User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


COVID-19 asymptomatic infection rate remains high
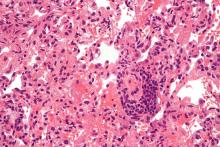
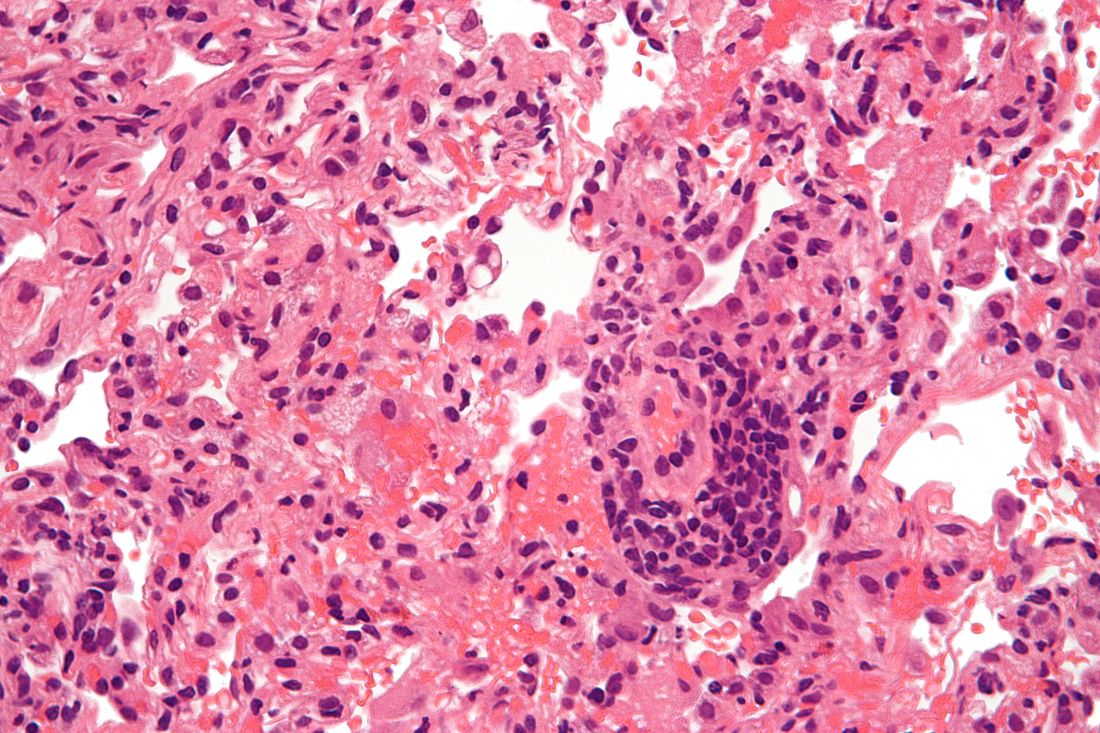
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases resume their climb
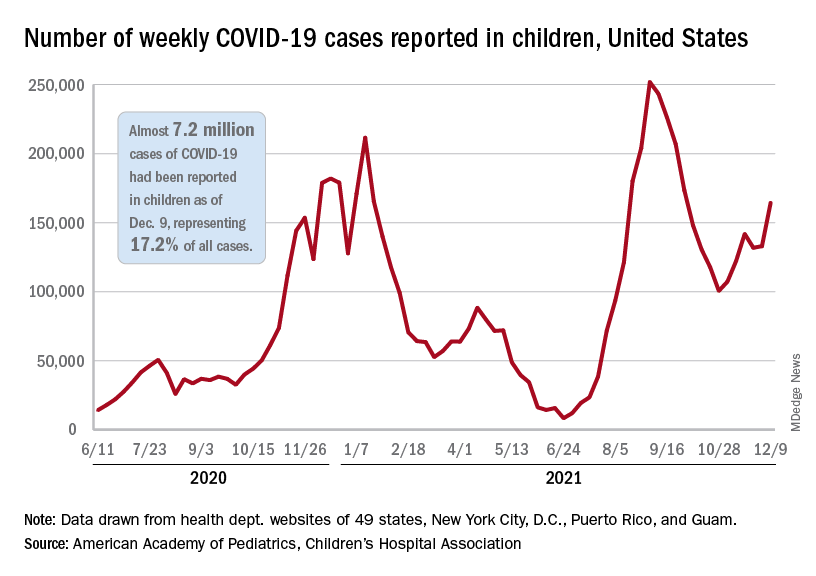
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
Physician gender pay gap isn’t news; health inequity is rampant
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
D-dimer thresholds rule out PE in meta-analysis
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
In a patient suspected to have a PE, “diagnosis is made radiographically, usually with CT pulmonary angiogram, or V/Q scan,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview.
