User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Miscommunication With Dermatology Patients: Are We Speaking the Same Language?
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
Resident Pearl
- It is not just the esoteric jargon and complex pathophysiologic concepts in dermatology that can challenge effective communication with our patients. We face potential for misunderstanding even in situations that may seem straightforward. Vigilance in avoiding ambiguity in all our exchanges with patients can help foster therapeutic relationships and optimize patient care.
February 2019 Highlights
Nail Psoriasis Tips
What does your patient need to know at the first visit?
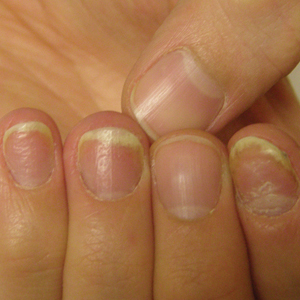
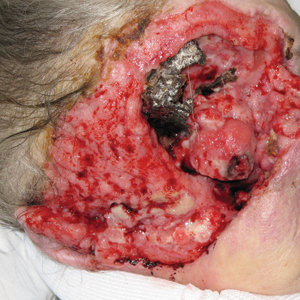
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.
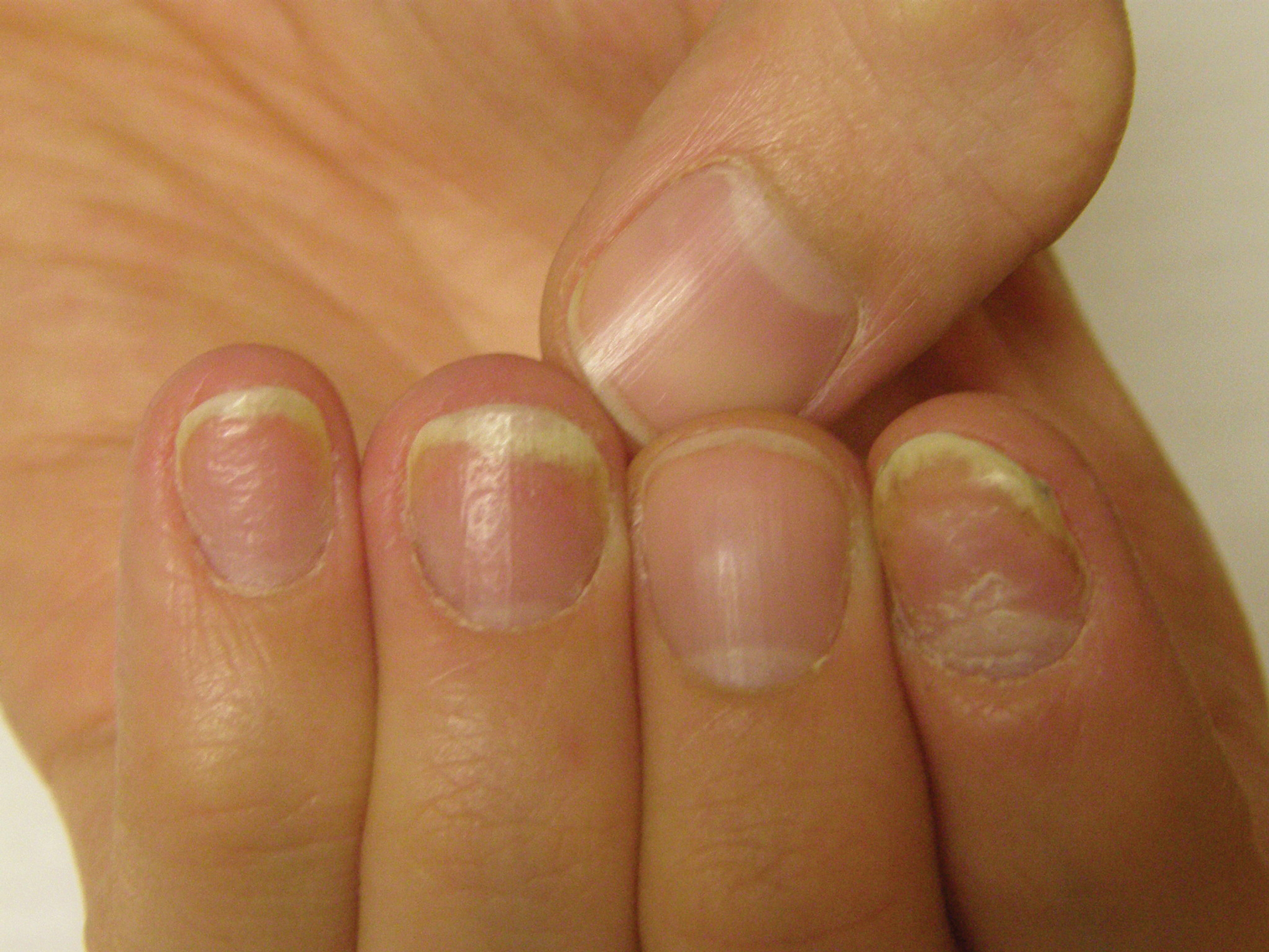
The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
What does your patient need to know at the first visit?
Patient education is important initially. There are several causes for nail dystrophy. Oftentimes, when patients present, they believe that they have onychomycosis. Therefore, it is important to counsel individuals with potential nail psoriasis (Figure) and to discuss the differential diagnosis of the condition.

