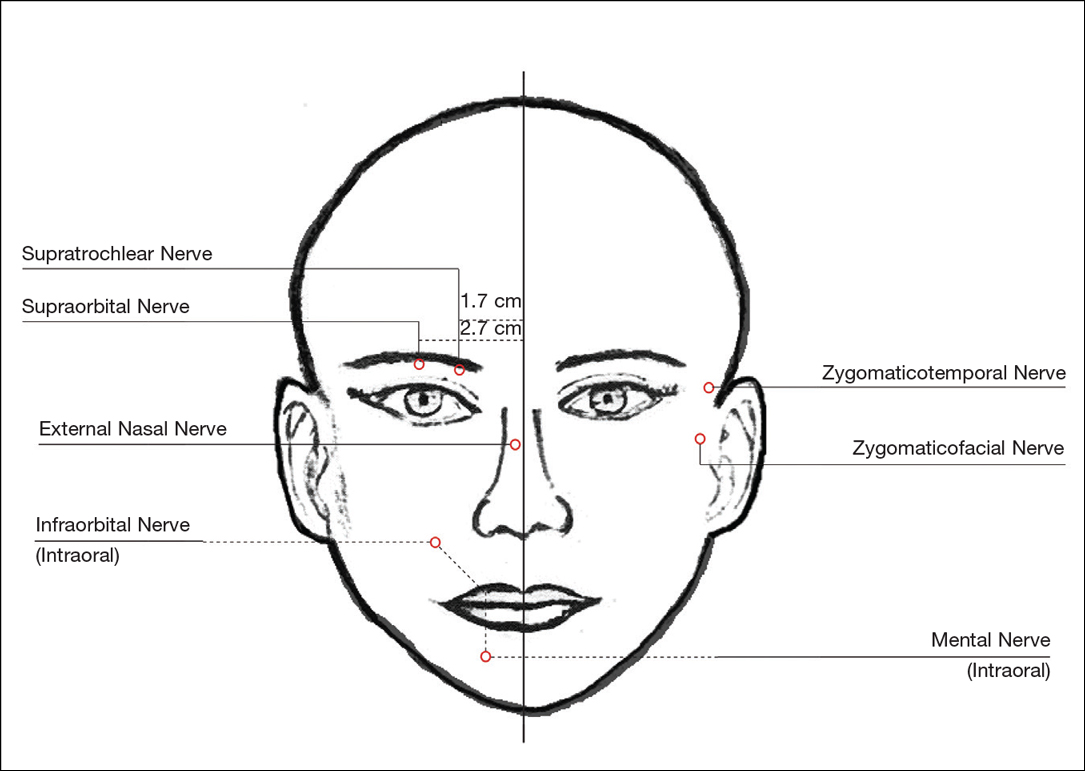
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).
Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
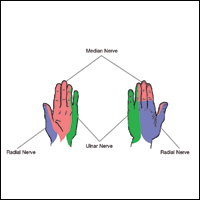
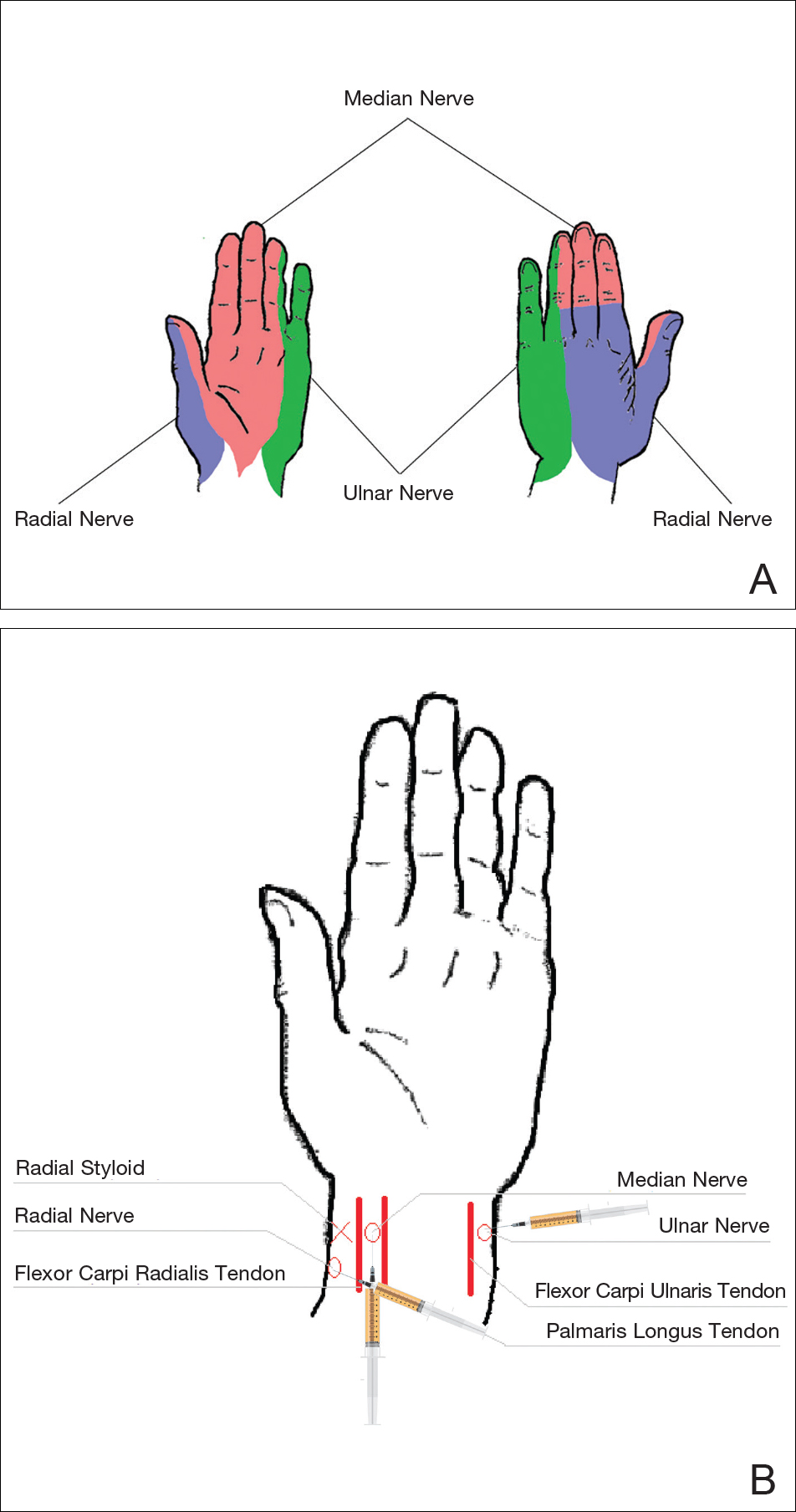
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.
Ankles
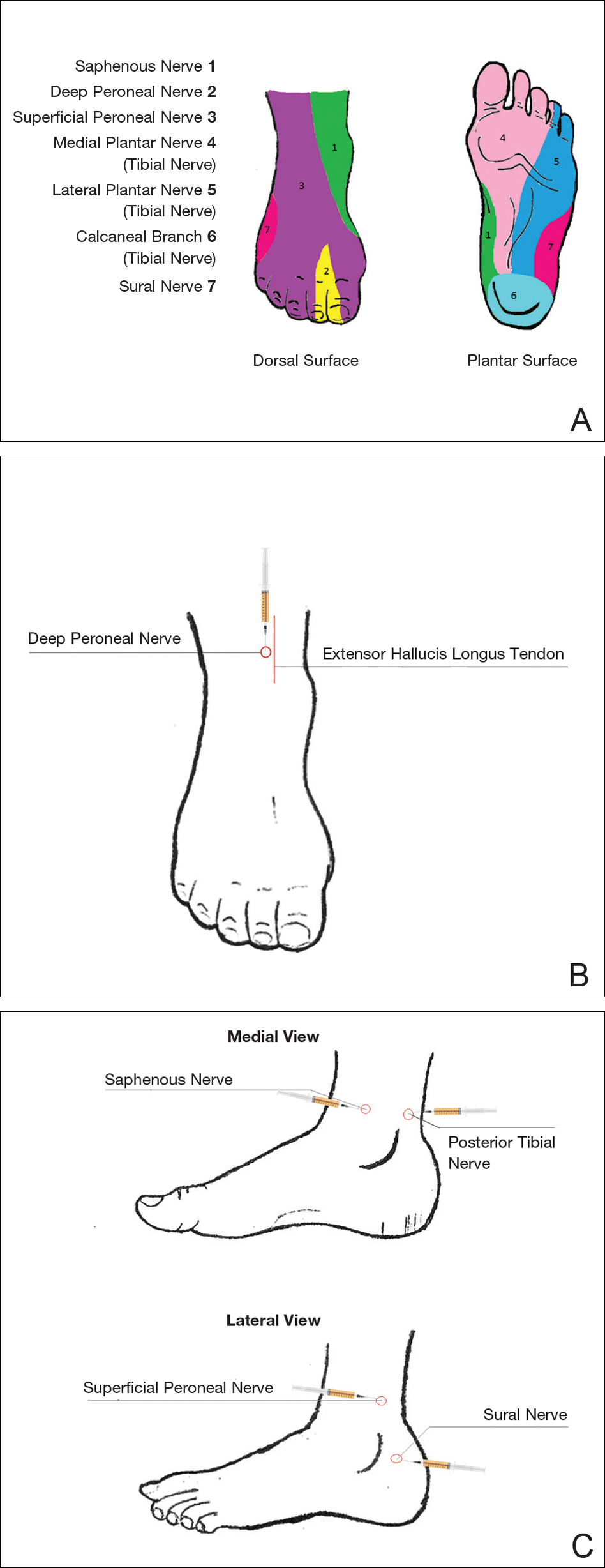
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).
Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).

Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.

Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).

Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).

Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.

Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).

Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
Practice Points
- The proper delivery of local anesthesia is integral to successful cosmetic interventions.
- Regional nerve blocks can provide effective analgesia while reducing the number of injections and preserving the architecture of the cosmetic field.
Product News: 06 2017
Avène Complexion Correcting Shield SPF 50+
Pierre Fabre Dermo-Cosmetique USA adds the Avène Complexion Correcting Shield SPF 50+ mineral sunscreen to its physician-dispensed sun care line. This tinted moisturizer, available in 3 shades, provides 24-hour hydration and an effective antioxidant defense against sun-induced free radicals. Avène Complexion Correcting Shield provides an instant blurring effect to camouflage skin imperfections such as large pores, uneven skin tone, redness, fine lines, and wrinkles. For more information, visit www.aveneusa.com.
Coppertone Clearly Sheer Whipped Sunscreen
Bayer introduces Coppertone Clearly Sheer Whipped Sunscreen, a rich and creamy formula available in sun protection factor 30 and 50. Coppertone Clearly Sheer Whipped Sunscreen absorbs quickly to leave skin feeling soft and smooth. It offers broad-spectrum UVA/UVB protection and is water resistant for up to 80 minutes.For more information, visit www.coppertone.com.
DerMend Mature Skin Solutions
Ferndale Healthcare launches DerMend Mature Skin Solutions, an over-the-counter line consisting of 3 products specifically designed for patients aged 50 years and older. The Fragile Skin Moisturizing Formula rejuvenates thin and fragile skin with hyaluronic acid, retinol, glycolic acid, niacinaminde, and 5 ceramides. The Moisturizing Anti-Itch Lotion is steroid free and contains
Jan Marini Sunscreens
Jan Marini Skin Research, Inc, introduces Antioxidant Daily Face Protectant SPF 33 and Marini Physical Protectant SPF 45, both providing broad-spectrum UVA/UVB protection. Antioxidant Daily Face Protectant provides oil control and advanced hydration for daily use to reduce and address damage caused by sun exposure. Marini Physical Protectant utilizes purely physical filters to decrease the risk of premature skin aging and features a universal tint with a sheer matte finish. For more information, visit www.janmarini.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène Complexion Correcting Shield SPF 50+
Pierre Fabre Dermo-Cosmetique USA adds the Avène Complexion Correcting Shield SPF 50+ mineral sunscreen to its physician-dispensed sun care line. This tinted moisturizer, available in 3 shades, provides 24-hour hydration and an effective antioxidant defense against sun-induced free radicals. Avène Complexion Correcting Shield provides an instant blurring effect to camouflage skin imperfections such as large pores, uneven skin tone, redness, fine lines, and wrinkles. For more information, visit www.aveneusa.com.
Coppertone Clearly Sheer Whipped Sunscreen
Bayer introduces Coppertone Clearly Sheer Whipped Sunscreen, a rich and creamy formula available in sun protection factor 30 and 50. Coppertone Clearly Sheer Whipped Sunscreen absorbs quickly to leave skin feeling soft and smooth. It offers broad-spectrum UVA/UVB protection and is water resistant for up to 80 minutes.For more information, visit www.coppertone.com.
DerMend Mature Skin Solutions
Ferndale Healthcare launches DerMend Mature Skin Solutions, an over-the-counter line consisting of 3 products specifically designed for patients aged 50 years and older. The Fragile Skin Moisturizing Formula rejuvenates thin and fragile skin with hyaluronic acid, retinol, glycolic acid, niacinaminde, and 5 ceramides. The Moisturizing Anti-Itch Lotion is steroid free and contains
Jan Marini Sunscreens
Jan Marini Skin Research, Inc, introduces Antioxidant Daily Face Protectant SPF 33 and Marini Physical Protectant SPF 45, both providing broad-spectrum UVA/UVB protection. Antioxidant Daily Face Protectant provides oil control and advanced hydration for daily use to reduce and address damage caused by sun exposure. Marini Physical Protectant utilizes purely physical filters to decrease the risk of premature skin aging and features a universal tint with a sheer matte finish. For more information, visit www.janmarini.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène Complexion Correcting Shield SPF 50+
Pierre Fabre Dermo-Cosmetique USA adds the Avène Complexion Correcting Shield SPF 50+ mineral sunscreen to its physician-dispensed sun care line. This tinted moisturizer, available in 3 shades, provides 24-hour hydration and an effective antioxidant defense against sun-induced free radicals. Avène Complexion Correcting Shield provides an instant blurring effect to camouflage skin imperfections such as large pores, uneven skin tone, redness, fine lines, and wrinkles. For more information, visit www.aveneusa.com.
Coppertone Clearly Sheer Whipped Sunscreen
Bayer introduces Coppertone Clearly Sheer Whipped Sunscreen, a rich and creamy formula available in sun protection factor 30 and 50. Coppertone Clearly Sheer Whipped Sunscreen absorbs quickly to leave skin feeling soft and smooth. It offers broad-spectrum UVA/UVB protection and is water resistant for up to 80 minutes.For more information, visit www.coppertone.com.
DerMend Mature Skin Solutions
Ferndale Healthcare launches DerMend Mature Skin Solutions, an over-the-counter line consisting of 3 products specifically designed for patients aged 50 years and older. The Fragile Skin Moisturizing Formula rejuvenates thin and fragile skin with hyaluronic acid, retinol, glycolic acid, niacinaminde, and 5 ceramides. The Moisturizing Anti-Itch Lotion is steroid free and contains
Jan Marini Sunscreens
Jan Marini Skin Research, Inc, introduces Antioxidant Daily Face Protectant SPF 33 and Marini Physical Protectant SPF 45, both providing broad-spectrum UVA/UVB protection. Antioxidant Daily Face Protectant provides oil control and advanced hydration for daily use to reduce and address damage caused by sun exposure. Marini Physical Protectant utilizes purely physical filters to decrease the risk of premature skin aging and features a universal tint with a sheer matte finish. For more information, visit www.janmarini.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
What’s Eating You? Chiggers
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
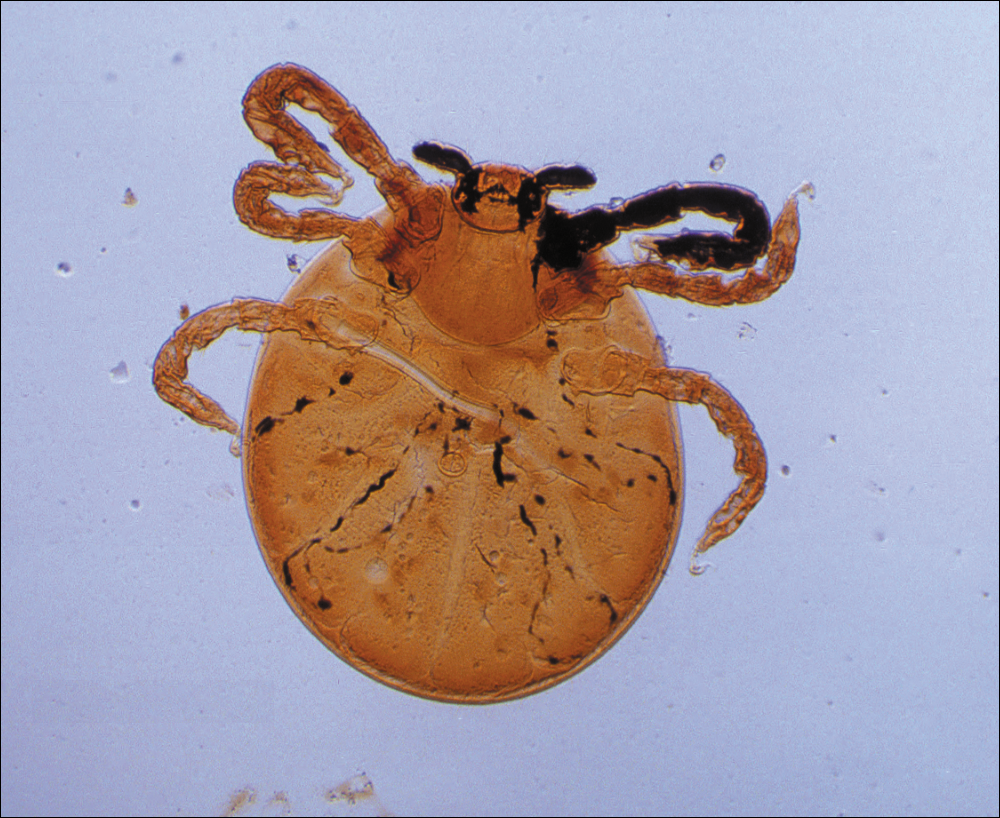
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).
The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
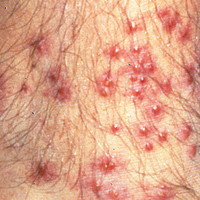
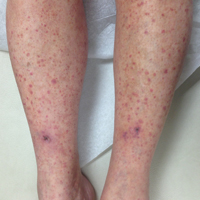
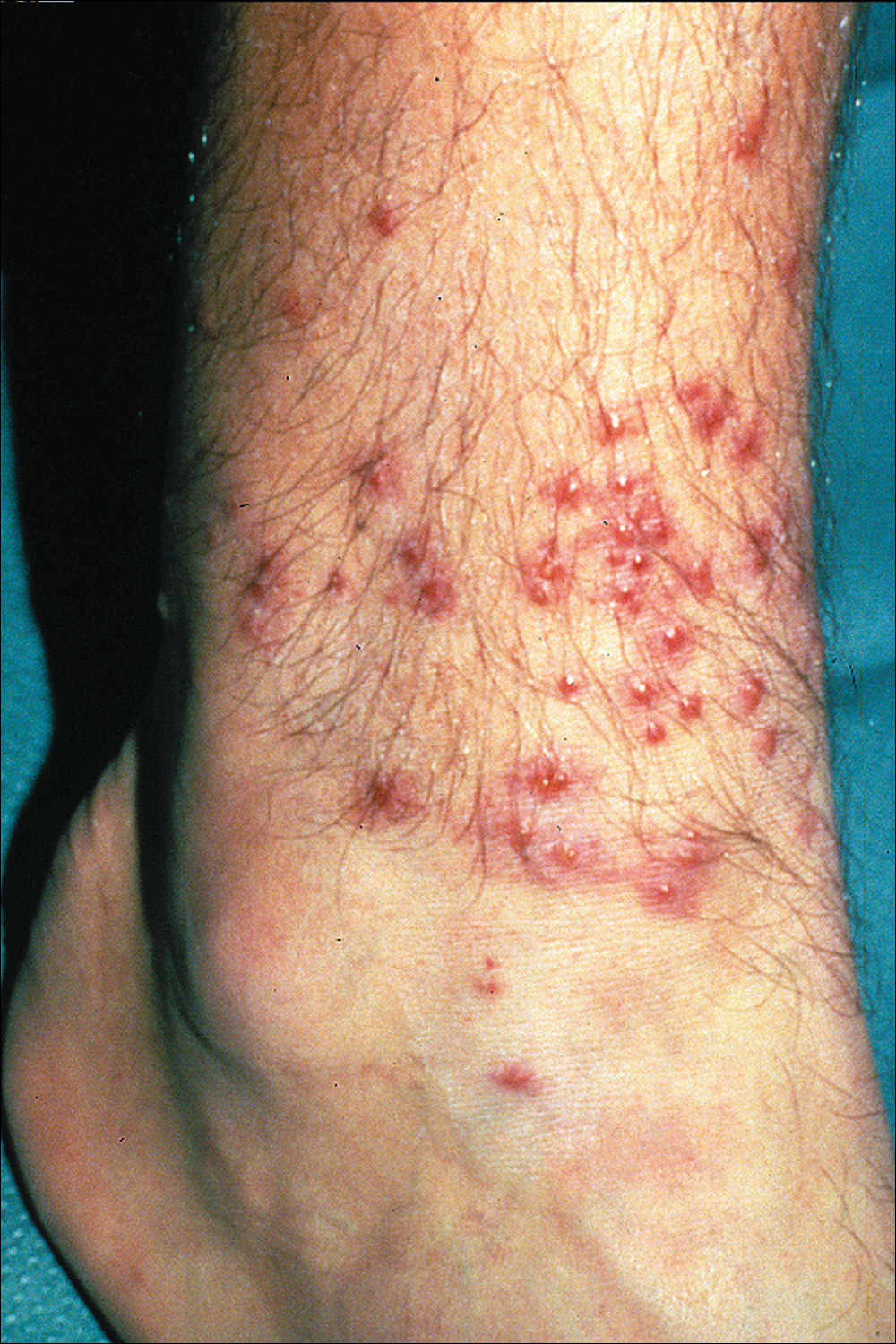
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.
Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).