“Validated clinical decision tools such as Wells’ score or Geneva score may be used to identify patients at low pretest probability of PE who may initially get a D-dimer level check, followed by imaging only if D-dimer level is elevated,” explained Dr. Pal, who was not involved with the new research, which was published in the Annals of Internal Medicine.
According to the authors of the new paper, while current diagnostic strategies in patients with suspected PE include use of a validated clinical decision rule (CDR) and D-dimer testing to rule out PE without imaging tests, the effectiveness of D-dimer tests in older patients, inpatients, cancer patients, and other high-risk groups has not been well-studied.
Lead author of the paper, Milou A.M. Stals, MD, and colleagues said their goal was to evaluate the safety and efficiency of the Wells rule and revised Geneva score in combination with D-dimer tests, and also the YEARS algorithm for D-dimer thresholds, in their paper.
Dr. Stals, of Leiden (the Netherlands) University Medical Center, and the coinvestigators conducted an international systemic review and individual patient data meta-analysis that included 16 studies and 20,553 patients, with all studies having been published between Jan. 1, 1995, and Jan. 1, 2021. Their primary outcomes were the safety and efficiency of each of these three strategies.
In the review, the researchers defined safety as the 3-month incidence of venous thromboembolism after PE was ruled out without imaging at baseline. They defined efficiency as the proportion patients for whom PE was ruled out based on D-dimer thresholds without imaging.
Overall, efficiency was highest in the subset of patients aged younger than 40 years, ranging from 47% to 68% in this group. Efficiency was lowest in patients aged 80 years and older (6.0%-23%), and in patients with cancer (9.6%-26%).
The efficiency was higher when D-dimer thresholds based on pretest probability were used, compared with when fixed or age-adjusted D-dimer thresholds were used.
The key finding was the significant variability in performance of the diagnostic strategies, the researchers said.
“The predicted failure rate was generally highest for strategies incorporating adapted D-dimer thresholds. However, at the same time, predicted overall efficiency was substantially higher with these strategies versus strategies with a fixed D-dimer threshold as well,” they said. Given that the benefits of each of the three diagnostic strategies depends on their correct application, the researchers recommended that an individual hospitalist choose one strategy for their institution.
“Whether clinicians should rely on the Wells rule, the YEARS algorithm, or the revised Geneva score becomes a matter of local preference and experience,” Dr. Stals and colleagues wrote.
The study findings were limited by several factors including between-study differences in scoring predictors and D-dimer assays. Another limitation was that differential verification biases for classifying fatal events and PE may have contributed to overestimation of failure rates of the adapted D-dimer thresholds.
Strengths of the study included its large sample size and original data on pretest probability, and that data support the use of any of the three strategies for ruling out PE in the identified subgroups without the need for imaging tests, the authors wrote.
“Pending the results of ongoing diagnostic randomized trials, physicians and guideline committees should balance the interlink between safety and efficiency of available diagnostic strategies,” they concluded.
Adapted D-dimer benefits some patients
“Clearly, increasing the D-dimer cutoff will lower the number of patients who require radiographic imaging (improved specificity), but this comes with a risk for missing PE (lower sensitivity). Is this risk worth taking?” Daniel J. Brotman, MD, of Johns Hopkins University, Baltimore, asked in an editorial accompanying the new study.
Dr. Brotman was not surprised by the study findings.
“Conditions that predispose to thrombosis through activated hemostasis – such as advanced age, cancer, inflammation, prolonged hospitalization, and trauma – drive D-dimer levels higher independent of the presence or absence of radiographically apparent thrombosis,” he said. However, these patients are unlikely to have normal D-dimer levels regardless of the cutoff used.
