User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Topical ivermectin study sheds light on dysbiosis in rosacea
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2023
A new long COVID explanation: Low serotonin levels?
Could antidepressants hold the key to treating long COVID? The study even points to a possible treatment.
Serotonin is a neurotransmitter that has many functions in the body and is targeted by the most commonly prescribed antidepressants – the selective serotonin reuptake inhibitors.
Serotonin is widely studied for its effects on the brain – it regulates the messaging between neurons, affecting sleep, mood, and memory. It is present in the gut, is found in cells along the gastrointestinal tract, and is absorbed by blood platelets. Gut serotonin, known as circulating serotonin, is responsible for a host of other functions, including the regulation of blood flow, body temperature, and digestion.
Low levels of serotonin could result in any number of seemingly unrelated symptoms, as in the case of long COVID, experts say. The condition affects about 7% of Americans and is associated with a wide range of health problems, including fatigue, shortness of breath, neurological symptoms, joint pain, blood clots, heart palpitations, and digestive problems.
Long COVID is difficult to treat because researchers haven’t been able to pinpoint the underlying mechanisms that cause prolonged illness after a SARS-CoV-2 infection, said study author Christoph A. Thaiss, PhD, an assistant professor of microbiology at the Perelman School of Medicine at the University of Pennsylvania.
The hope is that this study could have implications for new treatments, he said.
“Long COVID can have manifestations not only in the brain but in many different parts of the body, so it’s possible that serotonin reductions are involved in many different aspects of the disease,” said Dr. Thaiss.
Dr. Thaiss’s study, published in the journal Cell, found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but who fully recovered.
His team found that reductions in serotonin were driven by low levels of circulating SARS-CoV-2 virus that caused persistent inflammation as well as an inability of the body to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactive blood platelets were also shown to play a role; they serve as the primary means of serotonin absorption.
The study doesn’t make any recommendations for treatment, but understanding the role of serotonin in long COVID opens the door to a host of novel ideas that could set the stage for clinical trials and affect care.
“The study gives us a few possible targets that could be used in future clinical studies,” Dr. Thaiss said.
Persistent circulating virus is one of the drivers of low serotonin levels, said study author Michael Peluso, MD, an assistant research professor of infectious medicine at the University of California, San Francisco, School of Medicine. This points to the need to reduce viral load using antiviral medications like nirmatrelvir/ritonavir (Paxlovid), which is approved by the U.S. Food and Drug Administration for the treatment of COVID-19, and VV116, which has not yet been approved for use against COVID.
Research published in the New England Journal of Medicine found that the oral antiviral agent VV116 was as effective as nirmatrelvir/ritonavir in reducing the body’s viral load and aiding recovery from SARS-CoV-2 infection. Paxlovid has also been shown to reduce the likelihood of getting long COVID after an acute SARS-CoV-2 infection.
Researchers are investigating ways to target serotonin levels directly, potentially using SSRIs. But first they need to study whether improvement in serotonin level makes a difference.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said.
Indeed, the research did show that the SSRI fluoxetine, as well as a glycine-tryptophan supplement, improved cognitive function in SARS-CoV-2-infected rodent models, which were used in a portion of the study.
David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City, said the research is helping “to paint a biological picture” that’s in line with other research on the mechanisms that cause long COVID symptoms.
But Dr. Putrino, who was not involved in the study, cautions against treating long COVID patients with SSRIs or any other treatment that increases serotonin before testing patients to determine whether their serotonin levels are actually lower than those of healthy persons.
“We don’t want to assume that every patient with long COVID is going to have lower serotonin levels,” said Dr. Putrino.
What’s more, researchers need to investigate whether SSRIs increase levels of circulating serotonin. It’s important to note that researchers found lower levels of circulating serotonin but that serotonin levels in the brain remained normal.
Traditionally, SSRIs are used clinically for increasing the levels of serotonin in the brain, not the body.
“Whether that’s going to contribute to an increase in systemic levels of serotonin, that’s something that needs to be tested,” said Akiko Iwasaki, PhD, co-lead investigator of the Yale School of Medicine, New Haven, Conn., COVID-19 Recovery Study, who was not involved in the research.
Thus far, investigators have not identified one unifying biomarker that seems to cause long COVID in all patients, said Dr. Iwasaki. Some research has found higher levels of certain immune cells and biomarkers: for example, monocytes and activated B lymphocytes, indicating a stronger and ongoing antibody response to the virus. Other recent research conducted by Dr. Iwasaki, Dr. Putrino, and others, published in the journal Nature, showed that long COVID patients tend to have lower levels of cortisol, which could be a factor in the extreme fatigue experienced by many who suffer from the condition.
The findings in the study in The Cell are promising, but they need to be replicated in more people, said Dr. Iwasaki. And even if they’re replicated in a larger study population, this would still be just one biomarker that is associated with one subtype of the disease. There is a need to better understand which biomarkers go with which symptoms so that the most effective treatments can be identified, she said.
Both Dr. Putrino and Dr. Iwasaki contended that there isn’t a single factor that can explain all of long COVID. It’s a complex disease caused by a host of different mechanisms.
Still, low levels of serotonin could be an important piece of the puzzle. The next step, said Dr. Iwasaki, is to uncover how many of the millions of Americans with long COVID have this biomarker.
“People working in the field of long COVID should now be considering this pathway and thinking of ways to measure serotonin in their patients.”
A version of this article first appeared on Medscape.com.
Could antidepressants hold the key to treating long COVID? The study even points to a possible treatment.
Serotonin is a neurotransmitter that has many functions in the body and is targeted by the most commonly prescribed antidepressants – the selective serotonin reuptake inhibitors.
Serotonin is widely studied for its effects on the brain – it regulates the messaging between neurons, affecting sleep, mood, and memory. It is present in the gut, is found in cells along the gastrointestinal tract, and is absorbed by blood platelets. Gut serotonin, known as circulating serotonin, is responsible for a host of other functions, including the regulation of blood flow, body temperature, and digestion.
Low levels of serotonin could result in any number of seemingly unrelated symptoms, as in the case of long COVID, experts say. The condition affects about 7% of Americans and is associated with a wide range of health problems, including fatigue, shortness of breath, neurological symptoms, joint pain, blood clots, heart palpitations, and digestive problems.
Long COVID is difficult to treat because researchers haven’t been able to pinpoint the underlying mechanisms that cause prolonged illness after a SARS-CoV-2 infection, said study author Christoph A. Thaiss, PhD, an assistant professor of microbiology at the Perelman School of Medicine at the University of Pennsylvania.
The hope is that this study could have implications for new treatments, he said.
“Long COVID can have manifestations not only in the brain but in many different parts of the body, so it’s possible that serotonin reductions are involved in many different aspects of the disease,” said Dr. Thaiss.
Dr. Thaiss’s study, published in the journal Cell, found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but who fully recovered.
His team found that reductions in serotonin were driven by low levels of circulating SARS-CoV-2 virus that caused persistent inflammation as well as an inability of the body to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactive blood platelets were also shown to play a role; they serve as the primary means of serotonin absorption.
The study doesn’t make any recommendations for treatment, but understanding the role of serotonin in long COVID opens the door to a host of novel ideas that could set the stage for clinical trials and affect care.
“The study gives us a few possible targets that could be used in future clinical studies,” Dr. Thaiss said.
Persistent circulating virus is one of the drivers of low serotonin levels, said study author Michael Peluso, MD, an assistant research professor of infectious medicine at the University of California, San Francisco, School of Medicine. This points to the need to reduce viral load using antiviral medications like nirmatrelvir/ritonavir (Paxlovid), which is approved by the U.S. Food and Drug Administration for the treatment of COVID-19, and VV116, which has not yet been approved for use against COVID.
Research published in the New England Journal of Medicine found that the oral antiviral agent VV116 was as effective as nirmatrelvir/ritonavir in reducing the body’s viral load and aiding recovery from SARS-CoV-2 infection. Paxlovid has also been shown to reduce the likelihood of getting long COVID after an acute SARS-CoV-2 infection.
Researchers are investigating ways to target serotonin levels directly, potentially using SSRIs. But first they need to study whether improvement in serotonin level makes a difference.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said.
Indeed, the research did show that the SSRI fluoxetine, as well as a glycine-tryptophan supplement, improved cognitive function in SARS-CoV-2-infected rodent models, which were used in a portion of the study.
David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City, said the research is helping “to paint a biological picture” that’s in line with other research on the mechanisms that cause long COVID symptoms.
But Dr. Putrino, who was not involved in the study, cautions against treating long COVID patients with SSRIs or any other treatment that increases serotonin before testing patients to determine whether their serotonin levels are actually lower than those of healthy persons.
“We don’t want to assume that every patient with long COVID is going to have lower serotonin levels,” said Dr. Putrino.
What’s more, researchers need to investigate whether SSRIs increase levels of circulating serotonin. It’s important to note that researchers found lower levels of circulating serotonin but that serotonin levels in the brain remained normal.
Traditionally, SSRIs are used clinically for increasing the levels of serotonin in the brain, not the body.
“Whether that’s going to contribute to an increase in systemic levels of serotonin, that’s something that needs to be tested,” said Akiko Iwasaki, PhD, co-lead investigator of the Yale School of Medicine, New Haven, Conn., COVID-19 Recovery Study, who was not involved in the research.