The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.

Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
Identifying Characteristics and Disease Transmission
Chiggers belong to the Trombiculidae family of mites and also are referred to as harvest mites, harvest bugs, harvest lice, mower’s mites, and redbugs.1 The term chigger specifically describes the larval stage of this mite’s life cycle, as it is the only stage responsible for chigger bites. The nymph and adult phases feed on vegetable matter. Trombiculid mites are most often found in forests, grassy areas, gardens, and moist areas of soil near bodies of water. Trombicula alfreddugesi is the most common species in the United States, and these mites mainly live in the southeastern and south central regions of the country. Conversely, Trombicula autumnalis is most predominant in Western Europe and East Asia.1
The life cycle of the mite includes the egg, larval, nymphal, and adult stages.2 Due to their need for air humidity greater than 80%, mites lay their eggs on low leaves, blades of grass, or on the ground. They spend most of their lives on vegetation no more than 30 cm above ground level.3 Eggs remain dormant for approximately 6 days until the hatching of the prelarvae, which have 6 legs and are nonfeeding. It takes another 6 days for the prelarvae to mature into larvae. Measuring 0.15 to 0.3 mm in length, mite larvae are a mere fraction of the size of adult mites, which generally are 1 to 2 mm in length, and are bright red or brown-red in color (Figure 1).

The biting larvae have many acceptable hosts including turtles, toads, birds, small mammals, and humans, which act as accidental hosts. Larvae remain on vegetation waiting for a suitable host to pass by so they may attach to its skin and remain there for several days. In the exploration for an ideal area to begin feeding (eg, thin epidermis,4 localized increased air humidity5), larvae can travel extensively on the skin; however, they often are stopped by tight-fitting sections of clothing (eg, waistbands), so bites are mostly found in clusters. To feed, mite larvae latch onto the skin using chelicerae, jawlike appendages found in the front of the mouth in arachnids.6 They then inject digestive enzymes that liquefy epidermal cells on direct contact, which results in the formation of a stylostome from which the mites may suck up lymph fluid and broken down tissue.7 Although the actual initial bite is painless, this feeding process leads to the localized inflammation and irritation noticed by infested patients.8
The classic clinical presentation includes severe pruritus and cutaneous swelling as well as erythema caused by the combination of several factors, such as enzyme-induced cellular mechanical damage, human immune response, and sometimes a superimposed bacterial infection. Papules and papulovesicles appear in groups, most commonly affecting the legs and waistline (Figure 2).9 Itching generally occurs within hours of larval latching and subsides within 72 hours. Cutaneous lesions typically take 1 to 2 weeks to heal. In some rare cases, patients may react with urticarial, bullous, or morbilliform eruptions, and the inflammation and pruritus can last for weeks.6 Summer penile syndrome has been noted in boys who display a local hypersensitivity to chigger bites.10 This syndrome represents a triad of penile swelling, dysuria, and pruritus, which lasts for a few days to a few weeks.