The presence of psoriasis on other areas of the body and the absence of fungal infection on the soles of the feet and in between the toes increases the likelihood of nail psoriasis. The most accurate test to perform is a nail clipping with subsequent periodic acid–Schiff stain. It is important to remember, however, that nail psoriasis and fungal infection of the nail can coexist.
Once the diagnosis of nail psoriasis is established, it is important to review gentle care of the nails. A thorough discussion of therapeutic options is helpful. Patients also should be advised that the presence of nail psoriasis can increase the likelihood of the development of
psoriatic arthritis.
What are your go-to treatments?
Prior to the development of biologic therapies, topical treatments were the mainstay of treatment. Topical corticosteroid preparations can be used around and under the nail. Other therapeutic options include topical calcipotriene and topical retinoids.
Intralesional injection is another therapeutic option. Injection into the nail bed is useful for the treatment of nail bed symptoms of nail psoriasis such as onycholysis. Injection into the proximal nail fold can ameliorate signs of nail matrix psoriasis such as nail pitting. Although injection can be effective, it also can be painful; therefore, many patients do not opt to have this therapy performed.
Systemic therapy has been shown to be highly effective in improving nail psoriasis. There has been a good amount of data from studies specifically done in nail psoriasis and nail data that have been taken from larger phase 3 trials (Elewski et al; van de Kerkhof et al). Therefore, several of the biologics on the market as well apremilast are good options for the treatment of nail psoriasis. When using a systemic agent, it is important to carefully review the benefits and risks of each therapy with patients. Because the nail grows slowly, improvement can be gradual and take several months to peak.
How do you keep patients compliant with treatment?
Because nail psoriasis causes distress among patients, it generally is not too hard for them to be compliant. Of course, it is important to have regular follow-up to monitor progress and to reinforce the importance of continued therapy. At the end of the day, however, treatment success is the best asset to encourage continued compliance.
Resources for Patients
Managing nail psoriasis
http://www.psoriasis.org/about-psoriasis/specific-locations/hands-feet-nails/managing-nail-psoriasis
What is nail psoriasis, and how can I treat it?
http://www.aad.org/public/diseases/scaly-skin/psoriasis/diagnosis-and-treatment-of-psoriasis/what-is-nail-psoriasis-and-how-can-i-treat-it
Suggested Readings
Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of phase 3, randomized, placebo controlled trial. J Am Acad Dermatol. 2018;78:90.e1-99.e1.
Van de Kerkhof P, Guenther L, Gottlieb AB, et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled, and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31:477-482.
Yin N, Choudhary S, Nouri K. Pulsed dye laser for the treatment of nail psoriasis. Cutis. 2013;92:129-135.
Granuloma Annulare: A Retrospective Series of 133 Patients
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
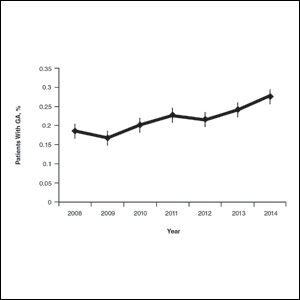
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).
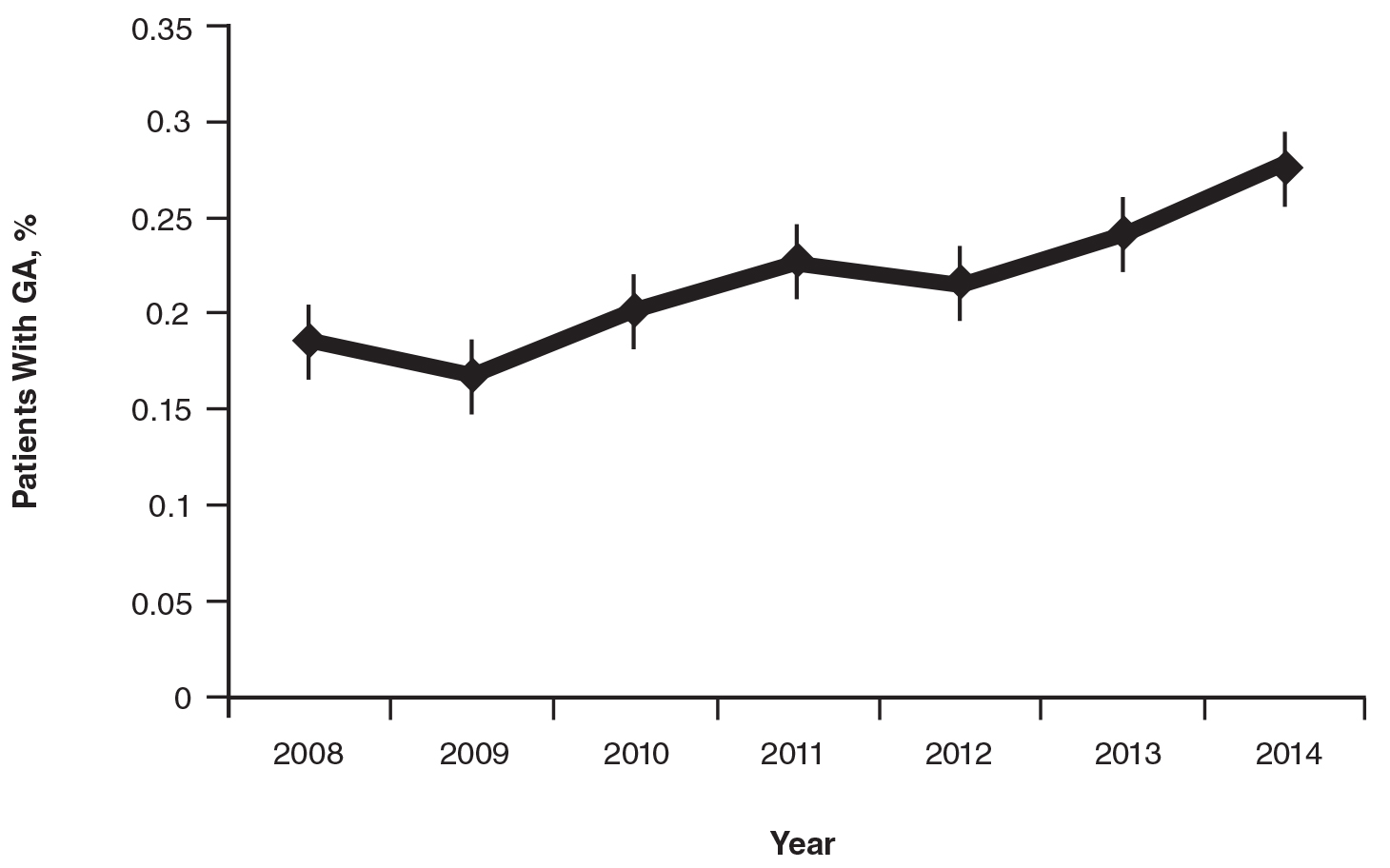
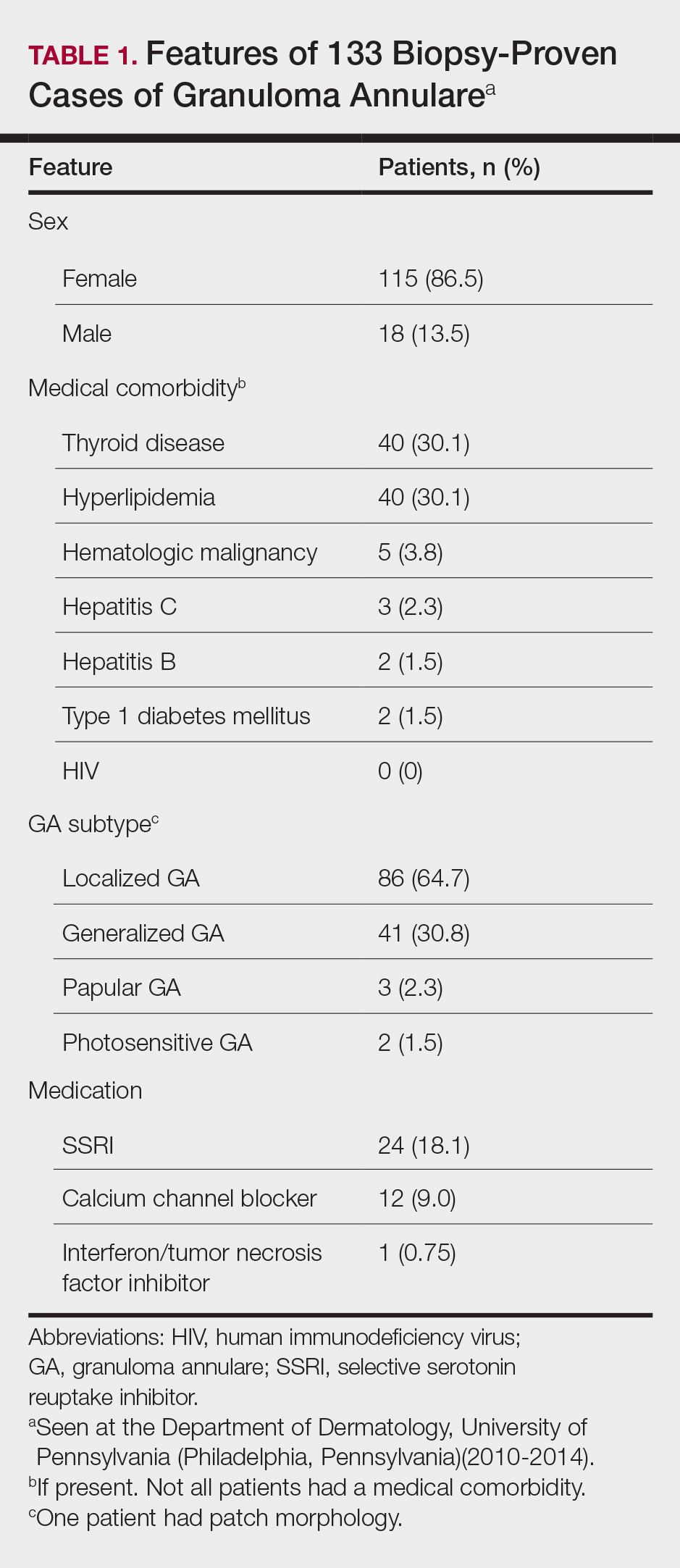
There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).
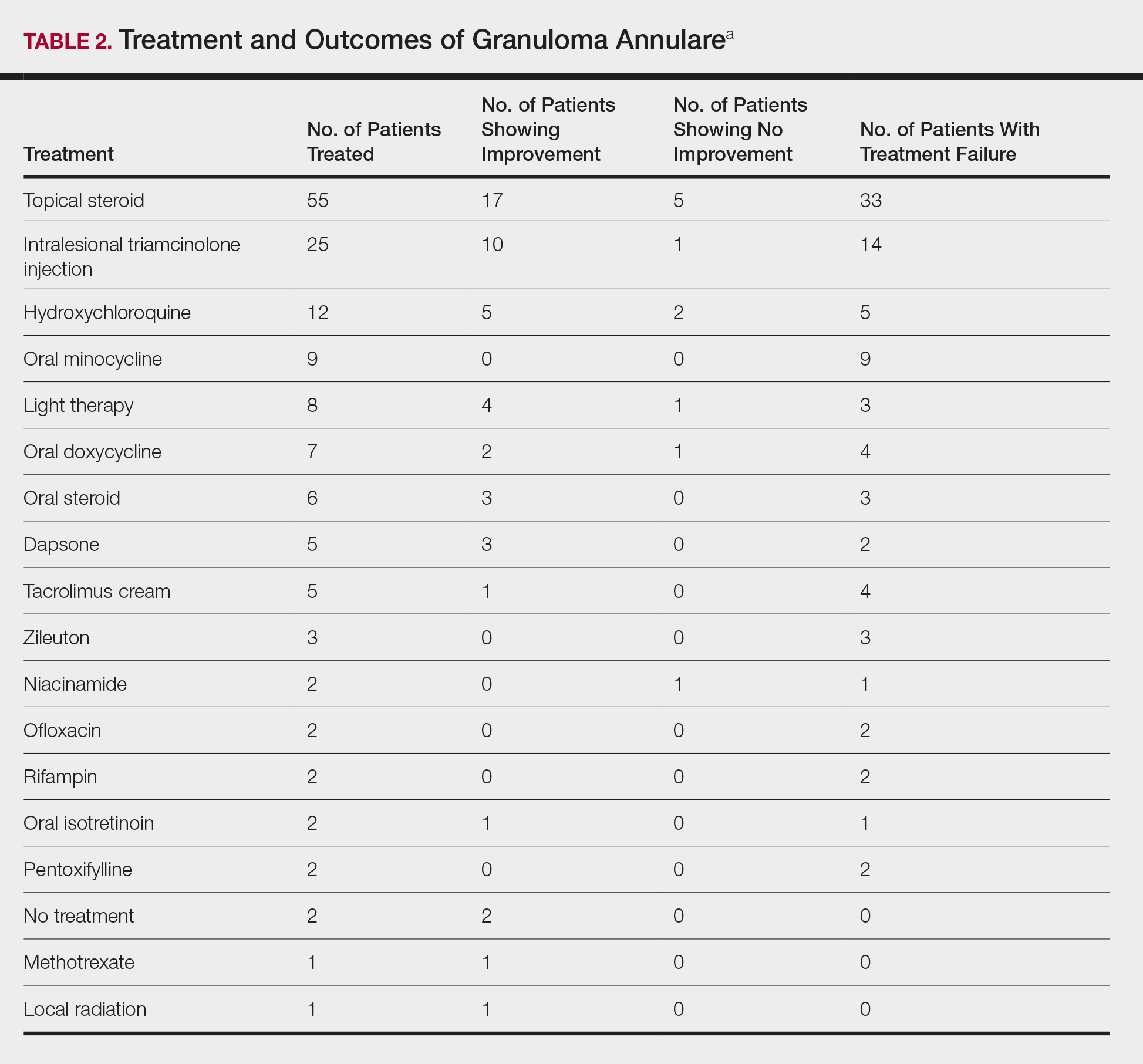
The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).
Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).

There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).

The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).

Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).

There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).

The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).

Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
Practice Points
- Although the pathogenesis of granuloma annulare (GA) is unknown, associations between the disorder and underlying systemic processes (eg, diabetes mellitus, hyperlipidemia, thyroid disease, human immunodeficiency virus) have been proposed.
- This study elicited a period prevalence of GA of 0.22% to 0.27%.
- The most commonly used treatments of GA were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine.
Clearance of Psoriasis After Ischemic Stroke
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
Practice Points
- Psoriasis is exacerbated in the presence of stress, and psoriatic lesions often have a symmetric distribution, which is evidence that the nervous system is involved in the pathophysiology of the condition.
- Various neuropeptides are involved in the pathophysiology of psoriasis, including substance P, nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide.
- Peripheral nerve damage results in decreased secretion of neuropeptides, which can lead to remission of psoriasis.
Large Hemorrhagic Plaque With Central Crusting
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.
Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.

Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
The Diagnosis: Bullous/Hemorrhagic Lichen Sclerosus et Atrophicus
Histopathologic examination revealed hyperkeratosis of the stratum corneum and thinning of the epidermis (Figure). Subepidermal edema and hemorrhage in the papillary dermis were seen. There were dilated vessels beneath the edema in the reticular dermis, as well as perivascular, perifollicular, and interstitial lymphocytic inflammation. No cytologic atypia characteristic of squamous cell carcinoma (SCC) and angiosarcoma or large lymphatic channels characteristic of lymphangioma were noted. Based on clinicopathologic correlation, the diagnosis of the bullous/hemorrhagic form of lichen sclerosus et atrophicus (LS&A) was made. The patient was treated with high-potency topical steroids with notable symptomatic improvement and rapid resolution of the hemorrhagic lesion.

Lichen sclerosus et atrophicus is a chronic inflammatory condition with a predilection for the anogenital region, though rare cases of extragenital involvement have been reported. It is seen in both sexes and across all age groups, with notably higher prevalence in females in the fifth and sixth decades of life.1,2 Lichen sclerosus et atrophicus can be difficult to diagnose, as these patients may present to a variety of specialists, may be embarrassed by the condition and reluctant for full evaluation, or may have asymptomatic lesions.2,3 Rare cases of isolated extragenital involvement and hemorrhagic or bullous lesions further complicate the diagnosis.1,2 Despite these difficulties, diagnosis is essential, as there is potential for cosmetically and functionally detrimental scarring as well as atrophy and development of overlying malignancies. Lichen sclerosus et atrophicus is not curable and rarely remits spontaneously, but appropriate treatment strategies can help control the symptoms of the condition as well as its most devastating sequelae.3
For females, classic LS&A is most common in theprepubertal, perimenopausal, or postmenopausal periods, commonly involving the vulva or perineum. Symptoms include pruritus, burning sensation, dysuria, dyspareunia, and labial stenosis, among others. For males, most cases involve the glans penis in prepubertal boys or middleaged men, and symptoms include pruritus, new-onset phimosis, decreased sensation, painful erections, dysuria, and urinary obstruction.1-3 An estimated 97% of patients have some form of genital involvement with only 2.5% showing isolated extragenital involvement, though the latter may be underdiagnosed, as this area is more likely to be asymptomatic.3-6 Extragenital LS&A most often involves the neck and shoulders. The classic appearance of LS&A includes shiny, white-red macules and papules that ultimately coalesce into atrophic plaques and can be accompanied by fissuring or scarring, especially in the genital area.2 There is an increased risk for SCC associated with genital LS&A.1
Bullous/hemorrhagic LS&A has been described as a rare phenotype. One case report cited an increased incidence of this subtype in patients with exclusively extragenital lesions, and the authors considered blister formation to be a characteristic feature of extragenital LS&A. The pathogenesis of blister formation and hemorrhage in LS&A is not completely understood, but trauma is thought to play a role due to decreased stress tolerance from atrophic skin.4 Furthermore, distortion of blood vessel architecture in LS&A has been described with loss of the capillary network and enlargement of vessels along the dermoepidermal junction, which also could play a role in hemorrhage. Differential diagnosis of the bullous/hemorrhagic type of LS&A includes bullous pemphigoid, bullous lichen planus, or bullous scleroderma.7 In our more exophytic hemorrhagic case, malignancies such as SCC or angiosarcoma also had to be considered. Unlike genital LS&A, extragenital LS&A including the bullous/hemorrhagic variant has not been linked to an increasedrisk for malignancy.1,5
The mainstay of treatment of all forms of LS&A is high-potency topical steroids, but topical retinoids, tacrolimus, and UVA phototherapy also have been used. Bullous/hemorrhagic lesions often resolve quickly with topical steroids, leaving behind more classic plaques in their place, which can be more refractory to treatment.5,7
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416.
- Pugliese JM, Morey AF, Peterson AC. Lichen sclerosus: review of the literature and current recommendations for management. J Urol. 2007;178:2268-2276.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Kimura A, Kambe N, Satoh T, et al. Follicular keratosis and bullous formation are typical signs of extragenital lichen sclerosus. J Dermatol. 2011;38:834-836.
- Khatu S, Vasani R. Isolated, localised extragenital bullous lichen sclerosus et atrophicus: a rare entity. Indian J Dermatol. 2013;58:409.
- Luzar B, Neil SM, Calonje E. Angiokeratoma-like changes in extragenital and genital lichen sclerosus. J Cutan Pathol. 2009;36:540-542.
- Lima RS, Maquine GA, Schettini AP, et al. Bullous and hemorrhagic lichen sclerosus—case report. An Bras Dermatol. 2015;90 (3 suppl 1):118-120.