Thus far, investigators have not identified one unifying biomarker that seems to cause long COVID in all patients, said Dr. Iwasaki. Some research has found higher levels of certain immune cells and biomarkers: for example, monocytes and activated B lymphocytes, indicating a stronger and ongoing antibody response to the virus. Other recent research conducted by Dr. Iwasaki, Dr. Putrino, and others, published in the journal Nature, showed that long COVID patients tend to have lower levels of cortisol, which could be a factor in the extreme fatigue experienced by many who suffer from the condition.
The findings in the study in The Cell are promising, but they need to be replicated in more people, said Dr. Iwasaki. And even if they’re replicated in a larger study population, this would still be just one biomarker that is associated with one subtype of the disease. There is a need to better understand which biomarkers go with which symptoms so that the most effective treatments can be identified, she said.
Both Dr. Putrino and Dr. Iwasaki contended that there isn’t a single factor that can explain all of long COVID. It’s a complex disease caused by a host of different mechanisms.
Still, low levels of serotonin could be an important piece of the puzzle. The next step, said Dr. Iwasaki, is to uncover how many of the millions of Americans with long COVID have this biomarker.
“People working in the field of long COVID should now be considering this pathway and thinking of ways to measure serotonin in their patients.”
A version of this article first appeared on Medscape.com.
Could antidepressants hold the key to treating long COVID? The study even points to a possible treatment.
Serotonin is a neurotransmitter that has many functions in the body and is targeted by the most commonly prescribed antidepressants – the selective serotonin reuptake inhibitors.
Serotonin is widely studied for its effects on the brain – it regulates the messaging between neurons, affecting sleep, mood, and memory. It is present in the gut, is found in cells along the gastrointestinal tract, and is absorbed by blood platelets. Gut serotonin, known as circulating serotonin, is responsible for a host of other functions, including the regulation of blood flow, body temperature, and digestion.
Low levels of serotonin could result in any number of seemingly unrelated symptoms, as in the case of long COVID, experts say. The condition affects about 7% of Americans and is associated with a wide range of health problems, including fatigue, shortness of breath, neurological symptoms, joint pain, blood clots, heart palpitations, and digestive problems.
Long COVID is difficult to treat because researchers haven’t been able to pinpoint the underlying mechanisms that cause prolonged illness after a SARS-CoV-2 infection, said study author Christoph A. Thaiss, PhD, an assistant professor of microbiology at the Perelman School of Medicine at the University of Pennsylvania.
The hope is that this study could have implications for new treatments, he said.
“Long COVID can have manifestations not only in the brain but in many different parts of the body, so it’s possible that serotonin reductions are involved in many different aspects of the disease,” said Dr. Thaiss.
Dr. Thaiss’s study, published in the journal Cell, found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but who fully recovered.
His team found that reductions in serotonin were driven by low levels of circulating SARS-CoV-2 virus that caused persistent inflammation as well as an inability of the body to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactive blood platelets were also shown to play a role; they serve as the primary means of serotonin absorption.
The study doesn’t make any recommendations for treatment, but understanding the role of serotonin in long COVID opens the door to a host of novel ideas that could set the stage for clinical trials and affect care.
“The study gives us a few possible targets that could be used in future clinical studies,” Dr. Thaiss said.
Persistent circulating virus is one of the drivers of low serotonin levels, said study author Michael Peluso, MD, an assistant research professor of infectious medicine at the University of California, San Francisco, School of Medicine. This points to the need to reduce viral load using antiviral medications like nirmatrelvir/ritonavir (Paxlovid), which is approved by the U.S. Food and Drug Administration for the treatment of COVID-19, and VV116, which has not yet been approved for use against COVID.
Research published in the New England Journal of Medicine found that the oral antiviral agent VV116 was as effective as nirmatrelvir/ritonavir in reducing the body’s viral load and aiding recovery from SARS-CoV-2 infection. Paxlovid has also been shown to reduce the likelihood of getting long COVID after an acute SARS-CoV-2 infection.
Researchers are investigating ways to target serotonin levels directly, potentially using SSRIs. But first they need to study whether improvement in serotonin level makes a difference.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said.
Indeed, the research did show that the SSRI fluoxetine, as well as a glycine-tryptophan supplement, improved cognitive function in SARS-CoV-2-infected rodent models, which were used in a portion of the study.
David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City, said the research is helping “to paint a biological picture” that’s in line with other research on the mechanisms that cause long COVID symptoms.
But Dr. Putrino, who was not involved in the study, cautions against treating long COVID patients with SSRIs or any other treatment that increases serotonin before testing patients to determine whether their serotonin levels are actually lower than those of healthy persons.
“We don’t want to assume that every patient with long COVID is going to have lower serotonin levels,” said Dr. Putrino.
What’s more, researchers need to investigate whether SSRIs increase levels of circulating serotonin. It’s important to note that researchers found lower levels of circulating serotonin but that serotonin levels in the brain remained normal.
Traditionally, SSRIs are used clinically for increasing the levels of serotonin in the brain, not the body.
“Whether that’s going to contribute to an increase in systemic levels of serotonin, that’s something that needs to be tested,” said Akiko Iwasaki, PhD, co-lead investigator of the Yale School of Medicine, New Haven, Conn., COVID-19 Recovery Study, who was not involved in the research.
Thus far, investigators have not identified one unifying biomarker that seems to cause long COVID in all patients, said Dr. Iwasaki. Some research has found higher levels of certain immune cells and biomarkers: for example, monocytes and activated B lymphocytes, indicating a stronger and ongoing antibody response to the virus. Other recent research conducted by Dr. Iwasaki, Dr. Putrino, and others, published in the journal Nature, showed that long COVID patients tend to have lower levels of cortisol, which could be a factor in the extreme fatigue experienced by many who suffer from the condition.
The findings in the study in The Cell are promising, but they need to be replicated in more people, said Dr. Iwasaki. And even if they’re replicated in a larger study population, this would still be just one biomarker that is associated with one subtype of the disease. There is a need to better understand which biomarkers go with which symptoms so that the most effective treatments can be identified, she said.
Both Dr. Putrino and Dr. Iwasaki contended that there isn’t a single factor that can explain all of long COVID. It’s a complex disease caused by a host of different mechanisms.
Still, low levels of serotonin could be an important piece of the puzzle. The next step, said Dr. Iwasaki, is to uncover how many of the millions of Americans with long COVID have this biomarker.
“People working in the field of long COVID should now be considering this pathway and thinking of ways to measure serotonin in their patients.”
A version of this article first appeared on Medscape.com.
FROM CELL
How to prescribe exercise in 5 steps
Clinicians are well aware of the benefits of physical activity and the consequences of inactivity.
Managing the diseases associated with inactivity – heart disease, type 2 diabetes, hypertension – falls to physicians. So one might assume they routinely prescribe exercise to their patients, just as they would statins, insulin, or beta-blockers.
But evidence indicates that doctors don’t routinely have those conversations. They may lack confidence in their ability to give effective advice, fear offending patients, or simply not know what to say.
That’s understandable. Many doctors receive little training on how to counsel patients to exercise, according to research over the past decade. Despite efforts to improve this, many medical students still feel unprepared to prescribe physical activity to patients.
But here’s the thing: Doctors are in a unique position to change things.
Only 28% of Americans meet physical activity guidelines, according to the U.S. Centers for Disease Control and Prevention. At the same time, other research suggests that patients want to be more active and would like help from their doctor.
“Patients are motivated to hear about physical activity from physicians and try to make a change,” says Jane Thornton, MD, PhD, an assistant professor in family medicine at Western University, Ont. “Just saying something, even if you don’t have specialized knowledge, makes a difference because of the credibility we have as physicians.”
Conveniently, just like exercise, the best way to get started is to ... get started.
Here’s how to break down the process into steps.
1. Ask patients about their physical activity
Think of this as taking any kind of patient history, only for physical activity.
Do they have a regular exercise routine? For how many minutes a day are they active? How many days a week?
“It takes less than a minute to ask and record,” Dr. Thornton says. Once you put it into the patient’s electronic record, you have something you can track.
2. Write an actual prescription
By giving the patient a written, printed prescription when they leave your office, “you’re showing it’s an important part of treatment or prevention,” Dr. Thornton explains. It puts physical activity on the level of a vital sign.
Include frequency, intensity, time, and type of exercise. The American College of Sports Medicine’s Exercise is Medicine initiative provides a prescription template you can use.
3. Measure what they do
Measurement helps the patient adopt the new behavior, and it helps the physician provide tailored advice going forward, Dr. Thornton says.
With the rise of health-monitoring wearables, tracking activity has never been easier. Of course, not everyone wants to (or can afford to) use a smartwatch or fitness tracker.
For tech-averse patients, ask if they’re willing to write something down, like how many minutes they spent walking, or how many yoga classes they attended. You may never get this from some patients, but it never hurts to ask.
4. Refer out when necessary
This brings us to a sticky issue for many physicians: lack of confidence in their ability to speak authoritatively about physical activity. “In most cases, you can absolutely say, ‘Start slow, go gradually,’ that kind of thing,” Dr. Thornton says. “As with anything, confidence will come with practice.”
For specific prescriptive advice, check out the Exercise is Medicine website, which also has handouts you can share with patients and information for specific conditions. If your patient has prediabetes, you can also point them toward the CDC’s diabetes prevention program, which is available in-person or online and may be free or covered by insurance.
