User login
Longer use of proton pump inhibitors tied to diabetes risk
Long-term use of a proton-pump inhibitor (PPI) was associated with an increased risk of being diagnosed with type 2 diabetes in a large, population-based case-control study in Italy.
The risk of diabetes increased from 19% to 56% as treatment duration increased from 8 weeks to more than 2 years, and prolonged treatment was associated with an even higher risk of diabetes in the youngest patients (age 40-65) and those with the most comorbidities.
The results suggest that “physicians should therefore avoid unnecessary prescription of this class of drugs, particularly for long-term use,” say Stefano Ciardullo, MD, University of Milano-Bicocca, Italy, and colleagues, in their article recently published online in the Journal of Clinical Endocrinology & Metabolism.
“Nonetheless, epidemiologic evidence on the topic remains conflicting,” they acknowledge, adding that “future studies are still needed to validate our findings.”
If the results are confirmed, these “may have important implications for both public health and clinical practice, given the high number of patients being treated with PPIs and the influence of diabetes on morbidity and mortality related to its possible micro- and macrovascular complications,” Dr. Ciardullo and colleagues conclude.
Not enough data to support a change in practice
The current findings align with a recent analysis of three prospective cohort studies of U.S. health care workers that showed a progressively increased risk of diabetes with longer treatment with PPIs, David A. Leiman, MD, MSHP, who was not involved with the current study, told this news organization in an email. “But the effect size remains relatively small and may be explained by residual or unmeasured confounding,” he cautioned.
“Ultimately, there do not seem to be enough data to support a change in clinical practice from this study alone, and, as a result, clinicians should continue to inform patients of the best available evidence regarding the benefits and risks of PPIs,” said Dr. Leiman, assistant professor of medicine, Division of Gastroenterology, Duke University Medical Center, Durham, N.C.
“Recent best practice advice from the American Gastroenterological Association does not recommend screening for insulin resistance among PPI users [and recommends that the decision to discontinue PPIs] should be based solely on the lack of an indication for PPI use, and not because of concern for PPI-associated adverse events,” he noted.
“Clinicians should be prepared to discuss the described risks associated with PPIs,” said Dr. Leiman, but they should “also feel comfortable affirming their safety profile and substantial efficacy in managing symptoms and preventing complications when prescribed for the appropriate indication.”
First-choice therapy for acid-related disorders
PPIs have become first-choice therapy for patients with acid-related disorders such as gastroesophageal reflux disease, Barrett esophagus, and peptic ulcer, and to prevent gastrointestinal bleeding while on nonsteroidal anti-inflammatory drugs (NSAIDs), Dr. Ciardullo and colleagues explain.
However, several studies have identified potential fractures, hypomagnesemia, gastric carcinoids, chronic kidney disease, dementia, and Clostridium difficile diarrhea with prolonged use of PPIs, and these agents can cause changes in the gut microbiome that may play a role in diabetes and other metabolic diseases.
To investigate a potential association between PPIs and type 2 diabetes, the researchers analyzed data from 777,420 patients age 40 and older who were newly treated with PPIs between 2010 and 2015 in Lombardy, Italy.
Of these, 50,540 patients were diagnosed with type 2 diabetes during follow-up until 2020 (a mean follow-up of 6.2 years and a diabetes incidence of 10.6 cases per 1,000 person-years).
The researchers matched 50,535 patients diagnosed with diabetes during follow-up with 50,535 control patients who had the same age, sex, and clinical status.
Patients were a mean age of 66 years and half were men. The most prescribed PPIs were pantoprazole and omeprazole, and the patients diagnosed with diabetes were more likely to use antihypertensives and lipid-lowering drugs.
Compared with patients who received PPIs for less than 8 weeks, those who received PPIs for 8 weeks to 6 months had a 19% increased risk of being diagnosed with diabetes during follow-up (odds ratio, 1.19; 95% confidence interval, 1.15-1.24), after adjusting for age, clinical profile, comorbidities, medical therapy, and PPI type.
Patients who received PPIs for 6 months to 2 years had a 43% increased risk of the outcome (OR, 1.43; 95% CI, 1.38-1.49), and those who received PPIs for more than 2 years had a 56% increased risk of the outcome (OR, 1.56; 95% CI, 1.49-1.64).
The researchers acknowledge limitations including that the study was not a randomized controlled trial, and it lacked information about over-the-counter medications and unmeasured confounders such as body mass index or family history of diabetes that may have affected the outcomes.
Dr. Leiman added that patients may have had prediabetes or undiagnosed diabetes and symptoms such as heartburn or dyspepsia arising from complications of insulin resistance, for which PPIs might have been prescribed.
The study was funded by a grant from the Italian Ministry of Education, University and Research. Dr. Ciardullo and Dr. Leiman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term use of a proton-pump inhibitor (PPI) was associated with an increased risk of being diagnosed with type 2 diabetes in a large, population-based case-control study in Italy.
The risk of diabetes increased from 19% to 56% as treatment duration increased from 8 weeks to more than 2 years, and prolonged treatment was associated with an even higher risk of diabetes in the youngest patients (age 40-65) and those with the most comorbidities.
The results suggest that “physicians should therefore avoid unnecessary prescription of this class of drugs, particularly for long-term use,” say Stefano Ciardullo, MD, University of Milano-Bicocca, Italy, and colleagues, in their article recently published online in the Journal of Clinical Endocrinology & Metabolism.
“Nonetheless, epidemiologic evidence on the topic remains conflicting,” they acknowledge, adding that “future studies are still needed to validate our findings.”
If the results are confirmed, these “may have important implications for both public health and clinical practice, given the high number of patients being treated with PPIs and the influence of diabetes on morbidity and mortality related to its possible micro- and macrovascular complications,” Dr. Ciardullo and colleagues conclude.
Not enough data to support a change in practice
The current findings align with a recent analysis of three prospective cohort studies of U.S. health care workers that showed a progressively increased risk of diabetes with longer treatment with PPIs, David A. Leiman, MD, MSHP, who was not involved with the current study, told this news organization in an email. “But the effect size remains relatively small and may be explained by residual or unmeasured confounding,” he cautioned.
“Ultimately, there do not seem to be enough data to support a change in clinical practice from this study alone, and, as a result, clinicians should continue to inform patients of the best available evidence regarding the benefits and risks of PPIs,” said Dr. Leiman, assistant professor of medicine, Division of Gastroenterology, Duke University Medical Center, Durham, N.C.
“Recent best practice advice from the American Gastroenterological Association does not recommend screening for insulin resistance among PPI users [and recommends that the decision to discontinue PPIs] should be based solely on the lack of an indication for PPI use, and not because of concern for PPI-associated adverse events,” he noted.
“Clinicians should be prepared to discuss the described risks associated with PPIs,” said Dr. Leiman, but they should “also feel comfortable affirming their safety profile and substantial efficacy in managing symptoms and preventing complications when prescribed for the appropriate indication.”
First-choice therapy for acid-related disorders
PPIs have become first-choice therapy for patients with acid-related disorders such as gastroesophageal reflux disease, Barrett esophagus, and peptic ulcer, and to prevent gastrointestinal bleeding while on nonsteroidal anti-inflammatory drugs (NSAIDs), Dr. Ciardullo and colleagues explain.
However, several studies have identified potential fractures, hypomagnesemia, gastric carcinoids, chronic kidney disease, dementia, and Clostridium difficile diarrhea with prolonged use of PPIs, and these agents can cause changes in the gut microbiome that may play a role in diabetes and other metabolic diseases.
To investigate a potential association between PPIs and type 2 diabetes, the researchers analyzed data from 777,420 patients age 40 and older who were newly treated with PPIs between 2010 and 2015 in Lombardy, Italy.
Of these, 50,540 patients were diagnosed with type 2 diabetes during follow-up until 2020 (a mean follow-up of 6.2 years and a diabetes incidence of 10.6 cases per 1,000 person-years).
The researchers matched 50,535 patients diagnosed with diabetes during follow-up with 50,535 control patients who had the same age, sex, and clinical status.
Patients were a mean age of 66 years and half were men. The most prescribed PPIs were pantoprazole and omeprazole, and the patients diagnosed with diabetes were more likely to use antihypertensives and lipid-lowering drugs.
Compared with patients who received PPIs for less than 8 weeks, those who received PPIs for 8 weeks to 6 months had a 19% increased risk of being diagnosed with diabetes during follow-up (odds ratio, 1.19; 95% confidence interval, 1.15-1.24), after adjusting for age, clinical profile, comorbidities, medical therapy, and PPI type.
Patients who received PPIs for 6 months to 2 years had a 43% increased risk of the outcome (OR, 1.43; 95% CI, 1.38-1.49), and those who received PPIs for more than 2 years had a 56% increased risk of the outcome (OR, 1.56; 95% CI, 1.49-1.64).
The researchers acknowledge limitations including that the study was not a randomized controlled trial, and it lacked information about over-the-counter medications and unmeasured confounders such as body mass index or family history of diabetes that may have affected the outcomes.
Dr. Leiman added that patients may have had prediabetes or undiagnosed diabetes and symptoms such as heartburn or dyspepsia arising from complications of insulin resistance, for which PPIs might have been prescribed.
The study was funded by a grant from the Italian Ministry of Education, University and Research. Dr. Ciardullo and Dr. Leiman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term use of a proton-pump inhibitor (PPI) was associated with an increased risk of being diagnosed with type 2 diabetes in a large, population-based case-control study in Italy.
The risk of diabetes increased from 19% to 56% as treatment duration increased from 8 weeks to more than 2 years, and prolonged treatment was associated with an even higher risk of diabetes in the youngest patients (age 40-65) and those with the most comorbidities.
The results suggest that “physicians should therefore avoid unnecessary prescription of this class of drugs, particularly for long-term use,” say Stefano Ciardullo, MD, University of Milano-Bicocca, Italy, and colleagues, in their article recently published online in the Journal of Clinical Endocrinology & Metabolism.
“Nonetheless, epidemiologic evidence on the topic remains conflicting,” they acknowledge, adding that “future studies are still needed to validate our findings.”
If the results are confirmed, these “may have important implications for both public health and clinical practice, given the high number of patients being treated with PPIs and the influence of diabetes on morbidity and mortality related to its possible micro- and macrovascular complications,” Dr. Ciardullo and colleagues conclude.
Not enough data to support a change in practice
The current findings align with a recent analysis of three prospective cohort studies of U.S. health care workers that showed a progressively increased risk of diabetes with longer treatment with PPIs, David A. Leiman, MD, MSHP, who was not involved with the current study, told this news organization in an email. “But the effect size remains relatively small and may be explained by residual or unmeasured confounding,” he cautioned.
“Ultimately, there do not seem to be enough data to support a change in clinical practice from this study alone, and, as a result, clinicians should continue to inform patients of the best available evidence regarding the benefits and risks of PPIs,” said Dr. Leiman, assistant professor of medicine, Division of Gastroenterology, Duke University Medical Center, Durham, N.C.
“Recent best practice advice from the American Gastroenterological Association does not recommend screening for insulin resistance among PPI users [and recommends that the decision to discontinue PPIs] should be based solely on the lack of an indication for PPI use, and not because of concern for PPI-associated adverse events,” he noted.
“Clinicians should be prepared to discuss the described risks associated with PPIs,” said Dr. Leiman, but they should “also feel comfortable affirming their safety profile and substantial efficacy in managing symptoms and preventing complications when prescribed for the appropriate indication.”
First-choice therapy for acid-related disorders
PPIs have become first-choice therapy for patients with acid-related disorders such as gastroesophageal reflux disease, Barrett esophagus, and peptic ulcer, and to prevent gastrointestinal bleeding while on nonsteroidal anti-inflammatory drugs (NSAIDs), Dr. Ciardullo and colleagues explain.
However, several studies have identified potential fractures, hypomagnesemia, gastric carcinoids, chronic kidney disease, dementia, and Clostridium difficile diarrhea with prolonged use of PPIs, and these agents can cause changes in the gut microbiome that may play a role in diabetes and other metabolic diseases.
To investigate a potential association between PPIs and type 2 diabetes, the researchers analyzed data from 777,420 patients age 40 and older who were newly treated with PPIs between 2010 and 2015 in Lombardy, Italy.
Of these, 50,540 patients were diagnosed with type 2 diabetes during follow-up until 2020 (a mean follow-up of 6.2 years and a diabetes incidence of 10.6 cases per 1,000 person-years).
The researchers matched 50,535 patients diagnosed with diabetes during follow-up with 50,535 control patients who had the same age, sex, and clinical status.
Patients were a mean age of 66 years and half were men. The most prescribed PPIs were pantoprazole and omeprazole, and the patients diagnosed with diabetes were more likely to use antihypertensives and lipid-lowering drugs.
Compared with patients who received PPIs for less than 8 weeks, those who received PPIs for 8 weeks to 6 months had a 19% increased risk of being diagnosed with diabetes during follow-up (odds ratio, 1.19; 95% confidence interval, 1.15-1.24), after adjusting for age, clinical profile, comorbidities, medical therapy, and PPI type.
Patients who received PPIs for 6 months to 2 years had a 43% increased risk of the outcome (OR, 1.43; 95% CI, 1.38-1.49), and those who received PPIs for more than 2 years had a 56% increased risk of the outcome (OR, 1.56; 95% CI, 1.49-1.64).
The researchers acknowledge limitations including that the study was not a randomized controlled trial, and it lacked information about over-the-counter medications and unmeasured confounders such as body mass index or family history of diabetes that may have affected the outcomes.
Dr. Leiman added that patients may have had prediabetes or undiagnosed diabetes and symptoms such as heartburn or dyspepsia arising from complications of insulin resistance, for which PPIs might have been prescribed.
The study was funded by a grant from the Italian Ministry of Education, University and Research. Dr. Ciardullo and Dr. Leiman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Vegan diet helps shed pounds but doesn’t dint diabetes
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
FROM ECO 2022
Breast cancer test recommended for extended endocrine therapy
In an updated clinical practice guideline, for women with early-stage, hormone receptor–positive breast cancer. The update applies to women who are node negative or have one to three positive nodes treated with 5 years of endocrine therapy and no sign of recurrence.
The update was published in the Journal of Clinical Oncology. It also gives more specific details on how to apply other, previously recommended, genomic tests to guide treatment choices.
More than half of breast cancer deaths occur after 5 years of tamoxifen therapy. The National Surgical Adjuvant Breast and Bowel Project (NSABP)- B14 trial, published in 2001, showed no benefit to extending tamoxifen therapy to 10 years, but other studies have produced mixed results.
Extended endocrine therapy may reduce the risk of recurrence, but significant side effects can impact quality of life, including osteoporosis, bone fractures, and joint pain. The uncertain benefits of extended endocrine therapy, combined with its side effects and impact on quality of life, has generated interest in genomic tests to identify patients most likely to benefit.
The BCI analyzes 11 genes from the tumor and delivers two results: the likelihood of recurrence 5-10 years after diagnosis, and whether a total of 10 years of endocrine therapy are likely to provide a survival benefit.
The 21-gene prognostic and predictive assay Oncotype DX Breast Recurrence Score, the 70-gene signature test Mammaprint, the 12-gene risk score EndoPredict, levels of Ki67 expression, and immunohistochemistry are also recommended for guiding decisions on endocrine therapy. The update included additional guidance on specific situations that each can be used. However, their usefulness for predicting recurrence at 5-10 years is unproven.
“The clinical decision to either extend or end adjuvant endocrine therapy after 5 years is a challenging decision for healthcare providers and their patients,” Mark Pegram, MD, said in a press release. He is chief medical consultant for breast oncology at Biotheranostics, a subsidiary of Hologic. “There is an extensive body of clinical evidence consistently proving the utility of BCI, and its addition to major oncology clinical guidelines like those from ASCO further underscores the test’s potential in clinical decision-making regarding extended adjuvant endocrine therapy.”
The practice update cited five previous studies showing the ability of BCI to predict benefit from extending endocrine therapy: From 5 years of tamoxifen to 5 more years of tamoxifen; from 5 years of tamoxifen to 5 years of an aromatase inhibitor, and from 5 years of an AI to another 5 years of a drug from the same class. Most of the trials included patients who were node negative or had one to three positive nodes, so there is limited evidence supporting BCI in patients with more than three positive lymph nodes. The recommendation also applies only to postmenopausal women, as the trials included fewer premenopausal and perimenopausal women.
Several of the guideline authors reported conflicts of interest with numerous sources.
In an updated clinical practice guideline, for women with early-stage, hormone receptor–positive breast cancer. The update applies to women who are node negative or have one to three positive nodes treated with 5 years of endocrine therapy and no sign of recurrence.
The update was published in the Journal of Clinical Oncology. It also gives more specific details on how to apply other, previously recommended, genomic tests to guide treatment choices.
More than half of breast cancer deaths occur after 5 years of tamoxifen therapy. The National Surgical Adjuvant Breast and Bowel Project (NSABP)- B14 trial, published in 2001, showed no benefit to extending tamoxifen therapy to 10 years, but other studies have produced mixed results.
Extended endocrine therapy may reduce the risk of recurrence, but significant side effects can impact quality of life, including osteoporosis, bone fractures, and joint pain. The uncertain benefits of extended endocrine therapy, combined with its side effects and impact on quality of life, has generated interest in genomic tests to identify patients most likely to benefit.
The BCI analyzes 11 genes from the tumor and delivers two results: the likelihood of recurrence 5-10 years after diagnosis, and whether a total of 10 years of endocrine therapy are likely to provide a survival benefit.
The 21-gene prognostic and predictive assay Oncotype DX Breast Recurrence Score, the 70-gene signature test Mammaprint, the 12-gene risk score EndoPredict, levels of Ki67 expression, and immunohistochemistry are also recommended for guiding decisions on endocrine therapy. The update included additional guidance on specific situations that each can be used. However, their usefulness for predicting recurrence at 5-10 years is unproven.
“The clinical decision to either extend or end adjuvant endocrine therapy after 5 years is a challenging decision for healthcare providers and their patients,” Mark Pegram, MD, said in a press release. He is chief medical consultant for breast oncology at Biotheranostics, a subsidiary of Hologic. “There is an extensive body of clinical evidence consistently proving the utility of BCI, and its addition to major oncology clinical guidelines like those from ASCO further underscores the test’s potential in clinical decision-making regarding extended adjuvant endocrine therapy.”
The practice update cited five previous studies showing the ability of BCI to predict benefit from extending endocrine therapy: From 5 years of tamoxifen to 5 more years of tamoxifen; from 5 years of tamoxifen to 5 years of an aromatase inhibitor, and from 5 years of an AI to another 5 years of a drug from the same class. Most of the trials included patients who were node negative or had one to three positive nodes, so there is limited evidence supporting BCI in patients with more than three positive lymph nodes. The recommendation also applies only to postmenopausal women, as the trials included fewer premenopausal and perimenopausal women.
Several of the guideline authors reported conflicts of interest with numerous sources.
In an updated clinical practice guideline, for women with early-stage, hormone receptor–positive breast cancer. The update applies to women who are node negative or have one to three positive nodes treated with 5 years of endocrine therapy and no sign of recurrence.
The update was published in the Journal of Clinical Oncology. It also gives more specific details on how to apply other, previously recommended, genomic tests to guide treatment choices.
More than half of breast cancer deaths occur after 5 years of tamoxifen therapy. The National Surgical Adjuvant Breast and Bowel Project (NSABP)- B14 trial, published in 2001, showed no benefit to extending tamoxifen therapy to 10 years, but other studies have produced mixed results.
Extended endocrine therapy may reduce the risk of recurrence, but significant side effects can impact quality of life, including osteoporosis, bone fractures, and joint pain. The uncertain benefits of extended endocrine therapy, combined with its side effects and impact on quality of life, has generated interest in genomic tests to identify patients most likely to benefit.
The BCI analyzes 11 genes from the tumor and delivers two results: the likelihood of recurrence 5-10 years after diagnosis, and whether a total of 10 years of endocrine therapy are likely to provide a survival benefit.
The 21-gene prognostic and predictive assay Oncotype DX Breast Recurrence Score, the 70-gene signature test Mammaprint, the 12-gene risk score EndoPredict, levels of Ki67 expression, and immunohistochemistry are also recommended for guiding decisions on endocrine therapy. The update included additional guidance on specific situations that each can be used. However, their usefulness for predicting recurrence at 5-10 years is unproven.
“The clinical decision to either extend or end adjuvant endocrine therapy after 5 years is a challenging decision for healthcare providers and their patients,” Mark Pegram, MD, said in a press release. He is chief medical consultant for breast oncology at Biotheranostics, a subsidiary of Hologic. “There is an extensive body of clinical evidence consistently proving the utility of BCI, and its addition to major oncology clinical guidelines like those from ASCO further underscores the test’s potential in clinical decision-making regarding extended adjuvant endocrine therapy.”
The practice update cited five previous studies showing the ability of BCI to predict benefit from extending endocrine therapy: From 5 years of tamoxifen to 5 more years of tamoxifen; from 5 years of tamoxifen to 5 years of an aromatase inhibitor, and from 5 years of an AI to another 5 years of a drug from the same class. Most of the trials included patients who were node negative or had one to three positive nodes, so there is limited evidence supporting BCI in patients with more than three positive lymph nodes. The recommendation also applies only to postmenopausal women, as the trials included fewer premenopausal and perimenopausal women.
Several of the guideline authors reported conflicts of interest with numerous sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Dodging potholes from cancer care to hospice transitions
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
Best antioxidants to prevent age-related dementia identified?
Investigators found that individuals with the highest serum levels of lutein + zeaxanthin and beta-cryptoxanthin at baseline were less likely to have dementia decades later than were their peers with lower levels of these antioxidants.
Lutein and zeaxanthin are found in green leafy vegetables such as kale, spinach, broccoli, and peas. Beta-cryptoxanthin is found in fruits such as oranges, papaya, tangerines, and persimmons.
“Antioxidants may help protect the brain from oxidative stress, which can cause cell damage,” first author May A. Beydoun, PhD, with the National Institute on Aging (NIA), said in a news release.
“This is the first nationally representative study to analyze blood levels of antioxidants in relation to dementia risk,” NIA scientific director Luigi Ferrucci, MD, said in an interview.
“Blood test results may be more representative of the actual antioxidant level than a person’s report of what kind of foods they regularly consume,” Dr. Ferrucci added.
The study was published online in Neurology.
Reduced dementia risk
The researchers tested associations and interactions of serum vitamins A, C and E, and total and individual serum carotenoids and interactions with incident Alzheimer’s disease (AD) and all-cause dementia.
They analyzed data from 7,283 participants in the Third National Health and Nutrition Examination Survey (NHANES III) who were at least 45 years old at baseline and followed for an average of 16-17 years.
They found serum levels of lutein + zeaxanthin were associated with reduced risk of all-cause dementia among people aged 65 and older in models adjusted for lifestyle.
For lutein + zeaxanthin, every standard deviation (SD) increase (roughly 15.4 µmol/liter) was associated with a 7% decrease in risk for dementia (hazard ratio [HR] 0.93; 95% confidence interval [CI], 0.87-0.99, P = .037). This association was attenuated somewhat after adjustment for socioeconomic status.
Serum levels of beta-cryptoxanthin showed a “strong” inverse relationship with all-cause dementia in age- and sex-adjusted models.
For beta-cryptoxanthin, every SD increase (roughly 8.6 µmol/liter) was associated with a 14% reduced risk for dementia in people aged 45 and older (HR, 0.86; 95% CI, 0.80-0.93, P < .001) and 65 and older (HR, 0.86; 95% CI, 0.80-0.93, P = .001).
This relationship remained strong in models adjusted for sociodemographic and socioeconomic factors but attenuated in subsequent models.
No associations were found for lycopene, alpha-carotene, beta-carotene, or vitamins A, C, or E in the fully adjusted models.
Antagonistic interactions were observed for vitamin A and alpha-carotene, vitamin A and beta-carotene, vitamin E and lycopene, and lycopene and beta-carotene, suggesting putative protective effects of one antioxidant at lower levels of the other, the researchers noted.
“This analysis of an observational study found that the most important carotenoids in potentially protecting the brain may be lutein + zeaxanthin and beta-cryptoxanthin. However, randomized controlled trials are needed to prove causality,” said Dr. Ferrucci.
“Experts do not yet know the daily level of antioxidant intake to promote healthy aging of the brain. More research is needed to establish the necessary level of antioxidant intake – through the diet and/or supplements – to promote brain health and healthy aging,” he added.
An important step forward
In an accompanying editorial, Babak Hooshmand, MD, PhD, and Miia Kivipelto, MD, PhD, with Karolinska Institute, Stockholm, noted that while nutrition and dietary components are “potential targets” for dementia risk reduction, observational studies to date have reported “inconsistent findings.”
This study is “an important step towards exploring the complex relationship between antioxidants and dementia because it accounts for factors that could possibly influence the associations and considers interactions between different components,” they wrote.
The findings are “challenging,” they added, because they may lead to the hypothesis that inhibition of oxidative damage by antioxidants might have beneficial effects on preventing dementia.
However, clinical trials of antioxidant supplementation have been mainly “disappointing” and a recent Cochrane review found a lack of evidence for supplement use to preserve cognitive function or prevent dementia, Dr. Hooshmand and Dr. Kivipelto noted.
They added that the study contributes to the belief that antioxidants don’t act independently of each other or other factors, including socioeconomic status and lifestyle, in the mediation of dementia risk.
“A careful examination of the evidence is required to learn how antioxidants influence the complex pathology of dementia, because it appears to be more to it than meets the eye,”they concluded.
The research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Institute on Aging. Dr. Beydoun, Dr. Ferrucci, and Dr. Hooshmand report no relevant disclosures. Dr. Kivipelto has supported advisory boards for Combinostics, Roche, and Biogen.
A version of this article first appeared on Medscape.com.
Investigators found that individuals with the highest serum levels of lutein + zeaxanthin and beta-cryptoxanthin at baseline were less likely to have dementia decades later than were their peers with lower levels of these antioxidants.
Lutein and zeaxanthin are found in green leafy vegetables such as kale, spinach, broccoli, and peas. Beta-cryptoxanthin is found in fruits such as oranges, papaya, tangerines, and persimmons.
“Antioxidants may help protect the brain from oxidative stress, which can cause cell damage,” first author May A. Beydoun, PhD, with the National Institute on Aging (NIA), said in a news release.
“This is the first nationally representative study to analyze blood levels of antioxidants in relation to dementia risk,” NIA scientific director Luigi Ferrucci, MD, said in an interview.
“Blood test results may be more representative of the actual antioxidant level than a person’s report of what kind of foods they regularly consume,” Dr. Ferrucci added.
The study was published online in Neurology.
Reduced dementia risk
The researchers tested associations and interactions of serum vitamins A, C and E, and total and individual serum carotenoids and interactions with incident Alzheimer’s disease (AD) and all-cause dementia.
They analyzed data from 7,283 participants in the Third National Health and Nutrition Examination Survey (NHANES III) who were at least 45 years old at baseline and followed for an average of 16-17 years.
They found serum levels of lutein + zeaxanthin were associated with reduced risk of all-cause dementia among people aged 65 and older in models adjusted for lifestyle.
For lutein + zeaxanthin, every standard deviation (SD) increase (roughly 15.4 µmol/liter) was associated with a 7% decrease in risk for dementia (hazard ratio [HR] 0.93; 95% confidence interval [CI], 0.87-0.99, P = .037). This association was attenuated somewhat after adjustment for socioeconomic status.
Serum levels of beta-cryptoxanthin showed a “strong” inverse relationship with all-cause dementia in age- and sex-adjusted models.
For beta-cryptoxanthin, every SD increase (roughly 8.6 µmol/liter) was associated with a 14% reduced risk for dementia in people aged 45 and older (HR, 0.86; 95% CI, 0.80-0.93, P < .001) and 65 and older (HR, 0.86; 95% CI, 0.80-0.93, P = .001).
This relationship remained strong in models adjusted for sociodemographic and socioeconomic factors but attenuated in subsequent models.
No associations were found for lycopene, alpha-carotene, beta-carotene, or vitamins A, C, or E in the fully adjusted models.
Antagonistic interactions were observed for vitamin A and alpha-carotene, vitamin A and beta-carotene, vitamin E and lycopene, and lycopene and beta-carotene, suggesting putative protective effects of one antioxidant at lower levels of the other, the researchers noted.
“This analysis of an observational study found that the most important carotenoids in potentially protecting the brain may be lutein + zeaxanthin and beta-cryptoxanthin. However, randomized controlled trials are needed to prove causality,” said Dr. Ferrucci.
“Experts do not yet know the daily level of antioxidant intake to promote healthy aging of the brain. More research is needed to establish the necessary level of antioxidant intake – through the diet and/or supplements – to promote brain health and healthy aging,” he added.
An important step forward
In an accompanying editorial, Babak Hooshmand, MD, PhD, and Miia Kivipelto, MD, PhD, with Karolinska Institute, Stockholm, noted that while nutrition and dietary components are “potential targets” for dementia risk reduction, observational studies to date have reported “inconsistent findings.”
This study is “an important step towards exploring the complex relationship between antioxidants and dementia because it accounts for factors that could possibly influence the associations and considers interactions between different components,” they wrote.
The findings are “challenging,” they added, because they may lead to the hypothesis that inhibition of oxidative damage by antioxidants might have beneficial effects on preventing dementia.
However, clinical trials of antioxidant supplementation have been mainly “disappointing” and a recent Cochrane review found a lack of evidence for supplement use to preserve cognitive function or prevent dementia, Dr. Hooshmand and Dr. Kivipelto noted.
They added that the study contributes to the belief that antioxidants don’t act independently of each other or other factors, including socioeconomic status and lifestyle, in the mediation of dementia risk.
“A careful examination of the evidence is required to learn how antioxidants influence the complex pathology of dementia, because it appears to be more to it than meets the eye,”they concluded.
The research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Institute on Aging. Dr. Beydoun, Dr. Ferrucci, and Dr. Hooshmand report no relevant disclosures. Dr. Kivipelto has supported advisory boards for Combinostics, Roche, and Biogen.
A version of this article first appeared on Medscape.com.
Investigators found that individuals with the highest serum levels of lutein + zeaxanthin and beta-cryptoxanthin at baseline were less likely to have dementia decades later than were their peers with lower levels of these antioxidants.
Lutein and zeaxanthin are found in green leafy vegetables such as kale, spinach, broccoli, and peas. Beta-cryptoxanthin is found in fruits such as oranges, papaya, tangerines, and persimmons.
“Antioxidants may help protect the brain from oxidative stress, which can cause cell damage,” first author May A. Beydoun, PhD, with the National Institute on Aging (NIA), said in a news release.
“This is the first nationally representative study to analyze blood levels of antioxidants in relation to dementia risk,” NIA scientific director Luigi Ferrucci, MD, said in an interview.
“Blood test results may be more representative of the actual antioxidant level than a person’s report of what kind of foods they regularly consume,” Dr. Ferrucci added.
The study was published online in Neurology.
Reduced dementia risk
The researchers tested associations and interactions of serum vitamins A, C and E, and total and individual serum carotenoids and interactions with incident Alzheimer’s disease (AD) and all-cause dementia.
They analyzed data from 7,283 participants in the Third National Health and Nutrition Examination Survey (NHANES III) who were at least 45 years old at baseline and followed for an average of 16-17 years.
They found serum levels of lutein + zeaxanthin were associated with reduced risk of all-cause dementia among people aged 65 and older in models adjusted for lifestyle.
For lutein + zeaxanthin, every standard deviation (SD) increase (roughly 15.4 µmol/liter) was associated with a 7% decrease in risk for dementia (hazard ratio [HR] 0.93; 95% confidence interval [CI], 0.87-0.99, P = .037). This association was attenuated somewhat after adjustment for socioeconomic status.
Serum levels of beta-cryptoxanthin showed a “strong” inverse relationship with all-cause dementia in age- and sex-adjusted models.
For beta-cryptoxanthin, every SD increase (roughly 8.6 µmol/liter) was associated with a 14% reduced risk for dementia in people aged 45 and older (HR, 0.86; 95% CI, 0.80-0.93, P < .001) and 65 and older (HR, 0.86; 95% CI, 0.80-0.93, P = .001).
This relationship remained strong in models adjusted for sociodemographic and socioeconomic factors but attenuated in subsequent models.
No associations were found for lycopene, alpha-carotene, beta-carotene, or vitamins A, C, or E in the fully adjusted models.
Antagonistic interactions were observed for vitamin A and alpha-carotene, vitamin A and beta-carotene, vitamin E and lycopene, and lycopene and beta-carotene, suggesting putative protective effects of one antioxidant at lower levels of the other, the researchers noted.
“This analysis of an observational study found that the most important carotenoids in potentially protecting the brain may be lutein + zeaxanthin and beta-cryptoxanthin. However, randomized controlled trials are needed to prove causality,” said Dr. Ferrucci.
“Experts do not yet know the daily level of antioxidant intake to promote healthy aging of the brain. More research is needed to establish the necessary level of antioxidant intake – through the diet and/or supplements – to promote brain health and healthy aging,” he added.
An important step forward
In an accompanying editorial, Babak Hooshmand, MD, PhD, and Miia Kivipelto, MD, PhD, with Karolinska Institute, Stockholm, noted that while nutrition and dietary components are “potential targets” for dementia risk reduction, observational studies to date have reported “inconsistent findings.”
This study is “an important step towards exploring the complex relationship between antioxidants and dementia because it accounts for factors that could possibly influence the associations and considers interactions between different components,” they wrote.
The findings are “challenging,” they added, because they may lead to the hypothesis that inhibition of oxidative damage by antioxidants might have beneficial effects on preventing dementia.
However, clinical trials of antioxidant supplementation have been mainly “disappointing” and a recent Cochrane review found a lack of evidence for supplement use to preserve cognitive function or prevent dementia, Dr. Hooshmand and Dr. Kivipelto noted.
They added that the study contributes to the belief that antioxidants don’t act independently of each other or other factors, including socioeconomic status and lifestyle, in the mediation of dementia risk.
“A careful examination of the evidence is required to learn how antioxidants influence the complex pathology of dementia, because it appears to be more to it than meets the eye,”they concluded.
The research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Institute on Aging. Dr. Beydoun, Dr. Ferrucci, and Dr. Hooshmand report no relevant disclosures. Dr. Kivipelto has supported advisory boards for Combinostics, Roche, and Biogen.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Misconceptions remain on gene signature use in breast cancer
BERLIN – , a European survey suggests.
The authors found, for instance, that while most specialists agreed that molecular intrinsic subtypes had clinical utility for understanding prognosis in early-stage hormone receptor (HR)–positive disease and for identifying patients for whom chemotherapy could be safely avoided, about 1 in 4 experts either disagreed or felt neutral about the use of signatures in these settings.
Similarly, almost 75% of respondents felt that these signatures were not useful in the triple-negative or metastatic setting, but a small percentage believed they were, and about 10% were neutral.
“Considering that breast cancer multigene signatures were developed in the post menopausal HR+/HER2- early breast cancer setting, the fact that some experts consider [them] useful in triple-negative, HER2+ breast cancer or in the metastatic setting corroborates a misunderstanding on how to interpret the results,” study author Giuseppe Curigliano, MD, PhD, associate professor of medical oncology at the University of Milan, and colleagues wrote.
Dr. Curigliano, who is also head of the Division of Early Drug Development at the European Institute of Oncology, presented the survey findings on May 4 at the European Society for Medical Oncology (ESMO BCC) Breast Cancer Congress.
Although several breast cancer multigene signatures are available to profile early breast cancer, little information exists on how these signatures should be used in clinical practice.
To investigate, Dr. Curigliano and colleagues convened a scientific committee of eight breast cancer experts to develop a Delphi questionnaire to examine respondents’ opinions and uses of these signatures.
The questionnaire asked about the clinical utility of multigene signatures in breast cancer and recommendations for their use in clinical practice.
In all, 133 breast cancer specialists from 11 European countries completed the questionnaire. Respondents were about 49 years old on average, and most (86.5%) worked in a teaching hospital. More than 72% were medical oncologists; 12% were pathologists.
Consensus was considered to be reached when 70% or more of the respondents were in agreement on a topic.
Participants had “extensive experience in the management of breast cancer patients and have been using breast cancer multigene signatures in clinical practice,” Dr. Curigliano said.
Almost all respondents (93.6%) reported using breast cancer multigene signatures routinely or in selected patients, and 73.4% had more than 5 years of experience with them.
Overall, more than 70% of respondents agreed that identifying tumor intrinsic subtype via gene expression profiling was important in making prognostic and treatment decisions; however, a consensus was not reached on the use of immunohistochemistry.
In addition, most respondents (76%) agreed that identifying breast cancer molecular intrinsic subtypes had clinical utility for prognosis in early-stage HR-positive disease and for identifying patients for whom chemotherapy can be safely avoided (75%). However, in both cases, about one-quarter of respondents either disagreed or felt neutral.
No consensus was reached on the clinical utility of these subtypes for selecting the most appropriate chemotherapy treatment – two-thirds disagreed, while 13% agreed and 17% felt neutral.
When deciding on the use of chemotherapy in the adjuvant setting in early node-negative breast cancer, 88% of respondents felt that breast cancer multigene signatures were important. Moreover, 75% considered such signatures important when deciding whether to use chemotherapy in the adjuvant setting for patients with one to three positive lymph nodes. However, no consensus was reached on the utility of signatures for deciding whether to extend endocrine therapy in either setting.
When examining the usefulness of signatures in more special settings, the authors found that the vast majority (90%) of respondents believed that multigene signatures had clinical utility for postmenopausal early breast cancer patients, and 82% did not consider signatures clinically useful in the early-stage HER2-overexpressed setting.
In addition, 74% thought that breast cancer multigene signatures were not useful in triple-negative disease or in the metastatic setting.
Respondents did not reach a consensus on the clinical utility of multigene signatures in the neoadjuvant setting – only 27% considered them useful, and almost half did not.
The “low percentage” of respondents using the signatures in the neoadjuvant setting and the “misconception regarding the predictive value of these tests on chemotherapy benefits suggest there is still room for training on results interpretation [for breast cancer multigene signatures],” the authors write.
The study was sponsored by Veracyte. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, and Veracyte. No other relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
This article was updated 5/9/22.
BERLIN – , a European survey suggests.
The authors found, for instance, that while most specialists agreed that molecular intrinsic subtypes had clinical utility for understanding prognosis in early-stage hormone receptor (HR)–positive disease and for identifying patients for whom chemotherapy could be safely avoided, about 1 in 4 experts either disagreed or felt neutral about the use of signatures in these settings.
Similarly, almost 75% of respondents felt that these signatures were not useful in the triple-negative or metastatic setting, but a small percentage believed they were, and about 10% were neutral.
“Considering that breast cancer multigene signatures were developed in the post menopausal HR+/HER2- early breast cancer setting, the fact that some experts consider [them] useful in triple-negative, HER2+ breast cancer or in the metastatic setting corroborates a misunderstanding on how to interpret the results,” study author Giuseppe Curigliano, MD, PhD, associate professor of medical oncology at the University of Milan, and colleagues wrote.
Dr. Curigliano, who is also head of the Division of Early Drug Development at the European Institute of Oncology, presented the survey findings on May 4 at the European Society for Medical Oncology (ESMO BCC) Breast Cancer Congress.
Although several breast cancer multigene signatures are available to profile early breast cancer, little information exists on how these signatures should be used in clinical practice.
To investigate, Dr. Curigliano and colleagues convened a scientific committee of eight breast cancer experts to develop a Delphi questionnaire to examine respondents’ opinions and uses of these signatures.
The questionnaire asked about the clinical utility of multigene signatures in breast cancer and recommendations for their use in clinical practice.
In all, 133 breast cancer specialists from 11 European countries completed the questionnaire. Respondents were about 49 years old on average, and most (86.5%) worked in a teaching hospital. More than 72% were medical oncologists; 12% were pathologists.
Consensus was considered to be reached when 70% or more of the respondents were in agreement on a topic.
Participants had “extensive experience in the management of breast cancer patients and have been using breast cancer multigene signatures in clinical practice,” Dr. Curigliano said.
Almost all respondents (93.6%) reported using breast cancer multigene signatures routinely or in selected patients, and 73.4% had more than 5 years of experience with them.
Overall, more than 70% of respondents agreed that identifying tumor intrinsic subtype via gene expression profiling was important in making prognostic and treatment decisions; however, a consensus was not reached on the use of immunohistochemistry.
In addition, most respondents (76%) agreed that identifying breast cancer molecular intrinsic subtypes had clinical utility for prognosis in early-stage HR-positive disease and for identifying patients for whom chemotherapy can be safely avoided (75%). However, in both cases, about one-quarter of respondents either disagreed or felt neutral.
No consensus was reached on the clinical utility of these subtypes for selecting the most appropriate chemotherapy treatment – two-thirds disagreed, while 13% agreed and 17% felt neutral.
When deciding on the use of chemotherapy in the adjuvant setting in early node-negative breast cancer, 88% of respondents felt that breast cancer multigene signatures were important. Moreover, 75% considered such signatures important when deciding whether to use chemotherapy in the adjuvant setting for patients with one to three positive lymph nodes. However, no consensus was reached on the utility of signatures for deciding whether to extend endocrine therapy in either setting.
When examining the usefulness of signatures in more special settings, the authors found that the vast majority (90%) of respondents believed that multigene signatures had clinical utility for postmenopausal early breast cancer patients, and 82% did not consider signatures clinically useful in the early-stage HER2-overexpressed setting.
In addition, 74% thought that breast cancer multigene signatures were not useful in triple-negative disease or in the metastatic setting.
Respondents did not reach a consensus on the clinical utility of multigene signatures in the neoadjuvant setting – only 27% considered them useful, and almost half did not.
The “low percentage” of respondents using the signatures in the neoadjuvant setting and the “misconception regarding the predictive value of these tests on chemotherapy benefits suggest there is still room for training on results interpretation [for breast cancer multigene signatures],” the authors write.
The study was sponsored by Veracyte. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, and Veracyte. No other relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
This article was updated 5/9/22.
BERLIN – , a European survey suggests.
The authors found, for instance, that while most specialists agreed that molecular intrinsic subtypes had clinical utility for understanding prognosis in early-stage hormone receptor (HR)–positive disease and for identifying patients for whom chemotherapy could be safely avoided, about 1 in 4 experts either disagreed or felt neutral about the use of signatures in these settings.
Similarly, almost 75% of respondents felt that these signatures were not useful in the triple-negative or metastatic setting, but a small percentage believed they were, and about 10% were neutral.
“Considering that breast cancer multigene signatures were developed in the post menopausal HR+/HER2- early breast cancer setting, the fact that some experts consider [them] useful in triple-negative, HER2+ breast cancer or in the metastatic setting corroborates a misunderstanding on how to interpret the results,” study author Giuseppe Curigliano, MD, PhD, associate professor of medical oncology at the University of Milan, and colleagues wrote.
Dr. Curigliano, who is also head of the Division of Early Drug Development at the European Institute of Oncology, presented the survey findings on May 4 at the European Society for Medical Oncology (ESMO BCC) Breast Cancer Congress.
Although several breast cancer multigene signatures are available to profile early breast cancer, little information exists on how these signatures should be used in clinical practice.
To investigate, Dr. Curigliano and colleagues convened a scientific committee of eight breast cancer experts to develop a Delphi questionnaire to examine respondents’ opinions and uses of these signatures.
The questionnaire asked about the clinical utility of multigene signatures in breast cancer and recommendations for their use in clinical practice.
In all, 133 breast cancer specialists from 11 European countries completed the questionnaire. Respondents were about 49 years old on average, and most (86.5%) worked in a teaching hospital. More than 72% were medical oncologists; 12% were pathologists.
Consensus was considered to be reached when 70% or more of the respondents were in agreement on a topic.
Participants had “extensive experience in the management of breast cancer patients and have been using breast cancer multigene signatures in clinical practice,” Dr. Curigliano said.
Almost all respondents (93.6%) reported using breast cancer multigene signatures routinely or in selected patients, and 73.4% had more than 5 years of experience with them.
Overall, more than 70% of respondents agreed that identifying tumor intrinsic subtype via gene expression profiling was important in making prognostic and treatment decisions; however, a consensus was not reached on the use of immunohistochemistry.
In addition, most respondents (76%) agreed that identifying breast cancer molecular intrinsic subtypes had clinical utility for prognosis in early-stage HR-positive disease and for identifying patients for whom chemotherapy can be safely avoided (75%). However, in both cases, about one-quarter of respondents either disagreed or felt neutral.
No consensus was reached on the clinical utility of these subtypes for selecting the most appropriate chemotherapy treatment – two-thirds disagreed, while 13% agreed and 17% felt neutral.
When deciding on the use of chemotherapy in the adjuvant setting in early node-negative breast cancer, 88% of respondents felt that breast cancer multigene signatures were important. Moreover, 75% considered such signatures important when deciding whether to use chemotherapy in the adjuvant setting for patients with one to three positive lymph nodes. However, no consensus was reached on the utility of signatures for deciding whether to extend endocrine therapy in either setting.
When examining the usefulness of signatures in more special settings, the authors found that the vast majority (90%) of respondents believed that multigene signatures had clinical utility for postmenopausal early breast cancer patients, and 82% did not consider signatures clinically useful in the early-stage HER2-overexpressed setting.
In addition, 74% thought that breast cancer multigene signatures were not useful in triple-negative disease or in the metastatic setting.
Respondents did not reach a consensus on the clinical utility of multigene signatures in the neoadjuvant setting – only 27% considered them useful, and almost half did not.
The “low percentage” of respondents using the signatures in the neoadjuvant setting and the “misconception regarding the predictive value of these tests on chemotherapy benefits suggest there is still room for training on results interpretation [for breast cancer multigene signatures],” the authors write.
The study was sponsored by Veracyte. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, and Veracyte. No other relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
This article was updated 5/9/22.
AT ESMO BCC 2022
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
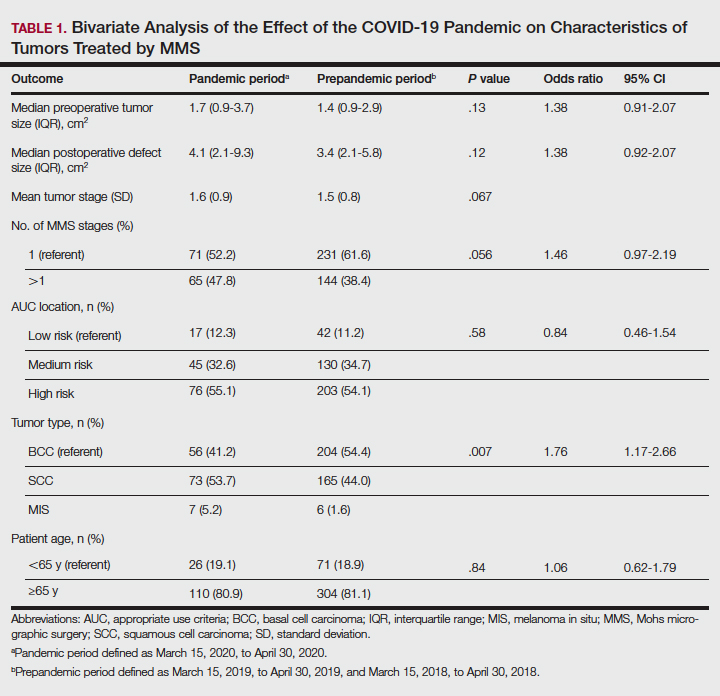
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
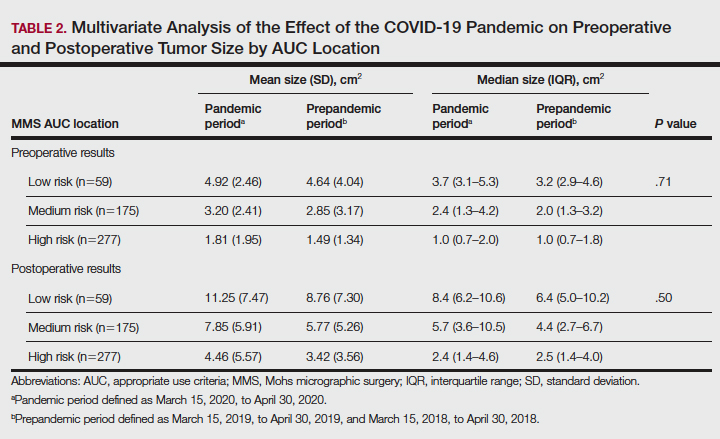
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Many state cancer plans drift from USPSTF breast cancer recommendations
When it comes to mammography recommendations state comprehensive cancer control (CCC) plans vary considerably and don’t always closely match the U.S. Preventive Services Task Force (USPSTF) recommendations for mammography frequency in women at average risk for breast cancer, according to a new cross-sectional study of CCC plans in all 50 states and the District of Columbia. The recommended age for initiation varied widely among CCC plans, and nearly one in five bore little resemblance at all to the USPTF recommendations.
According to the authors of the study, the variation among suggested ages of initiation may indicate a lack of consensus among state agencies. “For a recommendation tied to service coverage, this is a serious gap in public health policy,” they wrote in the study published online in JAMA Network Open.
CCC plans include goals, measurable objectives, and evidence-based strategies to combat cancers that are both common and preventable. They include input from multiple groups, frequently take 4-6 years to create, and should be updated regularly. Funding from the Centers for Disease Control and Prevention requires that the plans include data cancer screening prevalence rates and specific objectives and strategies.
Breast cancer is the most common cancer in women in the United States, and the second highest cause of cancer death. Regular, high-quality screening reduces breast cancer mortality by 25%-31% among women aged 50-69. As a result, the American Cancer Society, the USPSTF, the American College of Physicians, and the American Academy of Family Physicians have produced guidelines for mammography screening in women at average risk of breast cancer.
Although the benefits of screening are widely accepted, there is disagreement about the ages it should be initiated and ended. These inconsistencies stem from different evidence used to support recommendations, as well as different standards for benefits and harms from screening. Common concerns include overdiagnosis, false-positive results, and radiation damage from mammography.
Because these benefits and harms can vary based on age and values, there is an emphasis on shared decision-making between clinicians and women, especially those aged 40-49.
The most recent USPTF recommendation, issued in 2016, states that women aged 50-74 with average risk should be screened with mammography every 2 years. The choice of mammography in average-risk women under 50 should be approached on an individual basis. USPTF defines average risk as having no signs, symptoms, or previous diagnosis of breast cancer, and no family history or genetic causes for concern.
In the new study, researchers conducted a point-in-time evaluation of CCC plans from 50 states and the District of Columbia, between Nov. 1, 2019, and June 30, 2021.
Thirty-one percent of the plans included the complete USPTF recommendations of biennial mammography between ages 50 and 74; 51% included some, but not all of the USPTF recommendations; and 18% were not consistent at all with USPTF recommendations.
Overall, 59% of plans recommended initiation at age 50 and 37% at age 40, which is consistent with the older 2009 USPSTF recommendation. Eight percent of plans recommended starting at both 40 and 50, and 20% of plans had no recommended age of initiation.
Among the plans that were partially consistent with USPTF, 73% recommended initiation of mammography at age 40 and 31% at age 50. Eighty-five percent did not include an age to stop mammography; 15% did not include a recommended frequency; and 15% had an initiation age other than 40 or 50. Eighty-five percent of plans partially consistent with USPSTF included a recommendation that mammography should be conducted biennially.
The authors state that CCC plans could be improved by a unified emphasis on biennial screening of the general population of women aged 50-74, as well as clear differentiation between women at average risk and those at high risk, who could be screened at ages younger than 50 in consultation with their physician.
The study is limited by the fact that plans were reviewed a single time, while state CCC plans are updated with varying periodicity. The authors agree that implementation of population-based screening should be tailored to individual states and health care systems.
When it comes to mammography recommendations state comprehensive cancer control (CCC) plans vary considerably and don’t always closely match the U.S. Preventive Services Task Force (USPSTF) recommendations for mammography frequency in women at average risk for breast cancer, according to a new cross-sectional study of CCC plans in all 50 states and the District of Columbia. The recommended age for initiation varied widely among CCC plans, and nearly one in five bore little resemblance at all to the USPTF recommendations.
According to the authors of the study, the variation among suggested ages of initiation may indicate a lack of consensus among state agencies. “For a recommendation tied to service coverage, this is a serious gap in public health policy,” they wrote in the study published online in JAMA Network Open.
CCC plans include goals, measurable objectives, and evidence-based strategies to combat cancers that are both common and preventable. They include input from multiple groups, frequently take 4-6 years to create, and should be updated regularly. Funding from the Centers for Disease Control and Prevention requires that the plans include data cancer screening prevalence rates and specific objectives and strategies.
Breast cancer is the most common cancer in women in the United States, and the second highest cause of cancer death. Regular, high-quality screening reduces breast cancer mortality by 25%-31% among women aged 50-69. As a result, the American Cancer Society, the USPSTF, the American College of Physicians, and the American Academy of Family Physicians have produced guidelines for mammography screening in women at average risk of breast cancer.
Although the benefits of screening are widely accepted, there is disagreement about the ages it should be initiated and ended. These inconsistencies stem from different evidence used to support recommendations, as well as different standards for benefits and harms from screening. Common concerns include overdiagnosis, false-positive results, and radiation damage from mammography.
Because these benefits and harms can vary based on age and values, there is an emphasis on shared decision-making between clinicians and women, especially those aged 40-49.
The most recent USPTF recommendation, issued in 2016, states that women aged 50-74 with average risk should be screened with mammography every 2 years. The choice of mammography in average-risk women under 50 should be approached on an individual basis. USPTF defines average risk as having no signs, symptoms, or previous diagnosis of breast cancer, and no family history or genetic causes for concern.
In the new study, researchers conducted a point-in-time evaluation of CCC plans from 50 states and the District of Columbia, between Nov. 1, 2019, and June 30, 2021.
Thirty-one percent of the plans included the complete USPTF recommendations of biennial mammography between ages 50 and 74; 51% included some, but not all of the USPTF recommendations; and 18% were not consistent at all with USPTF recommendations.
Overall, 59% of plans recommended initiation at age 50 and 37% at age 40, which is consistent with the older 2009 USPSTF recommendation. Eight percent of plans recommended starting at both 40 and 50, and 20% of plans had no recommended age of initiation.
Among the plans that were partially consistent with USPTF, 73% recommended initiation of mammography at age 40 and 31% at age 50. Eighty-five percent did not include an age to stop mammography; 15% did not include a recommended frequency; and 15% had an initiation age other than 40 or 50. Eighty-five percent of plans partially consistent with USPSTF included a recommendation that mammography should be conducted biennially.
The authors state that CCC plans could be improved by a unified emphasis on biennial screening of the general population of women aged 50-74, as well as clear differentiation between women at average risk and those at high risk, who could be screened at ages younger than 50 in consultation with their physician.
The study is limited by the fact that plans were reviewed a single time, while state CCC plans are updated with varying periodicity. The authors agree that implementation of population-based screening should be tailored to individual states and health care systems.
When it comes to mammography recommendations state comprehensive cancer control (CCC) plans vary considerably and don’t always closely match the U.S. Preventive Services Task Force (USPSTF) recommendations for mammography frequency in women at average risk for breast cancer, according to a new cross-sectional study of CCC plans in all 50 states and the District of Columbia. The recommended age for initiation varied widely among CCC plans, and nearly one in five bore little resemblance at all to the USPTF recommendations.
According to the authors of the study, the variation among suggested ages of initiation may indicate a lack of consensus among state agencies. “For a recommendation tied to service coverage, this is a serious gap in public health policy,” they wrote in the study published online in JAMA Network Open.
CCC plans include goals, measurable objectives, and evidence-based strategies to combat cancers that are both common and preventable. They include input from multiple groups, frequently take 4-6 years to create, and should be updated regularly. Funding from the Centers for Disease Control and Prevention requires that the plans include data cancer screening prevalence rates and specific objectives and strategies.
Breast cancer is the most common cancer in women in the United States, and the second highest cause of cancer death. Regular, high-quality screening reduces breast cancer mortality by 25%-31% among women aged 50-69. As a result, the American Cancer Society, the USPSTF, the American College of Physicians, and the American Academy of Family Physicians have produced guidelines for mammography screening in women at average risk of breast cancer.
Although the benefits of screening are widely accepted, there is disagreement about the ages it should be initiated and ended. These inconsistencies stem from different evidence used to support recommendations, as well as different standards for benefits and harms from screening. Common concerns include overdiagnosis, false-positive results, and radiation damage from mammography.
Because these benefits and harms can vary based on age and values, there is an emphasis on shared decision-making between clinicians and women, especially those aged 40-49.
The most recent USPTF recommendation, issued in 2016, states that women aged 50-74 with average risk should be screened with mammography every 2 years. The choice of mammography in average-risk women under 50 should be approached on an individual basis. USPTF defines average risk as having no signs, symptoms, or previous diagnosis of breast cancer, and no family history or genetic causes for concern.
In the new study, researchers conducted a point-in-time evaluation of CCC plans from 50 states and the District of Columbia, between Nov. 1, 2019, and June 30, 2021.
Thirty-one percent of the plans included the complete USPTF recommendations of biennial mammography between ages 50 and 74; 51% included some, but not all of the USPTF recommendations; and 18% were not consistent at all with USPTF recommendations.
Overall, 59% of plans recommended initiation at age 50 and 37% at age 40, which is consistent with the older 2009 USPSTF recommendation. Eight percent of plans recommended starting at both 40 and 50, and 20% of plans had no recommended age of initiation.
Among the plans that were partially consistent with USPTF, 73% recommended initiation of mammography at age 40 and 31% at age 50. Eighty-five percent did not include an age to stop mammography; 15% did not include a recommended frequency; and 15% had an initiation age other than 40 or 50. Eighty-five percent of plans partially consistent with USPSTF included a recommendation that mammography should be conducted biennially.
The authors state that CCC plans could be improved by a unified emphasis on biennial screening of the general population of women aged 50-74, as well as clear differentiation between women at average risk and those at high risk, who could be screened at ages younger than 50 in consultation with their physician.
The study is limited by the fact that plans were reviewed a single time, while state CCC plans are updated with varying periodicity. The authors agree that implementation of population-based screening should be tailored to individual states and health care systems.
FROM JAMA NETWORK OPEN
New data confirm risk of Guillain-Barré with J&J COVID shot
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Air pollution is a seizure trigger for patients with epilepsy
, a unique new study suggests.
The link between daily outdoor CO exposure and seizure risk was particularly evident for subclinical seizures – those in patients with abnormal electroencephalography (EEG) signals but no clinical symptoms.
“Our findings suggest that people with epilepsy should avoid high CO exposure to reduce potential seizure risk,” said study investigator Zhuying Chen, PhD candidate, department of biomedical engineering, University of Melbourne.
The study was published online in Epilepsia.
Pollution’s impact on brain health
Emerging evidence indicates air pollution affects brain health and may increase the risk of hospitalization or outpatient visits for epilepsy. However, little is known about the effect of pollution on the occurrence of epileptic seizures.
The study used two independent long-term seizure datasets – the NeuroVista (NV) study and the Seer App seizure diary (SD). In the NeuroVista study, researchers recorded continuous intracranial iEEG from patients with refractory focal epilepsy who had been implanted with a personal seizure advisory device that wirelessly recorded seizures on an external device.
The SD dataset included diaries documenting self-reported seizures, seizure cycles, and medication adherence.
Researchers collected data on hourly concentrations of outdoor CO, nitrogen dioxide (NO2), particulate matter of 10 μm or less in diameter (PM10), ozone (O3), and sulfur dioxide (SO2). The levels were measured at air quality monitoring stations in Australia.
Investigators aggregated hourly observations into daily mean data. All daily concentrations of CO and SO2 and at least 95% of daily concentrations of NO2, O3, and PM10 were within Australian air quality standards, said Mr. Chen.
The study included 49 participants, with epilepsy data on 15 patients in the NeuroVista study and on 34 from the SD dataset.
Overall, 6,692 epileptic seizures on 3,639 seizure days were recorded during 23,349 follow-up days from 2010 to 2012 (NV dataset) and 2018 to 2021 (SD dataset).
The investigators found a significant positive association between CO concentrations and epileptic seizure risks. The relative risk (RR) was 1.04 (95% confidence interval, 1.01–1.07; P < .01) for an interquartile range (IQR) increase of CO (0.13 parts per million).
Sex differences
There were no significant relationships for the other four air pollutants. However, Mr. Chen noted that Australia has very low air pollution levels; most usually are within World Health Organization air quality guidelines.
“Our findings may not be generalized to other countries with high air pollution levels,” said Mr. Chen. He noted that the relatively small number of patients in the study may limit the statistical power to detect some associations.
The study showed that females had a significantly increased risk of epileptic seizures when exposed to elevated CO (RR, 1.05; 95% CI, 1.01–1.08; P < .05) and NO2 (RR, 1.09; 95% CI, 1.01–1.16; P < .05) concentrations. There were no significant associations in males for any air pollutants.
Differences in outdoor activities and behaviors such as smoking and exercise may lead to variations in environmental exposure and help explain the sex differences, said Mr. Chen. These differences may also be due to the study’s limited sample size.
Analyzing the two datasets separately, the researchers found there was a significant association between CO concentration and epileptic seizure risk in the NV dataset (RR, 1.10; 95% CI, 1.03–1.17; P < .01).
There were no significant associations in the SD dataset for any air pollutants. This may be because only clinical seizures – those associated with evident symptoms – are self-reported, said Mr. Chen. He also noted that seizure diaries may be unreliable.
In the NV dataset, the epileptic seizure risk was significantly increased when only subclinical seizures were considered (RR, 1.20; 95% CI, 1.12–1.28; P < .001) for an IQR increase of CO concentration.
The risk was significantly decreased by 13% for subclinical seizures with an IQR increase of PM10 and by 9% for subclinical seizures with an IQR increase of SO2 concentrations.
These negative associations should be interpreted with caution, inasmuch as the associations were not robust in subsequent subgroup and sensitivity analyses, said Mr. Chen.
There were no significant associations when considering clinical seizures for any air pollutants.
The positive association for subclinical but not clinical seizures suggests that low-level CO exposure may not be strong enough to directly trigger clinical seizures, said Mr. Chen.
Although previous research has demonstrated adverse neurologic effects of exposure to air pollutants, most studies were based on hospital databases or registers. Thus, they may have missed seizures that did not lead to hospital admission.
Unclear mechanism
The exact mechanisms linking air pollution to seizures are unclear but probably involve the synergistic interaction of multiple pathways, said Mr. Chen. “Air pollution may affect brain metabolism, alter the immune response of the brain, and induce oxidative stress and neuroinflammation, causing the brain to be more susceptible to seizures,” he noted.
This is the first study to investigate seizure rates through intracranial EEG signals and self-reported seizure diaries. It’s also the first to look into the impact of pollutants at low concentration levels on subclinical seizures.
However, the study has some limitations. Self-reported seizures in the SD dataset might underestimate the influence of air pollution on seizures. The study used postal codes as proxies for exposure to pollution, which could introduce measurement errors and underestimate associations.
In addition, Mr. Chen noted that seizures from the NeuroVista dataset were recorded from patients with drug-resistant focal epilepsy. “Whether our findings can be generalized to other epilepsy types needs further investigation.”
The study could have important clinical and public health implications. For example, said Mr. Chen, it’s possible that seizure risk could be reduced through behavioral interventions, such as avoiding being outside or using an air filtration system when pollutant levels are high.
“Clinicians could counsel their patients to avoid the potential risk of high carbon monoxide exposure,” he said.
CO exposure could be a new factor for seizure risk forecasting, which could reduce the uncertainty of seizures and help guide epilepsy management, Mr. Chen added.
The study was supported by the Melbourne Monash Consciousness Research Seed Funding and an Australian National Health and Medical Research Council Ideas grant. Mr. Chen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a unique new study suggests.
The link between daily outdoor CO exposure and seizure risk was particularly evident for subclinical seizures – those in patients with abnormal electroencephalography (EEG) signals but no clinical symptoms.
“Our findings suggest that people with epilepsy should avoid high CO exposure to reduce potential seizure risk,” said study investigator Zhuying Chen, PhD candidate, department of biomedical engineering, University of Melbourne.
The study was published online in Epilepsia.
Pollution’s impact on brain health
Emerging evidence indicates air pollution affects brain health and may increase the risk of hospitalization or outpatient visits for epilepsy. However, little is known about the effect of pollution on the occurrence of epileptic seizures.
The study used two independent long-term seizure datasets – the NeuroVista (NV) study and the Seer App seizure diary (SD). In the NeuroVista study, researchers recorded continuous intracranial iEEG from patients with refractory focal epilepsy who had been implanted with a personal seizure advisory device that wirelessly recorded seizures on an external device.
The SD dataset included diaries documenting self-reported seizures, seizure cycles, and medication adherence.
Researchers collected data on hourly concentrations of outdoor CO, nitrogen dioxide (NO2), particulate matter of 10 μm or less in diameter (PM10), ozone (O3), and sulfur dioxide (SO2). The levels were measured at air quality monitoring stations in Australia.
Investigators aggregated hourly observations into daily mean data. All daily concentrations of CO and SO2 and at least 95% of daily concentrations of NO2, O3, and PM10 were within Australian air quality standards, said Mr. Chen.
The study included 49 participants, with epilepsy data on 15 patients in the NeuroVista study and on 34 from the SD dataset.
Overall, 6,692 epileptic seizures on 3,639 seizure days were recorded during 23,349 follow-up days from 2010 to 2012 (NV dataset) and 2018 to 2021 (SD dataset).
The investigators found a significant positive association between CO concentrations and epileptic seizure risks. The relative risk (RR) was 1.04 (95% confidence interval, 1.01–1.07; P < .01) for an interquartile range (IQR) increase of CO (0.13 parts per million).
Sex differences
There were no significant relationships for the other four air pollutants. However, Mr. Chen noted that Australia has very low air pollution levels; most usually are within World Health Organization air quality guidelines.
“Our findings may not be generalized to other countries with high air pollution levels,” said Mr. Chen. He noted that the relatively small number of patients in the study may limit the statistical power to detect some associations.
The study showed that females had a significantly increased risk of epileptic seizures when exposed to elevated CO (RR, 1.05; 95% CI, 1.01–1.08; P < .05) and NO2 (RR, 1.09; 95% CI, 1.01–1.16; P < .05) concentrations. There were no significant associations in males for any air pollutants.
Differences in outdoor activities and behaviors such as smoking and exercise may lead to variations in environmental exposure and help explain the sex differences, said Mr. Chen. These differences may also be due to the study’s limited sample size.
Analyzing the two datasets separately, the researchers found there was a significant association between CO concentration and epileptic seizure risk in the NV dataset (RR, 1.10; 95% CI, 1.03–1.17; P < .01).
There were no significant associations in the SD dataset for any air pollutants. This may be because only clinical seizures – those associated with evident symptoms – are self-reported, said Mr. Chen. He also noted that seizure diaries may be unreliable.
In the NV dataset, the epileptic seizure risk was significantly increased when only subclinical seizures were considered (RR, 1.20; 95% CI, 1.12–1.28; P < .001) for an IQR increase of CO concentration.
The risk was significantly decreased by 13% for subclinical seizures with an IQR increase of PM10 and by 9% for subclinical seizures with an IQR increase of SO2 concentrations.
These negative associations should be interpreted with caution, inasmuch as the associations were not robust in subsequent subgroup and sensitivity analyses, said Mr. Chen.
There were no significant associations when considering clinical seizures for any air pollutants.
The positive association for subclinical but not clinical seizures suggests that low-level CO exposure may not be strong enough to directly trigger clinical seizures, said Mr. Chen.
Although previous research has demonstrated adverse neurologic effects of exposure to air pollutants, most studies were based on hospital databases or registers. Thus, they may have missed seizures that did not lead to hospital admission.
Unclear mechanism
The exact mechanisms linking air pollution to seizures are unclear but probably involve the synergistic interaction of multiple pathways, said Mr. Chen. “Air pollution may affect brain metabolism, alter the immune response of the brain, and induce oxidative stress and neuroinflammation, causing the brain to be more susceptible to seizures,” he noted.
This is the first study to investigate seizure rates through intracranial EEG signals and self-reported seizure diaries. It’s also the first to look into the impact of pollutants at low concentration levels on subclinical seizures.
However, the study has some limitations. Self-reported seizures in the SD dataset might underestimate the influence of air pollution on seizures. The study used postal codes as proxies for exposure to pollution, which could introduce measurement errors and underestimate associations.
In addition, Mr. Chen noted that seizures from the NeuroVista dataset were recorded from patients with drug-resistant focal epilepsy. “Whether our findings can be generalized to other epilepsy types needs further investigation.”
The study could have important clinical and public health implications. For example, said Mr. Chen, it’s possible that seizure risk could be reduced through behavioral interventions, such as avoiding being outside or using an air filtration system when pollutant levels are high.
“Clinicians could counsel their patients to avoid the potential risk of high carbon monoxide exposure,” he said.
CO exposure could be a new factor for seizure risk forecasting, which could reduce the uncertainty of seizures and help guide epilepsy management, Mr. Chen added.
The study was supported by the Melbourne Monash Consciousness Research Seed Funding and an Australian National Health and Medical Research Council Ideas grant. Mr. Chen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a unique new study suggests.
The link between daily outdoor CO exposure and seizure risk was particularly evident for subclinical seizures – those in patients with abnormal electroencephalography (EEG) signals but no clinical symptoms.
“Our findings suggest that people with epilepsy should avoid high CO exposure to reduce potential seizure risk,” said study investigator Zhuying Chen, PhD candidate, department of biomedical engineering, University of Melbourne.
The study was published online in Epilepsia.
Pollution’s impact on brain health
Emerging evidence indicates air pollution affects brain health and may increase the risk of hospitalization or outpatient visits for epilepsy. However, little is known about the effect of pollution on the occurrence of epileptic seizures.
The study used two independent long-term seizure datasets – the NeuroVista (NV) study and the Seer App seizure diary (SD). In the NeuroVista study, researchers recorded continuous intracranial iEEG from patients with refractory focal epilepsy who had been implanted with a personal seizure advisory device that wirelessly recorded seizures on an external device.
The SD dataset included diaries documenting self-reported seizures, seizure cycles, and medication adherence.
Researchers collected data on hourly concentrations of outdoor CO, nitrogen dioxide (NO2), particulate matter of 10 μm or less in diameter (PM10), ozone (O3), and sulfur dioxide (SO2). The levels were measured at air quality monitoring stations in Australia.
Investigators aggregated hourly observations into daily mean data. All daily concentrations of CO and SO2 and at least 95% of daily concentrations of NO2, O3, and PM10 were within Australian air quality standards, said Mr. Chen.
The study included 49 participants, with epilepsy data on 15 patients in the NeuroVista study and on 34 from the SD dataset.
Overall, 6,692 epileptic seizures on 3,639 seizure days were recorded during 23,349 follow-up days from 2010 to 2012 (NV dataset) and 2018 to 2021 (SD dataset).
The investigators found a significant positive association between CO concentrations and epileptic seizure risks. The relative risk (RR) was 1.04 (95% confidence interval, 1.01–1.07; P < .01) for an interquartile range (IQR) increase of CO (0.13 parts per million).
Sex differences
There were no significant relationships for the other four air pollutants. However, Mr. Chen noted that Australia has very low air pollution levels; most usually are within World Health Organization air quality guidelines.
“Our findings may not be generalized to other countries with high air pollution levels,” said Mr. Chen. He noted that the relatively small number of patients in the study may limit the statistical power to detect some associations.
The study showed that females had a significantly increased risk of epileptic seizures when exposed to elevated CO (RR, 1.05; 95% CI, 1.01–1.08; P < .05) and NO2 (RR, 1.09; 95% CI, 1.01–1.16; P < .05) concentrations. There were no significant associations in males for any air pollutants.
Differences in outdoor activities and behaviors such as smoking and exercise may lead to variations in environmental exposure and help explain the sex differences, said Mr. Chen. These differences may also be due to the study’s limited sample size.
Analyzing the two datasets separately, the researchers found there was a significant association between CO concentration and epileptic seizure risk in the NV dataset (RR, 1.10; 95% CI, 1.03–1.17; P < .01).
There were no significant associations in the SD dataset for any air pollutants. This may be because only clinical seizures – those associated with evident symptoms – are self-reported, said Mr. Chen. He also noted that seizure diaries may be unreliable.
In the NV dataset, the epileptic seizure risk was significantly increased when only subclinical seizures were considered (RR, 1.20; 95% CI, 1.12–1.28; P < .001) for an IQR increase of CO concentration.
The risk was significantly decreased by 13% for subclinical seizures with an IQR increase of PM10 and by 9% for subclinical seizures with an IQR increase of SO2 concentrations.
These negative associations should be interpreted with caution, inasmuch as the associations were not robust in subsequent subgroup and sensitivity analyses, said Mr. Chen.
There were no significant associations when considering clinical seizures for any air pollutants.
The positive association for subclinical but not clinical seizures suggests that low-level CO exposure may not be strong enough to directly trigger clinical seizures, said Mr. Chen.
Although previous research has demonstrated adverse neurologic effects of exposure to air pollutants, most studies were based on hospital databases or registers. Thus, they may have missed seizures that did not lead to hospital admission.
Unclear mechanism
The exact mechanisms linking air pollution to seizures are unclear but probably involve the synergistic interaction of multiple pathways, said Mr. Chen. “Air pollution may affect brain metabolism, alter the immune response of the brain, and induce oxidative stress and neuroinflammation, causing the brain to be more susceptible to seizures,” he noted.
This is the first study to investigate seizure rates through intracranial EEG signals and self-reported seizure diaries. It’s also the first to look into the impact of pollutants at low concentration levels on subclinical seizures.
However, the study has some limitations. Self-reported seizures in the SD dataset might underestimate the influence of air pollution on seizures. The study used postal codes as proxies for exposure to pollution, which could introduce measurement errors and underestimate associations.
In addition, Mr. Chen noted that seizures from the NeuroVista dataset were recorded from patients with drug-resistant focal epilepsy. “Whether our findings can be generalized to other epilepsy types needs further investigation.”
The study could have important clinical and public health implications. For example, said Mr. Chen, it’s possible that seizure risk could be reduced through behavioral interventions, such as avoiding being outside or using an air filtration system when pollutant levels are high.
“Clinicians could counsel their patients to avoid the potential risk of high carbon monoxide exposure,” he said.
CO exposure could be a new factor for seizure risk forecasting, which could reduce the uncertainty of seizures and help guide epilepsy management, Mr. Chen added.
The study was supported by the Melbourne Monash Consciousness Research Seed Funding and an Australian National Health and Medical Research Council Ideas grant. Mr. Chen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.

