User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Lung cancer CT scan is chance for ‘opportunistic’ osteoporosis check
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Low-dose chest CT for lung cancer screening provides the opportunity to simultaneously screen patients for osteoporosis, detecting notably higher rates of osteoporosis in men than the traditional tool of DXA, research published in the Journal of Bone and Mineral Research shows.
“Our large-scale, multicenter study of bone density measured from routine low-dose CT scans demonstrated the great potential of using low-dose CT for the opportunistic screening of osteoporosis as an alternative to standard DXA scans,” said senior author Wei Tian, MD, of the Chinese Academy of Engineering and Peking University, in a press statement from the journal.
“Our study revealed the unexpectedly high prevalence of osteoporosis in men, which may impact on the management strategy of men in the future,” Dr. Tian added.
Josephine Therkildsen, MD, of Herning Hospital, Denmark, who has conducted similar research using cardiac CT scans, said the findings add important new insights into the issue of opportunistic screening.
“The results are highly interesting, as they show that low-dose CT-based opportunistic screening could identify a substantial number of patients with low lumbar bone mineral density (BMD) with the future potential to diagnose osteoporosis and initiate relevant treatment before a fracture occurs,” she told this news organization.
Perry J. Pickhardt, MD, chief of gastrointestinal imaging at the University of Wisconsin School of Medicine and Public Health in Madison, agrees. He said in an interview that CT scans of the chest and abdomen, commonly performed for a variety of clinical indications and widespread in most developed countries, can in fact be essential for the detection of a multitude of other concerns – yet are underused for those other purposes.
Use of CT in this way “would likely be very cost effective and clinically efficacious,” he said, adding: “We are seeing greatly increased interest in leveraging this extra information that is contained within every CT scan.” And, “Importantly, artificial intelligence advances now allow for automated approaches, which should allow for expanded use.”
Lung cancer CT scans shed light on osteoporosis prevalence
In the study, led by Xiaoguang Cheng, MD, PhD, of the department of radiology, Beijing Jishuitan Hospital, China, researchers examined lung cancer CT screening data from the prospective China Biobank Project to determine the prevalence of osteoporosis in China.
This included the thoracic low-dose CT scans of 69,095 adults, including 40,733 men and 28,362 women, taken between 2018 and 2019.
To screen for osteoporosis, they used quantitative CT software to evaluate lumbar spine (L1-L2) trabecular volume BMD (vBMD) and diagnostic criteria from the American College of Radiology. Using the vBMD measures from the CT imaging, they found the prevalence of osteoporosis among those over 50 years of age in the Chinese population to be 29% for women (49 million) and 13.5% for men (22.8 million).
Interestingly, the osteoporosis prevalence rate among women was comparable to estimates in the population derived from DXA (29.1%); however, the rate in men was twice that estimated from DXA scans (6.5%).
Decreases in trabecular vBMD with age were observed in both genders. However, declines were steeper among women, who had higher peak trabecular vBMD (185.4 mg/cm3), compared with men (176.6 mg/cm3) at age 30-34 years, but significantly lower measures (62.4 mg/cm3) than men (92.1 mg/cm3) at age 80 years.
The prevalence of osteoporosis in women increased from 2.8% at age 50-54 years to 79.8% at age 85 or older, while in men, the prevalence was 3.2% at age 50-54 years and 44.1% at age 85 or older.
“This is the first study to establish Chinese reference data for vBMD using opportunistic screening from low-dose chest CT in a large population cohort,” the authors write.
“The opportunistic screening of osteoporosis using low-dose CT is clinically feasible and requires no additional exposure to ionizing radiation.”
In addition, no additional equipment or patient time was required, suggesting that “this approach has potential for opportunistic screening for osteoporosis.”
They note, however, that further cohort studies are needed to assess clinical utility of this method.
CT ‘likely a more accurate measure’ of volumetric BMD
Dr. Pickhardt said the differences in osteoporosis prevalence observed between DXA and CT-derived measures in men likely reflect the greater accuracy of CT.
“DXA is a planar technique with a number of drawbacks,” he said in an interview. “CT provides a more direct volumetric measure and is likely a more accurate method for BMD assessment.”
He speculated that the greater differences between DXA versus CT seen in men than women “may relate to sex differences in cortical bone of vertebral bodies, which cannot be separated from the underlying trabecular bone with DXA (whereas CT directly measures the inner trabecular bone).”