Disease Management
Because the lesions are self-healing, treatment is focused on symptomatic relief of itching by means of topical antipruritics (eg, camphor and menthol, pramoxine lotion) or oral antihistamines (eg, diphenhydramine, hydroxyzine). Potent topical corticosteroids may be used to alleviate inflammation and pruritus, especially when occluded under plastic wrap to increase absorption. In severe cases, an intralesional triamcinolone acetonide (2.5–5 mg/mL) injection may be required.9 The best practice, however, is to take preventative measures to avoid becoming a host for the mites. Patients should take special care when traveling in infested areas by completely covering their skin, tucking pant cuffs into their socks, and applying products containing DEET (N,N-diethyl-meta-toluamide or N,N-diethyl-3-methylbenzamide) to the skin and clothing. The odds of prevention are increased even further when clothing also is treated with permethrin.11
In parts of Asia and Australia, these mites may transmit Orientia tsutsugamushi, the organism responsible for scrub typhus, through their saliva during a bite.12 Scrub typhus is associated with an eschar, as well as fever, intense headache, and diffuse myalgia. It responds well to treatment with doxycycline 100 mg twice daily.13 Studies investigating genetic material found in trombiculid mites across the globe have detected Ehrlichia-specific DNA in Spain,14Borrelia-specific DNA in the Czech Republic,15,16 and Hantavirus-specific RNA in Texas.17 There is evidence that the mites play a role in maintenance of zoonotic reservoirs, while humans are infected via ingestion or inhalation of infectious rodent extreta.18
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
- McClain D, Dana AN, Goldenberg G. Mite infestations. Dermatol Ther. 2009;22:327-346.
- Lane RP, Crosskey RW. Medical Insects and Arachnids. London, England: Chapman & Hall; 1993.
- Gasser R, Wyniger R. Distribution and control of Trombiculidae with special reference to Trombicula autumnalis [article in German]. Acta Trop. 1955;12:308-326.
- Jones BM. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology. 1950;40:247-260.
- Farkas J. Concerning the predilected localisation of the manifestations of trombidiosis. predilected localisation and its relation to the ways of invasion [article in German]. Dermatol Monatsschr. 1979;165:858-861.
- Jones JG. Chiggers. Am Fam Physician. 1987;36:149-152.
- Shatrov AB. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol. 2009;49:261-280.
- Potts J. Eradication of ectoparasites in children. how to treat infestations of lice, scabies, and chiggers. Postgrad Med. 2001;110:57-59, 63-64.
- Elston DM. Arthropods and infestations. Infectious Diseases of the Skin. Boca Raton, FL; CRC Press; 2009:112-116.
- Smith GA, Sharma V, Knapp JF, et al. The summer penile syndrome: seasonal acute hypersensitivity reaction caused by chigger bites on the penis. Pediatr Emerg Care. 1998;14:116-118.
- Young GD, Evans S. Safety of DEET and permethrin in the prevention of arthropod attack. Military Med. 1998;163:324-330.
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429-436.
- Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2000;2:CD002150.
- Fernández-Soto P, Pérez-Sánchez R, Encinas-Grandes A. Molecular detection of Ehrlichia phagocytophila genogroup organisms in larvae of Neotrombicula autumnalis (Acari: Trombiculidae) captured in Spain. J Parasitol. 2001;87:1482-1483.
- Literak I, Stekolnikov AA, Sychra O, et al. Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 2008;44:307-314.
- Kampen H, Schöler A, Metzen M, et al. Neotrombicula autumnalis (Acari, Trombiculidae) as a vector for Borrelia burgdorferi sensu lato? Exp Appl Acarol. 2004;33:93-102.
- Houck MA, Qin H, Roberts HR. Hantavirus transmission: potential role of ectoparasites. Vector Borne Zoonotic Dis. 2001;1:75-79.
- Yu XJ, Tesh RB. The role of mites in the transmission and maintenance of Hantaan virus (Hantavirus: Bunyaviridae). J Infect Dis. 2014;210:1693-1699.
Practice Points
- The classic clinical presentation of chigger bites includes severe pruritus, cutaneous swelling, and erythematous papules and papulovesicles appearing in groups, most commonly affecting the legs and waistline.
- Because itching generally subsides within 72 hours of the chigger bite and cutaneous lesions typically heal within 1 to 2 weeks, treatment is focused on symptomatic relief.
- Symptomatic relief may be achieved by means of topical antipruritics or oral antihistamines as well as potent topical corticosteroids or an intralesional triamcinolone acetonide injection in severe cases.
Narrowband UVB Treatment Increases Serum 25-Hydroxyvitamin D Levels in Patients With Chronic Plaque Psoriasis
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).
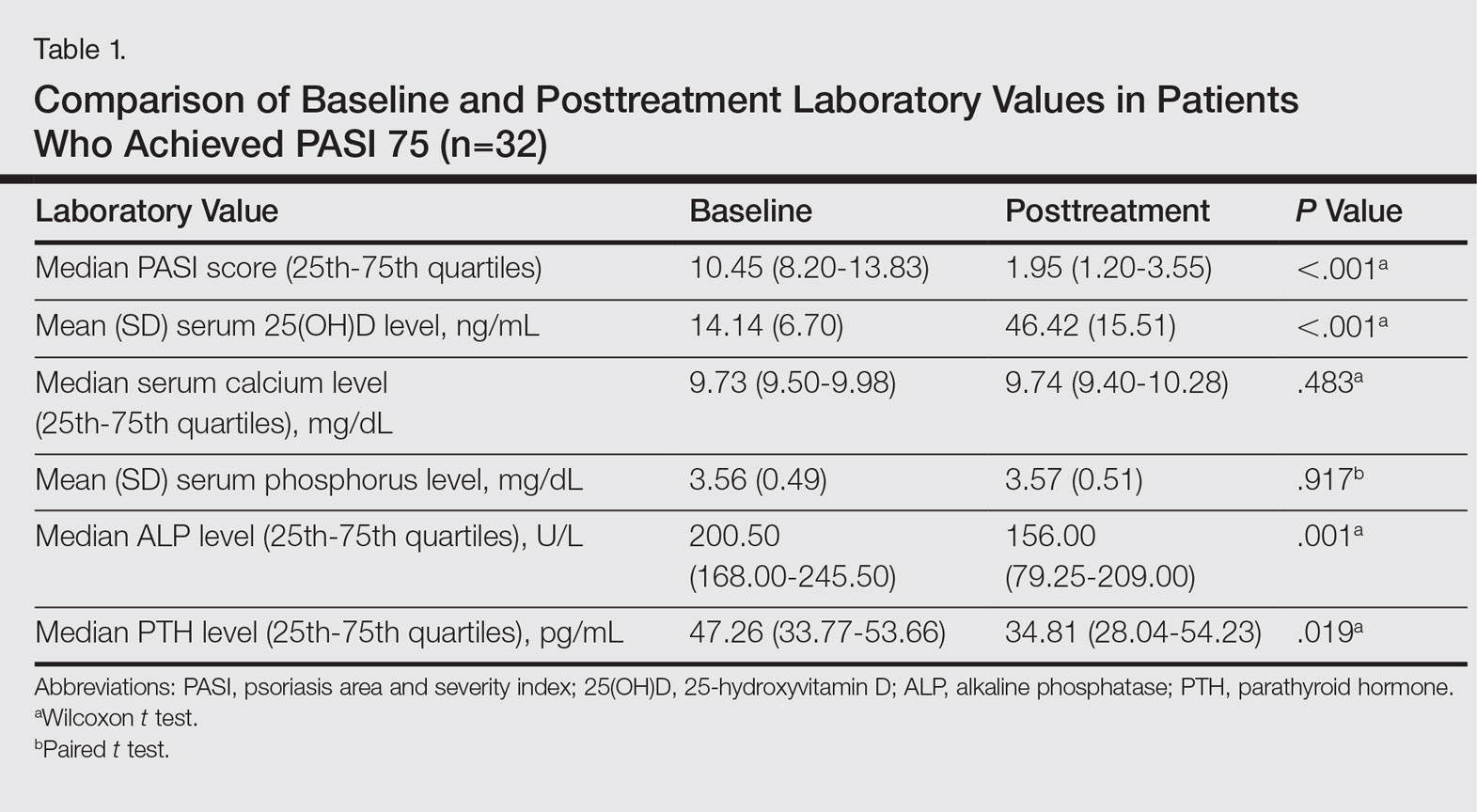
Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).
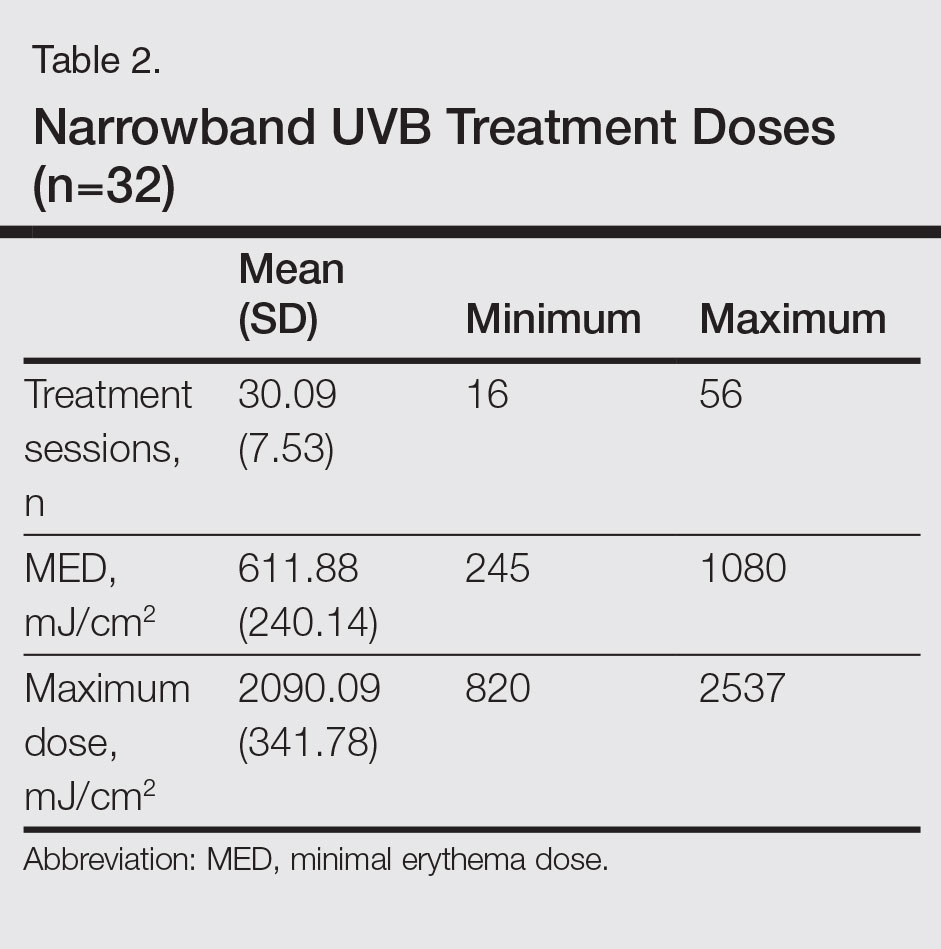
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).

Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).

Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).

Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).

Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
Practice Points
- The 25-hydroxyvitamin D (25[OH]D) levels are increased by narrowband UVB (NB-UVB) treatment in psoriasis patients.
- The number of sessions of NB-UVB is associated with increased 25(OH)D levels.
Leukocytoclastic Vasculitis Resolution With Topical Dapsone
Leukocytoclastic vasculitis (LCV) is a disease characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.1 Numerous etiologies have been described, but the disease commonly remains idiopathic.2,3 Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment. Chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids. These agents are effective but carry risks of serious side effects.4,5 These side effects and/or medical contraindications prevent some patients from taking systemic medications for LCV. We present a case of LCV that resolved after treatment with topical dapsone, highlighting a potential new treatment ofLCV with a markedly better side-effect profile.
Case Report
A 60-year-old woman with recent upper respiratory tract and sinus infections presented to our dermatology clinic with painful palpable purpura on the bilateral shins, thighs, and dorsal aspects of the feet of several months’ duration (Figure, A). Her primary care provider initiated treatment with amoxicillin and doxycycline for the infections. When the rash developed approximately 1.5 weeks following initiation of her symptoms, the patient was referred to the dermatology and rheumatology departments at our institution. The treating dermatologist (M.B.T.) obtained a 4-mm punch biopsy from the right lower leg and LCV was shown on histology. The patient completed a 14-day course of doxycycline and amoxicillin without resolution of the eruption. After an extensive investigation, the treating rheumatologist concluded that the LCV was idiopathic or secondary to an infection or drug exposure. The rheumatologist started the patient on oral prednisone for the chronic symptomatic LCV, but she was intolerant of this medication and discontinued it after 1 week. Our dermatology clinic started her on triamcinolone cream 0.1% twice daily, but she continued to experience new and worsening lesions. At her follow-up appointment 1 month later, triamcinolone cream was discontinued and dapsone gel 5% twice daily was started. She experienced resolution of her previously recalcitrant LCV within 3 weeks (Figure, B).
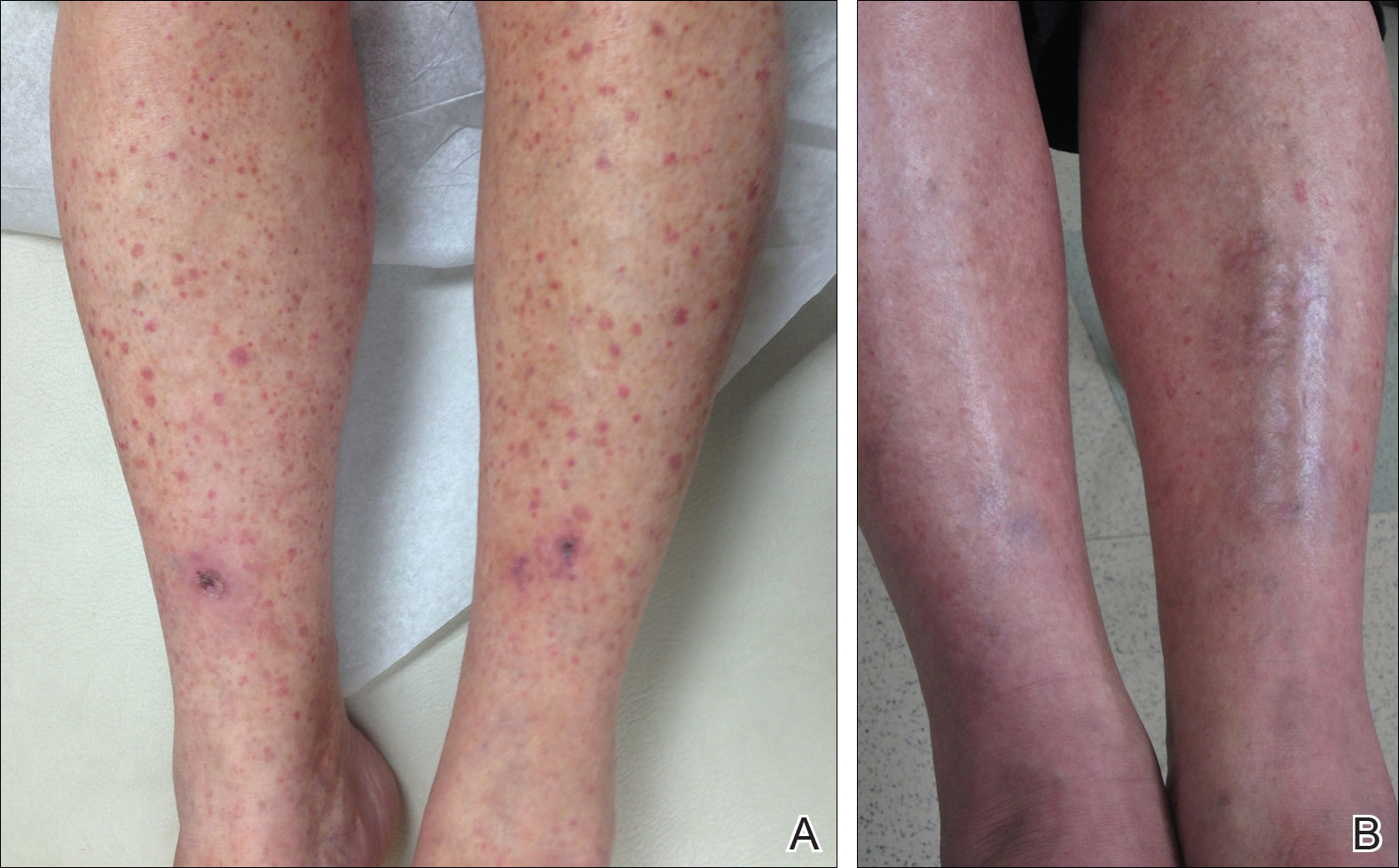
Comment
Established therapies for LCV carry serious side-effect profiles, which can preclude their use.5 Therefore, a topical therapeutic alternative for LCV would be ideal. Systemic prednisone is the first-line therapy for chronic and/or symptomatic LCV, but its side effects include suppression of the hypothalamic-pituitary-adrenal axis, immunosuppression, osteonecrosis, and glucose intolerance.5 Colchicine therapy carries risks for blood dyscrasia, immunosuppression, and gastrointestinal tract upset. Systemic dapsone also is an effective therapy for chronic and/or symptomatic LCV.5,6 However, systemic dapsone requires glucose-6-phosphate dehydrogenase deficiency screening and routine monitoring of blood counts, and it also carries the risk for serious adverse effects including neuropathy, blood dyscrasia, and hypersensitivity syndrome.5,6 Topical dapsone may provide similar efficacy with far fewer adverse effects and has proven to be a safe treatment of acne, even when used in patients with glucose-6-phosphate dehydrogenase deficiency. It displays low systemic absorption and does not accumulate over time once a steady state is reached.7 It also has been shown to be beneficial in other vasculopathies such as erythema elevatum diutinum and in other neutrophilic inflammatory disorders such as pyoderma gangrenosum.8,9 A case of methemoglobinemia due to topical dapsone has been reported.10 Although this effect is rare, clinicians should be aware of such adverse effects when using medications for off-label purposes.
Leukocytoclastic vasculitis can spontaneously resolve; however, our patient’s disease was chronic for several months, and she continued to develop new lesions without signs of resolution. After initiating topical dapsone, she experienced resolution within 3 weeks.
Conclusion
Topical dapsone is a novel approach for treating LCV. Given this drug’s favorable side-effect profile compared to the currently available therapeutic alternatives, we believe it is a reasonable option in select patients. Further investigation is needed to prove its efficacy, but it could be an ideal alternative for patients with contraindications to traditional therapies and/or for those unable to tolerate systemic therapy.
- Koutkia P, Mylonakis E, Rounds S, et al. Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol. 2001;30:315-322.
- Af Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis. clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120:484-489.
- Gyselbrecht L, de Keyser F, Ongenae K, et al. Etiological factors and underlying conditions in patientswith leucocytoclastic vasculitis. Clin Exp Rheumatol. 1996;14:665-668.
- Sais G, Vidaller A, Jucglà A, et al. Colchicine in the treatment of cutaneous leukocytoclastic vasculitis. results of a prospective, randomized controlled trial. Arch Dermatol. 1995;131:1399-1402.
- Sunderkotter C, Bonsmann G, Sindrilaru A, et al. Management of leukocytoclastic vasculitis: clinical review. J Dermatol Treat. 2005;16:193-206.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001;45:420-434.
- Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol. 2009;10:221-227.
- Frieling GW, Williams NL, Lim SJ, et al. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol. 2013;12:481-484.
- Handler MZ, Hamilton H, Aires D. Treatment of peristomal pyoderma gangrenosum with topical crushed dapsone. J Drugs Dermatol. 2011;10:1059-1061.
- Swartzentruber GS, Yanta JH, Pizon AF. Methemoglobi-nemia as a complication of topical dapsone. N Engl J Med. 2015;372:491-492.
Leukocytoclastic vasculitis (LCV) is a disease characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.1 Numerous etiologies have been described, but the disease commonly remains idiopathic.2,3 Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment. Chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids. These agents are effective but carry risks of serious side effects.4,5 These side effects and/or medical contraindications prevent some patients from taking systemic medications for LCV. We present a case of LCV that resolved after treatment with topical dapsone, highlighting a potential new treatment ofLCV with a markedly better side-effect profile.
Case Report
A 60-year-old woman with recent upper respiratory tract and sinus infections presented to our dermatology clinic with painful palpable purpura on the bilateral shins, thighs, and dorsal aspects of the feet of several months’ duration (Figure, A). Her primary care provider initiated treatment with amoxicillin and doxycycline for the infections. When the rash developed approximately 1.5 weeks following initiation of her symptoms, the patient was referred to the dermatology and rheumatology departments at our institution. The treating dermatologist (M.B.T.) obtained a 4-mm punch biopsy from the right lower leg and LCV was shown on histology. The patient completed a 14-day course of doxycycline and amoxicillin without resolution of the eruption. After an extensive investigation, the treating rheumatologist concluded that the LCV was idiopathic or secondary to an infection or drug exposure. The rheumatologist started the patient on oral prednisone for the chronic symptomatic LCV, but she was intolerant of this medication and discontinued it after 1 week. Our dermatology clinic started her on triamcinolone cream 0.1% twice daily, but she continued to experience new and worsening lesions. At her follow-up appointment 1 month later, triamcinolone cream was discontinued and dapsone gel 5% twice daily was started. She experienced resolution of her previously recalcitrant LCV within 3 weeks (Figure, B).

Comment
Established therapies for LCV carry serious side-effect profiles, which can preclude their use.5 Therefore, a topical therapeutic alternative for LCV would be ideal. Systemic prednisone is the first-line therapy for chronic and/or symptomatic LCV, but its side effects include suppression of the hypothalamic-pituitary-adrenal axis, immunosuppression, osteonecrosis, and glucose intolerance.5 Colchicine therapy carries risks for blood dyscrasia, immunosuppression, and gastrointestinal tract upset. Systemic dapsone also is an effective therapy for chronic and/or symptomatic LCV.5,6 However, systemic dapsone requires glucose-6-phosphate dehydrogenase deficiency screening and routine monitoring of blood counts, and it also carries the risk for serious adverse effects including neuropathy, blood dyscrasia, and hypersensitivity syndrome.5,6 Topical dapsone may provide similar efficacy with far fewer adverse effects and has proven to be a safe treatment of acne, even when used in patients with glucose-6-phosphate dehydrogenase deficiency. It displays low systemic absorption and does not accumulate over time once a steady state is reached.7 It also has been shown to be beneficial in other vasculopathies such as erythema elevatum diutinum and in other neutrophilic inflammatory disorders such as pyoderma gangrenosum.8,9 A case of methemoglobinemia due to topical dapsone has been reported.10 Although this effect is rare, clinicians should be aware of such adverse effects when using medications for off-label purposes.
Leukocytoclastic vasculitis can spontaneously resolve; however, our patient’s disease was chronic for several months, and she continued to develop new lesions without signs of resolution. After initiating topical dapsone, she experienced resolution within 3 weeks.
Conclusion
Topical dapsone is a novel approach for treating LCV. Given this drug’s favorable side-effect profile compared to the currently available therapeutic alternatives, we believe it is a reasonable option in select patients. Further investigation is needed to prove its efficacy, but it could be an ideal alternative for patients with contraindications to traditional therapies and/or for those unable to tolerate systemic therapy.
Leukocytoclastic vasculitis (LCV) is a disease characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.1 Numerous etiologies have been described, but the disease commonly remains idiopathic.2,3 Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment. Chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids. These agents are effective but carry risks of serious side effects.4,5 These side effects and/or medical contraindications prevent some patients from taking systemic medications for LCV. We present a case of LCV that resolved after treatment with topical dapsone, highlighting a potential new treatment ofLCV with a markedly better side-effect profile.
Case Report
A 60-year-old woman with recent upper respiratory tract and sinus infections presented to our dermatology clinic with painful palpable purpura on the bilateral shins, thighs, and dorsal aspects of the feet of several months’ duration (Figure, A). Her primary care provider initiated treatment with amoxicillin and doxycycline for the infections. When the rash developed approximately 1.5 weeks following initiation of her symptoms, the patient was referred to the dermatology and rheumatology departments at our institution. The treating dermatologist (M.B.T.) obtained a 4-mm punch biopsy from the right lower leg and LCV was shown on histology. The patient completed a 14-day course of doxycycline and amoxicillin without resolution of the eruption. After an extensive investigation, the treating rheumatologist concluded that the LCV was idiopathic or secondary to an infection or drug exposure. The rheumatologist started the patient on oral prednisone for the chronic symptomatic LCV, but she was intolerant of this medication and discontinued it after 1 week. Our dermatology clinic started her on triamcinolone cream 0.1% twice daily, but she continued to experience new and worsening lesions. At her follow-up appointment 1 month later, triamcinolone cream was discontinued and dapsone gel 5% twice daily was started. She experienced resolution of her previously recalcitrant LCV within 3 weeks (Figure, B).

