User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Calcium burden drives CV risk whether coronary disease is obstructive or not
Coronary artery calcium (CAC) score as a measure of plaque burden more reliably predicts future cardiovascular (CV) risk in patients with suspected coronary disease (CAD) than whether or not the disease is obstructive, a large retrospective study suggests.
Indeed, CV risk went up in tandem with growing plaque burden regardless of whether there was obstructive disease in any coronary artery, defined as a 50% or greater stenosis by computed tomographic angiography (CTA).
The findings argue for plaque burden as measured by CAC score, rather than percent-stenosis severity, for guiding further treatment decisions in such patients, researchers say.
The research was based on more than 20,000 symptomatic patients referred to diagnostic CTA in the Western Denmark Heart Registry who were then followed for about 4 years for major CV events, including death, myocardial infarction, or stroke.
“What we show is that CAC is important for prognosis, and that patients with no stenosis have similar high risk as patients with stenosis when CAC burden is similar,” Martin Bødtker Mortensen, MD, PhD, Aarhus (Denmark) University Hospital, said in an interview.
The guidelines “distinguish between primary and secondary prevention patients” based on the presence or absence of obstructive CAD, he said, but “our results challenge this long-held approach. We show that patients with nonobstructive CAD carry similar risk as patients with obstructive CAD.”
In practice, risk tends to be greater in patients with obstructive compared with nonobstructive CAD. But the reason “is simply that they normally have higher atherosclerosis burden,” Dr. Mortensen said. “When you stratify based on atherosclerosis burden, then patients with obstructive and nonobstructive CAD have similar risk.”
The analysis was published online Dec. 7 in the Journal of the American College of Cardiology with Mortensen as lead author.
Until recently, it had long been believed that CV-event risk was driven by ischemia – but “ischemia is just a surrogate for the extent of atherosclerotic disease,” Armin Arbab Zadeh, MD, PhD, MPH, who is not connected with the current study, said in an interview.
The finding that CV risk climbs with growing coronary plaque burden “essentially confirms” other recent studies, but with “added value in showing how well the calcium scores, compared to obstructive disease, track with risk. So it’s definitely a nice extension of the evidence,” said Dr. Zadeh, director of cardiac CT at Johns Hopkins University, Baltimore.
“This study clearly shows that there is no ischemia ‘threshold,’ that the risk starts from mild and goes up with the burden of atherosclerotic disease. We were essentially taught wrong for decades.”
Dr. Mortensen said that the new results “are in line with previous studies showing that atherosclerosis burden is very important for risk.” They also help explain why revascularization of patients with stable angina failed to cut the risk of MI or death in trials like COURAGE, FAME-2, and ISCHEMIA. It’s because “stenosis per se explains little of the risk compared to atherosclerosis burden.”
In the current analysis, for example, about 65% of events were in patients who did not show obstructive CAD at CTA. Its 23,759 patients with symptoms suggestive of CAD were referred for CTA from 2008 through 2017; 5,043 (21.2%) were found to have obstructive disease and 18,716 (78.8%) either had no CAD or nonobstructive disease.
About 4.4% of patients experienced a first major CV event over a median follow-up of 4.3 years. Only events occurring later than 90 days after CTA were counted in an effort to exclude any directly related to revascularization, Dr. Mortensen noted.
The risk of events went up proportionally with both CAC score and the number of coronaries with obstructive disease.
The number of major CV events per 1,000 person-years was 6.2 for patients with a CAC score of 0, of whom 87% had no CAD by CTA, 7% had nonobstructive CAD, and 6% had obstructive CAD.
The corresponding rate was 17.5 among patients with a CAC score >100-399 for a hazard ratio (HR) of 1.7 (95% confidence interval [CI] 1.4-2.1) vs. a CAC score of 0.
And it was 42.3 per 1,000 patient-years among patients with CAC score >1000, HR 3.4 (95% CI, 2.5-4.6) vs. a CAC score of 0. Among those with the highest-tier CAC score, none were without CAD by CTA, 17% had nonobstructive disease, and 83% had obstructive CAD.
The major CV event rate rose similarly by number of coronaries with obstructive disease. It was 6.1 per 1,000 person-years in patients with no CAD. But it was 12.3 in those with nonobstructive disease, HR 1.3 (95% CI 1.1-1.6), up to 34.7 in those with triple-vessel obstructive disease, HR 2.9 (95% CI 2.2-3.9), vs. no CAD.
However, in an analysis with stratification by CAC score tier (0, 1-99, 100-399, 400-1,000, and >1,000), obstructive CAD was not associated with increased major CV-event risk in any stratum. The findings were similar in each subgroup with 1-vessel, 2-vessel, or 3-vessel CAD when stratified by CAC score.
Nor did major CV event risk track with obstructive CAD in analyses by age or after excluding all patients who underwent coronary revascularization within 90 days of CTA, the group reported.
“I believe these results support the use of CTA as a first-line test in patients with symptoms suggestive of CAD, as it provides valuable information for both diagnosis and prognosis in symptomatic patients,” Dr. Mortensen said. Those found to have a higher burden of atherosclerosis, he added, should receive aggressive preventive therapy regardless of whether or not they have obstructive disease.
The evidence from this study and others “supports a CTA-based approach” in such patients, Dr. Zadeh said. “And I would go further to say that a stress test is really inadequate,” in that it “detects the disease at such a late stage, you’re missing the opportunity to identify these patients who have atherosclerotic disease while you can do something about it.”
Its continued use as a first-line test, Dr. Zadeh said, “is essentially, in my mind, dismissing the evidence.”
An accompanying editorial Todd C. Villines, MD, and Patricia Rodriguez Lozano, MD, of the University of Virginia, Charlottesville agreed that “it is time that the traditional definitions of primary and secondary prevention evolve to incorporate CAC and CTA measures of patient risk based on coronary artery plaque burden.”
But they pointed out some limitations of the current study.
“The authors compared CAC with ≥50% stenosis, not CAC to comprehensive, contemporary coronary CTA,” and so “did not assess numerous other well-validated measures of coronary plaque burden that are routinely obtained from coronary CTA that typically improve the prognostic accuracy of coronary CTA beyond stenosis alone.” Also not performed was “plaque quantification on coronary CTA, an emerging field of study.”