The authors note that, although areal BMD (aBMD) derived from DXA is required for osteoporosis diagnosis according to World Health Organization criteria, “trabecular vBMD derived from CT can be also used for diagnosis based on thresholds published by the American College of Radiology of 120 mg/cm3 and 80 mg/cm3 to define osteopenia and osteoporosis, respectively, thresholds that were subsequently confirmed for the Chinese population.”
Furthermore, vBMD has been shown in some studies to be more strongly related to fracture risk, compared with DXA aBMD measures.
Importantly, in another recent study involving 9,223 adults, Dr. Pickhardt and colleagues reported that bone and muscle biomarkers derived from CT were comparable to the Fracture Risk Assessment Tool score for the presymptomatic prediction of future osteoporotic fractures.
Dr. Pickhardt is an advisor to Bracco Imaging and Zebra Medical Vision. Dr. Therkildsen has reported no relevant financial relationships.
This article first appeared on Medscape.com.
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
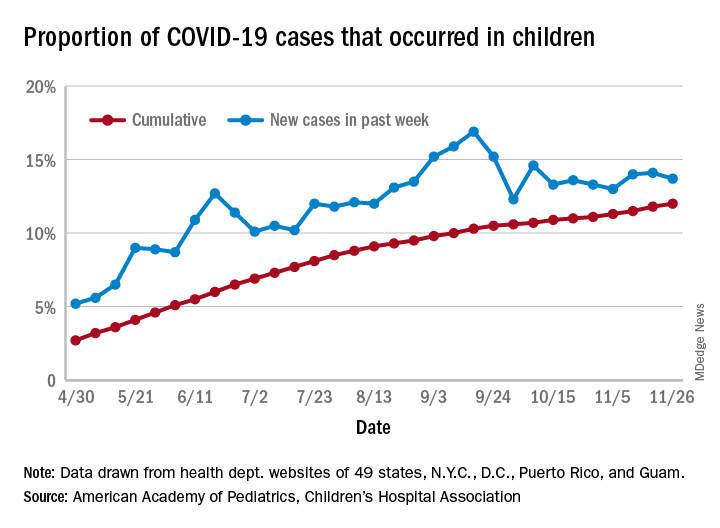
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
ACIP: Health workers, long-term care residents first tier for COVID-19 vaccine
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
Treating insomnia, anxiety in a pandemic
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
CMS launches hospital-at-home program to free up hospital capacity
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
Are more female physicians leaving medicine as pandemic surges?
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
Moderna filing for FDA emergency COVID-19 vaccine approval, reports 94.1% efficacy
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
Blood glucose on admission predicts COVID-19 severity in all
Hyperglycemia at hospital admission – regardless of diabetes status – is a key predictor of COVID-19-related death and severity among noncritical patients, new research from Spain finds.
The observational study, the largest to date to investigate this association, was published online Nov. 23 in Annals of Medicine by Francisco Javier Carrasco-Sánchez, MD, PhD, and colleagues.
Among more than 11,000 patients with confirmed COVID-19 from March to May 2020 in a nationwide Spanish registry involving 109 hospitals, admission hyperglycemia independently predicted progression from noncritical to critical condition and death, regardless of prior diabetes history.
Those with abnormally high glucose levels were more than twice as likely to die from the virus than those with normal readings (41.4% vs 15.7%). They also had an increased need for a ventilator and intensive care unit (ICU) admission.
“These results provided a simple and practical way to stratify risk of death in hospitalized patients with COVID-19. Hence, admission hyperglycemia should not be overlooked, but rather detected and appropriately treated to improve the outcomes of COVID-19 patients with and without diabetes,” Dr. Carrasco-Sánchez and colleagues wrote.
The findings confirm those of previous retrospective observational studies, but the current study “has, by far, the biggest number of patients involved in this kind of study [to date]. All conclusions are consistent to other studies,” Dr. Carrasco-Sánchez, of University Hospital Juan Ramón Jiménez, Huelva, Spain, said in an interview.
However, a surprising finding, he said, “was how hyperglycemia works in the nondiabetic population and [that] glucose levels over 140 [mg/dL] ... increase the risk of death.”
Pay attention to even mild hyperglycemia from admission
The study also differs from some of the prior observational ones in that it examines outcome by admission glycemia rather than during the hospital stay, therefore eliminating the effect of any inpatient treatment, such as dexamethasone, he noted.
Although blood glucose measurement at admission is routine for all patients in Spain, as it is in the United States and elsewhere, a mildly elevated level in a person without a diagnosis of diabetes may not be recognized as important.