If a patient has contraindications, refer out. If you don’t have exercise or rehab professionals in your network, Dr. Thornton recommends reaching out to your regional or national association of sports-medicine professionals. You should be able to find it with a quick Google search.
5. Follow up
Ask about physical activity during every contact, either in person or online.
Dr. Thornton says the second and fifth steps matter most to patients, especially when the prescription and follow-up come from their primary care physician, rather than a nurse or physician assistant to whom you’ve delegated the task.
“The value comes in having a physician emphasize the importance,” Dr. Thornton says. The more time you spend on it, the more that value comes through.
What NOT to say to patients about exercise
This might surprise you:
“I definitely don’t think telling people the official recommendations for physical activity is useful,” says Yoni Freedhoff, MD, an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. “If anything, I’d venture it’s counterproductive.”
It’s not that there’s anything wrong with the recommended minimum – 150 minutes of moderate-to-vigorous-intensity physical activity per week. The problem is what it says to a patient who doesn’t come close to those standards.
“Few real-world people have the interest, time, energy, or privilege to achieve them,” Dr. Freedhoff says. “Many will recognize that instantly and consequently feel [that] less than that is pointless.”
And that, Dr. Thornton says, is categorically not true. “Even minimal physical activity, in some cases, is beneficial.”
You also want to avoid any explicit connection between exercise and weight loss, Dr. Thornton says.
Though many people do connect the two, the link is often negative, notes a 2019 study from the University of Toronto., triggering painful memories that might go all the way back to gym class.
Try this pivot from Dr. Freedhoff: “Focus on the role of exercise in mitigating the risks of weight,” he says – like decreasing pain, increasing energy, and improving sleep.
How to motivate patients to move
New research backs up this more positive approach. In a study published in Annals of Internal Medicine, doctors in the United Kingdom who emphasized benefits and minimized health harms convinced more patients to join a weight management program than negative or neutral docs did. These doctors conveyed optimism and excitement, smiling and avoiding any mention of obesity or body mass index.
Exactly what benefits inspire change will be different for each patient. But in general, the more immediate the benefit, the more motivating it will be.
As the University of Toronto study noted, patients weren’t motivated by vague, distant goals like “increasing life expectancy or avoiding health problems many years in the future.”
They’re much more likely to take action to avoid surgery, reduce medications, or minimize the risk of falling.
For an older patient, Dr. Freedhoff says, “focusing on the preservation of functional independence can be extremely motivating.” That’s especially true if the patient has vivid memories of seeing a sedentary loved one decline late in life.
For patients who may be more focused on appearance, they could respond to the idea of improving their body composition. For that, “we talk about the quality of weight loss,” says Spencer Nadolsky, DO, an obesity and lipid specialist and medical director of WeightWatchers. “Ultimately, exercise helps shape the body instead of just changing the number on the scale.”
Reducing resistance to resistance training
A conversation about reshaping the body or avoiding age-related disabilities leads naturally to resistance training.
“I always frame resistance training as the single most valuable thing a person might do to try to preserve their functional independence,” Dr. Freedhoff says. If the patient is over 65, he won’t wait for them to show an interest. “I’ll absolutely bring it up with them directly.”
Dr. Freedhoff has an on-site training facility where trainers show patients how to work out at home with minimal equipment, like dumbbells and resistance bands.
Most doctors, however, don’t have those options. That can lead to a tricky conversation. Participants in the University of Toronto study told the authors they disliked the gym, finding it “boring, intimidating, or discouraging.”
And yet, “a common suggestion ... from health care providers was to join a gym.”
Many patients, Spencer Nadolsky, MD, says, associate strength training with “grunting, groaning, or getting ‘bulky’ vs. ‘toned.’ ” Memories of soreness from overzealous workouts are another barrier.
He recommends “starting small and slow,” with one or two full-body workouts a week. Those initial workouts might include just one to two sets of four to five exercises. “Consider if someone is exercising at home or in a gym to build a routine around equipment that’s available to them,” Dr. Nadolsky says.
Once you determine what you have to work with, help the patient choose exercises that fit their needs, goals, preferences, limitations, and prior injuries.
One more consideration: While Dr. Nadolsky tries to “stay away from telling a patient they need to do specific types of exercise to be successful,” he makes an exception for patients who’re taking a GLP-1 agonist. “There is a concern for muscle mass loss along with fat loss.”
Practicing, preaching, and checking privilege
When Dr. Thornton, Dr. Freedhoff, and Dr. Nadolsky discuss exercise, their patients know they practice what they preach.
Dr. Nadolsky, who was a nationally ranked wrestler at the University of North Carolina, hosts the Docs Who Lift podcast with his brother, Karl Nadolsky, MD.
Dr. Freedhoff is also a lifter and fitness enthusiast, and Dr. Thornton was a world-class rower whose team came within 0.8 seconds of a silver medal at the Beijing Olympics. (They finished fourth.)
But not all physicians follow their own lifestyle advice, Dr. Freedhoff says. That doesn’t make them bad doctors – it makes them human.
“I’ve done 300 minutes a week of exercise” – the recommended amount for weight maintenance – “to see what’s involved,” Dr. Freedhoff says. “That’s far, far, far from a trivial amount.”
That leads to this advice for his fellow physicians:
“The most important thing to know about exercise is that finding the time and having the health to do so is a privilege,” he says.
Understanding that is crucial for assessing your patient’s needs and providing the right help.
A version of this article first appeared on Medscape.com.
Clinicians are well aware of the benefits of physical activity and the consequences of inactivity.
Managing the diseases associated with inactivity – heart disease, type 2 diabetes, hypertension – falls to physicians. So one might assume they routinely prescribe exercise to their patients, just as they would statins, insulin, or beta-blockers.
But evidence indicates that doctors don’t routinely have those conversations. They may lack confidence in their ability to give effective advice, fear offending patients, or simply not know what to say.
That’s understandable. Many doctors receive little training on how to counsel patients to exercise, according to research over the past decade. Despite efforts to improve this, many medical students still feel unprepared to prescribe physical activity to patients.
But here’s the thing: Doctors are in a unique position to change things.
Only 28% of Americans meet physical activity guidelines, according to the U.S. Centers for Disease Control and Prevention. At the same time, other research suggests that patients want to be more active and would like help from their doctor.
“Patients are motivated to hear about physical activity from physicians and try to make a change,” says Jane Thornton, MD, PhD, an assistant professor in family medicine at Western University, Ont. “Just saying something, even if you don’t have specialized knowledge, makes a difference because of the credibility we have as physicians.”
Conveniently, just like exercise, the best way to get started is to ... get started.
Here’s how to break down the process into steps.
1. Ask patients about their physical activity
Think of this as taking any kind of patient history, only for physical activity.
Do they have a regular exercise routine? For how many minutes a day are they active? How many days a week?
“It takes less than a minute to ask and record,” Dr. Thornton says. Once you put it into the patient’s electronic record, you have something you can track.
2. Write an actual prescription
By giving the patient a written, printed prescription when they leave your office, “you’re showing it’s an important part of treatment or prevention,” Dr. Thornton explains. It puts physical activity on the level of a vital sign.
Include frequency, intensity, time, and type of exercise. The American College of Sports Medicine’s Exercise is Medicine initiative provides a prescription template you can use.
3. Measure what they do
Measurement helps the patient adopt the new behavior, and it helps the physician provide tailored advice going forward, Dr. Thornton says.
With the rise of health-monitoring wearables, tracking activity has never been easier. Of course, not everyone wants to (or can afford to) use a smartwatch or fitness tracker.
For tech-averse patients, ask if they’re willing to write something down, like how many minutes they spent walking, or how many yoga classes they attended. You may never get this from some patients, but it never hurts to ask.
4. Refer out when necessary
This brings us to a sticky issue for many physicians: lack of confidence in their ability to speak authoritatively about physical activity. “In most cases, you can absolutely say, ‘Start slow, go gradually,’ that kind of thing,” Dr. Thornton says. “As with anything, confidence will come with practice.”
For specific prescriptive advice, check out the Exercise is Medicine website, which also has handouts you can share with patients and information for specific conditions. If your patient has prediabetes, you can also point them toward the CDC’s diabetes prevention program, which is available in-person or online and may be free or covered by insurance.
If a patient has contraindications, refer out. If you don’t have exercise or rehab professionals in your network, Dr. Thornton recommends reaching out to your regional or national association of sports-medicine professionals. You should be able to find it with a quick Google search.
5. Follow up
Ask about physical activity during every contact, either in person or online.
Dr. Thornton says the second and fifth steps matter most to patients, especially when the prescription and follow-up come from their primary care physician, rather than a nurse or physician assistant to whom you’ve delegated the task.
“The value comes in having a physician emphasize the importance,” Dr. Thornton says. The more time you spend on it, the more that value comes through.
What NOT to say to patients about exercise
This might surprise you:
“I definitely don’t think telling people the official recommendations for physical activity is useful,” says Yoni Freedhoff, MD, an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. “If anything, I’d venture it’s counterproductive.”
It’s not that there’s anything wrong with the recommended minimum – 150 minutes of moderate-to-vigorous-intensity physical activity per week. The problem is what it says to a patient who doesn’t come close to those standards.
“Few real-world people have the interest, time, energy, or privilege to achieve them,” Dr. Freedhoff says. “Many will recognize that instantly and consequently feel [that] less than that is pointless.”