Adapted D-dimer cutoffs may benefit some patients, including those with contraindications or limited access to imaging, said Dr. Brotman. D-dimer may be used for risk stratification regardless of PE, since patients with marginally elevated D-dimers have better prognoses than those with higher D-dimer elevations, even if a small PE is missed.
Dr. Brotman wrote that increasing D-dimer cutoffs for high-risk patients in the subgroups analyzed may spare some patients radiographic testing, but doing so carries an increased risk for diagnostic failure. Overall, “the important work by Stals and colleagues offers reassurance that modifying D-dimer thresholds according to age or pretest probability is safe enough for widespread practice, even in high-risk groups.”
Focus on single strategy ‘based on local needs’
“Several validated clinical decision tools, along with age or pretest probability adjusted D-dimer threshold are currently in use as diagnostic strategies for ruling out pulmonary embolism,” Dr. Pal said in an interview.
The current study is important because of limited data on the performance of these strategies in specific subgroups of patients whose risk of PE may differ from the overall patient population, he noted.
“Different diagnostic strategies for PE have a variable performance in patients with differences of age, active cancer, and history of VTE,” said Dr. Pal. “However, in this study, no clear preference for one strategy over others could be established for these subgroups, and clinicians should continue to follow institution-specific guidance.
“A single strategy should be adopted at each institution based on local needs and used as the standard of care until further data are available,” he said.
“The use of D-dimer to rule out PE, either with fixed threshold or age-adjusted thresholds, can be confounded in clinical settings by other comorbid conditions such as sepsis, recent surgery, and more recently, COVID-19,” he said.
“Since the findings of this study do not show a clear benefit of one diagnostic strategy over others in the analyzed subgroups of patients, further prospective head-to-head comparison among the subgroups of interest would be helpful to guide clinical decision making,” Dr. Pal added.
YEARS-specific study supports D-dimer safety and value
A recent paper published in JAMA supported the results of the meta-analysis. In that study, Yonathan Freund, MD, of Sorbonne Université, Paris, and colleagues focused on the YEARS strategy combined with age-adjusted D-dimer thresholds as a way to rule out PE in PERC-positive ED patients.
The authors of this paper randomized 18 EDs to either a protocol of intervention followed by control, or control followed by intervention. The study population included 726 patients in the intervention group and 688 in the control group.
The intervention strategy to rule out PE consisted of assessing the YEARS criteria and D-dimer testing. PE was ruled out in patients with no YEARS criteria and a D-dimer level below 1,000 ng/mL and in patients with one or more YEARS criteria and D-dimers below an age-adjusted threshold (defined as age times 10 ng/mL in patients aged 50 years and older).
The control strategy consisted of D-dimer testing for all patients with the threshold at age-adjusted levels; D-dimers about these levels prompted chest imaging.
Overall, the risk of a missed VTE at 3 months was noninferior between the groups (0.15% in the intervention group and 0.80% in the controls).
“The intervention was associated with a statistically significant reduction in chest imaging use,” the researchers wrote.
This study’s findings were limited by randomization at the center level, rather than the patient level, and the use of imaging on some patients despite negative D-dimer tests, the researchers wrote. However, their findings support those of previous studies and especially support the safety of the intervention, in an emergency medicine setting, as no PEs occurred in patients with a YEARS score of zero who underwent the intervention.
Downsides to applying algorithms to every patient explained
In an editorial accompanying the JAMA study, Marcel Levi, MD, and Nick van Es, MD, of Amsterdam University Medical Center, emphasized the challenges of diagnosing PE given that many patients present with nonspecific clinical manifestations and without typical signs and symptoms. High-resolution CT pulmonary angiography allows for a fast and easy diagnosis in an emergency setting. However, efforts are ongoing to develop alternative strategies that avoid unnecessary scanning for potential PE patients, many of whom have alternative diagnoses such as pulmonary infections, cardiac conditions, pleural disease, or musculoskeletal problems.
On review of the JAMA study using the YEARS rule with adjusted D-dimer thresholds, the editorialists noted that the data were robust and indicated a 10% reduction in chest imaging. They also emphasized the potential to overwhelm busy clinicians with more algorithms.
“Blindly applying algorithms to every patient may be less appropriate or even undesirable in specific situations in which deviation from the rules on clinical grounds is indicated,” but a complex imaging approach may be time consuming and challenging in the acute setting, and a simple algorithm may be safe and efficient in many cases, they wrote. “From a patient perspective, a negative diagnostic algorithm for pulmonary embolism does not diminish the physician’s obligation to consider other diagnoses that explain the symptoms, for which chest CT scans may still be needed and helpful.”