The editorialists noted that noncontrast CT as used in the study for CAC scoring “is generally not recommended as a standalone test in symptomatic patients. Most studies have shown that coronary CTA, a test that accurately detects stenosis and identifies all types of coronary atherosclerosis (calcified and noncalcified), has significantly higher diagnostic and prognostic accuracy than CAC when performed in symptomatic patients without known coronary artery disease.”
Dr. Mortensen has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Villines and Dr. Rodriguez Lozano have disclosed no relevant financial relationships. Dr. Zadeh disclosed receiving grant support from Canon Medical Systems.
A version of this article originally appeared on Medscape.com.
Coronary artery calcium (CAC) score as a measure of plaque burden more reliably predicts future cardiovascular (CV) risk in patients with suspected coronary disease (CAD) than whether or not the disease is obstructive, a large retrospective study suggests.
Indeed, CV risk went up in tandem with growing plaque burden regardless of whether there was obstructive disease in any coronary artery, defined as a 50% or greater stenosis by computed tomographic angiography (CTA).
The findings argue for plaque burden as measured by CAC score, rather than percent-stenosis severity, for guiding further treatment decisions in such patients, researchers say.
The research was based on more than 20,000 symptomatic patients referred to diagnostic CTA in the Western Denmark Heart Registry who were then followed for about 4 years for major CV events, including death, myocardial infarction, or stroke.
“What we show is that CAC is important for prognosis, and that patients with no stenosis have similar high risk as patients with stenosis when CAC burden is similar,” Martin Bødtker Mortensen, MD, PhD, Aarhus (Denmark) University Hospital, said in an interview.
The guidelines “distinguish between primary and secondary prevention patients” based on the presence or absence of obstructive CAD, he said, but “our results challenge this long-held approach. We show that patients with nonobstructive CAD carry similar risk as patients with obstructive CAD.”
In practice, risk tends to be greater in patients with obstructive compared with nonobstructive CAD. But the reason “is simply that they normally have higher atherosclerosis burden,” Dr. Mortensen said. “When you stratify based on atherosclerosis burden, then patients with obstructive and nonobstructive CAD have similar risk.”
The analysis was published online Dec. 7 in the Journal of the American College of Cardiology with Mortensen as lead author.
Until recently, it had long been believed that CV-event risk was driven by ischemia – but “ischemia is just a surrogate for the extent of atherosclerotic disease,” Armin Arbab Zadeh, MD, PhD, MPH, who is not connected with the current study, said in an interview.
The finding that CV risk climbs with growing coronary plaque burden “essentially confirms” other recent studies, but with “added value in showing how well the calcium scores, compared to obstructive disease, track with risk. So it’s definitely a nice extension of the evidence,” said Dr. Zadeh, director of cardiac CT at Johns Hopkins University, Baltimore.
“This study clearly shows that there is no ischemia ‘threshold,’ that the risk starts from mild and goes up with the burden of atherosclerotic disease. We were essentially taught wrong for decades.”
Dr. Mortensen said that the new results “are in line with previous studies showing that atherosclerosis burden is very important for risk.” They also help explain why revascularization of patients with stable angina failed to cut the risk of MI or death in trials like COURAGE, FAME-2, and ISCHEMIA. It’s because “stenosis per se explains little of the risk compared to atherosclerosis burden.”
In the current analysis, for example, about 65% of events were in patients who did not show obstructive CAD at CTA. Its 23,759 patients with symptoms suggestive of CAD were referred for CTA from 2008 through 2017; 5,043 (21.2%) were found to have obstructive disease and 18,716 (78.8%) either had no CAD or nonobstructive disease.
About 4.4% of patients experienced a first major CV event over a median follow-up of 4.3 years. Only events occurring later than 90 days after CTA were counted in an effort to exclude any directly related to revascularization, Dr. Mortensen noted.
The risk of events went up proportionally with both CAC score and the number of coronaries with obstructive disease.
The number of major CV events per 1,000 person-years was 6.2 for patients with a CAC score of 0, of whom 87% had no CAD by CTA, 7% had nonobstructive CAD, and 6% had obstructive CAD.
The corresponding rate was 17.5 among patients with a CAC score >100-399 for a hazard ratio (HR) of 1.7 (95% confidence interval [CI] 1.4-2.1) vs. a CAC score of 0.
And it was 42.3 per 1,000 patient-years among patients with CAC score >1000, HR 3.4 (95% CI, 2.5-4.6) vs. a CAC score of 0. Among those with the highest-tier CAC score, none were without CAD by CTA, 17% had nonobstructive disease, and 83% had obstructive CAD.
The major CV event rate rose similarly by number of coronaries with obstructive disease. It was 6.1 per 1,000 person-years in patients with no CAD. But it was 12.3 in those with nonobstructive disease, HR 1.3 (95% CI 1.1-1.6), up to 34.7 in those with triple-vessel obstructive disease, HR 2.9 (95% CI 2.2-3.9), vs. no CAD.
However, in an analysis with stratification by CAC score tier (0, 1-99, 100-399, 400-1,000, and >1,000), obstructive CAD was not associated with increased major CV-event risk in any stratum. The findings were similar in each subgroup with 1-vessel, 2-vessel, or 3-vessel CAD when stratified by CAC score.
Nor did major CV event risk track with obstructive CAD in analyses by age or after excluding all patients who underwent coronary revascularization within 90 days of CTA, the group reported.
“I believe these results support the use of CTA as a first-line test in patients with symptoms suggestive of CAD, as it provides valuable information for both diagnosis and prognosis in symptomatic patients,” Dr. Mortensen said. Those found to have a higher burden of atherosclerosis, he added, should receive aggressive preventive therapy regardless of whether or not they have obstructive disease.
The evidence from this study and others “supports a CTA-based approach” in such patients, Dr. Zadeh said. “And I would go further to say that a stress test is really inadequate,” in that it “detects the disease at such a late stage, you’re missing the opportunity to identify these patients who have atherosclerotic disease while you can do something about it.”
Its continued use as a first-line test, Dr. Zadeh said, “is essentially, in my mind, dismissing the evidence.”
