User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
AI algorithm aids egg retrieval date during fertility treatment cycles
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
According to the researchers, such an algorithm is needed due to the increased demand for fertility treatments, as well as the high day-to-day variability in lab workload.
According to the study investigators, predicting retrieval dates in advance for ongoing cycles is of major importance for both patients and clinicians.
“The population requiring fertility treatments, including genetic testing and fertility preservation, has massively increased, and this causes many more cycles and a high day-to-day variability in IVF activity, especially in the lab workload,” said Rohi Hourvitz, MBA, from FertilAI, an Israeli health care company focused on developing technologies that improve fertility treatments.
“We also need to accommodate and reschedule for non-working days, which causes a big issue with managing the workload in many clinics around the world,” added Mr. Hourvitz, who presented the research highlighting AI’s growing role in reproductive medicine.
In addition, AI has recently emerged as an effective tool for assisting in clinical decision-making in assisted reproductive technology, prompting further research in this space, he said.
The new study used a dataset of 9,550 predictable antagonist cycles (defined as having all necessary data) gathered from one lab with over 50 physicians between August 2018 and October 2022. The data were split into two subsets: one for training the AI model and the other for prospective testing.
To train and test the AI model, data from nearly 6,000 predictable antagonist cycles were used. Key factors used for each cycle included estrogen levels, mean follicle size, primary follicle size, and various patient demographics. Other features were considered, but Mr. Hourvitz noted that primary follicle size influenced the algorithm most, “because that is what most of us use when we want to trigger.”
Mr. Hourvitz explained that these patient data were run through an algorithm that produced a graph predicting the most probable date for a cycle retrieval.
“We could accurately predict when those ‘peak days’ were going to be happening in the clinic, and we could also give a pretty good estimate on how many cycles you’re going to have every day,” Mr. Hourvitz said, explaining that this information could help clinics more efficiently allocate resources and manage patients.
According to Mr. Hourvitz, the predictions derived from this study could improve various aspects of fertility treatments and related procedures, including better staff planning and caseload management in IVF labs, as well as higher-quality eggs at retrieval. Patients would have a clearer timeline for their treatment cycles.
Nikica Zaninovic, PhD, MS, director of the embryology lab at Weill Cornell Medical College, New York City, cautioned that the new findings are not yet ready for clinical application but emphasized the importance of more AI research focusing on the quality of oocytes, not only embryos.
“We’re so focused on the end of the process: the embryo,” Dr. Zaninovic, who was not involved in the research, said in an interview. “I think the focus should be on the beginning – the quality of eggs and sperm, not just the quantity – because that’s what the embryos will depend on.”
He noted the increasing numbers of young women in the United States undergoing egg freezing.
“Cornell is the largest academic IVF center in the United States; 20%-30% of all of the patients that we treat are actually freezing their eggs,” he said. “It’s a huge population.”
“When they come to us, they ask how many eggs they’ll need to guarantee one or two children in the future,” Dr. Zaninovic continued. “We don’t have that answer, so we always tell them [we’ll retrieve] as many as we can. That’s not the answer; we need to be more precise. We’re still lacking these tools, and I think that’s where the research will go.”
The study was funded by FertilAI. Mr. Hourvitz is a shareholder and CEO of FertilAI. Dr. Zaninovic is president of the AI Fertility Society.
A version of this article appeared on Medscape.com.
FROM ASRM 2023
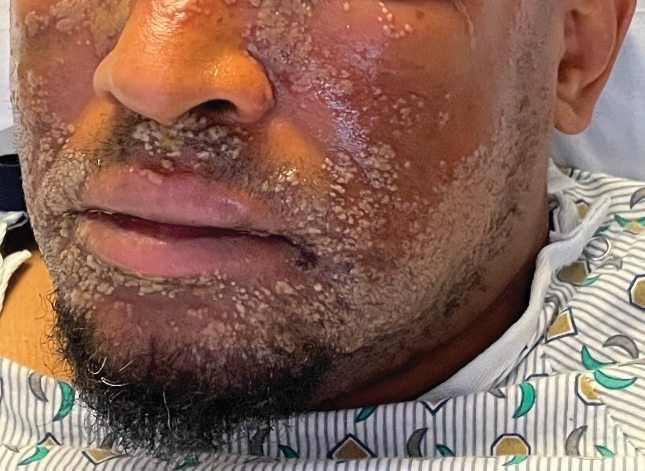
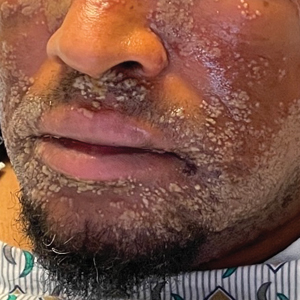
The challenges of palmoplantar pustulosis and other acral psoriatic disease
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
AT THE NPF RESEARCH SYMPOSIUM 2023
The steep costs of disrupting gut-barrier harmony
An interview with Elena Ivanina, DO, MPH
From Ayurveda to the teachings of Hippocrates, medicine’s earliest traditions advanced a belief that the gut was the foundation of all health and disease. It wasn’t until recently, however, that Western medicine has adopted the notion of gut-barrier dysfunction as a pathologic phenomenon critical to not only digestive health but also chronic allergic, inflammatory, and autoimmune disease.
To learn more, Medscape contributor Akash Goel, MD, interviewed Elena Ivanina, DO, MPH, an integrative gastroenterologist, on the role of the gut barrier. Dr. Ivanina is the founder of the Center for Integrative Gut Health and the former director of Neurogastroenterology and Motility at Lenox Hill Hospital in New York. She runs the educational platform for all things gut health, gutlove.com.
What is the role of the gut barrier in overall health and disease?
The gut contains the human body’s largest interface between a person and their external environment. The actual interface is at the gut barrier, where there needs to be an ideal homeostasis and selectivity mechanism to allow the absorption of healthy nutrients, but on the other hand prevent the penetration of harmful microbes, food antigens, and other proinflammatory factors and toxins.
The gut barrier is made up of the mucus layer, gut microbiome, epithelial cells, and immune cells in the lamina propria. When this apparatus is disrupted by factors such as infection, low-fiber diet, antibiotics, and alcohol, then it cannot function normally to selectively keep out the harmful intraluminal substances.
Gut-barrier disruption leads to translocation of dangerous intraluminal components, such as bacteria and their components, into the gut wall and, most importantly, exposes the immune system to them. This causes improper immune activation and dysregulation, which has been shown to lead to various diseases, including gastrointestinal inflammatory disorders such as inflammatory bowel disease (IBD) and celiac disease, systemic autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, and metabolic diseases such as obesity and diabetes.
Is disruption of this barrier what is usually referred to as “leaky gut”?
Leaky gut is a colloquial term for increased intestinal permeability or intestinal hyperpermeability. In a 2019 review article, Dr. Michael Camilleri exposes leaky gut as a term that can be misleading and confusing to the general population. It calls upon clinicians to have an increased awareness of the potential of barrier dysfunction in diseases, and to consider the barrier as a target for treatment.
Is leaky gut more of a mechanism of underlying chronic disease or is it a disease of its own?
Intestinal permeability is a pathophysiologic process in the gut with certain risk factors that in some conditions has been shown to precede chronic disease. There has not been any convincing evidence that it can be diagnosed and treated as its own entity, but research is ongoing.
In IBD, the Crohn’s and Colitis Canada Genetic, Environmental, Microbial Project research consortium has been studying individuals at increased risk for Crohn’s disease because of a first-degree family member with Crohn’s disease. They found an increased abundance of Ruminococcus torques in the microbiomes of at-risk individuals who went on to develop the disease. R. torques are mucin degraders that induce an increase in other mucin-using bacteria, which can contribute to gut-barrier compromise.
In other studies, patients have been found to have asymptomatic intestinal hyperpermeability years before their diagnosis of Crohn’s disease. This supports understanding more about the potential of intestinal hyperpermeability as its own diagnosis that, if addressed, could possibly prevent disease development.
The many possible sources of gut-barrier disruption
What causes leaky gut, and when should physicians and patients be suspicious if they have it?
