User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Avoiding excess oxygen in mechanically ventilated patients ‘seems sensible’
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
FROM SHM CONVERGE 2021
DOJ charges 14 with COVID-19–related fraud nearing $150M
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
Children aged 12-15 years continue to close COVID-19 vaccination gap
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.
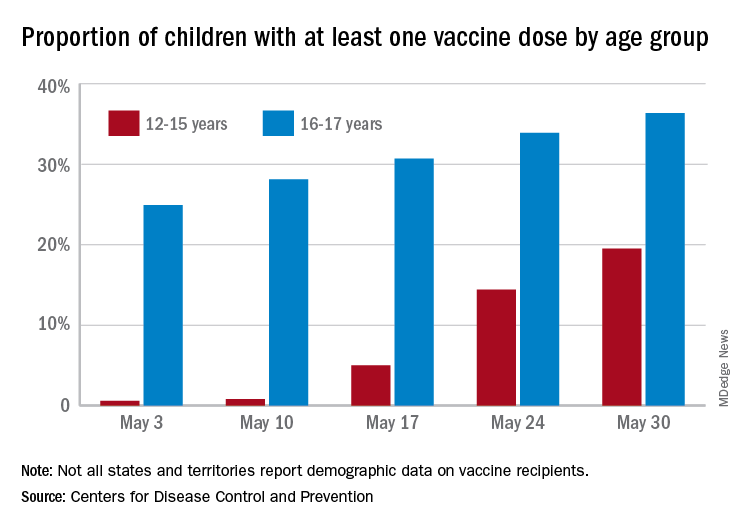
with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
Drug combo disappoints as second-line therapy for advanced NSCLC with EGFR and T790M mutations
Adding bevacizumab (Avastin) to second-line osimertinib (Tagrisso) provided no overall benefit versus osimertinib alone for advanced non–small cell lung cancer with epidermal growth factor receptor (EGFR) and T790M mutations in the randomized, open-label, phase 2 European Thoracic Oncology Platform (ETOP) BOOSTER trial.
The combination treatment did, however, show superiority over osimertinib alone in current and former smokers in the study, say the investigators.
“The use of osimertinib and bevacizumab was associated with longer progression-free survival in the subgroup of patients who were former or current smokers [hazard ratio, 0.57],” Ross Soo, MD, reported during a European Society of Medical Oncology virtual plenary session.
The findings were also published May 12, 2021, in Annals of Oncology.
Osimertinib, a third-generation EGFR tyrosine kinase inhibitor (TKI) with selective activity toward EGFR-sensitizing and T790M resistance mutations, is the standard treatment in this patient population, but progression inevitably occurs.
Based on preclinical studies suggesting that the angiogenic pathway is implicated in EGFR TKI resistance, the current study was designed to assess the efficacy and safety of combined osimertinib and the antiangiogenic agent bevacizumab versus osimertinib alone in patients who progressed on prior EGFR TKI therapy, explained Dr. Soo, a senior consultant in the department of hematology-oncology at the National University Cancer Institute, Singapore.
Median overall progression-free survival (PFS) at a median follow-up of 34 months was 15.4 months versus 12.3 months in 78 patients in the bevacizumab/osimertinib combination therapy group and 77 patients in the osimertinib monotherapy group, respectively – which translated into a nonstatistically significant difference (HR, 0.96).
In the current and former smoker subgroup, median PFS was 16.5 months and 8.4 months with combination versus monotherapy, respectively (HR, .57), Dr. Soo said.
An exploratory analysis showed that the effect of the combination therapy was statistically different in current/former smokers versus never-smokers (HR, 0.52 and 1.47, respectively), he noted.
For the secondary study endpoint of overall survival (OS), no significant difference was seen overall with the combination versus monotherapy (24.0 vs. 24.3 months; HR, 1.03) or the current or former smoker subgroup (HR, 0.54).
However, in the current and former smoker subgroup, the effect of the treatment combination “was in the same direction and similar in magnitude to progression-free survival, but did not reach statistical significance,” Dr. Soo noted.
The exploratory analysis showed OS HRs of 0.59 and 1.54 in the current/former smokers versus never-smokers, respectively.
