User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Why some infectious disease docs are ‘encouraged’ by new bivalent COVID vaccines
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
The LIVE CHEST Challenge Championship is back!
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
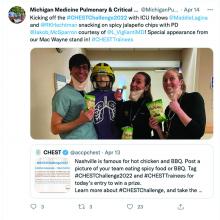
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
Absence does make the heart grow fonder. Three years have passed since our last in person CHEST Challenge Championship.
It was CHEST 2019 New Orleans when we last saw the enthusiasm and camaraderie of talented fellow teams cheered on by that irreplaceable, engaged audience, creating moments and memories through that magical combination of education and entertainment (“edutainment”). We were blissfully ignorant then to the terrible challenges that would soon come with the pandemic.
Fellows from across the country first compete in a challenging, secure online knowledge quiz from which top-performing programs are selected as finalists.
All along the way, the participants engage in social media challenges that build excitement and collegiality (see tweeted image above). A recent commentary in the CHEST® journal highlighted the competition’s important milestones,1 and organizers continue to innovate year after year.
Dr. William Kelly, creator of CHEST Challenge, noted, “Our 20th anniversary broadcast during CHEST 2021 was our most innovative, had the most generous prizes, and the largest, most interactive audience to date. Our team of amazing committee members, CHEST staff, and contributors are somehow going even bigger this year! When combining never-before-seen challenges, surprises, giveaways, and a special ‘opening act’ with the joy and energy of all of us being back together again in person – I just can’t wait.
“That necessary pivot to online-only events in 2020 and 2021 brought new challenges to the game but also provided lessons to be learned, inspired reflection, and gave us opportunities to interact, play, and learn together in new ways.
“As chair of the Training and Transitions Committee, I recall the innovations: CHEST Challenge has always been about innovation in medical education.
“Two decades of history allowed pushing the boundaries into the online arena, allowing competitors to play from their own institutions, audience to join from home, and the camaraderie and support characteristics of the CHEST community to transcend virtual barriers.
“Using advanced, remote video recordings with virtual proctoring by judges, we were able to offer more extensive skills challenges. Highly engaged online audiences had contagious and hilarious chat room banter. And, virtual watch parties allowed for greatly increased viewership. Leveraging social media, the audience became part of the competition, including winning substantial prizes for themselves.
“It takes an extraordinary number of dedicated individuals to deliver the experience.”
Dr. Matthew Miles, past chair of T&T Committee, comments: “One of the joys of working on CHEST Challenge is just being part of the production team. We have brilliant faculty who specialize in cutting-edge education, visionaries who concoct new and imaginative ways for fellows to compete, and incredible CHEST staff who somehow pull off an amazing event every year.
“I’m so thankful for the way that our CHEST community celebrates learning and prioritizes our fellows-in-training,” he added.
“Years after each in-person championship, the attendees still comment on the electrifying atmosphere they thoroughly enjoyed.
“It is literally a nail-biter – you can see people in the audience sitting at the edge of their seats, holding their breath while teams play to win big in surprise hands-on simulation-based challenges during the Championship,” says Dr. Subani Chandra, who helped implement surprise simulation challenges into the live CHEST Challenge Championship in 2017 that are now an integral part of the experience.
On October 18, at CHEST 2022, championship fellow teams from New York Presbyterian Brooklyn Methodist, Mayo Clinic, and Brooke Army Medical Center, cheered on live by all of us, will compete in order to hoist the Rosen Cup and be declared the CHEST Challenge Champions!
Come experience for yourself the rapid-fire pulmonary, critical care, and sleep medicine knowledge review, the thrill of competition, and see the energy of some of our best and brightest fellows.
Being together in person again to support and learn with each other will be a big win for all of us.
CHEST Challenge is sponsored by VIATRIS
Reference
1. Danckers M, et al. CHEST Challenge turns twenty. Chest. 2022;161(3):860.
What are we missing when it comes to obstructive sleep apnea and atrial fibrillation?
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
ACC/AHA issue chest pain data standards update to 2021 guideline
The American College of Cardiology/American Heart Association have issued a set of data standards for chest pain and acute myocardial infarction to accompany the 2021 guidelines for evaluation and diagnosis of chest pain.
In October 2021, the AHA/ACC issued a joint clinical practice guideline encouraging clinicians to use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain, as reported by this news organization.
The writing group underscored the need to reach a consensus for the definitions of chest pain. The new document standardizes related data elements for consistent reporting on chest pain syndromes.
“This is an appendix to the guidelines and a planned effort to try to harmonize and bring uniformity to the language applied,” writing committee chair H.V. “Skip” Anderson, MD, with UT Health Science Center, Houston, told this news organization.
“You want heart attack to mean the same thing in Miami Beach as in Western Pennsylvania, as in Oregon and Washington and every place in between,” Dr. Anderson explained. “You want everybody to be using the same language, so that’s what these data standards are meant to do.”
In the document, data elements are grouped into three broad categories: chest pain, myocardial injury, and MI.
“We deliberately followed the plans contained in the new guideline and focused on potentially serious cardiovascular causes of chest pain as might be encountered in emergency departments,” the writing group notes in the document.
The terms “typical” and “atypical” as descriptors of chest pain or anginal syndromes are not used in the new document, in line with the 2021 guidance to abandon these terms.
Instead, the new document divides chest pain syndromes into three categories: “cardiac,” “possible cardiac,” and “noncardiac” – again, in keeping with the chest pain guideline.
The document also includes data elements for risk stratification scoring according to several common risk scoring algorithms and for procedure-related myocardial injury and procedure-related MI.
Each year, chest pain sends more than 7 million adults to the emergency department in the United States. Although noncardiac causes of chest pain make up a large majority of these cases, there are several life-threatening causes of chest pain that must be identified and treated promptly.
Distinguishing between serious and nonserious causes of chest pain is an urgent imperative, the writing group says.