A 54-year-old woman with no notable medical history was referred to dermatology by her primary care provider for evaluation of a hematoma on the posterior neck that had developed gradually over 5 months. The lesion initially was asymptomatic but more recently had started to be painful and bleed intermittently. The patient denied any personal or family history of skin cancer. Physical examination revealed a large hemorrhagic plaque on the left side of the posterior neck with central brown-yellow crusting. There were few smaller, white, thin, sclerotic plaques with crinkling atrophy at the periphery of and inferolateral to the lesion. A punch biopsy specimen was obtained from the hemorrhagic plaque.
Managing Postinflammatory Hyperpigmentation in Pediatric Patients With Skin of Color
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
Postnflammatory hyperpigmentation (PIH) is an acquired hypermelanosis that can occur in children and adults following an inflammatory cutaneous disease or trauma. Postinflammatory hyperpigmentation may last for months to even years. Although PIH may occur in all skin types, it is more common and presents with greater severity and intensity in individuals with skin of color. By the year 2050, 1 in 3 US residents is projected to be Hispanic.1 It is projected that by 2044, non-Hispanic white individuals (all ages) will make up less than 50% of the US population.2 Currently, the majority of the US residents younger than 18 years are minorities. The majority minority population in the United States already exists in those younger than 18 years and is predicted to occur in the adult population by 2044.2
Effective treatment options and management strategies for PIH in adults with skin of color have been described in the literature.3 Due to a paucity of research, the approach to management of PIH in children with skin of color has been based on clinical experience and lessons learned from adult patients. This article focuses on management of PIH in pediatric patients with skin of color, which includes black/African American, African-Caribbean, Hispanic, Asian, Pacific Islander, and American Indian individuals.
Underlying Inflammatory Dermatoses Resulting in PIH
There are numerous conditions that may result in PIH, including but not limited to atopic dermatitis (AD), acne, arthropod bites, and injuries to the skin. Postinflammatory hyperpigmentation may have more of a psychological impact than the inciting disease or injury itself. The most important step in the approach to managing PIH is treating the underlying inflammatory condition that caused the pigmentation.
Parents/guardians may report a chief concern of dark spots, manchas (stains), blemishes, or stains on the skin, often with no mention of a coexisting inflammatory dermatosis. Parents/guardians of children with skin of color often have personally experienced PIH and may be determined to shield their children from similar angst associated with the condition. Although physicians may see just another pediatric patient with PIH, the child’s parents/guardians may see a condition that will be readily perceptible during major life events, such as the child’s prom or even his/her wedding day. Promptly diagnosing and instituting early treatment of inflammatory conditions associated with PIH may accelerate resolution and prevent worsening of the pigmentation.3
Select inflammatory dermatoses that are common in children with skin of color and may lead to PIH are highlighted below. Although this list is not comprehensive, the approach and management strategies should prompt creation of plans that keep PIH in mind when treating primary inflammatory skin diseases.
Atopic Dermatitis
Atopic dermatitis may induce PIH or hypopigmentation of the skin in children with skin of color. Developing a plan for AD flare prevention, as well as management of mild, moderate, and severe AD flares, is imperative in pediatric patients. Prevention plans should include gentle skin care, twice-daily application of emollients to the full body, and reduction of Staphylococcus aureus loads on the skin. The treatment action plan for mild to moderate flares may include topical corticosteroids, immunomodulators, and nonsteroidal agents. Treatment options for severe AD or patients who were unsuccessfully treated with other therapies may include phototherapy, biologics, and methotrexate, among others.4 Creating action plans for AD flares is a vital step in the prevention of PIH in patients with skin of color. Additionally, PIH should not be considered a sign of AD treatment failure.
Acne
Acne is a common skin disorder seen in patients with skin of color.5 A prospective observational study found that 34.3% of 683 children aged 9 to 14 years in a pediatric ambulatory clinic had acne.6 The number of preadolescents with acne is growing. Most cases are not associated with underlying endocrinopathy.7 With the growing population of children with skin of color in the United States along with the increasing childhood acne rate and subsequent inherent risk for hyperpigmentation, early acne interventions should be considered in pediatric acne patients with skin of color to reduce the impact of PIH in those at risk.