Smoking data may be important
Study participants were adults with a median age of 67 years who had exon 19 del or L858R and T790M mutation at progression on prior EGFR TKI therapy. Most (62%) were women and 40% were current or former smokers. They were enrolled between 2017 and 2019 from 22 centers in six countries and randomly assigned to receive bevacizumab at a dose of 15 mg/kg intravenously on day 1 every 3 weeks plus osimertinib at 80 mg daily or osimertinib alone.
The median time to treatment failure was 8.2 months in the combination therapy, (with TTF of 8.2 months for bevacizumab and 12.4 for osimertinib), compared with 10.8 months for osimertinib monotherapy.
Overall response was 55% in both groups, and disease control rates were 90% and 82% in the groups, respectively. Median duration of response was 14.5 months versus 16.6 months, Dr. Soo said.
Grade 3 or greater treatment-related adverse events occurred in 47% and 18% of patients in the combination and monotherapy groups. The most frequent adverse event in both groups was diarrhea. Proteinuria and hypertension occurred more often in the combination-therapy group.
Based on these findings, osimertinib remains the standard of care in patients with advanced NSCLC with acquired EGFR TKI resistance harboring EGFR T790M mutations, he concluded.
The findings are in line with those from prior smaller studies, and are “hypothesis generating,” said invited discussant Edward B. Garon, MD, professor and director of the thoracic oncology program at the University of California, Los Angeles.
The new data are hypothesis generating and will help in analyzing other studies to determine whether there is a difference based on smoking history, he said.
Dr. Garon also noted that there has been increasing interest in similar combination approaches in the frontline setting, but to date there is little to support frontline use.
“It is certainly a situation where there is room for studies exploring other approaches in the frontline setting,” he concluded.
This study was supported by Astra Zeneca and Roche. Dr. Soo reported financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, Merck, Novartis, Otsuka, Pfizer, Roche, Synthorx, Taiho, Takeda, and Yuhan. Dr. Garon reported relationships with ABL-Bio, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Dracen Pharmaceuticals, Dynavax Technologies, Eli Lilly, EMD Serono, Eisai, Genentech, GlaxoSmithKline, Iovance Biotherapeutics, Merck, Mirati Therapeutics, Natera, Neon, Novartis, Regeneron, Sanofi, Shionogi, and Xilio.
A version of this article first appeared on Medscape.com.
Adding bevacizumab (Avastin) to second-line osimertinib (Tagrisso) provided no overall benefit versus osimertinib alone for advanced non–small cell lung cancer with epidermal growth factor receptor (EGFR) and T790M mutations in the randomized, open-label, phase 2 European Thoracic Oncology Platform (ETOP) BOOSTER trial.
The combination treatment did, however, show superiority over osimertinib alone in current and former smokers in the study, say the investigators.
“The use of osimertinib and bevacizumab was associated with longer progression-free survival in the subgroup of patients who were former or current smokers [hazard ratio, 0.57],” Ross Soo, MD, reported during a European Society of Medical Oncology virtual plenary session.
The findings were also published May 12, 2021, in Annals of Oncology.
Osimertinib, a third-generation EGFR tyrosine kinase inhibitor (TKI) with selective activity toward EGFR-sensitizing and T790M resistance mutations, is the standard treatment in this patient population, but progression inevitably occurs.
Based on preclinical studies suggesting that the angiogenic pathway is implicated in EGFR TKI resistance, the current study was designed to assess the efficacy and safety of combined osimertinib and the antiangiogenic agent bevacizumab versus osimertinib alone in patients who progressed on prior EGFR TKI therapy, explained Dr. Soo, a senior consultant in the department of hematology-oncology at the National University Cancer Institute, Singapore.
Median overall progression-free survival (PFS) at a median follow-up of 34 months was 15.4 months versus 12.3 months in 78 patients in the bevacizumab/osimertinib combination therapy group and 77 patients in the osimertinib monotherapy group, respectively – which translated into a nonstatistically significant difference (HR, 0.96).
In the current and former smoker subgroup, median PFS was 16.5 months and 8.4 months with combination versus monotherapy, respectively (HR, .57), Dr. Soo said.
