User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


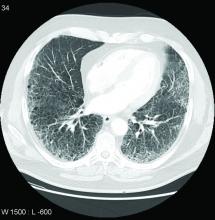
Nintedanib slows interstitial lung disease in RA patients
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
FROM THE EULAR 2021 CONGRESS
Medical licensing questions continue to violate ADA
With the COVID-19 pandemic, already high rates of suicide, depression, and burnout among physicians became even more acute. Yet, 3 years after the Federation of State Medical Boards issued recommendations on what questions about mental health status license applications should – or mostly should not – include, only North Carolina fully complies with all four recommendations, and most states comply with two or fewer, a study of state medical board applications has found (JAMA. 2021 May 18;325[19];2017-8).
Questions about mental health history or “its hypothetical effect on competency,” violate the Americans with Disabilities Act, the study authors stated. In a research letter to JAMA, the authors also reported that five state boards do not comply with any of the FSMB recommendations. Twenty-four states comply with three of the four recommendations.
Overall, the mean consistency score was 2.1, which means state medical licensing applications typically run afoul of the Americans With Disabilities Act when it comes to mental health history of applicants.
“No one should ever wonder, ‘Will I lose my job, or should I get help?’ ” said co–senior author Jessica A. Gold, MD, MS, a psychiatrist at Washington University in St. Louis. “This should absolutely never be a question on someone’s mind. And the fact that it is, in medicine, is a problem that needs to be solved. I hope that people are beginning to see that, and we can make a change to get people the help they need before it is too late.”
High rates of depression, suicide
She noted that before COVID-19, physicians already had higher rates of depression, burnout, and suicide than the general population. “Over COVID-19, it has become clear that the mental health of physicians has become additionally compounded,” Dr. Gold said.
One study found that physicians had a 44% higher rate of suicide (PLoS One. 2019 Dec;14[12]:e0226361), but they’re notoriously reluctant to seek out mental health care. A 2017 study reported that 40% of physicians would be reluctant to seek mental health care because of concerns about their licensure (Mayo Clin Proc. 2017;92[10]:1486-93).
As the pandemic went on, Dr. Gold and her colleagues decided to study whether state boards had improved their compliance with the FSMB recommendations issued in 2018. Those recommendations include these four limitations regarding questions about mental health conditions on license applications:
- Include only when they result in impairment.
- Include only when the mental health conditions are current – that is, when they’ve occurred within the past 2 years.
- Provide safe haven nonreporting – that is, allow physicians to not report previously diagnosed and treated mental health conditions if they’re being monitored and are in good standing with a physician health program.
- Include supportive or nonjudgmental language about seeking mental health care.
The study considered board applications that had questions about mental health status as consistent with the first three recommendations. Seventeen states complied.
Thirty-nine state boards complied with the first recommendation regarding impairment; 41 with the second recommendation about near-term history; 25 with safe-haven nonreporting. Only eight states were consistent with the recommendation on supportive language.
The ADA limits inquiries about an applicant’s impairment to only current conditions. In a 2017 study, only 21 state boards had limited questions to current impairment. “This is a significant improvement, but this still means the rest of the states are violating an actual law,” Dr. Gold said. “Another plus is that 17 states asked no questions at all that could require mental health disclosure. This, too is significant because it highlights change in thinking.”
But still, the fact that five states didn’t comply with any recommendation and only one followed all of them is “utterly unacceptable,” Dr. Gold said. “Instead, we should have universal adoption of FSMB recommendations.”
Time to remove stigma
Michael F. Myers, MD, a clinical psychiatrist at the State University of New York, Brooklyn, said removing the stigma of seeking help for mental health conditions is especially important for physicians. He’s written several books about physician mental health, including his latest, “Becoming a Doctor’s Doctor: A Memoir.”
“I would say at least 15% of the families that I interviewed who lost a physician loved one to suicide have said the doctor was petrified of going for professional help because of fears of what this could do to their medical license,” he said. “It is extremely important that those licensing questions will be either brought up to speed, or – the ones that are clearly violating the ADA – that they be removed.”
Applications for hospital privileges can also run afoul of the same ADA standard, Dr. Myers added. “Physicians have told me that when they go to get medical privileges at a medical center, they get asked all kinds of questions that are outdated, that are intrusive, that violate the ADA,” he said.
Credentialing is another area that Dr. Gold and her colleagues are interested in studying, she said. “Sometimes the licensing applications can be fine, but then the hospital someone is applying to work at can ask the same illegal questions anyway,” she said. “So it doesn’t matter that the state fixed the problem because the hospital asked them anyway. You feel your job is at risk in the same way, so you still don’t get help.”
Dr. Gold and Dr. Myers have no relevant financial relationships to disclose.
With the COVID-19 pandemic, already high rates of suicide, depression, and burnout among physicians became even more acute. Yet, 3 years after the Federation of State Medical Boards issued recommendations on what questions about mental health status license applications should – or mostly should not – include, only North Carolina fully complies with all four recommendations, and most states comply with two or fewer, a study of state medical board applications has found (JAMA. 2021 May 18;325[19];2017-8).
Questions about mental health history or “its hypothetical effect on competency,” violate the Americans with Disabilities Act, the study authors stated. In a research letter to JAMA, the authors also reported that five state boards do not comply with any of the FSMB recommendations. Twenty-four states comply with three of the four recommendations.
Overall, the mean consistency score was 2.1, which means state medical licensing applications typically run afoul of the Americans With Disabilities Act when it comes to mental health history of applicants.
“No one should ever wonder, ‘Will I lose my job, or should I get help?’ ” said co–senior author Jessica A. Gold, MD, MS, a psychiatrist at Washington University in St. Louis. “This should absolutely never be a question on someone’s mind. And the fact that it is, in medicine, is a problem that needs to be solved. I hope that people are beginning to see that, and we can make a change to get people the help they need before it is too late.”
High rates of depression, suicide
She noted that before COVID-19, physicians already had higher rates of depression, burnout, and suicide than the general population. “Over COVID-19, it has become clear that the mental health of physicians has become additionally compounded,” Dr. Gold said.
One study found that physicians had a 44% higher rate of suicide (PLoS One. 2019 Dec;14[12]:e0226361), but they’re notoriously reluctant to seek out mental health care. A 2017 study reported that 40% of physicians would be reluctant to seek mental health care because of concerns about their licensure (Mayo Clin Proc. 2017;92[10]:1486-93).
As the pandemic went on, Dr. Gold and her colleagues decided to study whether state boards had improved their compliance with the FSMB recommendations issued in 2018. Those recommendations include these four limitations regarding questions about mental health conditions on license applications:
- Include only when they result in impairment.
- Include only when the mental health conditions are current – that is, when they’ve occurred within the past 2 years.
- Provide safe haven nonreporting – that is, allow physicians to not report previously diagnosed and treated mental health conditions if they’re being monitored and are in good standing with a physician health program.
- Include supportive or nonjudgmental language about seeking mental health care.
The study considered board applications that had questions about mental health status as consistent with the first three recommendations. Seventeen states complied.
Thirty-nine state boards complied with the first recommendation regarding impairment; 41 with the second recommendation about near-term history; 25 with safe-haven nonreporting. Only eight states were consistent with the recommendation on supportive language.
The ADA limits inquiries about an applicant’s impairment to only current conditions. In a 2017 study, only 21 state boards had limited questions to current impairment. “This is a significant improvement, but this still means the rest of the states are violating an actual law,” Dr. Gold said. “Another plus is that 17 states asked no questions at all that could require mental health disclosure. This, too is significant because it highlights change in thinking.”
But still, the fact that five states didn’t comply with any recommendation and only one followed all of them is “utterly unacceptable,” Dr. Gold said. “Instead, we should have universal adoption of FSMB recommendations.”
Time to remove stigma
Michael F. Myers, MD, a clinical psychiatrist at the State University of New York, Brooklyn, said removing the stigma of seeking help for mental health conditions is especially important for physicians. He’s written several books about physician mental health, including his latest, “Becoming a Doctor’s Doctor: A Memoir.”
“I would say at least 15% of the families that I interviewed who lost a physician loved one to suicide have said the doctor was petrified of going for professional help because of fears of what this could do to their medical license,” he said. “It is extremely important that those licensing questions will be either brought up to speed, or – the ones that are clearly violating the ADA – that they be removed.”
Applications for hospital privileges can also run afoul of the same ADA standard, Dr. Myers added. “Physicians have told me that when they go to get medical privileges at a medical center, they get asked all kinds of questions that are outdated, that are intrusive, that violate the ADA,” he said.
Credentialing is another area that Dr. Gold and her colleagues are interested in studying, she said. “Sometimes the licensing applications can be fine, but then the hospital someone is applying to work at can ask the same illegal questions anyway,” she said. “So it doesn’t matter that the state fixed the problem because the hospital asked them anyway. You feel your job is at risk in the same way, so you still don’t get help.”
Dr. Gold and Dr. Myers have no relevant financial relationships to disclose.
With the COVID-19 pandemic, already high rates of suicide, depression, and burnout among physicians became even more acute. Yet, 3 years after the Federation of State Medical Boards issued recommendations on what questions about mental health status license applications should – or mostly should not – include, only North Carolina fully complies with all four recommendations, and most states comply with two or fewer, a study of state medical board applications has found (JAMA. 2021 May 18;325[19];2017-8).
Questions about mental health history or “its hypothetical effect on competency,” violate the Americans with Disabilities Act, the study authors stated. In a research letter to JAMA, the authors also reported that five state boards do not comply with any of the FSMB recommendations. Twenty-four states comply with three of the four recommendations.
Overall, the mean consistency score was 2.1, which means state medical licensing applications typically run afoul of the Americans With Disabilities Act when it comes to mental health history of applicants.
“No one should ever wonder, ‘Will I lose my job, or should I get help?’ ” said co–senior author Jessica A. Gold, MD, MS, a psychiatrist at Washington University in St. Louis. “This should absolutely never be a question on someone’s mind. And the fact that it is, in medicine, is a problem that needs to be solved. I hope that people are beginning to see that, and we can make a change to get people the help they need before it is too late.”
High rates of depression, suicide
She noted that before COVID-19, physicians already had higher rates of depression, burnout, and suicide than the general population. “Over COVID-19, it has become clear that the mental health of physicians has become additionally compounded,” Dr. Gold said.
One study found that physicians had a 44% higher rate of suicide (PLoS One. 2019 Dec;14[12]:e0226361), but they’re notoriously reluctant to seek out mental health care. A 2017 study reported that 40% of physicians would be reluctant to seek mental health care because of concerns about their licensure (Mayo Clin Proc. 2017;92[10]:1486-93).
As the pandemic went on, Dr. Gold and her colleagues decided to study whether state boards had improved their compliance with the FSMB recommendations issued in 2018. Those recommendations include these four limitations regarding questions about mental health conditions on license applications:
- Include only when they result in impairment.
- Include only when the mental health conditions are current – that is, when they’ve occurred within the past 2 years.
- Provide safe haven nonreporting – that is, allow physicians to not report previously diagnosed and treated mental health conditions if they’re being monitored and are in good standing with a physician health program.
- Include supportive or nonjudgmental language about seeking mental health care.
The study considered board applications that had questions about mental health status as consistent with the first three recommendations. Seventeen states complied.
Thirty-nine state boards complied with the first recommendation regarding impairment; 41 with the second recommendation about near-term history; 25 with safe-haven nonreporting. Only eight states were consistent with the recommendation on supportive language.
The ADA limits inquiries about an applicant’s impairment to only current conditions. In a 2017 study, only 21 state boards had limited questions to current impairment. “This is a significant improvement, but this still means the rest of the states are violating an actual law,” Dr. Gold said. “Another plus is that 17 states asked no questions at all that could require mental health disclosure. This, too is significant because it highlights change in thinking.”
But still, the fact that five states didn’t comply with any recommendation and only one followed all of them is “utterly unacceptable,” Dr. Gold said. “Instead, we should have universal adoption of FSMB recommendations.”
Time to remove stigma
Michael F. Myers, MD, a clinical psychiatrist at the State University of New York, Brooklyn, said removing the stigma of seeking help for mental health conditions is especially important for physicians. He’s written several books about physician mental health, including his latest, “Becoming a Doctor’s Doctor: A Memoir.”
“I would say at least 15% of the families that I interviewed who lost a physician loved one to suicide have said the doctor was petrified of going for professional help because of fears of what this could do to their medical license,” he said. “It is extremely important that those licensing questions will be either brought up to speed, or – the ones that are clearly violating the ADA – that they be removed.”
Applications for hospital privileges can also run afoul of the same ADA standard, Dr. Myers added. “Physicians have told me that when they go to get medical privileges at a medical center, they get asked all kinds of questions that are outdated, that are intrusive, that violate the ADA,” he said.
Credentialing is another area that Dr. Gold and her colleagues are interested in studying, she said. “Sometimes the licensing applications can be fine, but then the hospital someone is applying to work at can ask the same illegal questions anyway,” she said. “So it doesn’t matter that the state fixed the problem because the hospital asked them anyway. You feel your job is at risk in the same way, so you still don’t get help.”
Dr. Gold and Dr. Myers have no relevant financial relationships to disclose.
FROM JAMA
Medtronic yanks Heartware VAD, calls for halt to new implants
Medtronic has stopped the sale of its Heartware Ventricular Assist Device (HVAD) system and is advising that physicians cease implanting the device because problems with an internal pump can lead to death or serious injuries.
“There is an increased risk of neurological adverse events and mortality associated with the internal pump,” the U.S. Food and Drug Administration announced today.
There is also a potential for the internal pump to stop, and there may be delay or failure to restart. “Both problems may lead to death or serious injuries,” the agency said.
Between January 2009 and April 22, 2021, Medtronic received a total of 106 complaints involving delay or failure to restart with the HVAD pump. Of these, 26 complaints involved HVAD devices operating under normal conditions (dual stator mode) and 80 involved devices operating in a back-up mode (single stator mode) that allows for continued pump function if electrical continuity between the pump and controller is interrupted.
Of the 26 complaints that occurred under normal conditions, four resulted in patient death and five led to urgent explant. Of the 80 complaints that occurred in single stator mode, 10 deaths and eight explants were reported to Medtronic, according to an urgent medical device communication letter issued by the company today.
“Considering these findings and given the availability of alternative devices such as the Abbott HeartMate 3, Medtronic has made the decision to stop the distribution and sale of the HVAD System,” the letter says. “Medtronic advises that there be no further implantations of the HVAD System.”
Medtronic undertook a previous recall of the Heartware HVAD system in February, focusing on batteries, power, datalink cables, and other peripheral equipment, because of the “risk of wear and tear of the connector plugs (power sources, data cable, and alarm adapter), which could cause damage to the controller port metal pins (for example, bent pins).” The FDA deemed that recall Class I, the most serious category of safety alert, in April.
The company noted that patients who currently have an HVAD implant “may require support for many years,” and that it is moving as quickly as possible to create a plan to guide the ongoing support for patients, caregivers, and health care professionals.
In response to the restart failure issue and evolving data about neurologic risks associated with the HVAD pump, Medtronic said it engaged an Independent Practitioner Quality Panel (IPQP), composed of cardiologists, surgeons, and VAD coordinators, to advise on recommendations for appropriate patient management. Based on information collected to date and IPQP input, Medtronic is recommending that physicians continue following best clinical practices and manage patients implanted with the HVAD pump according to the recommendations in the Instructions for Use (IFU).
“Prophylactic explant of the HVAD™ device is not recommended, as risks associated with explantation may outweigh the potential benefits,” the letter says. “The decision regarding explant and exchange of the HVAD™ pump should be made by physicians on a case-by-case basis, considering the patient’s clinical condition and surgical risks. If a physician determines that pump exchange is appropriate, we recommend exchanging to an alternative commercial LVAD.”