There are many risk factors that have been associated with leaky gut in both human studies and animal studies, including acrolein (food toxin), aging, alcohol, antacid drugs, antibiotics, burn injury, chemotherapy, circadian rhythm disruption, corticosteroids, emulsifiers (food additives), strenuous exercise (≥ 2 hours) at 60% VO2 max, starvation, fructose, fructans, gliadin (wheat protein), high-fat diet, high-salt diet, high-sugar diet, hyperglycemia, low-fiber diet, nonsteroidal anti-inflammatory drugs, pesticide, proinflammatory cytokines, psychological stress, radiation, sleep deprivation, smoking, and sweeteners.
Patients may be completely asymptomatic with leaky gut. Physicians should be suspicious if there is a genetic predisposition to chronic disease or if any risk factors are unveiled after assessing diet and lifestyle exposures.
What is the role of the Western diet and processed food consumption in driving disruptions of the gut barrier?
The Western diet reduces gut-barrier mucus thickness, leading to increased gut permeability. People who consume a Western diet typically eat less than 15 grams of fiber per day, which is significantly less than many other cultures, including the hunter-gatherers of Tanzania (Hadza), who get 100 or more grams of fiber a day in their food.
With a fiber-depleted diet, gut microbiota that normally feed on fiber gradually disappear and other commensals shift their metabolism to degrade the gut-barrier mucus layer.
A low-fiber diet also decreases short-chain fatty acid production, which reduces production of mucus and affects tight junction regulation.
Emerging evidence on causality
New evidence is demonstrating that previous functional conditions of the gastrointestinal tract, like functional dyspepsia, are associated with abnormalities to the intestinal barrier. What is the association between conditions like functional dyspepsia and irritable bowel syndrome (IBS) with gut-barrier disruption?
Conditions such as functional dyspepsia and IBS are similar in that their pathophysiology is incompletely understood and likely attributable to contributions from many different underlying mechanisms. This makes it difficult for clinicians to explain the condition to patients and often to treat without specific therapeutic targets.
Emerging evidence with new diagnostic tools, such as confocal laser endomicroscopy, has demonstrated altered mucosal barrier function in both conditions.
In patients with IBS who have a suspected food intolerance, studies looking at exposure to the food antigens found that the food caused immediate breaks, increased intervillous spaces, and increased inflammatory cells in the gut mucosa. These changes were associated with patient responses to exclusion diets.
In functional dyspepsia, another study, using confocal laser endomicroscopy, has shown that affected patients have significantly greater epithelial gap density in the duodenum, compared with healthy controls. There was also impaired duodenal-epithelial barrier integrity and evidence of increased cellular pyroptosis in the duodenal mucosa.
These findings suggest that while IBS and functional dyspepsia are still likely multifactorial, there may be a common preclinical state that can be further investigated as far as preventing its development and using it as a therapeutic target.
What diagnostic testing are you using to determine whether patients have disruptions to the gut barrier? Are they validated or more experimental?
There are various testing strategies that have been used in research to diagnose intestinal hyperpermeability. In a 2021 analysis, Dr. Michael Camilleri found that the optimal probes for measuring small intestinal and colonic permeability are the mass excreted of 13C-mannitol at 0-2 hours and lactulose during 2-8 hours or sucralose during 8-24 hours. Studies looking at postinfectious IBS have incorporated elevated urinary lactulose/mannitol ratios. Dr. Alessio Fasano and others have looked at using zonulin as a biomarker of impaired gut-barrier function. These tests are still considered experimental.
Is there an association between alterations in the gut microbiome and gut-barrier disruption?
There is an integral relationship between the gut microbiome and gut-barrier function, and dysbiosis can disrupt gut-barrier functionality.
The microbiota produce a variety of metabolites in close proximity to the gut epithelium, impacting gut-barrier function and immune response. For example, short-chain fatty acids produced by Bifidobacterium, Bacteroides, Enterobacter, Faecalibacterium, and Roseburia species impact host immune cell differentiation and metabolism as well as influence susceptibility to pathogens.
Studies have shown that sodium butyrate significantly improves epithelial-barrier function. Other experiments have used transplantation of the intestinal microbiota to show that introduction of certain microbial phenotypes can significantly increase gut permeability.
Practical advice for clinicians and patients
How do you advise patients to avoid gut-barrier disruption?
It is important to educate and counsel patients about the long list of risk factors, many of which are closely related to a Western diet and lifestyle, which can increase their risk for leaky gut.
Once one has it, can it be repaired? Can you share a bit about your protocols in general terms?
Many interventions have been shown to improve intestinal permeability. They include berberine, butyrate, caloric restriction and fasting, curcumin, dietary fiber (prebiotics), moderate exercise, fermented food, fish oil, glutamine, quercetin, probiotics, vagus nerve stimulation, vitamin D, and zinc.
Protocols have to be tailored to patients and their risk factors, diet, and lifestyle.
What are some tips from a nutrition and lifestyle standpoint that patients can follow to ensure a robust gut barrier?
It is important to emphasize a high-fiber diet with naturally fermented food, incorporating time-restricted eating, such as eating an early dinner and nothing else before bedtime, a moderate exercise routine, and gut-brain modulation with techniques such as acupuncture that can incorporate vagus nerve stimulation. Limited safe precision supplementation can be discussed on an individual basis based on the patient’s interest, additional testing, and other existing health conditions.
Dr. Akash Goel is a clinical assistant professor of medicine at Weill Cornell in gastroenterology and hepatology. He has disclosed no relevant financial relationships. His work has appeared on networks and publications such as CNN, The New York Times, Time Magazine, and Financial Times. He has a deep interest in nutrition, food as medicine, and the intersection between the gut microbiome and human health.
A version of this article appeared on Medscape.com.
An interview with Elena Ivanina, DO, MPH
An interview with Elena Ivanina, DO, MPH
From Ayurveda to the teachings of Hippocrates, medicine’s earliest traditions advanced a belief that the gut was the foundation of all health and disease. It wasn’t until recently, however, that Western medicine has adopted the notion of gut-barrier dysfunction as a pathologic phenomenon critical to not only digestive health but also chronic allergic, inflammatory, and autoimmune disease.
To learn more, Medscape contributor Akash Goel, MD, interviewed Elena Ivanina, DO, MPH, an integrative gastroenterologist, on the role of the gut barrier. Dr. Ivanina is the founder of the Center for Integrative Gut Health and the former director of Neurogastroenterology and Motility at Lenox Hill Hospital in New York. She runs the educational platform for all things gut health, gutlove.com.
What is the role of the gut barrier in overall health and disease?
The gut contains the human body’s largest interface between a person and their external environment. The actual interface is at the gut barrier, where there needs to be an ideal homeostasis and selectivity mechanism to allow the absorption of healthy nutrients, but on the other hand prevent the penetration of harmful microbes, food antigens, and other proinflammatory factors and toxins.
The gut barrier is made up of the mucus layer, gut microbiome, epithelial cells, and immune cells in the lamina propria. When this apparatus is disrupted by factors such as infection, low-fiber diet, antibiotics, and alcohol, then it cannot function normally to selectively keep out the harmful intraluminal substances.
Gut-barrier disruption leads to translocation of dangerous intraluminal components, such as bacteria and their components, into the gut wall and, most importantly, exposes the immune system to them. This causes improper immune activation and dysregulation, which has been shown to lead to various diseases, including gastrointestinal inflammatory disorders such as inflammatory bowel disease (IBD) and celiac disease, systemic autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, and metabolic diseases such as obesity and diabetes.
Is disruption of this barrier what is usually referred to as “leaky gut”?
Leaky gut is a colloquial term for increased intestinal permeability or intestinal hyperpermeability. In a 2019 review article, Dr. Michael Camilleri exposes leaky gut as a term that can be misleading and confusing to the general population. It calls upon clinicians to have an increased awareness of the potential of barrier dysfunction in diseases, and to consider the barrier as a target for treatment.
Is leaky gut more of a mechanism of underlying chronic disease or is it a disease of its own?
Intestinal permeability is a pathophysiologic process in the gut with certain risk factors that in some conditions has been shown to precede chronic disease. There has not been any convincing evidence that it can be diagnosed and treated as its own entity, but research is ongoing.