And that, Dr. Thornton says, is categorically not true. “Even minimal physical activity, in some cases, is beneficial.”
You also want to avoid any explicit connection between exercise and weight loss, Dr. Thornton says.
Though many people do connect the two, the link is often negative, notes a 2019 study from the University of Toronto., triggering painful memories that might go all the way back to gym class.
Try this pivot from Dr. Freedhoff: “Focus on the role of exercise in mitigating the risks of weight,” he says – like decreasing pain, increasing energy, and improving sleep.
How to motivate patients to move
New research backs up this more positive approach. In a study published in Annals of Internal Medicine, doctors in the United Kingdom who emphasized benefits and minimized health harms convinced more patients to join a weight management program than negative or neutral docs did. These doctors conveyed optimism and excitement, smiling and avoiding any mention of obesity or body mass index.
Exactly what benefits inspire change will be different for each patient. But in general, the more immediate the benefit, the more motivating it will be.
As the University of Toronto study noted, patients weren’t motivated by vague, distant goals like “increasing life expectancy or avoiding health problems many years in the future.”
They’re much more likely to take action to avoid surgery, reduce medications, or minimize the risk of falling.
For an older patient, Dr. Freedhoff says, “focusing on the preservation of functional independence can be extremely motivating.” That’s especially true if the patient has vivid memories of seeing a sedentary loved one decline late in life.
For patients who may be more focused on appearance, they could respond to the idea of improving their body composition. For that, “we talk about the quality of weight loss,” says Spencer Nadolsky, DO, an obesity and lipid specialist and medical director of WeightWatchers. “Ultimately, exercise helps shape the body instead of just changing the number on the scale.”
Reducing resistance to resistance training
A conversation about reshaping the body or avoiding age-related disabilities leads naturally to resistance training.
“I always frame resistance training as the single most valuable thing a person might do to try to preserve their functional independence,” Dr. Freedhoff says. If the patient is over 65, he won’t wait for them to show an interest. “I’ll absolutely bring it up with them directly.”
Dr. Freedhoff has an on-site training facility where trainers show patients how to work out at home with minimal equipment, like dumbbells and resistance bands.
Most doctors, however, don’t have those options. That can lead to a tricky conversation. Participants in the University of Toronto study told the authors they disliked the gym, finding it “boring, intimidating, or discouraging.”
And yet, “a common suggestion ... from health care providers was to join a gym.”
Many patients, Spencer Nadolsky, MD, says, associate strength training with “grunting, groaning, or getting ‘bulky’ vs. ‘toned.’ ” Memories of soreness from overzealous workouts are another barrier.
He recommends “starting small and slow,” with one or two full-body workouts a week. Those initial workouts might include just one to two sets of four to five exercises. “Consider if someone is exercising at home or in a gym to build a routine around equipment that’s available to them,” Dr. Nadolsky says.
Once you determine what you have to work with, help the patient choose exercises that fit their needs, goals, preferences, limitations, and prior injuries.
One more consideration: While Dr. Nadolsky tries to “stay away from telling a patient they need to do specific types of exercise to be successful,” he makes an exception for patients who’re taking a GLP-1 agonist. “There is a concern for muscle mass loss along with fat loss.”
Practicing, preaching, and checking privilege
When Dr. Thornton, Dr. Freedhoff, and Dr. Nadolsky discuss exercise, their patients know they practice what they preach.
Dr. Nadolsky, who was a nationally ranked wrestler at the University of North Carolina, hosts the Docs Who Lift podcast with his brother, Karl Nadolsky, MD.
Dr. Freedhoff is also a lifter and fitness enthusiast, and Dr. Thornton was a world-class rower whose team came within 0.8 seconds of a silver medal at the Beijing Olympics. (They finished fourth.)
But not all physicians follow their own lifestyle advice, Dr. Freedhoff says. That doesn’t make them bad doctors – it makes them human.
“I’ve done 300 minutes a week of exercise” – the recommended amount for weight maintenance – “to see what’s involved,” Dr. Freedhoff says. “That’s far, far, far from a trivial amount.”
That leads to this advice for his fellow physicians:
“The most important thing to know about exercise is that finding the time and having the health to do so is a privilege,” he says.
Understanding that is crucial for assessing your patient’s needs and providing the right help.
A version of this article first appeared on Medscape.com.
Clinicians are well aware of the benefits of physical activity and the consequences of inactivity.
Managing the diseases associated with inactivity – heart disease, type 2 diabetes, hypertension – falls to physicians. So one might assume they routinely prescribe exercise to their patients, just as they would statins, insulin, or beta-blockers.
But evidence indicates that doctors don’t routinely have those conversations. They may lack confidence in their ability to give effective advice, fear offending patients, or simply not know what to say.
That’s understandable. Many doctors receive little training on how to counsel patients to exercise, according to research over the past decade. Despite efforts to improve this, many medical students still feel unprepared to prescribe physical activity to patients.
But here’s the thing: Doctors are in a unique position to change things.
Only 28% of Americans meet physical activity guidelines, according to the U.S. Centers for Disease Control and Prevention. At the same time, other research suggests that patients want to be more active and would like help from their doctor.
“Patients are motivated to hear about physical activity from physicians and try to make a change,” says Jane Thornton, MD, PhD, an assistant professor in family medicine at Western University, Ont. “Just saying something, even if you don’t have specialized knowledge, makes a difference because of the credibility we have as physicians.”
Conveniently, just like exercise, the best way to get started is to ... get started.
Here’s how to break down the process into steps.
1. Ask patients about their physical activity
Think of this as taking any kind of patient history, only for physical activity.
Do they have a regular exercise routine? For how many minutes a day are they active? How many days a week?
“It takes less than a minute to ask and record,” Dr. Thornton says. Once you put it into the patient’s electronic record, you have something you can track.
2. Write an actual prescription
By giving the patient a written, printed prescription when they leave your office, “you’re showing it’s an important part of treatment or prevention,” Dr. Thornton explains. It puts physical activity on the level of a vital sign.
Include frequency, intensity, time, and type of exercise. The American College of Sports Medicine’s Exercise is Medicine initiative provides a prescription template you can use.
3. Measure what they do
Measurement helps the patient adopt the new behavior, and it helps the physician provide tailored advice going forward, Dr. Thornton says.
With the rise of health-monitoring wearables, tracking activity has never been easier. Of course, not everyone wants to (or can afford to) use a smartwatch or fitness tracker.
For tech-averse patients, ask if they’re willing to write something down, like how many minutes they spent walking, or how many yoga classes they attended. You may never get this from some patients, but it never hurts to ask.
4. Refer out when necessary
This brings us to a sticky issue for many physicians: lack of confidence in their ability to speak authoritatively about physical activity. “In most cases, you can absolutely say, ‘Start slow, go gradually,’ that kind of thing,” Dr. Thornton says. “As with anything, confidence will come with practice.”
For specific prescriptive advice, check out the Exercise is Medicine website, which also has handouts you can share with patients and information for specific conditions. If your patient has prediabetes, you can also point them toward the CDC’s diabetes prevention program, which is available in-person or online and may be free or covered by insurance.
If a patient has contraindications, refer out. If you don’t have exercise or rehab professionals in your network, Dr. Thornton recommends reaching out to your regional or national association of sports-medicine professionals. You should be able to find it with a quick Google search.
5. Follow up
Ask about physical activity during every contact, either in person or online.
Dr. Thornton says the second and fifth steps matter most to patients, especially when the prescription and follow-up come from their primary care physician, rather than a nurse or physician assistant to whom you’ve delegated the task.
“The value comes in having a physician emphasize the importance,” Dr. Thornton says. The more time you spend on it, the more that value comes through.
What NOT to say to patients about exercise
This might surprise you:
“I definitely don’t think telling people the official recommendations for physical activity is useful,” says Yoni Freedhoff, MD, an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. “If anything, I’d venture it’s counterproductive.”
It’s not that there’s anything wrong with the recommended minimum – 150 minutes of moderate-to-vigorous-intensity physical activity per week. The problem is what it says to a patient who doesn’t come close to those standards.
“Few real-world people have the interest, time, energy, or privilege to achieve them,” Dr. Freedhoff says. “Many will recognize that instantly and consequently feel [that] less than that is pointless.”
And that, Dr. Thornton says, is categorically not true. “Even minimal physical activity, in some cases, is beneficial.”
You also want to avoid any explicit connection between exercise and weight loss, Dr. Thornton says.
Though many people do connect the two, the link is often negative, notes a 2019 study from the University of Toronto., triggering painful memories that might go all the way back to gym class.
Try this pivot from Dr. Freedhoff: “Focus on the role of exercise in mitigating the risks of weight,” he says – like decreasing pain, increasing energy, and improving sleep.
How to motivate patients to move
New research backs up this more positive approach. In a study published in Annals of Internal Medicine, doctors in the United Kingdom who emphasized benefits and minimized health harms convinced more patients to join a weight management program than negative or neutral docs did. These doctors conveyed optimism and excitement, smiling and avoiding any mention of obesity or body mass index.
Exactly what benefits inspire change will be different for each patient. But in general, the more immediate the benefit, the more motivating it will be.
As the University of Toronto study noted, patients weren’t motivated by vague, distant goals like “increasing life expectancy or avoiding health problems many years in the future.”
