User login
In high-risk patients, methylation strikes genes before psychosis hits
BERLIN – Researchers are honing in on several sets of genes that, when altered by as-yet-unknown factors, may signal conversion to full-blown psychosis in people at ultrahigh risk for the disorder.
If confirmed, these candidate markers might have potential as blood-based biomarkers to predict conversion risk and assist in clinical staging, Marie-Odile Krebs, MD, PhD, said at the meeting of the World Psychiatric Association.
The genes modulate three biologic pathways that also have been implicated in schizophrenia: glutathione metabolism, axonal targeting, and inflammation, said Dr. Krebs of Saint-Anne Hospital, Paris. “Knowing this may even help us to target some drugs that work in those pathways,” she said.
Several blood-based analyte screens have been investigated with mixed results, Dr. Krebs noted.
In 2015, researchers at the University of North Carolina at Chapel Hill, and Harvard Medical School, Boston, created a 15-analyte plasma panel that performed well in the North American Prodrome Longitudinal Study (NAPL-S) cohort. The project is a multisite endeavor that aims to better understand predictors and mechanisms for the development of psychosis. The panel separated 35 unaffected controls from 32 with high-risk symptoms who converted to psychosis and from 40 who did not, with an area under the curve (AUC) of 0.91 (Schizophr Bull. 2015 Mar;41[2]:419-28).
Selected from an initial group of 185 analytes, the candidate markers were inflammatory cytokines, proteins modulating blood-brain barrier inflammation, and hormones related to the hypothalamic-pituitary axes. Several also were involved in reacting to oxidative stress.
Earlier this year, members of that same group identified a set of nine microRNAs related to cortical thinning in patients who converted to psychosis. These microRNAs also have been implicated in brain development, synaptic plasticity, immune function, and schizophrenia (Neuropsychopharmacology. 2017 Feb 10. doi: 10.1038/npp.2017.34).
Although these studies are helpful signposts, Dr. Krebs said they do not reflect the dynamic interaction of disease risk, which includes not only the intrinsic factors of genetics, enzymes, and proteins, but the extrinsic risks imposed by other factors: stress, trauma, cannabis use, and other completely individual experiences. “This is a dynamic process, and we need a dynamic assessment,” she said.
To that end, Dr. Krebs and her colleagues decided to look at methylomic changes in a small group of 39 patients at ultrahigh risk for psychosis conversion. All of these patients (mean age, 22 years) were seen at Saint-Anne Hospital from 2009 to 2013. Using whole blood, Dr. Krebs performed a genomewide DNA methylation study to determine what genes – if any – were differently methylated between the converters and nonconverters. The mean follow-up was 1 year (Mol Psychiatry. 2017 Apr;22[4]:512-8).
Although no significant difference was found in global methylation associated with conversion, Dr. Krebs did find longitudinal changes associated with conversion in three regions.
A cluster of five genes in the glutathione S-transferase family was differently methylated between the converters and nonconverters. Two were related to the GSTM5 promoter gene, which encodes for cytosolic and membrane-bound glutathione S-transferase – an important antioxidant enzyme, the downregulation of which has been implicated in schizophrenia. These two regions appeared to be stable over time, suggesting that methylation occurred before conversion, Dr. Krebs said.
Oxidative stress has been implicated in schizophrenia, and GSTM5 is expressed in the brain, Dr. Krebs noted. Some researchers suggest the gene is involved in dopamine metabolism. It’s also underexpressed in the prefrontal cortex of schizophrenia patients.
Three other regions in the GST family changed with conversion: two on the glutathione S-transferase theta 1 gene and one on the glutathione S-transferase P gene. Since all of these have to do with production of the innate antioxidant glutathione, “these findings suggest a potential use for antioxidant drugs,” Dr. Krebs said.
She found two other differently methylated regions as well.
One was a cluster of eight genes that are all involved in axon guidance – the process by which axons branch out to their correct targets. The second cluster comprised seven genes, all of which are involved in regulating interleukin-17 signaling. This cytokine has been implicated in autoimmune disorders.
Finally, Dr. Krebs performed a transcriptome analysis looking at the brain-expressed messenger RNA in the samples. “The methylome seemed less dynamic than the transcriptome,” she said. “Some methylomic changes may have occurred several months before the conversion, whereas transcriptomic analysis may reflect more rapid changes.”
There was only a 22% concordance between the two analyses. However, the GSTM5 gene and the neuropilin 1 gene – one of those involved in axon guidance – were both methylated and downregulated in the converters. The transcriptome analysis also found significantly decreased expression (although not methylation) of another gene, carnitine palmitoyltransferase 1A. This is a key enzyme in oxidizing long-chain fatty acids and transporting them into the mitochondria.
Adapting these observed differences in gene expression into a useful clinical tool will be challenging, Dr. Krebs said. In addition to large-group validation, any risk prediction model would have to take into account the many other factors that influence psychosis conversion: cerebral and sexual maturation during adolescence, cannabis use, and stress and other completely individual life experiences.
Nevertheless, she concluded, “longitudinal ‘multi-omics’ may be a step toward a future of personalized molecular psychiatry.”
Dr. Krebs had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
BERLIN – Researchers are honing in on several sets of genes that, when altered by as-yet-unknown factors, may signal conversion to full-blown psychosis in people at ultrahigh risk for the disorder.
If confirmed, these candidate markers might have potential as blood-based biomarkers to predict conversion risk and assist in clinical staging, Marie-Odile Krebs, MD, PhD, said at the meeting of the World Psychiatric Association.
The genes modulate three biologic pathways that also have been implicated in schizophrenia: glutathione metabolism, axonal targeting, and inflammation, said Dr. Krebs of Saint-Anne Hospital, Paris. “Knowing this may even help us to target some drugs that work in those pathways,” she said.
Several blood-based analyte screens have been investigated with mixed results, Dr. Krebs noted.
In 2015, researchers at the University of North Carolina at Chapel Hill, and Harvard Medical School, Boston, created a 15-analyte plasma panel that performed well in the North American Prodrome Longitudinal Study (NAPL-S) cohort. The project is a multisite endeavor that aims to better understand predictors and mechanisms for the development of psychosis. The panel separated 35 unaffected controls from 32 with high-risk symptoms who converted to psychosis and from 40 who did not, with an area under the curve (AUC) of 0.91 (Schizophr Bull. 2015 Mar;41[2]:419-28).
Selected from an initial group of 185 analytes, the candidate markers were inflammatory cytokines, proteins modulating blood-brain barrier inflammation, and hormones related to the hypothalamic-pituitary axes. Several also were involved in reacting to oxidative stress.
Earlier this year, members of that same group identified a set of nine microRNAs related to cortical thinning in patients who converted to psychosis. These microRNAs also have been implicated in brain development, synaptic plasticity, immune function, and schizophrenia (Neuropsychopharmacology. 2017 Feb 10. doi: 10.1038/npp.2017.34).
Although these studies are helpful signposts, Dr. Krebs said they do not reflect the dynamic interaction of disease risk, which includes not only the intrinsic factors of genetics, enzymes, and proteins, but the extrinsic risks imposed by other factors: stress, trauma, cannabis use, and other completely individual experiences. “This is a dynamic process, and we need a dynamic assessment,” she said.
To that end, Dr. Krebs and her colleagues decided to look at methylomic changes in a small group of 39 patients at ultrahigh risk for psychosis conversion. All of these patients (mean age, 22 years) were seen at Saint-Anne Hospital from 2009 to 2013. Using whole blood, Dr. Krebs performed a genomewide DNA methylation study to determine what genes – if any – were differently methylated between the converters and nonconverters. The mean follow-up was 1 year (Mol Psychiatry. 2017 Apr;22[4]:512-8).
Although no significant difference was found in global methylation associated with conversion, Dr. Krebs did find longitudinal changes associated with conversion in three regions.
A cluster of five genes in the glutathione S-transferase family was differently methylated between the converters and nonconverters. Two were related to the GSTM5 promoter gene, which encodes for cytosolic and membrane-bound glutathione S-transferase – an important antioxidant enzyme, the downregulation of which has been implicated in schizophrenia. These two regions appeared to be stable over time, suggesting that methylation occurred before conversion, Dr. Krebs said.
Oxidative stress has been implicated in schizophrenia, and GSTM5 is expressed in the brain, Dr. Krebs noted. Some researchers suggest the gene is involved in dopamine metabolism. It’s also underexpressed in the prefrontal cortex of schizophrenia patients.
Three other regions in the GST family changed with conversion: two on the glutathione S-transferase theta 1 gene and one on the glutathione S-transferase P gene. Since all of these have to do with production of the innate antioxidant glutathione, “these findings suggest a potential use for antioxidant drugs,” Dr. Krebs said.
She found two other differently methylated regions as well.
One was a cluster of eight genes that are all involved in axon guidance – the process by which axons branch out to their correct targets. The second cluster comprised seven genes, all of which are involved in regulating interleukin-17 signaling. This cytokine has been implicated in autoimmune disorders.
Finally, Dr. Krebs performed a transcriptome analysis looking at the brain-expressed messenger RNA in the samples. “The methylome seemed less dynamic than the transcriptome,” she said. “Some methylomic changes may have occurred several months before the conversion, whereas transcriptomic analysis may reflect more rapid changes.”
There was only a 22% concordance between the two analyses. However, the GSTM5 gene and the neuropilin 1 gene – one of those involved in axon guidance – were both methylated and downregulated in the converters. The transcriptome analysis also found significantly decreased expression (although not methylation) of another gene, carnitine palmitoyltransferase 1A. This is a key enzyme in oxidizing long-chain fatty acids and transporting them into the mitochondria.
Adapting these observed differences in gene expression into a useful clinical tool will be challenging, Dr. Krebs said. In addition to large-group validation, any risk prediction model would have to take into account the many other factors that influence psychosis conversion: cerebral and sexual maturation during adolescence, cannabis use, and stress and other completely individual life experiences.
Nevertheless, she concluded, “longitudinal ‘multi-omics’ may be a step toward a future of personalized molecular psychiatry.”
Dr. Krebs had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
BERLIN – Researchers are honing in on several sets of genes that, when altered by as-yet-unknown factors, may signal conversion to full-blown psychosis in people at ultrahigh risk for the disorder.
If confirmed, these candidate markers might have potential as blood-based biomarkers to predict conversion risk and assist in clinical staging, Marie-Odile Krebs, MD, PhD, said at the meeting of the World Psychiatric Association.
The genes modulate three biologic pathways that also have been implicated in schizophrenia: glutathione metabolism, axonal targeting, and inflammation, said Dr. Krebs of Saint-Anne Hospital, Paris. “Knowing this may even help us to target some drugs that work in those pathways,” she said.
Several blood-based analyte screens have been investigated with mixed results, Dr. Krebs noted.
In 2015, researchers at the University of North Carolina at Chapel Hill, and Harvard Medical School, Boston, created a 15-analyte plasma panel that performed well in the North American Prodrome Longitudinal Study (NAPL-S) cohort. The project is a multisite endeavor that aims to better understand predictors and mechanisms for the development of psychosis. The panel separated 35 unaffected controls from 32 with high-risk symptoms who converted to psychosis and from 40 who did not, with an area under the curve (AUC) of 0.91 (Schizophr Bull. 2015 Mar;41[2]:419-28).
Selected from an initial group of 185 analytes, the candidate markers were inflammatory cytokines, proteins modulating blood-brain barrier inflammation, and hormones related to the hypothalamic-pituitary axes. Several also were involved in reacting to oxidative stress.
Earlier this year, members of that same group identified a set of nine microRNAs related to cortical thinning in patients who converted to psychosis. These microRNAs also have been implicated in brain development, synaptic plasticity, immune function, and schizophrenia (Neuropsychopharmacology. 2017 Feb 10. doi: 10.1038/npp.2017.34).
Although these studies are helpful signposts, Dr. Krebs said they do not reflect the dynamic interaction of disease risk, which includes not only the intrinsic factors of genetics, enzymes, and proteins, but the extrinsic risks imposed by other factors: stress, trauma, cannabis use, and other completely individual experiences. “This is a dynamic process, and we need a dynamic assessment,” she said.
To that end, Dr. Krebs and her colleagues decided to look at methylomic changes in a small group of 39 patients at ultrahigh risk for psychosis conversion. All of these patients (mean age, 22 years) were seen at Saint-Anne Hospital from 2009 to 2013. Using whole blood, Dr. Krebs performed a genomewide DNA methylation study to determine what genes – if any – were differently methylated between the converters and nonconverters. The mean follow-up was 1 year (Mol Psychiatry. 2017 Apr;22[4]:512-8).
Although no significant difference was found in global methylation associated with conversion, Dr. Krebs did find longitudinal changes associated with conversion in three regions.
A cluster of five genes in the glutathione S-transferase family was differently methylated between the converters and nonconverters. Two were related to the GSTM5 promoter gene, which encodes for cytosolic and membrane-bound glutathione S-transferase – an important antioxidant enzyme, the downregulation of which has been implicated in schizophrenia. These two regions appeared to be stable over time, suggesting that methylation occurred before conversion, Dr. Krebs said.
Oxidative stress has been implicated in schizophrenia, and GSTM5 is expressed in the brain, Dr. Krebs noted. Some researchers suggest the gene is involved in dopamine metabolism. It’s also underexpressed in the prefrontal cortex of schizophrenia patients.
Three other regions in the GST family changed with conversion: two on the glutathione S-transferase theta 1 gene and one on the glutathione S-transferase P gene. Since all of these have to do with production of the innate antioxidant glutathione, “these findings suggest a potential use for antioxidant drugs,” Dr. Krebs said.
She found two other differently methylated regions as well.
One was a cluster of eight genes that are all involved in axon guidance – the process by which axons branch out to their correct targets. The second cluster comprised seven genes, all of which are involved in regulating interleukin-17 signaling. This cytokine has been implicated in autoimmune disorders.
Finally, Dr. Krebs performed a transcriptome analysis looking at the brain-expressed messenger RNA in the samples. “The methylome seemed less dynamic than the transcriptome,” she said. “Some methylomic changes may have occurred several months before the conversion, whereas transcriptomic analysis may reflect more rapid changes.”
There was only a 22% concordance between the two analyses. However, the GSTM5 gene and the neuropilin 1 gene – one of those involved in axon guidance – were both methylated and downregulated in the converters. The transcriptome analysis also found significantly decreased expression (although not methylation) of another gene, carnitine palmitoyltransferase 1A. This is a key enzyme in oxidizing long-chain fatty acids and transporting them into the mitochondria.
Adapting these observed differences in gene expression into a useful clinical tool will be challenging, Dr. Krebs said. In addition to large-group validation, any risk prediction model would have to take into account the many other factors that influence psychosis conversion: cerebral and sexual maturation during adolescence, cannabis use, and stress and other completely individual life experiences.
Nevertheless, she concluded, “longitudinal ‘multi-omics’ may be a step toward a future of personalized molecular psychiatry.”