In IBD, the Crohn’s and Colitis Canada Genetic, Environmental, Microbial Project research consortium has been studying individuals at increased risk for Crohn’s disease because of a first-degree family member with Crohn’s disease. They found an increased abundance of Ruminococcus torques in the microbiomes of at-risk individuals who went on to develop the disease. R. torques are mucin degraders that induce an increase in other mucin-using bacteria, which can contribute to gut-barrier compromise.
In other studies, patients have been found to have asymptomatic intestinal hyperpermeability years before their diagnosis of Crohn’s disease. This supports understanding more about the potential of intestinal hyperpermeability as its own diagnosis that, if addressed, could possibly prevent disease development.
The many possible sources of gut-barrier disruption
What causes leaky gut, and when should physicians and patients be suspicious if they have it?
There are many risk factors that have been associated with leaky gut in both human studies and animal studies, including acrolein (food toxin), aging, alcohol, antacid drugs, antibiotics, burn injury, chemotherapy, circadian rhythm disruption, corticosteroids, emulsifiers (food additives), strenuous exercise (≥ 2 hours) at 60% VO2 max, starvation, fructose, fructans, gliadin (wheat protein), high-fat diet, high-salt diet, high-sugar diet, hyperglycemia, low-fiber diet, nonsteroidal anti-inflammatory drugs, pesticide, proinflammatory cytokines, psychological stress, radiation, sleep deprivation, smoking, and sweeteners.
Patients may be completely asymptomatic with leaky gut. Physicians should be suspicious if there is a genetic predisposition to chronic disease or if any risk factors are unveiled after assessing diet and lifestyle exposures.
What is the role of the Western diet and processed food consumption in driving disruptions of the gut barrier?
The Western diet reduces gut-barrier mucus thickness, leading to increased gut permeability. People who consume a Western diet typically eat less than 15 grams of fiber per day, which is significantly less than many other cultures, including the hunter-gatherers of Tanzania (Hadza), who get 100 or more grams of fiber a day in their food.
With a fiber-depleted diet, gut microbiota that normally feed on fiber gradually disappear and other commensals shift their metabolism to degrade the gut-barrier mucus layer.
A low-fiber diet also decreases short-chain fatty acid production, which reduces production of mucus and affects tight junction regulation.
Emerging evidence on causality
New evidence is demonstrating that previous functional conditions of the gastrointestinal tract, like functional dyspepsia, are associated with abnormalities to the intestinal barrier. What is the association between conditions like functional dyspepsia and irritable bowel syndrome (IBS) with gut-barrier disruption?
Conditions such as functional dyspepsia and IBS are similar in that their pathophysiology is incompletely understood and likely attributable to contributions from many different underlying mechanisms. This makes it difficult for clinicians to explain the condition to patients and often to treat without specific therapeutic targets.
Emerging evidence with new diagnostic tools, such as confocal laser endomicroscopy, has demonstrated altered mucosal barrier function in both conditions.
In patients with IBS who have a suspected food intolerance, studies looking at exposure to the food antigens found that the food caused immediate breaks, increased intervillous spaces, and increased inflammatory cells in the gut mucosa. These changes were associated with patient responses to exclusion diets.
In functional dyspepsia, another study, using confocal laser endomicroscopy, has shown that affected patients have significantly greater epithelial gap density in the duodenum, compared with healthy controls. There was also impaired duodenal-epithelial barrier integrity and evidence of increased cellular pyroptosis in the duodenal mucosa.
These findings suggest that while IBS and functional dyspepsia are still likely multifactorial, there may be a common preclinical state that can be further investigated as far as preventing its development and using it as a therapeutic target.
What diagnostic testing are you using to determine whether patients have disruptions to the gut barrier? Are they validated or more experimental?
There are various testing strategies that have been used in research to diagnose intestinal hyperpermeability. In a 2021 analysis, Dr. Michael Camilleri found that the optimal probes for measuring small intestinal and colonic permeability are the mass excreted of 13C-mannitol at 0-2 hours and lactulose during 2-8 hours or sucralose during 8-24 hours. Studies looking at postinfectious IBS have incorporated elevated urinary lactulose/mannitol ratios. Dr. Alessio Fasano and others have looked at using zonulin as a biomarker of impaired gut-barrier function. These tests are still considered experimental.
Is there an association between alterations in the gut microbiome and gut-barrier disruption?
There is an integral relationship between the gut microbiome and gut-barrier function, and dysbiosis can disrupt gut-barrier functionality.
The microbiota produce a variety of metabolites in close proximity to the gut epithelium, impacting gut-barrier function and immune response. For example, short-chain fatty acids produced by Bifidobacterium, Bacteroides, Enterobacter, Faecalibacterium, and Roseburia species impact host immune cell differentiation and metabolism as well as influence susceptibility to pathogens.
Studies have shown that sodium butyrate significantly improves epithelial-barrier function. Other experiments have used transplantation of the intestinal microbiota to show that introduction of certain microbial phenotypes can significantly increase gut permeability.
Practical advice for clinicians and patients
How do you advise patients to avoid gut-barrier disruption?
It is important to educate and counsel patients about the long list of risk factors, many of which are closely related to a Western diet and lifestyle, which can increase their risk for leaky gut.
Once one has it, can it be repaired? Can you share a bit about your protocols in general terms?
Many interventions have been shown to improve intestinal permeability. They include berberine, butyrate, caloric restriction and fasting, curcumin, dietary fiber (prebiotics), moderate exercise, fermented food, fish oil, glutamine, quercetin, probiotics, vagus nerve stimulation, vitamin D, and zinc.
Protocols have to be tailored to patients and their risk factors, diet, and lifestyle.
What are some tips from a nutrition and lifestyle standpoint that patients can follow to ensure a robust gut barrier?
It is important to emphasize a high-fiber diet with naturally fermented food, incorporating time-restricted eating, such as eating an early dinner and nothing else before bedtime, a moderate exercise routine, and gut-brain modulation with techniques such as acupuncture that can incorporate vagus nerve stimulation. Limited safe precision supplementation can be discussed on an individual basis based on the patient’s interest, additional testing, and other existing health conditions.
Dr. Akash Goel is a clinical assistant professor of medicine at Weill Cornell in gastroenterology and hepatology. He has disclosed no relevant financial relationships. His work has appeared on networks and publications such as CNN, The New York Times, Time Magazine, and Financial Times. He has a deep interest in nutrition, food as medicine, and the intersection between the gut microbiome and human health.
A version of this article appeared on Medscape.com.
From Ayurveda to the teachings of Hippocrates, medicine’s earliest traditions advanced a belief that the gut was the foundation of all health and disease. It wasn’t until recently, however, that Western medicine has adopted the notion of gut-barrier dysfunction as a pathologic phenomenon critical to not only digestive health but also chronic allergic, inflammatory, and autoimmune disease.
To learn more, Medscape contributor Akash Goel, MD, interviewed Elena Ivanina, DO, MPH, an integrative gastroenterologist, on the role of the gut barrier. Dr. Ivanina is the founder of the Center for Integrative Gut Health and the former director of Neurogastroenterology and Motility at Lenox Hill Hospital in New York. She runs the educational platform for all things gut health, gutlove.com.
What is the role of the gut barrier in overall health and disease?
The gut contains the human body’s largest interface between a person and their external environment. The actual interface is at the gut barrier, where there needs to be an ideal homeostasis and selectivity mechanism to allow the absorption of healthy nutrients, but on the other hand prevent the penetration of harmful microbes, food antigens, and other proinflammatory factors and toxins.
The gut barrier is made up of the mucus layer, gut microbiome, epithelial cells, and immune cells in the lamina propria. When this apparatus is disrupted by factors such as infection, low-fiber diet, antibiotics, and alcohol, then it cannot function normally to selectively keep out the harmful intraluminal substances.
Gut-barrier disruption leads to translocation of dangerous intraluminal components, such as bacteria and their components, into the gut wall and, most importantly, exposes the immune system to them. This causes improper immune activation and dysregulation, which has been shown to lead to various diseases, including gastrointestinal inflammatory disorders such as inflammatory bowel disease (IBD) and celiac disease, systemic autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, and metabolic diseases such as obesity and diabetes.
Is disruption of this barrier what is usually referred to as “leaky gut”?