They’re much more likely to take action to avoid surgery, reduce medications, or minimize the risk of falling.
For an older patient, Dr. Freedhoff says, “focusing on the preservation of functional independence can be extremely motivating.” That’s especially true if the patient has vivid memories of seeing a sedentary loved one decline late in life.
For patients who may be more focused on appearance, they could respond to the idea of improving their body composition. For that, “we talk about the quality of weight loss,” says Spencer Nadolsky, DO, an obesity and lipid specialist and medical director of WeightWatchers. “Ultimately, exercise helps shape the body instead of just changing the number on the scale.”
Reducing resistance to resistance training
A conversation about reshaping the body or avoiding age-related disabilities leads naturally to resistance training.
“I always frame resistance training as the single most valuable thing a person might do to try to preserve their functional independence,” Dr. Freedhoff says. If the patient is over 65, he won’t wait for them to show an interest. “I’ll absolutely bring it up with them directly.”
Dr. Freedhoff has an on-site training facility where trainers show patients how to work out at home with minimal equipment, like dumbbells and resistance bands.
Most doctors, however, don’t have those options. That can lead to a tricky conversation. Participants in the University of Toronto study told the authors they disliked the gym, finding it “boring, intimidating, or discouraging.”
And yet, “a common suggestion ... from health care providers was to join a gym.”
Many patients, Spencer Nadolsky, MD, says, associate strength training with “grunting, groaning, or getting ‘bulky’ vs. ‘toned.’ ” Memories of soreness from overzealous workouts are another barrier.
He recommends “starting small and slow,” with one or two full-body workouts a week. Those initial workouts might include just one to two sets of four to five exercises. “Consider if someone is exercising at home or in a gym to build a routine around equipment that’s available to them,” Dr. Nadolsky says.
Once you determine what you have to work with, help the patient choose exercises that fit their needs, goals, preferences, limitations, and prior injuries.
One more consideration: While Dr. Nadolsky tries to “stay away from telling a patient they need to do specific types of exercise to be successful,” he makes an exception for patients who’re taking a GLP-1 agonist. “There is a concern for muscle mass loss along with fat loss.”
Practicing, preaching, and checking privilege
When Dr. Thornton, Dr. Freedhoff, and Dr. Nadolsky discuss exercise, their patients know they practice what they preach.
Dr. Nadolsky, who was a nationally ranked wrestler at the University of North Carolina, hosts the Docs Who Lift podcast with his brother, Karl Nadolsky, MD.
Dr. Freedhoff is also a lifter and fitness enthusiast, and Dr. Thornton was a world-class rower whose team came within 0.8 seconds of a silver medal at the Beijing Olympics. (They finished fourth.)
But not all physicians follow their own lifestyle advice, Dr. Freedhoff says. That doesn’t make them bad doctors – it makes them human.
“I’ve done 300 minutes a week of exercise” – the recommended amount for weight maintenance – “to see what’s involved,” Dr. Freedhoff says. “That’s far, far, far from a trivial amount.”
That leads to this advice for his fellow physicians:
“The most important thing to know about exercise is that finding the time and having the health to do so is a privilege,” he says.
Understanding that is crucial for assessing your patient’s needs and providing the right help.
A version of this article first appeared on Medscape.com.
Even one night in the ED raises risk for death
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.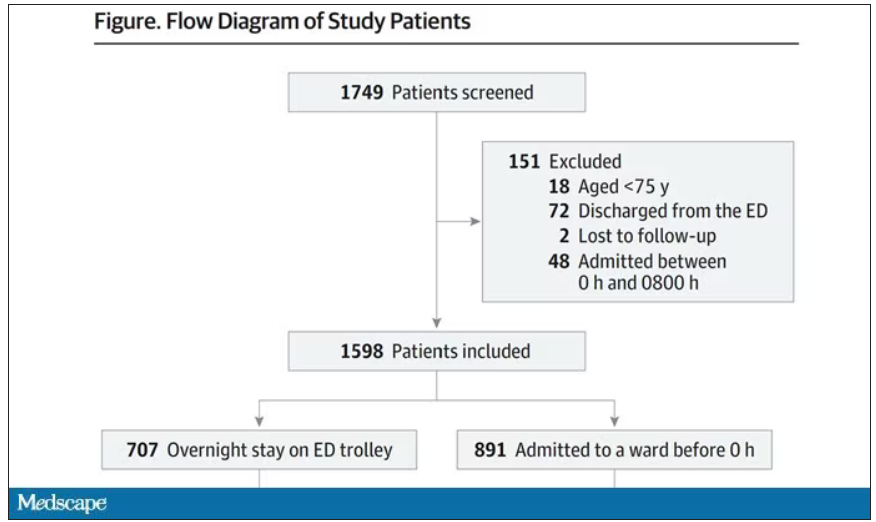
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.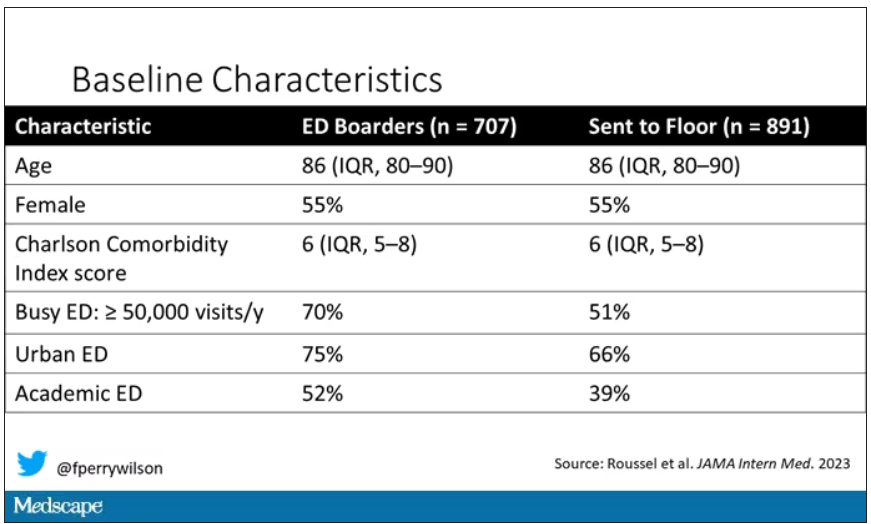
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Pervasive ‘forever chemicals’ linked to thyroid cancer?
The study suggests that higher exposure to per- and polyfluoroalkyl substances (PFAS), specifically perfluorooctanesulfonic acid (n-PFOS), may increase a person’s risk for thyroid cancer by 56%.
Several news outlets played up the findings, published online in eBioMedicine. “Dangerous ‘Forever Chemicals’ in Your Everyday Items Are Causing Cancer,” Newsweek reported.
But Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong (Australia), voiced his skepticism.
“While it’s possible that PFAS might be causing thyroid cancer, the evidence thus far is unconvincing and probably not worth worrying about,” said Dr. Meyerowitz-Katz, who was not involved in the research.
PFAS and thyroid cancer
PFAS are a class of widely used synthetic chemicals found in many consumer and industrial products, including nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam.
These substances have been dubbed “forever chemicals” because they do not degrade and are ubiquitous in the environment.
Exposure to endocrine-disrupting chemicals, including PFAS, has been identified as a potential risk factor for thyroid cancer, with some research linking PFAS exposure to thyroid dysfunction and carcinogenesis.
To investigate further, the researchers performed a nested case-control study of 86 patients with thyroid cancer using plasma samples collected at or before diagnosis and 86 controls without cancer who were matched on age, sex, race/ethnicity, body weight, smoking status, and year of sample collection.
Eighteen individual PFAS were measured in plasma samples; 10 were undetectable and were therefore excluded from the analysis. Of the remaining eight PFAS, only one showed a statistically significant correlation with thyroid cancer.
Specifically, the researchers found that exposure to n-PFOS was associated with a 56% increased risk for thyroid cancer among people who had a high level of the chemical in their blood (adjusted odds ratio, 1.56; P = .004). The results were similar when patients with papillary thyroid cancer only were included (aOR, 1.56; P = .009).
A separate longitudinal analysis of 31 patients diagnosed with thyroid cancer 1 year or more after plasma sample collection and 31 controls confirmed the positive association between n-PFOS and thyroid cancer (aOR, 2.67; P < .001). The longitudinal analysis also suggested correlations for a few other PFAS.
“This study supports the hypothesis that PFAS exposure may be associated with increased risk of thyroid cancer,” the authors concluded.
But in a Substack post, Dr. Meyerowitz-Katz said that it’s important to put the findings into “proper context before getting terrified about this all-new cancer risk.”
First, this study was “genuinely tiny,” with data on just 88 people with thyroid cancer and 88 controls, a limitation the researchers also acknowledged.
“That’s really not enough to do any sort of robust epidemiological analysis – you can generate interesting correlations, but what those correlations mean is anyone’s guess,” Dr. Meyerowitz-Katz said.
Even more importantly, one could easily argue that the results of this study show that most PFAS aren’t associated with thyroid cancer, given that there was no strong association for seven of the eight PFAS measured, he explained.
“There are no serious methodological concerns here, but equally there’s just not much you can reasonably gather from finding a single correlation among a vast ocean of possibilities,” Dr. Meyerowitz-Katz wrote. “Maybe there’s a correlation there, but you’d need to investigate this in much bigger samples, with more controls, and better data, to understand what that correlation means.”
