User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Intrahepatic Cholestasis of Pregnancy
To the Editor:
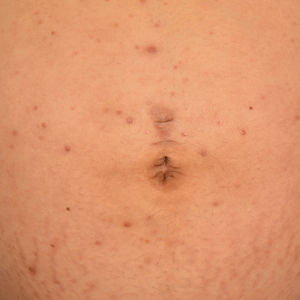
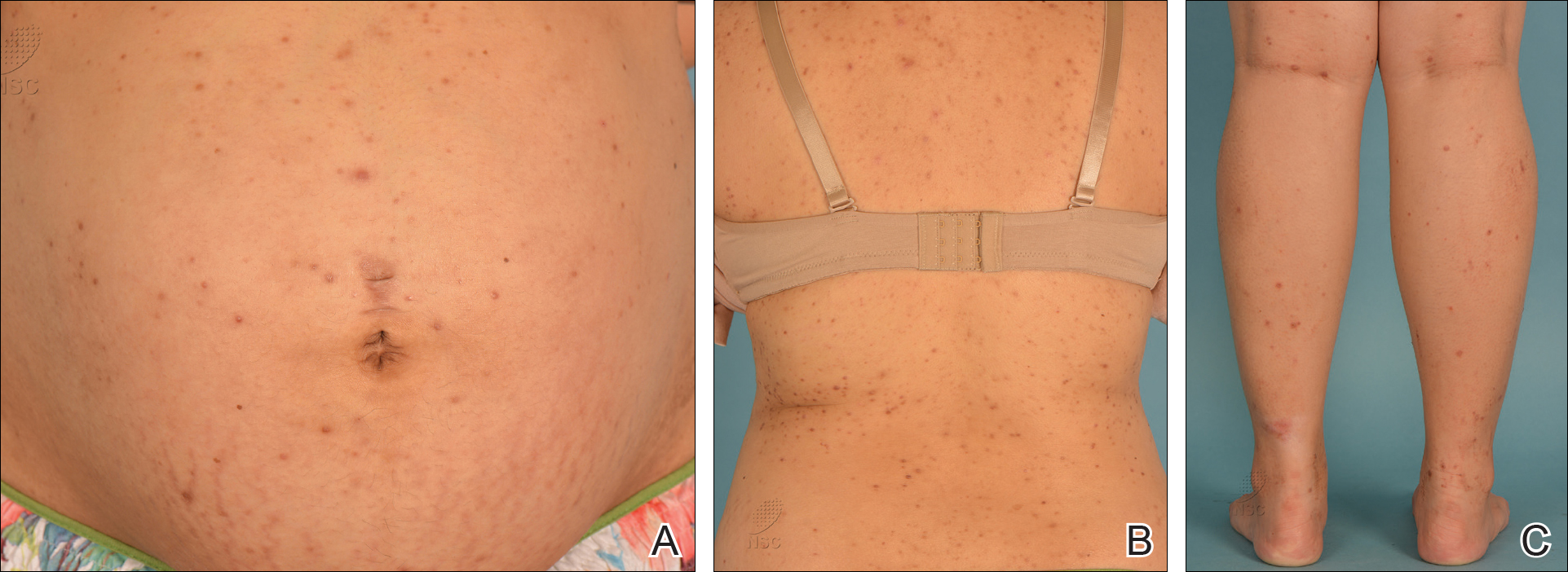
A 28-year-old primigravid woman at 32 weeks’ gestation presented to an outpatient dermatology clinic with a generalized rash and itch of 3 months’ duration. She was distressed with the itch and had tried antihistamines (eg, chlorpheniramine, cetirizine) without relief. She had no notable medical history. Physical examination revealed generalized erythematous papules and nodules on the chest, back, periumbilical region, arms, and legs (Figure). A few pustules were noted on the upper back. No wheals, plaques, vesicles, or bullae were seen.
Laboratory investigations revealed elevated alkaline phosphatase (187 U/L [reference range, 30–120 U/L]), aspartate aminotransferase (45 U/L [reference range, 10–30 U/L]), alanine aminotransferase (120 U/L [reference range, 10–40 U/L]), and γ-glutamyltransferase (48 U/L [reference range, 9–40 U/L]) levels. A fungal scrape of the papules on the upper back demonstrated spores. Subsequent tests included ultrasonography of the liver, which showed fatty changes, as well as rising levels of alkaline phosphatase. Fasting glucose and 2-hour oral glucose tolerance tests showed poorly controlled gestational diabetes mellitus (DM) as well as raised triglycerides.
Based on the patient’s reports of itch, signs of erythematous papules and nodules, and laboratory results of cholestasis, a diagnosis of intrahepatic cholestasis of pregnancy (ICP) was made. The finding of Pityrosporum folliculitis also prompted screening for gestational DM, which was positive.
Treatment with ursodeoxycholic acid (UDCA) 250 mg twice daily was prescribed, which led to some relief of the skin symptoms. Her cutaneous symptoms were discussed with her obstetrician, and a decision was made for emergency cesarean delivery at 37 weeks’ gestation in light of nonreassuring fetal status during her follow-up antenatal ultrasonograph. Her pruritus and poor liver function resolved within 2 weeks after delivery.
Intrahepatic cholestasis of pregnancy is a rare form of reversible cholestasis occurring in the second half of pregnancy. The incidence varies with geographical location and ethnicity.1 It is one of the specific dermatoses of pregnancy and usually presents in the third trimester. It is characterized by pruritus, elevation of serum total bile acids and mild elevations of other liver function tests, and increased rates of adverse fetal outcomes. A positive diagnosis is made by the elevation of the serum total bile acid levels (>11.0 μmol/L [reference range, 0.73–5.63 μmol/L]). It is important for clinicians to recognize ICP because it is associated with fetal prematurity, intrapartal fetal distress, and stillbirths.2
The pathogenesis of ICP is not fully understood. During pregnancy, estrogens interfere with bile acid secretion, and progestins inhibit hepatic glucuronyltransferase. Increased IFN-γ, natural killer cells, and natural killer T cells, as well as decreased T cells in decidua parietalis, also have been reported.3
Mutations in the ATP binding cassette subfamily B member 4 gene, ABCB4, which encodes the multidrug resistance protein 3, a canalicular phosphatidylcholine translocase, have been found in several women with ICP.4 Clinically, patients usually present with pruritus that may precede or follow laboratory abnormalities. The pruritus worsens as the pregnancy advances and can resolve within 48 hours of delivery. Pruritus usually affects the palms and soles but may extend to the legs and abdomen or become generalized.4
Generally, there are no cutaneous signs other than excoriation marks, contrary to primary skin lesions found in other specific dermatoses of pregnancy. Mild jaundice can develop 2 to 4 weeks after the onset of pruritus, which may be associated with subclinical steatorrhea and increased risk of intrapartum and postpartum hemorrhage.5 Of note, ICP may be associated with increased risk for gestational DM, as illustrated in our case.6
Ursodeoxycholic acid currently is the most effective pharmacologic treatment of ICP. It reduces bile acids in cord blood, colostrum, and amniotic fluid.7 A meta-analysis of randomized controlled trials demonstrated that UDCA (450–1200 mg daily) is highly effective in alleviating pruritus and normalizing laboratory abnormalities associated with ICP.8 No severe adverse events have been reported related to UDCA.9,10
Intrahepatic cholestasis of pregnancy has been associated with increased risk for preterm delivery (19%–60%), meconium staining of amniotic fluid (≤27%), fetal bradycardia (≤14%), fetal distress (22%–41%), and fetal loss (0.4%–4.1%).11 The risk for serious fetal complications in ICP makes intensive fetal surveillance mandatory, including weekly nonstress cardiotocography or biophysical assessment from 34 weeks’ gestation. Delivery at 36 weeks or earlier (if lung maturity is achieved and cervix favorable) should be considered for severe cases with jaundice, progressive elevations in serum total bile acids, and suspected fetal distress. At more than 36 weeks’ gestation, amniocentesis and delivery should be considered if cervix is favorable and fetal lung maturity satisfactory.12-14
Our case highlights the importance of diagnosing ICP when a pregnant patient presents with generalized itch associated with elevated liver function tests. Interdisciplinary management involving dermatologists, obstetricians, pediatricians, and gastroenterologists is mandatory to acquire a better outcome for the mother and the fetus.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-474.
- Ling B, Yao F, Zhou Y, et al. Cell-mediated immunity imbalance in patients with intrahepatic cholestasis of pregnancy. Cell Mol Immunol. 2007;4:71-75.
- Dixon PH, Weerasekera N, Linton KJ, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genetics. 2000;9:1209-1217.
- Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
- Martineau M, Raker C, Powrie R, et al. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. Eur J Obstet Gynecol Reprod Biol. 2014;176:80-85.
- Laifer SA, Stiller RJ, Siddiqui DS, et al. Ursodeoxycholic acid for the treatment of intrahepatic cholestasis of pregnancy. J Matern Fetal Med. 2001;10:131-135.
- Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
- Kondrackiene J, Beuers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129:894-901.
- Tan LK. Obstetric cholestasis: current opinions and management. Ann Acad Med Singapore. 2003;32:294-298.
- Ghosh S, Chaudhuri S. Intra-hepatic cholestasis of pregnancy: a comprehensive review. Indian J Dermatol. 2013;58:327.
- Rioseco AJ, Ivankovic MB, Manzur A, et al. Intrahepatic cholestasis of pregnancy: retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. 1994;170:890-895.
- Saleh MM, Abdo KR. Intrahepatic cholestasis of pregnancy: review of the literature and evaluation of current evidence. J Womens Health (Larchmt). 2007;16:833-841.
- Roncaglia N, Arreghini A, Locatelli A, et al. Obstetric cholestasis: outcome with active management. Eur J Obstet Gynecol Reprod Biol. 2002;100:167-170.
To the Editor:
A 28-year-old primigravid woman at 32 weeks’ gestation presented to an outpatient dermatology clinic with a generalized rash and itch of 3 months’ duration. She was distressed with the itch and had tried antihistamines (eg, chlorpheniramine, cetirizine) without relief. She had no notable medical history. Physical examination revealed generalized erythematous papules and nodules on the chest, back, periumbilical region, arms, and legs (Figure). A few pustules were noted on the upper back. No wheals, plaques, vesicles, or bullae were seen.