In a survey study of 313 adult acne patients with skin of color, 37.2% reported the presence of dark marks lasting 4 months or longer.5 Regardless of the severity of the acne, treatment should be initiated as tolerated in those with PIH. Adolescent acne patients with skin of color may develop PIH that is more severe and longer lasting than the acne itself.
The foundation for treatment of acne in adolescent skin of color patients is the same as those without skin of color, including topical retinoids, topical antibiotics, oral antibiotics, and isotretinoin when needed. Topical tretinoin, adapalene, azelaic acid, and tazarotene not only treat acne but also are a valuable part of the treatment armamentarium for PIH. Several studies in adults with skin of color have demonstrated improvement of PIH from the use of topical retinoids alone.8-10 Despite wanting to treat the acne aggressively, special guidance should be given to prevent retinoid dermatitis, which may lead to PIH.10 Demonstrating the application of the topical acne medications, discussing how to avoid potential side effects, and giving permission to skip applications, if needed, may empower families to make adjustments between visits to limit irritation that might prompt further PIH. Incorporating α-hydroxy acid–based cleansers, α-hydroxy acid–based chemical peels, or salicylic acid chemical peels may be warranted in the setting of intense PIH. Selecting treatments that not only help the inflammatory disease leading to the PIH but also can help improve the pigmentation are preferred; however, the risks and benefits have to be weighed because many treatments that work well for PIH also may cause irritation, leading to new or worsening PIH.
Arthropod Bites
Arthropod bites cause inflamed pruritic papules and nodules, and the resulting PIH in those with darker skin types may be quite dramatic. Parents/guardians should be instructed to have a low-potency topical corticosteroid on hand to use on bites for a few days when they appear, which will not only help with the inflammation associated with the bite but also will help decrease pruritus and subsequently skin injury from scratching. In homes with pets, checking animals routinely for fleas and other infestations is helpful. In the setting of repeated arthropod bites in the spring and summer, applying bug repellant with 10% to 30% DEET (N,N-diethyl-meta-toluamide) on the child’s clothing and exposed body areas before playing outside or in the morning before school or camp may prevent some bites. There are DEET alternatives, such as picaridin, that may be used. Product instructions should be followed when using insect repellants in the pediatric population.11
PIH Management Strategies
Gentle Skin Care Routine
There are misconceptions that areas of hyperpigmentation on the skin are caused by dirt and that scrubbing the skin harder may help to lighten the affected areas. Parents/guardians may report that the child’s skin looks dirty or, in the setting of acne, view dirt as the cause of the skin condition, which may prompt the patient to scrub the skin and the friction further worsens the PIH. Use of daily gentle cleansers and moisturizers is advised to keep the skin moisturized and free of further potential irritation and dryness that may prompt scratching or flares of the underlying condition.
Photoprotection
During the treatment course for PIH, using sun protection is helpful to prevent further darkening of the PIH areas. Sun protection may be in the form of broad-spectrum sunscreen, hats, or sun-protective clothing. Patients should be encouraged to apply sunscreen daily and to reapply every 2 hours and after water-based activities.12 For pediatric and adolescent populations, practicing sun-protective behaviors before school or outdoor activities also should be advised, as many families only think about sun protection in the setting of sunny vacation activities. Research has demonstrated that individuals with skin of color may not realize that they can be affected by skin cancer,13 thus they may not have any experience selecting, applying, or regularly using sunscreens. Products that do not leave a white hue on the skin are suggested for adolescents who may be sensitive about their appearance following sunscreen application.
Skin Lightening Treatments
Although the most important therapy for PIH is to treat the underlying inflammatory conditions, some parents/guardians may desire additional options due to the extent of involvement of the PIH, its psychological impact on the child, or adverse effect on the child’s quality of life.14 In adolescents, incorporating an α-hydroxy acid–based cleanser, glycolic acid chemical peels, salicylic acid chemical peels, and topical cosmeceuticals may be warranted in the setting of intense PIH and acne. However, irritation may lead to further dyspigmentation.
Topical ammonium lactate 12% is lactic acid neutralized with ammonium hydroxide that is formulated as a lotion or a cream. It is used to hydrate dry skin and may decrease corneocyte cohesion.15 Topical ammonium lactate also has been used anecdotally for PIH on the body during periods of watchful waiting.