For patients in urgent need of an LVAD, Medtronic said physicians should use an alternative commercial LVAD or, if one is not available, that “a Patient Information form is required to be completed by you and your patient to acknowledge the risks of an HVAD implant prior to implanting your HVAD inventory.”
Today’s letter also provides recommendations on blood pressure management goals and anticoagulation. For any other questions or concerns, physicians should contact the Medtronic Office of Medical Affairs at: [email protected].
Medtronic issued another urgent letter in December 2020, warning physicians that a subset of HVAD devices included an internal pump component from three specific lots that increased the risk for restart failure. At that time, the company had not been able to pinpoint a root cause of the pump restart failure.
Consistent with the December 2020 notice, the rate of failure among pumps outside of the subset of three specific lots currently remains at about 0.4%, according to today’s notice.
Although Medtronic has identified the root cause and mitigations for pumps within the three specific lots, it has not been able to identify a root cause of the other restart failures reported with the HVAD pumps, the company said.
A version of this article first appeared on Medscape.com.
Medtronic has stopped the sale of its Heartware Ventricular Assist Device (HVAD) system and is advising that physicians cease implanting the device because problems with an internal pump can lead to death or serious injuries.
“There is an increased risk of neurological adverse events and mortality associated with the internal pump,” the U.S. Food and Drug Administration announced today.
There is also a potential for the internal pump to stop, and there may be delay or failure to restart. “Both problems may lead to death or serious injuries,” the agency said.
Between January 2009 and April 22, 2021, Medtronic received a total of 106 complaints involving delay or failure to restart with the HVAD pump. Of these, 26 complaints involved HVAD devices operating under normal conditions (dual stator mode) and 80 involved devices operating in a back-up mode (single stator mode) that allows for continued pump function if electrical continuity between the pump and controller is interrupted.
Of the 26 complaints that occurred under normal conditions, four resulted in patient death and five led to urgent explant. Of the 80 complaints that occurred in single stator mode, 10 deaths and eight explants were reported to Medtronic, according to an urgent medical device communication letter issued by the company today.
“Considering these findings and given the availability of alternative devices such as the Abbott HeartMate 3, Medtronic has made the decision to stop the distribution and sale of the HVAD System,” the letter says. “Medtronic advises that there be no further implantations of the HVAD System.”
Medtronic undertook a previous recall of the Heartware HVAD system in February, focusing on batteries, power, datalink cables, and other peripheral equipment, because of the “risk of wear and tear of the connector plugs (power sources, data cable, and alarm adapter), which could cause damage to the controller port metal pins (for example, bent pins).” The FDA deemed that recall Class I, the most serious category of safety alert, in April.
The company noted that patients who currently have an HVAD implant “may require support for many years,” and that it is moving as quickly as possible to create a plan to guide the ongoing support for patients, caregivers, and health care professionals.
In response to the restart failure issue and evolving data about neurologic risks associated with the HVAD pump, Medtronic said it engaged an Independent Practitioner Quality Panel (IPQP), composed of cardiologists, surgeons, and VAD coordinators, to advise on recommendations for appropriate patient management. Based on information collected to date and IPQP input, Medtronic is recommending that physicians continue following best clinical practices and manage patients implanted with the HVAD pump according to the recommendations in the Instructions for Use (IFU).
“Prophylactic explant of the HVAD™ device is not recommended, as risks associated with explantation may outweigh the potential benefits,” the letter says. “The decision regarding explant and exchange of the HVAD™ pump should be made by physicians on a case-by-case basis, considering the patient’s clinical condition and surgical risks. If a physician determines that pump exchange is appropriate, we recommend exchanging to an alternative commercial LVAD.”
For patients in urgent need of an LVAD, Medtronic said physicians should use an alternative commercial LVAD or, if one is not available, that “a Patient Information form is required to be completed by you and your patient to acknowledge the risks of an HVAD implant prior to implanting your HVAD inventory.”
Today’s letter also provides recommendations on blood pressure management goals and anticoagulation. For any other questions or concerns, physicians should contact the Medtronic Office of Medical Affairs at: [email protected].
Medtronic issued another urgent letter in December 2020, warning physicians that a subset of HVAD devices included an internal pump component from three specific lots that increased the risk for restart failure. At that time, the company had not been able to pinpoint a root cause of the pump restart failure.
Consistent with the December 2020 notice, the rate of failure among pumps outside of the subset of three specific lots currently remains at about 0.4%, according to today’s notice.
Although Medtronic has identified the root cause and mitigations for pumps within the three specific lots, it has not been able to identify a root cause of the other restart failures reported with the HVAD pumps, the company said.
A version of this article first appeared on Medscape.com.
Medtronic has stopped the sale of its Heartware Ventricular Assist Device (HVAD) system and is advising that physicians cease implanting the device because problems with an internal pump can lead to death or serious injuries.
“There is an increased risk of neurological adverse events and mortality associated with the internal pump,” the U.S. Food and Drug Administration announced today.
There is also a potential for the internal pump to stop, and there may be delay or failure to restart. “Both problems may lead to death or serious injuries,” the agency said.
Between January 2009 and April 22, 2021, Medtronic received a total of 106 complaints involving delay or failure to restart with the HVAD pump. Of these, 26 complaints involved HVAD devices operating under normal conditions (dual stator mode) and 80 involved devices operating in a back-up mode (single stator mode) that allows for continued pump function if electrical continuity between the pump and controller is interrupted.
Of the 26 complaints that occurred under normal conditions, four resulted in patient death and five led to urgent explant. Of the 80 complaints that occurred in single stator mode, 10 deaths and eight explants were reported to Medtronic, according to an urgent medical device communication letter issued by the company today.
“Considering these findings and given the availability of alternative devices such as the Abbott HeartMate 3, Medtronic has made the decision to stop the distribution and sale of the HVAD System,” the letter says. “Medtronic advises that there be no further implantations of the HVAD System.”
Medtronic undertook a previous recall of the Heartware HVAD system in February, focusing on batteries, power, datalink cables, and other peripheral equipment, because of the “risk of wear and tear of the connector plugs (power sources, data cable, and alarm adapter), which could cause damage to the controller port metal pins (for example, bent pins).” The FDA deemed that recall Class I, the most serious category of safety alert, in April.
The company noted that patients who currently have an HVAD implant “may require support for many years,” and that it is moving as quickly as possible to create a plan to guide the ongoing support for patients, caregivers, and health care professionals.
In response to the restart failure issue and evolving data about neurologic risks associated with the HVAD pump, Medtronic said it engaged an Independent Practitioner Quality Panel (IPQP), composed of cardiologists, surgeons, and VAD coordinators, to advise on recommendations for appropriate patient management. Based on information collected to date and IPQP input, Medtronic is recommending that physicians continue following best clinical practices and manage patients implanted with the HVAD pump according to the recommendations in the Instructions for Use (IFU).
“Prophylactic explant of the HVAD™ device is not recommended, as risks associated with explantation may outweigh the potential benefits,” the letter says. “The decision regarding explant and exchange of the HVAD™ pump should be made by physicians on a case-by-case basis, considering the patient’s clinical condition and surgical risks. If a physician determines that pump exchange is appropriate, we recommend exchanging to an alternative commercial LVAD.”
For patients in urgent need of an LVAD, Medtronic said physicians should use an alternative commercial LVAD or, if one is not available, that “a Patient Information form is required to be completed by you and your patient to acknowledge the risks of an HVAD implant prior to implanting your HVAD inventory.”
Today’s letter also provides recommendations on blood pressure management goals and anticoagulation. For any other questions or concerns, physicians should contact the Medtronic Office of Medical Affairs at: [email protected].
Medtronic issued another urgent letter in December 2020, warning physicians that a subset of HVAD devices included an internal pump component from three specific lots that increased the risk for restart failure. At that time, the company had not been able to pinpoint a root cause of the pump restart failure.
Consistent with the December 2020 notice, the rate of failure among pumps outside of the subset of three specific lots currently remains at about 0.4%, according to today’s notice.
Although Medtronic has identified the root cause and mitigations for pumps within the three specific lots, it has not been able to identify a root cause of the other restart failures reported with the HVAD pumps, the company said.
A version of this article first appeared on Medscape.com.
NCAA athletes: ECG abnormalities persist after COVID-19
College athletes who have recently recovered from COVID-19 infection show cardiac abnormalities on electrocardiography.
In a small study of ECGs on National Collegiate Athletic Association Division II athletes, those who had been infected with COVID-19 had a prolonged PR interval, compared with matched athletes who had not been infected.
The study was presented at the 2021 Virtual American College of Sports Medicine Annual Meeting & World Congresses.
“The NCAA was requiring athletes to have an ECG for return to play after noting there could be some myocardial abnormalities following COVID-19 infection,” lead author Frank Wyatt, EdD, a sports physiologist and professor at Midwestern State University in Wichita Falls, Tex., told this news organization.
“Our head athletic trainer asked me if I could do ECGs on our COVID-19–recovered athletes, and I decided to do a matched pair–design study to see how our infected and noninfected athletes compared,” Dr. Wyatt said.
Research in the general population has suggested that COVID-19 can cause damage not only to the lungs, but also to the myocardium, he said. “Recent literature suggests COVID-19 is actually infusing itself into the cells of the myocardium and killing those cells, much the way it did in the lung, and possibly kidney and liver, so it’s going after those organs as well, not just the lungs.”
Dr. Wyatt presented results of ECGs that were done in seven COVID-infected athletes and in seven controls, who were free of infection.
The athletes’ recovery from COVID-19 infection was documented after two negative tests.
All subjects were matched by sport, gender, ethnicity, and anthropometry. Investigators obtained ECG recordings 2-4 weeks after the infected athletes had their recovery documented.
Study participants engaged in football, basketball, soccer, and volleyball, and Dr. Wyatt and associates were blinded as to their infected or control status.
Participants self-reported their ethnicity. Most were White or African American.
The main abnormality found was a prolonged PR interval. In the athletes who were recovered from COVID-19, the mean PR interval was 183.6 milliseconds (± 32.4 ms), compared with 141.7 ms (± 22.7 ms) among the controls.
Baseline ECGs for all young athletes?
Dr. Wyatt said he would like to see ECGs done at baseline as part of the physical exam NCAA athletes have to undergo at the start of each season. But that would be expensive.
“It has been suggested that they all need to have ECGs for baseline information, but they don’t do it because of money. If we had that baseline data on these athletes it would really give us a better picture of whether there was damage or not,” he said. “At our small university, if I wasn’t available to do these ECGs, our athletic department would then have to go to the cardiologist to do them, and that is tremendously expensive. It has also been suggested that high school athletes get ECGs as a preliminary test when they start their season, and I think that is warranted as well as for the NCAA athletes, but because of the expense, they’re not doing it.”
Dr. Wyatt has continued to do ECGs on athletes who have survived COVID-19 and to date has ECG data on 70 athletes. He plans further comparisons between the infected and noninfected athletes.
“We want to see if we can solidify the results we presented at ACSM. We had small numbers, so our follow up is to see if we can statistically show in a more robust manner whether or not there was widespread abnormality in the athletes who got infected. They were only 2-4 weeks post infected, and I don’t know what the long-term effects are going to be,” he said.
May be an important finding
“This may be an important finding, but needs many more athletes, as there were only seven in each group,” commented Curt J. Daniels, MD, director of the sports cardiology program and professor at Ohio State University Wexner Medical Center, Columbus.
“Plus, it will need some imaging correlate and recovery ECGs to see if this effect of PR interval prolongation correlates with myocardial changes and whether it persists or resolves,” added Dr. Daniels, who was not part of the study. “But I do find this interesting. ... I agree we are looking for any ECG sign that might help tell us who needs a cardiac MRI. The Big Ten COVID-19 Cardiac Registry has 1,597 ECGs on post COVID athletes we are analyzing, but preliminarily did not see any changes.”
Dr. Wyatt and Dr. Daniels reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
College athletes who have recently recovered from COVID-19 infection show cardiac abnormalities on electrocardiography.
In a small study of ECGs on National Collegiate Athletic Association Division II athletes, those who had been infected with COVID-19 had a prolonged PR interval, compared with matched athletes who had not been infected.
The study was presented at the 2021 Virtual American College of Sports Medicine Annual Meeting & World Congresses.
“The NCAA was requiring athletes to have an ECG for return to play after noting there could be some myocardial abnormalities following COVID-19 infection,” lead author Frank Wyatt, EdD, a sports physiologist and professor at Midwestern State University in Wichita Falls, Tex., told this news organization.
“Our head athletic trainer asked me if I could do ECGs on our COVID-19–recovered athletes, and I decided to do a matched pair–design study to see how our infected and noninfected athletes compared,” Dr. Wyatt said.
Research in the general population has suggested that COVID-19 can cause damage not only to the lungs, but also to the myocardium, he said. “Recent literature suggests COVID-19 is actually infusing itself into the cells of the myocardium and killing those cells, much the way it did in the lung, and possibly kidney and liver, so it’s going after those organs as well, not just the lungs.”
Dr. Wyatt presented results of ECGs that were done in seven COVID-infected athletes and in seven controls, who were free of infection.
The athletes’ recovery from COVID-19 infection was documented after two negative tests.
All subjects were matched by sport, gender, ethnicity, and anthropometry. Investigators obtained ECG recordings 2-4 weeks after the infected athletes had their recovery documented.
Study participants engaged in football, basketball, soccer, and volleyball, and Dr. Wyatt and associates were blinded as to their infected or control status.
Participants self-reported their ethnicity. Most were White or African American.
The main abnormality found was a prolonged PR interval. In the athletes who were recovered from COVID-19, the mean PR interval was 183.6 milliseconds (± 32.4 ms), compared with 141.7 ms (± 22.7 ms) among the controls.
Baseline ECGs for all young athletes?
Dr. Wyatt said he would like to see ECGs done at baseline as part of the physical exam NCAA athletes have to undergo at the start of each season. But that would be expensive.
“It has been suggested that they all need to have ECGs for baseline information, but they don’t do it because of money. If we had that baseline data on these athletes it would really give us a better picture of whether there was damage or not,” he said. “At our small university, if I wasn’t available to do these ECGs, our athletic department would then have to go to the cardiologist to do them, and that is tremendously expensive. It has also been suggested that high school athletes get ECGs as a preliminary test when they start their season, and I think that is warranted as well as for the NCAA athletes, but because of the expense, they’re not doing it.”
Dr. Wyatt has continued to do ECGs on athletes who have survived COVID-19 and to date has ECG data on 70 athletes. He plans further comparisons between the infected and noninfected athletes.
“We want to see if we can solidify the results we presented at ACSM. We had small numbers, so our follow up is to see if we can statistically show in a more robust manner whether or not there was widespread abnormality in the athletes who got infected. They were only 2-4 weeks post infected, and I don’t know what the long-term effects are going to be,” he said.
May be an important finding
“This may be an important finding, but needs many more athletes, as there were only seven in each group,” commented Curt J. Daniels, MD, director of the sports cardiology program and professor at Ohio State University Wexner Medical Center, Columbus.
“Plus, it will need some imaging correlate and recovery ECGs to see if this effect of PR interval prolongation correlates with myocardial changes and whether it persists or resolves,” added Dr. Daniels, who was not part of the study. “But I do find this interesting. ... I agree we are looking for any ECG sign that might help tell us who needs a cardiac MRI. The Big Ten COVID-19 Cardiac Registry has 1,597 ECGs on post COVID athletes we are analyzing, but preliminarily did not see any changes.”
Dr. Wyatt and Dr. Daniels reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
College athletes who have recently recovered from COVID-19 infection show cardiac abnormalities on electrocardiography.
In a small study of ECGs on National Collegiate Athletic Association Division II athletes, those who had been infected with COVID-19 had a prolonged PR interval, compared with matched athletes who had not been infected.
The study was presented at the 2021 Virtual American College of Sports Medicine Annual Meeting & World Congresses.