An exploratory analysis showed that the effect of the combination therapy was statistically different in current/former smokers versus never-smokers (HR, 0.52 and 1.47, respectively), he noted.
For the secondary study endpoint of overall survival (OS), no significant difference was seen overall with the combination versus monotherapy (24.0 vs. 24.3 months; HR, 1.03) or the current or former smoker subgroup (HR, 0.54).
However, in the current and former smoker subgroup, the effect of the treatment combination “was in the same direction and similar in magnitude to progression-free survival, but did not reach statistical significance,” Dr. Soo noted.
The exploratory analysis showed OS HRs of 0.59 and 1.54 in the current/former smokers versus never-smokers, respectively.
Smoking data may be important
Study participants were adults with a median age of 67 years who had exon 19 del or L858R and T790M mutation at progression on prior EGFR TKI therapy. Most (62%) were women and 40% were current or former smokers. They were enrolled between 2017 and 2019 from 22 centers in six countries and randomly assigned to receive bevacizumab at a dose of 15 mg/kg intravenously on day 1 every 3 weeks plus osimertinib at 80 mg daily or osimertinib alone.
The median time to treatment failure was 8.2 months in the combination therapy, (with TTF of 8.2 months for bevacizumab and 12.4 for osimertinib), compared with 10.8 months for osimertinib monotherapy.
Overall response was 55% in both groups, and disease control rates were 90% and 82% in the groups, respectively. Median duration of response was 14.5 months versus 16.6 months, Dr. Soo said.
Grade 3 or greater treatment-related adverse events occurred in 47% and 18% of patients in the combination and monotherapy groups. The most frequent adverse event in both groups was diarrhea. Proteinuria and hypertension occurred more often in the combination-therapy group.
Based on these findings, osimertinib remains the standard of care in patients with advanced NSCLC with acquired EGFR TKI resistance harboring EGFR T790M mutations, he concluded.
The findings are in line with those from prior smaller studies, and are “hypothesis generating,” said invited discussant Edward B. Garon, MD, professor and director of the thoracic oncology program at the University of California, Los Angeles.
The new data are hypothesis generating and will help in analyzing other studies to determine whether there is a difference based on smoking history, he said.
Dr. Garon also noted that there has been increasing interest in similar combination approaches in the frontline setting, but to date there is little to support frontline use.
“It is certainly a situation where there is room for studies exploring other approaches in the frontline setting,” he concluded.
This study was supported by Astra Zeneca and Roche. Dr. Soo reported financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, Merck, Novartis, Otsuka, Pfizer, Roche, Synthorx, Taiho, Takeda, and Yuhan. Dr. Garon reported relationships with ABL-Bio, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Dracen Pharmaceuticals, Dynavax Technologies, Eli Lilly, EMD Serono, Eisai, Genentech, GlaxoSmithKline, Iovance Biotherapeutics, Merck, Mirati Therapeutics, Natera, Neon, Novartis, Regeneron, Sanofi, Shionogi, and Xilio.
A version of this article first appeared on Medscape.com.
Adding bevacizumab (Avastin) to second-line osimertinib (Tagrisso) provided no overall benefit versus osimertinib alone for advanced non–small cell lung cancer with epidermal growth factor receptor (EGFR) and T790M mutations in the randomized, open-label, phase 2 European Thoracic Oncology Platform (ETOP) BOOSTER trial.
The combination treatment did, however, show superiority over osimertinib alone in current and former smokers in the study, say the investigators.
“The use of osimertinib and bevacizumab was associated with longer progression-free survival in the subgroup of patients who were former or current smokers [hazard ratio, 0.57],” Ross Soo, MD, reported during a European Society of Medical Oncology virtual plenary session.
The findings were also published May 12, 2021, in Annals of Oncology.
Osimertinib, a third-generation EGFR tyrosine kinase inhibitor (TKI) with selective activity toward EGFR-sensitizing and T790M resistance mutations, is the standard treatment in this patient population, but progression inevitably occurs.
