User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


FREEDOM COVID: Full-dose anticoagulation cut mortality but missed primary endpoint
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
Study conducted in noncritically ill
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
AT ACC 2023
Clinician violence: Virtual reality to the rescue?
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
Who can sue docs for wrongful death? Some states are trying to expand that group
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
‘Breakthrough’ study: Diabetes drug helps prevent long COVID
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
with The Lancet on SSRN. The preprint hasn’t yet been peer-reviewed or published in a journal.
In particular, metformin led to a 42% drop in long COVID among people who had a mild to moderate COVID-19 infection.
“Long COVID affects millions of people, and preventing long COVID through a treatment like metformin could prevent significant disruptions in people’s lives,” said lead author Carolyn Bramante, MD, assistant professor of internal medicine and pediatrics at the University of Minnesota, Minneapolis.
Between January 2021 and February 2022, Dr. Bramante and colleagues tested three oral medications – metformin (typically used to treat type 2 diabetes), ivermectin (an antiparasitic), and fluvoxamine (an antidepressant) – in a clinical trial across the United States called COVID-OUT. The people being studied, investigators, care providers, and others involved in the study were blinded to the randomized treatments. The trial was decentralized, with no in-person contact with participants.
The researchers included patients who were aged 30-85 with overweight or obesity, had documentation of a confirmed COVID-19 infection, had fewer than 7 days of symptoms, had no known prior infection, and joined the study within 3 days of their positive test. The study included monthly follow-up for 300 days, and participants indicated whether they received a long COVID diagnosis from a medical doctor, which the researchers confirmed in medical records after participants gave consent.
The medications were prepackaged into pill boxes for fast delivery to participants and to ensure they took the correct number of each type of pill. The packages were sent via same-day courier or overnight shipping.
The metformin doses were doled out over 14 days, with 500 milligrams on the first day, 500 milligrams twice a day for the next 4 days, and then 500 milligrams in the morning and 1,000 milligrams in the evening for the remaining 9 days.
Among the 1,323 people studied, 1,125 agreed to do long-term follow-up for long COVID: 564 in the metformin group and 561 in the blinded placebo group. The average age was 45, and 56% were women, including 7% who were pregnant.
The average time from the start of symptoms to starting medication was 5 days, and 47% began taking the drug within 4 days or less. About 55% had received the primary COVID-19 vaccination series, including 5.1% who received an initial booster, before enrolling in the study.
Overall, 8.4% of participants reported that a medical provider diagnosed them with long COVID. Of those who took metformin, 6.3% developed long COVID, compared to 10.6% among those who took the identical-matched placebo.
The risk reduction for metformin was 42% versus the placebo, which was consistent across subgroups, including vaccination status and different COVID-19 variants.
When metformin was started less than 4 days after COVID-19 symptoms started, the effect was potentially even greater, with a 64% reduction, as compared with a 36% reduction among those who started metformin after 4 or more days after symptoms.
Neither ivermectin nor fluvoxamine showed any benefits for preventing long COVID.
At the same time, the study authors caution that more research is needed.
“The COVID-OUT trial does not indicate whether or not metformin would be effective at preventing long COVID if started at the time of emergency department visit or hospitalization for COVID-19, nor whether metformin would be effective as treatment in persons who already have long COVID,” they wrote. “With the burden of long COVID on society, confirmation is urgently needed in a trial that addresses our study’s limitations in order to translate these results into practice and policy.”
Several risk factors for long COVID emerged in the analysis. About 11.1% of the women had a long COVID diagnosis, compared with 4.9% of the men. Also, those who had received at least the primary vaccine series had a lower risk of developing long COVID, at 6.6%, as compared with 10.5% among the unvaccinated. Only 1 of the 57 people who received a booster shot developed long COVID.
Notably, pregnant and lactating people were included in this study, which is important given that pregnant people face higher risks for poor COVID-19 outcomes and are excluded from most nonobstetric clinical trials, the study authors wrote. In this study, they were randomized to metformin or placebo but not ivermectin or fluvoxamine due to limited research about the safety of those drugs during pregnancy and lactation.
The results are now under journal review but show findings consistent with those from other recent studies. Also, in August 2022, the authors published results from COVID-OUT that showed metformin led to a 42% reduction in hospital visits, emergency department visits, and deaths related to severe COVID-19.
“Given the lack of side effects and cost for a 2-week course, I think these data support use of metformin now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief of Medscape, WebMD’s sister site for health care professionals.
Dr. Topol, who wasn’t involved with this study, has been a leading voice on COVID-19 research throughout the pandemic. He noted the need for more studies, including a factorial design trial to test metformin and Paxlovid, which has shown promise in preventing long COVID. Dr. Topol also wrote about the preprint in Ground Truths, his online newsletter.
“As I’ve written in the past, I don’t use the term ‘breakthrough’ lightly,” he wrote. “But to see such a pronounced benefit in the current randomized trial of metformin, in the context of its being so safe and low cost, I’d give it a breakthrough categorization.”
Another way to put it, Dr. Topol wrote, is that based on this study, he would take metformin if he became infected with COVID-19.
Jeremy Faust, MD, an emergency medicine doctor at Brigham and Women’s Hospital in Boston, also wrote about the study in his newsletter, Inside Medicine. He noted that the 42% reduction in long COVID means that 23 COVID-19 patients need to be treated with metformin to prevent one long COVID diagnosis, which is an “important reduction.”
“Bottom line: If a person who meets criteria for obesity or overweight status were to ask me if they should take metformin (for 2 weeks) starting as soon as they learn they have COVID-19, I would say yes in many if not most cases, based on this new data,” he wrote. “This is starting to look like a real win.”
A version of this article first appeared on WebMD.com.
The triple overlap: COPD-OSA-OHS. Is it time for new definitions?
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):
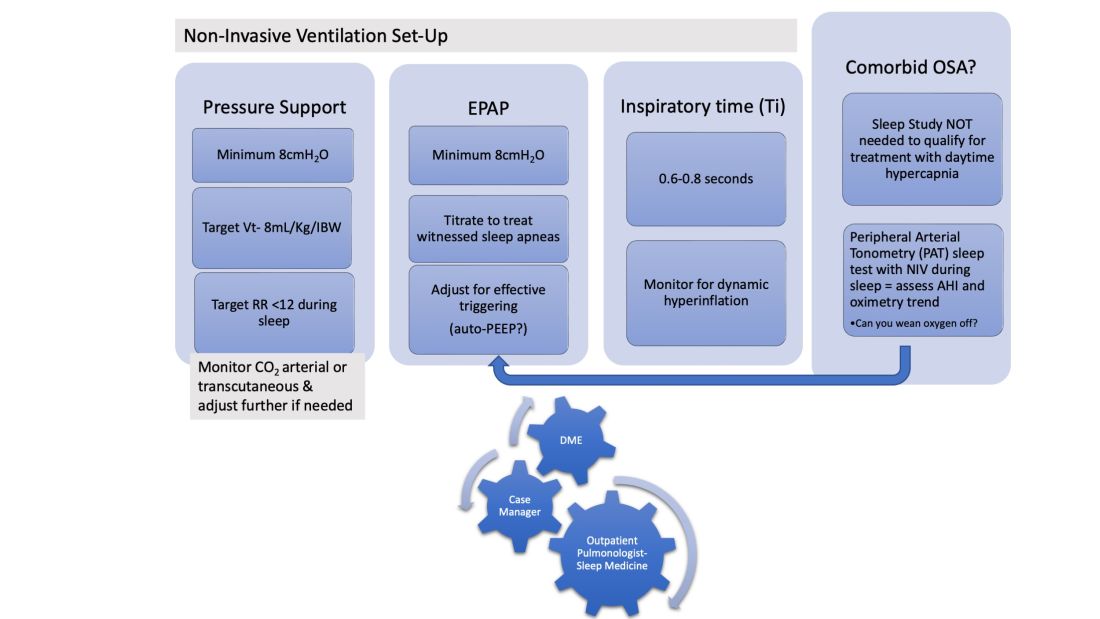
1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):

1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):

1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
Introducing CHEST President-Designate John A. Howington, MD, MBA, FCCP
John A. Howington, MD, MBA, FCCP, is a cardiothoracic surgeon currently serving as Chief of Oncology Services and Chair of Thoracic Surgery at Ascension Saint Thomas Health and a professor at the University of Tennessee Health Sciences Center in Nashville, Tennessee.
Dr. Howington received his undergraduate degree from Tennessee Technological University and medical degree from the University of Tennessee. He completed his general surgery residency at the University of Missouri, Kansas City and thoracic surgery residency at Vanderbilt University Medical Center.
Most recently, he received his Physician Executive MBA from the University of Tennessee.
As a passionate thoracic surgeon, he has lent his knowledge to the extensive CHEST lung cancer guideline portfolio for more than a decade. He offers regular leadership in multidisciplinary and executive forums and has spearheaded a series of quality improvement initiatives at Ascension. He has served in a variety of leadership roles with CHEST and with other national thoracic surgery societies.
Dr. Howington began his CHEST leadership journey with the Networks, as a member of the Interventional Chest Medicine Steering Committee and then as the Thoracic Oncology Network Chair (2008-2010).
Other leadership positions include serving as the President of the CHEST Foundation (2014-2016), member of the Scientific Program Committee and Membership Committee, and, recently, as the Chair of the Finance Committee from 2018-2021.
Since 2017, he has served on the Board of Regents as a Member at Large. Dr. Howington will serve as the 87th CHEST President in 2025.
John A. Howington, MD, MBA, FCCP, is a cardiothoracic surgeon currently serving as Chief of Oncology Services and Chair of Thoracic Surgery at Ascension Saint Thomas Health and a professor at the University of Tennessee Health Sciences Center in Nashville, Tennessee.
Dr. Howington received his undergraduate degree from Tennessee Technological University and medical degree from the University of Tennessee. He completed his general surgery residency at the University of Missouri, Kansas City and thoracic surgery residency at Vanderbilt University Medical Center.
Most recently, he received his Physician Executive MBA from the University of Tennessee.
As a passionate thoracic surgeon, he has lent his knowledge to the extensive CHEST lung cancer guideline portfolio for more than a decade. He offers regular leadership in multidisciplinary and executive forums and has spearheaded a series of quality improvement initiatives at Ascension. He has served in a variety of leadership roles with CHEST and with other national thoracic surgery societies.
Dr. Howington began his CHEST leadership journey with the Networks, as a member of the Interventional Chest Medicine Steering Committee and then as the Thoracic Oncology Network Chair (2008-2010).
Other leadership positions include serving as the President of the CHEST Foundation (2014-2016), member of the Scientific Program Committee and Membership Committee, and, recently, as the Chair of the Finance Committee from 2018-2021.
Since 2017, he has served on the Board of Regents as a Member at Large. Dr. Howington will serve as the 87th CHEST President in 2025.
John A. Howington, MD, MBA, FCCP, is a cardiothoracic surgeon currently serving as Chief of Oncology Services and Chair of Thoracic Surgery at Ascension Saint Thomas Health and a professor at the University of Tennessee Health Sciences Center in Nashville, Tennessee.
Dr. Howington received his undergraduate degree from Tennessee Technological University and medical degree from the University of Tennessee. He completed his general surgery residency at the University of Missouri, Kansas City and thoracic surgery residency at Vanderbilt University Medical Center.
Most recently, he received his Physician Executive MBA from the University of Tennessee.
As a passionate thoracic surgeon, he has lent his knowledge to the extensive CHEST lung cancer guideline portfolio for more than a decade. He offers regular leadership in multidisciplinary and executive forums and has spearheaded a series of quality improvement initiatives at Ascension. He has served in a variety of leadership roles with CHEST and with other national thoracic surgery societies.
Dr. Howington began his CHEST leadership journey with the Networks, as a member of the Interventional Chest Medicine Steering Committee and then as the Thoracic Oncology Network Chair (2008-2010).
Other leadership positions include serving as the President of the CHEST Foundation (2014-2016), member of the Scientific Program Committee and Membership Committee, and, recently, as the Chair of the Finance Committee from 2018-2021.
Since 2017, he has served on the Board of Regents as a Member at Large. Dr. Howington will serve as the 87th CHEST President in 2025.
In utero exposure to asthma medication not tied to risks of neurodevelopmental disorders
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
The drugs included in the study were leukotriene-receptor antagonists (LTRAs), which are often used to treat allergic airway diseases, including asthma and allergic rhinitis.
“Over the years, the U.S. Food and Drug Administration has monitored post-marketing data about the potential harm of neuropsychiatric events (NEs) associated with montelukast, the first type of LTRAs, and issued boxed warnings about serious mental health side effects for montelukast in 2020,” said corresponding author Tsung-Chieh Yao, MD, of Chang Gung Memorial Hospital, Taiwan, in an interview.
However, evidence of a link between NEs and LTRA use has been inconsistent, according to Dr. Yao and colleagues.
“To date, it remains totally unknown whether the exposure to LTRAs during pregnancy is associated with the risk of neuropsychiatric events in offspring,” said Dr. Yao.
To address this question, the researchers used data from National Health Insurance Research Database in Taiwan to identify pregnant women and their offspring from 2009 to 2019. The initial study population included 576,157 mother-offspring pairs, including 1,995 LTRA-exposed and 574,162 nonexposed children.
The women had a diagnosis of asthma or allergic rhinitis; multiple births and children with congenital malformations were excluded. LTRA exposure was defined as any dispensed prescription for LTRAs during pregnancy. Approximately two-thirds of the mothers were aged 30-40 years at the time of delivery.
The findings were published in a research letter in JAMA Network Open.
In the study population at large, the incidence of the three neurodevelopmental disorders ADHD, autism spectrum disorder (ASD), and Tourette syndrome was not significantly different between those children exposed to LTRAs and those not exposed to LTRAs in utero (1.25% vs. 1.32%; 3.31% vs. 4.36%; and 0.45% vs. 0.83%, respectively).
After propensity score matching, the study population included 1,988 LTRA-exposed children and 19,863 nonexposed children. In this group, no significant associations appeared between prenatal LTRA exposure and the risk of attention-deficit/hyperactivity disorder (adjusted hazard ratio, 1.03), autism spectrum disorder (AHR, 1.01), and Tourette syndrome (AHR, 0.63).
Neither duration nor cumulative dose of LTRA use during pregnancy showed an association with ADHD, ASD, or Tourette syndrome in offspring. Duration of LTRA use was categorized as shorter or longer periods of 1-4 weeks vs. more than 4 weeks; cumulative dose was categorized as 1-170 mg vs. 170 mg or higher.
The findings were limited by the lack of randomization, inability to detect long-term risk, and potential lack of generalizability to non-Asian populations, and more research is needed to replicate the results, the researchers noted. However, the current findings were strengthened by the large study population, and suggest that LTRA use in pregnancy does not present a significant risk for NEs in children, which should be reassuring to clinicians and patients, they concluded.
The current study is the first to use the whole of Taiwan population data and extends previous studies by examining the association between LTRA use during pregnancy and risk of neuropsychiatric events in offspring, Dr. Yao said in an interview. “The possibly surprising, but reassuring, finding is that prenatal LTRA exposure did not increase risk of ADHD, ASD, and Tourette syndrome in offspring,” he said.
“Clinicians prescribing LTRAs such as montelukast (Singulair and generics) to pregnant women with asthma or allergic rhinitis may be reassured by our findings,” Dr. Yao added. The results offer real-world evidence to help inform decision-making about the use of LTRAs during pregnancy, although additional research is needed to replicate the study findings in other populations, he said.
The study was supported by the National Health Research Institutes, Taiwan, the Ministry of Science and Technology of Taiwan, the National Science and Technology Council of Taiwan, and the Chang Gung Medical Foundation. The researchers had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Fixed-dose combo pill for PAH promises accelerated benefit: A DUE
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
Already commonly used in combination for the treatment of pulmonary arterial hypertension (PAH), macitentan and tadalafil are safe and effective in a fixed-dose combination even as first-line therapy, according to a randomized multicenter comparative trial.
The fixed-dose combination “led to a highly significant and marked improvement in pulmonary vascular resistance when compared to macitentan and tadalafil as monotherapies,” Kelly Chin, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Guidelines encourage rapid PVR reductions
In practice, it is common to start treatment with either the endothelial receptor antagonist (ERA) macitentan, the phosphodiesterase-5 (PDE-5) inhibitor tadalafil, or other frequently used medications for PAH, and to then add additional treatments, according to Dr. Chin. She pointed out, however, that guidelines, including those issued jointly by the European Society of Cardiology and the European Respiratory Society, encourage rapid escalation of therapy to quickly lower pulmonary vascular resistance (PVR).
In general, both macitentan and tadalafil are well tolerated, but the advantage and the safety of rapidly reducing PVR when these are initiated together in a single pill had not been evaluated previously in a major trial. In this double-blind phase III trial, called A DUE, 187 patients in functional class II or III PAH were randomized. The three-arm study included both treatment naive patients and patients who had been on stable doses (> 3 months) of an ERA or a PDE5 inhibitor, explained Dr. Chin, director of pulmonary hypertension at the UT Southwestern, Dallas.
Treatment naive patients, representing about 53% of the study population, were randomized to 10 mg macitentan monotherapy, 40 mg tadalafil monotherapy, or a fixed-dose, single-pill combination containing both. If on a stable dose of an ERA at trial entry, patients were randomized to 10 macitentan as a monotherapy or to the fixed dose combination. Patients entering the trial already on a stable dose of a PDE5 inhibitor were randomized to 40 mg tadalafil or the combination.
PVR reduced twofold on combination therapy
Relative to macitentan monotherapy, the percentage change from baseline in PVR by ratio of geometric mean, which was the primary outcome, was about twice as high on the combination (45% vs. 23%) at the end of the 16-week trial. This translates into a 29% PVR reduction (hazard ratio, 0.71; P < .0001).
For combination therapy relative to tadalafil monotherapy, the advantage for the fixed dose combination (44% vs. 22%) was about the same, also providing a nearly 30% relative reduction (HR, 0.72; P < .0001).
The increases in 6-minute walk distance (6MWD) at 16 weeks, a secondary endpoint, numerically favored the combination pill over both macitentan monotherapy (52.9 vs. 39.5 meters; P = .38) and tadalafil (43.4 vs. 15.9 meters; P = .059), but only the improvement relative to tadalafil monotherapy was considered a trend.
The proportion of patients who experienced at least one serious adverse event was higher in the combination arm (14.0%) relative to single agent macitentan (8.6%) or single agent tadalafil (9.1%). The adverse events and serious adverse events more common on the combination included hypotension, fluid retention, and anemia. This latter side effect occurred in 18.7%, 2.9%, and 2.3% in the combination, macitentan monotherapy, and tadalafil arms, respectively.
Several of those invited by the ACC to discuss the paper, including Lee R. Goldberg, MD, section chief of advanced heart failure and cardiac transplant, University of Pennsylvania, Philadelphia, raised concern about the increased rate of anemia among those in the combination pill. Two of the patients (2%) treated with the combination developed a hemoglobin < 8 g/dL.
Overall, nine (8.4%) of those on the fixed-dose combination, two (4.5%) of those randomized to tadalafil monotherapy, and none of the patients randomized to macitentan discontinued therapy due to side effects.
Anemia risk unexpected
Based on “the unexpected signal of an anemia risk,” Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, said that a larger scale trial with a longer follow-up is needed. While the concept of front-loading two drugs is attractive “for the very challenging PAH population,” she called for further evaluation of this safety signal before clinicians switch from the current practice of starting with one PAH therapy before adding others.
In addition, Dr. Bozkurt said a more definitive study would be helpful in determining whether starting with a fixed-pill combination is better than sequential treatment to improve quality of life. Dr. Bozkurt said it is likely that the lack of significant benefit on 6MWD in this study was due to the relatively small sample size, but an improvement in this measure would be another reason to consider a front-line fixed-dose combination.
Dr. Chin, in an interview, did not agree. She agreed that a larger sample size might have yielded a significant improvement in 6MWD, but she noted this outcome was moving in the right direction and was not the primary endpoint. In her opinion, this phase 3 trial does confirm that fixed-dose combination is well tolerated, has acceptable safety, and markedly improves PVR, fulfilling the guideline goal of controlling PAH more quickly.
Dr. Chin reports financial relationships with Altavant, Arena, Gossamer Bio, Janssen, Merck, ShouTi, and United Therapeutics. Dr. Goldberg reports financial relationships with Abbott, Respicardia/Zoll, and Viscardia. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
AT ACC 2023
Telehealth doctor indicted on health care fraud, opioid distribution charges
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
We have seen the future of healthy muffins, and its name is Roselle
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.
Get ‘em while they’re hot … for your health
Today on the Eating Channel, it’s a very special episode of “Much Ado About Muffin.”
The muffin. For some of us, it’s a good way to pretend we’re not having dessert for breakfast. A bran muffin can be loaded with calcium and fiber, and our beloved blueberry is full of yummy antioxidants and vitamins. Definitely not dessert.
Well, the muffin denial can stop there because there’s a new flavor on the scene, and research suggests it may actually be healthy. (Disclaimer: Muffin may not be considered healthy in Norway.) This new muffin has a name, Roselle, that comes from the calyx extract used in it, which is found in the Hibiscus sabdariffa plant of the same name.
Now, when it comes to new foods, especially ones that are supposed to be healthy, the No. 1 criteria is the same: It has to taste good. Researchers at the Norwegian University of Science and Technology and Amity University in India agreed, but they also set out to make it nutritionally valuable and give it a long shelf life without the addition of preservatives.
Sounds like a tall order, but they figured it out.