“The NCAA was requiring athletes to have an ECG for return to play after noting there could be some myocardial abnormalities following COVID-19 infection,” lead author Frank Wyatt, EdD, a sports physiologist and professor at Midwestern State University in Wichita Falls, Tex., told this news organization.
“Our head athletic trainer asked me if I could do ECGs on our COVID-19–recovered athletes, and I decided to do a matched pair–design study to see how our infected and noninfected athletes compared,” Dr. Wyatt said.
Research in the general population has suggested that COVID-19 can cause damage not only to the lungs, but also to the myocardium, he said. “Recent literature suggests COVID-19 is actually infusing itself into the cells of the myocardium and killing those cells, much the way it did in the lung, and possibly kidney and liver, so it’s going after those organs as well, not just the lungs.”
Dr. Wyatt presented results of ECGs that were done in seven COVID-infected athletes and in seven controls, who were free of infection.
The athletes’ recovery from COVID-19 infection was documented after two negative tests.
All subjects were matched by sport, gender, ethnicity, and anthropometry. Investigators obtained ECG recordings 2-4 weeks after the infected athletes had their recovery documented.
Study participants engaged in football, basketball, soccer, and volleyball, and Dr. Wyatt and associates were blinded as to their infected or control status.
Participants self-reported their ethnicity. Most were White or African American.
The main abnormality found was a prolonged PR interval. In the athletes who were recovered from COVID-19, the mean PR interval was 183.6 milliseconds (± 32.4 ms), compared with 141.7 ms (± 22.7 ms) among the controls.
Baseline ECGs for all young athletes?
Dr. Wyatt said he would like to see ECGs done at baseline as part of the physical exam NCAA athletes have to undergo at the start of each season. But that would be expensive.
“It has been suggested that they all need to have ECGs for baseline information, but they don’t do it because of money. If we had that baseline data on these athletes it would really give us a better picture of whether there was damage or not,” he said. “At our small university, if I wasn’t available to do these ECGs, our athletic department would then have to go to the cardiologist to do them, and that is tremendously expensive. It has also been suggested that high school athletes get ECGs as a preliminary test when they start their season, and I think that is warranted as well as for the NCAA athletes, but because of the expense, they’re not doing it.”
Dr. Wyatt has continued to do ECGs on athletes who have survived COVID-19 and to date has ECG data on 70 athletes. He plans further comparisons between the infected and noninfected athletes.
“We want to see if we can solidify the results we presented at ACSM. We had small numbers, so our follow up is to see if we can statistically show in a more robust manner whether or not there was widespread abnormality in the athletes who got infected. They were only 2-4 weeks post infected, and I don’t know what the long-term effects are going to be,” he said.
May be an important finding
“This may be an important finding, but needs many more athletes, as there were only seven in each group,” commented Curt J. Daniels, MD, director of the sports cardiology program and professor at Ohio State University Wexner Medical Center, Columbus.
“Plus, it will need some imaging correlate and recovery ECGs to see if this effect of PR interval prolongation correlates with myocardial changes and whether it persists or resolves,” added Dr. Daniels, who was not part of the study. “But I do find this interesting. ... I agree we are looking for any ECG sign that might help tell us who needs a cardiac MRI. The Big Ten COVID-19 Cardiac Registry has 1,597 ECGs on post COVID athletes we are analyzing, but preliminarily did not see any changes.”
Dr. Wyatt and Dr. Daniels reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dapagliflozin’s cost-effectiveness ‘intermediate’ for HFrEF
Although recent trial results have established the sodium glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin as a key new part of the recommended multidrug treatment regimen for patients with heart failure with reduced ejection fraction, the current U.S. cost for dapagliflozin means it has merely “intermediate” value when it comes to cost-effectiveness.
A typical regimen with dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) costs about $474/month or roughly $5,700/year based on Medicare pricing. After factoring in the incremental clinical benefits producing by dapagliflozin seen in the DAPA-HF pivotal trial that helped establish its role, this price produces a cost per quality-adjusted life-year (QALY) gain of about $84,000, which puts dapagliflozin squarely in the intermediate range for value set in 2014 by a task force of the American College of Cardiology and the American Heart Association.
This cost-effectiveness value depends largely on the proven efficacy of dapagliflozin (Farxiga) for decreasing the incidence of cardiovascular death among treated patients with HFrEF, and puts the drug’s value roughly on par with another agent recently approved to treat such patients, sacubitril/valsartan (Entresto), which carries a cost-effectiveness value of about $45,000/QALY.
The U.S. cost per QALY for dapagliflozin treatment of patients with HFrEF dwarfed the value numbers calculated for several other countries that were generally one-tenth this size. This disparity stemmed from both the relatively high price for dapagliflozin in the U.S. compared with other countries – nearly tenfold higher – and relatively higher costs for all types of U.S. medical care, Justin T. Parizo, MD, and coauthors said in a recent report. But the cost, and hence the cost per QALY, of dapagliflozin may soon drop because certain patents on the drug expired in October 2020, added Dr. Parizo, a cardiologist at Stanford (Calif.) University, and associates. Despite the expired patents, as of June 2021 no generic form of dapagliflozin appeared available for U.S. sale.
Medicare patients pay about $1,630/year out-of-pocket
“A key caveat” to this finding for dapagliflozin is that being cost-effective “is not by itself a mandate for routine clinical use,” Derek S. Chew, MD, and Daniel B. Mark, MD, said in an editorial that accompanied the report.
A major stumbling block for widespread U.S. prescribing of dapagliflozin to patients with HFrEF is its overall price tag for U.S. patients, estimated at $12 billion/year, as well as an out-of-pocket annual cost for individual Medicare patients of roughly $1,630/year. Adding this out-of-pocket cost to the copay for sacubitril/valsartan and two other much less expensive drug classes that together form the current mainstay, quadruple-drug regimen for HFrEF treatment means a potential annual cost paid by each Medicare patient of about $3,000, wrote Dr. Chew, a cardiologist, and Dr. Mark, a cardiologist and professor, both at Duke University, Durham, N.C.
They cited the precedent of the “unexpectedly slow” and “anemic” uptake of sacubitril/valsartan since its U.S. approval in 2015, a cost-effective agent with “comparable clinical effectiveness” to dapagliflozin. “Even with full inclusion [of sacubitril/valsartan] on formularies and elimination of preapproval requirements, use remains very low, and patient-borne out-of-pocket costs may be a key factor,” wrote Dr. Chew and Dr. Mark. They cited a results from a study that showed abandonment of new prescriptions at retail U.S. pharmacies spiked to a 60% rate when out-of-pocket cost exceeded $500.
More than what patients ‘can afford or are willing to spend’
The estimated $3,000-plus total out-of-pocket cost currently borne by some Medicare beneficiaries with HFrEF who have to shell out for both sacubitril/valsartan and dapagliflozin “appears to substantially exceed what many patients with heart failure can afford or are willing to spend,” wrote Dr. Chew and Dr. Mark.
Dr. Parizo and coauthors developed their cost-effectiveness model for dapagliflozin in treating HFrEF using primarily data collected in the DAPA-HF trial, which proved the efficacy of the drug for reducing cardiovascular deaths or acute heart failure events that led to hospitalization or intravenous outpatient treatment in more than 4,700 randomized patients with HFrEF. The trial enrolled roughly similar numbers of patients with or without type 2 diabetes.
The model showed an overall incremental cost-effectiveness ratio of $83,650/QALY, which was about the same regardless of whether patients also had type 2 diabetes. On a more granular level, the cost-effectiveness value estimate was $78,483/QALY in patients with mild health-status impairment due to their heart failure, and $97,608/QALY in patients with moderate impairment, a finding that underscores the importance of starting dapagliflozin treatment early in the course of HFrEF when disease effects are less severe. The analysis could not address value in patients with more advanced heart failure and in New York Heart Association functional class IV because fewer than 1% of patients in DAPA-HF were in this category.
Drug cost was a major determinant of cost-effectiveness. A 50% drop in cost from the Medicare benchmark of $473.64/month resulted in an incremental cost-effectiveness ratio of about $45,000/QALY (putting it into the high-value category based on the 2014 ACC/AHA formula), while a 50% rise in price yielded a value of nearly $123,000/QALY (still in the intermediate range, which spans from $50,000/QALY to $150,000/QALY). No other cost parameters had a meaningful effect on the cost-effectiveness calculation. The analyses also showed that using the basic cost assumptions, treatment with dapagliflozin needs to persist and remain effective for at least 44 months to produce a cost per QALY that’s less than $150,000. The authors stressed that their analysis considered heart failure effects and did not account for added benefit from treatment with dapagliflozin on preservation of renal function.
While it’s indisputable that treatment with dapagliflozin decreases health care costs by, for example, reducing hospitalizations for heart failure, each hospitalization costs just over $12,000, according to the assumptions made by Dr. Parizo and coauthors. But given dapagliflozin’s impact on this outcome, this cost saving translates into about $500/patient during 18 months on treatment (the median duration of treatment in DAPA-HF), which means the savings barely counterbalances the current cost of dapagliflozin treatment for 1 month, noted Dr. Chew and Dr. Mark.
The DAPA-HF trial was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Parizo had no disclosures and none of his coauthors had a relationship with AstraZeneca. Dr. Chew had no disclosures. Dr. Mark has received research grants from HeartFlow, Mayo Clinic, and Merck.
Although recent trial results have established the sodium glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin as a key new part of the recommended multidrug treatment regimen for patients with heart failure with reduced ejection fraction, the current U.S. cost for dapagliflozin means it has merely “intermediate” value when it comes to cost-effectiveness.
A typical regimen with dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) costs about $474/month or roughly $5,700/year based on Medicare pricing. After factoring in the incremental clinical benefits producing by dapagliflozin seen in the DAPA-HF pivotal trial that helped establish its role, this price produces a cost per quality-adjusted life-year (QALY) gain of about $84,000, which puts dapagliflozin squarely in the intermediate range for value set in 2014 by a task force of the American College of Cardiology and the American Heart Association.
This cost-effectiveness value depends largely on the proven efficacy of dapagliflozin (Farxiga) for decreasing the incidence of cardiovascular death among treated patients with HFrEF, and puts the drug’s value roughly on par with another agent recently approved to treat such patients, sacubitril/valsartan (Entresto), which carries a cost-effectiveness value of about $45,000/QALY.
The U.S. cost per QALY for dapagliflozin treatment of patients with HFrEF dwarfed the value numbers calculated for several other countries that were generally one-tenth this size. This disparity stemmed from both the relatively high price for dapagliflozin in the U.S. compared with other countries – nearly tenfold higher – and relatively higher costs for all types of U.S. medical care, Justin T. Parizo, MD, and coauthors said in a recent report. But the cost, and hence the cost per QALY, of dapagliflozin may soon drop because certain patents on the drug expired in October 2020, added Dr. Parizo, a cardiologist at Stanford (Calif.) University, and associates. Despite the expired patents, as of June 2021 no generic form of dapagliflozin appeared available for U.S. sale.
Medicare patients pay about $1,630/year out-of-pocket
“A key caveat” to this finding for dapagliflozin is that being cost-effective “is not by itself a mandate for routine clinical use,” Derek S. Chew, MD, and Daniel B. Mark, MD, said in an editorial that accompanied the report.
A major stumbling block for widespread U.S. prescribing of dapagliflozin to patients with HFrEF is its overall price tag for U.S. patients, estimated at $12 billion/year, as well as an out-of-pocket annual cost for individual Medicare patients of roughly $1,630/year. Adding this out-of-pocket cost to the copay for sacubitril/valsartan and two other much less expensive drug classes that together form the current mainstay, quadruple-drug regimen for HFrEF treatment means a potential annual cost paid by each Medicare patient of about $3,000, wrote Dr. Chew, a cardiologist, and Dr. Mark, a cardiologist and professor, both at Duke University, Durham, N.C.
They cited the precedent of the “unexpectedly slow” and “anemic” uptake of sacubitril/valsartan since its U.S. approval in 2015, a cost-effective agent with “comparable clinical effectiveness” to dapagliflozin. “Even with full inclusion [of sacubitril/valsartan] on formularies and elimination of preapproval requirements, use remains very low, and patient-borne out-of-pocket costs may be a key factor,” wrote Dr. Chew and Dr. Mark. They cited a results from a study that showed abandonment of new prescriptions at retail U.S. pharmacies spiked to a 60% rate when out-of-pocket cost exceeded $500.
More than what patients ‘can afford or are willing to spend’
The estimated $3,000-plus total out-of-pocket cost currently borne by some Medicare beneficiaries with HFrEF who have to shell out for both sacubitril/valsartan and dapagliflozin “appears to substantially exceed what many patients with heart failure can afford or are willing to spend,” wrote Dr. Chew and Dr. Mark.
Dr. Parizo and coauthors developed their cost-effectiveness model for dapagliflozin in treating HFrEF using primarily data collected in the DAPA-HF trial, which proved the efficacy of the drug for reducing cardiovascular deaths or acute heart failure events that led to hospitalization or intravenous outpatient treatment in more than 4,700 randomized patients with HFrEF. The trial enrolled roughly similar numbers of patients with or without type 2 diabetes.
The model showed an overall incremental cost-effectiveness ratio of $83,650/QALY, which was about the same regardless of whether patients also had type 2 diabetes. On a more granular level, the cost-effectiveness value estimate was $78,483/QALY in patients with mild health-status impairment due to their heart failure, and $97,608/QALY in patients with moderate impairment, a finding that underscores the importance of starting dapagliflozin treatment early in the course of HFrEF when disease effects are less severe. The analysis could not address value in patients with more advanced heart failure and in New York Heart Association functional class IV because fewer than 1% of patients in DAPA-HF were in this category.
Drug cost was a major determinant of cost-effectiveness. A 50% drop in cost from the Medicare benchmark of $473.64/month resulted in an incremental cost-effectiveness ratio of about $45,000/QALY (putting it into the high-value category based on the 2014 ACC/AHA formula), while a 50% rise in price yielded a value of nearly $123,000/QALY (still in the intermediate range, which spans from $50,000/QALY to $150,000/QALY). No other cost parameters had a meaningful effect on the cost-effectiveness calculation. The analyses also showed that using the basic cost assumptions, treatment with dapagliflozin needs to persist and remain effective for at least 44 months to produce a cost per QALY that’s less than $150,000. The authors stressed that their analysis considered heart failure effects and did not account for added benefit from treatment with dapagliflozin on preservation of renal function.
While it’s indisputable that treatment with dapagliflozin decreases health care costs by, for example, reducing hospitalizations for heart failure, each hospitalization costs just over $12,000, according to the assumptions made by Dr. Parizo and coauthors. But given dapagliflozin’s impact on this outcome, this cost saving translates into about $500/patient during 18 months on treatment (the median duration of treatment in DAPA-HF), which means the savings barely counterbalances the current cost of dapagliflozin treatment for 1 month, noted Dr. Chew and Dr. Mark.
The DAPA-HF trial was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Parizo had no disclosures and none of his coauthors had a relationship with AstraZeneca. Dr. Chew had no disclosures. Dr. Mark has received research grants from HeartFlow, Mayo Clinic, and Merck.
Although recent trial results have established the sodium glucose cotransporter 2 inhibitors dapagliflozin and empagliflozin as a key new part of the recommended multidrug treatment regimen for patients with heart failure with reduced ejection fraction, the current U.S. cost for dapagliflozin means it has merely “intermediate” value when it comes to cost-effectiveness.
A typical regimen with dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) costs about $474/month or roughly $5,700/year based on Medicare pricing. After factoring in the incremental clinical benefits producing by dapagliflozin seen in the DAPA-HF pivotal trial that helped establish its role, this price produces a cost per quality-adjusted life-year (QALY) gain of about $84,000, which puts dapagliflozin squarely in the intermediate range for value set in 2014 by a task force of the American College of Cardiology and the American Heart Association.
