User login
NCI mammography trial mostly a ‘waste,’ says expert
Funding for this trial is largely misspent money, it may produce misleading results, and it should be abandoned, he says.
Dr. Kopans has been an outspoken critic of the trial, describing it as a “huge waste of money” in comments made last year. Now he has set out his criticisms of the trial in an essay published in the October issue of Clinical Imaging, which outlines his objections and concerns for the first time in a peer-reviewed journal.
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) is comparing digital breast tomosynthesis (DBT), also known as 3-D mammography, with the older 2-D technology or full-field digital mammography (FFDM).
Dr. Kopans coined the term DBT and formerly held a now-expired patent on the first version of this technology.
“It could be argued that the imaging part of TMIST is a waste of valuable resources,” he writes in the essay.
The “imaging part” of the trial refers to the primary outcome measure and driving purpose of the trial, which is designed to learn which technology is better at finding – and reducing the rate of – potentially lethal “advanced” cancers.
These cancers include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease. These malignancies correlate with breast cancer mortality, TMIST’s principal investigator Etta Pisano, MD, of the American College of Radiology, has said in the past.
However, Dr. Kopans says that this surrogate endpoint is problematic. “TMIST will only investigate whether or not digital breast tomography results in a decline in advanced cancers, ignoring the fact that many women still die from cancers that are not advanced at the time of diagnosis,” he writes.
“Clearly reducing the rate of advanced cancers is not the only way that early detection saves lives. Lives are also saved by finding cancers at a smaller size within stages,” Dr. Kopans writes. He adds that DBT has been proven in observational cohort studies to find more smaller breast cancers than FFDM.
Dr. Kopans’ opinion that TMIST is largely a waste of resources is not shared by the National Cancer Institute. “We feel strongly that TMIST is a critical study,” an NCI spokesperson told this news organization.
Study power concerns
Another concern is that TMIST “may be underpowered,” Dr. Kopans writes. That concern arises in part from a recent review of TMIST by an advisory committee (that was prompted by low patient accrual rates), which proposed reducing the size of the trial. Dr. Kopans says this would result in “a reduction of the planned power of the trial.”
The NCI says that reducing the study size has been discussed but has not yet been implemented. “Any reduction in size would, of course, have appropriate statistical considerations in mind,” according to the NCI spokesperson.
Dr. Kopans’ concern about statistical power extends beyond downsizing the trial. An advanced cancer in TMIST is counted “if it occurs at any time while the participant is on study,” according to the NCI. Dr. Kopans says that is a problem.
“Since DBT cannot have any effect on advanced cancers in the prevalence year (they are already there), data from the first year (prevalence cancers are likely the largest number) will be unusable, and if used will, inappropriately, dilute the results,” he writes.
Dr. Kopans hopes that the investigators address the statistical power issues with the trial because, if not, “its results may be grossly misleading.”
American radiology practice
Dr. Kopans praises one aspect of TMIST – the trial’s effort to create a repository of blood and oral swab specimens, along with participant genetic data. The goal, say TMIST investigators, is to individualize or optimize screening strategies by tying molecular data to clinical outcomes in the trial.
However, apart from that one aspect, Dr. Kopans is highly critical of the trial.
It is now too late to compare the two technologies, he suggests, as DBT is already replacing FFDM for breast cancer screening in the U.S.
He notes that 76% of mammography facilities in the United States have 3-D devices (as of April 2021). That percentage has climbed steadily in recent years. “By the time the TMIST study is completed, DBT will, almost certainly, have become the ‘standard of care,’” he asserts, echoing others who have commented on the trial, including some participating physicians.
The money being spent on TMIST “should not be used for looking backwards,” says Dr. Kopans.
The NCI responded to that criticism. “TMIST is looking to clarify the best screening for women based on the science and is not solely about access. We are seeking to determine which technology is better and [are] providing access to the trial across the country in diverse practices and populations,” the NCI said in an email.
In his essay, Dr. Kopans says it is time to stop TMIST and put the money into other pressing breast cancer issues and questions. “... it makes no sense to continue this flawed trial whose results will be obsolete by the time they become available,” he writes.
Dr. Kopans reports consulting with DART Imaging in China, which is developing a digital breast tomosynthesis machine.
A version of this article first appeared on Medscape.com.
Funding for this trial is largely misspent money, it may produce misleading results, and it should be abandoned, he says.
Dr. Kopans has been an outspoken critic of the trial, describing it as a “huge waste of money” in comments made last year. Now he has set out his criticisms of the trial in an essay published in the October issue of Clinical Imaging, which outlines his objections and concerns for the first time in a peer-reviewed journal.
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) is comparing digital breast tomosynthesis (DBT), also known as 3-D mammography, with the older 2-D technology or full-field digital mammography (FFDM).
Dr. Kopans coined the term DBT and formerly held a now-expired patent on the first version of this technology.
“It could be argued that the imaging part of TMIST is a waste of valuable resources,” he writes in the essay.
The “imaging part” of the trial refers to the primary outcome measure and driving purpose of the trial, which is designed to learn which technology is better at finding – and reducing the rate of – potentially lethal “advanced” cancers.
These cancers include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease. These malignancies correlate with breast cancer mortality, TMIST’s principal investigator Etta Pisano, MD, of the American College of Radiology, has said in the past.
However, Dr. Kopans says that this surrogate endpoint is problematic. “TMIST will only investigate whether or not digital breast tomography results in a decline in advanced cancers, ignoring the fact that many women still die from cancers that are not advanced at the time of diagnosis,” he writes.
“Clearly reducing the rate of advanced cancers is not the only way that early detection saves lives. Lives are also saved by finding cancers at a smaller size within stages,” Dr. Kopans writes. He adds that DBT has been proven in observational cohort studies to find more smaller breast cancers than FFDM.
Dr. Kopans’ opinion that TMIST is largely a waste of resources is not shared by the National Cancer Institute. “We feel strongly that TMIST is a critical study,” an NCI spokesperson told this news organization.
Study power concerns
Another concern is that TMIST “may be underpowered,” Dr. Kopans writes. That concern arises in part from a recent review of TMIST by an advisory committee (that was prompted by low patient accrual rates), which proposed reducing the size of the trial. Dr. Kopans says this would result in “a reduction of the planned power of the trial.”
