User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Risk for Appendicitis, Cholecystitis, or Diverticulitis in Patients With Psoriasis
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
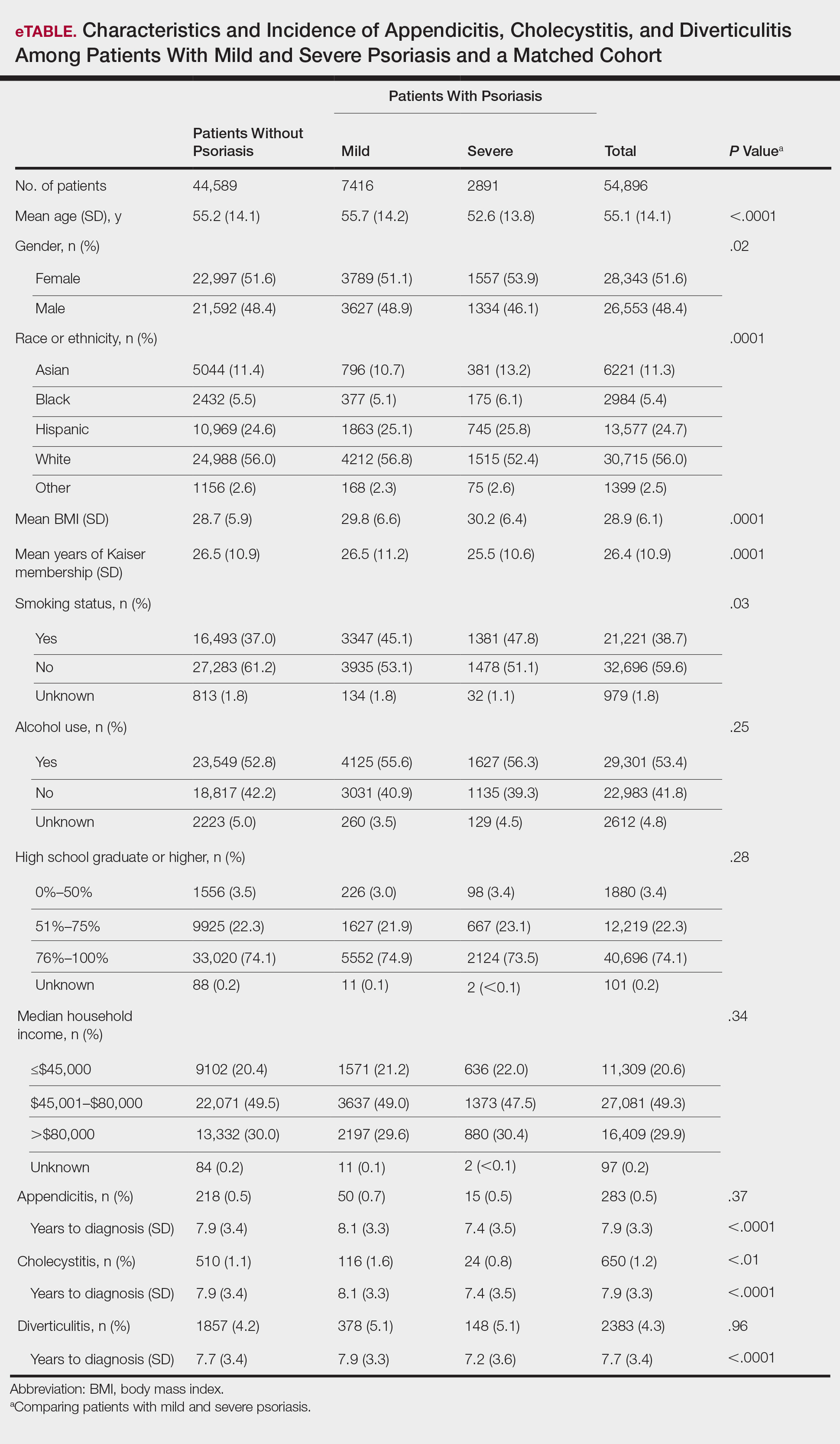
Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
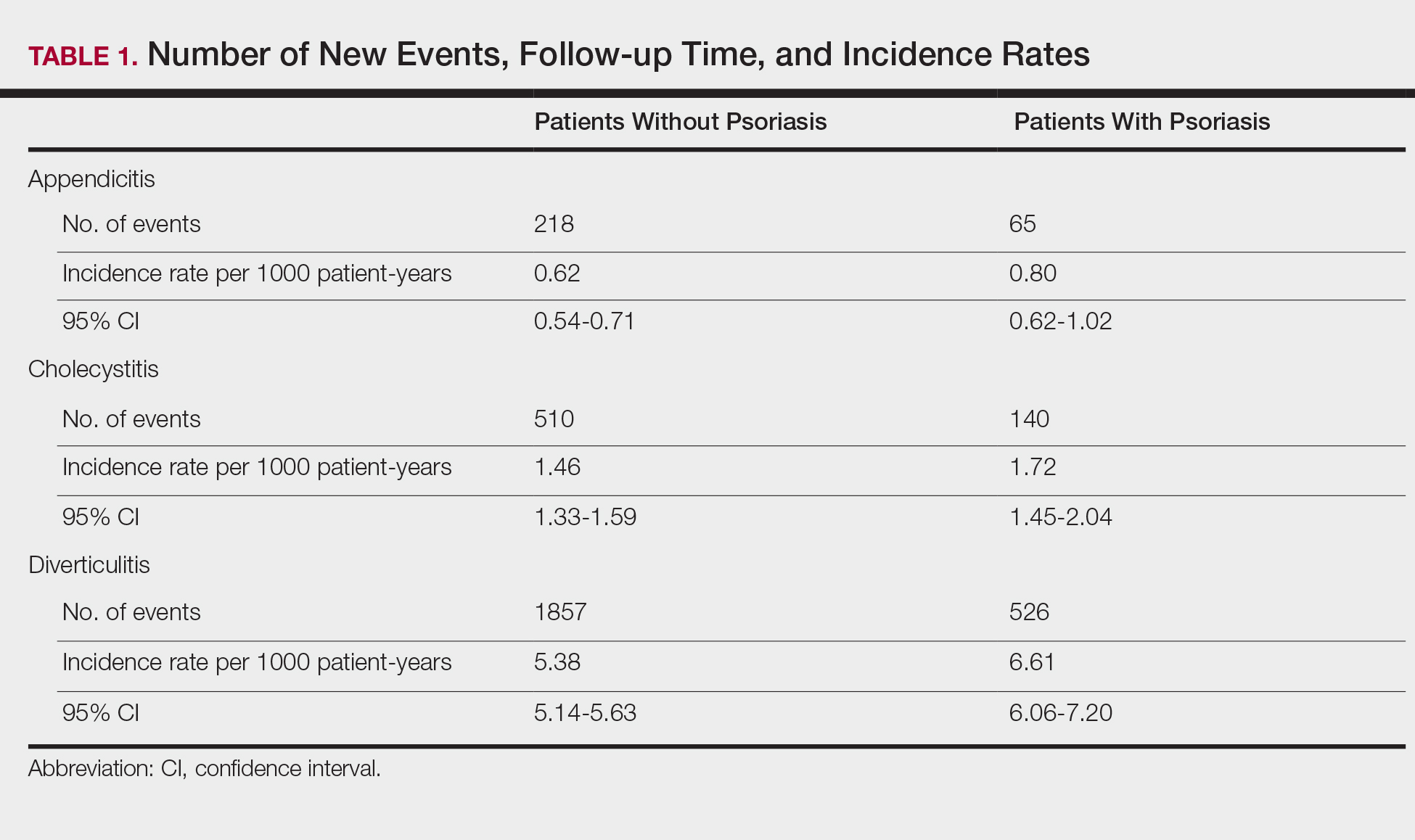
Cholecystitis
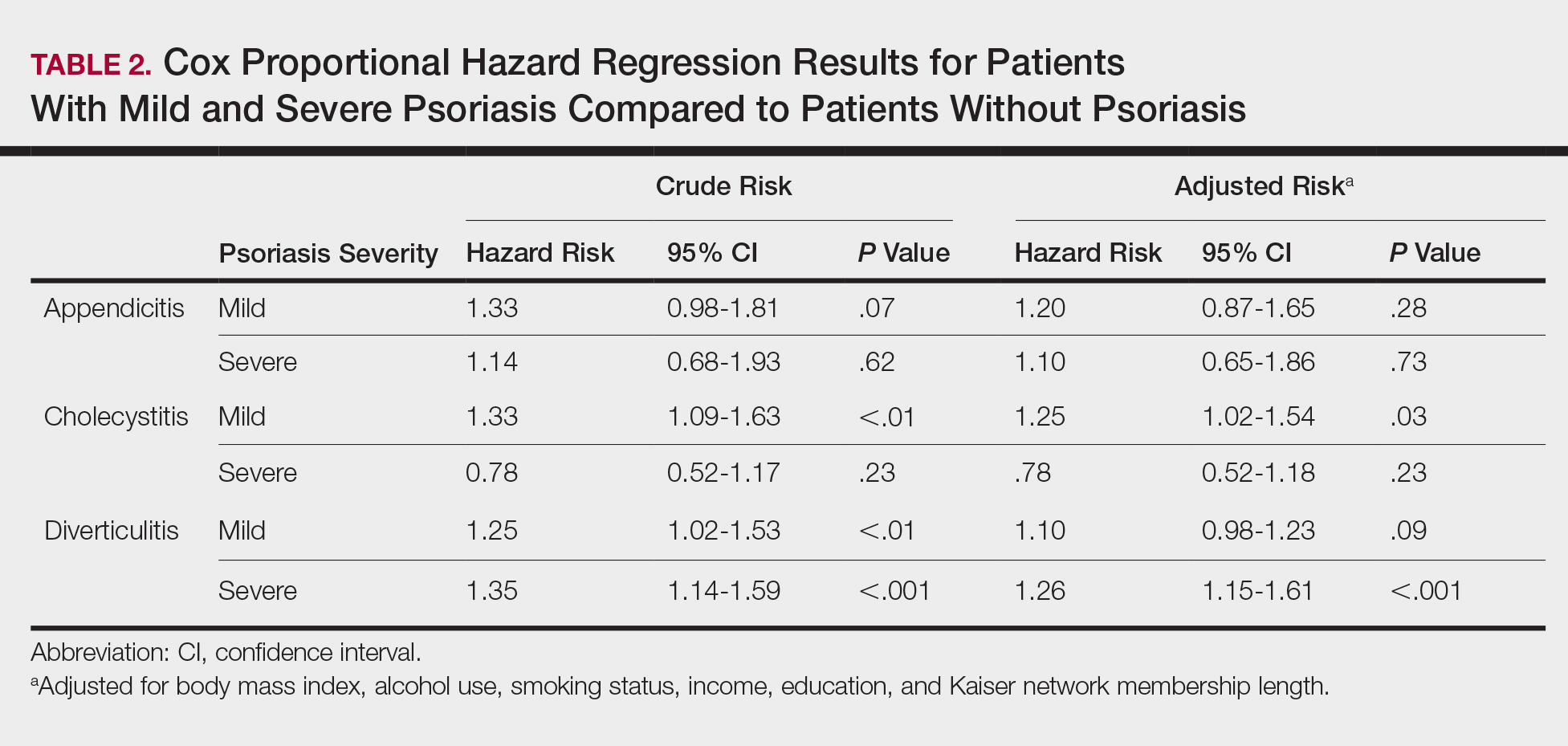
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
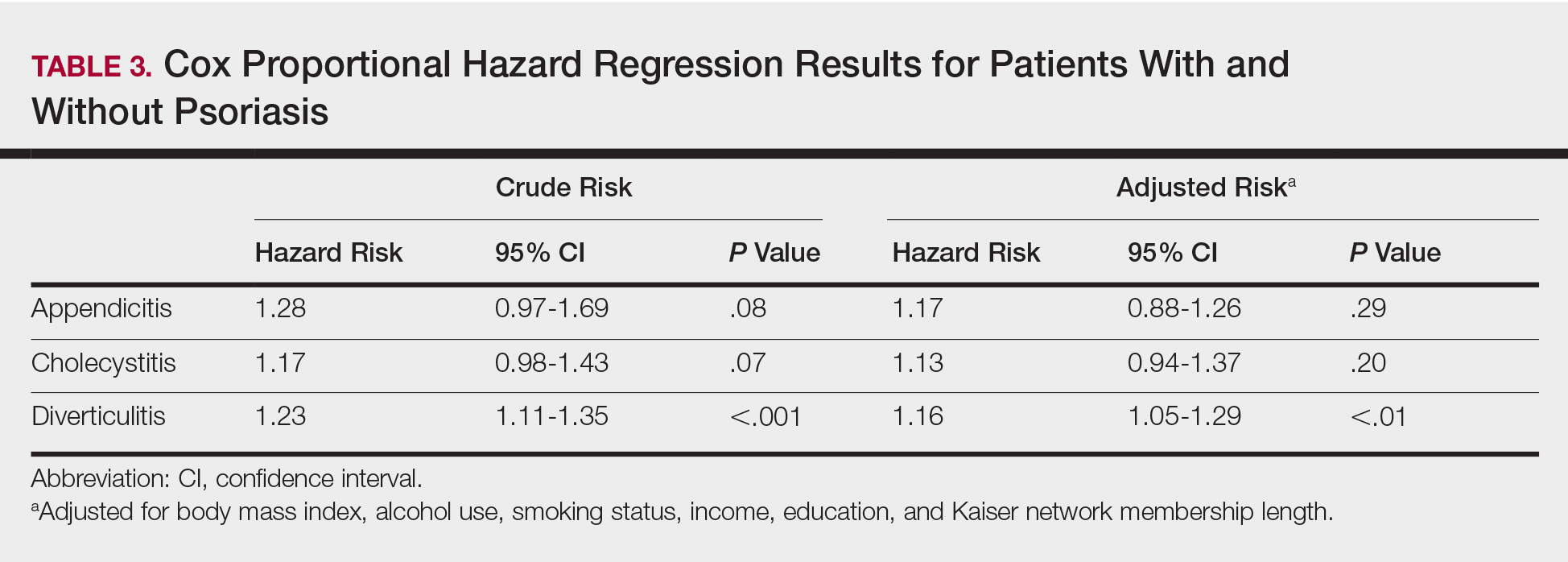
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Practice Points
- Patients with psoriasis may have elevated risk of diverticulitis compared to healthy patients. However, psoriasis patients do not appear to have increased risk of appendicitis or cholecystitis.
- Clinicians treating psoriasis patients should consider assessing for other risk factors of diverticulitis at regular intervals.
Cutaneous Rosai-Dorfman Disease
Case Report
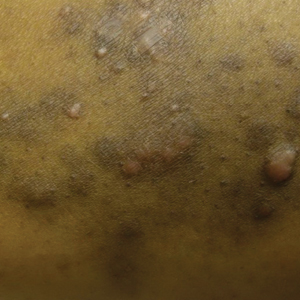
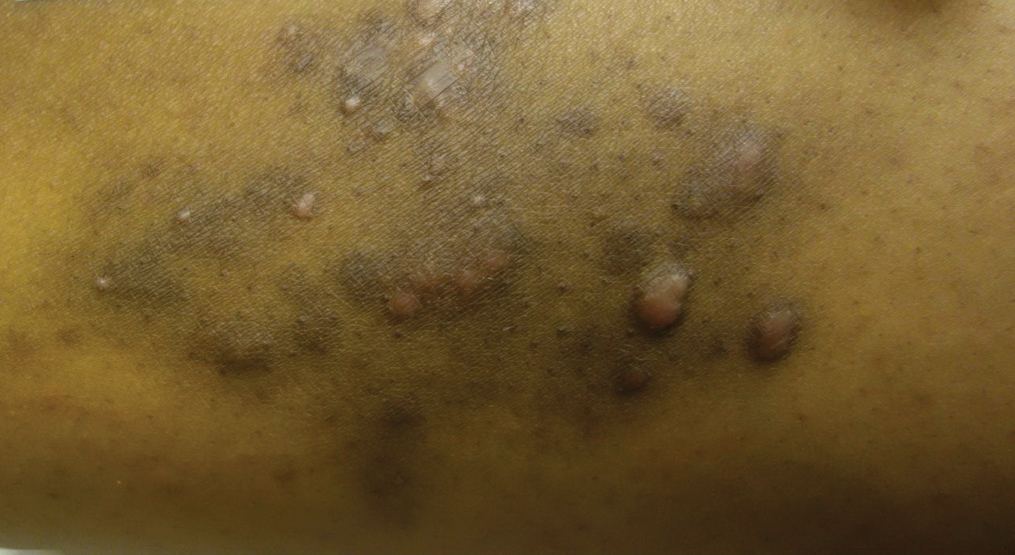
A 31-year-old black woman presented with a slow-spreading pruritic rash on the right thigh of 1 year’s duration. She had previously seen a dermatologist and was prescribed triamcinolone acetonide cream 0.1% and mupirocin ointment 2% but declined a biopsy. Review of symptoms was negative for any constitutional symptoms. Family history included hypertension and eczema with a personal history of anxiety. Clinical examination revealed grouped flesh-colored to light pink papules and plaques within a hyperpigmented patch on the right medial thigh (Figure 1).
Histopathology
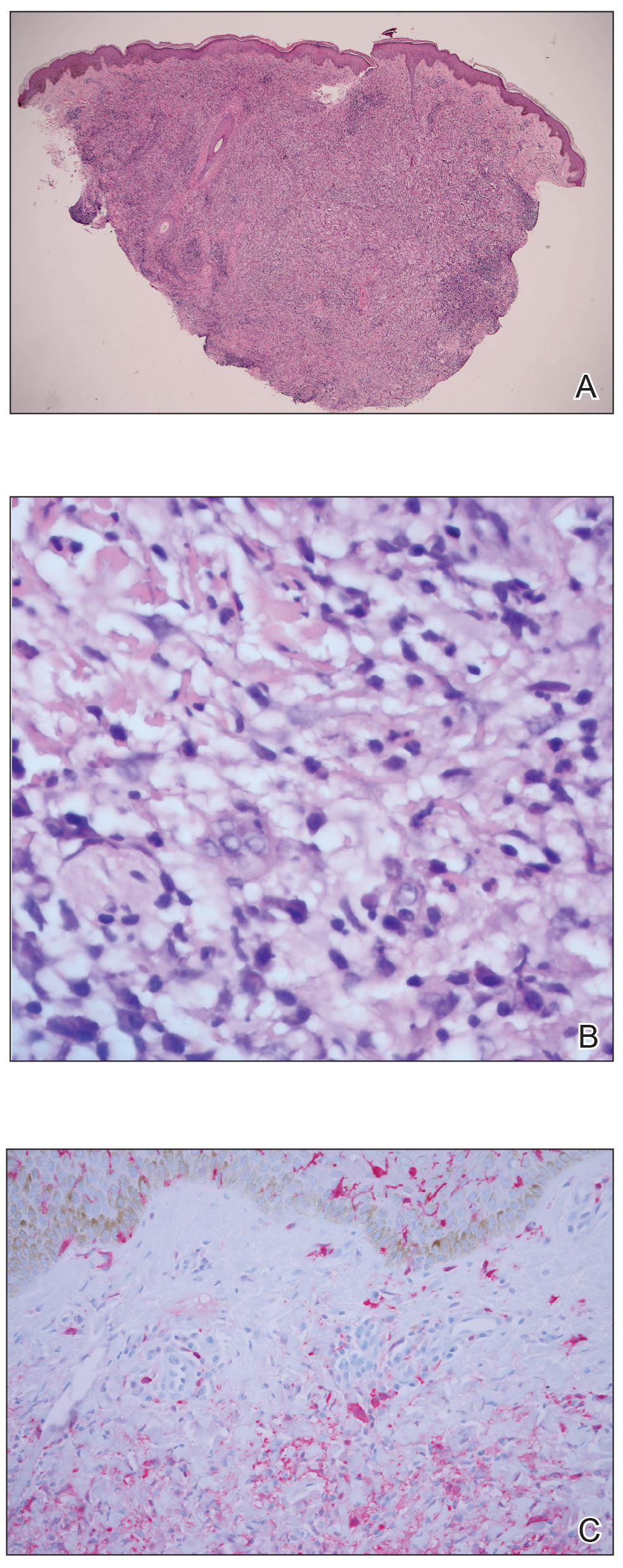
A punch biopsy was negative for fungal, bacterial, or acid-fast bacilli culture. Histopathologic evaluation demonstrated a dense dermal infiltrate of large histiocytes admixed with inflammatory cells composed predominantly of lymphocytes and plasma cells. The histiocytes within the inflammatory infiltrate had vesicular nuclei and abundant eosinophilic cytoplasm (Figure 2A). Areas of emperipolesis were noted (Figure 2B). The large histiocytes stained positive for S-100 protein (Figure 2C) and negative for CD1a.
Course and Treatment
Laboratory studies revealed leukopenia. Prior to histopathologic results, empiric treatment was started with doxycycline 100 mg twice daily for 2 weeks. Once pathology confirmed the diagnosis of Rosai-Dorfman disease (RDD), computed tomography of the chest, abdomen, and pelvis was performed and within normal limits. Due to the lack of systemic involvement, we diagnosed the rare form of purely cutaneous Rosai-Dorfman disease (CRDD). In subsequent visits, treatment with oral prednisone (40 mg daily for 1 week followed by 20 mg daily for 1 week) and intralesional triamcinolone acetonide (5 areas on the right medial thigh were injected with 1.0 mL of 10 mg/mL) was attempted with mild improvement, though the patient declined surgical excision.
Comment
Rosai-Dorfman disease (also known as sinus histiocytosis with massive lymphadenopathy) is a non–Langerhans cell histiocytosis.1 There are 2 main forms of RDD: one form that affects the lymph nodes and in certain cases the extranodal organs, and the other is purely CRDD. Cutaneous RDD is extremely rare and the etiology is unknown, though a number of viral and immune causes have been postulated. Cutaneous RDD presents as solitary or numerous papules, nodules, and/or plaques. Treatment options include steroids, methotrexate, dapsone, thalidomide, and isotretinoin, with varying efficacy reported.1
Extranodal forms occur in 43% of RDD cases, with the skin being the most common site.1 Other extranodal sites include the soft tissue, upper and lower respiratory tract, bones, genitourinary tract, oral cavity, gastrointestinal tract, orbits, testes, and rarely central nervous system involvement.2
Approximately 10% of RDD patients exhibit skin lesions, and in 3% it is contained solely in the skin.3 Pure CRDD was first documented in 1978 by Thawerani et al4 who presented the case of a 48-year-old man with a solitary nodule on the shoulder.
Cutaneous RDD and RDD may be distinct clinical entities. Cutaneous RDD has a later age of onset than RDD (median age, 43.5 years vs 20.6 years) and a female predominance (2:1 vs 1.4:1). It most commonly affects Asian and white individuals while the majority of patients with RDD are of African descent with rare reports in Asians.1
The etiology of CRDD remains unknown with hypotheses of viral and immune causes such as human herpesvirus 6, Epstein-Barr virus, and parvovirus B19. The polyclonal nature of the cell infiltrate and the clinical progression of RDD suggest a reactive process rather than a neoplastic disorder.1 Rosai-Dorfman disease has been hypothesized to be closely related to autoimmune lymphoproliferative syndrome, an inherited disorder associated with defects in Fas-mediated apoptosis.5
Histologic findings in CRDD are similar to those in RDD, with a superficial and deep perivascular infiltrate of lymphocytes and plasma cells. A diffuse and nodular dermal infiltrate of foamy histiocytes exists in a background infiltrate of lymphocytes and plasma cells. Foamy histiocytes may be seen in dermal lymphatics, and lymphoid follicles with reactive germinal centers also may be present. Emperipolesis, the presence of intact inflammatory cells within histiocytes, is common in CRDD. Less often, histiocytes may contain plasma cells, neutrophils, and red blood cells. Mitoses and nuclear atypia are rare. Cutaneous RDD histiocytes stain positive for S-100 protein, CD4, factor XIIIa, and CD68, and negative for CD1a. Birbeck granules are absent on electronic microscopy of CRDD tissue, eliminating Langerhans cell histiocytosis.1,3,5
The clinical diagnosis of CRDD is hard to confirm in the absence of lymphadenopathy. The lesions in CRDD may be solitary or numerous, usually presenting as papules, nodules, and/or plaques. More rarely, the lesions may present as pustules, acneform lesions, mimickers of vasculitis and panniculitis, macular erythema, large annular lesions resembling granuloma annulare, or even a breast mass.1,3 One case report with involvement of deep subcutaneous fat presented with flank swelling beneath papules and nodules.6
The most common site of lesions in CRDD is the face, with the eyelids and malar regions frequently involved, followed by the back, chest, thighs, flanks, and shoulders.1,5 Rarely, CRDD may be associated with other disorders, including bilateral uveitis, antinuclear antibody–positive lupus erythematosus, rheumatoid arthritis, hypothyroidism, lymphoma, and human immunodeficiency virus.1
Numerous treatments have been attempted, yet the response often is poor. Because RDD is a benign and self-limiting disease, less aggressive therapeutic approaches should be used, if possible. Surgical excision of the lesions has been helpful in certain cases.6 Cryotherapy and local radiation, topical steroids, or laser treatment also have been found to improve the condition.1,7 For refractory cases, dapsone and thalidomide have been effective. Mixed results have been observed with isotretinoin and imatinib; some patients improved whereas others did not. Utikal et al8 described a patient with complete remission of CRDD after receiving imatinib therapy; however, a different study reported a patient with CRDD who was completely resistant to this treatment.9 One case presenting on the breast did not respond to topical steroids, acitretin, and thalidomide but later responded to methotrexate.10
Conclusion
Cutaneous RDD is an unusual clinical entity with varied lesions. Generally, CRDD follows a benign clinical course, with a possibility of spontaneous remission. Further studies are required to confidently classify the etiology and variance between both RDD and CRDD.
- Fang S, Chen AJ. Facial cutaneous Rosai-Dorfman disease: a case report and literature review [published online February 5, 2015]. Exp Ther Med. 2015;9:1389-1392.
- Chen A, Fernedez A, Janik M, et al. Cutaneous Rosai-Dorfman disease. J Am Acad Dermatol. 2012;66:AB48.
- James WD, Berger T, Elston D, et al. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Thawerani H, Sanchez RL, Rosai J, et al. The cutaneous manifestations of sinus histiocytosis with massive lymphadenopathy. Arch Dermatol. 1978;114:191-197.
- Bolognia JL, Jorizzo JL, Schaffer JV, et al, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Al Salamah SM, Abdullah M, Al Salamah RA, et al. Cutaneous Rosai-Dorfman disease presenting as a flank swelling. Int J Health Sci. 2014;8:434-438.
- Khan A, Musbahi E, Suchak R, et al. Cutaneous Rosai-Dorfman disease treated by surgical excision and a review of the literature. J Am Acad Dermatol. 2015;72:AB259.
- Utikal J, Ugurel S, Kurzen H, et al. Imatinib as a treatment option forsystemic non-Langerhans cell histiocytosis. Arch Dermatol. 2007;143:736-740.
- Gebhardt C, Averbeck M, Paasch V, et al. A case of cutaneous Rosai-Dorfman disease refractory to imatinib therapy. Arch Dermatol. 2009;145:571-574.
- Nadal M, Kervarrec T, Machet MC, et al. Cutaneous Rosai-Dorfman disease located on the breast: rapid effectiveness of methotrexate after failure of topical corticosteroids, acitretin and thalidomide. Acta Derm Venereol. 2015;95:758-759.
Case Report
A 31-year-old black woman presented with a slow-spreading pruritic rash on the right thigh of 1 year’s duration. She had previously seen a dermatologist and was prescribed triamcinolone acetonide cream 0.1% and mupirocin ointment 2% but declined a biopsy. Review of symptoms was negative for any constitutional symptoms. Family history included hypertension and eczema with a personal history of anxiety. Clinical examination revealed grouped flesh-colored to light pink papules and plaques within a hyperpigmented patch on the right medial thigh (Figure 1).