This cost-effectiveness value depends largely on the proven efficacy of dapagliflozin (Farxiga) for decreasing the incidence of cardiovascular death among treated patients with HFrEF, and puts the drug’s value roughly on par with another agent recently approved to treat such patients, sacubitril/valsartan (Entresto), which carries a cost-effectiveness value of about $45,000/QALY.
The U.S. cost per QALY for dapagliflozin treatment of patients with HFrEF dwarfed the value numbers calculated for several other countries that were generally one-tenth this size. This disparity stemmed from both the relatively high price for dapagliflozin in the U.S. compared with other countries – nearly tenfold higher – and relatively higher costs for all types of U.S. medical care, Justin T. Parizo, MD, and coauthors said in a recent report. But the cost, and hence the cost per QALY, of dapagliflozin may soon drop because certain patents on the drug expired in October 2020, added Dr. Parizo, a cardiologist at Stanford (Calif.) University, and associates. Despite the expired patents, as of June 2021 no generic form of dapagliflozin appeared available for U.S. sale.
Medicare patients pay about $1,630/year out-of-pocket
“A key caveat” to this finding for dapagliflozin is that being cost-effective “is not by itself a mandate for routine clinical use,” Derek S. Chew, MD, and Daniel B. Mark, MD, said in an editorial that accompanied the report.
A major stumbling block for widespread U.S. prescribing of dapagliflozin to patients with HFrEF is its overall price tag for U.S. patients, estimated at $12 billion/year, as well as an out-of-pocket annual cost for individual Medicare patients of roughly $1,630/year. Adding this out-of-pocket cost to the copay for sacubitril/valsartan and two other much less expensive drug classes that together form the current mainstay, quadruple-drug regimen for HFrEF treatment means a potential annual cost paid by each Medicare patient of about $3,000, wrote Dr. Chew, a cardiologist, and Dr. Mark, a cardiologist and professor, both at Duke University, Durham, N.C.
They cited the precedent of the “unexpectedly slow” and “anemic” uptake of sacubitril/valsartan since its U.S. approval in 2015, a cost-effective agent with “comparable clinical effectiveness” to dapagliflozin. “Even with full inclusion [of sacubitril/valsartan] on formularies and elimination of preapproval requirements, use remains very low, and patient-borne out-of-pocket costs may be a key factor,” wrote Dr. Chew and Dr. Mark. They cited a results from a study that showed abandonment of new prescriptions at retail U.S. pharmacies spiked to a 60% rate when out-of-pocket cost exceeded $500.
More than what patients ‘can afford or are willing to spend’
The estimated $3,000-plus total out-of-pocket cost currently borne by some Medicare beneficiaries with HFrEF who have to shell out for both sacubitril/valsartan and dapagliflozin “appears to substantially exceed what many patients with heart failure can afford or are willing to spend,” wrote Dr. Chew and Dr. Mark.
Dr. Parizo and coauthors developed their cost-effectiveness model for dapagliflozin in treating HFrEF using primarily data collected in the DAPA-HF trial, which proved the efficacy of the drug for reducing cardiovascular deaths or acute heart failure events that led to hospitalization or intravenous outpatient treatment in more than 4,700 randomized patients with HFrEF. The trial enrolled roughly similar numbers of patients with or without type 2 diabetes.
The model showed an overall incremental cost-effectiveness ratio of $83,650/QALY, which was about the same regardless of whether patients also had type 2 diabetes. On a more granular level, the cost-effectiveness value estimate was $78,483/QALY in patients with mild health-status impairment due to their heart failure, and $97,608/QALY in patients with moderate impairment, a finding that underscores the importance of starting dapagliflozin treatment early in the course of HFrEF when disease effects are less severe. The analysis could not address value in patients with more advanced heart failure and in New York Heart Association functional class IV because fewer than 1% of patients in DAPA-HF were in this category.
Drug cost was a major determinant of cost-effectiveness. A 50% drop in cost from the Medicare benchmark of $473.64/month resulted in an incremental cost-effectiveness ratio of about $45,000/QALY (putting it into the high-value category based on the 2014 ACC/AHA formula), while a 50% rise in price yielded a value of nearly $123,000/QALY (still in the intermediate range, which spans from $50,000/QALY to $150,000/QALY). No other cost parameters had a meaningful effect on the cost-effectiveness calculation. The analyses also showed that using the basic cost assumptions, treatment with dapagliflozin needs to persist and remain effective for at least 44 months to produce a cost per QALY that’s less than $150,000. The authors stressed that their analysis considered heart failure effects and did not account for added benefit from treatment with dapagliflozin on preservation of renal function.
While it’s indisputable that treatment with dapagliflozin decreases health care costs by, for example, reducing hospitalizations for heart failure, each hospitalization costs just over $12,000, according to the assumptions made by Dr. Parizo and coauthors. But given dapagliflozin’s impact on this outcome, this cost saving translates into about $500/patient during 18 months on treatment (the median duration of treatment in DAPA-HF), which means the savings barely counterbalances the current cost of dapagliflozin treatment for 1 month, noted Dr. Chew and Dr. Mark.
The DAPA-HF trial was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Parizo had no disclosures and none of his coauthors had a relationship with AstraZeneca. Dr. Chew had no disclosures. Dr. Mark has received research grants from HeartFlow, Mayo Clinic, and Merck.
FROM JAMA CARDIOLOGY
In-hospital resuscitation: Focus on effective chest pumps, prompt shocks
The keys to effective resuscitation in the hospital setting include effective compression and early defibrillation, according to Jessica Nave Allen, MD, FHM, a hospitalist with Emory University Hospital in Atlanta. She spoke about best practices in resuscitation medicine recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know CPR [cardiopulmonary resuscitation] and shocking are the two biggest determinants of outcomes, so really strive to make those chest compressions really high quality,” said Dr. Allen. She urged hospitalists to consider mechanical piston compressions and even “reverse CPR” when appropriate.
Dr. Allen offered several other tips about effective in-hospital resuscitation.
Don’t overcrowd the hospital room
There shouldn’t be more than eight people inside the room during a code, she said. If you’re the code leader, “make sure that somebody has already started high-quality chest compressions. You want to make sure that somebody is already on the airway. It’s usually two people, one person to actually hold the mask down to make sure there’s a good seal, and the other person to deliver the breaths.”
Two to three people should be assigned to chest compressions, Dr. Allen said, “and you need one or two nurses for medication delivery and grabbing things from the runners. And then you need to have a recorder and the code leader. Everyone else who’s not in one of those formalized roles needs to be outside the room. That includes the pharmacist, who usually stands at the door if you don’t have a code pharmacist at your institution.”
A helpful mnemonic for the resuscitation process is I(CA)RAMBO, which was developed at Tufts Medical Center and published in 2020, she said. The mnemonic stands for the following:
- I: Identify yourself as code leader.
- CA: Compression, Airway.
- R: Roles (assign roles in the resuscitation).
- A: Access (intravenous access is preferred to intraosseous, per the American Heart Association’s , unless intravenous access is unavailable, Dr. Allen noted).
- M: Monitor (make sure pads are placed correctly; turn the defibrillator on).
- B: Backboard.
- O: Oxygen.
Focus on high-quality chest compressions
The number of chest compressions must be 100-120 per minute, Dr. Allen said. You can time them to the beat of a song, such as “Stayin’ Alive,” or with a metronome, she said, “but whatever it is, you need to stay in that window.”
The correct compression depth is 2-2.4 inches. “That’s very difficult to do during the middle of a code, which is why it’s important to allow full recoil,” she said. “This doesn’t mean taking your hands off of the chest: You should actually never take your hands off of the chest. But you should allow the chest wall to return to its normal state. Also, make sure you aren’t off the chest for more for 10 seconds whenever you’re doing a rhythm check.”
Audiovisual feedback devices can provide insight into the quality of chest compressions. For example, some defibrillators are equipped with sensors that urge users to push harder and faster when appropriate. “Studies have shown that the quality of chest compressions goes up when you use these devices,” she said.
Don’t be afraid of mechanical chest compression
Although early research raised questions about the quality of resuscitation outcomes when mechanical piston chest compression devices are used, a 2015 systematic review and meta-analysis found that “man was equal to machine,” Dr. Allen said. “The bottom line is that these devices may be a reasonable alternative to conventional CPR in specific settings.”
American Heart Association guidelines state that mechanical compressions may be appropriate in certain specific situations “where the delivery of high-quality manual compressions may be challenging or dangerous for the provider.”
According to Dr. Allen, “there are times when it’s useful,” such as for a patient with COVID-19, in the cath lab, or in a medical helicopter.
Move quickly to defibrillation
“Most of us know that you want to shock as early as possible in shockable rhythms,” Dr. Allen said. Support, she said, comes from a 2008 study that linked delayed defibrillation to lower survival rates. “We want to shock as soon as possible, because your chances of surviving go down for every minute you wait.”
Take special care for patients with confirmed or suspected COVID-19
“Not surprisingly, the goals here are to minimize exposure to staff,” Dr. Allen said.
Put on personal protective equipment before entering the room even if care is delayed, she advised, and reduce the number of staff members in the room below the typical maximum of eight. “In COVID, it should be a maximum of six, and some institutions have even gotten it down to four where the code leaders are outside the room with an iPad.”
Use mechanical compression devices, she advised, and place patients on ventilators as soon as possible. She added: “Use a HEPA [high-efficiency particulate air] filter for all your airway modalities.”
CPR may be challenging in some cases, such as when a large, intubated patient is prone and cannot be quickly or safely flipped over. In those cases, consider posterior chest compressions, also known as reverse CPR, at vertebral positions T7-T10. “We have done reverse CPR on several COVID patients throughout the Emory system,” she said.
Debrief right after codes
“You really want to debrief with the code team,” Dr. Allen said. “If you don’t already have a policy in place at your institution, you should help come up with one where you sit down with the team and talk about what could you have done better as a group. It’s not a time to place blame. It’s a time to learn.”
Dr. Allen has disclosed no relevant financial relationships.
This article was updated 7/26/21.
A version of this article first appeared on Medscape.com.
The keys to effective resuscitation in the hospital setting include effective compression and early defibrillation, according to Jessica Nave Allen, MD, FHM, a hospitalist with Emory University Hospital in Atlanta. She spoke about best practices in resuscitation medicine recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know CPR [cardiopulmonary resuscitation] and shocking are the two biggest determinants of outcomes, so really strive to make those chest compressions really high quality,” said Dr. Allen. She urged hospitalists to consider mechanical piston compressions and even “reverse CPR” when appropriate.
Dr. Allen offered several other tips about effective in-hospital resuscitation.
Don’t overcrowd the hospital room
There shouldn’t be more than eight people inside the room during a code, she said. If you’re the code leader, “make sure that somebody has already started high-quality chest compressions. You want to make sure that somebody is already on the airway. It’s usually two people, one person to actually hold the mask down to make sure there’s a good seal, and the other person to deliver the breaths.”
Two to three people should be assigned to chest compressions, Dr. Allen said, “and you need one or two nurses for medication delivery and grabbing things from the runners. And then you need to have a recorder and the code leader. Everyone else who’s not in one of those formalized roles needs to be outside the room. That includes the pharmacist, who usually stands at the door if you don’t have a code pharmacist at your institution.”
A helpful mnemonic for the resuscitation process is I(CA)RAMBO, which was developed at Tufts Medical Center and published in 2020, she said. The mnemonic stands for the following:
- I: Identify yourself as code leader.
- CA: Compression, Airway.
- R: Roles (assign roles in the resuscitation).
- A: Access (intravenous access is preferred to intraosseous, per the American Heart Association’s , unless intravenous access is unavailable, Dr. Allen noted).
- M: Monitor (make sure pads are placed correctly; turn the defibrillator on).
- B: Backboard.
- O: Oxygen.
Focus on high-quality chest compressions
The number of chest compressions must be 100-120 per minute, Dr. Allen said. You can time them to the beat of a song, such as “Stayin’ Alive,” or with a metronome, she said, “but whatever it is, you need to stay in that window.”
The correct compression depth is 2-2.4 inches. “That’s very difficult to do during the middle of a code, which is why it’s important to allow full recoil,” she said. “This doesn’t mean taking your hands off of the chest: You should actually never take your hands off of the chest. But you should allow the chest wall to return to its normal state. Also, make sure you aren’t off the chest for more for 10 seconds whenever you’re doing a rhythm check.”
Audiovisual feedback devices can provide insight into the quality of chest compressions. For example, some defibrillators are equipped with sensors that urge users to push harder and faster when appropriate. “Studies have shown that the quality of chest compressions goes up when you use these devices,” she said.
Don’t be afraid of mechanical chest compression
Although early research raised questions about the quality of resuscitation outcomes when mechanical piston chest compression devices are used, a 2015 systematic review and meta-analysis found that “man was equal to machine,” Dr. Allen said. “The bottom line is that these devices may be a reasonable alternative to conventional CPR in specific settings.”
American Heart Association guidelines state that mechanical compressions may be appropriate in certain specific situations “where the delivery of high-quality manual compressions may be challenging or dangerous for the provider.”
According to Dr. Allen, “there are times when it’s useful,” such as for a patient with COVID-19, in the cath lab, or in a medical helicopter.
Move quickly to defibrillation
“Most of us know that you want to shock as early as possible in shockable rhythms,” Dr. Allen said. Support, she said, comes from a 2008 study that linked delayed defibrillation to lower survival rates. “We want to shock as soon as possible, because your chances of surviving go down for every minute you wait.”
Take special care for patients with confirmed or suspected COVID-19
“Not surprisingly, the goals here are to minimize exposure to staff,” Dr. Allen said.
Put on personal protective equipment before entering the room even if care is delayed, she advised, and reduce the number of staff members in the room below the typical maximum of eight. “In COVID, it should be a maximum of six, and some institutions have even gotten it down to four where the code leaders are outside the room with an iPad.”
Use mechanical compression devices, she advised, and place patients on ventilators as soon as possible. She added: “Use a HEPA [high-efficiency particulate air] filter for all your airway modalities.”
CPR may be challenging in some cases, such as when a large, intubated patient is prone and cannot be quickly or safely flipped over. In those cases, consider posterior chest compressions, also known as reverse CPR, at vertebral positions T7-T10. “We have done reverse CPR on several COVID patients throughout the Emory system,” she said.
Debrief right after codes
“You really want to debrief with the code team,” Dr. Allen said. “If you don’t already have a policy in place at your institution, you should help come up with one where you sit down with the team and talk about what could you have done better as a group. It’s not a time to place blame. It’s a time to learn.”
Dr. Allen has disclosed no relevant financial relationships.
This article was updated 7/26/21.
A version of this article first appeared on Medscape.com.
The keys to effective resuscitation in the hospital setting include effective compression and early defibrillation, according to Jessica Nave Allen, MD, FHM, a hospitalist with Emory University Hospital in Atlanta. She spoke about best practices in resuscitation medicine recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know CPR [cardiopulmonary resuscitation] and shocking are the two biggest determinants of outcomes, so really strive to make those chest compressions really high quality,” said Dr. Allen. She urged hospitalists to consider mechanical piston compressions and even “reverse CPR” when appropriate.
Dr. Allen offered several other tips about effective in-hospital resuscitation.
Don’t overcrowd the hospital room
There shouldn’t be more than eight people inside the room during a code, she said. If you’re the code leader, “make sure that somebody has already started high-quality chest compressions. You want to make sure that somebody is already on the airway. It’s usually two people, one person to actually hold the mask down to make sure there’s a good seal, and the other person to deliver the breaths.”
Two to three people should be assigned to chest compressions, Dr. Allen said, “and you need one or two nurses for medication delivery and grabbing things from the runners. And then you need to have a recorder and the code leader. Everyone else who’s not in one of those formalized roles needs to be outside the room. That includes the pharmacist, who usually stands at the door if you don’t have a code pharmacist at your institution.”