“In patients with diabetes we start the protocol to control and treat hyperglycemia during hospitalization. However, in nondiabetic patients blood glucose levels under 180 [mg/dL], and even greater, are usually overlooked. This means there is not a correct follow-up of the patients during hospitalization.
“After this study we learned that we need to pay attention to this population ... who develop hyperglycemia from the beginning,” he said.
The study was limited in that patients who had previously undiagnosed diabetes couldn’t always be distinguished from those with acute “stress hyperglycemia.”
However, both need to be managed during hospitalization, he said. “Unfortunately, there is high variability in inpatient glucose management. The working group of diabetes of the Spanish Society of Internal Medicine is working on specific protocols,” said Dr. Carrasco-Sánchez.
All-cause death, progress to critical care higher with hyperglycemia
The retrospective, multicenter study was based on data from 11,312 adult patients with confirmed COVID-19 in 109 hospitals participating in Spain’s SEMI-COVID-19 registry as of May 29, 2020. They had a mean age of 67 years, 57% were male, and 19% had a diagnosis of diabetes. A total of 20% (n = 2,289) died during hospitalization.
Overall all-cause mortality was 41.1% among those with admission blood glucose levels above 180 mg/dL, 33.0% for those with glucose levels 140-180 mg/dL, and 15.7% for levels below 140 mg/dL. All differences were significant (P < .0001), but there were no differences in mortality rates within each blood glucose category between patients with or without a previous diagnosis of diabetes.
After adjustment for confounding factors, elevated admission blood glucose level remained a significant predictor of death. Compared to < 140 mg/dL, the hazard ratios for 140-180 mg/dL and > 180 mg/dL were 1.48 and 1.50, respectively (both P < .001). (Adjustments included age, gender, hypertension, diabetes, chronic obstructive pulmonary disease, lymphopenia, anemia (hemoglobin < 10 g/dL), serum creatinine, C-reactive protein > 60 mg/L, lactate dehydrogenase > 400 U/L and D-dimer >1000 ng/mL.)
Length of stay was 12, 11.5, and 11.1 days for those with admission blood glucose levels > 180, 140-180, and < 140 mg/dL, respectively (P = .011).
Use of mechanical ventilation and admission to intensive care also rose with higher admission blood glucose levels. For the composite of death, mechanical ventilation, and/or ICU admission, odds ratios for 140-180 mg/dL and > 180 mg/dL compared with < 140 mg/dL were 1.70 and 2.02, respectively (both P < .001).
The study was supported by the Spanish Federation of Internal Medicine. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hyperglycemia at hospital admission – regardless of diabetes status – is a key predictor of COVID-19-related death and severity among noncritical patients, new research from Spain finds.
The observational study, the largest to date to investigate this association, was published online Nov. 23 in Annals of Medicine by Francisco Javier Carrasco-Sánchez, MD, PhD, and colleagues.
Among more than 11,000 patients with confirmed COVID-19 from March to May 2020 in a nationwide Spanish registry involving 109 hospitals, admission hyperglycemia independently predicted progression from noncritical to critical condition and death, regardless of prior diabetes history.
Those with abnormally high glucose levels were more than twice as likely to die from the virus than those with normal readings (41.4% vs 15.7%). They also had an increased need for a ventilator and intensive care unit (ICU) admission.
“These results provided a simple and practical way to stratify risk of death in hospitalized patients with COVID-19. Hence, admission hyperglycemia should not be overlooked, but rather detected and appropriately treated to improve the outcomes of COVID-19 patients with and without diabetes,” Dr. Carrasco-Sánchez and colleagues wrote.
The findings confirm those of previous retrospective observational studies, but the current study “has, by far, the biggest number of patients involved in this kind of study [to date]. All conclusions are consistent to other studies,” Dr. Carrasco-Sánchez, of University Hospital Juan Ramón Jiménez, Huelva, Spain, said in an interview.
However, a surprising finding, he said, “was how hyperglycemia works in the nondiabetic population and [that] glucose levels over 140 [mg/dL] ... increase the risk of death.”
Pay attention to even mild hyperglycemia from admission
The study also differs from some of the prior observational ones in that it examines outcome by admission glycemia rather than during the hospital stay, therefore eliminating the effect of any inpatient treatment, such as dexamethasone, he noted.
Although blood glucose measurement at admission is routine for all patients in Spain, as it is in the United States and elsewhere, a mildly elevated level in a person without a diagnosis of diabetes may not be recognized as important.
“In patients with diabetes we start the protocol to control and treat hyperglycemia during hospitalization. However, in nondiabetic patients blood glucose levels under 180 [mg/dL], and even greater, are usually overlooked. This means there is not a correct follow-up of the patients during hospitalization.
