User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
What’s New in Topical Treatments for Psoriasis
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
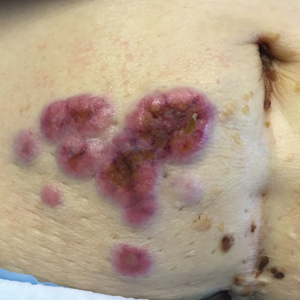
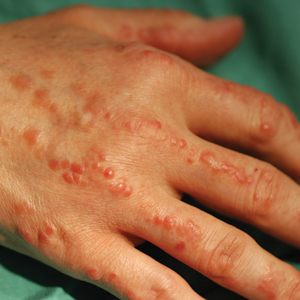
Erythematous Periumbilical Papules and Plaques
The Diagnosis: Metastatic Cancer
Further workup of patient 1 revealed an alkaline phosphatase level of 743 U/L (reference range, 30–120 U/L), total bilirubin level of 8.5 mg/dL (reference range, 0.3–1.2 mg/dL), and a white blood cell count of 14,000/μL (reference range, 4500–11,000/μL). Computed tomography of the abdomen and pelvis demonstrated cancer of unknown primary site that had metastasized to the colon, liver, and lungs. There was suspicion for potential colon cancer as the primary disease; however, based on the cutaneous findings, a skin biopsy was performed to confirm the diagnosis. Histology and immunohistochemistry revealed adenocarcinoma tumor cells positive for CDX2 (caudal type homeobox 2) and cytokeratin (CK) 7 with a subset positive for CK-20. The cells were negative for estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 (GATA binding protein 3). Immunohistochemistry was most consistent with pancreatic cancer. During palliative percutaneous transhepatic biliary drainage placement, a liver biopsy confirmed the skin biopsy results.
Further workup of patient 2 revealed a white blood cell count of 13,000/μL (reference range, 4500–11,000/μL). Computed tomography of the chest, abdomen, and pelvis revealed metastatic disease to the lungs with a suspicion for colon cancer as the primary site. Biopsy of the skin lesion revealed a mucin-producing adenocarcinoma, and immunohistochemistry was positive for keratin (AE1/AE3), CK-20, and CDX2, consistent with metastatic colon carcinoma. Immunohistochemistry of the biopsied skin lesion was nonreactive for CK-7. The patient had a colonoscopy that revealed a fungating, partially obstructing, circumferential large mass in the ascending colon.
Metastasis to the skin from visceral malignancies is not uncommon and may represent the first evidence of widespread disease, particularly in breast cancer or mucosal cancers of the head and neck.1 Cutaneous metastasis of colon cancer is uncommon and cutaneous metastasis of pancreatic cancer is rare. Furthermore, nonumbilical sites are much more common than umbilical sites for cutaneous metastatic disease.2 Pancreatic cancer is estimated to be the origin of a cutaneous umbilical metastasis, frequently termed Sister Mary Joseph nodule, in 7% to 9% of cases; colon cancer is estimated to account for 13% to 15% of cases.3 Sister Mary Joseph nodule or sign refers to a nodule often bulging into the umbilicus, signifying metastasis from a
malignant cancer.
In a study of cutaneous metastases, 10% (42/420) of patients with metastatic disease had cutaneous metastasis; 0.48% (2/420) were due to pancreatic cancer and 4.3% (18/420) were due to colon cancer.4 In another review, 63 cases of cutaneous metastasis of pancreatic cancer were found, 43 of which were nonumbilical.2
On immunohistochemistry, CK-7 positivity is highly specific for pancreatic cancer.2 Cytokeratin 7 often is used in conjunction with CK-20 to differentiate various types of glandular tumors. CDX2 is a highly sensitive and specific marker for adenocarcinomas of intestinal origin.5 The negative estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 stains are useful in excluding breast cancer (patient 1 had history of breast cancer).
When cutaneous involvement is present in pancreatic cancer, the disease usually is widespread. Multiple studies have reported involvement of other organs with cutaneous metastasis at rates of 88.9%,6 90.3%,7 and 93.5%.2 However, early recognition of metastatic cancerous lesions can lead to earlier diagnosis and earlier palliative treatment, perhaps prolonging median survival time in patients. In a review of 63 patients with cutaneous metastatic pancreatic cancer, the authors found a median survival time of 5 months, with surgery, chemotherapy, radiation therapy, or a combination helping to improve survival time from a median of 3.0 to 8.3 months.2
The location of lesions and duration of disease in both patients was atypical for arthropod assault. Acyclovir-resistant herpes zoster rarely is reported outside of human immunodeficiency patients; in addition, there was a lack of clear dermatomal distribution. Although cutaneous Crohn disease can manifest as pink papules, it is rare and unlikely as a presenting symptom. Cutaneous sarcoidosis can take many different skin manifestations, and patients can have cutaneous involvement without systemic manifestation. In both patients, medical history was more indicative of metastatic cancer than the other options in the differential diagnosis.
Cutaneous metastasis from colon cancer and pancreatic cancer is rare, and the prognosis is poor in these cases; however, in the appropriate clinical scenario, especially in a patient with a history of cancer, sinister etiologies should be considered for firm red papules of the umbilicus. Skin biopsy coupled with immunohistochemical staining can assist in identifying the primary malignancy.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-165.
- Zhou HY, Wang XB, Gao F, et al. Cutaneous metastasis from pancreatic cancer: a case report and systematic review of the literature [published online October 10, 2014]. Oncol Lett. 2014;8:2654-2660.
- Galvañ VG. Sister Mary Joseph's nodule. Ann Intern Med. 1998;128:410.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immnohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Takeuchi H, Kawano T, Toda T, et al. Cutaneous metastasis from pancreatic adenocarcinoma: a case report and a review of the literature. Hepatogastroenterology. 2003;50:275-277.
- Horino K, Hiraoka T, Kanemitsu K, et al. Subcutaneous metastases after curative resection for pancreatic carcinoma: a case report and review of the literature. Pancreas. 1999;19:406-408.
The Diagnosis: Metastatic Cancer
Further workup of patient 1 revealed an alkaline phosphatase level of 743 U/L (reference range, 30–120 U/L), total bilirubin level of 8.5 mg/dL (reference range, 0.3–1.2 mg/dL), and a white blood cell count of 14,000/μL (reference range, 4500–11,000/μL). Computed tomography of the abdomen and pelvis demonstrated cancer of unknown primary site that had metastasized to the colon, liver, and lungs. There was suspicion for potential colon cancer as the primary disease; however, based on the cutaneous findings, a skin biopsy was performed to confirm the diagnosis. Histology and immunohistochemistry revealed adenocarcinoma tumor cells positive for CDX2 (caudal type homeobox 2) and cytokeratin (CK) 7 with a subset positive for CK-20. The cells were negative for estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 (GATA binding protein 3). Immunohistochemistry was most consistent with pancreatic cancer. During palliative percutaneous transhepatic biliary drainage placement, a liver biopsy confirmed the skin biopsy results.
Further workup of patient 2 revealed a white blood cell count of 13,000/μL (reference range, 4500–11,000/μL). Computed tomography of the chest, abdomen, and pelvis revealed metastatic disease to the lungs with a suspicion for colon cancer as the primary site. Biopsy of the skin lesion revealed a mucin-producing adenocarcinoma, and immunohistochemistry was positive for keratin (AE1/AE3), CK-20, and CDX2, consistent with metastatic colon carcinoma. Immunohistochemistry of the biopsied skin lesion was nonreactive for CK-7. The patient had a colonoscopy that revealed a fungating, partially obstructing, circumferential large mass in the ascending colon.
Metastasis to the skin from visceral malignancies is not uncommon and may represent the first evidence of widespread disease, particularly in breast cancer or mucosal cancers of the head and neck.1 Cutaneous metastasis of colon cancer is uncommon and cutaneous metastasis of pancreatic cancer is rare. Furthermore, nonumbilical sites are much more common than umbilical sites for cutaneous metastatic disease.2 Pancreatic cancer is estimated to be the origin of a cutaneous umbilical metastasis, frequently termed Sister Mary Joseph nodule, in 7% to 9% of cases; colon cancer is estimated to account for 13% to 15% of cases.3 Sister Mary Joseph nodule or sign refers to a nodule often bulging into the umbilicus, signifying metastasis from a
malignant cancer.
In a study of cutaneous metastases, 10% (42/420) of patients with metastatic disease had cutaneous metastasis; 0.48% (2/420) were due to pancreatic cancer and 4.3% (18/420) were due to colon cancer.4 In another review, 63 cases of cutaneous metastasis of pancreatic cancer were found, 43 of which were nonumbilical.2
On immunohistochemistry, CK-7 positivity is highly specific for pancreatic cancer.2 Cytokeratin 7 often is used in conjunction with CK-20 to differentiate various types of glandular tumors. CDX2 is a highly sensitive and specific marker for adenocarcinomas of intestinal origin.5 The negative estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 stains are useful in excluding breast cancer (patient 1 had history of breast cancer).
When cutaneous involvement is present in pancreatic cancer, the disease usually is widespread. Multiple studies have reported involvement of other organs with cutaneous metastasis at rates of 88.9%,6 90.3%,7 and 93.5%.2 However, early recognition of metastatic cancerous lesions can lead to earlier diagnosis and earlier palliative treatment, perhaps prolonging median survival time in patients. In a review of 63 patients with cutaneous metastatic pancreatic cancer, the authors found a median survival time of 5 months, with surgery, chemotherapy, radiation therapy, or a combination helping to improve survival time from a median of 3.0 to 8.3 months.2
The location of lesions and duration of disease in both patients was atypical for arthropod assault. Acyclovir-resistant herpes zoster rarely is reported outside of human immunodeficiency patients; in addition, there was a lack of clear dermatomal distribution. Although cutaneous Crohn disease can manifest as pink papules, it is rare and unlikely as a presenting symptom. Cutaneous sarcoidosis can take many different skin manifestations, and patients can have cutaneous involvement without systemic manifestation. In both patients, medical history was more indicative of metastatic cancer than the other options in the differential diagnosis.
Cutaneous metastasis from colon cancer and pancreatic cancer is rare, and the prognosis is poor in these cases; however, in the appropriate clinical scenario, especially in a patient with a history of cancer, sinister etiologies should be considered for firm red papules of the umbilicus. Skin biopsy coupled with immunohistochemical staining can assist in identifying the primary malignancy.
The Diagnosis: Metastatic Cancer
Further workup of patient 1 revealed an alkaline phosphatase level of 743 U/L (reference range, 30–120 U/L), total bilirubin level of 8.5 mg/dL (reference range, 0.3–1.2 mg/dL), and a white blood cell count of 14,000/μL (reference range, 4500–11,000/μL). Computed tomography of the abdomen and pelvis demonstrated cancer of unknown primary site that had metastasized to the colon, liver, and lungs. There was suspicion for potential colon cancer as the primary disease; however, based on the cutaneous findings, a skin biopsy was performed to confirm the diagnosis. Histology and immunohistochemistry revealed adenocarcinoma tumor cells positive for CDX2 (caudal type homeobox 2) and cytokeratin (CK) 7 with a subset positive for CK-20. The cells were negative for estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 (GATA binding protein 3). Immunohistochemistry was most consistent with pancreatic cancer. During palliative percutaneous transhepatic biliary drainage placement, a liver biopsy confirmed the skin biopsy results.
Further workup of patient 2 revealed a white blood cell count of 13,000/μL (reference range, 4500–11,000/μL). Computed tomography of the chest, abdomen, and pelvis revealed metastatic disease to the lungs with a suspicion for colon cancer as the primary site. Biopsy of the skin lesion revealed a mucin-producing adenocarcinoma, and immunohistochemistry was positive for keratin (AE1/AE3), CK-20, and CDX2, consistent with metastatic colon carcinoma. Immunohistochemistry of the biopsied skin lesion was nonreactive for CK-7. The patient had a colonoscopy that revealed a fungating, partially obstructing, circumferential large mass in the ascending colon.