Leaky gut is a colloquial term for increased intestinal permeability or intestinal hyperpermeability. In a 2019 review article, Dr. Michael Camilleri exposes leaky gut as a term that can be misleading and confusing to the general population. It calls upon clinicians to have an increased awareness of the potential of barrier dysfunction in diseases, and to consider the barrier as a target for treatment.
Is leaky gut more of a mechanism of underlying chronic disease or is it a disease of its own?
Intestinal permeability is a pathophysiologic process in the gut with certain risk factors that in some conditions has been shown to precede chronic disease. There has not been any convincing evidence that it can be diagnosed and treated as its own entity, but research is ongoing.
In IBD, the Crohn’s and Colitis Canada Genetic, Environmental, Microbial Project research consortium has been studying individuals at increased risk for Crohn’s disease because of a first-degree family member with Crohn’s disease. They found an increased abundance of Ruminococcus torques in the microbiomes of at-risk individuals who went on to develop the disease. R. torques are mucin degraders that induce an increase in other mucin-using bacteria, which can contribute to gut-barrier compromise.
In other studies, patients have been found to have asymptomatic intestinal hyperpermeability years before their diagnosis of Crohn’s disease. This supports understanding more about the potential of intestinal hyperpermeability as its own diagnosis that, if addressed, could possibly prevent disease development.
The many possible sources of gut-barrier disruption
What causes leaky gut, and when should physicians and patients be suspicious if they have it?
There are many risk factors that have been associated with leaky gut in both human studies and animal studies, including acrolein (food toxin), aging, alcohol, antacid drugs, antibiotics, burn injury, chemotherapy, circadian rhythm disruption, corticosteroids, emulsifiers (food additives), strenuous exercise (≥ 2 hours) at 60% VO2 max, starvation, fructose, fructans, gliadin (wheat protein), high-fat diet, high-salt diet, high-sugar diet, hyperglycemia, low-fiber diet, nonsteroidal anti-inflammatory drugs, pesticide, proinflammatory cytokines, psychological stress, radiation, sleep deprivation, smoking, and sweeteners.
Patients may be completely asymptomatic with leaky gut. Physicians should be suspicious if there is a genetic predisposition to chronic disease or if any risk factors are unveiled after assessing diet and lifestyle exposures.
What is the role of the Western diet and processed food consumption in driving disruptions of the gut barrier?
The Western diet reduces gut-barrier mucus thickness, leading to increased gut permeability. People who consume a Western diet typically eat less than 15 grams of fiber per day, which is significantly less than many other cultures, including the hunter-gatherers of Tanzania (Hadza), who get 100 or more grams of fiber a day in their food.
With a fiber-depleted diet, gut microbiota that normally feed on fiber gradually disappear and other commensals shift their metabolism to degrade the gut-barrier mucus layer.
A low-fiber diet also decreases short-chain fatty acid production, which reduces production of mucus and affects tight junction regulation.
Emerging evidence on causality
New evidence is demonstrating that previous functional conditions of the gastrointestinal tract, like functional dyspepsia, are associated with abnormalities to the intestinal barrier. What is the association between conditions like functional dyspepsia and irritable bowel syndrome (IBS) with gut-barrier disruption?
Conditions such as functional dyspepsia and IBS are similar in that their pathophysiology is incompletely understood and likely attributable to contributions from many different underlying mechanisms. This makes it difficult for clinicians to explain the condition to patients and often to treat without specific therapeutic targets.
Emerging evidence with new diagnostic tools, such as confocal laser endomicroscopy, has demonstrated altered mucosal barrier function in both conditions.
In patients with IBS who have a suspected food intolerance, studies looking at exposure to the food antigens found that the food caused immediate breaks, increased intervillous spaces, and increased inflammatory cells in the gut mucosa. These changes were associated with patient responses to exclusion diets.
In functional dyspepsia, another study, using confocal laser endomicroscopy, has shown that affected patients have significantly greater epithelial gap density in the duodenum, compared with healthy controls. There was also impaired duodenal-epithelial barrier integrity and evidence of increased cellular pyroptosis in the duodenal mucosa.
These findings suggest that while IBS and functional dyspepsia are still likely multifactorial, there may be a common preclinical state that can be further investigated as far as preventing its development and using it as a therapeutic target.
What diagnostic testing are you using to determine whether patients have disruptions to the gut barrier? Are they validated or more experimental?
There are various testing strategies that have been used in research to diagnose intestinal hyperpermeability. In a 2021 analysis, Dr. Michael Camilleri found that the optimal probes for measuring small intestinal and colonic permeability are the mass excreted of 13C-mannitol at 0-2 hours and lactulose during 2-8 hours or sucralose during 8-24 hours. Studies looking at postinfectious IBS have incorporated elevated urinary lactulose/mannitol ratios. Dr. Alessio Fasano and others have looked at using zonulin as a biomarker of impaired gut-barrier function. These tests are still considered experimental.
Is there an association between alterations in the gut microbiome and gut-barrier disruption?
There is an integral relationship between the gut microbiome and gut-barrier function, and dysbiosis can disrupt gut-barrier functionality.
The microbiota produce a variety of metabolites in close proximity to the gut epithelium, impacting gut-barrier function and immune response. For example, short-chain fatty acids produced by Bifidobacterium, Bacteroides, Enterobacter, Faecalibacterium, and Roseburia species impact host immune cell differentiation and metabolism as well as influence susceptibility to pathogens.
Studies have shown that sodium butyrate significantly improves epithelial-barrier function. Other experiments have used transplantation of the intestinal microbiota to show that introduction of certain microbial phenotypes can significantly increase gut permeability.
Practical advice for clinicians and patients
How do you advise patients to avoid gut-barrier disruption?
It is important to educate and counsel patients about the long list of risk factors, many of which are closely related to a Western diet and lifestyle, which can increase their risk for leaky gut.
Once one has it, can it be repaired? Can you share a bit about your protocols in general terms?
Many interventions have been shown to improve intestinal permeability. They include berberine, butyrate, caloric restriction and fasting, curcumin, dietary fiber (prebiotics), moderate exercise, fermented food, fish oil, glutamine, quercetin, probiotics, vagus nerve stimulation, vitamin D, and zinc.
Protocols have to be tailored to patients and their risk factors, diet, and lifestyle.
What are some tips from a nutrition and lifestyle standpoint that patients can follow to ensure a robust gut barrier?
It is important to emphasize a high-fiber diet with naturally fermented food, incorporating time-restricted eating, such as eating an early dinner and nothing else before bedtime, a moderate exercise routine, and gut-brain modulation with techniques such as acupuncture that can incorporate vagus nerve stimulation. Limited safe precision supplementation can be discussed on an individual basis based on the patient’s interest, additional testing, and other existing health conditions.
Dr. Akash Goel is a clinical assistant professor of medicine at Weill Cornell in gastroenterology and hepatology. He has disclosed no relevant financial relationships. His work has appeared on networks and publications such as CNN, The New York Times, Time Magazine, and Financial Times. He has a deep interest in nutrition, food as medicine, and the intersection between the gut microbiome and human health.
A version of this article appeared on Medscape.com.
Risk calculator for early-stage CKD may soon enter U.S. market
PHILADELPHIA – The analyses offer the possibility of focusing intensified medical management of early-stage CKD on those patients who could potentially receive the most benefit.
The Klinrisk model predicts the risk of an adult with early-stage CKD developing either a 40% or greater drop in estimated glomerular filtration rate or kidney failure. It calculates risk based on 20 lab-measured variables that include serum creatinine, urine albumin-to-creatinine ratio, and other values taken from routinely ordered tests such as complete blood cell counts, chemistry panels, comprehensive metabolic panels, and urinalysis.
In the most recent and largest external validation study using data from 4.6 million American adults enrolled in commercial and Medicare insurance plans, the results showed Klinrisk correctly predicted CKD progression in 80%-83% of individuals over 2 years and in 78%-83% of individuals over 5 years, depending on the insurance provider, Navdeep Tangri, MD, PhD, reported at the annual meeting of the American Society of Nephrology. When urinalysis data were available, the model correctly predicted CKD progression in 81%-87% of individuals over 2 years and in 80%-87% of individuals over 5 years. These results follow prior reports of several other successful validations of Klinrisk.