Laboratory investigations revealed elevated alkaline phosphatase (187 U/L [reference range, 30–120 U/L]), aspartate aminotransferase (45 U/L [reference range, 10–30 U/L]), alanine aminotransferase (120 U/L [reference range, 10–40 U/L]), and γ-glutamyltransferase (48 U/L [reference range, 9–40 U/L]) levels. A fungal scrape of the papules on the upper back demonstrated spores. Subsequent tests included ultrasonography of the liver, which showed fatty changes, as well as rising levels of alkaline phosphatase. Fasting glucose and 2-hour oral glucose tolerance tests showed poorly controlled gestational diabetes mellitus (DM) as well as raised triglycerides.
Based on the patient’s reports of itch, signs of erythematous papules and nodules, and laboratory results of cholestasis, a diagnosis of intrahepatic cholestasis of pregnancy (ICP) was made. The finding of Pityrosporum folliculitis also prompted screening for gestational DM, which was positive.
Treatment with ursodeoxycholic acid (UDCA) 250 mg twice daily was prescribed, which led to some relief of the skin symptoms. Her cutaneous symptoms were discussed with her obstetrician, and a decision was made for emergency cesarean delivery at 37 weeks’ gestation in light of nonreassuring fetal status during her follow-up antenatal ultrasonograph. Her pruritus and poor liver function resolved within 2 weeks after delivery.
Intrahepatic cholestasis of pregnancy is a rare form of reversible cholestasis occurring in the second half of pregnancy. The incidence varies with geographical location and ethnicity.1 It is one of the specific dermatoses of pregnancy and usually presents in the third trimester. It is characterized by pruritus, elevation of serum total bile acids and mild elevations of other liver function tests, and increased rates of adverse fetal outcomes. A positive diagnosis is made by the elevation of the serum total bile acid levels (>11.0 μmol/L [reference range, 0.73–5.63 μmol/L]). It is important for clinicians to recognize ICP because it is associated with fetal prematurity, intrapartal fetal distress, and stillbirths.2
The pathogenesis of ICP is not fully understood. During pregnancy, estrogens interfere with bile acid secretion, and progestins inhibit hepatic glucuronyltransferase. Increased IFN-γ, natural killer cells, and natural killer T cells, as well as decreased T cells in decidua parietalis, also have been reported.3
Mutations in the ATP binding cassette subfamily B member 4 gene, ABCB4, which encodes the multidrug resistance protein 3, a canalicular phosphatidylcholine translocase, have been found in several women with ICP.4 Clinically, patients usually present with pruritus that may precede or follow laboratory abnormalities. The pruritus worsens as the pregnancy advances and can resolve within 48 hours of delivery. Pruritus usually affects the palms and soles but may extend to the legs and abdomen or become generalized.4
Generally, there are no cutaneous signs other than excoriation marks, contrary to primary skin lesions found in other specific dermatoses of pregnancy. Mild jaundice can develop 2 to 4 weeks after the onset of pruritus, which may be associated with subclinical steatorrhea and increased risk of intrapartum and postpartum hemorrhage.5 Of note, ICP may be associated with increased risk for gestational DM, as illustrated in our case.6
Ursodeoxycholic acid currently is the most effective pharmacologic treatment of ICP. It reduces bile acids in cord blood, colostrum, and amniotic fluid.7 A meta-analysis of randomized controlled trials demonstrated that UDCA (450–1200 mg daily) is highly effective in alleviating pruritus and normalizing laboratory abnormalities associated with ICP.8 No severe adverse events have been reported related to UDCA.9,10
Intrahepatic cholestasis of pregnancy has been associated with increased risk for preterm delivery (19%–60%), meconium staining of amniotic fluid (≤27%), fetal bradycardia (≤14%), fetal distress (22%–41%), and fetal loss (0.4%–4.1%).11 The risk for serious fetal complications in ICP makes intensive fetal surveillance mandatory, including weekly nonstress cardiotocography or biophysical assessment from 34 weeks’ gestation. Delivery at 36 weeks or earlier (if lung maturity is achieved and cervix favorable) should be considered for severe cases with jaundice, progressive elevations in serum total bile acids, and suspected fetal distress. At more than 36 weeks’ gestation, amniocentesis and delivery should be considered if cervix is favorable and fetal lung maturity satisfactory.12-14
Our case highlights the importance of diagnosing ICP when a pregnant patient presents with generalized itch associated with elevated liver function tests. Interdisciplinary management involving dermatologists, obstetricians, pediatricians, and gastroenterologists is mandatory to acquire a better outcome for the mother and the fetus.
To the Editor:
A 28-year-old primigravid woman at 32 weeks’ gestation presented to an outpatient dermatology clinic with a generalized rash and itch of 3 months’ duration. She was distressed with the itch and had tried antihistamines (eg, chlorpheniramine, cetirizine) without relief. She had no notable medical history. Physical examination revealed generalized erythematous papules and nodules on the chest, back, periumbilical region, arms, and legs (Figure). A few pustules were noted on the upper back. No wheals, plaques, vesicles, or bullae were seen.