“After this study we learned that we need to pay attention to this population ... who develop hyperglycemia from the beginning,” he said.
The study was limited in that patients who had previously undiagnosed diabetes couldn’t always be distinguished from those with acute “stress hyperglycemia.”
However, both need to be managed during hospitalization, he said. “Unfortunately, there is high variability in inpatient glucose management. The working group of diabetes of the Spanish Society of Internal Medicine is working on specific protocols,” said Dr. Carrasco-Sánchez.
All-cause death, progress to critical care higher with hyperglycemia
The retrospective, multicenter study was based on data from 11,312 adult patients with confirmed COVID-19 in 109 hospitals participating in Spain’s SEMI-COVID-19 registry as of May 29, 2020. They had a mean age of 67 years, 57% were male, and 19% had a diagnosis of diabetes. A total of 20% (n = 2,289) died during hospitalization.
Overall all-cause mortality was 41.1% among those with admission blood glucose levels above 180 mg/dL, 33.0% for those with glucose levels 140-180 mg/dL, and 15.7% for levels below 140 mg/dL. All differences were significant (P < .0001), but there were no differences in mortality rates within each blood glucose category between patients with or without a previous diagnosis of diabetes.
After adjustment for confounding factors, elevated admission blood glucose level remained a significant predictor of death. Compared to < 140 mg/dL, the hazard ratios for 140-180 mg/dL and > 180 mg/dL were 1.48 and 1.50, respectively (both P < .001). (Adjustments included age, gender, hypertension, diabetes, chronic obstructive pulmonary disease, lymphopenia, anemia (hemoglobin < 10 g/dL), serum creatinine, C-reactive protein > 60 mg/L, lactate dehydrogenase > 400 U/L and D-dimer >1000 ng/mL.)
Length of stay was 12, 11.5, and 11.1 days for those with admission blood glucose levels > 180, 140-180, and < 140 mg/dL, respectively (P = .011).
Use of mechanical ventilation and admission to intensive care also rose with higher admission blood glucose levels. For the composite of death, mechanical ventilation, and/or ICU admission, odds ratios for 140-180 mg/dL and > 180 mg/dL compared with < 140 mg/dL were 1.70 and 2.02, respectively (both P < .001).
The study was supported by the Spanish Federation of Internal Medicine. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hyperglycemia at hospital admission – regardless of diabetes status – is a key predictor of COVID-19-related death and severity among noncritical patients, new research from Spain finds.
The observational study, the largest to date to investigate this association, was published online Nov. 23 in Annals of Medicine by Francisco Javier Carrasco-Sánchez, MD, PhD, and colleagues.
Among more than 11,000 patients with confirmed COVID-19 from March to May 2020 in a nationwide Spanish registry involving 109 hospitals, admission hyperglycemia independently predicted progression from noncritical to critical condition and death, regardless of prior diabetes history.
Those with abnormally high glucose levels were more than twice as likely to die from the virus than those with normal readings (41.4% vs 15.7%). They also had an increased need for a ventilator and intensive care unit (ICU) admission.
“These results provided a simple and practical way to stratify risk of death in hospitalized patients with COVID-19. Hence, admission hyperglycemia should not be overlooked, but rather detected and appropriately treated to improve the outcomes of COVID-19 patients with and without diabetes,” Dr. Carrasco-Sánchez and colleagues wrote.
The findings confirm those of previous retrospective observational studies, but the current study “has, by far, the biggest number of patients involved in this kind of study [to date]. All conclusions are consistent to other studies,” Dr. Carrasco-Sánchez, of University Hospital Juan Ramón Jiménez, Huelva, Spain, said in an interview.
However, a surprising finding, he said, “was how hyperglycemia works in the nondiabetic population and [that] glucose levels over 140 [mg/dL] ... increase the risk of death.”
Pay attention to even mild hyperglycemia from admission
The study also differs from some of the prior observational ones in that it examines outcome by admission glycemia rather than during the hospital stay, therefore eliminating the effect of any inpatient treatment, such as dexamethasone, he noted.
Although blood glucose measurement at admission is routine for all patients in Spain, as it is in the United States and elsewhere, a mildly elevated level in a person without a diagnosis of diabetes may not be recognized as important.
“In patients with diabetes we start the protocol to control and treat hyperglycemia during hospitalization. However, in nondiabetic patients blood glucose levels under 180 [mg/dL], and even greater, are usually overlooked. This means there is not a correct follow-up of the patients during hospitalization.