Comment
Established therapies for LCV carry serious side-effect profiles, which can preclude their use.5 Therefore, a topical therapeutic alternative for LCV would be ideal. Systemic prednisone is the first-line therapy for chronic and/or symptomatic LCV, but its side effects include suppression of the hypothalamic-pituitary-adrenal axis, immunosuppression, osteonecrosis, and glucose intolerance.5 Colchicine therapy carries risks for blood dyscrasia, immunosuppression, and gastrointestinal tract upset. Systemic dapsone also is an effective therapy for chronic and/or symptomatic LCV.5,6 However, systemic dapsone requires glucose-6-phosphate dehydrogenase deficiency screening and routine monitoring of blood counts, and it also carries the risk for serious adverse effects including neuropathy, blood dyscrasia, and hypersensitivity syndrome.5,6 Topical dapsone may provide similar efficacy with far fewer adverse effects and has proven to be a safe treatment of acne, even when used in patients with glucose-6-phosphate dehydrogenase deficiency. It displays low systemic absorption and does not accumulate over time once a steady state is reached.7 It also has been shown to be beneficial in other vasculopathies such as erythema elevatum diutinum and in other neutrophilic inflammatory disorders such as pyoderma gangrenosum.8,9 A case of methemoglobinemia due to topical dapsone has been reported.10 Although this effect is rare, clinicians should be aware of such adverse effects when using medications for off-label purposes.
Leukocytoclastic vasculitis can spontaneously resolve; however, our patient’s disease was chronic for several months, and she continued to develop new lesions without signs of resolution. After initiating topical dapsone, she experienced resolution within 3 weeks.
Conclusion
Topical dapsone is a novel approach for treating LCV. Given this drug’s favorable side-effect profile compared to the currently available therapeutic alternatives, we believe it is a reasonable option in select patients. Further investigation is needed to prove its efficacy, but it could be an ideal alternative for patients with contraindications to traditional therapies and/or for those unable to tolerate systemic therapy.
- Koutkia P, Mylonakis E, Rounds S, et al. Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol. 2001;30:315-322.
- Af Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis. clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120:484-489.
- Gyselbrecht L, de Keyser F, Ongenae K, et al. Etiological factors and underlying conditions in patientswith leucocytoclastic vasculitis. Clin Exp Rheumatol. 1996;14:665-668.
- Sais G, Vidaller A, Jucglà A, et al. Colchicine in the treatment of cutaneous leukocytoclastic vasculitis. results of a prospective, randomized controlled trial. Arch Dermatol. 1995;131:1399-1402.
- Sunderkotter C, Bonsmann G, Sindrilaru A, et al. Management of leukocytoclastic vasculitis: clinical review. J Dermatol Treat. 2005;16:193-206.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001;45:420-434.
- Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol. 2009;10:221-227.
- Frieling GW, Williams NL, Lim SJ, et al. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol. 2013;12:481-484.
- Handler MZ, Hamilton H, Aires D. Treatment of peristomal pyoderma gangrenosum with topical crushed dapsone. J Drugs Dermatol. 2011;10:1059-1061.
- Swartzentruber GS, Yanta JH, Pizon AF. Methemoglobi-nemia as a complication of topical dapsone. N Engl J Med. 2015;372:491-492.
- Koutkia P, Mylonakis E, Rounds S, et al. Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol. 2001;30:315-322.
- Af Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis. clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120:484-489.
- Gyselbrecht L, de Keyser F, Ongenae K, et al. Etiological factors and underlying conditions in patientswith leucocytoclastic vasculitis. Clin Exp Rheumatol. 1996;14:665-668.
- Sais G, Vidaller A, Jucglà A, et al. Colchicine in the treatment of cutaneous leukocytoclastic vasculitis. results of a prospective, randomized controlled trial. Arch Dermatol. 1995;131:1399-1402.
- Sunderkotter C, Bonsmann G, Sindrilaru A, et al. Management of leukocytoclastic vasculitis: clinical review. J Dermatol Treat. 2005;16:193-206.
- Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001;45:420-434.
- Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: a review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol. 2009;10:221-227.
- Frieling GW, Williams NL, Lim SJ, et al. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol. 2013;12:481-484.
- Handler MZ, Hamilton H, Aires D. Treatment of peristomal pyoderma gangrenosum with topical crushed dapsone. J Drugs Dermatol. 2011;10:1059-1061.
- Swartzentruber GS, Yanta JH, Pizon AF. Methemoglobi-nemia as a complication of topical dapsone. N Engl J Med. 2015;372:491-492.
Practice Points
- Leukocytoclastic vasculitis is characterized by inflammation of small vessels with characteristic clinical findings of petechiae and palpable purpura.
- Leukocytoclastic vasculitis often spontaneously resolves within weeks and requires only symptomatic treatment, but chronic or severe disease can require systemic medical treatment with agents such as colchicine, dapsone, and corticosteroids.
Segmental Vitiligo–like Hypopigmentation Associated With Metastatic Melanoma
To the Editor:
Melanoma-associated hypopigmentation frequently has been reported during the disease course and can include different characteristics such as regression of the primary melanoma and/or its metastases as well as common vitiligolike hypopigmentation at sites distant from the melanoma.1,2 Among patients who present with hypopigmentation, the most common clinical presentation is hypopigmented patches in a bilateral symmetric distribution that is similar to vitiligo.1 We report a case of segmental vitiligo–like hypopigmentation associated with melanoma.
RELATED ARTICLE: Novel Melanoma Therapies and Their Side Effects
A 37-year-old man presented with achromic patches on the right side of the neck and lower face of 2 months’ duration. He had a history of melanoma (Breslow thickness, 1.37 mm; mitotic rate, 4/mm2) on the right retroauricular region that was treated by wide local excision 12 years prior; after 10 years, he began to have headaches. At that time, imaging studies including computed tomography, magnetic resonance imaging, and positron emission tomography–computed tomography revealed multiple nodules on the brain, lungs, pancreas, left scapula, and left suprarenal gland. A lung biopsy confirmed metastatic melanoma. Intr
On physical examination using a Wood lamp at the current presentation 2 months later, the achromic patches were linearly distributed on the inferior portion of the right cheek (Figure). A 2×3-cm atrophic scar was present on the right retroauricular region. No regional or distant lymph nodes were enlarged or hard on examination. Although vitiligo is diagnosed using clinical findings,3 a biopsy was performed and showed absence of melanocytes at the dermoepidermal junction (hematoxylin and eosin stain) and complete absence of melanin pigment (Fontana-Masson stain). The patient was treated with topical tacrolimus with poor improvement after 2 months.
The relationship between melanoma and vitiligolike hypopigmentation is a fascinating and controversial topic. Its association is considered to be a consequence of the immune-mediated response against antigens shared by normal melanocytes and melanoma cells.4 Vitiligolike hypopigmentation occurs in 2.8%2 of melanoma patients and is reported in metastatic disease1 as well as those undergoing immunotherapy with or without chemotherapy.5 Its development in patients with stage III or IV melanoma seems to represent an independent positive prognostic factor2 and correlates with a better therapeutic outcome in patients undergoing treatment with biotherapy.5
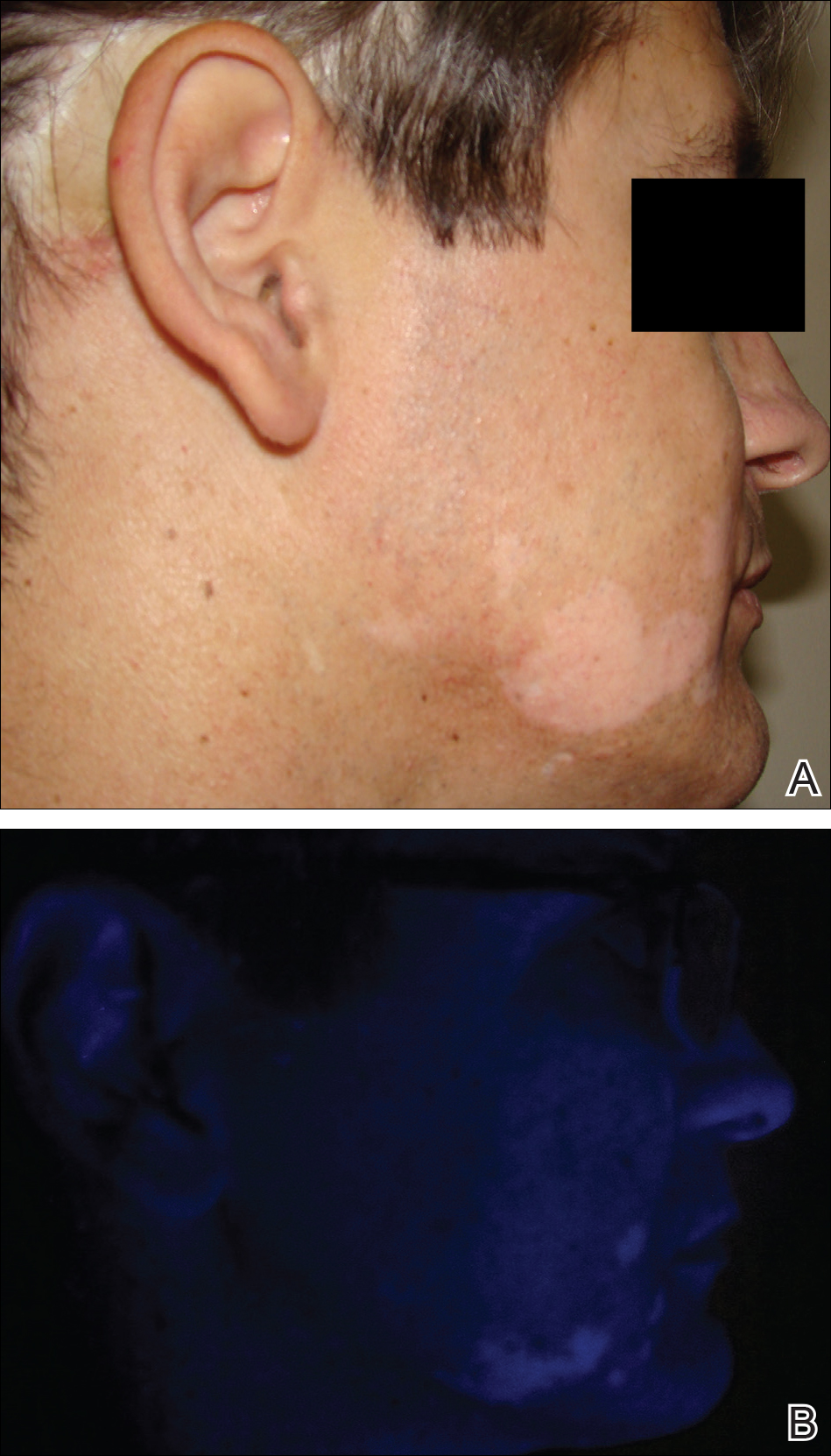
In most cases, the onset of achromic lesions follows the diagnosis of melanoma. Hypopigmentation appears on average 4.8 years after the initial diagnosis and approximately 1 to 2 years after lymph node or distant metastasis.1 In our case, it occurred 12 years after the initial diagnosis and 2 years after metastatic disease was diagnosed.
Despite having widespread metastatic melanoma, our patient only developed achromic patches on the area near the prior melanoma. However, most affected patients present with hypopigmented patches in a bilateral symmetric distribution pattern similar to common vitiligo. No correlation has been found between the hypopigmentation distribution and the location of the primary tumor.1
Because fotemustine is not likely to induce hypopigmentation, we believe that the vitiligolike hypopigmentation in our patient was related to an immune-mediated response associated with melanoma. To help explain our findings, one hypothesis considered was that cutaneous mosaicism may be involved in segmental vitiligo.6 The tumor may have triggered an immune response that affected a close susceptible area of mosaic vitiligo, leading to these clinical findings.
- Hartmann A, Bedenk C, Keikavoussi P, et al. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6:1053-1059.
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409-414.
- Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27-35.
- Becker JC, Guldberg P, Zeuthen J, et al. Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J Invest Dermatol. 1999;113:1033-1038.
- Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658-2663.
- Van Geel N, Speeckaert R, Melsens E, et al. The distribution pattern of segmental vitiligo: clues for somatic mosaicism. Br J Dermatol. 2013;168:56-64.
To the Editor:
Melanoma-associated hypopigmentation frequently has been reported during the disease course and can include different characteristics such as regression of the primary melanoma and/or its metastases as well as common vitiligolike hypopigmentation at sites distant from the melanoma.1,2 Among patients who present with hypopigmentation, the most common clinical presentation is hypopigmented patches in a bilateral symmetric distribution that is similar to vitiligo.1 We report a case of segmental vitiligo–like hypopigmentation associated with melanoma.
RELATED ARTICLE: Novel Melanoma Therapies and Their Side Effects
A 37-year-old man presented with achromic patches on the right side of the neck and lower face of 2 months’ duration. He had a history of melanoma (Breslow thickness, 1.37 mm; mitotic rate, 4/mm2) on the right retroauricular region that was treated by wide local excision 12 years prior; after 10 years, he began to have headaches. At that time, imaging studies including computed tomography, magnetic resonance imaging, and positron emission tomography–computed tomography revealed multiple nodules on the brain, lungs, pancreas, left scapula, and left suprarenal gland. A lung biopsy confirmed metastatic melanoma. Intr
On physical examination using a Wood lamp at the current presentation 2 months later, the achromic patches were linearly distributed on the inferior portion of the right cheek (Figure). A 2×3-cm atrophic scar was present on the right retroauricular region. No regional or distant lymph nodes were enlarged or hard on examination. Although vitiligo is diagnosed using clinical findings,3 a biopsy was performed and showed absence of melanocytes at the dermoepidermal junction (hematoxylin and eosin stain) and complete absence of melanin pigment (Fontana-Masson stain). The patient was treated with topical tacrolimus with poor improvement after 2 months.
The relationship between melanoma and vitiligolike hypopigmentation is a fascinating and controversial topic. Its association is considered to be a consequence of the immune-mediated response against antigens shared by normal melanocytes and melanoma cells.4 Vitiligolike hypopigmentation occurs in 2.8%2 of melanoma patients and is reported in metastatic disease1 as well as those undergoing immunotherapy with or without chemotherapy.5 Its development in patients with stage III or IV melanoma seems to represent an independent positive prognostic factor2 and correlates with a better therapeutic outcome in patients undergoing treatment with biotherapy.5