An accompanying editorial Todd C. Villines, MD, and Patricia Rodriguez Lozano, MD, of the University of Virginia, Charlottesville agreed that “it is time that the traditional definitions of primary and secondary prevention evolve to incorporate CAC and CTA measures of patient risk based on coronary artery plaque burden.”
But they pointed out some limitations of the current study.
“The authors compared CAC with ≥50% stenosis, not CAC to comprehensive, contemporary coronary CTA,” and so “did not assess numerous other well-validated measures of coronary plaque burden that are routinely obtained from coronary CTA that typically improve the prognostic accuracy of coronary CTA beyond stenosis alone.” Also not performed was “plaque quantification on coronary CTA, an emerging field of study.”
The editorialists noted that noncontrast CT as used in the study for CAC scoring “is generally not recommended as a standalone test in symptomatic patients. Most studies have shown that coronary CTA, a test that accurately detects stenosis and identifies all types of coronary atherosclerosis (calcified and noncalcified), has significantly higher diagnostic and prognostic accuracy than CAC when performed in symptomatic patients without known coronary artery disease.”
Dr. Mortensen has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Villines and Dr. Rodriguez Lozano have disclosed no relevant financial relationships. Dr. Zadeh disclosed receiving grant support from Canon Medical Systems.
A version of this article originally appeared on Medscape.com.
Coronary artery calcium (CAC) score as a measure of plaque burden more reliably predicts future cardiovascular (CV) risk in patients with suspected coronary disease (CAD) than whether or not the disease is obstructive, a large retrospective study suggests.
Indeed, CV risk went up in tandem with growing plaque burden regardless of whether there was obstructive disease in any coronary artery, defined as a 50% or greater stenosis by computed tomographic angiography (CTA).
The findings argue for plaque burden as measured by CAC score, rather than percent-stenosis severity, for guiding further treatment decisions in such patients, researchers say.
The research was based on more than 20,000 symptomatic patients referred to diagnostic CTA in the Western Denmark Heart Registry who were then followed for about 4 years for major CV events, including death, myocardial infarction, or stroke.
“What we show is that CAC is important for prognosis, and that patients with no stenosis have similar high risk as patients with stenosis when CAC burden is similar,” Martin Bødtker Mortensen, MD, PhD, Aarhus (Denmark) University Hospital, said in an interview.
The guidelines “distinguish between primary and secondary prevention patients” based on the presence or absence of obstructive CAD, he said, but “our results challenge this long-held approach. We show that patients with nonobstructive CAD carry similar risk as patients with obstructive CAD.”
In practice, risk tends to be greater in patients with obstructive compared with nonobstructive CAD. But the reason “is simply that they normally have higher atherosclerosis burden,” Dr. Mortensen said. “When you stratify based on atherosclerosis burden, then patients with obstructive and nonobstructive CAD have similar risk.”
The analysis was published online Dec. 7 in the Journal of the American College of Cardiology with Mortensen as lead author.
Until recently, it had long been believed that CV-event risk was driven by ischemia – but “ischemia is just a surrogate for the extent of atherosclerotic disease,” Armin Arbab Zadeh, MD, PhD, MPH, who is not connected with the current study, said in an interview.
The finding that CV risk climbs with growing coronary plaque burden “essentially confirms” other recent studies, but with “added value in showing how well the calcium scores, compared to obstructive disease, track with risk. So it’s definitely a nice extension of the evidence,” said Dr. Zadeh, director of cardiac CT at Johns Hopkins University, Baltimore.
“This study clearly shows that there is no ischemia ‘threshold,’ that the risk starts from mild and goes up with the burden of atherosclerotic disease. We were essentially taught wrong for decades.”
Dr. Mortensen said that the new results “are in line with previous studies showing that atherosclerosis burden is very important for risk.” They also help explain why revascularization of patients with stable angina failed to cut the risk of MI or death in trials like COURAGE, FAME-2, and ISCHEMIA. It’s because “stenosis per se explains little of the risk compared to atherosclerosis burden.”
In the current analysis, for example, about 65% of events were in patients who did not show obstructive CAD at CTA. Its 23,759 patients with symptoms suggestive of CAD were referred for CTA from 2008 through 2017; 5,043 (21.2%) were found to have obstructive disease and 18,716 (78.8%) either had no CAD or nonobstructive disease.
About 4.4% of patients experienced a first major CV event over a median follow-up of 4.3 years. Only events occurring later than 90 days after CTA were counted in an effort to exclude any directly related to revascularization, Dr. Mortensen noted.
The risk of events went up proportionally with both CAC score and the number of coronaries with obstructive disease.
The number of major CV events per 1,000 person-years was 6.2 for patients with a CAC score of 0, of whom 87% had no CAD by CTA, 7% had nonobstructive CAD, and 6% had obstructive CAD.
The corresponding rate was 17.5 among patients with a CAC score >100-399 for a hazard ratio (HR) of 1.7 (95% confidence interval [CI] 1.4-2.1) vs. a CAC score of 0.
And it was 42.3 per 1,000 patient-years among patients with CAC score >1000, HR 3.4 (95% CI, 2.5-4.6) vs. a CAC score of 0. Among those with the highest-tier CAC score, none were without CAD by CTA, 17% had nonobstructive disease, and 83% had obstructive CAD.
The major CV event rate rose similarly by number of coronaries with obstructive disease. It was 6.1 per 1,000 person-years in patients with no CAD. But it was 12.3 in those with nonobstructive disease, HR 1.3 (95% CI 1.1-1.6), up to 34.7 in those with triple-vessel obstructive disease, HR 2.9 (95% CI 2.2-3.9), vs. no CAD.
However, in an analysis with stratification by CAC score tier (0, 1-99, 100-399, 400-1,000, and >1,000), obstructive CAD was not associated with increased major CV-event risk in any stratum. The findings were similar in each subgroup with 1-vessel, 2-vessel, or 3-vessel CAD when stratified by CAC score.
Nor did major CV event risk track with obstructive CAD in analyses by age or after excluding all patients who underwent coronary revascularization within 90 days of CTA, the group reported.