Metastasis to the skin from visceral malignancies is not uncommon and may represent the first evidence of widespread disease, particularly in breast cancer or mucosal cancers of the head and neck.1 Cutaneous metastasis of colon cancer is uncommon and cutaneous metastasis of pancreatic cancer is rare. Furthermore, nonumbilical sites are much more common than umbilical sites for cutaneous metastatic disease.2 Pancreatic cancer is estimated to be the origin of a cutaneous umbilical metastasis, frequently termed Sister Mary Joseph nodule, in 7% to 9% of cases; colon cancer is estimated to account for 13% to 15% of cases.3 Sister Mary Joseph nodule or sign refers to a nodule often bulging into the umbilicus, signifying metastasis from a
malignant cancer.
In a study of cutaneous metastases, 10% (42/420) of patients with metastatic disease had cutaneous metastasis; 0.48% (2/420) were due to pancreatic cancer and 4.3% (18/420) were due to colon cancer.4 In another review, 63 cases of cutaneous metastasis of pancreatic cancer were found, 43 of which were nonumbilical.2
On immunohistochemistry, CK-7 positivity is highly specific for pancreatic cancer.2 Cytokeratin 7 often is used in conjunction with CK-20 to differentiate various types of glandular tumors. CDX2 is a highly sensitive and specific marker for adenocarcinomas of intestinal origin.5 The negative estrogen receptor, progesterone receptor, mammaglobin, gross cystic disease fluid protein, and GATA3 stains are useful in excluding breast cancer (patient 1 had history of breast cancer).
When cutaneous involvement is present in pancreatic cancer, the disease usually is widespread. Multiple studies have reported involvement of other organs with cutaneous metastasis at rates of 88.9%,6 90.3%,7 and 93.5%.2 However, early recognition of metastatic cancerous lesions can lead to earlier diagnosis and earlier palliative treatment, perhaps prolonging median survival time in patients. In a review of 63 patients with cutaneous metastatic pancreatic cancer, the authors found a median survival time of 5 months, with surgery, chemotherapy, radiation therapy, or a combination helping to improve survival time from a median of 3.0 to 8.3 months.2
The location of lesions and duration of disease in both patients was atypical for arthropod assault. Acyclovir-resistant herpes zoster rarely is reported outside of human immunodeficiency patients; in addition, there was a lack of clear dermatomal distribution. Although cutaneous Crohn disease can manifest as pink papules, it is rare and unlikely as a presenting symptom. Cutaneous sarcoidosis can take many different skin manifestations, and patients can have cutaneous involvement without systemic manifestation. In both patients, medical history was more indicative of metastatic cancer than the other options in the differential diagnosis.
Cutaneous metastasis from colon cancer and pancreatic cancer is rare, and the prognosis is poor in these cases; however, in the appropriate clinical scenario, especially in a patient with a history of cancer, sinister etiologies should be considered for firm red papules of the umbilicus. Skin biopsy coupled with immunohistochemical staining can assist in identifying the primary malignancy.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-165.
- Zhou HY, Wang XB, Gao F, et al. Cutaneous metastasis from pancreatic cancer: a case report and systematic review of the literature [published online October 10, 2014]. Oncol Lett. 2014;8:2654-2660.
- Galvañ VG. Sister Mary Joseph's nodule. Ann Intern Med. 1998;128:410.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immnohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Takeuchi H, Kawano T, Toda T, et al. Cutaneous metastasis from pancreatic adenocarcinoma: a case report and a review of the literature. Hepatogastroenterology. 2003;50:275-277.
- Horino K, Hiraoka T, Kanemitsu K, et al. Subcutaneous metastases after curative resection for pancreatic carcinoma: a case report and review of the literature. Pancreas. 1999;19:406-408.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-165.
- Zhou HY, Wang XB, Gao F, et al. Cutaneous metastasis from pancreatic cancer: a case report and systematic review of the literature [published online October 10, 2014]. Oncol Lett. 2014;8:2654-2660.
- Galvañ VG. Sister Mary Joseph's nodule. Ann Intern Med. 1998;128:410.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immnohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Takeuchi H, Kawano T, Toda T, et al. Cutaneous metastasis from pancreatic adenocarcinoma: a case report and a review of the literature. Hepatogastroenterology. 2003;50:275-277.
- Horino K, Hiraoka T, Kanemitsu K, et al. Subcutaneous metastases after curative resection for pancreatic carcinoma: a case report and review of the literature. Pancreas. 1999;19:406-408.

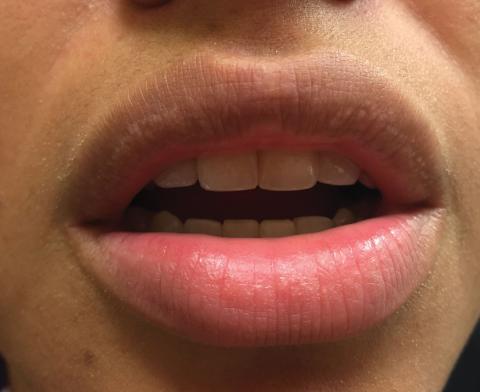
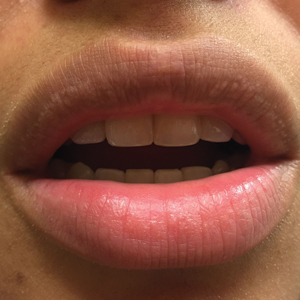
A 75-year-old woman (patient 1) with a history of localized invasive ductal breast cancer treated definitively with lumpectomy and radiation therapy more than a decade ago presented to the emergency department with jaundice, abdominal pain, weakness, and multiple periumbilical pink-red papules (top) of 2 weeks’ duration. Prior to presentation, the skin lesions did not improve with 10 days of acyclovir treatment prescribed by her primary care physician for presumed herpes zoster.
An 86-year-old man (patient 2) with chronic lymphocytic leukemia treated with ibrutinib presented to the emergency department with jaundice, abdominal pain, weakness, and multiple pink periumbilical papules (bottom) of 6 weeks’ duration. Prior to presentation, the skin lesions did not improve with 21 days of valacyclovir treatment prescribed by his oncologist for presumed herpes zoster.
The Dermatologist’s Role in Amputee Skin Care
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20
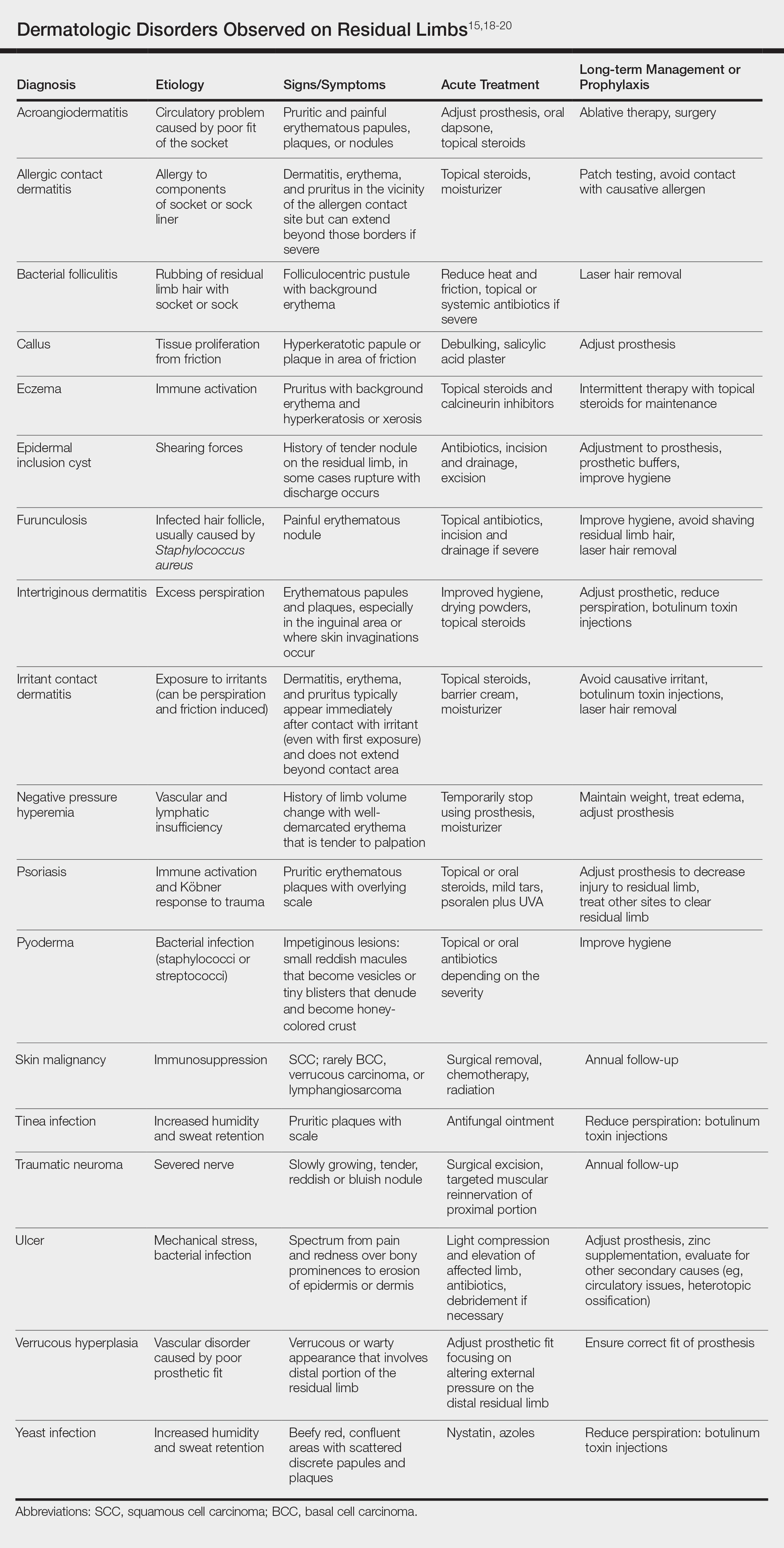
Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20

Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20

Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
Practice Points
- Amputees have an increased risk for skin disease occurring on residual limbs.
- It is important to educate patients about proper hygiene techniques for residual limbs and prostheses as well as common signs and symptoms of skin disease at the amputation site.
- Amputees should see a dermatologist within the first year after amputation and often benefit from annual follow-up examinations.
- Early referral to a dermatologist for skin disease affecting residual limbs is warranted.
Multicentric Reticulohistiocytosis With Arthralgia and Red-Orange Papulonodules
To the Editor:
A 50-year-old woman presented with an asymptomatic eruption on the dorsal aspect of the hands, abdomen, and face of 6 months’ duration. The eruption was associated with generalized arthralgia and fatigue. Within several weeks of onset of the cutaneous eruption, the patient developed swelling in the hands as well as worsening arthralgia. She was treated for presumed Lyme borreliosis but reported no improvement in the symptoms. She was then referred to dermatology for further management.
Physical examination revealed red-orange, edematous, monomorphic papulonodules scattered on the nasolabial folds, upper lip, and along the dorsal aspect of the hands and fingers (Figure 1). A brown rippled plaque was present on the left lower abdomen. The oral mucosa and nails were unremarkable. Laboratory studies showed elevated total cholesterol (244 mg/dL [reference range, <200 mg/dL]), low-density lipoproteins (130 mg/dL [reference range, 10–30 mg/dL]), aspartate aminotransferase (140 U/L [reference range, 10–30 U/L]), alanine aminotransferase (110 U/L [reference range, 10–40 U/L]), and total bilirubin (1.5 mg/dL [reference range, 0.3–1.2 mg/dL]). White blood cell count and C-reactive protein levels were within reference range. An antinuclear antibody titer of 1:80 with a homogenous pattern was found, and aldolase levels were elevated. Laboratory investigations for rheumatoid factor, Lyme disease, tuberculosis, hepatitis, and human immunodeficiency virus were negative. A chest radiograph was normal.
A punch biopsy from the right dorsal hand revealed a dermal proliferation of mononucleated and multinucleated epithelioid histiocytes with ample amounts of eosinophilic ground-glass cytoplasm (Figure 2). Immunohistochemistry revealed epithelioid histiocytes reactive for CD68, CD163, and factor XIIIA, and negative for S-100 and CD1a.
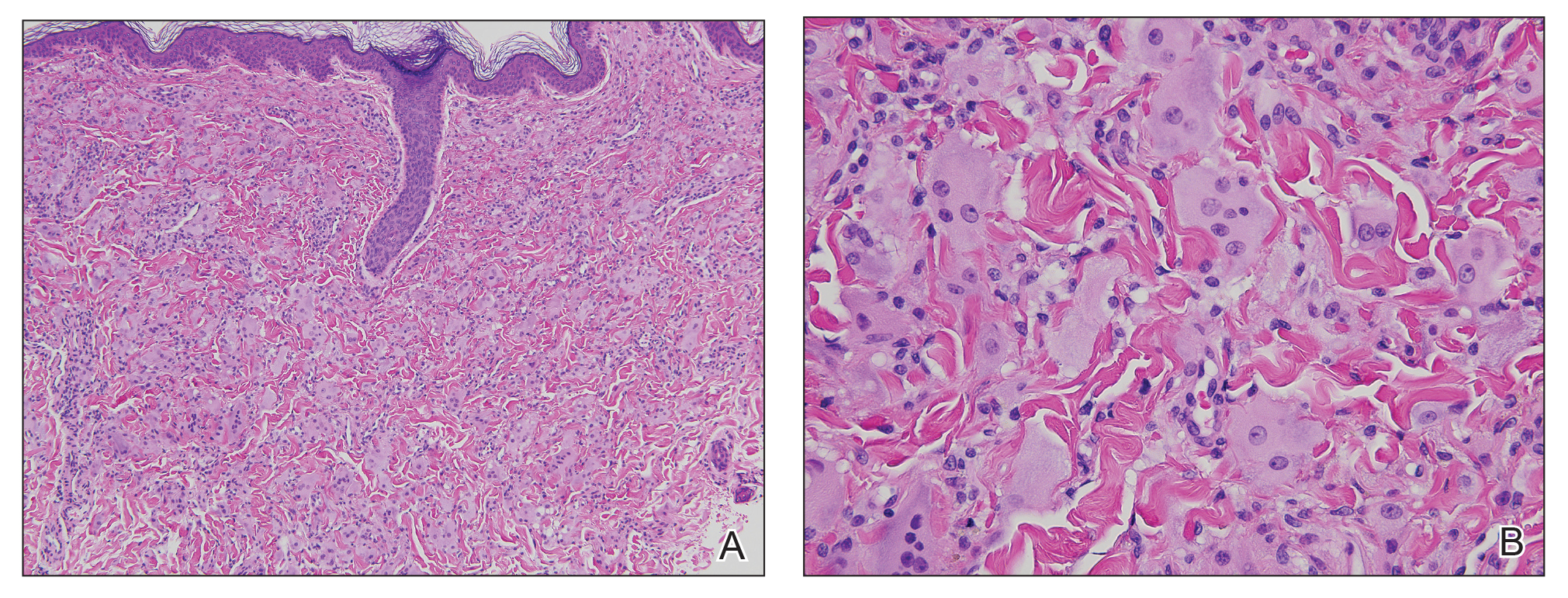
The patient was diagnosed with multicentric reticulohistiocytosis (MRH) and was initially treated with prednisone. Treatment was later augmented with etanercept and methotrexate with improvement in both the skin and joint symptoms.
Multicentric reticulohistiocytosis is a rare, non–Langerhans cell histiocytosis with both cutaneous and systemic features. Although case reports date back to the late 1800s, the term multicentric reticulohistiocytosis was first used in 1954.1 Multicentric reticulohistiocytosis is extremely uncommon and precludes thorough investigation of its etiology and management. The condition typically presents in the fifth to sixth decades of life and occurs more frequently in women with a female to male ratio estimated at 3 to 1.2,3 Pediatric cases have been reported but are exceedingly rare.4
Multicentric reticulohistiocytosis typically presents with a severe erosive arthropathy known as arthritis mutilans. Patients display a symmetric polyarthritis that commonly involves the elbows, wrists, and proximal and distal aspects of the interphalangeal joints. Onset and progression can be rapid, and the erosive nature leads to deformities in up to 45% of patients.2,5,6 Cutaneous findings arise an average of 3 years after the development of arthritis, though one-fifth of patients will initially present with cutaneous findings followed by the development of arthritis at any time.3,6 Clinical features include flesh-colored to reddish brown or yellow papulonodules that range in size from several millimeters to 2 cm. The lesions most commonly occur on the face (eg, ears, nose, paranasal cheeks), scalp, dorsal and lateral aspects of the hands and fingers, and overlying articular regions of the extremities. Characteristic periungual lesions classically are referred to as coral beads.4,6 Patients commonly report pruritus that may precede the development of the papules and nodules. Other cutaneous manifestations include xanthelasma, nail changes, and a photodistributed erythematous maculopapular eruption that may mimic dermatomyositis.6
Cutaneous findings of MRH can mimic rheumatoid nodules, gout, Gottron papules of dermatomyositis, lipoid proteinosis, sarcoidosis, lepromatous leprosy, granuloma annulare, xanthoma, xanthogranuloma, and fibroxanthoma.6,7 Histopathologic features may distinguish MRH from such entities. Findings include fairly well-circumscribed aggregates of large multinucleated giant cells with characteristic eosinophilic ground-glass cytoplasm. Histiocytes stain positively for CD68, HAM56, CD11b, and CD14, and variably for factor XIIIa. CD68, which is expressed by monocytes/macrophages, has been universally reported to be the most reliable marker of MRH. Negative staining for S-100 and CD1a supports a non-Langerhans origin for the involved histiocytes. If arthritic symptoms predominate, MRH must be distinguished from rheumatoid and psoriatic arthritis.6,7
Mucosal involvement occurs in approximately 50% of patients and includes the presence of nodules in the oral, nasal, and pharyngeal mucosae, as well as eye structures.2,3 Histiocytic infiltration has been documented in the heart, lungs, thyroid, liver, stomach, kidneys, muscle, bone marrow, and urogenital tract. Histiocytes also can invade the cartilage of the ears and nose causing disfigurement and characteristic leonine facies. Pathologic fractures may occur with bone involvement.5
Systemic features associated with MRH include hyperlipidemia, diabetes mellitus, thyroid disease, hypergammaglobulinemia, and various autoimmune diseases. Patients less frequently report fever and weight loss.2,5,6,8 Additionally, a positive tuberculin test occurs in 12% to 50% of patients.6 Various autoimmune diseases occur in 6% to 17% of cases including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, dermatomyositis, Sjögren syndrome, and primary biliary cirrhosis.2,5,6,8 The most clinically salient feature of MRH is its association with malignant conditions, which occur in up to 31% of patients. A variety of cancers have been reported in association with MRH, including breast, cervical, ovarian, stomach, penile, lymphoma, mesothelioma, and melanoma.7
The etiology of MRH is unclear. Although onset may precede the development of a malignant condition and regress with treatment, it cannot be considered a true paraneoplastic disorder, as it has no association with a specific cancer and does not typically parallel the disease course.6,9 Reports of increased levels of inflammatory mediators released from macrophages and endothelial cells, specifically IL-12, IL-1β, IL-6, and tumor necrosis factor α (TNF-α), have been thought to drive the destruction of bone and cartilage.6 In particular, TNF-α acts to indirectly induce destruction by stimulating proteolytic activity in macrophages, similar to the pathogenesis of joint damage in rheumatoid arthritis.8 Osteoclastic activity may play a role in the pathogenesis of MRH, as multinucleated giant cells in MRH can mature into osteoclasts by receptor activated nuclear factor–κB ligand signaling. In addition, patients treated with bisphosphonates have had decreased lacunar resorption.2,8
Initial management of MRH should include screening for hyperlipidemia, hypergammaglobulinemia, hyperglycemia, thyroid dysfunction, and autoimmune diseases, as well as age-appropriate cancer screening. Imaging studies should evaluate for the presence of erosive arthritis. There are no well-defined treatment algorithms for MRH due to the rarity of the disease, and recommendations largely rely on case reports. Although spontaneous remission typically occurs within 5 to 10 years, the risk for joint destruction argues for early pharmacologic intervention. Current management includes the use of nonsteroidal anti-inflammatory drugs and various immunosuppressants including oral glucocorticoids, cyclophosphamide, chlorambucil, methotrexate, or azathioprine.2 A combination of methotrexate with cyclophosphamide or glucocorticoids also has shown efficacy.10 Anti–TNF-α agents, such as etanercept, adalimumab, and infliximab, have been used with some success.2 Tumor necrosis factor α inhibitors used in combination with oral glucocorticoids and methotrexate may have an increased benefit.2,9,11 Evidence suggesting that TNF-α plays a role in the destruction of bone and cartilage led to the successful use of infliximab in combination with oral glucocorticoids and methotrexate, which prevented possible development of antibodies to infliximab and increased its efficacy.12 Bisphosphonate use in combination with glucocorticoids and methotrexate may prevent joint destruction without the serious adverse events associated with anti–TNF-α agents.2,9,13,14
- Goltz RW, Laymon CW. Multicentric reticulohistiocytosis of the skin and synovia; reticulohistiocytoma or ganglioneuroma. AMA Arch Derma Syphilol. 1954;69:717-731.
- Islam AD, Naguwa SM, Cheema GS, et al. Multicentric reticulohistiocytosis: a rare yet challenging disease. Clin Rev Allergy Immunol. 2013;45:281-289.
- West KL, Sporn T, Puri PK. Multicentric reticulohistiocytosis: a unique case with pulmonary fibrosis. Arch Dermatol. 2012;148:228-232.
- Outland JD, Keiran SJ, Schikler KN, et al. Multicentric reticulohistiocytosis in a 14-year-old girl. Pediatr Dermatol. 2002;19:527-531.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Trotta F, Castellino G, Lo Monaco A. Multicentric reticulohistiocytosis. Best Pract Res Clin Rheumatol. 2004;18:759-772.
- Kalajian AH, Callen JP. Multicentric reticulohistiocytosis successfully treated with infliximab: an illustrative case and evaluation of cytokine expression supporting anti-tumor necrosis factor therapy. Arch Derm. 2008;144:1360-1366.
- Liang GC, Granston AS. Complete remission of multicentric reticulohistiocytosis with combination therapy of steroid, cyclophosphamide, and low-dose pulse methotrexate. case report, review of the literature, and proposal for treatment. Arthritis Rheum. 1996;39:171-174.
- Lovelace K, Loyd A, Adelson D, et al. Etanercept and the treatment of multicentric reticulohistiocytosis. Arch Dermatol. 2005;141:1167-1168.
- Lee MW, Lee EY, Jeong YI, et al. Successful treatment of multicentric reticulohistiocytosis with a combination of infliximab, prednisolone and methotrexate. Acta Derm Venereol. 2004;84:478-479.
- Adamopoulos IE, Wordsworth PB, Edwards JR, et al. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol. 2006;37:1176-1185.