Topical hydroquinone, the gold standard for treating hyperpigmentation,3,16 is not approved in children, but some parents/guardians elect to utilize hydroquinone off label to accelerate the clearing of distressing PIH in adolescents. Careful consideration including a discussion of potential risks and alternatives (eg, watchful waiting) should be highlighted.
In the setting of chronic inflammatory conditions that recur and remit, potentially irritating topical treatments should be used only during periods when symptoms of inflammation such as itching or erythema are absent.
Conclusion
Despite the best management efforts, PIH in some patients with skin of color may be present for months to years. In the pediatric skin of color population, treatment of the underlying inflammatory condition, gentle skin care, use of photoprotection, and time may be all that is needed for PIH resolution. With their parent/guardians’ consent, adolescents distressed by PIH may decide to pursue more aggressive, potentially irritating treatments. Above all, the most important management in the setting of PIH is to treat the underlying inflammatory condition causing the PIH and set reasonable expectations. For challenging cases, pediatric dermatologists with special expertise in treating pediatric and adolescent patients with skin of color may be consulted.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
- Broughton A. Minorities expected to be majority in 2050. CNN. August 13, 2008. Accessed January 2, 2019.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014. Current Population Reports, P25-1143. Published March 2015. Accessed January 23, 2019.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49-S57.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Napolitano M, Ruggiero G, Monfrecola G, et al. Acne prevalence in 9 to 14-year-old patients attending pediatric ambulatory clinics in Italy. Int J Dermatol. 2018;57:1320-1323.
- Mancini AJ, Baldwin HE, Eichenfield LF. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg 2011;30:2-5.
- Lowe NJ, Rizk D, Grimes P, et al. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin Ther. 1998;20:945-959.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Bulengo-Ransby SM, Griffiths CE, Kimbrough-Green CK, et al. Topical tretinoin (retinoid acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- American Academy of Pediatrics. Choosing an insect repellent for your child. Healthy Children website. Updated July 18, 2018. Accessed January 8, 2019.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:312-317.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Downie J. Help prevent and reverse post-inflammatory hyperpigmentation. Pract Dermatol Pediatr. May/June 2011:12-14. Accessed January 18, 2019.
- Ammonium lactate lotion 12% [package insert]. Bronx, New York: Perrigo New York, Inc; 2006.
- Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77-85.
Practice Points
- The US population of children with skin of color is growing rapidly.
- Treating the underlying inflammatory dermatosis is the most important step in managing postinflammatory hyperpigmentation (PIH); however, many pediatric PIH patients and their parents/guardians presenting with a chief concern of pigmentary changes are unaware of the associated inflammatory condition.
- When appropriate, choose treatments for the underlying inflammatory condition that can simultaneously improve any existing PIH. Gentle skin care, avoidance of rubbing and scrubbing the skin, and photoprotection are essential to halt worsening of PIH.
- Patients’ parents/guardians may consent to more aggressive PIH treatment in select cases (eg, emotional distress in adolescents).
Safety and Efficacy of Halobetasol Propionate Lotion 0.01% in the Treatment of Moderate to Severe Plaque Psoriasis: A Pooled Analysis of 2 Phase 3 Studies
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
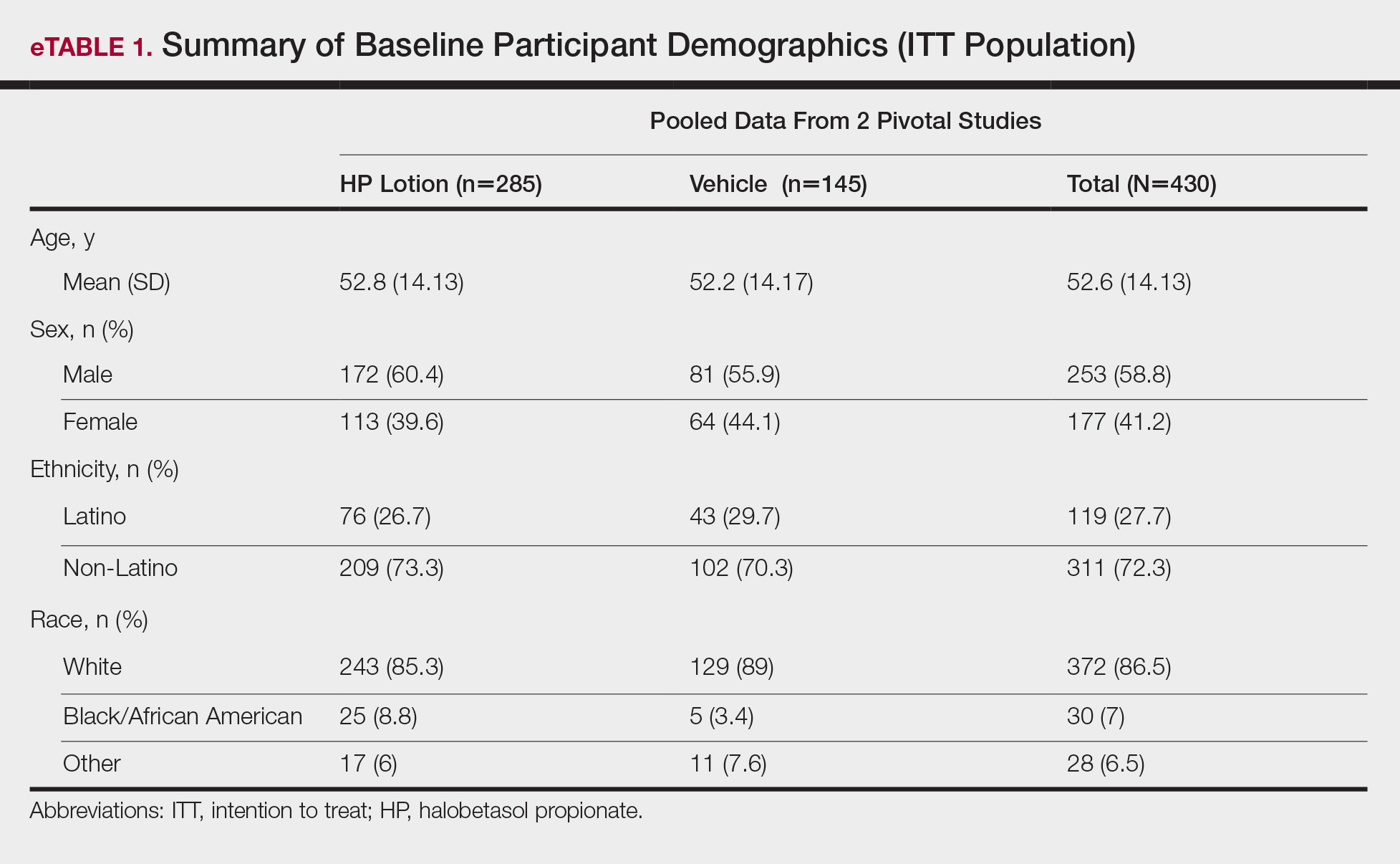
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
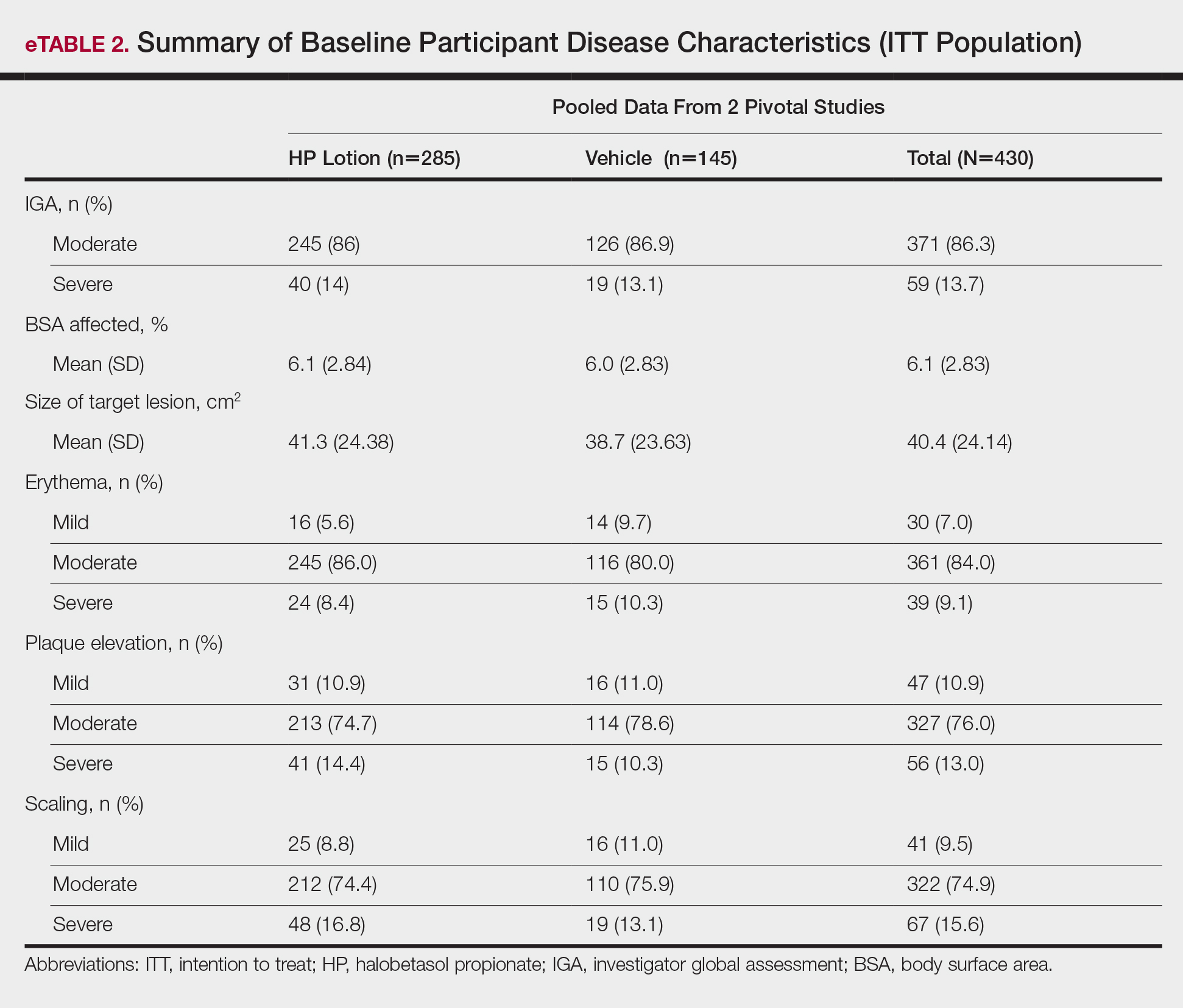
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
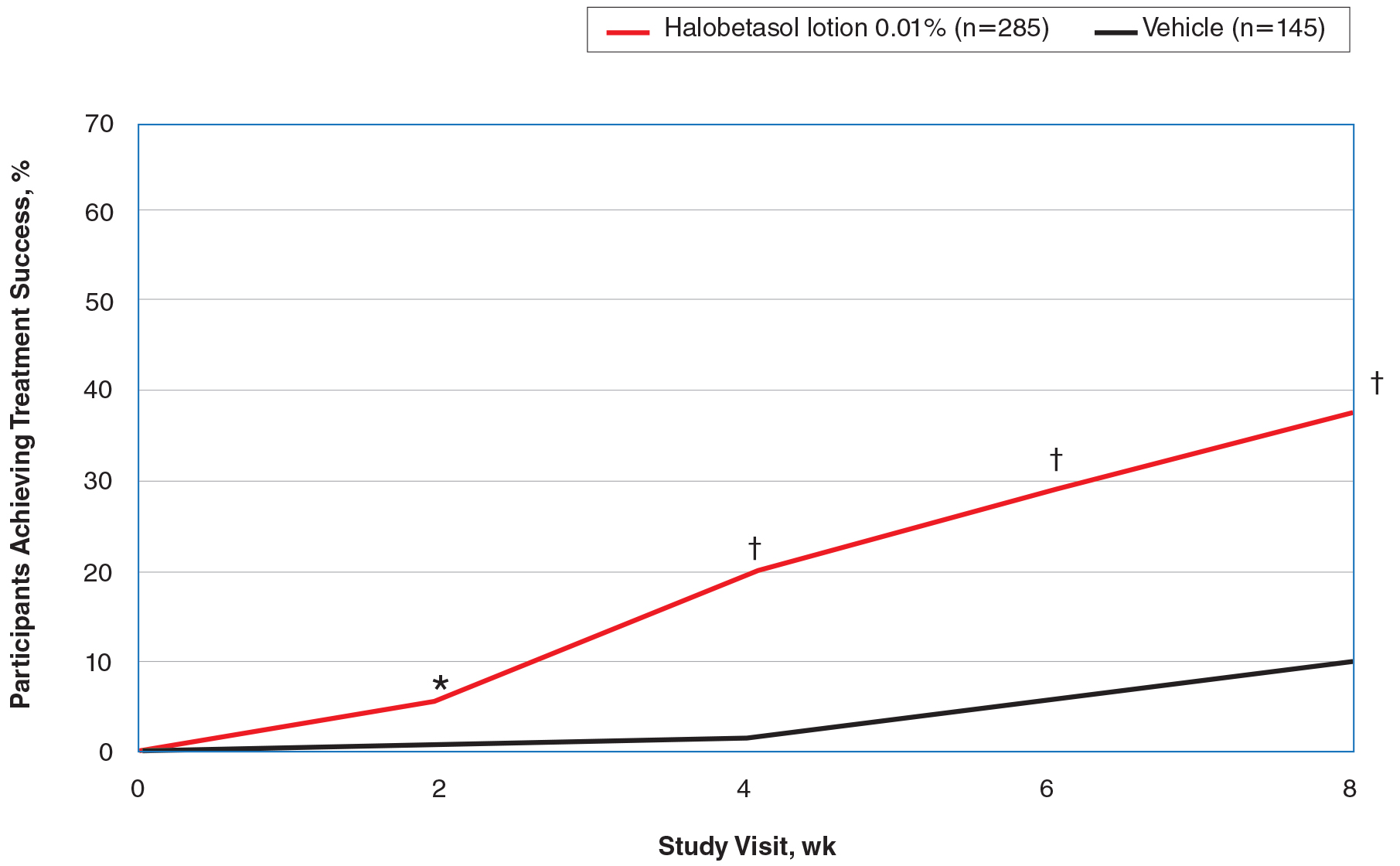
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
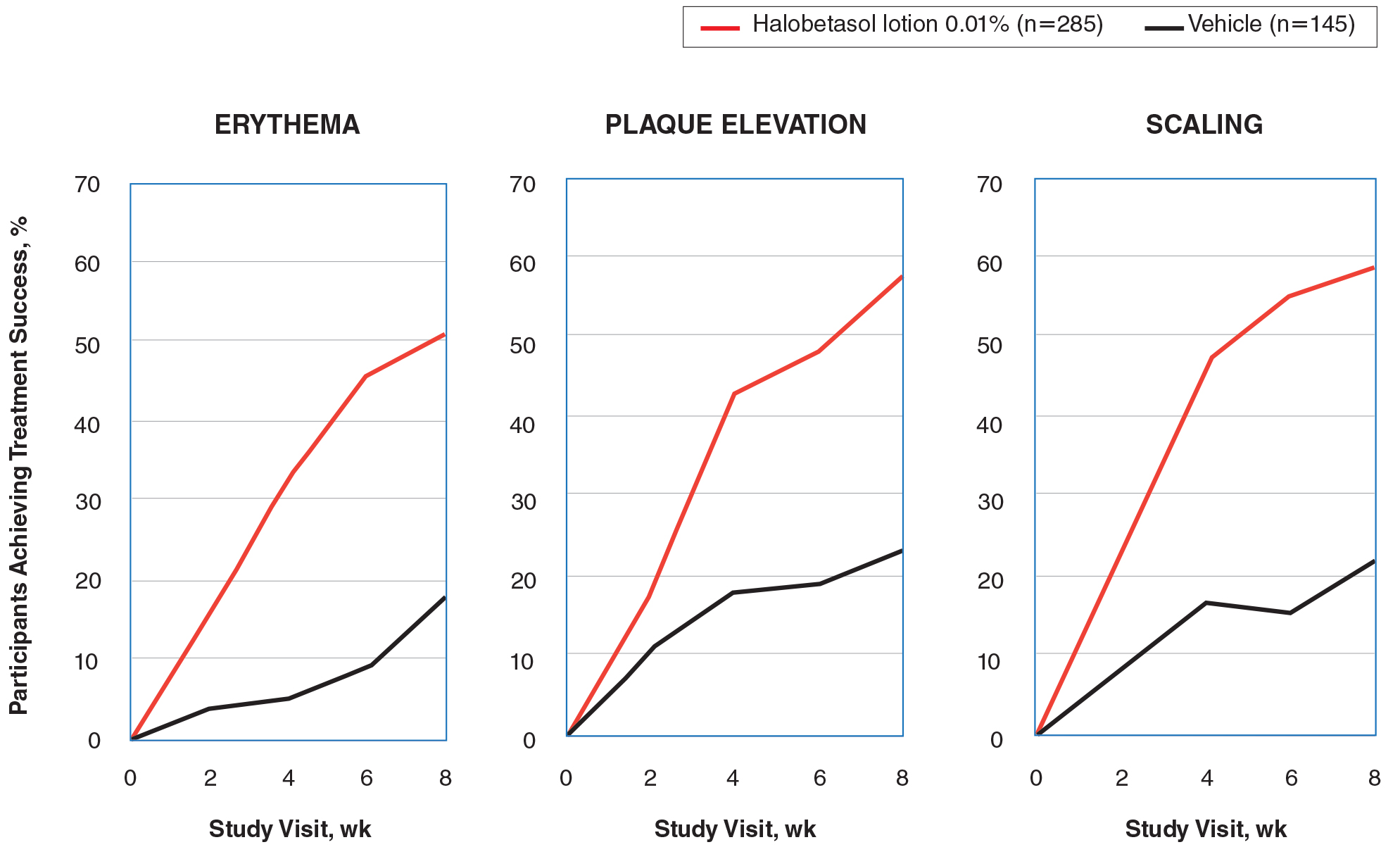
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
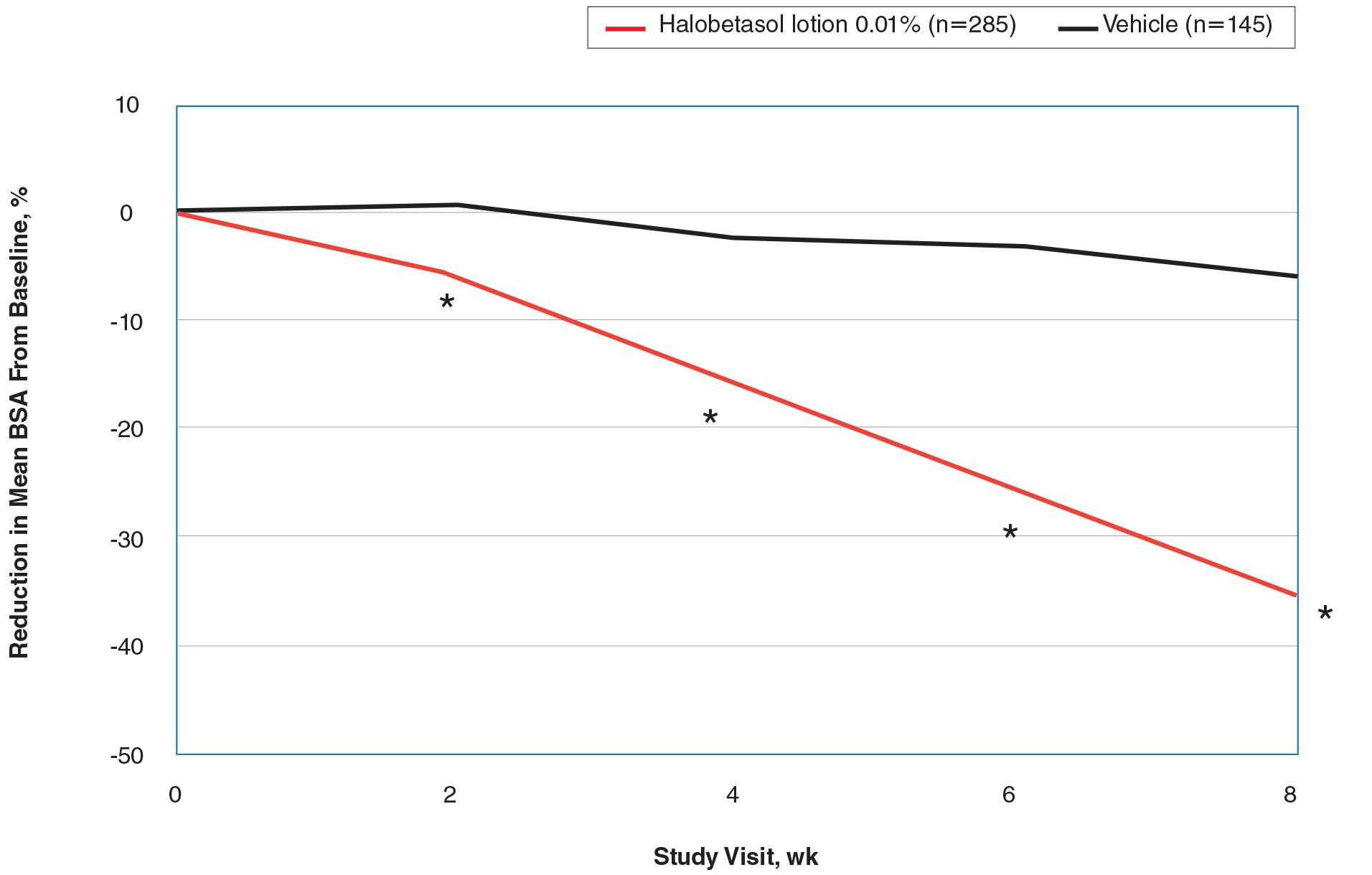
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
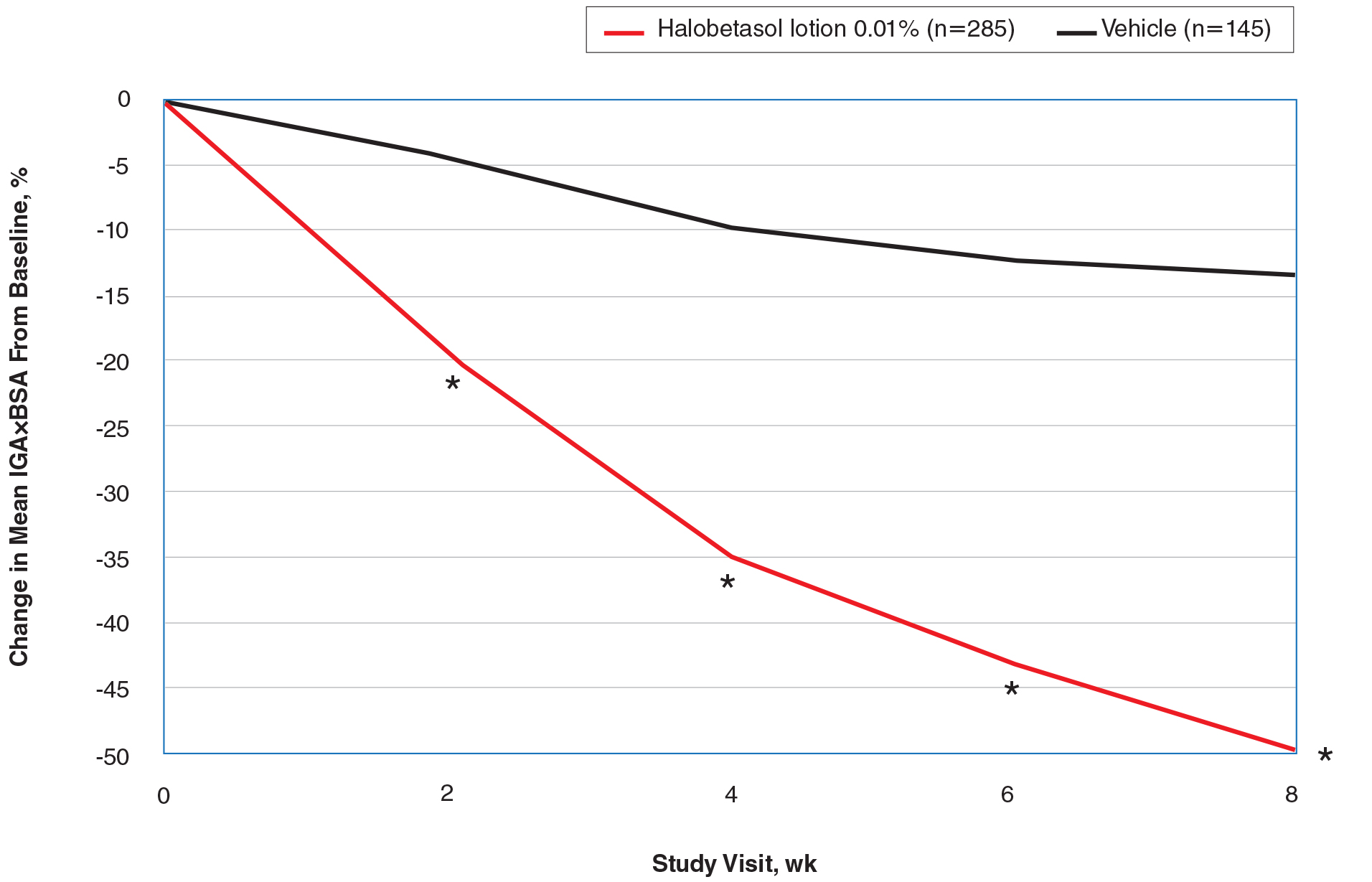
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
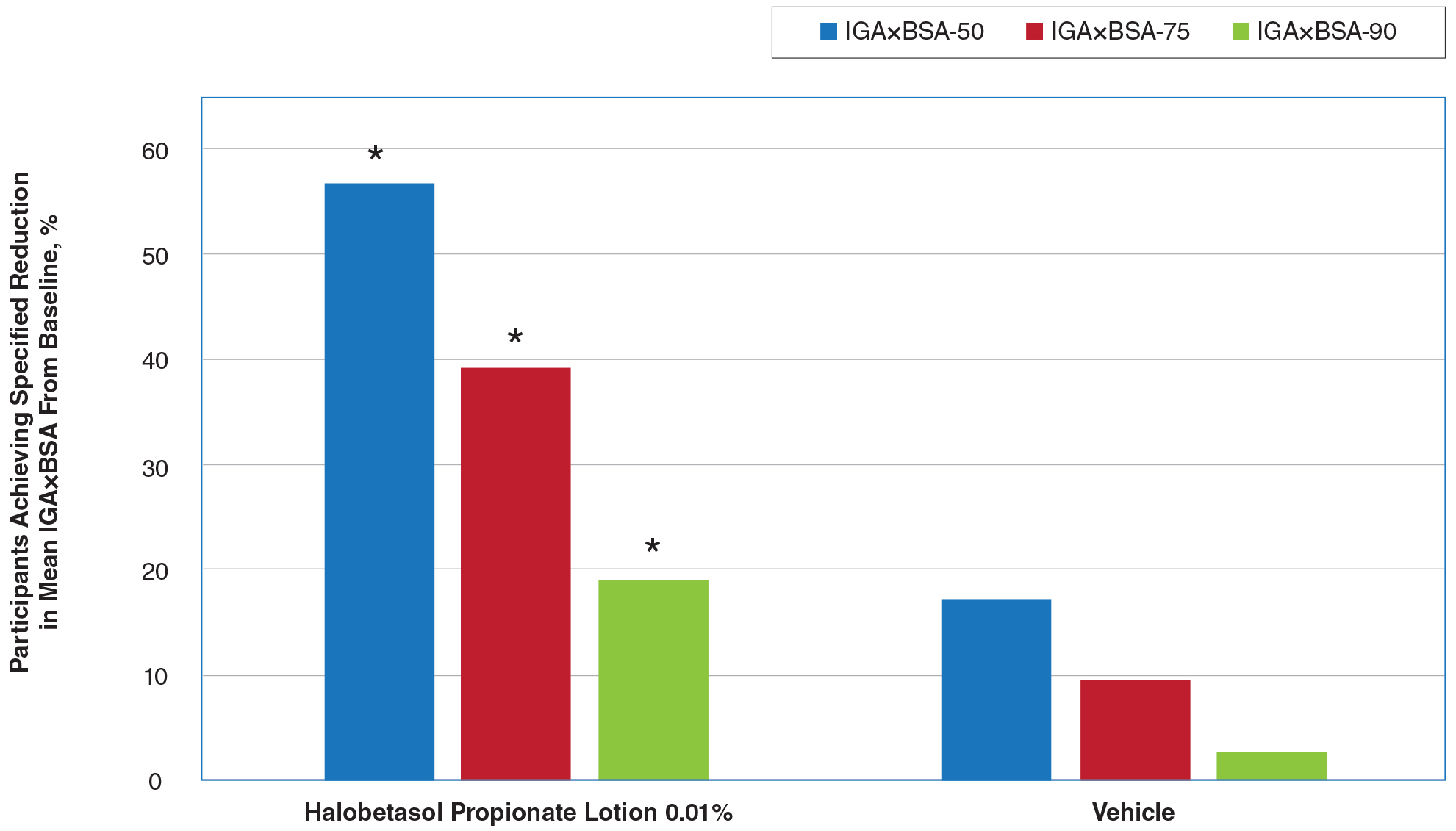
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
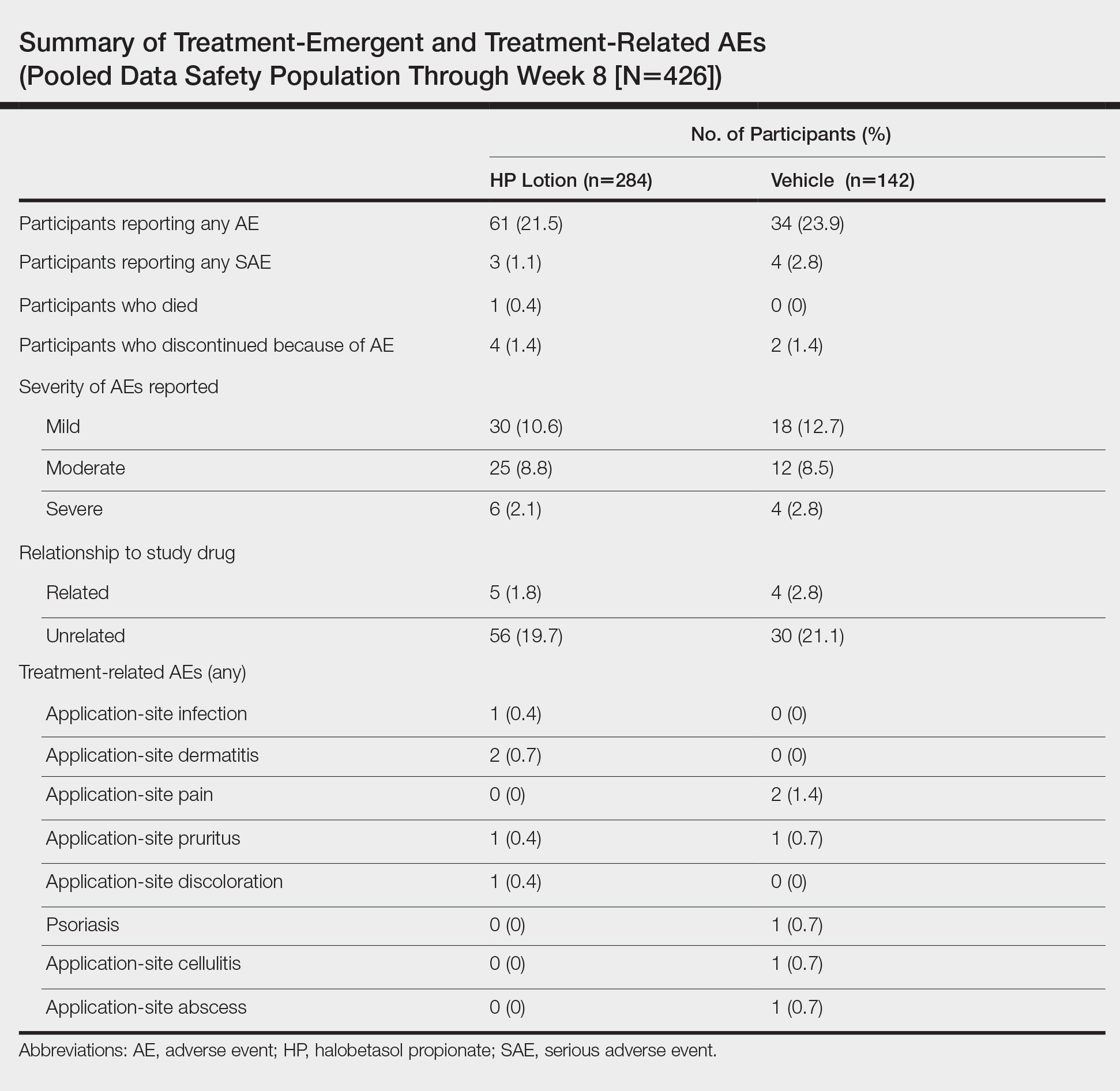
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis Treatment in Patients With Sickle Cell Disease
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Practice Points
• Tumor necrosis factor α contributes both to the vascular inflammatory state seen in sickle cell disease as well as the cycle of inflammation seen in the development of psoriasis.
• Tumor necrosis factor α inhibitors may be the drug of choice for patients with both psoriasis and sickle cell disease.
Locally Destructive Metastatic Basal Cell Carcinoma
To the Editor:
A 60-year-old woman with a history of lymphoma presented to the emergency department for evaluation of intermittent diarrhea and vomiting of 2 weeks’ duration. On presentation, a rather large dressing covering the entire right half of the face was noted. Removal of the bandage revealed a necrotic, extensively destructive, right-sided facial lesion with a fully exposed ocular globe (Figure 1). The patient lived alone and was accompanied by a neighbor, who disclosed that the lesion had been neglected and enlarged over the last 15 years. Moreover, the neighbor reported that the patient had recently experienced several episodes of vertigo and frequent falls.
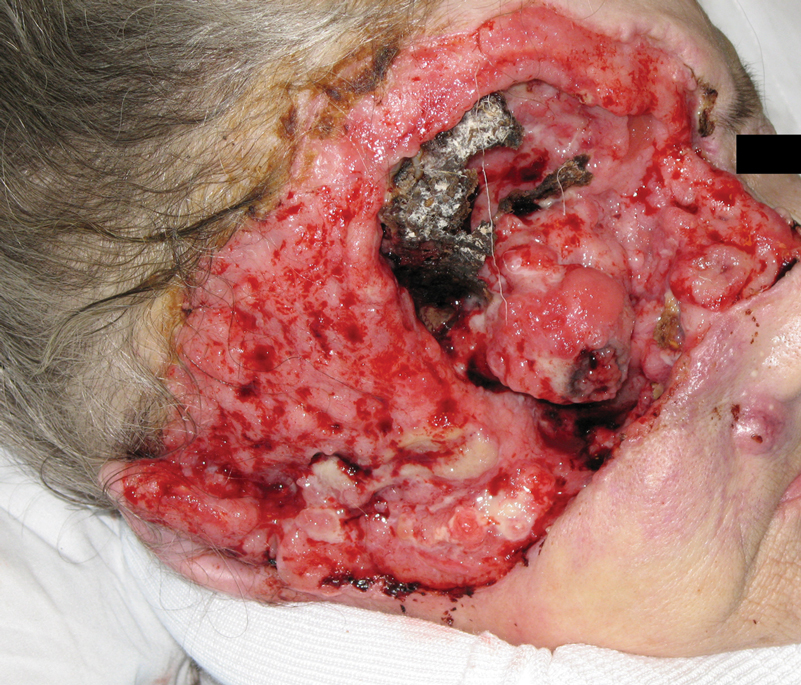
On admission to the hospital, dermatology was consulted and initial workup included computed tomography (CT) scan of the head and maxillofacial region, which showed a destructive process involving the right frontotemporal bone, maxillofacial region, sphenoid, and skull base with exposure of intracranial contents (Figure 2). An aggressive wound care regimen was instituted. Biopsy of the wound margin revealed nodular and focally infiltrative basal cell carcinoma (BCC) (Figure 3).
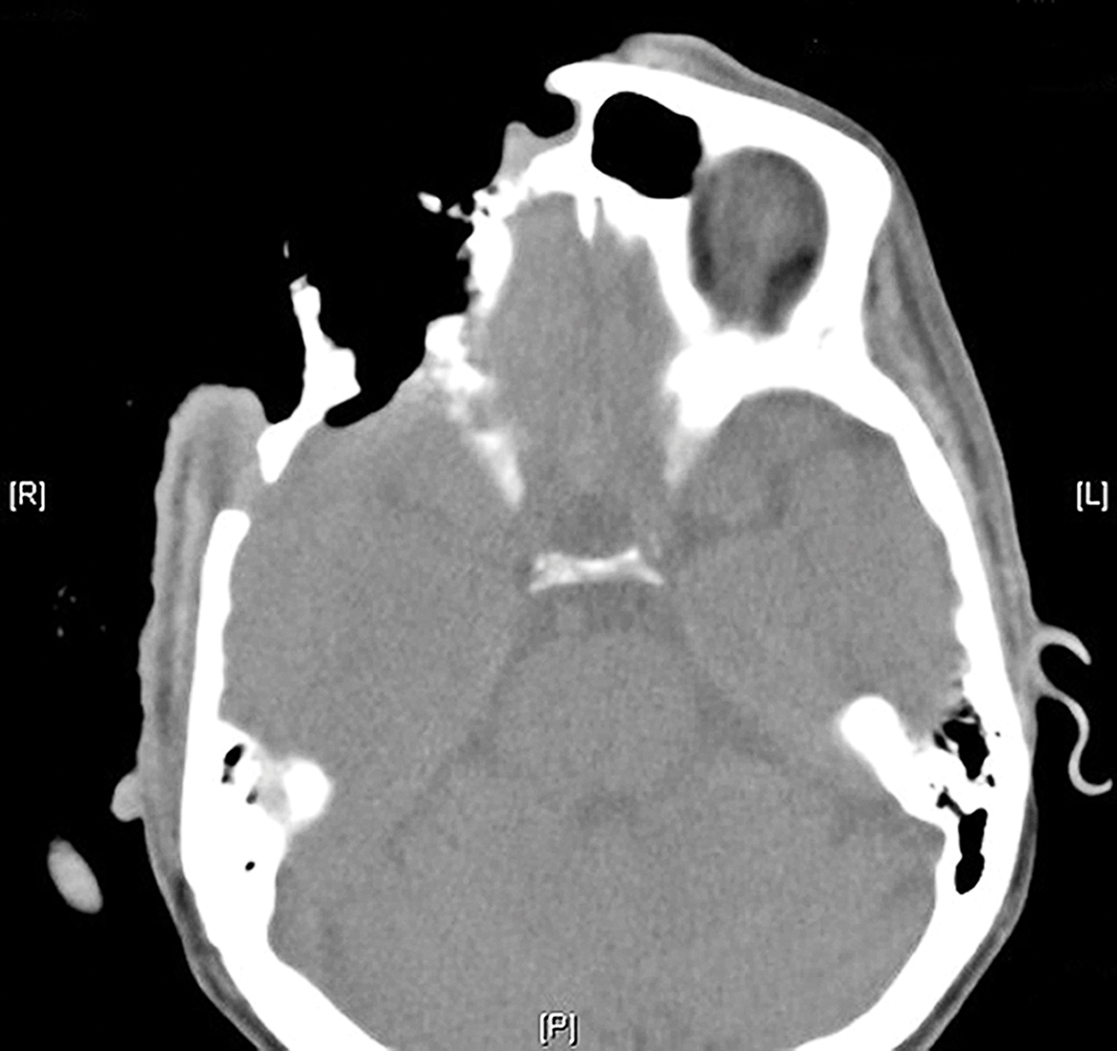
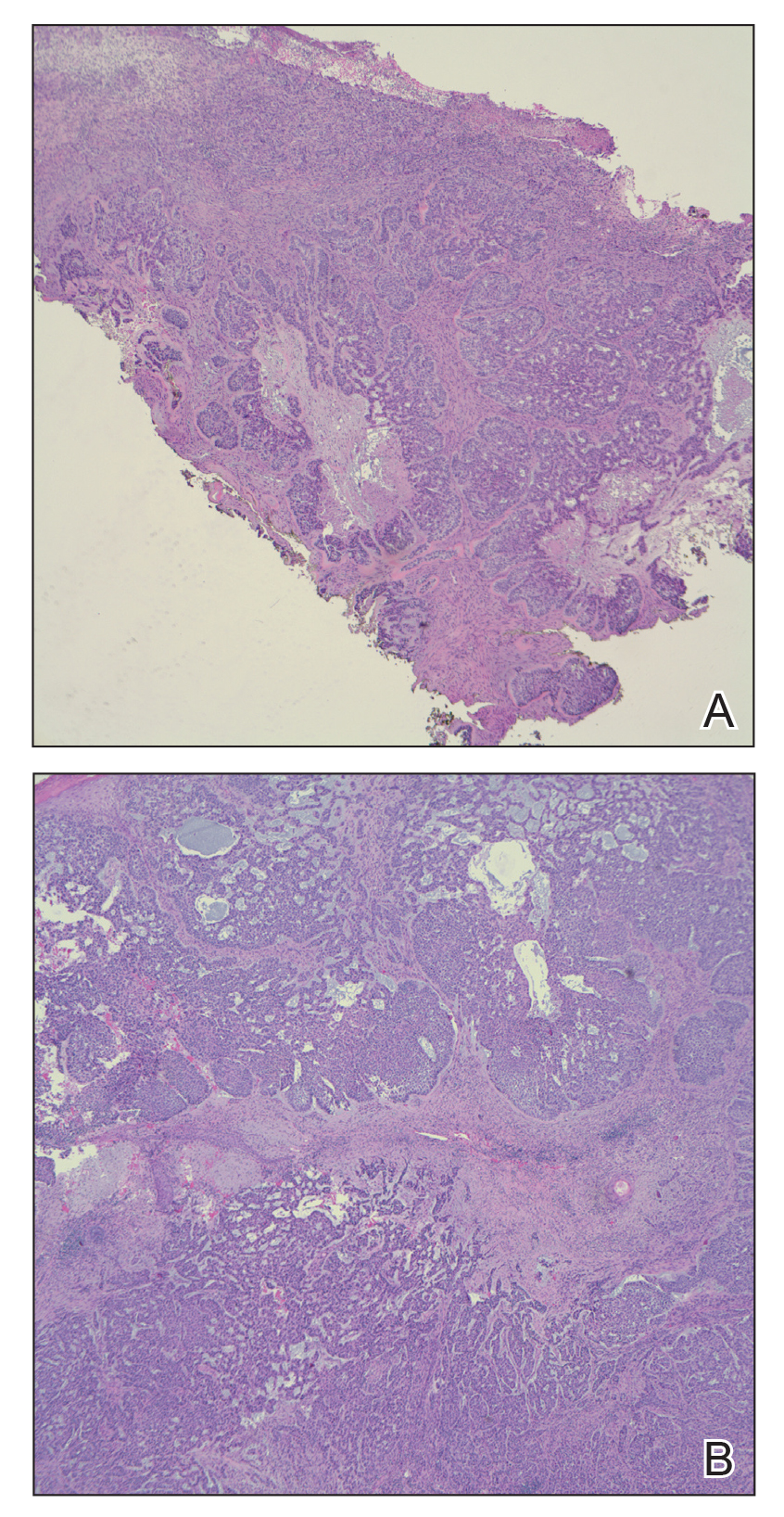
and focally infiltrative basal cell carcinoma (H&E, original magnifications×4 and ×10).
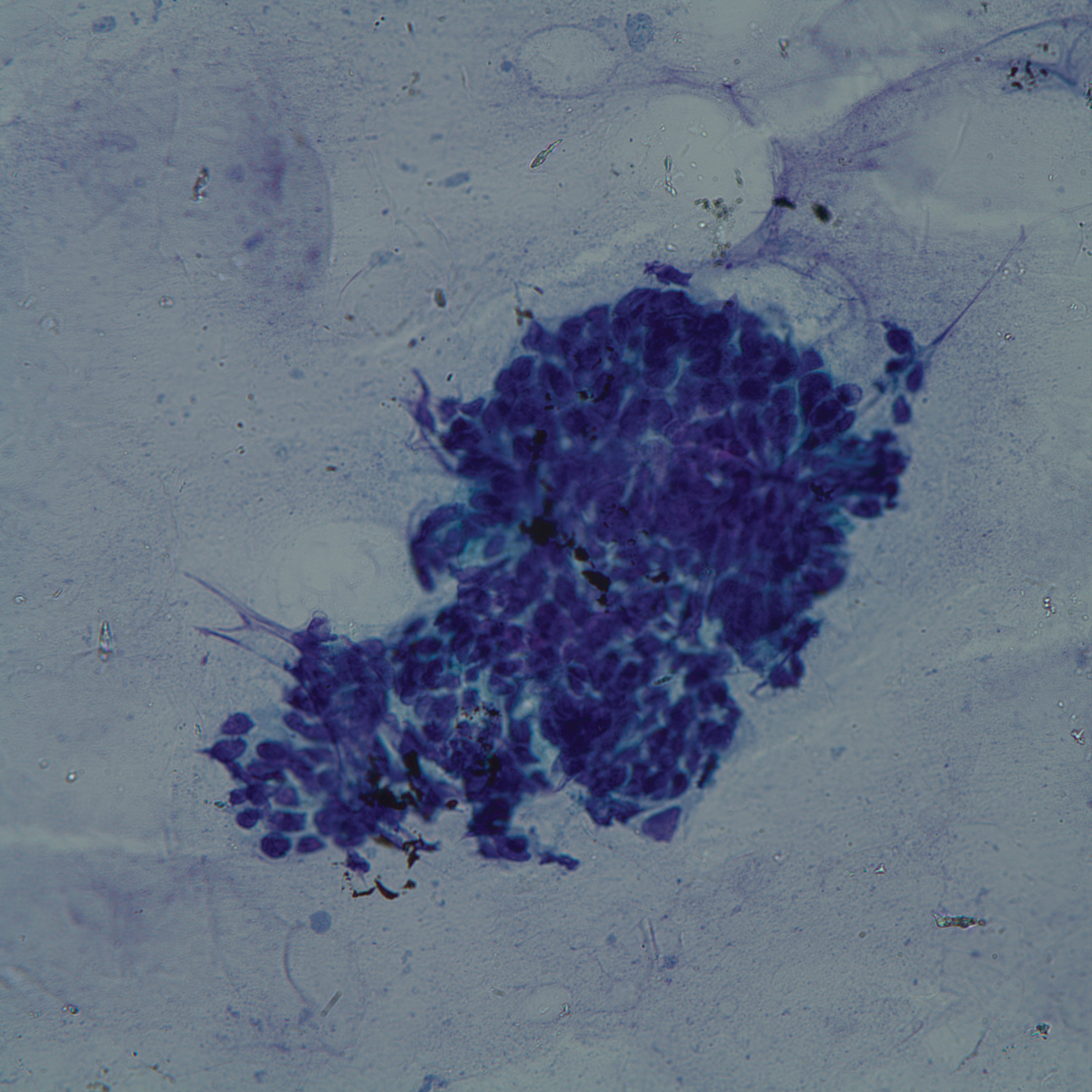
Several days into her hospitalization, the patient developed radicular pain in both arms and weakness in all 4 extremities. A CT scan of the neck revealed a pathologic fracture of the C7 vertebrae. Several medical and surgical services as well as psychiatry were consulted. Given the extensive nature of the disease involvement with limited treatment options, the patient sought to forego further interventions and was discharged to hospice care.
Basal cell carcinomas rarely metastasize, with a reported incidence of 0.0028% to 0.5%.1 The likelihood of metastasis is most closely related to tumor size and depth of invasion. Tumors greater than 3 cm in diameter have a 2% incidence of metastatic spread and/or death. The incidence of metastatic spread and/or death is estimated to be 25% for tumors with a diameter of 5 cm and 50% for tumors with a diameter of 10 cm or greater.2 Other risk factors for metastatic spread include long duration of disease, failure to respond to conventional treatment, and prior radiation treatment in the affected area.1 In one review, the median interval between onset of BCC and metastasis was 9 years.3 In our case, 15 years of neglect most likely led to the aggressiveness of the tumor. Although the workup in our patient was limited per her request, there was no evidence that her lymphoma had recurred or that she was in any other way immunocompromised. Unfortunately, in this patient’s case, the local destructiveness of the carcinoma with subsequent bony invasion and necrosis was complicated with secondary Rhizopus infection. A PubMed search of articles indexed for MEDLINE using the terms basal cell carcinoma and mucormycosis revealed no other reported cases of BCC associated with mucormycosis; therefore, our case represents a rare presentation of this association. Rhinocerebral mucormycosis is the most common manifestation of mucormycosis and more commonly occurs in diabetics with ketoacidosis and in severely debilitated or immunosuppressed individuals.4 The extensive bony destruction, especially of the nasal region, of our patient’s tumor likely led to secondary infection with Rhizopus.
Approximately 85% of all metastatic BCCs originate in the head and neck region, with lymph nodes being the first site of metastasis and involved in approximately half of all cases.1,4 Metastases to the lungs, bone, liver, and other viscera can occur with advanced disease. Metastasis generally portends a poor prognosis, with survival rarely exceeding 1.5 years. Until recently, therapeutic options for metastatic disease were limited, with marginal response to chemotherapy with methotrexate, fluorouracil, bleomycin, and cisplatin.4 Vismodegib, a novel smoothened receptor inhibitor that blocks the sonic hedgehog pathway implicated in BCC carcinogenesis, offers a new promising treatment for management and control of advanced disease.5
- Junior W, Ribeiro SC, Vieira SC, et al. Metastatic basal cell carcinoma: a case report. Dermatol Online J. 2003;9:18.
- Snow SN, Sahl WJ, Lo J, et al. Metastatic basal cell carcinoma: report of 5 cases. Cancer. 1994;73:328-335.
- Von Domarus H, Stevens PJ. Metastatic basal cell carcinoma: report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Kolekar JS. Rhinocerebral mucormycosis: a retrospective study. Indian J Otolaryngol Head Neck Surg. 2015;67:93-96.
- Koehlblinger P, Lang R. New developments in the treatment of basal cell carcinoma: update on current and emerging treatment options with a focus on vismodegib. Onc Targets Ther. 2018;11:8327-8340.
To the Editor:
A 60-year-old woman with a history of lymphoma presented to the emergency department for evaluation of intermittent diarrhea and vomiting of 2 weeks’ duration. On presentation, a rather large dressing covering the entire right half of the face was noted. Removal of the bandage revealed a necrotic, extensively destructive, right-sided facial lesion with a fully exposed ocular globe (Figure 1). The patient lived alone and was accompanied by a neighbor, who disclosed that the lesion had been neglected and enlarged over the last 15 years. Moreover, the neighbor reported that the patient had recently experienced several episodes of vertigo and frequent falls.