“I believe these results support the use of CTA as a first-line test in patients with symptoms suggestive of CAD, as it provides valuable information for both diagnosis and prognosis in symptomatic patients,” Dr. Mortensen said. Those found to have a higher burden of atherosclerosis, he added, should receive aggressive preventive therapy regardless of whether or not they have obstructive disease.
The evidence from this study and others “supports a CTA-based approach” in such patients, Dr. Zadeh said. “And I would go further to say that a stress test is really inadequate,” in that it “detects the disease at such a late stage, you’re missing the opportunity to identify these patients who have atherosclerotic disease while you can do something about it.”
Its continued use as a first-line test, Dr. Zadeh said, “is essentially, in my mind, dismissing the evidence.”
An accompanying editorial Todd C. Villines, MD, and Patricia Rodriguez Lozano, MD, of the University of Virginia, Charlottesville agreed that “it is time that the traditional definitions of primary and secondary prevention evolve to incorporate CAC and CTA measures of patient risk based on coronary artery plaque burden.”
But they pointed out some limitations of the current study.
“The authors compared CAC with ≥50% stenosis, not CAC to comprehensive, contemporary coronary CTA,” and so “did not assess numerous other well-validated measures of coronary plaque burden that are routinely obtained from coronary CTA that typically improve the prognostic accuracy of coronary CTA beyond stenosis alone.” Also not performed was “plaque quantification on coronary CTA, an emerging field of study.”
The editorialists noted that noncontrast CT as used in the study for CAC scoring “is generally not recommended as a standalone test in symptomatic patients. Most studies have shown that coronary CTA, a test that accurately detects stenosis and identifies all types of coronary atherosclerosis (calcified and noncalcified), has significantly higher diagnostic and prognostic accuracy than CAC when performed in symptomatic patients without known coronary artery disease.”
Dr. Mortensen has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Villines and Dr. Rodriguez Lozano have disclosed no relevant financial relationships. Dr. Zadeh disclosed receiving grant support from Canon Medical Systems.
A version of this article originally appeared on Medscape.com.
This month in the journal CHEST®
Editor’s picks
Power Outage: An Ignored Risk Factor for Chronic Obstructive Pulmonary Disease ExacerbationsBy Dr. Wangjian Zhang, et al.
PROPHETIC: Prospective Identification of Pneumonia in Hospitalized Patients in the ICU By Dr. Stephen P. Bergin, et al.
Chronic Beryllium Disease: Update on a Moving Target By Dr. Maeve MacMurdo, et al.
Development of Learning Curves for Bronchoscopy: Results of a Multicenter Study of Pulmonary Trainees By Dr. Nha Voduc, et al.
Bias and Racism Teaching Rounds at an Academic Medical Center By Dr. Quinn Capers, IV, et al.
Editor’s picks
Editor’s picks
Power Outage: An Ignored Risk Factor for Chronic Obstructive Pulmonary Disease ExacerbationsBy Dr. Wangjian Zhang, et al.
PROPHETIC: Prospective Identification of Pneumonia in Hospitalized Patients in the ICU By Dr. Stephen P. Bergin, et al.
Chronic Beryllium Disease: Update on a Moving Target By Dr. Maeve MacMurdo, et al.
Development of Learning Curves for Bronchoscopy: Results of a Multicenter Study of Pulmonary Trainees By Dr. Nha Voduc, et al.
Bias and Racism Teaching Rounds at an Academic Medical Center By Dr. Quinn Capers, IV, et al.
Power Outage: An Ignored Risk Factor for Chronic Obstructive Pulmonary Disease ExacerbationsBy Dr. Wangjian Zhang, et al.
PROPHETIC: Prospective Identification of Pneumonia in Hospitalized Patients in the ICU By Dr. Stephen P. Bergin, et al.
Chronic Beryllium Disease: Update on a Moving Target By Dr. Maeve MacMurdo, et al.
Development of Learning Curves for Bronchoscopy: Results of a Multicenter Study of Pulmonary Trainees By Dr. Nha Voduc, et al.
Bias and Racism Teaching Rounds at an Academic Medical Center By Dr. Quinn Capers, IV, et al.
Upper GI bleeds in COVID-19 not related to increased mortality
A Spanish survey of COVID-19 patients suggests that upper gastrointestinal bleeding (UGB) does not affect in-hospital mortality. It also found that fewer COVID-19–positive patients underwent endoscopies, but there was no statistically significant difference in in-hospital mortality outcome as a result of delays.
“In-hospital mortality in COVID-19 patients with upper-GI bleeding seemed to be more influenced by COVID-19 than by upper-GI bleeding, and that’s something I think is important for us to know,” Gyanprakash Ketwaroo, MD, associate professor at Baylor College of Medicine, Houston, said in an interview. Dr. Ketwaroo was not involved in the study.
The results weren’t a surprise, but they do provide some reassurance. “It’s probably what I expected. Initially, we thought there might be some COVID-19 related (GI) lesions, but that didn’t seem to be borne out. So we thought the bleeding was related to (the patient) being in a hospital or the typical reasons for bleeding. It’s also what I expected that less endoscopies would be performed in these patients, and even though fewer endoscopies were performed, the outcomes were still similar. I think it’s what most people expected,” said Dr. Ketwaroo.
The study was published online Nov. 25 in the Journal of Clinical Gastroenterology, and led by Rebeca González González, MD, of Severo Ochoa University Hospital in Leganés, Madrid, and Pascual Piñera-Salmerón, MD, of Reina Sofia University General Hospital in Murcia, Spain. The researchers retrospectively analyzed data on 71,904 COVID-19 patients at 62 emergency departments in Spain, and compared 83 patients who had COVID-19 and UGB to two control groups: 249 randomly selected COVID-19 patients without UGB, and 249 randomly selected non-COVID-19 patients with UGB.
They found that 1.11% of COVID-19 patients presented with UGB, compared with 1.78% of non-COVID-19 patients at emergency departments. In patients with COVID-19, risk of UGB was associated with hemoglobin values < 10 g/L (odds ratio [OR], 34.255, 95% confidence interval [CI], 12.752-92.021), abdominal pain (OR, 11.4; 95% CI, 5.092-25.944), and systolic blood pressure < 90 mm Hg (OR, 11.096; 95% CI, 2.975-41.390).
