User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


COVID-19 vaccine supply will be limited at first, ACIP says
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
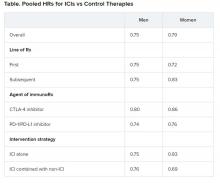
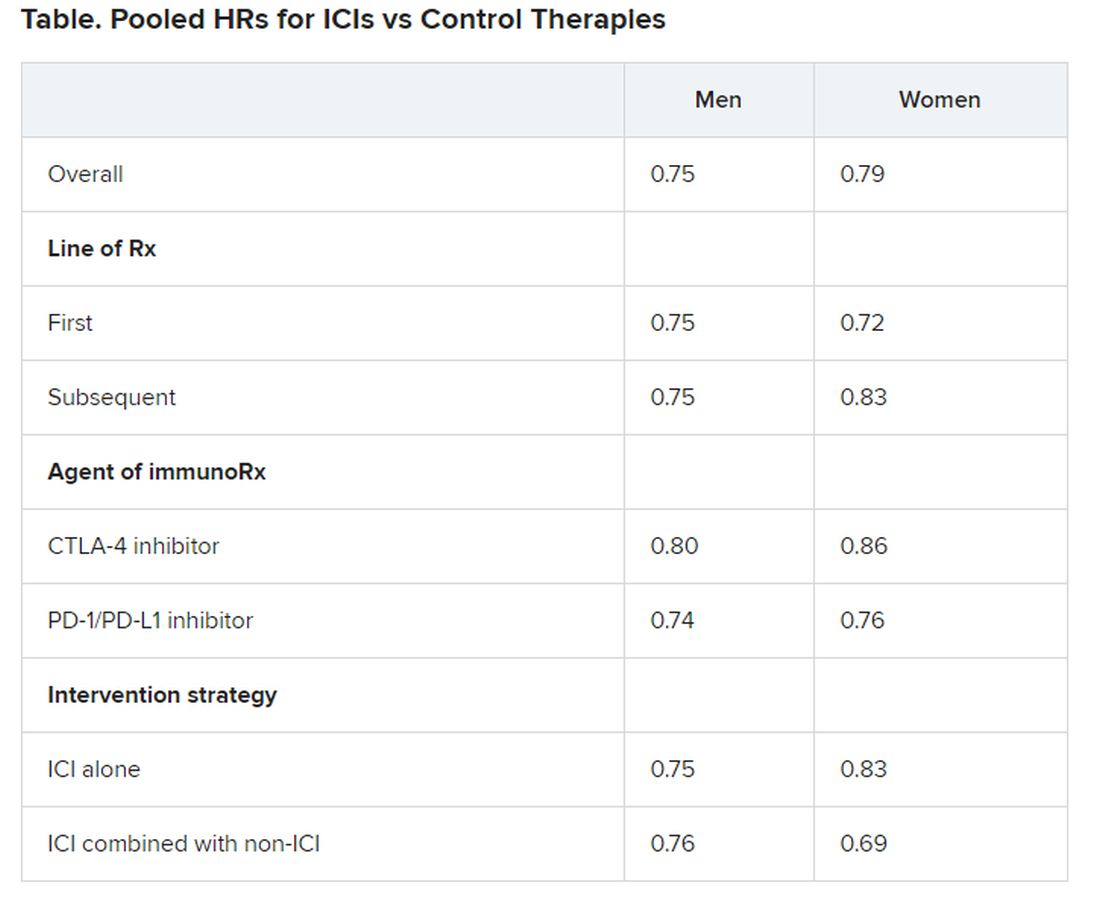
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Selpercatinib ‘poised to alter the landscape’ of RET+ cancers
Clinical data for the first-ever RET inhibitor, selpercatinib (Retevmo), show efficacy in two groups of patients with cancer – those with RET fusion–positive non–small cell lung cancer (NSCLC), and those with RET-mutant medullary thyroid cancer (MTC).
The drug showed “very good efficacy and also very good tolerability” in both groups, said lead author Lori J. Wirth, MD, medical director of head and neck cancers, Massachusetts General Hospital Cancer Center, Boston, in a statement.
“The response rates are high, responses are very durable, and overall, the drug does not cause a lot of toxicity,” she said.
“If you have a clean, RET-specific inhibitor such as selpercatinib, then you can really pound down RET very strongly and hit the driver alteration much harder, with a better side effect profile,” Dr. Wirth added.
Both groups of patients were part of the phase 1/2 LIBRETTO-001 study, which served as the basis for the recent accelerated approval of selpercatinib by the Food and Drug Administration.
Data from LIBRETTO-001 were published in the New England Journal of Medicine as two articles, one on NSCLC patients and one on MTC patients.
There has been a “remarkable increase” in the number of targeted agents that are effective in treating patients with advanced cancers that harbor specific genomic alterations, commented Razelle Kurzrock, MD, from the University of California, San Diego, in an accompanying editorial.
Selpercatinib, a potent RET inhibitor, “is now poised to alter the landscape of another genomic subgroup – RET-altered cancers,” she wrote.
Multikinase inhibitors such as vandetanib and cabozantinib have ancillary RET-inhibitor activity and are also active against RET-driven cancers. But these drugs are limited by off-target side effects, Dr. Krurzrock pointed out. “In contrast, next-generation, highly potent, and selective RET inhibitors such as selpercatinib offer the potential for improved efficacy and a more satisfactory side effect profile.”
In both parts of the study, selpercatinib produced durable responses in a majority of patients. Only about 3% of patients discontinued taking selpercatinib because of drug-related adverse events.