Overall, they say this new clinical lexicon and set of data standards should be “broadly applicable” in various settings, including clinical trials and observational studies, patient care, electronic health records (EHRs), quality and performance improvement initiatives, registries, and public reporting programs.
The 2022 ACC/AHA Key Data Elements and Definitions for Chest Pain and Acute Myocardial Infarction was simultaneously published online in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
It was developed in collaboration with the American College of Emergency Physicians and the Society for Cardiac Angiography and Interventions and endorsed by the Society for Academic Emergency Medicine.
Dr. Anderson noted that “almost all of the guidelines that come out now, certainly in the last few years, have been followed after a certain interval by a set of data standards applicable to the guidelines.”
“It would be really great if it could actually be attached as an appendix, but the nature of the development of these things is such that there will always be a bit of a time lag between the writing group that develops the guidelines and the work group that develops the data standards; you can’t really have them working in parallel at the same time,” Dr. Anderson said in an interview.
This research had no commercial funding. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
The American College of Cardiology/American Heart Association have issued a set of data standards for chest pain and acute myocardial infarction to accompany the 2021 guidelines for evaluation and diagnosis of chest pain.
In October 2021, the AHA/ACC issued a joint clinical practice guideline encouraging clinicians to use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain, as reported by this news organization.
The writing group underscored the need to reach a consensus for the definitions of chest pain. The new document standardizes related data elements for consistent reporting on chest pain syndromes.
“This is an appendix to the guidelines and a planned effort to try to harmonize and bring uniformity to the language applied,” writing committee chair H.V. “Skip” Anderson, MD, with UT Health Science Center, Houston, told this news organization.
“You want heart attack to mean the same thing in Miami Beach as in Western Pennsylvania, as in Oregon and Washington and every place in between,” Dr. Anderson explained. “You want everybody to be using the same language, so that’s what these data standards are meant to do.”
In the document, data elements are grouped into three broad categories: chest pain, myocardial injury, and MI.
“We deliberately followed the plans contained in the new guideline and focused on potentially serious cardiovascular causes of chest pain as might be encountered in emergency departments,” the writing group notes in the document.
The terms “typical” and “atypical” as descriptors of chest pain or anginal syndromes are not used in the new document, in line with the 2021 guidance to abandon these terms.
Instead, the new document divides chest pain syndromes into three categories: “cardiac,” “possible cardiac,” and “noncardiac” – again, in keeping with the chest pain guideline.
The document also includes data elements for risk stratification scoring according to several common risk scoring algorithms and for procedure-related myocardial injury and procedure-related MI.
Each year, chest pain sends more than 7 million adults to the emergency department in the United States. Although noncardiac causes of chest pain make up a large majority of these cases, there are several life-threatening causes of chest pain that must be identified and treated promptly.
Distinguishing between serious and nonserious causes of chest pain is an urgent imperative, the writing group says.
Overall, they say this new clinical lexicon and set of data standards should be “broadly applicable” in various settings, including clinical trials and observational studies, patient care, electronic health records (EHRs), quality and performance improvement initiatives, registries, and public reporting programs.
The 2022 ACC/AHA Key Data Elements and Definitions for Chest Pain and Acute Myocardial Infarction was simultaneously published online in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
It was developed in collaboration with the American College of Emergency Physicians and the Society for Cardiac Angiography and Interventions and endorsed by the Society for Academic Emergency Medicine.
Dr. Anderson noted that “almost all of the guidelines that come out now, certainly in the last few years, have been followed after a certain interval by a set of data standards applicable to the guidelines.”
“It would be really great if it could actually be attached as an appendix, but the nature of the development of these things is such that there will always be a bit of a time lag between the writing group that develops the guidelines and the work group that develops the data standards; you can’t really have them working in parallel at the same time,” Dr. Anderson said in an interview.
This research had no commercial funding. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
The American College of Cardiology/American Heart Association have issued a set of data standards for chest pain and acute myocardial infarction to accompany the 2021 guidelines for evaluation and diagnosis of chest pain.
In October 2021, the AHA/ACC issued a joint clinical practice guideline encouraging clinicians to use standardized risk assessments, clinical pathways, and tools to evaluate and communicate with patients who present with chest pain, as reported by this news organization.
The writing group underscored the need to reach a consensus for the definitions of chest pain. The new document standardizes related data elements for consistent reporting on chest pain syndromes.
“This is an appendix to the guidelines and a planned effort to try to harmonize and bring uniformity to the language applied,” writing committee chair H.V. “Skip” Anderson, MD, with UT Health Science Center, Houston, told this news organization.
“You want heart attack to mean the same thing in Miami Beach as in Western Pennsylvania, as in Oregon and Washington and every place in between,” Dr. Anderson explained. “You want everybody to be using the same language, so that’s what these data standards are meant to do.”
In the document, data elements are grouped into three broad categories: chest pain, myocardial injury, and MI.
“We deliberately followed the plans contained in the new guideline and focused on potentially serious cardiovascular causes of chest pain as might be encountered in emergency departments,” the writing group notes in the document.
The terms “typical” and “atypical” as descriptors of chest pain or anginal syndromes are not used in the new document, in line with the 2021 guidance to abandon these terms.
Instead, the new document divides chest pain syndromes into three categories: “cardiac,” “possible cardiac,” and “noncardiac” – again, in keeping with the chest pain guideline.
The document also includes data elements for risk stratification scoring according to several common risk scoring algorithms and for procedure-related myocardial injury and procedure-related MI.
Each year, chest pain sends more than 7 million adults to the emergency department in the United States. Although noncardiac causes of chest pain make up a large majority of these cases, there are several life-threatening causes of chest pain that must be identified and treated promptly.
Distinguishing between serious and nonserious causes of chest pain is an urgent imperative, the writing group says.
Overall, they say this new clinical lexicon and set of data standards should be “broadly applicable” in various settings, including clinical trials and observational studies, patient care, electronic health records (EHRs), quality and performance improvement initiatives, registries, and public reporting programs.
The 2022 ACC/AHA Key Data Elements and Definitions for Chest Pain and Acute Myocardial Infarction was simultaneously published online in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes.
It was developed in collaboration with the American College of Emergency Physicians and the Society for Cardiac Angiography and Interventions and endorsed by the Society for Academic Emergency Medicine.
Dr. Anderson noted that “almost all of the guidelines that come out now, certainly in the last few years, have been followed after a certain interval by a set of data standards applicable to the guidelines.”
“It would be really great if it could actually be attached as an appendix, but the nature of the development of these things is such that there will always be a bit of a time lag between the writing group that develops the guidelines and the work group that develops the data standards; you can’t really have them working in parallel at the same time,” Dr. Anderson said in an interview.
This research had no commercial funding. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Influenza vaccine may offer much more than flu prevention
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
CDC says 44% of people hospitalized with COVID had third dose or booster
, the Centers for Disease Control and Prevention says.
Unvaccinated adults were 3.4 times more likely to be hospitalized with COVID than those who were vaccinated, the CDC said.
The CDC report considered hospitalization numbers from March 20 to May 31, when the omicron subvariant BA.2 was the dominant strain. Researchers found 39.1% of patients had received a primary vaccination series and at least one booster or additional dose; 5% were fully vaccinated with two boosters.
“Adults should stay up to date with COVID-19 vaccination, including booster doses,” the CDC said. “Multiple nonpharmaceutical and medical prevention measures should be used to protect persons at high risk for severe SARS-CoV-2, regardless of vaccination status.”
Older adults and people with underlying medical conditions who become infected with the coronavirus are more likely to be hospitalized.
The study also found that hospitalization rates among people over 65 increased threefold over the study period. Rates among people under 65 rose 1.7 times.
A version of this article first appeared on WebMD.com.
, the Centers for Disease Control and Prevention says.
Unvaccinated adults were 3.4 times more likely to be hospitalized with COVID than those who were vaccinated, the CDC said.
The CDC report considered hospitalization numbers from March 20 to May 31, when the omicron subvariant BA.2 was the dominant strain. Researchers found 39.1% of patients had received a primary vaccination series and at least one booster or additional dose; 5% were fully vaccinated with two boosters.
“Adults should stay up to date with COVID-19 vaccination, including booster doses,” the CDC said. “Multiple nonpharmaceutical and medical prevention measures should be used to protect persons at high risk for severe SARS-CoV-2, regardless of vaccination status.”
Older adults and people with underlying medical conditions who become infected with the coronavirus are more likely to be hospitalized.
The study also found that hospitalization rates among people over 65 increased threefold over the study period. Rates among people under 65 rose 1.7 times.
A version of this article first appeared on WebMD.com.
, the Centers for Disease Control and Prevention says.
Unvaccinated adults were 3.4 times more likely to be hospitalized with COVID than those who were vaccinated, the CDC said.
The CDC report considered hospitalization numbers from March 20 to May 31, when the omicron subvariant BA.2 was the dominant strain. Researchers found 39.1% of patients had received a primary vaccination series and at least one booster or additional dose; 5% were fully vaccinated with two boosters.
“Adults should stay up to date with COVID-19 vaccination, including booster doses,” the CDC said. “Multiple nonpharmaceutical and medical prevention measures should be used to protect persons at high risk for severe SARS-CoV-2, regardless of vaccination status.”
Older adults and people with underlying medical conditions who become infected with the coronavirus are more likely to be hospitalized.
The study also found that hospitalization rates among people over 65 increased threefold over the study period. Rates among people under 65 rose 1.7 times.
A version of this article first appeared on WebMD.com.
FROM MMWR
New study supports safety of COVID-19 boosters during pregnancy
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Unvaccinated 10 times more likely to be hospitalized for Omicron
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
FROM JAMA INTERNAL MEDICINE
One fish, two fish, are good fish for you ... fish
Good news for pregnant women; bad news for fish
As soon as women find out they’re pregnant, doctors recommend they give up smoking, drinking, and eating certain types of fish. That last item may need to be reconsidered, since a recent study supports the idea that it doesn’t matter what type of fish pregnant women are eating, as long as they’re eating it.
Researchers collected data from two different studies that reviewed the mercury levels of mothers from Bristol, England, and the Seychelles, a island chain off East Africa where “fish consumption is high and prenatal mercury levels are 10 times higher than in the [United States],” they said in NeuroToxicology.
Those data showed that the mercury levels had no adverse effects on child development as long as the mother ate fish. The nutrients and vitamins in the fish – vitamin D, long-chain fatty acids, selenium, and iodine – provide protection against mercury. There’s also the already-known benefits to eyesight and intellectual abilities that have been associated with fish consumption.
This analysis goes starkly against the grain of what is commonly recommended to expectant mothers, which is to cut out fish altogether. The researchers suggested that governments should review and change those recommendations to focus on the benefits instead.
As long as women follow the researchers’ recommendation to eat “at least two portions of fish a week, one of which should be oily,” they may not have to lay off on the sushi after all.
We’ll show our gut worms the world
Never let it be said that mankind is not a generous species. Sure, we could maybe be kinder to our fellow human beings, maybe declare a little less war on each other, but for the past 50,000 years, we’ve been giving a free ride to millions upon millions to one of mankind’s closest companions: the whipworm.
This revelation into human kindness comes from Denmark, where researchers from Copenhagen conducted a genetic analysis of ancient preserved whipworm eggs found in old Viking and Norse settlements, some of which date back over 2,000 years. In normal conditions genetic material wouldn’t last very long, but these were Viking whipworms eggs with tiny little horned helmets, so the DNA within has remained unchanged. Or it may be the tough chitinous exterior of the eggs protecting the DNA from degrading, combined with their preservation in moist soil.
Once they had their Viking whipworm DNA, the researchers compared it with whipworm DNA from all over the world, tracing its history as it followed mankind from Africa. And it’s been a while: We brought whipworms with us during our initial migration into Asia and Europe over 50,000 years ago. When the Bering land bridge opened up and humanity moved into the Americas, the worms came as well.
This is all possible because the whipworm goes about its parasitic business quietly and cleverly. It mostly sits harmlessly in our digestive systems, producing thousands of eggs a day that get expelled through poop and picked up by another host (human or otherwise); whipworms only cause disease in those with compromised immune systems.
The researchers noted that their study, the first complete genetic analysis of the whipworm, could help combat the parasite, which to this day infects hundred of millions who don’t have access to modern medicine or sanitary conditions. Hopefully, though, the days of free rides will soon be over for the whipworm. After all, if we have to pay hundreds or thousands of dollars to visit other countries, it’s only fair that our parasites do as well.
From zero to vasectomy in 6.7 seconds
There’s an old saying that you’ve probably heard: When life gives you lemons, make lemonade. It’s meant to encourage optimism in the face of adversity. Then there’s the new saying we just made up: When life gives you a power outage, plug your surgical instruments into an electric pickup.
That’s what Dr. Christopher Yang did, and now we’re making the urologist from Austin, Tex., famous by sharing his surgical/electrical adventure with all 17 of LOTME’s regular readers. That’s some serious lemonade.
Dr. Yang’s tale begins when the electricity went out at his clinic, seemingly forcing him to cancel or reschedule several surgical procedures. Not so fast. Dr. Yang happens to own a Rivian R1T, an electric pickup truck that has four power outlets. A staff member suggested plugging the surgical instruments into the truck and, surprisingly, one of the day’s patients agreed to go ahead with his vasectomy.
“We were fortunate that my normal parking spot is close enough to a patient room to run an extension cord,” Dr. Yang said on TheDrive.com. That extension cord was attached to an electrocautery device, with a handheld device available as backup, and “after we were done, I told his family. We all had a good laugh together too,” Dr. Yang told radio station WGLT in Normal, Ill.
To us, anyway, this opens up all sorts of alternative energy possibilities. Can a windmill power a liposuction? Is a gerbil running in a wheel enough to do a colonoscopy? How many potatoes do you need to keep an EHR going?
Learning through random acts of not-exactly noisiness
First things first. Transcranial random noise stimulation (tRNS) is not really noise in the auditory sense of the word. For some people with learning disabilities, though, it can actually be very helpful. The technology, which uses electrodes attached to the head so a weak current can pass through specific parts of the brain, may help those with learning disabilities, perhaps even those with brain injuries and visual deficits, learn, said Dr. Onno van der Groen of Edith Cowan University in Perth, Australia.
“When you add this type of stimulation during learning, you get better performance, faster learning and better attention afterwards as well,” he said in a statement from the university.
The researchers say that tRNS can allow the brain to form new connections and pathways, which in turn help a person learn more effectively. “If you do 10 sessions of a visual perception task with the tRNS and then come back and do it again without it, you’ll find you perform better than the control group who hasn’t used it,” Dr. van der Groen noted.
Can this also work for the average person? It’s possible, but tRNS didn’t seem to improve the math skills of a top-level mathematician who underwent the process, according to a case study that Dr. van der Groen mentioned.
This line of work is still pretty new, though, so researchers don’t have all the answers yet. As always, we’re rooting for you, science!
Good news for pregnant women; bad news for fish
As soon as women find out they’re pregnant, doctors recommend they give up smoking, drinking, and eating certain types of fish. That last item may need to be reconsidered, since a recent study supports the idea that it doesn’t matter what type of fish pregnant women are eating, as long as they’re eating it.
Researchers collected data from two different studies that reviewed the mercury levels of mothers from Bristol, England, and the Seychelles, a island chain off East Africa where “fish consumption is high and prenatal mercury levels are 10 times higher than in the [United States],” they said in NeuroToxicology.
Those data showed that the mercury levels had no adverse effects on child development as long as the mother ate fish. The nutrients and vitamins in the fish – vitamin D, long-chain fatty acids, selenium, and iodine – provide protection against mercury. There’s also the already-known benefits to eyesight and intellectual abilities that have been associated with fish consumption.
This analysis goes starkly against the grain of what is commonly recommended to expectant mothers, which is to cut out fish altogether. The researchers suggested that governments should review and change those recommendations to focus on the benefits instead.
As long as women follow the researchers’ recommendation to eat “at least two portions of fish a week, one of which should be oily,” they may not have to lay off on the sushi after all.
We’ll show our gut worms the world
Never let it be said that mankind is not a generous species. Sure, we could maybe be kinder to our fellow human beings, maybe declare a little less war on each other, but for the past 50,000 years, we’ve been giving a free ride to millions upon millions to one of mankind’s closest companions: the whipworm.
This revelation into human kindness comes from Denmark, where researchers from Copenhagen conducted a genetic analysis of ancient preserved whipworm eggs found in old Viking and Norse settlements, some of which date back over 2,000 years. In normal conditions genetic material wouldn’t last very long, but these were Viking whipworms eggs with tiny little horned helmets, so the DNA within has remained unchanged. Or it may be the tough chitinous exterior of the eggs protecting the DNA from degrading, combined with their preservation in moist soil.
Once they had their Viking whipworm DNA, the researchers compared it with whipworm DNA from all over the world, tracing its history as it followed mankind from Africa. And it’s been a while: We brought whipworms with us during our initial migration into Asia and Europe over 50,000 years ago. When the Bering land bridge opened up and humanity moved into the Americas, the worms came as well.
This is all possible because the whipworm goes about its parasitic business quietly and cleverly. It mostly sits harmlessly in our digestive systems, producing thousands of eggs a day that get expelled through poop and picked up by another host (human or otherwise); whipworms only cause disease in those with compromised immune systems.
The researchers noted that their study, the first complete genetic analysis of the whipworm, could help combat the parasite, which to this day infects hundred of millions who don’t have access to modern medicine or sanitary conditions. Hopefully, though, the days of free rides will soon be over for the whipworm. After all, if we have to pay hundreds or thousands of dollars to visit other countries, it’s only fair that our parasites do as well.
From zero to vasectomy in 6.7 seconds
There’s an old saying that you’ve probably heard: When life gives you lemons, make lemonade. It’s meant to encourage optimism in the face of adversity. Then there’s the new saying we just made up: When life gives you a power outage, plug your surgical instruments into an electric pickup.
That’s what Dr. Christopher Yang did, and now we’re making the urologist from Austin, Tex., famous by sharing his surgical/electrical adventure with all 17 of LOTME’s regular readers. That’s some serious lemonade.
Dr. Yang’s tale begins when the electricity went out at his clinic, seemingly forcing him to cancel or reschedule several surgical procedures. Not so fast. Dr. Yang happens to own a Rivian R1T, an electric pickup truck that has four power outlets. A staff member suggested plugging the surgical instruments into the truck and, surprisingly, one of the day’s patients agreed to go ahead with his vasectomy.
“We were fortunate that my normal parking spot is close enough to a patient room to run an extension cord,” Dr. Yang said on TheDrive.com. That extension cord was attached to an electrocautery device, with a handheld device available as backup, and “after we were done, I told his family. We all had a good laugh together too,” Dr. Yang told radio station WGLT in Normal, Ill.
To us, anyway, this opens up all sorts of alternative energy possibilities. Can a windmill power a liposuction? Is a gerbil running in a wheel enough to do a colonoscopy? How many potatoes do you need to keep an EHR going?
Learning through random acts of not-exactly noisiness
First things first. Transcranial random noise stimulation (tRNS) is not really noise in the auditory sense of the word. For some people with learning disabilities, though, it can actually be very helpful. The technology, which uses electrodes attached to the head so a weak current can pass through specific parts of the brain, may help those with learning disabilities, perhaps even those with brain injuries and visual deficits, learn, said Dr. Onno van der Groen of Edith Cowan University in Perth, Australia.
“When you add this type of stimulation during learning, you get better performance, faster learning and better attention afterwards as well,” he said in a statement from the university.
The researchers say that tRNS can allow the brain to form new connections and pathways, which in turn help a person learn more effectively. “If you do 10 sessions of a visual perception task with the tRNS and then come back and do it again without it, you’ll find you perform better than the control group who hasn’t used it,” Dr. van der Groen noted.
Can this also work for the average person? It’s possible, but tRNS didn’t seem to improve the math skills of a top-level mathematician who underwent the process, according to a case study that Dr. van der Groen mentioned.
This line of work is still pretty new, though, so researchers don’t have all the answers yet. As always, we’re rooting for you, science!
Good news for pregnant women; bad news for fish
As soon as women find out they’re pregnant, doctors recommend they give up smoking, drinking, and eating certain types of fish. That last item may need to be reconsidered, since a recent study supports the idea that it doesn’t matter what type of fish pregnant women are eating, as long as they’re eating it.
Researchers collected data from two different studies that reviewed the mercury levels of mothers from Bristol, England, and the Seychelles, a island chain off East Africa where “fish consumption is high and prenatal mercury levels are 10 times higher than in the [United States],” they said in NeuroToxicology.
Those data showed that the mercury levels had no adverse effects on child development as long as the mother ate fish. The nutrients and vitamins in the fish – vitamin D, long-chain fatty acids, selenium, and iodine – provide protection against mercury. There’s also the already-known benefits to eyesight and intellectual abilities that have been associated with fish consumption.
This analysis goes starkly against the grain of what is commonly recommended to expectant mothers, which is to cut out fish altogether. The researchers suggested that governments should review and change those recommendations to focus on the benefits instead.
As long as women follow the researchers’ recommendation to eat “at least two portions of fish a week, one of which should be oily,” they may not have to lay off on the sushi after all.
We’ll show our gut worms the world
Never let it be said that mankind is not a generous species. Sure, we could maybe be kinder to our fellow human beings, maybe declare a little less war on each other, but for the past 50,000 years, we’ve been giving a free ride to millions upon millions to one of mankind’s closest companions: the whipworm.
This revelation into human kindness comes from Denmark, where researchers from Copenhagen conducted a genetic analysis of ancient preserved whipworm eggs found in old Viking and Norse settlements, some of which date back over 2,000 years. In normal conditions genetic material wouldn’t last very long, but these were Viking whipworms eggs with tiny little horned helmets, so the DNA within has remained unchanged. Or it may be the tough chitinous exterior of the eggs protecting the DNA from degrading, combined with their preservation in moist soil.
Once they had their Viking whipworm DNA, the researchers compared it with whipworm DNA from all over the world, tracing its history as it followed mankind from Africa. And it’s been a while: We brought whipworms with us during our initial migration into Asia and Europe over 50,000 years ago. When the Bering land bridge opened up and humanity moved into the Americas, the worms came as well.
This is all possible because the whipworm goes about its parasitic business quietly and cleverly. It mostly sits harmlessly in our digestive systems, producing thousands of eggs a day that get expelled through poop and picked up by another host (human or otherwise); whipworms only cause disease in those with compromised immune systems.
The researchers noted that their study, the first complete genetic analysis of the whipworm, could help combat the parasite, which to this day infects hundred of millions who don’t have access to modern medicine or sanitary conditions. Hopefully, though, the days of free rides will soon be over for the whipworm. After all, if we have to pay hundreds or thousands of dollars to visit other countries, it’s only fair that our parasites do as well.
From zero to vasectomy in 6.7 seconds
There’s an old saying that you’ve probably heard: When life gives you lemons, make lemonade. It’s meant to encourage optimism in the face of adversity. Then there’s the new saying we just made up: When life gives you a power outage, plug your surgical instruments into an electric pickup.
That’s what Dr. Christopher Yang did, and now we’re making the urologist from Austin, Tex., famous by sharing his surgical/electrical adventure with all 17 of LOTME’s regular readers. That’s some serious lemonade.
Dr. Yang’s tale begins when the electricity went out at his clinic, seemingly forcing him to cancel or reschedule several surgical procedures. Not so fast. Dr. Yang happens to own a Rivian R1T, an electric pickup truck that has four power outlets. A staff member suggested plugging the surgical instruments into the truck and, surprisingly, one of the day’s patients agreed to go ahead with his vasectomy.
“We were fortunate that my normal parking spot is close enough to a patient room to run an extension cord,” Dr. Yang said on TheDrive.com. That extension cord was attached to an electrocautery device, with a handheld device available as backup, and “after we were done, I told his family. We all had a good laugh together too,” Dr. Yang told radio station WGLT in Normal, Ill.
To us, anyway, this opens up all sorts of alternative energy possibilities. Can a windmill power a liposuction? Is a gerbil running in a wheel enough to do a colonoscopy? How many potatoes do you need to keep an EHR going?
Learning through random acts of not-exactly noisiness
First things first. Transcranial random noise stimulation (tRNS) is not really noise in the auditory sense of the word. For some people with learning disabilities, though, it can actually be very helpful. The technology, which uses electrodes attached to the head so a weak current can pass through specific parts of the brain, may help those with learning disabilities, perhaps even those with brain injuries and visual deficits, learn, said Dr. Onno van der Groen of Edith Cowan University in Perth, Australia.
“When you add this type of stimulation during learning, you get better performance, faster learning and better attention afterwards as well,” he said in a statement from the university.
The researchers say that tRNS can allow the brain to form new connections and pathways, which in turn help a person learn more effectively. “If you do 10 sessions of a visual perception task with the tRNS and then come back and do it again without it, you’ll find you perform better than the control group who hasn’t used it,” Dr. van der Groen noted.
Can this also work for the average person? It’s possible, but tRNS didn’t seem to improve the math skills of a top-level mathematician who underwent the process, according to a case study that Dr. van der Groen mentioned.
This line of work is still pretty new, though, so researchers don’t have all the answers yet. As always, we’re rooting for you, science!
Biomarker-guided steroid therapy shown safe for COPD
Eosinophil-guided corticosteroid therapy for patients with chronic obstructive pulmonary disease (COPD) is equivalent in efficacy to standard of care therapy, but the eosinophil-guided therapy may help mitigate the harmful side effects associated with even short courses of corticosteroids, investigators said in a primary care–based randomized trial.
Among patients in 14 primary care practices in the United Kingdom who experienced COPD exacerbations, the proportion of patients who experienced treatment failure at day 28 was 27% for those who were randomized to receive prednisolone only when blood eosinophil counts on a point-of-care assay equaled or exceeded 2%, compared with 34% of all patients randomized to standard of care.
The relative risk for treatment failure using the eosinophil-guided approach was 0.82, which did not reach statistical significance, but indicated noninferiority for the biomarker-based dosing method, Mona Bafadhel, MD, of King’s College London, reported on behalf of colleagues in the Stratified Treatment to Reduce Risk in COPD (STARR2) trial.
This is the largest primary care multicenter trial, and probably adds another 20% to the literature base for exacerbations in COPD,” she said in an oral abstract presentation at the European Respiratory Society 2022 Congress.
“A personalized endotype-based treatment with oral prednisolone is possible in patients with COPD and I think should be now part of clinical guidelines,” she added.
Too much of a good thing
Although systemic corticosteroids are the universal treatment for COPD exacerbations, the drugs are also known to increase harm, with studies showing that cumulative doses of oral corticosteroids in COPD patients is associated with an increased risk for death. In addition, systemic corticosteroids are the third most common cause of adverse events leading to hospitalization, behind only chemotherapy and antibiotic use leading to Clostridioides difficile infections, Dr. Bafadhel said.
“And of course, corticosteroids are associated with significant harmful effects, including a five-times increased risk of sepsis, three-times increased risk of [venous thromboembolism], and a twice-increased risk of fracture,” she said.
Dr. Bafadhel and colleagues had previously shown in the single-center BEAT-COPD study that peripheral blood eosinophils at the time of a moderate COPD exacerbation could be used to safely direct oral corticosteroid therapy. She also pointed to a 2019 multicenter open-label study showing that eosinophil-guided care was noninferior to standard prescribing of oral corticosteroids for patients with severe exacerbations.
Primary care study
The investigators conducted the current study to test whether eosinophil-guided therapy at the point of care in a primary practice setting was efficacious, with the ultimate goal of encouraging changes in guidelines.
They recruited patients with COPD exacerbations from 14 general practices in Oxfordshire and Buckinghamshire in the Thames Valley.
The patients were randomly assigned to receive either standard of care or the biomarker-guided intervention for 14 days. In this arm, patients with eosinophil counts of 2% or greater received matched prednisolone, while patients with counts below 2% received placebo. The patients were blinded to the assigned drug.
A total of 203 exacerbations among 152 patients were evenly allocated to treatment or control groups. The mean patient age was 71. Of the 102 exacerbations allocated to eosinophil-guided therapy, 34 were treated with placebo.
As noted before, in the intention-to-treat analysis the primary outcome of the treatment failure rate, defined as any need for antibiotics and/or steroids at one month, was 27% in the biomarker-guided arm and 34% in the standard care arm.