Compared with non-COVID-19 patients with UGB, those COVID-19 patients with UGB were more likely to have interstitial lung infiltrates (OR, 66.42; 95% CI, 15.364-287.223) and ground-glass opacities (OR, 21.27; 95% CI, 9.720-46.567) in chest radiograph, as well as fever (OR, 34.67; 95% CI, 11.719-102.572) and cough (OR, 26.4; 95% CI, 8.845-78.806).
Gastroscopy and endoscopic procedures were lower in patients with COVID-19 than in the general population (gastroscopy OR, 0.269; 95% CI, 0.160-0.453: endoscopy OR, 0.26; 95% CI, 0.165-0.623). There was no difference between the two groups with respect to endoscopic findings. After adjustment for age and sex, the only significant difference between COVID-19 patients with UGB and COVID-19 patients without UGB was a higher rate of intensive care unit admission (OR, 2.98; 95% CI, 1.16-7.65). Differences between COVID-19 patients with UGB and non–COVID-19 patients with UGB included higher rates of ICU admission (OR, 3.29; 95% CI, 1.28-8.47), prolonged hospitalizations (OR, 2.02; 95% CI, 1.15-3.55), and in-hospital mortality (OR, 2.05; 95% CI, 1.09-3.86).
UGB development was not associated with increased in-hospital mortality in COVID-19 patients (OR, 1.14; 95% CI, 0.59-2.19).
A limitation to the study it was that it was performed in Spain, where endoscopies are performed in the emergency department, and where there are different thresholds for admission to the intensive care unit than in the United States.
The authors did not report a funding source. Dr. Ketwaroo has no relevant financial disclosures.
SOURCE: González González R et al. J Clin Gastroenterol. 10.1097/MCG.0000000000001465.
A Spanish survey of COVID-19 patients suggests that upper gastrointestinal bleeding (UGB) does not affect in-hospital mortality. It also found that fewer COVID-19–positive patients underwent endoscopies, but there was no statistically significant difference in in-hospital mortality outcome as a result of delays.
“In-hospital mortality in COVID-19 patients with upper-GI bleeding seemed to be more influenced by COVID-19 than by upper-GI bleeding, and that’s something I think is important for us to know,” Gyanprakash Ketwaroo, MD, associate professor at Baylor College of Medicine, Houston, said in an interview. Dr. Ketwaroo was not involved in the study.
The results weren’t a surprise, but they do provide some reassurance. “It’s probably what I expected. Initially, we thought there might be some COVID-19 related (GI) lesions, but that didn’t seem to be borne out. So we thought the bleeding was related to (the patient) being in a hospital or the typical reasons for bleeding. It’s also what I expected that less endoscopies would be performed in these patients, and even though fewer endoscopies were performed, the outcomes were still similar. I think it’s what most people expected,” said Dr. Ketwaroo.
The study was published online Nov. 25 in the Journal of Clinical Gastroenterology, and led by Rebeca González González, MD, of Severo Ochoa University Hospital in Leganés, Madrid, and Pascual Piñera-Salmerón, MD, of Reina Sofia University General Hospital in Murcia, Spain. The researchers retrospectively analyzed data on 71,904 COVID-19 patients at 62 emergency departments in Spain, and compared 83 patients who had COVID-19 and UGB to two control groups: 249 randomly selected COVID-19 patients without UGB, and 249 randomly selected non-COVID-19 patients with UGB.
They found that 1.11% of COVID-19 patients presented with UGB, compared with 1.78% of non-COVID-19 patients at emergency departments. In patients with COVID-19, risk of UGB was associated with hemoglobin values < 10 g/L (odds ratio [OR], 34.255, 95% confidence interval [CI], 12.752-92.021), abdominal pain (OR, 11.4; 95% CI, 5.092-25.944), and systolic blood pressure < 90 mm Hg (OR, 11.096; 95% CI, 2.975-41.390).
Compared with non-COVID-19 patients with UGB, those COVID-19 patients with UGB were more likely to have interstitial lung infiltrates (OR, 66.42; 95% CI, 15.364-287.223) and ground-glass opacities (OR, 21.27; 95% CI, 9.720-46.567) in chest radiograph, as well as fever (OR, 34.67; 95% CI, 11.719-102.572) and cough (OR, 26.4; 95% CI, 8.845-78.806).
Gastroscopy and endoscopic procedures were lower in patients with COVID-19 than in the general population (gastroscopy OR, 0.269; 95% CI, 0.160-0.453: endoscopy OR, 0.26; 95% CI, 0.165-0.623). There was no difference between the two groups with respect to endoscopic findings. After adjustment for age and sex, the only significant difference between COVID-19 patients with UGB and COVID-19 patients without UGB was a higher rate of intensive care unit admission (OR, 2.98; 95% CI, 1.16-7.65). Differences between COVID-19 patients with UGB and non–COVID-19 patients with UGB included higher rates of ICU admission (OR, 3.29; 95% CI, 1.28-8.47), prolonged hospitalizations (OR, 2.02; 95% CI, 1.15-3.55), and in-hospital mortality (OR, 2.05; 95% CI, 1.09-3.86).
UGB development was not associated with increased in-hospital mortality in COVID-19 patients (OR, 1.14; 95% CI, 0.59-2.19).
A limitation to the study it was that it was performed in Spain, where endoscopies are performed in the emergency department, and where there are different thresholds for admission to the intensive care unit than in the United States.
The authors did not report a funding source. Dr. Ketwaroo has no relevant financial disclosures.
SOURCE: González González R et al. J Clin Gastroenterol. 10.1097/MCG.0000000000001465.
A Spanish survey of COVID-19 patients suggests that upper gastrointestinal bleeding (UGB) does not affect in-hospital mortality. It also found that fewer COVID-19–positive patients underwent endoscopies, but there was no statistically significant difference in in-hospital mortality outcome as a result of delays.
“In-hospital mortality in COVID-19 patients with upper-GI bleeding seemed to be more influenced by COVID-19 than by upper-GI bleeding, and that’s something I think is important for us to know,” Gyanprakash Ketwaroo, MD, associate professor at Baylor College of Medicine, Houston, said in an interview. Dr. Ketwaroo was not involved in the study.