A helpful mnemonic for the resuscitation process is I(CA)RAMBO, which was developed at Tufts Medical Center and published in 2020, she said. The mnemonic stands for the following:
- I: Identify yourself as code leader.
- CA: Compression, Airway.
- R: Roles (assign roles in the resuscitation).
- A: Access (intravenous access is preferred to intraosseous, per the American Heart Association’s , unless intravenous access is unavailable, Dr. Allen noted).
- M: Monitor (make sure pads are placed correctly; turn the defibrillator on).
- B: Backboard.
- O: Oxygen.
Focus on high-quality chest compressions
The number of chest compressions must be 100-120 per minute, Dr. Allen said. You can time them to the beat of a song, such as “Stayin’ Alive,” or with a metronome, she said, “but whatever it is, you need to stay in that window.”
The correct compression depth is 2-2.4 inches. “That’s very difficult to do during the middle of a code, which is why it’s important to allow full recoil,” she said. “This doesn’t mean taking your hands off of the chest: You should actually never take your hands off of the chest. But you should allow the chest wall to return to its normal state. Also, make sure you aren’t off the chest for more for 10 seconds whenever you’re doing a rhythm check.”
Audiovisual feedback devices can provide insight into the quality of chest compressions. For example, some defibrillators are equipped with sensors that urge users to push harder and faster when appropriate. “Studies have shown that the quality of chest compressions goes up when you use these devices,” she said.
Don’t be afraid of mechanical chest compression
Although early research raised questions about the quality of resuscitation outcomes when mechanical piston chest compression devices are used, a 2015 systematic review and meta-analysis found that “man was equal to machine,” Dr. Allen said. “The bottom line is that these devices may be a reasonable alternative to conventional CPR in specific settings.”
American Heart Association guidelines state that mechanical compressions may be appropriate in certain specific situations “where the delivery of high-quality manual compressions may be challenging or dangerous for the provider.”
According to Dr. Allen, “there are times when it’s useful,” such as for a patient with COVID-19, in the cath lab, or in a medical helicopter.
Move quickly to defibrillation
“Most of us know that you want to shock as early as possible in shockable rhythms,” Dr. Allen said. Support, she said, comes from a 2008 study that linked delayed defibrillation to lower survival rates. “We want to shock as soon as possible, because your chances of surviving go down for every minute you wait.”
Take special care for patients with confirmed or suspected COVID-19
“Not surprisingly, the goals here are to minimize exposure to staff,” Dr. Allen said.
Put on personal protective equipment before entering the room even if care is delayed, she advised, and reduce the number of staff members in the room below the typical maximum of eight. “In COVID, it should be a maximum of six, and some institutions have even gotten it down to four where the code leaders are outside the room with an iPad.”
Use mechanical compression devices, she advised, and place patients on ventilators as soon as possible. She added: “Use a HEPA [high-efficiency particulate air] filter for all your airway modalities.”
CPR may be challenging in some cases, such as when a large, intubated patient is prone and cannot be quickly or safely flipped over. In those cases, consider posterior chest compressions, also known as reverse CPR, at vertebral positions T7-T10. “We have done reverse CPR on several COVID patients throughout the Emory system,” she said.
Debrief right after codes
“You really want to debrief with the code team,” Dr. Allen said. “If you don’t already have a policy in place at your institution, you should help come up with one where you sit down with the team and talk about what could you have done better as a group. It’s not a time to place blame. It’s a time to learn.”
Dr. Allen has disclosed no relevant financial relationships.
This article was updated 7/26/21.
A version of this article first appeared on Medscape.com.
FROM SHM CONVERGE 2021
Neurologists brace and prepare for long-COVID fallout
“If there’s one universal truth amongst all the patients I’ve interviewed, it’s that they’re often brushed aside, pigeonholed, or, frankly, abandoned,” said Greg Vanichkachorn, MD, MPH, a family physician and founder of Mayo Clinic’s COVID-19 Activity Rehabilitation Program (CARP).
Take a nap. Tough it out. Push through it. Dr. Vanichkachorn describes the frustration voiced by thousands of patients whose lives continue to be disrupted and thrown into upheaval.
Brain fog. Cognitive dysfunction. Headaches. These are just a few of the manifestations of what the National Institutes of Health has termed post-acute sequelae of SARS COVID-2 (PASC), more commonly known as long-COVID.
PASC is loosely defined as symptoms and/or sequelae that persist for several weeks to months after the initial infection has cleared.
A total of 33.6% (95% confidence interval, 11.17-34.07) of patients with COVID-19 experience neurologic sequelae in the first 6 months following resolution of the infection. Almost half of cases (12.8%; 95% CI, 12.36-13.33) represented first-time diagnoses.
“Anecdotally, the longer we go into this, and the more people that, in the past, have been infected with COVID-19, the more patients will be seeing neurologists with some of these complaints,” said Ralph Sacco, MD, professor and Olemberg chair of neurology at University of Miami, and past president of the American Academy of Neurology.
Neurologic detritus
Further complicating the epidemiologic picture is the broad array of clinical and functional symptoms. “What we call long-haul COVID is not a single entity,” explained Michel Toledano, MD, a neurology consultant and a member of the CARP team at the Mayo Clinic in Rochester, Minnesota. Patients present with persistent or emergent polysymptomatic and multisystemic diseases that often include neurologic symptoms, he said. In many circumstances, they had an acute infection with either very mild symptoms or no symptoms at all.
“There’s no doubt that these people are experiencing significant neurologic symptoms, but it remains unclear whether the driving factor is mainly systemic or the nervous system independently of what is happening in the body,” he said.
Like patients with SARS-CoV-1 and Middle Eastern respiratory syndrome (MERS), patients recovering from confirmed or suspected SARS-CoV-2 infections experience a variety of self-reported neurologic symptoms that vary in terms of time frame, duration, and severity.
Take Jacqueline Jolly, for example, a 50-year-old single mother and construction permit contractor living outside of Tampa, Florida. She was diagnosed with COVID-19 in January 2021. Jolly explained that she was never sick enough to be admitted to the hospital and yet is still not close to full recovery. Lingering, debilitating symptoms include executive function challenges, anosmia, headaches, and paresthesia that frequently bring her to the edge of losing consciousness. She has not returned to work, despite multiple attempts.
Vicky Nunally, a 35-year-old single mother and medical office assistant who lives in the suburbs of Atlanta, Georgia, recounted that she landed in the intensive care unit with a severe SARS-CoV-2 infection. Roughly 6 months later, she continues to experience debilitating headaches, brain fog, and cognitive delays. Her endometriosis has flared up. She says that she is depressed, anxious, and has returned to therapy. “It makes you feel crazy,” she said.
Debilitating, pervasive symptoms
Findings from an international survey of 3,762 respondents that was published on April 21 in medRXiv underscore that PASC occurs predominantly in middle-aged women (78.9% were women; 31% were aged 40-49 years; 25.0% were aged 50-59 years). For the most part, it manifests similarly among people with prior confirmed or suspected SARS-CoV-2 infections. In the study, symptoms were reported in both cohorts well past the initial infection; symptoms persisted past 90 days in 96% of patients and for at least 6 months in 65.2%.
Similarly, a recent study showed that 68% of patients who were enrolled in Mayo Clinic’s CARP as of June 2020 were women, middle aged (mean age, 45 ± 14.2 years), and presented roughly 3 months (94.4 ± 65 days) post diagnosis. Of these patients, 75% had not been previously hospitalized.
In both studies, fatigue and cognitive dysfunction were consistently cited as the most debilitating and pervasive manifestations lasting more than 6 months. Others included postexertional (physical or mental) malaise, sensorimotor symptoms, headaches, and memory problems.
Reports of even more severe neurologic first-time diagnoses are emerging. Findings from the Lancet Psychiatry study showed there was a small but clinically relevant risk for a range of conditions that included intracranial hemorrhage (0.14%; 95% CI, 0.10-0.20), ischemic stroke (0.43%; 95% CI, 0.36-0.52), parkinsonism (0.07%; 95% CI, 0.05-0.12), and nerve root/plexus disorders (2.69%; 95% CI, 2.51-2.89).
Dr. Toledano noted that he’s also seen patients who developed autonomic/small-fiber dysfunction. Preliminary data from a retrospective chart review suggest that the most likely diagnosis is orthostatic intolerance without tachycardia or hypotension.
In the study, 63% (17) of the 27 participants who met the inclusion criteria had abnormal results on function testing, but Composite Autonomic Severity Score results indicated mostly mild disease (sudomotor range, 0-3 [median, 0]; cardiovagal, 0-3 [median, 0]; cardiovascular adrenergic, 0-4 [median, 0]).
“The pattern that’s emerging is consistent with deconditioning,” Dr. Toledano said. “However, a small proportion of patients do have evidence of damage to the nervous system, which is something we’ve seen with other viruses.”
Proliferation of long-COVID clinics
A few of these patients with orthostatic intolerance developed postural tachycardia syndrome or experienced exacerbations of preexisting sensory or autonomic small-fiber neuropathies. “These post-viral, autonomic neuropathies tend to be self-limiting, but we’re starting to see different levels of involvement,” Dr. Toledano explained. At present, causality and/or underlying mechanisms are unclear.
Speaking on behalf of the American Academy of Neurology, Dr. Sacco acknowledged the challenges that lie ahead. “Like any neurological symptom that continues to affect a patient’s quality of life, you may need to seek the expertise of a neurologist. The only issue is that some may still not be sure exactly what to do; we don’t have all the data yet,” he said.
On the flip side, he pointed to the lessons of the past year and how quickly health care systems were able to pivot to deal with the pandemic and critically ill patients, then pivot again to disseminate vaccines, and how they are pivoting yet again to address PASC.
Across the nation, numerous hospitals and health care systems and even small private clinics have launched clinics that focus on long-COVID, including Mayo.
The program has a multidisciplinary, collaborative framework and offers both face-to-face and video telemedicine consultations. The latter are geared toward ensuring that under-resourced populations can access needed care and assistance.
“At Mayo, we have a centralized triage system to help target patients’ visits so that appropriate subspecialties and studies can be preordered,” explained Dr. Toledano. During these visits, patients are assessed for underlying conditions and possible signs of decompensation, as well as functional and physical needs and psychosocial challenges. Thereafter, patients enter either CARP, which offers active rehabilitation for up to 3 months after resolution of the acute infection, or the Post-COVID Care Center, which focuses on patients whose condition is not improving or who are demonstrating signs of central sensitization.
An uphill battle
Both programs incorporate individually paced occupational and physical therapy aimed at ameliorating symptoms, restoring function, developing psychosocial coping skills, and, ultimately, facilitating a return to work. “The idea is, if we can meet with these patients sooner than later and help them recover in an appropriate fashion, they will exit the program faster,” Dr. Vanichkachorn said. “We do see a group of patients who tend to get better right around the 4-month period.”
Although telemedicine provides an opportunity for clinicians outside these hospital systems to engage patients, Dr. Vanichkachorn pointed out that almost all the initial treatments can be offered in the local community by a provider who has adequate time and knowledge of the condition.
He also acknowledged the potential for an uphill battle, especially among those who cling to the belief that PASC is simply a manifestation of anxiety or depression. “This is something that we’ve seen previously with SARS and MERS, as well as in conjunction with fibromyalgia and chronic fatigue, only now with greater magnitude,” he said. “This is a real condition that has important and huge ramifications on a person’s ability to function.”
The silver lining is that last December, the NIH announced that it was allocating $1.1 billion for research through its NIH PASC Initiative. Like other institutions, Mayo is waiting to hear what it has been awarded. In the meantime, it has developed a biorepository of patient samples to better understand pathophysiologic mechanisms underlying PASC and to identify possible biomarkers that differentiate these patients.
Despite the challenges, Dr. Vanichkachorn is hopeful. “If we can get a concrete understanding of what is occurring on the chemical level and develop diagnostic tests, then the education will follow. Providers won’t be able to ignore it any longer,” he said.
The interviewees have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“If there’s one universal truth amongst all the patients I’ve interviewed, it’s that they’re often brushed aside, pigeonholed, or, frankly, abandoned,” said Greg Vanichkachorn, MD, MPH, a family physician and founder of Mayo Clinic’s COVID-19 Activity Rehabilitation Program (CARP).
Take a nap. Tough it out. Push through it. Dr. Vanichkachorn describes the frustration voiced by thousands of patients whose lives continue to be disrupted and thrown into upheaval.
Brain fog. Cognitive dysfunction. Headaches. These are just a few of the manifestations of what the National Institutes of Health has termed post-acute sequelae of SARS COVID-2 (PASC), more commonly known as long-COVID.
PASC is loosely defined as symptoms and/or sequelae that persist for several weeks to months after the initial infection has cleared.
A total of 33.6% (95% confidence interval, 11.17-34.07) of patients with COVID-19 experience neurologic sequelae in the first 6 months following resolution of the infection. Almost half of cases (12.8%; 95% CI, 12.36-13.33) represented first-time diagnoses.
“Anecdotally, the longer we go into this, and the more people that, in the past, have been infected with COVID-19, the more patients will be seeing neurologists with some of these complaints,” said Ralph Sacco, MD, professor and Olemberg chair of neurology at University of Miami, and past president of the American Academy of Neurology.
Neurologic detritus
Further complicating the epidemiologic picture is the broad array of clinical and functional symptoms. “What we call long-haul COVID is not a single entity,” explained Michel Toledano, MD, a neurology consultant and a member of the CARP team at the Mayo Clinic in Rochester, Minnesota. Patients present with persistent or emergent polysymptomatic and multisystemic diseases that often include neurologic symptoms, he said. In many circumstances, they had an acute infection with either very mild symptoms or no symptoms at all.
“There’s no doubt that these people are experiencing significant neurologic symptoms, but it remains unclear whether the driving factor is mainly systemic or the nervous system independently of what is happening in the body,” he said.
Like patients with SARS-CoV-1 and Middle Eastern respiratory syndrome (MERS), patients recovering from confirmed or suspected SARS-CoV-2 infections experience a variety of self-reported neurologic symptoms that vary in terms of time frame, duration, and severity.
Take Jacqueline Jolly, for example, a 50-year-old single mother and construction permit contractor living outside of Tampa, Florida. She was diagnosed with COVID-19 in January 2021. Jolly explained that she was never sick enough to be admitted to the hospital and yet is still not close to full recovery. Lingering, debilitating symptoms include executive function challenges, anosmia, headaches, and paresthesia that frequently bring her to the edge of losing consciousness. She has not returned to work, despite multiple attempts.
Vicky Nunally, a 35-year-old single mother and medical office assistant who lives in the suburbs of Atlanta, Georgia, recounted that she landed in the intensive care unit with a severe SARS-CoV-2 infection. Roughly 6 months later, she continues to experience debilitating headaches, brain fog, and cognitive delays. Her endometriosis has flared up. She says that she is depressed, anxious, and has returned to therapy. “It makes you feel crazy,” she said.
Debilitating, pervasive symptoms
Findings from an international survey of 3,762 respondents that was published on April 21 in medRXiv underscore that PASC occurs predominantly in middle-aged women (78.9% were women; 31% were aged 40-49 years; 25.0% were aged 50-59 years). For the most part, it manifests similarly among people with prior confirmed or suspected SARS-CoV-2 infections. In the study, symptoms were reported in both cohorts well past the initial infection; symptoms persisted past 90 days in 96% of patients and for at least 6 months in 65.2%.
Similarly, a recent study showed that 68% of patients who were enrolled in Mayo Clinic’s CARP as of June 2020 were women, middle aged (mean age, 45 ± 14.2 years), and presented roughly 3 months (94.4 ± 65 days) post diagnosis. Of these patients, 75% had not been previously hospitalized.
In both studies, fatigue and cognitive dysfunction were consistently cited as the most debilitating and pervasive manifestations lasting more than 6 months. Others included postexertional (physical or mental) malaise, sensorimotor symptoms, headaches, and memory problems.