Bottom line, Dr. Meyerowitz-Katz explained, is that “the link between PFAS and thyroid cancer is, at best, incredibly weak.”
Funding for the study was provided by the National Institutes of Health and The Andrea and Charles Bronfman Philanthropies. One coauthor is cofounder of Linus Biotechnology and is owner of a license agreement with NIES (Japan); received honoraria and travel compensation for lectures for the Bio-Echo and Brin foundations; and has 22 patents at various stages. Dr. Meyerowitz-Katz has no relevant disclosures.
A version of this article appeared on Medscape.com.
The study suggests that higher exposure to per- and polyfluoroalkyl substances (PFAS), specifically perfluorooctanesulfonic acid (n-PFOS), may increase a person’s risk for thyroid cancer by 56%.
Several news outlets played up the findings, published online in eBioMedicine. “Dangerous ‘Forever Chemicals’ in Your Everyday Items Are Causing Cancer,” Newsweek reported.
But Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong (Australia), voiced his skepticism.
“While it’s possible that PFAS might be causing thyroid cancer, the evidence thus far is unconvincing and probably not worth worrying about,” said Dr. Meyerowitz-Katz, who was not involved in the research.
PFAS and thyroid cancer
PFAS are a class of widely used synthetic chemicals found in many consumer and industrial products, including nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam.
These substances have been dubbed “forever chemicals” because they do not degrade and are ubiquitous in the environment.
Exposure to endocrine-disrupting chemicals, including PFAS, has been identified as a potential risk factor for thyroid cancer, with some research linking PFAS exposure to thyroid dysfunction and carcinogenesis.
To investigate further, the researchers performed a nested case-control study of 86 patients with thyroid cancer using plasma samples collected at or before diagnosis and 86 controls without cancer who were matched on age, sex, race/ethnicity, body weight, smoking status, and year of sample collection.
Eighteen individual PFAS were measured in plasma samples; 10 were undetectable and were therefore excluded from the analysis. Of the remaining eight PFAS, only one showed a statistically significant correlation with thyroid cancer.
Specifically, the researchers found that exposure to n-PFOS was associated with a 56% increased risk for thyroid cancer among people who had a high level of the chemical in their blood (adjusted odds ratio, 1.56; P = .004). The results were similar when patients with papillary thyroid cancer only were included (aOR, 1.56; P = .009).
A separate longitudinal analysis of 31 patients diagnosed with thyroid cancer 1 year or more after plasma sample collection and 31 controls confirmed the positive association between n-PFOS and thyroid cancer (aOR, 2.67; P < .001). The longitudinal analysis also suggested correlations for a few other PFAS.
“This study supports the hypothesis that PFAS exposure may be associated with increased risk of thyroid cancer,” the authors concluded.
But in a Substack post, Dr. Meyerowitz-Katz said that it’s important to put the findings into “proper context before getting terrified about this all-new cancer risk.”
First, this study was “genuinely tiny,” with data on just 88 people with thyroid cancer and 88 controls, a limitation the researchers also acknowledged.
“That’s really not enough to do any sort of robust epidemiological analysis – you can generate interesting correlations, but what those correlations mean is anyone’s guess,” Dr. Meyerowitz-Katz said.
Even more importantly, one could easily argue that the results of this study show that most PFAS aren’t associated with thyroid cancer, given that there was no strong association for seven of the eight PFAS measured, he explained.
“There are no serious methodological concerns here, but equally there’s just not much you can reasonably gather from finding a single correlation among a vast ocean of possibilities,” Dr. Meyerowitz-Katz wrote. “Maybe there’s a correlation there, but you’d need to investigate this in much bigger samples, with more controls, and better data, to understand what that correlation means.”
Bottom line, Dr. Meyerowitz-Katz explained, is that “the link between PFAS and thyroid cancer is, at best, incredibly weak.”
Funding for the study was provided by the National Institutes of Health and The Andrea and Charles Bronfman Philanthropies. One coauthor is cofounder of Linus Biotechnology and is owner of a license agreement with NIES (Japan); received honoraria and travel compensation for lectures for the Bio-Echo and Brin foundations; and has 22 patents at various stages. Dr. Meyerowitz-Katz has no relevant disclosures.
A version of this article appeared on Medscape.com.
The study suggests that higher exposure to per- and polyfluoroalkyl substances (PFAS), specifically perfluorooctanesulfonic acid (n-PFOS), may increase a person’s risk for thyroid cancer by 56%.
Several news outlets played up the findings, published online in eBioMedicine. “Dangerous ‘Forever Chemicals’ in Your Everyday Items Are Causing Cancer,” Newsweek reported.
But Gideon Meyerowitz-Katz, PhD, an epidemiologist at the University of Wollongong (Australia), voiced his skepticism.
“While it’s possible that PFAS might be causing thyroid cancer, the evidence thus far is unconvincing and probably not worth worrying about,” said Dr. Meyerowitz-Katz, who was not involved in the research.
PFAS and thyroid cancer
PFAS are a class of widely used synthetic chemicals found in many consumer and industrial products, including nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam.
These substances have been dubbed “forever chemicals” because they do not degrade and are ubiquitous in the environment.
Exposure to endocrine-disrupting chemicals, including PFAS, has been identified as a potential risk factor for thyroid cancer, with some research linking PFAS exposure to thyroid dysfunction and carcinogenesis.
To investigate further, the researchers performed a nested case-control study of 86 patients with thyroid cancer using plasma samples collected at or before diagnosis and 86 controls without cancer who were matched on age, sex, race/ethnicity, body weight, smoking status, and year of sample collection.
Eighteen individual PFAS were measured in plasma samples; 10 were undetectable and were therefore excluded from the analysis. Of the remaining eight PFAS, only one showed a statistically significant correlation with thyroid cancer.
Specifically, the researchers found that exposure to n-PFOS was associated with a 56% increased risk for thyroid cancer among people who had a high level of the chemical in their blood (adjusted odds ratio, 1.56; P = .004). The results were similar when patients with papillary thyroid cancer only were included (aOR, 1.56; P = .009).
A separate longitudinal analysis of 31 patients diagnosed with thyroid cancer 1 year or more after plasma sample collection and 31 controls confirmed the positive association between n-PFOS and thyroid cancer (aOR, 2.67; P < .001). The longitudinal analysis also suggested correlations for a few other PFAS.
“This study supports the hypothesis that PFAS exposure may be associated with increased risk of thyroid cancer,” the authors concluded.
But in a Substack post, Dr. Meyerowitz-Katz said that it’s important to put the findings into “proper context before getting terrified about this all-new cancer risk.”
First, this study was “genuinely tiny,” with data on just 88 people with thyroid cancer and 88 controls, a limitation the researchers also acknowledged.
“That’s really not enough to do any sort of robust epidemiological analysis – you can generate interesting correlations, but what those correlations mean is anyone’s guess,” Dr. Meyerowitz-Katz said.
Even more importantly, one could easily argue that the results of this study show that most PFAS aren’t associated with thyroid cancer, given that there was no strong association for seven of the eight PFAS measured, he explained.
“There are no serious methodological concerns here, but equally there’s just not much you can reasonably gather from finding a single correlation among a vast ocean of possibilities,” Dr. Meyerowitz-Katz wrote. “Maybe there’s a correlation there, but you’d need to investigate this in much bigger samples, with more controls, and better data, to understand what that correlation means.”
Bottom line, Dr. Meyerowitz-Katz explained, is that “the link between PFAS and thyroid cancer is, at best, incredibly weak.”
Funding for the study was provided by the National Institutes of Health and The Andrea and Charles Bronfman Philanthropies. One coauthor is cofounder of Linus Biotechnology and is owner of a license agreement with NIES (Japan); received honoraria and travel compensation for lectures for the Bio-Echo and Brin foundations; and has 22 patents at various stages. Dr. Meyerowitz-Katz has no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM EBIOMEDICINE
No longer a death sentence, HIV diagnosis still hits hard
Veronica Brady and her team at the University of Texas Health Science Center, Houston, sat down with 37 people diagnosed with HIV or AIDS to ask them what that felt like.
“The results were really eye-opening and sad,” says Brady, PhD, RN, from the Cizik School of Nursing with UTHealth, Houston.
Many of the people Dr. Brady and her team spoke with were diagnosed through routine or random testing. They ranged in age from 21 years to 65 and said they did not know how they had been infected and felt shocked, freaked out, scared, and in a state of disbelief.
Their conversations about being diagnosed with HIV, presented at the annual meeting of the Association of Nurses in AIDS Care in New Orleans, also described how symptoms of the disease or side effects from treatment can have a huge impact on the daily lives of those affected.
Jesse Milan Jr., president of AIDS United, an HIV advocacy organization based in Washington, D.C., says he recognizes all of these feelings from his own experience with HIV after being diagnosed more than 40 years ago.
“All of those have come up over the years,” he says. “They are all relevant and important at different times.”
For Mr. Milan, less was known about the virus at the time of his diagnosis, and he watched loved ones die. He lived to see the introduction of antiretroviral therapies and receive treatment when his partner and many of his friends did not.