“In the per-protocol analysis we also demonstrated that there was a suggestion that there is possible superiority of using blood eosinophil-directed oral corticosteroid prescriptions at the time of acute exacerbation using the point-of-care eosinophil test,” Dr. Bafadhel said.
There were no significant differences in the secondary outcomes of mean change in forced expiratory volume in 1 second (FEV1), COPD Assessment Test scores from exacerbation to follow-up, and symptoms according to a visual analog scale.
Invited discussant Dave Singh, MD, of the University of Manchester, England, asked Dr. Bafadhel how the data she presented supported her conclusions about the potential benefits of eosinophil-guided therapy, given that the P values were nonsignificant.
“The primary outcome was powered on noninferiority, and of course what we’ve shown is that it’s not any worse, it’s not any better, but of course it’s the effect of how many courses of steroids you can reduce in that population,” Dr. Bafadhel replied.
She noted that although the investigators have not performed an economic analysis to determine how many adverse events might be avoided using the biomarker-guided approach, “we do know that some of these patients who are given prednisolone, their comorbidities of diabetes worsened, for example.”
In the online Q&A for the presentation, Sohail Ansari, MD, from the Mid and South Essex NHS Foundation Trust in the United Kingdom, said that many patients in primary care practices receive “rescue packs” containing antibiotics and steroids, but may not be equipped to know when they should use the steroids and therefore may overuse them.
“Perhaps community-based, adequately resourced respiratory teams [may] be a way forward, but it will need adequate investment and commitment,” he wrote.
The trial was supported by the University of Oxford and National Institute for Health and Care Research, UK. Dr. Bafadhel reported grant and research support from the National Institute for Health and Care Research, Asthma & Lung UK, AstraZeneca, and Roche, and honoraria or fees from others. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Ansari reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Eosinophil-guided corticosteroid therapy for patients with chronic obstructive pulmonary disease (COPD) is equivalent in efficacy to standard of care therapy, but the eosinophil-guided therapy may help mitigate the harmful side effects associated with even short courses of corticosteroids, investigators said in a primary care–based randomized trial.
Among patients in 14 primary care practices in the United Kingdom who experienced COPD exacerbations, the proportion of patients who experienced treatment failure at day 28 was 27% for those who were randomized to receive prednisolone only when blood eosinophil counts on a point-of-care assay equaled or exceeded 2%, compared with 34% of all patients randomized to standard of care.
The relative risk for treatment failure using the eosinophil-guided approach was 0.82, which did not reach statistical significance, but indicated noninferiority for the biomarker-based dosing method, Mona Bafadhel, MD, of King’s College London, reported on behalf of colleagues in the Stratified Treatment to Reduce Risk in COPD (STARR2) trial.
This is the largest primary care multicenter trial, and probably adds another 20% to the literature base for exacerbations in COPD,” she said in an oral abstract presentation at the European Respiratory Society 2022 Congress.
“A personalized endotype-based treatment with oral prednisolone is possible in patients with COPD and I think should be now part of clinical guidelines,” she added.
Too much of a good thing
Although systemic corticosteroids are the universal treatment for COPD exacerbations, the drugs are also known to increase harm, with studies showing that cumulative doses of oral corticosteroids in COPD patients is associated with an increased risk for death. In addition, systemic corticosteroids are the third most common cause of adverse events leading to hospitalization, behind only chemotherapy and antibiotic use leading to Clostridioides difficile infections, Dr. Bafadhel said.
“And of course, corticosteroids are associated with significant harmful effects, including a five-times increased risk of sepsis, three-times increased risk of [venous thromboembolism], and a twice-increased risk of fracture,” she said.
Dr. Bafadhel and colleagues had previously shown in the single-center BEAT-COPD study that peripheral blood eosinophils at the time of a moderate COPD exacerbation could be used to safely direct oral corticosteroid therapy. She also pointed to a 2019 multicenter open-label study showing that eosinophil-guided care was noninferior to standard prescribing of oral corticosteroids for patients with severe exacerbations.
Primary care study
The investigators conducted the current study to test whether eosinophil-guided therapy at the point of care in a primary practice setting was efficacious, with the ultimate goal of encouraging changes in guidelines.
They recruited patients with COPD exacerbations from 14 general practices in Oxfordshire and Buckinghamshire in the Thames Valley.
The patients were randomly assigned to receive either standard of care or the biomarker-guided intervention for 14 days. In this arm, patients with eosinophil counts of 2% or greater received matched prednisolone, while patients with counts below 2% received placebo. The patients were blinded to the assigned drug.
A total of 203 exacerbations among 152 patients were evenly allocated to treatment or control groups. The mean patient age was 71. Of the 102 exacerbations allocated to eosinophil-guided therapy, 34 were treated with placebo.
As noted before, in the intention-to-treat analysis the primary outcome of the treatment failure rate, defined as any need for antibiotics and/or steroids at one month, was 27% in the biomarker-guided arm and 34% in the standard care arm.
“In the per-protocol analysis we also demonstrated that there was a suggestion that there is possible superiority of using blood eosinophil-directed oral corticosteroid prescriptions at the time of acute exacerbation using the point-of-care eosinophil test,” Dr. Bafadhel said.
There were no significant differences in the secondary outcomes of mean change in forced expiratory volume in 1 second (FEV1), COPD Assessment Test scores from exacerbation to follow-up, and symptoms according to a visual analog scale.
Invited discussant Dave Singh, MD, of the University of Manchester, England, asked Dr. Bafadhel how the data she presented supported her conclusions about the potential benefits of eosinophil-guided therapy, given that the P values were nonsignificant.
“The primary outcome was powered on noninferiority, and of course what we’ve shown is that it’s not any worse, it’s not any better, but of course it’s the effect of how many courses of steroids you can reduce in that population,” Dr. Bafadhel replied.