Reports of even more severe neurologic first-time diagnoses are emerging. Findings from the Lancet Psychiatry study showed there was a small but clinically relevant risk for a range of conditions that included intracranial hemorrhage (0.14%; 95% CI, 0.10-0.20), ischemic stroke (0.43%; 95% CI, 0.36-0.52), parkinsonism (0.07%; 95% CI, 0.05-0.12), and nerve root/plexus disorders (2.69%; 95% CI, 2.51-2.89).
Dr. Toledano noted that he’s also seen patients who developed autonomic/small-fiber dysfunction. Preliminary data from a retrospective chart review suggest that the most likely diagnosis is orthostatic intolerance without tachycardia or hypotension.
In the study, 63% (17) of the 27 participants who met the inclusion criteria had abnormal results on function testing, but Composite Autonomic Severity Score results indicated mostly mild disease (sudomotor range, 0-3 [median, 0]; cardiovagal, 0-3 [median, 0]; cardiovascular adrenergic, 0-4 [median, 0]).
“The pattern that’s emerging is consistent with deconditioning,” Dr. Toledano said. “However, a small proportion of patients do have evidence of damage to the nervous system, which is something we’ve seen with other viruses.”
Proliferation of long-COVID clinics
A few of these patients with orthostatic intolerance developed postural tachycardia syndrome or experienced exacerbations of preexisting sensory or autonomic small-fiber neuropathies. “These post-viral, autonomic neuropathies tend to be self-limiting, but we’re starting to see different levels of involvement,” Dr. Toledano explained. At present, causality and/or underlying mechanisms are unclear.
Speaking on behalf of the American Academy of Neurology, Dr. Sacco acknowledged the challenges that lie ahead. “Like any neurological symptom that continues to affect a patient’s quality of life, you may need to seek the expertise of a neurologist. The only issue is that some may still not be sure exactly what to do; we don’t have all the data yet,” he said.
On the flip side, he pointed to the lessons of the past year and how quickly health care systems were able to pivot to deal with the pandemic and critically ill patients, then pivot again to disseminate vaccines, and how they are pivoting yet again to address PASC.
Across the nation, numerous hospitals and health care systems and even small private clinics have launched clinics that focus on long-COVID, including Mayo.
The program has a multidisciplinary, collaborative framework and offers both face-to-face and video telemedicine consultations. The latter are geared toward ensuring that under-resourced populations can access needed care and assistance.
“At Mayo, we have a centralized triage system to help target patients’ visits so that appropriate subspecialties and studies can be preordered,” explained Dr. Toledano. During these visits, patients are assessed for underlying conditions and possible signs of decompensation, as well as functional and physical needs and psychosocial challenges. Thereafter, patients enter either CARP, which offers active rehabilitation for up to 3 months after resolution of the acute infection, or the Post-COVID Care Center, which focuses on patients whose condition is not improving or who are demonstrating signs of central sensitization.
An uphill battle
Both programs incorporate individually paced occupational and physical therapy aimed at ameliorating symptoms, restoring function, developing psychosocial coping skills, and, ultimately, facilitating a return to work. “The idea is, if we can meet with these patients sooner than later and help them recover in an appropriate fashion, they will exit the program faster,” Dr. Vanichkachorn said. “We do see a group of patients who tend to get better right around the 4-month period.”
Although telemedicine provides an opportunity for clinicians outside these hospital systems to engage patients, Dr. Vanichkachorn pointed out that almost all the initial treatments can be offered in the local community by a provider who has adequate time and knowledge of the condition.
He also acknowledged the potential for an uphill battle, especially among those who cling to the belief that PASC is simply a manifestation of anxiety or depression. “This is something that we’ve seen previously with SARS and MERS, as well as in conjunction with fibromyalgia and chronic fatigue, only now with greater magnitude,” he said. “This is a real condition that has important and huge ramifications on a person’s ability to function.”
The silver lining is that last December, the NIH announced that it was allocating $1.1 billion for research through its NIH PASC Initiative. Like other institutions, Mayo is waiting to hear what it has been awarded. In the meantime, it has developed a biorepository of patient samples to better understand pathophysiologic mechanisms underlying PASC and to identify possible biomarkers that differentiate these patients.
Despite the challenges, Dr. Vanichkachorn is hopeful. “If we can get a concrete understanding of what is occurring on the chemical level and develop diagnostic tests, then the education will follow. Providers won’t be able to ignore it any longer,” he said.
The interviewees have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“If there’s one universal truth amongst all the patients I’ve interviewed, it’s that they’re often brushed aside, pigeonholed, or, frankly, abandoned,” said Greg Vanichkachorn, MD, MPH, a family physician and founder of Mayo Clinic’s COVID-19 Activity Rehabilitation Program (CARP).
Take a nap. Tough it out. Push through it. Dr. Vanichkachorn describes the frustration voiced by thousands of patients whose lives continue to be disrupted and thrown into upheaval.
Brain fog. Cognitive dysfunction. Headaches. These are just a few of the manifestations of what the National Institutes of Health has termed post-acute sequelae of SARS COVID-2 (PASC), more commonly known as long-COVID.
PASC is loosely defined as symptoms and/or sequelae that persist for several weeks to months after the initial infection has cleared.
A total of 33.6% (95% confidence interval, 11.17-34.07) of patients with COVID-19 experience neurologic sequelae in the first 6 months following resolution of the infection. Almost half of cases (12.8%; 95% CI, 12.36-13.33) represented first-time diagnoses.
“Anecdotally, the longer we go into this, and the more people that, in the past, have been infected with COVID-19, the more patients will be seeing neurologists with some of these complaints,” said Ralph Sacco, MD, professor and Olemberg chair of neurology at University of Miami, and past president of the American Academy of Neurology.
Neurologic detritus
Further complicating the epidemiologic picture is the broad array of clinical and functional symptoms. “What we call long-haul COVID is not a single entity,” explained Michel Toledano, MD, a neurology consultant and a member of the CARP team at the Mayo Clinic in Rochester, Minnesota. Patients present with persistent or emergent polysymptomatic and multisystemic diseases that often include neurologic symptoms, he said. In many circumstances, they had an acute infection with either very mild symptoms or no symptoms at all.
“There’s no doubt that these people are experiencing significant neurologic symptoms, but it remains unclear whether the driving factor is mainly systemic or the nervous system independently of what is happening in the body,” he said.
Like patients with SARS-CoV-1 and Middle Eastern respiratory syndrome (MERS), patients recovering from confirmed or suspected SARS-CoV-2 infections experience a variety of self-reported neurologic symptoms that vary in terms of time frame, duration, and severity.
Take Jacqueline Jolly, for example, a 50-year-old single mother and construction permit contractor living outside of Tampa, Florida. She was diagnosed with COVID-19 in January 2021. Jolly explained that she was never sick enough to be admitted to the hospital and yet is still not close to full recovery. Lingering, debilitating symptoms include executive function challenges, anosmia, headaches, and paresthesia that frequently bring her to the edge of losing consciousness. She has not returned to work, despite multiple attempts.
Vicky Nunally, a 35-year-old single mother and medical office assistant who lives in the suburbs of Atlanta, Georgia, recounted that she landed in the intensive care unit with a severe SARS-CoV-2 infection. Roughly 6 months later, she continues to experience debilitating headaches, brain fog, and cognitive delays. Her endometriosis has flared up. She says that she is depressed, anxious, and has returned to therapy. “It makes you feel crazy,” she said.
Debilitating, pervasive symptoms
Findings from an international survey of 3,762 respondents that was published on April 21 in medRXiv underscore that PASC occurs predominantly in middle-aged women (78.9% were women; 31% were aged 40-49 years; 25.0% were aged 50-59 years). For the most part, it manifests similarly among people with prior confirmed or suspected SARS-CoV-2 infections. In the study, symptoms were reported in both cohorts well past the initial infection; symptoms persisted past 90 days in 96% of patients and for at least 6 months in 65.2%.
Similarly, a recent study showed that 68% of patients who were enrolled in Mayo Clinic’s CARP as of June 2020 were women, middle aged (mean age, 45 ± 14.2 years), and presented roughly 3 months (94.4 ± 65 days) post diagnosis. Of these patients, 75% had not been previously hospitalized.
In both studies, fatigue and cognitive dysfunction were consistently cited as the most debilitating and pervasive manifestations lasting more than 6 months. Others included postexertional (physical or mental) malaise, sensorimotor symptoms, headaches, and memory problems.
Reports of even more severe neurologic first-time diagnoses are emerging. Findings from the Lancet Psychiatry study showed there was a small but clinically relevant risk for a range of conditions that included intracranial hemorrhage (0.14%; 95% CI, 0.10-0.20), ischemic stroke (0.43%; 95% CI, 0.36-0.52), parkinsonism (0.07%; 95% CI, 0.05-0.12), and nerve root/plexus disorders (2.69%; 95% CI, 2.51-2.89).
Dr. Toledano noted that he’s also seen patients who developed autonomic/small-fiber dysfunction. Preliminary data from a retrospective chart review suggest that the most likely diagnosis is orthostatic intolerance without tachycardia or hypotension.
In the study, 63% (17) of the 27 participants who met the inclusion criteria had abnormal results on function testing, but Composite Autonomic Severity Score results indicated mostly mild disease (sudomotor range, 0-3 [median, 0]; cardiovagal, 0-3 [median, 0]; cardiovascular adrenergic, 0-4 [median, 0]).
“The pattern that’s emerging is consistent with deconditioning,” Dr. Toledano said. “However, a small proportion of patients do have evidence of damage to the nervous system, which is something we’ve seen with other viruses.”
Proliferation of long-COVID clinics
A few of these patients with orthostatic intolerance developed postural tachycardia syndrome or experienced exacerbations of preexisting sensory or autonomic small-fiber neuropathies. “These post-viral, autonomic neuropathies tend to be self-limiting, but we’re starting to see different levels of involvement,” Dr. Toledano explained. At present, causality and/or underlying mechanisms are unclear.
Speaking on behalf of the American Academy of Neurology, Dr. Sacco acknowledged the challenges that lie ahead. “Like any neurological symptom that continues to affect a patient’s quality of life, you may need to seek the expertise of a neurologist. The only issue is that some may still not be sure exactly what to do; we don’t have all the data yet,” he said.
On the flip side, he pointed to the lessons of the past year and how quickly health care systems were able to pivot to deal with the pandemic and critically ill patients, then pivot again to disseminate vaccines, and how they are pivoting yet again to address PASC.
Across the nation, numerous hospitals and health care systems and even small private clinics have launched clinics that focus on long-COVID, including Mayo.
The program has a multidisciplinary, collaborative framework and offers both face-to-face and video telemedicine consultations. The latter are geared toward ensuring that under-resourced populations can access needed care and assistance.
“At Mayo, we have a centralized triage system to help target patients’ visits so that appropriate subspecialties and studies can be preordered,” explained Dr. Toledano. During these visits, patients are assessed for underlying conditions and possible signs of decompensation, as well as functional and physical needs and psychosocial challenges. Thereafter, patients enter either CARP, which offers active rehabilitation for up to 3 months after resolution of the acute infection, or the Post-COVID Care Center, which focuses on patients whose condition is not improving or who are demonstrating signs of central sensitization.
An uphill battle
Both programs incorporate individually paced occupational and physical therapy aimed at ameliorating symptoms, restoring function, developing psychosocial coping skills, and, ultimately, facilitating a return to work. “The idea is, if we can meet with these patients sooner than later and help them recover in an appropriate fashion, they will exit the program faster,” Dr. Vanichkachorn said. “We do see a group of patients who tend to get better right around the 4-month period.”
Although telemedicine provides an opportunity for clinicians outside these hospital systems to engage patients, Dr. Vanichkachorn pointed out that almost all the initial treatments can be offered in the local community by a provider who has adequate time and knowledge of the condition.
He also acknowledged the potential for an uphill battle, especially among those who cling to the belief that PASC is simply a manifestation of anxiety or depression. “This is something that we’ve seen previously with SARS and MERS, as well as in conjunction with fibromyalgia and chronic fatigue, only now with greater magnitude,” he said. “This is a real condition that has important and huge ramifications on a person’s ability to function.”
The silver lining is that last December, the NIH announced that it was allocating $1.1 billion for research through its NIH PASC Initiative. Like other institutions, Mayo is waiting to hear what it has been awarded. In the meantime, it has developed a biorepository of patient samples to better understand pathophysiologic mechanisms underlying PASC and to identify possible biomarkers that differentiate these patients.
Despite the challenges, Dr. Vanichkachorn is hopeful. “If we can get a concrete understanding of what is occurring on the chemical level and develop diagnostic tests, then the education will follow. Providers won’t be able to ignore it any longer,” he said.
The interviewees have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Trastuzumab deruxtecan-related lung disease in MBC patients can occur anytime in first year
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
Although rates are generally low, interstitial lung disease (ILD) can occur at any point in the first year of treatment with trastuzumab deruxtecan (T-DXd) for HER2-positive metastatic breast cancer (MBC).
That’s according to a pooled analysis of three early clinical trials with the drug that was reported at the European Society for Medical Oncology (ESMO): Breast Cancer virtual meeting.
Over a 5-year analysis period, the rate of any grade of ILD was 15.5%. The majority (79%) of those events were grade 1 or 2, observed pulmonologist Charles A. Powell, MD, of Icahn School of Medicine at Mount Sinai in New York, who presented the findings.
Of the 245 patients who were included in the analysis, 38 had an ILD event deemed related to treatment. A respective 9 (3.7%) and 21 (8.6%) had events graded as 1 or 2, 1 patient each (0.4%) had a grade 3 or 4 event, and 6 (2.4%) patients had a grade 5 event.
The timing of the first identified ILD event varied from 1.1 months to 20.8 months, given a median of 5.6 months overall. “This highlights an opportunity for more timely detection of ILD,” Dr. Powell suggested. He added that in almost all (97%) cases, ILD occurred before 12 months and the risk may even decrease over time “suggesting that the risk is not cumulative.”
He cautioned, however: “It is important to note that this analysis is exploratory and hypothesis generating in nature.”
ILD occurs with other cancer drugs
ILD is not just associated with T-DXd treatment, said the invited discussant for the trial, Harold J. Burstein, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“It’s important for clinicians to remember that ILD/pneumonitis is an uncommon, but potentially very serious side effect that affects many breast cancer treatments,” he said.
That not only includes T-DXd, but other newer drugs such the cyclin dependent kinase (CDK) 4/6 inhibitors and immune checkpoint inhibitors, as well as other older more established drugs including taxanes, cyclophosphamide and even the mTOR inhibitor everolimus.
“Both clinicians and patients need to be aware of this risk. It’s part of the differential diagnosis for any patient who develops either ground glass changes or other infiltrates on a CT scan, or who has symptoms,” Dr. Burstein added.
Investigating ILD in T-DXd trials
T-DXd (Enhertu) is an anti-HER2-antibody drug conjugate that contains a humanized anti-HER2 IgG1 monoclonal antibody akin to trastuzumab that is linked to DXd, a topoisomerase I inhibitor that is a derivative of exatecan.
It has been approved for use in patients with HER2-positive metastatic breast cancer after two other HER2 treatments fail in the United States and Europe, and after chemotherapy in Japan, noted Dr. Powell. This is largely due to the results from the phase 2, open-label DESTINY-Breast01 trial.
“In breast cancer, T-DXd continues to demonstrate clinically meaningful efficacy with a median duration of response of more than 20 months in a heavily pretreated population,” he said. Objective response rates seen in the DESTINY-Breast01 trial were around 60%, and the median progression-free survival was a little over 19 months.
To look at the issue of drug-related ILD events in patients treated with T-DXd for HER2-positive MBC, an independent adjudication committee was formed to look at all the imaging and clinical data from the DESTINY-Breast01 trial and two single-arm phase 1 trials (NCT02564900 and NCT03383692).