The Annals of Internal Medicine study was supported by the Dutch Research Council. The JAMA study was supported by the French Health Ministry. Dr. Stals, Dr. Freund, Dr. Pal, Dr. Levi, and Dr. van Es had no financial conflicts to disclose.
FROM THE ANNALS OF INTERNAL MEDICINE
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
12 state boards have disciplined docs for COVID misinformation
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
Lung transplantation in the era of COVID-19: New issues and paradigms
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Major COVID-19 case growth expected in coming weeks
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
Omicron may require fourth vaccine dose, Pfizer says
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
Idiopathic pulmonary fibrosis – a mortality predictor found?
A low lymphocyte-to-monocyte ratio (LMR) is associated with worse survival in newly-diagnosed patients with idiopathic pulmonary fibrosis (IPF), according to a new retrospective, single-center analysis. Patients with both IPF and lung cancer also had a lower LMR than patients with IPF alone.
The study, published online in Respiratory Medicine, was conducted among 77 newly diagnosed patients, 40 end-stage IPF patients, and 17 patients with IPF and lung cancer. All received at least 1 year of antifibrotic therapy (pirfenidone or nintedanib). The researchers collected demographic and clinical data between December 2014 and December 2020.
The disease course of IPF is difficult to predict, with some patients progressing slowly, and others suffer a rapid decline to respiratory failure. Previous studies found that higher levels of monocytes are associated with higher mortality in IPF and other fibrotic lung disease, and both neutrophil-to-lymphocyte ratio (NLR) and LMR have been shown to predict mortality in lung cancer.
A previous study found that IPF patients had a higher NLR and a lower LMR than controls, but that research did not consider the impact of antifibrotic treatment, which may improve outcomes.
There has been accumulating cellular and molecular evidence that leukocyte population abnormalities are associated with IPF outcomes, but that work was more discovery based and relied on tests that aren’t readily available clinically, said Erica L. Herzog, MD, PhD, who was asked to comment on the study. “It’s provided a lot of insight into potential new mechanisms and potential biomarkers, but their clinical utility for patients is limited. So the use of lymphocyte-to-monocyte ratios that can be obtained from a complete blood cell count, which is a test that can be done in any hospital, would really be a game-changer in terms of predictive algorithms for patients with IPF,” said Dr. Herzog, who is a professor of medicine and pathology at Yale University, New Haven, Conn., and director of the Yale ILD Center of Excellence.
In humans, abnormalities in circulating monocytes and lymphocytes have individually been linked worse IPF outcomes. Animal studies have implicated monocyte-derived cells in lung fibrosis, but because animal studies have shown that lymphocyte populations are not required for fibrosis, more work is needed.
“I think what we’re finding is that lymphocytes probably have a regulatory role, and there’s probably a protective population and potentially a pathogenic population. Something about the balance between adaptive immunity, which is reflected by your lymphocytes, and innate immunity, which is reflected by your monocytes. Something about that balance is important for tissue homeostasis, and then when it’s disrupted or perturbed, fibrosis ensues,” said Dr. Herzog.
Study details
The newly diagnosed patients were older (mean age, 70 years) than the end-stage IPF patients (mean age, 60 years) and patients with IPF and lung cancer (mean age, 64 years; P < .0001).
Among newly diagnosed IPF patients, a receiving operating characteristic analysis before antifibrotic treatment determined a cutoff LMR value of less than 4.18, with an area under the curve of 0.67 (P = .025). Values below 4.18 were associated with shorter survival (hazard ratio, 6.88; P = .027).
Patients with LMR less than 4.18 were more likely to be men (89% vs. 67%; P = .036), had a lower percent predicted forced vital capacity (76% vs. 87%; P = .023), and were more likely to die or undergo lung transplant (34% vs. 5%; P = .009).
Mortality results
A Kaplan-Meier curve illustrated a stark difference between patients with LMR of 4.18 or higher, nearly all of whom remained alive out to almost 100 months of follow-up. Around 30% of those with LMR less than 4.18 remained alive while close to 100% of the patients with LMR below this value showed worsened outcomes. “You don’t normally see curves like that,” said Dr. Herzog.