Taken together, these results show that selpercatinib “had marked and durable antitumor activity in most patients with RET-altered thyroid cancer or NSCLC,” wrote Dr. Krurzrock. “RET abnormalities now join other genomic alterations such as NTRK fusions, tumor mutational burden, and deficient mismatchrepair genes across cancers and ALK, BRAF, EGFR, MET, and ROS1 alterations in NSCLC that warrant molecular screening strategies.”
Results in patients with RET-mutated NSCLC
All patients enrolled in the LIBRETTO-001 trial received selpercatinib 160 mg orally twice daily until disease progression or unacceptable toxicity occurred.
Of 105 patients with NSCLC who had received at least one platinum-based chemotherapy regimen, the objective response rate was 64%. The median duration of response was 17.5 months.
At a median follow-up of 12.1 months, 63% of the responses were ongoing.
The cohort included 39 treatment-naive patients, among whom the response rate was even higher, at 85%; 90% of the responses were ongoing at 6 months. In addition, 11 patients had measurable central nervous system metastasis at study enrollment. Of this group, 91% achieved an intracranial response.
Common adverse events of grade 3 or higher included hypertension (in 14% of the patients), an increase in ALT level (in 12%), an increase in AST level (in 10%), hyponatremia (in 6%), and lymphopenia (in 6%). The drug was discontinued in 12 patients because of a drug-related adverse event.
Results in patients with RET-mutated MTC
Efficacy for MTC was evaluated in 55 patients with advanced or metastatic RET-mutant MTC who had previously been treated with cabozantinib, vandetanib, or both. The objective response rate was 69%. The 1-year progression-free survival rate was 82%.
For the 88 patients who had not previously received vandetanib or cabozantinib, the response rate was 73%. The 1-year progression-free survival rate was 92%.
In a subgroup of 19 patients with previously treated RET fusion–positive thyroid cancer, 79% responded to the therapy; 1-year progression-free survival was 64%.
The most common adverse events of grade 3 or higher were hypertension (in 21% of the patients), an increase in ALT level (in 11%), an increase in AST level (in 9%), hyponatremia (in 8%), and diarrhea (in 6%). Selpercatinib was discontinued by 12 patients because of drug-related adverse events.
The study was funded by Loxo Oncology (a wholly owned subsidiary of Eli Lilly) and by grants from the National Institutes of Health and the University of Texas MD Anderson Cancer Center. Kurzrock and Wirth report relationships with numerous pharmaceutical companies, as listed in the journal article.
This article first appeared on Medscape.com.
Clinical data for the first-ever RET inhibitor, selpercatinib (Retevmo), show efficacy in two groups of patients with cancer – those with RET fusion–positive non–small cell lung cancer (NSCLC), and those with RET-mutant medullary thyroid cancer (MTC).
The drug showed “very good efficacy and also very good tolerability” in both groups, said lead author Lori J. Wirth, MD, medical director of head and neck cancers, Massachusetts General Hospital Cancer Center, Boston, in a statement.
“The response rates are high, responses are very durable, and overall, the drug does not cause a lot of toxicity,” she said.
“If you have a clean, RET-specific inhibitor such as selpercatinib, then you can really pound down RET very strongly and hit the driver alteration much harder, with a better side effect profile,” Dr. Wirth added.
Both groups of patients were part of the phase 1/2 LIBRETTO-001 study, which served as the basis for the recent accelerated approval of selpercatinib by the Food and Drug Administration.
Data from LIBRETTO-001 were published in the New England Journal of Medicine as two articles, one on NSCLC patients and one on MTC patients.
There has been a “remarkable increase” in the number of targeted agents that are effective in treating patients with advanced cancers that harbor specific genomic alterations, commented Razelle Kurzrock, MD, from the University of California, San Diego, in an accompanying editorial.
Selpercatinib, a potent RET inhibitor, “is now poised to alter the landscape of another genomic subgroup – RET-altered cancers,” she wrote.
Multikinase inhibitors such as vandetanib and cabozantinib have ancillary RET-inhibitor activity and are also active against RET-driven cancers. But these drugs are limited by off-target side effects, Dr. Krurzrock pointed out. “In contrast, next-generation, highly potent, and selective RET inhibitors such as selpercatinib offer the potential for improved efficacy and a more satisfactory side effect profile.”
In both parts of the study, selpercatinib produced durable responses in a majority of patients. Only about 3% of patients discontinued taking selpercatinib because of drug-related adverse events.
Taken together, these results show that selpercatinib “had marked and durable antitumor activity in most patients with RET-altered thyroid cancer or NSCLC,” wrote Dr. Krurzrock. “RET abnormalities now join other genomic alterations such as NTRK fusions, tumor mutational burden, and deficient mismatchrepair genes across cancers and ALK, BRAF, EGFR, MET, and ROS1 alterations in NSCLC that warrant molecular screening strategies.”
Results in patients with RET-mutated NSCLC
All patients enrolled in the LIBRETTO-001 trial received selpercatinib 160 mg orally twice daily until disease progression or unacceptable toxicity occurred.
Of 105 patients with NSCLC who had received at least one platinum-based chemotherapy regimen, the objective response rate was 64%. The median duration of response was 17.5 months.
At a median follow-up of 12.1 months, 63% of the responses were ongoing.
The cohort included 39 treatment-naive patients, among whom the response rate was even higher, at 85%; 90% of the responses were ongoing at 6 months. In addition, 11 patients had measurable central nervous system metastasis at study enrollment. Of this group, 91% achieved an intracranial response.