In most cases, the onset of achromic lesions follows the diagnosis of melanoma. Hypopigmentation appears on average 4.8 years after the initial diagnosis and approximately 1 to 2 years after lymph node or distant metastasis.1 In our case, it occurred 12 years after the initial diagnosis and 2 years after metastatic disease was diagnosed.
Despite having widespread metastatic melanoma, our patient only developed achromic patches on the area near the prior melanoma. However, most affected patients present with hypopigmented patches in a bilateral symmetric distribution pattern similar to common vitiligo. No correlation has been found between the hypopigmentation distribution and the location of the primary tumor.1
Because fotemustine is not likely to induce hypopigmentation, we believe that the vitiligolike hypopigmentation in our patient was related to an immune-mediated response associated with melanoma. To help explain our findings, one hypothesis considered was that cutaneous mosaicism may be involved in segmental vitiligo.6 The tumor may have triggered an immune response that affected a close susceptible area of mosaic vitiligo, leading to these clinical findings.
To the Editor:
Melanoma-associated hypopigmentation frequently has been reported during the disease course and can include different characteristics such as regression of the primary melanoma and/or its metastases as well as common vitiligolike hypopigmentation at sites distant from the melanoma.1,2 Among patients who present with hypopigmentation, the most common clinical presentation is hypopigmented patches in a bilateral symmetric distribution that is similar to vitiligo.1 We report a case of segmental vitiligo–like hypopigmentation associated with melanoma.
RELATED ARTICLE: Novel Melanoma Therapies and Their Side Effects
A 37-year-old man presented with achromic patches on the right side of the neck and lower face of 2 months’ duration. He had a history of melanoma (Breslow thickness, 1.37 mm; mitotic rate, 4/mm2) on the right retroauricular region that was treated by wide local excision 12 years prior; after 10 years, he began to have headaches. At that time, imaging studies including computed tomography, magnetic resonance imaging, and positron emission tomography–computed tomography revealed multiple nodules on the brain, lungs, pancreas, left scapula, and left suprarenal gland. A lung biopsy confirmed metastatic melanoma. Intr
On physical examination using a Wood lamp at the current presentation 2 months later, the achromic patches were linearly distributed on the inferior portion of the right cheek (Figure). A 2×3-cm atrophic scar was present on the right retroauricular region. No regional or distant lymph nodes were enlarged or hard on examination. Although vitiligo is diagnosed using clinical findings,3 a biopsy was performed and showed absence of melanocytes at the dermoepidermal junction (hematoxylin and eosin stain) and complete absence of melanin pigment (Fontana-Masson stain). The patient was treated with topical tacrolimus with poor improvement after 2 months.
The relationship between melanoma and vitiligolike hypopigmentation is a fascinating and controversial topic. Its association is considered to be a consequence of the immune-mediated response against antigens shared by normal melanocytes and melanoma cells.4 Vitiligolike hypopigmentation occurs in 2.8%2 of melanoma patients and is reported in metastatic disease1 as well as those undergoing immunotherapy with or without chemotherapy.5 Its development in patients with stage III or IV melanoma seems to represent an independent positive prognostic factor2 and correlates with a better therapeutic outcome in patients undergoing treatment with biotherapy.5