On admission to the hospital, dermatology was consulted and initial workup included computed tomography (CT) scan of the head and maxillofacial region, which showed a destructive process involving the right frontotemporal bone, maxillofacial region, sphenoid, and skull base with exposure of intracranial contents (Figure 2). An aggressive wound care regimen was instituted. Biopsy of the wound margin revealed nodular and focally infiltrative basal cell carcinoma (BCC) (Figure 3).


and focally infiltrative basal cell carcinoma (H&E, original magnifications×4 and ×10).

Several days into her hospitalization, the patient developed radicular pain in both arms and weakness in all 4 extremities. A CT scan of the neck revealed a pathologic fracture of the C7 vertebrae. Several medical and surgical services as well as psychiatry were consulted. Given the extensive nature of the disease involvement with limited treatment options, the patient sought to forego further interventions and was discharged to hospice care.
Basal cell carcinomas rarely metastasize, with a reported incidence of 0.0028% to 0.5%.1 The likelihood of metastasis is most closely related to tumor size and depth of invasion. Tumors greater than 3 cm in diameter have a 2% incidence of metastatic spread and/or death. The incidence of metastatic spread and/or death is estimated to be 25% for tumors with a diameter of 5 cm and 50% for tumors with a diameter of 10 cm or greater.2 Other risk factors for metastatic spread include long duration of disease, failure to respond to conventional treatment, and prior radiation treatment in the affected area.1 In one review, the median interval between onset of BCC and metastasis was 9 years.3 In our case, 15 years of neglect most likely led to the aggressiveness of the tumor. Although the workup in our patient was limited per her request, there was no evidence that her lymphoma had recurred or that she was in any other way immunocompromised. Unfortunately, in this patient’s case, the local destructiveness of the carcinoma with subsequent bony invasion and necrosis was complicated with secondary Rhizopus infection. A PubMed search of articles indexed for MEDLINE using the terms basal cell carcinoma and mucormycosis revealed no other reported cases of BCC associated with mucormycosis; therefore, our case represents a rare presentation of this association. Rhinocerebral mucormycosis is the most common manifestation of mucormycosis and more commonly occurs in diabetics with ketoacidosis and in severely debilitated or immunosuppressed individuals.4 The extensive bony destruction, especially of the nasal region, of our patient’s tumor likely led to secondary infection with Rhizopus.
Approximately 85% of all metastatic BCCs originate in the head and neck region, with lymph nodes being the first site of metastasis and involved in approximately half of all cases.1,4 Metastases to the lungs, bone, liver, and other viscera can occur with advanced disease. Metastasis generally portends a poor prognosis, with survival rarely exceeding 1.5 years. Until recently, therapeutic options for metastatic disease were limited, with marginal response to chemotherapy with methotrexate, fluorouracil, bleomycin, and cisplatin.4 Vismodegib, a novel smoothened receptor inhibitor that blocks the sonic hedgehog pathway implicated in BCC carcinogenesis, offers a new promising treatment for management and control of advanced disease.5
To the Editor:
A 60-year-old woman with a history of lymphoma presented to the emergency department for evaluation of intermittent diarrhea and vomiting of 2 weeks’ duration. On presentation, a rather large dressing covering the entire right half of the face was noted. Removal of the bandage revealed a necrotic, extensively destructive, right-sided facial lesion with a fully exposed ocular globe (Figure 1). The patient lived alone and was accompanied by a neighbor, who disclosed that the lesion had been neglected and enlarged over the last 15 years. Moreover, the neighbor reported that the patient had recently experienced several episodes of vertigo and frequent falls.