Histopathology
A punch biopsy was negative for fungal, bacterial, or acid-fast bacilli culture. Histopathologic evaluation demonstrated a dense dermal infiltrate of large histiocytes admixed with inflammatory cells composed predominantly of lymphocytes and plasma cells. The histiocytes within the inflammatory infiltrate had vesicular nuclei and abundant eosinophilic cytoplasm (Figure 2A). Areas of emperipolesis were noted (Figure 2B). The large histiocytes stained positive for S-100 protein (Figure 2C) and negative for CD1a.

Course and Treatment
Laboratory studies revealed leukopenia. Prior to histopathologic results, empiric treatment was started with doxycycline 100 mg twice daily for 2 weeks. Once pathology confirmed the diagnosis of Rosai-Dorfman disease (RDD), computed tomography of the chest, abdomen, and pelvis was performed and within normal limits. Due to the lack of systemic involvement, we diagnosed the rare form of purely cutaneous Rosai-Dorfman disease (CRDD). In subsequent visits, treatment with oral prednisone (40 mg daily for 1 week followed by 20 mg daily for 1 week) and intralesional triamcinolone acetonide (5 areas on the right medial thigh were injected with 1.0 mL of 10 mg/mL) was attempted with mild improvement, though the patient declined surgical excision.
Comment
Rosai-Dorfman disease (also known as sinus histiocytosis with massive lymphadenopathy) is a non–Langerhans cell histiocytosis.1 There are 2 main forms of RDD: one form that affects the lymph nodes and in certain cases the extranodal organs, and the other is purely CRDD. Cutaneous RDD is extremely rare and the etiology is unknown, though a number of viral and immune causes have been postulated. Cutaneous RDD presents as solitary or numerous papules, nodules, and/or plaques. Treatment options include steroids, methotrexate, dapsone, thalidomide, and isotretinoin, with varying efficacy reported.1
Extranodal forms occur in 43% of RDD cases, with the skin being the most common site.1 Other extranodal sites include the soft tissue, upper and lower respiratory tract, bones, genitourinary tract, oral cavity, gastrointestinal tract, orbits, testes, and rarely central nervous system involvement.2
Approximately 10% of RDD patients exhibit skin lesions, and in 3% it is contained solely in the skin.3 Pure CRDD was first documented in 1978 by Thawerani et al4 who presented the case of a 48-year-old man with a solitary nodule on the shoulder.
Cutaneous RDD and RDD may be distinct clinical entities. Cutaneous RDD has a later age of onset than RDD (median age, 43.5 years vs 20.6 years) and a female predominance (2:1 vs 1.4:1). It most commonly affects Asian and white individuals while the majority of patients with RDD are of African descent with rare reports in Asians.1
The etiology of CRDD remains unknown with hypotheses of viral and immune causes such as human herpesvirus 6, Epstein-Barr virus, and parvovirus B19. The polyclonal nature of the cell infiltrate and the clinical progression of RDD suggest a reactive process rather than a neoplastic disorder.1 Rosai-Dorfman disease has been hypothesized to be closely related to autoimmune lymphoproliferative syndrome, an inherited disorder associated with defects in Fas-mediated apoptosis.5
Histologic findings in CRDD are similar to those in RDD, with a superficial and deep perivascular infiltrate of lymphocytes and plasma cells. A diffuse and nodular dermal infiltrate of foamy histiocytes exists in a background infiltrate of lymphocytes and plasma cells. Foamy histiocytes may be seen in dermal lymphatics, and lymphoid follicles with reactive germinal centers also may be present. Emperipolesis, the presence of intact inflammatory cells within histiocytes, is common in CRDD. Less often, histiocytes may contain plasma cells, neutrophils, and red blood cells. Mitoses and nuclear atypia are rare. Cutaneous RDD histiocytes stain positive for S-100 protein, CD4, factor XIIIa, and CD68, and negative for CD1a. Birbeck granules are absent on electronic microscopy of CRDD tissue, eliminating Langerhans cell histiocytosis.1,3,5
The clinical diagnosis of CRDD is hard to confirm in the absence of lymphadenopathy. The lesions in CRDD may be solitary or numerous, usually presenting as papules, nodules, and/or plaques. More rarely, the lesions may present as pustules, acneform lesions, mimickers of vasculitis and panniculitis, macular erythema, large annular lesions resembling granuloma annulare, or even a breast mass.1,3 One case report with involvement of deep subcutaneous fat presented with flank swelling beneath papules and nodules.6
The most common site of lesions in CRDD is the face, with the eyelids and malar regions frequently involved, followed by the back, chest, thighs, flanks, and shoulders.1,5 Rarely, CRDD may be associated with other disorders, including bilateral uveitis, antinuclear antibody–positive lupus erythematosus, rheumatoid arthritis, hypothyroidism, lymphoma, and human immunodeficiency virus.1
Numerous treatments have been attempted, yet the response often is poor. Because RDD is a benign and self-limiting disease, less aggressive therapeutic approaches should be used, if possible. Surgical excision of the lesions has been helpful in certain cases.6 Cryotherapy and local radiation, topical steroids, or laser treatment also have been found to improve the condition.1,7 For refractory cases, dapsone and thalidomide have been effective. Mixed results have been observed with isotretinoin and imatinib; some patients improved whereas others did not. Utikal et al8 described a patient with complete remission of CRDD after receiving imatinib therapy; however, a different study reported a patient with CRDD who was completely resistant to this treatment.9 One case presenting on the breast did not respond to topical steroids, acitretin, and thalidomide but later responded to methotrexate.10
Conclusion
Cutaneous RDD is an unusual clinical entity with varied lesions. Generally, CRDD follows a benign clinical course, with a possibility of spontaneous remission. Further studies are required to confidently classify the etiology and variance between both RDD and CRDD.
Case Report
A 31-year-old black woman presented with a slow-spreading pruritic rash on the right thigh of 1 year’s duration. She had previously seen a dermatologist and was prescribed triamcinolone acetonide cream 0.1% and mupirocin ointment 2% but declined a biopsy. Review of symptoms was negative for any constitutional symptoms. Family history included hypertension and eczema with a personal history of anxiety. Clinical examination revealed grouped flesh-colored to light pink papules and plaques within a hyperpigmented patch on the right medial thigh (Figure 1).

Histopathology
A punch biopsy was negative for fungal, bacterial, or acid-fast bacilli culture. Histopathologic evaluation demonstrated a dense dermal infiltrate of large histiocytes admixed with inflammatory cells composed predominantly of lymphocytes and plasma cells. The histiocytes within the inflammatory infiltrate had vesicular nuclei and abundant eosinophilic cytoplasm (Figure 2A). Areas of emperipolesis were noted (Figure 2B). The large histiocytes stained positive for S-100 protein (Figure 2C) and negative for CD1a.

Course and Treatment
Laboratory studies revealed leukopenia. Prior to histopathologic results, empiric treatment was started with doxycycline 100 mg twice daily for 2 weeks. Once pathology confirmed the diagnosis of Rosai-Dorfman disease (RDD), computed tomography of the chest, abdomen, and pelvis was performed and within normal limits. Due to the lack of systemic involvement, we diagnosed the rare form of purely cutaneous Rosai-Dorfman disease (CRDD). In subsequent visits, treatment with oral prednisone (40 mg daily for 1 week followed by 20 mg daily for 1 week) and intralesional triamcinolone acetonide (5 areas on the right medial thigh were injected with 1.0 mL of 10 mg/mL) was attempted with mild improvement, though the patient declined surgical excision.
Comment
Rosai-Dorfman disease (also known as sinus histiocytosis with massive lymphadenopathy) is a non–Langerhans cell histiocytosis.1 There are 2 main forms of RDD: one form that affects the lymph nodes and in certain cases the extranodal organs, and the other is purely CRDD. Cutaneous RDD is extremely rare and the etiology is unknown, though a number of viral and immune causes have been postulated. Cutaneous RDD presents as solitary or numerous papules, nodules, and/or plaques. Treatment options include steroids, methotrexate, dapsone, thalidomide, and isotretinoin, with varying efficacy reported.1
Extranodal forms occur in 43% of RDD cases, with the skin being the most common site.1 Other extranodal sites include the soft tissue, upper and lower respiratory tract, bones, genitourinary tract, oral cavity, gastrointestinal tract, orbits, testes, and rarely central nervous system involvement.2
Approximately 10% of RDD patients exhibit skin lesions, and in 3% it is contained solely in the skin.3 Pure CRDD was first documented in 1978 by Thawerani et al4 who presented the case of a 48-year-old man with a solitary nodule on the shoulder.
Cutaneous RDD and RDD may be distinct clinical entities. Cutaneous RDD has a later age of onset than RDD (median age, 43.5 years vs 20.6 years) and a female predominance (2:1 vs 1.4:1). It most commonly affects Asian and white individuals while the majority of patients with RDD are of African descent with rare reports in Asians.1
The etiology of CRDD remains unknown with hypotheses of viral and immune causes such as human herpesvirus 6, Epstein-Barr virus, and parvovirus B19. The polyclonal nature of the cell infiltrate and the clinical progression of RDD suggest a reactive process rather than a neoplastic disorder.1 Rosai-Dorfman disease has been hypothesized to be closely related to autoimmune lymphoproliferative syndrome, an inherited disorder associated with defects in Fas-mediated apoptosis.5
Histologic findings in CRDD are similar to those in RDD, with a superficial and deep perivascular infiltrate of lymphocytes and plasma cells. A diffuse and nodular dermal infiltrate of foamy histiocytes exists in a background infiltrate of lymphocytes and plasma cells. Foamy histiocytes may be seen in dermal lymphatics, and lymphoid follicles with reactive germinal centers also may be present. Emperipolesis, the presence of intact inflammatory cells within histiocytes, is common in CRDD. Less often, histiocytes may contain plasma cells, neutrophils, and red blood cells. Mitoses and nuclear atypia are rare. Cutaneous RDD histiocytes stain positive for S-100 protein, CD4, factor XIIIa, and CD68, and negative for CD1a. Birbeck granules are absent on electronic microscopy of CRDD tissue, eliminating Langerhans cell histiocytosis.1,3,5
The clinical diagnosis of CRDD is hard to confirm in the absence of lymphadenopathy. The lesions in CRDD may be solitary or numerous, usually presenting as papules, nodules, and/or plaques. More rarely, the lesions may present as pustules, acneform lesions, mimickers of vasculitis and panniculitis, macular erythema, large annular lesions resembling granuloma annulare, or even a breast mass.1,3 One case report with involvement of deep subcutaneous fat presented with flank swelling beneath papules and nodules.6
The most common site of lesions in CRDD is the face, with the eyelids and malar regions frequently involved, followed by the back, chest, thighs, flanks, and shoulders.1,5 Rarely, CRDD may be associated with other disorders, including bilateral uveitis, antinuclear antibody–positive lupus erythematosus, rheumatoid arthritis, hypothyroidism, lymphoma, and human immunodeficiency virus.1
Numerous treatments have been attempted, yet the response often is poor. Because RDD is a benign and self-limiting disease, less aggressive therapeutic approaches should be used, if possible. Surgical excision of the lesions has been helpful in certain cases.6 Cryotherapy and local radiation, topical steroids, or laser treatment also have been found to improve the condition.1,7 For refractory cases, dapsone and thalidomide have been effective. Mixed results have been observed with isotretinoin and imatinib; some patients improved whereas others did not. Utikal et al8 described a patient with complete remission of CRDD after receiving imatinib therapy; however, a different study reported a patient with CRDD who was completely resistant to this treatment.9 One case presenting on the breast did not respond to topical steroids, acitretin, and thalidomide but later responded to methotrexate.10
Conclusion
Cutaneous RDD is an unusual clinical entity with varied lesions. Generally, CRDD follows a benign clinical course, with a possibility of spontaneous remission. Further studies are required to confidently classify the etiology and variance between both RDD and CRDD.
- Fang S, Chen AJ. Facial cutaneous Rosai-Dorfman disease: a case report and literature review [published online February 5, 2015]. Exp Ther Med. 2015;9:1389-1392.
- Chen A, Fernedez A, Janik M, et al. Cutaneous Rosai-Dorfman disease. J Am Acad Dermatol. 2012;66:AB48.
- James WD, Berger T, Elston D, et al. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Thawerani H, Sanchez RL, Rosai J, et al. The cutaneous manifestations of sinus histiocytosis with massive lymphadenopathy. Arch Dermatol. 1978;114:191-197.
- Bolognia JL, Jorizzo JL, Schaffer JV, et al, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Al Salamah SM, Abdullah M, Al Salamah RA, et al. Cutaneous Rosai-Dorfman disease presenting as a flank swelling. Int J Health Sci. 2014;8:434-438.
- Khan A, Musbahi E, Suchak R, et al. Cutaneous Rosai-Dorfman disease treated by surgical excision and a review of the literature. J Am Acad Dermatol. 2015;72:AB259.
- Utikal J, Ugurel S, Kurzen H, et al. Imatinib as a treatment option forsystemic non-Langerhans cell histiocytosis. Arch Dermatol. 2007;143:736-740.
- Gebhardt C, Averbeck M, Paasch V, et al. A case of cutaneous Rosai-Dorfman disease refractory to imatinib therapy. Arch Dermatol. 2009;145:571-574.
- Nadal M, Kervarrec T, Machet MC, et al. Cutaneous Rosai-Dorfman disease located on the breast: rapid effectiveness of methotrexate after failure of topical corticosteroids, acitretin and thalidomide. Acta Derm Venereol. 2015;95:758-759.
- Fang S, Chen AJ. Facial cutaneous Rosai-Dorfman disease: a case report and literature review [published online February 5, 2015]. Exp Ther Med. 2015;9:1389-1392.
- Chen A, Fernedez A, Janik M, et al. Cutaneous Rosai-Dorfman disease. J Am Acad Dermatol. 2012;66:AB48.
- James WD, Berger T, Elston D, et al. Andrews’ Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2015.
- Thawerani H, Sanchez RL, Rosai J, et al. The cutaneous manifestations of sinus histiocytosis with massive lymphadenopathy. Arch Dermatol. 1978;114:191-197.
- Bolognia JL, Jorizzo JL, Schaffer JV, et al, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
- Al Salamah SM, Abdullah M, Al Salamah RA, et al. Cutaneous Rosai-Dorfman disease presenting as a flank swelling. Int J Health Sci. 2014;8:434-438.
- Khan A, Musbahi E, Suchak R, et al. Cutaneous Rosai-Dorfman disease treated by surgical excision and a review of the literature. J Am Acad Dermatol. 2015;72:AB259.
- Utikal J, Ugurel S, Kurzen H, et al. Imatinib as a treatment option forsystemic non-Langerhans cell histiocytosis. Arch Dermatol. 2007;143:736-740.
- Gebhardt C, Averbeck M, Paasch V, et al. A case of cutaneous Rosai-Dorfman disease refractory to imatinib therapy. Arch Dermatol. 2009;145:571-574.
- Nadal M, Kervarrec T, Machet MC, et al. Cutaneous Rosai-Dorfman disease located on the breast: rapid effectiveness of methotrexate after failure of topical corticosteroids, acitretin and thalidomide. Acta Derm Venereol. 2015;95:758-759.
Practice Points
- Rosai-Dorfman disease generally is characterized by painless cervical lymphadenopathy and systemic involvement.
- Cutaneous Rosai-Dorfman disease can clinically present with great variety, mimicking many other dermatologic conditions.
- Patients presenting with cutaneous lesions and lymphadenopathy warrant workup with systemic imaging.
Vaginal and Scrotal Rejuvenation: The Potential Role of Dermatologists in Genital Rejuvenation
To the Editor:
I read with interest the informative Cutis article by Hashim et al,1 which not only summarized the key features of vaginal rejuvenation but also concisely reviewed noninvasive treatments, including lasers and radiofrequency devices, that can be used to address this important issue. The authors emphasized that these treatments may represent a valuable addition to the cosmetic landscape. In addition, they also asserted that noninvasive vaginal rejuvenation is an expanding focus of cosmetic dermatology.1
Genital rejuvenation includes rejuvenation of the vagina in women and the scrotum in men. Since the term initially appeared in the literature in 2007,2 a PubMed search of articles indexed for MEDLINE yields an increasing number of articles on vaginal rejuvenation. More recently, the concept of scrotal rejuvenation was introduced in 2018.3
Similar to vaginal rejuvenation, scrotal rejuvenation includes procedures to remedy medical or cosmetic conditions of the scrotum. Hashim et al1 focused on morphology-associated vaginal changes for which rejuvenation techniques have been successful, such as excess clitoral hood; excess labia majora or minora; vaginal laxity; and vulvovaginal atrophy, which is considered to be a component of genitourinary syndrome of menopause. Morphology-associated changes of the scrotum include wrinkling (cutis scrotum gyratum or scrotum rugosum) and laxity (low-hanging or sagging scrotum or scrotomegaly
Intrinsic (aging) and extrinsic (trauma) alterations also can result in other changes that may be amenable to vaginal or scrotal rejuvenation; for example, in addition to changes in morphology, there are hair (eg, alopecia, hypertrichosis) and vascular (eg, angiokeratomas) conditions of the vagina and scrotum that may be suitable for rejuvenation.3-5 Dermatologists have the opportunity to provide treatment for the genital rejuvenation of their patients.
- Hashim PW, Nia JK, Zade J, et al. Noninvasive vaginal rejuvenation. Cutis. 2018;102:243-246.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 378: vaginal “rejuvenation” and cosmetic vaginal procedures. Obstet Gynecol. 2007;110:737-738.
- Cohen PR. Scrotal rejuvenation. Cureus. 2018;10:e2316.
- Cohen PR. Genital rejuvenation: the next frontier in medical and cosmetic dermatology. Dermatol Online J. 2018:24. http://escholarship.org/uc/item/27v774t5. Accessed February 15, 2018.
- Cohen PR. A case report of scrotal rejuvenation: laser treatment of angiokeratomas of the scrotum [published online November 26, 2018]. Dermatol Ther (Heidelb). doi:10.1007/s13555-018-0272-z.
To the Editor:
I read with interest the informative Cutis article by Hashim et al,1 which not only summarized the key features of vaginal rejuvenation but also concisely reviewed noninvasive treatments, including lasers and radiofrequency devices, that can be used to address this important issue. The authors emphasized that these treatments may represent a valuable addition to the cosmetic landscape. In addition, they also asserted that noninvasive vaginal rejuvenation is an expanding focus of cosmetic dermatology.1
Genital rejuvenation includes rejuvenation of the vagina in women and the scrotum in men. Since the term initially appeared in the literature in 2007,2 a PubMed search of articles indexed for MEDLINE yields an increasing number of articles on vaginal rejuvenation. More recently, the concept of scrotal rejuvenation was introduced in 2018.3
Similar to vaginal rejuvenation, scrotal rejuvenation includes procedures to remedy medical or cosmetic conditions of the scrotum. Hashim et al1 focused on morphology-associated vaginal changes for which rejuvenation techniques have been successful, such as excess clitoral hood; excess labia majora or minora; vaginal laxity; and vulvovaginal atrophy, which is considered to be a component of genitourinary syndrome of menopause. Morphology-associated changes of the scrotum include wrinkling (cutis scrotum gyratum or scrotum rugosum) and laxity (low-hanging or sagging scrotum or scrotomegaly
Intrinsic (aging) and extrinsic (trauma) alterations also can result in other changes that may be amenable to vaginal or scrotal rejuvenation; for example, in addition to changes in morphology, there are hair (eg, alopecia, hypertrichosis) and vascular (eg, angiokeratomas) conditions of the vagina and scrotum that may be suitable for rejuvenation.3-5 Dermatologists have the opportunity to provide treatment for the genital rejuvenation of their patients.
To the Editor:
I read with interest the informative Cutis article by Hashim et al,1 which not only summarized the key features of vaginal rejuvenation but also concisely reviewed noninvasive treatments, including lasers and radiofrequency devices, that can be used to address this important issue. The authors emphasized that these treatments may represent a valuable addition to the cosmetic landscape. In addition, they also asserted that noninvasive vaginal rejuvenation is an expanding focus of cosmetic dermatology.1
Genital rejuvenation includes rejuvenation of the vagina in women and the scrotum in men. Since the term initially appeared in the literature in 2007,2 a PubMed search of articles indexed for MEDLINE yields an increasing number of articles on vaginal rejuvenation. More recently, the concept of scrotal rejuvenation was introduced in 2018.3
Similar to vaginal rejuvenation, scrotal rejuvenation includes procedures to remedy medical or cosmetic conditions of the scrotum. Hashim et al1 focused on morphology-associated vaginal changes for which rejuvenation techniques have been successful, such as excess clitoral hood; excess labia majora or minora; vaginal laxity; and vulvovaginal atrophy, which is considered to be a component of genitourinary syndrome of menopause. Morphology-associated changes of the scrotum include wrinkling (cutis scrotum gyratum or scrotum rugosum) and laxity (low-hanging or sagging scrotum or scrotomegaly
Intrinsic (aging) and extrinsic (trauma) alterations also can result in other changes that may be amenable to vaginal or scrotal rejuvenation; for example, in addition to changes in morphology, there are hair (eg, alopecia, hypertrichosis) and vascular (eg, angiokeratomas) conditions of the vagina and scrotum that may be suitable for rejuvenation.3-5 Dermatologists have the opportunity to provide treatment for the genital rejuvenation of their patients.
- Hashim PW, Nia JK, Zade J, et al. Noninvasive vaginal rejuvenation. Cutis. 2018;102:243-246.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 378: vaginal “rejuvenation” and cosmetic vaginal procedures. Obstet Gynecol. 2007;110:737-738.
- Cohen PR. Scrotal rejuvenation. Cureus. 2018;10:e2316.
- Cohen PR. Genital rejuvenation: the next frontier in medical and cosmetic dermatology. Dermatol Online J. 2018:24. http://escholarship.org/uc/item/27v774t5. Accessed February 15, 2018.
- Cohen PR. A case report of scrotal rejuvenation: laser treatment of angiokeratomas of the scrotum [published online November 26, 2018]. Dermatol Ther (Heidelb). doi:10.1007/s13555-018-0272-z.
- Hashim PW, Nia JK, Zade J, et al. Noninvasive vaginal rejuvenation. Cutis. 2018;102:243-246.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 378: vaginal “rejuvenation” and cosmetic vaginal procedures. Obstet Gynecol. 2007;110:737-738.
- Cohen PR. Scrotal rejuvenation. Cureus. 2018;10:e2316.
- Cohen PR. Genital rejuvenation: the next frontier in medical and cosmetic dermatology. Dermatol Online J. 2018:24. http://escholarship.org/uc/item/27v774t5. Accessed February 15, 2018.
- Cohen PR. A case report of scrotal rejuvenation: laser treatment of angiokeratomas of the scrotum [published online November 26, 2018]. Dermatol Ther (Heidelb). doi:10.1007/s13555-018-0272-z.
Aquatic Antagonists: Stingray Injury Update
Incidence and Characteristics