Laboratory investigations revealed elevated alkaline phosphatase (187 U/L [reference range, 30–120 U/L]), aspartate aminotransferase (45 U/L [reference range, 10–30 U/L]), alanine aminotransferase (120 U/L [reference range, 10–40 U/L]), and γ-glutamyltransferase (48 U/L [reference range, 9–40 U/L]) levels. A fungal scrape of the papules on the upper back demonstrated spores. Subsequent tests included ultrasonography of the liver, which showed fatty changes, as well as rising levels of alkaline phosphatase. Fasting glucose and 2-hour oral glucose tolerance tests showed poorly controlled gestational diabetes mellitus (DM) as well as raised triglycerides.
Based on the patient’s reports of itch, signs of erythematous papules and nodules, and laboratory results of cholestasis, a diagnosis of intrahepatic cholestasis of pregnancy (ICP) was made. The finding of Pityrosporum folliculitis also prompted screening for gestational DM, which was positive.
Treatment with ursodeoxycholic acid (UDCA) 250 mg twice daily was prescribed, which led to some relief of the skin symptoms. Her cutaneous symptoms were discussed with her obstetrician, and a decision was made for emergency cesarean delivery at 37 weeks’ gestation in light of nonreassuring fetal status during her follow-up antenatal ultrasonograph. Her pruritus and poor liver function resolved within 2 weeks after delivery.
Intrahepatic cholestasis of pregnancy is a rare form of reversible cholestasis occurring in the second half of pregnancy. The incidence varies with geographical location and ethnicity.1 It is one of the specific dermatoses of pregnancy and usually presents in the third trimester. It is characterized by pruritus, elevation of serum total bile acids and mild elevations of other liver function tests, and increased rates of adverse fetal outcomes. A positive diagnosis is made by the elevation of the serum total bile acid levels (>11.0 μmol/L [reference range, 0.73–5.63 μmol/L]). It is important for clinicians to recognize ICP because it is associated with fetal prematurity, intrapartal fetal distress, and stillbirths.2
The pathogenesis of ICP is not fully understood. During pregnancy, estrogens interfere with bile acid secretion, and progestins inhibit hepatic glucuronyltransferase. Increased IFN-γ, natural killer cells, and natural killer T cells, as well as decreased T cells in decidua parietalis, also have been reported.3
Mutations in the ATP binding cassette subfamily B member 4 gene, ABCB4, which encodes the multidrug resistance protein 3, a canalicular phosphatidylcholine translocase, have been found in several women with ICP.4 Clinically, patients usually present with pruritus that may precede or follow laboratory abnormalities. The pruritus worsens as the pregnancy advances and can resolve within 48 hours of delivery. Pruritus usually affects the palms and soles but may extend to the legs and abdomen or become generalized.4
Generally, there are no cutaneous signs other than excoriation marks, contrary to primary skin lesions found in other specific dermatoses of pregnancy. Mild jaundice can develop 2 to 4 weeks after the onset of pruritus, which may be associated with subclinical steatorrhea and increased risk of intrapartum and postpartum hemorrhage.5 Of note, ICP may be associated with increased risk for gestational DM, as illustrated in our case.6
Ursodeoxycholic acid currently is the most effective pharmacologic treatment of ICP. It reduces bile acids in cord blood, colostrum, and amniotic fluid.7 A meta-analysis of randomized controlled trials demonstrated that UDCA (450–1200 mg daily) is highly effective in alleviating pruritus and normalizing laboratory abnormalities associated with ICP.8 No severe adverse events have been reported related to UDCA.9,10
Intrahepatic cholestasis of pregnancy has been associated with increased risk for preterm delivery (19%–60%), meconium staining of amniotic fluid (≤27%), fetal bradycardia (≤14%), fetal distress (22%–41%), and fetal loss (0.4%–4.1%).11 The risk for serious fetal complications in ICP makes intensive fetal surveillance mandatory, including weekly nonstress cardiotocography or biophysical assessment from 34 weeks’ gestation. Delivery at 36 weeks or earlier (if lung maturity is achieved and cervix favorable) should be considered for severe cases with jaundice, progressive elevations in serum total bile acids, and suspected fetal distress. At more than 36 weeks’ gestation, amniocentesis and delivery should be considered if cervix is favorable and fetal lung maturity satisfactory.12-14
Our case highlights the importance of diagnosing ICP when a pregnant patient presents with generalized itch associated with elevated liver function tests. Interdisciplinary management involving dermatologists, obstetricians, pediatricians, and gastroenterologists is mandatory to acquire a better outcome for the mother and the fetus.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-474.
- Ling B, Yao F, Zhou Y, et al. Cell-mediated immunity imbalance in patients with intrahepatic cholestasis of pregnancy. Cell Mol Immunol. 2007;4:71-75.
- Dixon PH, Weerasekera N, Linton KJ, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genetics. 2000;9:1209-1217.
- Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
- Martineau M, Raker C, Powrie R, et al. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. Eur J Obstet Gynecol Reprod Biol. 2014;176:80-85.
- Laifer SA, Stiller RJ, Siddiqui DS, et al. Ursodeoxycholic acid for the treatment of intrahepatic cholestasis of pregnancy. J Matern Fetal Med. 2001;10:131-135.
- Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
- Kondrackiene J, Beuers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129:894-901.
- Tan LK. Obstetric cholestasis: current opinions and management. Ann Acad Med Singapore. 2003;32:294-298.
- Ghosh S, Chaudhuri S. Intra-hepatic cholestasis of pregnancy: a comprehensive review. Indian J Dermatol. 2013;58:327.
- Rioseco AJ, Ivankovic MB, Manzur A, et al. Intrahepatic cholestasis of pregnancy: retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. 1994;170:890-895.
- Saleh MM, Abdo KR. Intrahepatic cholestasis of pregnancy: review of the literature and evaluation of current evidence. J Womens Health (Larchmt). 2007;16:833-841.
- Roncaglia N, Arreghini A, Locatelli A, et al. Obstetric cholestasis: outcome with active management. Eur J Obstet Gynecol Reprod Biol. 2002;100:167-170.
- Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066.
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-474.
- Ling B, Yao F, Zhou Y, et al. Cell-mediated immunity imbalance in patients with intrahepatic cholestasis of pregnancy. Cell Mol Immunol. 2007;4:71-75.
- Dixon PH, Weerasekera N, Linton KJ, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genetics. 2000;9:1209-1217.
- Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
- Martineau M, Raker C, Powrie R, et al. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. Eur J Obstet Gynecol Reprod Biol. 2014;176:80-85.
- Laifer SA, Stiller RJ, Siddiqui DS, et al. Ursodeoxycholic acid for the treatment of intrahepatic cholestasis of pregnancy. J Matern Fetal Med. 2001;10:131-135.
- Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
- Kondrackiene J, Beuers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129:894-901.
- Tan LK. Obstetric cholestasis: current opinions and management. Ann Acad Med Singapore. 2003;32:294-298.
- Ghosh S, Chaudhuri S. Intra-hepatic cholestasis of pregnancy: a comprehensive review. Indian J Dermatol. 2013;58:327.
- Rioseco AJ, Ivankovic MB, Manzur A, et al. Intrahepatic cholestasis of pregnancy: retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. 1994;170:890-895.
- Saleh MM, Abdo KR. Intrahepatic cholestasis of pregnancy: review of the literature and evaluation of current evidence. J Womens Health (Larchmt). 2007;16:833-841.
- Roncaglia N, Arreghini A, Locatelli A, et al. Obstetric cholestasis: outcome with active management. Eur J Obstet Gynecol Reprod Biol. 2002;100:167-170.
Practice Points
- Intrahepatic cholestasis of pregnancy is a rare form of reversible cholestasis occurring in the second half of pregnancy.
- Interdisciplinary management involving dermatologists, obstetricians, pediatricians, and gastroenterologists is mandatory to acquire a better outcome for the mother and the fetus.
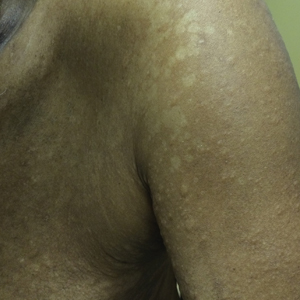
Bilateral Brown Plaques Behind the Ears
The Diagnosis: Terra Firma-Forme Dermatosis
Terra firma-forme dermatosis (TFFD), also known as Duncan dirty dermatosis, is an idiopathic benign cutaneous condition that is easily misdiagnosed or mismanaged. In 1987, Duncan et al1 first described the condition in children who had mothers that lamented over dirty skin spots that could not be washed off. The term terra firma translates in Latin to solid ground, which describes the characteristic dirtlike appearance of these lesions.
Terra firma-forme dermatosis most commonly affects children and young adults, though it can present in patients of any age without any known predisposing risk factors.1-4 The lesions have a predilection for the face, neck, shoulders, trunk, and ankles. Terra firma-forme dermatosis has no association with bathing and hygiene habits, and most patients describe unsuccessful removal of the lesions, even after vigorous scrubbing with soaps and detergents at home. The lesions are asymptomatic, and many patients present to dermatology for cosmetic concerns.1-8
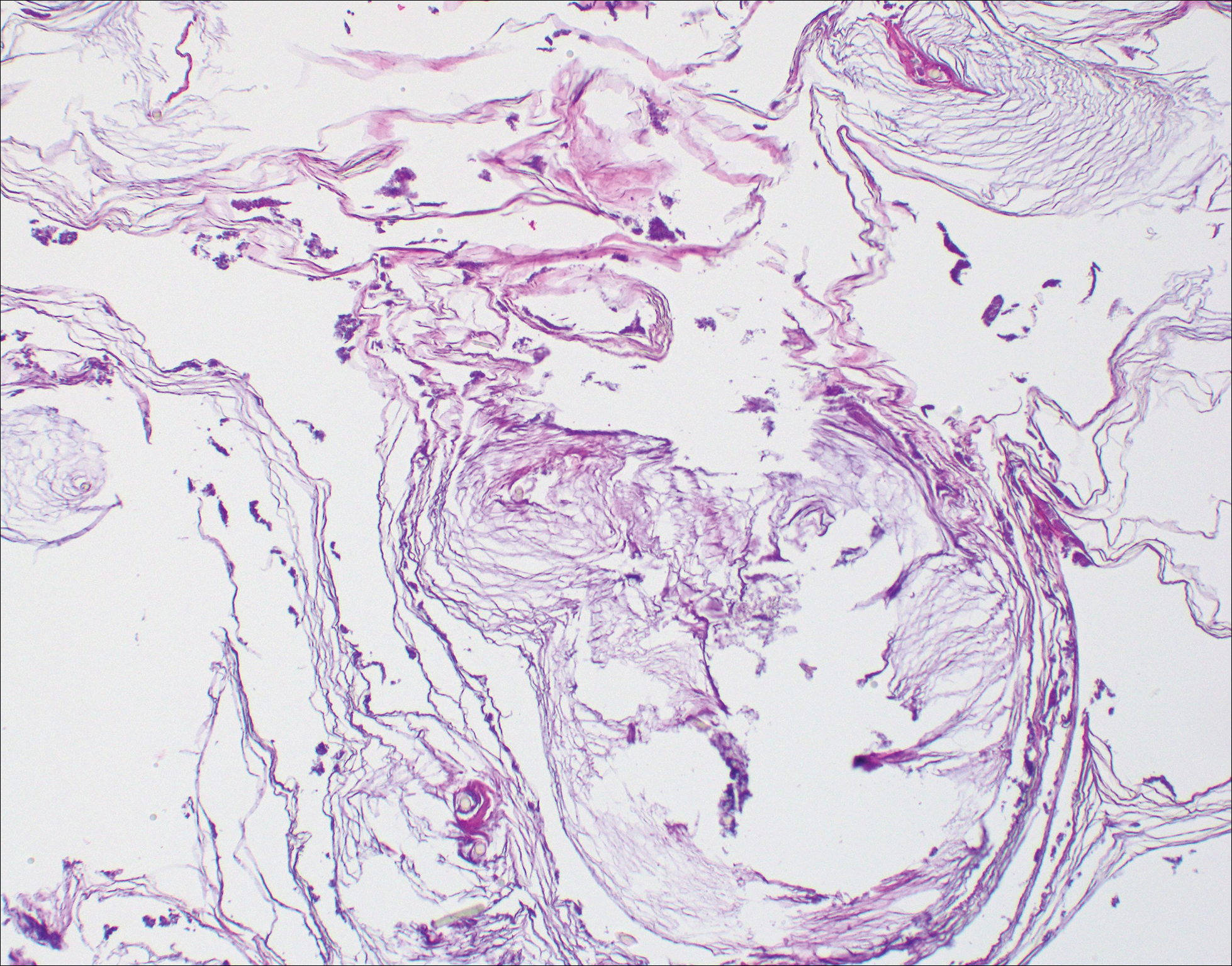
The etiology of TFFD is not well understood and is considered a retention hyperkeratosis. Duncan et al1 postulated that TFFD is the result of partial or improper maturation of keratinocytes leading to keratinocyte and melanin retention. Hematoxylin and eosin stains demonstrate lamellar hyperkeratosis of the stratum corneum without parakeratosis as well as keratin pearls scattered throughout. Mild acanthosis and papillomatosis also have been reported.1,5-7 Fontana-Masson stain shows excess melanin in these lesions, extending from the basal layer to the stratum corneum. Fungal and bacterial stains as well as cultures often have no notable findings.1,7 Similarly, histopathologic examination of our patient's biopsy with hematoxylin and eosin stain revealed hyperorthokeratosis with scattered naked vellus hair shafts and incidental yeast forms (Figure 1).
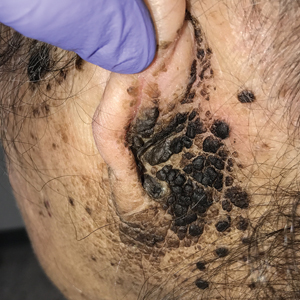
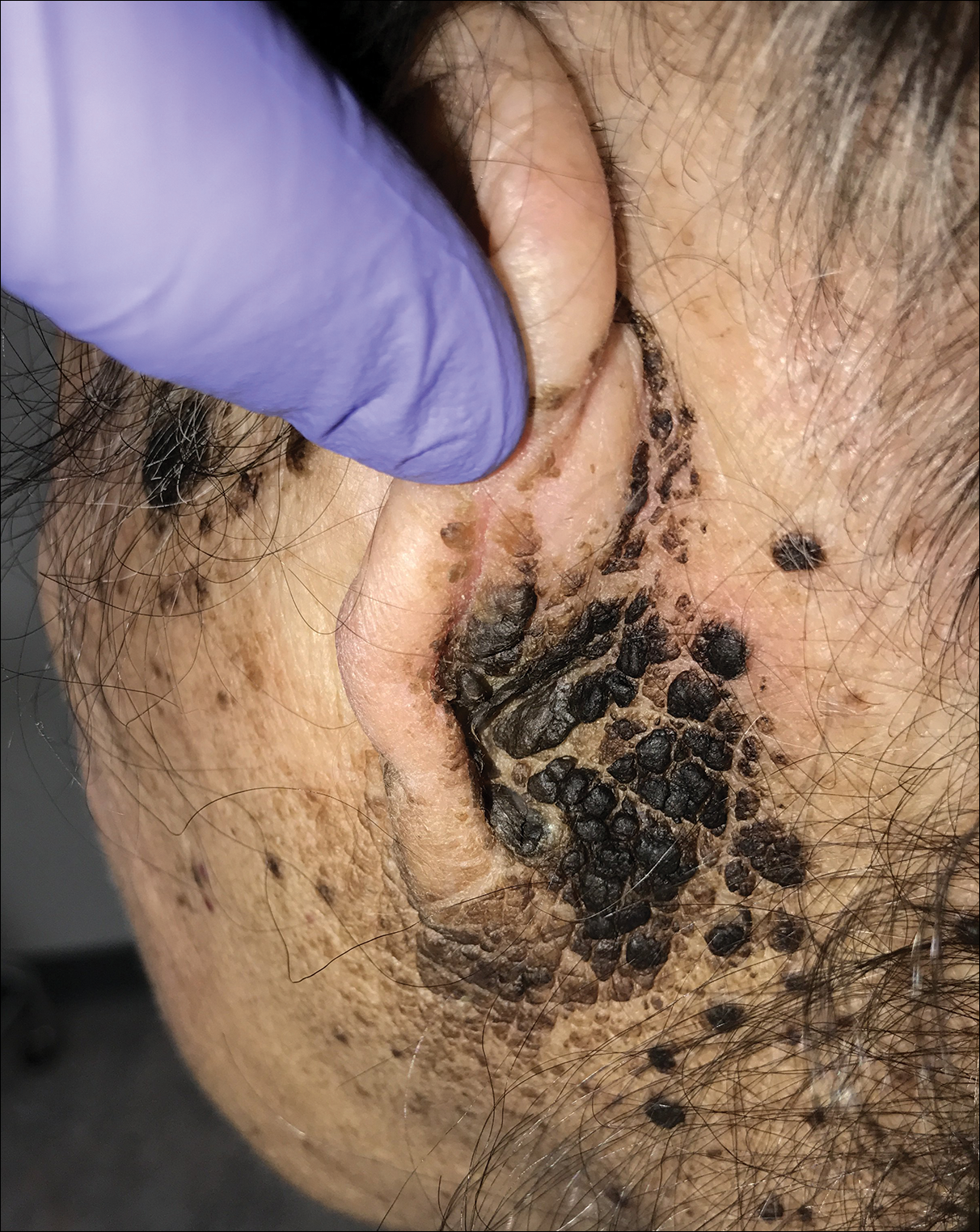
The differential diagnosis for TFFD may include pityriasis versicolor, confluent and reticulated papillomatosis, acanthosis nigricans, ichthyosis, malignant melanoma, and seborrheic keratosis. All of these diagnoses can be ruled out by the easy removal of the lesions with isopropyl alcohol 70%, which was performed on our patient by scrubbing the lesions with soaked gauze (Figure 2). Indeed, removal with isopropyl alcohol 70% is both the therapeutic and diagnostic procedure for TFFD.1-8 Of note, dermatitis neglecta is histologically and clinically identical to TFFD, albeit with a history of uncleanly habits or exposure to dirty environments.
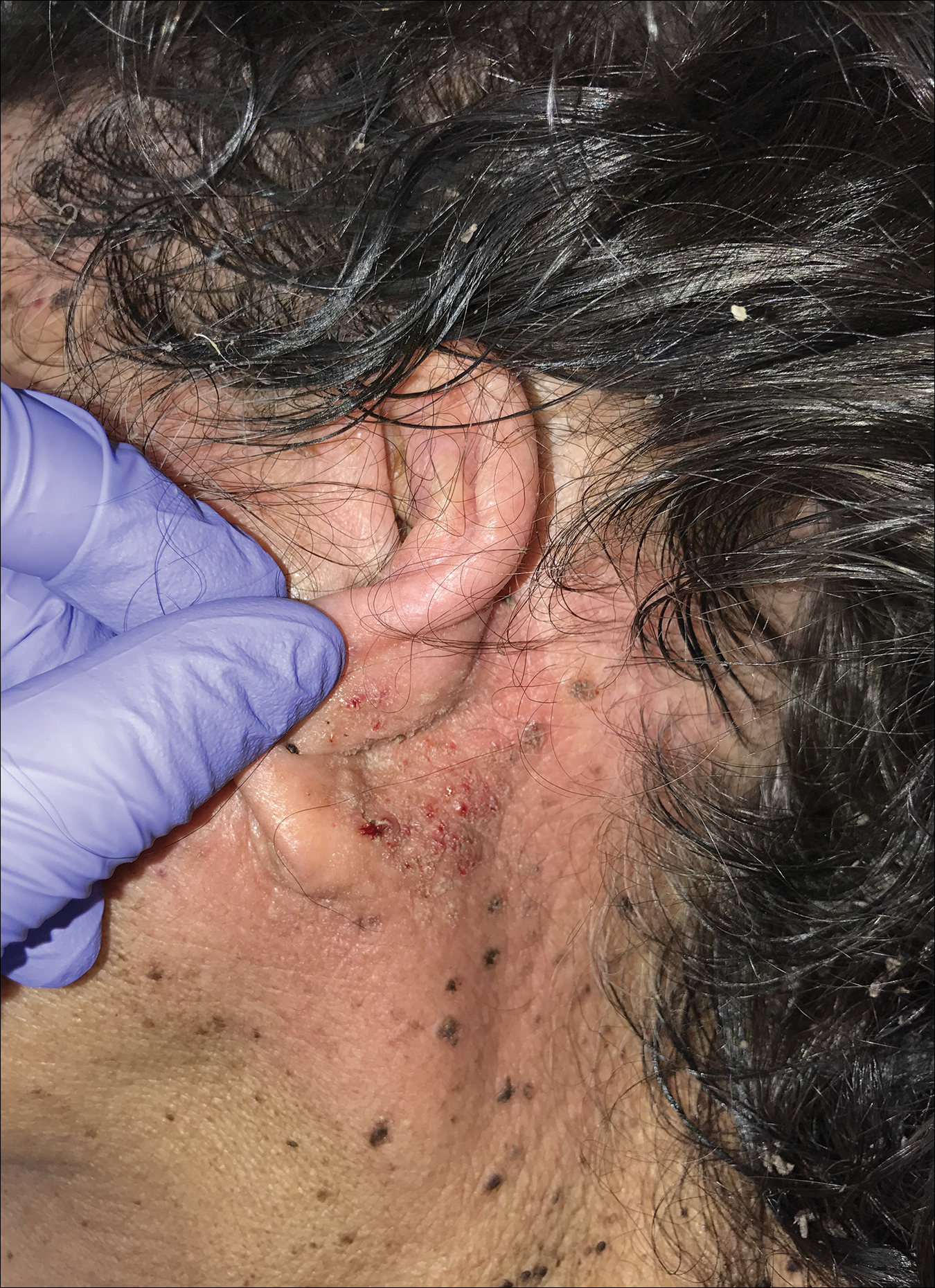
The diagnosis of TFFD often is discovered incidentally as physicians wipe the area with alcohol to prepare for biopsy.1 Occasionally, vigorous scrubbing is needed to completely remove the lesions, and without this effort the lesions may be easily mistaken for another cutaneous process.3 Failure to consider TFFD as a diagnosis has led to unnecessary endocrine workups and invasive biopsies.4 Therefore, physicians should have early clinical suspicion of TFFD and be aware of the bedside diagnostic procedure using isopropyl alcohol.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan's dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Moon J, Kim MW, Yoon HS, et al. A case of terra firma-forme dermatosis: differentiation from other dirty-appearing diseases. Ann Dermatol. 2016;28:413-415.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Chun SW, Lee SY, Kim JB, et al. A case of terra firma-forme dermatosis treated with salicylic acid alcohol peeling. Ann Dermatol. 2017;29:83-85.
- Aslan NC, Guler S, Demirci K, et al. Features of terra firma-forme dermatosis. Ann Fam Med. 2018;16:52-54.
The Diagnosis: Terra Firma-Forme Dermatosis
Terra firma-forme dermatosis (TFFD), also known as Duncan dirty dermatosis, is an idiopathic benign cutaneous condition that is easily misdiagnosed or mismanaged. In 1987, Duncan et al1 first described the condition in children who had mothers that lamented over dirty skin spots that could not be washed off. The term terra firma translates in Latin to solid ground, which describes the characteristic dirtlike appearance of these lesions.
Terra firma-forme dermatosis most commonly affects children and young adults, though it can present in patients of any age without any known predisposing risk factors.1-4 The lesions have a predilection for the face, neck, shoulders, trunk, and ankles. Terra firma-forme dermatosis has no association with bathing and hygiene habits, and most patients describe unsuccessful removal of the lesions, even after vigorous scrubbing with soaps and detergents at home. The lesions are asymptomatic, and many patients present to dermatology for cosmetic concerns.1-8
The etiology of TFFD is not well understood and is considered a retention hyperkeratosis. Duncan et al1 postulated that TFFD is the result of partial or improper maturation of keratinocytes leading to keratinocyte and melanin retention. Hematoxylin and eosin stains demonstrate lamellar hyperkeratosis of the stratum corneum without parakeratosis as well as keratin pearls scattered throughout. Mild acanthosis and papillomatosis also have been reported.1,5-7 Fontana-Masson stain shows excess melanin in these lesions, extending from the basal layer to the stratum corneum. Fungal and bacterial stains as well as cultures often have no notable findings.1,7 Similarly, histopathologic examination of our patient's biopsy with hematoxylin and eosin stain revealed hyperorthokeratosis with scattered naked vellus hair shafts and incidental yeast forms (Figure 1).