Based on preclinical studies suggesting that the angiogenic pathway is implicated in EGFR TKI resistance, the current study was designed to assess the efficacy and safety of combined osimertinib and the antiangiogenic agent bevacizumab versus osimertinib alone in patients who progressed on prior EGFR TKI therapy, explained Dr. Soo, a senior consultant in the department of hematology-oncology at the National University Cancer Institute, Singapore.
Median overall progression-free survival (PFS) at a median follow-up of 34 months was 15.4 months versus 12.3 months in 78 patients in the bevacizumab/osimertinib combination therapy group and 77 patients in the osimertinib monotherapy group, respectively – which translated into a nonstatistically significant difference (HR, 0.96).
In the current and former smoker subgroup, median PFS was 16.5 months and 8.4 months with combination versus monotherapy, respectively (HR, .57), Dr. Soo said.
An exploratory analysis showed that the effect of the combination therapy was statistically different in current/former smokers versus never-smokers (HR, 0.52 and 1.47, respectively), he noted.
For the secondary study endpoint of overall survival (OS), no significant difference was seen overall with the combination versus monotherapy (24.0 vs. 24.3 months; HR, 1.03) or the current or former smoker subgroup (HR, 0.54).
However, in the current and former smoker subgroup, the effect of the treatment combination “was in the same direction and similar in magnitude to progression-free survival, but did not reach statistical significance,” Dr. Soo noted.
The exploratory analysis showed OS HRs of 0.59 and 1.54 in the current/former smokers versus never-smokers, respectively.
Smoking data may be important
Study participants were adults with a median age of 67 years who had exon 19 del or L858R and T790M mutation at progression on prior EGFR TKI therapy. Most (62%) were women and 40% were current or former smokers. They were enrolled between 2017 and 2019 from 22 centers in six countries and randomly assigned to receive bevacizumab at a dose of 15 mg/kg intravenously on day 1 every 3 weeks plus osimertinib at 80 mg daily or osimertinib alone.
The median time to treatment failure was 8.2 months in the combination therapy, (with TTF of 8.2 months for bevacizumab and 12.4 for osimertinib), compared with 10.8 months for osimertinib monotherapy.
Overall response was 55% in both groups, and disease control rates were 90% and 82% in the groups, respectively. Median duration of response was 14.5 months versus 16.6 months, Dr. Soo said.
Grade 3 or greater treatment-related adverse events occurred in 47% and 18% of patients in the combination and monotherapy groups. The most frequent adverse event in both groups was diarrhea. Proteinuria and hypertension occurred more often in the combination-therapy group.
Based on these findings, osimertinib remains the standard of care in patients with advanced NSCLC with acquired EGFR TKI resistance harboring EGFR T790M mutations, he concluded.
The findings are in line with those from prior smaller studies, and are “hypothesis generating,” said invited discussant Edward B. Garon, MD, professor and director of the thoracic oncology program at the University of California, Los Angeles.
The new data are hypothesis generating and will help in analyzing other studies to determine whether there is a difference based on smoking history, he said.
Dr. Garon also noted that there has been increasing interest in similar combination approaches in the frontline setting, but to date there is little to support frontline use.
“It is certainly a situation where there is room for studies exploring other approaches in the frontline setting,” he concluded.
This study was supported by Astra Zeneca and Roche. Dr. Soo reported financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, Merck, Novartis, Otsuka, Pfizer, Roche, Synthorx, Taiho, Takeda, and Yuhan. Dr. Garon reported relationships with ABL-Bio, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Dracen Pharmaceuticals, Dynavax Technologies, Eli Lilly, EMD Serono, Eisai, Genentech, GlaxoSmithKline, Iovance Biotherapeutics, Merck, Mirati Therapeutics, Natera, Neon, Novartis, Regeneron, Sanofi, Shionogi, and Xilio.
A version of this article first appeared on Medscape.com.
First drug for lung cancer with KRAS mutation gains FDA approval
The first drug to target KRAS mutations in non–small cell lung cancer (NSCLC) has been approved by the Food and Drug Administration.
KRAS mutations are the most common mutations to occur in NSCLC tumors, accounting for about 25% of them, but for a long time they appeared to be resistant to drug therapy.