Stingrays are dorsoventrally flattened, diamond-shaped fish with light-colored ventral and dark-colored dorsal surfaces. They have strong pectoral wings that allow them to swim forward and backward and even launch off waves.3 Stingrays range in size from the palm of a human hand to 6.5 ft in width. They possess 1 or more spines (2.5 to >30 cm in length) that are disguised by much longer tails.6,7 They often are encountered accidentally because they bury themselves in the sand or mud of shallow coastal waters or rivers with only their eyes and tails exposed to fool prey and avoid predators.
Injury Clinical Presentation
Stingray injuries typically involve the lower legs, ankles, or feet after stepping on a stingray.8 Fishermen can present with injuries of the upper extremities after handling fish with their hands.9 Other rarer injuries occur when individuals are swimming alongside stingrays or when stingrays catapult off waves into moving boats.10,11 Stingrays impale victims by using their tails to direct a retroserrate barb composed of a strong cartilaginous material called vasodentin. The barb releases venom by breaking through the venom-containing integumentary sheath that encapsulates it. Stingray venom contains phosphodiesterase, serotonin, and 5′-nucleotidase. It causes severe pain, vasoconstriction, ischemia, and poor wound healing, along with systemic effects such as disorientation, syncope, seizures, salivation, nausea, vomiting, abdominal pain, diarrhea, muscle cramps or fasciculations, pruritus, allergic reaction, hypotension, cardiac arrhythmias, dyspnea, paralysis, and possibly death.1,8,12,13
Management
Pain Relief
As with many marine envenomations, immersion in hot but not scalding water can inactivate venom and reduce symptoms.8,9 In one retrospective review, 52 of 75 (69%) patients reporting to a California poison center with stingray injuries had improvement in pain within 1 hour of hot water immersion before any analgesics were instituted.8 In another review, 65 of 74 (88%) patients presenting to a California emergency department within 24 hours of sustaining a stingray injury had complete relief of pain within 30 minutes of hot water immersion. Patients who received analgesics in addition to hot water immersion did not require a second dose.9 In concordance with these studies, we suggest immersing areas affected by stingray injuries in hot water (temperature, 43.3°C to 46.1°C [110°F–115°F]; or as close to this range as tolerated) until pain subsides.8,9,14 Ice packs are an alternative to hot water immersion that may be more readily available to patients. If pain does not resolve following hot water immersion or application of an ice pack, additional analgesics and xylocaine without epinephrine may be helpful.9,15
Infection
One major complication of stingray injuries is infection.8,9 Many bacterial species reside in stingray mucus, the marine environment, or on human skin that may be introduced during a single injury. Marine envenomations can involve organisms such as Vibrio, Aeromonas, and Mycobacterium species, which often are resistant to antibiotic prophylaxis covering common causes of soft-tissue infection such as Staphylococcus and Streptococcus species.8,9,16,17 Additionally, physicians should cover for Clostridium species and ensure patients are up-to-date on vaccinations because severe cases of tetanus following stingray injuries have been reported.18 Lastly, fungal infections including fusariosis have been reported following stingray injuries and should be considered if a patient develops an infection.19
Several authors support the use of prophylactic broad-spectrum antibiotics in all but mild stingray injuries.8,9,20,21 Although no standardized definition exists, mild injuries generally represent patients with superficial lacerations or less, while deeper lacerations and puncture wounds require prophylaxis. Several authors agree on the use of fluoroquinolone antibiotics (eg, ciprofloxacin 500 mg twice daily) for 5 to 7 days following severe stingray injuries.1,9,13,22 Other proposed antibiotic regimens include trimethoprim-sulfamethoxazole (160/800 mg twice daily) or tetracycline (500 mg 4 times daily) for 7 days.13 Failure of ciprofloxacin therapy after 7 days has been reported, with resolution of infection after treatment with an intravenous cephalosporin for 7 days.20 Failure of trimethoprim-sulfamethoxazole therapy also has been reported, with one case requiring levofloxacin for a much longer course.21 Clinical follow-up remains essential after prescribing prophylactic antibiotics, as resistance is common.
Foreign Bodies
Stingray injuries also are often complicated by foreign bodies or retained spines.3,8 Although these complications are less severe than infection, all wounds should be explored for material under local anesthesia. Furthermore, there has been support for thorough debridement of necrotic tissue with referral to a hand specialist for deeper injuries to the hands as well as referral to a foot and ankle specialist for deeper injuries of the lower extremities.23,24 More serious injuries with penetration of vital structures, such as through the chest or abdomen, require immediate exploration in an operating room.1,24
Imaging
Routine imaging of stingray injuries remains controversial. In a case series of 119 patients presenting to a California emergency department with stingray injuries, Clark et al9 found that radiographs were not helpful. This finding likely is due in part to an inability to detect hypodense material such as integumentary or glandular tissue via radiography.3 However, radiographs have been used to identify retained stingray barbs in select cases in which retained barbs are suspected.2,25 Lastly, ultrasonography potentially may offer a better first choice when a barb is not readily apparent; magnetic resonance imaging may be indicated for more involved areas and for further visualization of suspected hypodense material, though at a higher expense.2,9
Biopsy
Biopsies of stingray injuries are rarely performed, and the findings are not well characterized. One case biopsied 2 months after injury showed a large zone of paucicellular necrosis with superficial ulceration and granulomatous inflammation. The stingray venom was most likely responsible for the pattern of necrosis noted in the biopsy.21
Avoidance and Prevention
Patients traveling to areas of the world inhabited by stingrays should receive counseling on how to avoid injury. Prior to entry, individuals can throw stones or use a long stick to clear their walking or swimming areas of venomous fish.26 Polarized sunglasses may help spot stingrays in shallow water. Furthermore, wading through water with a shuffling gait can help individuals avoid stepping directly on a stingray and also warns stingrays that someone is in the area. Individuals who spend more time in coastal waters or river systems inhabited by stingrays may invest in protective stingray gear such as leg guards or specialized wading boots.26 Lastly, fishermen should be advised to avoid handling stingrays with their hands and instead cut their fishing line to release the fish.
- Aurbach PS. Envenomations by aquatic vertebrates. In: Auerbach PS. Wilderness Medicine. 5th ed. St. Louis, MO: Mosby; 2007:1730-1749.
- Robins CR, Ray GC. A Field Guide to Atlantic Coast Fishes. New York, NY: Houghton Mifflin Company; 1986.
- Diaz JH. The evaluation, management, and prevention of stingray injuries in travelers. J Travel Med. 2008;15:102-109.
- Haddad V Jr, Neto DG, de Paula Neto JB, et al. Freshwater stingrays: study of epidemiologic, clinical and therapeutic aspects based on 84 envenomings in humans and some enzymatic activities of the venom. Toxicon. 2004;43:287-294.
- Marinkelle CJ. Accidents by venomous animals in Colombia. Ind Med Surg. 1966;35:988-992.
- Last PR, White WT, Caire JN, et al. Sharks and Rays of Borneo. Collingwood VIC, Australia: CSIRO Publishing; 2010.
- Mebs D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists. Boca Raton, FL: CRC Press; 2002.
- Clark AT, Clark RF, Cantrell FL. A retrospective review of the presentation and treatment of stingray stings reported to a poison control system. Am J Ther. 2017;24:E177-E180.
- Clark RF, Girard RH, Rao D, et al. Stingray envenomation: a retrospective review of clinical presentation and treatment in 119 cases. J Emerg Med. 2007;33:33-37.
- Mahjoubi L, Joyeux A, Delambre JF, et al. Near-death thoracic trauma caused by a stingray in the Indian Ocean. Semin Thorac Cardiovasc Surg. 2017;29:262-263.
- Parra MW, Constantini EN, Rodas EB. Surviving a transfixing cardiac injury caused by a stingray barb. J Thorac Cardiovasc Surg. 2010;139:E115-E116.
- Dos Santos JC, Grund LZ, Seibert CS, et al. Stingray venom activates IL-33 producing cardiomyocytes, but not mast cell, to promote acute neutrophil-mediated injury. Sci Rep. 2017;7:7912.
- Auerbach PS, Norris RL. Marine envenomation. In: Longo DL, Kasper SL, Jameson JL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:144-148.
- Cook MD, Matteucci MJ, Lall R, et al. Stingray envenomation. J Emerg Med. 2006;30:345-347.
- Bowers RC, Mustain MV. Disorders due to physical & environmental agents. In: Humphries RL, Stone C, eds. CURRENT Diagnosis & Treatment Emergency Medicine. 7th ed. New York, NY: McGraw-Hill; 2011:835-861.
- Domingos MO, Franzolin MR, dos Anjos MT, et al. The influence of environmental bacteria in freshwater stingray wound-healing. Toxicon. 2011;58:147-153.
- Auerbach PS, Yajko DM, Nassos PS, et al. Bacteriology of the marine environment: implications for clinical therapy. Ann Emerg Med. 1987;16:643-649.
- Torrez PP, Quiroga MM, Said R, et al. Tetanus after envenomations caused by freshwater stingrays. Toxicon. 2015;97:32-35.
- Hiemenz JW, Kennedy B, Kwon-Chung KJ. Invasive fusariosis associated with an injury by a stingray barb. J Med Vet Mycol. 1990;28:209-213.
- da Silva NJ Jr, Ferreira KR, Pinto RN, et al. A severe accident caused by an ocellate river stingray (Potamotrygon motoro) in central Brazil: how well do we really understand stingray venom chemistry, envenomation, and therapeutics? Toxins (Basel). 2015;7:2272-2288.
- Tartar D, Limova M, North J. Clinical and histopathologic findings in cutaneous sting ray wounds: a case report. Dermatol Online J. 2013;19:19261.
- Jarvis HC, Matheny LM, Clanton TO. Stingray injury to the webspace of the foot. Orthopedics. 2012;35:E762-E765.
- Trickett R, Whitaker IS, Boyce DE. Sting-ray injuries to the hand: case report, literature review and a suggested algorithm for management. J Plast Reconstruct Aesthet Surg. 2009;62:E270-E273.
- Fernandez I, Valladolid G, Varon J, et al. Encounters with venomous sea-life. J Emerg Med. 2011;40:103-112.
- O’Malley GF, O’Malley RN, Pham O, et al. Retained stingray barb and the importance of imaging. Wilderness Environ Med. 2015;26:375-379.
- How to protect yourself from stingrays. Howcast website. https://www.howcast.com/videos/228034-how-to-protect-yourself-from-stingrays/. Accessed July 12, 2018.
Incidence and Characteristics