The differential diagnosis for TFFD may include pityriasis versicolor, confluent and reticulated papillomatosis, acanthosis nigricans, ichthyosis, malignant melanoma, and seborrheic keratosis. All of these diagnoses can be ruled out by the easy removal of the lesions with isopropyl alcohol 70%, which was performed on our patient by scrubbing the lesions with soaked gauze (Figure 2). Indeed, removal with isopropyl alcohol 70% is both the therapeutic and diagnostic procedure for TFFD.1-8 Of note, dermatitis neglecta is histologically and clinically identical to TFFD, albeit with a history of uncleanly habits or exposure to dirty environments.

The diagnosis of TFFD often is discovered incidentally as physicians wipe the area with alcohol to prepare for biopsy.1 Occasionally, vigorous scrubbing is needed to completely remove the lesions, and without this effort the lesions may be easily mistaken for another cutaneous process.3 Failure to consider TFFD as a diagnosis has led to unnecessary endocrine workups and invasive biopsies.4 Therefore, physicians should have early clinical suspicion of TFFD and be aware of the bedside diagnostic procedure using isopropyl alcohol.
The Diagnosis: Terra Firma-Forme Dermatosis
Terra firma-forme dermatosis (TFFD), also known as Duncan dirty dermatosis, is an idiopathic benign cutaneous condition that is easily misdiagnosed or mismanaged. In 1987, Duncan et al1 first described the condition in children who had mothers that lamented over dirty skin spots that could not be washed off. The term terra firma translates in Latin to solid ground, which describes the characteristic dirtlike appearance of these lesions.
Terra firma-forme dermatosis most commonly affects children and young adults, though it can present in patients of any age without any known predisposing risk factors.1-4 The lesions have a predilection for the face, neck, shoulders, trunk, and ankles. Terra firma-forme dermatosis has no association with bathing and hygiene habits, and most patients describe unsuccessful removal of the lesions, even after vigorous scrubbing with soaps and detergents at home. The lesions are asymptomatic, and many patients present to dermatology for cosmetic concerns.1-8
The etiology of TFFD is not well understood and is considered a retention hyperkeratosis. Duncan et al1 postulated that TFFD is the result of partial or improper maturation of keratinocytes leading to keratinocyte and melanin retention. Hematoxylin and eosin stains demonstrate lamellar hyperkeratosis of the stratum corneum without parakeratosis as well as keratin pearls scattered throughout. Mild acanthosis and papillomatosis also have been reported.1,5-7 Fontana-Masson stain shows excess melanin in these lesions, extending from the basal layer to the stratum corneum. Fungal and bacterial stains as well as cultures often have no notable findings.1,7 Similarly, histopathologic examination of our patient's biopsy with hematoxylin and eosin stain revealed hyperorthokeratosis with scattered naked vellus hair shafts and incidental yeast forms (Figure 1).

The differential diagnosis for TFFD may include pityriasis versicolor, confluent and reticulated papillomatosis, acanthosis nigricans, ichthyosis, malignant melanoma, and seborrheic keratosis. All of these diagnoses can be ruled out by the easy removal of the lesions with isopropyl alcohol 70%, which was performed on our patient by scrubbing the lesions with soaked gauze (Figure 2). Indeed, removal with isopropyl alcohol 70% is both the therapeutic and diagnostic procedure for TFFD.1-8 Of note, dermatitis neglecta is histologically and clinically identical to TFFD, albeit with a history of uncleanly habits or exposure to dirty environments.

The diagnosis of TFFD often is discovered incidentally as physicians wipe the area with alcohol to prepare for biopsy.1 Occasionally, vigorous scrubbing is needed to completely remove the lesions, and without this effort the lesions may be easily mistaken for another cutaneous process.3 Failure to consider TFFD as a diagnosis has led to unnecessary endocrine workups and invasive biopsies.4 Therefore, physicians should have early clinical suspicion of TFFD and be aware of the bedside diagnostic procedure using isopropyl alcohol.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan's dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Moon J, Kim MW, Yoon HS, et al. A case of terra firma-forme dermatosis: differentiation from other dirty-appearing diseases. Ann Dermatol. 2016;28:413-415.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Chun SW, Lee SY, Kim JB, et al. A case of terra firma-forme dermatosis treated with salicylic acid alcohol peeling. Ann Dermatol. 2017;29:83-85.
- Aslan NC, Guler S, Demirci K, et al. Features of terra firma-forme dermatosis. Ann Fam Med. 2018;16:52-54.
- Duncan WC, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan's dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Moon J, Kim MW, Yoon HS, et al. A case of terra firma-forme dermatosis: differentiation from other dirty-appearing diseases. Ann Dermatol. 2016;28:413-415.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Chun SW, Lee SY, Kim JB, et al. A case of terra firma-forme dermatosis treated with salicylic acid alcohol peeling. Ann Dermatol. 2017;29:83-85.
- Aslan NC, Guler S, Demirci K, et al. Features of terra firma-forme dermatosis. Ann Fam Med. 2018;16:52-54.
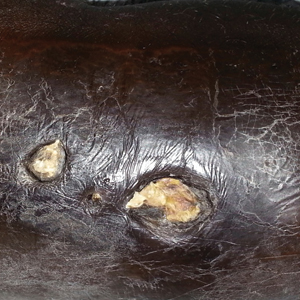
A 94-year-old woman was referred to the dermatology department for biopsy of pigmented tumors behind the ears of unknown duration. The growths were asymptomatic. Her medical history included the early stages of Alzheimer disease. On physical examination dark brown, smooth, coalescing papules and plaques were noted extending from the posterior neck to the conchal bowls and ear folds bilaterally. The nodules were removed by scrubbing with isopropyl alcohol 70%. A nodule was submitted for histopathologic review.
October 2018 Highlights
Sweat Regeneration Following CO2 Fractionated Laser Therapy
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).
A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).

A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
To the Editor:
It is not uncommon for patients with extensive dermal scarring to overheat due to the inability to regulate body temperature through evaporative heat loss, as lack of perspiration in areas of prior full-thickness skin injury is well known. One of the authors (C.M.H.) previously reported a case of a patient with considerable hypertrophic scarring after surviving an episode of toxic epidermal necrolysis that was likely precipitated by lamotrigine.1 The patient initially presented to our clinic in consultation for laser therapy to improve the pliability and cosmetic appearance of the scars; however, approximately 3 weeks after initiating treatment with a fractional CO2 laser, the patient noticed perspiration in areas where she once lacked the ability to perspire as well as improved functionality.1 It was speculated that scar remodeling stimulated by the CO2 fractional laser allowed new connections to form between eccrine ducts in the dermis and epidermis.2
These findings are even more notable in light of a study by Rittié et al3 that suggested the primary appendages of the skin involved in human wound healing are the eccrine sweat glands. The investigators were able to demonstrate that eccrine sweat glands are major contributors in reepithelialization and wound healing in humans; therefore, it is possible that stimulating these glands with the CO2 laser may promote enhanced reepithelialization in addition to the reestablishment of perspiration and wound healing.3 Considering inadequate wound repair represents a substantial disturbance to the patient and health care system, this finding offers promise as a potential means to decrease morbidity in patients with dermal scarring from burns and traumatic injuries. We have since evaluated and treated 3 patients who demonstrated sweat regeneration following treatment with the fractional CO2 laser (Table).

