User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Burnout and stress of today: How do we cope?
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Gout flares linked to transient jump in MI, stroke risk
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.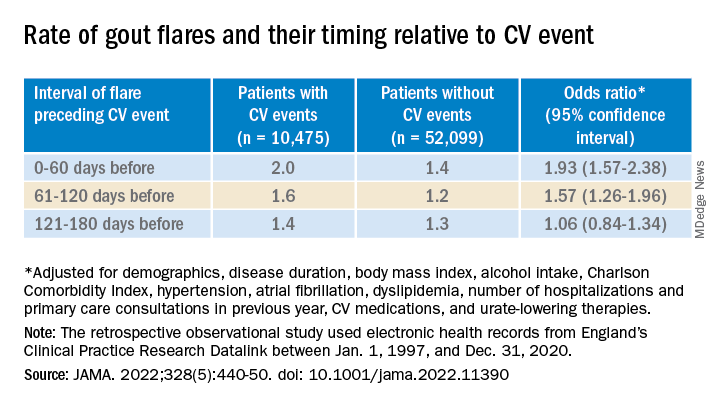
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
FROM JAMA
Is Lp(a) a marker for aortic calcium onset?
Lipoprotein(a) has long been thought to be a potential marker of aortic valve disease, and the population-based Rotterdam Study in the Netherlands has reported that Lp(a) has a strong association with new-onset aortic valve calcium (AVC), but not necessarily with progression of aortic valve disease.
Reporting in the European Heart Journal, the study authors analyzed data on 922 participants in the Rotterdam Study whose Lp(a) was measured along with a computed tomography scan upon enrollment, followed by CT scan 14 years later. At baseline, 702 participants didn’t have AVC, but the follow-up scan identified new-onset AVC in 415 (59.1%).
The investigators found an association between Lp(a) concentration and baseline AVC, with an odds ratio of 1.43 for each 50 mg/dL higher Lp(a) (95% confidence interval, 1.15-1.79), as well as new-onset AVC, with an OR of 1.30 for each 50 mg/dL increase in Lp(a) (95% CI, 1.02-1.65). However, the study found no association between rising Lp(a) levels and AVC progression; it found only an association between baseline AVC score and progression (P < .001).
‘Trigger’ for calcification but not progression
“This suggests that Lp(a) is an important trigger in the initiation of aortic valve calcification, but once the valve is calcified, disease progression may be primarily driven by other factors such as the baseline calcium burden of the valve and likely other unknown factors,” senior study author Daniel Bos, MD, PhD, said in e-mailed comments.
Dr. Bos and coauthors claim this is the first study to show that even minor AVC progresses independently of Lp(a).
“There are previous studies that showed a possible relationship between Lp(a) [and] progression of aortic valve calcium,” he said. “Our study suggests that the most meaningful benefit of Lp(a) lowering may actually be prior to the onset of aortic valve calcification.”
While no treatments have been approved for lowering Lp(a), the study findings could be meaningful if trials, including the ongoing phase 3 Lp(a) HORIZON trial of the investigational antisense agent pelacarsen (NCT04023552), show promising results, Dr. Bos said. Citing Lp(a) HORIZON, he said, “If the study shows Lp(a) lowering leads to a reduction in incident cardiovascular disease, similar strategies may be applied to prevent, rather than slow down, progression of aortic valve calcification.”
Dr. Bos called the Rotterdam Study results “an important first pointer into that direction.” He added, “We will need randomized trials to provide a definitive answer to the question whether Lp(a) lowering may prevent aortic valve calcium.”
Focus on AVC is study ‘weakness’
The study findings raise a key question for clinical trials of investigative Lp(a)-lowering therapies as well as how to use those therapies to treat aortic valve disease, said Christie Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston.
The findings could be “problematic” for these clinical trials, he said. “This study is just looking at calcium progression,” Dr. Ballantyne noted. “What we want to know about clinically is the progression to aortic stenosis, and then in particular to progression from mild disease to moderate or severe disease, because once you get into more severe disease, one has to do an intervention with either surgery or TAVR [transcatheter aortic valve replacement].”
He considered the study’s focus on AVC rather than aortic valve function a weakness and noted that only 14 study participants had TAVR. “We’re going to need much bigger numbers to look into this question of progression, including progression to severe diseases,” he said.
However, the Rotterdam Study showed the importance of CT in evaluating AVC, which can easily be done in other trials to further explore the association between Lp(a) and AVC, Dr. Ballantyne said.
Dr. Bos has no relevant disclosures. Study coauthors disclosed relationships with Amgen, Sanofi, Reservlogix, Athera, Experio, Novartis and Ionis Pharmaceuticals. Dr. Ballantyne disclosed relationships with Amgen and Novartis.
Lipoprotein(a) has long been thought to be a potential marker of aortic valve disease, and the population-based Rotterdam Study in the Netherlands has reported that Lp(a) has a strong association with new-onset aortic valve calcium (AVC), but not necessarily with progression of aortic valve disease.
Reporting in the European Heart Journal, the study authors analyzed data on 922 participants in the Rotterdam Study whose Lp(a) was measured along with a computed tomography scan upon enrollment, followed by CT scan 14 years later. At baseline, 702 participants didn’t have AVC, but the follow-up scan identified new-onset AVC in 415 (59.1%).
The investigators found an association between Lp(a) concentration and baseline AVC, with an odds ratio of 1.43 for each 50 mg/dL higher Lp(a) (95% confidence interval, 1.15-1.79), as well as new-onset AVC, with an OR of 1.30 for each 50 mg/dL increase in Lp(a) (95% CI, 1.02-1.65). However, the study found no association between rising Lp(a) levels and AVC progression; it found only an association between baseline AVC score and progression (P < .001).
‘Trigger’ for calcification but not progression
“This suggests that Lp(a) is an important trigger in the initiation of aortic valve calcification, but once the valve is calcified, disease progression may be primarily driven by other factors such as the baseline calcium burden of the valve and likely other unknown factors,” senior study author Daniel Bos, MD, PhD, said in e-mailed comments.
Dr. Bos and coauthors claim this is the first study to show that even minor AVC progresses independently of Lp(a).
“There are previous studies that showed a possible relationship between Lp(a) [and] progression of aortic valve calcium,” he said. “Our study suggests that the most meaningful benefit of Lp(a) lowering may actually be prior to the onset of aortic valve calcification.”
While no treatments have been approved for lowering Lp(a), the study findings could be meaningful if trials, including the ongoing phase 3 Lp(a) HORIZON trial of the investigational antisense agent pelacarsen (NCT04023552), show promising results, Dr. Bos said. Citing Lp(a) HORIZON, he said, “If the study shows Lp(a) lowering leads to a reduction in incident cardiovascular disease, similar strategies may be applied to prevent, rather than slow down, progression of aortic valve calcification.”
Dr. Bos called the Rotterdam Study results “an important first pointer into that direction.” He added, “We will need randomized trials to provide a definitive answer to the question whether Lp(a) lowering may prevent aortic valve calcium.”
Focus on AVC is study ‘weakness’
The study findings raise a key question for clinical trials of investigative Lp(a)-lowering therapies as well as how to use those therapies to treat aortic valve disease, said Christie Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston.
The findings could be “problematic” for these clinical trials, he said. “This study is just looking at calcium progression,” Dr. Ballantyne noted. “What we want to know about clinically is the progression to aortic stenosis, and then in particular to progression from mild disease to moderate or severe disease, because once you get into more severe disease, one has to do an intervention with either surgery or TAVR [transcatheter aortic valve replacement].”
He considered the study’s focus on AVC rather than aortic valve function a weakness and noted that only 14 study participants had TAVR. “We’re going to need much bigger numbers to look into this question of progression, including progression to severe diseases,” he said.
However, the Rotterdam Study showed the importance of CT in evaluating AVC, which can easily be done in other trials to further explore the association between Lp(a) and AVC, Dr. Ballantyne said.
Dr. Bos has no relevant disclosures. Study coauthors disclosed relationships with Amgen, Sanofi, Reservlogix, Athera, Experio, Novartis and Ionis Pharmaceuticals. Dr. Ballantyne disclosed relationships with Amgen and Novartis.
Lipoprotein(a) has long been thought to be a potential marker of aortic valve disease, and the population-based Rotterdam Study in the Netherlands has reported that Lp(a) has a strong association with new-onset aortic valve calcium (AVC), but not necessarily with progression of aortic valve disease.