Stingrays are dorsoventrally flattened, diamond-shaped fish with light-colored ventral and dark-colored dorsal surfaces. They have strong pectoral wings that allow them to swim forward and backward and even launch off waves.3 Stingrays range in size from the palm of a human hand to 6.5 ft in width. They possess 1 or more spines (2.5 to >30 cm in length) that are disguised by much longer tails.6,7 They often are encountered accidentally because they bury themselves in the sand or mud of shallow coastal waters or rivers with only their eyes and tails exposed to fool prey and avoid predators.
Injury Clinical Presentation
Stingray injuries typically involve the lower legs, ankles, or feet after stepping on a stingray.8 Fishermen can present with injuries of the upper extremities after handling fish with their hands.9 Other rarer injuries occur when individuals are swimming alongside stingrays or when stingrays catapult off waves into moving boats.10,11 Stingrays impale victims by using their tails to direct a retroserrate barb composed of a strong cartilaginous material called vasodentin. The barb releases venom by breaking through the venom-containing integumentary sheath that encapsulates it. Stingray venom contains phosphodiesterase, serotonin, and 5′-nucleotidase. It causes severe pain, vasoconstriction, ischemia, and poor wound healing, along with systemic effects such as disorientation, syncope, seizures, salivation, nausea, vomiting, abdominal pain, diarrhea, muscle cramps or fasciculations, pruritus, allergic reaction, hypotension, cardiac arrhythmias, dyspnea, paralysis, and possibly death.1,8,12,13
Management
Pain Relief
As with many marine envenomations, immersion in hot but not scalding water can inactivate venom and reduce symptoms.8,9 In one retrospective review, 52 of 75 (69%) patients reporting to a California poison center with stingray injuries had improvement in pain within 1 hour of hot water immersion before any analgesics were instituted.8 In another review, 65 of 74 (88%) patients presenting to a California emergency department within 24 hours of sustaining a stingray injury had complete relief of pain within 30 minutes of hot water immersion. Patients who received analgesics in addition to hot water immersion did not require a second dose.9 In concordance with these studies, we suggest immersing areas affected by stingray injuries in hot water (temperature, 43.3°C to 46.1°C [110°F–115°F]; or as close to this range as tolerated) until pain subsides.8,9,14 Ice packs are an alternative to hot water immersion that may be more readily available to patients. If pain does not resolve following hot water immersion or application of an ice pack, additional analgesics and xylocaine without epinephrine may be helpful.9,15
Infection
One major complication of stingray injuries is infection.8,9 Many bacterial species reside in stingray mucus, the marine environment, or on human skin that may be introduced during a single injury. Marine envenomations can involve organisms such as Vibrio, Aeromonas, and Mycobacterium species, which often are resistant to antibiotic prophylaxis covering common causes of soft-tissue infection such as Staphylococcus and Streptococcus species.8,9,16,17 Additionally, physicians should cover for Clostridium species and ensure patients are up-to-date on vaccinations because severe cases of tetanus following stingray injuries have been reported.18 Lastly, fungal infections including fusariosis have been reported following stingray injuries and should be considered if a patient develops an infection.19
Several authors support the use of prophylactic broad-spectrum antibiotics in all but mild stingray injuries.8,9,20,21 Although no standardized definition exists, mild injuries generally represent patients with superficial lacerations or less, while deeper lacerations and puncture wounds require prophylaxis. Several authors agree on the use of fluoroquinolone antibiotics (eg, ciprofloxacin 500 mg twice daily) for 5 to 7 days following severe stingray injuries.1,9,13,22 Other proposed antibiotic regimens include trimethoprim-sulfamethoxazole (160/800 mg twice daily) or tetracycline (500 mg 4 times daily) for 7 days.13 Failure of ciprofloxacin therapy after 7 days has been reported, with resolution of infection after treatment with an intravenous cephalosporin for 7 days.20 Failure of trimethoprim-sulfamethoxazole therapy also has been reported, with one case requiring levofloxacin for a much longer course.21 Clinical follow-up remains essential after prescribing prophylactic antibiotics, as resistance is common.
Foreign Bodies
Stingray injuries also are often complicated by foreign bodies or retained spines.3,8 Although these complications are less severe than infection, all wounds should be explored for material under local anesthesia. Furthermore, there has been support for thorough debridement of necrotic tissue with referral to a hand specialist for deeper injuries to the hands as well as referral to a foot and ankle specialist for deeper injuries of the lower extremities.23,24 More serious injuries with penetration of vital structures, such as through the chest or abdomen, require immediate exploration in an operating room.1,24
Imaging
Routine imaging of stingray injuries remains controversial. In a case series of 119 patients presenting to a California emergency department with stingray injuries, Clark et al9 found that radiographs were not helpful. This finding likely is due in part to an inability to detect hypodense material such as integumentary or glandular tissue via radiography.3 However, radiographs have been used to identify retained stingray barbs in select cases in which retained barbs are suspected.2,25 Lastly, ultrasonography potentially may offer a better first choice when a barb is not readily apparent; magnetic resonance imaging may be indicated for more involved areas and for further visualization of suspected hypodense material, though at a higher expense.2,9
Biopsy
Biopsies of stingray injuries are rarely performed, and the findings are not well characterized. One case biopsied 2 months after injury showed a large zone of paucicellular necrosis with superficial ulceration and granulomatous inflammation. The stingray venom was most likely responsible for the pattern of necrosis noted in the biopsy.21
Avoidance and Prevention
Patients traveling to areas of the world inhabited by stingrays should receive counseling on how to avoid injury. Prior to entry, individuals can throw stones or use a long stick to clear their walking or swimming areas of venomous fish.26 Polarized sunglasses may help spot stingrays in shallow water. Furthermore, wading through water with a shuffling gait can help individuals avoid stepping directly on a stingray and also warns stingrays that someone is in the area. Individuals who spend more time in coastal waters or river systems inhabited by stingrays may invest in protective stingray gear such as leg guards or specialized wading boots.26 Lastly, fishermen should be advised to avoid handling stingrays with their hands and instead cut their fishing line to release the fish.
Incidence and Characteristics


Stingrays are dorsoventrally flattened, diamond-shaped fish with light-colored ventral and dark-colored dorsal surfaces. They have strong pectoral wings that allow them to swim forward and backward and even launch off waves.3 Stingrays range in size from the palm of a human hand to 6.5 ft in width. They possess 1 or more spines (2.5 to >30 cm in length) that are disguised by much longer tails.6,7 They often are encountered accidentally because they bury themselves in the sand or mud of shallow coastal waters or rivers with only their eyes and tails exposed to fool prey and avoid predators.
Injury Clinical Presentation
Stingray injuries typically involve the lower legs, ankles, or feet after stepping on a stingray.8 Fishermen can present with injuries of the upper extremities after handling fish with their hands.9 Other rarer injuries occur when individuals are swimming alongside stingrays or when stingrays catapult off waves into moving boats.10,11 Stingrays impale victims by using their tails to direct a retroserrate barb composed of a strong cartilaginous material called vasodentin. The barb releases venom by breaking through the venom-containing integumentary sheath that encapsulates it. Stingray venom contains phosphodiesterase, serotonin, and 5′-nucleotidase. It causes severe pain, vasoconstriction, ischemia, and poor wound healing, along with systemic effects such as disorientation, syncope, seizures, salivation, nausea, vomiting, abdominal pain, diarrhea, muscle cramps or fasciculations, pruritus, allergic reaction, hypotension, cardiac arrhythmias, dyspnea, paralysis, and possibly death.1,8,12,13
Management
Pain Relief
As with many marine envenomations, immersion in hot but not scalding water can inactivate venom and reduce symptoms.8,9 In one retrospective review, 52 of 75 (69%) patients reporting to a California poison center with stingray injuries had improvement in pain within 1 hour of hot water immersion before any analgesics were instituted.8 In another review, 65 of 74 (88%) patients presenting to a California emergency department within 24 hours of sustaining a stingray injury had complete relief of pain within 30 minutes of hot water immersion. Patients who received analgesics in addition to hot water immersion did not require a second dose.9 In concordance with these studies, we suggest immersing areas affected by stingray injuries in hot water (temperature, 43.3°C to 46.1°C [110°F–115°F]; or as close to this range as tolerated) until pain subsides.8,9,14 Ice packs are an alternative to hot water immersion that may be more readily available to patients. If pain does not resolve following hot water immersion or application of an ice pack, additional analgesics and xylocaine without epinephrine may be helpful.9,15
Infection
One major complication of stingray injuries is infection.8,9 Many bacterial species reside in stingray mucus, the marine environment, or on human skin that may be introduced during a single injury. Marine envenomations can involve organisms such as Vibrio, Aeromonas, and Mycobacterium species, which often are resistant to antibiotic prophylaxis covering common causes of soft-tissue infection such as Staphylococcus and Streptococcus species.8,9,16,17 Additionally, physicians should cover for Clostridium species and ensure patients are up-to-date on vaccinations because severe cases of tetanus following stingray injuries have been reported.18 Lastly, fungal infections including fusariosis have been reported following stingray injuries and should be considered if a patient develops an infection.19
Several authors support the use of prophylactic broad-spectrum antibiotics in all but mild stingray injuries.8,9,20,21 Although no standardized definition exists, mild injuries generally represent patients with superficial lacerations or less, while deeper lacerations and puncture wounds require prophylaxis. Several authors agree on the use of fluoroquinolone antibiotics (eg, ciprofloxacin 500 mg twice daily) for 5 to 7 days following severe stingray injuries.1,9,13,22 Other proposed antibiotic regimens include trimethoprim-sulfamethoxazole (160/800 mg twice daily) or tetracycline (500 mg 4 times daily) for 7 days.13 Failure of ciprofloxacin therapy after 7 days has been reported, with resolution of infection after treatment with an intravenous cephalosporin for 7 days.20 Failure of trimethoprim-sulfamethoxazole therapy also has been reported, with one case requiring levofloxacin for a much longer course.21 Clinical follow-up remains essential after prescribing prophylactic antibiotics, as resistance is common.
Foreign Bodies
Stingray injuries also are often complicated by foreign bodies or retained spines.3,8 Although these complications are less severe than infection, all wounds should be explored for material under local anesthesia. Furthermore, there has been support for thorough debridement of necrotic tissue with referral to a hand specialist for deeper injuries to the hands as well as referral to a foot and ankle specialist for deeper injuries of the lower extremities.23,24 More serious injuries with penetration of vital structures, such as through the chest or abdomen, require immediate exploration in an operating room.1,24
Imaging
Routine imaging of stingray injuries remains controversial. In a case series of 119 patients presenting to a California emergency department with stingray injuries, Clark et al9 found that radiographs were not helpful. This finding likely is due in part to an inability to detect hypodense material such as integumentary or glandular tissue via radiography.3 However, radiographs have been used to identify retained stingray barbs in select cases in which retained barbs are suspected.2,25 Lastly, ultrasonography potentially may offer a better first choice when a barb is not readily apparent; magnetic resonance imaging may be indicated for more involved areas and for further visualization of suspected hypodense material, though at a higher expense.2,9
Biopsy
Biopsies of stingray injuries are rarely performed, and the findings are not well characterized. One case biopsied 2 months after injury showed a large zone of paucicellular necrosis with superficial ulceration and granulomatous inflammation. The stingray venom was most likely responsible for the pattern of necrosis noted in the biopsy.21
Avoidance and Prevention
Patients traveling to areas of the world inhabited by stingrays should receive counseling on how to avoid injury. Prior to entry, individuals can throw stones or use a long stick to clear their walking or swimming areas of venomous fish.26 Polarized sunglasses may help spot stingrays in shallow water. Furthermore, wading through water with a shuffling gait can help individuals avoid stepping directly on a stingray and also warns stingrays that someone is in the area. Individuals who spend more time in coastal waters or river systems inhabited by stingrays may invest in protective stingray gear such as leg guards or specialized wading boots.26 Lastly, fishermen should be advised to avoid handling stingrays with their hands and instead cut their fishing line to release the fish.
- Aurbach PS. Envenomations by aquatic vertebrates. In: Auerbach PS. Wilderness Medicine. 5th ed. St. Louis, MO: Mosby; 2007:1730-1749.
- Robins CR, Ray GC. A Field Guide to Atlantic Coast Fishes. New York, NY: Houghton Mifflin Company; 1986.
- Diaz JH. The evaluation, management, and prevention of stingray injuries in travelers. J Travel Med. 2008;15:102-109.
- Haddad V Jr, Neto DG, de Paula Neto JB, et al. Freshwater stingrays: study of epidemiologic, clinical and therapeutic aspects based on 84 envenomings in humans and some enzymatic activities of the venom. Toxicon. 2004;43:287-294.
- Marinkelle CJ. Accidents by venomous animals in Colombia. Ind Med Surg. 1966;35:988-992.
- Last PR, White WT, Caire JN, et al. Sharks and Rays of Borneo. Collingwood VIC, Australia: CSIRO Publishing; 2010.
- Mebs D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists. Boca Raton, FL: CRC Press; 2002.
- Clark AT, Clark RF, Cantrell FL. A retrospective review of the presentation and treatment of stingray stings reported to a poison control system. Am J Ther. 2017;24:E177-E180.
- Clark RF, Girard RH, Rao D, et al. Stingray envenomation: a retrospective review of clinical presentation and treatment in 119 cases. J Emerg Med. 2007;33:33-37.
- Mahjoubi L, Joyeux A, Delambre JF, et al. Near-death thoracic trauma caused by a stingray in the Indian Ocean. Semin Thorac Cardiovasc Surg. 2017;29:262-263.
- Parra MW, Constantini EN, Rodas EB. Surviving a transfixing cardiac injury caused by a stingray barb. J Thorac Cardiovasc Surg. 2010;139:E115-E116.
- Dos Santos JC, Grund LZ, Seibert CS, et al. Stingray venom activates IL-33 producing cardiomyocytes, but not mast cell, to promote acute neutrophil-mediated injury. Sci Rep. 2017;7:7912.
- Auerbach PS, Norris RL. Marine envenomation. In: Longo DL, Kasper SL, Jameson JL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:144-148.
- Cook MD, Matteucci MJ, Lall R, et al. Stingray envenomation. J Emerg Med. 2006;30:345-347.
- Bowers RC, Mustain MV. Disorders due to physical & environmental agents. In: Humphries RL, Stone C, eds. CURRENT Diagnosis & Treatment Emergency Medicine. 7th ed. New York, NY: McGraw-Hill; 2011:835-861.
- Domingos MO, Franzolin MR, dos Anjos MT, et al. The influence of environmental bacteria in freshwater stingray wound-healing. Toxicon. 2011;58:147-153.
- Auerbach PS, Yajko DM, Nassos PS, et al. Bacteriology of the marine environment: implications for clinical therapy. Ann Emerg Med. 1987;16:643-649.
- Torrez PP, Quiroga MM, Said R, et al. Tetanus after envenomations caused by freshwater stingrays. Toxicon. 2015;97:32-35.
- Hiemenz JW, Kennedy B, Kwon-Chung KJ. Invasive fusariosis associated with an injury by a stingray barb. J Med Vet Mycol. 1990;28:209-213.
- da Silva NJ Jr, Ferreira KR, Pinto RN, et al. A severe accident caused by an ocellate river stingray (Potamotrygon motoro) in central Brazil: how well do we really understand stingray venom chemistry, envenomation, and therapeutics? Toxins (Basel). 2015;7:2272-2288.
- Tartar D, Limova M, North J. Clinical and histopathologic findings in cutaneous sting ray wounds: a case report. Dermatol Online J. 2013;19:19261.
- Jarvis HC, Matheny LM, Clanton TO. Stingray injury to the webspace of the foot. Orthopedics. 2012;35:E762-E765.
- Trickett R, Whitaker IS, Boyce DE. Sting-ray injuries to the hand: case report, literature review and a suggested algorithm for management. J Plast Reconstruct Aesthet Surg. 2009;62:E270-E273.
- Fernandez I, Valladolid G, Varon J, et al. Encounters with venomous sea-life. J Emerg Med. 2011;40:103-112.
- O’Malley GF, O’Malley RN, Pham O, et al. Retained stingray barb and the importance of imaging. Wilderness Environ Med. 2015;26:375-379.
- How to protect yourself from stingrays. Howcast website. https://www.howcast.com/videos/228034-how-to-protect-yourself-from-stingrays/. Accessed July 12, 2018.
- Aurbach PS. Envenomations by aquatic vertebrates. In: Auerbach PS. Wilderness Medicine. 5th ed. St. Louis, MO: Mosby; 2007:1730-1749.
- Robins CR, Ray GC. A Field Guide to Atlantic Coast Fishes. New York, NY: Houghton Mifflin Company; 1986.
- Diaz JH. The evaluation, management, and prevention of stingray injuries in travelers. J Travel Med. 2008;15:102-109.
- Haddad V Jr, Neto DG, de Paula Neto JB, et al. Freshwater stingrays: study of epidemiologic, clinical and therapeutic aspects based on 84 envenomings in humans and some enzymatic activities of the venom. Toxicon. 2004;43:287-294.
- Marinkelle CJ. Accidents by venomous animals in Colombia. Ind Med Surg. 1966;35:988-992.
- Last PR, White WT, Caire JN, et al. Sharks and Rays of Borneo. Collingwood VIC, Australia: CSIRO Publishing; 2010.
- Mebs D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists. Boca Raton, FL: CRC Press; 2002.
- Clark AT, Clark RF, Cantrell FL. A retrospective review of the presentation and treatment of stingray stings reported to a poison control system. Am J Ther. 2017;24:E177-E180.
- Clark RF, Girard RH, Rao D, et al. Stingray envenomation: a retrospective review of clinical presentation and treatment in 119 cases. J Emerg Med. 2007;33:33-37.
- Mahjoubi L, Joyeux A, Delambre JF, et al. Near-death thoracic trauma caused by a stingray in the Indian Ocean. Semin Thorac Cardiovasc Surg. 2017;29:262-263.
- Parra MW, Constantini EN, Rodas EB. Surviving a transfixing cardiac injury caused by a stingray barb. J Thorac Cardiovasc Surg. 2010;139:E115-E116.
- Dos Santos JC, Grund LZ, Seibert CS, et al. Stingray venom activates IL-33 producing cardiomyocytes, but not mast cell, to promote acute neutrophil-mediated injury. Sci Rep. 2017;7:7912.
- Auerbach PS, Norris RL. Marine envenomation. In: Longo DL, Kasper SL, Jameson JL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:144-148.
- Cook MD, Matteucci MJ, Lall R, et al. Stingray envenomation. J Emerg Med. 2006;30:345-347.
- Bowers RC, Mustain MV. Disorders due to physical & environmental agents. In: Humphries RL, Stone C, eds. CURRENT Diagnosis & Treatment Emergency Medicine. 7th ed. New York, NY: McGraw-Hill; 2011:835-861.
- Domingos MO, Franzolin MR, dos Anjos MT, et al. The influence of environmental bacteria in freshwater stingray wound-healing. Toxicon. 2011;58:147-153.
- Auerbach PS, Yajko DM, Nassos PS, et al. Bacteriology of the marine environment: implications for clinical therapy. Ann Emerg Med. 1987;16:643-649.
- Torrez PP, Quiroga MM, Said R, et al. Tetanus after envenomations caused by freshwater stingrays. Toxicon. 2015;97:32-35.
- Hiemenz JW, Kennedy B, Kwon-Chung KJ. Invasive fusariosis associated with an injury by a stingray barb. J Med Vet Mycol. 1990;28:209-213.
- da Silva NJ Jr, Ferreira KR, Pinto RN, et al. A severe accident caused by an ocellate river stingray (Potamotrygon motoro) in central Brazil: how well do we really understand stingray venom chemistry, envenomation, and therapeutics? Toxins (Basel). 2015;7:2272-2288.
- Tartar D, Limova M, North J. Clinical and histopathologic findings in cutaneous sting ray wounds: a case report. Dermatol Online J. 2013;19:19261.
- Jarvis HC, Matheny LM, Clanton TO. Stingray injury to the webspace of the foot. Orthopedics. 2012;35:E762-E765.
- Trickett R, Whitaker IS, Boyce DE. Sting-ray injuries to the hand: case report, literature review and a suggested algorithm for management. J Plast Reconstruct Aesthet Surg. 2009;62:E270-E273.
- Fernandez I, Valladolid G, Varon J, et al. Encounters with venomous sea-life. J Emerg Med. 2011;40:103-112.
- O’Malley GF, O’Malley RN, Pham O, et al. Retained stingray barb and the importance of imaging. Wilderness Environ Med. 2015;26:375-379.
- How to protect yourself from stingrays. Howcast website. https://www.howcast.com/videos/228034-how-to-protect-yourself-from-stingrays/. Accessed July 12, 2018.
Practice Points
- Acute pain associated with stingray injuries can be treated with hot water immersion.
- Stingray injuries are prone to secondary infection and poor wound healing.
Eccrine Porocarcinoma Presenting as a Recurrent Wart
Eccrine porocarcinoma (EPC), originally described by Pinkus and Mehregan1 in 1963, is an exceedingly rare sweat gland tumor most commonly seen in older patients. Fewer than 300 cases have been reported in the literature, and it is believed to represent only 0.005% to 0.01% of cutaneous malignancies.2 In the absence of established guidelines, wide local excision (WLE) has traditionally been considered the standard of treatment; however, local recurrence and nodal metastasis rates associated with WLE have been reported as high as 20%.3 More recently, a number of case reports and small case series have demonstrated higher cure rates with Mohs micrographic surgery (MMS), though follow-up is limited.3-5 We describe a case of EPC presenting as a recurrent wart in a 36-year-old man that was successfully treated with MMS.
Case Report
A 36-year-old man with no notable medical history presented with a 0.5×0.5-cm, asymptomatic, flesh-colored, hyperkeratotic, polypoid papule on the right medial thigh (Figure 1). The lesion was diagnosed as a wart and treated with cryotherapy by another dermatologist several years prior to presentation. Dermatoscopic examination at the current presentation showed a homogenous yellow center with a few peripheral vessels and a faint pink-tan halo (Figure 2). Our differential diagnosis included a recurrent wart, fibrosed pyogenic granuloma, irritated intradermal nevus, skin tag, and adnexal neoplasm. A shave biopsy was performed. Histopathologic analysis revealed multiple aggregations of mildly pleomorphic epithelial cells emanating from the epidermis, with many aggregations containing ductal structures (Figure 3). Rare necrotic and pyknotic cells were present, but no mitotic figures or lymphovascular invasion were identified. Immunohistochemical staining was positive for carcinoembryonic antigen and epithelial membrane antigen but negative for Ber-EP4. These findings were consistent with a well-differentiated EPC.
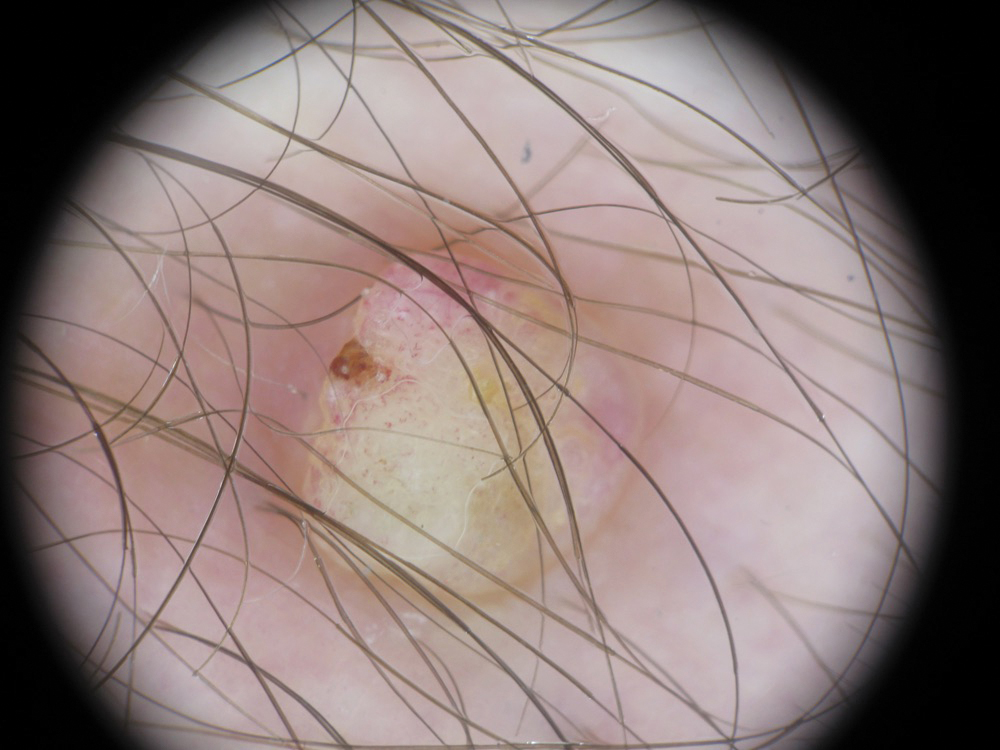
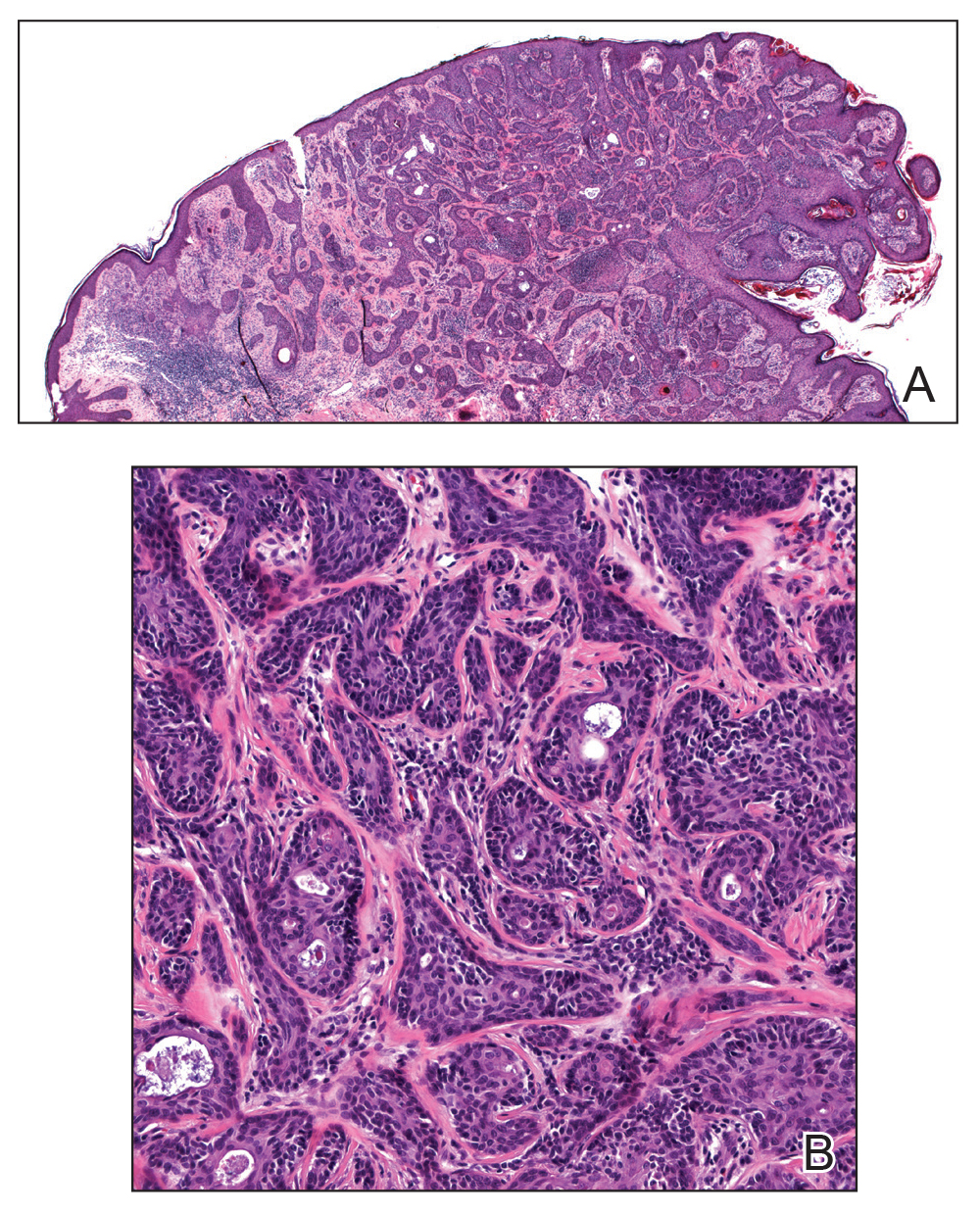
The patient was offered MMS or WLE, with or without sentinel lymph node biopsy (SLNB). He opted for MMS. The initial 1-cm margin taken during MMS was sufficient to achieve complete tumor extirpation, and the final 3.7×2.5-cm defect was closed primarily. The MMS debulking specimen was sent for permanent sectioning and showed a small focus of residual tumor cells, but no mitoses or lymphovascular invasion were seen. The patient was referred to surgical oncology to discuss the option of SLNB, which he ultimately declined. He also was offered regional or whole-body positron emission tomography–computed tomography (PET-CT) to rule out metastatic disease, which he also declined. There was no evidence of recurrence or lymphadenopathy 19 months postoperatively.
Comment
Eccrine porocarcinoma is an exceptionally rare adnexal neoplasm that most commonly affects older adults. The average age at diagnosis is 71 years in men and 75 years in women.2 Our case is rare because of the patient’s age. Benign eccrine poromas occur most frequently on the palms, soles, axillae, and forehead where eccrine density is highest; EPC occurs most frequently on the lower extremities.6 It may arise de novo or from malignant transformation of a preexisting benign poroma. Clinically, EPC may present as an asymptomatic pink-brown papule, plaque, or nodule and may have a polypoid or verrucous appearance, as in our patient. Ulceration is common.7 The differential diagnosis often includes nodular basal cell carcinoma, squamous cell carcinoma, pyogenic granuloma, and seborrheic keratosis.
Histologically, EPCs are characterized by aggregations of cohesive basaloid epithelial cells forming eccrine ductal structures.2 Cellular atypia may be extremely subtle but, if present, can be helpful in differentiating malignant from benign lesions. Features of basal and squamous cell carcinoma also may be present. Definitive diagnosis is frequently based on the overall invasive architectural pattern.5 Robson et al2 examined 69 cases of EPC for high-risk histologic features and concluded that tumor depth greater than 7 mm, mitoses greater than 14 per high-power field, and the presence of lymphovascular invasion were independently predictive of mortality. Moreover, after adjusting for mitosis and depth, an infiltrative border vs a pushing border was strongly predictive of local recurrence.2 Immunohistochemical stains, although not necessary for diagnosis, may have utility as adjunctive tools. Cells lining the ducts within EPCs commonly stain positive for carcinoembryonic antigen, though glandular myoepithelial cells stain positive for S-100. Negative Ber-EP4 staining helps to differentiate EPC from basal cell carcinoma. Abnormal expression of p53 and overexpression of p16 also has been described.4
The rarity of EPC has precluded the development of any evidence-based management guidelines. Historically, the standard of care has been WLE with 2- to 3-cm margins. A review of 105 cases of EPC treated with WLE showed 20% local recurrence, 20% regional metastases, and 12% distant metastasis rates.8 Mohs micrographic surgery, which allows examination of 100% of the surgical margin vs less than 1% for WLE with the standard bread-loafing technique, might be expected to achieve higher cure rates. A review of 29 cases treated with MMS monotherapy demonstrated no local recurrences, distant metastasis, or disease-specific deaths with follow-up ranging from 19 months to 6 years.5 One case was associated with regional lymph node metastases that were treated with completion lymphadenectomy and adjuvant radiation therapy.7 The high mortality rate of patients with nodal disease has led some to recommend PET-CT and SLNB for patients with EPC. However, the prognostic value of such procedures has not been clearly defined and there is no demonstrated survival benefit for treatment of widespread disease. Our patient declined both SLNB and PET-CT, and our plan was to follow him clinically with symptom-directed imaging only.
Conclusion
Patients with EPC generally have a favorable prognosis with prompt diagnosis and complete surgical excision. Although most commonly seen in elderly patients, EPC may present in younger patients and may be clinically and histologically nondescript with little cytologic atypia. Based on a small but growing body of literature, MMS appears to be at least as effective as WLE as a primary treatment modality for EPC, while offering the advantage of tissue sparing in cosmetically or functionally important areas.
- Pinkus H, Mehregan AH. Epidermatropic eccrine carcinoma. a case combining eccrine poroma and Paget’s dermatoses. Arch Dermatol. 1963;88:597-606.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: The Mayo Clinic Experience. Dermatol Surg. 2016;42:745-750.
- Tidwell WJ, Mayer JE, Malone J, et al. Treatment of eccrine porocarcinoma with Mohs micrographic surgery: a cases series and literature review. Int J Dermatol. 2015;54:1078-1083.
- Xu YG, Aylward J, Longley BJ, et al. Eccrine porocarcinoma treated by Mohs micrographic surgery: over 6-year follow-up of 12 cases and literature review. Dermatol Surg. 2015;41:685-692.
- D’Ambrosia RA, Ward H, Parry E. Eccrine porocarcinoma of the eyelid treated with Mohs micrographic surgery. Dermatol Surg. 2004;30:4:570-571.
- Vleugels FR, Girouard SD, Schmults CD, et al. Metastatic eccrine porocarcinoma after Mohs micrographic surgery: a case report. J Clin Oncol. 2012;30:188-191.
- Snow SN, Reizner GT. Eccrine porocarcinoma of the face. J Am Acad Dermatol. 1992;27:306-311.
Eccrine porocarcinoma (EPC), originally described by Pinkus and Mehregan1 in 1963, is an exceedingly rare sweat gland tumor most commonly seen in older patients. Fewer than 300 cases have been reported in the literature, and it is believed to represent only 0.005% to 0.01% of cutaneous malignancies.2 In the absence of established guidelines, wide local excision (WLE) has traditionally been considered the standard of treatment; however, local recurrence and nodal metastasis rates associated with WLE have been reported as high as 20%.3 More recently, a number of case reports and small case series have demonstrated higher cure rates with Mohs micrographic surgery (MMS), though follow-up is limited.3-5 We describe a case of EPC presenting as a recurrent wart in a 36-year-old man that was successfully treated with MMS.
Case Report
A 36-year-old man with no notable medical history presented with a 0.5×0.5-cm, asymptomatic, flesh-colored, hyperkeratotic, polypoid papule on the right medial thigh (Figure 1). The lesion was diagnosed as a wart and treated with cryotherapy by another dermatologist several years prior to presentation. Dermatoscopic examination at the current presentation showed a homogenous yellow center with a few peripheral vessels and a faint pink-tan halo (Figure 2). Our differential diagnosis included a recurrent wart, fibrosed pyogenic granuloma, irritated intradermal nevus, skin tag, and adnexal neoplasm. A shave biopsy was performed. Histopathologic analysis revealed multiple aggregations of mildly pleomorphic epithelial cells emanating from the epidermis, with many aggregations containing ductal structures (Figure 3). Rare necrotic and pyknotic cells were present, but no mitotic figures or lymphovascular invasion were identified. Immunohistochemical staining was positive for carcinoembryonic antigen and epithelial membrane antigen but negative for Ber-EP4. These findings were consistent with a well-differentiated EPC.