Dr. Krebs had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM WPA 2017
VIDEO: Researchers beginning to explore microbiome’s effect on surgical outcomes
SAN DIEGO – Surgery seems to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical-site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester, Minn. The path is not straightforward because the human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example), can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses. The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical-site infection.
Through diet or other presurgical interventions, Dr. Nelson said in a video interview, it might be possible to optimize the microbiome and reduce the chances of some of these occurrences.
She had no financial disclosures.
On Twitter @Alz_Gal
SAN DIEGO – Surgery seems to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical-site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester, Minn. The path is not straightforward because the human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example), can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses. The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical-site infection.
Through diet or other presurgical interventions, Dr. Nelson said in a video interview, it might be possible to optimize the microbiome and reduce the chances of some of these occurrences.
She had no financial disclosures.
On Twitter @Alz_Gal
SAN DIEGO – Surgery seems to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical-site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester, Minn. The path is not straightforward because the human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example), can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses. The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical-site infection.
Through diet or other presurgical interventions, Dr. Nelson said in a video interview, it might be possible to optimize the microbiome and reduce the chances of some of these occurrences.
She had no financial disclosures.
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
As nations advance economically, mental illnesses exact greater burdens
BERLIN – A vision perceived during America’s Great Depression has come to fruition across the globe, putting mental illness at the center of a devastating web of personal and economic costs.
In the 1930s, the Rockefeller Foundation’s director of medical science, Alan Gregg, MD, distilled an important notion from his decades of travel providing health care and advice to developing nations. As poor countries became richer, infectious diseases that had long ravaged their populations came under control. As people lived longer, however, they became subject to other disorders: chronic age-related illnesses for the old and, for the young, mental illnesses.
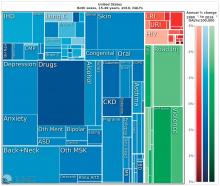
“We have a very, very low rate of infectious disease now, much as the Rockefeller Foundation predicted,” said Dr. Summergrad, the Dr. Frances S. Arkin Professor and chair of psychiatry at Tufts University, Boston. “But, in 2010, the biggest causes of morbidity and disability for U.S. residents aged 15-49 years old were major depressive disorder, dysthymia, drug and alcohol use, schizophrenia, and anxiety. These dwarf the impact of every other illness during that age period. …. They are the burdens of disease of the modern world.”
This shift from infectious disease to mental illness as a primary cause of disability has profound downstream health implications as well, Dr. Summergrad said. Mental disorders that emerge in adolescent and young adulthood are inextricably linked to the chronic diseases that develop in older people.
“Mental and behavioral disturbances are important risk factors for medical conditions that also exact a heavy burden,” he said, referring to a study in JAMA (2013 Aug 14;310[6]:591-608). The report, “The state of U.S. health, 1990-2010: Burden of diseases, injuries, and risk factors,” found that more than half of the of the Top 17 risk factors for morbidity and mortality were directly or indirectly related to mental or behavioral disorders. These included direct causes like alcohol and drug use, and indirect causes that are highly correlated with mental illnesses: physical inactivity, tobacco use, glycemic abnormalities, hypertension, and obesity.
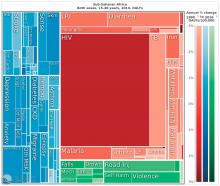
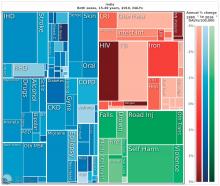
The Institute for Health Metrics and Evaluation at the University of Washington, Seattle, illustrated these global trends in September, with a report published in the Lancet (2017;390:1423-59). Produced in collaboration with the Bill and Melinda Gates Foundation, the report focused on the U.N. Sustainable Development Goals, measured 37 of the 50 health-related SDG indicators from 1990-2016 in 188 countries, and projected the indicators to 2030.
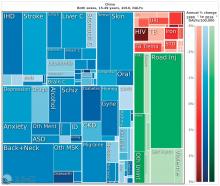
China, on the other hand, looked very much like North America. The proportion of infectious diseases was much smaller than in sub-Saharan Africa or India, a finding Dr. Summergrad attributed to the Chinese government’s post–World War II determination to eradicate communicable diseases.
Unfortunately, Dr. Summergrad said, most countries are ill equipped to handle this shift in the burden of illness. Even in the United States, there are limited mental health hospital beds and a dearth of psychiatrists to handle the burgeoning patient load. And the shift toward higher rates of mental illness will likely continue, at a shocking financial cost.
A Harvard School of Public Health policy report, issued in 2011, paints a stark picture. In 2010, mental illness cost high-income countries about $5.5 trillion in lost income and productivity, narrowly beating out the burden imposed by cardivoascular disease ($5.4 trillion). By 2030, lost wages and productivity tied to mental illiness is expected to cost the United States $7.3 trillion.
“Globally, by 2030, we can expect the direct economic impact of mental illnesses to reach $16 trillion,” Dr. Summergrad said. “We have a limited workforce, limited outpatient facilities, limited hospital beds, and limited money, even here in the U.S. All of this is almost nonexistent in much of the world. The integration of care and workforce and facilities will be a huge challenge as we move forward. We need to think about long-term investment here, much in the same way that the Rockefeller Foundation thought about this in the 1930s.”
Dr. Summergrad had no relevant financial disclosures.
BERLIN – A vision perceived during America’s Great Depression has come to fruition across the globe, putting mental illness at the center of a devastating web of personal and economic costs.
In the 1930s, the Rockefeller Foundation’s director of medical science, Alan Gregg, MD, distilled an important notion from his decades of travel providing health care and advice to developing nations. As poor countries became richer, infectious diseases that had long ravaged their populations came under control. As people lived longer, however, they became subject to other disorders: chronic age-related illnesses for the old and, for the young, mental illnesses.
“We have a very, very low rate of infectious disease now, much as the Rockefeller Foundation predicted,” said Dr. Summergrad, the Dr. Frances S. Arkin Professor and chair of psychiatry at Tufts University, Boston. “But, in 2010, the biggest causes of morbidity and disability for U.S. residents aged 15-49 years old were major depressive disorder, dysthymia, drug and alcohol use, schizophrenia, and anxiety. These dwarf the impact of every other illness during that age period. …. They are the burdens of disease of the modern world.”
This shift from infectious disease to mental illness as a primary cause of disability has profound downstream health implications as well, Dr. Summergrad said. Mental disorders that emerge in adolescent and young adulthood are inextricably linked to the chronic diseases that develop in older people.
“Mental and behavioral disturbances are important risk factors for medical conditions that also exact a heavy burden,” he said, referring to a study in JAMA (2013 Aug 14;310[6]:591-608). The report, “The state of U.S. health, 1990-2010: Burden of diseases, injuries, and risk factors,” found that more than half of the of the Top 17 risk factors for morbidity and mortality were directly or indirectly related to mental or behavioral disorders. These included direct causes like alcohol and drug use, and indirect causes that are highly correlated with mental illnesses: physical inactivity, tobacco use, glycemic abnormalities, hypertension, and obesity.
The Institute for Health Metrics and Evaluation at the University of Washington, Seattle, illustrated these global trends in September, with a report published in the Lancet (2017;390:1423-59). Produced in collaboration with the Bill and Melinda Gates Foundation, the report focused on the U.N. Sustainable Development Goals, measured 37 of the 50 health-related SDG indicators from 1990-2016 in 188 countries, and projected the indicators to 2030.
China, on the other hand, looked very much like North America. The proportion of infectious diseases was much smaller than in sub-Saharan Africa or India, a finding Dr. Summergrad attributed to the Chinese government’s post–World War II determination to eradicate communicable diseases.
Unfortunately, Dr. Summergrad said, most countries are ill equipped to handle this shift in the burden of illness. Even in the United States, there are limited mental health hospital beds and a dearth of psychiatrists to handle the burgeoning patient load. And the shift toward higher rates of mental illness will likely continue, at a shocking financial cost.
A Harvard School of Public Health policy report, issued in 2011, paints a stark picture. In 2010, mental illness cost high-income countries about $5.5 trillion in lost income and productivity, narrowly beating out the burden imposed by cardivoascular disease ($5.4 trillion). By 2030, lost wages and productivity tied to mental illiness is expected to cost the United States $7.3 trillion.
“Globally, by 2030, we can expect the direct economic impact of mental illnesses to reach $16 trillion,” Dr. Summergrad said. “We have a limited workforce, limited outpatient facilities, limited hospital beds, and limited money, even here in the U.S. All of this is almost nonexistent in much of the world. The integration of care and workforce and facilities will be a huge challenge as we move forward. We need to think about long-term investment here, much in the same way that the Rockefeller Foundation thought about this in the 1930s.”
Dr. Summergrad had no relevant financial disclosures.
BERLIN – A vision perceived during America’s Great Depression has come to fruition across the globe, putting mental illness at the center of a devastating web of personal and economic costs.
In the 1930s, the Rockefeller Foundation’s director of medical science, Alan Gregg, MD, distilled an important notion from his decades of travel providing health care and advice to developing nations. As poor countries became richer, infectious diseases that had long ravaged their populations came under control. As people lived longer, however, they became subject to other disorders: chronic age-related illnesses for the old and, for the young, mental illnesses.
“We have a very, very low rate of infectious disease now, much as the Rockefeller Foundation predicted,” said Dr. Summergrad, the Dr. Frances S. Arkin Professor and chair of psychiatry at Tufts University, Boston. “But, in 2010, the biggest causes of morbidity and disability for U.S. residents aged 15-49 years old were major depressive disorder, dysthymia, drug and alcohol use, schizophrenia, and anxiety. These dwarf the impact of every other illness during that age period. …. They are the burdens of disease of the modern world.”
This shift from infectious disease to mental illness as a primary cause of disability has profound downstream health implications as well, Dr. Summergrad said. Mental disorders that emerge in adolescent and young adulthood are inextricably linked to the chronic diseases that develop in older people.
“Mental and behavioral disturbances are important risk factors for medical conditions that also exact a heavy burden,” he said, referring to a study in JAMA (2013 Aug 14;310[6]:591-608). The report, “The state of U.S. health, 1990-2010: Burden of diseases, injuries, and risk factors,” found that more than half of the of the Top 17 risk factors for morbidity and mortality were directly or indirectly related to mental or behavioral disorders. These included direct causes like alcohol and drug use, and indirect causes that are highly correlated with mental illnesses: physical inactivity, tobacco use, glycemic abnormalities, hypertension, and obesity.
The Institute for Health Metrics and Evaluation at the University of Washington, Seattle, illustrated these global trends in September, with a report published in the Lancet (2017;390:1423-59). Produced in collaboration with the Bill and Melinda Gates Foundation, the report focused on the U.N. Sustainable Development Goals, measured 37 of the 50 health-related SDG indicators from 1990-2016 in 188 countries, and projected the indicators to 2030.
China, on the other hand, looked very much like North America. The proportion of infectious diseases was much smaller than in sub-Saharan Africa or India, a finding Dr. Summergrad attributed to the Chinese government’s post–World War II determination to eradicate communicable diseases.
Unfortunately, Dr. Summergrad said, most countries are ill equipped to handle this shift in the burden of illness. Even in the United States, there are limited mental health hospital beds and a dearth of psychiatrists to handle the burgeoning patient load. And the shift toward higher rates of mental illness will likely continue, at a shocking financial cost.
A Harvard School of Public Health policy report, issued in 2011, paints a stark picture. In 2010, mental illness cost high-income countries about $5.5 trillion in lost income and productivity, narrowly beating out the burden imposed by cardivoascular disease ($5.4 trillion). By 2030, lost wages and productivity tied to mental illiness is expected to cost the United States $7.3 trillion.
“Globally, by 2030, we can expect the direct economic impact of mental illnesses to reach $16 trillion,” Dr. Summergrad said. “We have a limited workforce, limited outpatient facilities, limited hospital beds, and limited money, even here in the U.S. All of this is almost nonexistent in much of the world. The integration of care and workforce and facilities will be a huge challenge as we move forward. We need to think about long-term investment here, much in the same way that the Rockefeller Foundation thought about this in the 1930s.”
Dr. Summergrad had no relevant financial disclosures.
EXPERT ANALYSIS FROM WPA 2017
From cells to socioeconomics, meth worsens HIV outcomes
BERLIN – From cellular pathology to socioeconomics, methamphetamine and HIV are a devastating combination.
Either one is enough to ruin a life on its own. But together they can become a fatal ouroboros, Jordi Blanch, MD, said at the meeting of the World Psychiatric Congress. The drug sparks dangerous sexual behavior that ups HIV risk. It increases HIV-vulnerable receptors on immune cells, priming them for viral invasion. It interferes with the metabolism of antiretroviral drugs and grinds medication adherence into the dust.
And even when faced with the facts about these interactions with a serious disease, meth users find it almost impossible to leave the drug behind.
Methamphetamine was once almost exclusively a North American problem, said Dr. Blanch of the University of Barcelona. But in the last decade, the drug has jumped the pond, storming the beaches of Western Europe. Bolstered by imports from Asia, it’s now spreading eastward and down into Africa. Meth is challenging and surpassing alcohol as the drug of choice for HIV high-risk groups (particularly men who have sex with men). Like alcohol, it’s cheap and easy to find. Unlike alcohol, it delivers an incredibly potent, nearly instantaneous brain hit that amps up sexual desire and capacity while decreasing inhibition and executive function.
“When we look at the use of meth in the context of sexual relationships, it’s not hard to understand how it leads to all kinds of sexually transmitted infections, including HIV,” Dr. Blanch said.
A potent dopamine agonist, meth not only increases the neurotransmitter’s release, it blocks reuptake. It reduces the expression of dopamine transporters on the cell surface. At the same time, meth inhibits monoamine oxidase, normally a prime metabolizer. It even creates more dopamine: Methamphetamine increases the activity of tyrosine hydroxylase, the enzyme that catalyzes tyrosine into the dopamine precursor, l-dopa.
The neurologic response to smoking crystal meth – still the most popular way of ingesting the drug – is practically instantaneous. “It’s a very fast and intense euphoric high that, as we know, can have a lot of really bad side effects, like anxiety, restlessness, and even psychosis,” Dr. Blanch said. Its other side effects, though, are what make meth such a potent driver of risky sexual behavior.
“Men who have sex with men use it because it dramatically facilitates sexual functioning. It allows them to have sex for much longer. It decreases pain sensation, so this makes it easier to engage in anal sex, which is likely to be unprotected,” Dr. Blanch said. At the same time, the drug decreases higher-order thinking and increases impulsivity, driving even more behaviors that increase the risk of HIV, including group sex and the use of alcohol and injectable drugs together.
It is not just a cognitive-behavioral problem, though. Animal studies have found some intriguing pathophysiologic links between HIV viral activity and meth.
“Meth actually facilitates the infection,” Dr. Blanch said. “The risk of getting it is much higher, and the risk of it progressing with a high viral load is much higher.”
A 2015 review paper by Ryan Colby Passaro and his associates touches on some of these animal models (J Neuroimmune Pharmacol. 2015 Sep;10[3]:477-86). One of the most intriguing is a mouse study, which found that methamphetamine upregulated the HIV-1 coreceptors, CXCR4 and CCR5, not only on CD4+ T cells, but on monocytes, macrophages, dendritic cells, and, to some extent, astrocytes.
Cat and rhesus monkey data implicated this meth-related effect on CXCR4 and CCR5 as well. But the drug also was implicated in other cellular pathways – all of which serve to make immune cells more vulnerable to HIV attack. These findings support the observation that methamphetamine users with the disease frequently have higher viral loads than nonusers.
After a diagnosis, users may continue to use as a way of avoiding confronting their illness, or even to combat the accompanying physical fatigue, Dr. Blanch said. Like many illicit drug users, meth users often show poor compliance with medical follow-up and poor medication adherence. But even if they do take their antiretroviral medications, methamphetamine still has a way of exerting its power. Ritonavir and cobicistat both inhibit the metabolic pathway that breaks down methamphetamine; using meth with either of those drugs can increase meth concentrations by up to 10-fold, a combination that has killed many patients.
Unfortunately, Dr. Blanch said, it’s terribly difficult for users to give up meth, even in the face of contracting such a serious illness.
“In the beginning, after a diagnosis, they may stop using for a while. But then many start again,” he said. “We see this in study after study. But we have not so many studies on how to treat these patients.”
Trials of antidepressants and antipsychotics, and of replacement therapy with amphetamines or methylphenidate, have had mixed results.
“In my own clinic, we try to explain these problems of the interaction of meth and HIV. We have tried even to motivate our patients to use just on the weekend, for example, but they didn’t accept that,” he said. “Usually, we end up trying to make an agreement that the patient will use as little as possible and let them know how much it interferes with their treatment. But in my clinical experience, it’s not so easy. It’s hard to make any change. … very difficult.”
Dr. Blanch had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
BERLIN – From cellular pathology to socioeconomics, methamphetamine and HIV are a devastating combination.
Either one is enough to ruin a life on its own. But together they can become a fatal ouroboros, Jordi Blanch, MD, said at the meeting of the World Psychiatric Congress. The drug sparks dangerous sexual behavior that ups HIV risk. It increases HIV-vulnerable receptors on immune cells, priming them for viral invasion. It interferes with the metabolism of antiretroviral drugs and grinds medication adherence into the dust.
And even when faced with the facts about these interactions with a serious disease, meth users find it almost impossible to leave the drug behind.
Methamphetamine was once almost exclusively a North American problem, said Dr. Blanch of the University of Barcelona. But in the last decade, the drug has jumped the pond, storming the beaches of Western Europe. Bolstered by imports from Asia, it’s now spreading eastward and down into Africa. Meth is challenging and surpassing alcohol as the drug of choice for HIV high-risk groups (particularly men who have sex with men). Like alcohol, it’s cheap and easy to find. Unlike alcohol, it delivers an incredibly potent, nearly instantaneous brain hit that amps up sexual desire and capacity while decreasing inhibition and executive function.
“When we look at the use of meth in the context of sexual relationships, it’s not hard to understand how it leads to all kinds of sexually transmitted infections, including HIV,” Dr. Blanch said.
A potent dopamine agonist, meth not only increases the neurotransmitter’s release, it blocks reuptake. It reduces the expression of dopamine transporters on the cell surface. At the same time, meth inhibits monoamine oxidase, normally a prime metabolizer. It even creates more dopamine: Methamphetamine increases the activity of tyrosine hydroxylase, the enzyme that catalyzes tyrosine into the dopamine precursor, l-dopa.
The neurologic response to smoking crystal meth – still the most popular way of ingesting the drug – is practically instantaneous. “It’s a very fast and intense euphoric high that, as we know, can have a lot of really bad side effects, like anxiety, restlessness, and even psychosis,” Dr. Blanch said. Its other side effects, though, are what make meth such a potent driver of risky sexual behavior.
“Men who have sex with men use it because it dramatically facilitates sexual functioning. It allows them to have sex for much longer. It decreases pain sensation, so this makes it easier to engage in anal sex, which is likely to be unprotected,” Dr. Blanch said. At the same time, the drug decreases higher-order thinking and increases impulsivity, driving even more behaviors that increase the risk of HIV, including group sex and the use of alcohol and injectable drugs together.
It is not just a cognitive-behavioral problem, though. Animal studies have found some intriguing pathophysiologic links between HIV viral activity and meth.
“Meth actually facilitates the infection,” Dr. Blanch said. “The risk of getting it is much higher, and the risk of it progressing with a high viral load is much higher.”
A 2015 review paper by Ryan Colby Passaro and his associates touches on some of these animal models (J Neuroimmune Pharmacol. 2015 Sep;10[3]:477-86). One of the most intriguing is a mouse study, which found that methamphetamine upregulated the HIV-1 coreceptors, CXCR4 and CCR5, not only on CD4+ T cells, but on monocytes, macrophages, dendritic cells, and, to some extent, astrocytes.
Cat and rhesus monkey data implicated this meth-related effect on CXCR4 and CCR5 as well. But the drug also was implicated in other cellular pathways – all of which serve to make immune cells more vulnerable to HIV attack. These findings support the observation that methamphetamine users with the disease frequently have higher viral loads than nonusers.
After a diagnosis, users may continue to use as a way of avoiding confronting their illness, or even to combat the accompanying physical fatigue, Dr. Blanch said. Like many illicit drug users, meth users often show poor compliance with medical follow-up and poor medication adherence. But even if they do take their antiretroviral medications, methamphetamine still has a way of exerting its power. Ritonavir and cobicistat both inhibit the metabolic pathway that breaks down methamphetamine; using meth with either of those drugs can increase meth concentrations by up to 10-fold, a combination that has killed many patients.
Unfortunately, Dr. Blanch said, it’s terribly difficult for users to give up meth, even in the face of contracting such a serious illness.
“In the beginning, after a diagnosis, they may stop using for a while. But then many start again,” he said. “We see this in study after study. But we have not so many studies on how to treat these patients.”
Trials of antidepressants and antipsychotics, and of replacement therapy with amphetamines or methylphenidate, have had mixed results.
“In my own clinic, we try to explain these problems of the interaction of meth and HIV. We have tried even to motivate our patients to use just on the weekend, for example, but they didn’t accept that,” he said. “Usually, we end up trying to make an agreement that the patient will use as little as possible and let them know how much it interferes with their treatment. But in my clinical experience, it’s not so easy. It’s hard to make any change. … very difficult.”
Dr. Blanch had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
BERLIN – From cellular pathology to socioeconomics, methamphetamine and HIV are a devastating combination.
Either one is enough to ruin a life on its own. But together they can become a fatal ouroboros, Jordi Blanch, MD, said at the meeting of the World Psychiatric Congress. The drug sparks dangerous sexual behavior that ups HIV risk. It increases HIV-vulnerable receptors on immune cells, priming them for viral invasion. It interferes with the metabolism of antiretroviral drugs and grinds medication adherence into the dust.
And even when faced with the facts about these interactions with a serious disease, meth users find it almost impossible to leave the drug behind.
Methamphetamine was once almost exclusively a North American problem, said Dr. Blanch of the University of Barcelona. But in the last decade, the drug has jumped the pond, storming the beaches of Western Europe. Bolstered by imports from Asia, it’s now spreading eastward and down into Africa. Meth is challenging and surpassing alcohol as the drug of choice for HIV high-risk groups (particularly men who have sex with men). Like alcohol, it’s cheap and easy to find. Unlike alcohol, it delivers an incredibly potent, nearly instantaneous brain hit that amps up sexual desire and capacity while decreasing inhibition and executive function.
“When we look at the use of meth in the context of sexual relationships, it’s not hard to understand how it leads to all kinds of sexually transmitted infections, including HIV,” Dr. Blanch said.
A potent dopamine agonist, meth not only increases the neurotransmitter’s release, it blocks reuptake. It reduces the expression of dopamine transporters on the cell surface. At the same time, meth inhibits monoamine oxidase, normally a prime metabolizer. It even creates more dopamine: Methamphetamine increases the activity of tyrosine hydroxylase, the enzyme that catalyzes tyrosine into the dopamine precursor, l-dopa.
The neurologic response to smoking crystal meth – still the most popular way of ingesting the drug – is practically instantaneous. “It’s a very fast and intense euphoric high that, as we know, can have a lot of really bad side effects, like anxiety, restlessness, and even psychosis,” Dr. Blanch said. Its other side effects, though, are what make meth such a potent driver of risky sexual behavior.
“Men who have sex with men use it because it dramatically facilitates sexual functioning. It allows them to have sex for much longer. It decreases pain sensation, so this makes it easier to engage in anal sex, which is likely to be unprotected,” Dr. Blanch said. At the same time, the drug decreases higher-order thinking and increases impulsivity, driving even more behaviors that increase the risk of HIV, including group sex and the use of alcohol and injectable drugs together.
It is not just a cognitive-behavioral problem, though. Animal studies have found some intriguing pathophysiologic links between HIV viral activity and meth.
“Meth actually facilitates the infection,” Dr. Blanch said. “The risk of getting it is much higher, and the risk of it progressing with a high viral load is much higher.”
A 2015 review paper by Ryan Colby Passaro and his associates touches on some of these animal models (J Neuroimmune Pharmacol. 2015 Sep;10[3]:477-86). One of the most intriguing is a mouse study, which found that methamphetamine upregulated the HIV-1 coreceptors, CXCR4 and CCR5, not only on CD4+ T cells, but on monocytes, macrophages, dendritic cells, and, to some extent, astrocytes.
Cat and rhesus monkey data implicated this meth-related effect on CXCR4 and CCR5 as well. But the drug also was implicated in other cellular pathways – all of which serve to make immune cells more vulnerable to HIV attack. These findings support the observation that methamphetamine users with the disease frequently have higher viral loads than nonusers.
After a diagnosis, users may continue to use as a way of avoiding confronting their illness, or even to combat the accompanying physical fatigue, Dr. Blanch said. Like many illicit drug users, meth users often show poor compliance with medical follow-up and poor medication adherence. But even if they do take their antiretroviral medications, methamphetamine still has a way of exerting its power. Ritonavir and cobicistat both inhibit the metabolic pathway that breaks down methamphetamine; using meth with either of those drugs can increase meth concentrations by up to 10-fold, a combination that has killed many patients.
Unfortunately, Dr. Blanch said, it’s terribly difficult for users to give up meth, even in the face of contracting such a serious illness.
“In the beginning, after a diagnosis, they may stop using for a while. But then many start again,” he said. “We see this in study after study. But we have not so many studies on how to treat these patients.”
Trials of antidepressants and antipsychotics, and of replacement therapy with amphetamines or methylphenidate, have had mixed results.
“In my own clinic, we try to explain these problems of the interaction of meth and HIV. We have tried even to motivate our patients to use just on the weekend, for example, but they didn’t accept that,” he said. “Usually, we end up trying to make an agreement that the patient will use as little as possible and let them know how much it interferes with their treatment. But in my clinical experience, it’s not so easy. It’s hard to make any change. … very difficult.”
Dr. Blanch had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM WPA 2017
CDC: Zika-exposed newborns need intensified eye, hearing, and neurological testing
Infants with possible prenatal Zika exposure who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurological evaluation with brain imaging within 1 month of birth, according to new interim guidance set forth by the Centers for Disease Control and Prevention.
The new clinical management guidelines, published in the Oct. 20 issue of the Morbidity and Mortality Weekly Report, supersede the most recent CDC guidance, issued in August 2016. The agency deemed the update necessary after a recent convocation sponsored by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. The meeting drew dozens of practicing clinicians and federal agency representatives, who reviewed the ever-evolving body of knowledge on how to best manage the care of these infants. Since Zika emerged as a public health threat, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, a developing microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
The guidance focuses on three groups: infants with clinical findings of Zika syndrome born to mothers with possible Zika exposure during pregnancy; infants without clinical findings of Zika syndrome whose mothers had lab-confirmed Zika exposure; and infants without symptoms whose mothers might have been exposed, but who did not have laboratory-confirmed infection (MMWR. 2017 Oct 20;66[41]:1089-120).
Infants with clinical findings consistent with Zika syndrome and mothers with possible prenatal Zika exposure
These infants should be tested for Zika virus with serum and urine tests. If those are negative and there is no other apparent cause of the symptoms, they should have a cerebrospinal fluid sample tested for Zika RNA and IgM Zika antibodies.
By 1 month, these infants need a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
A comprehensive neurological exam also is part of the recommendation. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify both obvious and subtle brain abnormalities: cortical thinning, corpus callosum abnormalities, calcifications at the white/gray matter junction, and ventricular enlargement are possible findings.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; this manifests by respiratory distress. Dysphagia that interferes with feeding can develop as well.
The complicated clinical picture calls for a team approach, Dr. Adebanjo said. “The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed.”
Infants without clinical findings, whose mothers have lab-confirmed Zika exposure
Initially, these infants should have the same early head ultrasound, hearing, and eye exams as those who display clinical findings. All of these infants also should be tested for Zika virus in the same way as those with clinical findings.
If tests return a positive result, they should have all the investigations and follow-ups recommended for babies with clinical findings. If lab testing is negative, and clinical findings are normal, Zika infection is highly unlikely and they can receive routine care, although clinicians and parents should be on the lookout for any new symptoms that might suggest postnatal Zika syndrome.
Infants without clinical findings, whose mothers had possible, but unconfirmed, Zika exposure
This is a varied and large group, which includes women who were never tested during pregnancy, as well as those who could have had a false negative test. “Because the latter issue is not easily discerned, all mothers with possible exposure to Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” Dr. Adebanjo said.
CDC does not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether any further evaluations would be helpful. But, Dr. Adebanjo said, “If findings consistent with congenital Zika syndrome are identified at any time, referrals to appropriate specialties should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika.”
CDC also reiterated its special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these remain unchanged from 2016, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis will improve and guidance will be updated.”
No one has yet identified the optimal timing for a Zika diagnostic ultrasound. CDC recommends serial ultrasounds be done every 3-4 weeks for women with lab-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggests that viral shedding into the amniotic fluid might be transient. If the procedure is done for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process is key when making screening decisions that should be individually weighed, Dr. Adebanjo said. “For example, serial ultrasounds might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients. Therefore, these decisions should be individualized.”
Neither Dr. Adebanjo nor any of the coauthors had any financial disclosures.
Infants with possible prenatal Zika exposure who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurological evaluation with brain imaging within 1 month of birth, according to new interim guidance set forth by the Centers for Disease Control and Prevention.
The new clinical management guidelines, published in the Oct. 20 issue of the Morbidity and Mortality Weekly Report, supersede the most recent CDC guidance, issued in August 2016. The agency deemed the update necessary after a recent convocation sponsored by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. The meeting drew dozens of practicing clinicians and federal agency representatives, who reviewed the ever-evolving body of knowledge on how to best manage the care of these infants. Since Zika emerged as a public health threat, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, a developing microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
The guidance focuses on three groups: infants with clinical findings of Zika syndrome born to mothers with possible Zika exposure during pregnancy; infants without clinical findings of Zika syndrome whose mothers had lab-confirmed Zika exposure; and infants without symptoms whose mothers might have been exposed, but who did not have laboratory-confirmed infection (MMWR. 2017 Oct 20;66[41]:1089-120).
Infants with clinical findings consistent with Zika syndrome and mothers with possible prenatal Zika exposure
These infants should be tested for Zika virus with serum and urine tests. If those are negative and there is no other apparent cause of the symptoms, they should have a cerebrospinal fluid sample tested for Zika RNA and IgM Zika antibodies.
By 1 month, these infants need a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
A comprehensive neurological exam also is part of the recommendation. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify both obvious and subtle brain abnormalities: cortical thinning, corpus callosum abnormalities, calcifications at the white/gray matter junction, and ventricular enlargement are possible findings.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; this manifests by respiratory distress. Dysphagia that interferes with feeding can develop as well.
The complicated clinical picture calls for a team approach, Dr. Adebanjo said. “The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed.”
Infants without clinical findings, whose mothers have lab-confirmed Zika exposure
Initially, these infants should have the same early head ultrasound, hearing, and eye exams as those who display clinical findings. All of these infants also should be tested for Zika virus in the same way as those with clinical findings.
If tests return a positive result, they should have all the investigations and follow-ups recommended for babies with clinical findings. If lab testing is negative, and clinical findings are normal, Zika infection is highly unlikely and they can receive routine care, although clinicians and parents should be on the lookout for any new symptoms that might suggest postnatal Zika syndrome.
Infants without clinical findings, whose mothers had possible, but unconfirmed, Zika exposure
This is a varied and large group, which includes women who were never tested during pregnancy, as well as those who could have had a false negative test. “Because the latter issue is not easily discerned, all mothers with possible exposure to Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” Dr. Adebanjo said.
CDC does not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether any further evaluations would be helpful. But, Dr. Adebanjo said, “If findings consistent with congenital Zika syndrome are identified at any time, referrals to appropriate specialties should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika.”
CDC also reiterated its special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these remain unchanged from 2016, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis will improve and guidance will be updated.”
No one has yet identified the optimal timing for a Zika diagnostic ultrasound. CDC recommends serial ultrasounds be done every 3-4 weeks for women with lab-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggests that viral shedding into the amniotic fluid might be transient. If the procedure is done for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process is key when making screening decisions that should be individually weighed, Dr. Adebanjo said. “For example, serial ultrasounds might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients. Therefore, these decisions should be individualized.”
Neither Dr. Adebanjo nor any of the coauthors had any financial disclosures.
Infants with possible prenatal Zika exposure who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurological evaluation with brain imaging within 1 month of birth, according to new interim guidance set forth by the Centers for Disease Control and Prevention.
The new clinical management guidelines, published in the Oct. 20 issue of the Morbidity and Mortality Weekly Report, supersede the most recent CDC guidance, issued in August 2016. The agency deemed the update necessary after a recent convocation sponsored by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. The meeting drew dozens of practicing clinicians and federal agency representatives, who reviewed the ever-evolving body of knowledge on how to best manage the care of these infants. Since Zika emerged as a public health threat, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, a developing microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
The guidance focuses on three groups: infants with clinical findings of Zika syndrome born to mothers with possible Zika exposure during pregnancy; infants without clinical findings of Zika syndrome whose mothers had lab-confirmed Zika exposure; and infants without symptoms whose mothers might have been exposed, but who did not have laboratory-confirmed infection (MMWR. 2017 Oct 20;66[41]:1089-120).
Infants with clinical findings consistent with Zika syndrome and mothers with possible prenatal Zika exposure
These infants should be tested for Zika virus with serum and urine tests. If those are negative and there is no other apparent cause of the symptoms, they should have a cerebrospinal fluid sample tested for Zika RNA and IgM Zika antibodies.
By 1 month, these infants need a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
A comprehensive neurological exam also is part of the recommendation. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify both obvious and subtle brain abnormalities: cortical thinning, corpus callosum abnormalities, calcifications at the white/gray matter junction, and ventricular enlargement are possible findings.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; this manifests by respiratory distress. Dysphagia that interferes with feeding can develop as well.
The complicated clinical picture calls for a team approach, Dr. Adebanjo said. “The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed.”
Infants without clinical findings, whose mothers have lab-confirmed Zika exposure
Initially, these infants should have the same early head ultrasound, hearing, and eye exams as those who display clinical findings. All of these infants also should be tested for Zika virus in the same way as those with clinical findings.
If tests return a positive result, they should have all the investigations and follow-ups recommended for babies with clinical findings. If lab testing is negative, and clinical findings are normal, Zika infection is highly unlikely and they can receive routine care, although clinicians and parents should be on the lookout for any new symptoms that might suggest postnatal Zika syndrome.
Infants without clinical findings, whose mothers had possible, but unconfirmed, Zika exposure
This is a varied and large group, which includes women who were never tested during pregnancy, as well as those who could have had a false negative test. “Because the latter issue is not easily discerned, all mothers with possible exposure to Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” Dr. Adebanjo said.
CDC does not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether any further evaluations would be helpful. But, Dr. Adebanjo said, “If findings consistent with congenital Zika syndrome are identified at any time, referrals to appropriate specialties should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika.”
CDC also reiterated its special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these remain unchanged from 2016, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis will improve and guidance will be updated.”
No one has yet identified the optimal timing for a Zika diagnostic ultrasound. CDC recommends serial ultrasounds be done every 3-4 weeks for women with lab-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggests that viral shedding into the amniotic fluid might be transient. If the procedure is done for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process is key when making screening decisions that should be individually weighed, Dr. Adebanjo said. “For example, serial ultrasounds might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients. Therefore, these decisions should be individualized.”
Neither Dr. Adebanjo nor any of the coauthors had any financial disclosures.
FROM MMWR
Early births stress dads too
BERLIN – The anxiety of a preterm birth affects fathers just as much as it does mothers, significantly increasing depression rates both before and after the baby arrives.
More than one-third of fathers developed depression after their partners were admitted to a hospital with signs of impending preterm labor – similar to the percentage of mothers who experienced depression during that time, Sally Schulze reported at the meeting of the World Psychiatric Association.
The increased prevalence of depression lingered, too, she said. Even at 6 months after the birth, the rate of depression among these men was 2.5 times higher than in the general population.
Ms. Schulze and her colleagues prospectively followed 69 couples in which the woman was admitted to the hospital at high risk of preterm birth. These women had a mean gestational age of 30 weeks and had symptoms of imminent preterm birth: shortening of the cervix, premature rupture of membranes, or active preterm labor. Ms. Schulze compared this group to 49 control couples with no signs of preterm labor, who had come to the hospital to register for a birth at a mean of 35 weeks’ gestation.
The majority of the pregnancies were singletons; there were two twin pregnancies, but no high-order multiples. Couples whose infant died were later excluded from the study.
Both mothers and fathers completed the Edinburg Postnatal Depression Scale at baseline, and at 6 weeks and 6 months after the birth. The survey has been validated for perinatal use. A score of 10 or higher is considered positive for depression.
She divided the preterm birth risk group into two subgroups: couples whose infant was born preterm (26) and couples who made it to term, either by staying in the hospital for treatment and observation, or after being stabilized and released home (27).
Upon admission to the hospital, 35% of the fathers in the preterm birth risk group scored positive for depression, compared with 8% of the fathers in the control group – a significant between-group difference.
“This is especially meaningful when we consider that the background rate of depression among men in Germany is 6%,” Ms. Schulze said. “So our control group fathers were right in line with that, but depression in the preterm birth fathers was significantly elevated.”
At 6 weeks’ postpartum, men in the preterm birth risk group still were experiencing significantly elevated rates of depression, compared with both the control group and the general population. The increase was apparent whether the infant had indeed been born early, or whether it made it to full term (12% and 15%, respectively). Both were significantly higher than the 5% rate among the control group fathers.
“We have to understand that these fathers are now 6 weeks at home with a healthy infant, but they are still experiencing the stress of being exposed to this risk of preterm birth,” Ms. Schulze said.
By 6 months, depression had eased off in fathers whose infants made it to term; at 5%, it was similar to the rate in the control group fathers (4%) and the general population. But many fathers whose babies came early still were experiencing depression (12%).
Ms. Schulze then compared the fathers’ experience to that of the mothers. At baseline, women at risk of preterm birth had exactly the same rate of depression as their partners (35%). However, depression also was elevated among women in the control group (18%). The background rate for depression among German women is 10%, Ms. Schulze said.
At 6 weeks’ postpartum, the timing of birth did not seem to matter as much to the mothers. Depression rates were similarly elevated in those who had a preterm birth and those who did not (25%, 28%). Both were significantly higher than the 17% rate in the control group.
At 6 months, things were leveling out some for these mothers, with depression present in 10% of the preterm birth group, 19% of the full-term birth group, and 13% of the control group.
“Men seem to suffer just as much stress from this experience as women do, although perhaps in a different trajectory,” Ms. Schulze said. “Although there may be different contributing factors, we believe that the psychological care offered to mothers at risk of preterm birth should also be extended to fathers.”
She had no financial disclosures.
[email protected]
On Twitter @alz_gal
BERLIN – The anxiety of a preterm birth affects fathers just as much as it does mothers, significantly increasing depression rates both before and after the baby arrives.
More than one-third of fathers developed depression after their partners were admitted to a hospital with signs of impending preterm labor – similar to the percentage of mothers who experienced depression during that time, Sally Schulze reported at the meeting of the World Psychiatric Association.
The increased prevalence of depression lingered, too, she said. Even at 6 months after the birth, the rate of depression among these men was 2.5 times higher than in the general population.
Ms. Schulze and her colleagues prospectively followed 69 couples in which the woman was admitted to the hospital at high risk of preterm birth. These women had a mean gestational age of 30 weeks and had symptoms of imminent preterm birth: shortening of the cervix, premature rupture of membranes, or active preterm labor. Ms. Schulze compared this group to 49 control couples with no signs of preterm labor, who had come to the hospital to register for a birth at a mean of 35 weeks’ gestation.
The majority of the pregnancies were singletons; there were two twin pregnancies, but no high-order multiples. Couples whose infant died were later excluded from the study.
Both mothers and fathers completed the Edinburg Postnatal Depression Scale at baseline, and at 6 weeks and 6 months after the birth. The survey has been validated for perinatal use. A score of 10 or higher is considered positive for depression.
She divided the preterm birth risk group into two subgroups: couples whose infant was born preterm (26) and couples who made it to term, either by staying in the hospital for treatment and observation, or after being stabilized and released home (27).
Upon admission to the hospital, 35% of the fathers in the preterm birth risk group scored positive for depression, compared with 8% of the fathers in the control group – a significant between-group difference.
“This is especially meaningful when we consider that the background rate of depression among men in Germany is 6%,” Ms. Schulze said. “So our control group fathers were right in line with that, but depression in the preterm birth fathers was significantly elevated.”
At 6 weeks’ postpartum, men in the preterm birth risk group still were experiencing significantly elevated rates of depression, compared with both the control group and the general population. The increase was apparent whether the infant had indeed been born early, or whether it made it to full term (12% and 15%, respectively). Both were significantly higher than the 5% rate among the control group fathers.
“We have to understand that these fathers are now 6 weeks at home with a healthy infant, but they are still experiencing the stress of being exposed to this risk of preterm birth,” Ms. Schulze said.
By 6 months, depression had eased off in fathers whose infants made it to term; at 5%, it was similar to the rate in the control group fathers (4%) and the general population. But many fathers whose babies came early still were experiencing depression (12%).
Ms. Schulze then compared the fathers’ experience to that of the mothers. At baseline, women at risk of preterm birth had exactly the same rate of depression as their partners (35%). However, depression also was elevated among women in the control group (18%). The background rate for depression among German women is 10%, Ms. Schulze said.
At 6 weeks’ postpartum, the timing of birth did not seem to matter as much to the mothers. Depression rates were similarly elevated in those who had a preterm birth and those who did not (25%, 28%). Both were significantly higher than the 17% rate in the control group.
At 6 months, things were leveling out some for these mothers, with depression present in 10% of the preterm birth group, 19% of the full-term birth group, and 13% of the control group.
“Men seem to suffer just as much stress from this experience as women do, although perhaps in a different trajectory,” Ms. Schulze said. “Although there may be different contributing factors, we believe that the psychological care offered to mothers at risk of preterm birth should also be extended to fathers.”
She had no financial disclosures.
[email protected]
On Twitter @alz_gal
BERLIN – The anxiety of a preterm birth affects fathers just as much as it does mothers, significantly increasing depression rates both before and after the baby arrives.
More than one-third of fathers developed depression after their partners were admitted to a hospital with signs of impending preterm labor – similar to the percentage of mothers who experienced depression during that time, Sally Schulze reported at the meeting of the World Psychiatric Association.
The increased prevalence of depression lingered, too, she said. Even at 6 months after the birth, the rate of depression among these men was 2.5 times higher than in the general population.
Ms. Schulze and her colleagues prospectively followed 69 couples in which the woman was admitted to the hospital at high risk of preterm birth. These women had a mean gestational age of 30 weeks and had symptoms of imminent preterm birth: shortening of the cervix, premature rupture of membranes, or active preterm labor. Ms. Schulze compared this group to 49 control couples with no signs of preterm labor, who had come to the hospital to register for a birth at a mean of 35 weeks’ gestation.
The majority of the pregnancies were singletons; there were two twin pregnancies, but no high-order multiples. Couples whose infant died were later excluded from the study.
Both mothers and fathers completed the Edinburg Postnatal Depression Scale at baseline, and at 6 weeks and 6 months after the birth. The survey has been validated for perinatal use. A score of 10 or higher is considered positive for depression.
She divided the preterm birth risk group into two subgroups: couples whose infant was born preterm (26) and couples who made it to term, either by staying in the hospital for treatment and observation, or after being stabilized and released home (27).
Upon admission to the hospital, 35% of the fathers in the preterm birth risk group scored positive for depression, compared with 8% of the fathers in the control group – a significant between-group difference.
“This is especially meaningful when we consider that the background rate of depression among men in Germany is 6%,” Ms. Schulze said. “So our control group fathers were right in line with that, but depression in the preterm birth fathers was significantly elevated.”
At 6 weeks’ postpartum, men in the preterm birth risk group still were experiencing significantly elevated rates of depression, compared with both the control group and the general population. The increase was apparent whether the infant had indeed been born early, or whether it made it to full term (12% and 15%, respectively). Both were significantly higher than the 5% rate among the control group fathers.
“We have to understand that these fathers are now 6 weeks at home with a healthy infant, but they are still experiencing the stress of being exposed to this risk of preterm birth,” Ms. Schulze said.
By 6 months, depression had eased off in fathers whose infants made it to term; at 5%, it was similar to the rate in the control group fathers (4%) and the general population. But many fathers whose babies came early still were experiencing depression (12%).
Ms. Schulze then compared the fathers’ experience to that of the mothers. At baseline, women at risk of preterm birth had exactly the same rate of depression as their partners (35%). However, depression also was elevated among women in the control group (18%). The background rate for depression among German women is 10%, Ms. Schulze said.
At 6 weeks’ postpartum, the timing of birth did not seem to matter as much to the mothers. Depression rates were similarly elevated in those who had a preterm birth and those who did not (25%, 28%). Both were significantly higher than the 17% rate in the control group.
At 6 months, things were leveling out some for these mothers, with depression present in 10% of the preterm birth group, 19% of the full-term birth group, and 13% of the control group.
“Men seem to suffer just as much stress from this experience as women do, although perhaps in a different trajectory,” Ms. Schulze said. “Although there may be different contributing factors, we believe that the psychological care offered to mothers at risk of preterm birth should also be extended to fathers.”
She had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT WPA 2017
Key clinical point:
Major finding: Depression developed in 35% of men whose wives were admitted to the hospital for imminent preterm birth; elevated depression rates lingered for up to 6 months in these fathers.
Data source: The prospective study comprised 69 couples admitted for preterm birth risk, and 49 control couples.
Disclosures: Ms. Schulze had no financial disclosures.
Revisions proposed for sexual behaviors in ICD-11
BERLIN – A move to depathologize paraphilias that do not adversely affect self or others is at the heart of changes suggested for the eleventh revision of the International Classification of Diseases and Related Health Problems.
The upcoming version of the World Health Organization document, slated for release in 2018, would reduce the number of named F65 classifications from nine to five, Richard B. Krueger, MD, said at the meeting of the World Psychiatric Association.
Three behaviors now included – fetishism, fetishistic transvestitism, and sadomasochism – would be dropped altogether, said Dr. Krueger of Columbia University, New York. Others would be streamlined into four more general diagnostic groupings (exhibitionism, voyeurism, pedophilia, and coercive sexual sadism disorderA fifth diagnosis, frotteuristic disorder, would be added in light of its fairly common presentation in clinical practice. Two new general categories would address unspecified behaviors that potentially are criminal, or which cause distress or danger to the individual who performs them, Dr. Krueger said.
The proposed changes would bring the document more in line with the DSM-5, said Dr. Krueger, who also leads the Working Group on the Classification of Sexual Disorders and Sexual Health. WHO charged the committee with reviewing the evidence and making recommendations for categories related to sexuality that are contained in the chapter of Mental and Behavioral Disorders in ICD-10.
This definition parallels the A criterion in the paraphilic definitions in the DSM-5, which identifies a pattern of “recurrent and intense sexual arousal” as well as the B criterion, which specifies that the person “has acted on these sexual urges with a nonconsenting person, or the sexual urges or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.”
The proposed revisions reflect a growing acceptance of the immense variation in human sexual behavior, and the depathologization of some of them, as long as they do not affect public health, said Dr. Krueger, a psychiatrist with expertise in forensic and sexual psychiatry.
“In a general sense, this is analogous to the situation that we faced with alcohol and drug users in the early 20th century, when the main treatment was incarceration. In the 1940s and 50s, if you admitted an alcoholic to the hospital for delirium tremens, you could risk losing your admitting privileges. This is similar to where we are now with the paraphilic disorders,” he said, with most of them still considered potentially criminal.
That paraphilic disorders will remain at all in ICD-11 is something of a compromise.
“At first, we considered outright deletion of this section. Many psychiatrists have suggested we have no business with these disorders, since paraphilias are largely dealt with in the legal system. Some feel that psychiatrists should not be involved with them at all. But there has been a lot of discussion about the public health impact of these behaviors. For example, about 50% of men incarcerated for crimes against children meet the diagnostic criteria for pedophilia, and about 10% of crimes that result in incarceration in the U.S. are sexual crimes. There is also forensic usage of these diagnoses in many countries in Europe, and in Great Britain, the U.S., and Canada, so we felt that it’s important to maintain them” as diagnosable disorders. Keeping official diagnostic codes alive also helps encourage research into epidemiology and potential treatments, he added.
The committee’s first recommendation is to change the name of the chapter from “Disorders of Sexual Preference” to “Paraphilic Disorders.”
“This better represents the content of the section, which involves atypical sexual interests,” Dr. Krueger said. “The word ‘disorders’ was added to clarify that these atypical interests have to be pathological. That is, they must result in action against a nonconsenting individual, or cause severe distress or significant risk of injury or death to the patient.”
This emphasis on a pathological aspect to the diagnoses leads directly to the elimination of the fetishism, transvestitism, and sadomasochism categories, which are considered largely benign. “These have no public health importance, generally no association with distress or functional impairment, and including them can result in stigmatization with no discernible health benefit,” Dr. Krueger said. “If you’re a sadomasochist and not bothered by it, and engage alone or with a consenting person, it’s not a problem. Why should these categories be retained if they are not associated with distress or dysfunction? If they are not, then clearly they are not disorders.”
The remaining diagnoses were not as cut-and-dried, he said. Voyeurism, exhibitionism, pedophilia, and frotteurism clearly can affect others who are either unwilling or unable to consent because of age or other circumstances – for example, children, unaware targets, and animals. These behaviors also present with different intensities, from solitary imaginings to well planned and executed acts.
Sadomasochism did retain an altered diagnostic code as a new entity: coercive sexual sadism disorder. While many practice sadomasochism with a willing partner and are not disturbed by it, the committee acknowledged that crimes of sexual sadism are a serious threat to public health and should be recognized as such.
The committee also added two general categories to cover unnamed paraphilias, of which there are many.
“Other paraphilic disorder involving nonconsenting individuals” may be used for a behavior in which the focus of arousal is unwilling or unable to consent, but not specified. Zoophilia would fall into this category, as would other potentially criminal acts. “We can capture a huge variety of paraphilic behaviors under this rubric,” Dr. Krueger said.
During discussion, some clinicians expressed concern that, in its effort to depathologize solitary behavior, the proposal shortchanges patients who suffer deeply from their sexual behaviors. An example, one British psychiatrist said, is the person who is distressed because he can’t experience sexual enjoyment without a particular object or behavior. The final diagnostic category, “Other paraphilic disorder involving solitary behavior or consenting individuals,” will serve that person’s needs, Dr. Krueger said.
“This is an attempt to address the concern if a behavior markedly distresses a person, outside of the rather normal fear of social rejection, or if the behavior poses a serious risk, such as asphyxiophilia.”
The draft proposal was published earlier this year in Archives of Sexual Behavior (2017 Jul;46[5]:1529-45).
Dr. Krueger had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
BERLIN – A move to depathologize paraphilias that do not adversely affect self or others is at the heart of changes suggested for the eleventh revision of the International Classification of Diseases and Related Health Problems.
The upcoming version of the World Health Organization document, slated for release in 2018, would reduce the number of named F65 classifications from nine to five, Richard B. Krueger, MD, said at the meeting of the World Psychiatric Association.
Three behaviors now included – fetishism, fetishistic transvestitism, and sadomasochism – would be dropped altogether, said Dr. Krueger of Columbia University, New York. Others would be streamlined into four more general diagnostic groupings (exhibitionism, voyeurism, pedophilia, and coercive sexual sadism disorderA fifth diagnosis, frotteuristic disorder, would be added in light of its fairly common presentation in clinical practice. Two new general categories would address unspecified behaviors that potentially are criminal, or which cause distress or danger to the individual who performs them, Dr. Krueger said.
The proposed changes would bring the document more in line with the DSM-5, said Dr. Krueger, who also leads the Working Group on the Classification of Sexual Disorders and Sexual Health. WHO charged the committee with reviewing the evidence and making recommendations for categories related to sexuality that are contained in the chapter of Mental and Behavioral Disorders in ICD-10.
This definition parallels the A criterion in the paraphilic definitions in the DSM-5, which identifies a pattern of “recurrent and intense sexual arousal” as well as the B criterion, which specifies that the person “has acted on these sexual urges with a nonconsenting person, or the sexual urges or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.”
The proposed revisions reflect a growing acceptance of the immense variation in human sexual behavior, and the depathologization of some of them, as long as they do not affect public health, said Dr. Krueger, a psychiatrist with expertise in forensic and sexual psychiatry.
“In a general sense, this is analogous to the situation that we faced with alcohol and drug users in the early 20th century, when the main treatment was incarceration. In the 1940s and 50s, if you admitted an alcoholic to the hospital for delirium tremens, you could risk losing your admitting privileges. This is similar to where we are now with the paraphilic disorders,” he said, with most of them still considered potentially criminal.
That paraphilic disorders will remain at all in ICD-11 is something of a compromise.
“At first, we considered outright deletion of this section. Many psychiatrists have suggested we have no business with these disorders, since paraphilias are largely dealt with in the legal system. Some feel that psychiatrists should not be involved with them at all. But there has been a lot of discussion about the public health impact of these behaviors. For example, about 50% of men incarcerated for crimes against children meet the diagnostic criteria for pedophilia, and about 10% of crimes that result in incarceration in the U.S. are sexual crimes. There is also forensic usage of these diagnoses in many countries in Europe, and in Great Britain, the U.S., and Canada, so we felt that it’s important to maintain them” as diagnosable disorders. Keeping official diagnostic codes alive also helps encourage research into epidemiology and potential treatments, he added.
The committee’s first recommendation is to change the name of the chapter from “Disorders of Sexual Preference” to “Paraphilic Disorders.”
“This better represents the content of the section, which involves atypical sexual interests,” Dr. Krueger said. “The word ‘disorders’ was added to clarify that these atypical interests have to be pathological. That is, they must result in action against a nonconsenting individual, or cause severe distress or significant risk of injury or death to the patient.”
This emphasis on a pathological aspect to the diagnoses leads directly to the elimination of the fetishism, transvestitism, and sadomasochism categories, which are considered largely benign. “These have no public health importance, generally no association with distress or functional impairment, and including them can result in stigmatization with no discernible health benefit,” Dr. Krueger said. “If you’re a sadomasochist and not bothered by it, and engage alone or with a consenting person, it’s not a problem. Why should these categories be retained if they are not associated with distress or dysfunction? If they are not, then clearly they are not disorders.”
The remaining diagnoses were not as cut-and-dried, he said. Voyeurism, exhibitionism, pedophilia, and frotteurism clearly can affect others who are either unwilling or unable to consent because of age or other circumstances – for example, children, unaware targets, and animals. These behaviors also present with different intensities, from solitary imaginings to well planned and executed acts.
Sadomasochism did retain an altered diagnostic code as a new entity: coercive sexual sadism disorder. While many practice sadomasochism with a willing partner and are not disturbed by it, the committee acknowledged that crimes of sexual sadism are a serious threat to public health and should be recognized as such.
The committee also added two general categories to cover unnamed paraphilias, of which there are many.
“Other paraphilic disorder involving nonconsenting individuals” may be used for a behavior in which the focus of arousal is unwilling or unable to consent, but not specified. Zoophilia would fall into this category, as would other potentially criminal acts. “We can capture a huge variety of paraphilic behaviors under this rubric,” Dr. Krueger said.
During discussion, some clinicians expressed concern that, in its effort to depathologize solitary behavior, the proposal shortchanges patients who suffer deeply from their sexual behaviors. An example, one British psychiatrist said, is the person who is distressed because he can’t experience sexual enjoyment without a particular object or behavior. The final diagnostic category, “Other paraphilic disorder involving solitary behavior or consenting individuals,” will serve that person’s needs, Dr. Krueger said.
“This is an attempt to address the concern if a behavior markedly distresses a person, outside of the rather normal fear of social rejection, or if the behavior poses a serious risk, such as asphyxiophilia.”
The draft proposal was published earlier this year in Archives of Sexual Behavior (2017 Jul;46[5]:1529-45).
Dr. Krueger had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
BERLIN – A move to depathologize paraphilias that do not adversely affect self or others is at the heart of changes suggested for the eleventh revision of the International Classification of Diseases and Related Health Problems.
The upcoming version of the World Health Organization document, slated for release in 2018, would reduce the number of named F65 classifications from nine to five, Richard B. Krueger, MD, said at the meeting of the World Psychiatric Association.
Three behaviors now included – fetishism, fetishistic transvestitism, and sadomasochism – would be dropped altogether, said Dr. Krueger of Columbia University, New York. Others would be streamlined into four more general diagnostic groupings (exhibitionism, voyeurism, pedophilia, and coercive sexual sadism disorderA fifth diagnosis, frotteuristic disorder, would be added in light of its fairly common presentation in clinical practice. Two new general categories would address unspecified behaviors that potentially are criminal, or which cause distress or danger to the individual who performs them, Dr. Krueger said.
The proposed changes would bring the document more in line with the DSM-5, said Dr. Krueger, who also leads the Working Group on the Classification of Sexual Disorders and Sexual Health. WHO charged the committee with reviewing the evidence and making recommendations for categories related to sexuality that are contained in the chapter of Mental and Behavioral Disorders in ICD-10.
This definition parallels the A criterion in the paraphilic definitions in the DSM-5, which identifies a pattern of “recurrent and intense sexual arousal” as well as the B criterion, which specifies that the person “has acted on these sexual urges with a nonconsenting person, or the sexual urges or fantasies cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.”
The proposed revisions reflect a growing acceptance of the immense variation in human sexual behavior, and the depathologization of some of them, as long as they do not affect public health, said Dr. Krueger, a psychiatrist with expertise in forensic and sexual psychiatry.
“In a general sense, this is analogous to the situation that we faced with alcohol and drug users in the early 20th century, when the main treatment was incarceration. In the 1940s and 50s, if you admitted an alcoholic to the hospital for delirium tremens, you could risk losing your admitting privileges. This is similar to where we are now with the paraphilic disorders,” he said, with most of them still considered potentially criminal.
That paraphilic disorders will remain at all in ICD-11 is something of a compromise.
“At first, we considered outright deletion of this section. Many psychiatrists have suggested we have no business with these disorders, since paraphilias are largely dealt with in the legal system. Some feel that psychiatrists should not be involved with them at all. But there has been a lot of discussion about the public health impact of these behaviors. For example, about 50% of men incarcerated for crimes against children meet the diagnostic criteria for pedophilia, and about 10% of crimes that result in incarceration in the U.S. are sexual crimes. There is also forensic usage of these diagnoses in many countries in Europe, and in Great Britain, the U.S., and Canada, so we felt that it’s important to maintain them” as diagnosable disorders. Keeping official diagnostic codes alive also helps encourage research into epidemiology and potential treatments, he added.
The committee’s first recommendation is to change the name of the chapter from “Disorders of Sexual Preference” to “Paraphilic Disorders.”
“This better represents the content of the section, which involves atypical sexual interests,” Dr. Krueger said. “The word ‘disorders’ was added to clarify that these atypical interests have to be pathological. That is, they must result in action against a nonconsenting individual, or cause severe distress or significant risk of injury or death to the patient.”
This emphasis on a pathological aspect to the diagnoses leads directly to the elimination of the fetishism, transvestitism, and sadomasochism categories, which are considered largely benign. “These have no public health importance, generally no association with distress or functional impairment, and including them can result in stigmatization with no discernible health benefit,” Dr. Krueger said. “If you’re a sadomasochist and not bothered by it, and engage alone or with a consenting person, it’s not a problem. Why should these categories be retained if they are not associated with distress or dysfunction? If they are not, then clearly they are not disorders.”
The remaining diagnoses were not as cut-and-dried, he said. Voyeurism, exhibitionism, pedophilia, and frotteurism clearly can affect others who are either unwilling or unable to consent because of age or other circumstances – for example, children, unaware targets, and animals. These behaviors also present with different intensities, from solitary imaginings to well planned and executed acts.
Sadomasochism did retain an altered diagnostic code as a new entity: coercive sexual sadism disorder. While many practice sadomasochism with a willing partner and are not disturbed by it, the committee acknowledged that crimes of sexual sadism are a serious threat to public health and should be recognized as such.
The committee also added two general categories to cover unnamed paraphilias, of which there are many.
“Other paraphilic disorder involving nonconsenting individuals” may be used for a behavior in which the focus of arousal is unwilling or unable to consent, but not specified. Zoophilia would fall into this category, as would other potentially criminal acts. “We can capture a huge variety of paraphilic behaviors under this rubric,” Dr. Krueger said.
During discussion, some clinicians expressed concern that, in its effort to depathologize solitary behavior, the proposal shortchanges patients who suffer deeply from their sexual behaviors. An example, one British psychiatrist said, is the person who is distressed because he can’t experience sexual enjoyment without a particular object or behavior. The final diagnostic category, “Other paraphilic disorder involving solitary behavior or consenting individuals,” will serve that person’s needs, Dr. Krueger said.
“This is an attempt to address the concern if a behavior markedly distresses a person, outside of the rather normal fear of social rejection, or if the behavior poses a serious risk, such as asphyxiophilia.”
The draft proposal was published earlier this year in Archives of Sexual Behavior (2017 Jul;46[5]:1529-45).
Dr. Krueger had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT WPA 2017
Bedside imaging finds best PEEP settings
A noninvasive bedside imaging technique can individually calibrate positive end-expiratory pressure settings in patients on extracorporeal membrane oxygenation (ECMO) for severe acute respiratory distress syndrome (ARDS), a study showed.
The step-down PEEP (positive end-expiratory pressure) trial could not identify a single PEEP setting that optimally balanced lung overdistension and lung collapse for all 15 patients. But, electrical impedance tomography (EIT) allowed investigators to individually titrate PEEP settings for each patient, Guillaume Franchineau, MD, wrote (Am J Respir Crit Care Med. 2017;196[4]:447-57. doi: 10.1164/rccm.201605-1055OC).
The 4-month study involved 15 patients (aged, 18-79 years) who were in acute respiratory distress syndrome for a variety of reasons, including influenza (7 patients), pneumonia (3), leukemia (2), and 1 case each of Pneumocystis, antisynthetase syndrome, and trauma. All patients were receiving ECMO with a constant driving pressure of 14 cm H2O. After verifying that the inspiratory flow was 0 at the end of inspiration, PEEP was increased to 20 cm H2O (PEEP 20) with a peak inspiratory pressure of 34 cm H2O. PEEP 20 was held for 20 minutes and then lowered by 5-cm H2O decrements with the potential of reaching PEEP 0.
The EIT device, consisting of a silicone belt with 16 surface electrodes, was placed around the thorax aligning with the sixth intercostal parasternal space and connected to a monitor. By measuring conductivity and impeditivity in the underlying tissues, the device generates a low-resolution, two-dimensional image. The image was sufficient to show lung distension and collapse as the PEEP settings changed. Investigators looked for the best compromise between overdistension and collapsed zones, which they defined as the lowest pressure able to limit EIT-assessed collapse to no more than 15% with the least overdistension.
There was no one-size-fits-all PEEP setting, the authors found. The setting that minimized both overdistension and collapse was PEEP 15 in seven patients, PEEP 10 in six patients, and PEEP 5 in two patients.
At each patient’s optimal PEEP setting, the median tidal volume was similar: 3.8 mL/kg ideal body weight for PEEP 15, 3.9 mL/kg ideal body weight for PEEP 10, and 4.3 mL/kg ideal body weight for PEEP 5.
Respiratory system compliance was also similar among the groups, at 20 mL/cm H2O, 18 mL/cm H2O, and 21 mL/cm H2O, respectively. However, arterial partial pressure of oxygen decreased as the PEEP setting decreased, dropping from 148 mm Hg to 128 mm Hg to 100 mm Hg, respectively. Conversely, arterial partial pressure of CO2 increased (32-41 mm Hg).
EIT also allowed clinicians to pinpoint areas of distension or collapse. As PEEP decreased, there was steady ventilation loss in the medial-dorsal and dorsal regions, which shifted to the medial-ventral and ventral regions.
“Most end-expiratory lung impedances were located in medial-dorsal and medial-ventral regions, whereas the dorsal region constantly contributed less than 10% of total end-expiratory lung impedance,” the authors noted.
“The broad variability of EIT-based best compromise PEEPs in these patients with severe ARDS reinforces the need to provide ventilation settings individually tailored to the regional ARDS-lesion distribution,” they concluded. “To achieve that goal, EIT seems to be an interesting bedside noninvasive tool to provide real-time monitoring of the PEEP effect and ventilation distribution on ECMO.”
Positive PEEP trial, but questions remain
This first study to examine EIT in patients under extracorporeal membrane oxygenation shows important clinical potential, but also raises important questions, Claude Guerin, MD, wrote in an accompanying editorial. (Am J Respir Crit Care Med. doi: 10.1164/rccm.201701-0167ed).
The ability to titrate PEEP settings to a patient’s individual needs could substantially reduce the risk of lung derecruitment or damage by overdistension.
The current study, however, has limitations that must be addressed in the next phase of research, before this technique can be adopted into clinical practice, noted Dr. Guerin, a pulmonologist at the Hospital de la Croix Rousse, Lyon, France. The 5-cm H20 PEEP steps may be too large to detect relevant changes, he said.
In several other studies, PEEP was reduced more gradually in 2- to 3-cm H2O increments. “Surprisingly, PEEP was reduced to 0 cm H2O in this study, with this step maintained for 20 minutes, raising the risk of derecruitment and further stretching once higher PEEP levels were resumed.”
The investigators did not perform any recruitment maneuvers before proceeding with PEEP adjustment. This is contrary to what has been done in prior animal and human studies.
The computation of driving pressure was done without taking total PEEP into account. “As total PEEP is frequently greater than PEEP in patients with [acute respiratory distress syndrome], driving pressure can be overestimated with the common computation.”
The optimal PEEP that the investigators aimed for was determined retrospectively from an offline analysis of the data; this technique would not be suitable for bedside management. “When ‘optimal’ PEEP was defined from [EIT criteria], from a higher PaO2 [arterial partial pressure of oxygen] or from a higher compliance of the respiratory system during the decremental PEEP trial, these three criteria were observed together in only four patients with [acute respiratory distress syndrome].”
The study was done only once and cannot comply with the need for regular PEEP-level assessments over time, as could be done with some other strategies.
“Further studies should also consider taking into account the role of chest wall mechanics,” Dr. Guerin said.
Nevertheless, he concluded, EIT-based PEEP titration for each individual patient represents a prospective tool for assisting with the treatment of acute respiratory distress syndrome, and should be fully investigated in a large, prospective trial.
Dr. Franchineau reported receiving speakers fees from Mapquet. Dr. Guerin had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
A noninvasive bedside imaging technique can individually calibrate positive end-expiratory pressure settings in patients on extracorporeal membrane oxygenation (ECMO) for severe acute respiratory distress syndrome (ARDS), a study showed.
The step-down PEEP (positive end-expiratory pressure) trial could not identify a single PEEP setting that optimally balanced lung overdistension and lung collapse for all 15 patients. But, electrical impedance tomography (EIT) allowed investigators to individually titrate PEEP settings for each patient, Guillaume Franchineau, MD, wrote (Am J Respir Crit Care Med. 2017;196[4]:447-57. doi: 10.1164/rccm.201605-1055OC).
The 4-month study involved 15 patients (aged, 18-79 years) who were in acute respiratory distress syndrome for a variety of reasons, including influenza (7 patients), pneumonia (3), leukemia (2), and 1 case each of Pneumocystis, antisynthetase syndrome, and trauma. All patients were receiving ECMO with a constant driving pressure of 14 cm H2O. After verifying that the inspiratory flow was 0 at the end of inspiration, PEEP was increased to 20 cm H2O (PEEP 20) with a peak inspiratory pressure of 34 cm H2O. PEEP 20 was held for 20 minutes and then lowered by 5-cm H2O decrements with the potential of reaching PEEP 0.
The EIT device, consisting of a silicone belt with 16 surface electrodes, was placed around the thorax aligning with the sixth intercostal parasternal space and connected to a monitor. By measuring conductivity and impeditivity in the underlying tissues, the device generates a low-resolution, two-dimensional image. The image was sufficient to show lung distension and collapse as the PEEP settings changed. Investigators looked for the best compromise between overdistension and collapsed zones, which they defined as the lowest pressure able to limit EIT-assessed collapse to no more than 15% with the least overdistension.
There was no one-size-fits-all PEEP setting, the authors found. The setting that minimized both overdistension and collapse was PEEP 15 in seven patients, PEEP 10 in six patients, and PEEP 5 in two patients.
At each patient’s optimal PEEP setting, the median tidal volume was similar: 3.8 mL/kg ideal body weight for PEEP 15, 3.9 mL/kg ideal body weight for PEEP 10, and 4.3 mL/kg ideal body weight for PEEP 5.
Respiratory system compliance was also similar among the groups, at 20 mL/cm H2O, 18 mL/cm H2O, and 21 mL/cm H2O, respectively. However, arterial partial pressure of oxygen decreased as the PEEP setting decreased, dropping from 148 mm Hg to 128 mm Hg to 100 mm Hg, respectively. Conversely, arterial partial pressure of CO2 increased (32-41 mm Hg).
EIT also allowed clinicians to pinpoint areas of distension or collapse. As PEEP decreased, there was steady ventilation loss in the medial-dorsal and dorsal regions, which shifted to the medial-ventral and ventral regions.
“Most end-expiratory lung impedances were located in medial-dorsal and medial-ventral regions, whereas the dorsal region constantly contributed less than 10% of total end-expiratory lung impedance,” the authors noted.
“The broad variability of EIT-based best compromise PEEPs in these patients with severe ARDS reinforces the need to provide ventilation settings individually tailored to the regional ARDS-lesion distribution,” they concluded. “To achieve that goal, EIT seems to be an interesting bedside noninvasive tool to provide real-time monitoring of the PEEP effect and ventilation distribution on ECMO.”
Positive PEEP trial, but questions remain
This first study to examine EIT in patients under extracorporeal membrane oxygenation shows important clinical potential, but also raises important questions, Claude Guerin, MD, wrote in an accompanying editorial. (Am J Respir Crit Care Med. doi: 10.1164/rccm.201701-0167ed).
The ability to titrate PEEP settings to a patient’s individual needs could substantially reduce the risk of lung derecruitment or damage by overdistension.
The current study, however, has limitations that must be addressed in the next phase of research, before this technique can be adopted into clinical practice, noted Dr. Guerin, a pulmonologist at the Hospital de la Croix Rousse, Lyon, France. The 5-cm H20 PEEP steps may be too large to detect relevant changes, he said.
In several other studies, PEEP was reduced more gradually in 2- to 3-cm H2O increments. “Surprisingly, PEEP was reduced to 0 cm H2O in this study, with this step maintained for 20 minutes, raising the risk of derecruitment and further stretching once higher PEEP levels were resumed.”
The investigators did not perform any recruitment maneuvers before proceeding with PEEP adjustment. This is contrary to what has been done in prior animal and human studies.
The computation of driving pressure was done without taking total PEEP into account. “As total PEEP is frequently greater than PEEP in patients with [acute respiratory distress syndrome], driving pressure can be overestimated with the common computation.”
The optimal PEEP that the investigators aimed for was determined retrospectively from an offline analysis of the data; this technique would not be suitable for bedside management. “When ‘optimal’ PEEP was defined from [EIT criteria], from a higher PaO2 [arterial partial pressure of oxygen] or from a higher compliance of the respiratory system during the decremental PEEP trial, these three criteria were observed together in only four patients with [acute respiratory distress syndrome].”
The study was done only once and cannot comply with the need for regular PEEP-level assessments over time, as could be done with some other strategies.
“Further studies should also consider taking into account the role of chest wall mechanics,” Dr. Guerin said.
Nevertheless, he concluded, EIT-based PEEP titration for each individual patient represents a prospective tool for assisting with the treatment of acute respiratory distress syndrome, and should be fully investigated in a large, prospective trial.
Dr. Franchineau reported receiving speakers fees from Mapquet. Dr. Guerin had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
A noninvasive bedside imaging technique can individually calibrate positive end-expiratory pressure settings in patients on extracorporeal membrane oxygenation (ECMO) for severe acute respiratory distress syndrome (ARDS), a study showed.
The step-down PEEP (positive end-expiratory pressure) trial could not identify a single PEEP setting that optimally balanced lung overdistension and lung collapse for all 15 patients. But, electrical impedance tomography (EIT) allowed investigators to individually titrate PEEP settings for each patient, Guillaume Franchineau, MD, wrote (Am J Respir Crit Care Med. 2017;196[4]:447-57. doi: 10.1164/rccm.201605-1055OC).
The 4-month study involved 15 patients (aged, 18-79 years) who were in acute respiratory distress syndrome for a variety of reasons, including influenza (7 patients), pneumonia (3), leukemia (2), and 1 case each of Pneumocystis, antisynthetase syndrome, and trauma. All patients were receiving ECMO with a constant driving pressure of 14 cm H2O. After verifying that the inspiratory flow was 0 at the end of inspiration, PEEP was increased to 20 cm H2O (PEEP 20) with a peak inspiratory pressure of 34 cm H2O. PEEP 20 was held for 20 minutes and then lowered by 5-cm H2O decrements with the potential of reaching PEEP 0.
The EIT device, consisting of a silicone belt with 16 surface electrodes, was placed around the thorax aligning with the sixth intercostal parasternal space and connected to a monitor. By measuring conductivity and impeditivity in the underlying tissues, the device generates a low-resolution, two-dimensional image. The image was sufficient to show lung distension and collapse as the PEEP settings changed. Investigators looked for the best compromise between overdistension and collapsed zones, which they defined as the lowest pressure able to limit EIT-assessed collapse to no more than 15% with the least overdistension.
There was no one-size-fits-all PEEP setting, the authors found. The setting that minimized both overdistension and collapse was PEEP 15 in seven patients, PEEP 10 in six patients, and PEEP 5 in two patients.
At each patient’s optimal PEEP setting, the median tidal volume was similar: 3.8 mL/kg ideal body weight for PEEP 15, 3.9 mL/kg ideal body weight for PEEP 10, and 4.3 mL/kg ideal body weight for PEEP 5.
Respiratory system compliance was also similar among the groups, at 20 mL/cm H2O, 18 mL/cm H2O, and 21 mL/cm H2O, respectively. However, arterial partial pressure of oxygen decreased as the PEEP setting decreased, dropping from 148 mm Hg to 128 mm Hg to 100 mm Hg, respectively. Conversely, arterial partial pressure of CO2 increased (32-41 mm Hg).
EIT also allowed clinicians to pinpoint areas of distension or collapse. As PEEP decreased, there was steady ventilation loss in the medial-dorsal and dorsal regions, which shifted to the medial-ventral and ventral regions.
“Most end-expiratory lung impedances were located in medial-dorsal and medial-ventral regions, whereas the dorsal region constantly contributed less than 10% of total end-expiratory lung impedance,” the authors noted.
“The broad variability of EIT-based best compromise PEEPs in these patients with severe ARDS reinforces the need to provide ventilation settings individually tailored to the regional ARDS-lesion distribution,” they concluded. “To achieve that goal, EIT seems to be an interesting bedside noninvasive tool to provide real-time monitoring of the PEEP effect and ventilation distribution on ECMO.”
Positive PEEP trial, but questions remain
This first study to examine EIT in patients under extracorporeal membrane oxygenation shows important clinical potential, but also raises important questions, Claude Guerin, MD, wrote in an accompanying editorial. (Am J Respir Crit Care Med. doi: 10.1164/rccm.201701-0167ed).
The ability to titrate PEEP settings to a patient’s individual needs could substantially reduce the risk of lung derecruitment or damage by overdistension.
The current study, however, has limitations that must be addressed in the next phase of research, before this technique can be adopted into clinical practice, noted Dr. Guerin, a pulmonologist at the Hospital de la Croix Rousse, Lyon, France. The 5-cm H20 PEEP steps may be too large to detect relevant changes, he said.
In several other studies, PEEP was reduced more gradually in 2- to 3-cm H2O increments. “Surprisingly, PEEP was reduced to 0 cm H2O in this study, with this step maintained for 20 minutes, raising the risk of derecruitment and further stretching once higher PEEP levels were resumed.”
The investigators did not perform any recruitment maneuvers before proceeding with PEEP adjustment. This is contrary to what has been done in prior animal and human studies.
The computation of driving pressure was done without taking total PEEP into account. “As total PEEP is frequently greater than PEEP in patients with [acute respiratory distress syndrome], driving pressure can be overestimated with the common computation.”
The optimal PEEP that the investigators aimed for was determined retrospectively from an offline analysis of the data; this technique would not be suitable for bedside management. “When ‘optimal’ PEEP was defined from [EIT criteria], from a higher PaO2 [arterial partial pressure of oxygen] or from a higher compliance of the respiratory system during the decremental PEEP trial, these three criteria were observed together in only four patients with [acute respiratory distress syndrome].”
The study was done only once and cannot comply with the need for regular PEEP-level assessments over time, as could be done with some other strategies.
“Further studies should also consider taking into account the role of chest wall mechanics,” Dr. Guerin said.
Nevertheless, he concluded, EIT-based PEEP titration for each individual patient represents a prospective tool for assisting with the treatment of acute respiratory distress syndrome, and should be fully investigated in a large, prospective trial.
Dr. Franchineau reported receiving speakers fees from Mapquet. Dr. Guerin had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
APOE affects tau pathology independent of amyloid-beta
Apolipoprotein E protein isoforms, particularly ApoE4, appear to accelerate brain-wide tau propagation that eventually leads to neuronal injury and death in a manner independent from amyloid-beta, according to findings from transgenic mouse model studies.
“We found ApoE itself, especially ApoE4, was essential to neuronal death,” wrote first author Yang Shi of Washington University, St. Louis, and her colleagues, led by David M. Holtzman, MD, in new research published in Nature. “With pathological tau accumulation, the presence of ApoE, especially ApoE4, may make neurons more susceptible to degeneration, whereas the absence of ApoE may protect neurons from death.”
The new research also illustrates a differential effect between the three APOE alleles. In the team’s in vivo study, tau-expressing mice with the APOE4 allele were most affected, and those with the E3 and E2 versions progressively less so. Mice that didn’t express the human gene at all showed no change in tau and no immune reaction (Nature. 2017;549:523-7).
This new picture of tauopathy – a common feature in Alzheimer’s, frontotemporal dementia, corticobasal degeneration, Pick disease, and progressive supranuclear palsy – suggests an expanded role for ApoE, which until now has been associated mostly with increased amyloid deposition in the Alzheimer’s disease (AD) brain.
“I think this paper is potentially very important, identifying what appear to be strong connections between tau and ApoE that we had no idea about before,” Dr. Wolfe said in an email. “While independent confirmation is needed, this new work is coming from a strong research team that has made other seminal discoveries in the field. Uncovering the molecular basis for ApoE’s effect on tau pathology and glial cell activation may suggest new targets for drug discovery for AD.”
Evidence of ApoE4’s greater impact
To examine ApoE’s effect on tau, the research team bred new lines of genetically modified mice, all of which expressed human tau. Some also expressed human ApoE4, E3, or E2 in place of mouse ApoE. A comparator mouse expressed tau, but not ApoE.
By the time the mice were 9 months old, the tau-E4 strain showed significantly more brain atrophy than did the tau-E3 and tau-E2 strains. The mice who expressed tau but were free of ApoE showed no brain changes.
A closer look showed that atrophy occurred primarily in areas associated with the cognitive changes seen in dementia: the hippocampus, piriform/entorhinal cortex, and amygdala. The ventricles were also enlarged.
“These results revealed an important role of ApoE in regulating tau-mediated neurodegeneration, with ApoE4 causing more severe damage and the absence of ApoE being protective,” the investigators wrote.
The E4/tau tango started early, too, the team noted. At 3 months old, tau-E4 mice had no obvious brain atrophy, but already had significantly more soluble tau than did any of the other strains. By 9 months, when tauopathy was obvious, the tau-E4 mice still had more of the protein, which had shifted from a soluble to an insoluble and hyperphosphorylated state. The tau-E4 mice didn’t appear to be making more tau than the others, though; rather, they were less able to clear it through the neurons’ clearing and recycling system of autophagy.
Drilling down further into the neurons’ pathophysiology, the team found that tau first appeared in the axons of dentate gyrus granule cells in the hippocampus, and then, at an early age, moved into the cell body. Again, there were APOE allele–specific patterns to tau propagation. The team saw four major tau staining patterns, which correlated with the level of brain atrophy. Types 1 and 2, associated with least atrophy, occurred most often in the tau-only, ApoE-negative mice; type 4, associated with the greatest atrophy, occurred most often in the tau-E4 mice.
“The featured distribution of these ... patterns, which either represent different tau structures or progressively more advanced pathological tau stages, indicate ApoE affects either tau conformation or tau pathology progression,” the investigators wrote.
Greatest neurodegeneration seen with ApoE4
Tau-mediated neurodegeneration initiated levels of inflammatory response that also depended on the type of ApoE isoform. Exposure to a culture of damaged neurons and mixed glial cells caused microglia to release a flood of inflammatory cytokines that called in a host of astrocytes to kill damaged tau-E4 neurons en masse, but attacked the tau-E3, tau-E2, and tau-only strains much less. This finding indicates that “ApoE itself was directly involved in inducing neurotoxicity in tau-expressing susceptible neurons,” the team wrote.
Finally, they investigated this model of neurodegeneration in postmortem brain samples of patients with corticobasal degeneration, Pick disease, and progressive supranuclear palsy – the three most common sporadic primary tauopathies. Patients with the E4 allele showed more severe neurodegeneration and a greater interaction of tau pathology and neurodegeneration. Amyloid deposition was associated with less severe neurodegeneration.
Taken together, the findings strongly suggest that the high-risk APOE4 allele is the linchpin that links neuroinflammation to neuronal death in the setting of tau pathology, the investigators concluded.
“The presence of degenerating neurons appeared to further induce neuroinflammation, which was augmented by ApoE4 owing to its inherently higher innate immune reactivity. While activated microglia may be protective to some extent in the setting of amyloid-beta pathology, by targeting plaques and reducing dystrophic neurites, they could be deleterious in tauopathy by directly targeting injured neurons and by activating toxic astrocytes. Enhanced neuroinflammation associated with ApoE4 may further exacerbate neurodegeneration.”
The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
[email protected]
On Twitter @Alz_Gal
The study by Shi et al. represents an important step forward in our understanding of Alzheimer’s disease (AD) pathogenesis and raises another challenge to the amyloid hypothesis: ApoE4 enhances tau pathology independent of amyloid in vivo, in vitro and – possibly – even in humans.
The discovery that the plaques seen in AD brains were composed of amyloid-beta (Abeta) peptide, and the discovery soon thereafter that mutations of genes involved in Abeta production caused dominantly inherited AD, made clear that there was something very important about amyloid and its relationship to AD.
ApoE4 is the next most potent genetic variant to predispose to AD, and much more prevalent than the dominant mutations. When first reported in the early 1990s, the gene was something of a mystery because it did not have an apparent link to amyloid. However, research suggests that ApoE4-mediated effects may be via either amyloid-dependent or amyloid-independent mechanisms. The latter have been particularly championed by investigators at the Gladstone Institute of Neurological Disease who are working to develop a structural modifier of the ApoE4 isoform to prevent the direct toxic effects of the intraneuronal carboxyl fragment that is generated during the production of Abeta. To date, no human trials have been conducted.
Turning to the study by Shi and colleagues, in addition to their convincing mouse and in vitro data, they present data from a collection of human tauopathy brains. These show that ApoE4 was associated with greater tau pathology in patients who died with corticobasal degeneration, Pick disease, and progressive supranuclear palsy. Incidental amyloid deposition in some members of this cohort was not associated with greater tauopathy burden, in contrast to what one might have predicted if Abeta was triggering tau hyperphosphorylation. For clinicians, the human observations are particularly intriguing and deserve replication and further study.
The study of Shi et al. does not invalidate the possibility that amyloid plays a key role in AD pathogenesis. Indeed, that relationship seems firm, since the dominantly inherited AD mutations all affect Abeta production or aggregation. However, it does raise questions as to whether the dominantly inherited cases are representative of the “sporadic” and ApoE4-associated cases. It also raises questions as to the exact role amyloid plays in AD pathogenesis. Abeta toxicity has been amply demonstrated, but that does not necessarily mean that Abeta toxicity is the cause of AD or the driving force behind disease progression.
Clearly, more work is needed to clarify what role amyloid is playing in AD pathogenesis beyond existing toxicity models. Hopefully, the study by Shi et al. will stimulate new models and therapeutic ideas.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic in Scottsdale, Ariz. He also is associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
The study by Shi et al. represents an important step forward in our understanding of Alzheimer’s disease (AD) pathogenesis and raises another challenge to the amyloid hypothesis: ApoE4 enhances tau pathology independent of amyloid in vivo, in vitro and – possibly – even in humans.
The discovery that the plaques seen in AD brains were composed of amyloid-beta (Abeta) peptide, and the discovery soon thereafter that mutations of genes involved in Abeta production caused dominantly inherited AD, made clear that there was something very important about amyloid and its relationship to AD.
ApoE4 is the next most potent genetic variant to predispose to AD, and much more prevalent than the dominant mutations. When first reported in the early 1990s, the gene was something of a mystery because it did not have an apparent link to amyloid. However, research suggests that ApoE4-mediated effects may be via either amyloid-dependent or amyloid-independent mechanisms. The latter have been particularly championed by investigators at the Gladstone Institute of Neurological Disease who are working to develop a structural modifier of the ApoE4 isoform to prevent the direct toxic effects of the intraneuronal carboxyl fragment that is generated during the production of Abeta. To date, no human trials have been conducted.
Turning to the study by Shi and colleagues, in addition to their convincing mouse and in vitro data, they present data from a collection of human tauopathy brains. These show that ApoE4 was associated with greater tau pathology in patients who died with corticobasal degeneration, Pick disease, and progressive supranuclear palsy. Incidental amyloid deposition in some members of this cohort was not associated with greater tauopathy burden, in contrast to what one might have predicted if Abeta was triggering tau hyperphosphorylation. For clinicians, the human observations are particularly intriguing and deserve replication and further study.
The study of Shi et al. does not invalidate the possibility that amyloid plays a key role in AD pathogenesis. Indeed, that relationship seems firm, since the dominantly inherited AD mutations all affect Abeta production or aggregation. However, it does raise questions as to whether the dominantly inherited cases are representative of the “sporadic” and ApoE4-associated cases. It also raises questions as to the exact role amyloid plays in AD pathogenesis. Abeta toxicity has been amply demonstrated, but that does not necessarily mean that Abeta toxicity is the cause of AD or the driving force behind disease progression.
Clearly, more work is needed to clarify what role amyloid is playing in AD pathogenesis beyond existing toxicity models. Hopefully, the study by Shi et al. will stimulate new models and therapeutic ideas.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic in Scottsdale, Ariz. He also is associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
The study by Shi et al. represents an important step forward in our understanding of Alzheimer’s disease (AD) pathogenesis and raises another challenge to the amyloid hypothesis: ApoE4 enhances tau pathology independent of amyloid in vivo, in vitro and – possibly – even in humans.
The discovery that the plaques seen in AD brains were composed of amyloid-beta (Abeta) peptide, and the discovery soon thereafter that mutations of genes involved in Abeta production caused dominantly inherited AD, made clear that there was something very important about amyloid and its relationship to AD.
ApoE4 is the next most potent genetic variant to predispose to AD, and much more prevalent than the dominant mutations. When first reported in the early 1990s, the gene was something of a mystery because it did not have an apparent link to amyloid. However, research suggests that ApoE4-mediated effects may be via either amyloid-dependent or amyloid-independent mechanisms. The latter have been particularly championed by investigators at the Gladstone Institute of Neurological Disease who are working to develop a structural modifier of the ApoE4 isoform to prevent the direct toxic effects of the intraneuronal carboxyl fragment that is generated during the production of Abeta. To date, no human trials have been conducted.
Turning to the study by Shi and colleagues, in addition to their convincing mouse and in vitro data, they present data from a collection of human tauopathy brains. These show that ApoE4 was associated with greater tau pathology in patients who died with corticobasal degeneration, Pick disease, and progressive supranuclear palsy. Incidental amyloid deposition in some members of this cohort was not associated with greater tauopathy burden, in contrast to what one might have predicted if Abeta was triggering tau hyperphosphorylation. For clinicians, the human observations are particularly intriguing and deserve replication and further study.
The study of Shi et al. does not invalidate the possibility that amyloid plays a key role in AD pathogenesis. Indeed, that relationship seems firm, since the dominantly inherited AD mutations all affect Abeta production or aggregation. However, it does raise questions as to whether the dominantly inherited cases are representative of the “sporadic” and ApoE4-associated cases. It also raises questions as to the exact role amyloid plays in AD pathogenesis. Abeta toxicity has been amply demonstrated, but that does not necessarily mean that Abeta toxicity is the cause of AD or the driving force behind disease progression.
Clearly, more work is needed to clarify what role amyloid is playing in AD pathogenesis beyond existing toxicity models. Hopefully, the study by Shi et al. will stimulate new models and therapeutic ideas.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic in Scottsdale, Ariz. He also is associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Apolipoprotein E protein isoforms, particularly ApoE4, appear to accelerate brain-wide tau propagation that eventually leads to neuronal injury and death in a manner independent from amyloid-beta, according to findings from transgenic mouse model studies.
“We found ApoE itself, especially ApoE4, was essential to neuronal death,” wrote first author Yang Shi of Washington University, St. Louis, and her colleagues, led by David M. Holtzman, MD, in new research published in Nature. “With pathological tau accumulation, the presence of ApoE, especially ApoE4, may make neurons more susceptible to degeneration, whereas the absence of ApoE may protect neurons from death.”
The new research also illustrates a differential effect between the three APOE alleles. In the team’s in vivo study, tau-expressing mice with the APOE4 allele were most affected, and those with the E3 and E2 versions progressively less so. Mice that didn’t express the human gene at all showed no change in tau and no immune reaction (Nature. 2017;549:523-7).
This new picture of tauopathy – a common feature in Alzheimer’s, frontotemporal dementia, corticobasal degeneration, Pick disease, and progressive supranuclear palsy – suggests an expanded role for ApoE, which until now has been associated mostly with increased amyloid deposition in the Alzheimer’s disease (AD) brain.
“I think this paper is potentially very important, identifying what appear to be strong connections between tau and ApoE that we had no idea about before,” Dr. Wolfe said in an email. “While independent confirmation is needed, this new work is coming from a strong research team that has made other seminal discoveries in the field. Uncovering the molecular basis for ApoE’s effect on tau pathology and glial cell activation may suggest new targets for drug discovery for AD.”
Evidence of ApoE4’s greater impact
To examine ApoE’s effect on tau, the research team bred new lines of genetically modified mice, all of which expressed human tau. Some also expressed human ApoE4, E3, or E2 in place of mouse ApoE. A comparator mouse expressed tau, but not ApoE.
By the time the mice were 9 months old, the tau-E4 strain showed significantly more brain atrophy than did the tau-E3 and tau-E2 strains. The mice who expressed tau but were free of ApoE showed no brain changes.
A closer look showed that atrophy occurred primarily in areas associated with the cognitive changes seen in dementia: the hippocampus, piriform/entorhinal cortex, and amygdala. The ventricles were also enlarged.
“These results revealed an important role of ApoE in regulating tau-mediated neurodegeneration, with ApoE4 causing more severe damage and the absence of ApoE being protective,” the investigators wrote.
The E4/tau tango started early, too, the team noted. At 3 months old, tau-E4 mice had no obvious brain atrophy, but already had significantly more soluble tau than did any of the other strains. By 9 months, when tauopathy was obvious, the tau-E4 mice still had more of the protein, which had shifted from a soluble to an insoluble and hyperphosphorylated state. The tau-E4 mice didn’t appear to be making more tau than the others, though; rather, they were less able to clear it through the neurons’ clearing and recycling system of autophagy.
Drilling down further into the neurons’ pathophysiology, the team found that tau first appeared in the axons of dentate gyrus granule cells in the hippocampus, and then, at an early age, moved into the cell body. Again, there were APOE allele–specific patterns to tau propagation. The team saw four major tau staining patterns, which correlated with the level of brain atrophy. Types 1 and 2, associated with least atrophy, occurred most often in the tau-only, ApoE-negative mice; type 4, associated with the greatest atrophy, occurred most often in the tau-E4 mice.
“The featured distribution of these ... patterns, which either represent different tau structures or progressively more advanced pathological tau stages, indicate ApoE affects either tau conformation or tau pathology progression,” the investigators wrote.
Greatest neurodegeneration seen with ApoE4
Tau-mediated neurodegeneration initiated levels of inflammatory response that also depended on the type of ApoE isoform. Exposure to a culture of damaged neurons and mixed glial cells caused microglia to release a flood of inflammatory cytokines that called in a host of astrocytes to kill damaged tau-E4 neurons en masse, but attacked the tau-E3, tau-E2, and tau-only strains much less. This finding indicates that “ApoE itself was directly involved in inducing neurotoxicity in tau-expressing susceptible neurons,” the team wrote.
Finally, they investigated this model of neurodegeneration in postmortem brain samples of patients with corticobasal degeneration, Pick disease, and progressive supranuclear palsy – the three most common sporadic primary tauopathies. Patients with the E4 allele showed more severe neurodegeneration and a greater interaction of tau pathology and neurodegeneration. Amyloid deposition was associated with less severe neurodegeneration.
Taken together, the findings strongly suggest that the high-risk APOE4 allele is the linchpin that links neuroinflammation to neuronal death in the setting of tau pathology, the investigators concluded.
“The presence of degenerating neurons appeared to further induce neuroinflammation, which was augmented by ApoE4 owing to its inherently higher innate immune reactivity. While activated microglia may be protective to some extent in the setting of amyloid-beta pathology, by targeting plaques and reducing dystrophic neurites, they could be deleterious in tauopathy by directly targeting injured neurons and by activating toxic astrocytes. Enhanced neuroinflammation associated with ApoE4 may further exacerbate neurodegeneration.”
The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
[email protected]
On Twitter @Alz_Gal
Apolipoprotein E protein isoforms, particularly ApoE4, appear to accelerate brain-wide tau propagation that eventually leads to neuronal injury and death in a manner independent from amyloid-beta, according to findings from transgenic mouse model studies.
“We found ApoE itself, especially ApoE4, was essential to neuronal death,” wrote first author Yang Shi of Washington University, St. Louis, and her colleagues, led by David M. Holtzman, MD, in new research published in Nature. “With pathological tau accumulation, the presence of ApoE, especially ApoE4, may make neurons more susceptible to degeneration, whereas the absence of ApoE may protect neurons from death.”
The new research also illustrates a differential effect between the three APOE alleles. In the team’s in vivo study, tau-expressing mice with the APOE4 allele were most affected, and those with the E3 and E2 versions progressively less so. Mice that didn’t express the human gene at all showed no change in tau and no immune reaction (Nature. 2017;549:523-7).
This new picture of tauopathy – a common feature in Alzheimer’s, frontotemporal dementia, corticobasal degeneration, Pick disease, and progressive supranuclear palsy – suggests an expanded role for ApoE, which until now has been associated mostly with increased amyloid deposition in the Alzheimer’s disease (AD) brain.
“I think this paper is potentially very important, identifying what appear to be strong connections between tau and ApoE that we had no idea about before,” Dr. Wolfe said in an email. “While independent confirmation is needed, this new work is coming from a strong research team that has made other seminal discoveries in the field. Uncovering the molecular basis for ApoE’s effect on tau pathology and glial cell activation may suggest new targets for drug discovery for AD.”
Evidence of ApoE4’s greater impact
To examine ApoE’s effect on tau, the research team bred new lines of genetically modified mice, all of which expressed human tau. Some also expressed human ApoE4, E3, or E2 in place of mouse ApoE. A comparator mouse expressed tau, but not ApoE.
By the time the mice were 9 months old, the tau-E4 strain showed significantly more brain atrophy than did the tau-E3 and tau-E2 strains. The mice who expressed tau but were free of ApoE showed no brain changes.
A closer look showed that atrophy occurred primarily in areas associated with the cognitive changes seen in dementia: the hippocampus, piriform/entorhinal cortex, and amygdala. The ventricles were also enlarged.
“These results revealed an important role of ApoE in regulating tau-mediated neurodegeneration, with ApoE4 causing more severe damage and the absence of ApoE being protective,” the investigators wrote.
The E4/tau tango started early, too, the team noted. At 3 months old, tau-E4 mice had no obvious brain atrophy, but already had significantly more soluble tau than did any of the other strains. By 9 months, when tauopathy was obvious, the tau-E4 mice still had more of the protein, which had shifted from a soluble to an insoluble and hyperphosphorylated state. The tau-E4 mice didn’t appear to be making more tau than the others, though; rather, they were less able to clear it through the neurons’ clearing and recycling system of autophagy.
Drilling down further into the neurons’ pathophysiology, the team found that tau first appeared in the axons of dentate gyrus granule cells in the hippocampus, and then, at an early age, moved into the cell body. Again, there were APOE allele–specific patterns to tau propagation. The team saw four major tau staining patterns, which correlated with the level of brain atrophy. Types 1 and 2, associated with least atrophy, occurred most often in the tau-only, ApoE-negative mice; type 4, associated with the greatest atrophy, occurred most often in the tau-E4 mice.
“The featured distribution of these ... patterns, which either represent different tau structures or progressively more advanced pathological tau stages, indicate ApoE affects either tau conformation or tau pathology progression,” the investigators wrote.
Greatest neurodegeneration seen with ApoE4
Tau-mediated neurodegeneration initiated levels of inflammatory response that also depended on the type of ApoE isoform. Exposure to a culture of damaged neurons and mixed glial cells caused microglia to release a flood of inflammatory cytokines that called in a host of astrocytes to kill damaged tau-E4 neurons en masse, but attacked the tau-E3, tau-E2, and tau-only strains much less. This finding indicates that “ApoE itself was directly involved in inducing neurotoxicity in tau-expressing susceptible neurons,” the team wrote.
Finally, they investigated this model of neurodegeneration in postmortem brain samples of patients with corticobasal degeneration, Pick disease, and progressive supranuclear palsy – the three most common sporadic primary tauopathies. Patients with the E4 allele showed more severe neurodegeneration and a greater interaction of tau pathology and neurodegeneration. Amyloid deposition was associated with less severe neurodegeneration.
Taken together, the findings strongly suggest that the high-risk APOE4 allele is the linchpin that links neuroinflammation to neuronal death in the setting of tau pathology, the investigators concluded.
“The presence of degenerating neurons appeared to further induce neuroinflammation, which was augmented by ApoE4 owing to its inherently higher innate immune reactivity. While activated microglia may be protective to some extent in the setting of amyloid-beta pathology, by targeting plaques and reducing dystrophic neurites, they could be deleterious in tauopathy by directly targeting injured neurons and by activating toxic astrocytes. Enhanced neuroinflammation associated with ApoE4 may further exacerbate neurodegeneration.”
The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
[email protected]
On Twitter @Alz_Gal
FROM NATURE
Key clinical point:
Major finding: The presence of the e4 allele was associated with brain atrophy and neurodegeneration in a transgenic mouse model, while the absence of any APOE allele was neuroprotective.
Data source: The in vivo study employed transgenic mice that expressed the human tau protein along with human ApoE variants.
Disclosures: The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
VIDEO: Keep index of suspicion high for groups at risk of suicide and substance abuse
WASHINGTON – , according to Alex Crosby, MD, senior adviser at the Centers for Disease Control and Prevention’s division of violence prevention at the National Center for Injury Prevention and Control.
Suicides increased by 25% during 2000-2015, and drug deaths – most notably, opioid-related deaths – have quadrupled since 1999. These increases strike the same groups: men, working-age adults, whites, Native Americans and Alaskan natives, and rural communities.
So consistent are the overlaps that primary care providers should view these demographics as red flags when such patients present with depression or pain complaints, Dr. Crosby said in an interview at an event sponsored by the Education Development Center and the National Alliance for Suicide Prevention. Vigilance in primary care settings is especially important, because many patients don’t have access to behavioral health specialists.
“As clinicians, we need to have a high index of suspicion, know what questions to ask, and to go a little bit deeper, beyond the presenting complaint,” he said. “Often, people with psychiatric illnesses [that predispose them to suicide and substance abuse] are going to their primary care provider first, because they don’t have access to behavioral health services. The family medicine practitioner, the internist, even the pediatrician, have to be aware of this. And clinicians need to have index of suspicion, and ask questions that go a little deeper than the presenting complaint was.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – , according to Alex Crosby, MD, senior adviser at the Centers for Disease Control and Prevention’s division of violence prevention at the National Center for Injury Prevention and Control.
Suicides increased by 25% during 2000-2015, and drug deaths – most notably, opioid-related deaths – have quadrupled since 1999. These increases strike the same groups: men, working-age adults, whites, Native Americans and Alaskan natives, and rural communities.
So consistent are the overlaps that primary care providers should view these demographics as red flags when such patients present with depression or pain complaints, Dr. Crosby said in an interview at an event sponsored by the Education Development Center and the National Alliance for Suicide Prevention. Vigilance in primary care settings is especially important, because many patients don’t have access to behavioral health specialists.
“As clinicians, we need to have a high index of suspicion, know what questions to ask, and to go a little bit deeper, beyond the presenting complaint,” he said. “Often, people with psychiatric illnesses [that predispose them to suicide and substance abuse] are going to their primary care provider first, because they don’t have access to behavioral health services. The family medicine practitioner, the internist, even the pediatrician, have to be aware of this. And clinicians need to have index of suspicion, and ask questions that go a little deeper than the presenting complaint was.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – , according to Alex Crosby, MD, senior adviser at the Centers for Disease Control and Prevention’s division of violence prevention at the National Center for Injury Prevention and Control.
Suicides increased by 25% during 2000-2015, and drug deaths – most notably, opioid-related deaths – have quadrupled since 1999. These increases strike the same groups: men, working-age adults, whites, Native Americans and Alaskan natives, and rural communities.
So consistent are the overlaps that primary care providers should view these demographics as red flags when such patients present with depression or pain complaints, Dr. Crosby said in an interview at an event sponsored by the Education Development Center and the National Alliance for Suicide Prevention. Vigilance in primary care settings is especially important, because many patients don’t have access to behavioral health specialists.
“As clinicians, we need to have a high index of suspicion, know what questions to ask, and to go a little bit deeper, beyond the presenting complaint,” he said. “Often, people with psychiatric illnesses [that predispose them to suicide and substance abuse] are going to their primary care provider first, because they don’t have access to behavioral health services. The family medicine practitioner, the internist, even the pediatrician, have to be aware of this. And clinicians need to have index of suspicion, and ask questions that go a little deeper than the presenting complaint was.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AN EXPERT PANEL ON SUICIDE AND OPIOID DEATHS