The NCI says that reducing the study size has been discussed but has not yet been implemented. “Any reduction in size would, of course, have appropriate statistical considerations in mind,” according to the NCI spokesperson.
Dr. Kopans’ concern about statistical power extends beyond downsizing the trial. An advanced cancer in TMIST is counted “if it occurs at any time while the participant is on study,” according to the NCI. Dr. Kopans says that is a problem.
“Since DBT cannot have any effect on advanced cancers in the prevalence year (they are already there), data from the first year (prevalence cancers are likely the largest number) will be unusable, and if used will, inappropriately, dilute the results,” he writes.
Dr. Kopans hopes that the investigators address the statistical power issues with the trial because, if not, “its results may be grossly misleading.”
American radiology practice
Dr. Kopans praises one aspect of TMIST – the trial’s effort to create a repository of blood and oral swab specimens, along with participant genetic data. The goal, say TMIST investigators, is to individualize or optimize screening strategies by tying molecular data to clinical outcomes in the trial.
However, apart from that one aspect, Dr. Kopans is highly critical of the trial.
It is now too late to compare the two technologies, he suggests, as DBT is already replacing FFDM for breast cancer screening in the U.S.
He notes that 76% of mammography facilities in the United States have 3-D devices (as of April 2021). That percentage has climbed steadily in recent years. “By the time the TMIST study is completed, DBT will, almost certainly, have become the ‘standard of care,’” he asserts, echoing others who have commented on the trial, including some participating physicians.
The money being spent on TMIST “should not be used for looking backwards,” says Dr. Kopans.
The NCI responded to that criticism. “TMIST is looking to clarify the best screening for women based on the science and is not solely about access. We are seeking to determine which technology is better and [are] providing access to the trial across the country in diverse practices and populations,” the NCI said in an email.
In his essay, Dr. Kopans says it is time to stop TMIST and put the money into other pressing breast cancer issues and questions. “... it makes no sense to continue this flawed trial whose results will be obsolete by the time they become available,” he writes.
Dr. Kopans reports consulting with DART Imaging in China, which is developing a digital breast tomosynthesis machine.
A version of this article first appeared on Medscape.com.
Funding for this trial is largely misspent money, it may produce misleading results, and it should be abandoned, he says.
Dr. Kopans has been an outspoken critic of the trial, describing it as a “huge waste of money” in comments made last year. Now he has set out his criticisms of the trial in an essay published in the October issue of Clinical Imaging, which outlines his objections and concerns for the first time in a peer-reviewed journal.
The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) is comparing digital breast tomosynthesis (DBT), also known as 3-D mammography, with the older 2-D technology or full-field digital mammography (FFDM).
Dr. Kopans coined the term DBT and formerly held a now-expired patent on the first version of this technology.
“It could be argued that the imaging part of TMIST is a waste of valuable resources,” he writes in the essay.
The “imaging part” of the trial refers to the primary outcome measure and driving purpose of the trial, which is designed to learn which technology is better at finding – and reducing the rate of – potentially lethal “advanced” cancers.
These cancers include larger HER2-positive and triple-negative malignancies; those associated with positive nodes; and metastatic disease. These malignancies correlate with breast cancer mortality, TMIST’s principal investigator Etta Pisano, MD, of the American College of Radiology, has said in the past.
However, Dr. Kopans says that this surrogate endpoint is problematic. “TMIST will only investigate whether or not digital breast tomography results in a decline in advanced cancers, ignoring the fact that many women still die from cancers that are not advanced at the time of diagnosis,” he writes.
“Clearly reducing the rate of advanced cancers is not the only way that early detection saves lives. Lives are also saved by finding cancers at a smaller size within stages,” Dr. Kopans writes. He adds that DBT has been proven in observational cohort studies to find more smaller breast cancers than FFDM.
Dr. Kopans’ opinion that TMIST is largely a waste of resources is not shared by the National Cancer Institute. “We feel strongly that TMIST is a critical study,” an NCI spokesperson told this news organization.
Study power concerns
Another concern is that TMIST “may be underpowered,” Dr. Kopans writes. That concern arises in part from a recent review of TMIST by an advisory committee (that was prompted by low patient accrual rates), which proposed reducing the size of the trial. Dr. Kopans says this would result in “a reduction of the planned power of the trial.”
The NCI says that reducing the study size has been discussed but has not yet been implemented. “Any reduction in size would, of course, have appropriate statistical considerations in mind,” according to the NCI spokesperson.
Dr. Kopans’ concern about statistical power extends beyond downsizing the trial. An advanced cancer in TMIST is counted “if it occurs at any time while the participant is on study,” according to the NCI. Dr. Kopans says that is a problem.
“Since DBT cannot have any effect on advanced cancers in the prevalence year (they are already there), data from the first year (prevalence cancers are likely the largest number) will be unusable, and if used will, inappropriately, dilute the results,” he writes.
Dr. Kopans hopes that the investigators address the statistical power issues with the trial because, if not, “its results may be grossly misleading.”
American radiology practice
Dr. Kopans praises one aspect of TMIST – the trial’s effort to create a repository of blood and oral swab specimens, along with participant genetic data. The goal, say TMIST investigators, is to individualize or optimize screening strategies by tying molecular data to clinical outcomes in the trial.
However, apart from that one aspect, Dr. Kopans is highly critical of the trial.
It is now too late to compare the two technologies, he suggests, as DBT is already replacing FFDM for breast cancer screening in the U.S.
He notes that 76% of mammography facilities in the United States have 3-D devices (as of April 2021). That percentage has climbed steadily in recent years. “By the time the TMIST study is completed, DBT will, almost certainly, have become the ‘standard of care,’” he asserts, echoing others who have commented on the trial, including some participating physicians.
The money being spent on TMIST “should not be used for looking backwards,” says Dr. Kopans.
The NCI responded to that criticism. “TMIST is looking to clarify the best screening for women based on the science and is not solely about access. We are seeking to determine which technology is better and [are] providing access to the trial across the country in diverse practices and populations,” the NCI said in an email.