- Satoh M, Oyama N, Yamada H, et al. Treatment trial of multicentric reticulohistiocytosis with a combination of predonisolone, methotrexate and alendronate. J Dermatol. 2008;35:168-171.
To the Editor:
A 50-year-old woman presented with an asymptomatic eruption on the dorsal aspect of the hands, abdomen, and face of 6 months’ duration. The eruption was associated with generalized arthralgia and fatigue. Within several weeks of onset of the cutaneous eruption, the patient developed swelling in the hands as well as worsening arthralgia. She was treated for presumed Lyme borreliosis but reported no improvement in the symptoms. She was then referred to dermatology for further management.
Physical examination revealed red-orange, edematous, monomorphic papulonodules scattered on the nasolabial folds, upper lip, and along the dorsal aspect of the hands and fingers (Figure 1). A brown rippled plaque was present on the left lower abdomen. The oral mucosa and nails were unremarkable. Laboratory studies showed elevated total cholesterol (244 mg/dL [reference range, <200 mg/dL]), low-density lipoproteins (130 mg/dL [reference range, 10–30 mg/dL]), aspartate aminotransferase (140 U/L [reference range, 10–30 U/L]), alanine aminotransferase (110 U/L [reference range, 10–40 U/L]), and total bilirubin (1.5 mg/dL [reference range, 0.3–1.2 mg/dL]). White blood cell count and C-reactive protein levels were within reference range. An antinuclear antibody titer of 1:80 with a homogenous pattern was found, and aldolase levels were elevated. Laboratory investigations for rheumatoid factor, Lyme disease, tuberculosis, hepatitis, and human immunodeficiency virus were negative. A chest radiograph was normal.

A punch biopsy from the right dorsal hand revealed a dermal proliferation of mononucleated and multinucleated epithelioid histiocytes with ample amounts of eosinophilic ground-glass cytoplasm (Figure 2). Immunohistochemistry revealed epithelioid histiocytes reactive for CD68, CD163, and factor XIIIA, and negative for S-100 and CD1a.

The patient was diagnosed with multicentric reticulohistiocytosis (MRH) and was initially treated with prednisone. Treatment was later augmented with etanercept and methotrexate with improvement in both the skin and joint symptoms.
Multicentric reticulohistiocytosis is a rare, non–Langerhans cell histiocytosis with both cutaneous and systemic features. Although case reports date back to the late 1800s, the term multicentric reticulohistiocytosis was first used in 1954.1 Multicentric reticulohistiocytosis is extremely uncommon and precludes thorough investigation of its etiology and management. The condition typically presents in the fifth to sixth decades of life and occurs more frequently in women with a female to male ratio estimated at 3 to 1.2,3 Pediatric cases have been reported but are exceedingly rare.4
Multicentric reticulohistiocytosis typically presents with a severe erosive arthropathy known as arthritis mutilans. Patients display a symmetric polyarthritis that commonly involves the elbows, wrists, and proximal and distal aspects of the interphalangeal joints. Onset and progression can be rapid, and the erosive nature leads to deformities in up to 45% of patients.2,5,6 Cutaneous findings arise an average of 3 years after the development of arthritis, though one-fifth of patients will initially present with cutaneous findings followed by the development of arthritis at any time.3,6 Clinical features include flesh-colored to reddish brown or yellow papulonodules that range in size from several millimeters to 2 cm. The lesions most commonly occur on the face (eg, ears, nose, paranasal cheeks), scalp, dorsal and lateral aspects of the hands and fingers, and overlying articular regions of the extremities. Characteristic periungual lesions classically are referred to as coral beads.4,6 Patients commonly report pruritus that may precede the development of the papules and nodules. Other cutaneous manifestations include xanthelasma, nail changes, and a photodistributed erythematous maculopapular eruption that may mimic dermatomyositis.6
Cutaneous findings of MRH can mimic rheumatoid nodules, gout, Gottron papules of dermatomyositis, lipoid proteinosis, sarcoidosis, lepromatous leprosy, granuloma annulare, xanthoma, xanthogranuloma, and fibroxanthoma.6,7 Histopathologic features may distinguish MRH from such entities. Findings include fairly well-circumscribed aggregates of large multinucleated giant cells with characteristic eosinophilic ground-glass cytoplasm. Histiocytes stain positively for CD68, HAM56, CD11b, and CD14, and variably for factor XIIIa. CD68, which is expressed by monocytes/macrophages, has been universally reported to be the most reliable marker of MRH. Negative staining for S-100 and CD1a supports a non-Langerhans origin for the involved histiocytes. If arthritic symptoms predominate, MRH must be distinguished from rheumatoid and psoriatic arthritis.6,7
Mucosal involvement occurs in approximately 50% of patients and includes the presence of nodules in the oral, nasal, and pharyngeal mucosae, as well as eye structures.2,3 Histiocytic infiltration has been documented in the heart, lungs, thyroid, liver, stomach, kidneys, muscle, bone marrow, and urogenital tract. Histiocytes also can invade the cartilage of the ears and nose causing disfigurement and characteristic leonine facies. Pathologic fractures may occur with bone involvement.5
Systemic features associated with MRH include hyperlipidemia, diabetes mellitus, thyroid disease, hypergammaglobulinemia, and various autoimmune diseases. Patients less frequently report fever and weight loss.2,5,6,8 Additionally, a positive tuberculin test occurs in 12% to 50% of patients.6 Various autoimmune diseases occur in 6% to 17% of cases including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, dermatomyositis, Sjögren syndrome, and primary biliary cirrhosis.2,5,6,8 The most clinically salient feature of MRH is its association with malignant conditions, which occur in up to 31% of patients. A variety of cancers have been reported in association with MRH, including breast, cervical, ovarian, stomach, penile, lymphoma, mesothelioma, and melanoma.7
The etiology of MRH is unclear. Although onset may precede the development of a malignant condition and regress with treatment, it cannot be considered a true paraneoplastic disorder, as it has no association with a specific cancer and does not typically parallel the disease course.6,9 Reports of increased levels of inflammatory mediators released from macrophages and endothelial cells, specifically IL-12, IL-1β, IL-6, and tumor necrosis factor α (TNF-α), have been thought to drive the destruction of bone and cartilage.6 In particular, TNF-α acts to indirectly induce destruction by stimulating proteolytic activity in macrophages, similar to the pathogenesis of joint damage in rheumatoid arthritis.8 Osteoclastic activity may play a role in the pathogenesis of MRH, as multinucleated giant cells in MRH can mature into osteoclasts by receptor activated nuclear factor–κB ligand signaling. In addition, patients treated with bisphosphonates have had decreased lacunar resorption.2,8
Initial management of MRH should include screening for hyperlipidemia, hypergammaglobulinemia, hyperglycemia, thyroid dysfunction, and autoimmune diseases, as well as age-appropriate cancer screening. Imaging studies should evaluate for the presence of erosive arthritis. There are no well-defined treatment algorithms for MRH due to the rarity of the disease, and recommendations largely rely on case reports. Although spontaneous remission typically occurs within 5 to 10 years, the risk for joint destruction argues for early pharmacologic intervention. Current management includes the use of nonsteroidal anti-inflammatory drugs and various immunosuppressants including oral glucocorticoids, cyclophosphamide, chlorambucil, methotrexate, or azathioprine.2 A combination of methotrexate with cyclophosphamide or glucocorticoids also has shown efficacy.10 Anti–TNF-α agents, such as etanercept, adalimumab, and infliximab, have been used with some success.2 Tumor necrosis factor α inhibitors used in combination with oral glucocorticoids and methotrexate may have an increased benefit.2,9,11 Evidence suggesting that TNF-α plays a role in the destruction of bone and cartilage led to the successful use of infliximab in combination with oral glucocorticoids and methotrexate, which prevented possible development of antibodies to infliximab and increased its efficacy.12 Bisphosphonate use in combination with glucocorticoids and methotrexate may prevent joint destruction without the serious adverse events associated with anti–TNF-α agents.2,9,13,14
To the Editor:
A 50-year-old woman presented with an asymptomatic eruption on the dorsal aspect of the hands, abdomen, and face of 6 months’ duration. The eruption was associated with generalized arthralgia and fatigue. Within several weeks of onset of the cutaneous eruption, the patient developed swelling in the hands as well as worsening arthralgia. She was treated for presumed Lyme borreliosis but reported no improvement in the symptoms. She was then referred to dermatology for further management.
Physical examination revealed red-orange, edematous, monomorphic papulonodules scattered on the nasolabial folds, upper lip, and along the dorsal aspect of the hands and fingers (Figure 1). A brown rippled plaque was present on the left lower abdomen. The oral mucosa and nails were unremarkable. Laboratory studies showed elevated total cholesterol (244 mg/dL [reference range, <200 mg/dL]), low-density lipoproteins (130 mg/dL [reference range, 10–30 mg/dL]), aspartate aminotransferase (140 U/L [reference range, 10–30 U/L]), alanine aminotransferase (110 U/L [reference range, 10–40 U/L]), and total bilirubin (1.5 mg/dL [reference range, 0.3–1.2 mg/dL]). White blood cell count and C-reactive protein levels were within reference range. An antinuclear antibody titer of 1:80 with a homogenous pattern was found, and aldolase levels were elevated. Laboratory investigations for rheumatoid factor, Lyme disease, tuberculosis, hepatitis, and human immunodeficiency virus were negative. A chest radiograph was normal.

A punch biopsy from the right dorsal hand revealed a dermal proliferation of mononucleated and multinucleated epithelioid histiocytes with ample amounts of eosinophilic ground-glass cytoplasm (Figure 2). Immunohistochemistry revealed epithelioid histiocytes reactive for CD68, CD163, and factor XIIIA, and negative for S-100 and CD1a.

The patient was diagnosed with multicentric reticulohistiocytosis (MRH) and was initially treated with prednisone. Treatment was later augmented with etanercept and methotrexate with improvement in both the skin and joint symptoms.