The patient was offered MMS or WLE, with or without sentinel lymph node biopsy (SLNB). He opted for MMS. The initial 1-cm margin taken during MMS was sufficient to achieve complete tumor extirpation, and the final 3.7×2.5-cm defect was closed primarily. The MMS debulking specimen was sent for permanent sectioning and showed a small focus of residual tumor cells, but no mitoses or lymphovascular invasion were seen. The patient was referred to surgical oncology to discuss the option of SLNB, which he ultimately declined. He also was offered regional or whole-body positron emission tomography–computed tomography (PET-CT) to rule out metastatic disease, which he also declined. There was no evidence of recurrence or lymphadenopathy 19 months postoperatively.
Comment
Eccrine porocarcinoma is an exceptionally rare adnexal neoplasm that most commonly affects older adults. The average age at diagnosis is 71 years in men and 75 years in women.2 Our case is rare because of the patient’s age. Benign eccrine poromas occur most frequently on the palms, soles, axillae, and forehead where eccrine density is highest; EPC occurs most frequently on the lower extremities.6 It may arise de novo or from malignant transformation of a preexisting benign poroma. Clinically, EPC may present as an asymptomatic pink-brown papule, plaque, or nodule and may have a polypoid or verrucous appearance, as in our patient. Ulceration is common.7 The differential diagnosis often includes nodular basal cell carcinoma, squamous cell carcinoma, pyogenic granuloma, and seborrheic keratosis.
Histologically, EPCs are characterized by aggregations of cohesive basaloid epithelial cells forming eccrine ductal structures.2 Cellular atypia may be extremely subtle but, if present, can be helpful in differentiating malignant from benign lesions. Features of basal and squamous cell carcinoma also may be present. Definitive diagnosis is frequently based on the overall invasive architectural pattern.5 Robson et al2 examined 69 cases of EPC for high-risk histologic features and concluded that tumor depth greater than 7 mm, mitoses greater than 14 per high-power field, and the presence of lymphovascular invasion were independently predictive of mortality. Moreover, after adjusting for mitosis and depth, an infiltrative border vs a pushing border was strongly predictive of local recurrence.2 Immunohistochemical stains, although not necessary for diagnosis, may have utility as adjunctive tools. Cells lining the ducts within EPCs commonly stain positive for carcinoembryonic antigen, though glandular myoepithelial cells stain positive for S-100. Negative Ber-EP4 staining helps to differentiate EPC from basal cell carcinoma. Abnormal expression of p53 and overexpression of p16 also has been described.4
The rarity of EPC has precluded the development of any evidence-based management guidelines. Historically, the standard of care has been WLE with 2- to 3-cm margins. A review of 105 cases of EPC treated with WLE showed 20% local recurrence, 20% regional metastases, and 12% distant metastasis rates.8 Mohs micrographic surgery, which allows examination of 100% of the surgical margin vs less than 1% for WLE with the standard bread-loafing technique, might be expected to achieve higher cure rates. A review of 29 cases treated with MMS monotherapy demonstrated no local recurrences, distant metastasis, or disease-specific deaths with follow-up ranging from 19 months to 6 years.5 One case was associated with regional lymph node metastases that were treated with completion lymphadenectomy and adjuvant radiation therapy.7 The high mortality rate of patients with nodal disease has led some to recommend PET-CT and SLNB for patients with EPC. However, the prognostic value of such procedures has not been clearly defined and there is no demonstrated survival benefit for treatment of widespread disease. Our patient declined both SLNB and PET-CT, and our plan was to follow him clinically with symptom-directed imaging only.
Conclusion
Patients with EPC generally have a favorable prognosis with prompt diagnosis and complete surgical excision. Although most commonly seen in elderly patients, EPC may present in younger patients and may be clinically and histologically nondescript with little cytologic atypia. Based on a small but growing body of literature, MMS appears to be at least as effective as WLE as a primary treatment modality for EPC, while offering the advantage of tissue sparing in cosmetically or functionally important areas.
Eccrine porocarcinoma (EPC), originally described by Pinkus and Mehregan1 in 1963, is an exceedingly rare sweat gland tumor most commonly seen in older patients. Fewer than 300 cases have been reported in the literature, and it is believed to represent only 0.005% to 0.01% of cutaneous malignancies.2 In the absence of established guidelines, wide local excision (WLE) has traditionally been considered the standard of treatment; however, local recurrence and nodal metastasis rates associated with WLE have been reported as high as 20%.3 More recently, a number of case reports and small case series have demonstrated higher cure rates with Mohs micrographic surgery (MMS), though follow-up is limited.3-5 We describe a case of EPC presenting as a recurrent wart in a 36-year-old man that was successfully treated with MMS.
Case Report
A 36-year-old man with no notable medical history presented with a 0.5×0.5-cm, asymptomatic, flesh-colored, hyperkeratotic, polypoid papule on the right medial thigh (Figure 1). The lesion was diagnosed as a wart and treated with cryotherapy by another dermatologist several years prior to presentation. Dermatoscopic examination at the current presentation showed a homogenous yellow center with a few peripheral vessels and a faint pink-tan halo (Figure 2). Our differential diagnosis included a recurrent wart, fibrosed pyogenic granuloma, irritated intradermal nevus, skin tag, and adnexal neoplasm. A shave biopsy was performed. Histopathologic analysis revealed multiple aggregations of mildly pleomorphic epithelial cells emanating from the epidermis, with many aggregations containing ductal structures (Figure 3). Rare necrotic and pyknotic cells were present, but no mitotic figures or lymphovascular invasion were identified. Immunohistochemical staining was positive for carcinoembryonic antigen and epithelial membrane antigen but negative for Ber-EP4. These findings were consistent with a well-differentiated EPC.