In all, data on 245 patients who had been treated with T-DXd at the approved dose of 5.4 mg/kg in those trials between August 2015 and June 2020 were analyzed.
Dealing with lung toxicity
“We are getting new drugs to improve the treatment of cancer, but they always come with a price in terms of toxicity,” observed David Cameron, MD, professor of medical oncology at Edinburgh University in Scotland. Dr. Cameron chaired the session.
“Several measures were taken to identify and mitigate ILD,” across all the T-DXd studies, Dr. Powell explained. As well as the independent adjudication committee, available guidelines were followed and updated on how to diagnose and treat drug-induced lung injuries, and a “safe use” campaign was run in 2019.
Many patients in the early MBC studies were recruited before these measures were in place, such as the use of systemic steroids to manage low-grade events.
The bottom line, however, is that if a patient develops ILD then treatment should be stopped, Dr. Powell said. “Patients with grade 1 events may restart once the ILD has resolved, but those with grade 2 to 4 events must discontinue treatment.”
Dr. Powell concluded: “The overall clinical data support the positive risk-benefit profile of T-DXd. Phase 3 randomized controlled trials in breast cancer are ongoing.”
ILD also seen in monarchE trial with abemaciclib
Data on ILD events seen in the phase 3 monarchE trial were also reported separately at the ESMO Breast Cancer virtual meeting. The analysis population included 2,971 patients who had been treated with the CDK 4/6 inhibitor abemaciclib (Verzenio) together with endocrine therapy and 2,800 who had received endocrine therapy alone in the early-stage, adjuvant advanced breast cancer setting.
Most ILD (97%) events that occurred were single occurrences, with any grade of ILD occurring in a higher percentage of patients treated with abemaciclib with endocrine therapy than endocrine therapy alone (2.9% vs. 1.2%). Grade 3 events occurred in a respective 0.4% and 0.0% of patients.
So who’s at risk?
The risk factors for ILD and pneumonitis are not well characterized with either of the two drugs discussed, Dr. Burstein observed.
“In the abemaciclib experience, it looked like obesity might be a predisposing factor, with trastuzumab deruxtecan, it looked like patients of Asian ancestry were greater risk, but we need more data to really understand who’s at jeopardy.”
Dr. Burstein observed: “This is something patients need to be aware of as they’re contemplating this treatment.”
While data to prove the benefit of the drug need to mature, Dr. Burstein “would likely discontinue therapy” if a patient were to develop ILD or pneumonitis and treat accordingly.
As for T-DXd, he said: “It’s important that patients know that lung disease is a potentially severe side effect of treatment and that any respiratory symptoms need to be jumped on quickly.”
While prospective studies are now needed, and the phase 3 data should help to better understand the risk of ILD with T-DXd, Dr. Burstein believes it will be important to develop algorithms to ensure the safe administration of the drug.
These algorithms should include “appropriate surveillance and monitoring, especially as we think about trying to move this drug forward into the early stage setting where we’re using it in women who have favorable prognosis, and potentially curative situations for breast cancer.”
The trastuzumab deruxtecan trials were cosponsored by Daiichi Sankyo and AstraZeneca. The monarchE trial was supported by Eli Lilly.
Dr. Powell acknowledged receiving personal fees for acting as an advisory or consultant to both companies as well as to Voluntis. Dr. Burstein had nothing to disclose, and Dr. Cameron had no relevant financial interests in the data being presented.
FROM ESMO BREAST CANCER 2021
Subclinical myocarditis found in some athletes post COVID
Myocarditis is present in a small percentage of competitive athletes after COVID-19 infection, even in those without symptoms, new research suggests.
In a cohort study of 1,597 competitive collegiate athletes undergoing comprehensive cardiovascular testing in the United States, the prevalence of clinical myocarditis based on a symptom-based screening strategy was only 0.31%.
But screening with cardiac MRI increased the prevalence of clinical and subclinical myocarditis by a factor of 7.4, to 2.3%, the authors reported.
The findings are published online May 27, 2021, in JAMA Cardiology.
“It was the largest study to evaluate college athletes who have had COVID with extensive cardiac testing, including MRI, and this gave us a very objective look at the cardiac findings, as they were not purely based upon a subjective evaluation of symptoms,” lead investigator Curt J. Daniels, MD, professor at Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Unfortunately, our study showed that athletes can be asymptomatic, or at least not report symptoms. This is a very subjective feature, and we don’t know if they don’t report symptoms because they didn’t want to get tested. That is why we took a very objective approach,” Dr. Daniels said.
The finding that more than half of the asymptomatic athletes had myocarditis, or as the investigators called it, “subclinical myocarditis,” was a surprise, he acknowledged.
“More than half of the athletes found to have myocarditis reported no symptoms, and yes, that was a surprise, because prior to this study, the protocols that had been published stated that you had to have symptoms to even enter into the protocol for cardiac MRI. But, as our ... paper shows, if we had followed that protocol, we only would have found about 5 cases of myocarditis, as opposed to the total of 37 we found with cardiac MRI,” Dr. Daniels said.
In October 2020, the American College of Cardiology’s Sports and Exercise Council recommended that cardiac MRI be limited to athletes who exhibited symptoms as part of their guide to ensuring a safe return to play.
As reported by this news organization the council recommended a tiered approach to screening based on the presence of symptoms, followed by electrocardiography, injury biomarkers, and echocardiography. Any abnormalities detected were to be further characterized by the selective use of cardiac MRI.
At the time, there were relatively few data to support the recommendations, and all stakeholders called for larger datasets to better drive informed recommendations in the future.
In the current study, Dr. Daniels and associates conducted comprehensive cardiac screening – including ECG, troponin testing, echocardiography, and cardiac MRI – of 1,597 college athlete survivors of COVID-19.
The athletes were part of the Big Ten athletic conference, which consists of 13 major American universities.
Cardiac MRI revealed that 37 (2.3%) of these athletes demonstrated diagnostic criteria for COVID-19 myocarditis; of these, 20 had no cardiovascular symptoms and had normal ECGs, echocardiography, and troponin test results.
“These patients would not have been identified without CMR imaging. If we were going according to the older protocol, we would not have made this discovery. Cardiac MRI is the most sensitive and specific test for myocardial inflammation, there is no argument about that,” Dr. Daniels said.
The catch is, cardiac MRI is expensive and often difficult to access, especially in remote, rural, or other underserviced areas.
“You can’t get an MRI for every person who has had COVID, it’s just not feasible,” Dr. Daniels said. “We are not advocating that everybody get an MRI. But we do hope that our study creates awareness among clinicians and athletes themselves that if you’ve had COVID, even if you’re asymptomatic, there may be some heart changes. So be aware when you start to exercise again, if you have any symptoms, pause and seek medical care.”
Kudos to the sports cardiology community
In an accompanying editorial, James E. Udelson, MD, Ethan J. Rowin, MD, and Barry J. Maron, MD, from the CardioVascular Center at Tufts Medical Center, Boston, applauded the sports cardiology community for its diligence in acquiring and publishing data about the post–COVID-19 prevalence of cardiac abnormalities in competitive athletes.
“It is a real tribute to the sports cardiology community. There has been an amazing growth of information, and they not only gathered this information, they analyzed and published it, starting out with a study of 29 or 30 athletes, and now thousands,” Dr. Udelson said in an interview.
At the start of the pandemic, it appeared that 15%-20% of athletes had myocarditis, and athletic conferences were discussing canceling sports events.
However, with greater numbers comes a more accurate picture of the extent of the problem.
“Once you get thousands of subjects in these studies, you can hone in on what the real number is, so now we understand that if you screen everybody with a cardiac MRI, 1%, 2%, or 3% will have some evidence of what looks like myocarditis,” he said.
Dr. Udelson agreed that doing cardiac imaging in everyone is not feasible.
“This study looked at a very large number of people who all had an MRI, but that doesn’t mean everyone should have them. If you just do an echo, an EKG, and a troponin test, and if everything is normal, which is kind of what current recommendations are, this paper tells us that we are going to miss one or two people out of a hundred, and that might be okay,” he said. “So, if you are at a huge university that has a large medical center and you want to screen all your athletes with MRI, great. But if you’re at a high school in a remote area, you know that the alternative, not having an MRI, isn’t so bad, either.”
A version of this article first appeared on Medscape.com.
Myocarditis is present in a small percentage of competitive athletes after COVID-19 infection, even in those without symptoms, new research suggests.
In a cohort study of 1,597 competitive collegiate athletes undergoing comprehensive cardiovascular testing in the United States, the prevalence of clinical myocarditis based on a symptom-based screening strategy was only 0.31%.
But screening with cardiac MRI increased the prevalence of clinical and subclinical myocarditis by a factor of 7.4, to 2.3%, the authors reported.
The findings are published online May 27, 2021, in JAMA Cardiology.
“It was the largest study to evaluate college athletes who have had COVID with extensive cardiac testing, including MRI, and this gave us a very objective look at the cardiac findings, as they were not purely based upon a subjective evaluation of symptoms,” lead investigator Curt J. Daniels, MD, professor at Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Unfortunately, our study showed that athletes can be asymptomatic, or at least not report symptoms. This is a very subjective feature, and we don’t know if they don’t report symptoms because they didn’t want to get tested. That is why we took a very objective approach,” Dr. Daniels said.
The finding that more than half of the asymptomatic athletes had myocarditis, or as the investigators called it, “subclinical myocarditis,” was a surprise, he acknowledged.
“More than half of the athletes found to have myocarditis reported no symptoms, and yes, that was a surprise, because prior to this study, the protocols that had been published stated that you had to have symptoms to even enter into the protocol for cardiac MRI. But, as our ... paper shows, if we had followed that protocol, we only would have found about 5 cases of myocarditis, as opposed to the total of 37 we found with cardiac MRI,” Dr. Daniels said.
In October 2020, the American College of Cardiology’s Sports and Exercise Council recommended that cardiac MRI be limited to athletes who exhibited symptoms as part of their guide to ensuring a safe return to play.
As reported by this news organization the council recommended a tiered approach to screening based on the presence of symptoms, followed by electrocardiography, injury biomarkers, and echocardiography. Any abnormalities detected were to be further characterized by the selective use of cardiac MRI.
At the time, there were relatively few data to support the recommendations, and all stakeholders called for larger datasets to better drive informed recommendations in the future.
In the current study, Dr. Daniels and associates conducted comprehensive cardiac screening – including ECG, troponin testing, echocardiography, and cardiac MRI – of 1,597 college athlete survivors of COVID-19.
The athletes were part of the Big Ten athletic conference, which consists of 13 major American universities.
Cardiac MRI revealed that 37 (2.3%) of these athletes demonstrated diagnostic criteria for COVID-19 myocarditis; of these, 20 had no cardiovascular symptoms and had normal ECGs, echocardiography, and troponin test results.
“These patients would not have been identified without CMR imaging. If we were going according to the older protocol, we would not have made this discovery. Cardiac MRI is the most sensitive and specific test for myocardial inflammation, there is no argument about that,” Dr. Daniels said.
The catch is, cardiac MRI is expensive and often difficult to access, especially in remote, rural, or other underserviced areas.
“You can’t get an MRI for every person who has had COVID, it’s just not feasible,” Dr. Daniels said. “We are not advocating that everybody get an MRI. But we do hope that our study creates awareness among clinicians and athletes themselves that if you’ve had COVID, even if you’re asymptomatic, there may be some heart changes. So be aware when you start to exercise again, if you have any symptoms, pause and seek medical care.”
Kudos to the sports cardiology community
In an accompanying editorial, James E. Udelson, MD, Ethan J. Rowin, MD, and Barry J. Maron, MD, from the CardioVascular Center at Tufts Medical Center, Boston, applauded the sports cardiology community for its diligence in acquiring and publishing data about the post–COVID-19 prevalence of cardiac abnormalities in competitive athletes.
“It is a real tribute to the sports cardiology community. There has been an amazing growth of information, and they not only gathered this information, they analyzed and published it, starting out with a study of 29 or 30 athletes, and now thousands,” Dr. Udelson said in an interview.
At the start of the pandemic, it appeared that 15%-20% of athletes had myocarditis, and athletic conferences were discussing canceling sports events.
However, with greater numbers comes a more accurate picture of the extent of the problem.
“Once you get thousands of subjects in these studies, you can hone in on what the real number is, so now we understand that if you screen everybody with a cardiac MRI, 1%, 2%, or 3% will have some evidence of what looks like myocarditis,” he said.
Dr. Udelson agreed that doing cardiac imaging in everyone is not feasible.
“This study looked at a very large number of people who all had an MRI, but that doesn’t mean everyone should have them. If you just do an echo, an EKG, and a troponin test, and if everything is normal, which is kind of what current recommendations are, this paper tells us that we are going to miss one or two people out of a hundred, and that might be okay,” he said. “So, if you are at a huge university that has a large medical center and you want to screen all your athletes with MRI, great. But if you’re at a high school in a remote area, you know that the alternative, not having an MRI, isn’t so bad, either.”
A version of this article first appeared on Medscape.com.
Myocarditis is present in a small percentage of competitive athletes after COVID-19 infection, even in those without symptoms, new research suggests.
In a cohort study of 1,597 competitive collegiate athletes undergoing comprehensive cardiovascular testing in the United States, the prevalence of clinical myocarditis based on a symptom-based screening strategy was only 0.31%.
But screening with cardiac MRI increased the prevalence of clinical and subclinical myocarditis by a factor of 7.4, to 2.3%, the authors reported.
The findings are published online May 27, 2021, in JAMA Cardiology.
“It was the largest study to evaluate college athletes who have had COVID with extensive cardiac testing, including MRI, and this gave us a very objective look at the cardiac findings, as they were not purely based upon a subjective evaluation of symptoms,” lead investigator Curt J. Daniels, MD, professor at Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Unfortunately, our study showed that athletes can be asymptomatic, or at least not report symptoms. This is a very subjective feature, and we don’t know if they don’t report symptoms because they didn’t want to get tested. That is why we took a very objective approach,” Dr. Daniels said.
The finding that more than half of the asymptomatic athletes had myocarditis, or as the investigators called it, “subclinical myocarditis,” was a surprise, he acknowledged.
“More than half of the athletes found to have myocarditis reported no symptoms, and yes, that was a surprise, because prior to this study, the protocols that had been published stated that you had to have symptoms to even enter into the protocol for cardiac MRI. But, as our ... paper shows, if we had followed that protocol, we only would have found about 5 cases of myocarditis, as opposed to the total of 37 we found with cardiac MRI,” Dr. Daniels said.
In October 2020, the American College of Cardiology’s Sports and Exercise Council recommended that cardiac MRI be limited to athletes who exhibited symptoms as part of their guide to ensuring a safe return to play.
As reported by this news organization the council recommended a tiered approach to screening based on the presence of symptoms, followed by electrocardiography, injury biomarkers, and echocardiography. Any abnormalities detected were to be further characterized by the selective use of cardiac MRI.
At the time, there were relatively few data to support the recommendations, and all stakeholders called for larger datasets to better drive informed recommendations in the future.
In the current study, Dr. Daniels and associates conducted comprehensive cardiac screening – including ECG, troponin testing, echocardiography, and cardiac MRI – of 1,597 college athlete survivors of COVID-19.
The athletes were part of the Big Ten athletic conference, which consists of 13 major American universities.
Cardiac MRI revealed that 37 (2.3%) of these athletes demonstrated diagnostic criteria for COVID-19 myocarditis; of these, 20 had no cardiovascular symptoms and had normal ECGs, echocardiography, and troponin test results.
“These patients would not have been identified without CMR imaging. If we were going according to the older protocol, we would not have made this discovery. Cardiac MRI is the most sensitive and specific test for myocardial inflammation, there is no argument about that,” Dr. Daniels said.
The catch is, cardiac MRI is expensive and often difficult to access, especially in remote, rural, or other underserviced areas.
“You can’t get an MRI for every person who has had COVID, it’s just not feasible,” Dr. Daniels said. “We are not advocating that everybody get an MRI. But we do hope that our study creates awareness among clinicians and athletes themselves that if you’ve had COVID, even if you’re asymptomatic, there may be some heart changes. So be aware when you start to exercise again, if you have any symptoms, pause and seek medical care.”
Kudos to the sports cardiology community
In an accompanying editorial, James E. Udelson, MD, Ethan J. Rowin, MD, and Barry J. Maron, MD, from the CardioVascular Center at Tufts Medical Center, Boston, applauded the sports cardiology community for its diligence in acquiring and publishing data about the post–COVID-19 prevalence of cardiac abnormalities in competitive athletes.
“It is a real tribute to the sports cardiology community. There has been an amazing growth of information, and they not only gathered this information, they analyzed and published it, starting out with a study of 29 or 30 athletes, and now thousands,” Dr. Udelson said in an interview.
At the start of the pandemic, it appeared that 15%-20% of athletes had myocarditis, and athletic conferences were discussing canceling sports events.
However, with greater numbers comes a more accurate picture of the extent of the problem.
“Once you get thousands of subjects in these studies, you can hone in on what the real number is, so now we understand that if you screen everybody with a cardiac MRI, 1%, 2%, or 3% will have some evidence of what looks like myocarditis,” he said.
Dr. Udelson agreed that doing cardiac imaging in everyone is not feasible.
“This study looked at a very large number of people who all had an MRI, but that doesn’t mean everyone should have them. If you just do an echo, an EKG, and a troponin test, and if everything is normal, which is kind of what current recommendations are, this paper tells us that we are going to miss one or two people out of a hundred, and that might be okay,” he said. “So, if you are at a huge university that has a large medical center and you want to screen all your athletes with MRI, great. But if you’re at a high school in a remote area, you know that the alternative, not having an MRI, isn’t so bad, either.”
A version of this article first appeared on Medscape.com.
Pericardial fat an independent risk factor for heart failure
Pericardial fat is associated with a heightened risk for heart failure, particularly in women, new research suggests.
In a prospective cohort study of nearly 7,000 individuals, excess pericardial fat was linked to a higher risk for heart failure, even after adjustment for established risk factors for heart failure.
Women with high pericardial fat volume (PFV), defined as more than 70 cm3 or 2.4 fluid ounces, had double the risk of developing heart failure. For men, high PFV, defined as more than 120 cm3 or 4.0 fluid ounces, was associated with a 50% increase in the risk for heart failure.
The findings were published in the Journal of the American College of Cardiology.
“People will ask why should they measure fat around the heart. Why can’t they just take the waist circumference or body mass index as a measure for increased risk?” lead author Satish Kenchaiah, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Yet, when we adjusted for waist circumference, hip circumference, waist to hip ratio, and other known variables, pericardial fat was still associated with an increased risk of heart failure. This tells me that it is not just overall fat in the body but something about its location around the heart that is playing a role,” Dr. Kenchaiah said.
“Now that we have found an association between any amount of fat around the pericardium and heart failure, it gives us an impetus to build future research on identifying how exactly these fat deposits influence the development of cardiomyopathy,” he said.
Dr. Kenchaiah and colleagues investigated the association of pericardial fat with incident heart failure by examining chest CT scans from 6,785 participants (3,584 women and 3,201 men aged 45-84 years) in the Multi-Ethnic Study of Atherosclerosis.
The participants were from four different ethnic groups: 38% were White; 28% were Black, 22% were Hispanic, and 12% were Chinese American. They were recruited between July 17, 2000, and Aug. 31, 2002, from six communities in the United States: Baltimore and Baltimore County; Chicago; Forsyth County, N.C.; Los Angeles County northern Manhattan and the Bronx, New York; and St. Paul, Minn.
All participants were free of cardiovascular disease at baseline.
The researchers followed participants for more than 17 years. During this time, 385 (5.7%; 164 women and 221 men) developed newly diagnosed heart failure.
In women, the hazard ratio for every 42 cm3 increase in PFV was 1.44 (95% confidence interval, 1.21-1.71; P < .001). In men, the HR was 1.13 (95% CI, 1.01-1.27; P = .03).
High PVF conferred a twofold greater risk for heart failure in women (HR, 2.06; 95% CI, 1.48-2.87; P < .001) and a 53% higher risk in men (HR, 1.53; 95% CI, 1.13-2.07; P = .006).
These associations remained significant after further adjustment for circulating markers of systemic inflammation (that is, C-reactive protein and interleukin-6), and abdominal subcutaneous or visceral fat.
They also found that the heightened risk persisted, even after adjustment for established risk factors for heart failure, such as age, cigarette smoking, alcohol consumption, sedentary lifestyle, high blood pressure, high blood sugar, high cholesterol, and myocardial infarction.
Results were similar among all of the ethnic groups studied.
A surprise finding
“The most surprising part of this study was that the risk for heart failure with increased pericardial fat does not seem to be explained by obesity and systemic inflammation alone,” Andreas P. Kalogeropoulos, MD, MPH, PhD, Stony Brook (N.Y.) University, said in an interview.
“If pericardial fat was merely a proxy for increased visceral fat, one would expect the association of pericardial fat with heart failure risk to go away after factoring in abdominal CT findings, which was not the case here. Also, accounting for inflammatory markers did not change things dramatically. However, we need to be careful here, as abdominal CT scans have not been done simultaneously with the pericardial fat scans in the study,” said Dr. Kalogeropoulos, who coauthored an accompanying editorial with Michael E. Hall, MD, University of Mississippi Medical Center, Jackson.
The other striking finding, although not entirely surprising, was the stronger association of pericardial fat with heart failure risk in women, he noted.
“Although several clues have been reported pointing to women being more sensitive to the adverse cardiac effects of pericardial fat, this is the first large prospective study to connect the dots and show much higher risk in women in a convincing way. For the record, this is the first prospective study to show the connection between pericardial fat and heart failure risk altogether,” Dr. Kalogeropoulos said.
“Obviously, we need to do more work to see how we can use the important findings of Kenchaiah and colleagues to reduce risk for heart failure among patients with increased pericardial fat, especially women. For starters, we would need a way to identify these patients,” he said. “In this aspect, it is encouraging that pericardial fat can be measured in low-radiation CT scans, similar to those used for coronary calcium, and that automation technology to speed up pericardial fat measurements is already in the pipeline.
“The next step would be to see what kind of interventions would reduce risk for heart failure in these patients,” he added. “Weight loss would be an obvious thing, but novel agents with favorable cardiometabolic effects, like newer antidiabetic medications, are intriguing options, too.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Kenchaiah and Dr. Kalogeropoulos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pericardial fat is associated with a heightened risk for heart failure, particularly in women, new research suggests.
In a prospective cohort study of nearly 7,000 individuals, excess pericardial fat was linked to a higher risk for heart failure, even after adjustment for established risk factors for heart failure.
Women with high pericardial fat volume (PFV), defined as more than 70 cm3 or 2.4 fluid ounces, had double the risk of developing heart failure. For men, high PFV, defined as more than 120 cm3 or 4.0 fluid ounces, was associated with a 50% increase in the risk for heart failure.
The findings were published in the Journal of the American College of Cardiology.
“People will ask why should they measure fat around the heart. Why can’t they just take the waist circumference or body mass index as a measure for increased risk?” lead author Satish Kenchaiah, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Yet, when we adjusted for waist circumference, hip circumference, waist to hip ratio, and other known variables, pericardial fat was still associated with an increased risk of heart failure. This tells me that it is not just overall fat in the body but something about its location around the heart that is playing a role,” Dr. Kenchaiah said.
“Now that we have found an association between any amount of fat around the pericardium and heart failure, it gives us an impetus to build future research on identifying how exactly these fat deposits influence the development of cardiomyopathy,” he said.
Dr. Kenchaiah and colleagues investigated the association of pericardial fat with incident heart failure by examining chest CT scans from 6,785 participants (3,584 women and 3,201 men aged 45-84 years) in the Multi-Ethnic Study of Atherosclerosis.
The participants were from four different ethnic groups: 38% were White; 28% were Black, 22% were Hispanic, and 12% were Chinese American. They were recruited between July 17, 2000, and Aug. 31, 2002, from six communities in the United States: Baltimore and Baltimore County; Chicago; Forsyth County, N.C.; Los Angeles County northern Manhattan and the Bronx, New York; and St. Paul, Minn.
All participants were free of cardiovascular disease at baseline.
The researchers followed participants for more than 17 years. During this time, 385 (5.7%; 164 women and 221 men) developed newly diagnosed heart failure.
In women, the hazard ratio for every 42 cm3 increase in PFV was 1.44 (95% confidence interval, 1.21-1.71; P < .001). In men, the HR was 1.13 (95% CI, 1.01-1.27; P = .03).
High PVF conferred a twofold greater risk for heart failure in women (HR, 2.06; 95% CI, 1.48-2.87; P < .001) and a 53% higher risk in men (HR, 1.53; 95% CI, 1.13-2.07; P = .006).
These associations remained significant after further adjustment for circulating markers of systemic inflammation (that is, C-reactive protein and interleukin-6), and abdominal subcutaneous or visceral fat.
They also found that the heightened risk persisted, even after adjustment for established risk factors for heart failure, such as age, cigarette smoking, alcohol consumption, sedentary lifestyle, high blood pressure, high blood sugar, high cholesterol, and myocardial infarction.
Results were similar among all of the ethnic groups studied.
A surprise finding
“The most surprising part of this study was that the risk for heart failure with increased pericardial fat does not seem to be explained by obesity and systemic inflammation alone,” Andreas P. Kalogeropoulos, MD, MPH, PhD, Stony Brook (N.Y.) University, said in an interview.
“If pericardial fat was merely a proxy for increased visceral fat, one would expect the association of pericardial fat with heart failure risk to go away after factoring in abdominal CT findings, which was not the case here. Also, accounting for inflammatory markers did not change things dramatically. However, we need to be careful here, as abdominal CT scans have not been done simultaneously with the pericardial fat scans in the study,” said Dr. Kalogeropoulos, who coauthored an accompanying editorial with Michael E. Hall, MD, University of Mississippi Medical Center, Jackson.
The other striking finding, although not entirely surprising, was the stronger association of pericardial fat with heart failure risk in women, he noted.
“Although several clues have been reported pointing to women being more sensitive to the adverse cardiac effects of pericardial fat, this is the first large prospective study to connect the dots and show much higher risk in women in a convincing way. For the record, this is the first prospective study to show the connection between pericardial fat and heart failure risk altogether,” Dr. Kalogeropoulos said.
“Obviously, we need to do more work to see how we can use the important findings of Kenchaiah and colleagues to reduce risk for heart failure among patients with increased pericardial fat, especially women. For starters, we would need a way to identify these patients,” he said. “In this aspect, it is encouraging that pericardial fat can be measured in low-radiation CT scans, similar to those used for coronary calcium, and that automation technology to speed up pericardial fat measurements is already in the pipeline.
“The next step would be to see what kind of interventions would reduce risk for heart failure in these patients,” he added. “Weight loss would be an obvious thing, but novel agents with favorable cardiometabolic effects, like newer antidiabetic medications, are intriguing options, too.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Kenchaiah and Dr. Kalogeropoulos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pericardial fat is associated with a heightened risk for heart failure, particularly in women, new research suggests.
In a prospective cohort study of nearly 7,000 individuals, excess pericardial fat was linked to a higher risk for heart failure, even after adjustment for established risk factors for heart failure.
Women with high pericardial fat volume (PFV), defined as more than 70 cm3 or 2.4 fluid ounces, had double the risk of developing heart failure. For men, high PFV, defined as more than 120 cm3 or 4.0 fluid ounces, was associated with a 50% increase in the risk for heart failure.
The findings were published in the Journal of the American College of Cardiology.
“People will ask why should they measure fat around the heart. Why can’t they just take the waist circumference or body mass index as a measure for increased risk?” lead author Satish Kenchaiah, MD, MPH, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Yet, when we adjusted for waist circumference, hip circumference, waist to hip ratio, and other known variables, pericardial fat was still associated with an increased risk of heart failure. This tells me that it is not just overall fat in the body but something about its location around the heart that is playing a role,” Dr. Kenchaiah said.
“Now that we have found an association between any amount of fat around the pericardium and heart failure, it gives us an impetus to build future research on identifying how exactly these fat deposits influence the development of cardiomyopathy,” he said.
Dr. Kenchaiah and colleagues investigated the association of pericardial fat with incident heart failure by examining chest CT scans from 6,785 participants (3,584 women and 3,201 men aged 45-84 years) in the Multi-Ethnic Study of Atherosclerosis.
The participants were from four different ethnic groups: 38% were White; 28% were Black, 22% were Hispanic, and 12% were Chinese American. They were recruited between July 17, 2000, and Aug. 31, 2002, from six communities in the United States: Baltimore and Baltimore County; Chicago; Forsyth County, N.C.; Los Angeles County northern Manhattan and the Bronx, New York; and St. Paul, Minn.
All participants were free of cardiovascular disease at baseline.
The researchers followed participants for more than 17 years. During this time, 385 (5.7%; 164 women and 221 men) developed newly diagnosed heart failure.
In women, the hazard ratio for every 42 cm3 increase in PFV was 1.44 (95% confidence interval, 1.21-1.71; P < .001). In men, the HR was 1.13 (95% CI, 1.01-1.27; P = .03).
High PVF conferred a twofold greater risk for heart failure in women (HR, 2.06; 95% CI, 1.48-2.87; P < .001) and a 53% higher risk in men (HR, 1.53; 95% CI, 1.13-2.07; P = .006).
These associations remained significant after further adjustment for circulating markers of systemic inflammation (that is, C-reactive protein and interleukin-6), and abdominal subcutaneous or visceral fat.
They also found that the heightened risk persisted, even after adjustment for established risk factors for heart failure, such as age, cigarette smoking, alcohol consumption, sedentary lifestyle, high blood pressure, high blood sugar, high cholesterol, and myocardial infarction.
Results were similar among all of the ethnic groups studied.
A surprise finding
“The most surprising part of this study was that the risk for heart failure with increased pericardial fat does not seem to be explained by obesity and systemic inflammation alone,” Andreas P. Kalogeropoulos, MD, MPH, PhD, Stony Brook (N.Y.) University, said in an interview.
“If pericardial fat was merely a proxy for increased visceral fat, one would expect the association of pericardial fat with heart failure risk to go away after factoring in abdominal CT findings, which was not the case here. Also, accounting for inflammatory markers did not change things dramatically. However, we need to be careful here, as abdominal CT scans have not been done simultaneously with the pericardial fat scans in the study,” said Dr. Kalogeropoulos, who coauthored an accompanying editorial with Michael E. Hall, MD, University of Mississippi Medical Center, Jackson.
The other striking finding, although not entirely surprising, was the stronger association of pericardial fat with heart failure risk in women, he noted.
“Although several clues have been reported pointing to women being more sensitive to the adverse cardiac effects of pericardial fat, this is the first large prospective study to connect the dots and show much higher risk in women in a convincing way. For the record, this is the first prospective study to show the connection between pericardial fat and heart failure risk altogether,” Dr. Kalogeropoulos said.
“Obviously, we need to do more work to see how we can use the important findings of Kenchaiah and colleagues to reduce risk for heart failure among patients with increased pericardial fat, especially women. For starters, we would need a way to identify these patients,” he said. “In this aspect, it is encouraging that pericardial fat can be measured in low-radiation CT scans, similar to those used for coronary calcium, and that automation technology to speed up pericardial fat measurements is already in the pipeline.
“The next step would be to see what kind of interventions would reduce risk for heart failure in these patients,” he added. “Weight loss would be an obvious thing, but novel agents with favorable cardiometabolic effects, like newer antidiabetic medications, are intriguing options, too.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Kenchaiah and Dr. Kalogeropoulos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.