User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


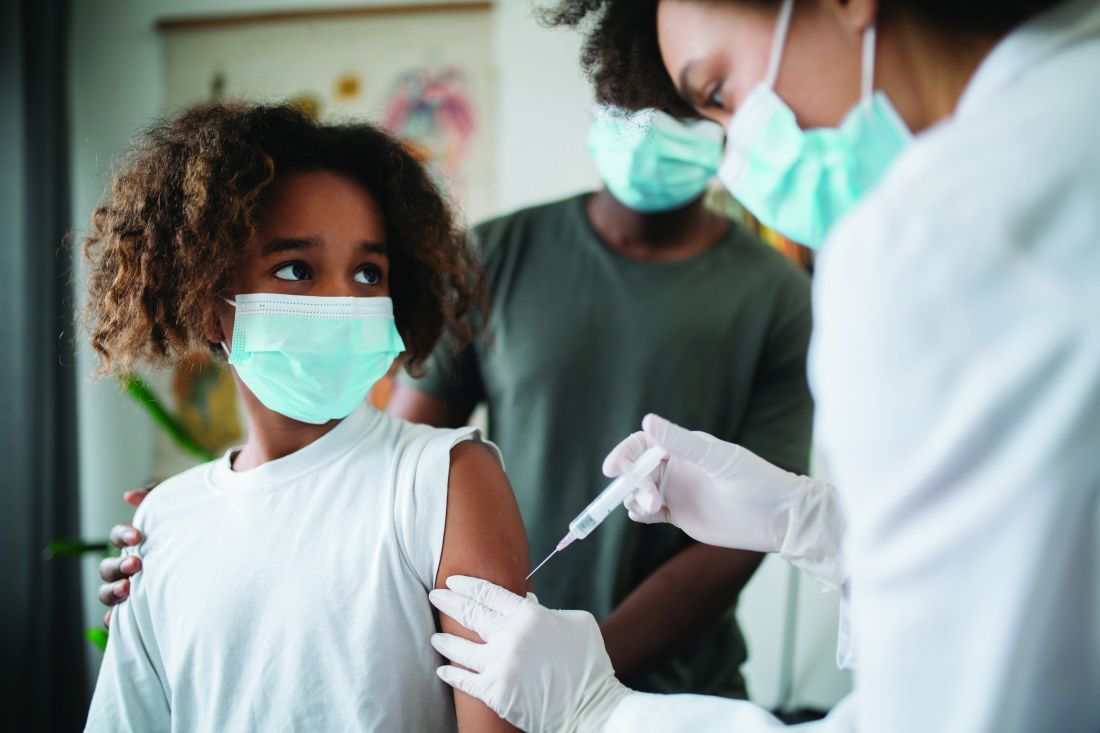
Pfizer COVID vaccine for younger children hits snag
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
COVID cases spike as questions remain about Omicron’s threat
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
Small myocarditis risk now seen for adenovirus-based COVID-19 vaccine
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
CDC panel backs mRNA COVID vaccines over J&J because of clot risk
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
Califf plans work on opioids, accelerated approvals on return to FDA
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
FDA updates risks, cautions for clotting-bleeding disorder on Janssen COVID-19 vaccine
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Doctors as trusted messengers
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
Even COVID-19 can’t stop a true optimist
Squeezing a little lemonade out of COVID-19
We like to think of ourselves as optimists here at LOTME. A glass is half full, the sky is partly sunny, and our motto is “Always look on the bright side of insanity.” Then again, our motto before that was “LOTME: Where science meets stupid,” so what do we know?
Anyway, it’s that upbeat, can-do attitude that allows us to say something positive – two somethings, actually – about the insanity that is COVID-19.
Our journey to the bright side begins, oddly enough, in the courtroom. Seems that our old friend, the face mask, is something of a lie-detector aid for juries. The authors of a recent literature review of studies on deception “found that facial expressions and other forms of nonverbal behaviour are an unreliable indicator of deceit,” according to a statement from the University of Portsmouth, where the analysis was conducted.
The one study that directly examined the role of face coverings in court proceedings showed that, “by taking away the distraction of nonverbal behaviours, observers had to rely on speech content, which turned out to be better for detecting lies,” the university said.
The second stage of our positivity trek brings us to the National Trends in Disability Employment monthly update, where we see a fourth consecutive month of gains for people with disabilities despite the larger trend of declines among those without disabilities.
Here are some numbers from the Kessler Foundation and the University of New Hampshire’s Institute on Disability to tell the story: From October to November, the employment-to-population ratio increased 4.2% for working-age people with disabilities, compared with 0.4% for people without disabilities. At the same time, the labor force participation rate rose 2.4% for working-age people with disabilities and just 0.1% for working-age people without disabilities.
Both indicators surpassed their historic highs, Andrew Houtenville, PhD, director of the Institute on Disability, said in the update. “These gains suggest that the restructuring resulting from the pandemic may be benefiting people with disabilities. Ironically, it may have taken a pandemic to shake the labor market loose for people with disabilities.”
And that is how a world-class optimist turns one gigantic lemon into lemonade.
Cut the cheese for better sleep
So, we’ve already talked about the TikTok lettuce tea hack that’s supposed to help us sleep better. Well, there’s another food that could have the opposite effect.
According to an article from the BBC, cheese has something of a reputation. Ever since the 1960s, when a researcher noted that one patient’s nightmares stopped after he quit eating an ounce or two of cheddar each night, there’s been speculation that cheese gives you weird dreams. Another study in 2005 suggested certain types of cheese cause certain types of dreams. Blue cheese for vivid dreams and cheddar cheese for celebrity cameos.
But is there any truth to it at all?
Regardless of what we eat, going to bed hungry could cause vivid dreams, according to research by Tore Nielsen, director of the University of Montreal’s dream and nightmare lab. The 2015 study showed that high lactose could have an effect on dreams.
In that study, 17% of participants said their dreams were influenced by what they ate, but the kicker was that dairy products were the foods most reported as causing the weird dreams, the BBC noted.
“It’s likely an indirect effect in that lactose produces symptoms like gas, bloating and diarrhoea and influences dreams, as dreams draw on somatic sources like this. And if you have certain kinds of intolerances, you still may be likely to eat those foods sometimes,” Mr. Nielsen told the BBC.
There’s also the theory that it’s all in the timing of consumption. Are you the type of person to sneak a slice of cheese from the fridge late at night? (Nods.) Same.
“One reason cheese and nightmares come about is that eating later before bed is more likely to disrupt sleep, and cheese can be hard to digest,” said Charlotte Gupta, a research fellow at Central Queensland University in Australia and a coauthor of a 2020 review on how diet affects our sleep.
So as tempting as it is, maybe skip sprinkling Parmesan cheese shreds into your mouth at the open fridge before bed.
Teeing up against Parkinson’s
For the nearly 1 million people in the United States with Parkinson’s disease, tai chi is one of the best ways to alleviate the symptoms. The average Parkinson’s patient, however, is going to be on the older side and more likely to view the martial art as some sort of communist plot. And would you participate in a communist plot? We don’t think so.
One group of researchers saw that patients weren’t keeping up with their therapy and decided to try a different activity, something that older people would be more likely to stick with. Something a bit more stereotypical. No, not shuffleboard. They tried golf.
“Golf is popular – the most popular sport for people over the age of 55 – which might encourage people to try it and stick with it,” study author Anne-Marie A. Wills, MD, of Massachusetts General Hospital, Boston, said in a Study Finds report.
In a small study, the investigators had a group of patients with Parkinson’s regularly go to a driving range for 10 weeks to hit golf balls (all expenses paid too, and that’s a big deal for golf), while another group continued with their tai chi.
At the end of the study, the 8 patients who went to the driving range had significantly better results in a Parkinson’s mobility test than those of the 12 patients in the tai chi group. In addition, the golf-group participants said they were more likely to continue with their therapy than were those who did tai chi.
Despite the small size of the study, the research team said the results certainly warrant further research. After all, the best sort of therapy is the kind that actually gets done. And golf just gets in your head. The eternal quest to add distance, to straighten out that annoying slice, to stop thinning half your chips, to make those annoying 4-footers. ... Maybe that’s just us.
Squeezing a little lemonade out of COVID-19
We like to think of ourselves as optimists here at LOTME. A glass is half full, the sky is partly sunny, and our motto is “Always look on the bright side of insanity.” Then again, our motto before that was “LOTME: Where science meets stupid,” so what do we know?
Anyway, it’s that upbeat, can-do attitude that allows us to say something positive – two somethings, actually – about the insanity that is COVID-19.
Our journey to the bright side begins, oddly enough, in the courtroom. Seems that our old friend, the face mask, is something of a lie-detector aid for juries. The authors of a recent literature review of studies on deception “found that facial expressions and other forms of nonverbal behaviour are an unreliable indicator of deceit,” according to a statement from the University of Portsmouth, where the analysis was conducted.
The one study that directly examined the role of face coverings in court proceedings showed that, “by taking away the distraction of nonverbal behaviours, observers had to rely on speech content, which turned out to be better for detecting lies,” the university said.
The second stage of our positivity trek brings us to the National Trends in Disability Employment monthly update, where we see a fourth consecutive month of gains for people with disabilities despite the larger trend of declines among those without disabilities.
Here are some numbers from the Kessler Foundation and the University of New Hampshire’s Institute on Disability to tell the story: From October to November, the employment-to-population ratio increased 4.2% for working-age people with disabilities, compared with 0.4% for people without disabilities. At the same time, the labor force participation rate rose 2.4% for working-age people with disabilities and just 0.1% for working-age people without disabilities.
Both indicators surpassed their historic highs, Andrew Houtenville, PhD, director of the Institute on Disability, said in the update. “These gains suggest that the restructuring resulting from the pandemic may be benefiting people with disabilities. Ironically, it may have taken a pandemic to shake the labor market loose for people with disabilities.”
And that is how a world-class optimist turns one gigantic lemon into lemonade.
Cut the cheese for better sleep
So, we’ve already talked about the TikTok lettuce tea hack that’s supposed to help us sleep better. Well, there’s another food that could have the opposite effect.
According to an article from the BBC, cheese has something of a reputation. Ever since the 1960s, when a researcher noted that one patient’s nightmares stopped after he quit eating an ounce or two of cheddar each night, there’s been speculation that cheese gives you weird dreams. Another study in 2005 suggested certain types of cheese cause certain types of dreams. Blue cheese for vivid dreams and cheddar cheese for celebrity cameos.
But is there any truth to it at all?
Regardless of what we eat, going to bed hungry could cause vivid dreams, according to research by Tore Nielsen, director of the University of Montreal’s dream and nightmare lab. The 2015 study showed that high lactose could have an effect on dreams.
In that study, 17% of participants said their dreams were influenced by what they ate, but the kicker was that dairy products were the foods most reported as causing the weird dreams, the BBC noted.
“It’s likely an indirect effect in that lactose produces symptoms like gas, bloating and diarrhoea and influences dreams, as dreams draw on somatic sources like this. And if you have certain kinds of intolerances, you still may be likely to eat those foods sometimes,” Mr. Nielsen told the BBC.
There’s also the theory that it’s all in the timing of consumption. Are you the type of person to sneak a slice of cheese from the fridge late at night? (Nods.) Same.
“One reason cheese and nightmares come about is that eating later before bed is more likely to disrupt sleep, and cheese can be hard to digest,” said Charlotte Gupta, a research fellow at Central Queensland University in Australia and a coauthor of a 2020 review on how diet affects our sleep.
So as tempting as it is, maybe skip sprinkling Parmesan cheese shreds into your mouth at the open fridge before bed.
Teeing up against Parkinson’s
For the nearly 1 million people in the United States with Parkinson’s disease, tai chi is one of the best ways to alleviate the symptoms. The average Parkinson’s patient, however, is going to be on the older side and more likely to view the martial art as some sort of communist plot. And would you participate in a communist plot? We don’t think so.
One group of researchers saw that patients weren’t keeping up with their therapy and decided to try a different activity, something that older people would be more likely to stick with. Something a bit more stereotypical. No, not shuffleboard. They tried golf.
“Golf is popular – the most popular sport for people over the age of 55 – which might encourage people to try it and stick with it,” study author Anne-Marie A. Wills, MD, of Massachusetts General Hospital, Boston, said in a Study Finds report.
In a small study, the investigators had a group of patients with Parkinson’s regularly go to a driving range for 10 weeks to hit golf balls (all expenses paid too, and that’s a big deal for golf), while another group continued with their tai chi.
At the end of the study, the 8 patients who went to the driving range had significantly better results in a Parkinson’s mobility test than those of the 12 patients in the tai chi group. In addition, the golf-group participants said they were more likely to continue with their therapy than were those who did tai chi.
Despite the small size of the study, the research team said the results certainly warrant further research. After all, the best sort of therapy is the kind that actually gets done. And golf just gets in your head. The eternal quest to add distance, to straighten out that annoying slice, to stop thinning half your chips, to make those annoying 4-footers. ... Maybe that’s just us.
Squeezing a little lemonade out of COVID-19
We like to think of ourselves as optimists here at LOTME. A glass is half full, the sky is partly sunny, and our motto is “Always look on the bright side of insanity.” Then again, our motto before that was “LOTME: Where science meets stupid,” so what do we know?
Anyway, it’s that upbeat, can-do attitude that allows us to say something positive – two somethings, actually – about the insanity that is COVID-19.
Our journey to the bright side begins, oddly enough, in the courtroom. Seems that our old friend, the face mask, is something of a lie-detector aid for juries. The authors of a recent literature review of studies on deception “found that facial expressions and other forms of nonverbal behaviour are an unreliable indicator of deceit,” according to a statement from the University of Portsmouth, where the analysis was conducted.
The one study that directly examined the role of face coverings in court proceedings showed that, “by taking away the distraction of nonverbal behaviours, observers had to rely on speech content, which turned out to be better for detecting lies,” the university said.
The second stage of our positivity trek brings us to the National Trends in Disability Employment monthly update, where we see a fourth consecutive month of gains for people with disabilities despite the larger trend of declines among those without disabilities.
Here are some numbers from the Kessler Foundation and the University of New Hampshire’s Institute on Disability to tell the story: From October to November, the employment-to-population ratio increased 4.2% for working-age people with disabilities, compared with 0.4% for people without disabilities. At the same time, the labor force participation rate rose 2.4% for working-age people with disabilities and just 0.1% for working-age people without disabilities.
Both indicators surpassed their historic highs, Andrew Houtenville, PhD, director of the Institute on Disability, said in the update. “These gains suggest that the restructuring resulting from the pandemic may be benefiting people with disabilities. Ironically, it may have taken a pandemic to shake the labor market loose for people with disabilities.”
And that is how a world-class optimist turns one gigantic lemon into lemonade.
Cut the cheese for better sleep
So, we’ve already talked about the TikTok lettuce tea hack that’s supposed to help us sleep better. Well, there’s another food that could have the opposite effect.
According to an article from the BBC, cheese has something of a reputation. Ever since the 1960s, when a researcher noted that one patient’s nightmares stopped after he quit eating an ounce or two of cheddar each night, there’s been speculation that cheese gives you weird dreams. Another study in 2005 suggested certain types of cheese cause certain types of dreams. Blue cheese for vivid dreams and cheddar cheese for celebrity cameos.
But is there any truth to it at all?
Regardless of what we eat, going to bed hungry could cause vivid dreams, according to research by Tore Nielsen, director of the University of Montreal’s dream and nightmare lab. The 2015 study showed that high lactose could have an effect on dreams.
In that study, 17% of participants said their dreams were influenced by what they ate, but the kicker was that dairy products were the foods most reported as causing the weird dreams, the BBC noted.
“It’s likely an indirect effect in that lactose produces symptoms like gas, bloating and diarrhoea and influences dreams, as dreams draw on somatic sources like this. And if you have certain kinds of intolerances, you still may be likely to eat those foods sometimes,” Mr. Nielsen told the BBC.
There’s also the theory that it’s all in the timing of consumption. Are you the type of person to sneak a slice of cheese from the fridge late at night? (Nods.) Same.
“One reason cheese and nightmares come about is that eating later before bed is more likely to disrupt sleep, and cheese can be hard to digest,” said Charlotte Gupta, a research fellow at Central Queensland University in Australia and a coauthor of a 2020 review on how diet affects our sleep.
So as tempting as it is, maybe skip sprinkling Parmesan cheese shreds into your mouth at the open fridge before bed.
Teeing up against Parkinson’s
For the nearly 1 million people in the United States with Parkinson’s disease, tai chi is one of the best ways to alleviate the symptoms. The average Parkinson’s patient, however, is going to be on the older side and more likely to view the martial art as some sort of communist plot. And would you participate in a communist plot? We don’t think so.
One group of researchers saw that patients weren’t keeping up with their therapy and decided to try a different activity, something that older people would be more likely to stick with. Something a bit more stereotypical. No, not shuffleboard. They tried golf.
“Golf is popular – the most popular sport for people over the age of 55 – which might encourage people to try it and stick with it,” study author Anne-Marie A. Wills, MD, of Massachusetts General Hospital, Boston, said in a Study Finds report.
In a small study, the investigators had a group of patients with Parkinson’s regularly go to a driving range for 10 weeks to hit golf balls (all expenses paid too, and that’s a big deal for golf), while another group continued with their tai chi.
At the end of the study, the 8 patients who went to the driving range had significantly better results in a Parkinson’s mobility test than those of the 12 patients in the tai chi group. In addition, the golf-group participants said they were more likely to continue with their therapy than were those who did tai chi.
Despite the small size of the study, the research team said the results certainly warrant further research. After all, the best sort of therapy is the kind that actually gets done. And golf just gets in your head. The eternal quest to add distance, to straighten out that annoying slice, to stop thinning half your chips, to make those annoying 4-footers. ... Maybe that’s just us.
Sputum biomarkers may predict COPD exacerbations
Examining sputum from patients with chronic obstructive pulmonary disease may help predict the course of the disease.
A mass spectrometric panel of biomarkers related to mucus hydration and inflammation examined in sputa showed elevated levels of metabolites from multiple pathways in patients with COPD. These correlated with sputum neutrophil counts and COPD exacerbations. In particular, sialic acid and hypoxanthine concentrations were strongly associated with disease severity, according to a study reported in the journal CHEST® authored by Charles R. Esther Jr. MD, PhD, and colleagues.
Given that an improved understanding of the pathways associated with airway pathophysiology in COPD will identify new predictive biomarkers and novel therapeutic targets, Dr. Esther and colleagues posed the question: Which physiologic pathways are altered and predict exacerbations in the airways of subjects with COPD?
They noted that in persons with COPD – characterized by dominant small airway obstruction associated with airway inflammation – multiple inflammatory pathways, as well as indices of oxidative stress (including oxidized glutathione and 8-isoprostane), are elevated in sputum. Because inflammation is a challenging therapeutic target, identification of other biologic pathways involved in COPD pathogenesis could point to novel biomarkers and therapeutic targets.
Using this approach in cystic fibrosis (CF), the authors have previously identified small molecule metabolites correlated with airway inflammation. Findings from that research supported development of a mass spectrometric biomarker panel for simultaneous measurement of inflammatory markers coupled to biomarkers of mucus hydration. The researchers applied this technology to sputum supernatants collected through the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), which included subjects with COPD, as well as relevant smoking and nonsmoking controls.
Addressing inflammation
“Inhaled steroids are really more effective for allergic inflammation as in asthma and less so for the neutrophilic inflammation that dominates in COPD. The challenge is that neutrophilic inflammation is also a key response to infection, and it’s really hard to find an anti-inflammatory that suppresses neutrophilic inflammation well enough to get clinical benefit but not so much that the patient becomes vulnerable to infection. Lots of clinical trials of anti-inflammatories in cystic fibrosis or COPD have been stopped because treated subjects had more trouble with infection,” Dr. Esther stated in an interview,
The investigators analyzed cell-free sputum supernatants from 980 subjects, including samples from 77 healthy nonsmokers (NS), 341 ever-smokers with preserved spirometry (SPS), and 562 subjects with COPD (178 GOLD [Global Initiative for Chronic Obstructive Lung Disease]1, 303 GOLD 2, and 81 GOLD 3). Among the subjects with COPD, elevated biomarkers from multiple pathways correlated with sputum neutrophil counts.
The most significant analytes (at FDR [False Discovery Rate] 0.1) were sialic acid (a mucin marker), hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione, with sialic acid and hypoxanthine strongly associated with measures of disease severity. Elevation of sialic acid and hypoxanthine were associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Study results
Sialic acid was elevated in all GOLD groups relative to NS healthy controls, with a 2.8-fold (0.44 log) increase in GOLD 2 and 3.7 fold (0.56 log) increase in GOLD 3 relative to NS. Sialic acid was also elevated in the most severe disease cohorts (GOLD 2 and GOLD 3) relative to smokers with preserved spirometry (SPS) and those with less severe disease (GOLD 1).
Because mucin secretion and inflammation are also related to the pathophysiology of pulmonary exacerbations, Dr. Esther and colleagues had hypothesized that sputum biomarkers would be predictive of future exacerbations. Within the full cohort, both sialic acid and hypoxanthine were significantly elevated in those who had multiple (two or more) pulmonary exacerbations relative to those who had none (P = .001). Similar, though less significant findings were observed for xanthine (P = .01), methylthioadenosine (P = .01), adenine (P = .01), and glutathione (P = .01).
Sputum tests needed
While tests still need to be developed, Dr. Esther noted in an interview that they would be based on well-established technologies commonly utilized in clinical laboratories. “Sputum biomarkers of mucus hydration and adenosine metabolism could help clinicians predict which patients with COPD are likely to experience multiple pulmonary exacerbations. Tests would be applied to patients with COPD at higher risk for exacerbations; for example, those who have low lung function or a history of prior exacerbations.”
Dr. Esther noted that these biomarkers could be helpful in developing novel therapies. “Using sialic acid to assess mucus concentrations is much easier than other methods, so it could help in developing mucolytic treatments. Also, adenosine metabolism represents a novel therapeutic target in COPD. Drugs that modify adenosine metabolism that have been approved for other diseases such as gout could be tested in COPD. As with mucus hydration, the biomarkers we identified (particularly hypoxanthine) could be utilized to make sure that novel therapies are having the intended impact on airway adenosine metabolism.”
The research was supported by SPIROMICS (funded by NIH and the COPD Foundation). Dr. Esther reported having no relevant disclosures.
Examining sputum from patients with chronic obstructive pulmonary disease may help predict the course of the disease.
A mass spectrometric panel of biomarkers related to mucus hydration and inflammation examined in sputa showed elevated levels of metabolites from multiple pathways in patients with COPD. These correlated with sputum neutrophil counts and COPD exacerbations. In particular, sialic acid and hypoxanthine concentrations were strongly associated with disease severity, according to a study reported in the journal CHEST® authored by Charles R. Esther Jr. MD, PhD, and colleagues.
Given that an improved understanding of the pathways associated with airway pathophysiology in COPD will identify new predictive biomarkers and novel therapeutic targets, Dr. Esther and colleagues posed the question: Which physiologic pathways are altered and predict exacerbations in the airways of subjects with COPD?
They noted that in persons with COPD – characterized by dominant small airway obstruction associated with airway inflammation – multiple inflammatory pathways, as well as indices of oxidative stress (including oxidized glutathione and 8-isoprostane), are elevated in sputum. Because inflammation is a challenging therapeutic target, identification of other biologic pathways involved in COPD pathogenesis could point to novel biomarkers and therapeutic targets.
Using this approach in cystic fibrosis (CF), the authors have previously identified small molecule metabolites correlated with airway inflammation. Findings from that research supported development of a mass spectrometric biomarker panel for simultaneous measurement of inflammatory markers coupled to biomarkers of mucus hydration. The researchers applied this technology to sputum supernatants collected through the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), which included subjects with COPD, as well as relevant smoking and nonsmoking controls.
Addressing inflammation
“Inhaled steroids are really more effective for allergic inflammation as in asthma and less so for the neutrophilic inflammation that dominates in COPD. The challenge is that neutrophilic inflammation is also a key response to infection, and it’s really hard to find an anti-inflammatory that suppresses neutrophilic inflammation well enough to get clinical benefit but not so much that the patient becomes vulnerable to infection. Lots of clinical trials of anti-inflammatories in cystic fibrosis or COPD have been stopped because treated subjects had more trouble with infection,” Dr. Esther stated in an interview,
The investigators analyzed cell-free sputum supernatants from 980 subjects, including samples from 77 healthy nonsmokers (NS), 341 ever-smokers with preserved spirometry (SPS), and 562 subjects with COPD (178 GOLD [Global Initiative for Chronic Obstructive Lung Disease]1, 303 GOLD 2, and 81 GOLD 3). Among the subjects with COPD, elevated biomarkers from multiple pathways correlated with sputum neutrophil counts.
The most significant analytes (at FDR [False Discovery Rate] 0.1) were sialic acid (a mucin marker), hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione, with sialic acid and hypoxanthine strongly associated with measures of disease severity. Elevation of sialic acid and hypoxanthine were associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Study results
Sialic acid was elevated in all GOLD groups relative to NS healthy controls, with a 2.8-fold (0.44 log) increase in GOLD 2 and 3.7 fold (0.56 log) increase in GOLD 3 relative to NS. Sialic acid was also elevated in the most severe disease cohorts (GOLD 2 and GOLD 3) relative to smokers with preserved spirometry (SPS) and those with less severe disease (GOLD 1).
Because mucin secretion and inflammation are also related to the pathophysiology of pulmonary exacerbations, Dr. Esther and colleagues had hypothesized that sputum biomarkers would be predictive of future exacerbations. Within the full cohort, both sialic acid and hypoxanthine were significantly elevated in those who had multiple (two or more) pulmonary exacerbations relative to those who had none (P = .001). Similar, though less significant findings were observed for xanthine (P = .01), methylthioadenosine (P = .01), adenine (P = .01), and glutathione (P = .01).
Sputum tests needed
While tests still need to be developed, Dr. Esther noted in an interview that they would be based on well-established technologies commonly utilized in clinical laboratories. “Sputum biomarkers of mucus hydration and adenosine metabolism could help clinicians predict which patients with COPD are likely to experience multiple pulmonary exacerbations. Tests would be applied to patients with COPD at higher risk for exacerbations; for example, those who have low lung function or a history of prior exacerbations.”
Dr. Esther noted that these biomarkers could be helpful in developing novel therapies. “Using sialic acid to assess mucus concentrations is much easier than other methods, so it could help in developing mucolytic treatments. Also, adenosine metabolism represents a novel therapeutic target in COPD. Drugs that modify adenosine metabolism that have been approved for other diseases such as gout could be tested in COPD. As with mucus hydration, the biomarkers we identified (particularly hypoxanthine) could be utilized to make sure that novel therapies are having the intended impact on airway adenosine metabolism.”
The research was supported by SPIROMICS (funded by NIH and the COPD Foundation). Dr. Esther reported having no relevant disclosures.
Examining sputum from patients with chronic obstructive pulmonary disease may help predict the course of the disease.
A mass spectrometric panel of biomarkers related to mucus hydration and inflammation examined in sputa showed elevated levels of metabolites from multiple pathways in patients with COPD. These correlated with sputum neutrophil counts and COPD exacerbations. In particular, sialic acid and hypoxanthine concentrations were strongly associated with disease severity, according to a study reported in the journal CHEST® authored by Charles R. Esther Jr. MD, PhD, and colleagues.
Given that an improved understanding of the pathways associated with airway pathophysiology in COPD will identify new predictive biomarkers and novel therapeutic targets, Dr. Esther and colleagues posed the question: Which physiologic pathways are altered and predict exacerbations in the airways of subjects with COPD?