The results weren’t a surprise, but they do provide some reassurance. “It’s probably what I expected. Initially, we thought there might be some COVID-19 related (GI) lesions, but that didn’t seem to be borne out. So we thought the bleeding was related to (the patient) being in a hospital or the typical reasons for bleeding. It’s also what I expected that less endoscopies would be performed in these patients, and even though fewer endoscopies were performed, the outcomes were still similar. I think it’s what most people expected,” said Dr. Ketwaroo.
The study was published online Nov. 25 in the Journal of Clinical Gastroenterology, and led by Rebeca González González, MD, of Severo Ochoa University Hospital in Leganés, Madrid, and Pascual Piñera-Salmerón, MD, of Reina Sofia University General Hospital in Murcia, Spain. The researchers retrospectively analyzed data on 71,904 COVID-19 patients at 62 emergency departments in Spain, and compared 83 patients who had COVID-19 and UGB to two control groups: 249 randomly selected COVID-19 patients without UGB, and 249 randomly selected non-COVID-19 patients with UGB.
They found that 1.11% of COVID-19 patients presented with UGB, compared with 1.78% of non-COVID-19 patients at emergency departments. In patients with COVID-19, risk of UGB was associated with hemoglobin values < 10 g/L (odds ratio [OR], 34.255, 95% confidence interval [CI], 12.752-92.021), abdominal pain (OR, 11.4; 95% CI, 5.092-25.944), and systolic blood pressure < 90 mm Hg (OR, 11.096; 95% CI, 2.975-41.390).
Compared with non-COVID-19 patients with UGB, those COVID-19 patients with UGB were more likely to have interstitial lung infiltrates (OR, 66.42; 95% CI, 15.364-287.223) and ground-glass opacities (OR, 21.27; 95% CI, 9.720-46.567) in chest radiograph, as well as fever (OR, 34.67; 95% CI, 11.719-102.572) and cough (OR, 26.4; 95% CI, 8.845-78.806).
Gastroscopy and endoscopic procedures were lower in patients with COVID-19 than in the general population (gastroscopy OR, 0.269; 95% CI, 0.160-0.453: endoscopy OR, 0.26; 95% CI, 0.165-0.623). There was no difference between the two groups with respect to endoscopic findings. After adjustment for age and sex, the only significant difference between COVID-19 patients with UGB and COVID-19 patients without UGB was a higher rate of intensive care unit admission (OR, 2.98; 95% CI, 1.16-7.65). Differences between COVID-19 patients with UGB and non–COVID-19 patients with UGB included higher rates of ICU admission (OR, 3.29; 95% CI, 1.28-8.47), prolonged hospitalizations (OR, 2.02; 95% CI, 1.15-3.55), and in-hospital mortality (OR, 2.05; 95% CI, 1.09-3.86).
UGB development was not associated with increased in-hospital mortality in COVID-19 patients (OR, 1.14; 95% CI, 0.59-2.19).
A limitation to the study it was that it was performed in Spain, where endoscopies are performed in the emergency department, and where there are different thresholds for admission to the intensive care unit than in the United States.
The authors did not report a funding source. Dr. Ketwaroo has no relevant financial disclosures.
SOURCE: González González R et al. J Clin Gastroenterol. 10.1097/MCG.0000000000001465.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Medical societies waive fees, weigh other options during pandemic
COVID-19’s toll on member facilities pushed the American Academy of Sleep Medicine (AASM) recently to take a sizable gamble.
AASM announced in September that it would waive facility fees at all 2,648 AASM-accredited sleep facilities for 2021.
At $1,800-$2,600 for each facility, that will mean lost revenue of between $4.8 million and $6.9 million, but it’s a risk the academy felt it had to take.
AASM President Kannan Ramar, MBBS, MD, said in an interview that they are betting on the future of the field.
An internal survey of members, he said, found that nearly half (46%) of the 551 respondents thought they might have to close by the end of the year.
In addition, 66% reported a lower patient volume in the past month, and 36% reported that their practice or facility had to apply for loans or other financial assistance because of COVID-19, AASM said in its press release.
“We are hoping that if we help our members through this, they will be there for our patients,” Dr. Ramar said.
Other medical societies also are weighing options, straddling the line between needing income to provide resources for members but being acutely aware of the financial toll the pandemic is taking, according to one sampling.
As previously reported, primary care practices are projected to lose more than $68,000 in revenue per full-time physician in 2020, after steep drops in office visits and the collection of fees from March to May, according to a study led by researchers in the Blavatnik Institute at Harvard Medical School, Boston.
Those losses were calculated without considering a potential second wave of COVID-19 this year, the authors noted.
‘We can survive this’
Although AASM waived fees for its member facilities, individual physician fees have not been reduced so far. But the group is looking for more ways to help lower the economic burden on members, Dr. Ramar said.
“I don’t think we’ve ever been in this situation in the 45 years of the academy. This is a once-in-a-lifetime event for challenges we’re going through,” he said. “The board and the leadership realized that, if we’re going to do something, this is the time to do it.”
In addition to waiving the fees, AASM and the AASM Foundation are offering relief funding to state and regional sleep societies and research award recipients through programs created in response to COVID-19.
Some societies said they are not making changes to their dues or fees, some are forgoing cost-of-living fee increases, and some are waiving registration fees for annual meetings.
The American College of Allergy, Asthma and Immunology (ACAAI) waived most members’ registration fees for its annual meeting in November. Typically, that fee would be $500-$800 per member, plus charges for some premium sessions, Michael Blaiss, MD, ACAAI executive medical director, said.
Dr. Blaiss said in an interview that the college thought offering its 6,000 members essentially 25 free hours of CME would benefit them more than waiving annual membership dues, which are about $425 for physicians in the United States.
If the pandemic stretches through 2021, Dr. Blaiss said, “We can survive this. I’m not worried about that at all.”
But he acknowledged the painful effect on medical societies.
“I don’t think any organization would tell you it’s not having an effect on their income,” he said. “I know it is for us and for virtually any medical organization. A high percentage of income comes from the annual meeting.”
Waiving dues has not been a high priority among members in communications so far, Blaiss said.
American Academy of Dermatology President Bruce H. Thiers, MD, said in an interview that there will be no cost-of-living increase for 2021 dues, and AAD members can request a reduction in dues, which will be considered on a case-by-case basis.