Multicentric reticulohistiocytosis is a rare, non–Langerhans cell histiocytosis with both cutaneous and systemic features. Although case reports date back to the late 1800s, the term multicentric reticulohistiocytosis was first used in 1954.1 Multicentric reticulohistiocytosis is extremely uncommon and precludes thorough investigation of its etiology and management. The condition typically presents in the fifth to sixth decades of life and occurs more frequently in women with a female to male ratio estimated at 3 to 1.2,3 Pediatric cases have been reported but are exceedingly rare.4
Multicentric reticulohistiocytosis typically presents with a severe erosive arthropathy known as arthritis mutilans. Patients display a symmetric polyarthritis that commonly involves the elbows, wrists, and proximal and distal aspects of the interphalangeal joints. Onset and progression can be rapid, and the erosive nature leads to deformities in up to 45% of patients.2,5,6 Cutaneous findings arise an average of 3 years after the development of arthritis, though one-fifth of patients will initially present with cutaneous findings followed by the development of arthritis at any time.3,6 Clinical features include flesh-colored to reddish brown or yellow papulonodules that range in size from several millimeters to 2 cm. The lesions most commonly occur on the face (eg, ears, nose, paranasal cheeks), scalp, dorsal and lateral aspects of the hands and fingers, and overlying articular regions of the extremities. Characteristic periungual lesions classically are referred to as coral beads.4,6 Patients commonly report pruritus that may precede the development of the papules and nodules. Other cutaneous manifestations include xanthelasma, nail changes, and a photodistributed erythematous maculopapular eruption that may mimic dermatomyositis.6
Cutaneous findings of MRH can mimic rheumatoid nodules, gout, Gottron papules of dermatomyositis, lipoid proteinosis, sarcoidosis, lepromatous leprosy, granuloma annulare, xanthoma, xanthogranuloma, and fibroxanthoma.6,7 Histopathologic features may distinguish MRH from such entities. Findings include fairly well-circumscribed aggregates of large multinucleated giant cells with characteristic eosinophilic ground-glass cytoplasm. Histiocytes stain positively for CD68, HAM56, CD11b, and CD14, and variably for factor XIIIa. CD68, which is expressed by monocytes/macrophages, has been universally reported to be the most reliable marker of MRH. Negative staining for S-100 and CD1a supports a non-Langerhans origin for the involved histiocytes. If arthritic symptoms predominate, MRH must be distinguished from rheumatoid and psoriatic arthritis.6,7
Mucosal involvement occurs in approximately 50% of patients and includes the presence of nodules in the oral, nasal, and pharyngeal mucosae, as well as eye structures.2,3 Histiocytic infiltration has been documented in the heart, lungs, thyroid, liver, stomach, kidneys, muscle, bone marrow, and urogenital tract. Histiocytes also can invade the cartilage of the ears and nose causing disfigurement and characteristic leonine facies. Pathologic fractures may occur with bone involvement.5
Systemic features associated with MRH include hyperlipidemia, diabetes mellitus, thyroid disease, hypergammaglobulinemia, and various autoimmune diseases. Patients less frequently report fever and weight loss.2,5,6,8 Additionally, a positive tuberculin test occurs in 12% to 50% of patients.6 Various autoimmune diseases occur in 6% to 17% of cases including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, dermatomyositis, Sjögren syndrome, and primary biliary cirrhosis.2,5,6,8 The most clinically salient feature of MRH is its association with malignant conditions, which occur in up to 31% of patients. A variety of cancers have been reported in association with MRH, including breast, cervical, ovarian, stomach, penile, lymphoma, mesothelioma, and melanoma.7
The etiology of MRH is unclear. Although onset may precede the development of a malignant condition and regress with treatment, it cannot be considered a true paraneoplastic disorder, as it has no association with a specific cancer and does not typically parallel the disease course.6,9 Reports of increased levels of inflammatory mediators released from macrophages and endothelial cells, specifically IL-12, IL-1β, IL-6, and tumor necrosis factor α (TNF-α), have been thought to drive the destruction of bone and cartilage.6 In particular, TNF-α acts to indirectly induce destruction by stimulating proteolytic activity in macrophages, similar to the pathogenesis of joint damage in rheumatoid arthritis.8 Osteoclastic activity may play a role in the pathogenesis of MRH, as multinucleated giant cells in MRH can mature into osteoclasts by receptor activated nuclear factor–κB ligand signaling. In addition, patients treated with bisphosphonates have had decreased lacunar resorption.2,8
Initial management of MRH should include screening for hyperlipidemia, hypergammaglobulinemia, hyperglycemia, thyroid dysfunction, and autoimmune diseases, as well as age-appropriate cancer screening. Imaging studies should evaluate for the presence of erosive arthritis. There are no well-defined treatment algorithms for MRH due to the rarity of the disease, and recommendations largely rely on case reports. Although spontaneous remission typically occurs within 5 to 10 years, the risk for joint destruction argues for early pharmacologic intervention. Current management includes the use of nonsteroidal anti-inflammatory drugs and various immunosuppressants including oral glucocorticoids, cyclophosphamide, chlorambucil, methotrexate, or azathioprine.2 A combination of methotrexate with cyclophosphamide or glucocorticoids also has shown efficacy.10 Anti–TNF-α agents, such as etanercept, adalimumab, and infliximab, have been used with some success.2 Tumor necrosis factor α inhibitors used in combination with oral glucocorticoids and methotrexate may have an increased benefit.2,9,11 Evidence suggesting that TNF-α plays a role in the destruction of bone and cartilage led to the successful use of infliximab in combination with oral glucocorticoids and methotrexate, which prevented possible development of antibodies to infliximab and increased its efficacy.12 Bisphosphonate use in combination with glucocorticoids and methotrexate may prevent joint destruction without the serious adverse events associated with anti–TNF-α agents.2,9,13,14
- Goltz RW, Laymon CW. Multicentric reticulohistiocytosis of the skin and synovia; reticulohistiocytoma or ganglioneuroma. AMA Arch Derma Syphilol. 1954;69:717-731.
- Islam AD, Naguwa SM, Cheema GS, et al. Multicentric reticulohistiocytosis: a rare yet challenging disease. Clin Rev Allergy Immunol. 2013;45:281-289.
- West KL, Sporn T, Puri PK. Multicentric reticulohistiocytosis: a unique case with pulmonary fibrosis. Arch Dermatol. 2012;148:228-232.
- Outland JD, Keiran SJ, Schikler KN, et al. Multicentric reticulohistiocytosis in a 14-year-old girl. Pediatr Dermatol. 2002;19:527-531.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Trotta F, Castellino G, Lo Monaco A. Multicentric reticulohistiocytosis. Best Pract Res Clin Rheumatol. 2004;18:759-772.
- Kalajian AH, Callen JP. Multicentric reticulohistiocytosis successfully treated with infliximab: an illustrative case and evaluation of cytokine expression supporting anti-tumor necrosis factor therapy. Arch Derm. 2008;144:1360-1366.
- Liang GC, Granston AS. Complete remission of multicentric reticulohistiocytosis with combination therapy of steroid, cyclophosphamide, and low-dose pulse methotrexate. case report, review of the literature, and proposal for treatment. Arthritis Rheum. 1996;39:171-174.
- Lovelace K, Loyd A, Adelson D, et al. Etanercept and the treatment of multicentric reticulohistiocytosis. Arch Dermatol. 2005;141:1167-1168.
- Lee MW, Lee EY, Jeong YI, et al. Successful treatment of multicentric reticulohistiocytosis with a combination of infliximab, prednisolone and methotrexate. Acta Derm Venereol. 2004;84:478-479.
- Adamopoulos IE, Wordsworth PB, Edwards JR, et al. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol. 2006;37:1176-1185.
- Satoh M, Oyama N, Yamada H, et al. Treatment trial of multicentric reticulohistiocytosis with a combination of predonisolone, methotrexate and alendronate. J Dermatol. 2008;35:168-171.
- Goltz RW, Laymon CW. Multicentric reticulohistiocytosis of the skin and synovia; reticulohistiocytoma or ganglioneuroma. AMA Arch Derma Syphilol. 1954;69:717-731.
- Islam AD, Naguwa SM, Cheema GS, et al. Multicentric reticulohistiocytosis: a rare yet challenging disease. Clin Rev Allergy Immunol. 2013;45:281-289.
- West KL, Sporn T, Puri PK. Multicentric reticulohistiocytosis: a unique case with pulmonary fibrosis. Arch Dermatol. 2012;148:228-232.
- Outland JD, Keiran SJ, Schikler KN, et al. Multicentric reticulohistiocytosis in a 14-year-old girl. Pediatr Dermatol. 2002;19:527-531.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Trotta F, Castellino G, Lo Monaco A. Multicentric reticulohistiocytosis. Best Pract Res Clin Rheumatol. 2004;18:759-772.
- Kalajian AH, Callen JP. Multicentric reticulohistiocytosis successfully treated with infliximab: an illustrative case and evaluation of cytokine expression supporting anti-tumor necrosis factor therapy. Arch Derm. 2008;144:1360-1366.
- Liang GC, Granston AS. Complete remission of multicentric reticulohistiocytosis with combination therapy of steroid, cyclophosphamide, and low-dose pulse methotrexate. case report, review of the literature, and proposal for treatment. Arthritis Rheum. 1996;39:171-174.
- Lovelace K, Loyd A, Adelson D, et al. Etanercept and the treatment of multicentric reticulohistiocytosis. Arch Dermatol. 2005;141:1167-1168.
- Lee MW, Lee EY, Jeong YI, et al. Successful treatment of multicentric reticulohistiocytosis with a combination of infliximab, prednisolone and methotrexate. Acta Derm Venereol. 2004;84:478-479.
- Adamopoulos IE, Wordsworth PB, Edwards JR, et al. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol. 2006;37:1176-1185.
- Satoh M, Oyama N, Yamada H, et al. Treatment trial of multicentric reticulohistiocytosis with a combination of predonisolone, methotrexate and alendronate. J Dermatol. 2008;35:168-171.
Practice Points
- Multicentric reticulohistiocytosis (MRH) is an important entity to recognize given its association with underlying malignancy and irreversible destructive arthritis.
- Diagnosis of MRH warrants extensive review of systems, age-appropriate cancer screening, and relevant systemic workup.
- Early pharmacologic intervention should be initiated with nonsteroidal anti-inflammatory agents or immunosuppressant agents.
Paraneoplastic Dermatomyositis Presenting With Interesting Cutaneous Findings
To the Editor:
We report an interesting clinical case of dermatomyositis (DM) that presented with an associated malignancy (small cell lung cancer). This patient also had an unusual clinical finding of predominantly unilateral, confluent, erythematous papules on the knee, a cutaneous sign that is seldom described in the DM literature. This case serves to reinforce the classic findings and associations of DM, in addition to the uncommon manifestation of predominantly unilateral papules on the knee.
A 68-year-old woman presented with several cutaneous manifestations including the classic findings of photo distributed erythema on the arms and face, a heliotrope rash, Gottron papules, and confluent pink papules on the left knee (Figure 1). The patient also had one of the more rare manifestations of DM, flagellate erythema on the back (Figure 2). She had a history of breast cancer and was found to have metastatic small cell lung cancer at the time of the DM diagnosis.
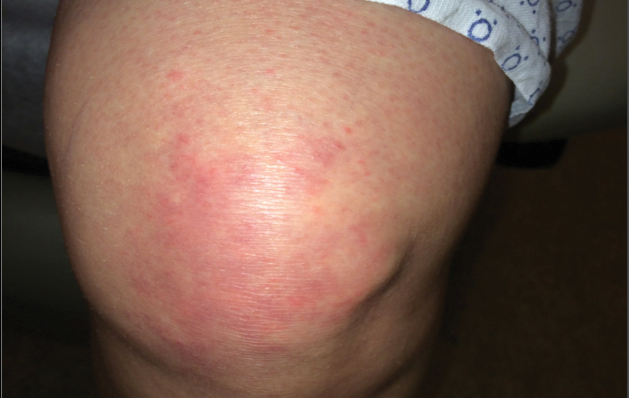
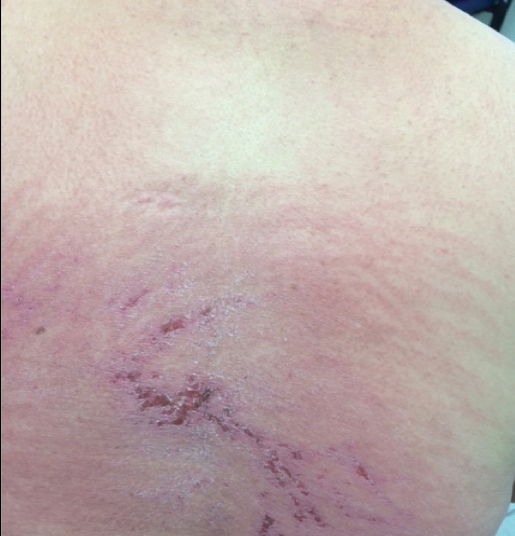
A punch biopsy from an area of flagellate erythema on the back revealed an interface dermatitis with a superficial, perivascular, lymphocyte-predominant inflammatory infiltrate (Figure 3). Alcian blue and colloidal iron stains revealed a marked increase in papillary dermal mucin. With the characteristic changes on skin biopsy and the classic skin findings present in our patient, we felt confident diagnosing her with DM. At the time of diagnosis, the patient also was found to have metastatic small cell lung cancer, suggesting a true paraneoplastic relationship.