On admission to the hospital, dermatology was consulted and initial workup included computed tomography (CT) scan of the head and maxillofacial region, which showed a destructive process involving the right frontotemporal bone, maxillofacial region, sphenoid, and skull base with exposure of intracranial contents (Figure 2). An aggressive wound care regimen was instituted. Biopsy of the wound margin revealed nodular and focally infiltrative basal cell carcinoma (BCC) (Figure 3).


and focally infiltrative basal cell carcinoma (H&E, original magnifications×4 and ×10).

Several days into her hospitalization, the patient developed radicular pain in both arms and weakness in all 4 extremities. A CT scan of the neck revealed a pathologic fracture of the C7 vertebrae. Several medical and surgical services as well as psychiatry were consulted. Given the extensive nature of the disease involvement with limited treatment options, the patient sought to forego further interventions and was discharged to hospice care.
Basal cell carcinomas rarely metastasize, with a reported incidence of 0.0028% to 0.5%.1 The likelihood of metastasis is most closely related to tumor size and depth of invasion. Tumors greater than 3 cm in diameter have a 2% incidence of metastatic spread and/or death. The incidence of metastatic spread and/or death is estimated to be 25% for tumors with a diameter of 5 cm and 50% for tumors with a diameter of 10 cm or greater.2 Other risk factors for metastatic spread include long duration of disease, failure to respond to conventional treatment, and prior radiation treatment in the affected area.1 In one review, the median interval between onset of BCC and metastasis was 9 years.3 In our case, 15 years of neglect most likely led to the aggressiveness of the tumor. Although the workup in our patient was limited per her request, there was no evidence that her lymphoma had recurred or that she was in any other way immunocompromised. Unfortunately, in this patient’s case, the local destructiveness of the carcinoma with subsequent bony invasion and necrosis was complicated with secondary Rhizopus infection. A PubMed search of articles indexed for MEDLINE using the terms basal cell carcinoma and mucormycosis revealed no other reported cases of BCC associated with mucormycosis; therefore, our case represents a rare presentation of this association. Rhinocerebral mucormycosis is the most common manifestation of mucormycosis and more commonly occurs in diabetics with ketoacidosis and in severely debilitated or immunosuppressed individuals.4 The extensive bony destruction, especially of the nasal region, of our patient’s tumor likely led to secondary infection with Rhizopus.
Approximately 85% of all metastatic BCCs originate in the head and neck region, with lymph nodes being the first site of metastasis and involved in approximately half of all cases.1,4 Metastases to the lungs, bone, liver, and other viscera can occur with advanced disease. Metastasis generally portends a poor prognosis, with survival rarely exceeding 1.5 years. Until recently, therapeutic options for metastatic disease were limited, with marginal response to chemotherapy with methotrexate, fluorouracil, bleomycin, and cisplatin.4 Vismodegib, a novel smoothened receptor inhibitor that blocks the sonic hedgehog pathway implicated in BCC carcinogenesis, offers a new promising treatment for management and control of advanced disease.5
- Junior W, Ribeiro SC, Vieira SC, et al. Metastatic basal cell carcinoma: a case report. Dermatol Online J. 2003;9:18.
- Snow SN, Sahl WJ, Lo J, et al. Metastatic basal cell carcinoma: report of 5 cases. Cancer. 1994;73:328-335.
- Von Domarus H, Stevens PJ. Metastatic basal cell carcinoma: report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Kolekar JS. Rhinocerebral mucormycosis: a retrospective study. Indian J Otolaryngol Head Neck Surg. 2015;67:93-96.
- Koehlblinger P, Lang R. New developments in the treatment of basal cell carcinoma: update on current and emerging treatment options with a focus on vismodegib. Onc Targets Ther. 2018;11:8327-8340.
- Junior W, Ribeiro SC, Vieira SC, et al. Metastatic basal cell carcinoma: a case report. Dermatol Online J. 2003;9:18.
- Snow SN, Sahl WJ, Lo J, et al. Metastatic basal cell carcinoma: report of 5 cases. Cancer. 1994;73:328-335.
- Von Domarus H, Stevens PJ. Metastatic basal cell carcinoma: report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10:1043-1060.
- Kolekar JS. Rhinocerebral mucormycosis: a retrospective study. Indian J Otolaryngol Head Neck Surg. 2015;67:93-96.
- Koehlblinger P, Lang R. New developments in the treatment of basal cell carcinoma: update on current and emerging treatment options with a focus on vismodegib. Onc Targets Ther. 2018;11:8327-8340.
Practice Points
- Risk factors associated with metastatic spread of basal cell carcinoma (BCC) include larger tumor size, greater depth of invasion, long duration of disease, failure to respond to conventional treatment, and prior radiation treatment in the affected area.
- The median interval between onset of BCC and metastasis has been shown to be approximately 9 years.
- Vismodegib can be an effective oral therapy for patients with metastatic BCC, locally advanced BCC, recurrence following surgery, or those who are not candidates for surgery or radiation.