The new drug, sotorasib (Lumakras), specifically targets the KRAS G12C mutation, which accounts for about 13% of NSCLC mutations.
It is considered to be something of a breakthrough in cancer research. When clinical data on the new drug (from 126 patients) were presented last year at the World Conference on Lung Cancer, lung cancer experts greeted the results enthusiastically, as reported by Medscape Medical News at the time.
“This is a historic milestone in lung cancer therapy. After four decades of scientific efforts in targeting KRAS, sotorasib has potential to be the first targeted treatment option for this patient population with a high unmet need,” Bob T. Li, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the time.
Now, in a press release from the manufacturer, Amgen, he said: “Sotorasib represents a major advancement in oncology and changes the treatment paradigm for patients with KRAS G12C-mutated non–small cell lung cancer.
“Patients with non–small cell lung cancer who have progressed beyond first-line treatment face a poor prognosis and have limited treatment options available to them. Sotorasib delivers a new option for these patients, and it is the first KRAS-targeted therapy to be approved after nearly four decades of research,” he added.
Details of clinical data
This is an accelerated approval based on response rate data.
The FDA notes that the clinical data come from a study of 124 patients with locally advanced or metastatic KRAS G12C-mutated NSCLC with disease progression after receiving an immune checkpoint inhibitor and/or platinum-based chemotherapy.
The major outcome measured was overall response rate (ORR), which was 36%. Of the patients who responded, 58% had a duration of response of 6 months or longer.
Sotorasib was approved at a dose of 960 mg, and this dose was based on available clinical data as well as pharmacokinetic and pharmacodynamic modeling, the FDA noted. As part of the evaluation for this accelerated approval, the agency is requiring a postmarketing trial to investigate whether a lower dose will have a similar clinical effect.
The most common side effects include diarrhea, musculoskeletal pain, nausea, fatigue, liver damage, and cough. Sotorasib should not be used if patients develop symptoms of interstitial lung disease, and should be permanently discontinued if interstitial lung disease is confirmed.
Patients on sotorasib should have liver function tests prior to starting and while taking the drug; if liver damage develops, the drug should be stopped or the dose reduced. Patients should avoid taking acid-reducing agents, drugs that induce or are substrates for certain enzymes in the liver, and drugs that are substrates of P-glycoprotein (P-gp).
Companion diagnostic tests also approved
Along with the new drug, the FDA approved two companion diagnostic tests – the QIAGEN therascreen KRAS RGQ PCR kit (approval granted to QIAGEN GmbH) for analyzing tumor tissue and the Guardant360 CDx (approval granted to Guardant Health) for analyzing plasma specimens to determine if the KRAS G12C mutation is present. The agency notes that if the plasma test comes back negative, the patient’s tumor tissue should be tested.
A version of this article first appeared on Medscape.com.
The first drug to target KRAS mutations in non–small cell lung cancer (NSCLC) has been approved by the Food and Drug Administration.
KRAS mutations are the most common mutations to occur in NSCLC tumors, accounting for about 25% of them, but for a long time they appeared to be resistant to drug therapy.
The new drug, sotorasib (Lumakras), specifically targets the KRAS G12C mutation, which accounts for about 13% of NSCLC mutations.
It is considered to be something of a breakthrough in cancer research. When clinical data on the new drug (from 126 patients) were presented last year at the World Conference on Lung Cancer, lung cancer experts greeted the results enthusiastically, as reported by Medscape Medical News at the time.
“This is a historic milestone in lung cancer therapy. After four decades of scientific efforts in targeting KRAS, sotorasib has potential to be the first targeted treatment option for this patient population with a high unmet need,” Bob T. Li, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the time.
Now, in a press release from the manufacturer, Amgen, he said: “Sotorasib represents a major advancement in oncology and changes the treatment paradigm for patients with KRAS G12C-mutated non–small cell lung cancer.
“Patients with non–small cell lung cancer who have progressed beyond first-line treatment face a poor prognosis and have limited treatment options available to them. Sotorasib delivers a new option for these patients, and it is the first KRAS-targeted therapy to be approved after nearly four decades of research,” he added.