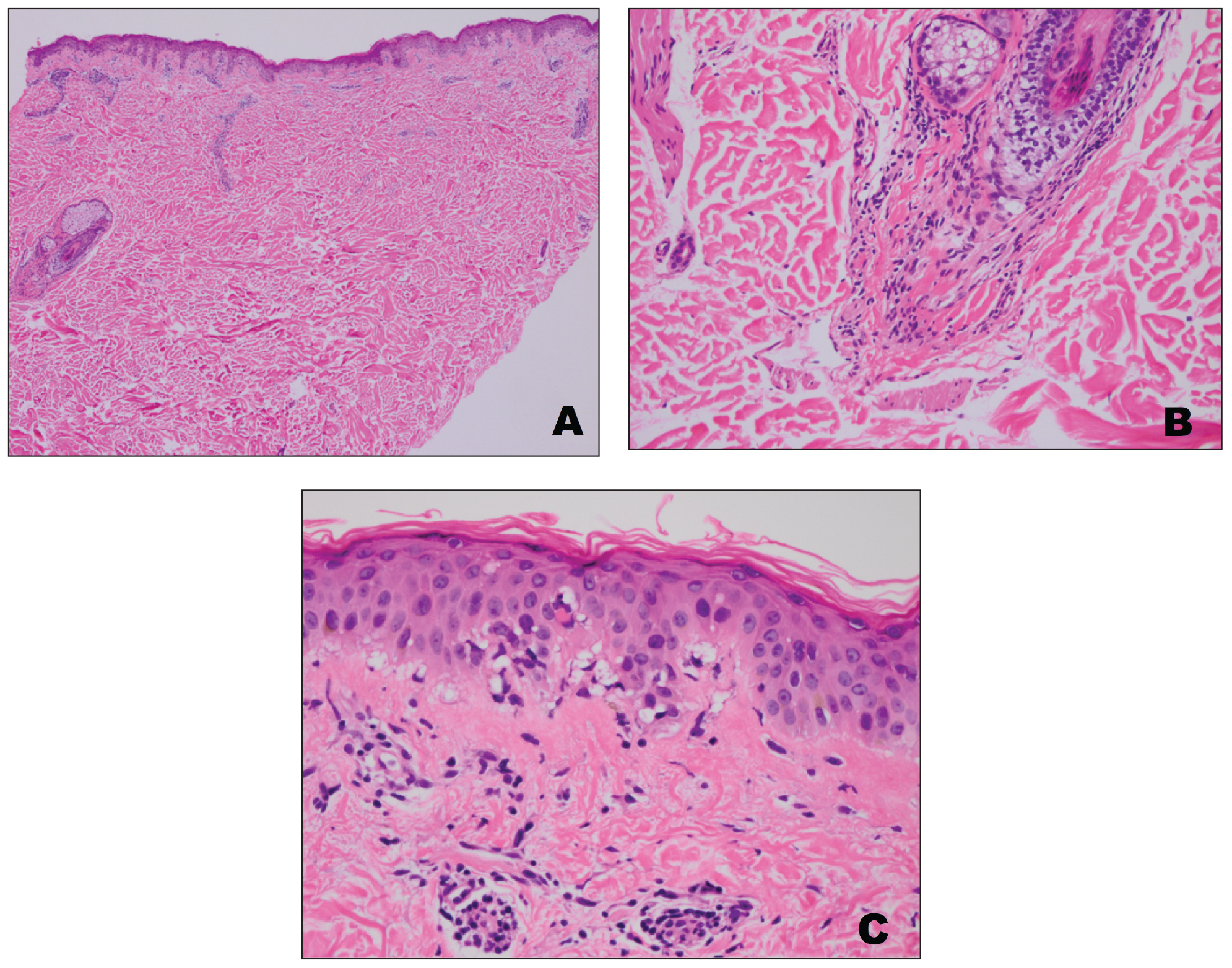
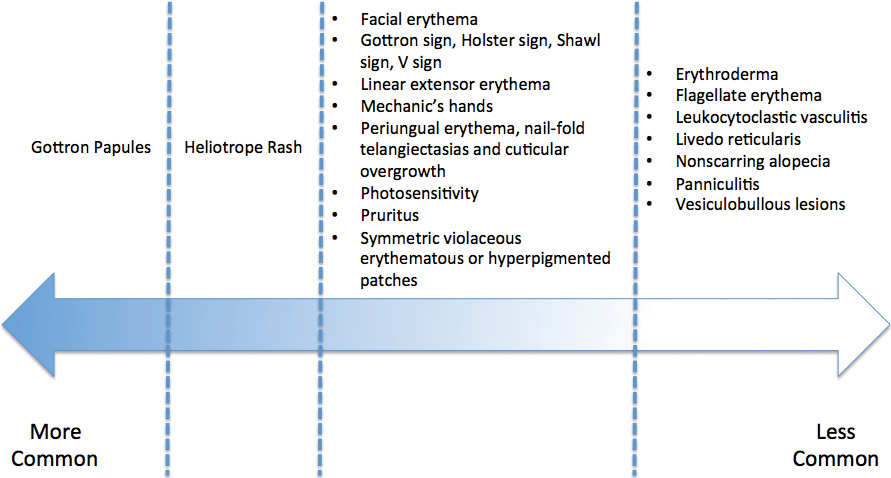
The association of DM and amyopathic DM with internal malignancy is well known. Bohan and Peter1 noted an overall figure ranging from 15% to 34% with an increased frequency in patients with skin and muscle involvement.1 Hill et al5 examined this link in a population-based study that identified corresponding malignancies. Specifically, they noted cancers to arise most frequently in the airway (eg, lung, trachea, bronchus), ovaries, breasts, colorectal region, and stomach.5 There also has been work performed to identify if certain dermatologic findings may be associated with a higher risk of malignancy.6,7 A meta-analysis by Wang et al6 showed that Gottron sign did not have an association with cancer, but findings of cutaneous necrosis did have an association. It is unknown if the specific cutaneous findings in our patient, including the predominantly unilateral papules on the knee, may have been a clue to the underlying malignancy.
In summary, we believe that our patient presented with the classic manifestations of DM in addition to the curious cutaneous sign of predominantly unilateral, confluent, erythematous papules on the knee, a clinical finding that may aid in the diagnosis of DM and also may alert the clinician to a possible underlying malignancy.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15:714-722.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Lister RK, Cooper ES, Paige DG. Papules and pustules of the elbows and knees: an uncommon clinical sign of dermatomyositis in oriental children. Pediatr Dermatol. 2000;17:37-40.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Wang J, Guo G, Chen G, et al. Meta‐analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case–control study. Br J Dermatol. 2001;144:825-831.
To the Editor:
We report an interesting clinical case of dermatomyositis (DM) that presented with an associated malignancy (small cell lung cancer). This patient also had an unusual clinical finding of predominantly unilateral, confluent, erythematous papules on the knee, a cutaneous sign that is seldom described in the DM literature. This case serves to reinforce the classic findings and associations of DM, in addition to the uncommon manifestation of predominantly unilateral papules on the knee.
A 68-year-old woman presented with several cutaneous manifestations including the classic findings of photo distributed erythema on the arms and face, a heliotrope rash, Gottron papules, and confluent pink papules on the left knee (Figure 1). The patient also had one of the more rare manifestations of DM, flagellate erythema on the back (Figure 2). She had a history of breast cancer and was found to have metastatic small cell lung cancer at the time of the DM diagnosis.


A punch biopsy from an area of flagellate erythema on the back revealed an interface dermatitis with a superficial, perivascular, lymphocyte-predominant inflammatory infiltrate (Figure 3). Alcian blue and colloidal iron stains revealed a marked increase in papillary dermal mucin. With the characteristic changes on skin biopsy and the classic skin findings present in our patient, we felt confident diagnosing her with DM. At the time of diagnosis, the patient also was found to have metastatic small cell lung cancer, suggesting a true paraneoplastic relationship.


The association of DM and amyopathic DM with internal malignancy is well known. Bohan and Peter1 noted an overall figure ranging from 15% to 34% with an increased frequency in patients with skin and muscle involvement.1 Hill et al5 examined this link in a population-based study that identified corresponding malignancies. Specifically, they noted cancers to arise most frequently in the airway (eg, lung, trachea, bronchus), ovaries, breasts, colorectal region, and stomach.5 There also has been work performed to identify if certain dermatologic findings may be associated with a higher risk of malignancy.6,7 A meta-analysis by Wang et al6 showed that Gottron sign did not have an association with cancer, but findings of cutaneous necrosis did have an association. It is unknown if the specific cutaneous findings in our patient, including the predominantly unilateral papules on the knee, may have been a clue to the underlying malignancy.
In summary, we believe that our patient presented with the classic manifestations of DM in addition to the curious cutaneous sign of predominantly unilateral, confluent, erythematous papules on the knee, a clinical finding that may aid in the diagnosis of DM and also may alert the clinician to a possible underlying malignancy.
To the Editor:
We report an interesting clinical case of dermatomyositis (DM) that presented with an associated malignancy (small cell lung cancer). This patient also had an unusual clinical finding of predominantly unilateral, confluent, erythematous papules on the knee, a cutaneous sign that is seldom described in the DM literature. This case serves to reinforce the classic findings and associations of DM, in addition to the uncommon manifestation of predominantly unilateral papules on the knee.
A 68-year-old woman presented with several cutaneous manifestations including the classic findings of photo distributed erythema on the arms and face, a heliotrope rash, Gottron papules, and confluent pink papules on the left knee (Figure 1). The patient also had one of the more rare manifestations of DM, flagellate erythema on the back (Figure 2). She had a history of breast cancer and was found to have metastatic small cell lung cancer at the time of the DM diagnosis.


A punch biopsy from an area of flagellate erythema on the back revealed an interface dermatitis with a superficial, perivascular, lymphocyte-predominant inflammatory infiltrate (Figure 3). Alcian blue and colloidal iron stains revealed a marked increase in papillary dermal mucin. With the characteristic changes on skin biopsy and the classic skin findings present in our patient, we felt confident diagnosing her with DM. At the time of diagnosis, the patient also was found to have metastatic small cell lung cancer, suggesting a true paraneoplastic relationship.


The association of DM and amyopathic DM with internal malignancy is well known. Bohan and Peter1 noted an overall figure ranging from 15% to 34% with an increased frequency in patients with skin and muscle involvement.1 Hill et al5 examined this link in a population-based study that identified corresponding malignancies. Specifically, they noted cancers to arise most frequently in the airway (eg, lung, trachea, bronchus), ovaries, breasts, colorectal region, and stomach.5 There also has been work performed to identify if certain dermatologic findings may be associated with a higher risk of malignancy.6,7 A meta-analysis by Wang et al6 showed that Gottron sign did not have an association with cancer, but findings of cutaneous necrosis did have an association. It is unknown if the specific cutaneous findings in our patient, including the predominantly unilateral papules on the knee, may have been a clue to the underlying malignancy.
In summary, we believe that our patient presented with the classic manifestations of DM in addition to the curious cutaneous sign of predominantly unilateral, confluent, erythematous papules on the knee, a clinical finding that may aid in the diagnosis of DM and also may alert the clinician to a possible underlying malignancy.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15:714-722.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Lister RK, Cooper ES, Paige DG. Papules and pustules of the elbows and knees: an uncommon clinical sign of dermatomyositis in oriental children. Pediatr Dermatol. 2000;17:37-40.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Wang J, Guo G, Chen G, et al. Meta‐analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case–control study. Br J Dermatol. 2001;144:825-831.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15:714-722.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Lister RK, Cooper ES, Paige DG. Papules and pustules of the elbows and knees: an uncommon clinical sign of dermatomyositis in oriental children. Pediatr Dermatol. 2000;17:37-40.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100.