The patient was offered MMS or WLE, with or without sentinel lymph node biopsy (SLNB). He opted for MMS. The initial 1-cm margin taken during MMS was sufficient to achieve complete tumor extirpation, and the final 3.7×2.5-cm defect was closed primarily. The MMS debulking specimen was sent for permanent sectioning and showed a small focus of residual tumor cells, but no mitoses or lymphovascular invasion were seen. The patient was referred to surgical oncology to discuss the option of SLNB, which he ultimately declined. He also was offered regional or whole-body positron emission tomography–computed tomography (PET-CT) to rule out metastatic disease, which he also declined. There was no evidence of recurrence or lymphadenopathy 19 months postoperatively.
Comment
Eccrine porocarcinoma is an exceptionally rare adnexal neoplasm that most commonly affects older adults. The average age at diagnosis is 71 years in men and 75 years in women.2 Our case is rare because of the patient’s age. Benign eccrine poromas occur most frequently on the palms, soles, axillae, and forehead where eccrine density is highest; EPC occurs most frequently on the lower extremities.6 It may arise de novo or from malignant transformation of a preexisting benign poroma. Clinically, EPC may present as an asymptomatic pink-brown papule, plaque, or nodule and may have a polypoid or verrucous appearance, as in our patient. Ulceration is common.7 The differential diagnosis often includes nodular basal cell carcinoma, squamous cell carcinoma, pyogenic granuloma, and seborrheic keratosis.
Histologically, EPCs are characterized by aggregations of cohesive basaloid epithelial cells forming eccrine ductal structures.2 Cellular atypia may be extremely subtle but, if present, can be helpful in differentiating malignant from benign lesions. Features of basal and squamous cell carcinoma also may be present. Definitive diagnosis is frequently based on the overall invasive architectural pattern.5 Robson et al2 examined 69 cases of EPC for high-risk histologic features and concluded that tumor depth greater than 7 mm, mitoses greater than 14 per high-power field, and the presence of lymphovascular invasion were independently predictive of mortality. Moreover, after adjusting for mitosis and depth, an infiltrative border vs a pushing border was strongly predictive of local recurrence.2 Immunohistochemical stains, although not necessary for diagnosis, may have utility as adjunctive tools. Cells lining the ducts within EPCs commonly stain positive for carcinoembryonic antigen, though glandular myoepithelial cells stain positive for S-100. Negative Ber-EP4 staining helps to differentiate EPC from basal cell carcinoma. Abnormal expression of p53 and overexpression of p16 also has been described.4
The rarity of EPC has precluded the development of any evidence-based management guidelines. Historically, the standard of care has been WLE with 2- to 3-cm margins. A review of 105 cases of EPC treated with WLE showed 20% local recurrence, 20% regional metastases, and 12% distant metastasis rates.8 Mohs micrographic surgery, which allows examination of 100% of the surgical margin vs less than 1% for WLE with the standard bread-loafing technique, might be expected to achieve higher cure rates. A review of 29 cases treated with MMS monotherapy demonstrated no local recurrences, distant metastasis, or disease-specific deaths with follow-up ranging from 19 months to 6 years.5 One case was associated with regional lymph node metastases that were treated with completion lymphadenectomy and adjuvant radiation therapy.7 The high mortality rate of patients with nodal disease has led some to recommend PET-CT and SLNB for patients with EPC. However, the prognostic value of such procedures has not been clearly defined and there is no demonstrated survival benefit for treatment of widespread disease. Our patient declined both SLNB and PET-CT, and our plan was to follow him clinically with symptom-directed imaging only.
Conclusion
Patients with EPC generally have a favorable prognosis with prompt diagnosis and complete surgical excision. Although most commonly seen in elderly patients, EPC may present in younger patients and may be clinically and histologically nondescript with little cytologic atypia. Based on a small but growing body of literature, MMS appears to be at least as effective as WLE as a primary treatment modality for EPC, while offering the advantage of tissue sparing in cosmetically or functionally important areas.
- Pinkus H, Mehregan AH. Epidermatropic eccrine carcinoma. a case combining eccrine poroma and Paget’s dermatoses. Arch Dermatol. 1963;88:597-606.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: The Mayo Clinic Experience. Dermatol Surg. 2016;42:745-750.
- Tidwell WJ, Mayer JE, Malone J, et al. Treatment of eccrine porocarcinoma with Mohs micrographic surgery: a cases series and literature review. Int J Dermatol. 2015;54:1078-1083.
- Xu YG, Aylward J, Longley BJ, et al. Eccrine porocarcinoma treated by Mohs micrographic surgery: over 6-year follow-up of 12 cases and literature review. Dermatol Surg. 2015;41:685-692.
- D’Ambrosia RA, Ward H, Parry E. Eccrine porocarcinoma of the eyelid treated with Mohs micrographic surgery. Dermatol Surg. 2004;30:4:570-571.
- Vleugels FR, Girouard SD, Schmults CD, et al. Metastatic eccrine porocarcinoma after Mohs micrographic surgery: a case report. J Clin Oncol. 2012;30:188-191.
- Snow SN, Reizner GT. Eccrine porocarcinoma of the face. J Am Acad Dermatol. 1992;27:306-311.
- Pinkus H, Mehregan AH. Epidermatropic eccrine carcinoma. a case combining eccrine poroma and Paget’s dermatoses. Arch Dermatol. 1963;88:597-606.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: The Mayo Clinic Experience. Dermatol Surg. 2016;42:745-750.
- Tidwell WJ, Mayer JE, Malone J, et al. Treatment of eccrine porocarcinoma with Mohs micrographic surgery: a cases series and literature review. Int J Dermatol. 2015;54:1078-1083.
- Xu YG, Aylward J, Longley BJ, et al. Eccrine porocarcinoma treated by Mohs micrographic surgery: over 6-year follow-up of 12 cases and literature review. Dermatol Surg. 2015;41:685-692.
- D’Ambrosia RA, Ward H, Parry E. Eccrine porocarcinoma of the eyelid treated with Mohs micrographic surgery. Dermatol Surg. 2004;30:4:570-571.
- Vleugels FR, Girouard SD, Schmults CD, et al. Metastatic eccrine porocarcinoma after Mohs micrographic surgery: a case report. J Clin Oncol. 2012;30:188-191.
- Snow SN, Reizner GT. Eccrine porocarcinoma of the face. J Am Acad Dermatol. 1992;27:306-311.
Practice Points
- Eccrine porocarcinoma is more common in older patients (age range, 71–75 years).
- Local recurrence and nodal metastasis are reported as high as 20% with wide local excision.
- Higher cure rates recently have been reported with Mohs micrographic surgery.
Hailey-Hailey Disease: A Diagnostic Challenge
Hailey-Hailey disease (HHD), also known as benign familial chronic pemphigus, is an autosomal-dominant genodermatosis caused by mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1.1 It is characterized by crusted macerated erosions and velvety, fissured, hypertrophic plaques classically involving the intertriginous areas. The diagnosis is suggested by characteristic clinical morphology, involvement of the intertriginous areas, and a positive family history. Histology often confirms the diagnosis and demonstrates a characteristic dilapidated brick wall appearance. If there is a need to distinguish HHD from pemphigus, direct immunofluorescence studies also should be performed, which would be negative.2,3 However, HHD often is misdiagnosed due to lack of knowledge of this uncommon disorder and its resemblance to other dermatoses of the intertriginous areas.4 We present an unusual presentation of HHD with late onset and involvement of the skin of the abdomen and foot.
Case Report
A 61-year-old woman presented with a 3×4-cm fissured plaque with erosions and a peripheral yellow crust on the left side of the anterior abdomen (Figure 1A). There was another fissured plaque with surrounding erythema and scaling on the fifth digit of the right foot (Figure 1B). For the last 11 years, she periodically experienced erosive and scabbing skin plaques under the breasts and on the axillae and groin. Her mother and maternal grandfather had a history of similar skin lesions. Due to a suspicion of HHD, a skin biopsy specimen of the abdominal plaque was performed, which demonstrated epidermal acanthosis and suprabasal acantholysis with lacunae formation (Figure 2). There was uneven thickening of the epidermal keratin layer with parakeratotic nests. The upper layer of the dermis demonstrated edema and focal fibrosis, enlarged capillaries, and pericapillary lymphohistiocytic infiltration with eosinophils and neutrophils. Accordingly, a diagnosis of HHD was established.
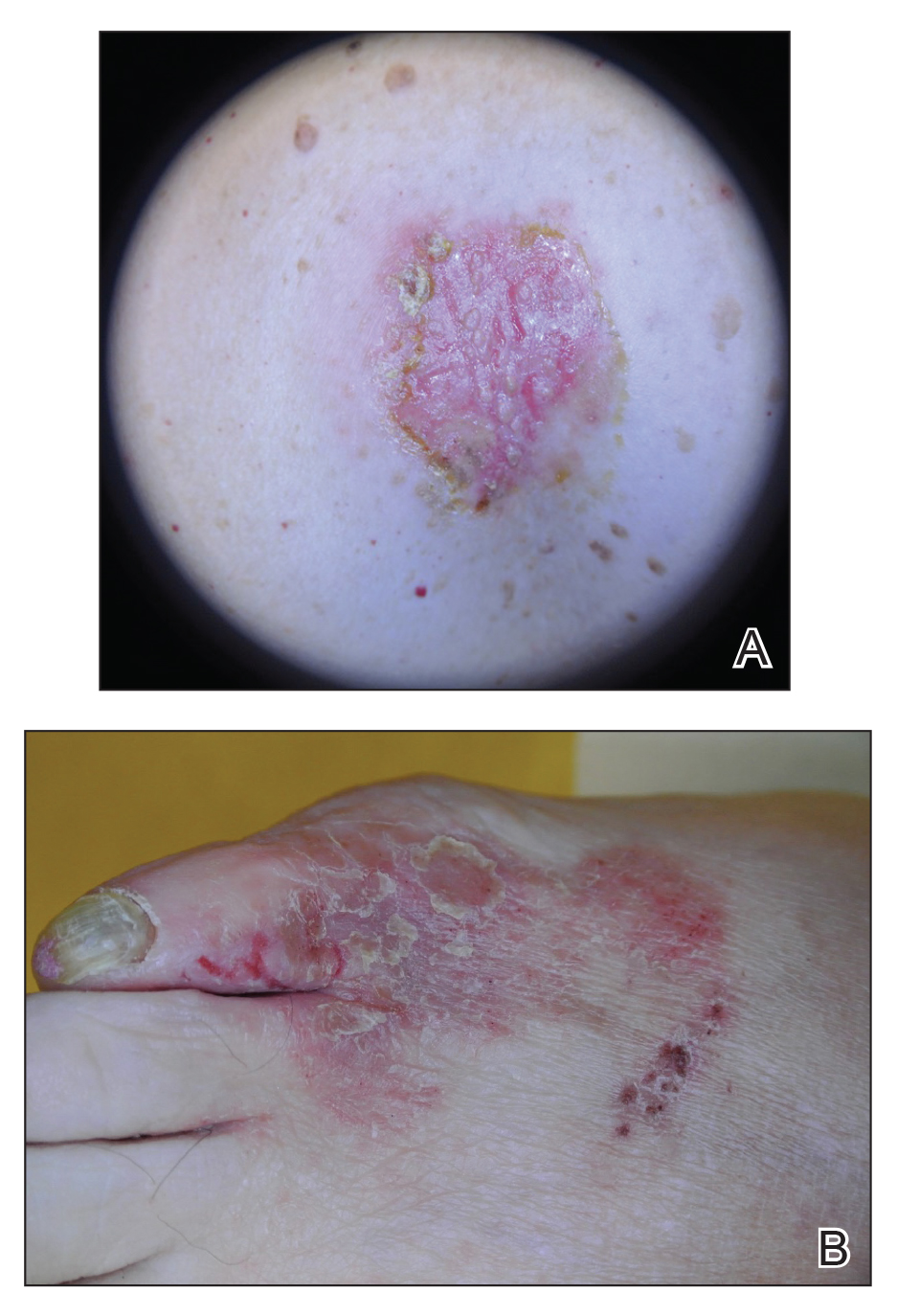
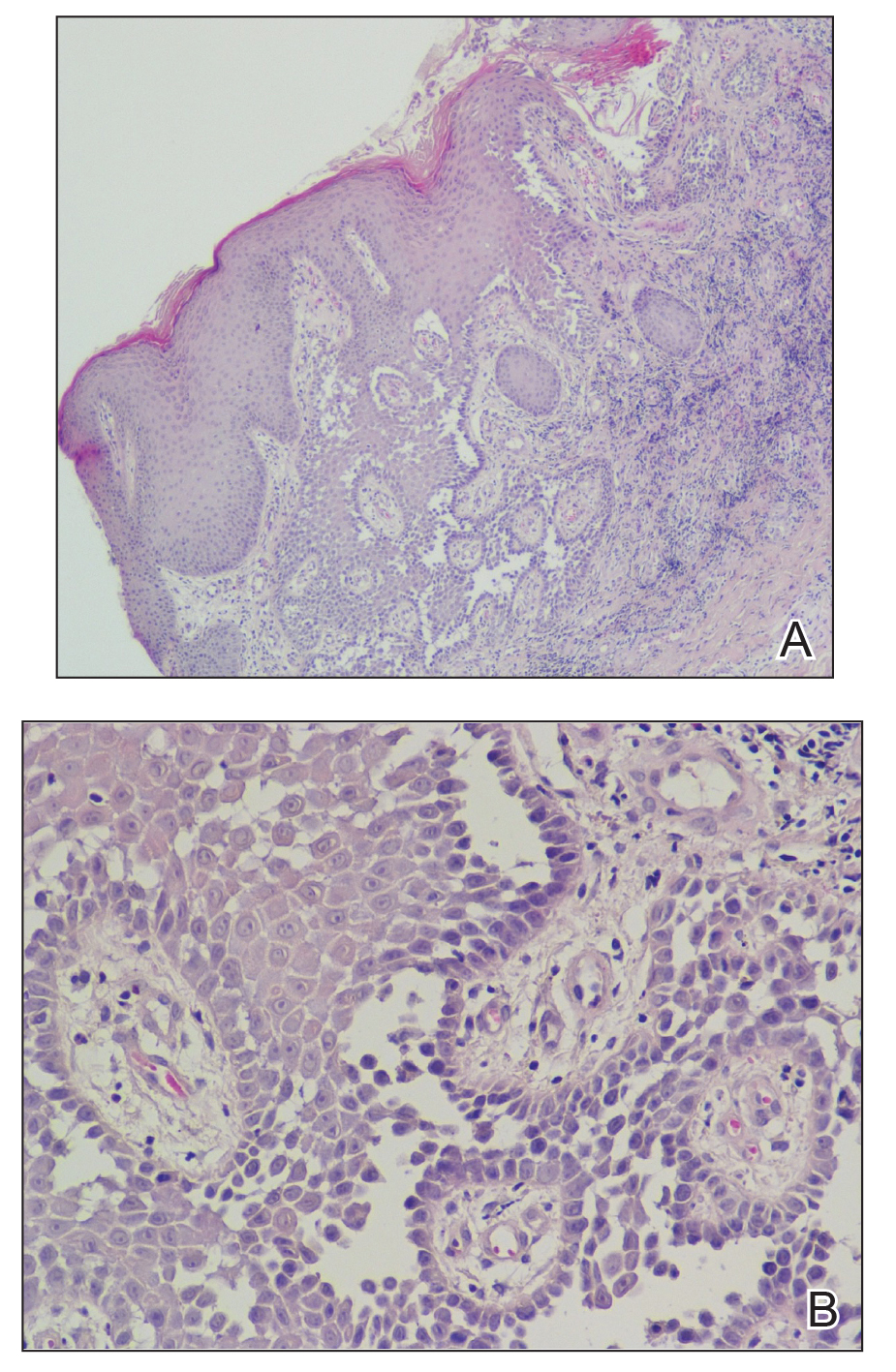
Comment
Hailey-Hailey disease occurs in 1 to 4 per 100,000 individuals without predilection for sex or ethnic group.5-9 Onset usually occurs after puberty, most commonly in the third decade of life.8,10-12 Mutations of the ATP2C1 gene on band 3q22.1 cause haploinsufficiency of Ca2+/Mn2+−ATPase protein 1 (hSPCA1) that alters the intracellular calcium gradient, leading to disruptions in assembly and trafficking of desmosomal proteins to the cell membrane. Consequently, altered intercellular connections and acantholysis of the epidermis occur.1,13-16
Hailey-Hailey disease initially manifests as grouped flaccid vesicles that rupture easily, leaving behind crusted erosions and dry, scaly, eczematous patches.17,18 Over time, velvety, fissured, and hypertrophic plaques develop. Up to 80% of patients experience secondary bacterial and fungal superinfections that may cause vegetative or malodorous plaques.9 Although HHD has no specific treatment, symptoms are managed with topical corticosteroids and antimicrobial agents. Patients should be advised to avoid irritants such as friction, sunlight, or sweat. For severe cases, botulinum toxin type A, laser therapy, dermabrasion, and surgery have been utilized with variable success.19-22 The responsiveness of HHD to corticosteroids and antimicrobial agents facilitates misdiagnosis as intertrigo, erythrasma, or dermatophytosis.
Our patient presented with late-onset HHD (age, 50 years) compared to the typical age of onset in the third decade of life.8 Furthermore, her presentation was atypical for HHD, which characteristically affects intertriginous areas due to sweat, heat, friction, and microorganisms. Hailey-Hailey disease involving the abdominal skin is unusual, as it typically occurs in regions of friction such as the belt area.23 Our patient lacked a history of friction or trauma at the site of the abdominal plaque. In addition, HHD involving the feet is exceedingly rare. It is plausible that friction and heat caused by footwear may have predisposed her to these skin changes.
Conclusion
This case highlights the difficulties of diagnosing HHD, especially if it appears in atypical locations.24 Obtaining a thorough family history and detailed dermatologic examination as well as maintaining a high level of suspicion can assist in diagnosing this uncommon disorder.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a alcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61-65.
- Ohata C. Hailey-Hailey disease. Cutis. 2014;94:33-34.
- Abdullah L, Abbas O. Dermacase. can you identify this condition? benign familial chronic pemphigus. Can Fam Physician. 2011;57:1157-1158.
- Le Donne M, Lentini M, Moretti G, et al. Chronic vulvocrural dermatitis with burning and itching. CMAJ. 2008;179:555-556.
- Hohl D. Darier disease and Hailey-Hailey disease. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012:887-897.
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105.
- Godic A, Miljkovic J, Kansky A, et al. Epidemiology of Darier’s disease in Slovenia. Acta Dermatovenerol Alp Pannonica Adriat. 2005;14:43-48.
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282.
- Benmously-Mlika R, Bchetnia M, Deghais S, et al. Hailey-Hailey disease in Tunisia. Int J Dermatol. 2010;49:396-401.
- Bessa GR, Grazziotin TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722.
- Gu H, Chang B, Chen W, et al. Clinical analysis of 69 patients with familial benign chronic pemphigus. Chin Med J (Engl). 1999;112:761-763.
- Dobson-Stone C, Fairclough R, Dunne E, et al. Hailey-Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002;118:338-343.
- Fairclough RJ, Lonie L, Van Baelen K, et al. Hailey-Hailey disease: identification of novel mutations in ATP2C1 and effect of missense mutation A528P on protein expression levels. J Invest Dermatol. 2004;123:6771.
- Shibata A, Sugiura K, Kimura U, et al. A novel ATP2C1 early truncation mutation suggests haploinsufficiency as a pathogenic mechanism in a patient with Hailey-Hailey disease. Acta Derm Venereol. 2013;93:719-720.
- Dhitavat J, Fairclough RJ, Hovnanian A, et al. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150:821-828.
- Raiko L, Siljamaki E, Mahoney MG, et al. Hailey-Hailey disease and tight junctions: claudins 1 and 4 are regulated by ATP2C1 gene encoding Ca(2+)/Mn(2+) ATPase SPCA1 in cultured keratinocytes. Exp Dermatol. 2012;21:586-591.
- Yadav N, Madke B, Kar S, et al. Hailey-Hailey disease. Indian Dermatol Online J. 2016;7:147-148.
- Vasudevan B, Verma R, Badwal S, et al. Hailey-Hailey disease with skin lesions at unusual sites and a good response to acitretin. Indian J Dermatol Venereol Leprol. 2015;81:88-91.
- Bagherani N, Smoller BR. The efficacy of botulinum toxin type A in the treatment of Hailey Hailey disease. Dermatol Ther. 2016;29:394-395.
- Hochwalt PC, Christensen KN, Cantwell SR, et al. Carbon dioxide laser treatment for Hailey-Hailey disease: a retrospective chart review with patient-reported outcomes. Int J Dermatol. 2015;54:1309-1314.
- Falto-Aizpurua LA, Griffith RD, Yazdani Abyaneh MA, et al. Laser therapy for the treatment of Hailey-Hailey disease: a systematic review with focus on carbon dioxide laser resurfacing. J Eur Acad Dermatol Venereol. 2015;29:1045-1052.
- Arora H, Bray FN, Cervantes J, et al. Management of familial benign chronic pemphigus. Clin Cosmet Investig Dermatol. 2016;9:281-290.
- Iijima S, Hamada T, Kanzaki M, et al. Sibling cases of Hailey-Hailey disease showing atypical clinical features and unique disease course. JAMA Dermatol. 2014;150:97-99.
- Saied NK, Schwartz RA, Hansen RC, et al. Atypical familial benign chronic pemphigus. Cutis. 1981;27:666-669.
Hailey-Hailey disease (HHD), also known as benign familial chronic pemphigus, is an autosomal-dominant genodermatosis caused by mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1.1 It is characterized by crusted macerated erosions and velvety, fissured, hypertrophic plaques classically involving the intertriginous areas. The diagnosis is suggested by characteristic clinical morphology, involvement of the intertriginous areas, and a positive family history. Histology often confirms the diagnosis and demonstrates a characteristic dilapidated brick wall appearance. If there is a need to distinguish HHD from pemphigus, direct immunofluorescence studies also should be performed, which would be negative.2,3 However, HHD often is misdiagnosed due to lack of knowledge of this uncommon disorder and its resemblance to other dermatoses of the intertriginous areas.4 We present an unusual presentation of HHD with late onset and involvement of the skin of the abdomen and foot.
Case Report
A 61-year-old woman presented with a 3×4-cm fissured plaque with erosions and a peripheral yellow crust on the left side of the anterior abdomen (Figure 1A). There was another fissured plaque with surrounding erythema and scaling on the fifth digit of the right foot (Figure 1B). For the last 11 years, she periodically experienced erosive and scabbing skin plaques under the breasts and on the axillae and groin. Her mother and maternal grandfather had a history of similar skin lesions. Due to a suspicion of HHD, a skin biopsy specimen of the abdominal plaque was performed, which demonstrated epidermal acanthosis and suprabasal acantholysis with lacunae formation (Figure 2). There was uneven thickening of the epidermal keratin layer with parakeratotic nests. The upper layer of the dermis demonstrated edema and focal fibrosis, enlarged capillaries, and pericapillary lymphohistiocytic infiltration with eosinophils and neutrophils. Accordingly, a diagnosis of HHD was established.


Comment
Hailey-Hailey disease occurs in 1 to 4 per 100,000 individuals without predilection for sex or ethnic group.5-9 Onset usually occurs after puberty, most commonly in the third decade of life.8,10-12 Mutations of the ATP2C1 gene on band 3q22.1 cause haploinsufficiency of Ca2+/Mn2+−ATPase protein 1 (hSPCA1) that alters the intracellular calcium gradient, leading to disruptions in assembly and trafficking of desmosomal proteins to the cell membrane. Consequently, altered intercellular connections and acantholysis of the epidermis occur.1,13-16
Hailey-Hailey disease initially manifests as grouped flaccid vesicles that rupture easily, leaving behind crusted erosions and dry, scaly, eczematous patches.17,18 Over time, velvety, fissured, and hypertrophic plaques develop. Up to 80% of patients experience secondary bacterial and fungal superinfections that may cause vegetative or malodorous plaques.9 Although HHD has no specific treatment, symptoms are managed with topical corticosteroids and antimicrobial agents. Patients should be advised to avoid irritants such as friction, sunlight, or sweat. For severe cases, botulinum toxin type A, laser therapy, dermabrasion, and surgery have been utilized with variable success.19-22 The responsiveness of HHD to corticosteroids and antimicrobial agents facilitates misdiagnosis as intertrigo, erythrasma, or dermatophytosis.
Our patient presented with late-onset HHD (age, 50 years) compared to the typical age of onset in the third decade of life.8 Furthermore, her presentation was atypical for HHD, which characteristically affects intertriginous areas due to sweat, heat, friction, and microorganisms. Hailey-Hailey disease involving the abdominal skin is unusual, as it typically occurs in regions of friction such as the belt area.23 Our patient lacked a history of friction or trauma at the site of the abdominal plaque. In addition, HHD involving the feet is exceedingly rare. It is plausible that friction and heat caused by footwear may have predisposed her to these skin changes.
Conclusion
This case highlights the difficulties of diagnosing HHD, especially if it appears in atypical locations.24 Obtaining a thorough family history and detailed dermatologic examination as well as maintaining a high level of suspicion can assist in diagnosing this uncommon disorder.
Hailey-Hailey disease (HHD), also known as benign familial chronic pemphigus, is an autosomal-dominant genodermatosis caused by mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1.1 It is characterized by crusted macerated erosions and velvety, fissured, hypertrophic plaques classically involving the intertriginous areas. The diagnosis is suggested by characteristic clinical morphology, involvement of the intertriginous areas, and a positive family history. Histology often confirms the diagnosis and demonstrates a characteristic dilapidated brick wall appearance. If there is a need to distinguish HHD from pemphigus, direct immunofluorescence studies also should be performed, which would be negative.2,3 However, HHD often is misdiagnosed due to lack of knowledge of this uncommon disorder and its resemblance to other dermatoses of the intertriginous areas.4 We present an unusual presentation of HHD with late onset and involvement of the skin of the abdomen and foot.
Case Report
A 61-year-old woman presented with a 3×4-cm fissured plaque with erosions and a peripheral yellow crust on the left side of the anterior abdomen (Figure 1A). There was another fissured plaque with surrounding erythema and scaling on the fifth digit of the right foot (Figure 1B). For the last 11 years, she periodically experienced erosive and scabbing skin plaques under the breasts and on the axillae and groin. Her mother and maternal grandfather had a history of similar skin lesions. Due to a suspicion of HHD, a skin biopsy specimen of the abdominal plaque was performed, which demonstrated epidermal acanthosis and suprabasal acantholysis with lacunae formation (Figure 2). There was uneven thickening of the epidermal keratin layer with parakeratotic nests. The upper layer of the dermis demonstrated edema and focal fibrosis, enlarged capillaries, and pericapillary lymphohistiocytic infiltration with eosinophils and neutrophils. Accordingly, a diagnosis of HHD was established.