Details of clinical data
This is an accelerated approval based on response rate data.
The FDA notes that the clinical data come from a study of 124 patients with locally advanced or metastatic KRAS G12C-mutated NSCLC with disease progression after receiving an immune checkpoint inhibitor and/or platinum-based chemotherapy.
The major outcome measured was overall response rate (ORR), which was 36%. Of the patients who responded, 58% had a duration of response of 6 months or longer.
Sotorasib was approved at a dose of 960 mg, and this dose was based on available clinical data as well as pharmacokinetic and pharmacodynamic modeling, the FDA noted. As part of the evaluation for this accelerated approval, the agency is requiring a postmarketing trial to investigate whether a lower dose will have a similar clinical effect.
The most common side effects include diarrhea, musculoskeletal pain, nausea, fatigue, liver damage, and cough. Sotorasib should not be used if patients develop symptoms of interstitial lung disease, and should be permanently discontinued if interstitial lung disease is confirmed.
Patients on sotorasib should have liver function tests prior to starting and while taking the drug; if liver damage develops, the drug should be stopped or the dose reduced. Patients should avoid taking acid-reducing agents, drugs that induce or are substrates for certain enzymes in the liver, and drugs that are substrates of P-glycoprotein (P-gp).
Companion diagnostic tests also approved
Along with the new drug, the FDA approved two companion diagnostic tests – the QIAGEN therascreen KRAS RGQ PCR kit (approval granted to QIAGEN GmbH) for analyzing tumor tissue and the Guardant360 CDx (approval granted to Guardant Health) for analyzing plasma specimens to determine if the KRAS G12C mutation is present. The agency notes that if the plasma test comes back negative, the patient’s tumor tissue should be tested.
A version of this article first appeared on Medscape.com.
The first drug to target KRAS mutations in non–small cell lung cancer (NSCLC) has been approved by the Food and Drug Administration.
KRAS mutations are the most common mutations to occur in NSCLC tumors, accounting for about 25% of them, but for a long time they appeared to be resistant to drug therapy.
The new drug, sotorasib (Lumakras), specifically targets the KRAS G12C mutation, which accounts for about 13% of NSCLC mutations.
It is considered to be something of a breakthrough in cancer research. When clinical data on the new drug (from 126 patients) were presented last year at the World Conference on Lung Cancer, lung cancer experts greeted the results enthusiastically, as reported by Medscape Medical News at the time.
“This is a historic milestone in lung cancer therapy. After four decades of scientific efforts in targeting KRAS, sotorasib has potential to be the first targeted treatment option for this patient population with a high unmet need,” Bob T. Li, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the time.
Now, in a press release from the manufacturer, Amgen, he said: “Sotorasib represents a major advancement in oncology and changes the treatment paradigm for patients with KRAS G12C-mutated non–small cell lung cancer.
“Patients with non–small cell lung cancer who have progressed beyond first-line treatment face a poor prognosis and have limited treatment options available to them. Sotorasib delivers a new option for these patients, and it is the first KRAS-targeted therapy to be approved after nearly four decades of research,” he added.
Details of clinical data
This is an accelerated approval based on response rate data.
The FDA notes that the clinical data come from a study of 124 patients with locally advanced or metastatic KRAS G12C-mutated NSCLC with disease progression after receiving an immune checkpoint inhibitor and/or platinum-based chemotherapy.
The major outcome measured was overall response rate (ORR), which was 36%. Of the patients who responded, 58% had a duration of response of 6 months or longer.
Sotorasib was approved at a dose of 960 mg, and this dose was based on available clinical data as well as pharmacokinetic and pharmacodynamic modeling, the FDA noted. As part of the evaluation for this accelerated approval, the agency is requiring a postmarketing trial to investigate whether a lower dose will have a similar clinical effect.
The most common side effects include diarrhea, musculoskeletal pain, nausea, fatigue, liver damage, and cough. Sotorasib should not be used if patients develop symptoms of interstitial lung disease, and should be permanently discontinued if interstitial lung disease is confirmed.
