User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


RETHINC takes air out of COPD-like therapy for smokers
Current or former smokers who have clinically significant respiratory symptoms but no spirometric evidence of airway obstruction are often treated with dual bronchodilators commonly prescribed for patients with chronic obstructive pulmonary disease (COPD).
But as results of the randomized RETHINC (Redefining Therapy In Early COPD for the Pulmonary Trials Cooperative) trial showed, bronchodilator therapy was no better than placebo at reducing respiratory symptoms in smokers, reported MeiLan K. Han, MD, from the University of Michigan, Ann Arbor, on behalf of colleagues in the RETHINC study group.
“Many tobacco-exposed symptomatic individuals are currently being treated. We don’t know if this is because physicians just aren’t doing spirometry and assuming COPD or they strongly believe that there’s a benefit, but the bottom line is that we really need to do spirometry to understand who benefits from bronchodilators, and we need further research to understand how to treat this specific group of patients because there truly is pathogenesis and disease burden,” Dr. Han said in an oral abstract presentation at the annual congress of the European Respiratory Society.
The study results were also published online in the New England Journal of Medicine to coincide with the presentation.
In an editorial accompanying the study, Don D. Sin, MD, MPH, from the University of British Columbia, Vancouver, commented that these medications should most likely be reserved for patients with COPD who have clinically significant airflow limitation,” and that “respiratory symptoms in tobacco-exposed persons are common but are highly variable over time.”
Dave Singh, MD, from the University of Manchester (England), the invited discussant, called it “a very important negative study.”
Not up to GOLD standard
Current or former smokers who are symptomatic, with COPD Assessment Test (CAT) scores of at least 10 despite having preserved function on spirometry, have been shown to have higher prospective rates of respiratory disease exacerbations and increased sputum total mucin concentrations. Approximately 43% of such patients are treated with bronchodilators, and 23% are treated with inhaled corticosteroids (ICS), Dr. Han noted.
Her group hypothesized that ever-smokers with spirometric values that fall within the normal range – that is, a postbronchodilator FEV1/FVC ratio of 70 or greater – would still derive benefit from long-acting bronchodilator therapy, even though these patients are currently excluded from Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations.
To test this, they conducted a 12-week, multicenter, randomized, parallel-group study in which patients were assigned to receive either indacaterol (27.5 mcg) and glycopyrrolate (15.6 mcg) inhaled twice daily or placebo.
They enrolled adults aged 40-80 years with a minimum of 10 pack-years of smoking history, postbronchodilator FEV1/FVC ratio of 70 or greater, and CAT scores of 10 or greater. Patients with known concomitant lung disease, a primary diagnosis of asthma, or body mass index lower than 15 or higher than 40 and those being concomitantly treated with long-acting beta2-agonists or muscarinic antagonists or a short-acting combination were excluded, although patients were allowed to be on a short-acting beta-agonist.
A total of 535 participants were randomized, but COVID-19 pandemic–imposed obstacles resulted in a modified intention-to-treat population of 277 patients assigned to receive the active treatment and 244 assigned to receive placebo.
There was no difference between the groups for the primary outcome of an at least 4-point decrease in St. George’s Respiratory Questionnaire scores in patients who did not experience treatment failure, defined as an increase in respiratory symptoms requiring treatment with active long-acting bronchodilators or ICS.
The primary endpoint was seen in 56.4% of patients in the bronchodilator group, and 59% of controls.
Although there was greater improvement in pulmonary function from baseline in the treatment group, compared with the placebo group, the improvements did not correlate with similar improvements in symptoms, Dr. Han said.
There were 4 serious adverse events in the bronchodilator group and 11 in the placebo group, but none of the events were deemed to be related to the assigned treatments.
Dr. Han acknowledged limitations of the study, which may have included symptoms driven by other factors such as cardiac disease, suggesting that if such patients had been identified and excluded, a stronger effect might have been seen for the active treatment.
In addition, the study was underpowered to look at the subgroup of participants with chronic bronchitis, and the 12 weeks of the study may have been too short to see improvements in symptoms.
In his editorial, Dr. Sin noted that the study showed that cough and sputum production rather than exertion dyspnea are the primary symptoms among ever-smokers.
“Although bronchodilators are effective in ameliorating breathlessness and improving exercise tolerance, they are generally ineffective for cough,” he wrote. “Existing drugs for the treatment of COPD, such as inhaled glucocorticoids or phosphodiesterase-4 inhibitors, or new therapeutics such as P2X3 receptor antagonists may be more effective for the treatment of cough and sputum production related to smoking and could be considered for future evaluations in this patient population.”
The study was supported by the National Heart, Lung, and Blood Institute, the National Center for Advancing Translational Sciences, and Sunovion Pharmaceuticals. Novartis Pharmaceuticals donated the trial medication and placebo. Dr. Han disclosed grant/research support and honoraria or consulting fees from various companies. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Sin reported having no conflicts of interest to disclose.
A version of this article first appeared on Medscape.com.
Current or former smokers who have clinically significant respiratory symptoms but no spirometric evidence of airway obstruction are often treated with dual bronchodilators commonly prescribed for patients with chronic obstructive pulmonary disease (COPD).
But as results of the randomized RETHINC (Redefining Therapy In Early COPD for the Pulmonary Trials Cooperative) trial showed, bronchodilator therapy was no better than placebo at reducing respiratory symptoms in smokers, reported MeiLan K. Han, MD, from the University of Michigan, Ann Arbor, on behalf of colleagues in the RETHINC study group.
“Many tobacco-exposed symptomatic individuals are currently being treated. We don’t know if this is because physicians just aren’t doing spirometry and assuming COPD or they strongly believe that there’s a benefit, but the bottom line is that we really need to do spirometry to understand who benefits from bronchodilators, and we need further research to understand how to treat this specific group of patients because there truly is pathogenesis and disease burden,” Dr. Han said in an oral abstract presentation at the annual congress of the European Respiratory Society.
The study results were also published online in the New England Journal of Medicine to coincide with the presentation.
In an editorial accompanying the study, Don D. Sin, MD, MPH, from the University of British Columbia, Vancouver, commented that these medications should most likely be reserved for patients with COPD who have clinically significant airflow limitation,” and that “respiratory symptoms in tobacco-exposed persons are common but are highly variable over time.”
Dave Singh, MD, from the University of Manchester (England), the invited discussant, called it “a very important negative study.”
Not up to GOLD standard
Current or former smokers who are symptomatic, with COPD Assessment Test (CAT) scores of at least 10 despite having preserved function on spirometry, have been shown to have higher prospective rates of respiratory disease exacerbations and increased sputum total mucin concentrations. Approximately 43% of such patients are treated with bronchodilators, and 23% are treated with inhaled corticosteroids (ICS), Dr. Han noted.
Her group hypothesized that ever-smokers with spirometric values that fall within the normal range – that is, a postbronchodilator FEV1/FVC ratio of 70 or greater – would still derive benefit from long-acting bronchodilator therapy, even though these patients are currently excluded from Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations.
To test this, they conducted a 12-week, multicenter, randomized, parallel-group study in which patients were assigned to receive either indacaterol (27.5 mcg) and glycopyrrolate (15.6 mcg) inhaled twice daily or placebo.
They enrolled adults aged 40-80 years with a minimum of 10 pack-years of smoking history, postbronchodilator FEV1/FVC ratio of 70 or greater, and CAT scores of 10 or greater. Patients with known concomitant lung disease, a primary diagnosis of asthma, or body mass index lower than 15 or higher than 40 and those being concomitantly treated with long-acting beta2-agonists or muscarinic antagonists or a short-acting combination were excluded, although patients were allowed to be on a short-acting beta-agonist.
A total of 535 participants were randomized, but COVID-19 pandemic–imposed obstacles resulted in a modified intention-to-treat population of 277 patients assigned to receive the active treatment and 244 assigned to receive placebo.
There was no difference between the groups for the primary outcome of an at least 4-point decrease in St. George’s Respiratory Questionnaire scores in patients who did not experience treatment failure, defined as an increase in respiratory symptoms requiring treatment with active long-acting bronchodilators or ICS.
The primary endpoint was seen in 56.4% of patients in the bronchodilator group, and 59% of controls.
Although there was greater improvement in pulmonary function from baseline in the treatment group, compared with the placebo group, the improvements did not correlate with similar improvements in symptoms, Dr. Han said.
There were 4 serious adverse events in the bronchodilator group and 11 in the placebo group, but none of the events were deemed to be related to the assigned treatments.
Dr. Han acknowledged limitations of the study, which may have included symptoms driven by other factors such as cardiac disease, suggesting that if such patients had been identified and excluded, a stronger effect might have been seen for the active treatment.
In addition, the study was underpowered to look at the subgroup of participants with chronic bronchitis, and the 12 weeks of the study may have been too short to see improvements in symptoms.
In his editorial, Dr. Sin noted that the study showed that cough and sputum production rather than exertion dyspnea are the primary symptoms among ever-smokers.
“Although bronchodilators are effective in ameliorating breathlessness and improving exercise tolerance, they are generally ineffective for cough,” he wrote. “Existing drugs for the treatment of COPD, such as inhaled glucocorticoids or phosphodiesterase-4 inhibitors, or new therapeutics such as P2X3 receptor antagonists may be more effective for the treatment of cough and sputum production related to smoking and could be considered for future evaluations in this patient population.”
The study was supported by the National Heart, Lung, and Blood Institute, the National Center for Advancing Translational Sciences, and Sunovion Pharmaceuticals. Novartis Pharmaceuticals donated the trial medication and placebo. Dr. Han disclosed grant/research support and honoraria or consulting fees from various companies. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Sin reported having no conflicts of interest to disclose.
A version of this article first appeared on Medscape.com.