“We understand that many members will have to make tough financial decisions,” he said.
In addition, AAD, which has more than 20,000 members, is exploring payment options to help members spread out the cost of membership.
ACP extends membership
The American College of Physicians, whose membership cycle starts in July, did not reduce dues but extended membership at no cost for 3 months through September to its 163,000 members, Phil Masters, MD, ACP’s vice president of membership, said in an interview.
It also expanded its educational offerings related to the pandemic, including webinars on physician wellness and issues regarding telemedicine.
He said expanding educational resources rather than waiving dues was an intentional decision after much discussion because “we’re primarily a services resource organization.”
Membership data are still being calculated, but early indications are that membership is not increasing this year, after seeing annual growth of about 2%-2.5%, Dr. Masters said. He noted that income is down “by several percent.” Annual membership dues average about $500 for physicians who have been practicing for 10 years.
“We’re well positioned to tolerate the ups and downs,” he said, but he acknowledged that “there’s no question the financial impact has been devastating on some practices.”
Like some other associations, ACP decided to cancel this year’s annual meeting, which had been planned for April. The 2021 annual meeting will be conducted online from April 29 to May 1.
Smaller organizations that rely heavily on income from the annual meeting will be severely challenged the longer the pandemic continues, Dr. Masters said.
The decision is not as simple as whether to reduce or eliminate dues, he noted. Organizations will have to reexamine their missions and structure their fees and offerings according to the needs of members.
“It’s a balance in doing things for the community at large and balancing the need to be sensitive to financial implications,” Dr. Masters said.
This article first appeared on Medscape.com.
COVID-19’s toll on member facilities pushed the American Academy of Sleep Medicine (AASM) recently to take a sizable gamble.
AASM announced in September that it would waive facility fees at all 2,648 AASM-accredited sleep facilities for 2021.
At $1,800-$2,600 for each facility, that will mean lost revenue of between $4.8 million and $6.9 million, but it’s a risk the academy felt it had to take.
AASM President Kannan Ramar, MBBS, MD, said in an interview that they are betting on the future of the field.
An internal survey of members, he said, found that nearly half (46%) of the 551 respondents thought they might have to close by the end of the year.
In addition, 66% reported a lower patient volume in the past month, and 36% reported that their practice or facility had to apply for loans or other financial assistance because of COVID-19, AASM said in its press release.
“We are hoping that if we help our members through this, they will be there for our patients,” Dr. Ramar said.
Other medical societies also are weighing options, straddling the line between needing income to provide resources for members but being acutely aware of the financial toll the pandemic is taking, according to one sampling.
As previously reported, primary care practices are projected to lose more than $68,000 in revenue per full-time physician in 2020, after steep drops in office visits and the collection of fees from March to May, according to a study led by researchers in the Blavatnik Institute at Harvard Medical School, Boston.
Those losses were calculated without considering a potential second wave of COVID-19 this year, the authors noted.
‘We can survive this’
Although AASM waived fees for its member facilities, individual physician fees have not been reduced so far. But the group is looking for more ways to help lower the economic burden on members, Dr. Ramar said.
“I don’t think we’ve ever been in this situation in the 45 years of the academy. This is a once-in-a-lifetime event for challenges we’re going through,” he said. “The board and the leadership realized that, if we’re going to do something, this is the time to do it.”
In addition to waiving the fees, AASM and the AASM Foundation are offering relief funding to state and regional sleep societies and research award recipients through programs created in response to COVID-19.
Some societies said they are not making changes to their dues or fees, some are forgoing cost-of-living fee increases, and some are waiving registration fees for annual meetings.
The American College of Allergy, Asthma and Immunology (ACAAI) waived most members’ registration fees for its annual meeting in November. Typically, that fee would be $500-$800 per member, plus charges for some premium sessions, Michael Blaiss, MD, ACAAI executive medical director, said.
Dr. Blaiss said in an interview that the college thought offering its 6,000 members essentially 25 free hours of CME would benefit them more than waiving annual membership dues, which are about $425 for physicians in the United States.
If the pandemic stretches through 2021, Dr. Blaiss said, “We can survive this. I’m not worried about that at all.”
But he acknowledged the painful effect on medical societies.
“I don’t think any organization would tell you it’s not having an effect on their income,” he said. “I know it is for us and for virtually any medical organization. A high percentage of income comes from the annual meeting.”
Waiving dues has not been a high priority among members in communications so far, Blaiss said.
American Academy of Dermatology President Bruce H. Thiers, MD, said in an interview that there will be no cost-of-living increase for 2021 dues, and AAD members can request a reduction in dues, which will be considered on a case-by-case basis.
“We understand that many members will have to make tough financial decisions,” he said.
In addition, AAD, which has more than 20,000 members, is exploring payment options to help members spread out the cost of membership.
ACP extends membership
The American College of Physicians, whose membership cycle starts in July, did not reduce dues but extended membership at no cost for 3 months through September to its 163,000 members, Phil Masters, MD, ACP’s vice president of membership, said in an interview.
It also expanded its educational offerings related to the pandemic, including webinars on physician wellness and issues regarding telemedicine.
He said expanding educational resources rather than waiving dues was an intentional decision after much discussion because “we’re primarily a services resource organization.”
Membership data are still being calculated, but early indications are that membership is not increasing this year, after seeing annual growth of about 2%-2.5%, Dr. Masters said. He noted that income is down “by several percent.” Annual membership dues average about $500 for physicians who have been practicing for 10 years.
“We’re well positioned to tolerate the ups and downs,” he said, but he acknowledged that “there’s no question the financial impact has been devastating on some practices.”
Like some other associations, ACP decided to cancel this year’s annual meeting, which had been planned for April. The 2021 annual meeting will be conducted online from April 29 to May 1.
Smaller organizations that rely heavily on income from the annual meeting will be severely challenged the longer the pandemic continues, Dr. Masters said.
The decision is not as simple as whether to reduce or eliminate dues, he noted. Organizations will have to reexamine their missions and structure their fees and offerings according to the needs of members.
“It’s a balance in doing things for the community at large and balancing the need to be sensitive to financial implications,” Dr. Masters said.