Patients on sotorasib should have liver function tests prior to starting and while taking the drug; if liver damage develops, the drug should be stopped or the dose reduced. Patients should avoid taking acid-reducing agents, drugs that induce or are substrates for certain enzymes in the liver, and drugs that are substrates of P-glycoprotein (P-gp).
Companion diagnostic tests also approved
Along with the new drug, the FDA approved two companion diagnostic tests – the QIAGEN therascreen KRAS RGQ PCR kit (approval granted to QIAGEN GmbH) for analyzing tumor tissue and the Guardant360 CDx (approval granted to Guardant Health) for analyzing plasma specimens to determine if the KRAS G12C mutation is present. The agency notes that if the plasma test comes back negative, the patient’s tumor tissue should be tested.
A version of this article first appeared on Medscape.com.
NSCLC survival on immunotherapy much lower in ‘real world’
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Clean indoor air is vital for infection control
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea linked to COVID-19 risk
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
FROM ATS 2021
Bill seeks to streamline prior authorization in Medicare Advantage plans
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
A group of bipartisan lawmakers intends to compel insurers to streamline prior authorization processes for Medicare Advantage plans, including a bid to end the use of faxes and develop systems that can allow for real-time decisions.
Rep. Suzan DelBene (D-Wash.); Rep. Mike Kelly (R-Pa.); Rep. Ami Bera, MD (D-Calif.); and Rep. Larry Bucshon, MD, (R-Ind.) on May 13 introduced a bill that would task federal officials with refining standards regarding prior authorization for Medicare Advantage. Titled the Improving Seniors’ Timely Access to Care Act of 2021, the bill would direct the Department of Health & Human Services to create rules intended to make prior authorization more transparent and speedy for the insurer-run Medicare plans. Known as Medicare Advantage, these plans cover about 24.1 million people of the 62 million enrolled in the giant federal health program, according to the nonprofit Kaiser Family Foundation.
These revamped prior authorization systems could not rely on faxes nor could they employ proprietary payer portals that did not meet HHS’ standards, says the text of the bill released by Rep. DelBene. Insurers would also have to report to the Centers for Medicare & Medicaid Services about the extent of their use of prior authorization and the rate of approvals or denials. The bill seeks to encourage plans to adopt prior authorization programs that adhere to evidence-based medical guidelines in consultation with physicians.
There were several reasons for focusing on Medicare Advantage plans, although prior authorization concerns extend more broadly in the U.S. health care system, said Susan Bailey, MD, president of the American Medical Association.
There’s an ample body of research about issues seen in the Medicare Advantage plans. Dr. Bailey also said that, in her experience, Medicare Advantage plans have had some of the most restrictive policies. And, by starting with Medicare Advantage, there’s a potential for a ripple effect in the industry, easing this issue when physicians work with other insurers as well.
“When Medicare adopts a policy whether it be a payment policy or a coverage policy, private insurers typically follow along,” she said.
Strong support among health care groups
There’s strong support for streamlining prior authorization both in the medical community and in Congress.
The bill has the support of about 70 health care organizations, including the AMA and the American Academy of Family Physicians, according to its sponsors. As of May 17, the bill had attracted the backing of 97 members of the House of Representatives, roughly evenly split among Democrats and Republicans.
Rep. DelBene’s previous version of this bill, the Improving Seniors’ Timely Access to Care Act of 2019, attracted 143 Democratic cosponsors and 137 Republican ones, or more than half of the members of the House. This bill was not completed during the previous session of Congress (January 2019–January 2021) because of the more urgent needs of pandemic response, said Rep. Bucshon, who practiced cardiothoracic surgery before joining Congress.
“It wasn’t quite on the radar as much as it might have been if we didn’t have COVID,” Rep. Bucshon said.
Rep. Bucshon added that he expects strong Senate support for a companion measure of the House bill, which could make the difference for efforts to pass it this year.
Insurers have become more aggressive over time in denying payments through prior authorization systems for services that physicians say their patients need, according to Rep. Bucshon. There may be some “bad actors” in medicine who would order unnecessary procedures, Rep. Bucshon allowed, but in most cases, the cumbersome prior authorization processes only put a hurdle for patients seeking needed treatments, he said.