Not only is it tasty, but the properties of it could rival your morning multivitamin. Hibiscus extract has huge amounts of antioxidants, like phenolics, which are believed to help prevent cell membrane damage. Foods like vegetables, flax seed, and whole grains also have these antioxidants, but why not just have a Roselle muffin instead? You also get a dose of ascorbic acid without the glass of OJ in the morning.
The ascorbic acid, however, is not there just to help you. It also helps to check the researcher’s third box, shelf life. These naturally rosy-colored pastries will stay mold-free for 6 days without refrigeration at room temperature and without added preservatives.
Our guess, though, is they won’t be on the kitchen counter long enough to find out.
A sobering proposition
If Hollywood is to be believed, there’s no amount of drunkenness that can’t be cured with a cup of coffee or a stern slap in the face. Unfortunately, here in the real world the only thing that can make you less drunk is time. Maybe next time you’ll stop after that seventh Manhattan.
But what if we could beat time? What if there’s an actual sobriety drug out there?
Say hello to fibroblast growth factor 21. Although the liver already does good work filtering out what is essentially poison, it then goes the extra mile and produces fibroblast growth factor 21 (or, as her friends call her, FGF21), a hormone that suppresses the desire to drink, makes you desire water, and protects the liver all at the same time.
Now, FGF21 in its current role is great, but if you’ve ever seen or been a drunk person before, you’ve experienced the lack of interest in listening to reason, especially when it comes from within our own bodies. Who are you to tell us what to do, body? You’re not the boss of us! So a group of scientists decided to push the limits of FGF21. Could it do more than it already does?
First off, they genetically altered a group of mice so that they didn’t produce FGF21 on their own. Then they got them drunk. We’re going to assume they built a scale model of the bar from Cheers and had the mice filter in through the front door as they served their subjects beer out of tiny little glasses.
Once the mice were nice and liquored up, some were given a treatment of FGF21 while others were given a placebo. Lo and behold, the mice given FGF21 recovered about 50% faster than those that received the control treatment. Not exactly instant, but 50% is nothing to sniff at.
Before you bring your FGF21 supplement to the bar, though, this research only applies to mice. We don’t know if it works in people. And make sure you stick to booze. If your choice of intoxication is a bit more exotic, FGF21 isn’t going to do anything for you. Yes, the scientists tried. Yes, those mice are living a very interesting life. And yes, we are jealous of drugged-up lab mice.
Supersize your imagination, shrink your snacks
Have you ever heard of the meal-recall effect? Did you know that, in England, a biscuit is really a cookie? Did you also know that the magazine Bon Appétit is not the same as the peer-reviewed journal Appetite? We do … now.
The meal-recall effect is the subsequent reduction in snacking that comes from remembering a recent meal. It was used to great effect in a recent study conducted at the University of Cambridge, which is in England, where they feed their experimental humans cookies but, for some reason, call them biscuits.
For the first part of the study, the participants were invited to dine at Che Laboratory, where they “were given a microwave ready meal of rice and sauce and a cup of water,” according to a statement from the university. As our Uncle Ernie would say, “Gourmet all the way.”
The test subjects were instructed not to eat anything for 3 hours and “then invited back to the lab to perform imagination tasks.” Those who did come back were randomly divided into five different groups, each with a different task:
- Imagine moving their recent lunch at the lab around a plate.
- Recall eating their recent lunch in detail.
- Imagine that the lunch was twice as big and filling as it really was.
- Look at a photograph of spaghetti hoops in tomato sauce and write a description of it before imagining moving the food around a plate.
- Look at a photo of paper clips and rubber bands and imagine moving them around.
Now, at last, we get to the biscuits/cookies, which were the subject of a taste test that “was simply a rouse for covertly assessing snacking,” the investigators explained. As part of that test, participants were told they could eat as many biscuits as they wanted.
When the tables were cleared and the leftovers examined, the group that imagined spaghetti hoops had eaten the most biscuits (75.9 g), followed by the group that imagined paper clips (75.5 g), the moving-their-lunch-around-the-plate group (72.0 g), and the group that relived eating their lunch (70.0 g).
In a victory for the meal-recall effect, the people who imagined their meal being twice as big ate the fewest biscuits (51.1 g). “Your mind can be more powerful than your stomach in dictating how much you eat,” lead author Joanna Szypula, PhD, said in the university statement.
Oh! One more thing. The study appeared in Appetite, which is a peer-reviewed journal, not in Bon Appétit, which is not a peer-reviewed journal. Thanks to the fine folks at both publications for pointing that out to us.