Common adverse events of grade 3 or higher included hypertension (in 14% of the patients), an increase in ALT level (in 12%), an increase in AST level (in 10%), hyponatremia (in 6%), and lymphopenia (in 6%). The drug was discontinued in 12 patients because of a drug-related adverse event.
Results in patients with RET-mutated MTC
Efficacy for MTC was evaluated in 55 patients with advanced or metastatic RET-mutant MTC who had previously been treated with cabozantinib, vandetanib, or both. The objective response rate was 69%. The 1-year progression-free survival rate was 82%.
For the 88 patients who had not previously received vandetanib or cabozantinib, the response rate was 73%. The 1-year progression-free survival rate was 92%.
In a subgroup of 19 patients with previously treated RET fusion–positive thyroid cancer, 79% responded to the therapy; 1-year progression-free survival was 64%.
The most common adverse events of grade 3 or higher were hypertension (in 21% of the patients), an increase in ALT level (in 11%), an increase in AST level (in 9%), hyponatremia (in 8%), and diarrhea (in 6%). Selpercatinib was discontinued by 12 patients because of drug-related adverse events.
The study was funded by Loxo Oncology (a wholly owned subsidiary of Eli Lilly) and by grants from the National Institutes of Health and the University of Texas MD Anderson Cancer Center. Kurzrock and Wirth report relationships with numerous pharmaceutical companies, as listed in the journal article.
This article first appeared on Medscape.com.
Clinical data for the first-ever RET inhibitor, selpercatinib (Retevmo), show efficacy in two groups of patients with cancer – those with RET fusion–positive non–small cell lung cancer (NSCLC), and those with RET-mutant medullary thyroid cancer (MTC).
The drug showed “very good efficacy and also very good tolerability” in both groups, said lead author Lori J. Wirth, MD, medical director of head and neck cancers, Massachusetts General Hospital Cancer Center, Boston, in a statement.
“The response rates are high, responses are very durable, and overall, the drug does not cause a lot of toxicity,” she said.
“If you have a clean, RET-specific inhibitor such as selpercatinib, then you can really pound down RET very strongly and hit the driver alteration much harder, with a better side effect profile,” Dr. Wirth added.
Both groups of patients were part of the phase 1/2 LIBRETTO-001 study, which served as the basis for the recent accelerated approval of selpercatinib by the Food and Drug Administration.
Data from LIBRETTO-001 were published in the New England Journal of Medicine as two articles, one on NSCLC patients and one on MTC patients.
There has been a “remarkable increase” in the number of targeted agents that are effective in treating patients with advanced cancers that harbor specific genomic alterations, commented Razelle Kurzrock, MD, from the University of California, San Diego, in an accompanying editorial.
Selpercatinib, a potent RET inhibitor, “is now poised to alter the landscape of another genomic subgroup – RET-altered cancers,” she wrote.
Multikinase inhibitors such as vandetanib and cabozantinib have ancillary RET-inhibitor activity and are also active against RET-driven cancers. But these drugs are limited by off-target side effects, Dr. Krurzrock pointed out. “In contrast, next-generation, highly potent, and selective RET inhibitors such as selpercatinib offer the potential for improved efficacy and a more satisfactory side effect profile.”
In both parts of the study, selpercatinib produced durable responses in a majority of patients. Only about 3% of patients discontinued taking selpercatinib because of drug-related adverse events.
Taken together, these results show that selpercatinib “had marked and durable antitumor activity in most patients with RET-altered thyroid cancer or NSCLC,” wrote Dr. Krurzrock. “RET abnormalities now join other genomic alterations such as NTRK fusions, tumor mutational burden, and deficient mismatchrepair genes across cancers and ALK, BRAF, EGFR, MET, and ROS1 alterations in NSCLC that warrant molecular screening strategies.”
Results in patients with RET-mutated NSCLC
All patients enrolled in the LIBRETTO-001 trial received selpercatinib 160 mg orally twice daily until disease progression or unacceptable toxicity occurred.
Of 105 patients with NSCLC who had received at least one platinum-based chemotherapy regimen, the objective response rate was 64%. The median duration of response was 17.5 months.
At a median follow-up of 12.1 months, 63% of the responses were ongoing.
The cohort included 39 treatment-naive patients, among whom the response rate was even higher, at 85%; 90% of the responses were ongoing at 6 months. In addition, 11 patients had measurable central nervous system metastasis at study enrollment. Of this group, 91% achieved an intracranial response.
Common adverse events of grade 3 or higher included hypertension (in 14% of the patients), an increase in ALT level (in 12%), an increase in AST level (in 10%), hyponatremia (in 6%), and lymphopenia (in 6%). The drug was discontinued in 12 patients because of a drug-related adverse event.
Results in patients with RET-mutated MTC
Efficacy for MTC was evaluated in 55 patients with advanced or metastatic RET-mutant MTC who had previously been treated with cabozantinib, vandetanib, or both. The objective response rate was 69%. The 1-year progression-free survival rate was 82%.
For the 88 patients who had not previously received vandetanib or cabozantinib, the response rate was 73%. The 1-year progression-free survival rate was 92%.
In a subgroup of 19 patients with previously treated RET fusion–positive thyroid cancer, 79% responded to the therapy; 1-year progression-free survival was 64%.
The most common adverse events of grade 3 or higher were hypertension (in 21% of the patients), an increase in ALT level (in 11%), an increase in AST level (in 9%), hyponatremia (in 8%), and diarrhea (in 6%). Selpercatinib was discontinued by 12 patients because of drug-related adverse events.
The study was funded by Loxo Oncology (a wholly owned subsidiary of Eli Lilly) and by grants from the National Institutes of Health and the University of Texas MD Anderson Cancer Center. Kurzrock and Wirth report relationships with numerous pharmaceutical companies, as listed in the journal article.