“The premise is that it controls health care costs but actually what it does is it helps insurance company’s bottom line,” Rep. Bucshon said.
In a prepared statement, former Pennsylvania representative Allyson Y. Schwartz, now CEO of the Better Medicare Alliance, said her group had spoken with sponsors of this legislation and appreciates “their receptiveness to feedback in this process.”
“Prior authorization ensures beneficiaries receive clinically appropriate care and reduces exposures to duplicative and unnecessary services,” Ms. Schwartz said. “We share an interest in ensuring prior authorization works as smoothly and effectively as possible for beneficiaries while protecting its essential function of facilitating safe, evidenced-based care.”
The Better Medicare Alliance said its funders include UnitedHealth, Humana, and CVS Health/Aetna, which run Advantage plans. The group also lists as its partners many medical organizations.
“Rationing care by hassling”
Like Rep. Bucshon, Dr. Bailey sees a different motivation in insurers’ persistence in keeping the prior authorization process cumbersome.
Phone calls and faxes remain the key methods for handling prior authorization for medical services, according to the results of a survey done by the AMA in December. Phone calls were always or often required for prior authorization for medical services (59%), with faxes the second-most common approach (46%), followed by health plans’ online portals (39%), electronic health records and practice management systems (29%), and email or U.S. mail (26%), according to the AMA’s report on the survey.
“It seems like every step in the process is designed to make the patient less likely to get the therapy that the doctor thinks that the patient needs,” Dr. Bailey said. “It’s almost like rationing care by hassling the patient and the physician.”
The findings of an investigation by HHS’ internal watchdog unit appear to support Dr. Bailey’s view, showing that insurer-run Medicare plans had a pattern of often walking back their initial rejections.
In 2018, the Office of the Inspector General for HHS reported that Medicare Advantage organizations (MAOs) overturned 75% of their own denials during 2014-16. In addition, independent reviewers within the appeals process overturned additional denials in favor of patients and clinicians, OIG said.
“The high number of overturned denials raises concerns that some Medicare Advantage beneficiaries and providers were initially denied services and payments that should have been provided,” the OIG said in the report. “This is especially concerning because beneficiaries and providers rarely used the appeals process, which is designed to ensure access to care and payment.”
During 2014-2016, patients and clinicians appealed only 1% of denials to the first level of appeal, OIG said. In the report, the watchdog group noted that CMS audits had highlighted “widespread and persistent MAO performance problems related to denials of care and payment.” In 2015, for example, CMS cited 56% of audited contracts for making inappropriate denials.
Dr. Bailey also said in an interview that she routinely encounters problems with prior authorization in her own practice as an allergist and immunologist in Fort Worth, Tex.
In late May, for example, a Medicare Advantage plan made a patient whose chronic asthma had been stable for years change to a new inhaler that resulted in him developing a yeast infection in his mouth, Dr. Bailey said.
“We treated the yeast infection, made some changes in the way he uses his inhaler, so hopefully he would tolerate it better,” Dr. Bailey said. “He had a reaction to the medication to treat the yeast infection and ended up in the hospital. How is that helping anyone? It certainly hasn’t helped my patient.”
Dr. Bailey said insurers have also asked to seek prior authorization to prescribe medications that have been generic for years and have used the process to challenge her on cases of what seem to be common sense in medical practice. This included a bid to have Dr. Bailey prescribe a medication in pill form for a 6-month-old baby who had no teeth.
“Every doctor has got absurd stories like that, but unfortunately, every doctor is going to have tragic stories where prior authorization has resulted in death and harm to the patients,” Dr. Bailey said.
Some physicians leave it to the patient to try to overcome insurers’ decisions on prior authorization, seeing this task as falling outside of their duties, Dr. Bailey said.
“I don’t do that. I fight. I spend a lot of time fighting. I don’t like to lose. I don’t like my patients to lose, so I will go to the mat for them,” Dr. Bailey said. “But I’m blessed to be in a specialty where I’ve got loads more control over my schedule than many other specialties do.”
A version of this article first appeared on Medscape.com.
New AHA/ASA guideline on secondary stroke prevention
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.