- Wang J, Guo G, Chen G, et al. Meta‐analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847.
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case–control study. Br J Dermatol. 2001;144:825-831.
Practice Points
- Dermatomyositis has myriad cutaneous features including the shawl sign, the heliotrope sign, and Gottron papules.
- Less commonly, patients can present with the Holster sign (poikiloderma of the lateral thighs).
- Even less commonly, as in this report, patients can present with a psoriasiform papular eruption on the knees or with flagellate erythema on the back.
Small White Spots on the Lips
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
Bedbugs in the Workplace



Emerging Roles of Social Media in Dermatology
As the residents on the podium ran through case presentations at the Texas Dermatological Society meeting this past fall (September 21-22, 2018; Galveston, Texas), I discretely surveyed the room. To no surprise, perhaps half of the attendees at some point during the hour-long presentation glanced down at their smartphones, and 2018 statistics suggest that approximately 74% of these Internet glances were made by engagers of social media sites.1 My FOMO (fear of missing out) kicked in. What was everyone looking at? I opened Instagram on my smartphone and plastered at the top of my home page were Texas Dermatological Society–related “stories” posted by other dermatology residents from across the state, one story featuring the very presentation I was attending. I peeked 2 rows ahead to find the social media “influencer” I have been following on Instagram for months in real life for the first time.
It is not just the younger population glued to their social media accounts. In fact, Facebook boasted a more than 80% increase in users 55 years and older between 2011 and 2014 and a 41% increase in users aged 35 to 54 years.2 In total, there were 3.2 billion social media users globally in 2018.3 With such a large portion of the population engaged in social media, it is no wonder that it has become a rapidly emerging presence within the field of dermatology.
#Ad
Social media has become a powerful marketing tool for the practicing dermatologist. In a recent survey, 41% of social media users reported that social media influenced their choice of a particular physician, facility, or medical practice.4 Corresponding to this behavior, dermatology practices also have used social media to educate patients on services offered, acquire new patients, engage existing patients, create brand loyalty, become a trusted source of medical information in a sea of digital misinformation, and facilitate positive word-of-mouth opportunities.5 In fact, 53% of physician practices in the United States operate a Facebook page.6 For these physicians, marketing through social media carries the advantages of low cost and rapid transmission of information to a wide audience.7 Furthermore, the development of business insights and statistics by some social media platforms, such as those available to users on business profiles on Instagram, enables practices and marketers to target their audiences and optimize reach.
#DermLife
The role of social media in dermatology extends far beyond marketing. Lifestyle blogs centered on daily life as a medical provider, even within the field of dermatology, are gaining popularity. Dermatology-centered lifestyle blogs often incorporate the root derm in their handle, enabling other users to identify the account holder and interact in meaningful ways. According to a post from one popular Instagram influencer Dr. Audrey Sue Cruz (@dr.audreyxsue), such profiles may serve to prevent burnout, provide a creative outlet, share life as a resident, develop a supportive community, provide mentorship, and spread inspiration.
#Hashtag
Another interesting utility of social media is the use of standardized hashtags to facilitate scientific and clinical dialogue among medical professionals. Standardization of hashtag ontology on Twitter and Instagram has been adopted by the urology and gastroenterology fields to filter out “noise” by individuals not intending to join academic discussion.8 In dermatology, standardized hashtags have not been adopted, to my knowledge; however, a search for esoteric dermatologic terms such as #dermatopathology or #mohssurgery directs users to specialty-specific discussions.
#DontFryDay
Another role of social media in dermatology is dissemination of information. One notable example is the reach on Twitter of the “Don’t Fry Day” campaign, an annual campaign by the National Council on Skin Cancer Prevention to promote sun safety awareness and sun protection behaviors. In a recent study by Nguyen et al,9 the hashtag #DontFryDay was tracked on Twitter to assess the reach of the campaign. They found that this campaign had an impressive reach of approximately 1200 contributors, resulting in more than 16.5 million impressions; 18 celebrities and verified individuals accounted for 8,735,549 impressions.9
Despite the large potential for dissemination of information on social media, in 2014 none of the top 10 dermatologic journals or professional dermatologic organizations maintained an Instagram account. Only one of the top 10 patient advocate groups related to dermatology conditions—the Melanoma Research Foundation—was found on Instagram as of 2014.10 Furthermore, none of the top 10 most popular dermatology journals, professional dermatology organizations, or dermatology-related patient advocate groups could be found on Tumblr as of 2014.11 Although some of the aforementioned organizations have since adopted social media accounts, such as Cutis and Dermatology News (@mdedgederm) on Instagram in 2018, these social media platforms remain largely untapped outlets for dissemination of information to the public by reputable sources.
#VerifyHealthcare
Although social media has offered many advantages to the field of dermatology, it also has brought about unique challenges such as blind authorship, lack of source citation, and presentation of opinion as fact.7 To compound the challenge, 90% of millennials aged 18 to 24 years reportedly trust health care information shared by others on social media.12 Do we, as dermatologists, have a duty to take to social media to provide reputable health information? In an effort to address this emerging problem, popular Instagram influencer Dr. Austin Chiang (@austinchiangmd) initiated the #VerifyHealthcare movement, which called for physicians active on social media to practice transparency by providing users with their credentials.13 The goal of the movement is to help users differentiate medical information disseminated by trained medical professionals from misinformation by disreputable sources.
Final Thoughts
Despite its shortcomings, the emerging roles of social media in dermatology have proven to be a prominent force here to stay, providing new and innovative opportunities to dermatologists for social networking, dissemination of health information, motivation and inspiration, and marketing.
- Warden C. 30 statistics on social media and healthcare. Referral MD website. https://getreferralmd.com/2017/01/30-facts-statistics-on-social-media-and-healthcare/. Accessed January 16, 2019.
- Saul DJ. 3 million teens leave Facebook in 3 years: the Facebook demographic report. ISL website. https://isl.co/2014/01/3-million-teens-leave-facebook-in-3-years-the-2014-facebook-demographic-report/. Published January 15, 2014. Accessed January 9, 2019.
- Chaffey D. Global social media research summary 2018. Smart Insights website. https://www.smartinsights.com/social-media-marketing/social-media-strategy/new-global-social-media-research/. Published November 23, 2018. Accessed January 3, 2019.
- Ottenhoff M. Infographic: rising use of social and mobile in healthcare. The Spark Report. December 17, 2012. http://thesparkreport.com/infographic-social-mobile-healthcare/. Accessed January 9, 2019.
- Benabio J. The value of social media for dermatologists. Cutis. 2013;91:269-270.
- The healthcare social media shakeup. CDW Healthcare website. http://www.cdwcommunit.com/resources/infographic/social-media/. Accessed January 9, 2019.
- Vance K, Howe W, Dellavelle RP. Social internet sites as a source of public health information. Dermatol Clin. 2009;27:133-136.
- Chang AL, Vartabedian B, Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroeneterol. 2016;111:1082-1084.
- Nguyen JL, Heckman C, Perna F. Analysis of the Twitter “Don’t Fry Day” campaign. JAMA Dermatol. 2018;154:961-962.
- Karimkhani C, Connett J, Boyars L, et al. Dermatology on Instagram. Dermatol Online J. 2014;20. pii:13030/qt71g178w9.
- Correnti C, Boyars L, Karimkhani C, et al. Dermatology on Tumblr. Dermatol Online J. 2014;20:22642.
- Honigman B. 24 outstanding statistics on how social media has impacted healthcare. Referral MD website. https://getreferralmd.com/2013/09/healthcare-social-media-statistics. Accessed January 16, 2019.
- Oliver E. #VerifyHealthcare campaign seeks to increase social media transparency—5 insights. Becker’s GI & Endoscopy website. https://www.beckersasc.com/gastroenterology-and-endoscopy/verifyhealthcare-campaign-seeks-to-increase-social-media-transparency-5-insights.html. Published September 24, 2018. Accessed January 16, 2019.
As the residents on the podium ran through case presentations at the Texas Dermatological Society meeting this past fall (September 21-22, 2018; Galveston, Texas), I discretely surveyed the room. To no surprise, perhaps half of the attendees at some point during the hour-long presentation glanced down at their smartphones, and 2018 statistics suggest that approximately 74% of these Internet glances were made by engagers of social media sites.1 My FOMO (fear of missing out) kicked in. What was everyone looking at? I opened Instagram on my smartphone and plastered at the top of my home page were Texas Dermatological Society–related “stories” posted by other dermatology residents from across the state, one story featuring the very presentation I was attending. I peeked 2 rows ahead to find the social media “influencer” I have been following on Instagram for months in real life for the first time.
It is not just the younger population glued to their social media accounts. In fact, Facebook boasted a more than 80% increase in users 55 years and older between 2011 and 2014 and a 41% increase in users aged 35 to 54 years.2 In total, there were 3.2 billion social media users globally in 2018.3 With such a large portion of the population engaged in social media, it is no wonder that it has become a rapidly emerging presence within the field of dermatology.
#Ad
Social media has become a powerful marketing tool for the practicing dermatologist. In a recent survey, 41% of social media users reported that social media influenced their choice of a particular physician, facility, or medical practice.4 Corresponding to this behavior, dermatology practices also have used social media to educate patients on services offered, acquire new patients, engage existing patients, create brand loyalty, become a trusted source of medical information in a sea of digital misinformation, and facilitate positive word-of-mouth opportunities.5 In fact, 53% of physician practices in the United States operate a Facebook page.6 For these physicians, marketing through social media carries the advantages of low cost and rapid transmission of information to a wide audience.7 Furthermore, the development of business insights and statistics by some social media platforms, such as those available to users on business profiles on Instagram, enables practices and marketers to target their audiences and optimize reach.
#DermLife
The role of social media in dermatology extends far beyond marketing. Lifestyle blogs centered on daily life as a medical provider, even within the field of dermatology, are gaining popularity. Dermatology-centered lifestyle blogs often incorporate the root derm in their handle, enabling other users to identify the account holder and interact in meaningful ways. According to a post from one popular Instagram influencer Dr. Audrey Sue Cruz (@dr.audreyxsue), such profiles may serve to prevent burnout, provide a creative outlet, share life as a resident, develop a supportive community, provide mentorship, and spread inspiration.
#Hashtag
Another interesting utility of social media is the use of standardized hashtags to facilitate scientific and clinical dialogue among medical professionals. Standardization of hashtag ontology on Twitter and Instagram has been adopted by the urology and gastroenterology fields to filter out “noise” by individuals not intending to join academic discussion.8 In dermatology, standardized hashtags have not been adopted, to my knowledge; however, a search for esoteric dermatologic terms such as #dermatopathology or #mohssurgery directs users to specialty-specific discussions.
#DontFryDay
Another role of social media in dermatology is dissemination of information. One notable example is the reach on Twitter of the “Don’t Fry Day” campaign, an annual campaign by the National Council on Skin Cancer Prevention to promote sun safety awareness and sun protection behaviors. In a recent study by Nguyen et al,9 the hashtag #DontFryDay was tracked on Twitter to assess the reach of the campaign. They found that this campaign had an impressive reach of approximately 1200 contributors, resulting in more than 16.5 million impressions; 18 celebrities and verified individuals accounted for 8,735,549 impressions.9
Despite the large potential for dissemination of information on social media, in 2014 none of the top 10 dermatologic journals or professional dermatologic organizations maintained an Instagram account. Only one of the top 10 patient advocate groups related to dermatology conditions—the Melanoma Research Foundation—was found on Instagram as of 2014.10 Furthermore, none of the top 10 most popular dermatology journals, professional dermatology organizations, or dermatology-related patient advocate groups could be found on Tumblr as of 2014.11 Although some of the aforementioned organizations have since adopted social media accounts, such as Cutis and Dermatology News (@mdedgederm) on Instagram in 2018, these social media platforms remain largely untapped outlets for dissemination of information to the public by reputable sources.
#VerifyHealthcare
Although social media has offered many advantages to the field of dermatology, it also has brought about unique challenges such as blind authorship, lack of source citation, and presentation of opinion as fact.7 To compound the challenge, 90% of millennials aged 18 to 24 years reportedly trust health care information shared by others on social media.12 Do we, as dermatologists, have a duty to take to social media to provide reputable health information? In an effort to address this emerging problem, popular Instagram influencer Dr. Austin Chiang (@austinchiangmd) initiated the #VerifyHealthcare movement, which called for physicians active on social media to practice transparency by providing users with their credentials.13 The goal of the movement is to help users differentiate medical information disseminated by trained medical professionals from misinformation by disreputable sources.
Final Thoughts
Despite its shortcomings, the emerging roles of social media in dermatology have proven to be a prominent force here to stay, providing new and innovative opportunities to dermatologists for social networking, dissemination of health information, motivation and inspiration, and marketing.
As the residents on the podium ran through case presentations at the Texas Dermatological Society meeting this past fall (September 21-22, 2018; Galveston, Texas), I discretely surveyed the room. To no surprise, perhaps half of the attendees at some point during the hour-long presentation glanced down at their smartphones, and 2018 statistics suggest that approximately 74% of these Internet glances were made by engagers of social media sites.1 My FOMO (fear of missing out) kicked in. What was everyone looking at? I opened Instagram on my smartphone and plastered at the top of my home page were Texas Dermatological Society–related “stories” posted by other dermatology residents from across the state, one story featuring the very presentation I was attending. I peeked 2 rows ahead to find the social media “influencer” I have been following on Instagram for months in real life for the first time.
It is not just the younger population glued to their social media accounts. In fact, Facebook boasted a more than 80% increase in users 55 years and older between 2011 and 2014 and a 41% increase in users aged 35 to 54 years.2 In total, there were 3.2 billion social media users globally in 2018.3 With such a large portion of the population engaged in social media, it is no wonder that it has become a rapidly emerging presence within the field of dermatology.
#Ad
Social media has become a powerful marketing tool for the practicing dermatologist. In a recent survey, 41% of social media users reported that social media influenced their choice of a particular physician, facility, or medical practice.4 Corresponding to this behavior, dermatology practices also have used social media to educate patients on services offered, acquire new patients, engage existing patients, create brand loyalty, become a trusted source of medical information in a sea of digital misinformation, and facilitate positive word-of-mouth opportunities.5 In fact, 53% of physician practices in the United States operate a Facebook page.6 For these physicians, marketing through social media carries the advantages of low cost and rapid transmission of information to a wide audience.7 Furthermore, the development of business insights and statistics by some social media platforms, such as those available to users on business profiles on Instagram, enables practices and marketers to target their audiences and optimize reach.
#DermLife
The role of social media in dermatology extends far beyond marketing. Lifestyle blogs centered on daily life as a medical provider, even within the field of dermatology, are gaining popularity. Dermatology-centered lifestyle blogs often incorporate the root derm in their handle, enabling other users to identify the account holder and interact in meaningful ways. According to a post from one popular Instagram influencer Dr. Audrey Sue Cruz (@dr.audreyxsue), such profiles may serve to prevent burnout, provide a creative outlet, share life as a resident, develop a supportive community, provide mentorship, and spread inspiration.
#Hashtag
Another interesting utility of social media is the use of standardized hashtags to facilitate scientific and clinical dialogue among medical professionals. Standardization of hashtag ontology on Twitter and Instagram has been adopted by the urology and gastroenterology fields to filter out “noise” by individuals not intending to join academic discussion.8 In dermatology, standardized hashtags have not been adopted, to my knowledge; however, a search for esoteric dermatologic terms such as #dermatopathology or #mohssurgery directs users to specialty-specific discussions.
#DontFryDay
Another role of social media in dermatology is dissemination of information. One notable example is the reach on Twitter of the “Don’t Fry Day” campaign, an annual campaign by the National Council on Skin Cancer Prevention to promote sun safety awareness and sun protection behaviors. In a recent study by Nguyen et al,9 the hashtag #DontFryDay was tracked on Twitter to assess the reach of the campaign. They found that this campaign had an impressive reach of approximately 1200 contributors, resulting in more than 16.5 million impressions; 18 celebrities and verified individuals accounted for 8,735,549 impressions.9
Despite the large potential for dissemination of information on social media, in 2014 none of the top 10 dermatologic journals or professional dermatologic organizations maintained an Instagram account. Only one of the top 10 patient advocate groups related to dermatology conditions—the Melanoma Research Foundation—was found on Instagram as of 2014.10 Furthermore, none of the top 10 most popular dermatology journals, professional dermatology organizations, or dermatology-related patient advocate groups could be found on Tumblr as of 2014.11 Although some of the aforementioned organizations have since adopted social media accounts, such as Cutis and Dermatology News (@mdedgederm) on Instagram in 2018, these social media platforms remain largely untapped outlets for dissemination of information to the public by reputable sources.
#VerifyHealthcare
Although social media has offered many advantages to the field of dermatology, it also has brought about unique challenges such as blind authorship, lack of source citation, and presentation of opinion as fact.7 To compound the challenge, 90% of millennials aged 18 to 24 years reportedly trust health care information shared by others on social media.12 Do we, as dermatologists, have a duty to take to social media to provide reputable health information? In an effort to address this emerging problem, popular Instagram influencer Dr. Austin Chiang (@austinchiangmd) initiated the #VerifyHealthcare movement, which called for physicians active on social media to practice transparency by providing users with their credentials.13 The goal of the movement is to help users differentiate medical information disseminated by trained medical professionals from misinformation by disreputable sources.
Final Thoughts
Despite its shortcomings, the emerging roles of social media in dermatology have proven to be a prominent force here to stay, providing new and innovative opportunities to dermatologists for social networking, dissemination of health information, motivation and inspiration, and marketing.
- Warden C. 30 statistics on social media and healthcare. Referral MD website. https://getreferralmd.com/2017/01/30-facts-statistics-on-social-media-and-healthcare/. Accessed January 16, 2019.
- Saul DJ. 3 million teens leave Facebook in 3 years: the Facebook demographic report. ISL website. https://isl.co/2014/01/3-million-teens-leave-facebook-in-3-years-the-2014-facebook-demographic-report/. Published January 15, 2014. Accessed January 9, 2019.
- Chaffey D. Global social media research summary 2018. Smart Insights website. https://www.smartinsights.com/social-media-marketing/social-media-strategy/new-global-social-media-research/. Published November 23, 2018. Accessed January 3, 2019.
- Ottenhoff M. Infographic: rising use of social and mobile in healthcare. The Spark Report. December 17, 2012. http://thesparkreport.com/infographic-social-mobile-healthcare/. Accessed January 9, 2019.
- Benabio J. The value of social media for dermatologists. Cutis. 2013;91:269-270.
- The healthcare social media shakeup. CDW Healthcare website. http://www.cdwcommunit.com/resources/infographic/social-media/. Accessed January 9, 2019.
- Vance K, Howe W, Dellavelle RP. Social internet sites as a source of public health information. Dermatol Clin. 2009;27:133-136.
- Chang AL, Vartabedian B, Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroeneterol. 2016;111:1082-1084.
- Nguyen JL, Heckman C, Perna F. Analysis of the Twitter “Don’t Fry Day” campaign. JAMA Dermatol. 2018;154:961-962.
- Karimkhani C, Connett J, Boyars L, et al. Dermatology on Instagram. Dermatol Online J. 2014;20. pii:13030/qt71g178w9.
- Correnti C, Boyars L, Karimkhani C, et al. Dermatology on Tumblr. Dermatol Online J. 2014;20:22642.
- Honigman B. 24 outstanding statistics on how social media has impacted healthcare. Referral MD website. https://getreferralmd.com/2013/09/healthcare-social-media-statistics. Accessed January 16, 2019.
- Oliver E. #VerifyHealthcare campaign seeks to increase social media transparency—5 insights. Becker’s GI & Endoscopy website. https://www.beckersasc.com/gastroenterology-and-endoscopy/verifyhealthcare-campaign-seeks-to-increase-social-media-transparency-5-insights.html. Published September 24, 2018. Accessed January 16, 2019.
- Warden C. 30 statistics on social media and healthcare. Referral MD website. https://getreferralmd.com/2017/01/30-facts-statistics-on-social-media-and-healthcare/. Accessed January 16, 2019.
- Saul DJ. 3 million teens leave Facebook in 3 years: the Facebook demographic report. ISL website. https://isl.co/2014/01/3-million-teens-leave-facebook-in-3-years-the-2014-facebook-demographic-report/. Published January 15, 2014. Accessed January 9, 2019.
- Chaffey D. Global social media research summary 2018. Smart Insights website. https://www.smartinsights.com/social-media-marketing/social-media-strategy/new-global-social-media-research/. Published November 23, 2018. Accessed January 3, 2019.
- Ottenhoff M. Infographic: rising use of social and mobile in healthcare. The Spark Report. December 17, 2012. http://thesparkreport.com/infographic-social-mobile-healthcare/. Accessed January 9, 2019.
- Benabio J. The value of social media for dermatologists. Cutis. 2013;91:269-270.
- The healthcare social media shakeup. CDW Healthcare website. http://www.cdwcommunit.com/resources/infographic/social-media/. Accessed January 9, 2019.
- Vance K, Howe W, Dellavelle RP. Social internet sites as a source of public health information. Dermatol Clin. 2009;27:133-136.
- Chang AL, Vartabedian B, Spiegel B. Harnessing the hashtag: a standard approach to GI dialogue on social media. Am J Gastroeneterol. 2016;111:1082-1084.
- Nguyen JL, Heckman C, Perna F. Analysis of the Twitter “Don’t Fry Day” campaign. JAMA Dermatol. 2018;154:961-962.
- Karimkhani C, Connett J, Boyars L, et al. Dermatology on Instagram. Dermatol Online J. 2014;20. pii:13030/qt71g178w9.
- Correnti C, Boyars L, Karimkhani C, et al. Dermatology on Tumblr. Dermatol Online J. 2014;20:22642.
- Honigman B. 24 outstanding statistics on how social media has impacted healthcare. Referral MD website. https://getreferralmd.com/2013/09/healthcare-social-media-statistics. Accessed January 16, 2019.
- Oliver E. #VerifyHealthcare campaign seeks to increase social media transparency—5 insights. Becker’s GI & Endoscopy website. https://www.beckersasc.com/gastroenterology-and-endoscopy/verifyhealthcare-campaign-seeks-to-increase-social-media-transparency-5-insights.html. Published September 24, 2018. Accessed January 16, 2019.
Resident Pearl
- The emerging presence of social media in dermatology provides opportunities for dermatologists to participate in dissemination and consumption of reliable health information, marketing, social networking among colleagues, and motivation and inspiration. It has been proposed that participation may serve to prevent resident burnout.
White Concretions on the Hair Shaft
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
A 35-year-old woman presented with possible nits on the hair of 1 year’s duration. She was previously evaluated by several outside medical providers and was unsuccessfully treated with topical and systemic medications for pediculosis. She reported sporadic scalp pruritus but denied hair loss, breakage, close contacts with similar symptoms, or recent travel outside the United States. She was otherwise healthy and was not taking any medications. Physical examination revealed small 1- to 2-mm, generalized, somewhat detachable, white concretions randomly distributed on the hair shafts. No broken hairs were observed. The eyebrows, eyelash hairs, and surrounding skin were normal. Potassium hydroxide mount was equivocal for nits.