“After this study we learned that we need to pay attention to this population ... who develop hyperglycemia from the beginning,” he said.
The study was limited in that patients who had previously undiagnosed diabetes couldn’t always be distinguished from those with acute “stress hyperglycemia.”
However, both need to be managed during hospitalization, he said. “Unfortunately, there is high variability in inpatient glucose management. The working group of diabetes of the Spanish Society of Internal Medicine is working on specific protocols,” said Dr. Carrasco-Sánchez.
All-cause death, progress to critical care higher with hyperglycemia
The retrospective, multicenter study was based on data from 11,312 adult patients with confirmed COVID-19 in 109 hospitals participating in Spain’s SEMI-COVID-19 registry as of May 29, 2020. They had a mean age of 67 years, 57% were male, and 19% had a diagnosis of diabetes. A total of 20% (n = 2,289) died during hospitalization.
Overall all-cause mortality was 41.1% among those with admission blood glucose levels above 180 mg/dL, 33.0% for those with glucose levels 140-180 mg/dL, and 15.7% for levels below 140 mg/dL. All differences were significant (P < .0001), but there were no differences in mortality rates within each blood glucose category between patients with or without a previous diagnosis of diabetes.
After adjustment for confounding factors, elevated admission blood glucose level remained a significant predictor of death. Compared to < 140 mg/dL, the hazard ratios for 140-180 mg/dL and > 180 mg/dL were 1.48 and 1.50, respectively (both P < .001). (Adjustments included age, gender, hypertension, diabetes, chronic obstructive pulmonary disease, lymphopenia, anemia (hemoglobin < 10 g/dL), serum creatinine, C-reactive protein > 60 mg/L, lactate dehydrogenase > 400 U/L and D-dimer >1000 ng/mL.)
Length of stay was 12, 11.5, and 11.1 days for those with admission blood glucose levels > 180, 140-180, and < 140 mg/dL, respectively (P = .011).
Use of mechanical ventilation and admission to intensive care also rose with higher admission blood glucose levels. For the composite of death, mechanical ventilation, and/or ICU admission, odds ratios for 140-180 mg/dL and > 180 mg/dL compared with < 140 mg/dL were 1.70 and 2.02, respectively (both P < .001).
The study was supported by the Spanish Federation of Internal Medicine. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Black patients with ES-SCLC get less chemo but have better survival
Black patients with extensive-stage small cell lung cancer (ES-SCLC) are less likely to receive chemotherapy but have better survival, compared with White patients, according to a study published in JTO Clinical Research and Reports.
This study provides a large-scale analysis of real-world data identifying racial and socioeconomic factors impacting systemic therapy delivery and survival in ES-SCLC.
“The most important finding was the significant disparity in receipt of chemotherapy,” said study author Umit Tapan, MD, of Boston Medical Center.
“Black individuals with ES-SCLC were less likely to receive chemotherapy compared to Whites and other racial groups. Similarly, elderly, uninsured patients, patients with nonprivate health insurance, and those with lower education levels were less likely to be treated with chemotherapy,” Dr. Tapan said.
Using the National Cancer Data Base (NCDB), Dr. Tapan and colleagues identified 148,961 patients who were diagnosed with stage IV ES-SCLC during 2004-2016. In all, 82,592 patients were included in the study.
Results: Treatment and survival
Compared with White patients, Black patients (adjusted odds ratio, 0.85; P = .0004) and patients from other racial groups (aOR, 0.87; P = .126) had lower odds of receiving chemotherapy on multivariate analysis.
However, survival was superior in Black patients (adjusted hazard ratio, 0.92; P < .0001) and other non-White patients (aHR 0.86; P < .0001).
“We speculate that additional factors, such as performance status, which is not captured by NCDB, might have accounted for better survival for Black patients,” Dr. Tapan said, noting that the analysis was adjusted for known possible confounding factors, such as age, gender, and comorbidity status.
Black patients had higher odds of receiving chemotherapy between 2010 and 2016 compared with 2004 and 2009. “This suggests a positive impact of the Patient Protection and Affordable Care Act (ACA) in 2010,” Dr. Tapan said.
Another surprising finding pertained to patients with nonprivate insurance. These patients had even lower odds of getting chemotherapy after the implementation of ACA, Dr. Tapan said. Patients who had private insurance had higher survival compared with those who were uninsured.
Higher level of education, measured by percentage of residents with a high school degree, increased the odds of receiving chemotherapy.
Age also had a significant impact on receipt of chemotherapy. About 83% of patients over age 80 years received chemotherapy, compared with 94% of patients aged 40-64 years.