This article first appeared on Medscape.com.
COVID-19’s toll on member facilities pushed the American Academy of Sleep Medicine (AASM) recently to take a sizable gamble.
AASM announced in September that it would waive facility fees at all 2,648 AASM-accredited sleep facilities for 2021.
At $1,800-$2,600 for each facility, that will mean lost revenue of between $4.8 million and $6.9 million, but it’s a risk the academy felt it had to take.
AASM President Kannan Ramar, MBBS, MD, said in an interview that they are betting on the future of the field.
An internal survey of members, he said, found that nearly half (46%) of the 551 respondents thought they might have to close by the end of the year.
In addition, 66% reported a lower patient volume in the past month, and 36% reported that their practice or facility had to apply for loans or other financial assistance because of COVID-19, AASM said in its press release.
“We are hoping that if we help our members through this, they will be there for our patients,” Dr. Ramar said.
Other medical societies also are weighing options, straddling the line between needing income to provide resources for members but being acutely aware of the financial toll the pandemic is taking, according to one sampling.
As previously reported, primary care practices are projected to lose more than $68,000 in revenue per full-time physician in 2020, after steep drops in office visits and the collection of fees from March to May, according to a study led by researchers in the Blavatnik Institute at Harvard Medical School, Boston.
Those losses were calculated without considering a potential second wave of COVID-19 this year, the authors noted.
‘We can survive this’
Although AASM waived fees for its member facilities, individual physician fees have not been reduced so far. But the group is looking for more ways to help lower the economic burden on members, Dr. Ramar said.
“I don’t think we’ve ever been in this situation in the 45 years of the academy. This is a once-in-a-lifetime event for challenges we’re going through,” he said. “The board and the leadership realized that, if we’re going to do something, this is the time to do it.”
In addition to waiving the fees, AASM and the AASM Foundation are offering relief funding to state and regional sleep societies and research award recipients through programs created in response to COVID-19.
Some societies said they are not making changes to their dues or fees, some are forgoing cost-of-living fee increases, and some are waiving registration fees for annual meetings.
The American College of Allergy, Asthma and Immunology (ACAAI) waived most members’ registration fees for its annual meeting in November. Typically, that fee would be $500-$800 per member, plus charges for some premium sessions, Michael Blaiss, MD, ACAAI executive medical director, said.
Dr. Blaiss said in an interview that the college thought offering its 6,000 members essentially 25 free hours of CME would benefit them more than waiving annual membership dues, which are about $425 for physicians in the United States.
If the pandemic stretches through 2021, Dr. Blaiss said, “We can survive this. I’m not worried about that at all.”
But he acknowledged the painful effect on medical societies.
“I don’t think any organization would tell you it’s not having an effect on their income,” he said. “I know it is for us and for virtually any medical organization. A high percentage of income comes from the annual meeting.”
Waiving dues has not been a high priority among members in communications so far, Blaiss said.
American Academy of Dermatology President Bruce H. Thiers, MD, said in an interview that there will be no cost-of-living increase for 2021 dues, and AAD members can request a reduction in dues, which will be considered on a case-by-case basis.
“We understand that many members will have to make tough financial decisions,” he said.
In addition, AAD, which has more than 20,000 members, is exploring payment options to help members spread out the cost of membership.
ACP extends membership
The American College of Physicians, whose membership cycle starts in July, did not reduce dues but extended membership at no cost for 3 months through September to its 163,000 members, Phil Masters, MD, ACP’s vice president of membership, said in an interview.
It also expanded its educational offerings related to the pandemic, including webinars on physician wellness and issues regarding telemedicine.
He said expanding educational resources rather than waiving dues was an intentional decision after much discussion because “we’re primarily a services resource organization.”
Membership data are still being calculated, but early indications are that membership is not increasing this year, after seeing annual growth of about 2%-2.5%, Dr. Masters said. He noted that income is down “by several percent.” Annual membership dues average about $500 for physicians who have been practicing for 10 years.
“We’re well positioned to tolerate the ups and downs,” he said, but he acknowledged that “there’s no question the financial impact has been devastating on some practices.”
Like some other associations, ACP decided to cancel this year’s annual meeting, which had been planned for April. The 2021 annual meeting will be conducted online from April 29 to May 1.
Smaller organizations that rely heavily on income from the annual meeting will be severely challenged the longer the pandemic continues, Dr. Masters said.
The decision is not as simple as whether to reduce or eliminate dues, he noted. Organizations will have to reexamine their missions and structure their fees and offerings according to the needs of members.
“It’s a balance in doing things for the community at large and balancing the need to be sensitive to financial implications,” Dr. Masters said.
This article first appeared on Medscape.com.
Prioritize COVID-19 vaccination in both types of diabetes, say docs
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FDA safety alert: Face masks with metal can burn during MRI
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
Demand for COVID vaccines expected to get heated – and fast
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
New child COVID-19 cases down in last weekly count
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 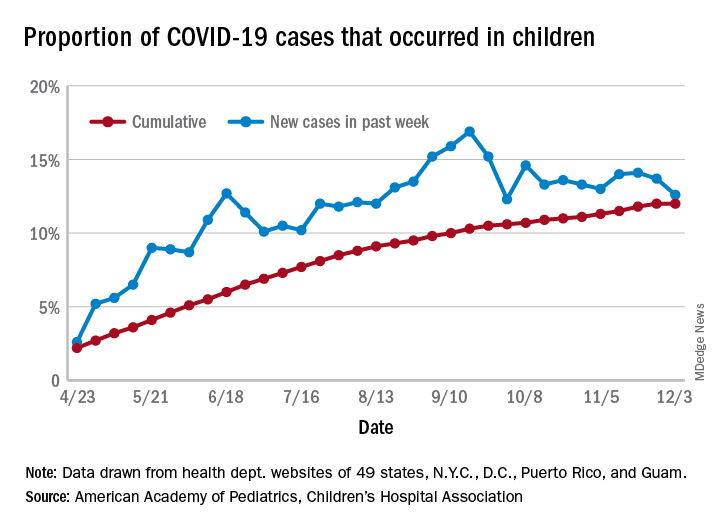
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
New residency matching sets record, says NRMP
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.