Comment
Hailey-Hailey disease occurs in 1 to 4 per 100,000 individuals without predilection for sex or ethnic group.5-9 Onset usually occurs after puberty, most commonly in the third decade of life.8,10-12 Mutations of the ATP2C1 gene on band 3q22.1 cause haploinsufficiency of Ca2+/Mn2+−ATPase protein 1 (hSPCA1) that alters the intracellular calcium gradient, leading to disruptions in assembly and trafficking of desmosomal proteins to the cell membrane. Consequently, altered intercellular connections and acantholysis of the epidermis occur.1,13-16
Hailey-Hailey disease initially manifests as grouped flaccid vesicles that rupture easily, leaving behind crusted erosions and dry, scaly, eczematous patches.17,18 Over time, velvety, fissured, and hypertrophic plaques develop. Up to 80% of patients experience secondary bacterial and fungal superinfections that may cause vegetative or malodorous plaques.9 Although HHD has no specific treatment, symptoms are managed with topical corticosteroids and antimicrobial agents. Patients should be advised to avoid irritants such as friction, sunlight, or sweat. For severe cases, botulinum toxin type A, laser therapy, dermabrasion, and surgery have been utilized with variable success.19-22 The responsiveness of HHD to corticosteroids and antimicrobial agents facilitates misdiagnosis as intertrigo, erythrasma, or dermatophytosis.
Our patient presented with late-onset HHD (age, 50 years) compared to the typical age of onset in the third decade of life.8 Furthermore, her presentation was atypical for HHD, which characteristically affects intertriginous areas due to sweat, heat, friction, and microorganisms. Hailey-Hailey disease involving the abdominal skin is unusual, as it typically occurs in regions of friction such as the belt area.23 Our patient lacked a history of friction or trauma at the site of the abdominal plaque. In addition, HHD involving the feet is exceedingly rare. It is plausible that friction and heat caused by footwear may have predisposed her to these skin changes.
Conclusion
This case highlights the difficulties of diagnosing HHD, especially if it appears in atypical locations.24 Obtaining a thorough family history and detailed dermatologic examination as well as maintaining a high level of suspicion can assist in diagnosing this uncommon disorder.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a alcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61-65.
- Ohata C. Hailey-Hailey disease. Cutis. 2014;94:33-34.
- Abdullah L, Abbas O. Dermacase. can you identify this condition? benign familial chronic pemphigus. Can Fam Physician. 2011;57:1157-1158.
- Le Donne M, Lentini M, Moretti G, et al. Chronic vulvocrural dermatitis with burning and itching. CMAJ. 2008;179:555-556.
- Hohl D. Darier disease and Hailey-Hailey disease. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012:887-897.
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105.
- Godic A, Miljkovic J, Kansky A, et al. Epidemiology of Darier’s disease in Slovenia. Acta Dermatovenerol Alp Pannonica Adriat. 2005;14:43-48.
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282.
- Benmously-Mlika R, Bchetnia M, Deghais S, et al. Hailey-Hailey disease in Tunisia. Int J Dermatol. 2010;49:396-401.
- Bessa GR, Grazziotin TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722.
- Gu H, Chang B, Chen W, et al. Clinical analysis of 69 patients with familial benign chronic pemphigus. Chin Med J (Engl). 1999;112:761-763.
- Dobson-Stone C, Fairclough R, Dunne E, et al. Hailey-Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002;118:338-343.
- Fairclough RJ, Lonie L, Van Baelen K, et al. Hailey-Hailey disease: identification of novel mutations in ATP2C1 and effect of missense mutation A528P on protein expression levels. J Invest Dermatol. 2004;123:6771.
- Shibata A, Sugiura K, Kimura U, et al. A novel ATP2C1 early truncation mutation suggests haploinsufficiency as a pathogenic mechanism in a patient with Hailey-Hailey disease. Acta Derm Venereol. 2013;93:719-720.
- Dhitavat J, Fairclough RJ, Hovnanian A, et al. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150:821-828.
- Raiko L, Siljamaki E, Mahoney MG, et al. Hailey-Hailey disease and tight junctions: claudins 1 and 4 are regulated by ATP2C1 gene encoding Ca(2+)/Mn(2+) ATPase SPCA1 in cultured keratinocytes. Exp Dermatol. 2012;21:586-591.
- Yadav N, Madke B, Kar S, et al. Hailey-Hailey disease. Indian Dermatol Online J. 2016;7:147-148.
- Vasudevan B, Verma R, Badwal S, et al. Hailey-Hailey disease with skin lesions at unusual sites and a good response to acitretin. Indian J Dermatol Venereol Leprol. 2015;81:88-91.
- Bagherani N, Smoller BR. The efficacy of botulinum toxin type A in the treatment of Hailey Hailey disease. Dermatol Ther. 2016;29:394-395.
- Hochwalt PC, Christensen KN, Cantwell SR, et al. Carbon dioxide laser treatment for Hailey-Hailey disease: a retrospective chart review with patient-reported outcomes. Int J Dermatol. 2015;54:1309-1314.
- Falto-Aizpurua LA, Griffith RD, Yazdani Abyaneh MA, et al. Laser therapy for the treatment of Hailey-Hailey disease: a systematic review with focus on carbon dioxide laser resurfacing. J Eur Acad Dermatol Venereol. 2015;29:1045-1052.
- Arora H, Bray FN, Cervantes J, et al. Management of familial benign chronic pemphigus. Clin Cosmet Investig Dermatol. 2016;9:281-290.
- Iijima S, Hamada T, Kanzaki M, et al. Sibling cases of Hailey-Hailey disease showing atypical clinical features and unique disease course. JAMA Dermatol. 2014;150:97-99.
- Saied NK, Schwartz RA, Hansen RC, et al. Atypical familial benign chronic pemphigus. Cutis. 1981;27:666-669.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a alcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61-65.
- Ohata C. Hailey-Hailey disease. Cutis. 2014;94:33-34.
- Abdullah L, Abbas O. Dermacase. can you identify this condition? benign familial chronic pemphigus. Can Fam Physician. 2011;57:1157-1158.
- Le Donne M, Lentini M, Moretti G, et al. Chronic vulvocrural dermatitis with burning and itching. CMAJ. 2008;179:555-556.
- Hohl D. Darier disease and Hailey-Hailey disease. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Saunders; 2012:887-897.
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105.
- Godic A, Miljkovic J, Kansky A, et al. Epidemiology of Darier’s disease in Slovenia. Acta Dermatovenerol Alp Pannonica Adriat. 2005;14:43-48.
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282.
- Benmously-Mlika R, Bchetnia M, Deghais S, et al. Hailey-Hailey disease in Tunisia. Int J Dermatol. 2010;49:396-401.
- Bessa GR, Grazziotin TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722.
- Gu H, Chang B, Chen W, et al. Clinical analysis of 69 patients with familial benign chronic pemphigus. Chin Med J (Engl). 1999;112:761-763.
- Dobson-Stone C, Fairclough R, Dunne E, et al. Hailey-Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002;118:338-343.
- Fairclough RJ, Lonie L, Van Baelen K, et al. Hailey-Hailey disease: identification of novel mutations in ATP2C1 and effect of missense mutation A528P on protein expression levels. J Invest Dermatol. 2004;123:6771.
- Shibata A, Sugiura K, Kimura U, et al. A novel ATP2C1 early truncation mutation suggests haploinsufficiency as a pathogenic mechanism in a patient with Hailey-Hailey disease. Acta Derm Venereol. 2013;93:719-720.
- Dhitavat J, Fairclough RJ, Hovnanian A, et al. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150:821-828.
- Raiko L, Siljamaki E, Mahoney MG, et al. Hailey-Hailey disease and tight junctions: claudins 1 and 4 are regulated by ATP2C1 gene encoding Ca(2+)/Mn(2+) ATPase SPCA1 in cultured keratinocytes. Exp Dermatol. 2012;21:586-591.
- Yadav N, Madke B, Kar S, et al. Hailey-Hailey disease. Indian Dermatol Online J. 2016;7:147-148.
- Vasudevan B, Verma R, Badwal S, et al. Hailey-Hailey disease with skin lesions at unusual sites and a good response to acitretin. Indian J Dermatol Venereol Leprol. 2015;81:88-91.
- Bagherani N, Smoller BR. The efficacy of botulinum toxin type A in the treatment of Hailey Hailey disease. Dermatol Ther. 2016;29:394-395.
- Hochwalt PC, Christensen KN, Cantwell SR, et al. Carbon dioxide laser treatment for Hailey-Hailey disease: a retrospective chart review with patient-reported outcomes. Int J Dermatol. 2015;54:1309-1314.
- Falto-Aizpurua LA, Griffith RD, Yazdani Abyaneh MA, et al. Laser therapy for the treatment of Hailey-Hailey disease: a systematic review with focus on carbon dioxide laser resurfacing. J Eur Acad Dermatol Venereol. 2015;29:1045-1052.
- Arora H, Bray FN, Cervantes J, et al. Management of familial benign chronic pemphigus. Clin Cosmet Investig Dermatol. 2016;9:281-290.
- Iijima S, Hamada T, Kanzaki M, et al. Sibling cases of Hailey-Hailey disease showing atypical clinical features and unique disease course. JAMA Dermatol. 2014;150:97-99.
- Saied NK, Schwartz RA, Hansen RC, et al. Atypical familial benign chronic pemphigus. Cutis. 1981;27:666-669.
Practice Points
- Hailey-Hailey disease may present atypically with a late age of onset, involvement of nonintertriginous areas, and lack of clear exacerbating factors such as friction.
- A detailed history and physical examination as well as a high degree of suspicion can aid in diagnosing this uncommon disorder.
The Role of Diet in Preventing Photoaging and Treating Common Skin Conditions
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
Practice Points
- Growing evidence indicates that diet plays a role in overall skin health as well as the pathophysiology of several common cutaneous diseases.
- Broadly, we advocate for a low-glycemic diet that is rich in fruits and vegetables. In addition, dietary supplements of beta-carotene, collagen peptides, zinc, and fat-soluble vitamins (eg, vitamins D and E) have shown promising results in various conditions.
Recurrence of Elevated Intracranial Pressure Following Tetracycline Antibiotic Use
To the Editor:
In 1995, one of the authors (A.G.L.) reported the case of a 14-year-old boy who was diagnosed with pseudotumor cerebri following treatment with isotretinoin and tetracycline, both of which were implicated in the development of elevated intracranial pressure (ICP). The patient subsequently underwent optic nerve sheath fenestration for decompression due to progressive deterioration of the visual field despite discontinuation of both drugs.1
This patient recently returned to our office 28 years after his initial presentation with a recurrence of similar symptoms. He was subsequently diagnosed with elevated ICP after a single dose of doxycycline. His vision was 20/20 with correction for distance. Pupil size and extraocular motility were within normal limits. Physical examination was normal, and a dilated fundus examination showed a Frisen stage 1 disc edema in the right eye and a Frisen stage 3 disc edema in the left eye at presentation. The visual field showed enlarged blind spots in both eyes consistent with papilledema. Optical coherence tomography for optic nerve was 93 µm in the right eye and 124 µm in the left eye compared to earlier measures of 66 and 68 µm in the right and left eyes, respectively, indicating pseudonormalization of the parameters (disc edema in the setting of prior optic atrophy). In the setting of optic atrophy, when the nerve develops any swelling the thickness measured on optical coherence tomography may reach normal values, which are in fact abnormal and elevated in this case. Magnetic resonance imaging and magnetic resonance venography were within normal limits. Cerebrospinal fluid opening pressure was 26 cm of water, and analysis revealed high levels of West Nile virus antibodies (IgM and IgG), suggesting a recent viral infection. In addition to an established predisposition to develop elevated ICP on tetracycline antibiotics, this patient also had the precipitating factor of recent viral infection contributing to his raised ICP. Prior to his most recent presentation, his condition was stable with evidence of mild optic atrophy in both eyes and stable visual fields.
Various case reports have linked the use of tetracycline antibiotics to increased ICP. Gardner et al2 reported a case of fraternal twins who developed elevated ICP while on tetracycline for acne, suggesting a possible genetic susceptibility. In one nested case-control study, it was found that the relative risk (RR) of developing elevated ICP with tetracycline antibiotics was increased (RR=2.68 [95% CI, 0.89-8.11] for 15 days of current use; RR=3.64 [95% CI, 1.67-7.91] for 30 days of current use).3 Retrospective studies have demonstrated that 9% of the population (N=207) had prior treatment with tetracylines in a cohort of patients diagnosed with elevated ICP.4
In this group of drugs, minocycline has been closely associated with development of elevated ICP. One retrospective study showed that 75% of patients (9/12) with minocycline-associated ICP developed symptoms of elevated ICP within 8 weeks of starting therapy; however, half of the patients included in the study were obese. The inclusion of obese patients in this study is a confounding variable because idiopathic intracranial hypertension (IIH) is a disease that predominantly affects obese young females. The diagnosis of IIH, however, should be considered a diagnosis of exclusion, and it is uncommon in thin elderly or male patients.5
Tetracyclines have a half-life of 6 to 11 hours, and usually the elevated ICP decreases once the offending agent is discontinued, though papilledema could take months to resolve.
We describe an inadvertent rechallenge
- Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis. 1995;55:165-168.
- Gardner K, Cox T, Digre KB. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review. Neurology. 1995;45:6-10.
- Sodhi M, Sheldon CA, Carleton B, et al. Oral fluoroquinolones and risk of secondary pseudotumor cerebri syndrome: nested case-control study. Neurology. 2017;89:792-795.
- Sundholm A, Burkill S, Sveinsson O, et al. Population‐based incidence and clinical characteristics of idiopathic intracranial hypertension. Acta Neurologica Scandinavica. 2017;136:427-433.
- Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126:116-121.
To the Editor:
In 1995, one of the authors (A.G.L.) reported the case of a 14-year-old boy who was diagnosed with pseudotumor cerebri following treatment with isotretinoin and tetracycline, both of which were implicated in the development of elevated intracranial pressure (ICP). The patient subsequently underwent optic nerve sheath fenestration for decompression due to progressive deterioration of the visual field despite discontinuation of both drugs.1
This patient recently returned to our office 28 years after his initial presentation with a recurrence of similar symptoms. He was subsequently diagnosed with elevated ICP after a single dose of doxycycline. His vision was 20/20 with correction for distance. Pupil size and extraocular motility were within normal limits. Physical examination was normal, and a dilated fundus examination showed a Frisen stage 1 disc edema in the right eye and a Frisen stage 3 disc edema in the left eye at presentation. The visual field showed enlarged blind spots in both eyes consistent with papilledema. Optical coherence tomography for optic nerve was 93 µm in the right eye and 124 µm in the left eye compared to earlier measures of 66 and 68 µm in the right and left eyes, respectively, indicating pseudonormalization of the parameters (disc edema in the setting of prior optic atrophy). In the setting of optic atrophy, when the nerve develops any swelling the thickness measured on optical coherence tomography may reach normal values, which are in fact abnormal and elevated in this case. Magnetic resonance imaging and magnetic resonance venography were within normal limits. Cerebrospinal fluid opening pressure was 26 cm of water, and analysis revealed high levels of West Nile virus antibodies (IgM and IgG), suggesting a recent viral infection. In addition to an established predisposition to develop elevated ICP on tetracycline antibiotics, this patient also had the precipitating factor of recent viral infection contributing to his raised ICP. Prior to his most recent presentation, his condition was stable with evidence of mild optic atrophy in both eyes and stable visual fields.
Various case reports have linked the use of tetracycline antibiotics to increased ICP. Gardner et al2 reported a case of fraternal twins who developed elevated ICP while on tetracycline for acne, suggesting a possible genetic susceptibility. In one nested case-control study, it was found that the relative risk (RR) of developing elevated ICP with tetracycline antibiotics was increased (RR=2.68 [95% CI, 0.89-8.11] for 15 days of current use; RR=3.64 [95% CI, 1.67-7.91] for 30 days of current use).3 Retrospective studies have demonstrated that 9% of the population (N=207) had prior treatment with tetracylines in a cohort of patients diagnosed with elevated ICP.4
In this group of drugs, minocycline has been closely associated with development of elevated ICP. One retrospective study showed that 75% of patients (9/12) with minocycline-associated ICP developed symptoms of elevated ICP within 8 weeks of starting therapy; however, half of the patients included in the study were obese. The inclusion of obese patients in this study is a confounding variable because idiopathic intracranial hypertension (IIH) is a disease that predominantly affects obese young females. The diagnosis of IIH, however, should be considered a diagnosis of exclusion, and it is uncommon in thin elderly or male patients.5
Tetracyclines have a half-life of 6 to 11 hours, and usually the elevated ICP decreases once the offending agent is discontinued, though papilledema could take months to resolve.
We describe an inadvertent rechallenge
To the Editor:
In 1995, one of the authors (A.G.L.) reported the case of a 14-year-old boy who was diagnosed with pseudotumor cerebri following treatment with isotretinoin and tetracycline, both of which were implicated in the development of elevated intracranial pressure (ICP). The patient subsequently underwent optic nerve sheath fenestration for decompression due to progressive deterioration of the visual field despite discontinuation of both drugs.1
This patient recently returned to our office 28 years after his initial presentation with a recurrence of similar symptoms. He was subsequently diagnosed with elevated ICP after a single dose of doxycycline. His vision was 20/20 with correction for distance. Pupil size and extraocular motility were within normal limits. Physical examination was normal, and a dilated fundus examination showed a Frisen stage 1 disc edema in the right eye and a Frisen stage 3 disc edema in the left eye at presentation. The visual field showed enlarged blind spots in both eyes consistent with papilledema. Optical coherence tomography for optic nerve was 93 µm in the right eye and 124 µm in the left eye compared to earlier measures of 66 and 68 µm in the right and left eyes, respectively, indicating pseudonormalization of the parameters (disc edema in the setting of prior optic atrophy). In the setting of optic atrophy, when the nerve develops any swelling the thickness measured on optical coherence tomography may reach normal values, which are in fact abnormal and elevated in this case. Magnetic resonance imaging and magnetic resonance venography were within normal limits. Cerebrospinal fluid opening pressure was 26 cm of water, and analysis revealed high levels of West Nile virus antibodies (IgM and IgG), suggesting a recent viral infection. In addition to an established predisposition to develop elevated ICP on tetracycline antibiotics, this patient also had the precipitating factor of recent viral infection contributing to his raised ICP. Prior to his most recent presentation, his condition was stable with evidence of mild optic atrophy in both eyes and stable visual fields.
Various case reports have linked the use of tetracycline antibiotics to increased ICP. Gardner et al2 reported a case of fraternal twins who developed elevated ICP while on tetracycline for acne, suggesting a possible genetic susceptibility. In one nested case-control study, it was found that the relative risk (RR) of developing elevated ICP with tetracycline antibiotics was increased (RR=2.68 [95% CI, 0.89-8.11] for 15 days of current use; RR=3.64 [95% CI, 1.67-7.91] for 30 days of current use).3 Retrospective studies have demonstrated that 9% of the population (N=207) had prior treatment with tetracylines in a cohort of patients diagnosed with elevated ICP.4
In this group of drugs, minocycline has been closely associated with development of elevated ICP. One retrospective study showed that 75% of patients (9/12) with minocycline-associated ICP developed symptoms of elevated ICP within 8 weeks of starting therapy; however, half of the patients included in the study were obese. The inclusion of obese patients in this study is a confounding variable because idiopathic intracranial hypertension (IIH) is a disease that predominantly affects obese young females. The diagnosis of IIH, however, should be considered a diagnosis of exclusion, and it is uncommon in thin elderly or male patients.5
Tetracyclines have a half-life of 6 to 11 hours, and usually the elevated ICP decreases once the offending agent is discontinued, though papilledema could take months to resolve.
We describe an inadvertent rechallenge
- Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis. 1995;55:165-168.
- Gardner K, Cox T, Digre KB. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review. Neurology. 1995;45:6-10.
- Sodhi M, Sheldon CA, Carleton B, et al. Oral fluoroquinolones and risk of secondary pseudotumor cerebri syndrome: nested case-control study. Neurology. 2017;89:792-795.
- Sundholm A, Burkill S, Sveinsson O, et al. Population‐based incidence and clinical characteristics of idiopathic intracranial hypertension. Acta Neurologica Scandinavica. 2017;136:427-433.
- Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126:116-121.
- Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis. 1995;55:165-168.
- Gardner K, Cox T, Digre KB. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review. Neurology. 1995;45:6-10.
- Sodhi M, Sheldon CA, Carleton B, et al. Oral fluoroquinolones and risk of secondary pseudotumor cerebri syndrome: nested case-control study. Neurology. 2017;89:792-795.
- Sundholm A, Burkill S, Sveinsson O, et al. Population‐based incidence and clinical characteristics of idiopathic intracranial hypertension. Acta Neurologica Scandinavica. 2017;136:427-433.
- Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126:116-121.
A Closer Look at the New Biopsy Codes
Effective January 1, 2019, the 2 long-standing Current Procedural Terminology (CPT) biopsy codes 11100 (first lesion) and 11101 (each additional lesion biopsied on the same date of service) were replaced by a series of new biopsy codes that are specific to the method of removal, including tangential (11102, +11103), punch (11104, +11105), and incisional biopsies (11106, +11107)(Table).1,2 If a biopsy is performed using multiple techniques, only a single primary code of the highest value would be reported (ie, incisional>punch>tangential). The add-on codes are to be used for each additional lesion biopsied using the same or a different technique on the same day.
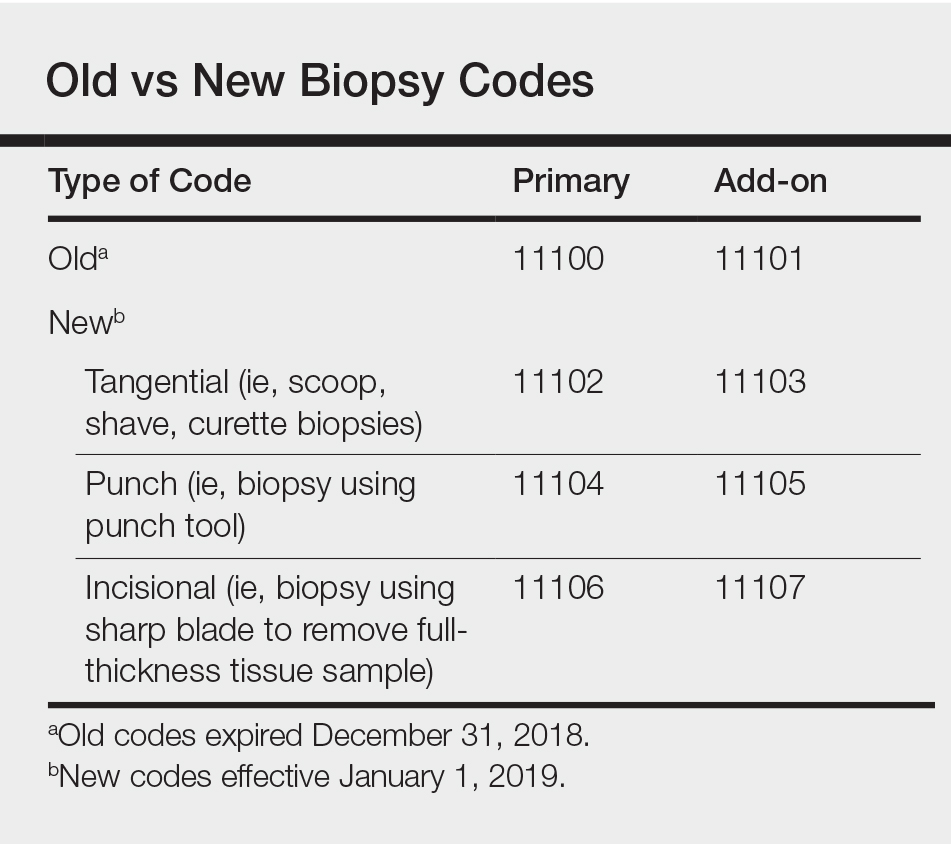
Tangential biopsies, performed with a sharp blade to remove epidermal tissue, include scoop, shave, and curette biopsies. Punch biopsies are performed using a punch tool, while incisional biopsies involve the use of a sharp blade to remove a full-thickness tissue sample.
Using Biopsy Codes Correctly
Only one primary biopsy code is to be reported on a given date. If 2 or more biopsies are performed using 2 or more techniques, then additional biopsies are to be reported using the relevant add-on codes. It is important to note that codes 11104 to 11107 include simple closures, which should not be coded separately.
In all cases, appropriate documentation is essential to support proper biopsy coding. Prior to any biopsy, patients should be educated on the associated risks, including bleeding, infection, and scarring, and patient consent should be obtained and documented in the medical record.
It bears noting the distinction between biopsies and excisions and their associated codes. Biopsies are performed for diagnostic purposes, with samples sent for histopathologic evaluation. Excisions are performed to entirely remove a lesion; it makes no difference whether or not the excised tissue is sent for histopathologic evaluation.
Reimbursement for biopsies has changed in 2019, with the rates for tangential biopsies decreasing relative to 2018 (−10.6%), while the rates for punch (+12.5%) and incisional (+35.5%) biopsies will be increasing.3
New Codes in Action
The following examples demonstrate how to use the new biopsy codes correctly in clinical practice.
A patient presents for evaluation of 3 lesions that he deems suspicious: 1 on the neck, 1 on the left upper arm, and 1 on the right lower arm. The dermatologist diagnoses the lesion on the neck as a seborrheic keratosis, a benign lesion, and the patient declines treatment. The lesions on each arm are suspicious for basal cell carcinoma, and the dermatologist performs shave biopsies at both sites to determine an accurate diagnosis. In this case, you would use CPT code 11102 (tangential biopsy of skin) for the first lesion and 11103 (tangential biopsy of skin, each additional lesion) for the second lesion.
A patient presents for evaluation of an itchy rash on both hands. On physical examination you observe small, firm, slightly erythematous papules in a ring formation on both hands. The patient says similar lesions have appeared and resolved in the past. She says she has sensitive skin and assumes the rash may have been caused by exposure to an irritating soap. The patient also points out a suspicious lesion on the right side of the upper back that seems to have grown in size over the last year. Based on the recurrence of the lesions on the hands and the characteristic formation of the papules, the dermatologist suspects granuloma annulare and performs a punch biopsy to confirm the diagnosis via histopathology. The lesion on the back is suspicious for melanoma, so the dermatologist performs an incisional biopsy of the lesion. For this patient, you would use CPT code 11106 (incisional biopsy) for the lesion on the back and 11105 (punch biopsy, each additional lesion) for the biopsy of the hand.
- Verhovshek J. CPT 2019 unveils tangential biopsy codes, more. American Academy of Professional Coders website. https://www.aapc.com/blog/44366-cpt-2019-unveils-tangential-biopsy-codes/. Published October 19, 2018. Accessed February 7, 2018.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 Monitor website. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies. Updated January 7, 2019. Accessed February 7, 2019.
- Kaufmann M. Coming soon: new biopsy codes. Practical Dermatol. 2018;15:18.
Effective January 1, 2019, the 2 long-standing Current Procedural Terminology (CPT) biopsy codes 11100 (first lesion) and 11101 (each additional lesion biopsied on the same date of service) were replaced by a series of new biopsy codes that are specific to the method of removal, including tangential (11102, +11103), punch (11104, +11105), and incisional biopsies (11106, +11107)(Table).1,2 If a biopsy is performed using multiple techniques, only a single primary code of the highest value would be reported (ie, incisional>punch>tangential). The add-on codes are to be used for each additional lesion biopsied using the same or a different technique on the same day.

Tangential biopsies, performed with a sharp blade to remove epidermal tissue, include scoop, shave, and curette biopsies. Punch biopsies are performed using a punch tool, while incisional biopsies involve the use of a sharp blade to remove a full-thickness tissue sample.
Using Biopsy Codes Correctly
Only one primary biopsy code is to be reported on a given date. If 2 or more biopsies are performed using 2 or more techniques, then additional biopsies are to be reported using the relevant add-on codes. It is important to note that codes 11104 to 11107 include simple closures, which should not be coded separately.
In all cases, appropriate documentation is essential to support proper biopsy coding. Prior to any biopsy, patients should be educated on the associated risks, including bleeding, infection, and scarring, and patient consent should be obtained and documented in the medical record.
It bears noting the distinction between biopsies and excisions and their associated codes. Biopsies are performed for diagnostic purposes, with samples sent for histopathologic evaluation. Excisions are performed to entirely remove a lesion; it makes no difference whether or not the excised tissue is sent for histopathologic evaluation.
Reimbursement for biopsies has changed in 2019, with the rates for tangential biopsies decreasing relative to 2018 (−10.6%), while the rates for punch (+12.5%) and incisional (+35.5%) biopsies will be increasing.3
New Codes in Action
The following examples demonstrate how to use the new biopsy codes correctly in clinical practice.
A patient presents for evaluation of 3 lesions that he deems suspicious: 1 on the neck, 1 on the left upper arm, and 1 on the right lower arm. The dermatologist diagnoses the lesion on the neck as a seborrheic keratosis, a benign lesion, and the patient declines treatment. The lesions on each arm are suspicious for basal cell carcinoma, and the dermatologist performs shave biopsies at both sites to determine an accurate diagnosis. In this case, you would use CPT code 11102 (tangential biopsy of skin) for the first lesion and 11103 (tangential biopsy of skin, each additional lesion) for the second lesion.
A patient presents for evaluation of an itchy rash on both hands. On physical examination you observe small, firm, slightly erythematous papules in a ring formation on both hands. The patient says similar lesions have appeared and resolved in the past. She says she has sensitive skin and assumes the rash may have been caused by exposure to an irritating soap. The patient also points out a suspicious lesion on the right side of the upper back that seems to have grown in size over the last year. Based on the recurrence of the lesions on the hands and the characteristic formation of the papules, the dermatologist suspects granuloma annulare and performs a punch biopsy to confirm the diagnosis via histopathology. The lesion on the back is suspicious for melanoma, so the dermatologist performs an incisional biopsy of the lesion. For this patient, you would use CPT code 11106 (incisional biopsy) for the lesion on the back and 11105 (punch biopsy, each additional lesion) for the biopsy of the hand.
Effective January 1, 2019, the 2 long-standing Current Procedural Terminology (CPT) biopsy codes 11100 (first lesion) and 11101 (each additional lesion biopsied on the same date of service) were replaced by a series of new biopsy codes that are specific to the method of removal, including tangential (11102, +11103), punch (11104, +11105), and incisional biopsies (11106, +11107)(Table).1,2 If a biopsy is performed using multiple techniques, only a single primary code of the highest value would be reported (ie, incisional>punch>tangential). The add-on codes are to be used for each additional lesion biopsied using the same or a different technique on the same day.

Tangential biopsies, performed with a sharp blade to remove epidermal tissue, include scoop, shave, and curette biopsies. Punch biopsies are performed using a punch tool, while incisional biopsies involve the use of a sharp blade to remove a full-thickness tissue sample.
Using Biopsy Codes Correctly
Only one primary biopsy code is to be reported on a given date. If 2 or more biopsies are performed using 2 or more techniques, then additional biopsies are to be reported using the relevant add-on codes. It is important to note that codes 11104 to 11107 include simple closures, which should not be coded separately.
In all cases, appropriate documentation is essential to support proper biopsy coding. Prior to any biopsy, patients should be educated on the associated risks, including bleeding, infection, and scarring, and patient consent should be obtained and documented in the medical record.
It bears noting the distinction between biopsies and excisions and their associated codes. Biopsies are performed for diagnostic purposes, with samples sent for histopathologic evaluation. Excisions are performed to entirely remove a lesion; it makes no difference whether or not the excised tissue is sent for histopathologic evaluation.
Reimbursement for biopsies has changed in 2019, with the rates for tangential biopsies decreasing relative to 2018 (−10.6%), while the rates for punch (+12.5%) and incisional (+35.5%) biopsies will be increasing.3
New Codes in Action
The following examples demonstrate how to use the new biopsy codes correctly in clinical practice.
A patient presents for evaluation of 3 lesions that he deems suspicious: 1 on the neck, 1 on the left upper arm, and 1 on the right lower arm. The dermatologist diagnoses the lesion on the neck as a seborrheic keratosis, a benign lesion, and the patient declines treatment. The lesions on each arm are suspicious for basal cell carcinoma, and the dermatologist performs shave biopsies at both sites to determine an accurate diagnosis. In this case, you would use CPT code 11102 (tangential biopsy of skin) for the first lesion and 11103 (tangential biopsy of skin, each additional lesion) for the second lesion.
A patient presents for evaluation of an itchy rash on both hands. On physical examination you observe small, firm, slightly erythematous papules in a ring formation on both hands. The patient says similar lesions have appeared and resolved in the past. She says she has sensitive skin and assumes the rash may have been caused by exposure to an irritating soap. The patient also points out a suspicious lesion on the right side of the upper back that seems to have grown in size over the last year. Based on the recurrence of the lesions on the hands and the characteristic formation of the papules, the dermatologist suspects granuloma annulare and performs a punch biopsy to confirm the diagnosis via histopathology. The lesion on the back is suspicious for melanoma, so the dermatologist performs an incisional biopsy of the lesion. For this patient, you would use CPT code 11106 (incisional biopsy) for the lesion on the back and 11105 (punch biopsy, each additional lesion) for the biopsy of the hand.
- Verhovshek J. CPT 2019 unveils tangential biopsy codes, more. American Academy of Professional Coders website. https://www.aapc.com/blog/44366-cpt-2019-unveils-tangential-biopsy-codes/. Published October 19, 2018. Accessed February 7, 2018.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 Monitor website. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies. Updated January 7, 2019. Accessed February 7, 2019.
- Kaufmann M. Coming soon: new biopsy codes. Practical Dermatol. 2018;15:18.
- Verhovshek J. CPT 2019 unveils tangential biopsy codes, more. American Academy of Professional Coders website. https://www.aapc.com/blog/44366-cpt-2019-unveils-tangential-biopsy-codes/. Published October 19, 2018. Accessed February 7, 2018.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 Monitor website. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies. Updated January 7, 2019. Accessed February 7, 2019.
- Kaufmann M. Coming soon: new biopsy codes. Practical Dermatol. 2018;15:18.
Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.
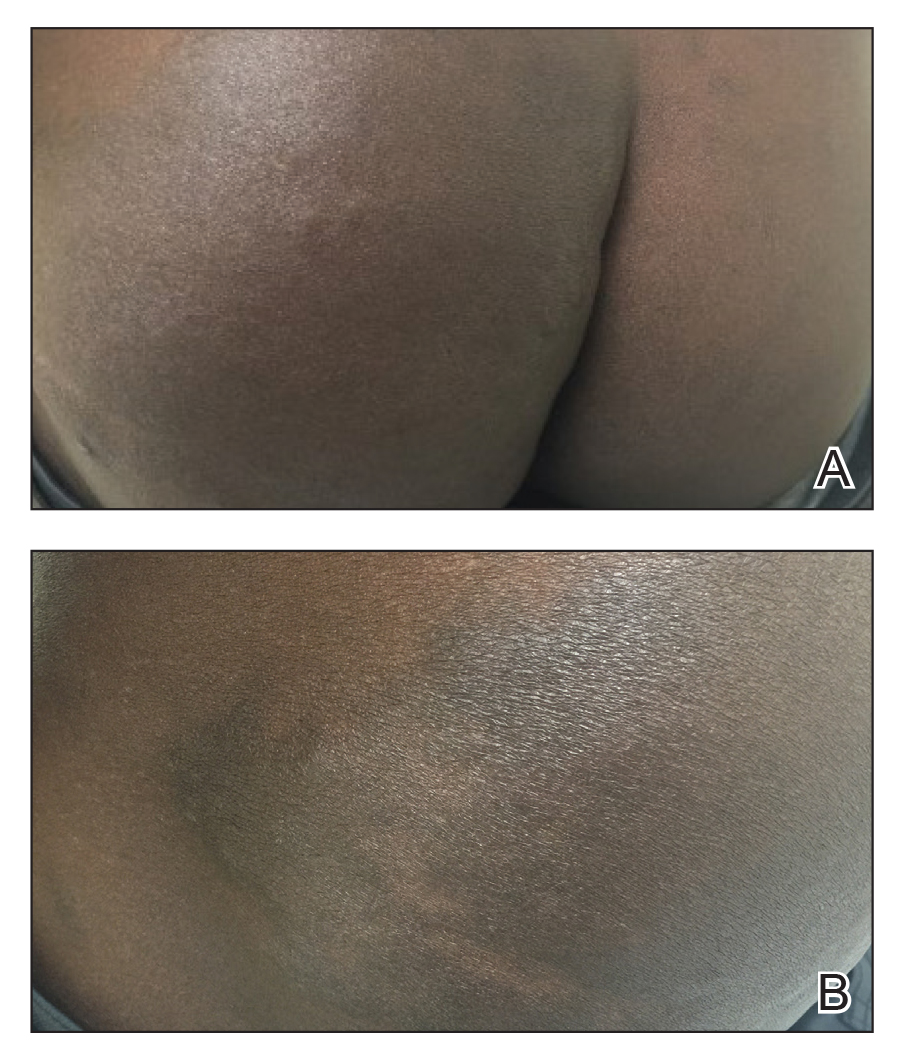
Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.
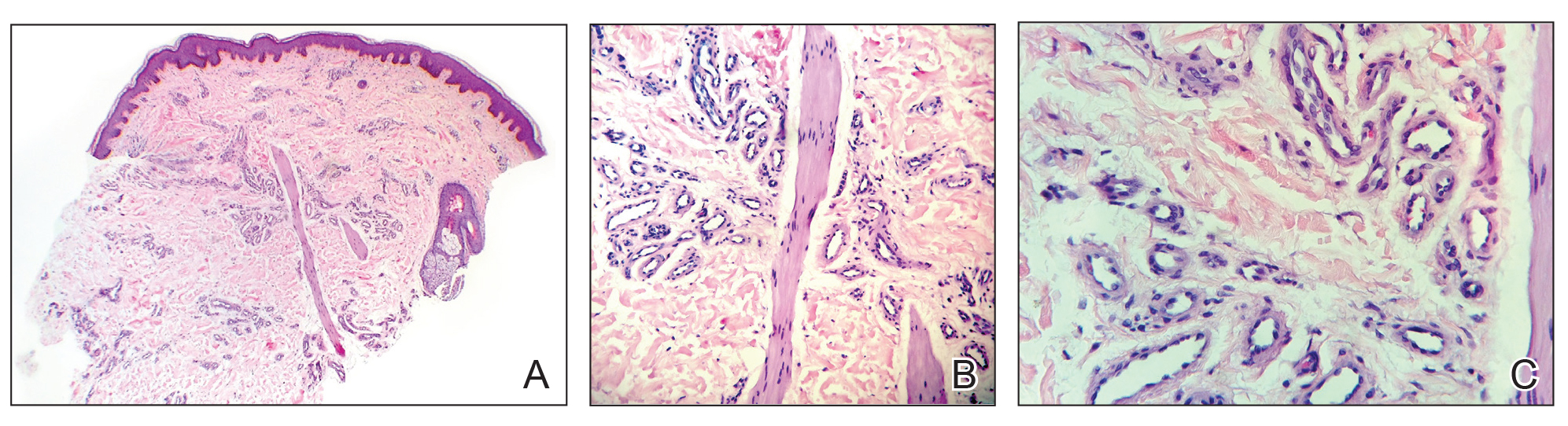
Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32
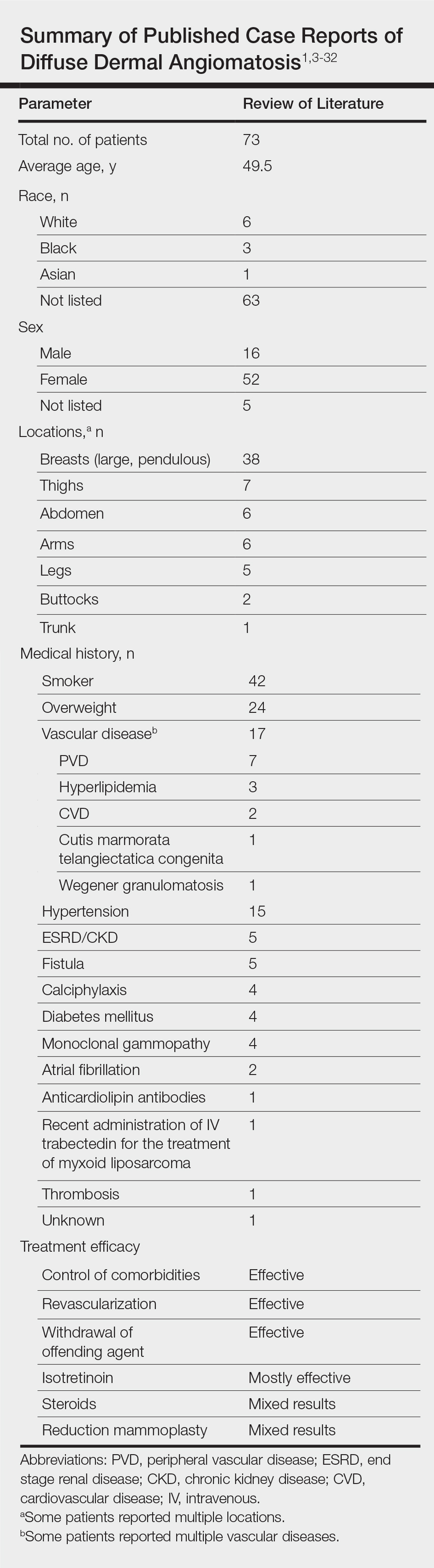
Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.

Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.

Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32

Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.

Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.

Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32

Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
Practice Points
- Diffuse dermal angiomatosis is commonly reported in patients with hypoxic comorbidities such as smoking or vascular disease as well as in women with large pendulous breasts.
- Effective treatments include control of comorbidities, revascularization, withdrawal of the offending agent, steroids, and isotretinoin.