Real-world data
Minorities are underrepresented in cancer clinical trials in the United States, with only 2% of National Cancer Institute trials having sufficient minority participants, Dr. Tapan said. A study published in Academic Medicine in 2018 showed that only 13% of 782 National Institute of Health–sponsored clinical trials reported outcomes by race and ethnicity.
As a result, we are missing data on patient care in minority populations, Dr. Tapan said. “Collecting and analyzing real-world data becomes critical to study treatment patterns and outcomes,” he added.
The current real-world study had a somewhat diverse patient population, but 90.6% of patients were White, 7.8% were Black, and 1.7% were other races.
“We would have expected a higher percentage of Black patients considering the most recent U.S. Census Bureau estimates that 76.3% of the U.S. population is White and 13.4% is Black,” Dr. Tapan said. “There are conflicting results in the literature regarding racial disparities in SCLC and survival. Many of these studies were performed via state-based cancer registries instead of on a national level, making prior reports less generalizable.”
‘More work to do’
While the new study showed patients with nonprivate insurance or those with no insurance were less likely to receive chemotherapy, studies have shown that chemotherapy administration was not impacted by insurance status in limited-stage SCLC.
This is in contrast to radiotherapy delivery. Studies have revealed a lower likelihood of radiotherapy delivery in limited-stage SCLC for patients with government health insurance such as Medicare/Medicaid, Dr. Tapan said.
“Access to cancer care has been shown to be one of the most important barriers in racial disparity. Studies analyzing outcomes in the equal access health systems, such as the Veteran Administration, have revealed less racial disparities,” Dr. Tapan said.
Even when Black patients have equal access to care, they might receive suboptimal treatment, Dr. Tapan noted.
“Studies have shown that Black patients are not only more likely to refuse surgery, but also are more likely to be given a negative recommendation by a surgeon as compared to Whites, suggesting potential involvement of miscommunication or bias during patient-physician encounters,” Dr. Tapan said. “In the same vein, physicians would need to acknowledge their patients’ beliefs. Not doing so may lead to unsatisfactory physician-patient interactions and suboptimal decision-making.”
“Measures to reduce physician bias are an important step to reduce disparities,” Dr. Tapan continued. “Studies have shown that Black patients are perceived to be less intelligent and educated, less likely to have social support, and more likely to be at risk of noncompliance. For some patients and oncologists, extra effort is needed so that every patient can access the best possible treatments and outcomes. It is the oncologist’s responsibility to advocate for patients, but, ultimately, further legislative actions are needed to mitigate the disparities around cancer care.”
Dr. Tapan noted that, in 1966, Martin Luther King Jr., PhD, stated that “of all the forms of inequality, injustice in health care is the most shocking and inhumane.”
Dr. Tapan said: “We have overcome some barriers since 1966, but we have more work to do.” He and colleagues had no disclosures related to this study.
SOURCE: Tapan U et al. JTO Clin Res Rep. 2020. doi: 10.1016/j.jtocrr.2020.100109.
Black patients with extensive-stage small cell lung cancer (ES-SCLC) are less likely to receive chemotherapy but have better survival, compared with White patients, according to a study published in JTO Clinical Research and Reports.
This study provides a large-scale analysis of real-world data identifying racial and socioeconomic factors impacting systemic therapy delivery and survival in ES-SCLC.
“The most important finding was the significant disparity in receipt of chemotherapy,” said study author Umit Tapan, MD, of Boston Medical Center.
“Black individuals with ES-SCLC were less likely to receive chemotherapy compared to Whites and other racial groups. Similarly, elderly, uninsured patients, patients with nonprivate health insurance, and those with lower education levels were less likely to be treated with chemotherapy,” Dr. Tapan said.
Using the National Cancer Data Base (NCDB), Dr. Tapan and colleagues identified 148,961 patients who were diagnosed with stage IV ES-SCLC during 2004-2016. In all, 82,592 patients were included in the study.
Results: Treatment and survival
Compared with White patients, Black patients (adjusted odds ratio, 0.85; P = .0004) and patients from other racial groups (aOR, 0.87; P = .126) had lower odds of receiving chemotherapy on multivariate analysis.
However, survival was superior in Black patients (adjusted hazard ratio, 0.92; P < .0001) and other non-White patients (aHR 0.86; P < .0001).
“We speculate that additional factors, such as performance status, which is not captured by NCDB, might have accounted for better survival for Black patients,” Dr. Tapan said, noting that the analysis was adjusted for known possible confounding factors, such as age, gender, and comorbidity status.