In most cases, the onset of achromic lesions follows the diagnosis of melanoma. Hypopigmentation appears on average 4.8 years after the initial diagnosis and approximately 1 to 2 years after lymph node or distant metastasis.1 In our case, it occurred 12 years after the initial diagnosis and 2 years after metastatic disease was diagnosed.
Despite having widespread metastatic melanoma, our patient only developed achromic patches on the area near the prior melanoma. However, most affected patients present with hypopigmented patches in a bilateral symmetric distribution pattern similar to common vitiligo. No correlation has been found between the hypopigmentation distribution and the location of the primary tumor.1
Because fotemustine is not likely to induce hypopigmentation, we believe that the vitiligolike hypopigmentation in our patient was related to an immune-mediated response associated with melanoma. To help explain our findings, one hypothesis considered was that cutaneous mosaicism may be involved in segmental vitiligo.6 The tumor may have triggered an immune response that affected a close susceptible area of mosaic vitiligo, leading to these clinical findings.
- Hartmann A, Bedenk C, Keikavoussi P, et al. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6:1053-1059.
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409-414.
- Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27-35.
- Becker JC, Guldberg P, Zeuthen J, et al. Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J Invest Dermatol. 1999;113:1033-1038.
- Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658-2663.
- Van Geel N, Speeckaert R, Melsens E, et al. The distribution pattern of segmental vitiligo: clues for somatic mosaicism. Br J Dermatol. 2013;168:56-64.
- Hartmann A, Bedenk C, Keikavoussi P, et al. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6:1053-1059.
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409-414.
- Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27-35.
- Becker JC, Guldberg P, Zeuthen J, et al. Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J Invest Dermatol. 1999;113:1033-1038.
- Boasberg PD, Hoon DS, Piro LD, et al. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658-2663.
- Van Geel N, Speeckaert R, Melsens E, et al. The distribution pattern of segmental vitiligo: clues for somatic mosaicism. Br J Dermatol. 2013;168:56-64.
Prac
- Melanoma-associated hypopigmentation usually manifests as common vitiligo; however, little is known about the pathophysiology of segmental vitiligo–like hypopigmentation associated with melanoma.
- This case of segmental vitiligo–like hypopigmentation associated with melanoma sheds light on possible autoimmune and mosaic disease etiology.
Isotretinoin for Acne: Tips for Prescribing and Managing Patient Concerns
What does your patient need to know at the first visit?
Most important is what you need to know before the first visit. As the prescribing physician, you must be familiar with the iPLEDGE program. Because of the complexity of the program, consider identifying a physician in your area to refer patients if you are not going to be a regular prescriber of the medication.
If you are enrolled in iPLEDGE, let your patients (and/or their parents/guardians) know that there is a great deal of misinformation on the Internet. Reiterate that you and your staff are available to discuss their concerns. Also, give them reliable sources of information, such as the American Academy of Dermatology's patient information sheet as well as the Mayo Clinic's acne information. Drugs.com is another resource.
All patients—males, females who cannot become pregnant, and females of childbearing potential (FCBPs)—must be aware that this medication can cause birth defects if taken during pregnancy. They must be informed that the medication is not to be shared with anyone and that they should not give blood while taking this medication.
What treatment course do you recommend?
My evidence-based approach is a course of isotretinoin totaling a minimum of 150 mg per kilogram body weight. Do not give a more abbreviated course unless the patient has cleared early; even then I tend to complete 150 mg when possible. There is published evidence that pushing the course to a total of 220 mg per kilogram body weight results in a longer remission.
Generally, I do few laboratory tests other than pretreatment lipid panels as well as 1 or 2 follow-up lipid panels at monthly intervals. To comply with the iPLEDGE program, FCBP patients must have a monthly pregnancy test, which is reported on the iPLEDGE website before the patient can be prescribed the drug and receive the drug from a pharmacist who is participating in the iPLEDGE program.
One of the defects of the iPLEDGE system is that although only a 30-day supply of pills can be prescribed, it is difficult to always bring a patient back in exactly 30 days; for example, we work on a 4-week cycle and 30 days brings us into the next week or uncommonly the weekend when we do not see patients. Our male patients or females not of childbearing potential are not affected, but for our FCBP patients, it means usually scheduling visits at 35-day intervals because the pregnancy tests must be performed at minimum 28-day intervals and the prescription cannot be written and the pregnancy test recorded until after at least 30 days.
What are the side effects?
The common side effects are what you would expect from a medicine that is supposed to dry up the oil on your skin: dryness of the lips, mouth, and skin, as well as rashes due to the dryness. There also can be minor swelling of the eyelids or lips, nosebleeds, upset stomach, and thinning of the hair; dryness of the scalp may occur. I recommend using a little petroleum jelly inside the nostrils at night to counteract the dryness that leads to nosebleeds, and saline drops or gel for the eyes, especially for contact lens wearers.
Joint aches and pains have been reported, though I rarely see those effects in patients who are physically active such as those participating in competitive sports. Mood changes have been reported, including suicidal ideation.
What do you do if patients refuse treatment?
There is so much false information on the Internet about the dangers of isotretinoin, leaving some patients (and parents/guardians) too afraid to use it. I sympathize with this anxiety, but I do endeavor to point out that the birth defects occur only in women taking the drug while pregnant and have not been reported to occur after the drug is out of the patient's system.
Similarly, I point out that almost all of the evidence-based studies failed to confirm any association between the use of isotretinoin and depression, teenage suicide, and subsequent inflammatory bowel disease. Nonetheless, I mention these issues and recommend that the parents/guardians observe the teenager; in the case of adult patients, they themselves must be sensitive to symptoms.
Suggested Readings
American Academy of Dermatology Association. Position statement on isotretinoin. https://www.aad.org/Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Published December 9, 2000. Updated November 13, 2010. Accessed May 18, 2017.
Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398.
What does your patient need to know at the first visit?
Most important is what you need to know before the first visit. As the prescribing physician, you must be familiar with the iPLEDGE program. Because of the complexity of the program, consider identifying a physician in your area to refer patients if you are not going to be a regular prescriber of the medication.
If you are enrolled in iPLEDGE, let your patients (and/or their parents/guardians) know that there is a great deal of misinformation on the Internet. Reiterate that you and your staff are available to discuss their concerns. Also, give them reliable sources of information, such as the American Academy of Dermatology's patient information sheet as well as the Mayo Clinic's acne information. Drugs.com is another resource.
All patients—males, females who cannot become pregnant, and females of childbearing potential (FCBPs)—must be aware that this medication can cause birth defects if taken during pregnancy. They must be informed that the medication is not to be shared with anyone and that they should not give blood while taking this medication.
What treatment course do you recommend?
My evidence-based approach is a course of isotretinoin totaling a minimum of 150 mg per kilogram body weight. Do not give a more abbreviated course unless the patient has cleared early; even then I tend to complete 150 mg when possible. There is published evidence that pushing the course to a total of 220 mg per kilogram body weight results in a longer remission.
Generally, I do few laboratory tests other than pretreatment lipid panels as well as 1 or 2 follow-up lipid panels at monthly intervals. To comply with the iPLEDGE program, FCBP patients must have a monthly pregnancy test, which is reported on the iPLEDGE website before the patient can be prescribed the drug and receive the drug from a pharmacist who is participating in the iPLEDGE program.
One of the defects of the iPLEDGE system is that although only a 30-day supply of pills can be prescribed, it is difficult to always bring a patient back in exactly 30 days; for example, we work on a 4-week cycle and 30 days brings us into the next week or uncommonly the weekend when we do not see patients. Our male patients or females not of childbearing potential are not affected, but for our FCBP patients, it means usually scheduling visits at 35-day intervals because the pregnancy tests must be performed at minimum 28-day intervals and the prescription cannot be written and the pregnancy test recorded until after at least 30 days.
What are the side effects?
The common side effects are what you would expect from a medicine that is supposed to dry up the oil on your skin: dryness of the lips, mouth, and skin, as well as rashes due to the dryness. There also can be minor swelling of the eyelids or lips, nosebleeds, upset stomach, and thinning of the hair; dryness of the scalp may occur. I recommend using a little petroleum jelly inside the nostrils at night to counteract the dryness that leads to nosebleeds, and saline drops or gel for the eyes, especially for contact lens wearers.
Joint aches and pains have been reported, though I rarely see those effects in patients who are physically active such as those participating in competitive sports. Mood changes have been reported, including suicidal ideation.
What do you do if patients refuse treatment?
There is so much false information on the Internet about the dangers of isotretinoin, leaving some patients (and parents/guardians) too afraid to use it. I sympathize with this anxiety, but I do endeavor to point out that the birth defects occur only in women taking the drug while pregnant and have not been reported to occur after the drug is out of the patient's system.
Similarly, I point out that almost all of the evidence-based studies failed to confirm any association between the use of isotretinoin and depression, teenage suicide, and subsequent inflammatory bowel disease. Nonetheless, I mention these issues and recommend that the parents/guardians observe the teenager; in the case of adult patients, they themselves must be sensitive to symptoms.
Suggested Readings
American Academy of Dermatology Association. Position statement on isotretinoin. https://www.aad.org/Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Published December 9, 2000. Updated November 13, 2010. Accessed May 18, 2017.
Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398.
What does your patient need to know at the first visit?
Most important is what you need to know before the first visit. As the prescribing physician, you must be familiar with the iPLEDGE program. Because of the complexity of the program, consider identifying a physician in your area to refer patients if you are not going to be a regular prescriber of the medication.
If you are enrolled in iPLEDGE, let your patients (and/or their parents/guardians) know that there is a great deal of misinformation on the Internet. Reiterate that you and your staff are available to discuss their concerns. Also, give them reliable sources of information, such as the American Academy of Dermatology's patient information sheet as well as the Mayo Clinic's acne information. Drugs.com is another resource.
All patients—males, females who cannot become pregnant, and females of childbearing potential (FCBPs)—must be aware that this medication can cause birth defects if taken during pregnancy. They must be informed that the medication is not to be shared with anyone and that they should not give blood while taking this medication.
What treatment course do you recommend?
My evidence-based approach is a course of isotretinoin totaling a minimum of 150 mg per kilogram body weight. Do not give a more abbreviated course unless the patient has cleared early; even then I tend to complete 150 mg when possible. There is published evidence that pushing the course to a total of 220 mg per kilogram body weight results in a longer remission.
Generally, I do few laboratory tests other than pretreatment lipid panels as well as 1 or 2 follow-up lipid panels at monthly intervals. To comply with the iPLEDGE program, FCBP patients must have a monthly pregnancy test, which is reported on the iPLEDGE website before the patient can be prescribed the drug and receive the drug from a pharmacist who is participating in the iPLEDGE program.
One of the defects of the iPLEDGE system is that although only a 30-day supply of pills can be prescribed, it is difficult to always bring a patient back in exactly 30 days; for example, we work on a 4-week cycle and 30 days brings us into the next week or uncommonly the weekend when we do not see patients. Our male patients or females not of childbearing potential are not affected, but for our FCBP patients, it means usually scheduling visits at 35-day intervals because the pregnancy tests must be performed at minimum 28-day intervals and the prescription cannot be written and the pregnancy test recorded until after at least 30 days.
What are the side effects?
The common side effects are what you would expect from a medicine that is supposed to dry up the oil on your skin: dryness of the lips, mouth, and skin, as well as rashes due to the dryness. There also can be minor swelling of the eyelids or lips, nosebleeds, upset stomach, and thinning of the hair; dryness of the scalp may occur. I recommend using a little petroleum jelly inside the nostrils at night to counteract the dryness that leads to nosebleeds, and saline drops or gel for the eyes, especially for contact lens wearers.
Joint aches and pains have been reported, though I rarely see those effects in patients who are physically active such as those participating in competitive sports. Mood changes have been reported, including suicidal ideation.
What do you do if patients refuse treatment?
There is so much false information on the Internet about the dangers of isotretinoin, leaving some patients (and parents/guardians) too afraid to use it. I sympathize with this anxiety, but I do endeavor to point out that the birth defects occur only in women taking the drug while pregnant and have not been reported to occur after the drug is out of the patient's system.
Similarly, I point out that almost all of the evidence-based studies failed to confirm any association between the use of isotretinoin and depression, teenage suicide, and subsequent inflammatory bowel disease. Nonetheless, I mention these issues and recommend that the parents/guardians observe the teenager; in the case of adult patients, they themselves must be sensitive to symptoms.
Suggested Readings
American Academy of Dermatology Association. Position statement on isotretinoin. https://www.aad.org/Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Published December 9, 2000. Updated November 13, 2010. Accessed May 18, 2017.
Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398.
In Vivo Reflectance Confocal Microscopy
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.
The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.

The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.

The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
Practice Points
- Reflectance confocal microscopy (RCM) recently received Category I Current Procedural Terminology codes for reimbursement comparable to a skin biopsy.
- When used in combination with dermoscopy, RCM has been shown to increase diagnostic accuracy of skin cancer.
- Reflectance confocal microscopy also is useful in surgical treatment planning and monitoring nonsurgical treatments over time.
- Limitations of RCM imaging include low imaging depth, difficulty in imaging certain areas of the skin, learning curve for interpreting these images, and the cost of equipment.