Reporting in the European Heart Journal, the study authors analyzed data on 922 participants in the Rotterdam Study whose Lp(a) was measured along with a computed tomography scan upon enrollment, followed by CT scan 14 years later. At baseline, 702 participants didn’t have AVC, but the follow-up scan identified new-onset AVC in 415 (59.1%).
The investigators found an association between Lp(a) concentration and baseline AVC, with an odds ratio of 1.43 for each 50 mg/dL higher Lp(a) (95% confidence interval, 1.15-1.79), as well as new-onset AVC, with an OR of 1.30 for each 50 mg/dL increase in Lp(a) (95% CI, 1.02-1.65). However, the study found no association between rising Lp(a) levels and AVC progression; it found only an association between baseline AVC score and progression (P < .001).
‘Trigger’ for calcification but not progression
“This suggests that Lp(a) is an important trigger in the initiation of aortic valve calcification, but once the valve is calcified, disease progression may be primarily driven by other factors such as the baseline calcium burden of the valve and likely other unknown factors,” senior study author Daniel Bos, MD, PhD, said in e-mailed comments.
Dr. Bos and coauthors claim this is the first study to show that even minor AVC progresses independently of Lp(a).
“There are previous studies that showed a possible relationship between Lp(a) [and] progression of aortic valve calcium,” he said. “Our study suggests that the most meaningful benefit of Lp(a) lowering may actually be prior to the onset of aortic valve calcification.”
While no treatments have been approved for lowering Lp(a), the study findings could be meaningful if trials, including the ongoing phase 3 Lp(a) HORIZON trial of the investigational antisense agent pelacarsen (NCT04023552), show promising results, Dr. Bos said. Citing Lp(a) HORIZON, he said, “If the study shows Lp(a) lowering leads to a reduction in incident cardiovascular disease, similar strategies may be applied to prevent, rather than slow down, progression of aortic valve calcification.”
Dr. Bos called the Rotterdam Study results “an important first pointer into that direction.” He added, “We will need randomized trials to provide a definitive answer to the question whether Lp(a) lowering may prevent aortic valve calcium.”
Focus on AVC is study ‘weakness’
The study findings raise a key question for clinical trials of investigative Lp(a)-lowering therapies as well as how to use those therapies to treat aortic valve disease, said Christie Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston.
The findings could be “problematic” for these clinical trials, he said. “This study is just looking at calcium progression,” Dr. Ballantyne noted. “What we want to know about clinically is the progression to aortic stenosis, and then in particular to progression from mild disease to moderate or severe disease, because once you get into more severe disease, one has to do an intervention with either surgery or TAVR [transcatheter aortic valve replacement].”
He considered the study’s focus on AVC rather than aortic valve function a weakness and noted that only 14 study participants had TAVR. “We’re going to need much bigger numbers to look into this question of progression, including progression to severe diseases,” he said.
However, the Rotterdam Study showed the importance of CT in evaluating AVC, which can easily be done in other trials to further explore the association between Lp(a) and AVC, Dr. Ballantyne said.
Dr. Bos has no relevant disclosures. Study coauthors disclosed relationships with Amgen, Sanofi, Reservlogix, Athera, Experio, Novartis and Ionis Pharmaceuticals. Dr. Ballantyne disclosed relationships with Amgen and Novartis.
FROM THE EUROPEAN HEART JOURNAL
Six specialties attracting the highest private equity acquisitions
While tracking the extent of physician practice acquisition by private equity firms may be difficult, new research highlights what specialties and U.S. regions are most affected by such purchases.
The study, supported by the National Institute for Health Care Management (NIHCM), examined 97,094 physicians practicing in six specialties, 4,738 of whom worked in private equity–acquired practices. Of these specialties, 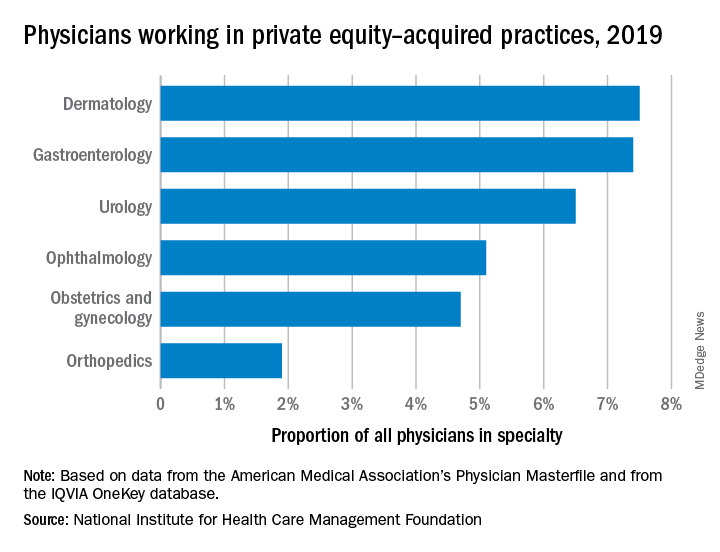
“These specialties offer private equity firms diverse revenue streams. You have a mix of commercially insured individuals with Medicare insurance and self-pay,” said Yashaswini Singh, MPA, a doctoral student at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and coauthor of the study, which was published in JAMA Health Forum as a research letter.
“In dermatology, you have a mix of surgical procedures that are covered under insurance, but also a lot of cosmetic procedures that are most likely to be self-pay procedures. This offers private equity several mechanisms to which they can increase their revenues.”
Ms. Singh’s coauthors were part of a previous study looking at private practice penetration by private equity firms. That research found such deals surged from 59 deals in 2013 representing 843 physicians, to 136 private equity acquisition deals representing 1,882 physicians in 2016.
The most recent study notes limited data and use of nondisclosure agreements during early negotiations as part of the difficulty in truly pinpointing private equity’s presence in health care. Monitoring private equity activity has become necessary across all industries, noted the authors of the study. If continued at this rate, long-term private equity acquisition has a multitude of potential pros and cons.
Ms. Singh explained that such specialties are highly fragmented and they allow for economies of scale and scope. In particular, an aging population increases demand for dermatology, ophthalmology, and gastroenterology services such as skin biopsies, cataracts, and colonoscopies. This makes these specialties very attractive to private equity firms. The same can be said for obstetrics and gynecology, as fertility clinics have attracted many private equity investments.
“This is another area where understanding changes to physician practice patterns and patient outcomes is critical as women continue to delay motherhood,” said Ms. Singh.
Reducing competition, increasing focus on patient care
Researchers found significant geographical trends for private equity penetration, as it varies across the country. It is highest in the Northeast, Florida, and Arizona in hospital referral regions. Researchers are still analyzing the cause of this occurrence.
Geographic concentration of private equity penetration likely reflects strategic selection of investment opportunities by private equity funds as the decision to invest in a practice does not happen at random, Ms. Singh noted.
Ms. Singh said she hopes that by documenting a variation and geographic concentration that the NIHCM is providing the first foundational step to tackle questions related to incentives and regulations that facilitate investment.
“Understanding the regulatory and economic environments that facilitate private equity activity is an interesting and important question to explore further,” she said in an interview. “This can include supply-side factors that can shape the business environment, e.g., taxation environment, regulatory burden to complete acquisitions, as well as demand-side factors that facilitate growth.”
Researchers found that continued growth of private equity penetration may lead to consolidation among independent practices facing financial pressures, as well as reduced competition and increased prices within each local health care market.
“Localized consolidation in certain markets has the potential for competition to reduce, [and] reduced competition has been shown in a variety of settings to be associated with increases in prices and reduced access for patients,” said Ms. Singh.
Conversely, Ms. Singh addressed several benefits of growing private equity presence. Companies can exploit their full potential through the addition of private equity expertise and contacts. Specifically, health care development of technological infrastructure is likely, along with reduced patient wait times and the expansion of business hours. It could also be a way for practices to offload administrative responsibilities and for physicians to focus more on the care delivery process.
A version of this article first appeared on Medscape.com.
While tracking the extent of physician practice acquisition by private equity firms may be difficult, new research highlights what specialties and U.S. regions are most affected by such purchases.
The study, supported by the National Institute for Health Care Management (NIHCM), examined 97,094 physicians practicing in six specialties, 4,738 of whom worked in private equity–acquired practices. Of these specialties, 
“These specialties offer private equity firms diverse revenue streams. You have a mix of commercially insured individuals with Medicare insurance and self-pay,” said Yashaswini Singh, MPA, a doctoral student at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and coauthor of the study, which was published in JAMA Health Forum as a research letter.
“In dermatology, you have a mix of surgical procedures that are covered under insurance, but also a lot of cosmetic procedures that are most likely to be self-pay procedures. This offers private equity several mechanisms to which they can increase their revenues.”
Ms. Singh’s coauthors were part of a previous study looking at private practice penetration by private equity firms. That research found such deals surged from 59 deals in 2013 representing 843 physicians, to 136 private equity acquisition deals representing 1,882 physicians in 2016.
The most recent study notes limited data and use of nondisclosure agreements during early negotiations as part of the difficulty in truly pinpointing private equity’s presence in health care. Monitoring private equity activity has become necessary across all industries, noted the authors of the study. If continued at this rate, long-term private equity acquisition has a multitude of potential pros and cons.
Ms. Singh explained that such specialties are highly fragmented and they allow for economies of scale and scope. In particular, an aging population increases demand for dermatology, ophthalmology, and gastroenterology services such as skin biopsies, cataracts, and colonoscopies. This makes these specialties very attractive to private equity firms. The same can be said for obstetrics and gynecology, as fertility clinics have attracted many private equity investments.
“This is another area where understanding changes to physician practice patterns and patient outcomes is critical as women continue to delay motherhood,” said Ms. Singh.
Reducing competition, increasing focus on patient care
Researchers found significant geographical trends for private equity penetration, as it varies across the country. It is highest in the Northeast, Florida, and Arizona in hospital referral regions. Researchers are still analyzing the cause of this occurrence.
Geographic concentration of private equity penetration likely reflects strategic selection of investment opportunities by private equity funds as the decision to invest in a practice does not happen at random, Ms. Singh noted.
Ms. Singh said she hopes that by documenting a variation and geographic concentration that the NIHCM is providing the first foundational step to tackle questions related to incentives and regulations that facilitate investment.
“Understanding the regulatory and economic environments that facilitate private equity activity is an interesting and important question to explore further,” she said in an interview. “This can include supply-side factors that can shape the business environment, e.g., taxation environment, regulatory burden to complete acquisitions, as well as demand-side factors that facilitate growth.”
Researchers found that continued growth of private equity penetration may lead to consolidation among independent practices facing financial pressures, as well as reduced competition and increased prices within each local health care market.
“Localized consolidation in certain markets has the potential for competition to reduce, [and] reduced competition has been shown in a variety of settings to be associated with increases in prices and reduced access for patients,” said Ms. Singh.
Conversely, Ms. Singh addressed several benefits of growing private equity presence. Companies can exploit their full potential through the addition of private equity expertise and contacts. Specifically, health care development of technological infrastructure is likely, along with reduced patient wait times and the expansion of business hours. It could also be a way for practices to offload administrative responsibilities and for physicians to focus more on the care delivery process.
A version of this article first appeared on Medscape.com.
While tracking the extent of physician practice acquisition by private equity firms may be difficult, new research highlights what specialties and U.S. regions are most affected by such purchases.
The study, supported by the National Institute for Health Care Management (NIHCM), examined 97,094 physicians practicing in six specialties, 4,738 of whom worked in private equity–acquired practices. Of these specialties, 
“These specialties offer private equity firms diverse revenue streams. You have a mix of commercially insured individuals with Medicare insurance and self-pay,” said Yashaswini Singh, MPA, a doctoral student at the Johns Hopkins Bloomberg School of Public Health, Baltimore, and coauthor of the study, which was published in JAMA Health Forum as a research letter.
“In dermatology, you have a mix of surgical procedures that are covered under insurance, but also a lot of cosmetic procedures that are most likely to be self-pay procedures. This offers private equity several mechanisms to which they can increase their revenues.”
Ms. Singh’s coauthors were part of a previous study looking at private practice penetration by private equity firms. That research found such deals surged from 59 deals in 2013 representing 843 physicians, to 136 private equity acquisition deals representing 1,882 physicians in 2016.
The most recent study notes limited data and use of nondisclosure agreements during early negotiations as part of the difficulty in truly pinpointing private equity’s presence in health care. Monitoring private equity activity has become necessary across all industries, noted the authors of the study. If continued at this rate, long-term private equity acquisition has a multitude of potential pros and cons.
Ms. Singh explained that such specialties are highly fragmented and they allow for economies of scale and scope. In particular, an aging population increases demand for dermatology, ophthalmology, and gastroenterology services such as skin biopsies, cataracts, and colonoscopies. This makes these specialties very attractive to private equity firms. The same can be said for obstetrics and gynecology, as fertility clinics have attracted many private equity investments.
“This is another area where understanding changes to physician practice patterns and patient outcomes is critical as women continue to delay motherhood,” said Ms. Singh.
Reducing competition, increasing focus on patient care
Researchers found significant geographical trends for private equity penetration, as it varies across the country. It is highest in the Northeast, Florida, and Arizona in hospital referral regions. Researchers are still analyzing the cause of this occurrence.
Geographic concentration of private equity penetration likely reflects strategic selection of investment opportunities by private equity funds as the decision to invest in a practice does not happen at random, Ms. Singh noted.
Ms. Singh said she hopes that by documenting a variation and geographic concentration that the NIHCM is providing the first foundational step to tackle questions related to incentives and regulations that facilitate investment.
“Understanding the regulatory and economic environments that facilitate private equity activity is an interesting and important question to explore further,” she said in an interview. “This can include supply-side factors that can shape the business environment, e.g., taxation environment, regulatory burden to complete acquisitions, as well as demand-side factors that facilitate growth.”
Researchers found that continued growth of private equity penetration may lead to consolidation among independent practices facing financial pressures, as well as reduced competition and increased prices within each local health care market.
“Localized consolidation in certain markets has the potential for competition to reduce, [and] reduced competition has been shown in a variety of settings to be associated with increases in prices and reduced access for patients,” said Ms. Singh.
Conversely, Ms. Singh addressed several benefits of growing private equity presence. Companies can exploit their full potential through the addition of private equity expertise and contacts. Specifically, health care development of technological infrastructure is likely, along with reduced patient wait times and the expansion of business hours. It could also be a way for practices to offload administrative responsibilities and for physicians to focus more on the care delivery process.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
Ezetimibe plus statin: Attractive bypass to high-dose monotherapy
More patients with established atherosclerotic cardiovascular disease (ASCVD) achieved a low-density lipoprotein (LDL) cholesterol of less than 70 mg/dL, and fewer discontinued treatment with ezetimibe plus a moderate-dose statin, than did those on high-intensity statin monotherapy, a noninferiority trial shows.
While it’s now established that drug combinations can achieve better efficacy with lower risks than high-dose monotherapy, the study is the first to show the benefits of the strategy for ASCVD in a randomized trial with long-term follow-up.
The primary endpoint – 3-year composite of cardiovascular death, major cardiovascular events, or nonfatal stroke – occurred in about 9% of patients in each group, showing non-inferiority.
Furthermore, the authors suggest that ezetimibe combination therapy be considered earlier in the treatment of those at high risk of adverse events, rather than doubling the statin dose.
The study was published online in The Lancet.
Less intolerance, less discontinuations
The open-label study, dubbed RACING, randomized 3,780 patients with ASCVD to receive moderate-intensity rosuvastatin 10 mg plus ezetimibe 10 mg or high-intensity 20 mg rosuvastatin monotherapy. Participants’ average age was 64 and 75% were men.
The primary endpoint occurred in 9.1% of patients in the combination therapy group and 9.9% in the high-intensity monotherapy group. The absolute between-group difference was −0.78% (90% confidence interval [CI], −2.39 to 0.83), well below the 2% noninferiority margin.
In the combination therapy group, LDL cholesterol concentrations of less than 70 mg/dL were achieved in 73% of patients at 1 year, 75% at 2 years, and 72% at 3 years. By contrast, in the monotherapy group, the lower concentrations were seen in 55% at 1 year, 60% at 2 years, and 58% at 3 years.