Black patients had higher odds of receiving chemotherapy between 2010 and 2016 compared with 2004 and 2009. “This suggests a positive impact of the Patient Protection and Affordable Care Act (ACA) in 2010,” Dr. Tapan said.
Another surprising finding pertained to patients with nonprivate insurance. These patients had even lower odds of getting chemotherapy after the implementation of ACA, Dr. Tapan said. Patients who had private insurance had higher survival compared with those who were uninsured.
Higher level of education, measured by percentage of residents with a high school degree, increased the odds of receiving chemotherapy.
Age also had a significant impact on receipt of chemotherapy. About 83% of patients over age 80 years received chemotherapy, compared with 94% of patients aged 40-64 years.
Real-world data
Minorities are underrepresented in cancer clinical trials in the United States, with only 2% of National Cancer Institute trials having sufficient minority participants, Dr. Tapan said. A study published in Academic Medicine in 2018 showed that only 13% of 782 National Institute of Health–sponsored clinical trials reported outcomes by race and ethnicity.
As a result, we are missing data on patient care in minority populations, Dr. Tapan said. “Collecting and analyzing real-world data becomes critical to study treatment patterns and outcomes,” he added.
The current real-world study had a somewhat diverse patient population, but 90.6% of patients were White, 7.8% were Black, and 1.7% were other races.
“We would have expected a higher percentage of Black patients considering the most recent U.S. Census Bureau estimates that 76.3% of the U.S. population is White and 13.4% is Black,” Dr. Tapan said. “There are conflicting results in the literature regarding racial disparities in SCLC and survival. Many of these studies were performed via state-based cancer registries instead of on a national level, making prior reports less generalizable.”
‘More work to do’
While the new study showed patients with nonprivate insurance or those with no insurance were less likely to receive chemotherapy, studies have shown that chemotherapy administration was not impacted by insurance status in limited-stage SCLC.
This is in contrast to radiotherapy delivery. Studies have revealed a lower likelihood of radiotherapy delivery in limited-stage SCLC for patients with government health insurance such as Medicare/Medicaid, Dr. Tapan said.
“Access to cancer care has been shown to be one of the most important barriers in racial disparity. Studies analyzing outcomes in the equal access health systems, such as the Veteran Administration, have revealed less racial disparities,” Dr. Tapan said.
Even when Black patients have equal access to care, they might receive suboptimal treatment, Dr. Tapan noted.
“Studies have shown that Black patients are not only more likely to refuse surgery, but also are more likely to be given a negative recommendation by a surgeon as compared to Whites, suggesting potential involvement of miscommunication or bias during patient-physician encounters,” Dr. Tapan said. “In the same vein, physicians would need to acknowledge their patients’ beliefs. Not doing so may lead to unsatisfactory physician-patient interactions and suboptimal decision-making.”
“Measures to reduce physician bias are an important step to reduce disparities,” Dr. Tapan continued. “Studies have shown that Black patients are perceived to be less intelligent and educated, less likely to have social support, and more likely to be at risk of noncompliance. For some patients and oncologists, extra effort is needed so that every patient can access the best possible treatments and outcomes. It is the oncologist’s responsibility to advocate for patients, but, ultimately, further legislative actions are needed to mitigate the disparities around cancer care.”
Dr. Tapan noted that, in 1966, Martin Luther King Jr., PhD, stated that “of all the forms of inequality, injustice in health care is the most shocking and inhumane.”
Dr. Tapan said: “We have overcome some barriers since 1966, but we have more work to do.” He and colleagues had no disclosures related to this study.
SOURCE: Tapan U et al. JTO Clin Res Rep. 2020. doi: 10.1016/j.jtocrr.2020.100109.
Black patients with extensive-stage small cell lung cancer (ES-SCLC) are less likely to receive chemotherapy but have better survival, compared with White patients, according to a study published in JTO Clinical Research and Reports.
This study provides a large-scale analysis of real-world data identifying racial and socioeconomic factors impacting systemic therapy delivery and survival in ES-SCLC.
“The most important finding was the significant disparity in receipt of chemotherapy,” said study author Umit Tapan, MD, of Boston Medical Center.
“Black individuals with ES-SCLC were less likely to receive chemotherapy compared to Whites and other racial groups. Similarly, elderly, uninsured patients, patients with nonprivate health insurance, and those with lower education levels were less likely to be treated with chemotherapy,” Dr. Tapan said.