Current or former smokers who have clinically significant respiratory symptoms but no spirometric evidence of airway obstruction are often treated with dual bronchodilators commonly prescribed for patients with chronic obstructive pulmonary disease (COPD).
But as results of the randomized RETHINC (Redefining Therapy In Early COPD for the Pulmonary Trials Cooperative) trial showed, bronchodilator therapy was no better than placebo at reducing respiratory symptoms in smokers, reported MeiLan K. Han, MD, from the University of Michigan, Ann Arbor, on behalf of colleagues in the RETHINC study group.
“Many tobacco-exposed symptomatic individuals are currently being treated. We don’t know if this is because physicians just aren’t doing spirometry and assuming COPD or they strongly believe that there’s a benefit, but the bottom line is that we really need to do spirometry to understand who benefits from bronchodilators, and we need further research to understand how to treat this specific group of patients because there truly is pathogenesis and disease burden,” Dr. Han said in an oral abstract presentation at the annual congress of the European Respiratory Society.
The study results were also published online in the New England Journal of Medicine to coincide with the presentation.
In an editorial accompanying the study, Don D. Sin, MD, MPH, from the University of British Columbia, Vancouver, commented that these medications should most likely be reserved for patients with COPD who have clinically significant airflow limitation,” and that “respiratory symptoms in tobacco-exposed persons are common but are highly variable over time.”
Dave Singh, MD, from the University of Manchester (England), the invited discussant, called it “a very important negative study.”
Not up to GOLD standard
Current or former smokers who are symptomatic, with COPD Assessment Test (CAT) scores of at least 10 despite having preserved function on spirometry, have been shown to have higher prospective rates of respiratory disease exacerbations and increased sputum total mucin concentrations. Approximately 43% of such patients are treated with bronchodilators, and 23% are treated with inhaled corticosteroids (ICS), Dr. Han noted.
Her group hypothesized that ever-smokers with spirometric values that fall within the normal range – that is, a postbronchodilator FEV1/FVC ratio of 70 or greater – would still derive benefit from long-acting bronchodilator therapy, even though these patients are currently excluded from Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations.
To test this, they conducted a 12-week, multicenter, randomized, parallel-group study in which patients were assigned to receive either indacaterol (27.5 mcg) and glycopyrrolate (15.6 mcg) inhaled twice daily or placebo.
They enrolled adults aged 40-80 years with a minimum of 10 pack-years of smoking history, postbronchodilator FEV1/FVC ratio of 70 or greater, and CAT scores of 10 or greater. Patients with known concomitant lung disease, a primary diagnosis of asthma, or body mass index lower than 15 or higher than 40 and those being concomitantly treated with long-acting beta2-agonists or muscarinic antagonists or a short-acting combination were excluded, although patients were allowed to be on a short-acting beta-agonist.
A total of 535 participants were randomized, but COVID-19 pandemic–imposed obstacles resulted in a modified intention-to-treat population of 277 patients assigned to receive the active treatment and 244 assigned to receive placebo.
There was no difference between the groups for the primary outcome of an at least 4-point decrease in St. George’s Respiratory Questionnaire scores in patients who did not experience treatment failure, defined as an increase in respiratory symptoms requiring treatment with active long-acting bronchodilators or ICS.
The primary endpoint was seen in 56.4% of patients in the bronchodilator group, and 59% of controls.
Although there was greater improvement in pulmonary function from baseline in the treatment group, compared with the placebo group, the improvements did not correlate with similar improvements in symptoms, Dr. Han said.
There were 4 serious adverse events in the bronchodilator group and 11 in the placebo group, but none of the events were deemed to be related to the assigned treatments.
Dr. Han acknowledged limitations of the study, which may have included symptoms driven by other factors such as cardiac disease, suggesting that if such patients had been identified and excluded, a stronger effect might have been seen for the active treatment.
In addition, the study was underpowered to look at the subgroup of participants with chronic bronchitis, and the 12 weeks of the study may have been too short to see improvements in symptoms.
In his editorial, Dr. Sin noted that the study showed that cough and sputum production rather than exertion dyspnea are the primary symptoms among ever-smokers.
“Although bronchodilators are effective in ameliorating breathlessness and improving exercise tolerance, they are generally ineffective for cough,” he wrote. “Existing drugs for the treatment of COPD, such as inhaled glucocorticoids or phosphodiesterase-4 inhibitors, or new therapeutics such as P2X3 receptor antagonists may be more effective for the treatment of cough and sputum production related to smoking and could be considered for future evaluations in this patient population.”
The study was supported by the National Heart, Lung, and Blood Institute, the National Center for Advancing Translational Sciences, and Sunovion Pharmaceuticals. Novartis Pharmaceuticals donated the trial medication and placebo. Dr. Han disclosed grant/research support and honoraria or consulting fees from various companies. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Sin reported having no conflicts of interest to disclose.
A version of this article first appeared on Medscape.com.
FROM ERS 2022
Children and COVID: Weekly cases close out August with a second straight increase
New cases rose by 4.6% for the week of Aug. 26 to Sept. 1, following a week in which cases increased by almost 9%, as the second half of August basically reversed the two consecutive weeks of decreases during the first half of the month, based on the AAP/CHA data collected from state and territorial health departments.
Similar trends can be seen for emergency department visits, with the exception of children aged 0-11 years, whose ED visit rates have continued to fall since late July. Children aged 12-15, however, had a 7-day average of 4.4% of ED visits with diagnosed COVID on Aug. 25, compared with 3.1% for Aug. 12. Children aged 16-17 years were at 3.4% on Aug. 27, compared with 3.1% as late as Aug. 15, the Centers for Disease Control and Prevention reported.
Hospital admissions with confirmed COVID-19, reported only for children aged 0-17 years, also reflect the late-August trend of increased cases. New hospitalizations dropped from 0.46 per 100,000 population on July 30 to 0.40 per 100,000 on Aug. 19 but have since risen to 0.44 per 100,000 as of Aug. 27, the CDC said on its COVID Data Tracker.
Initial vaccinations, meanwhile, have declined since early August for all children, according to a separate report from the AAP. A look at CDC data for two specific days – the first and last Mondays of the month – shows that those aged under 5 received 12,982 doses on Aug. 1, compared with 5,824 doses on Aug. 29. Over that same time, initial vaccinations in 5- to 11-year-olds went from 9,058 to 2,879, while among those aged 12-17 they dropped from 4,245 to 1,226.
Cumulatively, 5.5% of all children under age 5 had received at least one dose and 1.3% were fully vaccinated by Aug. 30, compared with 38.1% and 30.7%, respectively, of those aged 5-11 and 70.7% and 60.5% of 12- to 17-year-olds, the CDC said.
New cases rose by 4.6% for the week of Aug. 26 to Sept. 1, following a week in which cases increased by almost 9%, as the second half of August basically reversed the two consecutive weeks of decreases during the first half of the month, based on the AAP/CHA data collected from state and territorial health departments.
Similar trends can be seen for emergency department visits, with the exception of children aged 0-11 years, whose ED visit rates have continued to fall since late July. Children aged 12-15, however, had a 7-day average of 4.4% of ED visits with diagnosed COVID on Aug. 25, compared with 3.1% for Aug. 12. Children aged 16-17 years were at 3.4% on Aug. 27, compared with 3.1% as late as Aug. 15, the Centers for Disease Control and Prevention reported.
Hospital admissions with confirmed COVID-19, reported only for children aged 0-17 years, also reflect the late-August trend of increased cases. New hospitalizations dropped from 0.46 per 100,000 population on July 30 to 0.40 per 100,000 on Aug. 19 but have since risen to 0.44 per 100,000 as of Aug. 27, the CDC said on its COVID Data Tracker.
Initial vaccinations, meanwhile, have declined since early August for all children, according to a separate report from the AAP. A look at CDC data for two specific days – the first and last Mondays of the month – shows that those aged under 5 received 12,982 doses on Aug. 1, compared with 5,824 doses on Aug. 29. Over that same time, initial vaccinations in 5- to 11-year-olds went from 9,058 to 2,879, while among those aged 12-17 they dropped from 4,245 to 1,226.
Cumulatively, 5.5% of all children under age 5 had received at least one dose and 1.3% were fully vaccinated by Aug. 30, compared with 38.1% and 30.7%, respectively, of those aged 5-11 and 70.7% and 60.5% of 12- to 17-year-olds, the CDC said.
New cases rose by 4.6% for the week of Aug. 26 to Sept. 1, following a week in which cases increased by almost 9%, as the second half of August basically reversed the two consecutive weeks of decreases during the first half of the month, based on the AAP/CHA data collected from state and territorial health departments.
Similar trends can be seen for emergency department visits, with the exception of children aged 0-11 years, whose ED visit rates have continued to fall since late July. Children aged 12-15, however, had a 7-day average of 4.4% of ED visits with diagnosed COVID on Aug. 25, compared with 3.1% for Aug. 12. Children aged 16-17 years were at 3.4% on Aug. 27, compared with 3.1% as late as Aug. 15, the Centers for Disease Control and Prevention reported.
Hospital admissions with confirmed COVID-19, reported only for children aged 0-17 years, also reflect the late-August trend of increased cases. New hospitalizations dropped from 0.46 per 100,000 population on July 30 to 0.40 per 100,000 on Aug. 19 but have since risen to 0.44 per 100,000 as of Aug. 27, the CDC said on its COVID Data Tracker.
Initial vaccinations, meanwhile, have declined since early August for all children, according to a separate report from the AAP. A look at CDC data for two specific days – the first and last Mondays of the month – shows that those aged under 5 received 12,982 doses on Aug. 1, compared with 5,824 doses on Aug. 29. Over that same time, initial vaccinations in 5- to 11-year-olds went from 9,058 to 2,879, while among those aged 12-17 they dropped from 4,245 to 1,226.