Further, a post hoc analysis showed LDL concentrations of less than 55 mg/dL at 1, 2, and 3 years in 42%, 45%, and 42% of patients in the combination therapy group versus 25%, 29%, and 25% of those in the high-intensity statin monotherapy group.
Eighty-eight patients (4.8%) on combination therapy discontinued medication or received a dose reduction, versus 150 patients (8.2%) on monotherapy.
Rates of myonecrosis were similar in the combination therapy and high-intensity statin groups (11 vs. 13), whereas myalgia was more common with high-intensity statins (29 vs. 17). The open-label design could have led to bias in reporting of patient symptoms, the authors noted. All clinical events, however, were adjudicated by an independent committee masked to treatment assignment.
There might be “some level of difference” when extending the findings to other populations because the trial involved only Koreans, coauthor Myeong-Ki Hong, MD, Yonsei University, Seoul, South Korea, acknowledged in response to a query from this news organization. He thinks the findings can be applied broadly nonetheless, and his team is currently investigating whether certain patients might benefit more than others from the combination.
Various options for patients
“The field of hypertension changed [its] guidelines almost 20 years ago to consider the initial use of combination therapy in hard-to-treat patients,” Christie Mitchell Ballantyne, MD, Baylor College of Medicine, Houston, said in an interview. He coauthored an accompanying editorial with Baylor colleague Layla A. Abushamat, MD.
“We now have enough evidence of the efficacy and safety of combination therapy to consider early initiation of this approach in patients with challenging lipid disorders who are at increased risk of ASCVD events,” affirmed Dr. Ballantyne.
“This study reinforces important principles in the management and secondary prevention of cardiovascular disease, namely that LDL reduction and associated risk reduction can be achieved in various ways,” said Daniel Muñoz, MD, MPA, executive medical director of the Vanderbilt Heart & Vascular Institute, Vanderbilt University Medical Center, Nashville, Tenn.
However, he noted, “The high-intensity statin dose used as a comparator in this study was rosuvastatin 20 mg. In clinical practice, we often target maximally aggressive reduction of LDL via higher doses – that is, rosuvastatin 40 mg or atorvastatin 80 mg.”
The bottom line, said Dr. Muñoz, who was not involved in the study: “There are different ways to achieve LDL-lowering and associated risk reduction in patients with CVD. For patients who warrant but might not tolerate high-intensity statin therapy, this study supports the use of a moderate-intensity statin in combination with ezetimibe.”
The study was funded by Hanmi Pharmaceutical, Seoul, South Korea. One study coauthor received an institutional research grant from the company. No other authors reported relevant financial relationships, nor did Dr. Ballantyne, Dr. Abushamat, or Dr. Muñoz.
A version of this article first appeared on Medscape.com.
More patients with established atherosclerotic cardiovascular disease (ASCVD) achieved a low-density lipoprotein (LDL) cholesterol of less than 70 mg/dL, and fewer discontinued treatment with ezetimibe plus a moderate-dose statin, than did those on high-intensity statin monotherapy, a noninferiority trial shows.
While it’s now established that drug combinations can achieve better efficacy with lower risks than high-dose monotherapy, the study is the first to show the benefits of the strategy for ASCVD in a randomized trial with long-term follow-up.
The primary endpoint – 3-year composite of cardiovascular death, major cardiovascular events, or nonfatal stroke – occurred in about 9% of patients in each group, showing non-inferiority.
Furthermore, the authors suggest that ezetimibe combination therapy be considered earlier in the treatment of those at high risk of adverse events, rather than doubling the statin dose.
The study was published online in The Lancet.
Less intolerance, less discontinuations
The open-label study, dubbed RACING, randomized 3,780 patients with ASCVD to receive moderate-intensity rosuvastatin 10 mg plus ezetimibe 10 mg or high-intensity 20 mg rosuvastatin monotherapy. Participants’ average age was 64 and 75% were men.
The primary endpoint occurred in 9.1% of patients in the combination therapy group and 9.9% in the high-intensity monotherapy group. The absolute between-group difference was −0.78% (90% confidence interval [CI], −2.39 to 0.83), well below the 2% noninferiority margin.
In the combination therapy group, LDL cholesterol concentrations of less than 70 mg/dL were achieved in 73% of patients at 1 year, 75% at 2 years, and 72% at 3 years. By contrast, in the monotherapy group, the lower concentrations were seen in 55% at 1 year, 60% at 2 years, and 58% at 3 years.
Further, a post hoc analysis showed LDL concentrations of less than 55 mg/dL at 1, 2, and 3 years in 42%, 45%, and 42% of patients in the combination therapy group versus 25%, 29%, and 25% of those in the high-intensity statin monotherapy group.
Eighty-eight patients (4.8%) on combination therapy discontinued medication or received a dose reduction, versus 150 patients (8.2%) on monotherapy.
Rates of myonecrosis were similar in the combination therapy and high-intensity statin groups (11 vs. 13), whereas myalgia was more common with high-intensity statins (29 vs. 17). The open-label design could have led to bias in reporting of patient symptoms, the authors noted. All clinical events, however, were adjudicated by an independent committee masked to treatment assignment.
There might be “some level of difference” when extending the findings to other populations because the trial involved only Koreans, coauthor Myeong-Ki Hong, MD, Yonsei University, Seoul, South Korea, acknowledged in response to a query from this news organization. He thinks the findings can be applied broadly nonetheless, and his team is currently investigating whether certain patients might benefit more than others from the combination.
Various options for patients
“The field of hypertension changed [its] guidelines almost 20 years ago to consider the initial use of combination therapy in hard-to-treat patients,” Christie Mitchell Ballantyne, MD, Baylor College of Medicine, Houston, said in an interview. He coauthored an accompanying editorial with Baylor colleague Layla A. Abushamat, MD.
“We now have enough evidence of the efficacy and safety of combination therapy to consider early initiation of this approach in patients with challenging lipid disorders who are at increased risk of ASCVD events,” affirmed Dr. Ballantyne.
“This study reinforces important principles in the management and secondary prevention of cardiovascular disease, namely that LDL reduction and associated risk reduction can be achieved in various ways,” said Daniel Muñoz, MD, MPA, executive medical director of the Vanderbilt Heart & Vascular Institute, Vanderbilt University Medical Center, Nashville, Tenn.
However, he noted, “The high-intensity statin dose used as a comparator in this study was rosuvastatin 20 mg. In clinical practice, we often target maximally aggressive reduction of LDL via higher doses – that is, rosuvastatin 40 mg or atorvastatin 80 mg.”
The bottom line, said Dr. Muñoz, who was not involved in the study: “There are different ways to achieve LDL-lowering and associated risk reduction in patients with CVD. For patients who warrant but might not tolerate high-intensity statin therapy, this study supports the use of a moderate-intensity statin in combination with ezetimibe.”
The study was funded by Hanmi Pharmaceutical, Seoul, South Korea. One study coauthor received an institutional research grant from the company. No other authors reported relevant financial relationships, nor did Dr. Ballantyne, Dr. Abushamat, or Dr. Muñoz.
A version of this article first appeared on Medscape.com.
More patients with established atherosclerotic cardiovascular disease (ASCVD) achieved a low-density lipoprotein (LDL) cholesterol of less than 70 mg/dL, and fewer discontinued treatment with ezetimibe plus a moderate-dose statin, than did those on high-intensity statin monotherapy, a noninferiority trial shows.
While it’s now established that drug combinations can achieve better efficacy with lower risks than high-dose monotherapy, the study is the first to show the benefits of the strategy for ASCVD in a randomized trial with long-term follow-up.
The primary endpoint – 3-year composite of cardiovascular death, major cardiovascular events, or nonfatal stroke – occurred in about 9% of patients in each group, showing non-inferiority.
Furthermore, the authors suggest that ezetimibe combination therapy be considered earlier in the treatment of those at high risk of adverse events, rather than doubling the statin dose.
The study was published online in The Lancet.
Less intolerance, less discontinuations
The open-label study, dubbed RACING, randomized 3,780 patients with ASCVD to receive moderate-intensity rosuvastatin 10 mg plus ezetimibe 10 mg or high-intensity 20 mg rosuvastatin monotherapy. Participants’ average age was 64 and 75% were men.
The primary endpoint occurred in 9.1% of patients in the combination therapy group and 9.9% in the high-intensity monotherapy group. The absolute between-group difference was −0.78% (90% confidence interval [CI], −2.39 to 0.83), well below the 2% noninferiority margin.
In the combination therapy group, LDL cholesterol concentrations of less than 70 mg/dL were achieved in 73% of patients at 1 year, 75% at 2 years, and 72% at 3 years. By contrast, in the monotherapy group, the lower concentrations were seen in 55% at 1 year, 60% at 2 years, and 58% at 3 years.
Further, a post hoc analysis showed LDL concentrations of less than 55 mg/dL at 1, 2, and 3 years in 42%, 45%, and 42% of patients in the combination therapy group versus 25%, 29%, and 25% of those in the high-intensity statin monotherapy group.
Eighty-eight patients (4.8%) on combination therapy discontinued medication or received a dose reduction, versus 150 patients (8.2%) on monotherapy.
Rates of myonecrosis were similar in the combination therapy and high-intensity statin groups (11 vs. 13), whereas myalgia was more common with high-intensity statins (29 vs. 17). The open-label design could have led to bias in reporting of patient symptoms, the authors noted. All clinical events, however, were adjudicated by an independent committee masked to treatment assignment.
There might be “some level of difference” when extending the findings to other populations because the trial involved only Koreans, coauthor Myeong-Ki Hong, MD, Yonsei University, Seoul, South Korea, acknowledged in response to a query from this news organization. He thinks the findings can be applied broadly nonetheless, and his team is currently investigating whether certain patients might benefit more than others from the combination.
Various options for patients
“The field of hypertension changed [its] guidelines almost 20 years ago to consider the initial use of combination therapy in hard-to-treat patients,” Christie Mitchell Ballantyne, MD, Baylor College of Medicine, Houston, said in an interview. He coauthored an accompanying editorial with Baylor colleague Layla A. Abushamat, MD.
“We now have enough evidence of the efficacy and safety of combination therapy to consider early initiation of this approach in patients with challenging lipid disorders who are at increased risk of ASCVD events,” affirmed Dr. Ballantyne.
“This study reinforces important principles in the management and secondary prevention of cardiovascular disease, namely that LDL reduction and associated risk reduction can be achieved in various ways,” said Daniel Muñoz, MD, MPA, executive medical director of the Vanderbilt Heart & Vascular Institute, Vanderbilt University Medical Center, Nashville, Tenn.
However, he noted, “The high-intensity statin dose used as a comparator in this study was rosuvastatin 20 mg. In clinical practice, we often target maximally aggressive reduction of LDL via higher doses – that is, rosuvastatin 40 mg or atorvastatin 80 mg.”
The bottom line, said Dr. Muñoz, who was not involved in the study: “There are different ways to achieve LDL-lowering and associated risk reduction in patients with CVD. For patients who warrant but might not tolerate high-intensity statin therapy, this study supports the use of a moderate-intensity statin in combination with ezetimibe.”
The study was funded by Hanmi Pharmaceutical, Seoul, South Korea. One study coauthor received an institutional research grant from the company. No other authors reported relevant financial relationships, nor did Dr. Ballantyne, Dr. Abushamat, or Dr. Muñoz.
A version of this article first appeared on Medscape.com.
Do ICDs still ‘work’ in primary prevention given today’s recommended HF meds?
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
FROM JACC CLINICAL ELECTROPHYSIOLOGY
For patients with peripheral artery disease, pain can be gain
For people with peripheral artery disease (PAD), even short walks can be exercises in excruciation.
But a new study published in the Journal of the American Heart Association has found that patients who can push through the pain appear to reap significant benefits in ambulation, balance, and leg strength, which have been linked to increased longevity.
“You have to push yourself and get those uncomfortable symptoms, or else you probably won’t get gains,” said Mary McDermott, MD, professor of medicine at Northwestern University, Chicago, and the senior author of the study.
Walking for exercise is critical for people with lower-extremity PAD, Dr. McDermott said, but leg pain dissuades many people with the condition from doing so. She said her group hopes that showing the payoff of the “no pain, no gain” approach gives people with PAD the resolve to walk regularly, even when it’s hard.
The new study, a post hoc analysis of the LITE (Low-Intensity Exercise Intervention in PAD) trial, found that low-intensity exercise did not improve the symptoms of PAD but high-intensity exercise did.
Dr. McDermott and her colleagues compared 109 people with PAD who walked fast enough to cause discomfort versus 101 people who walked at a comfortable pace and 54 people who did not exercise at all. The average age was 69 years, 48% of participants were women, and 61% were Black.
Everyone in the exercise groups walked at home, with visits to a medical center early in the study to get exercise tips and then phone support from exercise coaches throughout the remainder of the study. Researchers encouraged those in the discomfort group to walk fast enough to cause significant pain in their legs, for up to 10 minutes or as long as they could. They then rested before walking again, ideally up to five times per day for 5 days per week.
At 6 months, people in the discomfort group were walking 0.056 m/sec faster than those in the comfort group during a 4-meter walking test (95% confidence interval [CI], 0.19-0.094 m/sec; P < .01), a gap that had grown by 12 months to 0.084 m/sec (95% CI, 0.049-0.120 m/sec; P <.01), according to the researchers. A statistically significant gap also emerged between the discomfort and nonexercising group at 6 months, but it eventually closed.
“It’s a question that people have asked for some time: Is it necessary to get that ischemic pain when you walk?” Dr. McDermott said. “This is the first well-powered clinical trial to provide a definitive answer on that, and the answer is that you do need that discomfort. It wasn’t even close.” Indeed, Dr. McDermott said, it’s possible that walking merely to the point of comfort and never pushing beyond it may harm people with PAD.
At the 6-month mark, the researchers found no statistical difference between the discomfort and comfort groups on a cumulative scale of usual walking speed, ability to rise from a chair, and ability to maintain balance in several positions. By 12 months, the two groups had diverged, with the discomfort group improving by almost 1 point on the scale, whereas the performance of the comfort group declined. No significant differences emerged between the discomfort and nonexercising groups, the researchers reported.
The investigators found, counterintuitively, that some people in the study who did not record exercising did as well as those in the discomfort group,
Dr. McDermott noted that the nonexercise group was smaller than the discomfort group, making firm comparisons between the two challenging to draw. In addition, people whose exercise was not recorded were not asked to take it easy whenever they walked, unlike those in the comfort group. As a result, she said, some people in this group may have walked vigorously.
Dr. McDermott emphasized that these benefits occurred at home rather than at medical centers that can be difficult for some people to visit regularly.
“It’s always good to have this kind of information for patients, to show them that it’s possible for them to continue to improve,” said Jonathan Ehrman, PhD, associate director of preventive cardiology at Henry Ford Medical Center, Detroit. Dr. Ehrman was not involved in this study but said that he is contemplating running a similar home-based study that would use video rather than telephone support for patients.
“There’s emerging data about walking speed being related to longevity and predicting better outcomes in cardiac surgeries,” Dr. Ehrman said. “It seems to be, if you can get people walking faster or they have a better walking pace, related to better health outcomes.”
Dr. McDermott reported relationships with Regeneron, Helixmith, Mars, ArtAssist, ReserveAge, and Hershey. Dr. Ehrman reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
For people with peripheral artery disease (PAD), even short walks can be exercises in excruciation.
But a new study published in the Journal of the American Heart Association has found that patients who can push through the pain appear to reap significant benefits in ambulation, balance, and leg strength, which have been linked to increased longevity.
“You have to push yourself and get those uncomfortable symptoms, or else you probably won’t get gains,” said Mary McDermott, MD, professor of medicine at Northwestern University, Chicago, and the senior author of the study.
Walking for exercise is critical for people with lower-extremity PAD, Dr. McDermott said, but leg pain dissuades many people with the condition from doing so. She said her group hopes that showing the payoff of the “no pain, no gain” approach gives people with PAD the resolve to walk regularly, even when it’s hard.
The new study, a post hoc analysis of the LITE (Low-Intensity Exercise Intervention in PAD) trial, found that low-intensity exercise did not improve the symptoms of PAD but high-intensity exercise did.
Dr. McDermott and her colleagues compared 109 people with PAD who walked fast enough to cause discomfort versus 101 people who walked at a comfortable pace and 54 people who did not exercise at all. The average age was 69 years, 48% of participants were women, and 61% were Black.
Everyone in the exercise groups walked at home, with visits to a medical center early in the study to get exercise tips and then phone support from exercise coaches throughout the remainder of the study. Researchers encouraged those in the discomfort group to walk fast enough to cause significant pain in their legs, for up to 10 minutes or as long as they could. They then rested before walking again, ideally up to five times per day for 5 days per week.
At 6 months, people in the discomfort group were walking 0.056 m/sec faster than those in the comfort group during a 4-meter walking test (95% confidence interval [CI], 0.19-0.094 m/sec; P < .01), a gap that had grown by 12 months to 0.084 m/sec (95% CI, 0.049-0.120 m/sec; P <.01), according to the researchers. A statistically significant gap also emerged between the discomfort and nonexercising group at 6 months, but it eventually closed.
“It’s a question that people have asked for some time: Is it necessary to get that ischemic pain when you walk?” Dr. McDermott said. “This is the first well-powered clinical trial to provide a definitive answer on that, and the answer is that you do need that discomfort. It wasn’t even close.” Indeed, Dr. McDermott said, it’s possible that walking merely to the point of comfort and never pushing beyond it may harm people with PAD.
At the 6-month mark, the researchers found no statistical difference between the discomfort and comfort groups on a cumulative scale of usual walking speed, ability to rise from a chair, and ability to maintain balance in several positions. By 12 months, the two groups had diverged, with the discomfort group improving by almost 1 point on the scale, whereas the performance of the comfort group declined. No significant differences emerged between the discomfort and nonexercising groups, the researchers reported.
The investigators found, counterintuitively, that some people in the study who did not record exercising did as well as those in the discomfort group,
Dr. McDermott noted that the nonexercise group was smaller than the discomfort group, making firm comparisons between the two challenging to draw. In addition, people whose exercise was not recorded were not asked to take it easy whenever they walked, unlike those in the comfort group. As a result, she said, some people in this group may have walked vigorously.
Dr. McDermott emphasized that these benefits occurred at home rather than at medical centers that can be difficult for some people to visit regularly.
“It’s always good to have this kind of information for patients, to show them that it’s possible for them to continue to improve,” said Jonathan Ehrman, PhD, associate director of preventive cardiology at Henry Ford Medical Center, Detroit. Dr. Ehrman was not involved in this study but said that he is contemplating running a similar home-based study that would use video rather than telephone support for patients.
“There’s emerging data about walking speed being related to longevity and predicting better outcomes in cardiac surgeries,” Dr. Ehrman said. “It seems to be, if you can get people walking faster or they have a better walking pace, related to better health outcomes.”
Dr. McDermott reported relationships with Regeneron, Helixmith, Mars, ArtAssist, ReserveAge, and Hershey. Dr. Ehrman reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
For people with peripheral artery disease (PAD), even short walks can be exercises in excruciation.
But a new study published in the Journal of the American Heart Association has found that patients who can push through the pain appear to reap significant benefits in ambulation, balance, and leg strength, which have been linked to increased longevity.
“You have to push yourself and get those uncomfortable symptoms, or else you probably won’t get gains,” said Mary McDermott, MD, professor of medicine at Northwestern University, Chicago, and the senior author of the study.
Walking for exercise is critical for people with lower-extremity PAD, Dr. McDermott said, but leg pain dissuades many people with the condition from doing so. She said her group hopes that showing the payoff of the “no pain, no gain” approach gives people with PAD the resolve to walk regularly, even when it’s hard.
The new study, a post hoc analysis of the LITE (Low-Intensity Exercise Intervention in PAD) trial, found that low-intensity exercise did not improve the symptoms of PAD but high-intensity exercise did.
Dr. McDermott and her colleagues compared 109 people with PAD who walked fast enough to cause discomfort versus 101 people who walked at a comfortable pace and 54 people who did not exercise at all. The average age was 69 years, 48% of participants were women, and 61% were Black.
Everyone in the exercise groups walked at home, with visits to a medical center early in the study to get exercise tips and then phone support from exercise coaches throughout the remainder of the study. Researchers encouraged those in the discomfort group to walk fast enough to cause significant pain in their legs, for up to 10 minutes or as long as they could. They then rested before walking again, ideally up to five times per day for 5 days per week.
At 6 months, people in the discomfort group were walking 0.056 m/sec faster than those in the comfort group during a 4-meter walking test (95% confidence interval [CI], 0.19-0.094 m/sec; P < .01), a gap that had grown by 12 months to 0.084 m/sec (95% CI, 0.049-0.120 m/sec; P <.01), according to the researchers. A statistically significant gap also emerged between the discomfort and nonexercising group at 6 months, but it eventually closed.
“It’s a question that people have asked for some time: Is it necessary to get that ischemic pain when you walk?” Dr. McDermott said. “This is the first well-powered clinical trial to provide a definitive answer on that, and the answer is that you do need that discomfort. It wasn’t even close.” Indeed, Dr. McDermott said, it’s possible that walking merely to the point of comfort and never pushing beyond it may harm people with PAD.
At the 6-month mark, the researchers found no statistical difference between the discomfort and comfort groups on a cumulative scale of usual walking speed, ability to rise from a chair, and ability to maintain balance in several positions. By 12 months, the two groups had diverged, with the discomfort group improving by almost 1 point on the scale, whereas the performance of the comfort group declined. No significant differences emerged between the discomfort and nonexercising groups, the researchers reported.
The investigators found, counterintuitively, that some people in the study who did not record exercising did as well as those in the discomfort group,
Dr. McDermott noted that the nonexercise group was smaller than the discomfort group, making firm comparisons between the two challenging to draw. In addition, people whose exercise was not recorded were not asked to take it easy whenever they walked, unlike those in the comfort group. As a result, she said, some people in this group may have walked vigorously.
Dr. McDermott emphasized that these benefits occurred at home rather than at medical centers that can be difficult for some people to visit regularly.
“It’s always good to have this kind of information for patients, to show them that it’s possible for them to continue to improve,” said Jonathan Ehrman, PhD, associate director of preventive cardiology at Henry Ford Medical Center, Detroit. Dr. Ehrman was not involved in this study but said that he is contemplating running a similar home-based study that would use video rather than telephone support for patients.
“There’s emerging data about walking speed being related to longevity and predicting better outcomes in cardiac surgeries,” Dr. Ehrman said. “It seems to be, if you can get people walking faster or they have a better walking pace, related to better health outcomes.”
Dr. McDermott reported relationships with Regeneron, Helixmith, Mars, ArtAssist, ReserveAge, and Hershey. Dr. Ehrman reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
‘Striking’ disparities in CVD deaths persist across COVID waves
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MAYO CLINIC PROCEEDINGS
Remnant cholesterol captures residual CV risk in patients with T2D
Adding to a growing body of evidence that elevated remnant cholesterol (remnant-C) provides additional and independent risk prediction for major cardiovascular events (MACE), a new analysis has this shown this biomarker has prognostic value specifically in patients with type 2 diabetes (T2D).
In a post hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, each standard-deviation increase in remnant-C was associated with a 7% increased risk in MACE (P = .004) after adjustment for several risk factors including other cholesterol values.
“In type 2 diabetes, remnant-C levels are associated with MACE regardless of LDL-C,” reported a team of investigators led by Liyao Fu, MD, Second Xiangya Hospital of Central South University, Changsha, China .
Remnant-C is one component of triglyceride-rich lipoproteins. Within triglyceride-rich lipoproteins, remnant-C has become a major focus of efforts to explain cardiovascular (CV) residual risk, according to the investigators.
Residual risk is a term used to explain why cardiovascular events occur after all known modifiable factors, such as LDL cholesterol (LDL-C), are controlled.
“Our primary findings indicate that baseline estimated remnant-C levels were associated with MACE regardless of clinical phenotypes, lifestyle confounders relative to CV risk, and lipid-lowering treatment,” said the authors of the analysis.
In the post hoc analysis of the ACCORD trial, which evaluated the effects of intensive glucose lowering in T2D more than 10 years ago, there were data on remnant-C over a median of 8.8 years of follow-up in 9,650 T2D patients. Over this period, 1,815 (17.8%) developed MACE.
Multiple analyses support prognostic value of remnant-C
In addition to the 7% rise in MACE for each standard-deviation increase in remnant-C when calculated as a continuous variable, other analyses told the same story.
This included an assessment by remnant-C tertiles. Not only was there a significant trend (P < .001) for greater risk with each higher baseline tertile of remnant-C, those in the highest tertile had a 38% greater risk of MACE relative to those in the lowest tertile (hazard ratio, 1.38; P < .001) after adjustment for confounders.
The same pattern was seen for several components of MACE, such as CV death and nonfatal myocardial infarction, when remnant-C tertiles were compared.
Visit-to-visit variability in remnant-C over the course of follow-up was also associated with greater risk of MACE. In logarithmic calculations, the risk of MACE climbed about 40% across all three models of risk adjustment. These models included adjustments for different sets of confounders, such as sex, age, blood pressure, CV disease history, and glucose levels. On an unadjusted basis, the risk was increased about 50% (HR, 1.52; P < .001).
For visit-to-visit variability in remnant-C, the greatest effect was on risk of nonfatal MI across models. In model 3, for example, which adjusted for the most confounders, the risk was nearly doubled (HR, 1.92; P < .001). In contrast, there did not appear to be a link between visit-to-visit variability and nonfatal stroke.
In a discordant analysis that was conducted to examine the relative risk of remnant-C independent of LDL-C, those who had a remnant-C level of at least 31 mg/dL were found to have a higher risk of MACE regardless of LDL-C level. Yet, the risk was higher if both remnant-C and LDL-C were elevated. For example, the risk was increased 22% for those with LDL-C at or below 100 mg/dL and remnant-C levels of at least 31 mg/dL (HR, 1.22; P = .015) but climbed to 37% for those with LDL-C above 100 mg/dL if remnant-C was at least 31 mg/dL (HR, 1.38; P = .007).
Remnant-C shows prognostic value in other risk groups
Although this study suggests an important prognostic value for remnant-C in T2D, there are numerous studies suggesting that it has prognostic value in other risk groups, such as those with a history of CV disease. This includes a study published earlier this year with 10 years of follow-up in 41,928 patients in Denmark. When combined with other risk factors, remnant-C substantially improved the accuracy of risk of events over time.
The investigators from this previous study, like the new study in patients with T2D, predict that remnant-C will be eventually included in guidelines.
According to Shi Tai, MD, a coauthor of the T2D study, remnant-C “may allow for the development of specific preventive and therapeutic approaches” to CV risk in patients with T2D.
T2D patients “with elevated plasma remnant-C levels represent a special population that deserves more attention regarding residual risk,” said Dr. Tai of the department of cardiovascular medicine at the Hospital of South Central China.
Great interest, but ready for guidelines?
This is an important direction of ongoing research, according to Christie M. Ballantyne, MD, professor of medicine, Baylor College of Medicine, Houston.
“There is a great deal of interest from both clinicians and trialists to find a simple way to identify patients with high residual risk who are on statin therapy,” he said. He thinks remnant-C has promise in this regard.
“Remnant-C is not in current guidelines,” he said in an interview, but he suggested that there is now a substantial body of evidence to suggest that it might be added if validated in further studies.
“Remnant-C is easy to calculate and may be helpful in practice now to identify patients who need more aggressive therapy to reduce risk and may be useful to identify patients for clinical trials who will benefit from new therapies that are in development,” he said.
However, the clinical relevance of therapies addressed at triglyceride-rich lipoproteins in general or their components, including triglycerides or remnant-C, has never been demonstrated, pointed out Peter W.F. Wilson, MD, PhD.
“Higher fasting or nonfasting triglyceride levels or their surrogates have been shown to be associated with increased risk for cardiovascular disease events in observational studies, but the importance of such measurements in persons already treated with very aggressive LDL-C lowering therapy is not known,” commented Dr. Wilson, director of epidemiology and genomic medicine, Emory School of Medicine, Atlanta.
Dr. Wilson was the coauthor of an editorial that accompanied the previously published Danish study of remnant-C. In his editorial, he suggested that remnant-C has promise for better understanding residual risk, but when contacted about these latest data he emphasized a lack of support so far for clinical relevance.
“Unfortunately, clinical trials have generally not shown that triglyceride lowering [to favorably alter remnant-C] in this situation favorably affects the risk of CV disease events,” he said in an interview. This does not preclude remnant-C as a targetable risk factor, but these data are needed.
Dr. Fu, Dr. Tai, and Dr. Wilson report no potential conflicts of interest. Dr. Ballantyne has financial relationships with more than 25 pharmaceutical companies, including several that produce products employed for the treatment of lipid abnormalities.
Adding to a growing body of evidence that elevated remnant cholesterol (remnant-C) provides additional and independent risk prediction for major cardiovascular events (MACE), a new analysis has this shown this biomarker has prognostic value specifically in patients with type 2 diabetes (T2D).
In a post hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, each standard-deviation increase in remnant-C was associated with a 7% increased risk in MACE (P = .004) after adjustment for several risk factors including other cholesterol values.
“In type 2 diabetes, remnant-C levels are associated with MACE regardless of LDL-C,” reported a team of investigators led by Liyao Fu, MD, Second Xiangya Hospital of Central South University, Changsha, China .
Remnant-C is one component of triglyceride-rich lipoproteins. Within triglyceride-rich lipoproteins, remnant-C has become a major focus of efforts to explain cardiovascular (CV) residual risk, according to the investigators.
Residual risk is a term used to explain why cardiovascular events occur after all known modifiable factors, such as LDL cholesterol (LDL-C), are controlled.
“Our primary findings indicate that baseline estimated remnant-C levels were associated with MACE regardless of clinical phenotypes, lifestyle confounders relative to CV risk, and lipid-lowering treatment,” said the authors of the analysis.
In the post hoc analysis of the ACCORD trial, which evaluated the effects of intensive glucose lowering in T2D more than 10 years ago, there were data on remnant-C over a median of 8.8 years of follow-up in 9,650 T2D patients. Over this period, 1,815 (17.8%) developed MACE.
Multiple analyses support prognostic value of remnant-C
In addition to the 7% rise in MACE for each standard-deviation increase in remnant-C when calculated as a continuous variable, other analyses told the same story.
This included an assessment by remnant-C tertiles. Not only was there a significant trend (P < .001) for greater risk with each higher baseline tertile of remnant-C, those in the highest tertile had a 38% greater risk of MACE relative to those in the lowest tertile (hazard ratio, 1.38; P < .001) after adjustment for confounders.
The same pattern was seen for several components of MACE, such as CV death and nonfatal myocardial infarction, when remnant-C tertiles were compared.
Visit-to-visit variability in remnant-C over the course of follow-up was also associated with greater risk of MACE. In logarithmic calculations, the risk of MACE climbed about 40% across all three models of risk adjustment. These models included adjustments for different sets of confounders, such as sex, age, blood pressure, CV disease history, and glucose levels. On an unadjusted basis, the risk was increased about 50% (HR, 1.52; P < .001).
For visit-to-visit variability in remnant-C, the greatest effect was on risk of nonfatal MI across models. In model 3, for example, which adjusted for the most confounders, the risk was nearly doubled (HR, 1.92; P < .001). In contrast, there did not appear to be a link between visit-to-visit variability and nonfatal stroke.
In a discordant analysis that was conducted to examine the relative risk of remnant-C independent of LDL-C, those who had a remnant-C level of at least 31 mg/dL were found to have a higher risk of MACE regardless of LDL-C level. Yet, the risk was higher if both remnant-C and LDL-C were elevated. For example, the risk was increased 22% for those with LDL-C at or below 100 mg/dL and remnant-C levels of at least 31 mg/dL (HR, 1.22; P = .015) but climbed to 37% for those with LDL-C above 100 mg/dL if remnant-C was at least 31 mg/dL (HR, 1.38; P = .007).
Remnant-C shows prognostic value in other risk groups
Although this study suggests an important prognostic value for remnant-C in T2D, there are numerous studies suggesting that it has prognostic value in other risk groups, such as those with a history of CV disease. This includes a study published earlier this year with 10 years of follow-up in 41,928 patients in Denmark. When combined with other risk factors, remnant-C substantially improved the accuracy of risk of events over time.
The investigators from this previous study, like the new study in patients with T2D, predict that remnant-C will be eventually included in guidelines.
According to Shi Tai, MD, a coauthor of the T2D study, remnant-C “may allow for the development of specific preventive and therapeutic approaches” to CV risk in patients with T2D.
T2D patients “with elevated plasma remnant-C levels represent a special population that deserves more attention regarding residual risk,” said Dr. Tai of the department of cardiovascular medicine at the Hospital of South Central China.
Great interest, but ready for guidelines?
This is an important direction of ongoing research, according to Christie M. Ballantyne, MD, professor of medicine, Baylor College of Medicine, Houston.
“There is a great deal of interest from both clinicians and trialists to find a simple way to identify patients with high residual risk who are on statin therapy,” he said. He thinks remnant-C has promise in this regard.
“Remnant-C is not in current guidelines,” he said in an interview, but he suggested that there is now a substantial body of evidence to suggest that it might be added if validated in further studies.
“Remnant-C is easy to calculate and may be helpful in practice now to identify patients who need more aggressive therapy to reduce risk and may be useful to identify patients for clinical trials who will benefit from new therapies that are in development,” he said.
However, the clinical relevance of therapies addressed at triglyceride-rich lipoproteins in general or their components, including triglycerides or remnant-C, has never been demonstrated, pointed out Peter W.F. Wilson, MD, PhD.
“Higher fasting or nonfasting triglyceride levels or their surrogates have been shown to be associated with increased risk for cardiovascular disease events in observational studies, but the importance of such measurements in persons already treated with very aggressive LDL-C lowering therapy is not known,” commented Dr. Wilson, director of epidemiology and genomic medicine, Emory School of Medicine, Atlanta.
Dr. Wilson was the coauthor of an editorial that accompanied the previously published Danish study of remnant-C. In his editorial, he suggested that remnant-C has promise for better understanding residual risk, but when contacted about these latest data he emphasized a lack of support so far for clinical relevance.
“Unfortunately, clinical trials have generally not shown that triglyceride lowering [to favorably alter remnant-C] in this situation favorably affects the risk of CV disease events,” he said in an interview. This does not preclude remnant-C as a targetable risk factor, but these data are needed.
Dr. Fu, Dr. Tai, and Dr. Wilson report no potential conflicts of interest. Dr. Ballantyne has financial relationships with more than 25 pharmaceutical companies, including several that produce products employed for the treatment of lipid abnormalities.
Adding to a growing body of evidence that elevated remnant cholesterol (remnant-C) provides additional and independent risk prediction for major cardiovascular events (MACE), a new analysis has this shown this biomarker has prognostic value specifically in patients with type 2 diabetes (T2D).
In a post hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, each standard-deviation increase in remnant-C was associated with a 7% increased risk in MACE (P = .004) after adjustment for several risk factors including other cholesterol values.
“In type 2 diabetes, remnant-C levels are associated with MACE regardless of LDL-C,” reported a team of investigators led by Liyao Fu, MD, Second Xiangya Hospital of Central South University, Changsha, China .
Remnant-C is one component of triglyceride-rich lipoproteins. Within triglyceride-rich lipoproteins, remnant-C has become a major focus of efforts to explain cardiovascular (CV) residual risk, according to the investigators.
Residual risk is a term used to explain why cardiovascular events occur after all known modifiable factors, such as LDL cholesterol (LDL-C), are controlled.
“Our primary findings indicate that baseline estimated remnant-C levels were associated with MACE regardless of clinical phenotypes, lifestyle confounders relative to CV risk, and lipid-lowering treatment,” said the authors of the analysis.
In the post hoc analysis of the ACCORD trial, which evaluated the effects of intensive glucose lowering in T2D more than 10 years ago, there were data on remnant-C over a median of 8.8 years of follow-up in 9,650 T2D patients. Over this period, 1,815 (17.8%) developed MACE.
Multiple analyses support prognostic value of remnant-C
In addition to the 7% rise in MACE for each standard-deviation increase in remnant-C when calculated as a continuous variable, other analyses told the same story.
This included an assessment by remnant-C tertiles. Not only was there a significant trend (P < .001) for greater risk with each higher baseline tertile of remnant-C, those in the highest tertile had a 38% greater risk of MACE relative to those in the lowest tertile (hazard ratio, 1.38; P < .001) after adjustment for confounders.
The same pattern was seen for several components of MACE, such as CV death and nonfatal myocardial infarction, when remnant-C tertiles were compared.
Visit-to-visit variability in remnant-C over the course of follow-up was also associated with greater risk of MACE. In logarithmic calculations, the risk of MACE climbed about 40% across all three models of risk adjustment. These models included adjustments for different sets of confounders, such as sex, age, blood pressure, CV disease history, and glucose levels. On an unadjusted basis, the risk was increased about 50% (HR, 1.52; P < .001).
For visit-to-visit variability in remnant-C, the greatest effect was on risk of nonfatal MI across models. In model 3, for example, which adjusted for the most confounders, the risk was nearly doubled (HR, 1.92; P < .001). In contrast, there did not appear to be a link between visit-to-visit variability and nonfatal stroke.
In a discordant analysis that was conducted to examine the relative risk of remnant-C independent of LDL-C, those who had a remnant-C level of at least 31 mg/dL were found to have a higher risk of MACE regardless of LDL-C level. Yet, the risk was higher if both remnant-C and LDL-C were elevated. For example, the risk was increased 22% for those with LDL-C at or below 100 mg/dL and remnant-C levels of at least 31 mg/dL (HR, 1.22; P = .015) but climbed to 37% for those with LDL-C above 100 mg/dL if remnant-C was at least 31 mg/dL (HR, 1.38; P = .007).
Remnant-C shows prognostic value in other risk groups
Although this study suggests an important prognostic value for remnant-C in T2D, there are numerous studies suggesting that it has prognostic value in other risk groups, such as those with a history of CV disease. This includes a study published earlier this year with 10 years of follow-up in 41,928 patients in Denmark. When combined with other risk factors, remnant-C substantially improved the accuracy of risk of events over time.
The investigators from this previous study, like the new study in patients with T2D, predict that remnant-C will be eventually included in guidelines.
According to Shi Tai, MD, a coauthor of the T2D study, remnant-C “may allow for the development of specific preventive and therapeutic approaches” to CV risk in patients with T2D.
T2D patients “with elevated plasma remnant-C levels represent a special population that deserves more attention regarding residual risk,” said Dr. Tai of the department of cardiovascular medicine at the Hospital of South Central China.
Great interest, but ready for guidelines?
This is an important direction of ongoing research, according to Christie M. Ballantyne, MD, professor of medicine, Baylor College of Medicine, Houston.
“There is a great deal of interest from both clinicians and trialists to find a simple way to identify patients with high residual risk who are on statin therapy,” he said. He thinks remnant-C has promise in this regard.
“Remnant-C is not in current guidelines,” he said in an interview, but he suggested that there is now a substantial body of evidence to suggest that it might be added if validated in further studies.
“Remnant-C is easy to calculate and may be helpful in practice now to identify patients who need more aggressive therapy to reduce risk and may be useful to identify patients for clinical trials who will benefit from new therapies that are in development,” he said.
However, the clinical relevance of therapies addressed at triglyceride-rich lipoproteins in general or their components, including triglycerides or remnant-C, has never been demonstrated, pointed out Peter W.F. Wilson, MD, PhD.
“Higher fasting or nonfasting triglyceride levels or their surrogates have been shown to be associated with increased risk for cardiovascular disease events in observational studies, but the importance of such measurements in persons already treated with very aggressive LDL-C lowering therapy is not known,” commented Dr. Wilson, director of epidemiology and genomic medicine, Emory School of Medicine, Atlanta.
Dr. Wilson was the coauthor of an editorial that accompanied the previously published Danish study of remnant-C. In his editorial, he suggested that remnant-C has promise for better understanding residual risk, but when contacted about these latest data he emphasized a lack of support so far for clinical relevance.
“Unfortunately, clinical trials have generally not shown that triglyceride lowering [to favorably alter remnant-C] in this situation favorably affects the risk of CV disease events,” he said in an interview. This does not preclude remnant-C as a targetable risk factor, but these data are needed.
Dr. Fu, Dr. Tai, and Dr. Wilson report no potential conflicts of interest. Dr. Ballantyne has financial relationships with more than 25 pharmaceutical companies, including several that produce products employed for the treatment of lipid abnormalities.
FROM DIABETES CARE
RADIANCE II: Positive signal for ultrasound renal denervation
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.