A 42-year-old man was our first patient to demonstrate functional scar improvement following bone marrow transplant for acute lymphoblastic leukemia complicated by chronic sclerodermoid graft-versus-host disease and subsequent extensive scarring on the chest and arms. Approximately 2 weeks after the first treatment with the fractional CO2 laser, the patient began to notice the presence of sweat beads in the treated areas. In addition to the reestablishment of perspiration, the patient had perceived increased mobility with improved pliability and “softness” (as described by family members) in treated areas likely related to scar remodeling.
A 36-year-old wounded army veteran presented with burns to the face, arms, and chest affecting 49% of the body surface area. After only 1 treatment, the patient reported that he could subjectively tolerate 10°F more ambient temperature and work all day outside in south Texas when heat intolerance previously would allow him to work only 2 to 3 hours. Additionally, he noted increased mobility and chest wall expansion, which in combination contributed to overall increased exercise tolerance and enhanced quality of life.
A 35-year-old US Marine and firefighter with burns primarily on the chest and arms involving 35% body surface area experienced increased exercise tolerance and sweat regeneration, particularly on the chest after a single treatment with the fractional CO2 laser but continued to experience improvement after a total of 3 treatments. Additionally, the cosmetic improvement was so substantial that the physician (C.M.H) had to review older photographs to verify the location of the scars.
We have now treated 3 patients with various mechanisms of injury and extensive scarring who noticed improved heat tolerance from sweat regeneration following fractional CO2 laser therapy. At this point, we only have anecdotal evidence of subjective functional improvement, and further research is warranted to elucidate the exact mechanism of action to support our findings.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
- Neiner J, Whittemore D, Hivnor C. Buried alive: functional eccrine coils buried under scar tissue? J Am Acad Dermatol. 2011;65:661-663.
- Waibel J, Beer K, Narurkar V, et al. Preliminary observations on fractional ablative resurfacing devices: clinical impressions. J Drugs Dermatol. 2009;8:481-485.
- Rittié L, Sachs D, Orringer J, et al. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol. 2013;1:163-171.
Practice Points
- Treatment of dermal scarring with fractional CO2 laser may contribute to eccrine sweat gland regeneration during the remodeling process in addition to increased skin pliability.
- Sweat regeneration has been demonstrated following treatment with fractional CO2 laser in patients with extensive scarring; this case shows sweat regeneration secondary to burns and chronic sclerodermoid graft-versus-host disease.
Primary Cutaneous Apocrine Carcinoma Arising Within a Nevus Sebaceus
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
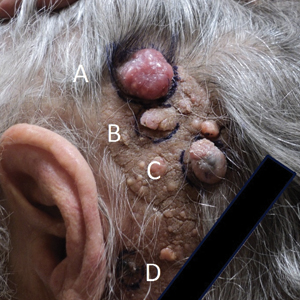
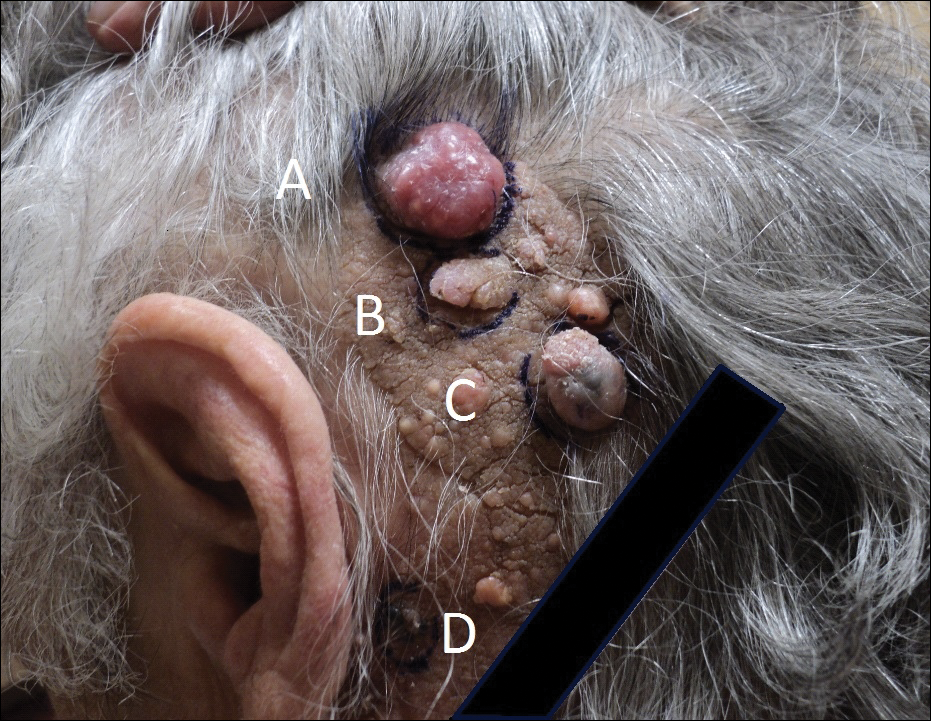
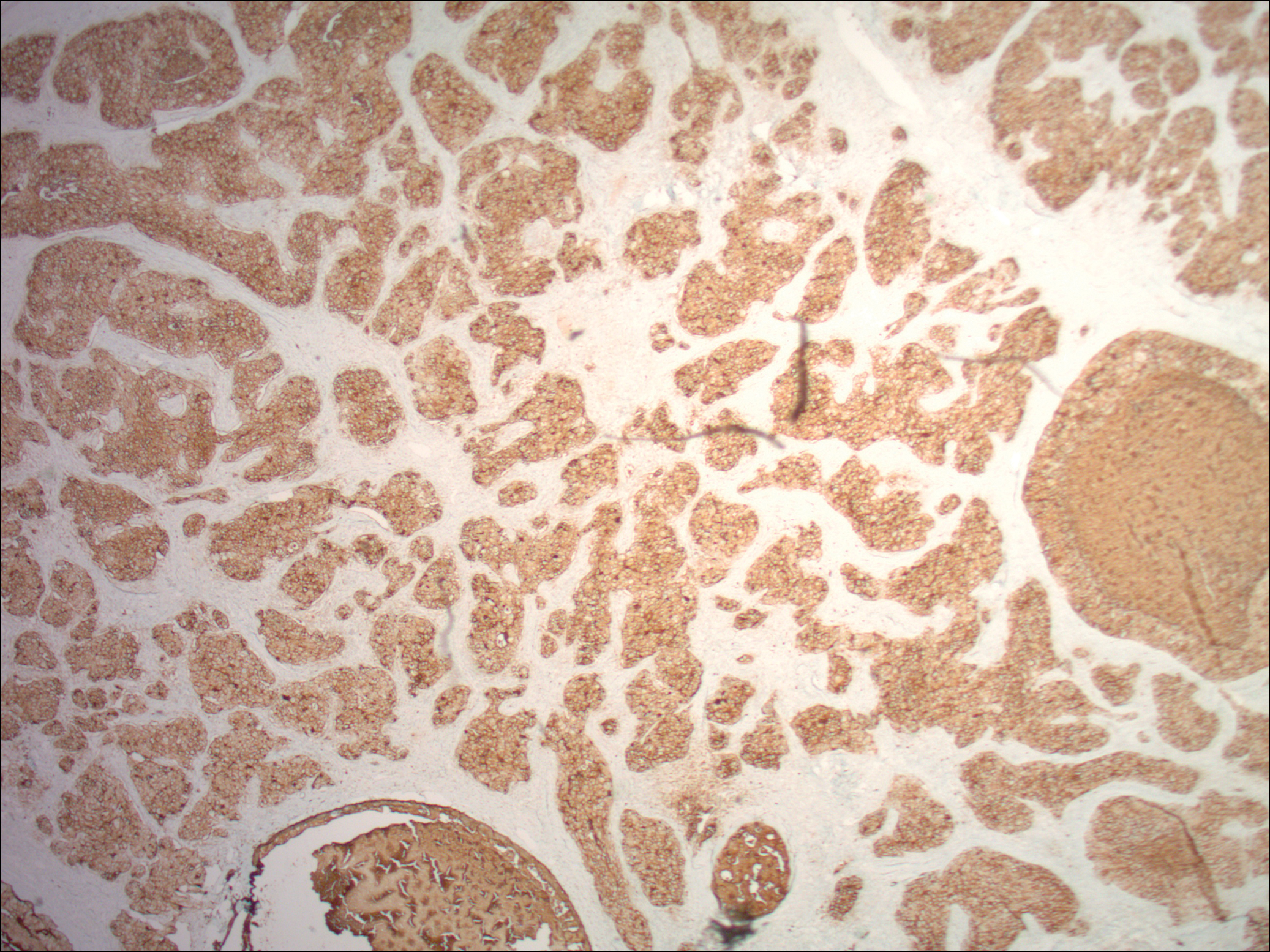
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.

Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.



Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.



Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
Practice Points
- Nevus sebaceus (NS) in the centrofacial region has been correlated with a higher risk for neurological abnormalities, including intellectual disability and seizures.
- Historically, basal cell carcinomas (BCCs) were considered a common occurrence arising from an NS, prompting prophylactic surgical excision of such lesions.
- More recently, it has been recognized that the most common tumor to arise from NS is trichoblastoma rather than BCC; in fact, BCC and other malignancies have been found to be relatively rare compared to their benign counterparts.
- In light of this discovery, observation of NS may be a more prudent course of treatment versus prophylactic surgical excision.
Xanthogranulomatous Reaction to Trametinib for Metastatic Malignant Melanoma
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.
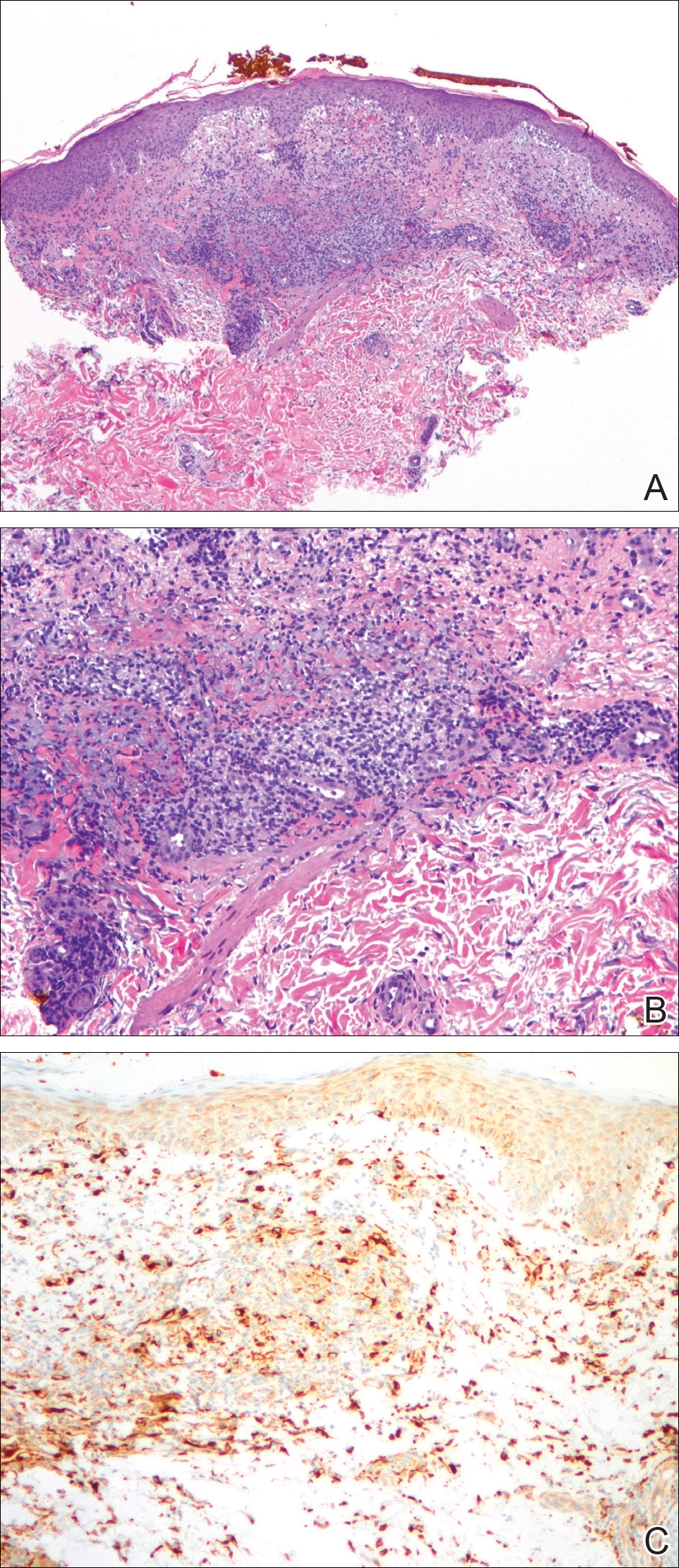
Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.
Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9
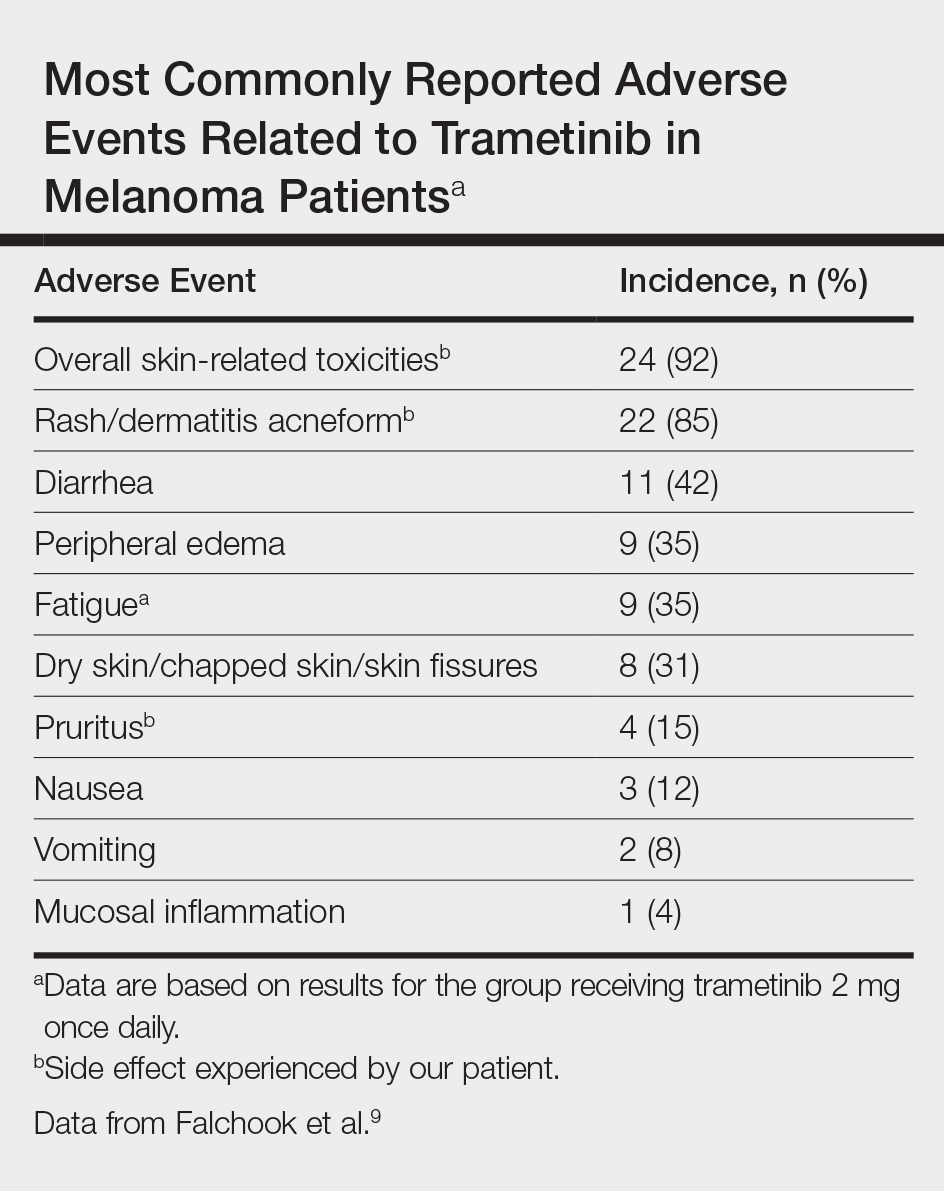
Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9

Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9

Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
Practice Points
- With the discovery of molecular targeting in melanoma, BRAF and MEK inhibitors have been increasingly utilized as therapies in metastatic melanoma management.
- Trametinib, a MEK inhibitor, is commonly associated with cutaneous adverse reactions, particularly acneform eruptions.
- We report a patient on trametinib who developed an eruption with an unusual xanthogranulomatous reaction pattern noted on histology.
Acquired Perforating Dermatosis in a Skin Graft
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.
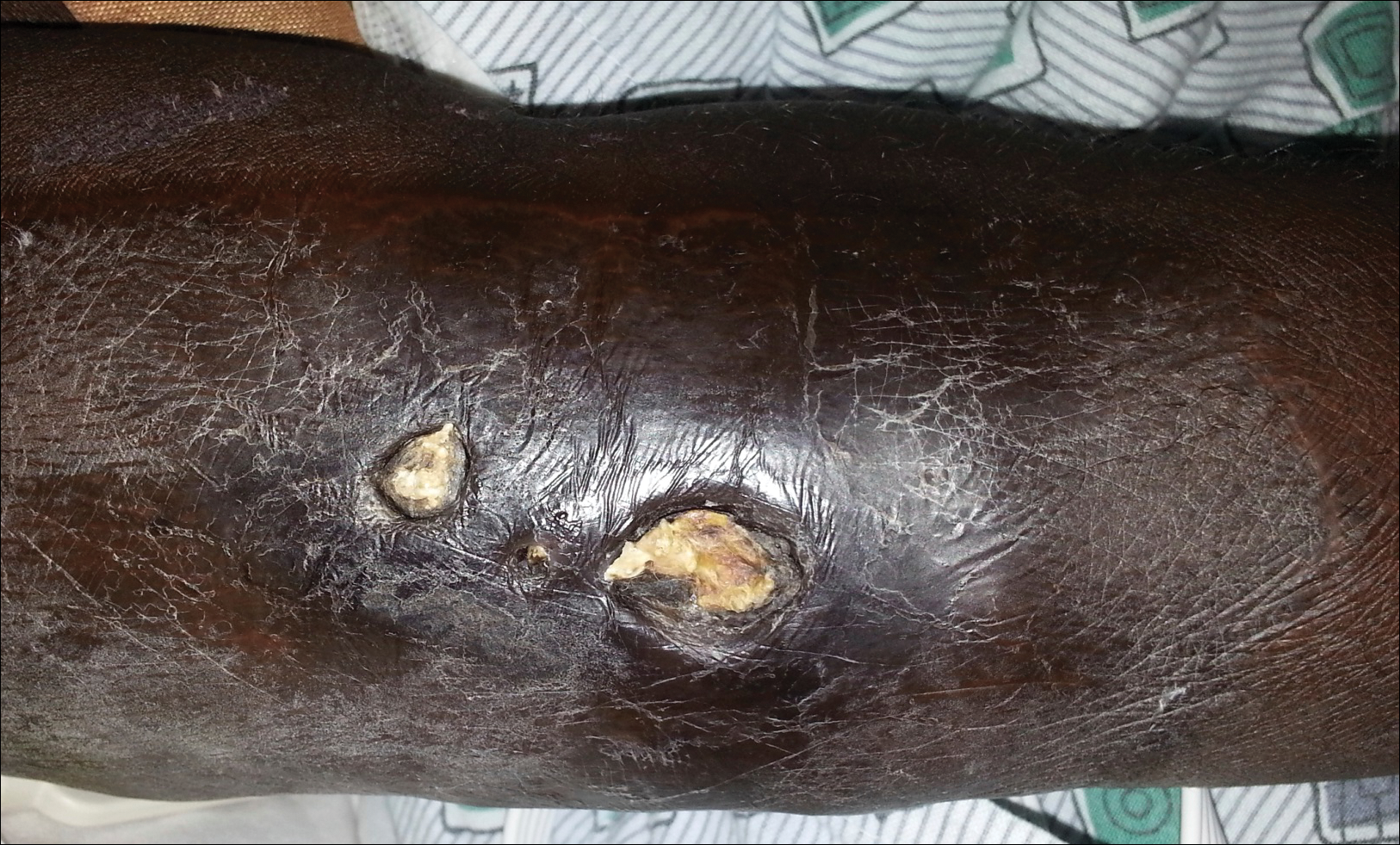
A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).
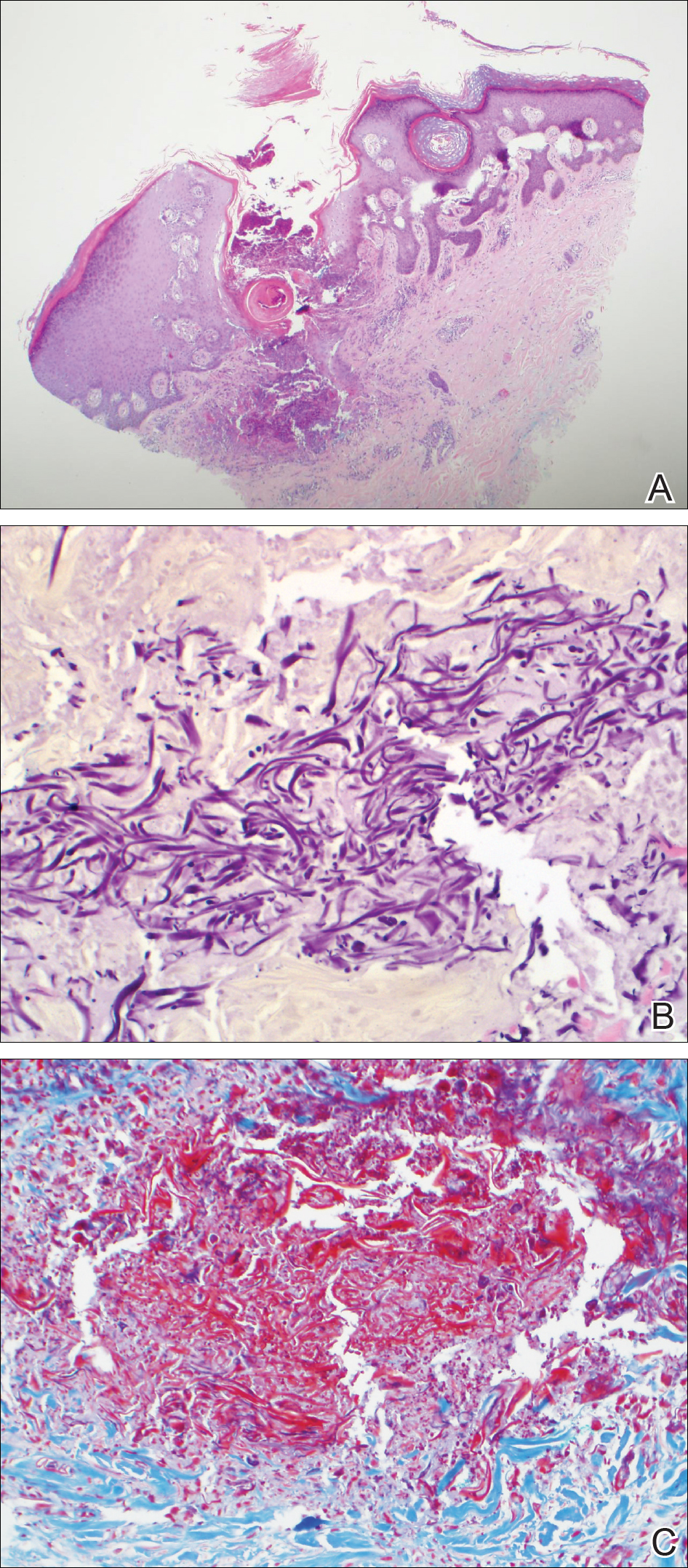
Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.

A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).

Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.

A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).

Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
Practice Points
- Acquired perforating dermatosis (APD) presents as pruritic crateriform papules and plaques with central keratotic plugs.
- A medical history of diabetes mellitus and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
Leukemia Cutis in Acute Myeloid Leukemia Signifies a Poor Prognosis
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).
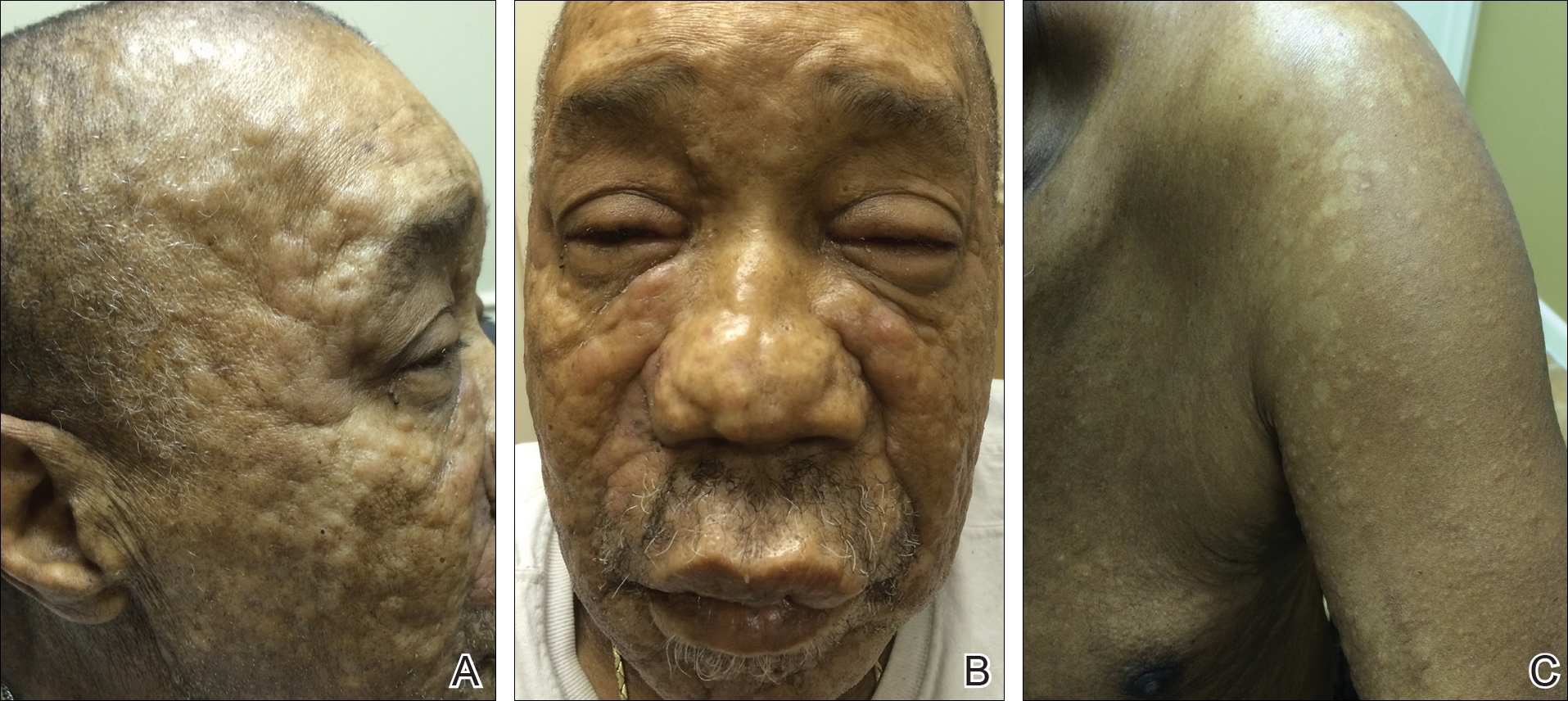
Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.
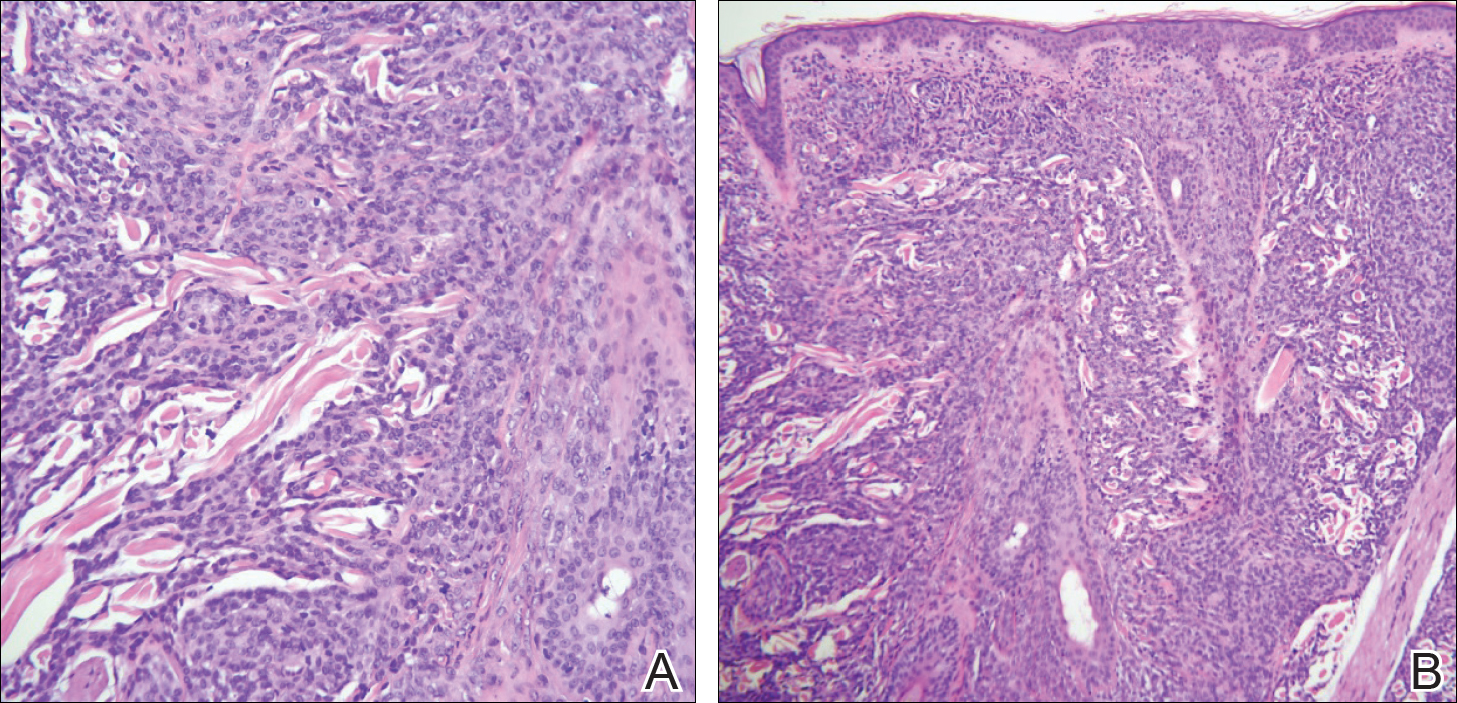
Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).

Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.

Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).

Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.

Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
Practice Points
- Leukemia cutis (LC) describes cutaneous and/or subcutaneous infiltration by leukemic cells and most commonly occurs in patients with acute myeloid leukemia.
- The vast majority of patients presenting with LC already have systemic involvement.
- Cutaneous presentation of LC is diverse, thus diagnosis often is dependent on immunohisto-chemical findings.
Trichodysplasia Spinulosa in the Setting of Colon Cancer
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).
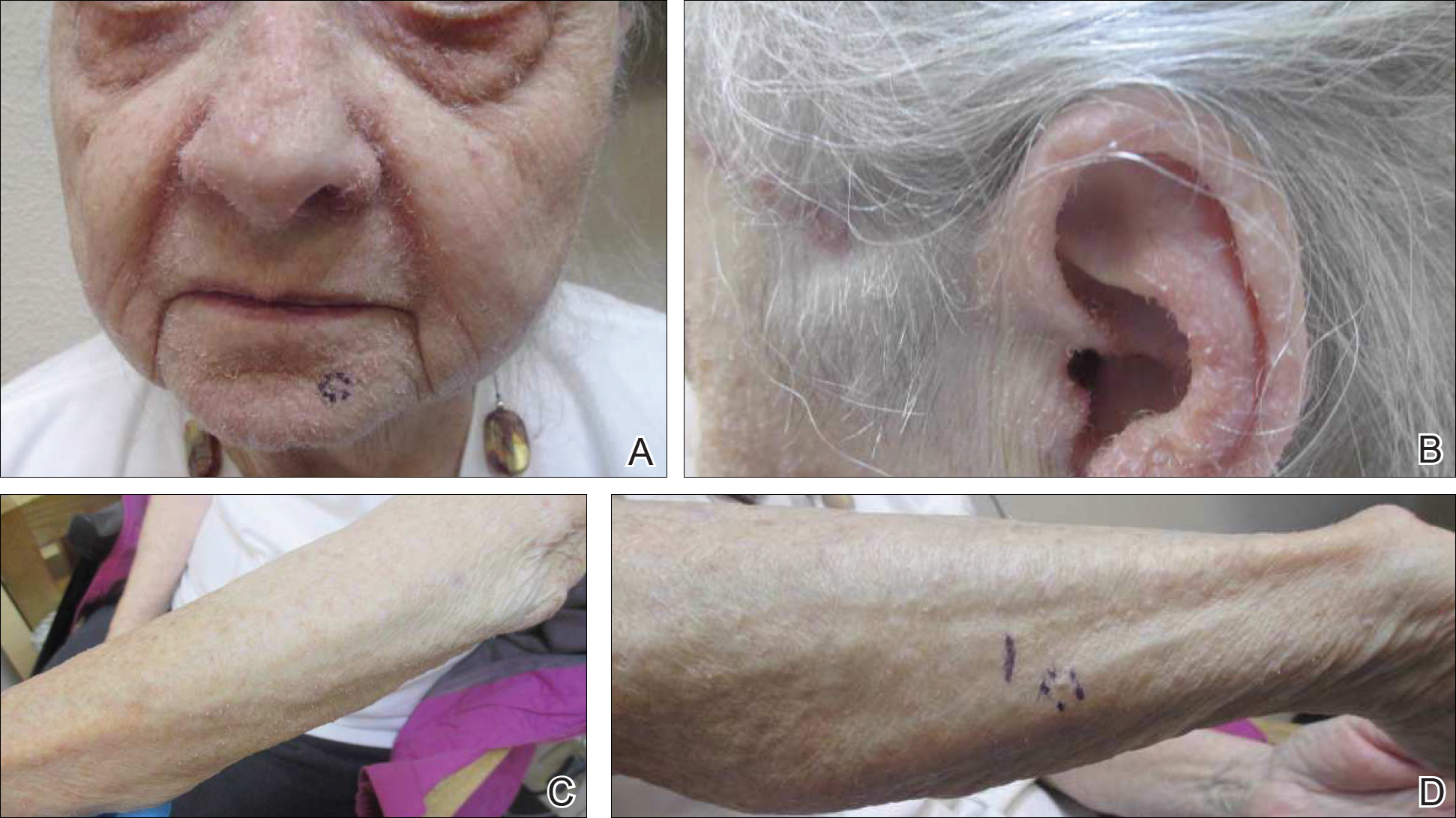
A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.
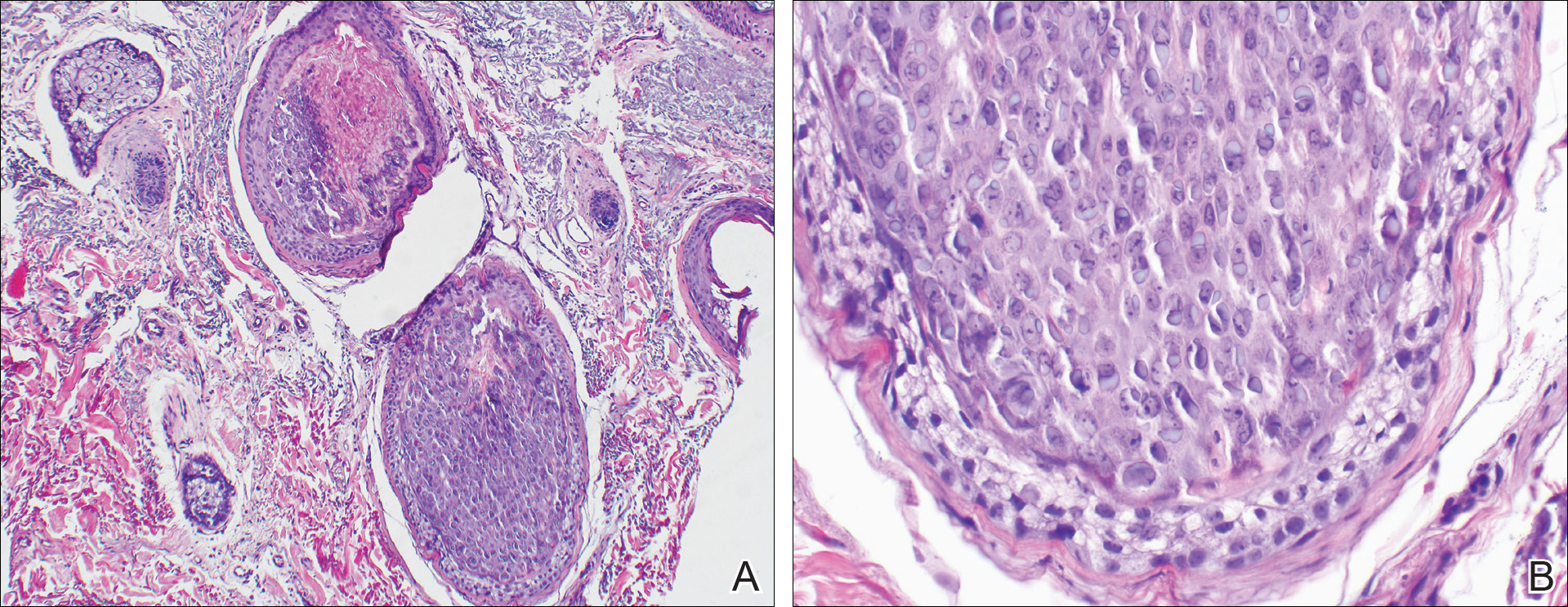
The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
Case Report
An 82-year-old woman presented to the clinic with a rash on the face that had been present for a few months. She denied any treatment or prior occurrence. Her medical history was remarkable for non-Hodgkin lymphoma that had been successfully treated with chemotherapy 4 years prior. Additionally, she recently had been diagnosed with stage IV colon cancer. She reported that surgery had been scheduled and she would start adjuvant chemotherapy soon after.
On physical examination she exhibited perioral and perinasal erythematous papules with sparing of the vermilion border. A diagnosis of perioral dermatitis was made, and she was started on topical metronidazole. At 1-month follow-up, her condition had slightly worsened and she was subsequently started on doxycycline. When she returned to the clinic again the following month, physical examination revealed agminated folliculocentric papules with central spicules on the face, nose, ears, upper extremities (Figure 1), and trunk. The differential diagnosis included multiple minute digitate hyperkeratosis, spiculosis of multiple myeloma, and trichodysplasia spinulosa (TS).