Cumulatively, 5.5% of all children under age 5 had received at least one dose and 1.3% were fully vaccinated by Aug. 30, compared with 38.1% and 30.7%, respectively, of those aged 5-11 and 70.7% and 60.5% of 12- to 17-year-olds, the CDC said.
Cannabis industry cribs Big Tobacco’s social responsibility initiatives
according to recent data.
A qualitative study of cannabis companies’ CSR practices over 10 years found, for example, that dispensary Trulieve provided $15,000 for internships and $20,000 for scholarships to prepare Black students for careers in the cannabis industry. The tobacco industry has used similar initiatives to foster good will and market its products to minority populations.
“The main message from this paper is that this is an industry selling a product with health impacts,” said study author Tanner Wakefield, an associate specialist at the Center for Tobacco Control Research and Education at the University of California, San Francisco. “We have seen how the tobacco industry in the past has used corporate social responsibility practices to insulate itself politically, engender public good will, and encourage consumption of tobacco products with harmful health effects.”
The study was published in JAMA Network Open.
A double agenda
The investigators identified 9 of the 10 largest publicly traded cannabis companies in the United States and Canada and examined the CSR activities that they conducted between Jan. 1, 2012, and Dec. 31, 2021. The investigators also conducted a systematic review of corporate websites and Nexis Uni articles that identified 153 news stories, press releases, and web pages that communicated about cannabis companies’ charitable and philanthropic activities.
Investigators identified themes in CSR activities by categorizing the language and informational patterns in the evidence they collected. They divided CSR practices into five categories, consisting of campaigns supposedly mitigating the harmful effects of past cannabis prohibition; initiatives characterized as promoting or increasing diversity, equity, and inclusion; charitable contributions; researching therapeutic cannabis uses and increasing medical access; and efforts claiming to address harms related to cannabis legalization.
The investigators observed that Green Thumb Industries and Cresco Labs set up “business incubators” and licensing assistance programs targeted toward members of racial and ethnic minority populations and communities most affected by cannabis prohibition. Canopy Growth Corporation supported research into whether medical cannabis could alleviate sleep disorders or treat mental health conditions. The company also collaborated with Canadian Students for Sensible Drug Policy and Parent Action on Drugs to create materials for preventing cannabis abuse among youth.
“I think we need to remember that this is an industry selling a product,” said Mr. Wakefield. “And just because there is merit to addressing certain issues or harms, that doesn’t mean we should forget that they are businesses seeking to make a profit. While CSR activities may have some potential benefits or apparent legitimacy, we have to remember that CSR is also a form of marketing and political influence.”
The investigators concluded that these CSR activities were similar to CSR strategies that the tobacco industry previously had used to encourage consumption, target marginalized communities, influence regulation, and advance corporate interests.
“A similarity to the tobacco industry is that they would provide funding or assistance to nonprofit groups that are not necessarily tied to cannabis or tobacco,” said Mr. Wakefield. For example, the investigators noted that cannabis companies contributed funding for breast cancer research and for veterans.
Moreover, the investigators observed “similarities in terms of focus and orientation toward special interest populations,” said Mr. Wakefield. Those special populations included the LGBTQ communities, and activities included sponsoring or participating in pride celebrations and releasing limited-edition pride products.
Overall, the cannabis industry engages in CSR activities that appear to mitigate the harmful effects of its products and operations, said Mr. Wakefield.
‘Incomplete information’
Jason W. Busse, DC, PhD, associate director of the Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., described the study as rigorous, but with limitations acknowledged by the authors.
“Understanding how these companies are promoting themselves and justifying themselves as good corporate citizens is important,” said Dr. Busse. “The investigators have undertaken a comprehensive study and identified CSR activities that cannabis companies are engaging in. We know from past experiences with tobacco companies that these activities may be used in part to encourage less regulation and increase market access.”
One constraint of the study is that the investigators used documents that were in the public domain, as opposed to internal company information. “The investigators were limited to the information that they were able to access,” said Dr. Busse. “Unless you use the Freedom of Information Act to compel companies to release internal documents, you don’t have that information. They haven’t done that, and it is almost a certainty that the authors had to work with incomplete information.”
The investigators suggest a need for oversight of the cannabis industry’s CSR practices, and Dr. Busse agreed with this assessment. “While engagement in social responsibility activities by cannabis corporations may have positive results, there should be independent assessment of outcomes. For example, sponsoring research may be problematic if such support comes with strings attached, such as suppressing or modifying unfavorable findings.”
The study was supported by the National Institutes of Health. Dr. Wakefield and Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to recent data.
A qualitative study of cannabis companies’ CSR practices over 10 years found, for example, that dispensary Trulieve provided $15,000 for internships and $20,000 for scholarships to prepare Black students for careers in the cannabis industry. The tobacco industry has used similar initiatives to foster good will and market its products to minority populations.
“The main message from this paper is that this is an industry selling a product with health impacts,” said study author Tanner Wakefield, an associate specialist at the Center for Tobacco Control Research and Education at the University of California, San Francisco. “We have seen how the tobacco industry in the past has used corporate social responsibility practices to insulate itself politically, engender public good will, and encourage consumption of tobacco products with harmful health effects.”
The study was published in JAMA Network Open.
A double agenda
The investigators identified 9 of the 10 largest publicly traded cannabis companies in the United States and Canada and examined the CSR activities that they conducted between Jan. 1, 2012, and Dec. 31, 2021. The investigators also conducted a systematic review of corporate websites and Nexis Uni articles that identified 153 news stories, press releases, and web pages that communicated about cannabis companies’ charitable and philanthropic activities.
Investigators identified themes in CSR activities by categorizing the language and informational patterns in the evidence they collected. They divided CSR practices into five categories, consisting of campaigns supposedly mitigating the harmful effects of past cannabis prohibition; initiatives characterized as promoting or increasing diversity, equity, and inclusion; charitable contributions; researching therapeutic cannabis uses and increasing medical access; and efforts claiming to address harms related to cannabis legalization.
The investigators observed that Green Thumb Industries and Cresco Labs set up “business incubators” and licensing assistance programs targeted toward members of racial and ethnic minority populations and communities most affected by cannabis prohibition. Canopy Growth Corporation supported research into whether medical cannabis could alleviate sleep disorders or treat mental health conditions. The company also collaborated with Canadian Students for Sensible Drug Policy and Parent Action on Drugs to create materials for preventing cannabis abuse among youth.
“I think we need to remember that this is an industry selling a product,” said Mr. Wakefield. “And just because there is merit to addressing certain issues or harms, that doesn’t mean we should forget that they are businesses seeking to make a profit. While CSR activities may have some potential benefits or apparent legitimacy, we have to remember that CSR is also a form of marketing and political influence.”
The investigators concluded that these CSR activities were similar to CSR strategies that the tobacco industry previously had used to encourage consumption, target marginalized communities, influence regulation, and advance corporate interests.
“A similarity to the tobacco industry is that they would provide funding or assistance to nonprofit groups that are not necessarily tied to cannabis or tobacco,” said Mr. Wakefield. For example, the investigators noted that cannabis companies contributed funding for breast cancer research and for veterans.
Moreover, the investigators observed “similarities in terms of focus and orientation toward special interest populations,” said Mr. Wakefield. Those special populations included the LGBTQ communities, and activities included sponsoring or participating in pride celebrations and releasing limited-edition pride products.
Overall, the cannabis industry engages in CSR activities that appear to mitigate the harmful effects of its products and operations, said Mr. Wakefield.
‘Incomplete information’
Jason W. Busse, DC, PhD, associate director of the Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., described the study as rigorous, but with limitations acknowledged by the authors.
“Understanding how these companies are promoting themselves and justifying themselves as good corporate citizens is important,” said Dr. Busse. “The investigators have undertaken a comprehensive study and identified CSR activities that cannabis companies are engaging in. We know from past experiences with tobacco companies that these activities may be used in part to encourage less regulation and increase market access.”
One constraint of the study is that the investigators used documents that were in the public domain, as opposed to internal company information. “The investigators were limited to the information that they were able to access,” said Dr. Busse. “Unless you use the Freedom of Information Act to compel companies to release internal documents, you don’t have that information. They haven’t done that, and it is almost a certainty that the authors had to work with incomplete information.”
The investigators suggest a need for oversight of the cannabis industry’s CSR practices, and Dr. Busse agreed with this assessment. “While engagement in social responsibility activities by cannabis corporations may have positive results, there should be independent assessment of outcomes. For example, sponsoring research may be problematic if such support comes with strings attached, such as suppressing or modifying unfavorable findings.”
The study was supported by the National Institutes of Health. Dr. Wakefield and Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to recent data.
A qualitative study of cannabis companies’ CSR practices over 10 years found, for example, that dispensary Trulieve provided $15,000 for internships and $20,000 for scholarships to prepare Black students for careers in the cannabis industry. The tobacco industry has used similar initiatives to foster good will and market its products to minority populations.
“The main message from this paper is that this is an industry selling a product with health impacts,” said study author Tanner Wakefield, an associate specialist at the Center for Tobacco Control Research and Education at the University of California, San Francisco. “We have seen how the tobacco industry in the past has used corporate social responsibility practices to insulate itself politically, engender public good will, and encourage consumption of tobacco products with harmful health effects.”
The study was published in JAMA Network Open.
A double agenda
The investigators identified 9 of the 10 largest publicly traded cannabis companies in the United States and Canada and examined the CSR activities that they conducted between Jan. 1, 2012, and Dec. 31, 2021. The investigators also conducted a systematic review of corporate websites and Nexis Uni articles that identified 153 news stories, press releases, and web pages that communicated about cannabis companies’ charitable and philanthropic activities.
Investigators identified themes in CSR activities by categorizing the language and informational patterns in the evidence they collected. They divided CSR practices into five categories, consisting of campaigns supposedly mitigating the harmful effects of past cannabis prohibition; initiatives characterized as promoting or increasing diversity, equity, and inclusion; charitable contributions; researching therapeutic cannabis uses and increasing medical access; and efforts claiming to address harms related to cannabis legalization.
The investigators observed that Green Thumb Industries and Cresco Labs set up “business incubators” and licensing assistance programs targeted toward members of racial and ethnic minority populations and communities most affected by cannabis prohibition. Canopy Growth Corporation supported research into whether medical cannabis could alleviate sleep disorders or treat mental health conditions. The company also collaborated with Canadian Students for Sensible Drug Policy and Parent Action on Drugs to create materials for preventing cannabis abuse among youth.
“I think we need to remember that this is an industry selling a product,” said Mr. Wakefield. “And just because there is merit to addressing certain issues or harms, that doesn’t mean we should forget that they are businesses seeking to make a profit. While CSR activities may have some potential benefits or apparent legitimacy, we have to remember that CSR is also a form of marketing and political influence.”
The investigators concluded that these CSR activities were similar to CSR strategies that the tobacco industry previously had used to encourage consumption, target marginalized communities, influence regulation, and advance corporate interests.
“A similarity to the tobacco industry is that they would provide funding or assistance to nonprofit groups that are not necessarily tied to cannabis or tobacco,” said Mr. Wakefield. For example, the investigators noted that cannabis companies contributed funding for breast cancer research and for veterans.
Moreover, the investigators observed “similarities in terms of focus and orientation toward special interest populations,” said Mr. Wakefield. Those special populations included the LGBTQ communities, and activities included sponsoring or participating in pride celebrations and releasing limited-edition pride products.
Overall, the cannabis industry engages in CSR activities that appear to mitigate the harmful effects of its products and operations, said Mr. Wakefield.
‘Incomplete information’
Jason W. Busse, DC, PhD, associate director of the Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., described the study as rigorous, but with limitations acknowledged by the authors.
“Understanding how these companies are promoting themselves and justifying themselves as good corporate citizens is important,” said Dr. Busse. “The investigators have undertaken a comprehensive study and identified CSR activities that cannabis companies are engaging in. We know from past experiences with tobacco companies that these activities may be used in part to encourage less regulation and increase market access.”
One constraint of the study is that the investigators used documents that were in the public domain, as opposed to internal company information. “The investigators were limited to the information that they were able to access,” said Dr. Busse. “Unless you use the Freedom of Information Act to compel companies to release internal documents, you don’t have that information. They haven’t done that, and it is almost a certainty that the authors had to work with incomplete information.”
The investigators suggest a need for oversight of the cannabis industry’s CSR practices, and Dr. Busse agreed with this assessment. “While engagement in social responsibility activities by cannabis corporations may have positive results, there should be independent assessment of outcomes. For example, sponsoring research may be problematic if such support comes with strings attached, such as suppressing or modifying unfavorable findings.”
The study was supported by the National Institutes of Health. Dr. Wakefield and Dr. Busse reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
CDC gives final approval to Omicron COVID-19 vaccine boosters
The Centers for Disease Control and Prevention on Sept. 1 approved the use of vaccines designed to target both Omicron and the older variants of the coronavirus, a step that may aid a goal of a widespread immunization campaign before winter arrives in the United States.
The CDC’s Advisory Committee on Immunization Practices voted 13-1 on two separate questions. One sought the panel’s backing for the use of a single dose of a new version of the Pfizer COVID-19 vaccines for people aged 12 and older. The second question dealt with a single dose of the reworked Moderna vaccine for people aged 18 and older.
The federal government wants to speed use of revamped COVID-19 shots, which the Food and Drug Administration on Sept. 1 cleared for use in the United States. Hours later, CDC Director Rochelle Walensky, MD, agreed with the panel’s recommendation.
“The updated COVID-19 boosters are formulated to better protect against the most recently circulating COVID-19 variant,” Dr. Walensky said in a statement. “They can help restore protection that has waned since previous vaccination and were designed to provide broader protection against newer variants. This recommendation followed a comprehensive scientific evaluation and robust scientific discussion. If you are eligible, there is no bad time to get your COVID-19 booster and I strongly encourage you to receive it.”
The FDA vote on Aug. 31 expanded the emergency use authorization EUA for both Moderna and Pfizer’s original COVID-19 vaccines. The new products are also called “updated boosters.” Both contain two mRNA components of SARS-CoV-2 virus, one of the original strain and another that is found in the BA.4 and BA.5 strains of the Omicron variant, the FDA said.
Basically, the FDA cleared the way for these new boosters after it relied heavily on results of certain blood tests that suggested an immune response boost from the new formulas, plus 18 months of mostly safe use of the original versions of the shots.
What neither the FDA nor the CDC has, however, is evidence from studies in humans on how well these new vaccines work or whether they are as safe as the originals. But the FDA did consider clinical evidence for the older shots and results from studies on the new boosters that were done in mice.
ACIP Committee member Pablo Sanchez, MD, of Ohio State University was the sole “no” vote on each question.
“It’s a new vaccine, it’s a new platform. There’s a lot of hesitancy already. We need the human data,” Dr. Sanchez said.
Dr. Sanchez did not doubt that the newer versions of the vaccine would prove safe.
“I personally am in the age group where I’m at high risk and I’m almost sure that I will receive it,” Dr. Sanchez said. “I just feel that this was a bit premature, and I wish that we had seen that data. Having said that, I am comfortable that the vaccine will likely be safe like the others.”
Dr. Sanchez was not alone in raising concerns about backing new COVID-19 shots for which there is not direct clinical evidence from human studies.
Committee member Sarah Long, MD, of Drexel University in Philadelphia, said during the discussion she would “reluctantly” vote in favor of the updated vaccines. She said she believes they will have the potential to reduce hospitalizations and even deaths, even with questions remaining about the data.
Dr. Long joined other committee members in pointing to the approach to updating flu vaccines as a model. In an attempt to keep ahead of influenza, companies seek to defeat new strains through tweaks to their FDA-approved vaccines. There is not much clinical information available about these revised products, Dr. Long said. She compared it to remodeling an existing home.
“It is the same scaffolding, part of the same roof, we’re just putting in some dormers and windows,” with the revisions to the flu vaccine, she said.
Earlier in the day, committee member Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., also used changes to the annual flu shots as the model for advancing COVID-19 shots.
“So after thinking about it, I am comfortable even though we don’t have human data,” he said.
There were several questions during the meeting about why the FDA had not convened a meeting of its Vaccines and Related Biological Products Advisory Committee (regarding these specific bivalent vaccines). Typically, the FDA committee of advisers considers new vaccines before the agency authorizes their use. In this case, however, the agency acted on its own.
The FDA said the committee considered the new, bivalent COVID-19 boosters in earlier meetings and that was enough outside feedback.
But holding a meeting of advisers on these specific products could have helped build public confidence in these medicines, Dorit Reiss, PhD, of the University of California Hastings College of Law, said during the public comment session of the CDC advisers’ meeting.
“We could wish the vaccines were more effective against infection, but they’re safe and they prevent hospitalization and death,” she said.
The Department of Health and Human Services anticipated the backing of ACIP. The Administration for Strategic Preparedness and Response on Aug. 31 began distributing “millions of doses of the updated booster to tens of thousands of sites nationwide,” Jason Roos, PhD, chief operating officer for HHS Coordination Operations and Response Element, wrote in a blog.
“These boosters will be available at tens of thousands of vaccination sites ... including local pharmacies, their physicians’ offices, and vaccine centers operated by state and local health officials,”Dr. Roos wrote.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention on Sept. 1 approved the use of vaccines designed to target both Omicron and the older variants of the coronavirus, a step that may aid a goal of a widespread immunization campaign before winter arrives in the United States.
The CDC’s Advisory Committee on Immunization Practices voted 13-1 on two separate questions. One sought the panel’s backing for the use of a single dose of a new version of the Pfizer COVID-19 vaccines for people aged 12 and older. The second question dealt with a single dose of the reworked Moderna vaccine for people aged 18 and older.
The federal government wants to speed use of revamped COVID-19 shots, which the Food and Drug Administration on Sept. 1 cleared for use in the United States. Hours later, CDC Director Rochelle Walensky, MD, agreed with the panel’s recommendation.
“The updated COVID-19 boosters are formulated to better protect against the most recently circulating COVID-19 variant,” Dr. Walensky said in a statement. “They can help restore protection that has waned since previous vaccination and were designed to provide broader protection against newer variants. This recommendation followed a comprehensive scientific evaluation and robust scientific discussion. If you are eligible, there is no bad time to get your COVID-19 booster and I strongly encourage you to receive it.”
The FDA vote on Aug. 31 expanded the emergency use authorization EUA for both Moderna and Pfizer’s original COVID-19 vaccines. The new products are also called “updated boosters.” Both contain two mRNA components of SARS-CoV-2 virus, one of the original strain and another that is found in the BA.4 and BA.5 strains of the Omicron variant, the FDA said.
Basically, the FDA cleared the way for these new boosters after it relied heavily on results of certain blood tests that suggested an immune response boost from the new formulas, plus 18 months of mostly safe use of the original versions of the shots.
What neither the FDA nor the CDC has, however, is evidence from studies in humans on how well these new vaccines work or whether they are as safe as the originals. But the FDA did consider clinical evidence for the older shots and results from studies on the new boosters that were done in mice.
ACIP Committee member Pablo Sanchez, MD, of Ohio State University was the sole “no” vote on each question.
“It’s a new vaccine, it’s a new platform. There’s a lot of hesitancy already. We need the human data,” Dr. Sanchez said.
Dr. Sanchez did not doubt that the newer versions of the vaccine would prove safe.
“I personally am in the age group where I’m at high risk and I’m almost sure that I will receive it,” Dr. Sanchez said. “I just feel that this was a bit premature, and I wish that we had seen that data. Having said that, I am comfortable that the vaccine will likely be safe like the others.”
Dr. Sanchez was not alone in raising concerns about backing new COVID-19 shots for which there is not direct clinical evidence from human studies.
Committee member Sarah Long, MD, of Drexel University in Philadelphia, said during the discussion she would “reluctantly” vote in favor of the updated vaccines. She said she believes they will have the potential to reduce hospitalizations and even deaths, even with questions remaining about the data.
Dr. Long joined other committee members in pointing to the approach to updating flu vaccines as a model. In an attempt to keep ahead of influenza, companies seek to defeat new strains through tweaks to their FDA-approved vaccines. There is not much clinical information available about these revised products, Dr. Long said. She compared it to remodeling an existing home.
“It is the same scaffolding, part of the same roof, we’re just putting in some dormers and windows,” with the revisions to the flu vaccine, she said.
Earlier in the day, committee member Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., also used changes to the annual flu shots as the model for advancing COVID-19 shots.
“So after thinking about it, I am comfortable even though we don’t have human data,” he said.
There were several questions during the meeting about why the FDA had not convened a meeting of its Vaccines and Related Biological Products Advisory Committee (regarding these specific bivalent vaccines). Typically, the FDA committee of advisers considers new vaccines before the agency authorizes their use. In this case, however, the agency acted on its own.
The FDA said the committee considered the new, bivalent COVID-19 boosters in earlier meetings and that was enough outside feedback.
But holding a meeting of advisers on these specific products could have helped build public confidence in these medicines, Dorit Reiss, PhD, of the University of California Hastings College of Law, said during the public comment session of the CDC advisers’ meeting.
“We could wish the vaccines were more effective against infection, but they’re safe and they prevent hospitalization and death,” she said.
The Department of Health and Human Services anticipated the backing of ACIP. The Administration for Strategic Preparedness and Response on Aug. 31 began distributing “millions of doses of the updated booster to tens of thousands of sites nationwide,” Jason Roos, PhD, chief operating officer for HHS Coordination Operations and Response Element, wrote in a blog.
“These boosters will be available at tens of thousands of vaccination sites ... including local pharmacies, their physicians’ offices, and vaccine centers operated by state and local health officials,”Dr. Roos wrote.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention on Sept. 1 approved the use of vaccines designed to target both Omicron and the older variants of the coronavirus, a step that may aid a goal of a widespread immunization campaign before winter arrives in the United States.
The CDC’s Advisory Committee on Immunization Practices voted 13-1 on two separate questions. One sought the panel’s backing for the use of a single dose of a new version of the Pfizer COVID-19 vaccines for people aged 12 and older. The second question dealt with a single dose of the reworked Moderna vaccine for people aged 18 and older.
The federal government wants to speed use of revamped COVID-19 shots, which the Food and Drug Administration on Sept. 1 cleared for use in the United States. Hours later, CDC Director Rochelle Walensky, MD, agreed with the panel’s recommendation.
“The updated COVID-19 boosters are formulated to better protect against the most recently circulating COVID-19 variant,” Dr. Walensky said in a statement. “They can help restore protection that has waned since previous vaccination and were designed to provide broader protection against newer variants. This recommendation followed a comprehensive scientific evaluation and robust scientific discussion. If you are eligible, there is no bad time to get your COVID-19 booster and I strongly encourage you to receive it.”
The FDA vote on Aug. 31 expanded the emergency use authorization EUA for both Moderna and Pfizer’s original COVID-19 vaccines. The new products are also called “updated boosters.” Both contain two mRNA components of SARS-CoV-2 virus, one of the original strain and another that is found in the BA.4 and BA.5 strains of the Omicron variant, the FDA said.
Basically, the FDA cleared the way for these new boosters after it relied heavily on results of certain blood tests that suggested an immune response boost from the new formulas, plus 18 months of mostly safe use of the original versions of the shots.
What neither the FDA nor the CDC has, however, is evidence from studies in humans on how well these new vaccines work or whether they are as safe as the originals. But the FDA did consider clinical evidence for the older shots and results from studies on the new boosters that were done in mice.
ACIP Committee member Pablo Sanchez, MD, of Ohio State University was the sole “no” vote on each question.
“It’s a new vaccine, it’s a new platform. There’s a lot of hesitancy already. We need the human data,” Dr. Sanchez said.
Dr. Sanchez did not doubt that the newer versions of the vaccine would prove safe.
“I personally am in the age group where I’m at high risk and I’m almost sure that I will receive it,” Dr. Sanchez said. “I just feel that this was a bit premature, and I wish that we had seen that data. Having said that, I am comfortable that the vaccine will likely be safe like the others.”
Dr. Sanchez was not alone in raising concerns about backing new COVID-19 shots for which there is not direct clinical evidence from human studies.
Committee member Sarah Long, MD, of Drexel University in Philadelphia, said during the discussion she would “reluctantly” vote in favor of the updated vaccines. She said she believes they will have the potential to reduce hospitalizations and even deaths, even with questions remaining about the data.
Dr. Long joined other committee members in pointing to the approach to updating flu vaccines as a model. In an attempt to keep ahead of influenza, companies seek to defeat new strains through tweaks to their FDA-approved vaccines. There is not much clinical information available about these revised products, Dr. Long said. She compared it to remodeling an existing home.
“It is the same scaffolding, part of the same roof, we’re just putting in some dormers and windows,” with the revisions to the flu vaccine, she said.
Earlier in the day, committee member Jamie Loehr, MD, of Cayuga Family Medicine in Ithaca, N.Y., also used changes to the annual flu shots as the model for advancing COVID-19 shots.
“So after thinking about it, I am comfortable even though we don’t have human data,” he said.
There were several questions during the meeting about why the FDA had not convened a meeting of its Vaccines and Related Biological Products Advisory Committee (regarding these specific bivalent vaccines). Typically, the FDA committee of advisers considers new vaccines before the agency authorizes their use. In this case, however, the agency acted on its own.
The FDA said the committee considered the new, bivalent COVID-19 boosters in earlier meetings and that was enough outside feedback.
But holding a meeting of advisers on these specific products could have helped build public confidence in these medicines, Dorit Reiss, PhD, of the University of California Hastings College of Law, said during the public comment session of the CDC advisers’ meeting.
“We could wish the vaccines were more effective against infection, but they’re safe and they prevent hospitalization and death,” she said.
The Department of Health and Human Services anticipated the backing of ACIP. The Administration for Strategic Preparedness and Response on Aug. 31 began distributing “millions of doses of the updated booster to tens of thousands of sites nationwide,” Jason Roos, PhD, chief operating officer for HHS Coordination Operations and Response Element, wrote in a blog.
“These boosters will be available at tens of thousands of vaccination sites ... including local pharmacies, their physicians’ offices, and vaccine centers operated by state and local health officials,”Dr. Roos wrote.
A version of this article first appeared on WebMD.com.
U.S. life expectancy drops to lowest in decades
according to new CDC data.
In 2021, the average American could expect to live until age 76, which fell from 77 in 2020 and 79 in 2019. That marks the lowest age since 1996 and the largest 2-year decline since 1923.
“Even small declines in life expectancy of a tenth or two-tenths of a year mean that on a population level, a lot more people are dying prematurely,” Robert Anderson, PhD, chief of mortality statistics at the National Center for Health Statistics, which produced the report, told The New York Times.
“This signals a huge impact on the population in terms of increased mortality,” he said.
COVID-19 played a major role, with excess death from the coronavirus contributing to half of the decline during the past 2 years. Drug overdose deaths also reached a record high in 2021, rising to about 109,000 people. Unintentional injuries, with about half due to drug overdose, were a leading cause of the decline in life expectancy, along with deaths from heart disease, chronic liver disease, cirrhosis, and suicide.
The decrease has been particularly devastating among Native Americans and Alaska Natives. Average life expectancy dropped by 4 years in 2020 alone and more than 6.5 years since the beginning of the pandemic. Now their life expectancy is 65, which was the average for all Americans in 1944.
“When I saw that in the report, I just – my jaw dropped,” Dr. Anderson told CNN.
“It was hard enough to fathom a 2.7-year decline over 2 years overall,” he said. “But then to see a 6.6-year decline for the American Indian population, it just shows the substantial impact that the pandemic has had on that population.”
Longstanding health issues and systemic problems, such as poverty, discrimination, and poor access to health care, led to the major declines among Native Americans and Alaska Natives, CNN reported.
“A lot of the talk is going to be around the pandemic, but we need to think about what has driven the conditions that have allowed certain communities to be more vulnerable,” Ruben Cantu, an associate program director with Prevention Institute, a nonprofit focused on health equity, told CNN.
The gap in life expectancy between women and men also became wider in 2021, growing to 5.9 years and marking the largest gap since 1996. The life expectancy for men in 2021 was 73.2, as compared with 79.1 for women.
The decline in overall U.S. life expectancy would have been even greater if there weren’t “offsetting effects,” the researchers wrote, such as declines in death due to the flu, pneumonia, chronic lower respiratory diseases, and Alzheimer’s disease.
The drop in U.S. life expectancy is “historic,” Steven Woolf, MD, retired director of the Center on Society and Health and Virginia Commonwealth University, told the Times.
Other high-income countries also saw a drop in life expectancy in 2020 due to the pandemic, but most began to recover last year due to major vaccine campaigns and behavior changes such as wearing masks, he said.
“None of them experienced a continuing fall in life expectancy like the U.S. did, and a good number of them saw life expectancy start inching back to normal,” he said. “The U.S. is clearly an outlier.”
A version of this article first appeared on WebMD.com.
according to new CDC data.
In 2021, the average American could expect to live until age 76, which fell from 77 in 2020 and 79 in 2019. That marks the lowest age since 1996 and the largest 2-year decline since 1923.
“Even small declines in life expectancy of a tenth or two-tenths of a year mean that on a population level, a lot more people are dying prematurely,” Robert Anderson, PhD, chief of mortality statistics at the National Center for Health Statistics, which produced the report, told The New York Times.
“This signals a huge impact on the population in terms of increased mortality,” he said.
COVID-19 played a major role, with excess death from the coronavirus contributing to half of the decline during the past 2 years. Drug overdose deaths also reached a record high in 2021, rising to about 109,000 people. Unintentional injuries, with about half due to drug overdose, were a leading cause of the decline in life expectancy, along with deaths from heart disease, chronic liver disease, cirrhosis, and suicide.
The decrease has been particularly devastating among Native Americans and Alaska Natives. Average life expectancy dropped by 4 years in 2020 alone and more than 6.5 years since the beginning of the pandemic. Now their life expectancy is 65, which was the average for all Americans in 1944.
“When I saw that in the report, I just – my jaw dropped,” Dr. Anderson told CNN.
“It was hard enough to fathom a 2.7-year decline over 2 years overall,” he said. “But then to see a 6.6-year decline for the American Indian population, it just shows the substantial impact that the pandemic has had on that population.”
Longstanding health issues and systemic problems, such as poverty, discrimination, and poor access to health care, led to the major declines among Native Americans and Alaska Natives, CNN reported.
“A lot of the talk is going to be around the pandemic, but we need to think about what has driven the conditions that have allowed certain communities to be more vulnerable,” Ruben Cantu, an associate program director with Prevention Institute, a nonprofit focused on health equity, told CNN.
The gap in life expectancy between women and men also became wider in 2021, growing to 5.9 years and marking the largest gap since 1996. The life expectancy for men in 2021 was 73.2, as compared with 79.1 for women.
The decline in overall U.S. life expectancy would have been even greater if there weren’t “offsetting effects,” the researchers wrote, such as declines in death due to the flu, pneumonia, chronic lower respiratory diseases, and Alzheimer’s disease.
The drop in U.S. life expectancy is “historic,” Steven Woolf, MD, retired director of the Center on Society and Health and Virginia Commonwealth University, told the Times.
Other high-income countries also saw a drop in life expectancy in 2020 due to the pandemic, but most began to recover last year due to major vaccine campaigns and behavior changes such as wearing masks, he said.
“None of them experienced a continuing fall in life expectancy like the U.S. did, and a good number of them saw life expectancy start inching back to normal,” he said. “The U.S. is clearly an outlier.”
A version of this article first appeared on WebMD.com.
according to new CDC data.
In 2021, the average American could expect to live until age 76, which fell from 77 in 2020 and 79 in 2019. That marks the lowest age since 1996 and the largest 2-year decline since 1923.
“Even small declines in life expectancy of a tenth or two-tenths of a year mean that on a population level, a lot more people are dying prematurely,” Robert Anderson, PhD, chief of mortality statistics at the National Center for Health Statistics, which produced the report, told The New York Times.
“This signals a huge impact on the population in terms of increased mortality,” he said.
COVID-19 played a major role, with excess death from the coronavirus contributing to half of the decline during the past 2 years. Drug overdose deaths also reached a record high in 2021, rising to about 109,000 people. Unintentional injuries, with about half due to drug overdose, were a leading cause of the decline in life expectancy, along with deaths from heart disease, chronic liver disease, cirrhosis, and suicide.
The decrease has been particularly devastating among Native Americans and Alaska Natives. Average life expectancy dropped by 4 years in 2020 alone and more than 6.5 years since the beginning of the pandemic. Now their life expectancy is 65, which was the average for all Americans in 1944.
“When I saw that in the report, I just – my jaw dropped,” Dr. Anderson told CNN.
“It was hard enough to fathom a 2.7-year decline over 2 years overall,” he said. “But then to see a 6.6-year decline for the American Indian population, it just shows the substantial impact that the pandemic has had on that population.”
Longstanding health issues and systemic problems, such as poverty, discrimination, and poor access to health care, led to the major declines among Native Americans and Alaska Natives, CNN reported.
“A lot of the talk is going to be around the pandemic, but we need to think about what has driven the conditions that have allowed certain communities to be more vulnerable,” Ruben Cantu, an associate program director with Prevention Institute, a nonprofit focused on health equity, told CNN.
The gap in life expectancy between women and men also became wider in 2021, growing to 5.9 years and marking the largest gap since 1996. The life expectancy for men in 2021 was 73.2, as compared with 79.1 for women.
The decline in overall U.S. life expectancy would have been even greater if there weren’t “offsetting effects,” the researchers wrote, such as declines in death due to the flu, pneumonia, chronic lower respiratory diseases, and Alzheimer’s disease.
The drop in U.S. life expectancy is “historic,” Steven Woolf, MD, retired director of the Center on Society and Health and Virginia Commonwealth University, told the Times.
Other high-income countries also saw a drop in life expectancy in 2020 due to the pandemic, but most began to recover last year due to major vaccine campaigns and behavior changes such as wearing masks, he said.
“None of them experienced a continuing fall in life expectancy like the U.S. did, and a good number of them saw life expectancy start inching back to normal,” he said. “The U.S. is clearly an outlier.”
A version of this article first appeared on WebMD.com.
Real medical news: Many teens trust fake medical news
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
Many young kids with COVID may show no symptoms
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
FROM JAMA NETWORK OPEN
Inhaled, systemic steroids linked to changes in brain structure
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Majority of muscle symptoms with statins not caused by treatment
In the vast majority of people who experience muscle pain or weakness while taking a statin, those symptoms are not related to the statin, a new individual patient data meta-analysis of randomized controlled trials shows.
The Cholesterol Trialists Collaboration meta-analysis examined 19 large randomized double-blind trials that compared statin therapy with placebo and involved almost 124,000 patients.
“Our results show that, in people who experience muscle symptoms in the first year of taking a statin, those symptoms are actually due to the statin in only 1 of 15 of those people. For the other 14 of the 15 people who experience muscle symptoms in the first year of taking a statin, that muscle pain is not due to the statin,” lead investigator Colin Baigent, MD, said.
After the first year, there was no difference in muscle symptoms between patients taking a statin or those taking placebo.
Dr. Baigent, who is director of the Population Health Research Unit at the University of Oxford (England), presented the data on Aug. 29 at the European Society of Cardiology 2022 Congress.
It was also simultaneously published online in The Lancet.
Dr. Baigent explained that statins very rarely cause serious muscle adverse effects with biochemical evidence of cellular damage, such as myopathy (which occurs in less than 1 in 10,000 patients per year) and rhabdomyolysis (which occurs in about 0.2 per 10,000 patients per year).
The effect of statins on other less serious muscle symptoms without biochemical evidence of cellular damage is less clear, but misinformation about the risks have arisen from nonrandomized studies, with social media and press reports suggesting that the risk for muscle symptoms with statins is extremely common, Dr. Baigent said.
In response to this, the Cholesterol Trialists Collaboration put together a new program of data collection, validation, and analysis to provide reliable information from large double-blind randomized trials that are free from bias and confounding.
“Overall, when we look at all these data, we find there is about a 3% relative increase in the risks of experiencing muscle pain or weakness with a statin versus with placebo,” Dr. Baigent reported.
Muscle pain or weakness was reported by 16,835 of 62,028 patients taking a statin, (27.1%), compared with 16,446 of 61,912 patients taking placebo (26.6%), for a rate ratio of 1.03 (95% confidence interval, 1.01-1.06).
In absolute terms, the results show a rate of 166 reports of muscle symptoms per 1,000 patient-years in those taking a statin, compared with 155 per 1,000-patient-years in those taking placebo in the first year. This gives a rate ratio of 1.07 and an excess of 11 cases of muscle pain or weakness per 1,000 patients in the first year of statin therapy.
“The very small excess of muscle symptoms in the statin patients were generally mild, with most patients able to continue treatment,” Dr. Baigent added.
After the first year, the rate of muscle pain or weakness was exactly the same in the statin and placebo groups, at 50 per 1,000 patient-years.
“Therefore, for the vast majority of people who experience muscle pain or weakness on a statin, those symptoms are not due to the statin itself. It is due to something else, which could be ageing, thyroid disease, or exercise,” Dr. Baigent said. “After the first year of taking a statin, there is no excess risk of muscle pain or weakness at all.”
“To summarize, the excess risk of muscle pain or weakness with statin use is tiny, and almost nonexistent after the first year,” he added.
“Muscle pain is very common in the general population, and it was very common in both patients taking a statin and those given placebo in these randomized trials. We can only detect a difference by looking at all the data combined in this enormous study. And we now know for sure that over 90% of cases of muscle symptoms experienced by people taking a statin are not due to the statin.”
The researchers also looked at statin intensity and found that the more intense statins tend to cause slightly more muscle pain. “There was also some evidence, although this was not very clear, that the muscle pain with the more intensive statins may persist for longer than 1 year,” Dr. Baigent said.
But in terms of different moderate-intensity and high-intensity statins, there was no evidence of differences in muscle pain between the individual statin brands, he added.
Better patient information needed
Dr. Baigent called for better information in statin package inserts about the real risk for muscle symptoms with these drugs.
“We need to do a better job of communicating the real risk of muscle symptom to patients who are taking statins and to their doctors. At the moment, doctors often stop statins if patients complain of muscle pain, but our data show that in 14 out of 15 times, they would be wrong for doing that. Stopping the statin is nearly always a mistake,” he commented.
“At present, the package inserts include a whole load of rubbish from observational studies, which are completely unreliable,” he added. “This is of no value to patients. They go through this information and find several symptoms they are experiencing, which they attribute to the drugs. We really need to divide up the information into the evidence that we really know for sure and then the more speculative stuff.”
Dr. Baigent also highlighted the large benefits of statins, compared with the small risk for muscle symptoms.
“While statins may cause 11 patients per 1,000 to experience some mild muscle pain in the first year of taking these drugs, and this was reduced to none in subsequent years, statins, when used for the primary prevention of cardiovascular disease, prevent 25 cardiovascular events per 1,000 patients every year they are taken. And for secondary prevention this rises to 50 events prevented per 1,000 patients each year,” he noted.
The individual participant data meta-analysis involved 23 trials with information on almost 155,000 patients. All trials included at least 1,000 patients and at least 2 years of scheduled treatment. Adverse-event data were collected for all individual participants in 19 large randomized double-blind trials comparing statin therapy with placebo (123,940 patients) and in four randomized double-blind trials comparing more-intensive with less-intensive statin therapy (30,724 patients).
In the four trials of more-intensive versus less-intensive statin therapy, high-intensity regimens (atorvastatin 40-80 mg daily or rosuvastatin 20-40 mg daily) resulted in a larger relative increase in the rate of muscle pain or weakness than moderate-intensity regimens, with rate ratios of 1.08 (95% CI, 1.04-1.13) and 1.02 (95% CI, 1.00-1.05), respectively.
‘Reassuring information’
Discussant of the study at the ESC Hotline session, Erin Bohula, MD, Brigham and Women’s Hospital, Boston, said this new analysis had many strengths and used a rigorous approach to look at the issue of muscle symptoms with statins.
She pointed out some challenges, including the fact that the definition of adverse muscle events has changed over time and differed in the various trials, with heterogeneous data capture across trials. “So, this was a Herculean task to harmonize this very complicated dataset.”
Dr. Bohula concluded: “I think this is a very significant undertaking, resulting in a rich dataset that enhances our understanding of muscle symptoms related to statin use. The take-home for me is that muscle symptoms are a common complaint in the general population but are very rarely attributable to statins. This is very reassuring to me, and I hope it is reassuring to patients and can help us encourage them with adherence, given the clear cardiovascular benefits of statins.”
Chair of the ESC Hotline session at which the study was presented, Gabriel Steg, MD, Hôpital Bichat, Paris, asked whether some statin patients who experienced muscle symptoms with the drugs in active run-in periods in the trials may have been excluded from the main trials, so that this information might not have been captured, but Dr. Baigent replied that they also examined those data, which had been accounted for in the analysis.
“That’s really good news,” Dr. Steg commented. “This study is going to be one more tool in our response to statin skeptics and I think, as such, this work is a really a service to public health.”
The meta-analysis was funded by the British Heart Foundation, the U.K. Medical Research Council, and the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
In the vast majority of people who experience muscle pain or weakness while taking a statin, those symptoms are not related to the statin, a new individual patient data meta-analysis of randomized controlled trials shows.
The Cholesterol Trialists Collaboration meta-analysis examined 19 large randomized double-blind trials that compared statin therapy with placebo and involved almost 124,000 patients.
“Our results show that, in people who experience muscle symptoms in the first year of taking a statin, those symptoms are actually due to the statin in only 1 of 15 of those people. For the other 14 of the 15 people who experience muscle symptoms in the first year of taking a statin, that muscle pain is not due to the statin,” lead investigator Colin Baigent, MD, said.
After the first year, there was no difference in muscle symptoms between patients taking a statin or those taking placebo.
Dr. Baigent, who is director of the Population Health Research Unit at the University of Oxford (England), presented the data on Aug. 29 at the European Society of Cardiology 2022 Congress.
It was also simultaneously published online in The Lancet.
Dr. Baigent explained that statins very rarely cause serious muscle adverse effects with biochemical evidence of cellular damage, such as myopathy (which occurs in less than 1 in 10,000 patients per year) and rhabdomyolysis (which occurs in about 0.2 per 10,000 patients per year).
The effect of statins on other less serious muscle symptoms without biochemical evidence of cellular damage is less clear, but misinformation about the risks have arisen from nonrandomized studies, with social media and press reports suggesting that the risk for muscle symptoms with statins is extremely common, Dr. Baigent said.
In response to this, the Cholesterol Trialists Collaboration put together a new program of data collection, validation, and analysis to provide reliable information from large double-blind randomized trials that are free from bias and confounding.
“Overall, when we look at all these data, we find there is about a 3% relative increase in the risks of experiencing muscle pain or weakness with a statin versus with placebo,” Dr. Baigent reported.
Muscle pain or weakness was reported by 16,835 of 62,028 patients taking a statin, (27.1%), compared with 16,446 of 61,912 patients taking placebo (26.6%), for a rate ratio of 1.03 (95% confidence interval, 1.01-1.06).
In absolute terms, the results show a rate of 166 reports of muscle symptoms per 1,000 patient-years in those taking a statin, compared with 155 per 1,000-patient-years in those taking placebo in the first year. This gives a rate ratio of 1.07 and an excess of 11 cases of muscle pain or weakness per 1,000 patients in the first year of statin therapy.
“The very small excess of muscle symptoms in the statin patients were generally mild, with most patients able to continue treatment,” Dr. Baigent added.
After the first year, the rate of muscle pain or weakness was exactly the same in the statin and placebo groups, at 50 per 1,000 patient-years.
“Therefore, for the vast majority of people who experience muscle pain or weakness on a statin, those symptoms are not due to the statin itself. It is due to something else, which could be ageing, thyroid disease, or exercise,” Dr. Baigent said. “After the first year of taking a statin, there is no excess risk of muscle pain or weakness at all.”
“To summarize, the excess risk of muscle pain or weakness with statin use is tiny, and almost nonexistent after the first year,” he added.
“Muscle pain is very common in the general population, and it was very common in both patients taking a statin and those given placebo in these randomized trials. We can only detect a difference by looking at all the data combined in this enormous study. And we now know for sure that over 90% of cases of muscle symptoms experienced by people taking a statin are not due to the statin.”
The researchers also looked at statin intensity and found that the more intense statins tend to cause slightly more muscle pain. “There was also some evidence, although this was not very clear, that the muscle pain with the more intensive statins may persist for longer than 1 year,” Dr. Baigent said.
But in terms of different moderate-intensity and high-intensity statins, there was no evidence of differences in muscle pain between the individual statin brands, he added.
Better patient information needed
Dr. Baigent called for better information in statin package inserts about the real risk for muscle symptoms with these drugs.
“We need to do a better job of communicating the real risk of muscle symptom to patients who are taking statins and to their doctors. At the moment, doctors often stop statins if patients complain of muscle pain, but our data show that in 14 out of 15 times, they would be wrong for doing that. Stopping the statin is nearly always a mistake,” he commented.
“At present, the package inserts include a whole load of rubbish from observational studies, which are completely unreliable,” he added. “This is of no value to patients. They go through this information and find several symptoms they are experiencing, which they attribute to the drugs. We really need to divide up the information into the evidence that we really know for sure and then the more speculative stuff.”
Dr. Baigent also highlighted the large benefits of statins, compared with the small risk for muscle symptoms.
“While statins may cause 11 patients per 1,000 to experience some mild muscle pain in the first year of taking these drugs, and this was reduced to none in subsequent years, statins, when used for the primary prevention of cardiovascular disease, prevent 25 cardiovascular events per 1,000 patients every year they are taken. And for secondary prevention this rises to 50 events prevented per 1,000 patients each year,” he noted.
The individual participant data meta-analysis involved 23 trials with information on almost 155,000 patients. All trials included at least 1,000 patients and at least 2 years of scheduled treatment. Adverse-event data were collected for all individual participants in 19 large randomized double-blind trials comparing statin therapy with placebo (123,940 patients) and in four randomized double-blind trials comparing more-intensive with less-intensive statin therapy (30,724 patients).
In the four trials of more-intensive versus less-intensive statin therapy, high-intensity regimens (atorvastatin 40-80 mg daily or rosuvastatin 20-40 mg daily) resulted in a larger relative increase in the rate of muscle pain or weakness than moderate-intensity regimens, with rate ratios of 1.08 (95% CI, 1.04-1.13) and 1.02 (95% CI, 1.00-1.05), respectively.
‘Reassuring information’
Discussant of the study at the ESC Hotline session, Erin Bohula, MD, Brigham and Women’s Hospital, Boston, said this new analysis had many strengths and used a rigorous approach to look at the issue of muscle symptoms with statins.
She pointed out some challenges, including the fact that the definition of adverse muscle events has changed over time and differed in the various trials, with heterogeneous data capture across trials. “So, this was a Herculean task to harmonize this very complicated dataset.”
Dr. Bohula concluded: “I think this is a very significant undertaking, resulting in a rich dataset that enhances our understanding of muscle symptoms related to statin use. The take-home for me is that muscle symptoms are a common complaint in the general population but are very rarely attributable to statins. This is very reassuring to me, and I hope it is reassuring to patients and can help us encourage them with adherence, given the clear cardiovascular benefits of statins.”
Chair of the ESC Hotline session at which the study was presented, Gabriel Steg, MD, Hôpital Bichat, Paris, asked whether some statin patients who experienced muscle symptoms with the drugs in active run-in periods in the trials may have been excluded from the main trials, so that this information might not have been captured, but Dr. Baigent replied that they also examined those data, which had been accounted for in the analysis.
“That’s really good news,” Dr. Steg commented. “This study is going to be one more tool in our response to statin skeptics and I think, as such, this work is a really a service to public health.”
The meta-analysis was funded by the British Heart Foundation, the U.K. Medical Research Council, and the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
In the vast majority of people who experience muscle pain or weakness while taking a statin, those symptoms are not related to the statin, a new individual patient data meta-analysis of randomized controlled trials shows.
The Cholesterol Trialists Collaboration meta-analysis examined 19 large randomized double-blind trials that compared statin therapy with placebo and involved almost 124,000 patients.
“Our results show that, in people who experience muscle symptoms in the first year of taking a statin, those symptoms are actually due to the statin in only 1 of 15 of those people. For the other 14 of the 15 people who experience muscle symptoms in the first year of taking a statin, that muscle pain is not due to the statin,” lead investigator Colin Baigent, MD, said.
After the first year, there was no difference in muscle symptoms between patients taking a statin or those taking placebo.
Dr. Baigent, who is director of the Population Health Research Unit at the University of Oxford (England), presented the data on Aug. 29 at the European Society of Cardiology 2022 Congress.
It was also simultaneously published online in The Lancet.
Dr. Baigent explained that statins very rarely cause serious muscle adverse effects with biochemical evidence of cellular damage, such as myopathy (which occurs in less than 1 in 10,000 patients per year) and rhabdomyolysis (which occurs in about 0.2 per 10,000 patients per year).
The effect of statins on other less serious muscle symptoms without biochemical evidence of cellular damage is less clear, but misinformation about the risks have arisen from nonrandomized studies, with social media and press reports suggesting that the risk for muscle symptoms with statins is extremely common, Dr. Baigent said.
In response to this, the Cholesterol Trialists Collaboration put together a new program of data collection, validation, and analysis to provide reliable information from large double-blind randomized trials that are free from bias and confounding.
“Overall, when we look at all these data, we find there is about a 3% relative increase in the risks of experiencing muscle pain or weakness with a statin versus with placebo,” Dr. Baigent reported.
Muscle pain or weakness was reported by 16,835 of 62,028 patients taking a statin, (27.1%), compared with 16,446 of 61,912 patients taking placebo (26.6%), for a rate ratio of 1.03 (95% confidence interval, 1.01-1.06).
In absolute terms, the results show a rate of 166 reports of muscle symptoms per 1,000 patient-years in those taking a statin, compared with 155 per 1,000-patient-years in those taking placebo in the first year. This gives a rate ratio of 1.07 and an excess of 11 cases of muscle pain or weakness per 1,000 patients in the first year of statin therapy.
“The very small excess of muscle symptoms in the statin patients were generally mild, with most patients able to continue treatment,” Dr. Baigent added.
After the first year, the rate of muscle pain or weakness was exactly the same in the statin and placebo groups, at 50 per 1,000 patient-years.
“Therefore, for the vast majority of people who experience muscle pain or weakness on a statin, those symptoms are not due to the statin itself. It is due to something else, which could be ageing, thyroid disease, or exercise,” Dr. Baigent said. “After the first year of taking a statin, there is no excess risk of muscle pain or weakness at all.”
“To summarize, the excess risk of muscle pain or weakness with statin use is tiny, and almost nonexistent after the first year,” he added.
“Muscle pain is very common in the general population, and it was very common in both patients taking a statin and those given placebo in these randomized trials. We can only detect a difference by looking at all the data combined in this enormous study. And we now know for sure that over 90% of cases of muscle symptoms experienced by people taking a statin are not due to the statin.”
The researchers also looked at statin intensity and found that the more intense statins tend to cause slightly more muscle pain. “There was also some evidence, although this was not very clear, that the muscle pain with the more intensive statins may persist for longer than 1 year,” Dr. Baigent said.
But in terms of different moderate-intensity and high-intensity statins, there was no evidence of differences in muscle pain between the individual statin brands, he added.
Better patient information needed
Dr. Baigent called for better information in statin package inserts about the real risk for muscle symptoms with these drugs.
“We need to do a better job of communicating the real risk of muscle symptom to patients who are taking statins and to their doctors. At the moment, doctors often stop statins if patients complain of muscle pain, but our data show that in 14 out of 15 times, they would be wrong for doing that. Stopping the statin is nearly always a mistake,” he commented.
“At present, the package inserts include a whole load of rubbish from observational studies, which are completely unreliable,” he added. “This is of no value to patients. They go through this information and find several symptoms they are experiencing, which they attribute to the drugs. We really need to divide up the information into the evidence that we really know for sure and then the more speculative stuff.”
Dr. Baigent also highlighted the large benefits of statins, compared with the small risk for muscle symptoms.
“While statins may cause 11 patients per 1,000 to experience some mild muscle pain in the first year of taking these drugs, and this was reduced to none in subsequent years, statins, when used for the primary prevention of cardiovascular disease, prevent 25 cardiovascular events per 1,000 patients every year they are taken. And for secondary prevention this rises to 50 events prevented per 1,000 patients each year,” he noted.
The individual participant data meta-analysis involved 23 trials with information on almost 155,000 patients. All trials included at least 1,000 patients and at least 2 years of scheduled treatment. Adverse-event data were collected for all individual participants in 19 large randomized double-blind trials comparing statin therapy with placebo (123,940 patients) and in four randomized double-blind trials comparing more-intensive with less-intensive statin therapy (30,724 patients).
In the four trials of more-intensive versus less-intensive statin therapy, high-intensity regimens (atorvastatin 40-80 mg daily or rosuvastatin 20-40 mg daily) resulted in a larger relative increase in the rate of muscle pain or weakness than moderate-intensity regimens, with rate ratios of 1.08 (95% CI, 1.04-1.13) and 1.02 (95% CI, 1.00-1.05), respectively.
‘Reassuring information’
Discussant of the study at the ESC Hotline session, Erin Bohula, MD, Brigham and Women’s Hospital, Boston, said this new analysis had many strengths and used a rigorous approach to look at the issue of muscle symptoms with statins.
She pointed out some challenges, including the fact that the definition of adverse muscle events has changed over time and differed in the various trials, with heterogeneous data capture across trials. “So, this was a Herculean task to harmonize this very complicated dataset.”
Dr. Bohula concluded: “I think this is a very significant undertaking, resulting in a rich dataset that enhances our understanding of muscle symptoms related to statin use. The take-home for me is that muscle symptoms are a common complaint in the general population but are very rarely attributable to statins. This is very reassuring to me, and I hope it is reassuring to patients and can help us encourage them with adherence, given the clear cardiovascular benefits of statins.”
Chair of the ESC Hotline session at which the study was presented, Gabriel Steg, MD, Hôpital Bichat, Paris, asked whether some statin patients who experienced muscle symptoms with the drugs in active run-in periods in the trials may have been excluded from the main trials, so that this information might not have been captured, but Dr. Baigent replied that they also examined those data, which had been accounted for in the analysis.
“That’s really good news,” Dr. Steg commented. “This study is going to be one more tool in our response to statin skeptics and I think, as such, this work is a really a service to public health.”
The meta-analysis was funded by the British Heart Foundation, the U.K. Medical Research Council, and the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Children and COVID: New cases increase; hospital admissions could follow
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.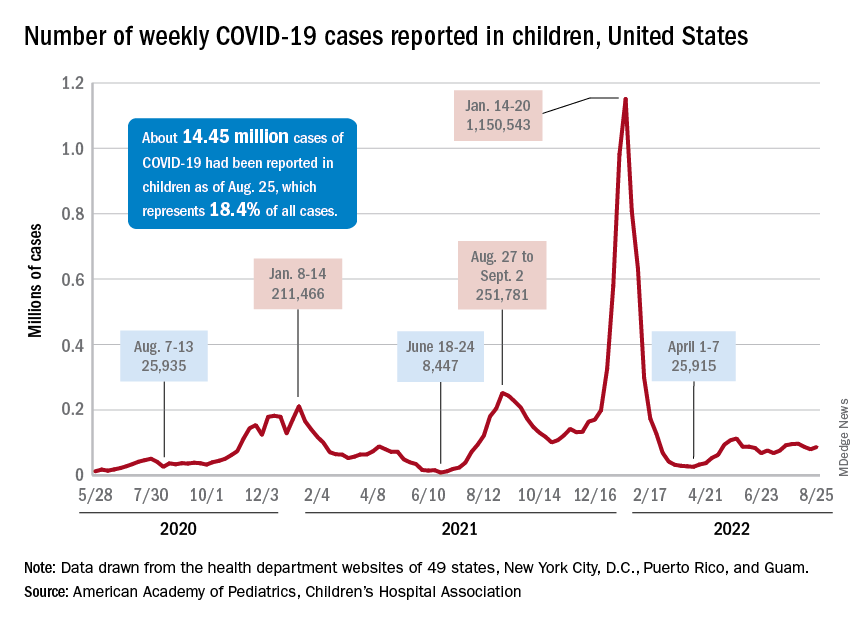
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.
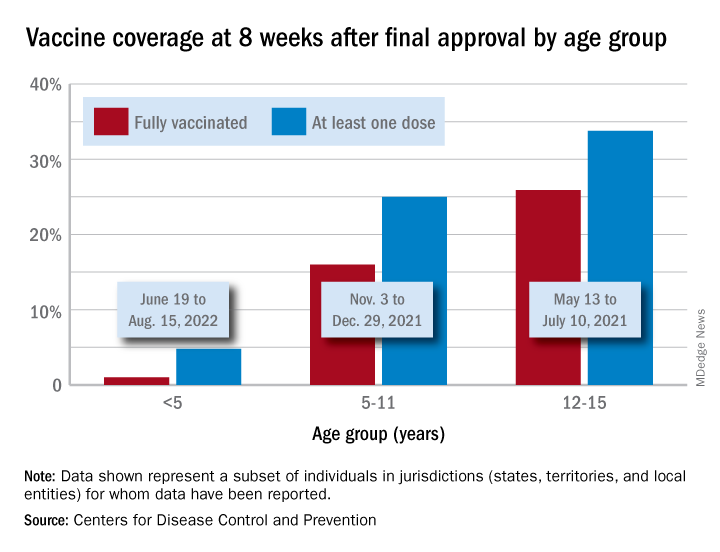
Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.