‘Ready to implement’
“The Klinrisk model is ready to implement by any payer, health system, or clinic where the needed lab data are available,” said Dr. Tangri, a nephrologist and professor at the University of Manitoba, Winnipeg, and founder of Klinrisk Inc., the company developing and commercializing the Klinrisk assessment tool.
For the time being, Dr. Tangri sees Klinrisk as a population health device that can allow insurers and health systems to track management quality and quality improvement and to target patients who stand to benefit most from relatively expensive resources. This includes prescriptions for finerenone (Kerendia, Bayer) for people who also have type 2 diabetes, and agents from the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors such as dapagliflozin (Farxiga, AstraZeneca) and empagliflozin (Jardiance, Boehringer Ingelheim and Lilly).
He has also begun discussions with the Food and Drug Administration about the data the agency will need to consider Klinrisk for potential approval as a new medical device, perhaps in 2025. That’s how he envisions getting a Klinrisk assessment into the hands of caregivers that they could use with individual patients to create an appropriate treatment plan.
Results from his new analysis showed that “all the kidney disease action is in the 10%-20% of people with the highest risk on Klinrisk, while not much happens in those in the bottom half,” Dr. Tangri said during his presentation.
“We’re trying to find the patients who get the largest [absolute] benefit from intensified treatment,” he added in an interview. “Klinrisk finds people with high-risk kidney disease early on, when kidney function is still normal or near normal. High-risk patients are often completely unrecognized. Risk-based management” that identifies the early-stage CKD patients who would benefit most from treatment with an SGLT2 inhibitor, finerenone, and other foundational treatments to slow CKD progression “is better than the free-for-all that occurs today.”
Simplified data collection
“Klinrisk is very effective,” but requires follow-up by clinicians and health systems to implement its findings, commented Josef Coresh, MD, a professor of clinical epidemiology at Johns Hopkins Bloomberg, Baltimore. Dr. Coresh compared it with a free equation that estimates a person’s risk for a 40% drop in kidney function over the next 3 years developed by Dr. Tangri, Dr. Coresh, and many collaborators led by Morgan C. Grams, MD, PhD, of New York University that they published in 2022, and posted on a website of the CKD Prognosis Consortium.
The CKD Prognosis Consortium formula “takes a different approach” from Klinrisk. The commercial formula “is simpler, only using lab measures, and avoids inputs taken from physical examination such as systolic blood pressure and body mass index and health history data such as smoking, noted Dr. Coresh. He also speculated that “a commercial formula that must be paid for may counterintuitively result in better follow-up for making management changes if it uses some of the resources for education and system changes.”
Using data from multiple sources, like the CKD Prognosis Consortium equation, can create implementation challenges, said Dr. Tangri. “Lab results don’t vary much,” which makes Klinrisk “quite an improvement for implementation. It’s easier to implement.”
Other findings from the newest validation study that Dr. Tangri presented were that the people studied with Klinrisk scores in the top 10% had, over the next 2 years of follow-up and compared with people in the bottom half for Klinrisk staging, a 3- to 5-fold higher rate of all-cause medical costs, a 13-30-fold increase in CKD-related costs, and a 5- to 10-fold increase in hospitalizations and ED visits.
Early identification of CKD and early initiation of intensified treatment for high-risk patients can reduce the rate of progression to dialysis, reduce hospitalizations for heart failure, and lower the cost of care, Dr. Tangri said.
The validation study in 4.6 million Americans was sponsored by Boehringer Ingelheim. Dr. Tangri founded and has an ownership interest in Klinrisk. He has also received honoraria from, has ownership interests in, and has been a consultant to multiple pharmaceutical companies. Dr. Coresh had no disclosures.
PHILADELPHIA – The analyses offer the possibility of focusing intensified medical management of early-stage CKD on those patients who could potentially receive the most benefit.
The Klinrisk model predicts the risk of an adult with early-stage CKD developing either a 40% or greater drop in estimated glomerular filtration rate or kidney failure. It calculates risk based on 20 lab-measured variables that include serum creatinine, urine albumin-to-creatinine ratio, and other values taken from routinely ordered tests such as complete blood cell counts, chemistry panels, comprehensive metabolic panels, and urinalysis.
In the most recent and largest external validation study using data from 4.6 million American adults enrolled in commercial and Medicare insurance plans, the results showed Klinrisk correctly predicted CKD progression in 80%-83% of individuals over 2 years and in 78%-83% of individuals over 5 years, depending on the insurance provider, Navdeep Tangri, MD, PhD, reported at the annual meeting of the American Society of Nephrology. When urinalysis data were available, the model correctly predicted CKD progression in 81%-87% of individuals over 2 years and in 80%-87% of individuals over 5 years. These results follow prior reports of several other successful validations of Klinrisk.
‘Ready to implement’
“The Klinrisk model is ready to implement by any payer, health system, or clinic where the needed lab data are available,” said Dr. Tangri, a nephrologist and professor at the University of Manitoba, Winnipeg, and founder of Klinrisk Inc., the company developing and commercializing the Klinrisk assessment tool.
For the time being, Dr. Tangri sees Klinrisk as a population health device that can allow insurers and health systems to track management quality and quality improvement and to target patients who stand to benefit most from relatively expensive resources. This includes prescriptions for finerenone (Kerendia, Bayer) for people who also have type 2 diabetes, and agents from the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors such as dapagliflozin (Farxiga, AstraZeneca) and empagliflozin (Jardiance, Boehringer Ingelheim and Lilly).
He has also begun discussions with the Food and Drug Administration about the data the agency will need to consider Klinrisk for potential approval as a new medical device, perhaps in 2025. That’s how he envisions getting a Klinrisk assessment into the hands of caregivers that they could use with individual patients to create an appropriate treatment plan.
Results from his new analysis showed that “all the kidney disease action is in the 10%-20% of people with the highest risk on Klinrisk, while not much happens in those in the bottom half,” Dr. Tangri said during his presentation.
“We’re trying to find the patients who get the largest [absolute] benefit from intensified treatment,” he added in an interview. “Klinrisk finds people with high-risk kidney disease early on, when kidney function is still normal or near normal. High-risk patients are often completely unrecognized. Risk-based management” that identifies the early-stage CKD patients who would benefit most from treatment with an SGLT2 inhibitor, finerenone, and other foundational treatments to slow CKD progression “is better than the free-for-all that occurs today.”
Simplified data collection
“Klinrisk is very effective,” but requires follow-up by clinicians and health systems to implement its findings, commented Josef Coresh, MD, a professor of clinical epidemiology at Johns Hopkins Bloomberg, Baltimore. Dr. Coresh compared it with a free equation that estimates a person’s risk for a 40% drop in kidney function over the next 3 years developed by Dr. Tangri, Dr. Coresh, and many collaborators led by Morgan C. Grams, MD, PhD, of New York University that they published in 2022, and posted on a website of the CKD Prognosis Consortium.
The CKD Prognosis Consortium formula “takes a different approach” from Klinrisk. The commercial formula “is simpler, only using lab measures, and avoids inputs taken from physical examination such as systolic blood pressure and body mass index and health history data such as smoking, noted Dr. Coresh. He also speculated that “a commercial formula that must be paid for may counterintuitively result in better follow-up for making management changes if it uses some of the resources for education and system changes.”
Using data from multiple sources, like the CKD Prognosis Consortium equation, can create implementation challenges, said Dr. Tangri. “Lab results don’t vary much,” which makes Klinrisk “quite an improvement for implementation. It’s easier to implement.”
Other findings from the newest validation study that Dr. Tangri presented were that the people studied with Klinrisk scores in the top 10% had, over the next 2 years of follow-up and compared with people in the bottom half for Klinrisk staging, a 3- to 5-fold higher rate of all-cause medical costs, a 13-30-fold increase in CKD-related costs, and a 5- to 10-fold increase in hospitalizations and ED visits.
Early identification of CKD and early initiation of intensified treatment for high-risk patients can reduce the rate of progression to dialysis, reduce hospitalizations for heart failure, and lower the cost of care, Dr. Tangri said.
The validation study in 4.6 million Americans was sponsored by Boehringer Ingelheim. Dr. Tangri founded and has an ownership interest in Klinrisk. He has also received honoraria from, has ownership interests in, and has been a consultant to multiple pharmaceutical companies. Dr. Coresh had no disclosures.