A punch biopsy of 2 separate papules on the face and upper extremity revealed dilated follicles with enlarged trichohyalin granules and dyskeratosis (Figure 2), consistent with TS. Additional testing such as electron microscopy or polymerase chain reaction was not performed to keep the patient’s medical costs down; also, the strong clinical and histopathologic evidence did not warrant further testing.

The plan was to start split-face treatment with topical acyclovir and a topical retinoid to see which agent was more effective, but the patient declined until her chemotherapy regimen had concluded. Unfortunately, the patient died 3 months later due to colon cancer.
Comment
History and Presentation
Trichodysplasia spinulosa was first recognized as hairlike hyperkeratosis.1 The name by which it is currently known was later championed by Haycox et al.2 They reported a case of a 44-year-old man who underwent a combined renal-pancreas transplant and while taking immunosuppressive medication developed erythematous papules with follicular spinous processes and progressive alopecia.2 Other synonymous terms used for this condition include pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, virus-associated trichodysplasia,3 and follicular dystrophy of immunosuppression.4 Trichodysplasia spinulosa can affect both adult and pediatric immunocompromised patients, including organ transplant recipients on immunosuppressants and cancer patients on chemotherapy.3 The condition also has been reported to precede the recurrence of lymphoma.5
Etiology
The connection of TS with a viral etiology was first demonstrated in 1999, and subsequently it was confirmed to be a polyomavirus.2 The family name of Polyomaviridae possesses a Greek derivation with poly- meaning many and -oma meaning cancer.3 This name was given after the polyomavirus induced multiple tumors in mice.3,6 This viral family consists of multiple naked viruses with a surrounding icosahedral capsid containing 3 structural proteins known as VP1, VP2, and VP3. Their life cycle is characterized by early and late phases with respective early and late protein formation.3
Polyomavirus infections maintain an asymptomatic and latent course in immunocompetent patients.7 The prevalence and manifestation of these viruses change when the host’s immune system is altered. The first identified JC virus and BK virus of the same family have been found at increased frequencies in blood and lymphoid tissue during host immunosuppression.6 Moreover, the Merkel cell polyomavirus detected in Merkel cell carcinoma is well documented in the dermatologic literature.6,8
A specific polyomavirus has been implicated in the majority of TS cases and has subsequently received the name of TS polyomavirus.9 As a polyomavirus, it similarly produces capsid antigens and large/small T antigens. Among the viral protein antigens produced, the large tumor or LT antigen represents one of the most potent viral proteins. It has been postulated to inhibit the retinoblastoma family of proteins, leading to increased inner root sheath cells that allow for further viral replication.9,10
The disease presents with folliculocentric papules localized mainly on the central face and ears, which grow central keratin spines or spicules that can become 1 to 3 mm in length. Coinciding alopecia and madarosis also may be present.9
Diagnosis
Histologic examination reveals abnormal follicular maturation and distension. Additionally, increased proliferation and amount of trichohyalin is seen within the inner root sheath cells. Further testing via viral culture, polymerase chain reaction, electron microscopy, or immunohistochemical stains can confirm the diagnosis. Such testing may not be warranted in all cases given that classic clinical findings coupled with routine histopathology staining can provide enough evidence.10,11
Management
Currently, a universal successful treatment for TS does not exist. There have been anecdotal successes reported with topical medications such as cidofovir ointment 1%, acyclovir combined with 2-deoxy-D-glucose and epigallocatechin, corticosteroids, topical tacrolimus, topical retinoids, and imiquimod. Additionally, success has been seen with oral minocycline, oral retinoids, valacyclovir, and valganciclovir, with the latter showing the best results. Patients also have shown improvement after modifying their immunosuppressive treatment regimen.10,12
Conclusion
Given the previously published case of TS preceding the recurrence of lymphoma,5 we notified our patient’s oncologist of this potential risk. Her history of lymphoma and immunosuppressive treatment 4 years prior may represent the etiology of the cutaneous presentation; however, the TS with concurrent colon cancer presented prior to starting immunosuppressive therapy, suggesting that it also may have been a paraneoplastic process and not just a sign of immunosuppression. Therefore, we recommend that patients who present with TS should be evaluated for underlying malignancy if not already diagnosed.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
- Linke M, Geraud C, Sauer C, et al. Follicular erythematous papules with keratotic spicules. Acta Derm Venereol . 2014;94:493-494.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- Moens U, Ludvigsen M, Van Ghelue M. Human polyomaviruses in skin diseases [published online September 12, 2011]. Patholog Res Int. 2011;2011:123491.
- Matthews MR, Wang RC, Reddick RL, et al. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa–associated human polyomavirus. J Cutan Pathol. 2011;38:420-431.
- Osswald SS, Kulick KB, Tomaszewski MM, et al. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721-725.
- Dalianis T, Hirsch HH. Human polyomavirus in disease and cancer. Virology. 2013;437:63-72.
- Tsuzuki S, Fukumoto H, Mine S, et al. Detection of trichodysplasia spinulosa–associated polyomavirus in a fatal case of myocarditis in a seven-month-old girl. Int J Clin Exp Pathol. 2014;7:5308-5312.
- Sadeghi M, Aronen M, Chen T, et al. Merkel cell polyomavirus and trichodysplasia spinulosa–associated polyomavirus DNAs and antibodies in blood among the elderly. BMC Infect Dis. 2012;12:383.
- Van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- Krichhof MG, Shojania K, Hull MW, et al. Trichodysplasia spinulosa: rare presentation of polyomavirus infection in immunocompromised patients. J Cutan Med Surg. 2014;18:430-435.
- Rianthavorn P, Posuwan N, Payungporn S, et al. Polyomavirus reactivation in pediatric patients with systemic lupus erythematosus. Tohoku J Exp Med. 2012;228:197-204.
- Wanat KA, Holler PD, Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219-223.
Practice Points
- Rashes have a life span and can evolve with time.
- If apparent straightforward conditions do not appear to respond to standard therapy, start to think outside the box for underlying potential causes.
Mobile App Rankings in Dermatology
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
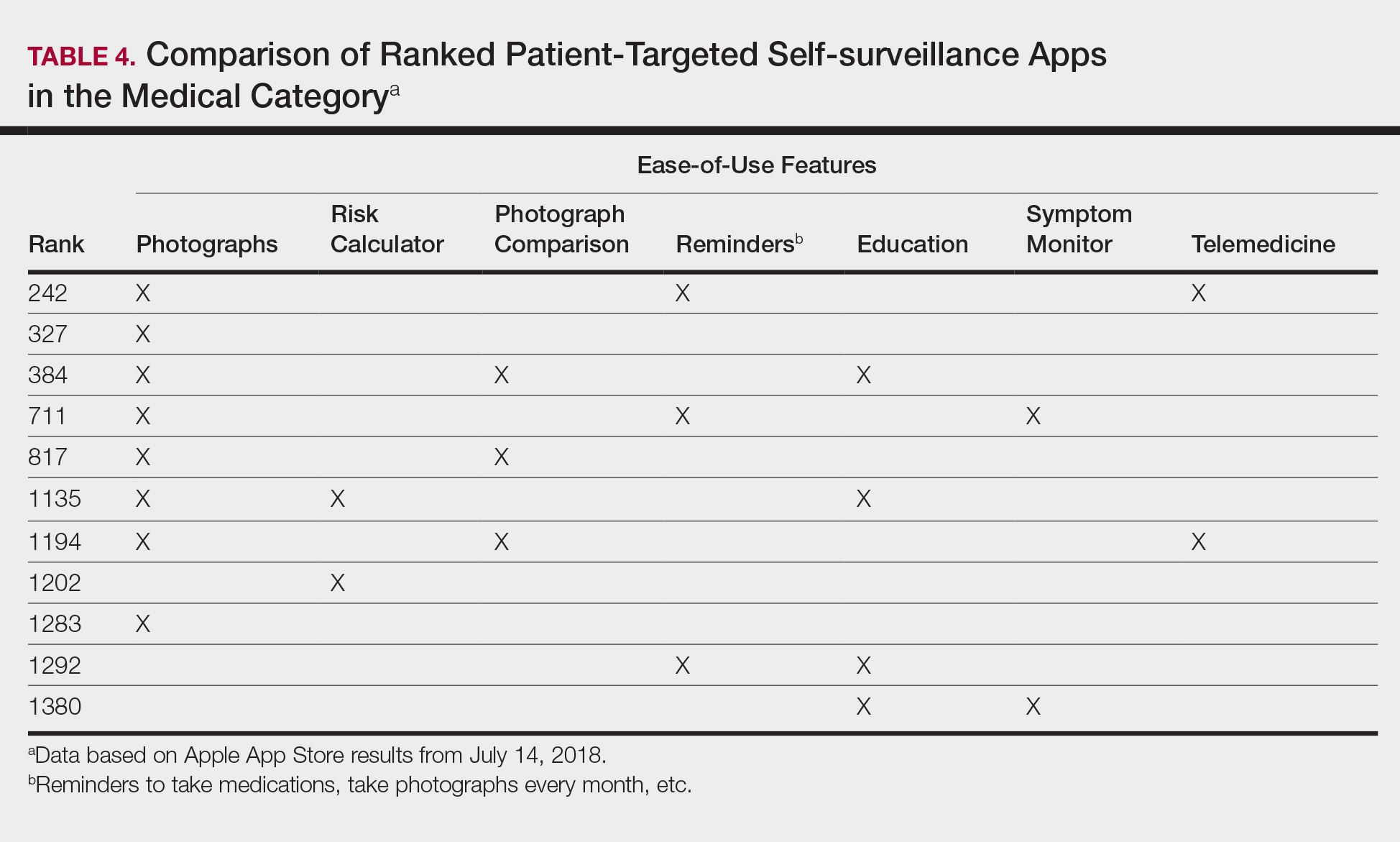
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
Practice Points
- As mobile application (app) usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in dermatology education. As such, it will become more critical to develop formal scientific standards.
- The most desired dermatology apps for patients were apps that allowed them to be proactive with their health.
- There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps.