User login
Herpes Zoster Following a Nucleoside-Modified Messenger RNA COVID-19 Vaccine
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
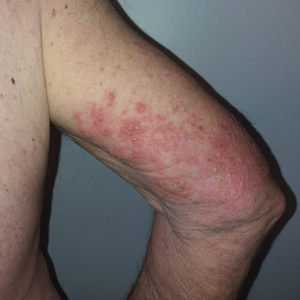
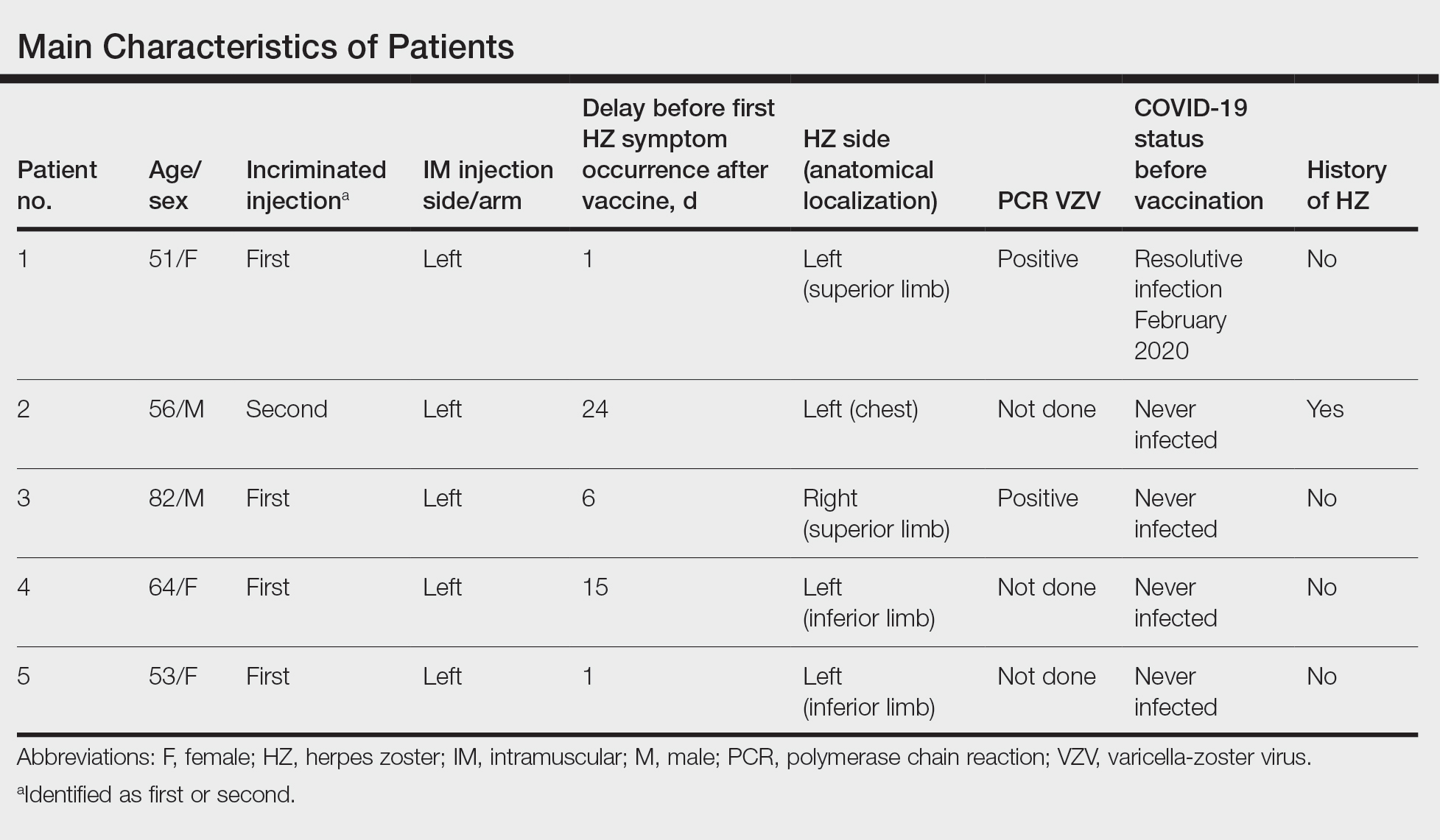
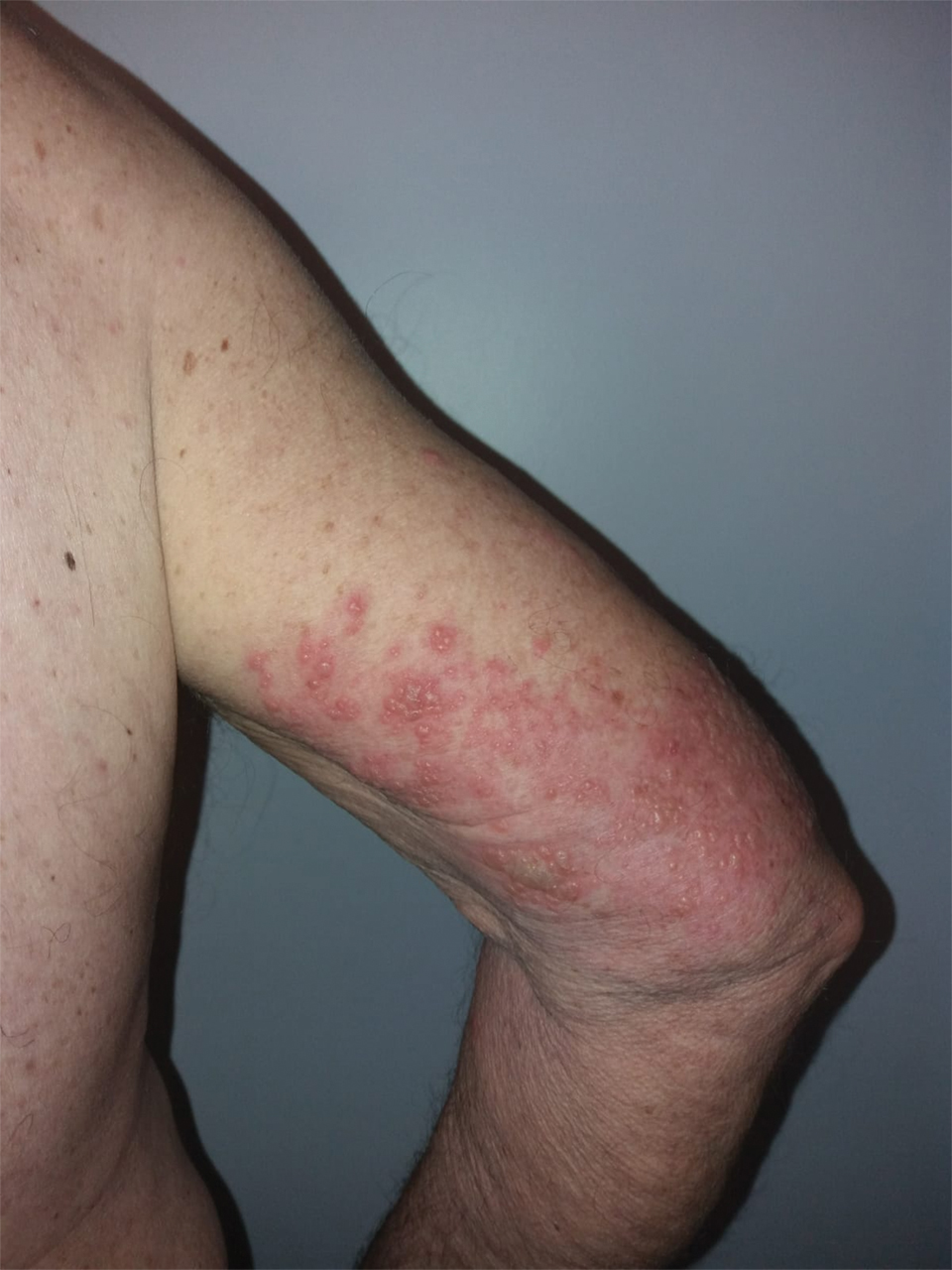
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.
Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5
Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9
In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Practice Points
- Herpes zoster (HZ) has been reported following COVID-19 vaccination.
- Postinjection pain is common with COVID-19 vaccination, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay in onset between the injection and the symptoms.
- When indicated, the second vaccine dose should not be avoided in patients who are diagnosed with HZ.
Serious problems rare in ages 5-11 from COVID vaccine
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
Breast cancer treatment worse for incarcerated patients
The study was presented at the 2021 San Antonio Breast Cancer Symposium on Dec. 10 (Abstract P5-14-10).
Examining the records of more than 4,300 patients with breast cancer who were treated between 2014 and 2020 in North Carolina, researchers identified 34 who were either incarcerated at the time of diagnosis or who were diagnosed before they were imprisoned.
They found that neoadjuvant therapy was not given to incarcerated breast cancer patients as compared to 8% of women who were never incarcerated and 20% of women incarcerated later. Incarcerated patients treated with surgery upfront had to wait on average more than 3 weeks longer than other patients for their procedure. Their findings were followed by a recently published study in JAMA Network Open indicating that young people with a history of incarceration were significantly more likely to experience early mortality and that mortality was higher among Black prisoners.
“These findings are concerning for missed treatment opportunities within the carceral system,” wrote researchers who were led by Oluwadamilola “Lola” Fayanju, MD, MPHS, FACS, chief of breast surgery for the University of Pennsylvania Health System, Philadelphia.
Dr. Fayanju told this news organization that she was “not surprised by the finding that there was no neoadjuvant chemotherapy given to patients at all. Even in the practice of care outside of the carceral system it is striking how much variation there is in regards to treatment sequence if it is not approached in an evidence-based way. Many of the social ills that contribute to incarceration also contribute to this variation in care, and it’s not surprising that in women who are experiencing incarceration, there is geometric escalation of disparities with regards to their opportunities for treatment.”
Erica L. Mayer, MD, MPH, a medical oncologist and clinical investigator in the Breast Oncology Center at the Dana-Faber Cancer Institute, Boston, said “this is really interesting and important work showing some worrisome trends. On the one hand, this is a very small experience and such a small sample size is always vulnerable to bias or skew from factors that become more important. However, this is not the first observation that there are disparities of care in incarcerated populations,”said Dr. Mayer, who was not involved in the study. “This is a topic that has been studied in diseases outside of oncology, such as heart disease and diabetes. There is a theme that patients who are incarcerated have a disparity and inequity of care compared to those who are not.”
The current findings “fit in with general themes,” she said. As rates of cancer are expected to grow in the coming years, “understanding how to provide the best possible care in those settings is very important. This is early data but it’s an important signal and is suggesting to us that a greater understanding of health care access for incarcerated individuals is a very important area of study, and hopefully an area for which one could provide interventions that might help to reduce these disparities.”
Dr. Fayanju and associates. set out to determine the disease and treatment characteristics of individuals with breast cancer and a history of incarceration. They focused on women who had a breast cancer diagnosis at the University of North Carolina Hospitals between April 2014 and December 2020. They gathered data on patient demographics, incarceration status, disease characteristics, treatment types, and dates of receipt of treatment, but there were few data available. “It is really striking how little data there is available. This is a very small study and is the best we could glean from a large state-wide dataset,” she said.
Of 4,332 breast cancer cases, 34 (0.8%) were diagnosed while incarcerated (70.6%) or before incarceration (29.4%). Those who were diagnosed during incarceration were significantly more likely to be single (P < .001), use illicit drugs at the time of diagnosis (P = .01), and have a family history of breast cancer (P = .03) as compared with patients who were never incarcerated and those who were diagnosed before incarceration.
The results also showed that patients diagnosed with breast cancer during incarceration were significantly less likely to receive neoadjuvant therapy at 0% versus 8.2% for those who were never incarcerated, and 20% for those who were diagnosed before incarceration (P = .01 for trend).
“Further research is needed to understand the full scope of cancer inequities and identify factors that contribute to them among patients who experience incarceration,” Dr. Fayanju said.
No funding or relevant financial relationships were declared for this featured study.
The study was presented at the 2021 San Antonio Breast Cancer Symposium on Dec. 10 (Abstract P5-14-10).
Examining the records of more than 4,300 patients with breast cancer who were treated between 2014 and 2020 in North Carolina, researchers identified 34 who were either incarcerated at the time of diagnosis or who were diagnosed before they were imprisoned.
They found that neoadjuvant therapy was not given to incarcerated breast cancer patients as compared to 8% of women who were never incarcerated and 20% of women incarcerated later. Incarcerated patients treated with surgery upfront had to wait on average more than 3 weeks longer than other patients for their procedure. Their findings were followed by a recently published study in JAMA Network Open indicating that young people with a history of incarceration were significantly more likely to experience early mortality and that mortality was higher among Black prisoners.
“These findings are concerning for missed treatment opportunities within the carceral system,” wrote researchers who were led by Oluwadamilola “Lola” Fayanju, MD, MPHS, FACS, chief of breast surgery for the University of Pennsylvania Health System, Philadelphia.
Dr. Fayanju told this news organization that she was “not surprised by the finding that there was no neoadjuvant chemotherapy given to patients at all. Even in the practice of care outside of the carceral system it is striking how much variation there is in regards to treatment sequence if it is not approached in an evidence-based way. Many of the social ills that contribute to incarceration also contribute to this variation in care, and it’s not surprising that in women who are experiencing incarceration, there is geometric escalation of disparities with regards to their opportunities for treatment.”
Erica L. Mayer, MD, MPH, a medical oncologist and clinical investigator in the Breast Oncology Center at the Dana-Faber Cancer Institute, Boston, said “this is really interesting and important work showing some worrisome trends. On the one hand, this is a very small experience and such a small sample size is always vulnerable to bias or skew from factors that become more important. However, this is not the first observation that there are disparities of care in incarcerated populations,”said Dr. Mayer, who was not involved in the study. “This is a topic that has been studied in diseases outside of oncology, such as heart disease and diabetes. There is a theme that patients who are incarcerated have a disparity and inequity of care compared to those who are not.”
The current findings “fit in with general themes,” she said. As rates of cancer are expected to grow in the coming years, “understanding how to provide the best possible care in those settings is very important. This is early data but it’s an important signal and is suggesting to us that a greater understanding of health care access for incarcerated individuals is a very important area of study, and hopefully an area for which one could provide interventions that might help to reduce these disparities.”
Dr. Fayanju and associates. set out to determine the disease and treatment characteristics of individuals with breast cancer and a history of incarceration. They focused on women who had a breast cancer diagnosis at the University of North Carolina Hospitals between April 2014 and December 2020. They gathered data on patient demographics, incarceration status, disease characteristics, treatment types, and dates of receipt of treatment, but there were few data available. “It is really striking how little data there is available. This is a very small study and is the best we could glean from a large state-wide dataset,” she said.
Of 4,332 breast cancer cases, 34 (0.8%) were diagnosed while incarcerated (70.6%) or before incarceration (29.4%). Those who were diagnosed during incarceration were significantly more likely to be single (P < .001), use illicit drugs at the time of diagnosis (P = .01), and have a family history of breast cancer (P = .03) as compared with patients who were never incarcerated and those who were diagnosed before incarceration.
The results also showed that patients diagnosed with breast cancer during incarceration were significantly less likely to receive neoadjuvant therapy at 0% versus 8.2% for those who were never incarcerated, and 20% for those who were diagnosed before incarceration (P = .01 for trend).
“Further research is needed to understand the full scope of cancer inequities and identify factors that contribute to them among patients who experience incarceration,” Dr. Fayanju said.
No funding or relevant financial relationships were declared for this featured study.
The study was presented at the 2021 San Antonio Breast Cancer Symposium on Dec. 10 (Abstract P5-14-10).
Examining the records of more than 4,300 patients with breast cancer who were treated between 2014 and 2020 in North Carolina, researchers identified 34 who were either incarcerated at the time of diagnosis or who were diagnosed before they were imprisoned.
They found that neoadjuvant therapy was not given to incarcerated breast cancer patients as compared to 8% of women who were never incarcerated and 20% of women incarcerated later. Incarcerated patients treated with surgery upfront had to wait on average more than 3 weeks longer than other patients for their procedure. Their findings were followed by a recently published study in JAMA Network Open indicating that young people with a history of incarceration were significantly more likely to experience early mortality and that mortality was higher among Black prisoners.
“These findings are concerning for missed treatment opportunities within the carceral system,” wrote researchers who were led by Oluwadamilola “Lola” Fayanju, MD, MPHS, FACS, chief of breast surgery for the University of Pennsylvania Health System, Philadelphia.
Dr. Fayanju told this news organization that she was “not surprised by the finding that there was no neoadjuvant chemotherapy given to patients at all. Even in the practice of care outside of the carceral system it is striking how much variation there is in regards to treatment sequence if it is not approached in an evidence-based way. Many of the social ills that contribute to incarceration also contribute to this variation in care, and it’s not surprising that in women who are experiencing incarceration, there is geometric escalation of disparities with regards to their opportunities for treatment.”
Erica L. Mayer, MD, MPH, a medical oncologist and clinical investigator in the Breast Oncology Center at the Dana-Faber Cancer Institute, Boston, said “this is really interesting and important work showing some worrisome trends. On the one hand, this is a very small experience and such a small sample size is always vulnerable to bias or skew from factors that become more important. However, this is not the first observation that there are disparities of care in incarcerated populations,”said Dr. Mayer, who was not involved in the study. “This is a topic that has been studied in diseases outside of oncology, such as heart disease and diabetes. There is a theme that patients who are incarcerated have a disparity and inequity of care compared to those who are not.”
The current findings “fit in with general themes,” she said. As rates of cancer are expected to grow in the coming years, “understanding how to provide the best possible care in those settings is very important. This is early data but it’s an important signal and is suggesting to us that a greater understanding of health care access for incarcerated individuals is a very important area of study, and hopefully an area for which one could provide interventions that might help to reduce these disparities.”
Dr. Fayanju and associates. set out to determine the disease and treatment characteristics of individuals with breast cancer and a history of incarceration. They focused on women who had a breast cancer diagnosis at the University of North Carolina Hospitals between April 2014 and December 2020. They gathered data on patient demographics, incarceration status, disease characteristics, treatment types, and dates of receipt of treatment, but there were few data available. “It is really striking how little data there is available. This is a very small study and is the best we could glean from a large state-wide dataset,” she said.
Of 4,332 breast cancer cases, 34 (0.8%) were diagnosed while incarcerated (70.6%) or before incarceration (29.4%). Those who were diagnosed during incarceration were significantly more likely to be single (P < .001), use illicit drugs at the time of diagnosis (P = .01), and have a family history of breast cancer (P = .03) as compared with patients who were never incarcerated and those who were diagnosed before incarceration.
The results also showed that patients diagnosed with breast cancer during incarceration were significantly less likely to receive neoadjuvant therapy at 0% versus 8.2% for those who were never incarcerated, and 20% for those who were diagnosed before incarceration (P = .01 for trend).
“Further research is needed to understand the full scope of cancer inequities and identify factors that contribute to them among patients who experience incarceration,” Dr. Fayanju said.
No funding or relevant financial relationships were declared for this featured study.
FROM SABCS 2021
Seventeen percent of breast cancer patients reclassified after risk score reassessment
Elisha Hughes, PhD, director of research biostatistics at Myriad Genetics (which funded the study), and colleagues combined a risk model containing 149 single-nucleotide polymorphisms (SNPs), of which just over one-third were related to genetic ancestry, with the Tyrer-Cuzick (TC) breast cancer risk model.
The resulting combined risk score, which was developed in a cohort of over 145,000 women and validated in another group of almost 69,000 women, was not only well calibrated, but also able to reclassify just over 17% of women into a different risk group versus the clinical model.
The research (abstract P2-11-21) was presented at the San Antonio Breast Cancer Symposium on Dec. 8.
“This is the first breast cancer risk model based on a polygenic score, the 149-SNP PRS, that incorporates genetically determined ancestral composition and is validated for diverse ancestries,” the team reported.
The combined model substantially improved risk stratification over TC alone and may “lead to enhanced breast cancer risk reduction strategies, such as increased surveillance and use of preventive medications,” the researchers reported.
Breast cancer has a substantial genetic component that can “inform risk prediction and personalized preventive measures.” However, polygenic risk scores are largely derived from studies of women of European descent and tend to have poor performance in non-European ancestries.
Combined score substantially improved risk stratification over TC alone
The research team developed a polygenic risk score based on 149 SNPs for women of diverse backgrounds who did not have pathologic variants in breast cancer susceptibility genes, and included 56 ancestry-informative variants with 93 BC-associated variants. They combined the 149-SNP polygenic risk score with the TC risk model to create a combined risk score that was developed in a cohort of 145,786 women who were unaffected by breast cancer, following a fixed-stratified model to avoid double counting between confounded factors.
Of the women included in the cohort, 69.1% were of European descent, while 10.2% were Hispanic, 10.0% Black/African, 1.9% Asian, and 8.8% all other groups.
An independent cohort of 68,803 women of a similar ethnic distribution was then used to evaluate the calibration of the combined risk score against the TC risk model alone, and to examine the relative contributions of the 149-SNP PRS, family history, and other clinical factors.
The results showed that, overall, the combined risk score was well calibrated across ancestries and percentiles of risks, and the absolute lifetime risks were similar to those derived from the TC risk model alone. The only exception was Hispanic carriers of a protective Amerindian SNP who had a lower score on the combined risk score than the TC model.
Using an ANOVA model, the team found that family history contributed 48% to the lifetime risk of breast cancer, while the 149-SNP PRS contributed 35% and other factors 17%. Family history was weakly, but significantly correlated with the 149-SNP PRS.
Determining the impact of adding the 149-SNP PRS to the TC risk model on risk classification, the team showed that across all ancestries, 17.3% of women were reclassified by the combined risk score versus the TC model alone, with 10.8% having their lifetime risk increased to high risk and 29.1% having their risk decreased by the combined model to low risk.
The largest reclassifications were seen for women of European descent, while the smallest were for Black/African women.
Study may have ‘cracked the code’
“What’s exciting is that I think we kind-of ‘cracked the code’ to some extent of how to do this across diseases for all ancestries,” Thomas P. Slavin, MD, chief medical officer at Myriad Genetics, said in an interview. “The adaptation for breast cancer risk stratification and the new panel [is] for breast cancer across all ancestries, but what we developed is something that could be used across diabetes, or colon cancer, or anything.”
He explained that they realized that “for each one of these little hot spots” in the SNPs, “that make one person different from another, you really need to find out where in the world that originated from. So, if you have genetic ancestry on an individual, you can say this spot in the genome has more of an African ancestry to it, or a European ancestry, and then you can weight it appropriately by the population.”
Dr. Slavin said that standard PRSs that simply add up SNPs are “pretty good” and “add a lot” to risk stratification, “but to fine-tune it a little bit and make the best risk model, you really do need to bring in clinical and family history factors.”
Montserrat García-Closas, MD, DrPH, deputy director of the cancer epidemiology and genetics for the National Cancer Institute, said the study is of interest, but “does not give information on how ancestry was considered in the models used to derive the scores.” She also cautioned that the method used in the study to calibrate the model seems “to mean a comparison of scores, rather than comparing the observed and expected risk in prospective cohorts by ancestry groups. This would be a way to estimate bias in risk prediction by ancestry.”
Nevertheless, Dr. García-Closas said the degree of risk reclassification seen with the combined risk score is as expected and pointed to recent work by her and her colleagues in which they tested an integrated model incorporating classical risk factors and a 313-variant PRS to predict breast-cancer risk and achieved similar results.
Several study authors disclosed ties with Myriad Genetics, as well as AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Helix BioPharma, Konica Minolta, Ambry Genetics, Invitae, Stryker, GAIL, Phenogen Sciences, Novartis, Pfizer, CancerIQ, Tempus, 54gene, Color Genetics, Roche/Genentech, ImpediMed, Prelude Therapeutics, BD, Agendia, Targeted Medical Education, Cerebrotech Medical Systems, Integra LifeSciences, Puma Biotechnology, GeneDX/BioReference, Change Health Care, Research to Practice, Clinical Care Options, Physician Education Resource, and Daiichi Sankyo.
The headline for this article was updated on 1/6/22.
Elisha Hughes, PhD, director of research biostatistics at Myriad Genetics (which funded the study), and colleagues combined a risk model containing 149 single-nucleotide polymorphisms (SNPs), of which just over one-third were related to genetic ancestry, with the Tyrer-Cuzick (TC) breast cancer risk model.
The resulting combined risk score, which was developed in a cohort of over 145,000 women and validated in another group of almost 69,000 women, was not only well calibrated, but also able to reclassify just over 17% of women into a different risk group versus the clinical model.
The research (abstract P2-11-21) was presented at the San Antonio Breast Cancer Symposium on Dec. 8.
“This is the first breast cancer risk model based on a polygenic score, the 149-SNP PRS, that incorporates genetically determined ancestral composition and is validated for diverse ancestries,” the team reported.
The combined model substantially improved risk stratification over TC alone and may “lead to enhanced breast cancer risk reduction strategies, such as increased surveillance and use of preventive medications,” the researchers reported.
Breast cancer has a substantial genetic component that can “inform risk prediction and personalized preventive measures.” However, polygenic risk scores are largely derived from studies of women of European descent and tend to have poor performance in non-European ancestries.
Combined score substantially improved risk stratification over TC alone
The research team developed a polygenic risk score based on 149 SNPs for women of diverse backgrounds who did not have pathologic variants in breast cancer susceptibility genes, and included 56 ancestry-informative variants with 93 BC-associated variants. They combined the 149-SNP polygenic risk score with the TC risk model to create a combined risk score that was developed in a cohort of 145,786 women who were unaffected by breast cancer, following a fixed-stratified model to avoid double counting between confounded factors.
Of the women included in the cohort, 69.1% were of European descent, while 10.2% were Hispanic, 10.0% Black/African, 1.9% Asian, and 8.8% all other groups.
An independent cohort of 68,803 women of a similar ethnic distribution was then used to evaluate the calibration of the combined risk score against the TC risk model alone, and to examine the relative contributions of the 149-SNP PRS, family history, and other clinical factors.
The results showed that, overall, the combined risk score was well calibrated across ancestries and percentiles of risks, and the absolute lifetime risks were similar to those derived from the TC risk model alone. The only exception was Hispanic carriers of a protective Amerindian SNP who had a lower score on the combined risk score than the TC model.
Using an ANOVA model, the team found that family history contributed 48% to the lifetime risk of breast cancer, while the 149-SNP PRS contributed 35% and other factors 17%. Family history was weakly, but significantly correlated with the 149-SNP PRS.
Determining the impact of adding the 149-SNP PRS to the TC risk model on risk classification, the team showed that across all ancestries, 17.3% of women were reclassified by the combined risk score versus the TC model alone, with 10.8% having their lifetime risk increased to high risk and 29.1% having their risk decreased by the combined model to low risk.
The largest reclassifications were seen for women of European descent, while the smallest were for Black/African women.
Study may have ‘cracked the code’
“What’s exciting is that I think we kind-of ‘cracked the code’ to some extent of how to do this across diseases for all ancestries,” Thomas P. Slavin, MD, chief medical officer at Myriad Genetics, said in an interview. “The adaptation for breast cancer risk stratification and the new panel [is] for breast cancer across all ancestries, but what we developed is something that could be used across diabetes, or colon cancer, or anything.”
He explained that they realized that “for each one of these little hot spots” in the SNPs, “that make one person different from another, you really need to find out where in the world that originated from. So, if you have genetic ancestry on an individual, you can say this spot in the genome has more of an African ancestry to it, or a European ancestry, and then you can weight it appropriately by the population.”
Dr. Slavin said that standard PRSs that simply add up SNPs are “pretty good” and “add a lot” to risk stratification, “but to fine-tune it a little bit and make the best risk model, you really do need to bring in clinical and family history factors.”
Montserrat García-Closas, MD, DrPH, deputy director of the cancer epidemiology and genetics for the National Cancer Institute, said the study is of interest, but “does not give information on how ancestry was considered in the models used to derive the scores.” She also cautioned that the method used in the study to calibrate the model seems “to mean a comparison of scores, rather than comparing the observed and expected risk in prospective cohorts by ancestry groups. This would be a way to estimate bias in risk prediction by ancestry.”
Nevertheless, Dr. García-Closas said the degree of risk reclassification seen with the combined risk score is as expected and pointed to recent work by her and her colleagues in which they tested an integrated model incorporating classical risk factors and a 313-variant PRS to predict breast-cancer risk and achieved similar results.
Several study authors disclosed ties with Myriad Genetics, as well as AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Helix BioPharma, Konica Minolta, Ambry Genetics, Invitae, Stryker, GAIL, Phenogen Sciences, Novartis, Pfizer, CancerIQ, Tempus, 54gene, Color Genetics, Roche/Genentech, ImpediMed, Prelude Therapeutics, BD, Agendia, Targeted Medical Education, Cerebrotech Medical Systems, Integra LifeSciences, Puma Biotechnology, GeneDX/BioReference, Change Health Care, Research to Practice, Clinical Care Options, Physician Education Resource, and Daiichi Sankyo.
The headline for this article was updated on 1/6/22.
Elisha Hughes, PhD, director of research biostatistics at Myriad Genetics (which funded the study), and colleagues combined a risk model containing 149 single-nucleotide polymorphisms (SNPs), of which just over one-third were related to genetic ancestry, with the Tyrer-Cuzick (TC) breast cancer risk model.
The resulting combined risk score, which was developed in a cohort of over 145,000 women and validated in another group of almost 69,000 women, was not only well calibrated, but also able to reclassify just over 17% of women into a different risk group versus the clinical model.
The research (abstract P2-11-21) was presented at the San Antonio Breast Cancer Symposium on Dec. 8.
“This is the first breast cancer risk model based on a polygenic score, the 149-SNP PRS, that incorporates genetically determined ancestral composition and is validated for diverse ancestries,” the team reported.
The combined model substantially improved risk stratification over TC alone and may “lead to enhanced breast cancer risk reduction strategies, such as increased surveillance and use of preventive medications,” the researchers reported.
Breast cancer has a substantial genetic component that can “inform risk prediction and personalized preventive measures.” However, polygenic risk scores are largely derived from studies of women of European descent and tend to have poor performance in non-European ancestries.
Combined score substantially improved risk stratification over TC alone
The research team developed a polygenic risk score based on 149 SNPs for women of diverse backgrounds who did not have pathologic variants in breast cancer susceptibility genes, and included 56 ancestry-informative variants with 93 BC-associated variants. They combined the 149-SNP polygenic risk score with the TC risk model to create a combined risk score that was developed in a cohort of 145,786 women who were unaffected by breast cancer, following a fixed-stratified model to avoid double counting between confounded factors.
Of the women included in the cohort, 69.1% were of European descent, while 10.2% were Hispanic, 10.0% Black/African, 1.9% Asian, and 8.8% all other groups.
An independent cohort of 68,803 women of a similar ethnic distribution was then used to evaluate the calibration of the combined risk score against the TC risk model alone, and to examine the relative contributions of the 149-SNP PRS, family history, and other clinical factors.
The results showed that, overall, the combined risk score was well calibrated across ancestries and percentiles of risks, and the absolute lifetime risks were similar to those derived from the TC risk model alone. The only exception was Hispanic carriers of a protective Amerindian SNP who had a lower score on the combined risk score than the TC model.
Using an ANOVA model, the team found that family history contributed 48% to the lifetime risk of breast cancer, while the 149-SNP PRS contributed 35% and other factors 17%. Family history was weakly, but significantly correlated with the 149-SNP PRS.
Determining the impact of adding the 149-SNP PRS to the TC risk model on risk classification, the team showed that across all ancestries, 17.3% of women were reclassified by the combined risk score versus the TC model alone, with 10.8% having their lifetime risk increased to high risk and 29.1% having their risk decreased by the combined model to low risk.
The largest reclassifications were seen for women of European descent, while the smallest were for Black/African women.
Study may have ‘cracked the code’
“What’s exciting is that I think we kind-of ‘cracked the code’ to some extent of how to do this across diseases for all ancestries,” Thomas P. Slavin, MD, chief medical officer at Myriad Genetics, said in an interview. “The adaptation for breast cancer risk stratification and the new panel [is] for breast cancer across all ancestries, but what we developed is something that could be used across diabetes, or colon cancer, or anything.”
He explained that they realized that “for each one of these little hot spots” in the SNPs, “that make one person different from another, you really need to find out where in the world that originated from. So, if you have genetic ancestry on an individual, you can say this spot in the genome has more of an African ancestry to it, or a European ancestry, and then you can weight it appropriately by the population.”
Dr. Slavin said that standard PRSs that simply add up SNPs are “pretty good” and “add a lot” to risk stratification, “but to fine-tune it a little bit and make the best risk model, you really do need to bring in clinical and family history factors.”
Montserrat García-Closas, MD, DrPH, deputy director of the cancer epidemiology and genetics for the National Cancer Institute, said the study is of interest, but “does not give information on how ancestry was considered in the models used to derive the scores.” She also cautioned that the method used in the study to calibrate the model seems “to mean a comparison of scores, rather than comparing the observed and expected risk in prospective cohorts by ancestry groups. This would be a way to estimate bias in risk prediction by ancestry.”
Nevertheless, Dr. García-Closas said the degree of risk reclassification seen with the combined risk score is as expected and pointed to recent work by her and her colleagues in which they tested an integrated model incorporating classical risk factors and a 313-variant PRS to predict breast-cancer risk and achieved similar results.
Several study authors disclosed ties with Myriad Genetics, as well as AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Helix BioPharma, Konica Minolta, Ambry Genetics, Invitae, Stryker, GAIL, Phenogen Sciences, Novartis, Pfizer, CancerIQ, Tempus, 54gene, Color Genetics, Roche/Genentech, ImpediMed, Prelude Therapeutics, BD, Agendia, Targeted Medical Education, Cerebrotech Medical Systems, Integra LifeSciences, Puma Biotechnology, GeneDX/BioReference, Change Health Care, Research to Practice, Clinical Care Options, Physician Education Resource, and Daiichi Sankyo.
The headline for this article was updated on 1/6/22.
FROM SABCS 2021
Omega-3 supplementation improves sleep, mood in breast cancer patients on hormone therapy
After 4 weeks of treatment, patients who received omega-3 reported better sleep, depression, and mood outcomes than those who received placebo.
Estrogen-receptor inhibitors are used to treat breast cancer with positive hormone receptors in combination with other therapies. However, the drugs can lead to long-term side effects, including hot flashes, night sweats, and changes to mood and sleep.
These side effects are often treated with selective serotonin reuptake inhibitors and some anticonvulsant drugs. Omega-3 supplements contain various polyunsaturated fatty acids, which influence cell signaling and contribute to the production of bioactive fat mediators that counter inflammation. They are widely used in cardiovascular disease, breast cancer, rheumatoid arthritis, depression, and other cognitive disorders. They also appear to amplify the antitumor efficacy of tamoxifen through the inhibition of proliferative and antiapoptotic pathways that that are influenced by estrogen-receptor signaling.
“This study showed that omega-3 supplementation can improve mood and sleep disorder in women suffering from breast cancer while they (are) managing with antihormone drugs. … this supplement can be proposed for the treatment of these patients,” wrote researchers led by Azadeh Moghaddas, MD, PhD, who is an associate professor of clinical pharmacy and pharmacy practice at Isfahan (Iran) University of Medical Sciences.
The study was made available as a preprint on ResearchSquare and has not yet been peer reviewed. It included 60 patients who were screened for baseline mood disorders using the hospital anxiety and depression scale (HADS), then randomized to 2 mg omega-3 per day for 4 weeks, or placebo.
Studies have shown that omega-3 supplementation improves menopause and mood symptoms in postmenopausal women without cancer.
Omega-3 supplementation has neuroprotective effects and improved brain function and mood in rats, and a 2019 review suggested that the evidence is strong enough to warrant clinical studies.
To determine if the supplement was also safe and effective in women with breast cancer undergoing hormone therapy, the researchers analyzed data from 32 patients in the intervention group and 28 patients in the placebo group.
At 4 weeks of follow-up, patients in the intervention group had significantly lower values on the Center for Epidemiological Studies-Depression scale (mean, 22.8 vs. 30.8; P < .001), Profile of Mood State (mean, 30.8 versus 39.5; P<.001), and Pittsburgh Sleep Quality Index (mean, 4.6 vs. 5.9; P = .04). There were no statistically significant changes in these values in the placebo group.
At 4 weeks, paired samples t-test comparisons between the intervention and the placebo groups revealed lower scores in the intervention group for mean scores in the PSQI subscales subjective sleep quality (0.8 vs. 1.4; P = .002), delay in falling asleep (1.1 vs. 1.6; P = .02), and sleep disturbances (0.8 vs. 1.1; P = .005).
There were no significant adverse reactions in either group.
The study is limited by its small sample size and the short follow-up period.
The study was funded by Isfahan University of Medical Sciences. The authors declare no other conflicts of interest.
After 4 weeks of treatment, patients who received omega-3 reported better sleep, depression, and mood outcomes than those who received placebo.
Estrogen-receptor inhibitors are used to treat breast cancer with positive hormone receptors in combination with other therapies. However, the drugs can lead to long-term side effects, including hot flashes, night sweats, and changes to mood and sleep.
These side effects are often treated with selective serotonin reuptake inhibitors and some anticonvulsant drugs. Omega-3 supplements contain various polyunsaturated fatty acids, which influence cell signaling and contribute to the production of bioactive fat mediators that counter inflammation. They are widely used in cardiovascular disease, breast cancer, rheumatoid arthritis, depression, and other cognitive disorders. They also appear to amplify the antitumor efficacy of tamoxifen through the inhibition of proliferative and antiapoptotic pathways that that are influenced by estrogen-receptor signaling.
“This study showed that omega-3 supplementation can improve mood and sleep disorder in women suffering from breast cancer while they (are) managing with antihormone drugs. … this supplement can be proposed for the treatment of these patients,” wrote researchers led by Azadeh Moghaddas, MD, PhD, who is an associate professor of clinical pharmacy and pharmacy practice at Isfahan (Iran) University of Medical Sciences.
The study was made available as a preprint on ResearchSquare and has not yet been peer reviewed. It included 60 patients who were screened for baseline mood disorders using the hospital anxiety and depression scale (HADS), then randomized to 2 mg omega-3 per day for 4 weeks, or placebo.
Studies have shown that omega-3 supplementation improves menopause and mood symptoms in postmenopausal women without cancer.
Omega-3 supplementation has neuroprotective effects and improved brain function and mood in rats, and a 2019 review suggested that the evidence is strong enough to warrant clinical studies.
To determine if the supplement was also safe and effective in women with breast cancer undergoing hormone therapy, the researchers analyzed data from 32 patients in the intervention group and 28 patients in the placebo group.
At 4 weeks of follow-up, patients in the intervention group had significantly lower values on the Center for Epidemiological Studies-Depression scale (mean, 22.8 vs. 30.8; P < .001), Profile of Mood State (mean, 30.8 versus 39.5; P<.001), and Pittsburgh Sleep Quality Index (mean, 4.6 vs. 5.9; P = .04). There were no statistically significant changes in these values in the placebo group.
At 4 weeks, paired samples t-test comparisons between the intervention and the placebo groups revealed lower scores in the intervention group for mean scores in the PSQI subscales subjective sleep quality (0.8 vs. 1.4; P = .002), delay in falling asleep (1.1 vs. 1.6; P = .02), and sleep disturbances (0.8 vs. 1.1; P = .005).
There were no significant adverse reactions in either group.
The study is limited by its small sample size and the short follow-up period.
The study was funded by Isfahan University of Medical Sciences. The authors declare no other conflicts of interest.
After 4 weeks of treatment, patients who received omega-3 reported better sleep, depression, and mood outcomes than those who received placebo.
Estrogen-receptor inhibitors are used to treat breast cancer with positive hormone receptors in combination with other therapies. However, the drugs can lead to long-term side effects, including hot flashes, night sweats, and changes to mood and sleep.
These side effects are often treated with selective serotonin reuptake inhibitors and some anticonvulsant drugs. Omega-3 supplements contain various polyunsaturated fatty acids, which influence cell signaling and contribute to the production of bioactive fat mediators that counter inflammation. They are widely used in cardiovascular disease, breast cancer, rheumatoid arthritis, depression, and other cognitive disorders. They also appear to amplify the antitumor efficacy of tamoxifen through the inhibition of proliferative and antiapoptotic pathways that that are influenced by estrogen-receptor signaling.
“This study showed that omega-3 supplementation can improve mood and sleep disorder in women suffering from breast cancer while they (are) managing with antihormone drugs. … this supplement can be proposed for the treatment of these patients,” wrote researchers led by Azadeh Moghaddas, MD, PhD, who is an associate professor of clinical pharmacy and pharmacy practice at Isfahan (Iran) University of Medical Sciences.
The study was made available as a preprint on ResearchSquare and has not yet been peer reviewed. It included 60 patients who were screened for baseline mood disorders using the hospital anxiety and depression scale (HADS), then randomized to 2 mg omega-3 per day for 4 weeks, or placebo.
Studies have shown that omega-3 supplementation improves menopause and mood symptoms in postmenopausal women without cancer.
Omega-3 supplementation has neuroprotective effects and improved brain function and mood in rats, and a 2019 review suggested that the evidence is strong enough to warrant clinical studies.
To determine if the supplement was also safe and effective in women with breast cancer undergoing hormone therapy, the researchers analyzed data from 32 patients in the intervention group and 28 patients in the placebo group.
At 4 weeks of follow-up, patients in the intervention group had significantly lower values on the Center for Epidemiological Studies-Depression scale (mean, 22.8 vs. 30.8; P < .001), Profile of Mood State (mean, 30.8 versus 39.5; P<.001), and Pittsburgh Sleep Quality Index (mean, 4.6 vs. 5.9; P = .04). There were no statistically significant changes in these values in the placebo group.
At 4 weeks, paired samples t-test comparisons between the intervention and the placebo groups revealed lower scores in the intervention group for mean scores in the PSQI subscales subjective sleep quality (0.8 vs. 1.4; P = .002), delay in falling asleep (1.1 vs. 1.6; P = .02), and sleep disturbances (0.8 vs. 1.1; P = .005).
There were no significant adverse reactions in either group.
The study is limited by its small sample size and the short follow-up period.
The study was funded by Isfahan University of Medical Sciences. The authors declare no other conflicts of interest.
FROM RESEARCHSQUARE
Clinical Edge Journal Scan Commentary: Breast Cancer January 2022
Oocyte and embryo cryopreservation are standard fertility preservation techniques, and gonadotropin-releasing hormone agonist (GnRHa) administration during chemotherapy is another strategy to preserve ovarian function. The phase 3 POEMS/S0230 study demonstrated higher pregnancy rates (5-year cumulative incidence 23.1% vs 12.2%, P = 0.03) among premenopausal patients with HR-negative early breast cancer who received GnRHa (goserelin) during chemotherapy vs chemotherapy alone. Furthermore, there was a trend towards improvement in survival outcomes with GnRHa + chemotherapy. Hypothetical concerns have existed regarding the safety of this approach, particularly in HR+ breast cancer. The PROMISE-GIM6 trial randomized 281 patients to receive chemotherapy alone or with GnRHa triptorelin (Lambertini et al) and found no difference in disease-free survival (DFS) or overall survival (OS) between GnRHa vs control groups (12-year DFS 65.7% vs 69.2%, HR 1.16; 12-year OS 81.2% vs 81.3%, HR 1.17). In patients with HR+ disease (80.4%), HR for DFS and OS was 1.02 and 1.12, respectively. The 12-year cumulative incidence of pregnancy was also higher in the GnRHa vs control group (6.5% vs 3.2%). These studies suggest no detrimental effect of GnRHa use during chemotherapy on long-term outcomes, including patients with HR+ disease, and support its role in ovarian protection.
COVID-19 has had various implications on breast cancer care, reflecting institutional policies, resources and patient preferences and potential concerns during the pandemic. A retrospective chart review of patients diagnosed at Mayo Clinic Rochester with a new breast cancer during vs pre-COVID-19, examined trends in diagnosis and treatment approaches during these times (Tonneson et al). Among 573 patients, there was no significant difference in clinical prognostic stage, although a slightly higher percentage of patients who presented with stage II-IV disease during COVID-19 vs pre-COVID-19 (29% vs 26%, P = 0.42). The use of neoadjuvant endocrine therapy (NET) significantly increased during COVID-19, and notably in patients with HR+/HER2- breast cancer (10% pre-COVID-19 vs 23% during COVID-19 (P = 0.001)) with a significant increase in stage I patients (7% vs 22%, P < 0.001). Various societies provided language to support neoadjuvant therapy as a bridge to surgical intervention during COVID-19 in the appropriate clinical scenarios. Extended follow-up of studies examining approaches utilized during the pandemic are desired to further define long-term impact on outcomes.
A pooled analysis of the PALOMA trials demonstrated progression-free survival benefit with palbociclib + endocrine therapy vs endocrine therapy alone in patients ≥65 years, and although myelosuppression was more common in patients ≥75 years, the combination remained well-tolerated. Ismail et al described real-world experience of palbociclib in older patients with advanced HR+ breast cancer. Among 598 patients, palbociclib dose reductions occurred in 33%, and those requiring a dose reduction were older vs those without dose reduction (median age 67 vs 63 years, P = 0.004). Despite higher frequency of dose reductions in older patients, this did not appear to compromise outcomes; time to next treatment was significantly longer (16.9 vs 11.6 months, P = 0.013) than younger patients but OS was similar (20.7 vs 26.7 months, P = 0.051). Although older patients may be at higher risk of toxicities due to co-morbidities or performance status limitations, palbociclib remains a valuable therapeutic option combined with endocrine therapy for advanced HR+/HER2- breast cancer.
References:
Francis PA, Pagani O, Fleming GF, et al; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122-137.
Moore HCF, Unger JM, Phillips K-A, et al. Final analysis of the prevention of early menopause study (POEMS)/SWOG Intergroup S0230. J Natl Cancer Inst. 2019;111(2):210–213.
Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487–97.
Rugo HS, Turner NC, Finn RS, et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer. 2018;101:123e33.
Oocyte and embryo cryopreservation are standard fertility preservation techniques, and gonadotropin-releasing hormone agonist (GnRHa) administration during chemotherapy is another strategy to preserve ovarian function. The phase 3 POEMS/S0230 study demonstrated higher pregnancy rates (5-year cumulative incidence 23.1% vs 12.2%, P = 0.03) among premenopausal patients with HR-negative early breast cancer who received GnRHa (goserelin) during chemotherapy vs chemotherapy alone. Furthermore, there was a trend towards improvement in survival outcomes with GnRHa + chemotherapy. Hypothetical concerns have existed regarding the safety of this approach, particularly in HR+ breast cancer. The PROMISE-GIM6 trial randomized 281 patients to receive chemotherapy alone or with GnRHa triptorelin (Lambertini et al) and found no difference in disease-free survival (DFS) or overall survival (OS) between GnRHa vs control groups (12-year DFS 65.7% vs 69.2%, HR 1.16; 12-year OS 81.2% vs 81.3%, HR 1.17). In patients with HR+ disease (80.4%), HR for DFS and OS was 1.02 and 1.12, respectively. The 12-year cumulative incidence of pregnancy was also higher in the GnRHa vs control group (6.5% vs 3.2%). These studies suggest no detrimental effect of GnRHa use during chemotherapy on long-term outcomes, including patients with HR+ disease, and support its role in ovarian protection.
COVID-19 has had various implications on breast cancer care, reflecting institutional policies, resources and patient preferences and potential concerns during the pandemic. A retrospective chart review of patients diagnosed at Mayo Clinic Rochester with a new breast cancer during vs pre-COVID-19, examined trends in diagnosis and treatment approaches during these times (Tonneson et al). Among 573 patients, there was no significant difference in clinical prognostic stage, although a slightly higher percentage of patients who presented with stage II-IV disease during COVID-19 vs pre-COVID-19 (29% vs 26%, P = 0.42). The use of neoadjuvant endocrine therapy (NET) significantly increased during COVID-19, and notably in patients with HR+/HER2- breast cancer (10% pre-COVID-19 vs 23% during COVID-19 (P = 0.001)) with a significant increase in stage I patients (7% vs 22%, P < 0.001). Various societies provided language to support neoadjuvant therapy as a bridge to surgical intervention during COVID-19 in the appropriate clinical scenarios. Extended follow-up of studies examining approaches utilized during the pandemic are desired to further define long-term impact on outcomes.
A pooled analysis of the PALOMA trials demonstrated progression-free survival benefit with palbociclib + endocrine therapy vs endocrine therapy alone in patients ≥65 years, and although myelosuppression was more common in patients ≥75 years, the combination remained well-tolerated. Ismail et al described real-world experience of palbociclib in older patients with advanced HR+ breast cancer. Among 598 patients, palbociclib dose reductions occurred in 33%, and those requiring a dose reduction were older vs those without dose reduction (median age 67 vs 63 years, P = 0.004). Despite higher frequency of dose reductions in older patients, this did not appear to compromise outcomes; time to next treatment was significantly longer (16.9 vs 11.6 months, P = 0.013) than younger patients but OS was similar (20.7 vs 26.7 months, P = 0.051). Although older patients may be at higher risk of toxicities due to co-morbidities or performance status limitations, palbociclib remains a valuable therapeutic option combined with endocrine therapy for advanced HR+/HER2- breast cancer.
References:
Francis PA, Pagani O, Fleming GF, et al; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122-137.
Moore HCF, Unger JM, Phillips K-A, et al. Final analysis of the prevention of early menopause study (POEMS)/SWOG Intergroup S0230. J Natl Cancer Inst. 2019;111(2):210–213.
Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487–97.
Rugo HS, Turner NC, Finn RS, et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer. 2018;101:123e33.
Oocyte and embryo cryopreservation are standard fertility preservation techniques, and gonadotropin-releasing hormone agonist (GnRHa) administration during chemotherapy is another strategy to preserve ovarian function. The phase 3 POEMS/S0230 study demonstrated higher pregnancy rates (5-year cumulative incidence 23.1% vs 12.2%, P = 0.03) among premenopausal patients with HR-negative early breast cancer who received GnRHa (goserelin) during chemotherapy vs chemotherapy alone. Furthermore, there was a trend towards improvement in survival outcomes with GnRHa + chemotherapy. Hypothetical concerns have existed regarding the safety of this approach, particularly in HR+ breast cancer. The PROMISE-GIM6 trial randomized 281 patients to receive chemotherapy alone or with GnRHa triptorelin (Lambertini et al) and found no difference in disease-free survival (DFS) or overall survival (OS) between GnRHa vs control groups (12-year DFS 65.7% vs 69.2%, HR 1.16; 12-year OS 81.2% vs 81.3%, HR 1.17). In patients with HR+ disease (80.4%), HR for DFS and OS was 1.02 and 1.12, respectively. The 12-year cumulative incidence of pregnancy was also higher in the GnRHa vs control group (6.5% vs 3.2%). These studies suggest no detrimental effect of GnRHa use during chemotherapy on long-term outcomes, including patients with HR+ disease, and support its role in ovarian protection.
COVID-19 has had various implications on breast cancer care, reflecting institutional policies, resources and patient preferences and potential concerns during the pandemic. A retrospective chart review of patients diagnosed at Mayo Clinic Rochester with a new breast cancer during vs pre-COVID-19, examined trends in diagnosis and treatment approaches during these times (Tonneson et al). Among 573 patients, there was no significant difference in clinical prognostic stage, although a slightly higher percentage of patients who presented with stage II-IV disease during COVID-19 vs pre-COVID-19 (29% vs 26%, P = 0.42). The use of neoadjuvant endocrine therapy (NET) significantly increased during COVID-19, and notably in patients with HR+/HER2- breast cancer (10% pre-COVID-19 vs 23% during COVID-19 (P = 0.001)) with a significant increase in stage I patients (7% vs 22%, P < 0.001). Various societies provided language to support neoadjuvant therapy as a bridge to surgical intervention during COVID-19 in the appropriate clinical scenarios. Extended follow-up of studies examining approaches utilized during the pandemic are desired to further define long-term impact on outcomes.
A pooled analysis of the PALOMA trials demonstrated progression-free survival benefit with palbociclib + endocrine therapy vs endocrine therapy alone in patients ≥65 years, and although myelosuppression was more common in patients ≥75 years, the combination remained well-tolerated. Ismail et al described real-world experience of palbociclib in older patients with advanced HR+ breast cancer. Among 598 patients, palbociclib dose reductions occurred in 33%, and those requiring a dose reduction were older vs those without dose reduction (median age 67 vs 63 years, P = 0.004). Despite higher frequency of dose reductions in older patients, this did not appear to compromise outcomes; time to next treatment was significantly longer (16.9 vs 11.6 months, P = 0.013) than younger patients but OS was similar (20.7 vs 26.7 months, P = 0.051). Although older patients may be at higher risk of toxicities due to co-morbidities or performance status limitations, palbociclib remains a valuable therapeutic option combined with endocrine therapy for advanced HR+/HER2- breast cancer.
References:
Francis PA, Pagani O, Fleming GF, et al; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122-137.
Moore HCF, Unger JM, Phillips K-A, et al. Final analysis of the prevention of early menopause study (POEMS)/SWOG Intergroup S0230. J Natl Cancer Inst. 2019;111(2):210–213.
Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487–97.
Rugo HS, Turner NC, Finn RS, et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer. 2018;101:123e33.
Last call? Moderate alcohol’s health benefits look increasingly doubtful
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
Pandemic poses short- and long-term risks to babies, especially boys
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The pandemic has created a hostile environment for pregnant people and their babies.
Stress levels among expectant mothers have soared. Pregnant women with COVID are 5 times as likely as uninfected pregnant people to require intensive care and 22 times as likely to die. Infected moms are four times as likely to have a stillborn child.
Yet some of the pandemic’s greatest threats to infants’ health may not be apparent for years or even decades.
That’s because babies of COVID-infected moms are 60% more likely to be born very prematurely, which increases the danger of infant mortality and long-term disabilities such as cerebral palsy, asthma, and hearing loss, as well as a child’s risk of adult disease, including depression, anxiety, heart disease, and kidney disease.
Studies have linked fever and infection during pregnancy to developmental and psychiatric conditions such as autism, depression, and schizophrenia.
“Some of these conditions do not show up until middle childhood or early adult life, but they have their origins in fetal life,” said Evdokia Anagnostou, MD, a child neurologist at Holland Bloorview Kids Rehabilitation Hospital and a pediatrics professor at the University of Toronto.
For fetuses exposed to COVID, the greatest danger is usually not the coronavirus itself, but the mother’s immune system.
Both severe COVID infections and the strain of the pandemic can expose fetuses to harmful inflammation, which can occur when a mother’s immune system is fighting a virus or when stress hormones send nonstop alarm signals.
Prenatal inflammation “changes the way the brain develops and, depending on the timing of the infection, it can change the way the heart or kidneys develop,” Dr. Anagnostou said.
Although health officials have strongly recommended COVID vaccines for pregnant people, only 35% are fully vaccinated.
At least 150,000 pregnant women have been diagnosed with COVID; more than 25,000 of them have been hospitalized, and 249 have died, according to the Centers for Disease Control and Prevention.
Although most babies will be fine, even a small increase in the percentage of children with special medical or educational needs could have a large effect on the population, given the huge number of COVID infections, Dr. Anagnostou said.
“If someone has a baby who is doing well, that is what they should focus on,” Dr. Anagnostou said. “But from a public health point of view, we need to follow women who experienced severe COVID and their babies to understand the impact.”
Learning from history
Researchers in the United States and other countries are already studying “the COVID generation” to see whether these children have more health issues than those conceived or born before 2020.
Previous crises have shown that the challenges fetuses face in the womb – such as maternal infections, hunger, stress, and hormone-disrupting chemicals – can leave a lasting imprint on their health, as well as that of their children and grandchildren, said Frederick Kaskel, MD, director of pediatric nephrology at the Children’s Hospital at Montefiore, New York.
People whose mothers were pregnant during surges in the 1918 influenza pandemic, for example, had poorer health throughout their lives, compared with Americans born at other times, said John McCarthy, who is a medical student at Albert Einstein College of Medicine, New York, and cowrote a recent review in JAMA Pediatrics with Dr. Kaskel.
Researchers don’t know exactly which moms were infected with pandemic flu, Mr. McCarthy said. But women who were pregnant during major surges – when infection was widespread – had children with higher rates of heart disease or diabetes. These children were also less successful in school, less economically productive, and more likely to live with a disability.
Because organ systems develop during different periods of pregnancy, fetuses exposed during the first trimester may face different risks than those exposed toward the end of pregnancy, Mr. McCarthy said. For example, people born in the fall of 1918 were 50% more likely than others to develop kidney disease; that may reflect an exposure to the pandemic in the third trimester, while the kidneys were still developing.
Nearly 2 years into the COVID pandemic, researchers have begun to publish preliminary observations of infants exposed to COVID infections and stress before birth.
Although Dr. Anagnostou noted that it’s too early to reach definitive conclusions, “there is evidence that babies born to moms with severe COVID infections have changes to their immune system,” she said. “It’s enough to make us worry a little bit.”
Damaging a fetal security system
The good news about the coronavirus is that it seldom crosses the placenta, the organ tasked with protecting a developing fetus from infections and providing it with oxygen. So moms with COVID rarely give the virus to their children before birth.
That’s important, because some viruses that directly infect the fetus – such as Zika – can cause devastating birth defects, said Karin Nielsen-Saines, MD, a specialist in pediatric infectious diseases at University of California, Los Angeles.
But studies also suggest that inflammation from a mother’s COVID infection can injure the placenta, said Jeffery Goldstein, MD, an assistant professor of pathology at Northwestern University, Chicago. In a study published in American Journal of Clinical Pathology , Dr. Goldstein and his coauthors found that placentas from COVID-infected moms had more abnormal blood vessels than placentas from patients without COVID, making it harder for them to deliver sufficient oxygen to the fetus.
Placental damage can also lead to preeclampsia, a serious complication of pregnancy that can cause a mother’s blood pressure to spike.
Preeclampsia occurs when blood vessels in the placenta don’t develop or function properly, forcing the mother’s heart to work harder to get blood to the fetus, which may not receive enough oxygen and nutrients. Preeclampsia also predisposes women to heart attacks and strokes later in life.
Rewiring the immune system
In some cases, COVID also appears to rewire a baby’s immune response, Dr. Nielsen-Saines said.
In an October study in the journal Cell Reports Medicine, Dr. Nielsen-Saines and her coauthors found that infants born to people with severe COVID infections had a different mix of immune cells and proteins than other babies. None of the newborns tested positive for the coronavirus.
The immune changes are concerning, Dr. Nielsen-Saines said, because this pattern of immune cells and proteins has previously been found in infants with respiratory problems and in some cases poor neurodevelopment.
Notably, all the babies in her study appear healthy, said Dr. Nielsen-Saines, who plans to follow them for 3 years to see whether these early signals translate into developmental delays, such as problems talking, walking, or interacting with others.
“How big of a difference does any of this make in the baby?” asked Dr. Anagnostou. “We won’t know for a few years. All we can do is try to be as prepared as possible.”
Increasing the risk for boys
Boys could face higher risks from COVID, even before birth.
Males are generally more vulnerable than females as fetuses and newborns; they’re more likely to be born prematurely and to die as infants. Preterm boys also have a higher risk of disability and death.
But coronavirus infection poses special dangers, said Sabra Klein, PhD, a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore.
That’s because boys are disproportionately affected by conditions linked to maternal infections. Boys are four times as likely as girls to be diagnosed with autism or attention-deficit/hyperactivity disorder, for example, while men are 75% more likely than women to develop schizophrenia.
Scientists don’t fully understand why boys appear more fragile in the womb, although testosterone – which can dampen immune response – may play a role, said Kristina Adams Waldorf, MD, a professor of obstetrics and gynecology at the University of Washington.
Men generally mount weaker immune responses than women and more often develop severe COVID infections. Recent research suggests boys with COVID are more likely than girls to become seriously ill or develop a rare inflammatory condition called multisystem inflammatory syndrome.
New research on COVID could help illuminate this vulnerability.
In a study published in October, researchers found that the sex of a fetus influences the way its placenta responds to COVID, as well as how its mother’s immune system responds.
Pregnant people infected with COVID made fewer antibodies against the coronavirus if they were carrying male fetuses than if they were carrying females. Mothers also transferred fewer antibodies to boys than to girls, said Andrea Edlow, MD, senior author of the study and a maternal-fetal medicine specialist at Massachusetts General Hospital, Boston.
When examining the placentas of male fetuses after delivery, researchers found changes that could leave boys less protected against damaging inflammation.
The sex of a fetus can influence its mother’s response to other illnesses, as well.
For example, research shows that pregnant women with asthma have worse symptoms if they’re carrying a female. Women carrying males are slightly more likely to develop gestational diabetes.
Dr. Edlow said her findings raise questions about the “cross talk” between mother and baby. “The mom’s immune system is sensing there is a male fetus,” Dr. Edlow said. “And the fetus is actively communicating with the mom’s immune system.”
Boosting toxic stress
Rates of depression and stress among pregnant women have increased dramatically during the pandemic.
That’s concerning because chronic stress can lead to inflammation, affecting the babies of both infected and uninfected women, Dr. Anagnostou said.
Studies consistently show that infants born to mothers who experience significant stress during pregnancy have higher rates of short- and long-term health damage – including heart defects and obesity – than babies born to women with less stress.
“We know that inflammation directly influences the way a baby’s brain develops,” said Elinor Sullivan, PhD, an associate professor in psychiatry at Oregon Health & Science University, Portland.
Lockdowns, travel restrictions and physical distancing left many pregnant women without the support of family and friends. The stress of losing a loved one, a job, or a home further heightens the risks to moms and babies, said Dr. Sullivan, who is following children born during the pandemic for 5 years.
In research that has not yet been published, Dr. Sullivan found that babies of women who were pregnant during the pandemic showed more sadness and negative emotions in the first year of life, compared with infants of women who were pregnant before the pandemic.
The findings show the importance of helping and protecting pregnant people before and after delivery, said Dr. Sullivan, who conducted a separate study that found women who received more social support were less depressed.
Italian researchers are also studying the effect of maternal stress on infants’ behavior, as well as the way their genes are regulated.
Although stress-related inflammation doesn’t alter the structure of a baby’s genes, it can influence whether they’re turned on and off, said Livio Provenzi, PhD, a psychologist at the C. Mondino National Institute of Neurology Foundation in Pavia, Italy.
In Dr. Provenzi’s study of 163 mother-baby pairs he found differences in how genes that regulate the stress response were activated. Genes that help people respond to stress were more likely to be turned off in babies whose moms reported the most stress during pregnancy. The same moms also reported that their babies cried more and were fussier when they were 3 months old.
Researchers usually prefer to make in-person observations of babies as they interact with their mothers, Dr. Provenzi said. But because of the pandemic, Dr. Provenzi asked mothers to fill out questionnaires about infant behavior. He plans to observe mothers and babies in person when the children are 12 months old.
While vaccinating pregnant people is the best way to protect them and their fetuses from the virus, Dr. Anagnostou said, society needs to do more to preserve expectant mothers’ mental health.
“We can’t escape the fact that we’ve lived through 2 years of a pandemic,” Dr. Anagnostou said. “But we can think about opportunities for reducing the risk.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Bariatric surgery can lead to diabetes remission, cut cancer risk
Patients with obesity and type 2 diabetes who underwent bariatric surgery and had 10-year durable diabetes remission had a 60% lower risk of incident cancer than patients who had usual obesity care.
And women who had bariatric surgery had a 42% lower risk of having cancer during a median 21-year follow-up, compared with women who had usual obesity care.
These findings from 701 patients in the Swedish Obese Subjects (SOS) study who had type 2 diabetes were recently published in Diabetes Care.
The results illustrate the “connection between glucose control and cancer prevention” and suggest that “among patients with type 2 diabetes, many cancer cases are preventable,” lead author Kajsa Sjöholm, PhD, associate professor of molecular medicine at Sahlgrenska Academy, University of Gothenburg (Sweden), said in a press release from the university.
“The global epidemic of both obesity and diabetes leads to an increased risk of cancer, as well as an increased risk of premature death,” added senior author Magdalena Taube, PhD, associate professor of molecular medicine in the same academy.
“It has been estimated that, over the next 10-15 years, obesity may cause more cancer cases than smoking in several countries,” she noted. Therefore, “strategies are needed to prevent this development, and our results can provide vital guidance for prevention of cancer in patients with obesity and type 2 diabetes.”
Durable diabetes remission seems key
Two-thirds of the patients in the bariatric surgery group had vertical banded gastroplasty (65%), and the rest had adjustable or nonadjustable gastric banding (18%) or gastric bypass (17%).
Each type of bariatric surgery was associated with higher diabetes remission rates, compared with usual care, in a previous study by these researchers, Dr. Taube said in an interview.
“In our present study,” she added, “we observed a nonsignificant trend, where patients with obesity and type 2 diabetes in the highest weight loss tertile (average weight loss, –44.8 kg) had somewhat lower risk of cancer compared to the lowest tertile [average weight loss, –14.9 kg].”
This might suggest, Dr. Taube continued, that with respect to cancer risk, surgery techniques resulting in greater weight loss (for example, Roux-en-Y gastric bypass and sleeve gastrectomy) should be recommended in patients with obesity and diabetes.
“However, it should also be noted that long-term diabetes remission seems imperative for cancer risk reduction,” she said, “and in a recent meta-analysis by McTigue et al., published in JAMA Surgery, it was shown that patients who had Roux-en-Y gastric bypass had greater weight loss, a slightly higher type 2 diabetes remission rate, less type 2 diabetes relapse, and better long-term glycemic control, compared with those who had sleeve gastrectomy.
“The observed cancer reduction in women with obesity and type 2 diabetes is in line with previous findings showing that cancer risk reduction following bariatric surgery in patients with obesity is more marked among women than men,” Dr. Taube noted. This may be because cancer rates are higher in women with diabetes than in men with diabetes, and common cancer types associated with obesity are female specific.
The main cancers in women were breast cancer, followed by endometrial and colorectal cancer. In men, the main cancers were colorectal, prostate, and urothelial/malignant skin cancer.
Study design and findings
It is well established that obesity is a risk factor for 13 types of cancer, and some of these cancers (liver, pancreatic, endometrial, colon and rectal, breast, and bladder) may be related to type 2 diabetes. And bariatric surgery has been shown to reduce cancer risk in patients with obesity.
However, it is not clear how bariatric surgery may affect cancer risk in patients with obesity and type 2 diabetes.
To study this, the researchers examined data from 393 patients who underwent bariatric surgery and 308 patients who received usual obesity treatment, who were part of the SOS study.
The SOS study enrolled men with a body mass index of at least 34 kg/m2, and women with a BMI of at least 38 kg/m2 who were aged 37-60 years between 1987 and 2001.
The current study outcome – cancer incidence in patients with obesity and type 2 diabetes – was not a prespecified outcome
The intervention groups were matched on 18 variables, including age, sex, serum insulin, alcohol, education, and smoking.
At baseline, the patients had a mean age of about 49 and 60% were women. They had a mean BMI of about 42 and a mean hemoglobin A1c of 7.8%.
On average, patients in the surgery group had lost 27.5 kg and 22.7 kg, and patients in the usual care group had lost 3.2 kg and 4.8 kg, at 2 years and 10 years, respectively.
During a median follow-up of 21 years, there were 74 incident cancers in the control group and 68 cancers in the bariatric surgery group.
The risk of cancer during follow-up was 37% lower in the surgery group than in the usual care group, after multivariable adjustment (adjusted hazard ratio, 0.63; 95% confidence interval, 0.44-0.89; P = .008).
A deeper dive showed that there were 86 incident cancers in women and 56 cancers in men. The risk of cancer was significantly lower in women who had bariatric surgery, compared with those who had usual care (aHR, 0.58; 95% CI 0.38-0.90, P = .016). However, the risk of cancer was not significantly lower in men who had bariatric surgery versus those who had usual care (aHR 0.79, 95% CI, 0.46-1.38; P = .413).
Diabetes remission at 10 years was associated with a 60% reduced cancer incidence (aHR, 0.40; 95% CI, 0.22-0.74, P = .003).
The study was funded by the Swedish state (under an agreement between the Swedish government and the county councils), the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Heart-Lung Foundation, and the Swedish Diabetes Foundation. One author received consulting fees from Johnson & Johnson. The other authors had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with obesity and type 2 diabetes who underwent bariatric surgery and had 10-year durable diabetes remission had a 60% lower risk of incident cancer than patients who had usual obesity care.
And women who had bariatric surgery had a 42% lower risk of having cancer during a median 21-year follow-up, compared with women who had usual obesity care.
These findings from 701 patients in the Swedish Obese Subjects (SOS) study who had type 2 diabetes were recently published in Diabetes Care.
The results illustrate the “connection between glucose control and cancer prevention” and suggest that “among patients with type 2 diabetes, many cancer cases are preventable,” lead author Kajsa Sjöholm, PhD, associate professor of molecular medicine at Sahlgrenska Academy, University of Gothenburg (Sweden), said in a press release from the university.
“The global epidemic of both obesity and diabetes leads to an increased risk of cancer, as well as an increased risk of premature death,” added senior author Magdalena Taube, PhD, associate professor of molecular medicine in the same academy.
“It has been estimated that, over the next 10-15 years, obesity may cause more cancer cases than smoking in several countries,” she noted. Therefore, “strategies are needed to prevent this development, and our results can provide vital guidance for prevention of cancer in patients with obesity and type 2 diabetes.”
Durable diabetes remission seems key
Two-thirds of the patients in the bariatric surgery group had vertical banded gastroplasty (65%), and the rest had adjustable or nonadjustable gastric banding (18%) or gastric bypass (17%).
Each type of bariatric surgery was associated with higher diabetes remission rates, compared with usual care, in a previous study by these researchers, Dr. Taube said in an interview.
“In our present study,” she added, “we observed a nonsignificant trend, where patients with obesity and type 2 diabetes in the highest weight loss tertile (average weight loss, –44.8 kg) had somewhat lower risk of cancer compared to the lowest tertile [average weight loss, –14.9 kg].”
This might suggest, Dr. Taube continued, that with respect to cancer risk, surgery techniques resulting in greater weight loss (for example, Roux-en-Y gastric bypass and sleeve gastrectomy) should be recommended in patients with obesity and diabetes.
“However, it should also be noted that long-term diabetes remission seems imperative for cancer risk reduction,” she said, “and in a recent meta-analysis by McTigue et al., published in JAMA Surgery, it was shown that patients who had Roux-en-Y gastric bypass had greater weight loss, a slightly higher type 2 diabetes remission rate, less type 2 diabetes relapse, and better long-term glycemic control, compared with those who had sleeve gastrectomy.
“The observed cancer reduction in women with obesity and type 2 diabetes is in line with previous findings showing that cancer risk reduction following bariatric surgery in patients with obesity is more marked among women than men,” Dr. Taube noted. This may be because cancer rates are higher in women with diabetes than in men with diabetes, and common cancer types associated with obesity are female specific.
The main cancers in women were breast cancer, followed by endometrial and colorectal cancer. In men, the main cancers were colorectal, prostate, and urothelial/malignant skin cancer.
Study design and findings
It is well established that obesity is a risk factor for 13 types of cancer, and some of these cancers (liver, pancreatic, endometrial, colon and rectal, breast, and bladder) may be related to type 2 diabetes. And bariatric surgery has been shown to reduce cancer risk in patients with obesity.
However, it is not clear how bariatric surgery may affect cancer risk in patients with obesity and type 2 diabetes.
To study this, the researchers examined data from 393 patients who underwent bariatric surgery and 308 patients who received usual obesity treatment, who were part of the SOS study.
The SOS study enrolled men with a body mass index of at least 34 kg/m2, and women with a BMI of at least 38 kg/m2 who were aged 37-60 years between 1987 and 2001.
The current study outcome – cancer incidence in patients with obesity and type 2 diabetes – was not a prespecified outcome
The intervention groups were matched on 18 variables, including age, sex, serum insulin, alcohol, education, and smoking.
At baseline, the patients had a mean age of about 49 and 60% were women. They had a mean BMI of about 42 and a mean hemoglobin A1c of 7.8%.
On average, patients in the surgery group had lost 27.5 kg and 22.7 kg, and patients in the usual care group had lost 3.2 kg and 4.8 kg, at 2 years and 10 years, respectively.
During a median follow-up of 21 years, there were 74 incident cancers in the control group and 68 cancers in the bariatric surgery group.
The risk of cancer during follow-up was 37% lower in the surgery group than in the usual care group, after multivariable adjustment (adjusted hazard ratio, 0.63; 95% confidence interval, 0.44-0.89; P = .008).
A deeper dive showed that there were 86 incident cancers in women and 56 cancers in men. The risk of cancer was significantly lower in women who had bariatric surgery, compared with those who had usual care (aHR, 0.58; 95% CI 0.38-0.90, P = .016). However, the risk of cancer was not significantly lower in men who had bariatric surgery versus those who had usual care (aHR 0.79, 95% CI, 0.46-1.38; P = .413).
Diabetes remission at 10 years was associated with a 60% reduced cancer incidence (aHR, 0.40; 95% CI, 0.22-0.74, P = .003).
The study was funded by the Swedish state (under an agreement between the Swedish government and the county councils), the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Heart-Lung Foundation, and the Swedish Diabetes Foundation. One author received consulting fees from Johnson & Johnson. The other authors had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with obesity and type 2 diabetes who underwent bariatric surgery and had 10-year durable diabetes remission had a 60% lower risk of incident cancer than patients who had usual obesity care.
And women who had bariatric surgery had a 42% lower risk of having cancer during a median 21-year follow-up, compared with women who had usual obesity care.
These findings from 701 patients in the Swedish Obese Subjects (SOS) study who had type 2 diabetes were recently published in Diabetes Care.
The results illustrate the “connection between glucose control and cancer prevention” and suggest that “among patients with type 2 diabetes, many cancer cases are preventable,” lead author Kajsa Sjöholm, PhD, associate professor of molecular medicine at Sahlgrenska Academy, University of Gothenburg (Sweden), said in a press release from the university.
“The global epidemic of both obesity and diabetes leads to an increased risk of cancer, as well as an increased risk of premature death,” added senior author Magdalena Taube, PhD, associate professor of molecular medicine in the same academy.
“It has been estimated that, over the next 10-15 years, obesity may cause more cancer cases than smoking in several countries,” she noted. Therefore, “strategies are needed to prevent this development, and our results can provide vital guidance for prevention of cancer in patients with obesity and type 2 diabetes.”
Durable diabetes remission seems key
Two-thirds of the patients in the bariatric surgery group had vertical banded gastroplasty (65%), and the rest had adjustable or nonadjustable gastric banding (18%) or gastric bypass (17%).
Each type of bariatric surgery was associated with higher diabetes remission rates, compared with usual care, in a previous study by these researchers, Dr. Taube said in an interview.
“In our present study,” she added, “we observed a nonsignificant trend, where patients with obesity and type 2 diabetes in the highest weight loss tertile (average weight loss, –44.8 kg) had somewhat lower risk of cancer compared to the lowest tertile [average weight loss, –14.9 kg].”
This might suggest, Dr. Taube continued, that with respect to cancer risk, surgery techniques resulting in greater weight loss (for example, Roux-en-Y gastric bypass and sleeve gastrectomy) should be recommended in patients with obesity and diabetes.
“However, it should also be noted that long-term diabetes remission seems imperative for cancer risk reduction,” she said, “and in a recent meta-analysis by McTigue et al., published in JAMA Surgery, it was shown that patients who had Roux-en-Y gastric bypass had greater weight loss, a slightly higher type 2 diabetes remission rate, less type 2 diabetes relapse, and better long-term glycemic control, compared with those who had sleeve gastrectomy.
“The observed cancer reduction in women with obesity and type 2 diabetes is in line with previous findings showing that cancer risk reduction following bariatric surgery in patients with obesity is more marked among women than men,” Dr. Taube noted. This may be because cancer rates are higher in women with diabetes than in men with diabetes, and common cancer types associated with obesity are female specific.
The main cancers in women were breast cancer, followed by endometrial and colorectal cancer. In men, the main cancers were colorectal, prostate, and urothelial/malignant skin cancer.
Study design and findings
It is well established that obesity is a risk factor for 13 types of cancer, and some of these cancers (liver, pancreatic, endometrial, colon and rectal, breast, and bladder) may be related to type 2 diabetes. And bariatric surgery has been shown to reduce cancer risk in patients with obesity.
However, it is not clear how bariatric surgery may affect cancer risk in patients with obesity and type 2 diabetes.
To study this, the researchers examined data from 393 patients who underwent bariatric surgery and 308 patients who received usual obesity treatment, who were part of the SOS study.
The SOS study enrolled men with a body mass index of at least 34 kg/m2, and women with a BMI of at least 38 kg/m2 who were aged 37-60 years between 1987 and 2001.
The current study outcome – cancer incidence in patients with obesity and type 2 diabetes – was not a prespecified outcome
The intervention groups were matched on 18 variables, including age, sex, serum insulin, alcohol, education, and smoking.
At baseline, the patients had a mean age of about 49 and 60% were women. They had a mean BMI of about 42 and a mean hemoglobin A1c of 7.8%.
On average, patients in the surgery group had lost 27.5 kg and 22.7 kg, and patients in the usual care group had lost 3.2 kg and 4.8 kg, at 2 years and 10 years, respectively.
During a median follow-up of 21 years, there were 74 incident cancers in the control group and 68 cancers in the bariatric surgery group.
The risk of cancer during follow-up was 37% lower in the surgery group than in the usual care group, after multivariable adjustment (adjusted hazard ratio, 0.63; 95% confidence interval, 0.44-0.89; P = .008).
A deeper dive showed that there were 86 incident cancers in women and 56 cancers in men. The risk of cancer was significantly lower in women who had bariatric surgery, compared with those who had usual care (aHR, 0.58; 95% CI 0.38-0.90, P = .016). However, the risk of cancer was not significantly lower in men who had bariatric surgery versus those who had usual care (aHR 0.79, 95% CI, 0.46-1.38; P = .413).
Diabetes remission at 10 years was associated with a 60% reduced cancer incidence (aHR, 0.40; 95% CI, 0.22-0.74, P = .003).
The study was funded by the Swedish state (under an agreement between the Swedish government and the county councils), the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Heart-Lung Foundation, and the Swedish Diabetes Foundation. One author received consulting fees from Johnson & Johnson. The other authors had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Axilla swelling after COVID booster puts focus on mammogram timing
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.