There was no significant difference in blood cell counts and ratios at the time of IPF diagnosis and after 1 year of antifibrotic treatment, which suggests that the risk profile is independent of treatment, according to the study authors.
Among patient subgroups, those with IPF and lung cancer had the lowest mean LMR (2.2), followed by newly diagnosed patients (3.5), and those with end-stage disease (3.6; P < .0001).
The study authors reported financial relationships with various pharmaceutical companies. Dr. Herzog had no relevant financial disclosures.
A low lymphocyte-to-monocyte ratio (LMR) is associated with worse survival in newly-diagnosed patients with idiopathic pulmonary fibrosis (IPF), according to a new retrospective, single-center analysis. Patients with both IPF and lung cancer also had a lower LMR than patients with IPF alone.
The study, published online in Respiratory Medicine, was conducted among 77 newly diagnosed patients, 40 end-stage IPF patients, and 17 patients with IPF and lung cancer. All received at least 1 year of antifibrotic therapy (pirfenidone or nintedanib). The researchers collected demographic and clinical data between December 2014 and December 2020.
The disease course of IPF is difficult to predict, with some patients progressing slowly, and others suffer a rapid decline to respiratory failure. Previous studies found that higher levels of monocytes are associated with higher mortality in IPF and other fibrotic lung disease, and both neutrophil-to-lymphocyte ratio (NLR) and LMR have been shown to predict mortality in lung cancer.
A previous study found that IPF patients had a higher NLR and a lower LMR than controls, but that research did not consider the impact of antifibrotic treatment, which may improve outcomes.
There has been accumulating cellular and molecular evidence that leukocyte population abnormalities are associated with IPF outcomes, but that work was more discovery based and relied on tests that aren’t readily available clinically, said Erica L. Herzog, MD, PhD, who was asked to comment on the study. “It’s provided a lot of insight into potential new mechanisms and potential biomarkers, but their clinical utility for patients is limited. So the use of lymphocyte-to-monocyte ratios that can be obtained from a complete blood cell count, which is a test that can be done in any hospital, would really be a game-changer in terms of predictive algorithms for patients with IPF,” said Dr. Herzog, who is a professor of medicine and pathology at Yale University, New Haven, Conn., and director of the Yale ILD Center of Excellence.
In humans, abnormalities in circulating monocytes and lymphocytes have individually been linked worse IPF outcomes. Animal studies have implicated monocyte-derived cells in lung fibrosis, but because animal studies have shown that lymphocyte populations are not required for fibrosis, more work is needed.
“I think what we’re finding is that lymphocytes probably have a regulatory role, and there’s probably a protective population and potentially a pathogenic population. Something about the balance between adaptive immunity, which is reflected by your lymphocytes, and innate immunity, which is reflected by your monocytes. Something about that balance is important for tissue homeostasis, and then when it’s disrupted or perturbed, fibrosis ensues,” said Dr. Herzog.
Study details
The newly diagnosed patients were older (mean age, 70 years) than the end-stage IPF patients (mean age, 60 years) and patients with IPF and lung cancer (mean age, 64 years; P < .0001).
Among newly diagnosed IPF patients, a receiving operating characteristic analysis before antifibrotic treatment determined a cutoff LMR value of less than 4.18, with an area under the curve of 0.67 (P = .025). Values below 4.18 were associated with shorter survival (hazard ratio, 6.88; P = .027).
Patients with LMR less than 4.18 were more likely to be men (89% vs. 67%; P = .036), had a lower percent predicted forced vital capacity (76% vs. 87%; P = .023), and were more likely to die or undergo lung transplant (34% vs. 5%; P = .009).
Mortality results
A Kaplan-Meier curve illustrated a stark difference between patients with LMR of 4.18 or higher, nearly all of whom remained alive out to almost 100 months of follow-up. Around 30% of those with LMR less than 4.18 remained alive while close to 100% of the patients with LMR below this value showed worsened outcomes. “You don’t normally see curves like that,” said Dr. Herzog.
There was no significant difference in blood cell counts and ratios at the time of IPF diagnosis and after 1 year of antifibrotic treatment, which suggests that the risk profile is independent of treatment, according to the study authors.