They noted that in persons with COPD – characterized by dominant small airway obstruction associated with airway inflammation – multiple inflammatory pathways, as well as indices of oxidative stress (including oxidized glutathione and 8-isoprostane), are elevated in sputum. Because inflammation is a challenging therapeutic target, identification of other biologic pathways involved in COPD pathogenesis could point to novel biomarkers and therapeutic targets.
Using this approach in cystic fibrosis (CF), the authors have previously identified small molecule metabolites correlated with airway inflammation. Findings from that research supported development of a mass spectrometric biomarker panel for simultaneous measurement of inflammatory markers coupled to biomarkers of mucus hydration. The researchers applied this technology to sputum supernatants collected through the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), which included subjects with COPD, as well as relevant smoking and nonsmoking controls.
Addressing inflammation
“Inhaled steroids are really more effective for allergic inflammation as in asthma and less so for the neutrophilic inflammation that dominates in COPD. The challenge is that neutrophilic inflammation is also a key response to infection, and it’s really hard to find an anti-inflammatory that suppresses neutrophilic inflammation well enough to get clinical benefit but not so much that the patient becomes vulnerable to infection. Lots of clinical trials of anti-inflammatories in cystic fibrosis or COPD have been stopped because treated subjects had more trouble with infection,” Dr. Esther stated in an interview,
The investigators analyzed cell-free sputum supernatants from 980 subjects, including samples from 77 healthy nonsmokers (NS), 341 ever-smokers with preserved spirometry (SPS), and 562 subjects with COPD (178 GOLD [Global Initiative for Chronic Obstructive Lung Disease]1, 303 GOLD 2, and 81 GOLD 3). Among the subjects with COPD, elevated biomarkers from multiple pathways correlated with sputum neutrophil counts.
The most significant analytes (at FDR [False Discovery Rate] 0.1) were sialic acid (a mucin marker), hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione, with sialic acid and hypoxanthine strongly associated with measures of disease severity. Elevation of sialic acid and hypoxanthine were associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Study results
Sialic acid was elevated in all GOLD groups relative to NS healthy controls, with a 2.8-fold (0.44 log) increase in GOLD 2 and 3.7 fold (0.56 log) increase in GOLD 3 relative to NS. Sialic acid was also elevated in the most severe disease cohorts (GOLD 2 and GOLD 3) relative to smokers with preserved spirometry (SPS) and those with less severe disease (GOLD 1).
Because mucin secretion and inflammation are also related to the pathophysiology of pulmonary exacerbations, Dr. Esther and colleagues had hypothesized that sputum biomarkers would be predictive of future exacerbations. Within the full cohort, both sialic acid and hypoxanthine were significantly elevated in those who had multiple (two or more) pulmonary exacerbations relative to those who had none (P = .001). Similar, though less significant findings were observed for xanthine (P = .01), methylthioadenosine (P = .01), adenine (P = .01), and glutathione (P = .01).
Sputum tests needed
While tests still need to be developed, Dr. Esther noted in an interview that they would be based on well-established technologies commonly utilized in clinical laboratories. “Sputum biomarkers of mucus hydration and adenosine metabolism could help clinicians predict which patients with COPD are likely to experience multiple pulmonary exacerbations. Tests would be applied to patients with COPD at higher risk for exacerbations; for example, those who have low lung function or a history of prior exacerbations.”
Dr. Esther noted that these biomarkers could be helpful in developing novel therapies. “Using sialic acid to assess mucus concentrations is much easier than other methods, so it could help in developing mucolytic treatments. Also, adenosine metabolism represents a novel therapeutic target in COPD. Drugs that modify adenosine metabolism that have been approved for other diseases such as gout could be tested in COPD. As with mucus hydration, the biomarkers we identified (particularly hypoxanthine) could be utilized to make sure that novel therapies are having the intended impact on airway adenosine metabolism.”
The research was supported by SPIROMICS (funded by NIH and the COPD Foundation). Dr. Esther reported having no relevant disclosures.
FROM THE JOURNAL CHEST®
More Americans skipping medical care because of cost, survey says
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.