PHILADELPHIA – The analyses offer the possibility of focusing intensified medical management of early-stage CKD on those patients who could potentially receive the most benefit.
The Klinrisk model predicts the risk of an adult with early-stage CKD developing either a 40% or greater drop in estimated glomerular filtration rate or kidney failure. It calculates risk based on 20 lab-measured variables that include serum creatinine, urine albumin-to-creatinine ratio, and other values taken from routinely ordered tests such as complete blood cell counts, chemistry panels, comprehensive metabolic panels, and urinalysis.
In the most recent and largest external validation study using data from 4.6 million American adults enrolled in commercial and Medicare insurance plans, the results showed Klinrisk correctly predicted CKD progression in 80%-83% of individuals over 2 years and in 78%-83% of individuals over 5 years, depending on the insurance provider, Navdeep Tangri, MD, PhD, reported at the annual meeting of the American Society of Nephrology. When urinalysis data were available, the model correctly predicted CKD progression in 81%-87% of individuals over 2 years and in 80%-87% of individuals over 5 years. These results follow prior reports of several other successful validations of Klinrisk.
‘Ready to implement’
“The Klinrisk model is ready to implement by any payer, health system, or clinic where the needed lab data are available,” said Dr. Tangri, a nephrologist and professor at the University of Manitoba, Winnipeg, and founder of Klinrisk Inc., the company developing and commercializing the Klinrisk assessment tool.
For the time being, Dr. Tangri sees Klinrisk as a population health device that can allow insurers and health systems to track management quality and quality improvement and to target patients who stand to benefit most from relatively expensive resources. This includes prescriptions for finerenone (Kerendia, Bayer) for people who also have type 2 diabetes, and agents from the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors such as dapagliflozin (Farxiga, AstraZeneca) and empagliflozin (Jardiance, Boehringer Ingelheim and Lilly).
He has also begun discussions with the Food and Drug Administration about the data the agency will need to consider Klinrisk for potential approval as a new medical device, perhaps in 2025. That’s how he envisions getting a Klinrisk assessment into the hands of caregivers that they could use with individual patients to create an appropriate treatment plan.
Results from his new analysis showed that “all the kidney disease action is in the 10%-20% of people with the highest risk on Klinrisk, while not much happens in those in the bottom half,” Dr. Tangri said during his presentation.
“We’re trying to find the patients who get the largest [absolute] benefit from intensified treatment,” he added in an interview. “Klinrisk finds people with high-risk kidney disease early on, when kidney function is still normal or near normal. High-risk patients are often completely unrecognized. Risk-based management” that identifies the early-stage CKD patients who would benefit most from treatment with an SGLT2 inhibitor, finerenone, and other foundational treatments to slow CKD progression “is better than the free-for-all that occurs today.”
Simplified data collection
“Klinrisk is very effective,” but requires follow-up by clinicians and health systems to implement its findings, commented Josef Coresh, MD, a professor of clinical epidemiology at Johns Hopkins Bloomberg, Baltimore. Dr. Coresh compared it with a free equation that estimates a person’s risk for a 40% drop in kidney function over the next 3 years developed by Dr. Tangri, Dr. Coresh, and many collaborators led by Morgan C. Grams, MD, PhD, of New York University that they published in 2022, and posted on a website of the CKD Prognosis Consortium.
The CKD Prognosis Consortium formula “takes a different approach” from Klinrisk. The commercial formula “is simpler, only using lab measures, and avoids inputs taken from physical examination such as systolic blood pressure and body mass index and health history data such as smoking, noted Dr. Coresh. He also speculated that “a commercial formula that must be paid for may counterintuitively result in better follow-up for making management changes if it uses some of the resources for education and system changes.”
Using data from multiple sources, like the CKD Prognosis Consortium equation, can create implementation challenges, said Dr. Tangri. “Lab results don’t vary much,” which makes Klinrisk “quite an improvement for implementation. It’s easier to implement.”
Other findings from the newest validation study that Dr. Tangri presented were that the people studied with Klinrisk scores in the top 10% had, over the next 2 years of follow-up and compared with people in the bottom half for Klinrisk staging, a 3- to 5-fold higher rate of all-cause medical costs, a 13-30-fold increase in CKD-related costs, and a 5- to 10-fold increase in hospitalizations and ED visits.
Early identification of CKD and early initiation of intensified treatment for high-risk patients can reduce the rate of progression to dialysis, reduce hospitalizations for heart failure, and lower the cost of care, Dr. Tangri said.
The validation study in 4.6 million Americans was sponsored by Boehringer Ingelheim. Dr. Tangri founded and has an ownership interest in Klinrisk. He has also received honoraria from, has ownership interests in, and has been a consultant to multiple pharmaceutical companies. Dr. Coresh had no disclosures.
AT KIDNEY WEEK 2023
MASLD, MASH projected to grow by 23% in the U.S. through 2050
BOSTON – The nomenclature may have changed, but the steady rise in the most common form of liver disease – metabolic dysfunction–associated steatotic liver disease (MASLD, formerly known as NAFLD) – is predicted to continue into the middle of this century.
That’s according to Phuc Le, PhD, MPH, and colleagues at the Cleveland Clinic. They created a mathematical model incorporating data on the growth of the U.S. population and the natural history of MASLD/NAFLD. The model projected a relative 23% increase in MASLD among U.S. adults from 2020 to 2050.
“Our model forecasts a substantial clinical burden of NAFLD over the next 3 decades. In the absence of effective treatments, health systems should plan for large increases in the number of liver cancer cases and the need for liver transplant,” Dr. Le said in a media briefing held on Nov. 7 prior to her presentation of the data at the annual meeting of the American Association for the Study of Liver Diseases.
The estimated worldwide prevalence of MASLD is 38%. In the United States, an estimated 27.8% of adults had MASLD as of 2020.
Dr. Le and colleagues wanted to get a clearer picture of the expected increase in the clinical burden of MASLD in the coming decades. The researchers used data from the medical literature to create an individual-level state transition model. They took into account projections of the growth of the U.S. population and the progression of MASLD and metabolic dysfunction–associated steatohepatitis (MASH, formerly NASH) through stages of fibrosis to decompensation, hepatocellular carcinoma (HCC), transplant, and liver-related death as a proportion of all-cause mortality.
Validated model
They validated the model by testing it against liver outcomes from 2000 through 2018 and published data on the U.S. population. The model closely matched trends in MASLD prevalence, MASH proportion, HCC and liver transplant incidences, and overall survival rates for patients with MASLD.
As noted, the model predicted a steady increase in MASLD prevalence, from 27.8% in 2020 to 34.3% by 2050, a relative increase of about 23%. The model also predicted a slight uptick in the proportion of MASH among patients with MASLD, from 20% to 21.8%.
The investigators said that the prevalence of MASLD/MASH would likely remain relatively stable among people aged 18-29 years but would increase significantly for all other age groups.
In addition, the model predicted an increase in the proportion of cirrhosis in patients with MASLD from 1.9% to 3.1%, as well as a rise in liver-related deaths from 0.4% of all deaths in 2020 to 1% by 2050.
The investigators also foresaw a rise in HCC cases, from 10,400 annually to 19,300 by 2050 and a more than twofold increase in liver transplants, from 1,700 in 2020 to 4,200 in 2050.
A “tsunami” of liver disease
In the question-and-answer portion of the briefing, Norah Terrault, MD, AASLD president and chief of gastroenterology and hepatology at the University of Southern California, Los Angeles, commented on the study findings and “the frightening trajectory in terms of disease burden.
“I’m thinking to myself there’s no way we’re going to be able to transplant our way out of this tsunami of disease that’s coming our way,” she said, and asked Dr. Le what policy or societal approaches might be implemented to help stem the tide.
“This is a really huge question,” Dr. Le acknowledged. The study only provides estimates of what the future burden of disease might be if there are no changes in clinical care for patients with MASLD or if the trajectory of contributing factors, such as obesity, diabetes, and other metabolic diseases, continued to increase, she cautioned.
Raising awareness of MASLD/MASH and working to improve collaboration among liver specialists and general practitioners could help to flatten the curve, she suggested.
The study was supported by a grant from the Agency for Healthcare Research and Quality. Dr. Le and Dr. Terrault have disclosed no relevant financial relations.