Effective treatments
There is a marked difference between the reaction of people diagnosed with HIV years ago and those diagnosed more recently, Dr. Brady explains. Those diagnosed before much was known about the virus and before there were effective treatments were more frightened, she says, whereas people hearing the news recently are much less worried and understand that if they take their medication, they will be fine.
Still, Mr. Milan says when he talks to people diagnosed now, they seem to experience more shame and embarrassment than before. Because it is long known how to prevent HIV infection, they often worry what people will think if they disclose their status. “It makes things harder for people diagnosed today,” says Mr. Milan. “There is a different level of embarrassment tinged with, ‘Why was I so stupid?’ ”
Diagnosis can also be hard on health care professionals, says Dr. Brady. “You never want to tell anyone they’re sick with a chronic disease, especially younger people,” she adds. “You know you’re adding a burden to someone’s life.”
Symptoms and side effects of treatment also had an important impact on the people in this report, with most aspects of their lives affected, including work, relationships, mood, and daily activities.
Clinicians should be supportive and spend some time sitting with patients as they come to terms with the diagnosis and its implications. They should help them understand what to expect and talk about how – or whether – to talk about their status with family and friends. “You need to show you care about the person and that they are not alone,” Dr. Brady says.
And most of all, clinicians need to explain that patients can live a long and healthy life and go on to become whoever they want to be. “Twenty years ago, we wouldn’t have as hopeful a message as we do now,” she says.
Hope is the most important thing for doctors and nurses to communicate to their patients. “There are medications available, and it will be okay. You don’t have to die,” Mr. Milan says. “That’s the core message that everyone needs to hear, whether they were diagnosed 30 years ago or 30 minutes ago.”
A version of this article appeared on Medscape.com.
Veronica Brady and her team at the University of Texas Health Science Center, Houston, sat down with 37 people diagnosed with HIV or AIDS to ask them what that felt like.
“The results were really eye-opening and sad,” says Brady, PhD, RN, from the Cizik School of Nursing with UTHealth, Houston.
Many of the people Dr. Brady and her team spoke with were diagnosed through routine or random testing. They ranged in age from 21 years to 65 and said they did not know how they had been infected and felt shocked, freaked out, scared, and in a state of disbelief.
Their conversations about being diagnosed with HIV, presented at the annual meeting of the Association of Nurses in AIDS Care in New Orleans, also described how symptoms of the disease or side effects from treatment can have a huge impact on the daily lives of those affected.
Jesse Milan Jr., president of AIDS United, an HIV advocacy organization based in Washington, D.C., says he recognizes all of these feelings from his own experience with HIV after being diagnosed more than 40 years ago.
“All of those have come up over the years,” he says. “They are all relevant and important at different times.”
For Mr. Milan, less was known about the virus at the time of his diagnosis, and he watched loved ones die. He lived to see the introduction of antiretroviral therapies and receive treatment when his partner and many of his friends did not.
Effective treatments
There is a marked difference between the reaction of people diagnosed with HIV years ago and those diagnosed more recently, Dr. Brady explains. Those diagnosed before much was known about the virus and before there were effective treatments were more frightened, she says, whereas people hearing the news recently are much less worried and understand that if they take their medication, they will be fine.
Still, Mr. Milan says when he talks to people diagnosed now, they seem to experience more shame and embarrassment than before. Because it is long known how to prevent HIV infection, they often worry what people will think if they disclose their status. “It makes things harder for people diagnosed today,” says Mr. Milan. “There is a different level of embarrassment tinged with, ‘Why was I so stupid?’ ”
Diagnosis can also be hard on health care professionals, says Dr. Brady. “You never want to tell anyone they’re sick with a chronic disease, especially younger people,” she adds. “You know you’re adding a burden to someone’s life.”
Symptoms and side effects of treatment also had an important impact on the people in this report, with most aspects of their lives affected, including work, relationships, mood, and daily activities.
Clinicians should be supportive and spend some time sitting with patients as they come to terms with the diagnosis and its implications. They should help them understand what to expect and talk about how – or whether – to talk about their status with family and friends. “You need to show you care about the person and that they are not alone,” Dr. Brady says.
And most of all, clinicians need to explain that patients can live a long and healthy life and go on to become whoever they want to be. “Twenty years ago, we wouldn’t have as hopeful a message as we do now,” she says.
Hope is the most important thing for doctors and nurses to communicate to their patients. “There are medications available, and it will be okay. You don’t have to die,” Mr. Milan says. “That’s the core message that everyone needs to hear, whether they were diagnosed 30 years ago or 30 minutes ago.”
A version of this article appeared on Medscape.com.
Veronica Brady and her team at the University of Texas Health Science Center, Houston, sat down with 37 people diagnosed with HIV or AIDS to ask them what that felt like.
“The results were really eye-opening and sad,” says Brady, PhD, RN, from the Cizik School of Nursing with UTHealth, Houston.
Many of the people Dr. Brady and her team spoke with were diagnosed through routine or random testing. They ranged in age from 21 years to 65 and said they did not know how they had been infected and felt shocked, freaked out, scared, and in a state of disbelief.
Their conversations about being diagnosed with HIV, presented at the annual meeting of the Association of Nurses in AIDS Care in New Orleans, also described how symptoms of the disease or side effects from treatment can have a huge impact on the daily lives of those affected.
Jesse Milan Jr., president of AIDS United, an HIV advocacy organization based in Washington, D.C., says he recognizes all of these feelings from his own experience with HIV after being diagnosed more than 40 years ago.
“All of those have come up over the years,” he says. “They are all relevant and important at different times.”
For Mr. Milan, less was known about the virus at the time of his diagnosis, and he watched loved ones die. He lived to see the introduction of antiretroviral therapies and receive treatment when his partner and many of his friends did not.
Effective treatments
There is a marked difference between the reaction of people diagnosed with HIV years ago and those diagnosed more recently, Dr. Brady explains. Those diagnosed before much was known about the virus and before there were effective treatments were more frightened, she says, whereas people hearing the news recently are much less worried and understand that if they take their medication, they will be fine.
Still, Mr. Milan says when he talks to people diagnosed now, they seem to experience more shame and embarrassment than before. Because it is long known how to prevent HIV infection, they often worry what people will think if they disclose their status. “It makes things harder for people diagnosed today,” says Mr. Milan. “There is a different level of embarrassment tinged with, ‘Why was I so stupid?’ ”
Diagnosis can also be hard on health care professionals, says Dr. Brady. “You never want to tell anyone they’re sick with a chronic disease, especially younger people,” she adds. “You know you’re adding a burden to someone’s life.”
Symptoms and side effects of treatment also had an important impact on the people in this report, with most aspects of their lives affected, including work, relationships, mood, and daily activities.
Clinicians should be supportive and spend some time sitting with patients as they come to terms with the diagnosis and its implications. They should help them understand what to expect and talk about how – or whether – to talk about their status with family and friends. “You need to show you care about the person and that they are not alone,” Dr. Brady says.
And most of all, clinicians need to explain that patients can live a long and healthy life and go on to become whoever they want to be. “Twenty years ago, we wouldn’t have as hopeful a message as we do now,” she says.
Hope is the most important thing for doctors and nurses to communicate to their patients. “There are medications available, and it will be okay. You don’t have to die,” Mr. Milan says. “That’s the core message that everyone needs to hear, whether they were diagnosed 30 years ago or 30 minutes ago.”
A version of this article appeared on Medscape.com.
Aprocitentan reduces resistant hypertension in CKD
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
AT KIDNEY WEEK 2023
Marijuana use dramatically increases risk of heart problems, stroke
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
FROM AHA 2023
Second infection hikes long COVID risk: Expert Q&A
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
EMR prompt boosts albuminuria measurement in T2D
PHILADELPHIA – An electronic medical record alert to primary care physicians that their adult patients with type 2 diabetes were due for an albuminuria and renal-function check boosted screening for chronic kidney disease (CKD) by roughly half compared with the preintervention rate in a single U.S. academic health system.
“Screening rates for CKD more rapidly improved after implementation” of the EMR alert, said Maggy M. Spolnik, MD, at Kidney Week 2023, organized by the American Society of Nephrology.
“There was an immediate and ongoing effect over a year,” said Dr. Spolnik, a nephrologist at Indiana University in Indianapolis.
However, CKD screening rates in the primary care setting remain a challenge. In the study, the EMR alert produced a urine albumin-to-creatinine ratio (UACR) screening rate of about 26% of patient encounters, she reported. While this was significantly above the roughly 17% rate that had persisted for months before the intervention, it still fell short of the universal annual screening for adults with type 2 diabetes not previously diagnosed with CKD recommended by medical groups such as the American Diabetes Association and the Kidney Disease: Improving Global Outcomes organization. The U.S. Preventive Services Task Force’s assessment in 2012 concluded inadequate information existed at that time to make recommendations about CKD screening, but the group is now revisiting the issue.
‘Albuminuria is an earlier marker’ than eGFR
“Primary care physicians need to regularly monitor albuminuria in adults with type 2 diabetes,” commented Karen A. Griffin, MD, a nephrologist and professor at Loyola University in Maywood, Ill. “By the time you diagnose CKD based on reduced estimated glomerular filtration rate (eGFR), a patient has already lost more than half their renal function. Albuminuria is an earlier marker of a problem,” Dr. Griffin said in an interview.