This article first appeared on Medscape.com.
Asymptomatic SARS-CoV-2 infections in kids tied to local rates
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Convalescent plasma actions spark trial recruitment concerns
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
e-Interview With CHEST President-Elect Steven Q. Simpson, MD, FCCP
CHEST President-Elect Steven Q. Simpson, MD, FCCP, is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services.
As we greet our new incoming CHEST President, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would you like to accomplish as President of CHEST?
This is an interesting question, because a global pandemic and other developments in our world dictate that our organizational goals must adapt to a landscape that has shifted in recent months. My goals as President are somewhat different from what I stated when I ran for the office.
1. First, I will build on the efforts of my predecessors to ensure that CHEST is an inclusive and anti-racist organization. All CHEST members must have equal opportunities within our organization to advance their lives and their careers, regardless of race, ethnicity, sex, or gender. My goal is to examine our structures for participation and advancement to positions of leadership in the organization and to evaluate our educational and research offerings, all with the purpose of discovering and remedying places where we have been blind to our own systematic bias. Further, CHEST must advocate for and lead others to advocate for equality, for equal access to medical care, and for policies that promote them. We must be leaders in this arena, through both our voice and our actions.
2. We will build on CHEST’s new initiative to support the wellness of our members and to help us all perform at our best, day in and day out. I hope for our newly established Wellness Center to become a frequent stop for all CHEST members, myself included, to help us to sustain ourselves through the pandemic and beyond.
3. We must maintain both the quality and the feel of our educational and research offerings during this time when we cannot come together in person. My goal for us is that we use this time to embrace remote and nontemporally synchronous education, ie, web-based education, to make CHEST’s offerings the best anywhere. In the remainder of the 21st century, digital transformation of teaching and learning will advance tremendously, and our creative use of technology will become a norm. I hope that we never abandon in-person meetings, but using technology to improve information transfer and augmenting our members’ continuing education are clearly here to stay. My goal for us is that we maintain an atmosphere to both our in-person meetings and our remotely delivered meetings that makes generating new knowledge and learning what we generate enjoyable, even fun. I believe our digital transformation will make some interesting things possible over time.
4. My overall goal for CHEST in the coming year is not that we “make it through” the current pandemic, but that we emerge stronger, smarter, and better for the experience, and prepared for the next challenge(s).
Before COVID-19, I had goals for my presidency, and these issues have not disappeared. CHEST needs to be user- friendly for our members, from our website, to the ways in which we deliver education, to the type of research we develop and promote. On the research side, our members have long been interested in clinical research that informs and improves our patient care. My goal is to double down on promoting, supporting, and presenting research that serves exactly this purpose. We are growing our team-based education, and I have a special goal for CHEST to become the home for pulmonary, critical care, and sleep advanced practice providers. I care tremendously about our international members, and I will promote both international growth and catering of CHEST’s offerings to benefit our international members.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
There is zero doubt that CHEST’s greatest strength is the people who gravitate to our organization. From pure clinicians to academicians; from clinical researchers to clinical educators to outcomes mavens—all levels of the health-care team. At every level of this organization are members who all want to be better at what we do, who want to figure out the ways for doing that, who want to explore the boundaries of what that means, and who want to help others to do the same. That goes, as well, for the professional staff who support the members, and who have adopted the motto, “CRUSH lung disease,” because they share our mission and are here to help us do it better.
The absolutely most enjoyable thing about leadership is having the opportunity to survey the landscape and see who’s looking for opportunity, who’s a rising star, who’s looking for people to mentor, then matching those people with opportunities and with jobs to do. Good people who are motivated by the right principles rise to the occasion. My job as President is to help ensure that the organization via the CHEST Board of Regents is addressing the correct problems with the right vision, to identify the right talented and dedicated members for the jobs, and then to support and stay out of their way as they make the vision a reality.
What are some challenges facing CHEST, and how will you address these challenges?
The major immediate challenges facing CHEST are pandemic-related, in terms of helping to ensure the well-being of our members, and in helping them to address the inequities and disparities in care for our patients of color, who have been hardest hit by the emergence of SARS-CoV-2. I addressed these with my goals, above. To be more specific, though, our board will be using various techniques, including dialogue with our members of color, to understand and address our own implicit biases, so that we can achieve the correct vision and tone of inclusion for all of our members. Also addressed in my goals is the isolation from one another that we are all experiencing because of the pandemic. This situation makes it difficult for us to maintain the style and tone of live learning experiences that our CHEST members are accustomed to. The challenge is to develop materials that can be interactive at a distance, and this likely includes gamification of educational content and employing virtual reality. CHEST Innovations is already working in this arena, and it will be our job as member volunteers to support those efforts. The isolation affects our international members, as well, and our ability to travel to maintain relationships. The nice thing is that web conferencing works just as well for international meetings as for meetings in the US, although somebody often has to go to bed very late or get up very early in the morning to make them work! The efforts are worth our time. Again, we will be working in various arenas to maintain and grow our international relationships.
And finally, what is your charge to the members and new Fellows (FCCPs) of CHEST?
We do not yet see clearly whether to expect a massive winter surge of COVID-19 infections. However, it is a reasonably likely possibility. My charge to our members and our new Fellows is first to stay safe, yourself, and to take care of your mental and physical well-being, so that you can be present and functioning at peak levels for your patients. Make sure your family is, likewise, being safe. Secondly, keep doing what you do, which is excellent patient care, excellent teaching, excellent research to push the boundaries of our knowledge. And finally, you’ve seen my ideas of the challenges facing CHEST. I want you to survey, yourself, and tell me what you think our challenges, goals, and responsibilities should be. And if anything I’ve said resonates with you, volunteer to help us address our challenges and keep CHEST the professional home that you deserve and that you will never want to leave. CHEST wants you and needs you. We are so happy you are with us!
CHEST President-Elect Steven Q. Simpson, MD, FCCP, is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services.
As we greet our new incoming CHEST President, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would you like to accomplish as President of CHEST?
This is an interesting question, because a global pandemic and other developments in our world dictate that our organizational goals must adapt to a landscape that has shifted in recent months. My goals as President are somewhat different from what I stated when I ran for the office.
1. First, I will build on the efforts of my predecessors to ensure that CHEST is an inclusive and anti-racist organization. All CHEST members must have equal opportunities within our organization to advance their lives and their careers, regardless of race, ethnicity, sex, or gender. My goal is to examine our structures for participation and advancement to positions of leadership in the organization and to evaluate our educational and research offerings, all with the purpose of discovering and remedying places where we have been blind to our own systematic bias. Further, CHEST must advocate for and lead others to advocate for equality, for equal access to medical care, and for policies that promote them. We must be leaders in this arena, through both our voice and our actions.
2. We will build on CHEST’s new initiative to support the wellness of our members and to help us all perform at our best, day in and day out. I hope for our newly established Wellness Center to become a frequent stop for all CHEST members, myself included, to help us to sustain ourselves through the pandemic and beyond.
3. We must maintain both the quality and the feel of our educational and research offerings during this time when we cannot come together in person. My goal for us is that we use this time to embrace remote and nontemporally synchronous education, ie, web-based education, to make CHEST’s offerings the best anywhere. In the remainder of the 21st century, digital transformation of teaching and learning will advance tremendously, and our creative use of technology will become a norm. I hope that we never abandon in-person meetings, but using technology to improve information transfer and augmenting our members’ continuing education are clearly here to stay. My goal for us is that we maintain an atmosphere to both our in-person meetings and our remotely delivered meetings that makes generating new knowledge and learning what we generate enjoyable, even fun. I believe our digital transformation will make some interesting things possible over time.
4. My overall goal for CHEST in the coming year is not that we “make it through” the current pandemic, but that we emerge stronger, smarter, and better for the experience, and prepared for the next challenge(s).
Before COVID-19, I had goals for my presidency, and these issues have not disappeared. CHEST needs to be user- friendly for our members, from our website, to the ways in which we deliver education, to the type of research we develop and promote. On the research side, our members have long been interested in clinical research that informs and improves our patient care. My goal is to double down on promoting, supporting, and presenting research that serves exactly this purpose. We are growing our team-based education, and I have a special goal for CHEST to become the home for pulmonary, critical care, and sleep advanced practice providers. I care tremendously about our international members, and I will promote both international growth and catering of CHEST’s offerings to benefit our international members.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
There is zero doubt that CHEST’s greatest strength is the people who gravitate to our organization. From pure clinicians to academicians; from clinical researchers to clinical educators to outcomes mavens—all levels of the health-care team. At every level of this organization are members who all want to be better at what we do, who want to figure out the ways for doing that, who want to explore the boundaries of what that means, and who want to help others to do the same. That goes, as well, for the professional staff who support the members, and who have adopted the motto, “CRUSH lung disease,” because they share our mission and are here to help us do it better.
The absolutely most enjoyable thing about leadership is having the opportunity to survey the landscape and see who’s looking for opportunity, who’s a rising star, who’s looking for people to mentor, then matching those people with opportunities and with jobs to do. Good people who are motivated by the right principles rise to the occasion. My job as President is to help ensure that the organization via the CHEST Board of Regents is addressing the correct problems with the right vision, to identify the right talented and dedicated members for the jobs, and then to support and stay out of their way as they make the vision a reality.
What are some challenges facing CHEST, and how will you address these challenges?
The major immediate challenges facing CHEST are pandemic-related, in terms of helping to ensure the well-being of our members, and in helping them to address the inequities and disparities in care for our patients of color, who have been hardest hit by the emergence of SARS-CoV-2. I addressed these with my goals, above. To be more specific, though, our board will be using various techniques, including dialogue with our members of color, to understand and address our own implicit biases, so that we can achieve the correct vision and tone of inclusion for all of our members. Also addressed in my goals is the isolation from one another that we are all experiencing because of the pandemic. This situation makes it difficult for us to maintain the style and tone of live learning experiences that our CHEST members are accustomed to. The challenge is to develop materials that can be interactive at a distance, and this likely includes gamification of educational content and employing virtual reality. CHEST Innovations is already working in this arena, and it will be our job as member volunteers to support those efforts. The isolation affects our international members, as well, and our ability to travel to maintain relationships. The nice thing is that web conferencing works just as well for international meetings as for meetings in the US, although somebody often has to go to bed very late or get up very early in the morning to make them work! The efforts are worth our time. Again, we will be working in various arenas to maintain and grow our international relationships.
And finally, what is your charge to the members and new Fellows (FCCPs) of CHEST?
We do not yet see clearly whether to expect a massive winter surge of COVID-19 infections. However, it is a reasonably likely possibility. My charge to our members and our new Fellows is first to stay safe, yourself, and to take care of your mental and physical well-being, so that you can be present and functioning at peak levels for your patients. Make sure your family is, likewise, being safe. Secondly, keep doing what you do, which is excellent patient care, excellent teaching, excellent research to push the boundaries of our knowledge. And finally, you’ve seen my ideas of the challenges facing CHEST. I want you to survey, yourself, and tell me what you think our challenges, goals, and responsibilities should be. And if anything I’ve said resonates with you, volunteer to help us address our challenges and keep CHEST the professional home that you deserve and that you will never want to leave. CHEST wants you and needs you. We are so happy you are with us!
CHEST President-Elect Steven Q. Simpson, MD, FCCP, is Professor of Medicine in the Division of Pulmonary and Critical Care Medicine at the University of Kansas. He is also senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the US Department of Health and Human Services.
As we greet our new incoming CHEST President, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would you like to accomplish as President of CHEST?
This is an interesting question, because a global pandemic and other developments in our world dictate that our organizational goals must adapt to a landscape that has shifted in recent months. My goals as President are somewhat different from what I stated when I ran for the office.
1. First, I will build on the efforts of my predecessors to ensure that CHEST is an inclusive and anti-racist organization. All CHEST members must have equal opportunities within our organization to advance their lives and their careers, regardless of race, ethnicity, sex, or gender. My goal is to examine our structures for participation and advancement to positions of leadership in the organization and to evaluate our educational and research offerings, all with the purpose of discovering and remedying places where we have been blind to our own systematic bias. Further, CHEST must advocate for and lead others to advocate for equality, for equal access to medical care, and for policies that promote them. We must be leaders in this arena, through both our voice and our actions.
2. We will build on CHEST’s new initiative to support the wellness of our members and to help us all perform at our best, day in and day out. I hope for our newly established Wellness Center to become a frequent stop for all CHEST members, myself included, to help us to sustain ourselves through the pandemic and beyond.
3. We must maintain both the quality and the feel of our educational and research offerings during this time when we cannot come together in person. My goal for us is that we use this time to embrace remote and nontemporally synchronous education, ie, web-based education, to make CHEST’s offerings the best anywhere. In the remainder of the 21st century, digital transformation of teaching and learning will advance tremendously, and our creative use of technology will become a norm. I hope that we never abandon in-person meetings, but using technology to improve information transfer and augmenting our members’ continuing education are clearly here to stay. My goal for us is that we maintain an atmosphere to both our in-person meetings and our remotely delivered meetings that makes generating new knowledge and learning what we generate enjoyable, even fun. I believe our digital transformation will make some interesting things possible over time.
4. My overall goal for CHEST in the coming year is not that we “make it through” the current pandemic, but that we emerge stronger, smarter, and better for the experience, and prepared for the next challenge(s).
Before COVID-19, I had goals for my presidency, and these issues have not disappeared. CHEST needs to be user- friendly for our members, from our website, to the ways in which we deliver education, to the type of research we develop and promote. On the research side, our members have long been interested in clinical research that informs and improves our patient care. My goal is to double down on promoting, supporting, and presenting research that serves exactly this purpose. We are growing our team-based education, and I have a special goal for CHEST to become the home for pulmonary, critical care, and sleep advanced practice providers. I care tremendously about our international members, and I will promote both international growth and catering of CHEST’s offerings to benefit our international members.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
There is zero doubt that CHEST’s greatest strength is the people who gravitate to our organization. From pure clinicians to academicians; from clinical researchers to clinical educators to outcomes mavens—all levels of the health-care team. At every level of this organization are members who all want to be better at what we do, who want to figure out the ways for doing that, who want to explore the boundaries of what that means, and who want to help others to do the same. That goes, as well, for the professional staff who support the members, and who have adopted the motto, “CRUSH lung disease,” because they share our mission and are here to help us do it better.
The absolutely most enjoyable thing about leadership is having the opportunity to survey the landscape and see who’s looking for opportunity, who’s a rising star, who’s looking for people to mentor, then matching those people with opportunities and with jobs to do. Good people who are motivated by the right principles rise to the occasion. My job as President is to help ensure that the organization via the CHEST Board of Regents is addressing the correct problems with the right vision, to identify the right talented and dedicated members for the jobs, and then to support and stay out of their way as they make the vision a reality.
What are some challenges facing CHEST, and how will you address these challenges?
The major immediate challenges facing CHEST are pandemic-related, in terms of helping to ensure the well-being of our members, and in helping them to address the inequities and disparities in care for our patients of color, who have been hardest hit by the emergence of SARS-CoV-2. I addressed these with my goals, above. To be more specific, though, our board will be using various techniques, including dialogue with our members of color, to understand and address our own implicit biases, so that we can achieve the correct vision and tone of inclusion for all of our members. Also addressed in my goals is the isolation from one another that we are all experiencing because of the pandemic. This situation makes it difficult for us to maintain the style and tone of live learning experiences that our CHEST members are accustomed to. The challenge is to develop materials that can be interactive at a distance, and this likely includes gamification of educational content and employing virtual reality. CHEST Innovations is already working in this arena, and it will be our job as member volunteers to support those efforts. The isolation affects our international members, as well, and our ability to travel to maintain relationships. The nice thing is that web conferencing works just as well for international meetings as for meetings in the US, although somebody often has to go to bed very late or get up very early in the morning to make them work! The efforts are worth our time. Again, we will be working in various arenas to maintain and grow our international relationships.
And finally, what is your charge to the members and new Fellows (FCCPs) of CHEST?
We do not yet see clearly whether to expect a massive winter surge of COVID-19 infections. However, it is a reasonably likely possibility. My charge to our members and our new Fellows is first to stay safe, yourself, and to take care of your mental and physical well-being, so that you can be present and functioning at peak levels for your patients. Make sure your family is, likewise, being safe. Secondly, keep doing what you do, which is excellent patient care, excellent teaching, excellent research to push the boundaries of our knowledge. And finally, you’ve seen my ideas of the challenges facing CHEST. I want you to survey, yourself, and tell me what you think our challenges, goals, and responsibilities should be. And if anything I’ve said resonates with you, volunteer to help us address our challenges and keep CHEST the professional home that you deserve and that you will never want to leave. CHEST wants you and needs you. We are so happy you are with us!