Among patient subgroups, those with IPF and lung cancer had the lowest mean LMR (2.2), followed by newly diagnosed patients (3.5), and those with end-stage disease (3.6; P < .0001).
The study authors reported financial relationships with various pharmaceutical companies. Dr. Herzog had no relevant financial disclosures.
A low lymphocyte-to-monocyte ratio (LMR) is associated with worse survival in newly-diagnosed patients with idiopathic pulmonary fibrosis (IPF), according to a new retrospective, single-center analysis. Patients with both IPF and lung cancer also had a lower LMR than patients with IPF alone.
The study, published online in Respiratory Medicine, was conducted among 77 newly diagnosed patients, 40 end-stage IPF patients, and 17 patients with IPF and lung cancer. All received at least 1 year of antifibrotic therapy (pirfenidone or nintedanib). The researchers collected demographic and clinical data between December 2014 and December 2020.
The disease course of IPF is difficult to predict, with some patients progressing slowly, and others suffer a rapid decline to respiratory failure. Previous studies found that higher levels of monocytes are associated with higher mortality in IPF and other fibrotic lung disease, and both neutrophil-to-lymphocyte ratio (NLR) and LMR have been shown to predict mortality in lung cancer.
A previous study found that IPF patients had a higher NLR and a lower LMR than controls, but that research did not consider the impact of antifibrotic treatment, which may improve outcomes.
There has been accumulating cellular and molecular evidence that leukocyte population abnormalities are associated with IPF outcomes, but that work was more discovery based and relied on tests that aren’t readily available clinically, said Erica L. Herzog, MD, PhD, who was asked to comment on the study. “It’s provided a lot of insight into potential new mechanisms and potential biomarkers, but their clinical utility for patients is limited. So the use of lymphocyte-to-monocyte ratios that can be obtained from a complete blood cell count, which is a test that can be done in any hospital, would really be a game-changer in terms of predictive algorithms for patients with IPF,” said Dr. Herzog, who is a professor of medicine and pathology at Yale University, New Haven, Conn., and director of the Yale ILD Center of Excellence.
In humans, abnormalities in circulating monocytes and lymphocytes have individually been linked worse IPF outcomes. Animal studies have implicated monocyte-derived cells in lung fibrosis, but because animal studies have shown that lymphocyte populations are not required for fibrosis, more work is needed.
“I think what we’re finding is that lymphocytes probably have a regulatory role, and there’s probably a protective population and potentially a pathogenic population. Something about the balance between adaptive immunity, which is reflected by your lymphocytes, and innate immunity, which is reflected by your monocytes. Something about that balance is important for tissue homeostasis, and then when it’s disrupted or perturbed, fibrosis ensues,” said Dr. Herzog.
Study details
The newly diagnosed patients were older (mean age, 70 years) than the end-stage IPF patients (mean age, 60 years) and patients with IPF and lung cancer (mean age, 64 years; P < .0001).
Among newly diagnosed IPF patients, a receiving operating characteristic analysis before antifibrotic treatment determined a cutoff LMR value of less than 4.18, with an area under the curve of 0.67 (P = .025). Values below 4.18 were associated with shorter survival (hazard ratio, 6.88; P = .027).
Patients with LMR less than 4.18 were more likely to be men (89% vs. 67%; P = .036), had a lower percent predicted forced vital capacity (76% vs. 87%; P = .023), and were more likely to die or undergo lung transplant (34% vs. 5%; P = .009).
Mortality results
A Kaplan-Meier curve illustrated a stark difference between patients with LMR of 4.18 or higher, nearly all of whom remained alive out to almost 100 months of follow-up. Around 30% of those with LMR less than 4.18 remained alive while close to 100% of the patients with LMR below this value showed worsened outcomes. “You don’t normally see curves like that,” said Dr. Herzog.
There was no significant difference in blood cell counts and ratios at the time of IPF diagnosis and after 1 year of antifibrotic treatment, which suggests that the risk profile is independent of treatment, according to the study authors.
Among patient subgroups, those with IPF and lung cancer had the lowest mean LMR (2.2), followed by newly diagnosed patients (3.5), and those with end-stage disease (3.6; P < .0001).
The study authors reported financial relationships with various pharmaceutical companies. Dr. Herzog had no relevant financial disclosures.
FROM RESPIRATORY MEDICINE