In his essay, Dr. Kopans says it is time to stop TMIST and put the money into other pressing breast cancer issues and questions. “... it makes no sense to continue this flawed trial whose results will be obsolete by the time they become available,” he writes.
Dr. Kopans reports consulting with DART Imaging in China, which is developing a digital breast tomosynthesis machine.
A version of this article first appeared on Medscape.com.
‘First reliable estimate’ of breast cancer metastasis
The data come from a massive meta-analysis of more than 400 studies conducted around the world, involving tens of thousands of women.
It found that the overall risk of metastasis is between 6% and 22%, with younger women having a higher risk.
While women aged 50 years or older when they were diagnosed with breast cancer have a risk of developing metastasis that ranged from 3.7% to 28.6%, women diagnosed with breast cancer before age 35 had a higher risk – 12.7% to 38%. The investigators speculate that this may be because younger women have a more aggressive form of breast cancer or because they are diagnosed at a later stage.
The risk of metastasis also varies by tumor type, with luminal B cancers having a 4.2% to 35.5% risk of metastasis versus a 2.3% to 11.8% risk with luminal A tumors.
“The quantification of recurrence and disease progression is important to assess the effectiveness of treatment, evaluate prognosis, and allocate resources,” commented lead investigator Eileen Morgan, PhD, of the International Agency for Research on Cancer.
Dr. Morgan and colleagues presented the new meta-analysis at the virtual Advanced Breast Cancer Sixth International Consensus Conference.
She added that this information has not been available until now “because cancer registries have not been routinely collecting this data.”
In fact, the U.S. National Cancer Institute began a project earlier this year to track this information, after 48 years of not doing so.
Reacting to the findings, Shani Paluch-Shimon, MBBS, director of the Breast Unit at Hadassah University Hospital, Jerusalem, commented that this work “provides the first reliable estimate of how many breast cancer patients go on to develop advanced disease in contemporary cohorts.”
“This information is, of course, important for patients who want to understand their prognosis,” she continued.
“But it’s also vital at a public health level for those of us working to treat and prevent advanced breast cancer, to help us understand the scale of the disease around the world,” she said. “It will help us identify at-risk groups across different populations and demonstrate how disease course is changing with contemporary treatments.”
“It will also help us understand what resources are needed and where, to ensure we can collect and analyze quality data in real-time as this is key for resource allocation and planning future studies.”
The work was funded by a grant from the Susan G. Komen Foundation.
A version of this article first appeared on Medscape.com.
The data come from a massive meta-analysis of more than 400 studies conducted around the world, involving tens of thousands of women.
It found that the overall risk of metastasis is between 6% and 22%, with younger women having a higher risk.
While women aged 50 years or older when they were diagnosed with breast cancer have a risk of developing metastasis that ranged from 3.7% to 28.6%, women diagnosed with breast cancer before age 35 had a higher risk – 12.7% to 38%. The investigators speculate that this may be because younger women have a more aggressive form of breast cancer or because they are diagnosed at a later stage.
The risk of metastasis also varies by tumor type, with luminal B cancers having a 4.2% to 35.5% risk of metastasis versus a 2.3% to 11.8% risk with luminal A tumors.
“The quantification of recurrence and disease progression is important to assess the effectiveness of treatment, evaluate prognosis, and allocate resources,” commented lead investigator Eileen Morgan, PhD, of the International Agency for Research on Cancer.
Dr. Morgan and colleagues presented the new meta-analysis at the virtual Advanced Breast Cancer Sixth International Consensus Conference.
She added that this information has not been available until now “because cancer registries have not been routinely collecting this data.”
In fact, the U.S. National Cancer Institute began a project earlier this year to track this information, after 48 years of not doing so.
Reacting to the findings, Shani Paluch-Shimon, MBBS, director of the Breast Unit at Hadassah University Hospital, Jerusalem, commented that this work “provides the first reliable estimate of how many breast cancer patients go on to develop advanced disease in contemporary cohorts.”
“This information is, of course, important for patients who want to understand their prognosis,” she continued.
“But it’s also vital at a public health level for those of us working to treat and prevent advanced breast cancer, to help us understand the scale of the disease around the world,” she said. “It will help us identify at-risk groups across different populations and demonstrate how disease course is changing with contemporary treatments.”
“It will also help us understand what resources are needed and where, to ensure we can collect and analyze quality data in real-time as this is key for resource allocation and planning future studies.”
The work was funded by a grant from the Susan G. Komen Foundation.
A version of this article first appeared on Medscape.com.
The data come from a massive meta-analysis of more than 400 studies conducted around the world, involving tens of thousands of women.
It found that the overall risk of metastasis is between 6% and 22%, with younger women having a higher risk.
While women aged 50 years or older when they were diagnosed with breast cancer have a risk of developing metastasis that ranged from 3.7% to 28.6%, women diagnosed with breast cancer before age 35 had a higher risk – 12.7% to 38%. The investigators speculate that this may be because younger women have a more aggressive form of breast cancer or because they are diagnosed at a later stage.
The risk of metastasis also varies by tumor type, with luminal B cancers having a 4.2% to 35.5% risk of metastasis versus a 2.3% to 11.8% risk with luminal A tumors.
“The quantification of recurrence and disease progression is important to assess the effectiveness of treatment, evaluate prognosis, and allocate resources,” commented lead investigator Eileen Morgan, PhD, of the International Agency for Research on Cancer.
Dr. Morgan and colleagues presented the new meta-analysis at the virtual Advanced Breast Cancer Sixth International Consensus Conference.
She added that this information has not been available until now “because cancer registries have not been routinely collecting this data.”
In fact, the U.S. National Cancer Institute began a project earlier this year to track this information, after 48 years of not doing so.
Reacting to the findings, Shani Paluch-Shimon, MBBS, director of the Breast Unit at Hadassah University Hospital, Jerusalem, commented that this work “provides the first reliable estimate of how many breast cancer patients go on to develop advanced disease in contemporary cohorts.”
“This information is, of course, important for patients who want to understand their prognosis,” she continued.
“But it’s also vital at a public health level for those of us working to treat and prevent advanced breast cancer, to help us understand the scale of the disease around the world,” she said. “It will help us identify at-risk groups across different populations and demonstrate how disease course is changing with contemporary treatments.”
“It will also help us understand what resources are needed and where, to ensure we can collect and analyze quality data in real-time as this is key for resource allocation and planning future studies.”
The work was funded by a grant from the Susan G. Komen Foundation.
A version of this article first appeared on Medscape.com.
Severe COVID two times higher for cancer patients
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
A new systematic review and meta-analysis finds that unvaccinated cancer patients who contracted COVID-19 last year, were more than two times more likely – than people without cancer – to develop a case of COVID-19 so severe it required hospitalization in an intensive care unit.
“Our study provides the most precise measure to date of the effect of COVID-19 in cancer patients,” wrote researchers who were led by Paolo Boffetta, MD, MPH, a specialist in population science with the Stony Brook Cancer Center in New York.
Dr. Boffetta and colleagues also found that patients with hematologic neoplasms had a higher mortality rate from COVID-19 comparable to that of all cancers combined.
Cancer patients have long been considered to be among those patients who are at high risk of developing COVID-19, and if they contract the disease, they are at high risk of having poor outcomes. Other high-risk patients include those with hypertension, diabetes, chronic kidney disease, or COPD, or the elderly. But how high the risk of developing severe COVID-19 disease is for cancer patients hasn’t yet been documented on a wide scale.
The study, which was made available as a preprint on medRxiv on Oct. 23, is based on an analysis of COVID-19 cases that were documented in 35 reviews, meta-analyses, case reports, and studies indexed in PubMed from authors in North America, Europe, and Asia.
In this study, the pooled odds ratio for mortality for all patients with any cancer was 2.32 (95% confidence interval, 1.82-2.94; 24 studies). For ICU admission, the odds ratio was 2.39 (95% CI, 1.90-3.02; I2 0.0%; 5 studies). And, for disease severity or hospitalization, it was 2.08 (95% CI, 1.60-2.72; I2 92.1%; 15 studies). The pooled mortality odds ratio for hematologic neoplasms was 2.14 (95% CI, 1.87-2.44; I2 20.8%; 8 studies).
Their findings, which have not yet been peer reviewed, confirmed the results of a similar analysis from China published as a preprint in May 2020. The analysis included 181,323 patients (23,736 cancer patients) from 26 studies reported an odds ratio of 2.54 (95% CI, 1.47-4.42). “Cancer patients with COVID-19 have an increased likelihood of death compared to non-cancer COVID-19 patients,” Venkatesulu et al. wrote. And a systematic review and meta-analysis of five studies of 2,619 patients published in October 2020 in Medicine also found a significantly higher risk of death from COVID-19 among cancer patients (odds ratio, 2.63; 95% confidence interval, 1.14-6.06; P = .023; I2 = 26.4%).
Fakih et al., writing in the journal Hematology/Oncology and Stem Cell Therapy conducted a meta-analysis early last year finding a threefold increase for admission to the intensive care unit, an almost fourfold increase for a severe SARS-CoV-2 infection, and a fivefold increase for being intubated.
The three studies show that mortality rates were higher early in the pandemic “when diagnosis and treatment for SARS-CoV-2 might have been delayed, resulting in higher death rate,” Boffetta et al. wrote, adding that their analysis showed only a twofold increase most likely because it was a year-long analysis.
“Future studies will be able to better analyze this association for the different subtypes of cancer. Furthermore, they will eventually be able to evaluate whether the difference among vaccinated population is reduced,” Boffetta et al. wrote.
The authors noted several limitations for the study, including the fact that many of the studies included in the analysis did not include sex, age, comorbidities, and therapy. Nor were the authors able to analyze specific cancers other than hematologic neoplasms.
The authors declared no conflicts of interest.
FROM MEDRXIV
Liquid biopsy in metastatic breast cancer management: Where does it stand in clinical practice?
Tissue biopsy remains the gold standard for characterizing tumor biology and guiding therapeutic decisions, but liquid biopsies — blood analyses that allow oncologists to detect circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) in the blood — are increasingly demonstrating their value. Last year, the U.S. Food and Drug Administration (FDA) approved two liquid biopsy tests, Guardant360 CDx and FoundationOne Liquid CDx, that can identify more than 300 cancer-related genes in the blood. In 2019, the FDA also approved the first companion diagnostic test, therascreen, to pinpoint PIK3CA gene mutations in patients’ ctDNA and determine whether patients should receive the PI3K inhibitor alpelisib along with fulvestrant.
Here’s an overview of how liquid biopsy is being used in monitoring MBC progression and treatment — and what some oncologists think of it.
What we do and don’t know
“Identifying a patient’s targetable mutations, most notably PIK3CA mutations, is currently the main use of liquid biopsy,” said Pedram Razavi, MD, PhD, a medical oncologist who leads the liquid biopsy program for breast cancer at Memorial Sloan Kettering (MSK) Cancer Center in New York City. “Patients who come to MSK are offered a tumor and liquid biopsy at the time of metastatic diagnosis as part of the standard of care.”
Liquid and tissue biopsy analyses can provide a more complete picture of a patient’s condition. Whereas tissue biopsy allows oncologists to target a more saturated sample of the cancer ecosystem and a wider array of biomarkers, liquid biopsy offers important advantages as well, including a less invasive way to sequence a sample, monitor patients’ treatment response, or track tumor evolution. Liquid biopsy also provides a bigger picture view of tumor heterogeneity by pooling information from many tumor locations as opposed to one.
But, cautioned Yuan Yuan, MD, PhD, liquid biopsy technology is not always sensitive enough to detect CTCs, ctDNA, or all relevant mutations. “When you collect a small tube of blood, you’re essentially trying to catch a small fish in a big sea and wading through a lot of background noise,” said Dr. Yuan, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County. “The results may be hard to interpret or come back inconclusive.”
And although emerging data suggest that liquid biopsy provides important insights about tumor dynamics — including mapping disease progression, predicting survival, and even detecting signs of cancer recurrence before metastasis develops — the tool has limited utility in clinical practice outside of identifying sensitivity to various therapies or drugs.
“Right now, a lot of research is being done to understand how to use CTC and ctDNA in particular as a means of surveillance in breast cancer, but we’re still in the beginning stages of applying that outside of clinical trials,” said Joseph A. Sparano, MD, deputy director of the Tisch Cancer Institute and chief of the division of hematology and medical oncology, Icahn School of Medicine at Mount Sinai, New York City.
Personalizing treatment
The companion diagnostic test therascreen marked the beginning stages of using liquid biopsy to match treatments to genetic abnormalities in MBC. The SOLAR-1 phase 3 trial, which led to the approval of alpelisib and therascreen, found that the PI3K inhibitor plus fulvestrant almost doubled progression-free survival (PFS) (11 months vs 5.7 months in placebo-fulvestrant group) in patients with PIK3CA-mutated, HR-positive, HER2-negative advanced breast cancer.
More recent studies have shown that liquid biopsy tests can also identify ESR1 mutations and predict responses to inhibitors that target AKT1 and HER2. Investigators presenting at the 2021 American Society of Clinical Oncology meeting reported that next-generation sequencing of ctDNA in patients with HR-positive MBC, HER-positive MBC, or triple-negative breast cancer detected ESR1 mutations in 14% of patients (71 of 501). Moreover, ESR1 mutations were found only in HR-positive patients who had already received endocrine therapy. (The study also examined PIK3CA mutations, which occurred in about one third of patients). A more in-depth look revealed that ESR1 mutations were strongly associated with liver and bone metastases and that mutations along specific codons negatively affected overall survival (OS) and PFS: codons 537 and 538 for OS and codons 380 and 536 for PFS.
According to Debasish Tripathy, MD, professor and chairman of the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston, in addition to tumor sequencing, “liquid biopsy has become a great research tool to track patients in real time and predict, for instance, who will respond to a treatment and identify emerging resistance.”
In terms of predicting responses to treatment, the plasmaMATCH trial assessed ctDNA in 1,034 patients with advanced breast cancer for mutations in ESR1, HER2, and AKT1 using digital droplet polymerase chain reaction (PCR) and Guardant360. Results showed that 357 (34.5%) of these patients had potentially targetable aberrations, including 222 patients with ESR1 mutations, 36 patients with HER2 mutations, and 30 patients with AKT1 mutations.
Agreement between digital droplet PCR and Guardant360 testing was 96%-99%, and liquid biopsy showed 93% sensitivity compared with tumor samples. The investigators also used liquid biopsy findings to match patients’ mutations to targeted treatments: fulvestrant for those with ESR1 mutations, neratinib for HER2 (ERBB2) mutations, and the selective AKT inhibitor capivasertib for estrogen receptor–positive tumors with AKT1 mutations.
Overall, the investigators concluded that ctDNA testing offers “accurate tumor genotyping” in line with tissue-based testing and is ready for routine clinical practice to identify common as well as rare genetic alterations, such as HER2 and AKT1 mutations, that affect only about 5% of patients with advanced disease.
Predicting survival and recurrence
A particularly promising area for liquid biopsy is its usefulness in helping to predict survival outcomes and monitor patients for early signs of recurrence before metastasis occurs. But the data to support this are still in their infancy.
A highly cited study, published over 15 years ago in the New England Journal of Medicine, found that patients with MBC who had five or more CTCs per 7.5 mL of whole blood before receiving first-line therapy exhibited significantly shorter median PFS (2.7 vs 7.0 months) and OS (10 vs > 18 months) compared with patients with fewer than five CTCs. Subsequent analyses performed more than a decade later, including a meta-analysis published last year, helped validate these early findings that levels of CTCs detected in the blood independently and strongly predicted PFS and OS in patients with MBC.
In addition, ctDNA can provide important information about patients’ survival odds. In a retrospective study published last year, investigators tracked changes in ctDNA in 291 plasma samples from 84 patients with locally advanced breast cancer who participated in the I-SPY trial. Patients who remained ctDNA-positive after 3 weeks of neoadjuvant chemotherapy were significantly more likely to have residual disease after completing their treatment compared with patients who cleared ctDNA at that early stage (83% for those with nonpathologic complete response vs 52%). Notably, the presence of ctDNA between therapy initiation and completion was associated with a significantly greater risk for metastatic recurrence, whereas clearance of ctDNA after neoadjuvant therapy was linked to improved survival.
“The study is important because it highlights how tracking circulating ctDNA status in neoadjuvant-treated breast cancer can expose a patient’s risk for distant metastasis,” said Dr. Yuan. But, she added, “I think the biggest attraction of liquid biopsy will be the ability to detect molecular disease even before imaging can, and identify who has a high risk for recurrence.”
Dr. Razavi agreed that the potential to prevent metastasis by finding minimal residual disease (MRD) is the most exciting area of liquid biopsy research. “If we can find tumor DNA early before tumors have a chance to establish themselves, we could potentially change the trajectory of the disease for patients,” he said.
Several studies suggest that monitoring patients’ ctDNA levels after neoadjuvant treatment and surgery may help predict their risk for relapse and progression to metastatic disease. A 2015 analysis, which followed 20 patients with breast cancer after surgery, found that ctDNA monitoring accurately differentiated those who ultimately developed metastatic disease from those who didn’t (sensitivity, 93%; specificity, 100%) and detected metastatic disease 11 months earlier, on average, than imaging did. Another 2015 study found that the presence of ctDNA in plasma after neoadjuvant chemotherapy and surgery predicted metastatic relapse a median of almost 8 months before clinical detection. Other recent data show the power of ultrasensitive blood tests to detect MRD and potentially find metastatic disease early.
Although an increasing number of studies show that ctDNA and CTCs are prognostic for breast cancer recurrence, a major question remains: For patients with ctDNA or CTCs but no overt disease after imaging, will initiating therapy prevent or delay the development of metastatic disease?
“We still have to do those clinical trials to determine whether detecting MRD and treating patients early actually positively affects their survival and quality of life,” Dr. Razavi said.
Tissue biopsy remains the gold standard for characterizing tumor biology and guiding therapeutic decisions, but liquid biopsies — blood analyses that allow oncologists to detect circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) in the blood — are increasingly demonstrating their value. Last year, the U.S. Food and Drug Administration (FDA) approved two liquid biopsy tests, Guardant360 CDx and FoundationOne Liquid CDx, that can identify more than 300 cancer-related genes in the blood. In 2019, the FDA also approved the first companion diagnostic test, therascreen, to pinpoint PIK3CA gene mutations in patients’ ctDNA and determine whether patients should receive the PI3K inhibitor alpelisib along with fulvestrant.
Here’s an overview of how liquid biopsy is being used in monitoring MBC progression and treatment — and what some oncologists think of it.
What we do and don’t know
“Identifying a patient’s targetable mutations, most notably PIK3CA mutations, is currently the main use of liquid biopsy,” said Pedram Razavi, MD, PhD, a medical oncologist who leads the liquid biopsy program for breast cancer at Memorial Sloan Kettering (MSK) Cancer Center in New York City. “Patients who come to MSK are offered a tumor and liquid biopsy at the time of metastatic diagnosis as part of the standard of care.”
Liquid and tissue biopsy analyses can provide a more complete picture of a patient’s condition. Whereas tissue biopsy allows oncologists to target a more saturated sample of the cancer ecosystem and a wider array of biomarkers, liquid biopsy offers important advantages as well, including a less invasive way to sequence a sample, monitor patients’ treatment response, or track tumor evolution. Liquid biopsy also provides a bigger picture view of tumor heterogeneity by pooling information from many tumor locations as opposed to one.
But, cautioned Yuan Yuan, MD, PhD, liquid biopsy technology is not always sensitive enough to detect CTCs, ctDNA, or all relevant mutations. “When you collect a small tube of blood, you’re essentially trying to catch a small fish in a big sea and wading through a lot of background noise,” said Dr. Yuan, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County. “The results may be hard to interpret or come back inconclusive.”
And although emerging data suggest that liquid biopsy provides important insights about tumor dynamics — including mapping disease progression, predicting survival, and even detecting signs of cancer recurrence before metastasis develops — the tool has limited utility in clinical practice outside of identifying sensitivity to various therapies or drugs.
“Right now, a lot of research is being done to understand how to use CTC and ctDNA in particular as a means of surveillance in breast cancer, but we’re still in the beginning stages of applying that outside of clinical trials,” said Joseph A. Sparano, MD, deputy director of the Tisch Cancer Institute and chief of the division of hematology and medical oncology, Icahn School of Medicine at Mount Sinai, New York City.
Personalizing treatment
The companion diagnostic test therascreen marked the beginning stages of using liquid biopsy to match treatments to genetic abnormalities in MBC. The SOLAR-1 phase 3 trial, which led to the approval of alpelisib and therascreen, found that the PI3K inhibitor plus fulvestrant almost doubled progression-free survival (PFS) (11 months vs 5.7 months in placebo-fulvestrant group) in patients with PIK3CA-mutated, HR-positive, HER2-negative advanced breast cancer.
More recent studies have shown that liquid biopsy tests can also identify ESR1 mutations and predict responses to inhibitors that target AKT1 and HER2. Investigators presenting at the 2021 American Society of Clinical Oncology meeting reported that next-generation sequencing of ctDNA in patients with HR-positive MBC, HER-positive MBC, or triple-negative breast cancer detected ESR1 mutations in 14% of patients (71 of 501). Moreover, ESR1 mutations were found only in HR-positive patients who had already received endocrine therapy. (The study also examined PIK3CA mutations, which occurred in about one third of patients). A more in-depth look revealed that ESR1 mutations were strongly associated with liver and bone metastases and that mutations along specific codons negatively affected overall survival (OS) and PFS: codons 537 and 538 for OS and codons 380 and 536 for PFS.
According to Debasish Tripathy, MD, professor and chairman of the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston, in addition to tumor sequencing, “liquid biopsy has become a great research tool to track patients in real time and predict, for instance, who will respond to a treatment and identify emerging resistance.”
In terms of predicting responses to treatment, the plasmaMATCH trial assessed ctDNA in 1,034 patients with advanced breast cancer for mutations in ESR1, HER2, and AKT1 using digital droplet polymerase chain reaction (PCR) and Guardant360. Results showed that 357 (34.5%) of these patients had potentially targetable aberrations, including 222 patients with ESR1 mutations, 36 patients with HER2 mutations, and 30 patients with AKT1 mutations.
Agreement between digital droplet PCR and Guardant360 testing was 96%-99%, and liquid biopsy showed 93% sensitivity compared with tumor samples. The investigators also used liquid biopsy findings to match patients’ mutations to targeted treatments: fulvestrant for those with ESR1 mutations, neratinib for HER2 (ERBB2) mutations, and the selective AKT inhibitor capivasertib for estrogen receptor–positive tumors with AKT1 mutations.
Overall, the investigators concluded that ctDNA testing offers “accurate tumor genotyping” in line with tissue-based testing and is ready for routine clinical practice to identify common as well as rare genetic alterations, such as HER2 and AKT1 mutations, that affect only about 5% of patients with advanced disease.
Predicting survival and recurrence
A particularly promising area for liquid biopsy is its usefulness in helping to predict survival outcomes and monitor patients for early signs of recurrence before metastasis occurs. But the data to support this are still in their infancy.
A highly cited study, published over 15 years ago in the New England Journal of Medicine, found that patients with MBC who had five or more CTCs per 7.5 mL of whole blood before receiving first-line therapy exhibited significantly shorter median PFS (2.7 vs 7.0 months) and OS (10 vs > 18 months) compared with patients with fewer than five CTCs. Subsequent analyses performed more than a decade later, including a meta-analysis published last year, helped validate these early findings that levels of CTCs detected in the blood independently and strongly predicted PFS and OS in patients with MBC.
In addition, ctDNA can provide important information about patients’ survival odds. In a retrospective study published last year, investigators tracked changes in ctDNA in 291 plasma samples from 84 patients with locally advanced breast cancer who participated in the I-SPY trial. Patients who remained ctDNA-positive after 3 weeks of neoadjuvant chemotherapy were significantly more likely to have residual disease after completing their treatment compared with patients who cleared ctDNA at that early stage (83% for those with nonpathologic complete response vs 52%). Notably, the presence of ctDNA between therapy initiation and completion was associated with a significantly greater risk for metastatic recurrence, whereas clearance of ctDNA after neoadjuvant therapy was linked to improved survival.
“The study is important because it highlights how tracking circulating ctDNA status in neoadjuvant-treated breast cancer can expose a patient’s risk for distant metastasis,” said Dr. Yuan. But, she added, “I think the biggest attraction of liquid biopsy will be the ability to detect molecular disease even before imaging can, and identify who has a high risk for recurrence.”
Dr. Razavi agreed that the potential to prevent metastasis by finding minimal residual disease (MRD) is the most exciting area of liquid biopsy research. “If we can find tumor DNA early before tumors have a chance to establish themselves, we could potentially change the trajectory of the disease for patients,” he said.
Several studies suggest that monitoring patients’ ctDNA levels after neoadjuvant treatment and surgery may help predict their risk for relapse and progression to metastatic disease. A 2015 analysis, which followed 20 patients with breast cancer after surgery, found that ctDNA monitoring accurately differentiated those who ultimately developed metastatic disease from those who didn’t (sensitivity, 93%; specificity, 100%) and detected metastatic disease 11 months earlier, on average, than imaging did. Another 2015 study found that the presence of ctDNA in plasma after neoadjuvant chemotherapy and surgery predicted metastatic relapse a median of almost 8 months before clinical detection. Other recent data show the power of ultrasensitive blood tests to detect MRD and potentially find metastatic disease early.
Although an increasing number of studies show that ctDNA and CTCs are prognostic for breast cancer recurrence, a major question remains: For patients with ctDNA or CTCs but no overt disease after imaging, will initiating therapy prevent or delay the development of metastatic disease?
“We still have to do those clinical trials to determine whether detecting MRD and treating patients early actually positively affects their survival and quality of life,” Dr. Razavi said.
Tissue biopsy remains the gold standard for characterizing tumor biology and guiding therapeutic decisions, but liquid biopsies — blood analyses that allow oncologists to detect circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) in the blood — are increasingly demonstrating their value. Last year, the U.S. Food and Drug Administration (FDA) approved two liquid biopsy tests, Guardant360 CDx and FoundationOne Liquid CDx, that can identify more than 300 cancer-related genes in the blood. In 2019, the FDA also approved the first companion diagnostic test, therascreen, to pinpoint PIK3CA gene mutations in patients’ ctDNA and determine whether patients should receive the PI3K inhibitor alpelisib along with fulvestrant.
Here’s an overview of how liquid biopsy is being used in monitoring MBC progression and treatment — and what some oncologists think of it.
What we do and don’t know
“Identifying a patient’s targetable mutations, most notably PIK3CA mutations, is currently the main use of liquid biopsy,” said Pedram Razavi, MD, PhD, a medical oncologist who leads the liquid biopsy program for breast cancer at Memorial Sloan Kettering (MSK) Cancer Center in New York City. “Patients who come to MSK are offered a tumor and liquid biopsy at the time of metastatic diagnosis as part of the standard of care.”
Liquid and tissue biopsy analyses can provide a more complete picture of a patient’s condition. Whereas tissue biopsy allows oncologists to target a more saturated sample of the cancer ecosystem and a wider array of biomarkers, liquid biopsy offers important advantages as well, including a less invasive way to sequence a sample, monitor patients’ treatment response, or track tumor evolution. Liquid biopsy also provides a bigger picture view of tumor heterogeneity by pooling information from many tumor locations as opposed to one.
But, cautioned Yuan Yuan, MD, PhD, liquid biopsy technology is not always sensitive enough to detect CTCs, ctDNA, or all relevant mutations. “When you collect a small tube of blood, you’re essentially trying to catch a small fish in a big sea and wading through a lot of background noise,” said Dr. Yuan, medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County. “The results may be hard to interpret or come back inconclusive.”
And although emerging data suggest that liquid biopsy provides important insights about tumor dynamics — including mapping disease progression, predicting survival, and even detecting signs of cancer recurrence before metastasis develops — the tool has limited utility in clinical practice outside of identifying sensitivity to various therapies or drugs.
“Right now, a lot of research is being done to understand how to use CTC and ctDNA in particular as a means of surveillance in breast cancer, but we’re still in the beginning stages of applying that outside of clinical trials,” said Joseph A. Sparano, MD, deputy director of the Tisch Cancer Institute and chief of the division of hematology and medical oncology, Icahn School of Medicine at Mount Sinai, New York City.
Personalizing treatment
The companion diagnostic test therascreen marked the beginning stages of using liquid biopsy to match treatments to genetic abnormalities in MBC. The SOLAR-1 phase 3 trial, which led to the approval of alpelisib and therascreen, found that the PI3K inhibitor plus fulvestrant almost doubled progression-free survival (PFS) (11 months vs 5.7 months in placebo-fulvestrant group) in patients with PIK3CA-mutated, HR-positive, HER2-negative advanced breast cancer.
More recent studies have shown that liquid biopsy tests can also identify ESR1 mutations and predict responses to inhibitors that target AKT1 and HER2. Investigators presenting at the 2021 American Society of Clinical Oncology meeting reported that next-generation sequencing of ctDNA in patients with HR-positive MBC, HER-positive MBC, or triple-negative breast cancer detected ESR1 mutations in 14% of patients (71 of 501). Moreover, ESR1 mutations were found only in HR-positive patients who had already received endocrine therapy. (The study also examined PIK3CA mutations, which occurred in about one third of patients). A more in-depth look revealed that ESR1 mutations were strongly associated with liver and bone metastases and that mutations along specific codons negatively affected overall survival (OS) and PFS: codons 537 and 538 for OS and codons 380 and 536 for PFS.
According to Debasish Tripathy, MD, professor and chairman of the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston, in addition to tumor sequencing, “liquid biopsy has become a great research tool to track patients in real time and predict, for instance, who will respond to a treatment and identify emerging resistance.”
In terms of predicting responses to treatment, the plasmaMATCH trial assessed ctDNA in 1,034 patients with advanced breast cancer for mutations in ESR1, HER2, and AKT1 using digital droplet polymerase chain reaction (PCR) and Guardant360. Results showed that 357 (34.5%) of these patients had potentially targetable aberrations, including 222 patients with ESR1 mutations, 36 patients with HER2 mutations, and 30 patients with AKT1 mutations.