Prognosis for rural hospitals worsens with pandemic
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
When viruses collide: Flu season during pandemic
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
The medical community is about to find out how prepared it is for the double whammy of influenza and COVID-19 that has been predicted for the fall of 2020. The complexities of diagnosis, management of vulnerable patients, and overflowing medical centers that have made the COVID-19 crisis so brutal may all be exacerbated by the arrival of seasonal influenza.
Lewis Jay Kaplan, MD, FCCP, a critical care surgeon at the University of Pennsylvania, Philadelphia, has seen his share of critically ill COVID-19 patients in the surgical ICU that he oversees. He’s approaching the upcoming flu season, poised to collide with the ongoing COVID-19 pandemic, ready to listen to each patient’s story to distinguish one from the other and determine treatment.
“The patients that have underlying comorbidities all have a story, and it’s up to you to figure out which chapter you’re in and how far along you happen to be,” he said. “It’s a very interesting approach to care, medical storytelling.”
With flu season closing in, pulmonologists are ruminating about how they’ll distinguish symptoms of COVID-19 and traditional influenza and how they’ll manage the most vulnerable patients, namely those with underlying respiratory disease and children. Influenza kills 12,000-61,000 people a year, according to the Centers for Disease Control, and results in 140,000-810,00 hospitalizations. Having a flu season in the midst of a pandemic of a disease with multiple overlapping symptoms threatens to overwhelm practitioners, hospitals, and the health system.
Dr. Kaplan said each patient’s story can point to the correct clinical approach. “Instead of just sharing data when you are on rounds, you’re really telling someone’s story.” It arises from a series of questions about how the disease has impacted them, specifics of their presentation, how their signs and symptoms differ from the usual, and how they responded to treatment. “It also helps you to then take what you’re doing, which can seem very, very complicated to individuals who are not medically sophisticated, and then help them to understand why you’re doing what you’re doing at this point.”
That can help get through to a patient with respiratory disease who insists he or she has or doesn’t have COVID-19 rather than the flu. “They form a different group that brings with them different fears and concerns, and you have to help them navigate that, too: all of this data and your decision-making around testing and admissions, and what you can omit doing and what you must do help them to navigate their own story,” Dr. Kaplan said.
Benjamin D. Singer, MD, a pulmonologist at Northwestern University, Chicago, authored an editorial in Science Advances that addressed four factors that will determine the scope of flu spread in the upcoming season: rate of transmission; vaccination rates; coinfection rates; and health disparities in minority populations, which are prone to higher rates of flu as well as COVID-19.
Flu vaccine ‘extra important’
The convergence of COVID-19 and influenza has the potential to overwhelm the health system, said Daniel A. Solomon, MD, of Brigham and Women’s in Boston. He coauthored a JAMA Insights clinical update on flu season during the COVID-19 pandemic that lists distinguishing and overlapping signs and symptoms of the two diseases.
The flu vaccine, he said, is “extra important this year,” especially in patients with existing respiratory disease, but COVID-19 has thrown up barriers to vaccination. Telemedicine has supplanted office visits. “People may miss that easy-touch opportunity to get the flu vaccine, so we have to be creative about making the flu vaccine highly accessible, maybe in nontraditional ways,” Dr. Solomon said. Some ideas he offered are pop-up vaccine fairs at schools and churches.
But just as COVID-19 may hinder flu vaccines, it may also be helping to mitigate flu transmission. “The interesting thing about transmission of the flu is that it’s transmitted the same way COVID is, so if we actually know how to decrease transmission of COVID, which we do – we’ve done it – we can actually decrease transmission of influenza as well,” Dr. Solomon said. Studies out of Hong Kong and Japan have reported a reduction in influenza cases during COVID-19 outbreaks in those places (Lancet Public Health. 2020;5:e279-88; JAMA. 2020;323:1969-71).
Risks of coinfection
About one in four COVID-19 patients have been diagnosed with an additional respiratory infection, including influenza (JAMA. 2020:323:2085-6). Pulmonologists must keep that in mind when managing COVID-19 suspects, said Dr. Singer.
“While it is true that most of the time COVID-19 travels alone, we have numerous examples in the literature and in our own experience that COVID-19 is accompanied by either another virus or another bacterial infection, including influenza,” Dr. Singer said. “The distinction is important. One is just for diagnostic reasons and public reporting reasons, but also because flu and COVID-19 have different requirements for how you care for patients in terms of the health system.”
Clinical suspicion for coinfection should remain high if the community spread of both COVID-19 and influenza is high, said Megan Conroy, MD, chief pulmonary and critical care fellow at Ohio State University, Columbus. “As the coronavirus first took hold in the United States in March 2020, we were at the tail end of influenza season, so it’s hard to predict what the upcoming influenza season will really look like with regards to coinfection.”
Distinguishing COVID-19 from flu
Multiple signs and symptoms between COVID-19 and the flu overlap. They include fever, chills, headache, myalgia, cough, and fatigue. Nasal congestion and sore throat are characteristic of the flu; shortness of breath and loss of the sense of smell have been widely reported in COVID-19. “While many upper respiratory infections can result in loss of smell, this may be more prevalent in COVID-19,” Dr. Conroy said. Other symptoms unique to COVID-19 are GI symptoms such as diarrhea and skin rashes such as acral ischemia.
Testing, however, is the cornerstone of the differential diagnosis. “You can’t confidently distinguish between them on symptoms alone,” Dr. Conroy added.
“I think the challenge we’ll face as clinicians, is caring for people with nonspecific symptoms of a respiratory viral illness, especially in the early phase of the illness,” said Dr. Solomon.
But even after that, symptoms can be difficult to distinguish.
“Later in the illness, COVID is more associated with a hypercoagulable state,” he said. “It is more associated with viral pneumonia on chest imaging, like the diffuse ground-glass infiltrates that we’ve all gotten used to seeing – but flu can do both of those things as well. So, without a test, it’s impossible to distinguish between the two infections in the clinic.”
But testing can have its shortcomings when flu season clashes with the COVID-19 pandemic. “Getting the test is not the same as getting the test results,” Dr. Solomon added. “Though a lot of people can get a test, if it takes 7 or 8 days to get the test result back, the result is useless.”
Widespread, rapid testing also depends on having adequate supplies of viral media transport and swabs. “I think that this is what we should be focusing on now: scaling up access to rapid turnaround testing,” he said. Distinguishing between the two is also important to preserve hospital resources. COVID-19 has more rigorous standards than flu for personal protective equipment and isolation of patients within the hospital.
Having chronic lung disease isn’t necessarily a risk factor for contracting COVID-19 or the flu, or both, Dr. Solomon said. “It’s a risk factor for having severe disease.” Again, he noted that flu vaccines are still necessary in these patients, as well as patients of advanced age and underlying medical conditions such as heart disease, diabetes, and obesity.
In managing children, it’s important to keep in mind that they communicate differently about their illnesses than adults, said Dr. Kaplan. “They may not have the words to tell you the same kind of thing that the adult tells you.” That’s where family members can help to flesh out the history. “They may present with an initially much milder form, if you will, where they’re not as critical up front, but then that small proportion of them comes back with the multi-inflammatory syndrome and then they are profoundly ill.”
Younger people make up a larger share of COVID-19 patients now, compared with the initial wave that hit the Northeast in the spring, Dr. Kaplan said. “We don’t know if that’s because the virus is a little different or the people that are getting sick are a little bit different.”
The COVID-19 strain now emerging may be less virulent than the strain that hit in early spring, he said. “That doesn’t mean that there aren’t still profoundly critical ill people with COVID of many different age ranges, that is true, but there are a lot of people that we now see will test positive, but aren’t really as profoundly ill as when it first landed here in the United States.”
That may be somewhat welcome as flu season arrives.
The physicians interviewed have no relevant disclosures.
As COVID-19 cases increase in children, deaths remain low
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
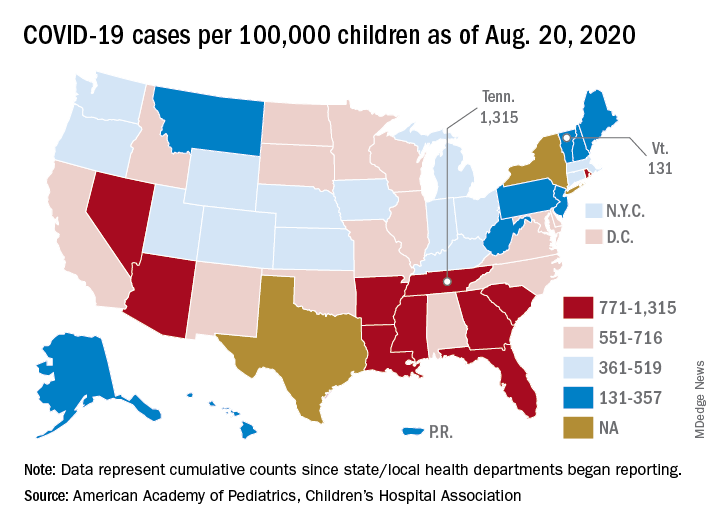
The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The cumulative number of pediatric cases reported up to that date was 442,785, or 9.3% of the total COVID-19 case load of more than 4.76 million among all ages. There have been only 92 pediatric deaths, however, which works out to just 0.06% of the 154,279 reported for all ages, the AAP and the CHA said Aug. 24 in their most recent update.
Child hospitalizations also were on the low side, representing 1.7% (4,062) of the cumulative total of 234,810 admissions among all ages as of Aug. 20, based on data from 21 states and New York City.
Nationally, the cumulative number of reported child cases is now up to 583 per 100,000 children, and that figure covers 49 states, Washington, D.C., Guam, New York City, and Puerto Rico.
There is some disagreement among the states, though, about the definition of “child.” Most states use an age range of 0-17, 0-18, or 0-19, but Florida and Utah go with a range of 0-14 years while South Carolina and Tennessee consider humans aged 0-20 years to be children. Other data limitations involve Texas, which has reported age distribution for only 8% of all cases, and New York, which is not reporting the age distribution of statewide cases, the AAP/CHA report noted.
The definition of child isn’t the only thing that varies between the states. The cumulative case rate for Tennessee, the highest in the country at 1,315 per 100,000 children, is 10 times that of Vermont, which is the lowest at 131 per 100,000, the AAP and CHA said. Vermont reports child COVID-19 cases using an age range of 0-19 years.
The other states with rates over 1,000 cases per 100,000 children are Arizona (1,300), which had the highest rate a week ago; South Carolina (1,214); Louisiana (1,127); Mississippi (1,120); and Nevada (1,068). Those with rates below 200 cases per 100,000 children are Maine (150), New Hampshire (175), and Hawaii (188), according to this week’s report.