Primary care physicians have been slow to adopt at least annual checks on both eGFR and the urinary albumin-to-creatinine ratio (UACR) in their adult patients with type 2 diabetes. Dr. Spolnik cited reasons such as the brief 15-minute consultation that primary care physicians have when seeing a patient, and an often confusing ordering menu that gives a UACR test various other names such as tests for microalbuminuria or macroalbuminuria.
To simplify ordering, the EMR prompt assessed in Dr. Spolnik’s study called the test “kidney screening” that automatically bundled an order for both eGFR calculation with UACR measurement. Another limitation is that UACR measurement requires a urine sample, which patients often find inconvenient to provide at the time of their examination.
The study run by Dr. Spolnik involved 10,744 adults with type 2 diabetes without an existing diagnosis of CKD seen in an outpatient, primary care visit to the UVA Health system centered in Charlottesville, Va. during April 2021–April 2022. A total of 23,419 encounters served as usual-care controls. The intervention period with active EMR alerts for kidney screening included 10,204 similar patients seen during April 2022–April 2023 in a total of 20,358 encounters. The patients averaged about 61-62 years old, and about 45% were men.
Bundling alerts into a single pop-up
The primary care clinicians who received the prompts were generally receptive to them, but they asked the researchers to bundle the UACR and eGFR measurement prompts along with any other alerts they received in the EMR into a single on-screen pop-up.
Dr. Spolnik acknowledged the need for further research and refinement to the prompt. For example, she wants to assess prompts for patients identified as having CKD that would promote best-practice management, including lifestyle and medical interventions. She also envisions expanding the prompts to also include other, related disorders such as hypertension.
But she and her colleagues were convinced enough by the results that they have not only continued the program at UVA Health but they also expanded it, starting in October 2023, to the academic primary care practice at Indiana University.
If the Indiana University trial confirms the efficacy seen in Virginia, the next step might be inclusion by Epic of the CKD screening alert as a routine option in the EMR software it distributes to its U.S. clients, Dr. Spolnik said in an interview.
Dr. Spolnik and Dr. Griffin had no disclosures.
PHILADELPHIA – An electronic medical record alert to primary care physicians that their adult patients with type 2 diabetes were due for an albuminuria and renal-function check boosted screening for chronic kidney disease (CKD) by roughly half compared with the preintervention rate in a single U.S. academic health system.
“Screening rates for CKD more rapidly improved after implementation” of the EMR alert, said Maggy M. Spolnik, MD, at Kidney Week 2023, organized by the American Society of Nephrology.
“There was an immediate and ongoing effect over a year,” said Dr. Spolnik, a nephrologist at Indiana University in Indianapolis.
However, CKD screening rates in the primary care setting remain a challenge. In the study, the EMR alert produced a urine albumin-to-creatinine ratio (UACR) screening rate of about 26% of patient encounters, she reported. While this was significantly above the roughly 17% rate that had persisted for months before the intervention, it still fell short of the universal annual screening for adults with type 2 diabetes not previously diagnosed with CKD recommended by medical groups such as the American Diabetes Association and the Kidney Disease: Improving Global Outcomes organization. The U.S. Preventive Services Task Force’s assessment in 2012 concluded inadequate information existed at that time to make recommendations about CKD screening, but the group is now revisiting the issue.
‘Albuminuria is an earlier marker’ than eGFR
“Primary care physicians need to regularly monitor albuminuria in adults with type 2 diabetes,” commented Karen A. Griffin, MD, a nephrologist and professor at Loyola University in Maywood, Ill. “By the time you diagnose CKD based on reduced estimated glomerular filtration rate (eGFR), a patient has already lost more than half their renal function. Albuminuria is an earlier marker of a problem,” Dr. Griffin said in an interview.
Primary care physicians have been slow to adopt at least annual checks on both eGFR and the urinary albumin-to-creatinine ratio (UACR) in their adult patients with type 2 diabetes. Dr. Spolnik cited reasons such as the brief 15-minute consultation that primary care physicians have when seeing a patient, and an often confusing ordering menu that gives a UACR test various other names such as tests for microalbuminuria or macroalbuminuria.
To simplify ordering, the EMR prompt assessed in Dr. Spolnik’s study called the test “kidney screening” that automatically bundled an order for both eGFR calculation with UACR measurement. Another limitation is that UACR measurement requires a urine sample, which patients often find inconvenient to provide at the time of their examination.
The study run by Dr. Spolnik involved 10,744 adults with type 2 diabetes without an existing diagnosis of CKD seen in an outpatient, primary care visit to the UVA Health system centered in Charlottesville, Va. during April 2021–April 2022. A total of 23,419 encounters served as usual-care controls. The intervention period with active EMR alerts for kidney screening included 10,204 similar patients seen during April 2022–April 2023 in a total of 20,358 encounters. The patients averaged about 61-62 years old, and about 45% were men.
Bundling alerts into a single pop-up
The primary care clinicians who received the prompts were generally receptive to them, but they asked the researchers to bundle the UACR and eGFR measurement prompts along with any other alerts they received in the EMR into a single on-screen pop-up.
Dr. Spolnik acknowledged the need for further research and refinement to the prompt. For example, she wants to assess prompts for patients identified as having CKD that would promote best-practice management, including lifestyle and medical interventions. She also envisions expanding the prompts to also include other, related disorders such as hypertension.
But she and her colleagues were convinced enough by the results that they have not only continued the program at UVA Health but they also expanded it, starting in October 2023, to the academic primary care practice at Indiana University.
If the Indiana University trial confirms the efficacy seen in Virginia, the next step might be inclusion by Epic of the CKD screening alert as a routine option in the EMR software it distributes to its U.S. clients, Dr. Spolnik said in an interview.
Dr. Spolnik and Dr. Griffin had no disclosures.
PHILADELPHIA – An electronic medical record alert to primary care physicians that their adult patients with type 2 diabetes were due for an albuminuria and renal-function check boosted screening for chronic kidney disease (CKD) by roughly half compared with the preintervention rate in a single U.S. academic health system.
“Screening rates for CKD more rapidly improved after implementation” of the EMR alert, said Maggy M. Spolnik, MD, at Kidney Week 2023, organized by the American Society of Nephrology.
“There was an immediate and ongoing effect over a year,” said Dr. Spolnik, a nephrologist at Indiana University in Indianapolis.
However, CKD screening rates in the primary care setting remain a challenge. In the study, the EMR alert produced a urine albumin-to-creatinine ratio (UACR) screening rate of about 26% of patient encounters, she reported. While this was significantly above the roughly 17% rate that had persisted for months before the intervention, it still fell short of the universal annual screening for adults with type 2 diabetes not previously diagnosed with CKD recommended by medical groups such as the American Diabetes Association and the Kidney Disease: Improving Global Outcomes organization. The U.S. Preventive Services Task Force’s assessment in 2012 concluded inadequate information existed at that time to make recommendations about CKD screening, but the group is now revisiting the issue.
‘Albuminuria is an earlier marker’ than eGFR
“Primary care physicians need to regularly monitor albuminuria in adults with type 2 diabetes,” commented Karen A. Griffin, MD, a nephrologist and professor at Loyola University in Maywood, Ill. “By the time you diagnose CKD based on reduced estimated glomerular filtration rate (eGFR), a patient has already lost more than half their renal function. Albuminuria is an earlier marker of a problem,” Dr. Griffin said in an interview.
Primary care physicians have been slow to adopt at least annual checks on both eGFR and the urinary albumin-to-creatinine ratio (UACR) in their adult patients with type 2 diabetes. Dr. Spolnik cited reasons such as the brief 15-minute consultation that primary care physicians have when seeing a patient, and an often confusing ordering menu that gives a UACR test various other names such as tests for microalbuminuria or macroalbuminuria.
To simplify ordering, the EMR prompt assessed in Dr. Spolnik’s study called the test “kidney screening” that automatically bundled an order for both eGFR calculation with UACR measurement. Another limitation is that UACR measurement requires a urine sample, which patients often find inconvenient to provide at the time of their examination.
The study run by Dr. Spolnik involved 10,744 adults with type 2 diabetes without an existing diagnosis of CKD seen in an outpatient, primary care visit to the UVA Health system centered in Charlottesville, Va. during April 2021–April 2022. A total of 23,419 encounters served as usual-care controls. The intervention period with active EMR alerts for kidney screening included 10,204 similar patients seen during April 2022–April 2023 in a total of 20,358 encounters. The patients averaged about 61-62 years old, and about 45% were men.
Bundling alerts into a single pop-up
The primary care clinicians who received the prompts were generally receptive to them, but they asked the researchers to bundle the UACR and eGFR measurement prompts along with any other alerts they received in the EMR into a single on-screen pop-up.
Dr. Spolnik acknowledged the need for further research and refinement to the prompt. For example, she wants to assess prompts for patients identified as having CKD that would promote best-practice management, including lifestyle and medical interventions. She also envisions expanding the prompts to also include other, related disorders such as hypertension.
But she and her colleagues were convinced enough by the results that they have not only continued the program at UVA Health but they also expanded it, starting in October 2023, to the academic primary care practice at Indiana University.
If the Indiana University trial confirms the efficacy seen in Virginia, the next step might be inclusion by Epic of the CKD screening alert as a routine option in the EMR software it distributes to its U.S. clients, Dr. Spolnik said in an interview.
Dr. Spolnik and Dr. Griffin had no disclosures.
REPORTING FROM KIDNEY WEEK 2023