Using the National Cancer Data Base (NCDB), Dr. Tapan and colleagues identified 148,961 patients who were diagnosed with stage IV ES-SCLC during 2004-2016. In all, 82,592 patients were included in the study.
Results: Treatment and survival
Compared with White patients, Black patients (adjusted odds ratio, 0.85; P = .0004) and patients from other racial groups (aOR, 0.87; P = .126) had lower odds of receiving chemotherapy on multivariate analysis.
However, survival was superior in Black patients (adjusted hazard ratio, 0.92; P < .0001) and other non-White patients (aHR 0.86; P < .0001).
“We speculate that additional factors, such as performance status, which is not captured by NCDB, might have accounted for better survival for Black patients,” Dr. Tapan said, noting that the analysis was adjusted for known possible confounding factors, such as age, gender, and comorbidity status.
Black patients had higher odds of receiving chemotherapy between 2010 and 2016 compared with 2004 and 2009. “This suggests a positive impact of the Patient Protection and Affordable Care Act (ACA) in 2010,” Dr. Tapan said.
Another surprising finding pertained to patients with nonprivate insurance. These patients had even lower odds of getting chemotherapy after the implementation of ACA, Dr. Tapan said. Patients who had private insurance had higher survival compared with those who were uninsured.
Higher level of education, measured by percentage of residents with a high school degree, increased the odds of receiving chemotherapy.
Age also had a significant impact on receipt of chemotherapy. About 83% of patients over age 80 years received chemotherapy, compared with 94% of patients aged 40-64 years.
Real-world data
Minorities are underrepresented in cancer clinical trials in the United States, with only 2% of National Cancer Institute trials having sufficient minority participants, Dr. Tapan said. A study published in Academic Medicine in 2018 showed that only 13% of 782 National Institute of Health–sponsored clinical trials reported outcomes by race and ethnicity.
As a result, we are missing data on patient care in minority populations, Dr. Tapan said. “Collecting and analyzing real-world data becomes critical to study treatment patterns and outcomes,” he added.
The current real-world study had a somewhat diverse patient population, but 90.6% of patients were White, 7.8% were Black, and 1.7% were other races.
“We would have expected a higher percentage of Black patients considering the most recent U.S. Census Bureau estimates that 76.3% of the U.S. population is White and 13.4% is Black,” Dr. Tapan said. “There are conflicting results in the literature regarding racial disparities in SCLC and survival. Many of these studies were performed via state-based cancer registries instead of on a national level, making prior reports less generalizable.”
‘More work to do’
While the new study showed patients with nonprivate insurance or those with no insurance were less likely to receive chemotherapy, studies have shown that chemotherapy administration was not impacted by insurance status in limited-stage SCLC.
This is in contrast to radiotherapy delivery. Studies have revealed a lower likelihood of radiotherapy delivery in limited-stage SCLC for patients with government health insurance such as Medicare/Medicaid, Dr. Tapan said.
“Access to cancer care has been shown to be one of the most important barriers in racial disparity. Studies analyzing outcomes in the equal access health systems, such as the Veteran Administration, have revealed less racial disparities,” Dr. Tapan said.
Even when Black patients have equal access to care, they might receive suboptimal treatment, Dr. Tapan noted.
“Studies have shown that Black patients are not only more likely to refuse surgery, but also are more likely to be given a negative recommendation by a surgeon as compared to Whites, suggesting potential involvement of miscommunication or bias during patient-physician encounters,” Dr. Tapan said. “In the same vein, physicians would need to acknowledge their patients’ beliefs. Not doing so may lead to unsatisfactory physician-patient interactions and suboptimal decision-making.”
“Measures to reduce physician bias are an important step to reduce disparities,” Dr. Tapan continued. “Studies have shown that Black patients are perceived to be less intelligent and educated, less likely to have social support, and more likely to be at risk of noncompliance. For some patients and oncologists, extra effort is needed so that every patient can access the best possible treatments and outcomes. It is the oncologist’s responsibility to advocate for patients, but, ultimately, further legislative actions are needed to mitigate the disparities around cancer care.”
Dr. Tapan noted that, in 1966, Martin Luther King Jr., PhD, stated that “of all the forms of inequality, injustice in health care is the most shocking and inhumane.”
Dr. Tapan said: “We have overcome some barriers since 1966, but we have more work to do.” He and colleagues had no disclosures related to this study.
SOURCE: Tapan U et al. JTO Clin Res Rep. 2020. doi: 10.1016/j.jtocrr.2020.100109.
FROM JTO CLINICAL AND RESEARCH REPORTS