She noted that although the investigators have not performed an economic analysis to determine how many adverse events might be avoided using the biomarker-guided approach, “we do know that some of these patients who are given prednisolone, their comorbidities of diabetes worsened, for example.”
In the online Q&A for the presentation, Sohail Ansari, MD, from the Mid and South Essex NHS Foundation Trust in the United Kingdom, said that many patients in primary care practices receive “rescue packs” containing antibiotics and steroids, but may not be equipped to know when they should use the steroids and therefore may overuse them.
“Perhaps community-based, adequately resourced respiratory teams [may] be a way forward, but it will need adequate investment and commitment,” he wrote.
The trial was supported by the University of Oxford and National Institute for Health and Care Research, UK. Dr. Bafadhel reported grant and research support from the National Institute for Health and Care Research, Asthma & Lung UK, AstraZeneca, and Roche, and honoraria or fees from others. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Ansari reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Eosinophil-guided corticosteroid therapy for patients with chronic obstructive pulmonary disease (COPD) is equivalent in efficacy to standard of care therapy, but the eosinophil-guided therapy may help mitigate the harmful side effects associated with even short courses of corticosteroids, investigators said in a primary care–based randomized trial.
Among patients in 14 primary care practices in the United Kingdom who experienced COPD exacerbations, the proportion of patients who experienced treatment failure at day 28 was 27% for those who were randomized to receive prednisolone only when blood eosinophil counts on a point-of-care assay equaled or exceeded 2%, compared with 34% of all patients randomized to standard of care.
The relative risk for treatment failure using the eosinophil-guided approach was 0.82, which did not reach statistical significance, but indicated noninferiority for the biomarker-based dosing method, Mona Bafadhel, MD, of King’s College London, reported on behalf of colleagues in the Stratified Treatment to Reduce Risk in COPD (STARR2) trial.
This is the largest primary care multicenter trial, and probably adds another 20% to the literature base for exacerbations in COPD,” she said in an oral abstract presentation at the European Respiratory Society 2022 Congress.
“A personalized endotype-based treatment with oral prednisolone is possible in patients with COPD and I think should be now part of clinical guidelines,” she added.
Too much of a good thing
Although systemic corticosteroids are the universal treatment for COPD exacerbations, the drugs are also known to increase harm, with studies showing that cumulative doses of oral corticosteroids in COPD patients is associated with an increased risk for death. In addition, systemic corticosteroids are the third most common cause of adverse events leading to hospitalization, behind only chemotherapy and antibiotic use leading to Clostridioides difficile infections, Dr. Bafadhel said.
“And of course, corticosteroids are associated with significant harmful effects, including a five-times increased risk of sepsis, three-times increased risk of [venous thromboembolism], and a twice-increased risk of fracture,” she said.
Dr. Bafadhel and colleagues had previously shown in the single-center BEAT-COPD study that peripheral blood eosinophils at the time of a moderate COPD exacerbation could be used to safely direct oral corticosteroid therapy. She also pointed to a 2019 multicenter open-label study showing that eosinophil-guided care was noninferior to standard prescribing of oral corticosteroids for patients with severe exacerbations.
Primary care study
The investigators conducted the current study to test whether eosinophil-guided therapy at the point of care in a primary practice setting was efficacious, with the ultimate goal of encouraging changes in guidelines.
They recruited patients with COPD exacerbations from 14 general practices in Oxfordshire and Buckinghamshire in the Thames Valley.
The patients were randomly assigned to receive either standard of care or the biomarker-guided intervention for 14 days. In this arm, patients with eosinophil counts of 2% or greater received matched prednisolone, while patients with counts below 2% received placebo. The patients were blinded to the assigned drug.
A total of 203 exacerbations among 152 patients were evenly allocated to treatment or control groups. The mean patient age was 71. Of the 102 exacerbations allocated to eosinophil-guided therapy, 34 were treated with placebo.
As noted before, in the intention-to-treat analysis the primary outcome of the treatment failure rate, defined as any need for antibiotics and/or steroids at one month, was 27% in the biomarker-guided arm and 34% in the standard care arm.
“In the per-protocol analysis we also demonstrated that there was a suggestion that there is possible superiority of using blood eosinophil-directed oral corticosteroid prescriptions at the time of acute exacerbation using the point-of-care eosinophil test,” Dr. Bafadhel said.
There were no significant differences in the secondary outcomes of mean change in forced expiratory volume in 1 second (FEV1), COPD Assessment Test scores from exacerbation to follow-up, and symptoms according to a visual analog scale.
Invited discussant Dave Singh, MD, of the University of Manchester, England, asked Dr. Bafadhel how the data she presented supported her conclusions about the potential benefits of eosinophil-guided therapy, given that the P values were nonsignificant.
“The primary outcome was powered on noninferiority, and of course what we’ve shown is that it’s not any worse, it’s not any better, but of course it’s the effect of how many courses of steroids you can reduce in that population,” Dr. Bafadhel replied.
She noted that although the investigators have not performed an economic analysis to determine how many adverse events might be avoided using the biomarker-guided approach, “we do know that some of these patients who are given prednisolone, their comorbidities of diabetes worsened, for example.”
In the online Q&A for the presentation, Sohail Ansari, MD, from the Mid and South Essex NHS Foundation Trust in the United Kingdom, said that many patients in primary care practices receive “rescue packs” containing antibiotics and steroids, but may not be equipped to know when they should use the steroids and therefore may overuse them.
“Perhaps community-based, adequately resourced respiratory teams [may] be a way forward, but it will need adequate investment and commitment,” he wrote.
The trial was supported by the University of Oxford and National Institute for Health and Care Research, UK. Dr. Bafadhel reported grant and research support from the National Institute for Health and Care Research, Asthma & Lung UK, AstraZeneca, and Roche, and honoraria or fees from others. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Ansari reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ERS 2022 CONGRESS