A version of this article first appeared on Medscape.com.
BOSTON – The nomenclature may have changed, but the steady rise in the most common form of liver disease – metabolic dysfunction–associated steatotic liver disease (MASLD, formerly known as NAFLD) – is predicted to continue into the middle of this century.
That’s according to Phuc Le, PhD, MPH, and colleagues at the Cleveland Clinic. They created a mathematical model incorporating data on the growth of the U.S. population and the natural history of MASLD/NAFLD. The model projected a relative 23% increase in MASLD among U.S. adults from 2020 to 2050.
“Our model forecasts a substantial clinical burden of NAFLD over the next 3 decades. In the absence of effective treatments, health systems should plan for large increases in the number of liver cancer cases and the need for liver transplant,” Dr. Le said in a media briefing held on Nov. 7 prior to her presentation of the data at the annual meeting of the American Association for the Study of Liver Diseases.
The estimated worldwide prevalence of MASLD is 38%. In the United States, an estimated 27.8% of adults had MASLD as of 2020.
Dr. Le and colleagues wanted to get a clearer picture of the expected increase in the clinical burden of MASLD in the coming decades. The researchers used data from the medical literature to create an individual-level state transition model. They took into account projections of the growth of the U.S. population and the progression of MASLD and metabolic dysfunction–associated steatohepatitis (MASH, formerly NASH) through stages of fibrosis to decompensation, hepatocellular carcinoma (HCC), transplant, and liver-related death as a proportion of all-cause mortality.
Validated model
They validated the model by testing it against liver outcomes from 2000 through 2018 and published data on the U.S. population. The model closely matched trends in MASLD prevalence, MASH proportion, HCC and liver transplant incidences, and overall survival rates for patients with MASLD.
As noted, the model predicted a steady increase in MASLD prevalence, from 27.8% in 2020 to 34.3% by 2050, a relative increase of about 23%. The model also predicted a slight uptick in the proportion of MASH among patients with MASLD, from 20% to 21.8%.
The investigators said that the prevalence of MASLD/MASH would likely remain relatively stable among people aged 18-29 years but would increase significantly for all other age groups.
In addition, the model predicted an increase in the proportion of cirrhosis in patients with MASLD from 1.9% to 3.1%, as well as a rise in liver-related deaths from 0.4% of all deaths in 2020 to 1% by 2050.
The investigators also foresaw a rise in HCC cases, from 10,400 annually to 19,300 by 2050 and a more than twofold increase in liver transplants, from 1,700 in 2020 to 4,200 in 2050.
A “tsunami” of liver disease
In the question-and-answer portion of the briefing, Norah Terrault, MD, AASLD president and chief of gastroenterology and hepatology at the University of Southern California, Los Angeles, commented on the study findings and “the frightening trajectory in terms of disease burden.
“I’m thinking to myself there’s no way we’re going to be able to transplant our way out of this tsunami of disease that’s coming our way,” she said, and asked Dr. Le what policy or societal approaches might be implemented to help stem the tide.
“This is a really huge question,” Dr. Le acknowledged. The study only provides estimates of what the future burden of disease might be if there are no changes in clinical care for patients with MASLD or if the trajectory of contributing factors, such as obesity, diabetes, and other metabolic diseases, continued to increase, she cautioned.
Raising awareness of MASLD/MASH and working to improve collaboration among liver specialists and general practitioners could help to flatten the curve, she suggested.
The study was supported by a grant from the Agency for Healthcare Research and Quality. Dr. Le and Dr. Terrault have disclosed no relevant financial relations.
A version of this article first appeared on Medscape.com.
BOSTON – The nomenclature may have changed, but the steady rise in the most common form of liver disease – metabolic dysfunction–associated steatotic liver disease (MASLD, formerly known as NAFLD) – is predicted to continue into the middle of this century.
That’s according to Phuc Le, PhD, MPH, and colleagues at the Cleveland Clinic. They created a mathematical model incorporating data on the growth of the U.S. population and the natural history of MASLD/NAFLD. The model projected a relative 23% increase in MASLD among U.S. adults from 2020 to 2050.
“Our model forecasts a substantial clinical burden of NAFLD over the next 3 decades. In the absence of effective treatments, health systems should plan for large increases in the number of liver cancer cases and the need for liver transplant,” Dr. Le said in a media briefing held on Nov. 7 prior to her presentation of the data at the annual meeting of the American Association for the Study of Liver Diseases.
The estimated worldwide prevalence of MASLD is 38%. In the United States, an estimated 27.8% of adults had MASLD as of 2020.
Dr. Le and colleagues wanted to get a clearer picture of the expected increase in the clinical burden of MASLD in the coming decades. The researchers used data from the medical literature to create an individual-level state transition model. They took into account projections of the growth of the U.S. population and the progression of MASLD and metabolic dysfunction–associated steatohepatitis (MASH, formerly NASH) through stages of fibrosis to decompensation, hepatocellular carcinoma (HCC), transplant, and liver-related death as a proportion of all-cause mortality.
Validated model
They validated the model by testing it against liver outcomes from 2000 through 2018 and published data on the U.S. population. The model closely matched trends in MASLD prevalence, MASH proportion, HCC and liver transplant incidences, and overall survival rates for patients with MASLD.
As noted, the model predicted a steady increase in MASLD prevalence, from 27.8% in 2020 to 34.3% by 2050, a relative increase of about 23%. The model also predicted a slight uptick in the proportion of MASH among patients with MASLD, from 20% to 21.8%.
The investigators said that the prevalence of MASLD/MASH would likely remain relatively stable among people aged 18-29 years but would increase significantly for all other age groups.
In addition, the model predicted an increase in the proportion of cirrhosis in patients with MASLD from 1.9% to 3.1%, as well as a rise in liver-related deaths from 0.4% of all deaths in 2020 to 1% by 2050.
The investigators also foresaw a rise in HCC cases, from 10,400 annually to 19,300 by 2050 and a more than twofold increase in liver transplants, from 1,700 in 2020 to 4,200 in 2050.
A “tsunami” of liver disease
In the question-and-answer portion of the briefing, Norah Terrault, MD, AASLD president and chief of gastroenterology and hepatology at the University of Southern California, Los Angeles, commented on the study findings and “the frightening trajectory in terms of disease burden.
“I’m thinking to myself there’s no way we’re going to be able to transplant our way out of this tsunami of disease that’s coming our way,” she said, and asked Dr. Le what policy or societal approaches might be implemented to help stem the tide.
“This is a really huge question,” Dr. Le acknowledged. The study only provides estimates of what the future burden of disease might be if there are no changes in clinical care for patients with MASLD or if the trajectory of contributing factors, such as obesity, diabetes, and other metabolic diseases, continued to increase, she cautioned.
Raising awareness of MASLD/MASH and working to improve collaboration among liver specialists and general practitioners could help to flatten the curve, she suggested.
The study was supported by a grant from the Agency for Healthcare Research and Quality. Dr. Le and Dr. Terrault have disclosed no relevant financial relations.
A version of this article first appeared on Medscape.com.
AT THE LIVER MEETING
Standing BP measures improve hypertension diagnosis
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FDA approves tirzepatide for treating obesity
Eli Lilly will market tirzepatide injections for weight management under the trade name Zepbound. It was approved in May 2022 for treating type 2 diabetes. The new indication is for adults with either obesity, defined as a body mass index of 30 kg/m2 or greater, or overweight, with a BMI of 27 or greater with at least one weight-related comorbidity, including hypertension, type 2 diabetes, or dyslipidemia.
“Obesity and overweight are serious conditions that can be associated with some of the leading causes of death, such as heart disease, stroke, and diabetes,” said John Sharretts, MD, director of the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research. “In light of increasing rates of both obesity and overweight in the United States, today’s approval addresses an unmet medical need.”
A once-weekly injection, tirzepatide reduces appetite by activating two gut hormones, glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The dosage is increased over 4-20 weeks to achieve a weekly dose target of 5 mg, 10 mg, or 15 mg maximum.
Efficacy was established in two pivotal randomized, double-blind, placebo-controlled trials of adults with obesity or overweight plus another condition. One trial measured weight reduction after 72 weeks in a total of 2,519 patients without diabetes who received either 5 mg, 10 mg or 15 mg of tirzepatide once weekly. Those who received the 15-mg dose achieved on average 18% of their initial body weight, compared with placebo.