Agreement between digital droplet PCR and Guardant360 testing was 96%-99%, and liquid biopsy showed 93% sensitivity compared with tumor samples. The investigators also used liquid biopsy findings to match patients’ mutations to targeted treatments: fulvestrant for those with ESR1 mutations, neratinib for HER2 (ERBB2) mutations, and the selective AKT inhibitor capivasertib for estrogen receptor–positive tumors with AKT1 mutations.
Overall, the investigators concluded that ctDNA testing offers “accurate tumor genotyping” in line with tissue-based testing and is ready for routine clinical practice to identify common as well as rare genetic alterations, such as HER2 and AKT1 mutations, that affect only about 5% of patients with advanced disease.
Predicting survival and recurrence
A particularly promising area for liquid biopsy is its usefulness in helping to predict survival outcomes and monitor patients for early signs of recurrence before metastasis occurs. But the data to support this are still in their infancy.
A highly cited study, published over 15 years ago in the New England Journal of Medicine, found that patients with MBC who had five or more CTCs per 7.5 mL of whole blood before receiving first-line therapy exhibited significantly shorter median PFS (2.7 vs 7.0 months) and OS (10 vs > 18 months) compared with patients with fewer than five CTCs. Subsequent analyses performed more than a decade later, including a meta-analysis published last year, helped validate these early findings that levels of CTCs detected in the blood independently and strongly predicted PFS and OS in patients with MBC.
In addition, ctDNA can provide important information about patients’ survival odds. In a retrospective study published last year, investigators tracked changes in ctDNA in 291 plasma samples from 84 patients with locally advanced breast cancer who participated in the I-SPY trial. Patients who remained ctDNA-positive after 3 weeks of neoadjuvant chemotherapy were significantly more likely to have residual disease after completing their treatment compared with patients who cleared ctDNA at that early stage (83% for those with nonpathologic complete response vs 52%). Notably, the presence of ctDNA between therapy initiation and completion was associated with a significantly greater risk for metastatic recurrence, whereas clearance of ctDNA after neoadjuvant therapy was linked to improved survival.
“The study is important because it highlights how tracking circulating ctDNA status in neoadjuvant-treated breast cancer can expose a patient’s risk for distant metastasis,” said Dr. Yuan. But, she added, “I think the biggest attraction of liquid biopsy will be the ability to detect molecular disease even before imaging can, and identify who has a high risk for recurrence.”
Dr. Razavi agreed that the potential to prevent metastasis by finding minimal residual disease (MRD) is the most exciting area of liquid biopsy research. “If we can find tumor DNA early before tumors have a chance to establish themselves, we could potentially change the trajectory of the disease for patients,” he said.
Several studies suggest that monitoring patients’ ctDNA levels after neoadjuvant treatment and surgery may help predict their risk for relapse and progression to metastatic disease. A 2015 analysis, which followed 20 patients with breast cancer after surgery, found that ctDNA monitoring accurately differentiated those who ultimately developed metastatic disease from those who didn’t (sensitivity, 93%; specificity, 100%) and detected metastatic disease 11 months earlier, on average, than imaging did. Another 2015 study found that the presence of ctDNA in plasma after neoadjuvant chemotherapy and surgery predicted metastatic relapse a median of almost 8 months before clinical detection. Other recent data show the power of ultrasensitive blood tests to detect MRD and potentially find metastatic disease early.
Although an increasing number of studies show that ctDNA and CTCs are prognostic for breast cancer recurrence, a major question remains: For patients with ctDNA or CTCs but no overt disease after imaging, will initiating therapy prevent or delay the development of metastatic disease?
“We still have to do those clinical trials to determine whether detecting MRD and treating patients early actually positively affects their survival and quality of life,” Dr. Razavi said.
Dogs show potential as medical detectives in breast cancer
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
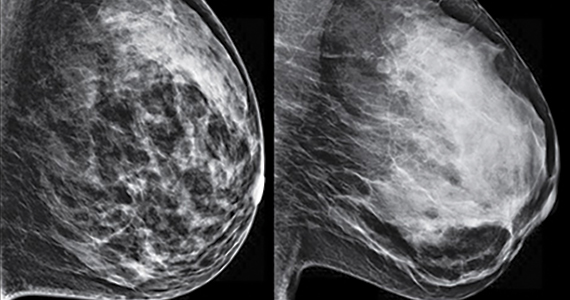
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
FROM BIOLOGY
COVID-19 has brought more complex, longer office visits
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Small fiber neuropathy is rising in the U.S., but why is a mystery
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Success of HPV vaccination: ‘Dramatic’ reduction in cervical cancer
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
3D vs 2D mammography for detecting cancer in dense breasts
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.
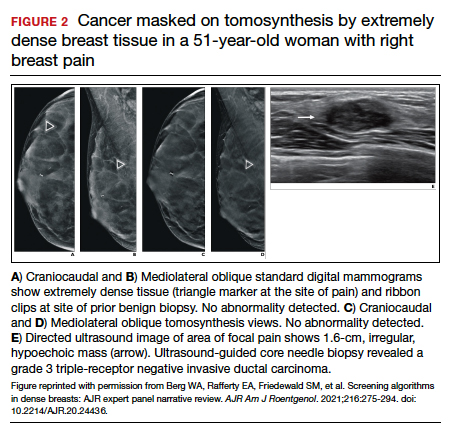
In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Quiz developed in collaboration with 
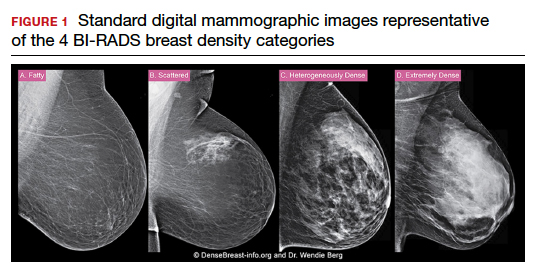
Q&A: Meeting the challenge of giving COVID vaccines to younger kids
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.