The other pivotal trial enrolled a total of 938 patients with type 2 diabetes. These patients achieved an average weight loss of 12% with once-weekly tirzepatide compared to placebo.
Another trial, which was presented at the 2023 Obesity Week meeting and was published in Nature Medicine, showed clinically meaningful added weight loss for adults with obesity who did not have diabetes and who had already experienced weight loss of at least 5% after a 12-week intensive lifestyle intervention.
Another trial, which was reported at the 2023 annual meeting of the European Association for the Study of Diabetes, found that tirzepatide continued to produce “highly significant weight loss” when the drug was continued in a 1-year follow-up trial. Those who discontinued taking the drug regained some weight but not all.
Tirzepatide can cause gastrointestinal side effects, such as nausea, diarrhea, vomiting, constipation, and abdominal pain or discomfort. Site reactions, hypersensitivity, hair loss, burping, and gastrointestinal reflux disease have also been reported.
The medication should not be used by patients with a personal or family history of medullary thyroid cancer or by patients with multiple endocrine neoplasia syndrome type 2. It should also not be used in combination with Mounjaro or another GLP-1 receptor agonist. The safety and effectiveness of the coadministration of tirzepatide with other medications for weight management have not been established.
Zepbound should go to market in the United States by the end of 2023, with an anticipated monthly list price of $1,060, according to a news release from Eli Lilly.
A version of this article first appeared on Medscape.com.
Eli Lilly will market tirzepatide injections for weight management under the trade name Zepbound. It was approved in May 2022 for treating type 2 diabetes. The new indication is for adults with either obesity, defined as a body mass index of 30 kg/m2 or greater, or overweight, with a BMI of 27 or greater with at least one weight-related comorbidity, including hypertension, type 2 diabetes, or dyslipidemia.
“Obesity and overweight are serious conditions that can be associated with some of the leading causes of death, such as heart disease, stroke, and diabetes,” said John Sharretts, MD, director of the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research. “In light of increasing rates of both obesity and overweight in the United States, today’s approval addresses an unmet medical need.”
A once-weekly injection, tirzepatide reduces appetite by activating two gut hormones, glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The dosage is increased over 4-20 weeks to achieve a weekly dose target of 5 mg, 10 mg, or 15 mg maximum.
Efficacy was established in two pivotal randomized, double-blind, placebo-controlled trials of adults with obesity or overweight plus another condition. One trial measured weight reduction after 72 weeks in a total of 2,519 patients without diabetes who received either 5 mg, 10 mg or 15 mg of tirzepatide once weekly. Those who received the 15-mg dose achieved on average 18% of their initial body weight, compared with placebo.
The other pivotal trial enrolled a total of 938 patients with type 2 diabetes. These patients achieved an average weight loss of 12% with once-weekly tirzepatide compared to placebo.
Another trial, which was presented at the 2023 Obesity Week meeting and was published in Nature Medicine, showed clinically meaningful added weight loss for adults with obesity who did not have diabetes and who had already experienced weight loss of at least 5% after a 12-week intensive lifestyle intervention.
Another trial, which was reported at the 2023 annual meeting of the European Association for the Study of Diabetes, found that tirzepatide continued to produce “highly significant weight loss” when the drug was continued in a 1-year follow-up trial. Those who discontinued taking the drug regained some weight but not all.
Tirzepatide can cause gastrointestinal side effects, such as nausea, diarrhea, vomiting, constipation, and abdominal pain or discomfort. Site reactions, hypersensitivity, hair loss, burping, and gastrointestinal reflux disease have also been reported.
The medication should not be used by patients with a personal or family history of medullary thyroid cancer or by patients with multiple endocrine neoplasia syndrome type 2. It should also not be used in combination with Mounjaro or another GLP-1 receptor agonist. The safety and effectiveness of the coadministration of tirzepatide with other medications for weight management have not been established.
Zepbound should go to market in the United States by the end of 2023, with an anticipated monthly list price of $1,060, according to a news release from Eli Lilly.
A version of this article first appeared on Medscape.com.
Eli Lilly will market tirzepatide injections for weight management under the trade name Zepbound. It was approved in May 2022 for treating type 2 diabetes. The new indication is for adults with either obesity, defined as a body mass index of 30 kg/m2 or greater, or overweight, with a BMI of 27 or greater with at least one weight-related comorbidity, including hypertension, type 2 diabetes, or dyslipidemia.
“Obesity and overweight are serious conditions that can be associated with some of the leading causes of death, such as heart disease, stroke, and diabetes,” said John Sharretts, MD, director of the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research. “In light of increasing rates of both obesity and overweight in the United States, today’s approval addresses an unmet medical need.”
A once-weekly injection, tirzepatide reduces appetite by activating two gut hormones, glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The dosage is increased over 4-20 weeks to achieve a weekly dose target of 5 mg, 10 mg, or 15 mg maximum.
Efficacy was established in two pivotal randomized, double-blind, placebo-controlled trials of adults with obesity or overweight plus another condition. One trial measured weight reduction after 72 weeks in a total of 2,519 patients without diabetes who received either 5 mg, 10 mg or 15 mg of tirzepatide once weekly. Those who received the 15-mg dose achieved on average 18% of their initial body weight, compared with placebo.
The other pivotal trial enrolled a total of 938 patients with type 2 diabetes. These patients achieved an average weight loss of 12% with once-weekly tirzepatide compared to placebo.
Another trial, which was presented at the 2023 Obesity Week meeting and was published in Nature Medicine, showed clinically meaningful added weight loss for adults with obesity who did not have diabetes and who had already experienced weight loss of at least 5% after a 12-week intensive lifestyle intervention.
Another trial, which was reported at the 2023 annual meeting of the European Association for the Study of Diabetes, found that tirzepatide continued to produce “highly significant weight loss” when the drug was continued in a 1-year follow-up trial. Those who discontinued taking the drug regained some weight but not all.
Tirzepatide can cause gastrointestinal side effects, such as nausea, diarrhea, vomiting, constipation, and abdominal pain or discomfort. Site reactions, hypersensitivity, hair loss, burping, and gastrointestinal reflux disease have also been reported.
The medication should not be used by patients with a personal or family history of medullary thyroid cancer or by patients with multiple endocrine neoplasia syndrome type 2. It should also not be used in combination with Mounjaro or another GLP-1 receptor agonist. The safety and effectiveness of the coadministration of tirzepatide with other medications for weight management have not been established.
Zepbound should go to market in the United States by the end of 2023, with an anticipated monthly list price of $1,060, according to a news release from Eli Lilly.
A version of this article first appeared on Medscape.com.
Review estimates acne risk with JAK inhibitor therapy
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
Newer antiobesity meds lower the body’s defended fat mass
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
FROM OBESITYWEEK® 2023
Pustular Eruption on the Face
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
A 52-year-old man developed a sudden eruption of small pustules on background erythema and edema covering the forehead, nasal bridge, periorbital region, cheeks, and perioral region on day 3 of hospitalization in the intensive care unit for management of septic shock secondary to a complicated urinary tract infection. He had a medical history of benign prostatic hyperplasia, sarcoidosis, and atopic dermatitis. He initially presented to the emergency department with fever, chills, and dysuria of 2 days’ duration. Because he received ceftriaxone, vancomycin, ciprofloxacin, and tamsulosin while hospitalized for the infection, the primary medical team suspected a drug reaction and empirically started applying hydrocortisone cream 2.5%. The rash continued to spread over the ensuing day, prompting a dermatology consultation to rule out a drug eruption and to help guide further management. The patient was in substantial distress and pain. Physical examination revealed numerous discrete and confluent monomorphic pustules on background erythema with faint collarettes of scale covering most of the face. Substantial periorbital and facial edema forced the eyes closed. There was no mucous membrane involvement. A review of systems was negative for dyspnea and dysphagia, and the rash was not present elsewhere on the body. Ophthalmologic evaluation revealed no ocular involvement or vision changes. Laboratory studies demonstrated neutrophilia (17.27×109 cells/L [reference range, 2.0–6.9×109 cells/L]). The eosinophil count, blood urea nitrogen/creatinine, and liver function tests were within reference range.